- Department of Obstetrics and Gynecology, University of Toyama, Toyama, Japan
Semi-allogenic fetuses are not rejected by the maternal immune system because feto-maternal tolerance induced by CD4+CD25+FoxP3+ regulatory T (Treg) cells is established during pregnancy. Paternal antigen-specific Treg cells accumulate during pregnancy, and seminal plasma priming plays an important role in expanding paternal antigen-specific Treg cells in mouse models. Although paternal-antigen specific Treg cells have not been identified in humans, recent studies suggest that antigen-specific Treg cells exist and expand at the feto-maternal interface in humans. Studies have also revealed that reduction of decidual functional Treg cells occurs during miscarriage with normal fetal chromosomal content, whereas insufficient clonal expansion of decidual Treg cells is observed in preeclampsia. In this review, we will discuss the recent advances in the investigation of mechanisms underlying Treg cell-dependent maintenance of feto-maternal tolerance.
Introduction
Feto-maternal tolerance protects the fetal tissues from rejection and leads to a successful pregnancy (1–7). After implantation of the blastocyst in the uterine endometrium, trophoblasts start to invade the endometrial tissue, and uterine spiral artery. Maternal lymphocytes such as CD4+ T cells, CD8+ T cells, and CD16−CD56bright natural killer (NK) cells express activation markers on their surfaces, suggesting that maternal lymphocytes recognize trophoblasts or fetuses (8). Interaction with maternal immune regulation and trophoblast-derived tolerogenic molecules induces a tolerogenic environment at the feto-maternal interface. Considering the maternal immune system, regulatory T cells (Treg cells) play an essential role in the maintenance of allogenic pregnancy (9–12). CD4+CD25+Foxp3+ regulatory T (Treg) cells regulate the T cell response. Treg cells are necessary to sustain tissue homeostasis and establish immune tolerance (13), and are also related to tumor growth and organ transplantation tolerance (14). Previous studies in mouse models have demonstrated that paternal antigen-specific Treg cells are expanded systemically and locally during pregnancy (15–17). Seminal plasma primes the induction of paternal antigen-specific Treg cells (17, 18). Treg cells also increase systemically and locally during human pregnancies (12, 19), whereas paternal antigen-specific Treg cells have not been identified in humans. Recent studies show that target-specific, clonally expanded Treg cells are expanded at the feto-maternal interface in human pregnancies (20). In the first part of this review, we discuss mechanisms by which Treg cells induce feto-maternal tolerance and highlight antigen-specific Treg cells by introducing recent important findings. Following that, we will attempt to analyze the relationship between maldistribution and dysfunction of Treg cells and implantation failure, recurrent pregnancy loss, and preeclampsia in humans.
Maternal Immune Cells at the Feto-Maternal Interface
Maternal immune cells in the reproductive tissues first come into contact with paternal antigens when seminal fluid is ejaculated into the vagina during intercourse. Seminal fluid is composed of seminal plasma and sperm. Maternal immune cells recognize paternal antigens which are contained in the seminal plasma. Sperm reach the fallopian tube and fertilize the oocyte present there. After fertilization, the blastocyst migrates to the uterus while undergoing cell cleavage and finally attaches to the decidua. During the implantation period, the blastocyst adheres to and starts invading the uterine endometrium. In human pregnancy, the cells of the trophoblast differentiate into villous and extravillous trophoblasts (EVTs), forming the placenta. EVTs invade the decidua and myometrium. Subsequent to implantation, EVTs further penetrate the maternal spiral artery and finally replace the vascular lumen (21, 22). The feto-maternal interface is thereby formed, and EVTs and maternal immune cells contact each other (23). EVTs escape from maternal immune cells by controlling the major histocompatibility complex (MHC) and expressing immune suppressive molecules. The maternal immune system also dynamically changes to induce tolerance against fetal tissues (Figure 1).
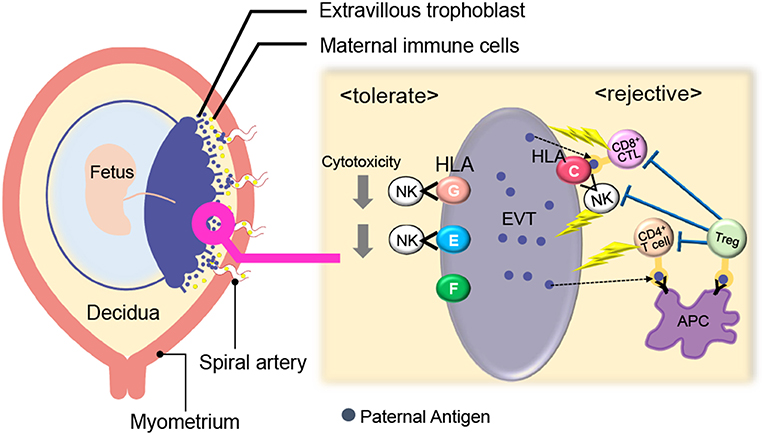
Figure 1. Immunological balance at the feto-maternal interface during early pregnancy. EVTs did not express polymorphic HLA-A, B whereas HLA-C and non-polymorphic HLA-E, G, and F were expressed. Maternal CD8+ T cells and NK cells can directly recognize paternal HLA-C and CD4+ T cells can indirectly recognize it. HLA- E and G protect EVTs from NK-cell mediated cytotoxicity. Treg cells can recognize fetal antigens via maternal antigen presenting cells (APCs) and induce tolerance in an antigen-specific manner. EVT, Extravillous trophoblast; NK, natural killer cell; Treg; regulatory T cell; APC, antigen-presenting cell.
Villous trophoblasts lack the surface expression of MHC class I and class II. EVTs do not express polymorphic HLA-A, B, whereas they express HLA-C and non-polymorphic HLA-E, G, and F (24–29). Maternal CD8+ T cells and NK cells can directly recognize paternal HLA-C, and CD4+ T cells can indirectly recognize it. On the other hand, HLA- E and G protect EVTs from NK-cell mediated cytotoxicity (30, 31). HLA-G positive EVTs regulate T cell activation through the induction of tolerogenic dendritic cells (DCs) (32) and directly cause the expansion of Treg cells (33). Furthermore, trophoblasts suppress maternal immune cells via the expression of indoleamine 2,3-dioxygenase (IDO) (34, 35), the secretion of inhibitory cytokines, such as IL-10 and TGF-β (36), and the expression of programmed death ligand (PD-L I) (37).
Considering maternal immune cells in the decidua, Treg cells and CD56brightCD16−uterine NK (uNK) cells play an important role in the maintenance of feto-maternal tolerance (3, 4, 38–41) (Figure 1). Treg cells, which are discussed in detail at a later part, can recognize fetal antigens via maternal antigen presenting cells (APCs) and induce tolerance in an antigen-specific manner. Compared with ordinary peripheral blood (pb) NK cells that have high cytotoxicity, uNK cells (CD56brightCD16− NK cells) produce many cytokines and their cytotoxic activities are low (42). Rather, uNK cells play an important role in uterine angiogenesis and spiral artery remodeling (23, 38, 43–46). The expression patterns of cell surface NK receptors in uNK cells differ from those of pbNK cells. For example, killer immunoglobulin-like receptor (KIR) and natural killer group 2 (NKG2) receptors are expressed at high levels on uNK cells (47). When the NKG2A receptor recognizes HLA-E on EVTs, an inhibitory signal suppresses the cytotoxicity of uNK cells (26). When KIR2DL on uNK cells interacts with HLA-G, the uNK cell activity is suppressed (30, 33).
