- Department of Obstetrics and Gynecology, Fertility Center of CHA Gangnam Medical Center, CHA University School of Medicine, Seoul, South Korea
Despite the large number of studies on blastocyst transfers, it is unclear whether day 6 blastocysts have similar pregnancy rates and safety with day 5 blastocysts. Thus, this study aimed to compare the obstetric, neonatal, and clinical outcomes of day 5 and day 6 vitrified blastocyst transfers (VBT). In this retrospective cohort study with propensity score matching, we evaluated 1,313 cycles of VBT performed between January 2014 and December 2015 at the Fertility Center of CHA Gangnam Medical Center. All cycles underwent natural endometrial preparation. We used propensity score matching to compare day 5 and day 6 VBTs in a matched comparison. After propensity score matching, there were 465 cycles of day 5 VBT and 155 cycles of day 6 VBT. Implantation rate (IR), clinical pregnancy rate (CPR), and live birth rate (LBR) were significantly lower in day 6 VBTs (44.2 vs. 53.1%, p = 0.023; 48.4 vs. 60.4%, p = 0.009; 33.5 vs. 51.8%, p < 0.001). Miscarriage rate was significantly higher in day 6 VBTs (29.3 vs. 10.7%, p < 0.001). Rate of multiple gestations was similar between the two groups (29.3 vs. 30.2%, p = 0.816). Assessing 241 and 52 babies from day 5 and day 6 VBTs, no differences were found in neonatal outcomes including rates of low birth weight, preterm birth, and congenital malformations. In propensity score-matched analysis, obstetric, and neonatal outcomes between day 5 and day 6 VBTs were similar so that day 6 VBTs are as safe as day 5 VBTs. IR, CPR, and LBR were are all significantly lower in day 6 VBTs. Therefore, if there are no differences in the morphological grade between day 5 and day 6 blastocysts, transfer of day 5 vitrified blastocysts should be considered first.
Introduction
As techniques for in vitro fertilization (IVF) and embryo culture have become advanced, many IVF centers can transfer embryos at the blastocyst stage. Some embryos have reached blastocyst stage by day 5 and others not until day 6 or even day 7. Recently, a study reported that the blastulation rate was 66% on day 5, 29% on day 6, and 6% on day 7 (1). Compared with normally growing embryos, there were increased number of abnormal mitotic spindle (2), decreased expression of mitotic spindle (3), and more molecular abnormalities (4) in growth-retarded embryos. These phenomena have raised the question: Does blastocysts with a delayed blastulation maintain acceptable pregnancy rates with safety?
To answer this question, several studies have compared IVF outcomes of day 5 and day 6 blastocyst embryo transfers. However, results of these studies are conflicting. In fresh IVF cycles, many studies suggest that day 5 blastocysts give rise to higher pregnancy rates than day 6 blastocysts (5–8). Theoretically, controlled ovarian hyperstimulation advances endometrial maturation by 1–2.5 days compared with the expected chronological date from oocyte retrieval. It causes asynchronous uterine environment with poor endometrial receptivity and may decrease pregnancy rates (9–11). However, it is difficult to determine whether poor endometrial receptivity is due to impaired embryo quality of day 6 blastocysts or asynchronous uterine environment with poor endometrial receptivity (5, 12).
Therefore, most available studies explore frozen-thawed blastocyst transfer (FBT) cycles or vitrified-warmed blastocyst transfer (VBT) cycles that have a more synchronized endometrium using artificial or medicated endometrial preparations. However, these studies also have conflicting results. Some studies suggest that day 6 blastocysts have comparable clinical outcomes with day 5 blastocysts (5, 13–18), while other studies suggest that clinical outcomes are better with day 5 blastocysts (19–26).
Despite the large number of studies on this field, it is unclear whether day 6 blastocysts have similar pregnancy rates and safety with day 5 blastocysts. Above all, no well-matched cohort study has been conducted yet. Therefore, in this study, we aimed to compare pregnancy and neonatal outcomes of day 5 and day 6 blastocysts in VBT cycles with propensity-score matching.
Materials and Methods
Patient Characteristics
We performed a retrospective cohort study to evaluate the outcomes of 1,313 VBT cycles of women under 40 years between January 2014 and December 2015 at the Fertility Center of CHA Gangnam Medical Center. Pregnancy outcomes, including neonatal data, are recorded continuously in the CHA Gangnam Medical Center database. In cases of missing data, telephone surveys were conducted.
