- 1Department of Endocrinology and Metabolism, Adrenal Center, West China Hospital of Sichuan University, Chengdu, China
- 2Department of Endocrinology and Metabolism, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, China
Background: Currently, increasing evidence shows that excess aldosterone may have an impact on bone health, and primary aldosteronism (PA) may be a secondary cause of osteoporosis. This problem is worthy of attention because secondary osteoporosis is always potentially reversible, which affects the selection of treatment for PA to some extent. The present systematic review will assess and summarize the available data regarding the relationship between PA and osteoporosis.
Methods: Pubmed and Embase were searched for clinical trials related to the association between PA and bone metabolism. The results were limited to full-text articles published in English, without restrictions for the publication time. The quality of clinical trials was appraised, and the data were extracted. Biochemical parameters of bone turnover in PA patients were assessed using random-effect meta-analysis. Descriptive analysis was performed for other parameters, for data is insufficient.
Results: A final total of 15 articles were included in this review. The meta-analysis of six studies showed that subjects with PA had higher serum PTH levels (MD=21.50 pg/ml, 95% CI (15.63, 27.37), P<0.00001) and slightly increased urinary calcium levels (MD = 1.65 mmol/24 h, 95% CI (1.24, 2.06), P < 0.00001) than the EH controls. PA is associated with an increased risk of bone fracture. Bone loss in patients with PA may be reversed by MR antagonists or adrenal surgery.
Conclusions: PA may be a secondary cause of osteoporosis and is associated with an increased risk of bone fracture. The clarification of the relationships between PA and bone metabolism requires additional prospective randomized controlled studies in a large sample.
Introduction
Primary aldosteronism (PA) is a major cause of secondary hypertension, accounting for approximately 5% to 11% of all cases of hypertension (1–3). Osteoporosis is a disease that requires comprehensive management because many risk factors may affect it. Secondary osteoporosis is always potentially reversible. The possibility of skeletal damage caused by glucocorticoids has been well known for many years (4–7); however, less is known about the link between hyperaldosteronism and osteoporosis. Currently, increasing evidence shows that excess aldosterone may have an impact on bone health, and PA may be a secondary cause of osteoporosis (8, 9). Some studies have indicated that subjects with PA had higher serum parathyroid hormone (PTH) levels, lower serum calcium levels, and higher urinary calcium levels than controls; however, not all studies have supported this finding (10–13). On the other hand, discrepancies also exist in the changes in biochemical parameters after surgery or spironolactone treatment in PA patients (13–15). Moreover, for bone mineral density (BMD) and bone turnover markers, the results of a limited number of studies are controversial. Some researchers have suggested that it is not appropriate to use dual X-ray absorptiometry (DXA) to detect bone damage because PA appear to impair the bone microarchitecture rather than bone density (12, 15–18). Only a few studies have focused on fracture, which is the endpoint event of osteoporosis (15, 17, 19).
Therefore, we conducted this study to systematically review the relationship between primary aldosteronism and bone metabolism according to the available data regarding the biochemical parameters, bone mineral density, bone quality, and risk of fractures of patients with PA. In addition, changes after surgery or spironolactone treatment are reviewed. Finally, the mechanism influencing the effects of excessive aldosterone on bone metabolism will be summarized.
Methods
Literature Search
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (20). PubMed and Embase were searched systematically for articles relating to PA and bone metabolism. Searching term was “(primary aldosteronism) AND (osteoporosis OR BMD OR PTH)”. There was no time restriction made on our search. The cutoff time for retrieval was July 2019. All reference lists from the main studies and relevant reviews were screened manually for additional eligible studies. The results were limited to full-text articles published in English.
Selection Criteria and Quality Assessment
The systematic review question was whether PA was associated with osteoporosis. Two authors independently screened the titles and abstracts for inclusion of all the potential studies identified before. The full-text was retrieved if necessary. All the human studies were included in this evaluation. The literature quality evaluation was conducted by means of the Newcastle-Ottawa Quality Assessment Scale by the two authors independently. NOS included eight items, with total score being nine, and focused on three areas, including participant selection, comparability of study groups, and ascertainment of exposure (21).
During the study selection process for Meta-analysis, inclusion criteria included: participants were patients with PA; PA was confirmed by a saline infusion test or captopril challenge test; the comparison between patients with PA and essential hypertension; studies provided data of serum PTH or calcium levels; case-control study, observational study, cohort study, or randomized clinical trial. Exclusion criteria included: in vitro or laboratory study; animal study; review or case report; an abstract or conference; and not meet the inclusion criteria.
Data Extraction
Data extraction was conducted by the two authors using a standardized data collection form. The following information is obtained from included studies: type of study, country, first author’s name, and year of publication, target population, comparison, all data related to bone metabolism, including biochemical parameters, parameters of bone damage and fractures, and follow-up data after treatment.
Statistical Analysis
We systematically analyzed all the related parameters of bone metabolism in PA patients both before and after treatment. When parameters were focused only by few studies, descriptive analysis was performed. However, if data is sufficient, Meta-analysis was performed using ReMan5.4. The mean differences (MD) were calculated using random-effects models for the presence of heterogeneity (I2 >50%, P<0.05). P value < 0.05 was considered as statistically significant.