CD8+ cytotoxic T cells can recognize the fetal antigen directly via HLA-C on EVTs and indirectly via maternal APCs (Figure 1). A previous report showed that fetal antigen-specific CD8+ cytotoxic T cells (CTLs) are detected in maternal peripheral blood during human pregnancies (48, 49). Viral antigen-specific decidual CTLs that can cross-react against allo-antigens are also reported (50, 51). CTLs in the decidua have distinct phenotypes and functions compared with those in peripheral blood. T-cell immunoglobulin mucin-3 (Tim-3) and programmed cell death-1 (PD-1) are negative immune regulatory molecules. The expression of Tim-3+PD-1+CD8+ T cells was higher in the human decidua than in peripheral blood. EVTs promote enrichment of Tim-3+PD-1+CD8+ T cells in an HLA-C dependent manner, suggesting that decidual CD8+ T cells would not attack trophoblasts. Furthermore, maternal Tim-3+PD-1+CD8+ T cells recognize PD-L I expressed on EVTs, resulting in trophoblast antigen-specific tolerance (52). Highly differentiated resident memory CD8+ T cells are observed in the decidua. This subset shows a lower expression of perforin and granzyme B (53). These reports suggest that antigen-specific CTLs exist at the feto-maternal interface, but their cytotoxic activity is controlled by the placental tissue (53).
How Do Paternal Antigen-Specific Treg Cells Function in Allogenic Pregnancy?
Previous studies suggest that Treg cells play an essential role in the induction of paternal antigen-specific tolerance in allogenic pregnancy in mice. Paternal MHC-specific tolerance during allogenic pregnancy was demonstrated by Tafuri et al. where a paternal MHC-bearing tumor graft was not rejected during pregnancy with the conceptus MHC being identical to the tumor graft, but was rejected in the postpartum period (54).
Aluvihare et al. (11) demonstrated that Treg cells are necessary for allogenic pregnancy in mice, but not necessary for syngeneic pregnancy. When T cell-depleted BALB/C nu/nu female mice were mated with C57BL/6 male mice (allogenic pregnancy) after transfer of total lymphocytes, pregnancies were normally maintained. On the other hand, mating after transfer of Treg cell-depleted lymphocytes resulted in fetal loss, suggesting that Treg cells are essential for the maintenance of allogenic pregnancies (11). Adoptive transfer of CD4+CD25+ Treg cells from mice with an allogenic pregnancy prevented fetal rejection in an abortion-prone mouse model during allogenic pregnancy, if the transfer was conducted before day 4.5 of gestation (9). Furthermore, depletion of Treg cells using anti-CD25 monoclonal antibodies induced implantation failure and abortion in allogenic pregnancies, but did not induce any pregnancy complications during the late stages of pregnancy (10). These findings suggest that Treg cells induce allo-antigen-specific tolerance and are necessary from implantation through early pregnancy periods in mice.
Where and how do fetal antigen-specific Treg cells expand during pregnancy? Previous studies have demonstrated the existence of fetal antigen-specific Treg cells and their distribution in mouse models (Table 1). Kahn and Baltimore showed that the H-Y-specific suppressive capability of Treg cells in splenocytes escalated more during pregnancy than before pregnancy (15). Rowe et al. demonstrated that Treg cells specific for the 2W1S antigen, which is derived from the mouse MHC-Eα chain, expanded in the systemic lymph nodes during the 1st pregnancy and rapidly re-accumulated during the 2nd pregnancy (16). Furthermore, Shima et al. reported the local distribution of paternal antigen-specific Treg cells during the implantation period and after pregnancy (Figure 2). DBA/2 mice have the Mls 1a super antigen, which is recognized by Vβ6 of the T cell receptor β chain. When BALB/C female mice were mated with DBA/2 male mice, CD4+CD25+Vβ6+ Treg cells, which can be regarded as Mls 1a-specific Treg cells, increased in the uterine-draining lymph nodes one day before implantation. This phenomenon was not observed when BALB/C female mice were mated with seminal vesicle-excised DBA/2 male mice. The local fetal antigen-specific Treg cells might be expanded at the draining lymph nodes by seminal plasma-priming and migrate to the uterus after pregnancy. After implantation, the Vβ6+ Treg cell population in the uterus increased day by day during pregnancy, but that in peripheral lymph nodes and spleen did not (17). Therefore, accumulation of paternal antigen-specific Treg cells is regulated in an organ-specific manner.
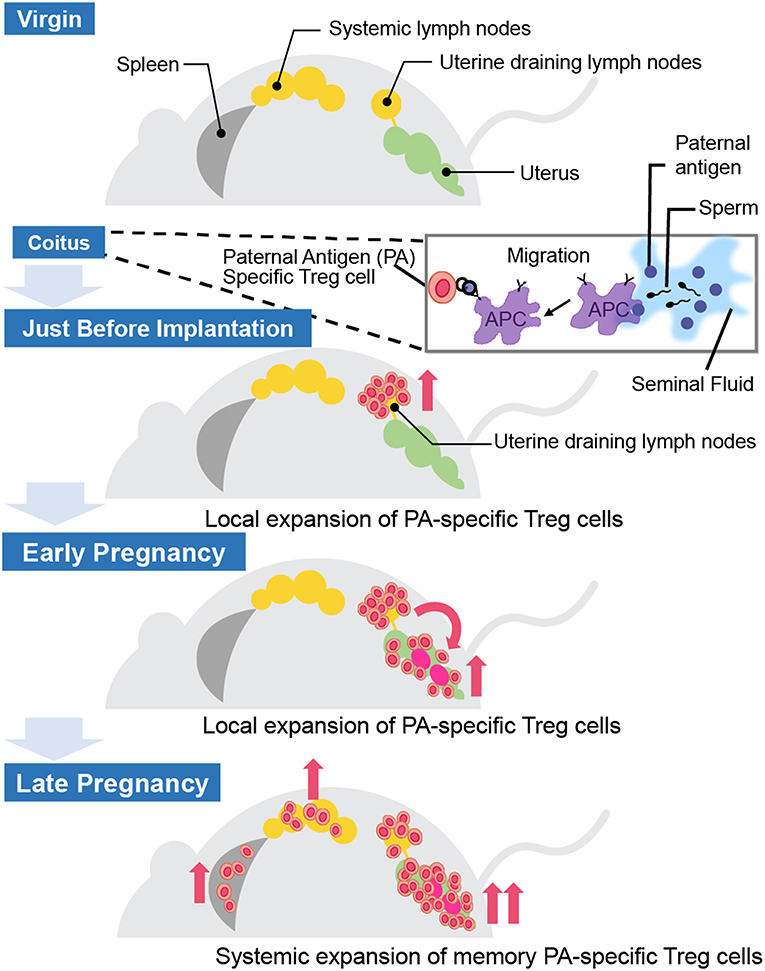
Figure 2. Distribution of paternal antigen-specific Treg cells in mice. When a female mouse is mated with an allogenic male mouse, paternal antigen-specific Treg cells increase in the uterine-draining lymph nodes one day before implantation due to seminal plasma priming. After implantation, the paternal antigen-specific Treg cells population in the uterus increases day by day during the course of the pregnancy, but not in the peripheral lymph nodes and the spleen. APC, antigen-presenting cell.
Paternal Antigen-Specific Treg Cells in Human Pregnancies
During pregnancy in humans, the systemic and local expansion of the Treg cell pool is observed from the 1st trimester and reaches a maximum in the 2nd trimester (12, 19). Although fetal antigen-specific regulatory T cells and their systemic and local expansion were observed in a mouse model, direct detection of fetal antigen-specific Treg cells is difficult in humans due to heterogenic MHC expression and limited knowledge concerning the physiological target peptide of Treg cells (55). However, the existence of fetal antigen-specific Treg cells in human pregnancies was indirectly suggested in some reports.
