- 1College of Animal Science and Veterinary Medicine, Henan Agricultural University, Zhengzhou, China
- 2College of Veterinary Medicine, China Agricultural University, Beijing, China
Toxoplasma gondii as a food-borne pathogen, the infection of it in food animals has relation with human toxoplasmosis, but the trends and epidemiological features of T. gondii infections in food animals are rarely studied in China. The aimed of this study was to assess the epidemiology and risks of T. gondii in sheep, goats, swines, chickens, yaks, cattle and humans from 2000 to 2017 and to explore prevention and control strategies. The overall seroprevalence of T. gondii infections in food animals is 23.7% (39,194/165,417, 95%CI, 23.49–23.90%), which is significantly higher than that in humans (8.2%, 95%CI, 8.06–8.39%, 8,502/103,383) (P < 0.0001). Compared the prevalence of T. gondii infections in animals and humans sampled from 2000 to 2010, it was significantly increased in the period 2011 to 2017 (P < 0.0001). Compared the food animals from non-Yangtze River, animals from regions of the Yangtze River have high seroprevalence rates for T. gondii (P < 0.0001). Furthermore, samples from the western to eastern regions of the Yellow River showed an increase in seroprevalence for T. gondii (P < 0.0001). It was speculated that T. gondii oocysts may be transmitted by water and annual precipitation possible help the oocyst spread and retain accessible for potential hosts. Effective prevention and control strategies are including water filtration or water boiling, inactivating oocysts from feline’s feces, monitoring birds and rodents. Chinese 1 (ToxoDB#9) is the predominant genotype in food animals from China.
Background
Toxoplasmosis, which is caused by Toxoplasma gondii, is one of the most common zoonoses around the world, affecting warm-blooded animals, including humans (Zhou et al., 2008; Dubey, 2010). It is estimated that about one-third of the world’s population has been infected with T. gondii (Montoya and Liesenfeld, 2004; Weiss and Dubey, 2009). In general, T. gondii infections are subclinical or asymptomatic in immunocompetent individuals (Wang et al., 2017). However, T. gondii infections involving pregnant women and small ruminants induce abortion or fetal developmental disorders (Dubey, 2010). T. gondii also poses a high risk in immunocompromised individuals for severe health problems (Weiss and Dubey, 2009; Wang et al., 2017).
Oocysts, bradyzoites, and tachyzoites are the three infectious stages of T. gondii. The transmission route of T. gondii may be vertical and horizontal. Vertical transmission involves tachyzoite infection through the placenta or semen (de Moraes et al., 2010; Dubey, 2010; Lopes et al., 2013). Horizontal transmission involves ingestion of fruits, vegetables, or water contaminated with oocysts, tachyzoite infection by blood transfusion or ingestion of raw milk and cheese (Dubey et al., 2014), or consumption of undercooked meat containing bradyzoites. The China annual production of pork, chicken, mutton, and beef has been estimated at 55, 18, 4 and 7 million tones, respectively (National Bureau of Statistics of China, 2015 update). Meats have become the major and favored food in the past decades (Jiang et al., 2013; Wang et al., 2015, 2016; Yang et al., 2017b). Simmered meat, rice, noodles, and mass-cooked vegetables are the main ingredients in the traditional Chinese diet. Raw or undercooked meat is not common in the daily diets of most Chinese. However, undercooked animal products such as hotpot, barbecue, and raw milk are popular dishes in certain regions (Li et al., 2009; Gao and Tang, 2018).
Raw or undercooked meat consumption is significantly associated with human T. gondii infections (Kapperud et al., 1996; Tao et al., 2011; Belluco et al., 2017). This is dependent on parasite prevalence, dietary habits, and cultural habits. However, no meat inspection strategy for T. gondii contamination has been established, and no performance standards for processing T. gondii-positive meat have been developed to date. National surveys have found that the seroprevalence of T. gondii infection in humans was increased from 5.2% (1988–1992) to 7.9% (2001–2004) (Yu et al., 1994; Zhou et al., 2008, 2011). The prevalence of T. gondii in pregnant Chinese women is less than 10%, indicating that a high proportion of women are susceptible to infection during pregnancy. Approximately 0.3% of pregnant women have been diagnosed with an acute infected of T. gondii during pregnancy (1990–2010) (Gao et al., 2012). Pan et al. (2017) have shown that the prevalence of animal T. gondii infections in sheep, swines, and chickens in China from 2010 to 2017 is significantly higher than that in humans. Most reports about T. gondii from China have been published in local journals, which are generally inaccessible to readers around the world. Therefore, this review aimed to collect all the data about food animals (sheep, goats, swines, chickens, cattle) and humans with T. gondii infection, mainly focusing on epidemiological data such as seroprevalence, risk factors, and genotypes in order to provide suggestions on the prevention and control of this foodborne pathogen.
Data Collection and Statistical Analysis
All the data about food animals and human with T. gondii were collected (2000–2017). The references were obtained from Baidu Scholar, China National Knowledge Infrastructure (CNKI), Wan Fang, VIP Chinese Journal Database, PubMed, and Google Scholar. The literature includes criteria were published in English and Chinese, the seroprevalence of T. gondii reports was limited to the detection of specific anti-Toxoplasma immunoglobulin (Ig) G in serum. The criteria used to exclude the reviewed studies and duplicated reports in the same research group, incomplete original data and the reports published before 2000.
Serological detection of specific anti-Toxoplasma immunoglobulin (Ig) G in serum is currently the most widely used method of detecting T. gondii in China, which include indirect hemagglutination (IHA), enzyme-linked immunoabsorbent assay (ELISA), modified agglutination test (MAT), latex agglutination test (LAT), and test paper. However, there were few studies compared the sensitivity and specificity of serologic assays for T. gondii in different hosts and serological detection methods. Apparent seroprevalence was used to estimate the epidemiological regularity of T. gondii infection. The results only represent rough estimates of T. gondii infection in China.
To further show epidemiological regularity with geographical differences in China, the country was divided into seven geographical areas. Statistical analysis was performed by the GraphPad Prism 4.0 software (GraphPad Software Inc., San Diego, CA, United States). The data were analyzed by the Chi-square test to determine the association between T. gondii seroprevalence with geographic locations and years. P < 0.05 was considered statistically significant. Graphs were generated using Graph Pad Prism 7.0 software (GraphPad Software, Inc., San Diego, CA, United States).
T. gondii Prevalence and Risk Assessment in Food Animals and Humans
Felids were the definitive host of T. gondii, which spread oocysts by fecal excretion (Dubey, 2010; Dubey et al., 2012). Ding reviewed the published articles from 1995 to 2016 and reported that the seroprevalence in cats was 24.5%, which involved 7,285 cats from 15 provinces in China (Ding et al., 2017). This finding indicates that T. gondii oocysts are widely distributed in the country. This viewpoint was also verified by other reports (Pan et al., 2017; Yang et al., 2017a). Furthermore, the popularity of hotpot with undercooked mutton and pork increases the risk of human T. gondii infection in China, with other food animals also remaining as threats to public health (Guo et al., 2015; Yang et al., 2017b).
Sheep
Sheep and goats are highly susceptible to T. gondii infections. Due to the subtropical climate and geographical environment, little sheep were breed in South China, mutton comes mainly from goats. Mutton, viscera, blood, exudates, and milk from sheep and goats that carry T. gondii parasites may infect other animals and humans (Dubey et al., 2014; Ding et al., 2017; Tonouhewa et al., 2017). Based on the data and the findings of Yang (Yang et al., 2017b), an epidemiological map of T. gondii in China was generated (Figure 1). The seroprevalence for T. gondii in sheep has been estimated to be 11.8% (95%CI, 11.33–12.23%, 2,305/19,565), which is lower than that in other countries (Dubey, 2010) (Supplementary Table S1).