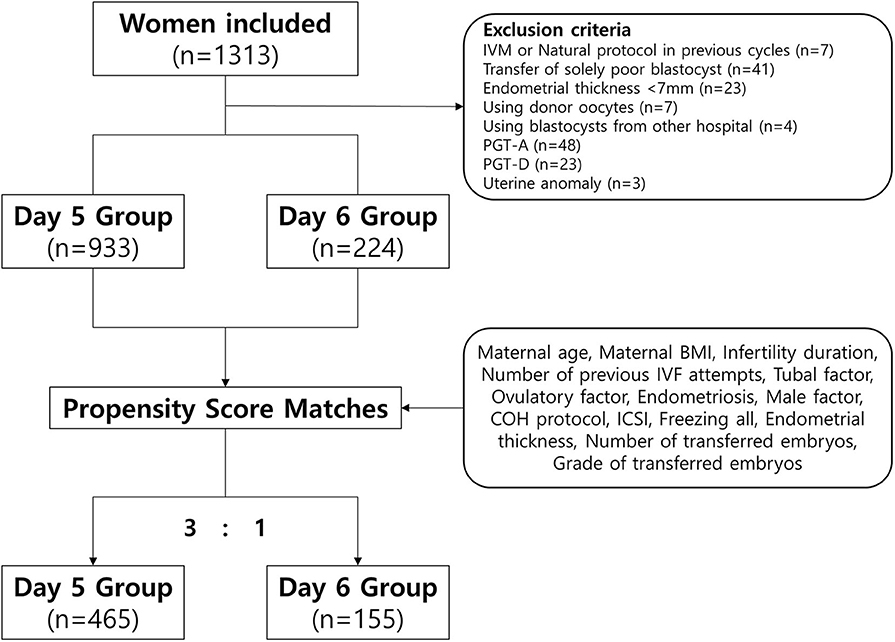
Among 1,313 VBT cycles, we excluded women who underwent VBT using donor oocytes, a single poor-quality blastocyst, blastocysts that underwent a preimplantation genetic test (PGT), blastocysts from other IVF centers, and those who underwent a natural protocol, modified natural protocol, or in vitro maturation protocol in previous fresh cycles. We also excluded women who had a thin endometrium (<7 mm) or uterine anomalies. Finally, we included 1,157 VBT cycles (Figure 1).
All VBT cycles were divided into two groups according to the day of blastulation: day 5 group (blastulation on day 5 of culture) and day 6 group (blastulation on day 6 of culture). The study was approved by the Institutional Review Board of CHA Gangnam Medical Center (IRB approval number: GCI 18–30). Informed consent was waived because of the retrospective study design.
Embryo Culture and Grading
To suppress ovulation until follicle maturity was attained, patients were treated with either a gonadotropin-releasing hormone (GnRH) agonist or a GnRH antagonist. The final follicular maturation was triggered with human chorionic gonadotropin or a GnRH agonist when the mean diameter at least two leading (largest and second largest) follicles was 18 mm. Oocytes were retrieved 36 h later by transvaginal ultrasound-guided needle aspiration of follicles.
Conventional IVF or intracytoplasmic sperm injection (ICSI) was used for embryo fertilization. Fertilization was confirmed when two pronuclear (2PN) zygotes were observed after 16–18 h in ICSI and 18–20 h in conventional IVF. Cleavage-stage embryos were cultured in a cleavage medium (Cook, Queensland, Australia), while blastocyst-stage embryos were cultured on a blastocyst medium (Cook). Embryos were cultured in HERA cell 240 incubators (Thermo Fisher Scientific, MA, USA) in an environment with 5% O2, 6% CO2, at 37°C. The oil-drop culture method was applied using a four-well dish (NUNC™, Thermo Fisher Scientific). At 10-μl drops, the embryos were cultured individually to observe their development. Then, light paraffin oil (OVOIL, Vitrolife AB, Sweden) was dropped onto the media to prevent the medium from drying and undergoing fast pH change.
Blastocysts were morphologically graded according to the Gardner classification, which takes into its expansion, inner cell mass (ICM) constitution, and trophectoderm composition prior to freezing on day 5 or 6 (27). Blastocysts had good quality if all of the following criteria were met: the blastocyst expanded and filled the embryo completely (grade 3), the ICM was composed of several loosely grouped cells (grade B), and the trophectoderm contained few cells that formed loose epithelium (grade B). Blastocysts had poor quality if any of these criteria were not met.
Vitrification and Warming of Blastocysts
For blastocysts vitrification, artificial shrinkage was performed on all blastocysts, and assisted hatching was facilitated with a laser. The blastocysts were pre-equilibrated in hydroxyethyl piperazine-ethanesulfonic acid (HEPES) medium (Quinn's-HEPES; SAGE, in-vitro Fertilization, Inc.) supplemented with 7.5% ethylene glycol and 7.5% dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO, USA) for 2.5 min and then transferred in 15% ethylene glycol, 15% dimethyl sulfoxide, and 0.5-M sucrose for the final equilibration. Thereafter, the blastocysts were loaded onto an electron microscopic (EM) gold grid (EM Grid, SPI Supplies) using a fine glass pipette. The EM grids containing the blastocysts were immediately plunged into slush liquid nitrogen using VitMaster, a vitrification device (IMT Ltd., Ness Ziona, Israel).
For vitrified blastocysts warming, the EM grids were sequentially transferred to culture dishes containing HEPES medium supplemented with 0.5-, 0.25-, 0.125-, and 0.0625-M sucrose at 2.5-min intervals, with 20% human serum albumin (SAGE BioPharma, Bedminster, NJ). The vitrified-warmed blastocysts were washed with blastocyst medium (Cook Medical, Bloomington, IN, USA) in a 37°C environment with 6% CO2, 5% O2, and 89% N2 and then cultured overnight.
VBT Protocol
All women underwent natural endometrial preparation. They were closely monitored for signs of dominant follicle collapse by transvaginal ultrasonography from days 10 to 12 of the menstrual cycle. Ovulation was confirmed if the follicular wall lost its clear appearance (28). Then, luteal support was initiated using Crinone 8% w/w Progesterone Vaginal Gel (Merck Serono Ltd., Middlesex, UK) or vaginal progesterone Utrogestan 600 mg (Hanhwa Pharmaceuticals, Seoul, Korea). An embryo replacement catheter (Cook) was used, and the warmed blastocysts were transferred under abdominal ultrasound guidance on day 5 after ovulation was observed. Finally, the embryo transfer catheter was checked to confirm that the embryo was no longer in the catheter.