Decidual Treg cells, but not peripheral blood Treg cells, showed higher suppression toward self-fetal cord blood than 3rd party cord blood, suggesting that fetal antigen-specific Treg cells might exist at the feto-maternal interface during human pregnancy (56). Among human Treg cell subsets, CD4+CD45RA−FoxP3high comprises effector Treg cells which are memory type T cells with a high suppressive capability, and CD4+CD45RA+FoxP3low comprises naïve Treg cells with a relatively lower suppressive capability (57, 58). Effector Treg cells are the most dominant among Treg cells in both peripheral blood and decidua in the late gestation stage of human pregnancies (59). To demonstrate if expansion of the effector Treg cell pool is a reflection of clonal expansion of antigen-specific Treg cells, we conducted single-cell-based T cell receptor (TCR) repertoire analysis of CD4+CD25+CD45RA−CD127low effector Treg cells in human pregnancies (Figure 3). Our study was the first to reveal that clonally expanded effector Treg cells were observed only in the decidua, but not in the peripheral blood (Figure 4A). Clonally expanded effector Treg cells were higher in the 3rd trimester than in the 1st trimester (Figure 4A). On the other hand, the common clonotypic effector Treg cells between the decidua and peripheral blood were rarely observed (20). Therefore, decidual effector Treg cells might recognize some antigens expressed at the feto-maternal interface and proliferate upon antigen stimulation. However, effector Treg cells in the peripheral blood expand nonspecifically. Interestingly, the same clonotypic decidual effector Treg cells repeatedly appeared in previous and subsequent pregnancies in three cases: two ended with paired normal term deliveries and the third ended with paired miscarriages (20). TCRβ varies, with over 2 × 107 patterns estimated in young humans (60), thus these same clonotypic Treg cells might be repeatedly recruited by the same antigens at the feto-maternal interface rather than by accidental coincidence. Furthermore, clonal populations of decidual effector Treg cells were lower in the 3rd trimester in preeclampsia cases than in normal pregnancies (Figure 4B). However, while the effector Treg cell pool was reduced, clonal populations were not reduced in 1st trimester miscarriage cases (20) (Figure 4B). Taken together, these data indicate that fetal antigen-specific Treg cells might be recruited and expand in a fetal antigen-specific manner at the feto-maternal interface, and polyclonally expand in systemic circulation during human pregnancy. Clonally expanded decidual Treg cells might be important in the maintenance of feto-maternal tolerance, especially in the 3rd trimester.
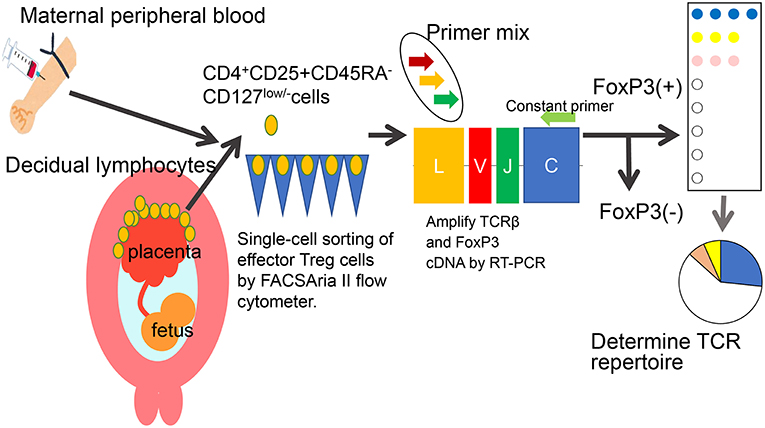
Figure 3. Single-cell based TCR repertoire analysis method. To study the clonality of effector Treg cells, a single-cell based T cell receptor (TCR) repertoire analysis method was used. Paired samples of maternal peripheral blood mononuclear cells and decidual lymphocytes were obtained. CD4+CD25+CD45RA-CD127low/- effector Treg cells were single-cell sorted. The cDNAs of complementarity determining lesion 3 (CDR3) in TCRβ chain and FoxP3 were amplified by RT-PCR. The nucleotides and amino acid sequences of CDR3 were analyzed.
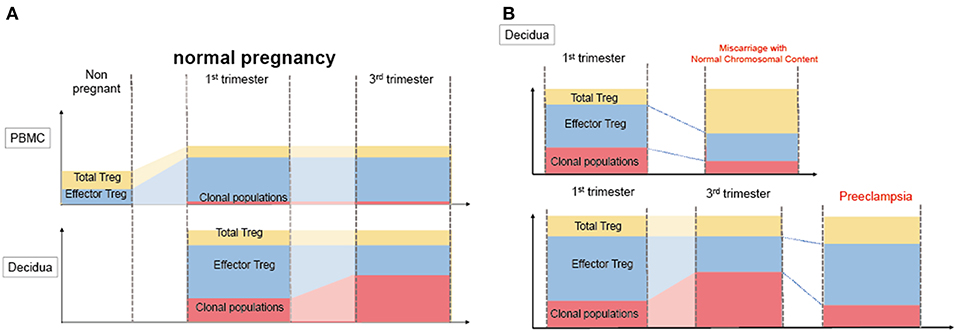
Figure 4. Distribution of clonally expanded effector Treg cells in humans. (A) Frequencies of total Treg cells, effector Treg cells and clonal populations of effector Treg cells in normal pregnancy. The systemic and local expansion of the Treg cells and effector Treg cells pool is observed during pregnancies in humans. Clonally expanded effector Treg cells increase in decidua, but not in peripheral blood. Clonal populations of effector Treg cells more increase in 3rd trimester than 1st trimester. (B) Clonally expanded decidual effector Treg cells in miscarriage and preeclampsia. In decidua, effector Treg cells pool decreased in miscarriage with normal chromosomal content than 1st trimester normal pregnancy, whereas the frequency of clonal populations of effector Treg cells does not significantly decrease. On the other hand, clonal populations of decidual effector Treg cells decreased preeclampsia than 3rd trimester normal pregnancies.
Which Peptides Are Recognized by Treg Cells at the Feto-Maternal Interface?
Paternal MHC and minor antigen-derived peptide-specific Treg cells were identified in mouse models as previously described (15–17). A recent study showed that non-inherited maternal antigen (NIMA)-specific Treg cells enforce tolerance in a mouse model (61). NIMAs are peptides derived from polymorphic genes such as MHC and are expressed in the mother but not in offspring. Microchimerism, developed during pregnancy and breast feeding, enables transfer of maternal cells to the offspring. Microchimeric maternal cells persist for a long time and tolerance for NIMAs persists during this time. Kinder et al. demonstrated that the paternal MHC-derived antigen was identical to the NIMA, and the rapid expansion of NIMA-specific Treg cells contributed to successful pregnancy (61). NIMA-matched organ transplantation presents a lower risk of rejection than NIMA-mismatched transplantation in humans (62–65). Thus, theoretically, NIMA-specific tolerance might be induced during human pregnancy.
In humans, a polymorphic HLA-C mismatch pregnancy indicates T cell activation and Treg cell expansion (66). In an oocyte donation (OD) pregnancy, in which the fetus is a total allograft, the match level of HLA-A, B, C, DR, and DQ between the mother and offspring was higher, and fewer pregnancy complications were observed (67). Trophoblasts lack surface expression of HLA-class II molecules, but contain them intracellularly. Considering microchimerism between the mother and fetus, peptides derived from HLA-class II can act as epitopes presented by maternal APCs. Trophoblasts contain intracellular HLA-class II and release HLA-DR molecules upon stimulation by IFN-γ (68). Cell surface expression of HLA-DR in syncytiotrophoblasts and the presence of HLA-DR in syncytiotrophoblast-derived extracellular vesicles were observed in preeclampsia (69). Seminal plasma also contains soluble HLA molecules (70–72). Additionally, human EVTs express minor histocompatibility antigens, such as HY, HA, and ACC (73). HY antigen-specific CD8+ T cells were observed during human pregnancy (48). Even these minor histocompatibility antigens mediate graft-vs.-host disease after organ transplantation (73); however, fetal tissues are not rejected. Taken together, allogenic-HLA-derived, and minor histocompatibility antigen-derived peptides presented by maternal APCs might be recognized by CD4+ conventional T cells and Treg cells. The main target antigens of decidual CD4+ conventional T cells, CTLs, and Treg cells are yet unclear. Further investigation is required to reveal the antigen-specificity of each T cell type and their regulation at the feto- maternal interface.