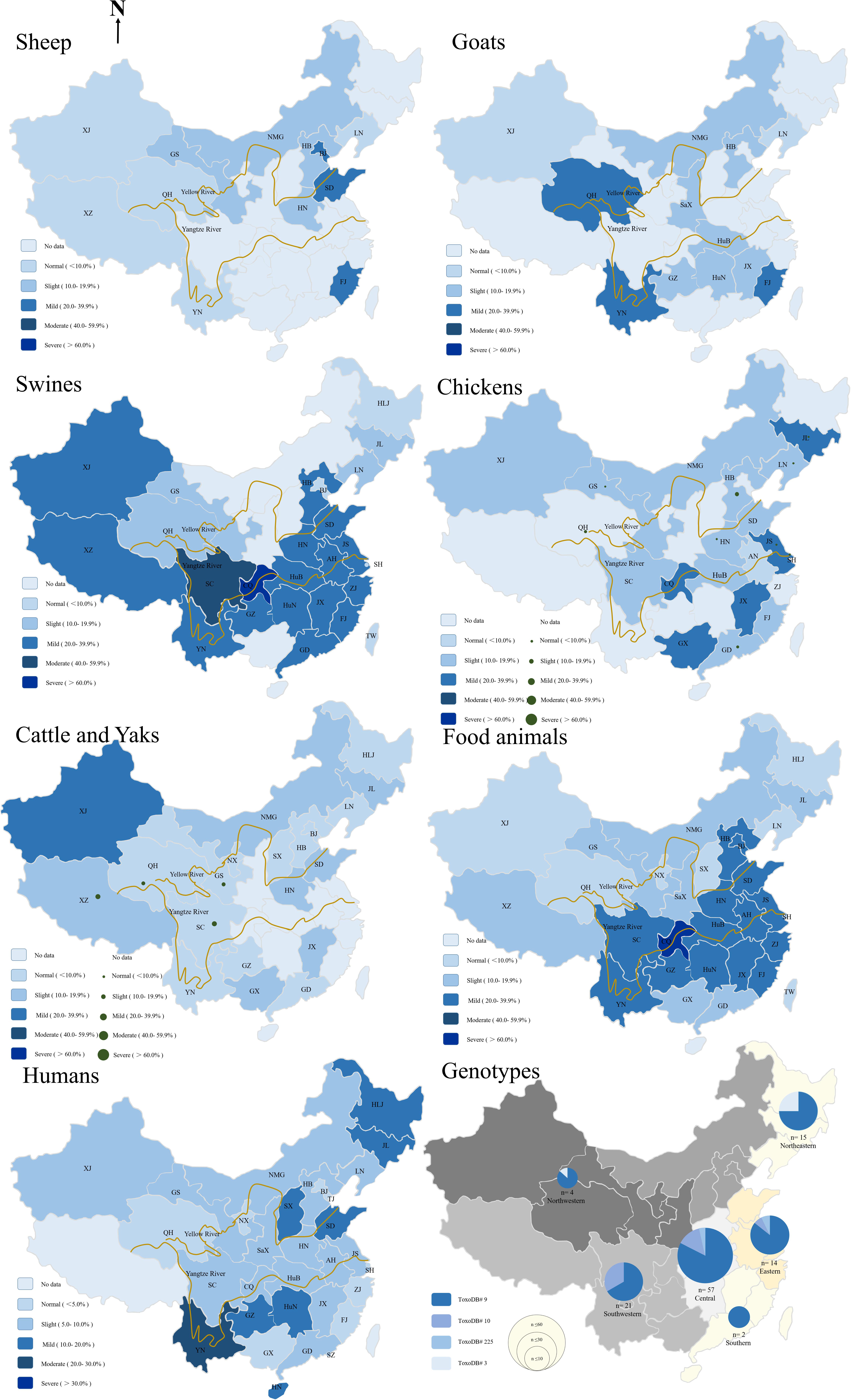
FIGURE 1. Geographical distribution map of epidemiology of Toxoplasma gondii in sheep, goats, swines, chickens, cattle and yaks, food animals summary, humans, and genotypes of T. gondii in food animals from 2000–2017 in China. HLJ: HeiLongJiang; JL: JiLin; LN: LiaoNing; HB: HeBei; GS: GanSu; QH: QingHai; XJ: XinJiang; XZ: Tibet; SC: SiChuan; CQ: ChongQing municipality; GZ: GuiZhou; HN: HeNan; HuB: HuBei; HuN: HuNan; SD: ShanDong; JS: JiangShu; ZJ: ZheJiang; AH: AnHui; JX: JiangXi; FJ: FuJian, YN: YunNan; GD: GuangDong; GX: GuangXi; TW: TaiWan; NX: NingXia; NMG: Inner Mongolia; SX: ShanXi; SaX: ShaanXi; SH: ShangHai; BJ: BeiJing.
Toxoplasma gondii seroprevalence in sheep varies with altitude, year, climate, geographic regions, abortion history, and age. Compared the prevalence of T. gondii infections in sheep sampled from 2000 to 2010, it was significantly increased in the period of 2011–2017 (P < 0.0001, OR = 1.578). Figure 1 shows that sheep from eastern coastal locations have the highest prevalence rates, higher than that of sheep from the west part (P < 0.0001, OR = 2.509) (Supplementary Table S2). The altitude of eastern coastal provinces is lower than western provinces, and most rivers flow from west to east, into the sea. A previous survey suggested that T. gondii oocysts could thus be transported via freshwater runoff into the ocean (Dubey, 2010; Cong et al., 2012; Gao et al., 2016). This might explain the high prevalence of T. gondii infection in the east compared with that in the west of China. Therefore, the high prevalence rate of T. gondii infections in eastern coastal provinces may be related to the accumulation of oocysts in rivers. Furthermore, the other possible reasons may be that residents in eastern part like to keep pet cats because better economic conditions or the T. gondii oocysts benefits from the development under warm climate and moist environment in the east. Abortion history, age, and geographical origin were the main risk factors associated with T. gondii infections (Yang et al., 2017b).
Hide (2016) reported that high prevalence of T. gondii infection in sheep is due to transplacental transmission rather than oocyst original infection. The seroprevalence of T. gondii in sheep from different areas in China was within the range of 0.8–39.3%, indicating sheep could be infected with T. gondii with oocysts from contaminated environmental or reactivation T. gondii cysts in chronic infection sheep during pregnancy. Most cooking methods for mutton meat were boiling for several hours, even overnight boiling for soup, which may effectively inactivate T. gondii cysts (Dubey, 2010). Therefore, the seroprevalence of T. gondii in sheep is not directly related to the prevalence of viable T. gondii in mutton. Lamb and mutton are very popular in certain parts of China (Gansu, Neimenggu, and Xinjiang), where pork may be eschewed for religious or economic reasons. Mutton is the main ingredient of hotpot, wherein the meat is frozen, sliced, and boiled 3 s to 2 min for eating. No reports describing that hotpot could effectively inactivate T. gondii were identified.