Results
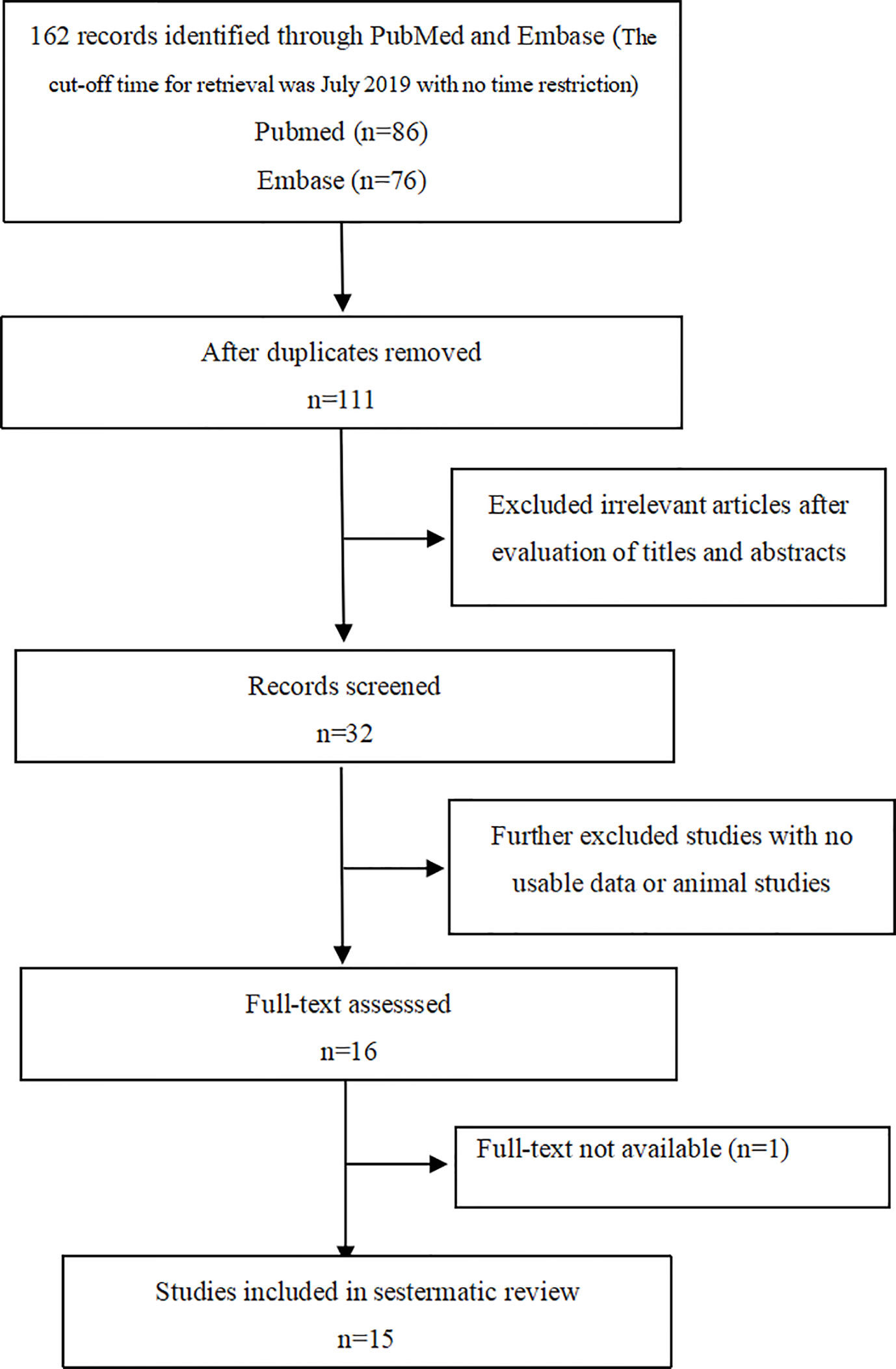
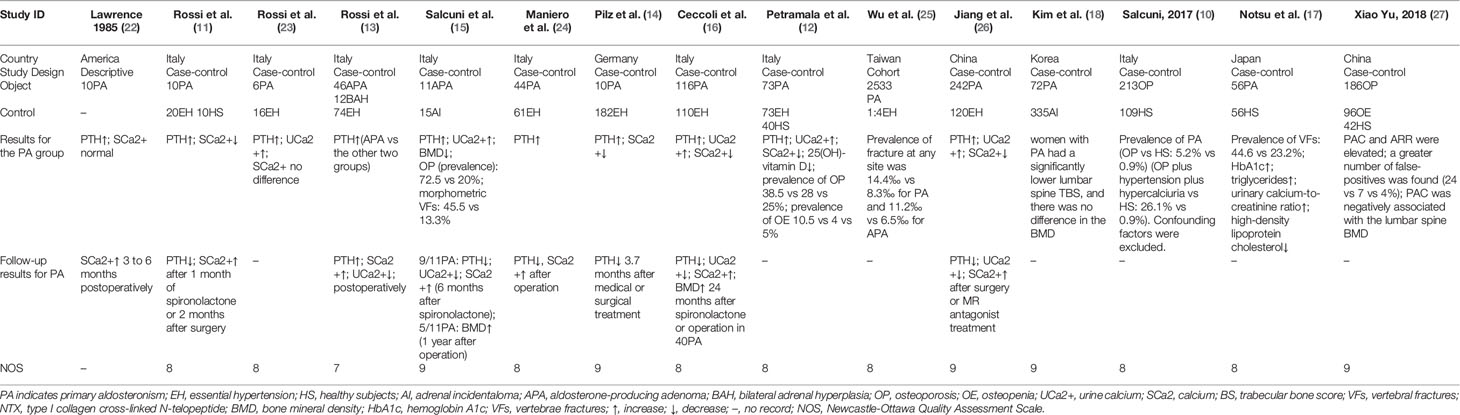
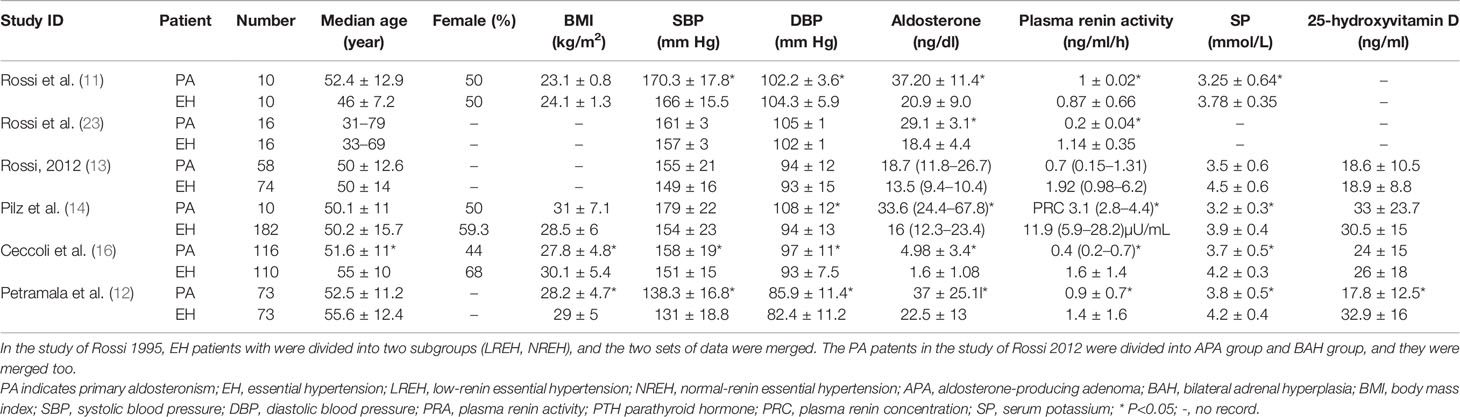
A total of 162 papers were identified, of which, 16 studies were included by screening the titles and abstracts. 1 full-text is not available, and finally, 15 available studies regarding the association between PA and bone metabolism were included in the systematic review (as shown in Figure 1) and are summarized in Table 1, which included 1 cohort, 1 descriptive, and 13 case-control studies. Participants in these studies came from different regions, including America, Italy, Germany, Japan, Korea, and China. 6 studies were included in the meta-analysis, and the clinical and biochemical parameters of the patients of the 6 studies were shown in Table 2.