Outcome Measures
Clinical and obstetric outcomes were as follows: implantation rate (IR), clinical pregnancy rate (CPR), multiple pregnancy rate (MPR), ectopic pregnancy rate, miscarriage rate, live birth rate (LBR). IR was calculated as the number of gestational sacs seen by ultrasonography divided by the total number of transferred blastocysts. Clinical pregnancy was defined as the presence of a fetal heartbeat on ultrasonogram. Miscarriage was defined as the spontaneous cessation of a clinical pregnancy before 20 gestational weeks. LBR was defined as delivery of a viable infant at >28 gestational weeks. Neonatal outcomes were as follows: birth weight, gestational age at delivery, and presence of malformations.
Statistical Analyses
We compared pregnancy and neonatal outcomes for the day 5 and day 6 groups in a propensity score-matched cohort to minimize potential biases (Figure 1) (29). The propensity scores were calculated using binary logistic regression analyses based on the following patient and menstrual cycle variables at baseline: maternal age, maternal body mass index, infertility duration, number of previous IVF attempts, presence of tubal factor (as diagnosed by tubal obstruction, tubal adhesion, or previous salpingitis), polycystic ovarian syndrome, endometriosis, male factors (defined as oligoasthenoteratozoospermia or sperm concentration <15 × 106/mL, vitality <40%, motility <32%, normal morphology <4%), protocol of controlled ovarian hyperstimulation, intracytoplasmic sperm injection, use of a freeze-all strategy, endometrial thickness, number of transferred embryos, and quality of transferred embryos. The matched ratio for day 5 vs. day 6 was 3:1. Quantitative variables are expressed as means ± standard deviations (SD) and were analyzed using Student's t-test. Qualitative variables are expressed as frequencies and percentages and were analyzed using the χ2-test. All statistical analyses were performed using SPSS version 23 (IBM). P < 0.05 was considered to indicate statistical significance.
Results
Patient Demographics and Previous IVF Cycle Characteristics
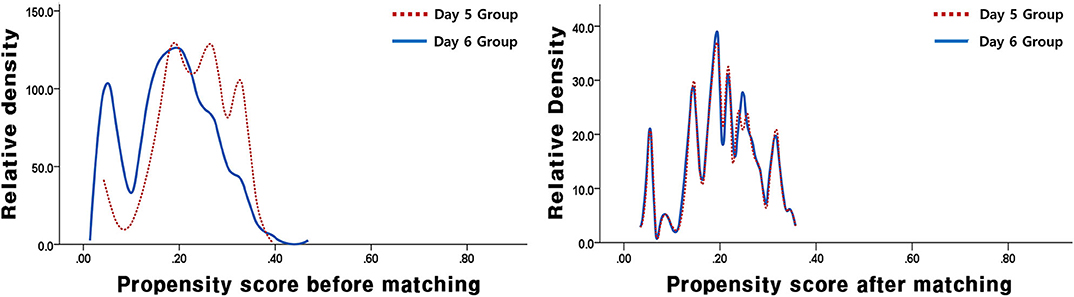
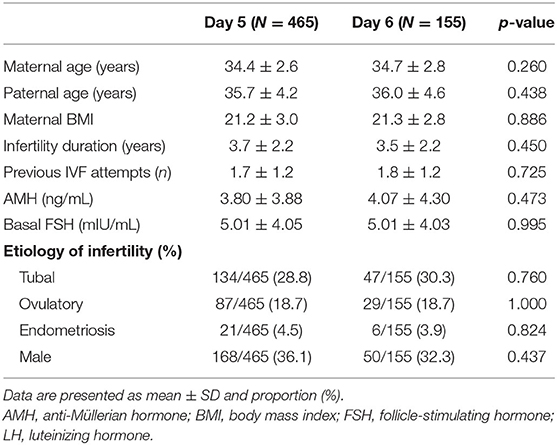
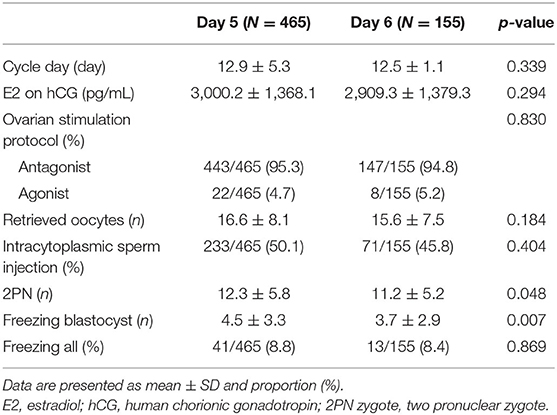
In this study, a total of 1,157 VBT cycles were analyzed, including 933 VBT cycles of day 5 group and 224 VBT cycles of day 6 group. Day 5 and 6 VBT cycles were matched at 3:1, that is, 155 triplet cycles in day 5 vs. day 6 cohorts (Figure 1). The distribution of propensity scores before and after matching is shown in Figure 2. The demographic characteristics of the patients are presented in Table 1. No statistical difference was found in maternal age at the time of oocyte retrieval between the two groups. All other variables such as previous IVF attempts and etiology of infertility were comparable. Previous IVF cycle characteristics are summarized in Table 2. The number of 2PN zygote decreased in the day 6 group (p = 0.048), and the number of freezing blastocysts was lower in the day 6 group (p = 0.007).