Goats
Toxoplasma gondii could cause abortion and neonatal mortality in goats (Dubey, 2010). Although more information and high prevalence rates of T. gondii have been reported in goats from around the world (Dubey, 2010), investigations on cases of toxoplasmosis in goats from China are limited. The overall estimated seroprevalence for T. gondii in goats is 17.6% (95%CI, 17.02–18.12%, 3,260/18,556), which is higher than that of sheep (Figure 1 and Supplementary Table S3). In East China, mutton mainly comes from sheep, and very little breeding for goats, so there were few available reports on the prevalence of T. gondii in goat from East China. The seroprevalence rates for T. gondii infection in goats from south-west of China was higher than other parts (P < 0.0001, OR = 1.130). Compared the prevalence of T. gondii infection in goats sampled from 2000 to 2010 (19.32%, 95%CI, 18.55–20.12%), the prevalence rate was decreased from 2011 to 2017 (14.09%, 95%CI, 13.38–14.83%) (P < 0.0001, OR = 1.460, Figure 2). Gender, season, age, geographical origin, the presence of cats, hygiene, and abortion history are risks for T. gondii infections in goats. Goat meat and milk contaminated T. gondii may have potential threaten for healthy consumers.
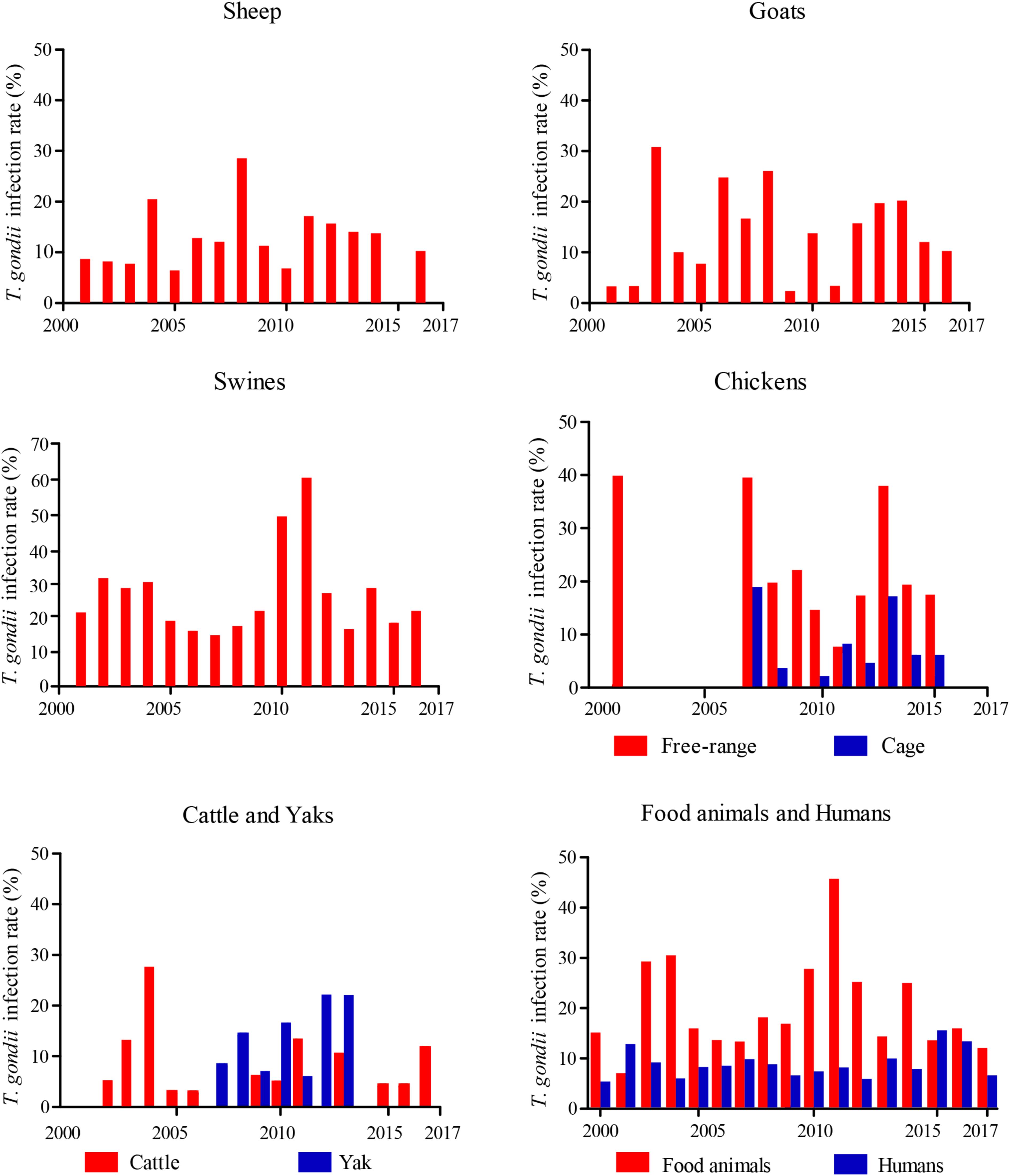
FIGURE 2. Year distribution map of epidemiology of Toxoplasma gondii in food animals and human, from 2000–2017 in China.
Swines
China is the largest consumer and a global producer of pork. T. gondii was isolated from retail pork samples (Dubey et al., 2005; Wang et al., 2012). The overall estimated seroprevalence for T. gondii in swine is 32.9% (95%CI, 32.54–33.16%, 29,559/89,978) (Supplementary Table S4). The prevalence of T. gondii varies widely among sample sources. Overall, the infection of T. gondii in swine from free-ranging and animal hospitals is higher than that of farms and slaughterhouses (P < 0.0001, OR = 1.201). The pigs from Chongqing were the most severely infected with T. gondii, whereas those from Sichuan, Guizhou provinces showed moderate degrees of infection (Figure 1). In general, the level of T. gondii infection in most areas of China is moderate and may be related to the presence of cats and dogs, the size of farm, age, breeding density, and insects, the frequency of scavenging, and management and transport of pigs (Wang et al., 2012; Liu et al., 2017).
The overall seroprevalence for T. gondii in swine is similar to that of American countries (Assadi-Rad et al., 1995; Suarez-Aranda et al., 2000). Further investigation indicated that these geographic surveys for T. gondii infections vary in terms of serological testing method used and cutoff values (Dubey, 2010). Nevertheless, a higher prevalence (63.3%) has been reported in Argentina (Omata et al., 1994), and lower seroprevalence rates have been reported in Mexico (8.9%) (Garcia-Vazquez et al., 1993) and Canada (9.4%) (Smith, 1991). In China, compared the prevalence of T. gondii infections in swines sampled from 2000 to 2010 (27.58%, 95%CI, 27.13–28.03%), it was significantly increased in the period of 2011–2017 (36.64%, 95%CI, 36.22–37.05%) (P < 0.0001, OR = 1.518, Figure 2). This may be related to the increased number of cats and the underestimate of T. gondii oocysts in the environment.
Chickens
Viable T. gondii strains have been isolated from chickens (Dubey, 2010), and T. gondii tachyzoites have been observed in red blood cells of birds (Dubey, 2002). Chickens play a crucial role in the transmission of T. gondii, free-ranging chickens are infected with T. gondii by ingesting oocysts from soil or food (Feng et al., 2016). Chicken may be utilized as an indicator of environmental and soil contamination with T. gondii oocysts. Although chickens are susceptible to T. gondii infections, they do not always exhibit clinical symptoms, and only a few reports have described the clinical symptoms of chicken toxoplasmosis. Although the consumption of raw eggs is not significantly associated with T. gondii infections (Dubey, 2002), it could not rule out the possibility of infection. However, no direct evidence has been reported.
