- 1Graduate Program in Biotechnology, University of Taquari Valley - Univates, Lajeado, Brazil
- 2Department of Genetics, Institute of Biosciences, Federal University of Rio Grande do Sul (UFRGS), Porto Alegre, Brazil
Chemical Fertilizers × Plant Growth-Promoting Rhizobacteria
Plant growth-promoting rhizobacteria (PGPR) is a well-known group of microorganisms able to promote plant growth through enhanced biological nitrogen fixation (BNF), synthesis of plant hormones, soil nutrient solubilization (as phosphorus [P] and potassium [K]; Gupta et al., 2015), besides preventing deleterious effects of soil-borne phytopathogens (Compant et al., 2005). Due to the high importance of nitrogen (N) for plant development and the low persistence time that synthetic N fertilizer presents in the soil (Galloway et al., 2003), most of the studies are focused on microorganisms able to biologically fix atmospheric N. BNF is performed by symbiotic PGPR, which are restricted to association of leguminous plants and rhizobial isolates (e.g., Rhizobium spp., Bradyrhizobium spp., Mesorhizobium spp., and Allorhizobium spp.), or by free-living bacterial isolates (e.g., Azospirillum spp., Pseudomonas spp., Burkholderia spp., Gluconacetobacter spp., and Herbaspirillum spp.; Remigi et al., 2016). However, the research focused only in BNF neglects the high biotechnological potential of PGPR to agriculture.
Overuse of synthetic fertilizers and agrochemical pesticides has sustained the high crop yield and, consequently, the population growth in the last century (Stewart et al., 2005). However, environment does not sustain these practices any more. The consequences are already observed as high eutrophication of rivers, groundwater contamination, atmospheric pollution, and losses of soil quality (Stewart et al., 2005; Mondal et al., 2017). These scenarios have stimulated several agricultural researches. Replacement of synthetic N inputs by PGPR inoculation has been possible only due to the deep knowledge about BNF. It is interesting to farmers, since it reduces production costs besides being an environmental-friendly technique. However, PGPR inoculation can go further, since it presents a potential to reduce the amount of the most important synthetic inputs applied on crops, which is of paramount importance regarding fertilizers obtained from finite sources.
Soil Phosphorus (P) and P-Fertilization
Phosphorus (P) is a good example of an essential nutrient for plant development derived from finite resources. P fertilizer is extracted from P-rich rock in the form of phosphate. Morocco, China, South Africa and the U.S. account for approximately 83% of the world's reserves of exploitable phosphate rock (Vaccari, 2009). Therefore, P deficiency is one of the major limitations to crop production and it is estimated that 5.7 billion hectares of land worldwide are deficient in P (Mouazen and Kuang, 2016). These numbers highlight the high importance of P fertilizers for achieving optimal crop production. Bouwman et al. (2013) estimated that annual P consumption in agriculture will increase around 2.5% per year. Considering the finite sources of P, this data and other studies indicated that a global P crisis is near (Abelson, 1999; Vaccari, 2009; Jones et al., 2015). However, none of these studies have considered the residual P in the soil (Sattari et al., 2012).
Some tropical agricultural soils are P-fixing, and the vast majority of P fertilizer added to them are adsorbed onto soil minerals [metal oxides (mainly iron and aluminum) and clay minerals], precipitated as P minerals (predominantly apatite-like minerals), and immobilized as organic P compounds (soil organic matter and phytate), making its residual P less available to crops (Martinez-Viveros et al., 2010; Hinsinger et al., 2011). Due to such P immobilization and environmental losses, producers need to apply twice or more P fertilizers than are actually needed for optimal yield production (Roy et al., 2016). It is estimated that 2–8 million tons of P fertilizer are applied to the soils every year, and ~1–4 million tons remain in the soil as a residual part. In a future scenario (2050), 4–14 million tons will be applied, and 2–7 million tons will remain in the soils (Roy et al., 2016). Considering that P fertilizer costs approximately US$ 400 per ton, around US$ 400 million to US$ 1.6 billion are lost with P fertilizers in crops around the world every year. It certainly means a substantial increase on the food prices for consumers.
Is Phosphorus Solubilization the Forgotten Child of PGPR?