Biochemical Parameters, Bone Strength, and Risk of Fractures of Patients With PA
Biochemical Parameters of Bone Metabolism
We conducted a random effect meta-analysis using the available studies to assess the biochemical parameters of bone turnover in PA patients compared with those in essential hypertension (EH) patients. Six studies including 283 PA patients and 475 EH patients were included in the meta-analysis to assess the changes of serum PTH, and the analysis indicated that subjects with PA had higher serum PTH levels than EH patients (MD=21.50 pg/ml, 95% CI (15.63, 27.37), P<0.00001) (Figure 2). Meta-analysis of five studies with urinary calcium levels suggested that urinary calcium was slightly increased in PA patients compared with EH patients (MD = 1.65 mmol/24 h, 95% CI (1.24, 2.06), P<0.00001) (Figure 3), while five studies with serum calcium levels indicated that serum calcium levels were not significantly different between PA and EH patients (MD = 0.00 mmol/L, 95% CI (−0.08, 0.08), P = 1.0) (Figure 4). In the meta-analysis, we only included studies comparing PA patients with EH patients. However, the following three studies compared PA patients with HS (healthy subjects). In 2017, Salicuni compared 12 PA patients with 310 HS and showed that PTH and urinary calcium excretion were significantly higher in PA patients (10), which is in line with the results of previous studies performed by Rossi et al. in 1995 (11) and Petramala et al. in 2014 (12). For the anthropometric parameters and 25(OH)-vitamin D levels, no differences were found between the PA group and the control group in most studies.
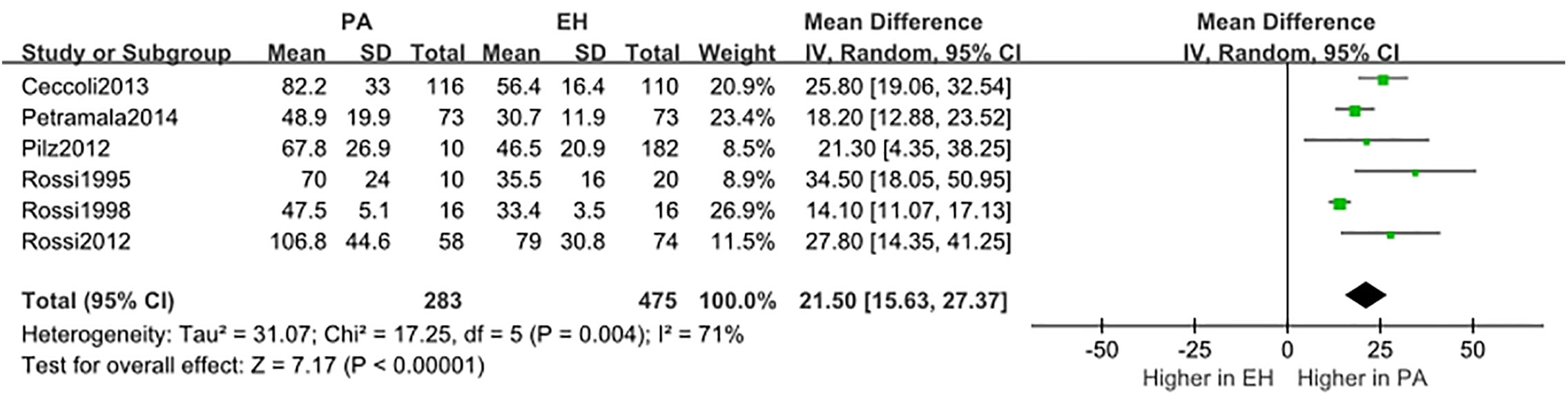
Figure 2 Comparison of PTH in the PA group vs the EH group (pg/ml). Vertical line indicates invalid line; horizontal lines, confidence interval; central squares, statistics; diamond, combined statistic and confidence interval of multiple studies.