The estimated seroprevalence for T. gondii was 17.9% (95%CI, 16.93–18.83%, 1,116/6,242) in free-ranging chicken, 6.3% (95%CI, 5.63–6.99, 307/4,866) in caged chickens, and overall prevalence in chickens of 19 provinces is 12.8% (95%CI, 12.19–13.43%, 1,423/11,108) (Supplementary Table S5). The epidemiological data of T. gondii are presented in Figure 1. In general, the level of T. gondii infection in most areas of China is slight and may be related to the source of chickens; the prevalence of T. gondii infections in most free-ranging chickens is higher than that of caged chicken. Compared the prevalence of T. gondii infections in chickens sampled from 2000 to 2010 (15.56%, 95%CI, 14.53–16.58%), the prevalence rate was decreased dramatically in the period of 2011–2017 (10.73%, 95%CI, 9.97–11.49%) (P < 0.0001, OR = 1.533) (Figure 2), which may be related to improved management and intensive farming.
Cattle and Yaks
Yak (Bos grunniens) is a long-haired bovid that is distributed in the Himalayan region of south-central Asia. China has 1.3 million yaks in the Qinghai-Tibetan Plateau, which include wild yak and domestic yak, accounting for 90% of the world’s yak population (Liu et al., 2008; Li et al., 2014). In addition, China is one of the largest countries that engage in cattle farming around the world, raising an estimated 50 million in 20151.
The overall estimated seroprevalence of T. gondii in cattle and yak was 9.1% (95%CI, 8.65–9.52%, 1,560/17,168) and 13.5% (95%CI, 12.80–14.21%, 1,221/9,042) respectively from China’s 20 Provinces (Supplementary Table S6 and Figure 1). The overall prevalence of the T. gondii was 10.6% (95%CI, 10.24–10.98%, 2,781/26,210) in cattle from China, which is lower than that reported in Estonia 16.8% (743/3991) (P < 0.001) (Robert-Gangneux and Darde, 2012), and similar to that reported in Czech Republic 9.7% (53/546) (P = 0.5723) (Bartova et al., 2015).
Although cattle are considered as poor hosts for T. gondii and good hosts for N. caninum, T. gondii infections could cause abortion, resulting in substantial economic losses and an increased potential in transmission to other animals and humans (Dubey and Jones, 2008). The observed higher seroprevalence of T. gondii in cattle from Xinjiang (Wang et al., 2011) and Qinghai (Liu et al., 2011) may possibly be due to geographical factors such as rivers, rainfall, wild animals, and differences in landscape. Compared to cattle that do not have a history of abortion, T. gondii infections are a risk for abortion in cattle from the north, northeast, and central China (Sun et al., 2015).
The cattle and yaks from the pastoral area have higher prevalence rates than those in farms, and yaks had higher prevalence rates than cattle raised in pastures (Dong et al., 2011). Ecological and geographical factors, pregnancies, ages, history of abortion, and breed style (graze, barns) are risks for T. gondii infections in cattle. T. gondii indirectly infects humans through the consumption of raw or undercooked beef or milk (Zhou et al., 2012; Xu et al., 2015).
Humans
T. gondii widely occurs among the Chinese and its prevalence rate widely varies among provinces (Supplementary Table S7). For 2000–2017, the overall seroprevalence of T. gondii in Chinese is 8.2% (95%CI, 8.06–8.39%, 8,502 /103,383) (Figure 1), which is relatively lower than that of France (61.0%), Brazil (84.5%), United States (38.0%), and India (24.0%) (Dubey, 2010). The prevalence rate of T. gondii is 8.6% in pregnant women or women diagnosed with gynecological diseases, 16.8% in cancer patients. Furthermore, specific professions (butchers, zookeepers, animal traffickers), low level of education, non-Han people, eating raw and undercooked meat were the most important risk factors. In addition, the prevalence of human T. gondii infection increased from West China to East China, which coincides with the incidence of T. gondii infection in food animals. Further, consistent with that in food animals, compared the prevalence of T. gondii infections in human from 2000 to 2010 (7.49%, 95%CI, 7.29–7.68%), the seroprevalence rate for human T. gondii infection significantly increased in the period of 2011–2017 (9.69%, 95%CI, 9.38–10.01%), (P < 0.0001, OR = 1.326), which may be related to changes in eating habits (steaks, vegetable salads, and barbecues were more popular), increased proportion of meat in people’s diet, and the increased number of pet cats in the past few decades. A recent review of T. gondii infections in China also showed the same trend (Pan et al., 2017).
Clinical Toxoplasmosis in Naturally Infected Food Animals
Most T. gondii infection in food animals from China was subclinical. Cases reports with evidence of toxoplasmosis were only found in swine (He et al., 2001; Liao et al., 2006; Li et al., 2010; Deng et al., 2012), few reports in other food animals. There have been three reports of clinical toxoplasmosis in pregnant sow and in fattening swine from Jiangxi Province (42% infected and 8% died) (He et al., 2001; Deng et al., 2012), Gansu Province (57% infected, 2% died) (Li et al., 2010) and Guangdong Province (33% infected, 2% died) (Liao et al., 2006). The case reports found clinicopathological changes in T. gondii infected swines were fever, dyspnea, loss appetite, skin cyanosis, abortions or stillbirths. Pulmonary congestion, lymphadenectasis, liver, heart, and spleen were enlarged and necrosis was observed by postmortem examination and histology inspection. Furthermore, T. gondii tachyzoites were found in the smear of tracheal lymph node, lung, liver, and spleen under the light microscope, and T. gondii could be recovered from mice inoculated with tissue of died swines or sows (He et al., 2001; Liao et al., 2006; Li et al., 2010; Deng et al., 2012). How most swine acquire infection is unknown, uncooked kitchen garbage, birds, rodent or cats may be the source of infection. It was speculated that the ingestion of T. gondii oocysts contaminated feed might contribute to the outbreak of toxoplasmosis (He et al., 2001; Li et al., 2010).
The Molecular Epidemiology of T. gondii in Food Animals and Humans
Isolation and the Genotypes Distribution of T. gondii
T. gondii is genetically diverse, exhibiting regional differences around the world (Lehmann et al., 2006; Su et al., 2012). T. gondii strains isolated from humans and animals are classified into three clonal lineages, namely, types I, II, and III (Howe and Sibley, 1995). A total of 231 genotypes have been identified around the world, which comprises 1,457 T. gondii strains2. Among 142 viable T. gondii isolates from animals and humans in China, most isolates (85 strains, 69.7%) were derived from the cats (Fu et al., 2015). The prevalence and T. gondii isolates from cats in China was previously summarized by Yang et al. (Yang et al., 2015). Three T. gondii strains were isolated from sheep and genotyped as ToxoDB#9 (Yang et al., 2017b) and ToxoDB#1 (Chen et al., 1988; Lu et al., 2014). Two T. gondii strains were isolated from chickens and were genotyped as ToxoDB#225 and Type I (Zhao et al., 2012; Wang et al., 2015). Forty-five T. gondii strains were isolated from swines and genotyped as ToxoDB#9, ToxoDB#3, and ToxoDB#1 (Zhou et al., 2009; Jiang et al., 2013, 2015; Li et al., 2015; Wang et al., 2015, 2016). Seven T. gondii strains were isolated from humans and genotyped as ToxoDB#1, ToxoDB#9, ToxoDB#3, ToxoDB#10, ToxoDB#204, and ToxoDB#4 (Fu et al., 2015). The number of T. gondii isolates from China is relatively small in terms of the broad land of the country. It may be related to the China predominant genotype ToxoDB#9, the virulence and cyst-forming capability differ in the strains sharing the same genotype (Li et al., 2014; Gao et al., 2017). Furthermore, commercial breeding of experimental animal cats and γ-IFN knockout mice in China is limited. T. gondii isolations are thus at a bottleneck, and only virulent strains or those with high rates of cyst formation have been successfully isolated. The genotypes of T. gondii from samples of food animals are summarized in Figure 1. Currently, a total of 112 samples (31 isolates and 81 DNAs) were characterized (Supplementary Table S8). Four genotypes have been identified in China, ToxoDB#9 (Chinese1) was the predominant genotype (78.57%), and ToxoDB#9 T. gondii also appears in Colombia (Dubey et al., 2007a), Vietnam (Dubey et al., 2007b), and Sri Lanka (Dubey et al., 2007c). ToxoDB#10 (type I) is the second most common genotype (14.29%), whereas ToxoDB#3 (type II variant) (3.58) are rarely detected. These findings indicate that T. gondii in food animals from China have limited genetic polymorphisms.