Recently, Roy et al. (2016) made a tricky question: is it possible that the increasing amount of immobilized P in the tropical agricultural soils eventually become available to plants and support crop productivity? In the case we keep using the same fertilization strategies used for many years, the answer is certainly no. However, we do believe that using adequate biotechnological approaches, the immobilized P could return to the plants in a soluble and available form. Screening of new PGP isolates for inoculant production aiming to optimize plant growth and BNF comprise an essential stage of in vitro phosphate solubilization analysis (Collavino et al., 2010; Souza et al., 2013, 2015; Walitang et al., 2017; Marag et al., 2018). These studies identified several bacterial isolates able to promote plant growth, improve rhizosphere area and solubilize different sources of immobilized P. Given the low mobility of P in soils, the enlargement of volume and geometry of the rhizosphere provided by PGPR inoculation determines the amount of P available to plants (Richardson et al., 2009). Therefore, inoculation of PGPR seems to be a reasonable tool to maximize such approach. Microorganisms increase the availability of inorganic P through the production of protons, organic acids, and ligands, which are ubiquitous among rhizosphere P-solubilizing microorganisms (Hinsinger et al., 2011), and also mobilize phytate (organic P) probably by phytase production (Jorquera et al., 2008). However, in greenhouse and/or field conditions, most of the studies do not evaluate different P-fertilization levels, phosphate solubilization in the soil and P uptake by the plants. The majority of the studies considers only plant agronomic parameters and plant N content in conditions with or without N fertilization.
Reduction of P-Fertilization Through PGPR Inoculation
Increasing P efficiency in crops without increasing or even decreasing P inputs requires a more efficient exploitation of soil microbial resources in agroecosystems. Some studies clearly report that plant inoculation with new PGPR can improve P uptake. Rudresh et al. (2005) showed that chickpea plants inoculated with Rhizobium sp. and Bacillus sp. present higher yield (two-fold) and higher P content (four-fold) in the grain. Vyas and Gulati (2009) and Granada et al. (2013) demonstrated that inoculation of maize (Zea mays) with Pseudomonas spp., and Lupinus albescens plants with free-living Sphingomonas sp. results in almost three-fold increases in their shoot P contents, respectively. Studying wheat (Triticum aestivum L.) plants, Kumar et al. (2014) showed that inoculation of Bacillus megaterium, Arthrobacter chlorophenolicus, and Enterobacter improves grain yield and the amount of P in the straw and grain up to two-fold in greenhouse and field experiments. Thus, it is already known that inoculation of efficient P-solubilizer bacteria significantly improve P absorption by plants, even though most of the experiments use the recommended P fertilizer dose, and reduction of the P-fertilization has not been evaluated.
Khalafallah et al. (1982) developed an important work inoculating Vicia faba plants with P-solubilizing bacteria. This work showed the possibility of reducing the P-fertilization up to 50%, once plants that received half of the recommended P-fertilizer dose presented similar plant dry weight and P-uptake when compared to plants that received usual P-fertilizer dose. More recently, Lavakush et al. (2014) observed the same potential in rice plants inoculated with the P-solubilizing bacteria Azotobacter chroococcum, Azospirillum brasilense, and combined Pseudomonas spp. culture. Inoculated rice plants presented similar performance in plant height, panicle length, grain number per panicle and grain yield when fertilized with 30 and 60 kg P ha−1 in a greenhouse experiment. Dutta and Bandyopadhyay (2009) showed that reduction of up to one-third in P-fertilization of chickpea plants (inoculated with P-solubilizing Pseudomonas sp.) did not cause any decrease in plant development parameters.