What Is the Origin of Treg Cells at the Feto-Maternal Interface?
There are two types of Treg cells in terms of origin: naturally occurring Treg (nTreg) cells, which originate in the thymus, and inducible Treg (iTreg) cells, which arise from conventional CD4+ T cells in peripheral tissues (74). Conserved noncoding sequence 1 (CNS1) is a FoxP3 enhancer and is necessary for developing iTreg cells. Interestingly, only placental mammals have CNS-1, while marsupials and monotremes do not. CNS-1 knockout female mice with allogenic pregnancies showed increased fetal resorption (75). Thus, iTreg cells might be necessary to maintain allogenic pregnancy in placental mammals. On the other hand, nTreg cells are the dominant population (~95%) among decidual Treg cells in the 1st trimester of human pregnancy, and the proportion of nTreg cells is similar between normal pregnancy and miscarriage (76). However, decidual iTreg cells significantly decreased in preeclampsia (77). To maintain human pregnancy, nTreg cells might be important in early stage pregnancy, and iTreg cells might also be important in late-stage gestation. Further study is necessary to confirm this possibility.
Pregnancy Complications and Treg Cells in Humans
Maldistribution and functional impairment of Treg cells were reported in implantation failure, miscarriage, and preeclampsia in humans. Contrarily, Treg cells are necessary in the implantation period and early gestation, but not in late gestation in mice (10).
Multiple factors, including Treg cell impairment, are thought to be related to implantation failure in humans. Treg cell transcription factor FoxP3 mRNA expression in the uterine endometrium is decreased in primary unexplained infertility (78, 79). A decrease in Treg cells in the peripheral blood in the late follicular phase predicts failure of artificial insemination by the donor (AID) sperm (80). These findings support the hypothesis that maldistribution of Treg cells impairs implantation in humans. Additionally, exposure to seminal plasma raises the success rate of IVF-ET pregnancy (81). Further investigation can validate the evidence that priming with the seminal plasma results in Treg cell-mediated tolerance and can rescue implantation failure.
Disturbance of Treg cell-mediated tolerance might be one of the etiologies of miscarriage. Previous studies reported that Treg cells were decreased in the peripheral blood and decidua in miscarriage cases (12, 82–84). Impaired suppressive capability of Treg cells in recurrent miscarriage cases has also been observed (85–88). Effector Treg cells and nTreg cells were decreased in the case of miscarriage with a normal karyotyped fetus compared to that in the 1st trimester of a normal pregnancy or in the case of miscarriage with an abnormal karyotyped fetus (76, 89). On the other hand, the clonally expanded population of Treg cells showed no significant difference between these groups (20) (Figure 5). These findings suggest that the number of nTreg cells and effector Treg cells is more important than antigen-specific Treg cell recruitment during the 1st trimester. The total Treg cell volume that regulates excessive inflammation might be important for the maintenance of the early gestation phase of pregnancy. A previous report demonstrated that high dose immunoglobulin therapy improved the live birth rate for refractory recurrent miscarriage cases with four or more consecutive miscarriages (90). Other studies also showed the effectiveness of anti-TNF-alfa inhibitor therapy (91). These medications have the potential to suppress immune activity in an antigen-nonspecific manner; therefore, these therapies might benefit patients with recurrent miscarriage with a reduced decidual effector Treg cell pool. In humans, peripheral blood Treg cells and decidual Treg cells form clonotypically different populations, and the migration of Treg cells from systemic circulation to the decidua has not yet been shown (20). Thus, these findings might explain why immunization therapy using white blood cells from the patient's partner is not effective for treating recurrent miscarriage (92).
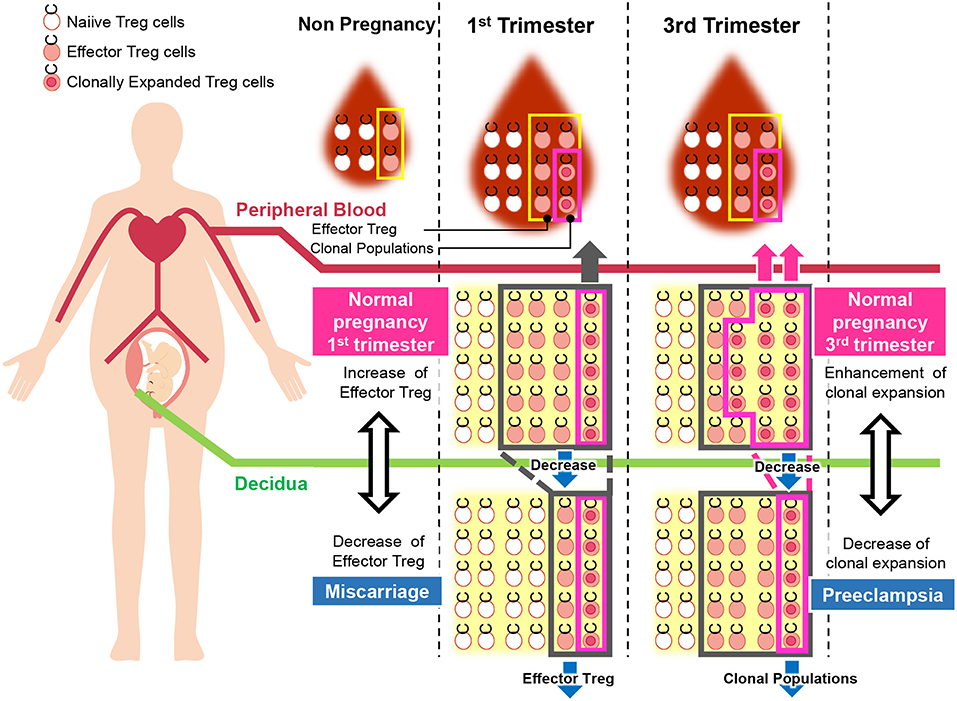
Figure 5. Pathological change in Treg cells during pregnancy in humans. During pregnancy, Treg cell pools both in the peripheral blood and decidua expand. Clonally expanded effector Treg cells are observed only in the decidua, but not in the peripheral blood. Clonally expanded effector Treg cells are higher in the 3rd trimester than in the 1st trimester. In miscarriage cases with normal chromosomal fetal content, the number of decidual effector Treg cells decreases. On the other hand, clonal populations of decidual effector Treg cells decrease in cases of preeclampsia.
Preeclampsia, which is defined as hypertension concomitant with proteinuria or placental dysfunction occurring in mid to late gestation, is a major cause of maternal and fetal morbidity and mortality. Chronic inflammation due to activation of neutrophils and NK cells, elevation of pro-inflammatory cytokines, and dysfunction of Treg cells is also thought to contribute to the pathogenesis of preeclampsia (93). Epidemiological findings provide the hypothesis that failure in maintaining paternal antigen-specific tolerance is related to the development of preeclampsia. First pregnancy, pregnancy following a partner change, and a pregnancy interval of more than ten years raise the risk of preeclampsia (94–96). Long-term condom usage and AID pregnancy also elevate the risk of preeclampsia, suggesting insufficient paternal antigen-specific tolerance mediated by seminal plasma priming (70, 97, 98). OD pregnancy, in which the fetus is completely allogenic and no priming effect has occurred, is associated with a high risk of preeclampsia (6, 98). Basic research on Treg cells supports this hypothesis.