Information on the genotypes of T. gondii in food animals from China was summarized up in this article. What factors influence ToxoDB#9 to become the predominant genotype and how did this particular genotype emerge? ToxoDB#3 strains are predominant in Europe yet also occur in Northwest and Northeast China (Chaichan et al., 2017). The atypical genotypes may be evolved from the archetypical lineages of types I, II, and III. Two studies supporting the hypothesis of Chinese 1 preceded Type II T. gondii, both of them sharing a common ancestor (Lorenzi et al., 2016; Bertranpetit et al., 2017). According to the geographical distribution of T. gondii strains in China (Figure 1), it was showed East–West genotype gradient of Chinese 1. The geographical distribution of T. gondii genotypes may reflect that the continuum with West China for East China and the circulation of strains though Silk Road or maritime coastal road. More epidemiological studies are required to confirm this hypothesis and to clarify the route of propagation of T. gondii genotypes in China.
The Seroepidemiology of T. gondii From China
Toxoplasma gondii is responsible for 20.7% of foodborne deaths due to known infectious agents (Ortega, 2006). The distribution and regularity of the epidemiology of T. gondii in food animals from China are summarized in Figure 1. The overall estimated seroprevalence of T. gondii in food animals in China is 23.7% (39,194/165,417, 95%CI, 23.49–23.90%), which include 31 provinces (Supplementary Table S9). The Yangtze, Yellow and Pearl River are three of the longest rivers in China and thus are the most important water source for the Chinese. The Yellow River runs from west to east, and the food animals from these regions showed increased seroprevalence for T. gondii. Compared the prevalence of T. gondii infection in food animals from Yellow River upstream (12.20%, 95%CI, 13.50–14.28%), the seroprevalence rate for T. gondii infection were increased in food animals from Yellow River midstream (21.80%, 95%CI, 27.09–28.67%) and downstream (21.40%, 95%CI, 25.37–29.07) (P < 0.0001). Meanwhile, during 2000–2010 annual accumulated, Yellow River downstream region maximum, minimum and average precipitation (1090, 456.6, and 693.5 mm) was higher than midstream region (995.3, 349.9, and 621.4 mm) and upstream region (800.4, 348, and 564.4 mm). (3National meteorological information center, China).
The Yangtze runs from the west to the east across central China, and food animals from these regions (28.76%, 95%CI, 39.93–40.80%) have higher seroprevalence for T. gondii than non-Yangtze River regions (20.37%, 95%CI, 25.27–25.89%), and much prefer Yellow River regions (15.57%, 95%CI, 18.09–18.80%) (P < 0.0001, OR = 2.189) and Pearl River regions (25.78%, 95%CI, 34.05–35.43%) (P < 0.0001, OR = 1.162). Moreover, food animals from Pearl River regions have higher seroprevalence for T. gondii than Yellow River regions (P < 0.0001, OR = 1.883). During 2000–2010 annual accumulated, Pearl River region maximum, minimum and average precipitation (2090.9, 1034.4, and 1463.8 mm) was higher than Yangtze River region (1514.7, 713.8, and 1082.7 mm) and Yellow River region (897.5, 364.0, and 599.1 mm). (3National meteorological information center, China). Results suggested that T. gondii oocysts may be transmitted by water, and annual precipitation possible help the oocyst spread and retain accessible for potential hosts, which in turn has fueled efforts in designing prevention and control strategies, including water filtration or water boiling, inactivating oocysts from felines, and monitoring birds and rodents.
In this paper, an only apparent seroprevalence of T. gondii infection from food animals and humans was showed and analyzed, the sensitivity and specificity of serologic assays in different hosts and different serological detection methods were not evaluated. In order to get a clearer picture of the true prevalence of T. gondii infection in China, apparent seroprevalence need proceed by Bayesian statistics for all unknown parameters (different samples, different hosts, different serological test methods, and different test kits) in the future (Basanez et al., 2004).
Conclusion and Perspective
Toxoplasma gondii can pose a high risk in immunocompromised individuals for serious health problems and result in destructive consequences. In recently two decades, Chinese scientists have obtained lots of viable T. gondii isolates from chickens, swines, sheep, and cats, indicating T. gondii is widespread in China. This review collected the data on T. gondii infection in food animals (sheep, goats, swine, chickens, cattle) and humans from China, and showing the epidemiological distribution of T. gondii. The predominant genotype of T. gondii in food animals from China is Chinese 1 (ToxoDB#9). The overall seroprevalence of T. gondii infections in food animals is 23.7%, which is three times of that in humans. The seroprevalence of T. gondii infections in animals and humans was significantly increased in the period of 2011–2017 when compared with data from 2000 to 2010 (P < 0.0001). Further, food animals from regions of the Yangtze River have higher seroprevalence rates for T. gondii than ones from non-Yangtze River (P < 0.0001), suggesting T. gondii oocysts may be transmitted by water and annual precipitation possible help the oocyst spread and retain accessible for potential hosts. This suggestion was confirmed by data within the Yellow River regions, where an increasing trend in seroprevalence for T. gondii (P < 0.0001) was found from upstream western to midstream and downstream eastern regions. Therefore, effective prevention and control strategies are proposed to include water filtration or water boiling, inactivating oocysts from feline’s feces, monitoring birds and rodents.
Author Contributions
YY conceived and designed the review. HD and YY drafted the manuscript. All authors contributed to the writing of the manuscript, critically reviewed the draft, and approved the final version of the manuscript.