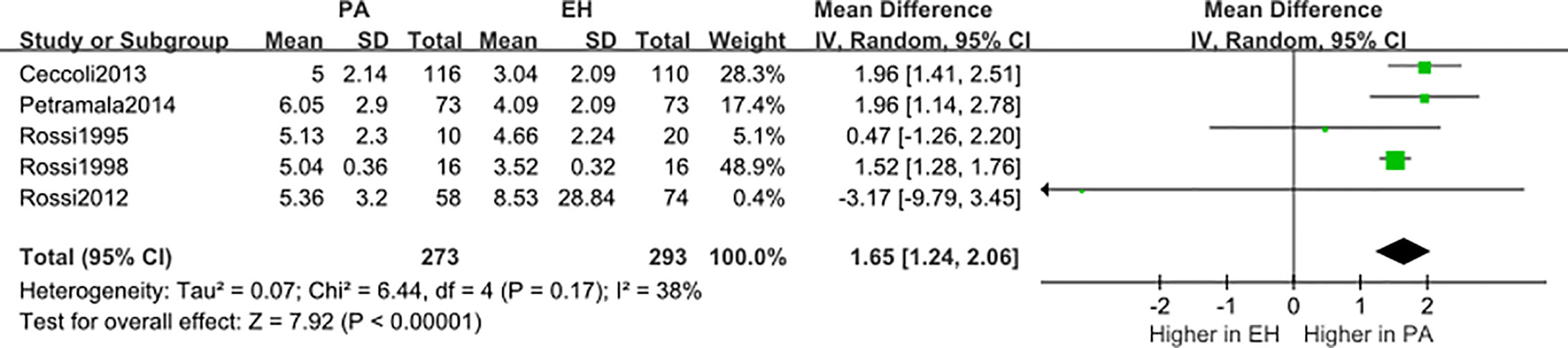
Figure 3 Comparison of urinary calcium in the PA group vs the EH group (mmol/24 h). Vertical line indicates invalid line; Horizontal lines, confidence interval; Central squares, statistics; Diamond, combined statistic and confidence interval of multiple studies.
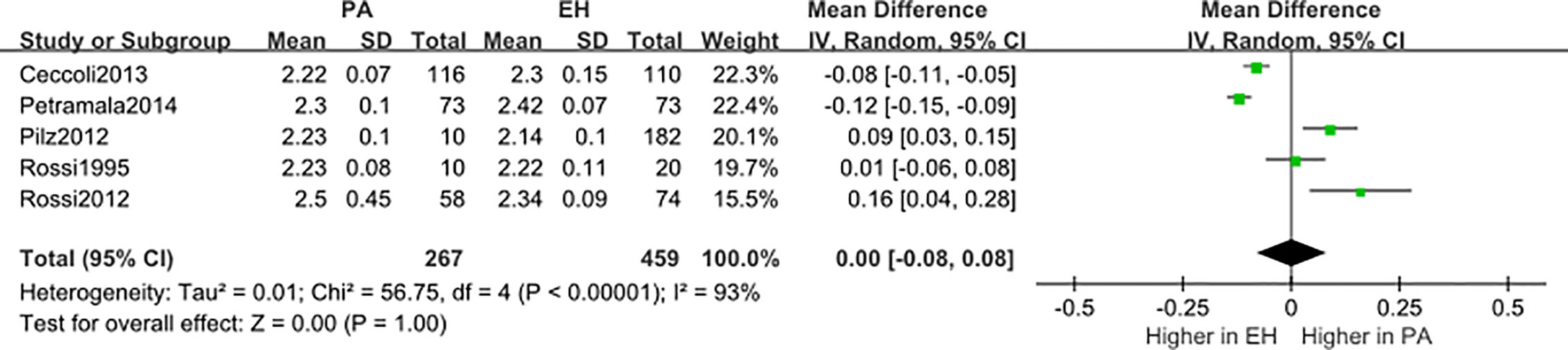
Figure 4 Comparison of blood calcium in the PA group vs the EH group (mmol/L). Vertical line indicates invalid line; horizontal lines, confidence interval; central squares, statistics; diamond, combined statistic and confidence interval of multiple studies.
Bone turnover markers (BTMs) have only been mentioned in two studies. Notsu et al. showed that the level of urinary type I collagen cross-linked N-telopeptide was not significantly different in 56 PA patients compared with that in 56 controls (17). Ceccoli et al. showed that the blood level of C-terminal telopeptide of type I collagen I (CTX) tended to be lower and the level of bone-specific alkaline phosphatase (B-ALP) tended to be higher in 40 PA patients after targeted treatment, but these differences were not statistically significant (16).
In summary, PA patients have increased serum PTH levels, and the discrepancies between the studies and the lack of significant changes in serum and urinary calcium levels may be due to insufficient sample sizes and the low impact of aldosterone on bone. Moreover, the laboratory testing methods were inconsistent in different studies, which may affect the accuracy of the conclusion. BTMs did not significantly differ between PA patients and controls, apart from the possible causes of the differences mentioned above. A potential explanation for this is that BTMs reflect only short-term bone transition states rather than chronic cumulative loss.
Parameters of Bone Strength
Only a few studies have examined the association of BMD with the prevalence of osteoporosis. Salcuni et al. first reported the association between hyperaldosteronism and osteoporosis in patients with APA. In 11 APA patients, there was decreased BMD in the lumbar spine, total hip, and femoral neck and an increased prevalence of osteoporosis (73% vs 20%) in comparison to those in 15 adrenal incidentaloma (AI) patients (15). Petramala et al. compared 73 PA patients with 73 EH patients and 40 HS and showed that the PA subjects had a significantly higher prevalence of osteopenia/osteoporosis (38.5% and 10.5%) than the EH patients (28% and 4%) and HS (25% and 5%, respectively) (12).
However, there was no difference in the BMD in the lumbar spine and femoral neck between 56 PA patients and 56 HS according to another study (17). Likewise, Kim et al. confirmed that there was no difference in the BMD between 72 PA patients and 335 HS patients (18). Controversial evidence supported the possibility that excess aldosterone may mainly affect bone quality rather than bone mass. Therefore, BMD may not be an ideal index to reflect bone involvement caused by aldosterone. Therefore, the trabecular bone score (TBS), as a parameter representing the bone microarchitecture that is determined by quantifying pixel-level grayscale variations in lumbar spine dual-energy X-ray absorptiometry scans, has been introduced. The TBS is related to the bone microarchitecture and provides information about the skeleton that is not captured by the standard BMD measurement (28). However, TBS itself is also a controversial parameter, which has limited application value in clinical practice. Kim et al. subsequently showed that women with PA had a significantly decreased lumbar spine TBS, and the PAC was inversely correlated with the lumbar spine TBS in women after adjustment for potential confounders. Importantly, all these observations in women remained statistically significant following an additional adjustment for the lumbar spine BMD in the multivariable model. However, similar correlations were not observed in men (18).