Therefore, PGPR inoculation can probably be used to reduce P-fertilization, being an excellent biotechnological tool. However, this research area is neglected by researches and certainly needs more investigation. All plant species are able to establish a relationship with some PGPR, and the selection of new bacterial isolates, able to solubilize different forms of P in vitro, is an important and necessary first step. We hope the results obtained in greenhouse and field inoculation experiments with selected P-solubilizing bacterial isolates and plant species subjected to reduced amounts of P-fertilizer could serve as an alert to producers about the high costs of normally used fertilization strategies, the concerns about finite P sources, and the environmentally friend biotechnological option of using PGPR. Based on previous works which addressed P-solubilization potential by PGPR inoculation in plants (mainly Khalafallah et al., 1982; Dutta and Bandyopadhyay, 2009; Kumar et al., 2014; Lavakush et al., 2014; Anzuay et al., 2015; Kaur and Reddy, 2015), we consider that an average reduction of 33% in P-fertilization could be achieved with the use of high efficient P-solubilizing bacterial isolates as crop inoculants, as indicated on the proposed biotechnological approach in Figure 1. Therefore, future experiments need to be specifically designed for such purposes. Considering the complexity of these mechanisms, an interdisciplinary approach taking into account molecular, biochemical, physiological, and agronomic parameters has a good probability to generate positive results. We have a long way to cross until reaching similar knowledge and applicability achieved by bacterial inoculants regarding the reduction of N-fertilizers. However, reasonable use of environmental resources should be the basis for modern and sustainable agriculture development.
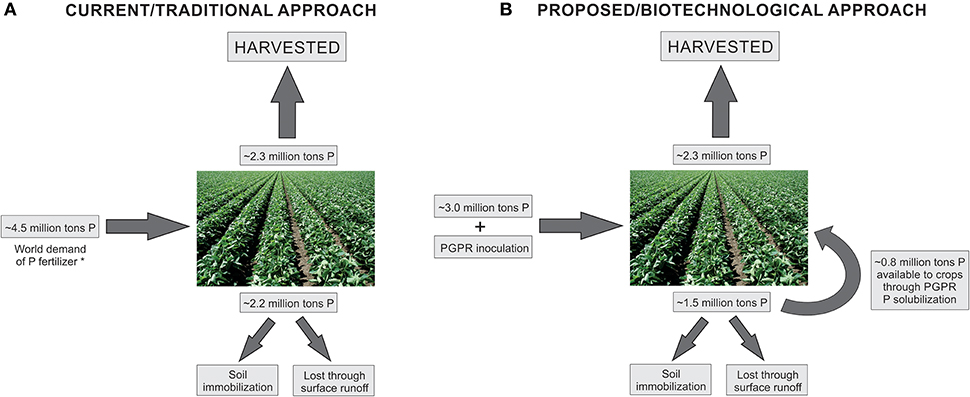
Figure 1. Schematic model of current/traditional approach (A) and proposed/biotechnological approach (B). *On the current agricultural approach, world demand of P fertilizer is approximately 4.5 million tons, according to FAO (http://www.fao.org/3/a-i6895e.pdf). From these, 2.2 million tons are unavailable to crops (soil immobilization or surface runoff), and 2.3 million tons are harvested with the crops. On the proposed biotechnological approach, we suggest the reduction of up to 33% on the P fertilizer dose applied on the soil, along with PGPR inoculation. Such reduction on P fertilizer together with PGPR inoculation would result in less P unavailable to the crops. Nearly half of such unavailable P can be further solubilized by PGPR and uptaked by the crops, resulting in the same 2.3 million tons of harvested P (adapted from Roy et al., 2016).
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abelson, P. H. (1999). A potential phosphate crisis. Science 283:2015. doi: 10.1126/science.283.5410.2015
Anzuay, M. S., Ludueña, L. M., Angelini, J. G., Fabra, A., and Taurian, T. (2015). Beneficial effects of native phosphate solubilizing bacteria on peanut (Arachis hypogaea L) growth and phosphorus acquisition. Symbiosis 66, 89–97. doi: 10.1007/s13199-015-0337-z
Bouwman, L., Goldewijk, K. K., Van Der Hoek, K. W., Beusen, A. H. W., Van Vuuren, D. P., Willems, J., et al. (2013). Exploring global changes in nitrogen and phosphorus cycles in agriculture induced by livestock production over the 1900-2050 period. Proc. Natl. Acad. Sci. U.S.A. 110, 20882–20887. doi: 10.1073/pnas.1012878108
Collavino, M. M., Sansberro, P. A., Mroginski, L. A., and Aguilar, O. M. (2010). Comparison of in vitro solubilization activity of diverse phosphate-solubilizing bacteria native to acid soil and their ability to promote Phaseolus vulgaris growth. Biol. Fertil. Soils 46, 727–738. doi: 10.1007/s00374-010-0480-x
Compant, S., Duffy, B., Nowak, J., Cle, C., and Barka, E. A. (2005). Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 71, 4951–4959. doi: 10.1128/AEM.71.9.4951-4959.2005
Dutta, D., and Bandyopadhyay, P. (2009). Performance of chickpea (Cicer arietinum L.) to application of phosphorus and bio-fertilizer in laterite soil. Arch. Agron. Soil Sci. 55, 147–155. doi: 10.1080/03650340802398864