Funding
This study was financed by the China Postdoctoral Science Foundation (2016M600577), the Program for Science and Technology Innovation Talents in Universities of Henan Province (17HASTIT038), China Agriculture Research System (CARS-36), and National Key Research and Development Program of China (2016YFD0500707). The design of the study and collection, analysis, and interpretation of data and in writing the manuscript were supported by these funding.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Chunlei Su at the University of Tennessee, Knoxville, United States for reviewing this manuscript and provided constructive comments. We thank Kun Yang (Jiangsu Institute of Parasitic Diseases) for kindly help for statistics and analysis. We also thank Chunfeng Wang, Jian Li, Hu Liang, and Zifu Zhu (Henan Agricultural University, Zhengzhou, China) for part of data collection.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.02108/full#supplementary-material
Footnotes
References
Assadi-Rad, A. M., New, J. C., and Patton, S. (1995). Risk factors associated with transmission of Toxoplasma gondii to sows kept in different management systems in Tennessee. Vet. Parasitol. 57, 289–297. doi: 10.1016/0304-4017(94)00677-5
Bartova, E., Sedlak, K., and Budikova, M. (2015). A study of Neospora caninum and Toxoplasma gondii antibody seroprevalence in healthy cattle in the Czech Republic. Ann. Agric. Environ. Med. 22, 32–34. doi: 10.5604/12321966.1141365
Basanez, M. G., Marshall, C., Carabin, H., Gyorkos, T., and Joseph, L. (2004). Bayesian statistics for parasitologists. Trends Parasitol. 20, 85–91. doi: 10.1016/j.pt.2003.11.008
Belluco, S., Simonato, G., Mancin, M., Pietrobelli, M., and Ricci, A. (2017). Toxoplasma gondii infection and food consumption: a systematic review and meta-analysis of case-controlled studies. Crit. Rev. Food Sci. Nutr. 11, 1–12. doi: 10.1080/10408398.2017.1352563
Bertranpetit, E., Jombart, T., Paradis, E., Pena, H., Dubey, J., Su, C., et al. (2017). Phylogeography of Toxoplasma gondii points to a South American origin. Infect. Genet. Evol. 48, 150–155. doi: 10.1016/j.meegid.2016.12.020
Chaichan, P., Mercier, A., Galal, L., Mahittikorn, A., Ariey, F., Morand, S., et al. (2017). Geographical distribution of Toxoplasma gondii genotypes in Asia: a link with neighboring continents. Infect. Genet. Evol. 53, 227–238. doi: 10.1016/j.meegid.2017.06.002
Chen, Y. M., Ma, J. W., Du, Z. B., and Li, G. D. (1988). A avirulent Toxoplasma gondii isolated from sheep. Chin. Vet. Sci. 5, 38–40.
Cong, W., Huang, S. Y., Zhou, D. H., Xu, M. J., Wu, S. M., Yan, C., et al. (2012). First report of Toxoplasma gondii infection in market-sold adult chickens, ducks and pigeons in northwest China. Parasit. Vectors 5:110. doi: 10.1186/1756-3305-5-110
de Moraes, E. P., Batista, A. M., Faria, E. B., Freire, R. L., Freitas, A. C., Silva, M. A., et al. (2010). Experimental infection by Toxoplasma gondii using contaminated semen containing different doses of tachyzoites in sheep. Vet. Parasitol. 170, 318–322. doi: 10.1016/j.vetpar.2010.02.017
Deng, S. Z., Zhang, W. B., Leng, C., Lu, G. Z., Xu, C. M., Zou, L., et al. (2012). Diagnosis of porcine toxoplasmosis and isolation and identification of Toxoplasma gondii. Chin Anim. Husb. Vet. Med. 39, 166–168. doi: 10.1016/j.exppara.2013.09.019
Ding, H., Gao, Y. M., Deng, Y., Lamberton, P. H., and Lu, D. B. (2017). A systematic review and meta-analysis of the seroprevalence of Toxoplasma gondii in cats in mainland China. Parasit. Vectors 10: 27. doi: 10.1186/s13071-017-1970-6
Dong, Y. S., Luo, Z. Q., Zhang, G. C., and Liu, X. Q. (2011). Epidemiological survey of toxoplasmosis in cattle and sheep in Qinghai Province. Chin. J. Zoonoses 27:359. doi: 10.3969/j.issn.1002-2694.2011.04.023
Dubey, J. P. (2002). A review of toxoplasmosis in wild birds. Vet. Parasitol. 106, 121–153. doi: 10.1016/S0304-4017(02)00034-1
Dubey, J. P., Cortes-Vecino, J. A., Vargas-Duarte, J. J., Sundar, N., Velmurugan, G. V., Bandini, L. M., et al. (2007a). Prevalence of Toxoplasma gondii in dogs from Colombia, South America and genetic characterization of T. gondii isolates. Vet. Parasitol. 145, 45–50. doi: 10.1016/j.vetpar.2006.12.001
Dubey, J. P., Huong, L. T., Sundar, N., and Su, C. (2007b). Genetic characterization of Toxoplasma gondii isolates in dogs from Vietnam suggests their South American origin. Vet. Parasitol. 146, 347–351. doi: 10.1016/j.vetpar.2007.03.008
Dubey, J. P., Rajapakse, R. P., Wijesundera, R. R., Sundar, N., Velmurugan, G. V., Kwok, O. C., et al. (2007c). Prevalence of Toxoplasma gondii in dogs from Sri Lanka and genetic characterization of the parasite isolates. Vet. Parasitol. 146, 341–346. doi: 10.1016/j.vetpar.2007.03.009
Dubey, J. P., Ferreira, L. R., Martins, J., and McLeod, R. (2012). Oral oocyst-induced mouse model of toxoplasmosis: effect of infection with Toxoplasma gondii strains of different genotypes, dose, and mouse strains (transgenic, out-bred, in-bred) on pathogenesis and mortality. Parasitology 139, 1–13. doi: 10.1017/S0031182011001673
Dubey, J. P., Hill, D. E., Jones, J. L., Hightower, A. W., Kirkland, E., Roberts, J. M., et al. (2005). Prevalence of viable Toxoplasma gondii in beef, chicken, and pork from retail meat stores in the United States: risk assessment to consumers. J. Parasitol. 91, 1082–1093. doi: 10.1645/GE-683.1
Dubey, J. P., and Jones, J. L. (2008). Toxoplasma gondii infection in humans and animals in the United States. J. Parasitol. 38, 1257–1278. doi: 10.1016/j.ijpara.2008.03.007
Dubey, J. P., Verma, S. K., Ferreira, L. R., Oliveira, S., Cassinelli, A. B., Ying, Y., et al. (2014). Detection and survival of Toxoplasma gondii in milk and cheese from experimentally infected goats. J. Food Prot. 77, 1747–1753. doi: 10.4315/0362-028X.JFP-14-167
Feng, Y., Lu, Y., Wang, Y., Liu, J., Zhang, L. X., and Yang, Y. R. (2016). Toxoplasma gondii and Neospora caninum in free-range chickens in Henan Province of China. Biomed. Res. Int. 2016:8290536. doi: 10.1155/2016/8290536
Fu, X. Y., Feng, Y. J., Liang, H. D., and Yang, Y. R. (2015). Genotypes and pathogenesis of Toxoplasma gondii isolates in China. Chin. J. Zoonoses 31, 669–672. doi: 10.3969/cjz.j.issn.1002-2694.2015.07.016
Gao, J., and Tang, Z. (2018). Changes in Chinese residents food consumption based on food equivalent. Food Nutr. China 24, 63–67.
