- 1Department of Laboratory Medicine, Wuhan Children’s Hospital (Wuhan Maternal and Child Healthcare Hospital), Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2State Key Laboratory of Virology, College of Life Sciences, Wuhan University, Wuhan, China
- 3Institute of Maternal and Child Health, Wuhan Children’s Hospital (Wuhan Maternal and Child Healthcare Hospital), Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Background and aims: Respiratory syncytial virus (RSV) is a major respiratory pathogen afflicting both infants and the elderly. Although three RSV vaccines have been approved for adults over the age of 60 or pregnant individuals, there are ongoing efforts to develop novel vaccines against RSV infection. This study was designed to develop and evaluate virus-like particles (VLPs) as potential RSV subunit vaccine candidates, with the goal of balancing immunogenicity, protective efficacy, and safety.
Methods: Two types of VLPs were constructed using a recombinant baculovirus (rBV)-insect cell expression system: GECD-VLPs (containing the extracellular domain [GECD] of RSV G protein) and GECD/M282-90-VLPs (containing GECD fused with the CTL epitope M282-90 of M2 protein). BALB/c mice were vaccinated with these VLPs, and immune responses were assessed via RSV-specific IgG and neutralizing antibody titers, cytokine profiles (IFN-γ, IL-2, TNF-α, IL-10, IL-4, IL-5), and lung T-cell subsets (CD25+FoxP3+ Treg and Th17 cells). Protective efficacy against RSV infection and immunopathology was further evaluated post-challenge.
Results: Vaccination with both VLPs induced robust RSV-specific IgG and neutralizing antibodies, conferring defense against RSV infection. Compared with the UV-RSV control group, both GECD/M282-90-VLPs and GECD-VLPs groups exhibited significantly increased Th1-type cytokine levels and decreased Th2-type cytokine concentrations (P<0.05, P<0.001). Importantly, compared to GECD-VLPs, GECD/M282-90-VLPs further significantly upregulated the expression of Th1-type cytokines (IFN-γ, IL-2) and regulatory cytokine IL-10, while significantly downregulating Th2-type cytokine IL-4 (all P<0.05). Post-RSV challenge, mice vaccinated with GECD/M282-90-VLPs exhibited a substantially increased proportion of CD25+FoxP3+ Treg cells and a decreased percentage of Th17 cells in the lungs. Notably, GECD/M282-90-VLP vaccination prevented RSV-induced immunopathology.
Discussion: Our findings demonstrate that vaccination with GECD/M282-90-VLPs elicited a balanced immune response and conferred protection against RSV infection without immunopathology. These data demonstrate that the GECD/M282-90-VLPs are a potential RSV subunit vaccine candidate.
1 Introduction
Respiratory syncytial virus (RSV) infection leads to a serious health concern for both infants and the elderly populations worldwide (1–3). Natural immunity to RSV is incomplete and reinfection occurs throughout life (4, 5). In the 1960s, the administration of formalin-inactivated RSV (FI-RSV) vaccines resulted in vaccine-enhanced disease (VED) upon subsequent RSV infection. Studies revealed that VED is closely related to an excessive Th2-type immune response and production of low-affinity antibodies to RSV (6–8). Although three RSV vaccines have been currently approved for the older and/or pregnant (9–11), it is still imperative to exploit effective vaccines preventing RSV infection with a balanced immune response and high-affinity antibodies (12, 13).
The development of mRNA vaccines was previously considered a promising approach; however, due to potential adverse reactions in certain cases, the development of some mRNA vaccines has been temporarily suspended (14). In this context, virus-like particles (VLPs) vaccines have garnered attention due to their potential in immunogenicity and safety.
The fusion (F) and the attachment (G) glycoproteins expressed on virion surfaces serve as the primary targets for RSV vaccine designs (15–19). The F protein has been the subject of extensive study for many vaccine candidates, as it is more conserved among RSV strains than the G protein. The F protein is characterized by two significant structures, known as pre-fusion (Pre-F) and post-fusion (Post-F) forms. The stabilized configuration of Pre-F protein induces potent RSV-neutralizing antibodies without pulmonary pathological injury (20–23). Consequently, RSV vaccine candidate studies have mainly targeted the immunogenicity of F protein (24–26). As the other major antigen on virion surfaces, the central conserved domain (CCD) of G protein is highly conserved in circulating strains (27). The G protein facilitates RSV infection by binding the CX3C chemokine receptor (CX3CR1) in human bronchial epithelial cells via its CX3C motif (28, 29). Monoclonal antibodies (mAbs) targeting the G protein can neutralize RSV infection, reduce viral loads, decrease pulmonary inflammation, and restore balanced Th1/Th2 response (30–33). Anti-G protein antibodies can enhance the host’s interferon response, improving protective early antiviral responses, which is of great significance for vaccine and therapeutic design (34). Importantly, the presence of the G protein cements the immunogenicity of F protein in VLP vaccine candidates, leading to better protection from RSV infection with higher neutralizing antibody titers (35). Therefore, RSV vaccines containing the G protein represent a promising strategy for enhancing immunity. Several RSV G protein-based subunit vaccine candidates have been reported (17, 36–38).
Virus-like particles (VLPs) produced through recombinant baculovirus expression systems have been widely regarded as effective vaccine platforms (39). The VLP-based vaccines against Hepatitis B Virus (HBV) and Human Papilloma Virus (HPV) are commercially available (40). VLPs containing RSV G protein, which displayed high immunogenicity without observed pathogenic enhancement, induce protection against RSV in vivo (35, 37, 41), and are one of promising RSV vaccine candidates. In this study, we generated VLPs containing H1N1 matrix 1 protein and the extracellular domain of RSV G protein (GECD-VLPs) or the GECD integrated with the M282-90 CTL epitope (GECD/M282-90-VLPs) using a recombinant baculovirus (rBV)-insect cell expression system, respectively. We further characterized these VLPs and investigated their potential as RSV vaccine candidates in BALB/c mice.
2 Materials and methods
2.1 Cells, viruses and preparation of UV-inactivated virus
HEp-2 and Vero cells were sourced from the China Center for Type Culture Collection (CCTCC; Wuhan, China) and maintained in Dulbecco’s modified Eagle’s medium (DMEM) with an addition of 10% fetal bovine serum (FBS, Gibco, NY, USA). HEp-2 and Vero cells were maintained at 37°C in an atmosphere of 5% CO2. Spodoptera frugiperda 9 (Sf9) cells, maintained in our laboratory, were grown in SF-900 II serum-free medium (SFM) at 27°C (Invitrogen, Carlsbad, CA, USA). The RSV A2 strain was propagated in HEp-2 cells, and the viral titers were determined in Vero cells. RSV was purified and inactivated as previously described (18, 22). The efficiency of RSV inactivation by UV radiation was measured by examining the infectivity of the inactivated virus using a plaque assay.
2.2 Construction of plasmids and recombinant baculoviruses
To produce virus-like particles (VLPs) expressing the G protein as the main antigen on their surface, we optimized the construction by replacing the cytoplasmic tail (CT) and transmembrane (TM) of the RSV G protein with the CT and TM of hemagglutinin (HA) of H1N1 (GenBank: MK159419.1), which were then ligated to the N-terminus of the GECD fragment (amino acids 65-298; Gene bank:KT992094.1) (37, 42). To improve the efficacy of GECD-VLPs vaccine, we incorporated the aa82-90 epitope from RSV M2 protein into the design, which is a protective antigen in H-2d mice (43). A flexible linker (GGGGS)3 was used to construct RSV Gaa65-298 in tandem with M2aa 82-90, forming GECD/M282-90-VLPs (Figure 1A). After codon optimization for insect cell use, the chimeric genes encoding either GECD or GECD/M282-90 were synthesized by Sangon Biotech (Shanghai, China).
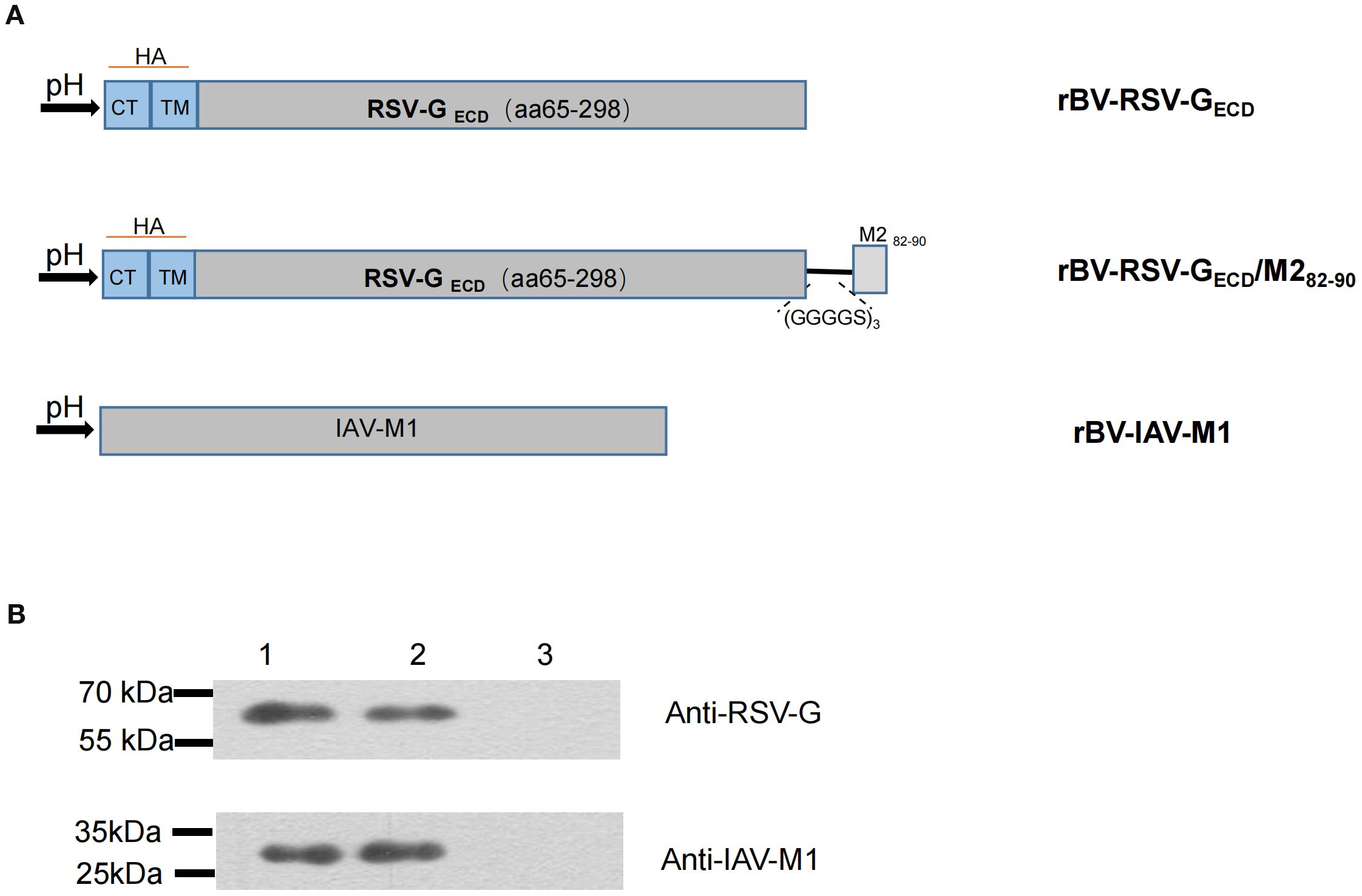
Figure 1. Construction of recombinant baculovirus and protein expression in Sf9 cells. (A) Schematic representation of the constructs. The cytoplasmic tail (CT) and transmembrane (TM) domain of RSV G protein gene were replaced with those of influenza virus HA protein. Subsequently, the M282-90 peptide was linked to the G protein ectodomain of RSV A2 strain via a linker (GSGSGRS)3; (B) Coexpression of the RSV GECD and IAV M1 proteins in Sf9 cells. The proteins were confirmed by western blotting. M:Molecular marker; Lane 1: rBV-RSV-GECD/M1; Lane 2: rBV-RSV-GECD/M282-90/M1; Lane 3: Sf9 cells.