Clinical, Obstetric, and Neonatal Outcomes of VBT Cycles
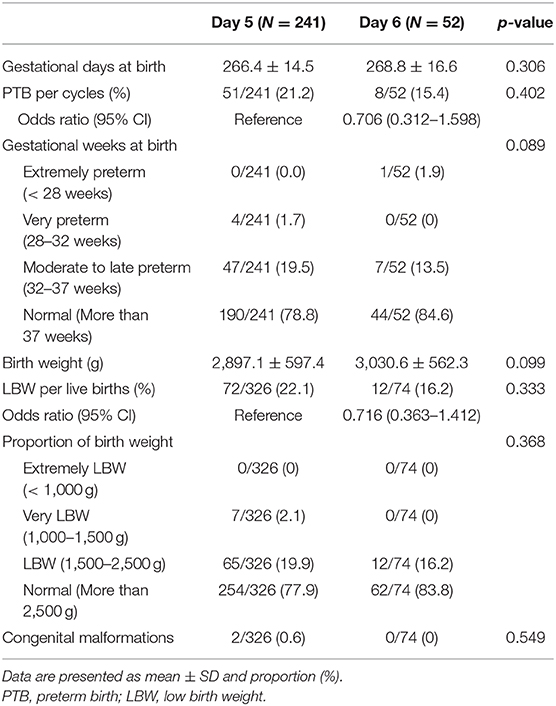
The clinical and obstetric outcomes of the VBT cycles are shown in Table 3. The IR, CPR, and LBR were significantly lower in the day 6 group than in the day 5 group (IR 44.2 vs. 53.1%, p = 0.023; CPR 48.4 vs. 60.4%, p = 0.009; LBR 33.5 vs. 51.8%, p < 0.001). The miscarriage rate was higher in the day 6 group than in the day 5 group (29.3 vs. 10.7%, p < 0.001). No significant differences were found in the ectopic pregnancy rate (1.3 vs. 2.8%, p = 0.458) and MPR (29.3 vs. 30.2%, p = 0.816). Table 4 displays the neonatal outcomes. No significant differences were observed in gestational age (days) at birth (268.8 d ± 16.6 d vs. 266.4 d ± 14.5 d, p = 0.306) and birth weight (3,030.6 g ± 562.3 g vs. 2,897.1 g ± 597.4 g, p = 0.099) between the two groups. Rates of preterm birth (PTB), low birth weight (LBW), and congenital malformations were all comparable (PTB 15.4 vs. 21.2%, p = 0.402; LBW 16.2 vs. 22.1%, p = 0.333; congenital malformations 0 vs. 0.6%, p = 0.549, respectively).
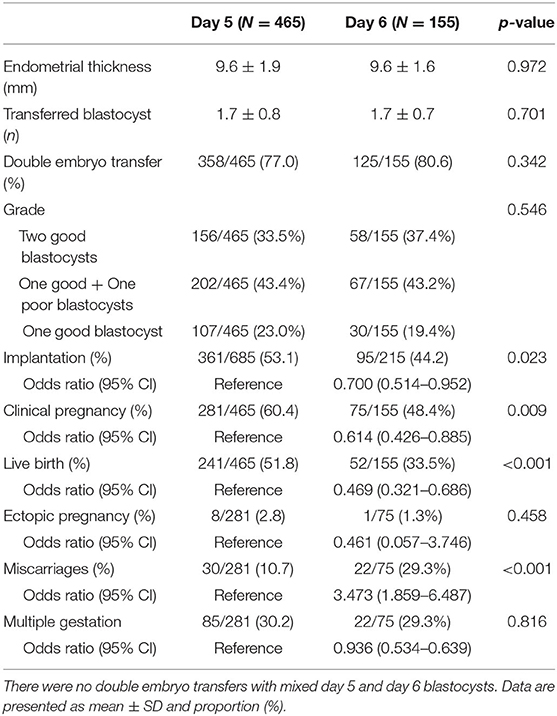
Table 3. Clinical and obstetric outcomes of vitrified-warmed blastocyst transfer cycles by day of blastulation.
Discussion
In this study, we compared the pregnancy and neonatal outcomes of day 5 and day 6 blastocysts in VBT cycles. Our present result indicates that VBT cycles with day 6 blastocysts were significantly inferior to those with day 5 blastocysts in terms of IR, CPR, and LBR. Additionally, the miscarriage rate was higher in VBT cycles with day 6 blastocysts. However, no significant differences were found in the neonatal outcomes between the two groups. Considering these findings, if there were no differences in the morphological grade between day 5 and day 6 blastocysts, transfer of day 5 vitrified blastocysts should be considered first.
Our results are consistent with previous studies that reported significantly lower clinical outcomes from FBT or VBT cycles with day 6 blastocysts (19–24). Among them, Ferreux et al. (23) reported a significantly lower LBR of day 6 blastocysts, regardless of the grades of embryos. Baseline characteristics were not significantly different between the study groups, and the blastocysts have similar grades using the grading scale proposed by Gardner et al. (27). However, they did not match the two groups, although the day 5 group had three times more cycles than the day 6 group. Moreover, our miscarriage rates in the day 5 and day 6 groups were similar to those in their study. Haas et al. also reported a significantly lower clinical outcomes including IR and CPR (22). Interestingly, they compared day 5 blastocysts with good-quality day 6 blastocysts (≥3 BB). However, they warmed day 5 blastocysts 20–24 h before the embryo transfer, while they warmed day 6 blastocysts 2–4 h prior to embryo transfer.