Previous reports show that Treg cell pools decrease in the peripheral blood and decidua in preeclampsia (77, 99–108). Some reports demonstrate functionally impaired Treg cells in preeclampsia, where Treg cell apoptosis can be easily induced (109). Other reports showed that effector Treg cells decreased in the peripheral blood (108). Hsu et al. demonstrated that the function of decidual APCs was impaired in preeclampsia, resulting in fewer peripherally induced Treg cells (iTreg cells) than in normal pregnancies (77). Elevation of soluble endoglin (sEND), which is a co-receptor of TGFβ, results in the capture of circulating TGFβ, resulting in a systemic decrease of the Treg cell pool. It might also disturb the conversion of conventional Treg cells to iTreg cells (6).
So far, it has not been clarified whether a decreased total volume of Treg cells or decreased paternal antigen-specific Treg cells are related to the pathogenesis of preeclampsia. Our study reported for the first time that clonal expansion of decidual Treg cells was impaired in preeclampsia, suggesting that paternal antigen-specific tolerance might be insufficient. The frequencies of clonal populations of decidual effector Treg cells were 20.9% (15.4–28.1%) in the 3rd trimester during normal pregnancy and 9.3% (4.4–14.5%) in pregnancies with preeclampsia (Figure 5). Both early onset and late onset preeclampsia showed the same tendency (20). Our result is compatible with epidemiological evidence that inadequate paternal antigen-specific tolerance raises the risk of preeclampsia. Paternal antigen-specific Treg cells are more important in the late gestation period of pregnancy than in early gestation (Figure 3). Decidual iTreg cells and clonal populations of decidual effector Treg cells decreased in preeclampsia (20, 77). The main population of decidual effector Treg cells during early pregnancy was that of nTreg (76), and the clonal population of decidual effector Treg cells did not decrease. These findings suggest that clonally expanded Treg cells might be iTreg cells. This point requires clarification in the future.
In terms of the clinical applications of these findings, oocyte donation after HLA matching with maternal or paternal HLA might reduce the risk of preeclampsia. Encouraging seminal plasma exposure might play a protective role for high risk patients.
Conclusion
Treg cell-mediated feto-maternal tolerance is important in the maintenance of allogenic pregnancy. Paternal antigen-specific Treg cells are expanded systemically and locally during mouse pregnancy. Seminal plasma priming induces paternal antigen-specific Treg cells. Although paternal antigen-specific Treg cells have not been identified in humans, clonal expansion of decidual effector Treg cells implies that antigen-specific tolerance by Treg cells might be induced during human pregnancy. A reduced amount of decidual Treg cells might be related to the pathogenesis of miscarriage, and the failure of decidual Treg cell clonal expansion might be related to the pathogenesis of preeclampsia in humans.
Author Contributions
SS, ST, TS, and AN conception and design, drafting manuscript and revision of the manuscript for important intellectual content.
Funding
This work was supported by grants from Ministry of Education, Culture, Sports, Science, and Technology in Japan [KAKENHI Grant Number 15H04980 (SS), 17K11221(TS)].
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing.
References
1. Saito S, Shima T, Nakashima A, Shiozaki A, Ito M, Sasaki Y. What is the role of regulatory T cells in the success of implantation and early pregnancy? J Assist Reprod Genet. (2007) 24:379–86. doi: 10.1007/s10815-007-9140-y
2. Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol. (2013) 13:23–33. doi: 10.1038/nri3361
3. Robertson SA, Prins JR, Sharkey DJ, Moldenhauer LM. Seminal fluid and the generation of regulatory T cells for embryo implantation. Am J Reprod Immunol. (2013) 69:315–30. doi: 10.1111/aji.12107
4. Zenclussen AC. Adaptive immune responses during pregnancy. Am J Reprod Immunol. (2013) 69:291–303. doi: 10.1111/aji.12097
5. PrabhuDas M, Bonney E, Caron K, Dey SCA, Fazleabas A, et al. Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat Immunol. (2015) 16:328–34. doi: 10.1038/ni.3131
6. Saito S, Nakabayashi Y, Nakashima A, Shima T, Yoshino O. A new era in reproductive medicine: consequences of third-party oocyte donation for maternal and fetal health. Semin Immunopathol. (2016) 38:687–97. doi: 10.1007/s00281-016-0577-x
7. Saito S, Shima T, Nakashima A, Inada K, Yoshino O. Role of paternal antigen-specific treg cells in successful implantation. Am J Reprod Immunol. (2016) 75:310–6. doi: 10.1111/aji.12469
8. Saito S, Nishikawa K, Morii T, Narita N, Enomoto M, Ichijo M. Expression of activation antigens CD69, HLA-DR, interleukin-2 receptor-alpha (IL-2R alpha) and IL-2R beta on T cells of human decidua at an early stage of pregnancy. Immunology. (1992) 75:710–2.
9. Zenclussen AC, Gerlof K, Zenclussen ML, Sollwedel A, Bertoja AZ, Ritter T, et al. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol. (2005) 166:811–22. doi: 10.1016/S0002-9440(10)62302-4
10. Shima T, Sasaki Y, Itoh M, Nakashima A, Ishii N, Sugamura K, et al. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J Reprod Immunol. (2010) 85:121–9. doi: 10.1016/j.jri.2010.02.006
11. Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. (2004) 5:266–71. doi: 10.1038/ni1037
12. Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. (2004) 10:347–53. doi: 10.1093/molehr/gah044
13. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. (1995) 155:1151–64.
14. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. (2008) 133:775–87. doi: 10.1016/j.cell.2008.05.009
15. Kahn DA, Baltimore D. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci USA. (2010) 107:9299–304. doi: 10.1073/pnas.1003909107
16. Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. (2012) 490:102–6. doi: 10.1038/nature11462
17. Shima T, Inada K, Nakashima A, Ushijima A, Ito M, Yoshino O, et al. Paternal antigen-specific proliferating regulatory T cells are increased in uterine-draining lymph nodes just before implantation and in pregnant uterus just after implantation by seminal plasma-priming in allogeneic mouse pregnancy. J Reprod Immunol. (2015) 108:72–82. doi: 10.1016/j.jri.2015.02.005
18. Robertson SA, Guerin LR, Bromfield JJ, Branson KM, Ahlstrom AC, Care AS. Seminal fluid drives expansion of the T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol Reprod. (2009) 80:1036–45. doi: 10.1095/biolreprod.108.074658
19. Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive regulatory T-cell subset. Immunology. (2004) 112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x
20. Tsuda S, Zhang X, Hamana H, Shima T, Ushijima A, Tsuda K, et al. Clonally expanded decidual effector regulatory T cells increase in late gestation of normal pregnancy, but not in preeclampsia, in humans. Front Immunol. (2018) 9:1934. doi: 10.3389/fimmu.2018.01934
21. Fisher SJ, Damsky CH. Human cytotrophoblast invasion. Semin Cell Biol. (1993) 4:183–8. doi: 10.1006/scel.1993.1022
22. Fisher SJ. Why is placentation abnormal in preeclampsia? Am J Obstet Gynecol. (2015) 213:S115–22. doi: 10.1016/j.ajog.2015.08.042
23. Wallace AE, Fraser R, Cartwright JE. Extravillous trophoblast and decidual natural killer cells: a remodelling partnership. Hum Reprod Update. (2012) 18:458–71. doi: 10.1093/humupd/dms015
24. Kovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, DeMars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science. (1990) 248:220–3. doi: 10.1126/science.2326636
25. McMaster MT, Librach CL, Zhou Y, Lim KH, Janatpour MJ, DeMars R, et al. Human placental HLA-G expression is restricted to differentiated cytotrophoblasts. J Immunol. (1995) 154:3771–8.