The chimeric GECD fragment was amplified by PCR using the synthesized plasmid pUC-GECD as a template with primers GECD-F/GECD-R. After EcoR I and Xbal I digestion, the digested-GECD fragment was cloned into pFBDM vector to create the plasmid pFBDM-GECD. Similarly, the GECD/M282-90 fragment was inserted into pFBDM to generate the plasmid pFBDM-GECD/M282-90. To construct the rBV expressing H1N1 M1 protein, the M1 gene was cloned into pFBDM to obtain pFBDM-M1 using the same method. The constructed plasmids were identified by sequencing. The specific primers are listed in Table 1. Recombinant baculoviruses rBV-RSV-GECD, rBV-RSV-GECD/M282-90, and rBV-IAV-M1 were generated as previously described (22, 44). Briefly, the construct pFBDM-GECD, pFBDM-GECD/M282-90 or pFBDM-M1 was separately transformed into competent E.coli DH10 MultiBac cells to generate the baculovirus plasmid DNA (bacmid). The resultant bacmid DNA was transfected into Sf9 cells to produce recombinant baculovirus rBV-RSV-GECD, rBV-RSV-GECD/M282-90, or rBV-IAV-M1, respectively.
Plaque assays were used to determine baculovirus titers. In brief, different dilutions of baculovirus solution were added to six-well plates containing Sf9 cells to infection, and then the Sf9 cells were covered with a 4% sterile low-melting point agarose gel was melted in a water bath at 70°C, and an equal volume of 2×SFM medium was added to achieve a final concentration of 2% agarose gel. After 4-6 days of incubation, the number of plaques was observed under a microscope to determine the viral titer.
2.3 Production and purification of VLPs
Sf9 cells were co-infected (MOI=1.0 for each rBV) with rBV-IAV-M1 and either rBV-RSV-GECD or rBV-RSV-GECD/M282-90, respectively. After 3 days of incubation, the Sf9 cell cultures were harvested and kept at -80°C for subsequent experiments. After VLP formation analysis, the cell cultures were lysed and resultant lysate was clarified at 5000 rpm for 30 min. The supernatant containing VLPs was then ultracentrifuged at 60,000×g for 6 h at 4°C. The precipitate was resuspended in a phosphate buffer containing 0.15 M NaCl and 0.05 M phosphate (PBS, pH 7.2). The VLPs were separated utilizing HiPrep Sephacryl S-500 HR (GE Healthcare, Germany) with a flow rate of 0.5 mL/min at room temperature as recommended-protocol by the manufacturer. Fractions with a protein absorbance value of A280>0.1 were collected as samples, and the fraction collection volume was about 10 mL. The purified VLPs were subsequently measured by a Bradford protein assay kit (Sangon Co., Ltd.).
2.4 SDS-PAGE, western blot and electron microscopy
VLP formation was analyzed by western blot, and the purified VLPs were confirmed by SDS-PAGE and electron microscopy, as described previously (22).Cells infected with recombinant baculovirus for 72 hours were collected, with uninfected cells used as a negative control. After treatment with lysis buffer, a certain ratio of loading buffer was added, and samples were mixed thoroughly and boiled for 10 minutes. The prepared samples were analyzed by SDS-PAGE to detect the expression of the target protein.
Expression of the target protein was identified using a mouse anti-RSV G mAb (Sino Biological, China) or an anti-M1 mAb clone 36H4 (Immune Tech, USA) by Western blot analysis. The purified samples are diluted to 200 μg/mL, and 10 μL of the protein samples are dropped onto the carbon film side of a copper grid, followed by incubation at room temperature for 5 minutes to allow adsorption. Residual protein solution is then absorbed with filter paper, and 10 μL of 2% phosphotungstic acid is added to the same location for negative staining at room temperature for 1-2 minutes. Afterward, the remaining negative staining solution is absorbed with filter paper, and the grid is dried at room temperature before storage or direct observation under the transmission electron microscope. Set the voltage to 100 kV and observe the virus-like particles at a magnification of 120,000. The morphology of VLPs were examined bytransmission electron microscopy (JEM-2100, JEOL, Tokyo, Japan).
2.5 Animal experiments
Animal experiments were performed as described previously (18, 22). Groups of five 6- to 8-week-old specific-pathogen-free (SPF) female BALB/c mice (Wuhan University Center for Animal Experiments) were immunized intramuscularly (i.m.) three times with 10 μg VLPs in 100 μL volume at a 2-week interval. The UV-RSV group of five animals were immunized i.m. with purified UV-RSV (1×105 PFU) in 100 μL, and the negative control was i.m. with the same volume of PBS. Sera collected at day 14 after immunization were used for the detection of antibody titers. Cytokines were assayed from isolated splenic lymphocytes.
For histological analysis, immunized mice were intranasally (i.n.) challenged with 3×106 PFU/100 μL of RSV at day 14 following the final immunization. At 4 days post-challenge (dpc), the lungs of mice were stained with haematoxylin and eosin (H&E) or with periodic acid-Schiff (PAS) for routine histology or mucus secretion, respectively. Pulmonary inflammation scores were graded as described previously (22, 45).
2.6 Neutralization antibody assays
Neutralizing antibody titers against RSV were assessed through a 50% plaque reduction neutralization test (PRNT50), as previously described (22). Mouse sera were serially diluted 2-fold in DMEM. Around 100 PFU RSV in 100 μL aliquots was mixed with the diluted sera for incubation. Subsequently. the mixture was added to pre-washed Vero cells. The mixture was replaced with methylcellulose after a 2-hour incubation and then incubated for 3 to 5 days. The plaques were stained using described previously (22, 37). The neutralizing antibody titers was defined as the log2 of the highest dilution of serum that resulted in 50% plaque reduction in virus titer (PRNT50).
2.7 Enzyme-linked immunosorbent assay
Virus-specific IgG, IgG2a, and IgG1 antibodies were assessed by ELISA with RSV serving as the coating antigen (46). Inactivated RSV (1×105 PFU/well) was placed in a 96-well plate as the coating antigen, followed by the addition of gradient dilutions of immunized mouse serum. After 1-hour incubation at 37°C, the secondary antibody, which was HRP-conjugated IgG, IgG2a or IgG1 (Abclonal), was added to 96-well plates. Then, TMB (3,3′,5,5′tetramethylbenzidine) was added as substrate for detection (Sigma). The antibody titer was determined based on the absorbance value at 450 nm.
Cytokine concentrations were quantified using ELISA as described previously (17, 22). Th1 (TNF-α, IL-2, IFN-γ), Th2 (IL-4, IL-5), and Th17-related (IL-17A) cytokines in supernatants of cultured cells or lung homogenates were quantitatively determined using ELISA kits (BioLegend, San Diego, CA, USA).
2.8 ELISpot
The ELISpot Kit (Mabtech, Sweden) was used to detect IFN-γ levels, following the manufacturer’s instructions. After washing the ELISpot plates four times with PBS, RPMI 1640 medium supplemented with 10% fetal bovine serum was added for a 1-hour incubation. Then, the splenic lymphocyte suspension (1×106) was added, and cells were stimulated with 10 µg/mL M2 82-90 (SYIGSINNI) for 24 hours at 37°C and 5% CO2. After stimulation, the plates were washed four times with wash buffer (0.05% Tween-20 in PBS). Subsequently, anti-mouse IFN-γ detection antibodies were added, followed by an overnight incubation of the plates at 4°C. The detection antibodies were then removed, followed by a thorough plate wash. Finally, spots were quantified using an ELISPOT reader. The calculation of RSV-specific ELISpot numbers involved subtracting the average ELISpots in unstimulated wells from those in triplicate wells for each cell type.