Gao, J. M., Xie, Y. T., Xu, Z. S., Chen, H., Hide, G., Yang, T. B., et al. (2017). Genetic analyses of Chinese isolates of Toxoplasma gondii reveal a new genotype with high virulence to murine hosts. Vet. Parasitol. 241, 52–60. doi: 10.1016/j.vetpar.2017.05.007
Gao, X. J., Zhao, Z. J., He, Z. H., Wang, T., Yang, T. B., Chen, X. G., et al. (2012). Toxoplasma gondii infection in pregnant women in China. Parasitology 139, 139–147. doi: 10.1017/S0031182011001880
Gao, Y.-M., Ding, H., Lamberton, P. H. L., and Lu, D. B. (2016). Prevalence of Toxoplasma gondii in pet dogs in mainland China: a meta-analysis. Vet. Parasitol. 229, 126–130. doi: 10.1016/j.vetpar.2016.10.009
Garcia-Vazquez, Z., Rosario-Crus, R., Diaz-Garcia, G., and Hernandez-Baumgarten, O. (1993). Seroprevalence of Toxoplasma gondii infection in cattle, swine and goats in four Mexican states. Prev. Vet. Med. 17, 127–132. doi: 10.1016/0167-5877(93)90061-W
Guo, M., Dubey, J. P., Hill, D., Buchanan, R. L., Gamble, H. R., Jones, J. L., et al. (2015). Prevalence and risk factors for Toxoplasma gondii infection in meat animals and meat products destined for human consumption. J. Food Prot. 78, 457–476. doi: 10.4315/0362-028X.JFP-14-328
He, H. J., Deng, S. Z., Luo, J. R., Wu, X. D., and Hua, X. B. (2001). Diagnosis of toxoplasmosis and T. gondii isolation in swine. Chin. J. Pre. Vet. Med. 23, 63–65. doi: 10.3969/j.issn.1008-0589.2001.01.020
Hide, G. (2016). Role of vertical transmission of Toxoplasma gondii in prevalence of infection. Expert Rev. Anti Infect. Ther. 14, 335–344. doi: 10.1586/14787210.2016.1146131
Howe, D. K., and Sibley, L. D. (1995). Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J. Infect. Dis. 172, 1561–1566. doi: 10.1093/infdis/172.6.1561
Jiang, H. H., Huang, S. Y., Zhou, D. H., Zhang, X. X., Su, C., Deng, S. Z., et al. (2013). Genetic characterization of Toxoplasma gondii from pigs from different localities in China by PCR-RFLP. Parasit. Vectors 6:227. doi: 10.1186/1756-3305-6-227
Jiang, H. H., Wang, S. C., Huang, S. Y., Zhao, L., Wang, Z. D., Zhu, X. Q., et al. (2015). Genetic Characterization of Toxoplasma gondii isolates from pigs in Jilin Province, Northeastern China. Foodborne Pathog Dis 13: 88. doi: 10.1089/fpd.2015.2043
Kapperud, G., Jenum, P. A., Stray-Pedersen, B., Melby, K. K., Eskild, A., and Eng, J. (1996). Risk factors for Toxoplasma gondii infection in pregnancy. Results of a prospective case-control study in Norway. Am. J. Epidemiol. 144, 405–412. doi: 10.1093/oxfordjournals.aje.a008942
Lehmann, T., Marcet, P. L., Graham, D. H., Dahl, E. R., and Dubey, J. P. (2006). Globalization and the population structure of Toxoplasma gondii. Proc. Natl. Acad. Sci. U.S.A. 103, 11423–11428. doi: 10.1073/pnas.0601438103
Li, K., Gao, J., Shahzad, M., Han, Z., Nabi, F., Liu, M., et al. (2014). Seroprevalence of Toxoplasma gondii infection in yaks (Bos grunniens) on the Qinghai-Tibetan Plateau of China. Vet. Parasitol. 205, 354–356. doi: 10.1016/j.vetpar.2014.07.014
Li, M., Mo, X. W., Wang, L., Chen, H., Luo, Q. L., Wen, H. Q., et al. (2014). Phylogeny and virulence divergency analyses of Toxoplasma gondii isolates from China. Parasit. Vectors 7:133. doi: 10.1186/1756-3305-7-133
Li, X., Wang, Y., Yu, F., Li, T., and Zhang, D. (2010). An outbreak of lethal toxoplasmosis in pigs in the Gansu province of China. J. Vet. Diagn. Invest. 22, 442–444. doi: 10.1177/104063871002200318
Li, X. M., Ouyang, Y., Yang, Y. C., Lin, R., Xu, H. B., Xie, Z. Y., et al. (2009). Distribution of food-borne parasitic diseases and dietary habits in human population in Guangxi. Chin. J. Parasitol. Parasit. Dis. 27, 151–155.
Li, Y. N., Nie, X. W., Peng, Q. Y., Mu, X. Q., Zhang, M., Tian, M. Y., et al. (2015). Seroprevalence and genotype of Toxoplasma gondii, in pigs, dogs and cats from Guizhou province. Southwest China. Parasit. Vectors 8:214 doi: 10.1186/s13071-015-0809-2
Liao, S. Q., Weng, Y. B., Song, H. Q., Yang, A. B., Cui, J. X., Zhang, H., et al. (2006). Diagnosis of swine toxoplasmosis by specific PCR assay and the isolation of Toxoplasma gondii strains. J. Trop. Med. 6, 969–971.
Liu, J., Cai, J. Z., Zhang, W., Liu, Q., Chen, D., Han, J. P., et al. (2008). Seroepidemiology of Neospora caninum and Toxoplasma gondii infection in yaks (Bos grunniens) in Qinghai. China Vet. Parasitol. 152, 330–332. doi: 10.1016/j.vetpar.2007.12.010
Liu, Q., Cai, J., Zhao, Q., Shang, L., Ma, R., Wang, X., et al. (2011). Seroprevalence of Toxoplasma gondii infection in yaks (Bos grunniens) in northwestern China. Trop. Anim. Health Prod. 43, 741–743. doi: 10.1007/s11250-010-9711-2
Liu, Q., Mao, K. B., Ma, Y., Tan, X. L., Han, J. Q., Li, L. P., et al. (2017). Pig’s circulation pattern based on agricultural big data visualization method in China. Sci. Geogr. Sin. 37, 118–124. doi: 10.13249/j.cnki.sgs.2017.01.014
Lopes, W. D., Rodriguez, J. D., Souza, F. A., dos Santos, T. R., dos Santos, R. S., Rosanese, W. M., et al. (2013). Sexual transmission of Toxoplasma gondii in sheep. Vet. Parasitol. 195, 47–56. doi: 10.1016/j.vetpar.2012.12.056
Lorenzi, H., Khan, A., Behnke, M. S., Namasivayam, S., Swapna, L. S., Hadjithomas, M., et al. (2016). Local admixture of amplified and diversified secreted pathogenesis determinants shapes mosaic Toxoplasma gondii genomes. Nat. Commun. 7:10147. doi: 10.1038/ncomms10147
Lu, J., Zhou, D. H., Chen, J., Zhang, N. Z., Wang, R. A., Weng, Y. B., et al. (2014). Characterization of the Toxoplasma gondii hsp60 gene sequences from different hosts and geographical locations. Genet. Mol. Res. 13, 6906–6911. doi: 10.4238/2014.August.29.13
Montoya, J. G., and Liesenfeld, O. (2004). Toxoplasmosis. Lancet 363, 1965–1976. doi: 10.1016/S0140-6736(04)16412-X
Omata, Y., Dilorenzo, C., Venturini, C., Venturini, L., Igarashi, I., Saito, A., et al. (1994). Correlation between antibody levels in Toxoplasma gondii infected pigs and pathogenicity of the isolated parasite. Vet. Parasitol. 51, 205–210. doi: 10.1016/0304-4017(94)90157-0
Ortega, Y. R. (2006). “Toxoplasmosis,” in Foodborne Parasites. Food Microbiology and Food Safety Series, ed. Y. R. Ortega (Boston, MA: Springer), doi: 10.1007/0-387-31197-1_5
Pan, M., Lyu, C., Zhao, J., and Shen, B. (2017). Sixty years (1957-2017) of research on toxoplasmosis in China-an overview. Front. Microbiol. 8:1825. doi: 10.3389/fmicb.2017.01825
Robert-Gangneux, F., and Darde, M. L. (2012). Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 25, 264–296. doi: 10.1128/CMR.05013-11
Smith, H. J. (1991). Seroprevalence of anti-Toxoplasma IgG in Canadian swine. Can. J. Vet. Res. 55:380.