26. King A, Allan DS, Bowen M, Powis SJ, Joseph S, Verma S, et al. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur J Immunol. (2000) 30:1623–31. doi: 10.1002/1521-4141(200006)30:6<1623::AID-IMMU1623>3.0.CO;2-M
27. King A, Burrows TD, Hiby SE, Bowen JM, Joseph S, Verma S, et al. Surface expression of HLA-C antigen by human extravillous trophoblast. Placenta. (2000) 21:376–87. doi: 10.1053/plac.1999.0496
28. Barakonyi A, Kovacs KT, Miko E, Szereday L, Varga P, Szekeres-Bartho J. Recognition of nonclassical HLA class I antigens by gamma delta T cells during pregnancy. J Immunol. (2002) 168:2683–8. doi: 10.4049/jimmunol.168.6.2683
29. Ishitani A, Sageshima N, Lee N, Dorofeeva N, Hatake K, Marquardt H, et al. Protein expression and peptide binding suggest unique and interacting functional roles for HLA-E, F, and G in maternal-placental immune recognition. J Immunol. (2003) 171:1376–84. doi: 10.4049/jimmunol.171.3.1376
30. Rajagopalan S, Long EO. A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. J Exp Med. (1999) 189:1093–100. doi: 10.1084/jem.189.7.1093
31. Li C, Houser BL, Nicotra ML, Strominger JL. HLA-G homodimer-induced cytokine secretion through HLA-G receptors on human decidual macrophages and natural killer cells. Proc Natl Acad Sci USA. (2009) 106:5767–72. doi: 10.1073/pnas.0901173106
32. Ristich V, Liang S, Zhang W, Wu J, Horuzsko A. Tolerization of dendritic cells by HLA-G. Eur J Immunol. (2005) 35:1133–42. doi: 10.1002/eji.200425741
33. Tilburgs T, Crespo AC, van der Zwan A, Rybalov B, Raj T, Stranger B, et al. Human HLA-G+ extravillous trophoblasts: Immune-activating cells that interact with decidual leukocytes. Proc Natl Acad Sci USA. (2015) 112:7219–24. doi: 10.1073/pnas.1507977112
34. Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. (1998) 281:1191–3. doi: 10.1126/science.281.5380.1191
35. Chang RQ, Li DJ, Li MQ. The role of indoleamine-2,3-dioxygenase in normal and pathological pregnancies. Am J Reprod Immunol. (2018) 79:e12786. doi: 10.1111/aji.12786
36. Guzeloglu-Kayisli O, Kayisli UA, Taylor HS. The role of growth factors and cytokines during implantation: endocrine and paracrine interactions. Semin Reprod Med. (2009) 27:62–79. doi: 10.1055/s-0028-1108011
37. Nagamatsu T, Schust DJ, Sugimoto J, Barrier BF. Human decidual stromal cells suppress cytokine secretion by allogenic CD4+ T cells via PD-1 ligand interactions. Hum Reprod. (2009) 24:3160–71. doi: 10.1093/humrep/dep308
38. King A, Wooding P, Gardner L, Loke YW. Expression of perforin, granzyme A and TIA-1 by human uterine CD56+ NK cells implies they are activated and capable of effector functions. Hum Reprod. (1993) 8:2061–7. doi: 10.1093/oxfordjournals.humrep.a137982
39. Higuma-Myojo S, Sasaki Y, Miyazaki S, Sakai M, Siozaki A, Miwa N, et al. Cytokine profile of natural killer cells in early human pregnancy. Am J Reprod Immunol. (2005) 54:21–9. doi: 10.1111/j.1600-0897.2005.00279.x
40. Saito S, Nakashima A, Myojo-Higuma S, Shiozaki A. The balance between cytotoxic NK cells and regulatory NK cells in human pregnancy. J Reprod Immunol. (2008) 77:14–22. doi: 10.1016/j.jri.2007.04.007
41. Moffett A, Colucci F. Uterine NK cells: active regulators at the maternal-fetal interface. J Clin Invest. (2014) 124:1872–9. doi: 10.1172/JCI68107
42. Saito S, Nishikawa K, Morii T, Enomoto M, Narita N, Motoyoshi K, et al. Cytokine production by CD16-CD56bright natural killer cells in the human early pregnancy decidua. Int Immunol. (1993) 5:559–63. doi: 10.1093/intimm/5.5.559
43. King A, Birkby C, Loke YW. Early human decidual cells exhibit NK activity against the K562 cell line but not against first trimester trophoblast. Cell Immunol. (1989) 118:337–44. doi: 10.1016/0008-8749(89)90382-1
44. Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. (2006) 12:1065–74. doi: 10.1038/nm1452
45. Lash GE, Schiessl B, Kirkley M, Innes BA, Cooper A, Searle RF, et al. Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. J Leukoc Biol. (2006) 80:572–80. doi: 10.1189/jlb.0406250
46. Pollheimer J, Vondra S, Baltayeva J, Beristain AG, Knofler M. Regulation of placental extravillous trophoblasts by the maternal uterine environment. Front Immunol. (2018) 9:2597. doi: 10.3389/fimmu.2018.02597
47. Cerdeira AS, Rajakumar A, Royle CM, Lo A, Husain Z, Thadhani RI, et al. Conversion of peripheral blood NK cells to a decidual NK-like phenotype by a cocktail of defined factors. J Immunol. (2013) 190:3939–48. doi: 10.4049/jimmunol.1202582
48. Lissauer D, Piper K, Goodyear O, Kilby MD, Moss PA. Fetal-specific CD8+ cytotoxic T cell responses develop during normal human pregnancy and exhibit broad functional capacity. J Immunol. (2012) 189:1072–80. doi: 10.4049/jimmunol.1200544
49. Tilburgs T, Strominger JL. CD8+ effector T cells at the fetal-maternal interface, balancing fetal tolerance and antiviral immunity. Am J Reprod Immunol. (2013) 69:395–407. doi: 10.1111/aji.12094
50. van Egmond A, van der Keur C, Swings GM, Scherjon SA, Claas FH. The possible role of virus-specific CD8(+) memory T cells in decidual tissue. J Reprod Immunol. (2016) 113:1–8. doi: 10.1016/j.jri.2015.09.073
51. van der Zwan A, van der Meer-Prins EMW, van Miert P, van den Heuvel H, Anholts JDH, Roelen DL, et al. Cross-reactivity of virus-specific CD8+ T cells against allogeneic HLA-C: possible implications for pregnancy outcome. Front Immunol. (2018) 9:2880. doi: 10.3389/fimmu.2018.02880
52. Wang SC, Li YH, Piao HL, Hong XW, Zhang D, Xu YY, et al. PD-1 and Tim-3 pathways are associated with regulatory CD8+ T-cell function in decidua and maintenance of normal pregnancy. Cell Death Dis. (2015) 6:e1738. doi: 10.1038/cddis.2015.112
53. Tilburgs T, Schonkeren D, Eikmans M, Nagtzaam NM, Datema G, Swings GM, et al. Human decidual tissue contains differentiated CD8+ effector-memory T cells with unique properties. J Immunol. (2010) 185:4470–77. doi: 10.4049/jimmunol.0903597
54. Tafuri A, Alferink J, Moller P, Hammerling GJ, Arnold B. T cell awareness of paternal alloantigens during pregnancy. Science. (1995) 270:630–3. doi: 10.1126/science.270.5236.630
55. Bacher P, Scheffold A. New technologies for monitoring human antigen-specific T cells and regulatory T cells by flow-cytometry. Curr Opin Pharmacol. (2015) 23:17–24. doi: 10.1016/j.coph.2015.04.005