2.9 Flow cytometry
Cell staining was performed as previously outlined (22, 45, 47, 48). For Treg analysis, cells were surface-stained with mAbs specific for CD4 (clone RM4-5, FITC-labeled, BioLegend, USA) and CD25 (clone PC61, APC-labeled, BioLegend). After fixation and permeabilization, intracellular staining was performed using PE-labeled anti-mouse Foxp3 antibody (clone MF-14, BioLegend). For Th17 analysis, cells were stained for surface or intracellular proteins following ex vivo stimulation. Cells were stimulated for 6 h at 37°C with Cell Activation Cocktail (containing PMA, ionomycin, and Brefeldin A; BioLegend) in RPMI supplemented with 10% FCS. They were then surface-stained for CD4, fixed, and permeabilized in intracellular staining perm/wash buffer, followed by intracellular staining with PE-labeled mAbs specific for IL-17A (clone TC11-18H10.1, BioLegend). Stained cells were analyzed by flow cytometry (BD FACSCanto™ II, USA). The gating strategies used for flow cytometric analysis are shown in Supplementary Figure S1. Data are presented as the percentage of CD25+Foxp3+ (Treg) cells or IL-17A–producing (Th17) cells within the CD4+ T cell population.
2.10 Real-time reverse transcription quantitative PCR
RSV load in the lung was quantified using RT-qPCR according to prior descriptions (18, 22). Lung tissues were used to extract total RNA with RNA Pure reagent (Aidlab, Beijing, China), and the RNA was reverse transcribed into cDNA using a Toyobo kit (Osaka, Japan). Using a 2×SYBR Green Master Mix (Novoprotein, China), RSV N gene copies were performed on a 7500 Real-Time PCR System by Applied Biosystems. The primer sequences for RT-qPCR were listed in Table 1.
2.11 Statistical analysis
The Student’s t tests or Analysis of Variance (ANOVA) was used for comparisons among different groups. Further analysis was performed using the Tukey test or the nonparametric Kruskal-Wallis test. P values below 0.05 was considered statistical significance.
3 Results
3.1 Preparation and characterization of self-assembled GECD-VLPs and GECD/M282-90-VLPs
To produce GECD-VLPs, Sf9 cells were co-infected with rBV-RSV-GECD and rBV-IAV-M1. After a 72-hours culture, the infected Sf9 cell lysate supernatant was collected for the subsequent assays. Western blot analysis showed that both RSV GECD and IAV M1 proteins were efficiently expressed in the infected Sf9 cells. Similarly, RSV GECD/M282-90 and IAV M1 proteins were observed in the supernatants of Sf9 cells co-infected with rBV-RSV-GECD/M282-90 and rBV-IAV-M1 (Figure 1B). VLPs were then purified from the supernatants of rBV-infected Sf9 cells as described in the Materials and Methods. SDS-PAGE analysis showed that the purified GECD-VLPs were composed of GECD and M1 and GECD/M282-90-VLPs contained GECD/M282-90 and M1 proteins (Figure 2A). An electron microscope displayed spherical self-assembling VLPs with sizes between 50 and 100 nm (Figure 2B). The results indicated that the GECD-VLPs and GECD/M282-90-VLPs were prepared from supernatants of rBVs-infected Sf9 cells.
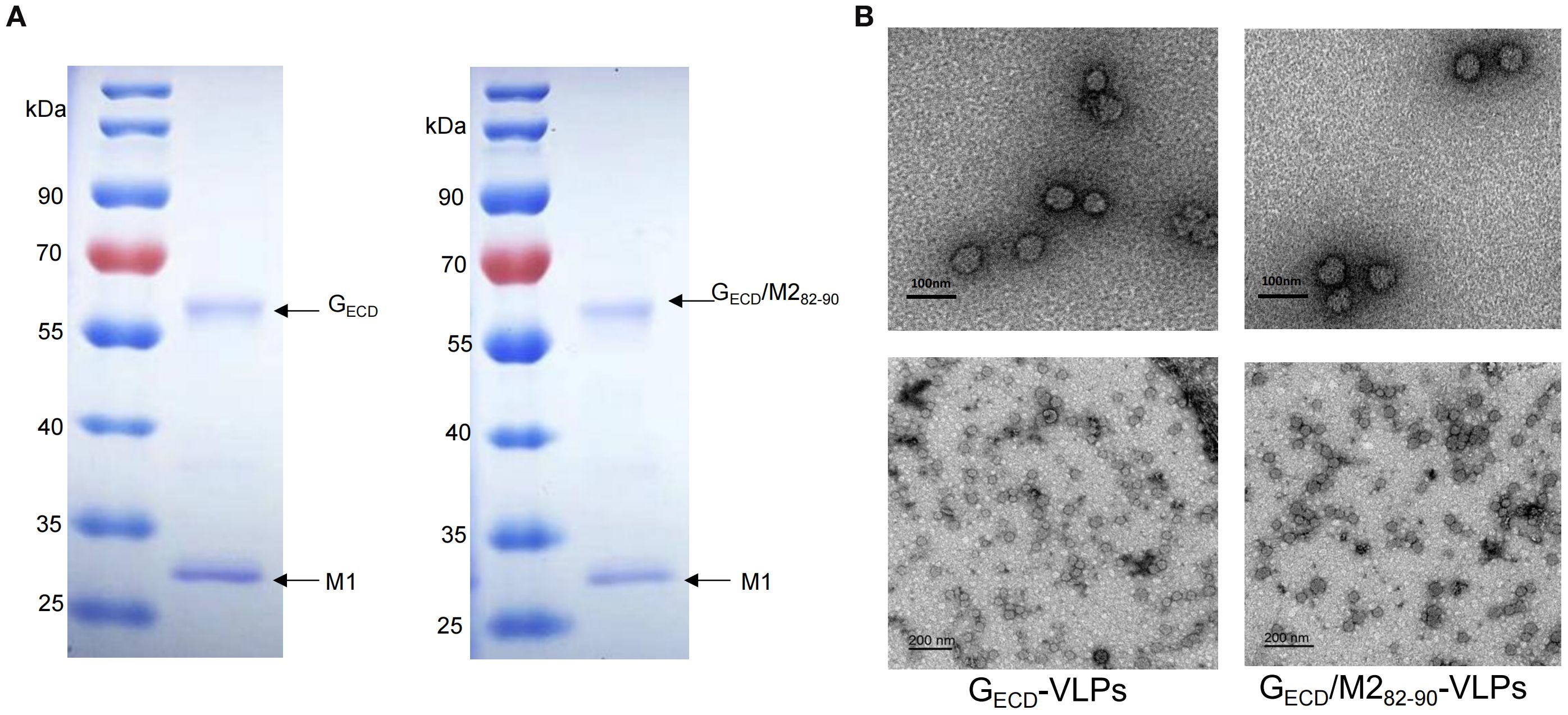
Figure 2. SDS-PAGE analysis of the purified proteins and observation of assembled VLPs. (A) The purified proteins GECD-VLPs or GECD/M282-90 -VLPs were examined by SDS-PAGE. (B) Electron microscopic analysis of GECD-VLPs and GECD/M282-90 -VLPs. Scale bar, 100nm or 200 nm.
3.2 Vaccination with GECD-VLPs and GECD/M282-90-VLPs elicited RSV-specific humoral responses
To evaluate the humoral responses induced by chimeric VLPs, RSV-specific antibody levels in the vaccinated mice sera were measured. The data showed that vaccination with GECD-VLPs and GECD/M282-90-VLPs effectively induced production of RSV-specific antibodies in mice (Figure 3). Compared to UV-RSV, vaccination with GECD-VLPs or GECD/M282-90-VLPs induced similar RSV-specific IgG levels (p>0.05) (Figure 3A). The IgG2a/IgG1 ratios were 1.10 and 1.20 in mice vaccinated with GECD-VLPs and GECD/M282-90-VLPs, respectively, while UV-RSV immunization produced a Th2-skewed response with a ratio of 0.96 (Figure 3B). This shift toward higher IgG2a/IgG1 ratios indicates a stronger Th1 polarization in the VLP-induced immune responses, which is desirable for effective antiviral protection. Vaccination with GECD-VLPs or GECD/M282-90-VLPs induced similar RSV neutralizing antibody (NAb) compared to UV-RSV group (p>0.05) (Figure 3C). These findings indicated that GECD-VLPs and GECD/M282-90-VLPs elicited a balanced immune response to RSV. The combination of GECD with the CTL epitope M282-90 did not enhance the neutralizing antibody levelin mice elicited by VLPs. By enhancing Th1-type immunity, the GECD/M282-90-VLPs formulation may reduce the risk of VED while maintaining robust protection against RSV infection.

Figure 3. Humoral responses in vaccinated mice. Female BALB/c mice were intramuscularly (i.m.) inoculated 3 times at 2-week interval with 10 μg of GECD -VLPs, GECD/M282-90 -VLPs, 1×105PFU of UV-inactivated RSV or 100 μL PBS. Sera of vaccinated mice were collected at 2 weeks after the last immunization. (A, B) RSV-specific IgG, IgG1 and IgG2a were determined by ELISA. (C) RSV neutralizing antibodies were measured by the plaque reduction neutralization test (PRNT50). Data shown are the mean values of 5 mice in each group with standard deviations. ***P < 0.001; **P < 0.01; *P <0.05; ns, not significant.
3.3 Cellular immune responses induced by GECD-VLPs and GECD/M282-90-VLPs
To determine the cellular responses induced by GECD-VLPs and GECD/M282-90-VLPs, splenocyte suspensions of vaccinated mice were prepared, cultured, and then exposed to 105 PFU heat-inactivated RSV virus. Th1 type cytokines (TNF-α, IL-2, IFN-γ) and Th2 type cytokines (IL-4, IL-5) were detected. Both the GECD-VLPs group and the GECD/M282-90-VLPs group were able to induce specific cellular immune responses. Compared with the UV-RSV group, the GECD/M282-90-VLPs group resulted in increased levels of TNF-α, IL-2, IFN-γ, and reduced IL-5 concentration. The data indicated an increased Th1 type response and reduced Th2 response induced by GECD/M282-90-VLPs (Figure 4A).