In summary, controversial evidence indicated that the BMD evaluated by DXA may not be an early index to reflect the bone involvement caused by excessive aldosterone; therefore, more studies focused on the BMD (e.g., QCT) and bone quality may be needed.
Risk of Fractures
Unlike the data on biochemical parameters and BMD, data regarding the risk of fractures are fully concordant and show that PA patients have a higher fracture risk compared with controls. Salcuni et al. first reported that the prevalence of vertebral fractures tended to be higher in PA patients than in controls (45.5% vs 13.3%) (15). Subsequently, Notsu et al. showed that the prevalence of vertebral fractures was significantly higher in 56 PA patients than that in 56 HS patients (44.6% vs 23.2%, P <0.05). Patients with PA suffered severe fractures more frequently than controls (17). However, both of these studies focused on morphometric vertebral fractures rather than fractures with clinical symptoms. Nevertheless, Wu et al. focused on clinical fractures. They used a longitudinal population database from the Taiwan National Health Insurance system that included 2533 PA patients and controls (1:4) and showed that the incidence rate of fracture at any site was 14.4‰ vs 8.3‰ for PA patients and 11.2‰ vs 6.5‰ for APA patients compared with that for controls (P<0.05) (19).
In summary, limited evidence has shown that PA is associated with an increased risk of bone fracture, which is of the greatest concern as the end-point event of osteoporosis, but few studies have focused directly on this association, highlighting the necessity of more research.
Changes in Biochemical Parameters of Bone Metabolism and BMD After Surgery or Spironolactone Treatment
It is well known that secondary osteoporosis is always potentially reversible, so some researchers have examined whether bone involvement can be reversed in PA patients after surgery or spironolactone treatment. Lawrence et al. first observed in 1985 that the serum calcium level was elevated postoperatively in six APA patients (P<0.05) (22). In 1995, Rossi et al. showed an increase in the serum ionized calcium level and a decrease in the PTH level after spironolactone administration or surgical treatment in ten PA patients (P<0.05) without collecting data on calcium excretion (11). In 2012, another study by Rossi et al. confirmed these results in 46 APA patients and found a significant decrease in urinary calcium excretion after adrenalectomy (5.6 ± 3.4 vs 4.1 ± 2.3 mmol/24 h, P = 0.038) (13). Subsequently, Maniero et al. also showed that adrenalectomy normalized PTH levels and markedly raised the serum ionized calcium level in 44 APA patients (24). Another study showed that changes in PTH levels appeared to be more pronounced in 5 patients with APA treated by surgery compared with those in 5 patients with idiopathic adrenal hyperplasia (IAH) treated by MR antagonists, but the serum calcium level was not significantly changed. However, this result should be interpreted cautiously since it was obtained in very small subgroups (14).
Studies assessing the change in the BMD over time in PA patients are scarce. In accordance with previous data, Salcuni et al. showed the same changes in urinary calcium excretion and PTH in 9 of 11 PA patients 6 months after the beginning of treatment (surgery or spironolactone), whereas in 5 of 11 PA patients 1 year after the beginning of treatment, the BMD was significantly increased in the lumbar spine (p < 0.01) (15). Subsequently, Ceccoli et al. also observed a significant improvement in the BMD in the lumbar spine and the Z-scores of the femoral neck and total hip after a mean follow-up of 24 months after treatment of 40 patients with PA, and a significant change in the PTH levels but not in the serum or urinary calcium levels were also observed (16). However, to date, no studies have assessed changes in fracture risk after surgery or spironolactone treatment for PA patients. All of these studies were compared the parameters before-and-after treatment of PA in same patients, and did not compare the results with those of EH patients. So, it is possible to ignore that the reduction in blood pressure itself may have a beneficial effect on bone metabolism.
In summary, bone loss in PA patients may be reversed by MR antagonists or adrenal surgery, but the results are limited by the relatively small sample size. Regarding the changes in serum and urinary calcium levels after treatment, inconsistent results has been observed, which may be due to the significant heterogeneity of studies (inconsistencies in object selection, drug doses, surgical approaches, follow-up time, and laboratory detection methods).
Serum Aldosterone in Patients With Osteoporosis
The available data show that PA may be a secondary cause of osteoporosis. Given the detrimental effects of excess aldosterone in bone tissue, attention has been focused on whether patients with osteoporosis have a high prevalence of PA and whether bone metabolism influences the renin angiotensin-aldosterone system (RAAS). Two recent studies targeted patients with osteoporosis (OP). Salcuni et al. observed a higher prevalence of PA in 213 OP patients than that in 109 controls (5.2% vs 0.9%) after excluding confounding factors. The prevalence of PA was even higher in patients with the concomitant presence of osteoporosis, hypertension, and hypercalciuria (26.1%) (10). Another recent study by Wu et al. showed that the PAC and ARR were elevated in 186 normotensive postmenopausal women with postmenopausal osteoporosis (PMO) compared with those in 96 osteopenia patients and 42 HS. An increase in false-positive plasma aldosterone/renin ratios (ARRs) was found in the PMO group. PAC was negatively associated with the BMD T-score of the lumbar spine, femur, neck, and total hip (27). Both studies considered the potential interactions between PA and bone from another perspective.
Discussion
How Does Excessive Aldosterone Affect Bone Metabolism?