56. Tilburgs T, Roelen DL, van der Mast BJ, de Groot-Swings GM, Kleijburg C, Scherjon SA, et al. Evidence for a selective migration of fetus-specific regulatory T cells from the peripheral blood to the decidua in human pregnancy. J Immunol. (2008) 180:5737–45. doi: 10.4049/jimmunol.180.8.5737
57. Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. (2009) 30:899–911. doi: 10.1016/j.immuni.2009.03.019
58. Miyara M, Chader D, Sage E, Sugiyama D, Nishikawa H, Bouvry D, et al. Sialyl Lewis x (CD15s) identifies highly differentiated and most suppressive FOXP3high regulatory T cells in humans. Proc Natl Acad Sci USA. (2015) 112:7225–30. doi: 10.1073/pnas.1508224112
59. Loewendorf AI, Nguyen TA, Yesayan MN, Kahn DA. Normal human pregnancy results in maternal immune activation in the periphery and at the uteroplacental interface. PLoS ONE. (2014) 9:e96723. doi: 10.1371/journal.pone.0096723
60. Qi Q, Liu Y, Cheng Y, Glanville J, Zhang D, Lee JY, et al. Diversity and clonal selection in the human T-cell repertoire. Proc Natl Acad Sci USA. (2014) 111:13139–44. doi: 10.1073/pnas.1409155111
61. Kinder JM, Jiang TT, Ertelt JM, Xin L, Strong BS, Shaaban AF, et al. Cross-generational reproductive fitness enforced by microchimeric maternal cells. Cell. (2015) 162:505–15. doi: 10.1016/j.cell.2015.07.006
62. Burlingham WJ, Grailer AP, Heisey DM, Claas FH, Norman D, Mohanakumar T, et al. The effect of tolerance to noninherited maternal HLA antigens on the survival of renal transplants from sibling donors. N Engl J Med. (1998) 339:1657–64. doi: 10.1056/NEJM199812033392302
63. van Rood JJ, Claas F. Both self and non-inherited maternal HLA antigens influence the immune response. Immunol Today. (2000) 21:269–73. doi: 10.1016/S0167-5699(00)01628-5
64. van Rood JJ, Loberiza FR Jr, Zhang MJ, Oudshoorn M, Claas F, Cairo MS, et al. Effect of tolerance to noninherited maternal antigens on the occurrence of graft-versus-host disease after bone marrow transplantation from a parent or an HLA-haploidentical sibling. Blood. (2002) 99:1572–7. doi: 10.1182/blood.V99.5.1572
65. Nijagal A, Fleck S, MacKenzie TC. Maternal microchimerism in patients with biliary atresia: implications for allograft tolerance. Chimerism. (2012) 3:37–9. doi: 10.4161/chim.20152
66. Tilburgs T, Scherjon SA, van der Mast BJ, Haasnoot GW, Versteeg VDV-MM, Roelen DL, et al. Fetal-maternal HLA-C mismatch is associated with decidual T cell activation and induction of functional T regulatory cells. J Reprod Immunol. (2009) 82:148–57. doi: 10.1016/j.jri.2009.05.003
67. Lashley LE, Haasnoot GW, Spruyt-Gerritse M, Claas FH. Selective advantage of HLA matching in successful uncomplicated oocyte donation pregnancies. J Reprod Immunol. (2015) 112:29–33. doi: 10.1016/j.jri.2015.05.006
68. Ranella A, Vassiliadis S, Mastora C, Valentina M, Dionyssopoulou E, Athanassakis I. Constitutive intracellular expression of human leukocyte antigen (HLA)-DO and HLA-DR but not HLA-DM in trophoblast cells. Hum Immunol. (2005) 66:43–55. doi: 10.1016/j.humimm.2004.10.002
69. Tersigni C, Redman CW, Dragovic R, Tannetta D, Scambia G, Di Simone N, et al. HLA-DR is aberrantly expressed at fetomaternal interface in pre-eclampsia. J Reprod Immunol. (2018) 129:48–52. doi: 10.1016/j.jri.2018.06.024
70. Koelman CA, Coumans AB, Nijman HW, Doxiadis II, Dekker GA, Claas FH. Correlation between oral sex and a low incidence of preeclampsia: a role for soluble HLA in seminal fluid? J Reprod Immunol. (2000) 46:155–66. doi: 10.1016/S0165-0378(99)00062-5
71. Larsen MH, Bzorek M, Pass MB, Larsen LG, Nielsen MW, Svendsen SG, et al. Human leukocyte antigen-G in the male reproductive system and in seminal plasma. Mol Hum Reprod. (2011) 17:727–38. doi: 10.1093/molehr/gar052
72. Craenmehr MH, Heidt S, Eikmans M, Claas FH. What is wrong with the regulatory T cells and foetomaternal tolerance in women with recurrent miscarriages? Hla. (2016) 87:69–78. doi: 10.1111/tan.12737
73. Linscheid C, Petroff MG. Minor histocompatibility antigens and the maternal immune response to the fetus during pregnancy. Am J Reprod Immunol. (2013) 69:304–14. doi: 10.1111/aji.12075
74. Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. (2003) 198:1875–86. doi: 10.1084/jem.20030152
75. Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. (2012) 150:29–38. doi: 10.1016/j.cell.2012.05.031
76. Inada K, Shima T, Ito M, Ushijima A, Saito S. Helios-positive functional regulatory T cells are decreased in decidua of miscarriage cases with normal fetal chromosomal content. J Reprod Immunol. (2015) 107:10–9. doi: 10.1016/j.jri.2014.09.053
77. Hsu P, Santner-Nanan B, Dahlstrom JE, Fadia M, Chandra A, Peek M, et al. Altered decidual DC-SIGN+ antigen-presenting cells and impaired regulatory T-cell induction in preeclampsia. Am J Pathol. (2012) 181:2149–60. doi: 10.1016/j.ajpath.2012.08.032
78. Zhou J, Wang Z, Zhao X, Wang J, Sun H, Hu Y. An increase of Treg cells in the peripheral blood is associated with a better in vitro fertilization treatment outcome. Am J Reprod Immunol. (2012) 68:100–6. doi: 10.1111/j.1600-0897.2012.01153.x
79. Jasper MJ, Tremellen KP, Robertson SA. Primary unexplained infertility is associated with reduced expression of the Tregulatory cell transcription factor Foxp3 in endometrial tissue. Mol Hum Reprod. (2006) 12:301–8. doi: 10.1093/molehr/gal032
80. Lu Y, Zhang F, Zhang Y, Zeng B, Hu L, Liao A. Quantitative reduction of peripheral regulatory T cells in reproductive failure after artificial insemination by donor sperm. Am J Reprod Immunol. (2013) 69:188–93. doi: 10.1111/aji.12041
81. Tremellen KP, Valbuena D, Landeras J, Ballesteros A, Martinez J, Mendoza S, et al. The effect of intercourse on pregnancy rates during assisted human reproduction. Hum Reprod. (2000) 15:2653–8. doi: 10.1093/humrep/15.12.2653
82. Yang H, Qiu L, Chen G, Ye Z, Lu C, Lin Q. Proportional change of regulatory T cells in decidua and peripheral blood in unexplained recurrent spontaneous abortion patients. Fertil Steril. (2008) 89:656–61. doi: 10.1016/j.fertnstert.2007.03.037