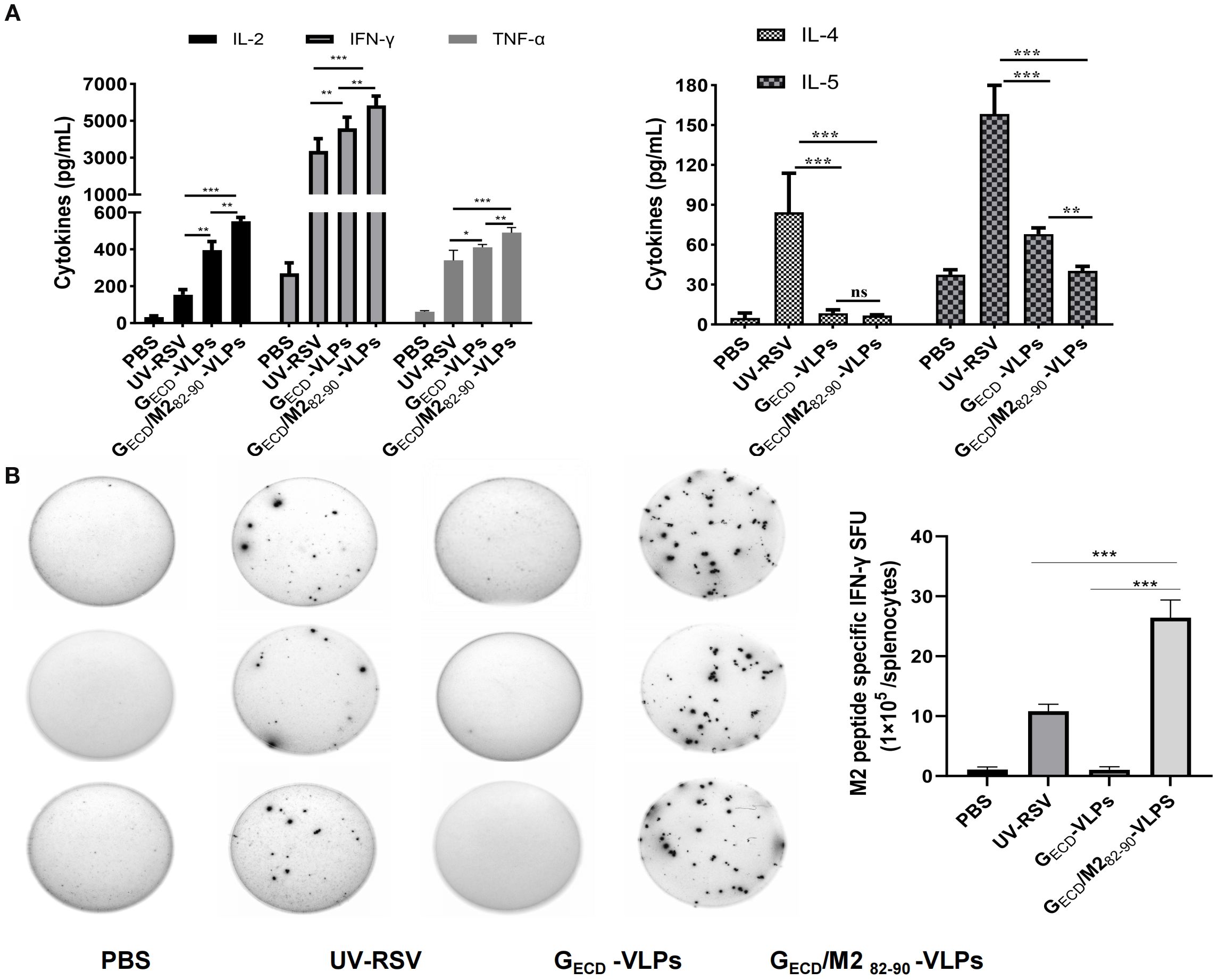
Figure 4. VLPs elicited cellular immune responses in mice. (A) Cytokine concentrations in the splenocytes. Splenocytes were isolated at 2 weeks after the last immunization and stimulated with 1×105 PFU of UV-inactivated RSV. The supernatants were collected after 72-h incubation, and Th1 cytokines (IFN-γ, IL-2 and TNF-α) and Th2 cytokines (IL-4 and IL-5) concentrations were measured by ELISA. (B) The experimental group was immunized three times, and splenic lymphocytes were subsequently isolated from the mice. The number of immune cells producing IFN-γ in response to M282-90 peptide (SYIGSINNI) stimulation was quantified using an ELISpot assay. Data are presented as the mean values ± standard deviations from 5 mice per group.***P < 0.001; **P < 0.01; *P <0.05; ns, not significant.
According to the literature reports, the M2 protein of RSV is a protective antigen in H-2d mice, but not in H-2b or H-2k mice (43). Data showed that the M2 (82–87) peptide specific IFN-γ production in both the UV-RSV group and the GECD/M282-90-VLPs group was observed. In the GECD/M282-90-VLPs group, IFN-γ levels were considerably elevated compared to level in the UV-RSV group. In contrast, no M2 peptide specific IFN-γ cytokine was measured in the GECD-VLPs group. The data indicated that the CTL epitope M282-90 in GECD/M282-90-VLPs specifically activates T cells (Figure 4B).
3.4 Viral load and pathology in lungs of mice vaccinated with VLPs following RSV challenge
To evaluate protection against RSV infection and pulmonary pathology induced by VLPs, we measured RSV concentrations in the lungs from vaccinated mice at 4 dpc (Figure 5A). The RSV N gene copy number was detected by RT-qPCR in mouse lungs. Data showed that approximately 106 RSV N gene copies were detected in the PBS group. However, the pulmonary RSV concentrations of mice vaccinated with GECD-VLPs or GECD/M282-90-VLPs exhibited extremely low N gene copies (similar to background level, P>0.05) (Figure 5B).
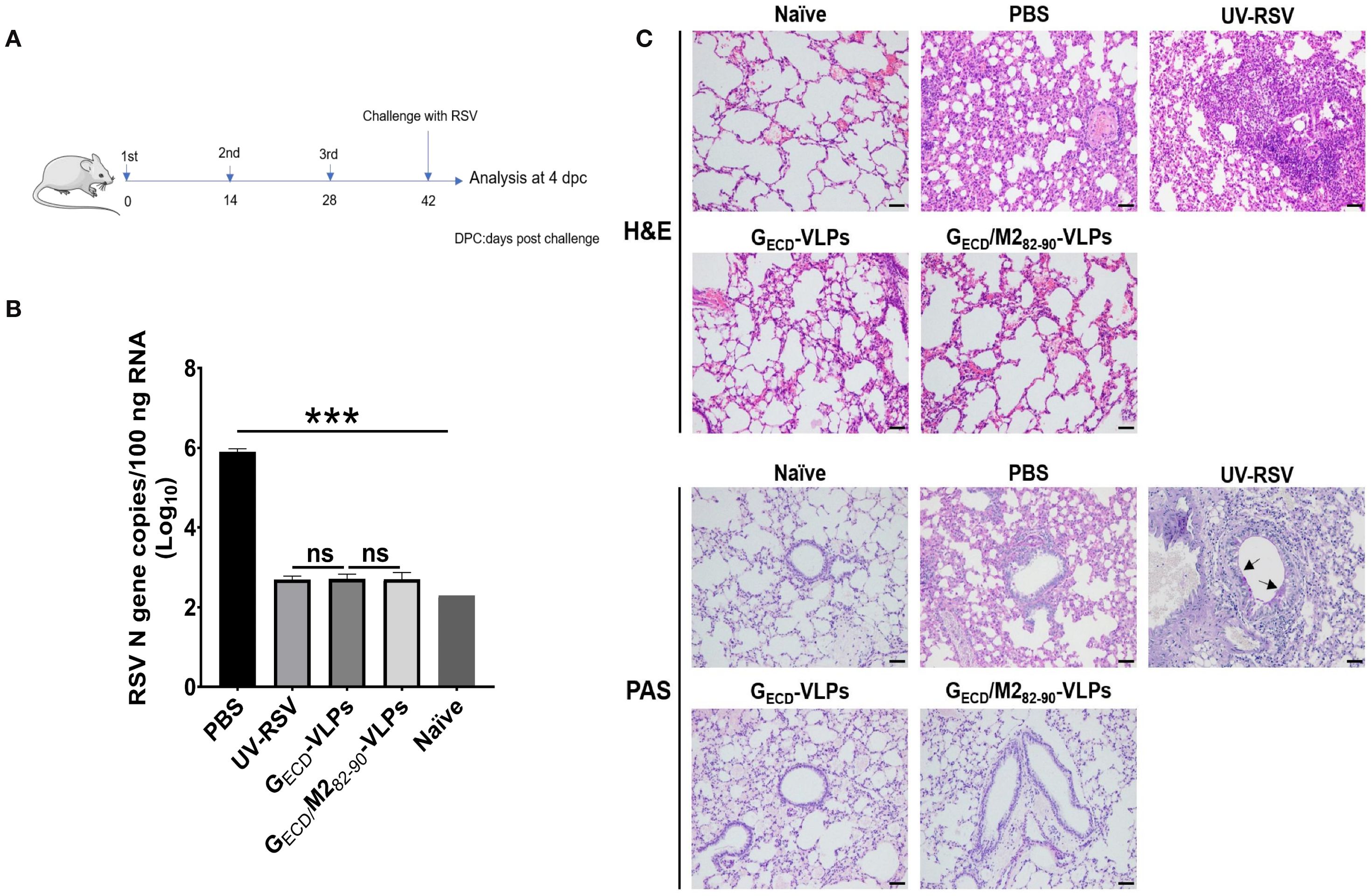
Figure 5. RSV load and histopathological analysis of lung tissues from vaccinated mice upon RSV challenge. (A) The scheme of the vaccination and challenge. Female BALB/c were immunized thrice with the indicated VLPs and challenged with RSV. Lungs were harvested on Day 4 p.c. (B) RSV N gene copy numbers in lung tissues were measured by RT-qPCR. Data are presented as mean values ± SDs for five mice in each group. Pairwise comparisons of values were performed using one-way ANOVA followed by the Tukey test. ***P < 0.001; **P < 0.01; *P < 0.05; ns, not significant. (C) Histopathological scores of lungs from immunized mice on Day 4 p.c. Representative histopathological section of lung from immunized mice by haematoxylin-eosin (H&E) (upper) and periodic acid-Schiff (PAS) (down) staining for each experimental group.