Galloway, J. N., Aber, J. D., Erisman, J. W., Seitzinger, S. P., Howarth, R. W., Cowling, E. B., et al. (2003). The nitrogen cascade. Bioscience 53, 341–356. doi: 10.1641/0006-3568(2003)053[0341:TNC]2.0.CO;2
Granada, C., da Costa, P. B., Lisboa, B. B., Vargas, L. K., and Passaglia, L. M. P. (2013). Comparison among bacterial communities present in arenized and adjacent areas subjected to different soil management regimes. Plant Soil 373, 339–358. doi: 10.1007/s11104-013-1796-8
Gupta, G., Parihar, S. S., Ahirwar, N. K., Snehi, S. K., and Singh, V. (2015). Plant growth promoting rhizobacteria (PGPR): current and future prospects for development of sustainable agriculture. J. Microb. Biochem. Technol. 7, 96–102. doi: 10.4172/1948-5948.1000188
Hinsinger, P., Betencourt, E., Bernard, L., Brauman, A., Plassard, C., Shen, J., et al. (2011). P for two, sharing a scarce resource: soil phosphorus acquisition in the rhizosphere of intercropped species. Plant Physiol. 156, 1078–1086. doi: 10.1104/pp.111.175331
Jones, C., Nomosatryo, S., Crowe, S. A., Bjerrum, C. J., and Canfield, D. E. (2015). Iron oxides, divalent cations, silica, and the early earth phosphorus crisis. Geology 43, 135–138. doi: 10.1130/G36044.1
Jorquera, M. A., Hernández, M. T., Rengel, Z., Marschner, P., and De La Luz Mora, M. (2008). Isolation of culturable phosphobacteria with both phytate-mineralization and phosphate-solubilization activity from the rhizosphere of plants grown in a volcanic soil. Biol. Fertil. Soils 44, 1025–1034. doi: 10.1007/s00374-008-0288-0
Kaur, G., and Reddy, M. S. (2015). Effects of phosphate-solubilizing bacteria, rock phosphate and chemical fertilizers on maize-wheat cropping cycle and economics. Pedosphere 25, 428–437. doi: 10.1016/S1002-0160(15)30010-2
Khalafallah, M. A., Saber, M. S. M., and Abd-El-Maksoun, H. K. (1982). Influence of phosphate dissolving bacteria on the efficiency of superphosphate in a calcareous soil cultivated with Vicia faba. Z. Pflanzenernaehr. Bodenk. 145, 455–459. doi: 10.1002/jpln.19821450505
Kumar, A., Maurya, B. R., and Raghuwanshi, R. (2014). Isolation and characterization of PGPR and their effect on growth, yield and nutrient content in wheat (Triticum aestivum L.). Biocatal. Agric. Biotechnol. 3, 121–128. doi: 10.1016/j.bcab.2014.08.003
Lavakush, Y. J., Verma, J. P., Jaiswal, D. K., and Kumar, A. (2014). Evaluation of PGPR and different concentration of phosphorus level on plant growth, yield and nutrient content of rice (Oryza sativa). Ecol. Engin. 62, 123–128. doi: 10.1016/j.ecoleng.2013.10.013
Marag, P. S., Suman, A., and Gond, S. (2018). Prospecting endophytic bacterial colonization and their potential plant growth promoting attributes in hybrid maize (Zea mays L.). Int. J. Curr. Microbiol. Appl. Sci. 7, 1292–1304. doi: 10.20546/ijcmas.2018.703.154
Martinez-Viveros, O., Jorquera, M. A., Crowley, D. E., Gajardo, G., and Mora, M. L. (2010). Mechanisms and practical considerations involved in plant growth promotion by Rhizobacteria. J. Soil Sci. Plant Nutr. 10, 293–319. doi: 10.4067/S0718-95162010000100006
Mondal, T., Datta, J. K., and Mondal, N. K. (2017). Chemical fertilizer in conjunction with biofertilizer and vermicompost induced changes in morpho-physiological and bio-chemical traits of mustard crop. J. Saudi Soc. Agric. Sci. 16, 135–144. doi: 10.1016/j.jssas.2015.05.001
Mouazen, A. M., and Kuang, B. (2016). On-line visible and near infrared spectroscopy for in-field phosphorous management. Soil Tillage Res. 155, 471–477. doi: 10.1016/j.still.2015.04.003
Remigi, P., Zhu, J., Young, J. P., and Masson-Boivin, C. (2016). Symbiosis within symbiosis: evolving nitrogen-fixing legume symbionts. Trends Microbiol. 24, 63–75. doi: 10.1016/j.tim.2015.10.007
Richardson, A. E., Baréa, J. M., McNeill, A. M., and Prigent-Combaret, C. (2009). Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321, 305–339. doi: 10.1007/s11104-009-9895-2
Roy, E. D., Richards, P. D., Martinelli, L. A., Coletta, L. D., Lins, S. R. M., Vazquez, F. F., et al. (2016). The phosphorus cost of agricultural intensification in the tropics. Nat. Plants 43, 1–6.