83. Mei S, Tan J, Chen H, Chen Y, Zhang J. Changes of regulatory T cells and FOXP3 expression in unexplained recurrent spontaneous abortion patients. Fertil Steril. (2010) 94:2244–7. doi: 10.1016/j.fertnstert.2009.11.020
84. Wang WJ, Hao CF, Yi L, Yin GJ, Bao SH, Qiu LH, et al. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J Reprod Immunol. (2010) 84:164–70. doi: 10.1016/j.jri.2009.12.003
85. Jin LP, Chen QY, Zhang T, Guo PF, Li DJ. The CD4+CD25 bright regulatory T cells and CTLA-4 expression in peripheral and decidual lymphocytes are down-regulated in human miscarriage. Clin Immunol. (2009) 133:402–10. doi: 10.1016/j.clim.2009.08.009
86. Wang WJ, Hao CF, Qu QL, Wang X, Qiu LH, Lin QD. The deregulation of regulatory T cells on interleukin-17-producing T helper cells in patients with unexplained early recurrent miscarriage. Hum Reprod. (2010) 25:2591–6. doi: 10.1093/humrep/deq198
87. Liu C, Wang XZ, Sun XB. Assessment of sperm antigen specific T regulatory cells in women with recurrent miscarriage. Early Hum Dev. (2013) 89:95–100. doi: 10.1016/j.earlhumdev.2012.08.003
88. Arruvito L, Sotelo AI, Billordo A, Fainboim L. A physiological role for inducible FOXP3(+) Treg cells. Lessons from women with reproductive failure. Clin Immunol. (2010) 136:432–41. doi: 10.1016/j.clim.2010.05.002
89. Inada K, Shima T, Nakashima A, Aoki K, Ito M, Saito S. Characterization of regulatory T cells in decidua of miscarriage cases with abnormal or normal fetal chromosomal content. J Reprod Immunol. (2013) 97:104–11. doi: 10.1016/j.jri.2012.12.001
90. Yamada H, Takeda M, Maezawa Y, Ebina Y, Hazama R, Tanimura K, et al. A high dose intravenous immunoglobulin therapy for women with four or more recurrent spontaneous abortions. ISRN Obstet Gynecol. (2012) 2012:512732. doi: 10.5402/2012/512732
91. Winger EE, Reed JL. Treatment with tumor necrosis factor inhibitors and intravenous immunoglobulin improves live birth rates in women with recurrent spontaneous abortion. Am J Reprod Immunol. (2008) 60:8–16. doi: 10.1111/j.1600-0897.2008.00585.x
92. Wong LF, Porter TF, Scott JR. Immunotherapy for recurrent miscarriage. Cochrane Database Syst Rev. (2014) Cd000112. doi: 10.1002/14651858.CD000112.pub3
93. Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. (1999) 180(Pt 1):499–506.
94. Robillard PY, Hulsey TC, Alexander GR, Keenan A, de Caunes F, Papiernik E. Paternity patterns and risk of preeclampsia in the last pregnancy in multiparae. J Reprod Immunol. (1993) 24:1–12.
95. Trupin LS, Simon LP, Eskenazi B. Change in paternity: a risk factor for preeclampsia in multiparas. Epidemiology. (1996) 7:240–4.
96. Skjaerven R, Wilcox AJ, Lie RT. The interval between pregnancies and the risk of preeclampsia. N Engl J Med. (2002) 346:33–8. doi: 10.1056/NEJMoa011379
97. Klonoff-Cohen HS, Savitz DA, Cefalo RC, McCann MF. An epidemiologic study of contraception and preeclampsia. JAMA. (1989) 262:3143–7.
98. Salha O, Sharma V, Dada T, Nugent D, Rutherford AJ, Tomlinson AJ, et al. The influence of donated gametes on the incidence of hypertensive disorders of pregnancy. Hum Reprod. (1999) 14:2268–73.
99. Darmochwal-Kolarz D, Saito S, Rolinski J, Tabarkiewicz J, Kolarz B, Leszczynska-Gorzelak B, et al. Activated T lymphocytes in pre-eclampsia. Am J Reprod Immunol. (2007) 58:39–45. doi: 10.1111/j.1600-0897.2007.00489.x
100. Sasaki Y, Darmochwal-Kolarz D, Suzuki D, Sakai M, Ito M, Shima T, et al. Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in pre-eclampsia. Clin Exp Immunol. (2007) 149:139–45. doi: 10.1111/j.1365-2249.2007.03397.x
101. Toldi G, Svec P, Vasarhelyi B, Meszaros G, Rigo J, Tulassay T, et al. Decreased number of FoxP3+ regulatory T cells in preeclampsia. Acta Obstet Gynecol Scand. (2008) 87:1229–33. doi: 10.1080/00016340802389470
102. Miko E, Szereday L, Barakonyi A, Jarkovich A, Varga P, Szekeres-Bartho A. Immunoactivation in preeclampsia: Vd2+ and regulatory T cells during the inflammatory stage of disease. J Reprod Immunol. (2009) 80:100–8. doi: 10.1016/j.jri.2009.01.003
103. Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas de St Groth B, et al. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol. (2009) 183:7023–30. doi: 10.4049/jimmunol.0901154
104. Toldi G, Rigo J Jr, Stenczer B, Vasarhelyi B, Molvarec A. Increased prevalence of IL-17-producing peripheral blood lymphocytes in pre-eclampsia. Am J Reprod Immunol. (2011) 66:223–9. doi: 10.1111/j.1600-0897.2011.00987.x
105. Darmochwal-Kolarz D, Kludka-Sternik M, Tabarkiewicz J, Kolarz B, Rolinski J, Leszczynska-Gorzelak B, et al. The predominance of Th17 lymphocytes and decreased number and function of Treg cells in preeclampsia. J Reprod Immunol. (2012) 93:75–81. doi: 10.1016/j.jri.2012.01.006
106. Zeng B, Kwak-Kim J, Liu Y, Liao AH. Treg cells are negatively correlated with increased memory B cells in pre-eclampsia while maintaining suppressive function on autologous B-cell proliferation. Am J Reprod Immunol. (2013) 70:454–63. doi: 10.1111/aji.12154
107. Moreno-Eutimio MA, Tovar-Rodriguez JM, Vargas-Avila K, Nieto-Velazquez NG, Frias-De-Leon MG, Sierra-Martinez M, et al. Increased serum levels of inflammatory mediators and low frequency of regulatory T cells in the peripheral blood of preeclamptic Mexican women. Biomed Res Int. (2014) 2014:413249. doi: 10.1155/2014/413249
108. Toldi G, Vasarhelyi ZE, Rigo J Jr, Orban C, Tamassy Z, Bajnok A, et al. Prevalence of Regulatory T-Cell Subtypes in Preeclampsia. Am J Reprod Immunol. (2015) 74:110–15. doi: 10.1111/aji.12380.
Keywords: miscarriage, preeclampsia, pregnancy, regulatory T cells, seminal plasma
Citation: Tsuda S, Nakashima A, Shima T and Saito S (2019) New Paradigm in the Role of Regulatory T Cells During Pregnancy. Front. Immunol. 10:573. doi: 10.3389/fimmu.2019.00573
Received: 28 December 2018; Accepted: 04 March 2019;
Published: 26 March 2019.
Edited by:
Julia Szekeres-Bartho, University of Pécs, HungaryReviewed by:
Attila Molvarec, Semmelweis University, HungaryBaojun Zhang, Duke University, United States
Copyright © 2019 Tsuda, Nakashima, Shima and Saito. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shigeru Saito, czMwc2FpdG9AbWVkLnUtdG95YW1hLmFjLmpw
 Sayaka Tsuda
Sayaka Tsuda Akitoshi Nakashima
Akitoshi Nakashima Tomoko Shima
Tomoko Shima Shigeru Saito
Shigeru Saito