The pulmonary histopathological modifications in vaccinated mice following RSV infection were assessed. The group of UV-RSV-immunized mice displayed severe lymphocyte infiltration around the alveolar hemorrhage, blood vessels and interstitial spaces (Figure 5C, upper), along with the generation of PAS-positive mucus in the lungs (Figure 5C, lower panel). In contrast, the GECD-VLPs vaccinated mice exhibited only mild inflammation in their lungs, and the GECD/M282-90-VLPs group showed no apparent signs of cellular infiltration or mucus production around the airway and interstitial spaces (Figure 5C). Vaccinated mice showed the following average scores for lung inflammation severity: UV-RSV > PBS > GECD-VLPs > GECD/M282-90-VLPs (Table 2). These findings demonstrated that vaccination with GECD/M282-90-VLPs successfully shielded mice from RSV infection without triggering vaccine-enhanced immunopathology, suggesting that GECD/M282-90-VLPs could be a promising RSV vaccine candidate.

Table 2. Histopathology scorea.
3.5 Lung tissue cellular immune responses induced by GECD-VLPs and GECD/M282-90-VLPs
Specific subsets of CD4+ T cells and Th2 type cytokines are vital in the worsening of immunopathology enhanced by RSV vaccines (49). After RSV challenge, the CD4+CD25+Foxp3+ regulatory T cells (Tregs) and IL-17A-producing CD4+ T cell subsets of vaccinated mice were examined. The data showed that vaccination with VLPs elicited notably boosted CD4+CD25+Foxp3+ Tregs and significantly decreased IL-17A-producing CD4+ T cells compared with UV-RSV (P < 0.001) (Figures 6A, B). Similarly, GECD fused with M2 epitope induced a significantly increased pulmonary CD4+CD25+Foxp3+Tregs and a significantly reduced CD4+IL-17A+T cells in mice following RSV challenge (P < 0.05) (Figures 6A, B), suggesting that mice vaccinated with the GECD/M282-90-VLPs were able to induce higher Treg cell levels and reduced Th17 cell production after RSV infection, resulting in a more balanced cellular immune response to Treg/Th17 in vivo.
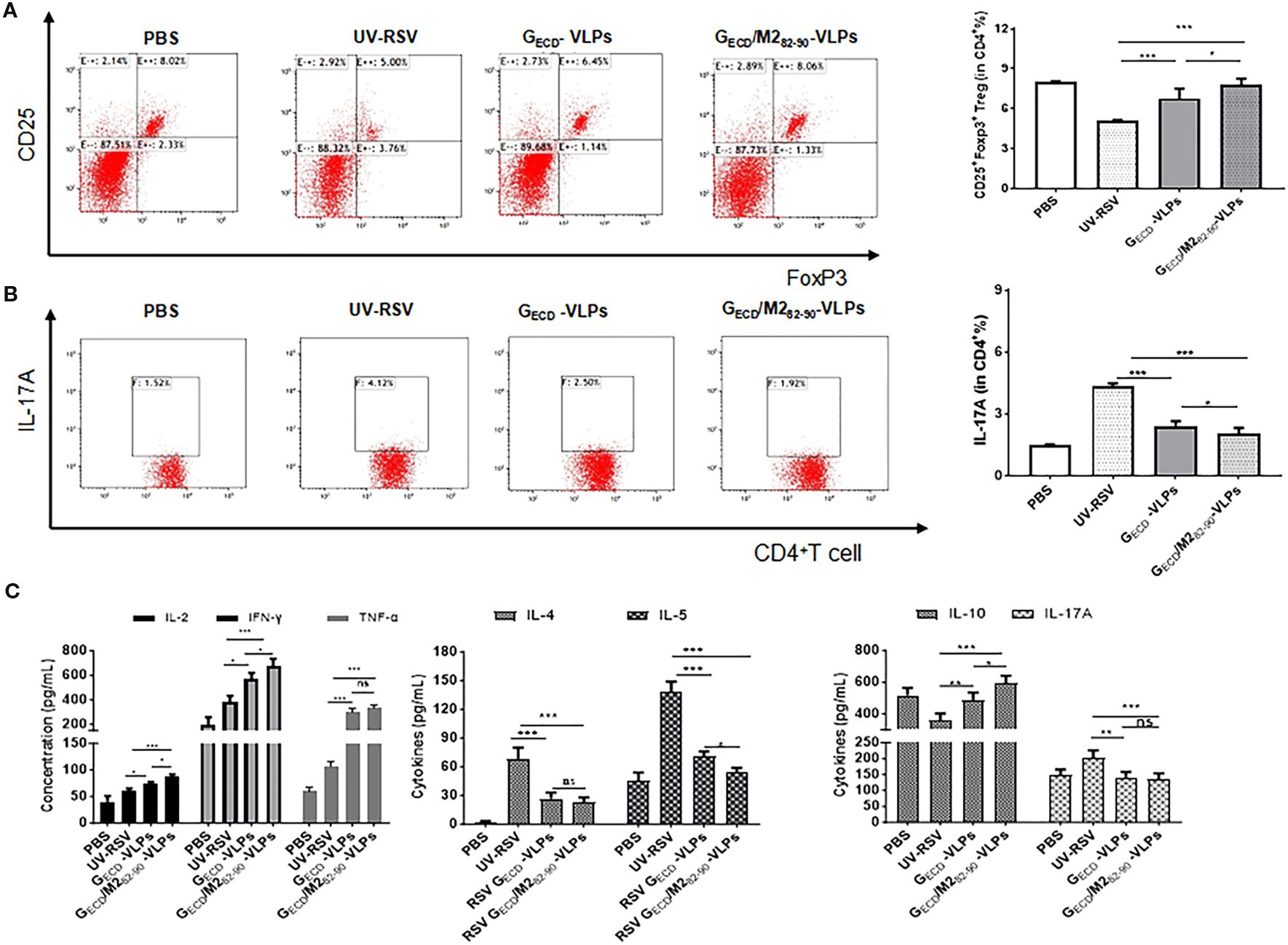
Figure 6. The percentage of Treg and IL-17A in CD4+ T cells in lungs Female BALB/c were immunized thrice with the indicated VLPs and challenged i.n. with RSV. Lungs were harvested on Day 4 p.c. The CD25+Foxp3+Treg cells (A) and IL-17A (B) in CD4+T cells from lungs were measured by flow cytometry with specific antibody staining. (C) Cytokines concentrations were measured by ELISA in the lung homogenizes on Day 4 p.c. Data shown are the mean values of 5 mice per group with standard deviations. P values were calculated with Student’s t-test or one-way ANOVA. ***P < 0.001; **P < 0.01; *P <0.05; ns, not significant.
We further investigated representative cytokines in lung homogenates of mice vaccinated with GECD-VLPs and GECD/M282-90-VLPs at 4 dpc. (Figure 6C). Compared with the UV-RSV, significantly increased Th1 cytokine and significantly decreased Th2 cytokine concentrations were observed in GECD/M282-90-VLPs group and GECD-VLPs group (P<0.05, P<0.001). Importantly, significantly increased IFN-γ, IL-2 and significantly decreased IL-5 cytokines in lung homogenates of mice vaccinated with GECD/M282-90-VLPs compared to the GECD-VLPs (P<0.05). Compared with UV-RSV, vaccination with GECD/M282-90-VLPs and GECD-VLPs induced more IL-10 secretion and reduced IL-17A content (Figure 6C), which confirmed the flow cytometry data (Figure 6B).
4 Discussion
There are ongoing efforts to develop vaccine or immunoprophylaxis measures for RSV infection (50). Notably, an early trial of a formalin-inactivated RSV (FI-RSV) vaccine in the late 1960s failed to demonstrate efficacy and safety (51, 52). Despite this setback, recent advances have led to the approval of several vaccines and monoclonal antibodies, marking a significant shift in RSV prevention strategies (14). However, a universally effective RSV vaccine for children remains unavailable. One major obstacle in developing safe and effective RSV vaccines has been vaccine-enhanced disease (VED). VED is characterized by pulmonary eosinophil infiltration, low affinity antibody and Th2 -biased responses (8, 18, 45, 53). Following the failure of the FI-RSV vaccine, subsequent vaccine development has focused on achieving robust immunogenicity without inducing VED. This has led to the exploration of optimized vaccine designs aimed at eliciting high-affinity antibodies and a Th1-biased immune response. A growing number of various RSV vaccine candidates including subunit, particle, and vector-based vaccines based on RSV F, G, and/or M proteins. These candidates have been extensively researched across various platforms (17, 37, 54–56).
RSV neutralizing antibodies are triggered by two key surface glycoproteins, F and G proteins, which are widely utilized in the development of RSV vaccine candidates. Among different RSV strains, the F protein is more conserved and expected to elicit a wider spectrum of protective immunity (57). Palivizumab, a licensed monoclonal antibody drug for RSV, identifies an epitope in the RSV F protein (58, 59). Consequently, the F protein is the primary focus for most vaccine candidates currently in preclinical and clinical development (22, 25, 60, 61). However, the glycoprotein G is also an important target antigen for RSV vaccine development as it can induce long-term neutralizing activities and block CX3C-CX3CR1 interaction (62–64). Importantly, in the ectodomain of the G protein, a highly conserved domain (CCD) with a CX3C chemokine motif is present, which elicits production of broadly effective neutralizing antibodies (33, 34, 65–68).