Compared with the association between excess cortisol secretion and bone, less is known about the link between hyperaldosteronism and osteoporosis. However, the detrimental effects of excess aldosterone on bone may be associated with the interaction and co-secretion of aldosterone with cortisol (29–31). In a recent study, Arlt et al. (29) performed a mass spectrometry–based analysis of 24-hour urine steroid in 174 newly diagnosed patients with PA in comparison to that in controls. This suggested that the secretion of cortisol in patients with PA is significantly increased and is only exceeded by the glucocorticoid output of patients with clinically overt adrenal Cushing syndrome. On the one hand, excess cortisol has a direct detrimental effect on bone health, and the underlying mechanisms are beyond the scope of this review and have been reviewed elsewhere (4). On the other hand, excess aldosterone or cortisone activates MR receptors, and subsequent expansion of the intravascular volume results in decreased proximal tubular resorption, thereby increasing the distal delivery of Na+, Mg2+ and Ca2+; however, mineralocorticoids promote distal tubular Na+ resorption without increasing Mg2+ and Ca2+ excretion. Thus, a high urinary calcium level may be present. A similar response was also found in feces; however, only milligram quantities of Ca2+ and Mg2+ were excreted through this route (32). The decrease in Ca2+ and Mg2+ led to secondary hyperparathyroidism, with increases in bone resorption to ensure Ca2+ homeostasis. Spironolactone treatment attenuated the loss of Ca2+ at each site (33).
In addition, several possible mechanisms may explain the direct effects of aldosterone on bone health. First, mineralocorticoid receptors are expressed in human and rat osteoclasts, osteocytes, and osteoblasts (34) as well as in normal and adenomatous parathyroid tissue (24), and treatment with MR antagonists was shown to attenuate bone loss (15, 16), suggesting a direct effect of aldosterone on bone health and the potential bidirectional interaction between aldosterone and PTH. Second, aldosteronism may account for oxidative/nitrosative stress in a rat model by resulting in a proinflammatory phenotype. Oxidative stress and inflammation lead to systemic effects, including the increased excretion of divalent cations, diminished osteogenesis (35), increased osteoblast and osteocyte apoptosis, and reduced bone formation. spironolactone treatment prevented the development of the proinflammatory phenotype (33). Third, a genome‐wide study focused on identifying new candidate genes associated with osteoporosis indicated that genes involved in aldosterone signaling in epithelial cells may be linked to osteoporosis, supporting the potential relationship between aldosterone and bone (36). Moreover, a very recent study immunolocalized Ca metabolism-related receptors (calcium sensitive receptor, vitamin D receptor, and PTH receptor) in normal adrenal glands and aldosterone-producing adenomas, which demonstrated that aldosterone biosynthesis was regulated by calcium (37). Therefore, excess aldosterone biosynthesis may be related to bone resorption.
Primary Aldosteronism and Hyperparathyroidism
In the present study, subjects with PA had higher serum PTH levels compared with those patients with essential hypertension or normal subjects. So whether the prevalence of hyperparathyroidism in patients with PA was affected? In fact, reports about coincident PA and primary hyperparathyroidism are increasing (38). In a recent study, Asbach et al. reported the prevalence of primary hyperparathyroidism was 1.2% in a retrospective series of 503 patients with PA, and was 2.1% in 141 prospective PA patients, higher than in normal subjects (2.33‰ in women, 0.85‰ in women). They also observed that secondary hyperparathyroidism was common in patients with PA (39, 40). Meanwhile, Concistre et al. reported the prevalence of primary hyperparathyroidism in patients with PA was 2.6% (8/306) (41). As we know, very rarely, PA and hyperparathyroidism can occur together in Multiple Endocrine Neoplasia Type 1 (MEN1), which is inherited in an autosomal dominant manner with the prevalence 2–20/100,000 (42). Nevertheless, it must be noted that MEN1 could not be ruled out in the above two studies. Moreover, few papers focused on the change of aldosterone in primary hyperparathyroidism. In two case reports, the hypertension and secondary aldosteronism were resolved after the surgical treatment of primary hyperparathyroidism (43, 44), and in another study, decreased aldosterone after the extirpation of the parathyroid adenoma was observed in 16 patients (45), which indicated that aldosteronism might be caused by primary hyperparathyroidism occasionally. Above evidences indicated a bidirectional interaction between aldosterone and PTH, but details and concrete mechanisms need more researches to elucidate.
Strengths and Limitation
To our knowledge, this is the first systematic review aimed to assess the association between PA and osteoporosis. However, some limitations must be pointed out: First, the reliability of the data was limited by the small sample and the observational design of this study. Second, the results included were limited to full-text articles published in English, the problem of incompletely retrieval may exist, and there may be possible bias due to the heterogeneity of the analyzed studies. Third, laboratory testing methods were inconsistent in different studies, which may affect the accuracy of the study. Forth, the time of follow-up was not long enough. Fifth, few data can be acquired with regard to BMD and fractures, so only descriptive analyses had been conducted.
Conclusion
Excess aldosterone may be associated with increased serum PTH levels, hypercalciuria, which can be reversed by adrenalectomy or spironolactone treatment. BMD evaluated by DXA may not be an ideal index to reflect the bone involvement caused by aldosterone. PA may be a secondary cause of osteoporosis and is associated with an increased risk of bone fracture. The clarification of the relationships between PA and bone metabolism requires additional prospective randomized controlled studies in a large sample.
Data Availability Statement
All datasets presented in this study are included in the article/supplementary material.
Author Contributions
SS and TC designed the study and participated in data collection. SS performed the meta-analysis and drafted the manuscript. HT and YR partially conceived the research idea. CL edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by a grant from the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYGD18022).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Wen Wang, professor of Sichuan University Evidence-based Medicine Center, for her assistance in the study design and statistical analysis.