Considering the lower clinical outcomes of day 6 blastocysts, there is still a degree of controversy in previous studies (5, 13–18). Yang et al. (15) reported that high-quality (≥3 BB) day 6 blastocysts in VBT had similar developmental potential and pregnancy outcomes to those of high-quality day 5 blastocysts. However, they did not match day 5 and 6 groups, although the day 5 group had five times more cycles than the day 6 group. Moreover, they did not report LBR. In a meta-analysis, Sunkara et al. (18) compared the clinical outcomes of FBT with day 5 blastocysts and those with day 6 blastocysts. They included 2,502 cycles from 15 controlled studies and concluded that FBT with day 6 blastocysts have similar CPR and LBR to FBT with day 5 blastocyst, if the morphological grade is the same. However, this meta-analysis had clinical heterogeneity and limited consideration of the confounders in the included studies.
Although we did not perform preimplantation genetic testing for aneuploidy, chromosomal abnormality could explain the significant difference in clinical outcomes including miscarriage rate between day 5 and 6 VBT groups. Indeed, there have been studies reporting that slower developing blastocysts have higher aneuploidy rate (25, 26). Taylor et al. (26) reported that day 5 blastocysts had a higher chance of being euploid than day 6 blastocysts. The risk of aneuploidy of day 6 blastocysts was 10% higher than that of day 5 blastocysts. To reduce bias, they used a sibling embryo model, that is, they included patient who had biopsy on both day 5 and day 6 blastocysts in the same IVF cycles. From time-lapse culture systems, some studies revealed the close relationship between timely cell division and developmental competence with kinetic data in accordance with our results (30–32). Campbell et al. (33) reported that embryos having single or multiple aneuploidy had delayed initiation of blastulation compared with euploid embryos in time-lapse culture systems.
An increase of spindle abnormalities in day 6 blastocysts could explain our significant results. Hashimoto et al. (2) conducted a cytoskeletal analysis of day 5 and 6 blastocysts. They found that the incidence of abnormal spindles was significantly higher in day 6 blastocysts, and IR and CPR were significantly higher in VBT of day 5 blastocysts. Interestingly, they evaluated the incidence of chromosomal abnormalities of the abortus and reported no differences between day 5 and 6 groups. They hypothesized that most blastomeres with abnormal spindles are eliminated before implantation. This hypothesis may support the safety of VBT with day 6 blastocysts, as revealed in our results.
In our study, the MPR of day 5 VBT was similar with that of day 6 VBT. In the day 5 VBT group, 2 good blastocyst transfers occurred in 42.8% (57/133) of multiple pregnancies, and one good and one poor blastocyst transfer occurred in 32.5% (28/86) of multiple pregnancies. Similarly, day 6 VBT had a 37.8% (14/37) of multiple pregnancies with 2 good blastocysts transfers and 29.1% (7/24) of multiple pregnancies with one good and one poor blastocyst transfer. This suggests that the quality of embryos, as well as the day of blastulation, is important for clinical outcomes. This is consistent with past reports that the morphological grades of embryos are one of the most important prognostic factors in IVF (34–37).
In contrast to the centers at which previous studies were conducted, our center used a natural endometrial preparation. Compared with artificial or medicated endometrial preparations, the clinical outcomes of VBT with natural endometrial preparations are not to be inferior (38, 39). Artificial endometrial preparations have been linked to a high miscarriage rate (40, 41). In addition, IVF is covered by the national health insurance system in Korea, so it is possible to perform daily ultrasonograms at low cost. For natural endometrial preparations, some clinicians use serial LH tests. However, the role of serial LH monitoring with ultrasonogram has been a subject of much debate in natural endometrial preparations and there is no clear definition of or consensus regarding LH surge (42–44). Furthermore, serial LH tests are not covered by the national health insurance. Because of these reasons, our IVF center prefers to perform natural endometrial preparations without serial LH tests.
The primary strength of our study is that we performed analysis using propensity score matching to control for potential confounders between the study groups. It is impossible to perform randomized controlled study for comparing day 5 and 6 blastocysts; thus, a prospective observational study or well-designed matched study is more appropriate. Therefore, a propensity score matching analysis, like this study, would be an adequate design for comparing day 5 blastocysts with day 6 blastocysts. Propensity score matching analysis is used for observational studies wherein allocation is not random, and it can also be viewed as an approach seeking to replicate the random assignment of study populations in conventional randomized controlled trials (45). Additionally, we included VBT cycles that are able to promote better embryo-endometrial synchrony, so we eliminate endometrial receptivity bias from controlled ovarian hyperstimulation. Moreover, this study has a single-center design; therefore, all IVF cycles were done under uniform conditions, and embryos were cultured in the same media using the same techniques by the same embryologists. By this, we can minimize not only observational biases but also the influence of varying culture media on neonatal birth weights (46, 47). Finally, all our patients were of the same ethnicity.