VLPs are regarded as promising, safe, and effective platforms for vaccines (39, 69, 70). In this study, we prepared the VLPs composed of RSV GECD or GECD/M282-90 and influenza virus matrix (M1) proteins using the rBV-Sf9 insect cell expression system. Influenza virus M1 is utilized as a core protein to provide structural support for the VLPs, as RSV is more pleomorphic than influenza virus, which is essential for viral budding (70, 71). The transmembrane region and the cytoplasmic tail of RSV G protein were substituted with the corresponding regions of influenza virus HA to facilitate the targeting of M1 protein to the cell membrane and contributing to budding particle formation (42, 72–74). The sequence of 82-90 in RSV M2 serves as a key CTL epitope which elicits CD8+ T cell responses (75). Our results demonstrate that the M282-90 epitope exhibits MHC restriction, as it induces robust CD8+ T cell responses only in specific MHC haplotype backgrounds. This finding aligns with previous studies on RSV antigens, where MHC restriction was shown to influence immune response quality and vaccine efficacy (76, 77). The chimeric tHBc ΔG/M282-90 or tHBc/FE1E2/M282-90 VLPs of truncated G or F fragment containing M2 CTL epitope, resulted in lessened mouse lung damage with a balanced immune response (17, 46). The measurement of RSV-specific total IgG antibody levels provides critical insights into the overall magnitude of the humoral immune response. In this study, the comparable total IgG levels observed between the GECD-VLPs, GECD/M282-90-VLPs, and UV-RSV groups suggest that both VLP formulations effectively mimicked the antibody responses elicited by UV-inactivated RSV. This indicates that the VLP-based vaccines are capable of inducing robust humoral immunity comparable to that of a traditional inactivated vaccine.The subclass analysis revealed distinct patterns of IgG1 and IgG2a antibody production, which are indicative of the polarization of the immune response (78). The GECD-VLPs and GECD/M282-90-VLPs groups exhibited significantly higher IgG2a levels compared to the UV-RSV group, while IgG1 levels were significantly lower. These differences were reflected in the IgG2a/IgG1 ratios, which were higher for the VLP groups compared to the UV-RSV group. This shift toward higher IgG2a/IgG1 ratios indicates a stronger Th1 polarization in the VLP-induced immune responses, which is desirable for effective antiviral protection (38).
In this study, immunization with GECD-VLPs or GECD/M282-90-VLPs effectively induced RSV-neutralizing antibody and an immune response favoring Th1 without causing VED. Importantly, vaccination with GECD/M282-90-VLPs led to a notable rise in the IgG2a/IgG1 ratio, cytokines IL-2 and IFN-γ, while significantly reducing IL-5 secretion. Our results demonstrate that the M282-90 epitope exhibits MHC restriction, as it induces robust CD8+ T cell responses only in specific MHC haplotype backgrounds. This finding aligns with previous studies on RSV antigens, where MHC restriction was shown to influence immune response quality and vaccine efficacy (76, 77).
Regulatory T cells (Tregs) mitigate vaccine-enhanced disease in lungs of patients infected with RSV by inhibiting a Th2-type immune response (79, 80). Th17 cells play a critical role in many inflammatory diseases. For RSV infection, Th17 cells are vital in increased mucus secretion, impaired viral clearance, and induction of Th2-type responses (81, 82). Our results showed that vaccination with VLPs displaying the extracellular domain of RSV G protein led to an increase in CD4+CD25+FoxP3+Tregs and a decrease in CD4+ IL-17A+Th17 cells. Interestingly, GECD/M282-90-VLPs induced significantly higher levels of pulmonary Tregs and lower levels of Th17 cells compared with GECD-VLPs, suggesting that GECD/M282-90-VLPs are a promising novel RSV vaccine candidate. These results suggest that M282-90 epitopes may play a role in vaccine vaccination by affecting the Treg/Th17 immune balance after RSV infection. The findings indicated that the RSV G protein enhances the immune responses to the pre-F protein, resulting in notably higher neutralizing antibody titers and enhanced protection against RSV infection in a VLP vaccine candidate (35). Our previous study demonstrated that VLPs incorporating a stabilized pre-F protein induced a balanced immune response and offered defense against RSV infection (22). This study presents a novel RSV vaccine candidate based on chimeric GECD/M282-90 -VLPs. RSV vaccines, although already approved for use in the elderly and pregnant women, remain a critical need for protection in children older than six months. Various vaccine strategies, including subunit vaccines, attenuated live vaccines, viral vector vaccines, and mRNA vaccines, are currently being developed for different population groups. Notably, subunit vaccines and mRNA vaccines utilizing the pre-fusion conformation of the F protein have been demonstrated to induce robust immune responses (83). To address this issue, virus-like particle (VLP) vaccines are considered a promising complementary strategy. VLP vaccines, due to their high immunogenicity and safety profile, are capable of effectively inducing both humoral and cell-mediated immune responses. By modifying the surface of VLPs, their half-life and targeting efficiency within the host can be further enhanced, thereby demonstrating significant potential in preventing RSV infections (84). Specifically, the combined use of G-protein VLP vaccines with F-protein is considered an effective strategy. G and F proteins are the major antigenic components of RSV, capable of inducing strong neutralizing antibody responses. Research has demonstrated that the co-administration of G-protein VLP vaccines with F-protein not only enhances immune responses but also provides broader protective efficacy (85, 86).
Researchers are continuously working to overcome these challenges and make preventive measures widely available to everyone in need (87). Future work will be focused on investigating that co-administration with both pre-F VLPs and GECD/M282-90 -VLPs provide the synergistic immunity for RSV infection.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Animal Ethics Committee of Wuhan University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
HQ: Formal Analysis, Writing – review & editing, Validation, Data curation, Methodology, Writing – original draft, Conceptualization, Investigation, Software. JL: Validation, Writing – review & editing, Methodology, Data curation, Resources, Writing – original draft, Investigation, Formal analysis, Software, Funding acquisition. ZP: Methodology, Validation, Writing – review & editing, Supervision, Resources, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (11831015), the National key R&D program of China (2017YFA0505801) and the Hubei Provincial Natural Science Foundation of China (2024AFB475).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1625670/full#supplementary-material
References
1. Shang Z, Tan S, and Ma D. Respiratory syncytial virus: from pathogenesis to potential therapeutic strategies. Int J Biol Sci. (2021) 17:4073–91. doi: 10.7150/ijbs.64762
2. Nam HH and Ison MG. Respiratory syncytial virus infection in adults. BMJ. (2019) 366:l5021. doi: 10.1136/bmj.l5021
3. Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. (2022) 399:2047–64. doi: 10.1016/S0140-6736(22)00478-0
4. Glezen WP, Taber LH, Frank AL, and Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. (1986) 140:543–6. doi: 10.1001/archpedi.1986.02140200053026
5. Johnson KM, Bloom HH, Mufson MA, and Chanock RM. Natural reinfection of adults by respiratory syncytial virus. Possible relation to mild upper respiratory disease. N Engl J Med. (1962) 267:68–72. doi: 10.1056/NEJM196207122670204
6. Bigay J, Le Grand R, Martinon F, and Maisonnasse P. Vaccine-associated enhanced disease in humans and animal models: Lessons and challenges for vaccine development. Front Microbiol. (2022) 13:932408. doi: 10.3389/fmicb.2022.932408
7. Collarini EJ, Lee FE, Foord O, Park M, Sperinde G, Wu H, et al. Potent high-affinity antibodies for treatment and prophylaxis of respiratory syncytial virus derived from B cells of infected patients. J Immunol. (2009) 183:6338–45. doi: 10.4049/jimmunol.0901373
8. Delgado MF, Coviello S, Monsalvo AC, Melendi GA, Hernandez JZ, Batalle JP, et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. (2009) 15:34–41. doi: 10.1038/nm.1894
9. Battles MB and McLellan JS. Respiratory syncytial virus entry and how to block it. Nat Rev Microbiol. (2019) 17:233–45. doi: 10.1038/s41579-019-0149-x
10. Papi A, Ison MG, Langley JM, Lee DG, Leroux-Roels I, Martinon-Torres F, et al. Respiratory syncytial virus prefusion F protein vaccine in older adults. N Engl J Med. (2023) 388:595–608. doi: 10.1056/NEJMoa2209604
11. Kampmann B, Madhi SA, Munjal I, Simoes EAF, Pahud BA, Llapur C, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. (2023) 388:1451–64. doi: 10.1056/NEJMoa2216480
12. Ruckwardt TJ, Morabito KM, and Graham BS. Immunological lessons from respiratory syncytial virus vaccine development. Immunity. (2019) 51:429–42. doi: 10.1016/j.immuni.2019.08.007
13. Rossey I and Saelens X. Vaccines against human respiratory syncytial virus in clinical trials, where are we now? Expert Rev Vaccines. (2019) 18:1053–67. doi: 10.1080/14760584.2019.1675520