References
1. Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol (2006) 48(11):2293–300. doi: 10.1016/j.jacc.2006.07.059
2. Loh K-C, Koay ES, Khaw M-C, Emmanuel SC, Young WF Jr. Prevalence of primary aldosteronism among Asian hypertensive patients in Singapore. J Clin Endocrinol Metab (2000) 85:2854–9. doi: 10.1210/jcem.85.8.6752
3. Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol (2017) 69:1811–20. doi: 10.1016/j.jacc.2017.01.052
4. Lenore Buckley MD MPH, Mary B, Humphrey MD. Glucocorticoid-Induced Osteoporosis. N Engl J Med (2018) 379:2547–56. doi: 10.1056/NEJMcp1800214
5. Buckley L, Humphrey MB. Glucocorticoid-Induced Osteoporosis. New Engl J Med (2018) 379:2547–56. doi: 10.1056/NEJMcp1800214
6. van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatol (Oxford Engl) (2000) 39:1383–9. doi: 10.1093/rheumatology/39.12.1383
7. Hardy RS, Zhou H, Seibel MJ, Cooper MS. Glucocorticoids and Bone: Consequences of Endogenous and Exogenous Excess and Replacement Therapy. Endoc Rev (2018) 39:519–48. doi: 10.1210/er.2018-00097
8. Altieri B, Muscogiuri G, Paschou SA, Vryonidou A, Della Casa S, Pontecorvi A, et al. Adrenocortical incidentalomas and bone: from molecular insights to clinical perspectives. Endocrine (2018) 62:506–16. doi: 10.1007/s12020-018-1696-z
9. Kim BJ, Lee SH. Bone Health in Adrenal Disorders. Endocrinol Metab (Seoul). (2018) 33(1):1–8. doi: 10.3803/EnM.2018.33.1.1
10. Salcuni AS, Carnevale V, Battista C, Palmieri S, Eller-Vainicher C, Guarnieri V, et al. Primary aldosteronism as a cause of secondary osteoporosis. Eur J Endocrinol (2017) 177:431–7. doi: 10.1530/EJE-17-0417
11. Rossi E, Sani C, Perazzoli F, Casoli MC, Negro A, Dotti C. Alterations of calcium metabolism and of parathyroid function in primary aldosteronism, and their reversal by spironolactone or by surgical removal of aldosterone-producing adenomas. Am J Hypertens (1995) 8:884–93. doi: 10.1016/0895-7061(95)00182-O
12. Petramala L, Zinnamosca L, Settevendemmie A, Marinelli C, Nardi M, Concistrè A, et al. Bone and mineral metabolism in patients with primary aldosteronism. Int J Endocrinol (2014) 2014. doi: 10.1155/2014/836529
13. Rossi Gian P, Ragazzo F, Seccia Teresa M, Maniero C, Barisa M, Calò Lorenzo A, et al. Hyperparathyroidism Can Be Useful in the Identification of Primary Aldosteronism Due To Aldosterone-Producing Adenoma. Hypertension (2012) 60:431–6. doi: 10.1161/HYPERTENSIONAHA.112.195891
14. Pilz S, Kienreich K, Drechsler C, Ritz E, Fahrleitner-Pammer A, Gaksch M, et al. Hyperparathyroidism in patients with primary aldosteronism: cross-sectional and interventional data from the GECOH study. J Clin Endocrinol Metab (2012) 97:E75–9. doi: 10.1210/jc.2011-2183
15. Salcuni AS, Palmieri S, Carnevale V, Morelli V, Battista C, Guarnieri V, et al. Bone involvement in aldosteronism. J Bone mineral Res Off J Am Soc Bone Mineral Res (2012) 27:2217–22. doi: 10.1002/jbmr.1660
16. Ceccoli L, Ronconi V, Giovannini L, Marcheggiani M, Turchi F, Boscaro M, et al. Bone health and aldosterone excess. Osteoporosis Int (2013) 24:2801–7. doi: 10.1007/s00198-013-2399-1
17. Notsu M, Yamauchi M, Yamamoto M, Nawata K, Sugimoto T. Primary aldosteronism as a risk factor for vertebral fracture. J Clin Endocrinol Metab (2017) 102:1237–43. doi: 10.1210/jc.2016-3206
18. Kim B-J, Kwak MK, Ahn SH, Kim H, Lee SH, Koh J-M. Lower trabecular bone score in patients with primary aldosteronism: human skeletal deterioration by aldosterone excess. J Clin Endocrinol Metab (2017) 103:615–21. doi: 10.1210/jc.2017-02043
19. Wu VC, Chang CH, Wang CY, Lin YH, Kao TW, Lin PC, et al. Risk of Fracture in Primary Aldosteronism: A Population-Based Cohort Study. J Bone Mineral Res (2017) 32:743–52. doi: 10.1002/jbmr.3033
20. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ (2015) 349:1–25. doi: 10.1136/bmj.g7647
21. Wells GA, Shea B, Higgins JP, Sterne J, Tugwell P, Reeves BC. Checklists of methodological issues for review authors to consider when including non-randomized studies in systematic reviews. Res Synth Methods (2013) 4:63–77. doi: 10.1002/jrsm.1077
22. Resnick LM, Laragh JH. Calcium metabolism and parathyroid function in primary aldosteronism. Am J Med (1985) 78:385–90. doi: 10.1016/0002-9343(85)90328-6
23. Rossi E, Perazzoli F, Negro A, Sani C, Davoli S, Dotti C, et al. Acute effects of intravenous sodium chloride load on calcium metabolism and on parathyroid function in patients with primary aldosteronism compared with subjects with essential hypertension. Am J Hypertension (1998) 11:8–13. doi: 10.1016/S0895-7061(97)00366-X
24. Maniero C, Fassinab A, Secciaa TM, Toniatod A, Iacobonee M, Plebanic M, et al. Mild hyperparathyroidism: a novel surgically correctable feature of primary aldosteronism. J Hypertension (2012) 30:390–5. doi: 10.1097/HJH.0b013e32834f0451
25. Wu VC, Chang CH, Wang CY, Lin YH, Kao TW, Lin PC, et al. Risk of Fracture in Primary Aldosteronism: A Population-Based Cohort Study. J Bone mineral Res Off J Am Soc Bone Mineral Res (2017) 32:743–52. doi: 10.1002/jbmr.3033