This study has some limitations. In performing propensity score matching, which controls for multiple confounding variables, the sample size decreased from 1,313 cycles to 620 cycles. This increases the risk of a type 2 error. Since the study had a retrospective design, there were not enough data that could be associated with PTB and LBW, including a previous history of PTB or LBW, underlying maternal disease, or pregnancy-associated diseases. In addition, we did not include data from VBTs with single poor-quality or double poor-quality blastocysts. Although there were 41 cases of VBT with a single poor-quality blastocyst during the study period, this number was considered too small to be included.
In conclusion, with the ever-evolving IVF technology, especially vitrification-warming technique, defining safety, and clinical outcomes of growth-retarded blastocysts compared with timely growing blastocysts is essential. Although VBT with day 6 blastocysts can lead to acceptable clinical outcomes and safety, our propensity score-matched study suggests that day 5 blastocysts for VBT offer significant favorable clinical outcomes by reducing miscarriage rate, if the morphological grades are not different between day 5 and 6 blastocysts. In the future, well-designed prospective study, especially focusing on the euploidy of growth-retarded blastocysts, is needed.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The study was approved by the Institutional Review Board of CHA Gangnam Medical Center (IRB Approval Number: GCI 18–30). Informed consent was waived because of the retrospective study design.
Author Contributions
SL contributed to the conception and design. DP, JK, and EC collected data and conducted analysis. WL and TY were responsible for data interpretation. DP drafted the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the Technology Innovation Program (or Industrial Strategic Technology Development Program, 20003838) funded by the Ministry of Trade, Industry, & Energy (MI, Korea).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Editage (www.editage.co.kr) for English language editing.
References
1. Kovalevsky G, Carney SM, Morrison LS, Boylan CF, Neithardt AB, Feinberg RF. Should embryos developing to blastocysts on day 7 be cryopreserved and transferred: an analysis of pregnancy and implantation rates. Fertil Steril. (2013) 100:1008–12. doi: 10.1016/j.fertnstert.2013.06.021
2. Hashimoto S, Amo A, Hama S, Ito K, Nakaoka Y, Morimoto Y. Growth retardation in human blastocysts increases the incidence of abnormal spindles and decreases implantation potential after vitrification. Hum Reprod. (2013) 28:1528–35. doi: 10.1093/humrep/det059
3. Hsieh RH, Au HK, Yeh TS, Chang SJ, Cheng YF, Tzeng CR. Decreased expression of mitochondrial genes in human unfertilized oocytes and arrested embryos. Fertil Steril. (2004) 81:912–8. doi: 10.1016/j.fertnstert.2003.11.013
4. Wood JR, Dumesic DA, Abbott DH, Strauss JF. Molecular abnormalities in oocytes from women with polycystic ovary syndrome revealed by microarray analysis. J Clin Endocrinol Metab. (2007) 92:705–13. doi: 10.1210/jc.2006-2123
5. Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Ross R. Contrasting patterns in in vitro fertilization pregnancy rates among fresh autologous, fresh oocyte donor, and cryopreserved cycles with the use of day 5 or day 6 blastocysts may reflect differences in embryo-endometrium synchrony. Fertil Steril. (2008) 89:20–6. doi: 10.1016/j.fertnstert.2006.08.092
6. Shapiro BS, Richter KS, Harris DC, Daneshmand ST. A comparison of day 5 and day 6 blastocyst transfers. Fertil Steril. (2001) 75:1126–30. doi: 10.1016/S0015-0282(01)01771-X
7. Barrenetxea G, Delarruzea A, Ganzabal T, Jimenez R, Carbonero K, Mandiola M. Blastocyst culture after repeated failure of cleavage-stage embryo transfers: a comparison of day 5 and day 6 transfers. Fertil Steril. (2005) 83:49–53. doi: 10.1016/j.fertnstert.2004.06.049
8. Khorram O, Shapiro SS, Jones JM. Transfer of nonassisted hatched and hatching human blastocysts after in vitro fertilization. Fertil Steril. (2000) 74:163–5. doi: 10.1016/S0015-0282(00)00567-7
9. Mirkin S, Nikas G, Hsiu JG, Díaz J, Oehninger S. Gene expression profiles and structural/functional features of the peri-implantation endometrium in natural and gonadotropin-stimulated cycles. J Clin Endocrinol Metab. (2004) 89:5742–52. doi: 10.1210/jc.2004-0605