Su, C., Khan, A., Zhou, P., Majumdar, D., Ajzenberg, D., Dardé, M. L., et al. (2012). Globally diverse Toxoplasma gondii isolates comprise six major clades originating from a small number of distinct ancestral lineages. Proc. Natl. Acad. Sci. U.S.A. 109, 5844–5849. doi: 10.1073/pnas.1203190109
Suarez-Aranda, F., Galisteo, A. J., Hiramoto, R. M., Cardoso, R. P., Meireles, L. R., Miguel, O., et al. (2000). The prevalence and avidity of Toxoplasma gondii IgG antibodies in pigs from Brazil and Peru. Vet. Parasitol. 91, 23–32. doi: 10.1016/S0304-4017(00)00249-1
Sun, W. W., Meng, Q. F., Cong, W., Shan, X. F., Wang, C. F., and Qian, A. D. (2015). Herd-level prevalence and associated risk factors for Toxoplasma gondii, Neospora caninum, Chlamydia abortus and bovine viral diarrhoea virus in commercial dairy and beef cattle in eastern, northern and northeastern China. Parasitol. Res. 114, 4211–4218. doi: 10.1007/s00436-015-4655-0
Tao, Q., Wang, Z. S., Feng, H. H., Fang, R., Hao, N., Hu, M., et al. (2011). Seroprevalence and risk factors for Toxoplasma gondii infection on pig farms in central China. J. Parasitol. 97, 262–264. doi: 10.1645/GE-2646.1
Tonouhewa, A. B., Akpo, Y., Sessou, P., Adoligbe, C., Yessinou, E., Hounmanou, Y. G., et al. (2017). Toxoplasma gondii infection in meat animals from Africa: systematic review and meta-analysis of sero-epidemiological studies. Vet. World 10, 194–208. doi: 10.14202/vetworld.2017.194-208
Wang, D., Liu, Y., Jiang, T., Zhang, G., Yuan, G., He, J., et al. (2016). Seroprevalence and genotypes of Toxoplasma gondii isolated from pigs intended for human consumption in Liaoning Province, northeastern China. Parasit. Vectors 9:248. doi: 10.1186/s13071-016-1525-2
Wang, H., Wang, T., Luo, Q., Huo, X., Wang, L., Liu, T., et al. (2012). Prevalence and genotypes of Toxoplasma gondii in pork from retail meat stores in Eastern China. Int. J. Food Microbiol. 157, 393–397. doi: 10.1016/j.ijfoodmicro.2012.06.011
Wang, L., Cheng, H. W., Huang, K. Q., Xu, Y. H., Li, Y. N., Du, J., et al. (2015). Toxoplasma gondii prevalence in food animals and rodents in different regions of China: isolation, genotyping and mouse pathogenicity. Parasit. Vectors 6:273. doi: 10.1186/1756-3305-6-273
Wang, W. S., Zhang, J. S., Chen, W., Wang, J. W., Meng, Q. L., and Qiao, J. (2011). Serological survey of Toxoplasma gondii infection among human and animals in Shihezi region. Prog. Vet. Med. 32, 120–122. doi: 10.3969/j.issn.1007-5038.2011.02.030
Wang, Z. D., Liu, H. H., Ma, Z. X., Ma, H. Y., Li, Z. Y., Yang, Z. B., et al. (2017). Toxoplasma gondii infection in immunocompromised patients: a systematic review and Meta-Analysis. Front. Microbiol. 8:389. doi: 10.3389/fmicb.2017.00389
Weiss, L. M., and Dubey, J. P. (2009). Toxoplasmosis: a history of clinical observations. Int. J. Parasitol. 39, 895–901. doi: 10.1016/j.ijpara.2009.02.004
Xu, P. P., Liu, F., Xu, J. J., Li, H., Niu, H. Q., He, J., et al. (2015). Investigation on Toxoplasma gondii infection of commercial beef and mutton in Xinxiang, Henan Province. Int. J. Med. Parasit. Dis. 42, 101–103.
Yang, Y. R., Feng, Y. J., Lu, Y. Y., Dong, H., Li, T. Y., Jiang, Y. B., et al. (2017a). Antibody detection, isolation, genotyping, and virulence of Toxoplasma gondii in captive felids from China. Front. Microbiol. 8:1414. doi: 10.3389/fmicb.2017.01414
Yang, Y. R., Feng, Y. J., Yao, Q., Wang, Y., Lu, Y. Y., Liang, H. D., et al. (2017b). Seroprevalence, isolation, genotyping, and pathogenicity of Toxoplasma gondii strains from sheep in China. Front. Microbiol. 8:136. doi: 10.3389/fmicb.2017.00136
Yang, Y. R., Ying, Y. Q., Verma, S. K., Cassinelli, A. B., Kwok, O. C., Liang, H. D., et al. (2015). Isolation and genetic characterization of viable Toxoplasma gondii from tissues and feces of cats from the central region of China. Vet. Parasitol. 211, 283–288. doi: 10.1016/j.vetpar.2015.05.006
Yu, S., Xu, L., Jiang, Z., Xu, S., Han, J., Zhu, Y., et al. (1994). Report on the first nationwide survey of the distribution of human parasites in China. 1. Regional distribution of parasite species. Chin. J. Parasitol. Paras. Dis. 12, 241–247.
Zhao, G. W., Shen, B., Xie, Q., Xu, L. X., Yan, R. F., Song, X. K., et al. (2012). Isolation and molecular characterization of Toxoplasma gondii from chickens in China. J. Integr. Agr. 11, 1347–1353. doi: 10.1645/GE-2221.1
Zhou, D. H., Zhao, F. R., Lu, P., Xia, H. Y., Xu, M. J., Yuan, L. G., et al. (2012). Seroprevalence of Toxoplasma gondii infection in dairy cattle in southern China. Parasit. Vectors 5:48. doi: 10.1186/1756-3305-5-48
Zhou, P., Chen, N., Zhang, R. L., Lin, R. Q., and Zhu, X. Q. (2008). Food-borne parasitic zoonoses in China: perspective for control. Trends Parasitol. 24, 190–196. doi: 10.1016/j.pt.2008.01.001
Zhou, P., Chen, Z. G., Li, H. L., Zheng, H. H., He, S. Y., Lin, R. Q., et al. (2011). Toxoplasma gondii infection in humans in China. Parasit. Vectors 4, 1–9. doi: 10.1186/1756-3305-4-165
Keywords: Toxoplasma gondii, sheep, goats, swines, chickens, cattle, humans, China
Citation: Dong H, Su R, Lu Y, Wang M, Liu J, Jian F and Yang Y (2018) Prevalence, Risk Factors, and Genotypes of Toxoplasma gondii in Food Animals and Humans (2000–2017) From China. Front. Microbiol. 9:2108. doi: 10.3389/fmicb.2018.02108
Received: 27 May 2018; Accepted: 20 August 2018;
Published: 11 September 2018.
Edited by:
Zhihong Sun, Inner Mongolia Agricultural University, ChinaReviewed by:
Tecia Maria Ulisses Carvalho, Universidade Federal do Rio de Janeiro, BrazilDe-hua Lai, Sun Yat-sen University, China
Copyright © 2018 Dong, Su, Lu, Wang, Liu, Jian and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yurong Yang, eWFuZ3l1NzcxMkBzaW5hLmNvbQ==
†Present address: Yurong Yang, Laboratory of Veterinary Pathology, College of Animal Science and Veterinary Medicine, Henan Agricultural University, Zhengzhou, China
 Hui Dong
Hui Dong Ruijing Su1
Ruijing Su1 Yaoyao Lu
Yaoyao Lu Jing Liu
Jing Liu Yurong Yang
Yurong Yang