26. Jiang Y, Zhang C, Ye L, Su T, Zhou W, Jiang L, et al. Factors affecting parathyroid hormone levels in different types of primary aldosteronism. Clin Endocrinol (2016) 85:267–74. doi: 10.1111/cen.12981
27. Shu X, Mei M, Ma L, Wang Z, Yang S, Hu J, et al. Postmenopausal osteoporosis is associated with elevated aldosterone/renin ratio. J Hum Hypertens (2018) 32:524–30. doi: 10.1038/s41371-018-0069-7
28. Silva BC, Leslie WD, Resch H, Lamy O, Lesnyak O, Binkley N, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Mineral Res (2014) 29:518–30. doi: 10.1002/jbmr.2176
29. Arlt W, Lang K, Sitch AJ, Dietz AS, Rhayem Y, Bancos I, et al. Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI Insight (2017) 2(8). doi: 10.1172/jci.insight.93136
30. Tang L, Li X, Wang B, Ma X, Li H, Gao Y, et al. Clinical characteristics of aldosterone-and cortisol-coproducing adrenal adenoma in primary aldosteronism. Int J Endocrinol (2018) 2018. doi: 10.1155/2018/4920841
31. Shifman BM, Platonova NM, Molashenko NV, Troshina EA, Romanova NY, Kolesnikova GS. Aldosterone- and cortisol-co-secreting adrenal tumors: an uneasy sum of well-known parts (review). Problemy Endokrinol (2019) 65:113–23. doi: 10.14341/probl10036
32. Sun Y, Ahokas RA, Bhattacharya SK, Gerling IC, Carbone LD, Weber KT. Oxidative stress in aldosteronism. Cardiovasc Res (2006) 71:300–9. doi: 10.1016/j.cardiores.2006.03.007
33. Chhokar VS, Sun Y, Bhattacharya SK, Ahokas RA, Myers LK, Xing Z, et al. Hyperparathyroidism and the calcium paradox of aldosteronism. Circulation (2005) 111:871–8. doi: 10.1161/01.CIR.0000155621.10213.06
34. Beavan S, Horner A, Bord S, Ireland D, Compston J. Colocalization of glucocorticoid and mineralocorticoid receptors in human bone. J Bone Mineral Res (2001) 16:1496–504. doi: 10.1359/jbmr.2001.16.8.1496
35. Atashi F, Modarressi A, Pepper MS. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a review. Stem Cells Dev (2015) 24:1150–63. doi: 10.1089/scd.2014.0484
36. Gupta M, Cheung CL, Hsu YH, Demissie S, Cupples LA, Kiel DP, et al. Identification of homogeneous genetic architecture of multiple genetically correlated traits by block clustering of genome-wide associations. J Bone Mineral Res (2011) 26:1261–71. doi: 10.1002/jbmr.333
37. Gao X, Yamazaki Y, Tezuka Y, Onodera Y, Ogata H, Omata K, et al. The crosstalk between aldosterone and calcium metabolism in primary aldosteronism: A possible calcium metabolism-associated aberrant “neoplastic” steroidogenesis in adrenals. J Steroid Biochem Mol Biol (2019) 193:105434. doi: 10.1016/j.jsbmb.2019.105434
38. Asbach E, Bekeran M, Reincke M, Lang K, Hanslik G, Treitl M. Parathyroid Gland Function in Primary Aldosteronism. Hormone Metab Res (2015) 47:994–9. doi: 10.1055/s-0035-1565224
39. Asbach E, Bekeran M, Konig A, Lang K, Hanslik G, Treitl M, et al. Primary and Secondary Hyperparathyroidism in Patients with Primary Aldosteronism – Findings From the German Conn’s Registry. Exp Clin Endocrinol Diabetes (2019). doi: 10.1055/a-1027-6472
40. Yeh MW, Ituarte PHG, Zhou HC, Nishimoto S, Liu IA, Harari A, et al. Incidence and Prevalence of Primary Hyperparathyroidism in a Racially Mixed Population. J Clin Endocrinol Metab (2013) 98:1122–9. doi: 10.1210/jc.2012-4022
41. Concistre A, Petramala L, Zinnamosca L, Settevendemmie A, Corpaci F, Marinelli C, et al. Primary aldosteronism with concurrent primary hyperparathyroidism: clinical case load in a single centre. Eur Rev Med Pharmacol Sci (2015) 19:971–6. doi: 10.1136/bcr-2017-221530
42. Thakker RV, Newey PJ, Walls GV, Bilezikian JP, Dralle H, Ebeling PR, et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab (2012) 97:2990–3011. doi: 10.1210/jc.2012-1230
43. Sabbadin C, Cavedon E, Zanon P, Iacobone M, Armanini D. Resolution of hypertension and secondary aldosteronism after surgical treatment of primary hyperparathyroidism. J Endocrinol Invest (2013) 36:665–6. doi: 10.3275/8960
44. Barkan A, Marilus R, Winkelsberg G, Yeshurun D, Blum I. Primary Hyperparathyroidism: Possible Cause of Primary Hyperaldosteronism in a 60-Year-Old Woman*. J Clin Endocrinol Metab (1980) 51:144–7. doi: 10.1210/jcem-51-1-144
Keywords: primary aldosteronism, osteoporosis, bone metabolism, fracture, systematic review
Citation: Shi S, Lu C, Tian H, Ren Y and Chen T (2020) Primary Aldosteronism and Bone Metabolism: A Systematic Review and Meta-Analysis. Front. Endocrinol. 11:574151. doi: 10.3389/fendo.2020.574151
Received: 19 June 2020; Accepted: 09 September 2020;
Published: 25 September 2020.
Edited by:
Elaine Dennison, MRC Lifecourse Epidemiology Unit (MRC), United KingdomReviewed by:
Luigi Petramala, Sapienza University of Rome, ItalyClaudio Letizia, Sapienza University of Rome, Italy
Copyright © 2020 Shi, Lu, Tian, Ren and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Chen, ZHIuY2hlbnRhb0BxcS5jb20=; Yan Ren, cmVueWFuQHNjdS5lZHUuY24=
 Shaomin Shi
Shaomin Shi Chunyan Lu1
Chunyan Lu1 Tao Chen
Tao Chen