10. Kolb BA, Paulson RJ. The luteal phase of cycles utilizing controlled ovarian hyperstimulation and the possible impact of this hyperstimulation on embryo implantation. Am J Obstet Gynecol. (1997) 176:1262–9. doi: 10.1016/S0002-9378(97)70344-2
11. Kolibianakis E, Bourgain C, Albano C, Osmanagaoglu K, Smitz J, Van Steirteghem A, et al. Effect of ovarian stimulation with recombinant follicle-stimulating hormone, gonadotropin releasing hormone antagonists, and human chorionic gonadotropin on endometrial maturation on the day of oocyte pick-up. Fertil Steril. (2002) 78:1025–9. doi: 10.1016/S0015-0282(02)03323-X
12. Richter KS, Shipley SK, McVearry I, Tucker MJ, Widra EA. Cryopreserved embryo transfers suggest that endometrial receptivity may contribute to reduced success rates of later developing embryos. Fertil Steril. (2006) 86:862–6. doi: 10.1016/j.fertnstert.2006.02.114
13. Behr B, Gebhardt J, Lyon J, Milki AA. Factors relating to a successful cryopreserved blastocyst transfer program. Fertil Steril. (2002) 77:697–9. doi: 10.1016/S0015-0282(01)03267-8
14. Hiraoka K, Hiraoka K, Kinutani M, Kinutani K. Blastocoele collapse by micropipetting prior to vitrification gives excellent survival and pregnancy outcomes for human day 5 and 6 expanded blastocysts. Hum Reprod. (2004) 19:2884–8. doi: 10.1093/humrep/deh504
15. Yang H, Yang Q, Dai S, Li G, Jin H, Yao G, et al. Comparison of differences in development potentials between frozen-thawed D5 and D6 blastocysts and their relationship with pregnancy outcomes. J Assist Reprod Genet. (2016) 33:865–72. doi: 10.1007/s10815-016-0712-6
16. Mukaida T. Vitrification of human blastocysts using cryoloops: clinical outcome of 223 cycles. Hum Reprod. (2003) 18:384–91. doi: 10.1093/humrep/deg047
17. El-Toukhy T, Wharf E, Walavalkar R, Singh A, Bolton V, Khalaf Y, et al. Delayed blastocyst development does not influence the outcome of frozen-thawed transfer cycles. BJOG. (2011) 118:1551–6. doi: 10.1111/j.1471-0528.2011.03101.x
18. Sunkara SK, Siozos A, Bolton VN, Khalaf Y, Braude PR, El-Toukhy T. The influence of delayed blastocyst formation on the outcome of frozen-thawed blastocyst transfer: a systematic review and meta-analysis. Hum Reprod. (2010) 25:1906–15. doi: 10.1093/humrep/deq143
19. Richter KS, Ginsburg DK, Shipley SK, Lim J, Tucker MJ, Graham JR, et al. Factors associated with birth outcomes from cryopreserved blastocysts: experience from 4,597 autologous transfers of 7,597 cryopreserved blastocysts. Fertil Steril. (2016) 106:354–62.e2. doi: 10.1016/j.fertnstert.2016.04.022
20. Levens ED, Whitcomb BW, Hennessy S, James AN, Yauger BJ, Larsen FW. Blastocyst development rate impacts outcome in cryopreserved blastocyst transfer cycles. Fertil Steril. (2008) 90:2138–43. doi: 10.1016/j.fertnstert.2007.10.029
21. Kang SM, Lee SW, Yoon SH, Kim JC, Lim JH, Lee SG. Comparison of clinical outcomes between single and double vitrified-warmed blastocyst embryo transfer according to the day of vitrification. J Assist Reprod Genet. (2013) 30:779–85. doi: 10.1007/s10815-013-0017-y
22. Haas J, Meriano J, Laskin C, Bentov Y, Barzilay E, Casper RF, et al. Clinical pregnancy rate following frozen embryo transfer is higher with blastocysts vitrified on day 5 than on day 6. J Assist Reprod Genet. (2016) 33:1553–7. doi: 10.1007/s10815-016-0818-x
23. Ferreux L, Bourdon M, Sallem A, Santulli P, Barraud-Lange V, Le Foll N, et al. Live birth rate following frozen–thawed blastocyst transfer is higher with blastocysts expanded on day 5 than on day 6. Hum Reprod. (2018) 33:390–8. doi: 10.1093/humrep/dey004
24. Desai N, Ploskonka S, Goodman L, Attaran M, Goldberg JM, Austin C, et al. Delayed blastulation, multinucleation, and expansion grade are independently associated with live-birth rates in frozen blastocyst transfer cycles. Fertil Steril. (2016) 106:1370–8. doi: 10.1016/j.fertnstert.2016.07.1095
25. Alfarawati S, Fragouli E, Colls P, Stevens J, Gutiérrez-Mateo C, Schoolcraft WB, et al. The relationship between blastocyst morphology, chromosomal abnormality, and embryo gender. Fertil Steril. (2011) 95:520–4. doi: 10.1016/j.fertnstert.2010.04.003
26. Taylor TH, Patrick JL, Gitlin SA, Wilson JM, Crain JL, Griffin DK. Comparison of aneuploidy, pregnancy and live birth rates between day 5 and day 6 blastocysts. Reprod Biomed Online. (2014) 29:305–10. doi: 10.1016/j.rbmo.2014.06.001