Rudresh, D. L., Shivaprakash, M. K., and Prasad, R. D. (2005). Effect of combined application of Rhizobium, phosphate solubilizing bacterium and Trichoderma spp. on growth, nutrient uptake and yield of chickpea (Cicer aritenium L.). Appl. Soil Ecol. 28, 139–146. doi: 10.1016/j.apsoil.2004.07.005
Sattari, S. Z., Bouwman, A. F., Giller, K. E., and van Ittersum, M. K. (2012). Residual soil phosphorus as the missing piece in the global phosphorus crisis puzzle. Proc. Natl. Acad. Sci. U.S.A. 109, 6348–6353. doi: 10.1073/pnas.1113675109
Souza, R., Beneduzi, A., Ambrosini, A., Costa, P. B., Meyer, J., Vargas, L. K., et al. (2013). The effect of plant growth-promoting rhizobacteria on the growth of rice (Oryza sativa L.) cropped in southern Brazilian fields. Plant Soil 366, 585–603. doi: 10.1038/nplants.2016.43
Souza, R., Meyer, J., Schoenfeld, R., Costa, P. B., and Passaglia, L. M. P. (2015). Characterization of plant growth-promoting bacteria associated with rice cropped in iron-stressed soils. Ann. Microbiol. 65, 951–964. doi: 10.1007/s13213-014-0939-3
Stewart, W. M., Dibb, D. W., Johnston, A. E., and Smyth, T. J. (2005). The contribution of commercial fertilizer nutrients to food production. Agron. J. 97, 1–6. doi: 10.2134/agronj2005.0001
Vaccari, D. A. (2009). Phosphorus: a looming crisis. Scientific American 300, 54–59. doi: 10.1038/scientificamerican0609-54
Vyas, P., and Gulati, A. (2009). Organic acid production in vitro and plant growth promotion in maize under controlled environment by phosphate-solubilizing fluorescent Pseudomonas. BMC Microbiol. 9:174. doi: 10.1186/1471-2180-9-174
Keywords: P-fertilizers, inoculation, P-solubilization, rhizobacteria, sustainable agriculture
Citation: Granada CE, Passaglia LMP, de Souza EM and Sperotto RA (2018) Is Phosphate Solubilization the Forgotten Child of Plant Growth-Promoting Rhizobacteria? Front. Microbiol. 9:2054. doi: 10.3389/fmicb.2018.02054
Received: 09 July 2018; Accepted: 13 August 2018;
Published: 03 September 2018.
Edited by:
Youssef Rouphael, Università degli Studi di Napoli Federico II, ItalyReviewed by:
Claudio Valverde, Universidad Nacional de Quilmes (UNQ), ArgentinaGyöngyvér Mara, Sapientia Hungarian University of Transylvania, Romania
Copyright © 2018 Granada, Passaglia, de Souza and Sperotto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Camille E. Granada, Y2VncmFuYWRhQHVuaXZhdGVzLmJy
 Camille E. Granada
Camille E. Granada Luciane M. P. Passaglia
Luciane M. P. Passaglia Eduardo M. de Souza1
Eduardo M. de Souza1 Raul A. Sperotto
Raul A. Sperotto