14. Lin M, Yin Y, Zhao X, Wang C, Zhu X, Zhan L, et al. A truncated pre-F protein mRNA vaccine elicits an enhanced immune response and protection against respiratory syncytial virus. Nat Commun. (2025) 16:1386. doi: 10.1038/s41467-025-56302-1
15. Cheng X, Zhao G, Dong A, He Z, Wang J, Jiang B, et al. A first-in-human trial to evaluate the safety and immunogenicity of a G protein-based recombinant respiratory syncytial virus vaccine in healthy adults 18-45 years of age. Vaccines. (2023) 11:999. doi: 10.3390/vaccines11050999
16. Crank MC, Ruckwardt TJ, Chen M, Morabito KM, Phung E, Costner PJ, et al. A proof of concept for structure-based vaccine design targeting RSV in humans. Science. (2019) 365:505–9. doi: 10.1126/science.aav9033
17. Qiao L, Zhang Y, Chai F, Tan Y, Huo C, and Pan Z. Chimeric virus-like particles containing a conserved region of the G protein in combination with a single peptide of the M2 protein confer protection against respiratory syncytial virus infection. Antiviral Res. (2016) 131:131–40. doi: 10.1016/j.antiviral.2016.05.001
18. Yang J, Ma C, Zhao Y, Fan A, Zou X, and Pan Z. Hepatitis B Virus Core Particles Containing a Conserved Region of the G Protein Combined with Interleukin-35 Protected Mice against Respiratory Syncytial Virus Infection without Vaccine-Enhanced Immunopathology. J Virol. (2020) 94:e00007–20. doi: 10.1128/JVI.00007-20
19. Zhang Y, Qiao L, Hu X, Zhao K, Zhang Y, Chai F, et al. Baculovirus vectors expressing F proteins in combination with virus-induced signaling adaptor (VISA) molecules confer protection against respiratory syncytial virus infection. Vaccine. (2016) 34:252–60. doi: 10.1016/j.vaccine.2015.11.027
20. McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. (2013) 340:1113–7. doi: 10.1126/science.1234914
21. Krarup A, Truan D, Furmanova-Hollenstein P, Bogaert L, Bouchier P, Bisschop IJM, et al. A highly stable prefusion RSV F vaccine derived from structural analysis of the fusion mechanism. Nat Commun. (2015) 6:8143. doi: 10.1038/ncomms9143
22. Luo J, Qin H, Lei L, Lou W, Li R, and Pan Z. Virus-like particles containing a prefusion-stabilized F protein induce a balanced immune response and confer protection against respiratory syncytial virus infection in mice. Front Immunol. (2022) 13:1054005. doi: 10.3389/fimmu.2022.1054005
23. Palomo C, Mas V, Thom M, Vazquez M, Cano O, Terron MC, et al. Influence of respiratory syncytial virus F glycoprotein conformation on induction of protective immune responses. J Virol. (2016) 90:5485–98. doi: 10.1128/JVI.00338-16
24. Shan J, Britton PN, King CL, and Booy R. The immunogenicity and safety of respiratory syncytial virus vaccines in development: A systematic review. Influenza other Respir viruses. (2021) 15:539–51. doi: 10.1111/irv.12850
25. Nussbaum J, Cao X, Railkar RA, Sachs JR, Spellman DS, Luk J, et al. Evaluation of a stabilized RSV pre-fusion F mRNA vaccine: Preclinical studies and Phase 1 clinical testing in healthy adults. Vaccine. (2023) 41:6488–501. doi: 10.1016/j.vaccine.2023.05.062
26. Ngwuta JO, Chen M, Modjarrad K, Joyce MG, Kanekiyo M, Kumar A, et al. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci Trans Med. (2015) 7:309ra162. doi: 10.1126/scitranslmed.aac4241
27. Kauvar LM, Harcourt JL, Haynes LM, and Tripp RA. Therapeutic targeting of respiratory syncytial virus G-protein. Immunotherapy. (2010) 2:655–61. doi: 10.2217/imt.10.53
28. Chirkova T, Lin S, Oomens AGP, Gaston KA, Boyoglu-Barnum S, Meng J, et al. CX3CR1 is an important surface molecule for respiratory syncytial virus infection in human airway epithelial cells. J Gen virology. (2015) 96:2543–56. doi: 10.1099/vir.0.000218
29. Johnson SM, McNally BA, Ioannidis I, Flano E, Teng MN, Oomens AG, et al. Respiratory syncytial virus uses CX3CR1 as a receptor on primary human airway epithelial cultures. PloS pathogens. (2015) 11:e1005318. doi: 10.1371/journal.ppat.1005318
30. Haynes LM, Caidi H, Radu GU, Miao C, Harcourt JL, Tripp RA, et al. Therapeutic monoclonal antibody treatment targeting respiratory syncytial virus (RSV) G protein mediates viral clearance and reduces the pathogenesis of RSV infection in BALB/c mice. J Infect diseases. (2009) 200:439–47. doi: 10.1086/600108
31. Radu GU, Caidi H, Miao C, Tripp RA, Anderson LJ, and Haynes LM. Prophylactic treatment with a G glycoprotein monoclonal antibody reduces pulmonary inflammation in respiratory syncytial virus (RSV)-challenged naive and formalin-inactivated RSV-immunized BALB/c mice. J Virol. (2010) 84:9632–6. doi: 10.1128/JVI.00451-10
32. Lee HJ, Lee JY, Park MH, Kim JY, and Chang J. Monoclonal antibody against G glycoprotein increases respiratory syncytial virus clearance in vivo and prevents vaccine-enhanced diseases. PloS One. (2017) 12:e0169139. doi: 10.1371/journal.pone.0169139
33. Boyoglu-Barnum S, Chirkova T, Todd SO, Barnum TR, Gaston KA, Jorquera P, et al. Prophylaxis with a respiratory syncytial virus (RSV) anti-G protein monoclonal antibody shifts the adaptive immune response to RSV rA2-line19F infection from Th2 to Th1 in BALB/c mice. J Virol. (2014) 88:10569–83. doi: 10.1128/JVI.01503-14
34. Bergeron HC, Kauvar LM, and Tripp RA. Anti-G protein antibodies targeting the RSV G protein CX3C chemokine region improve the interferon response. Ther Adv Infect Dis. (2023) 10:20499361231161157. doi: 10.1177/20499361231161157
35. McGinnes Cullen L, Luo B, Wen Z, Zhang L, Durr E, and Morrison TG. The respiratory syncytial virus (RSV) G protein enhances the immune responses to the RSV F protein in an enveloped virus-like particle vaccine candidate. J Virol. (2023) 97:e0190022. doi: 10.1128/jvi.01900-22
36. Bergeron HC, Murray J, Nunez Castrejon AM, DuBois RM, and Tripp RA. Respiratory syncytial virus (RSV) G protein vaccines with central conserved domain mutations induce CX3C-CX3CR1 blocking antibodies. Viruses. (2021) 13:352. doi: 10.3390/v13020352
37. Murawski MR, McGinnes LW, Finberg RW, Kurt-Jones EA, Massare MJ, Smith G, et al. Newcastle disease virus-like particles containing respiratory syncytial virus G protein induced protection in BALB/c mice, with no evidence of immunopathology. J Virol. (2010) 84:1110–23. doi: 10.1128/JVI.01709-09
38. Jo YM, Kim J, and Chang J. Vaccine containing G protein fragment and recombinant baculovirus expressing M2 protein induces protective immunity to respiratory syncytial virus. Clin Exp Vaccine Res. (2019) 8:43–53. doi: 10.7774/cevr.2019.8.1.43
39. Mena JA and Kamen AA. Insect cell technology is a versatile and robust vaccine manufacturing platform. Expert Rev Vaccines. (2011) 10:1063–81. doi: 10.1586/erv.11.24
40. Mohsen MO, Zha L, Cabral-Miranda G, and Bachmann MF. Major findings and recent advances in virus-like particle (VLP)-based vaccines. Semin Immunol. (2017) 34:123–32. doi: 10.1016/j.smim.2017.08.014
41. Gu H, Li T, Han L, Zhu P, Zhang P, Zhang S, et al. Protection conferred by virus-like particle vaccines against respiratory syncytial virus infection in mice by intranasal vaccination. Hum Vaccin Immunother. (2015) 11:1057–64. doi: 10.1080/21645515.2015.1011993
42. Siche S, Brett K, Moller L, Kordyukova LV, Mintaev RR, Alexeevski AV, et al. Two cytoplasmic acylation sites and an adjacent hydrophobic residue, but no other conserved amino acids in the cytoplasmic tail of HA from influenza A virus are crucial for virus replication. Viruses. (2015) 7:6458–75. doi: 10.3390/v7122950
43. Crowe JE Jr., Collins PL, London WT, Chanock RM, and Murphy BR. A comparison in chimpanzees of the immunogenicity and efficacy of live attenuated respiratory syncytial virus (RSV) temperature-sensitive mutant vaccines and vaccinia virus recombinants that express the surface glycoproteins of RSV. Vaccine. (1993) 11:1395–404. doi: 10.1016/0264-410X(93)90168-W
44. Cai M, Wang C, Li Y, Gu H, Sun S, Duan Y, et al. Virus-like particle vaccine by intranasal vaccination elicits protective immunity against respiratory syncytial viral infection in mice. Acta Biochim Biophys Sin (Shanghai). (2017) 49:74–82. doi: 10.1093/abbs/gmw118
45. Zhao Y, Ma C, Yang J, Zou X, and Pan Z. Dynamic host immune and transcriptomic responses to respiratory syncytial virus infection in a vaccination-challenge mouse model. Virol Sin. (2021) 36:1327–40. doi: 10.1007/s12250-021-00418-3
46. Lei L, Qin H, Luo J, Tan Y, Yang J, and Pan Z. Construction and immunological evaluation of hepatitis B virus core virus-like particles containing multiple antigenic peptides of respiratory syncytial virus. Virus Res. (2021) 298:198410. doi: 10.1016/j.virusres.2021.198410
47. Wang J, Kong L, Luo Q, Li B, Wu J, Liu B, et al. Dual effects of respiratory syncytial virus infections on airway inflammation by regulation of Th17/Treg responses in ovalbumin-challenged mice. Inflammation. (2014) 37:1984–2005. doi: 10.1007/s10753-014-9931-0
48. Serve R, Sturm R, Schimunek L, Störmann P, Heftrig D, Teuben MPJ, et al. Comparative analysis of the regulatory T cells dynamics in peripheral blood in human and porcine polytrauma. Front Immunol. (2018) 9:435. doi: 10.3389/fimmu.2018.00435
49. Galvez NMS, Soto JA, and Kalergis AM. New insights contributing to the development of effective vaccines and therapies to reduce the pathology caused by hRSV. Int J Mol Sci. (2017) 18:1753. doi: 10.3390/ijms18081753
50. Mazur NI, Terstappen J, Baral R, Bardaji A, Beutels P, Buchholz UJ, et al. Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. Lancet Infect Dis. (2023) 23:e2–e21. doi: 10.1016/S1473-3099(22)00291-2
51. Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. (1969) 89:422–34. doi: 10.1093/oxfordjournals.aje.a120955
52. Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, and Stewart CE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. (1969) 89:405–21. doi: 10.1093/oxfordjournals.aje.a120954
53. Ebenig A, Muraleedharan S, Kazmierski J, Todt D, Auste A, Anzaghe M, et al. Vaccine-associated enhanced respiratory pathology in COVID-19 hamsters after T(H)2-biased immunization. Cell Rep. (2022) 40:111214. doi: 10.1016/j.celrep.2022.111214
54. Quan FS, Kim Y, Lee S, Yi H, Kang SM, Bozja J, et al. Viruslike particle vaccine induces protection against respiratory syncytial virus infection in mice. J Infect diseases. (2011) 204:987–95. doi: 10.1093/infdis/jir474
55. Biagi C, Dondi A, Scarpini S, Rocca A, Vandini S, Poletti G, et al. Current state and challenges in developing respiratory syncytial virus vaccines. Vaccines. (2020) 8:672. doi: 10.3390/vaccines8040672
56. McGinnes Cullen L, Schmidt MR, and Morrison TG. Effect of previous respiratory syncytial virus infection on murine immune responses to F and G protein-containing virus-like particles. J Virol. (2019) 93:e00087–19. doi: 10.1128/JVI.00087-19
57. Melero JA and Moore ML. Influence of respiratory syncytial virus strain differences on pathogenesis and immunity. Curr topics Microbiol Immunol. (2013) 372:59–82. doi: 10.1007/978-3-642-38919-13
58. McLellan JS, Yang Y, Graham BS, and Kwong PD. Structure of respiratory syncytial virus fusion glycoprotein in the postfusion conformation reveals preservation of neutralizing epitopes. J Virol. (2011) 85:7788–96. doi: 10.1128/JVI.00555-11
59. Arbiza J, Taylor G, Lopez JA, Furze J, Wyld S, Whyte P, et al. Characterization of two antigenic sites recognized by neutralizing monoclonal antibodies directed against the fusion glycoprotein of human respiratory syncytial virus. J Gen Virol. (1992) 73:2225–34. doi: 10.1099/0022-1317-73-9-2225
60. Steff AM, Monroe J, Friedrich K, Chandramouli S, Nguyen TL, Tian S, et al. Pre-fusion RSV F strongly boosts pre-fusion specific neutralizing responses in cattle pre-exposed to bovine RSV. Nat Commun. (2017) 8:1085. doi: 10.1038/s41467-017-01092-4
61. Falsey AR, Williams K, Gymnopoulou E, Bart S, Ervin J, Bastian AR, et al. Efficacy and safety of an ad26.RSV.preF-RSV preF protein vaccine in older adults. N Engl J Med. (2023) 388:609–20. doi: 10.1056/NEJMoa2207566
62. Boyoglu-Barnum S, Todd SO, Chirkova T, Barnum TR, Gaston KA, Haynes LM, et al. An anti-G protein monoclonal antibody treats RSV disease more effectively than an anti-F monoclonal antibody in BALB/c mice. Virology. (2015) 483:117–25. doi: 10.1016/j.virol.2015.02.035
63. Choi Y, Mason CS, Jones LP, Crabtree J, Jorquera PA, and Tripp RA. Antibodies to the central conserved region of respiratory syncytial virus (RSV) G protein block RSV G protein CX3C-CX3CR1 binding and cross-neutralize RSV A and B strains. Viral Immunol. (2012) 25:193–203. doi: 10.1089/vim.2011.0094
64. Li C, Zhou X, Zhong Y, Li C, Dong A, He Z, et al. A Recombinant G Protein Plus Cyclosporine A-Based Respiratory Syncytial Virus Vaccine Elicits Humoral and Regulatory T Cell Responses against Infection without Vaccine-Enhanced Disease. J Immunol. (2016) 196:1721–31. doi: 10.4049/jimmunol.1502103
65. Powell TJ, Jacobs A, Tang J, Cardenas E, Palath N, Daniels J, et al. Microparticle RSV vaccines presenting the G protein CX3C chemokine motif in the context of TLR signaling induce protective th1 immune responses and prevent pulmonary eosinophilia post-challenge. Vaccines. (2022) 10:2078. doi: 10.3390/vaccines10122078
66. Rainho-Tomko JN, Pavot V, Kishko M, Swanson K, Edwards D, Yoon H, et al. Immunogenicity and protective efficacy of RSV G central conserved domain vaccine with a prefusion nanoparticle. NPJ Vaccines. (2022) 7:74. doi: 10.1038/s41541-022-00487-9
67. Bergeron HC, Murray J, Arora A, Nunez Castrejon AM, DuBois RM, Anderson LJ, et al. Immune prophylaxis targeting the respiratory syncytial virus (RSV) G protein. Viruses. (2023) 15:1067. doi: 10.3390/v15051067
68. Fedechkin SO, George NL, Wolff JT, Kauvar LM, and DuBois RM. Structures of respiratory syncytial virus G antigen bound to broadly neutralizing antibodies. Sci Immunol. (2018) 3:3534. doi: 10.1126/sciimmunol.aar3534
69. Jennings GT and Bachmann MF. The coming of age of virus-like particle vaccines. Biol Chem. (2008) 389:521–36. doi: 10.1515/BC.2008.064
70. Ruzzi F, Semprini MS, Scalambra L, Palladini A, Angelicola S, Cappello C, et al. Virus-like particle (VLP) vaccines for cancer immunotherapy. Int J Mol Sci. (2023) 24:12963. doi: 10.3390/ijms241612963
71. Schwarz B, Morabito KM, Ruckwardt TJ, Patterson DP, Avera J, Miettinen HM, et al. Viruslike particles encapsidating respiratory syncytial virus M and M2 proteins induce robust T cell responses. ACS biomaterials Sci engineering. (2016) 2:2324–32. doi: 10.1021/acsbiomaterials.6b00532
72. Gomez-Puertas P, Albo C, Perez-Pastrana E, Vivo A, and Portela A. Influenza virus matrix protein is the major driving force in virus budding. J Virol. (2000) 74:11538–47. doi: 10.1128/JVI.74.24.11538-11547.2000
73. Nayak DP, Hui EK, and Barman S. Assembly and budding of influenza virus. Virus Res. (2004) 106:147–65. doi: 10.1016/j.virusres.2004.08.012
74. Melikyan GB, Lin S, Roth MG, and Cohen FS. Amino acid sequence requirements of the transmembrane and cytoplasmic domains of influenza virus hemagglutinin for viable membrane fusion. Mol Biol Cell. (1999) 10:1821–36. doi: 10.1091/mbc.10.6.1821
75. Kulkarni AB, Collins PL, Bacik I, Yewdell JW, Bennink JR, Crowe JE Jr., et al. Cytotoxic T cells specific for a single peptide on the M2 protein of respiratory syncytial virus are the sole mediators of resistance induced by immunization with M2 encoded by a recombinant vaccinia virus. J Virol. (1995) 69:1261–4. doi: 10.1128/jvi.69.2.1261-1264.1995
76. Hancock GE, Tebbey PW, Scheuer CA, Pryharski KS, Heers KM, and LaPierre NA. Immune responses to the nonglycosylated ectodomain of respiratory syncytial virus attachment glycoprotein mediate pulmonary eosinophilia in inbred strains of mice with different MHC haplotypes. J Med virology. (2003) 70:301–8. doi: 10.1002/jmv.10395
77. McDermott DS, Knudson CJ, and Varga SM. Determining the breadth of the respiratory syncytial virus-specific T cell response. J Virol. (2014) 88:3135–43. doi: 10.1128/JVI.02139-13
78. Freitas GR, Silva DA, Yokosawa J, Paula NT, Costa LF, Carneiro BM, et al. Antibody response and avidity of respiratory syncytial virus-specific total IgG, IgG1, and IgG3 in young children. J Med virology. (2011) 83:1826–33. doi: 10.1002/jmv.22134
79. Durant LR, Makris S, Voorburg CM, Loebbermann J, Johansson C, and Openshaw PJ. Regulatory T cells prevent Th2 immune responses and pulmonary eosinophilia during respiratory syncytial virus infection in mice. J Virol. (2013) 87:10946–54. doi: 10.1128/JVI.01295-13
80. Loebbermann J, Durant L, Thornton H, Johansson C, and Openshaw PJ. Defective immunoregulation in RSV vaccine-augmented viral lung disease restored by selective chemoattraction of regulatory T cells. Proc Natl Acad Sci U S A. (2013) 110:2987–92. doi: 10.1073/pnas.1217580110
81. Bystrom J, Al-Adhoubi N, Al-Bogami M, Jawad AS, and Mageed RA. Th17 lymphocytes in respiratory syncytial virus infection. Viruses. (2013) 5:777–91. doi: 10.3390/v5030777
82. Sahu U, Biswas D, Prajapati VK, Singh AK, Samant M, and Khare P. Interleukin-17-A multifaceted cytokine in viral infections. J Cell Physiol. (2021) 236:8000–19. doi: 10.1002/jcp.30471
83. Silva GSD, Borges SG, Pozzebon BB, and Souza APD. Immune responses to respiratory syncytial virus vaccines: advances and challenges. Microorganisms. (2024) 12:2305. doi: 10.3390/microorganisms12112305
84. Nooraei S, Bahrulolum H, Hoseini ZS, Katalani C, Hajizade A, Easton AJ, et al. Virus-like particles: preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J nanobiotechnology. (2021) 19:59. doi: 10.1186/s12951-021-00806-7
85. Blanco JCG, Pletneva LM, McGinnes-Cullen L, Otoa RO, Patel MC, Fernando LR, et al. Efficacy of a respiratory syncytial virus vaccine candidate in a maternal immunization model. Nat Commun. (2018) 9:1904. doi: 10.1038/s41467-018-04216-6
86. Graham BS, Modjarrad K, and McLellan JS. Novel antigens for RSV vaccines. Curr Opin Immunol. (2015) 35:30–8. doi: 10.1016/j.coi.2015.04.005
Keywords: respiratory syncytial virus, virus-like particles, vaccine, G protein, baculovirus-insect cell expression system
Citation: Qin H, Luo J and Pan Z (2025) Virus-like particles containing the extracellular domain of G protein in combination with a CTL peptide of M2 elicit protection against respiratory syncytial virus infection without pulmonary disease. Front. Immunol. 16:1625670. doi: 10.3389/fimmu.2025.1625670
Received: 09 May 2025; Accepted: 29 August 2025;
Published: 12 September 2025.
Edited by:
Qiang Chen, Arizona State University, United StatesReviewed by:
Wayne Robert Thomas, University of Western Australia, AustraliaHarshad Patil, Bharati Vidyapeeth Deemed University, India
Copyright © 2025 Qin, Luo and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zishu Pan, enNwYW5Ad2h1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Huan Qin
Huan Qin Jin Luo
Jin Luo Zishu Pan
Zishu Pan