27. Gardner D, Lane M. Handbook of In Vitro Fertilization. 2nd Edn. Boca Raton, FL: CRC Press (2000).
28. Jaffe R, Ben Aderet N. Ultrasonic screening in predicting the time of ovulation. Gynecol Obstet Invest. (1984) 18:303–5. doi: 10.1159/000299097
29. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. (1983) 70:41–55. doi: 10.1093/biomet/70.1.41
30. Desai N, Ploskonka S, Goodman LR, Austin C, Goldberg J, Falcone T. Analysis of embryo morphokinetics, multinucleation and cleavage anomalies using continuous time-lapse monitoring in blastocyst transfer cycles. Reprod Biol Endocrinol. (2014) 12:54. doi: 10.1186/1477-7827-12-54
31. Meseguer M, Herrero J, Tejera A, Hilligsøe KM, Ramsing NB, Remoh J. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod. (2011) 26:2658–71. doi: 10.1093/humrep/der256
32. Wong CC, Loewke KE, Bossert NL, Behr B, De Jonge CJ, Baer TM, et al. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol. (2010) 28:1115–21. doi: 10.1038/nbt.1686
33. Campbell A, Fishel S, Bowman N, Duffy S, Sedler M, Hickman CFL. Modelling a risk classification of aneuploidy in human embryos using non-invasive morphokinetics. Reprod Biomed Online. (2013) 26:477–85. doi: 10.1016/j.rbmo.2013.02.006
34. Oron G, Son WY, Buckett W, Tulandi T, Holzer H. The association between embryo quality and perinatal outcome of singletons born after single embryo transfers: a pilot study. Hum Reprod. (2014) 29:1444–51. doi: 10.1093/humrep/deu079
35. Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. (2000) 73:1155–8. doi: 10.1016/S0015-0282(00)00518-5
36. El-Danasouri I, Rinaldi L, Pacchiarotti A, Desanto M, Selman H. Effect of transferring a morphologically impaired embryo with a good quality embryo on the pregnancy and implantation rates. Eur Rev Med Pharmacol Sci. (2016) 20:394–8.
37. Balaban B, Urman B, Sertac A, Alatas C, Aksoy S, Mercan R. Blastocyst quality affects the success of blastocyst-stage embryo transfer. Fertil Steril. (2000) 74:282–7. doi: 10.1016/S0015-0282(00)00645-2
38. Groenewoud ER, Cantineau AEP, Kollen BJ, Macklon NS, Cohlen BJ. What is the optimal means of preparing the endometrium in frozen–thawed embryo transfer cycles? A systematic review and meta-analysis. Hum Reprod Update. (2013) 19:458–70. doi: 10.1093/humupd/dmt030
39. Mackens S, Santos-Ribeiro S, van de Vijver A, Racca A, Van Landuyt L, Tournaye H, et al. Frozen embryo transfer: a review on the optimal endometrial preparation and timing. Hum Reprod. (2017) 32:2234–42. doi: 10.1093/humrep/dex285
40. Veleva Z, Tiitinen A, Vilska S, Hyden-Granskog C, Tomas C, Martikainen H, et al. High and low BMI increase the risk of miscarriage after IVF/ICSI and FET. Hum Reprod. (2008) 23:878–84. doi: 10.1093/humrep/den017
41. Tomás C, Alsbjerg B, Martikainen H, Humaidan P. Pregnancy loss after frozen-embryo transfer—a comparison of three protocols. Fertil Steril. (2012) 98:1165–9. doi: 10.1016/j.fertnstert.2012.07.1058
42. Groenewoud ER, Kollen BJ, Macklon NS, Cohlen BJ. Spontaneous LH surges prior to HCG administration in unstimulated-cycle frozen–thawed embryo transfer do not influence pregnancy rates. Reprod Biomed Online. (2012) 24:191–6. doi: 10.1016/j.rbmo.2011.11.003
43. Groenewoud ER, Macklon NS, Cohlen BJ, Al-Oraiby A, Brinkhuis EA, Broekmans FJM, et al. The effect of elevated progesterone levels before HCG triggering in modified natural cycle frozen-thawed embryo transfer cycles. Reprod Biomed Online. (2017) 34:546–54. doi: 10.1016/j.rbmo.2017.02.008
44. Lee VCY, Li RHW, Chai J, Yeung TWY, Yeung WSB, Ho PC, et al. Effect of preovulatory progesterone elevation and duration of progesterone elevation on the pregnancy rate of frozen–thawed embryo transfer in natural cycles. Fertil Steril. (2014) 101:1288–93. doi: 10.1016/j.fertnstert.2014.01.040
45. Whittaker W, Anselmi L, Kristensen SR, Lau YS, Bailey S, Bower P, et al. Associations between extending access to primary care and emergency department visits: a difference-in-differences analysis. PLOS Med. (2016) 13:e1002113. doi: 10.1371/journal.pmed.1002113
46. Vergouw CG, Hanna Kostelijk E, Doejaaren E, Hompes PGA, Lambalk CB, Schats R. The influence of the type of embryo culture medium on neonatal birthweight after single embryo transfer in IVF. Hum Reprod. (2012) 27:2619–26. doi: 10.1093/humrep/des252
Keywords: blastocyst, embryo transfer, in vitro fertilization, day 5 blastocyst transfer, day 6 blastocyst transfer, delayed blastulation, live birth rate, pregnancy outcome
Citation: Park DS, Kim JW, Chang EM, Lee WS, Yoon TK and Lyu SW (2020) Obstetric, Neonatal, and Clinical Outcomes of Day 6 vs. Day 5 Vitrified-Warmed Blastocyst Transfers: Retrospective Cohort Study With Propensity Score Matching. Front. Endocrinol. 11:499. doi: 10.3389/fendo.2020.00499
Received: 25 March 2020; Accepted: 23 June 2020;
Published: 04 August 2020.
Edited by:
Katja Teerds, Wageningen University, NetherlandsReviewed by:
Alan Decherney, National Institutes of Health Clinical Center (NIH), United StatesTae Hoon Kim, Michigan State University, United States
Copyright © 2020 Park, Kim, Chang, Lee, Yoon and Lyu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sang Woo Lyu, ZHVuZzUwMzhAY2hhLmFjLmty
 Dong Soo Park
Dong Soo Park Ji Won Kim
Ji Won Kim Sang Woo Lyu
Sang Woo Lyu