- 1Marine Ecology Department, Faculty of Biology and Chemistry, University of Bremen, Bremen, Germany
- 2Coral Reef Ecology Group, Leibniz Centre for Tropical Marine Research, Bremen, Germany
- 3Department of Integrative Marine Ecology, Stazione Zoologica Anton Dohrn, Naples, Italy
- 4Centre Scientifique de Monaco, Monaco, Monaco
- 5School of Biological Sciences, The University of Queensland, Brisbane, QLD, Australia
- 6Marine Science Station, The University of Jordan, Amman, Jordan
The northern Red Sea experiences strong annual differences in environmental conditions due to its relative high-latitude location for coral reefs. This allows the study of regulatory effects by key environmental parameters (i.e., temperature, inorganic nutrient, and organic matter concentrations) on reef primary production and dinitrogen (N2) fixation, but related knowledge is scarce. Therefore, this study measured environmental parameters, primary production and N2 fixation of phytoplankton groups in the water overlying a coral reef in the Gulf of Aqaba. To this end, we used a comparative approach between mixed and stratified water column scenarios in a full year of seasonal observations. Findings revealed that inorganic nutrient concentrations were significantly higher in the mixed compared to the stratified period. While gross photosynthesis and N2 fixation rates remained similar, net photosynthesis decreased from mixed to stratified period. Net heterotrophic activity of the planktonic community increased significantly during the stratified compared to the mixed period. While inorganic nitrogen (N) availability was correlated with net photosynthesis over the year, N2 fixation only correlated with N availability during the mixed period. This emphasizes the complexity of planktonic trophodynamics in northern Red Sea coral reefs. Comparing mixed and stratified planktonic N2 fixation rates with those of benthic organisms and substrates revealed a close seasonal activity similarity between free-living pelagic and benthic diazotrophs. During the mixed period, N2 fixation potentially contributed up to 3% of planktonic primary production N demand. This contribution increased by ca. one order of magnitude to 21% during the stratified period. Planktonic N2 fixation is likely a significant N source for phytoplankton to maintain high photosynthesis under oligotrophic conditions in coral reefs, especially during stratified conditions.
Introduction
Coral reefs thrive under oligotrophic conditions, particularly due to autochthonous generation of organic carbon (C) and nitrogen (N) through photosynthetic primary production, prokaryotic dinitrogen (N2) fixation and efficient internal recycling of those materials within the ecosystem (Hatcher, 1997). Internal recycling can occur through strong benthic-pelagic coupling of dissolved and particulate organic matter (POM) and nutrients (Yahel et al., 1998; Wild et al., 2004; Chipman et al., 2012). Benthic-pelagic coupling is mediated by benthic organisms such as corals, algae, and sponges (Haas et al., 2010; Naumann et al., 2010; de Goeij et al., 2013), but also by reef sediment and framework microbiota (Rasheed et al., 2002; Wild et al., 2009). Furthermore, reefs receive allochthonous energy and nutrients in the form of inorganic nutrients, plankton, and particulate/dissolved matters from offshore and/or riverine inflow. Since reefs are often N limited, diazotrophs (i.e., prokaryotes capable of N2 fixation) can facilitate primary production, particularly under oligotrophic conditions such as those found in the Gulf of Aqaba (Capone et al., 1997; Karl et al., 2002; Furnas et al., 2011).
The Gulf of Aqaba forms one of the northern tips of the Red Sea. Its desert coasts are bordered by fringing coral reefs that experience relatively strong variation in light availability and water temperature for warm-water coral reefs due to its relative high latitude location. The annual fluctuation in sea surface temperature (21–29°C) combined with relatively warm deeper water layers (year round ∼21°C for water depth >200 m) result in an annual cycle of deep water mixing from December until May and stratification down to 200 m water depth from June until ∼November (Carlson et al., 2014) with slow destratification from October and onwards (Biton and Gildor, 2011a,b,c). Inorganic nutrients are brought up to surface water layers during deep water mixing but are trapped in deeper waters during stratification, creating extreme oligotrophic conditions in coral reef surrounding surface waters (Rasheed et al., 2002, 2012; Silverman et al., 2007). These local physico-chemical conditions offer the rare opportunity to study the effects of variation in key environmental factors on important processes such as primary production and diazotrophy within coral reefs.
Planktonic primary production in the Gulf of Aqaba and northern Red Sea is dominated by photoautotrophic nano- and picoplankton. The plankton fraction <20 μm performs on average 81% of planktonic photosynthesis in the upper water layer (discrete depth) (Qurban et al., 2014) ranging from 0.02 to 3.38 μmol C L-1 d-1 (Levanon-Spanier et al., 1979; Qurban et al., 2014), assuming a 12 h period of daylight. The phytoplankton community in the Gulf of Aqaba is characterized by a strong shift in its composition between annual periods, i.e., mixed and stratified. During the mixed period, eukaryotic algae account for up to 95% of phytoplankton biomass, while during stratification >60% of the biomass consists of prokaryotes, in particular Prochlorococcus sp. which may comprise up to 50% of the biomass (Al-Najjar et al., 2007). The main groups of planktonic diazotrophs in the Gulf of Aqaba are Cyanobacteria and Proteobacteria, and they are responsible for water column N2 fixation rates ranging from 0.01 to 1.9 nmol N L-1 d-1 (Foster et al., 2009; Rahav et al., 2015). Planktonic photosynthesis rates in reef-surrounding waters can be 1–2 orders of magnitude higher than in oceanic waters offshore (D’Elia and Wiebe, 1990; Adey, 1998). Moreover, primary production and N2 fixation can interact synergistically within or between planktonic organisms; photosynthesis (and the subsequent organic C released to the surrounding water) may fuel the energy-demanding process of N2 fixation, which in turn can support primary production by supplying the bioavailable N required to synthesize proteins for photosynthesis (e.g., Foster et al., 2011).
Seasonality in the Gulf of Aqaba affects benthic primary production and N2 fixation in a range of common organisms and substrates including corals (Bednarz et al., 2015a; Cardini et al., 2015), algae (Rix et al., 2015; Tilstra et al., 2017), and sediments (Bednarz et al., 2015b). Some of these studies found positive relationships between the two processes, as well as with light intensity, temperature and a negative relationship with N availability (Bednarz et al., 2015a; Rix et al., 2015; Tilstra et al., 2017). While studies exist for open water planktonic primary production and/or N2 fixation in the Gulf of Aqaba (e.g., Foster et al., 2009; Rahav et al., 2015; Shiozaki et al., 2018), to our knowledge, there are no studies relating these two processes of planktonic communities directly overlying a coral reef, where strong benthic pelagic coupling is evident. Thus, to increase our understanding of the factors regulating planktonic primary production and N2 fixation, the objectives of this study were (i) to measure environmental parameters in a Gulf of Aqaba fringing coral reef over the two distinct periods (i.e., mixed and stratified), (ii) to quantify primary production and N2 fixation of the planktonic community, in water directly overlying a coral reef, over the two periods, (iii) to investigate functional relationships between primary production, N2 fixation and environmental parameters, and (iv) to compare the pattern of primary production and N2 fixation with benthic organisms and substrates investigated in parallel.
Materials and Methods
Study Site and Environmental Monitoring
The fieldwork for this study was conducted at the Marine Science Station (MSS) of The University of Jordan, located 10 km south of Aqaba, Jordan. The MSS is situated adjacent to a marine protected area encompassing a crescent shaped fringing coral reef with a length of ca. 1 km. All water column sampling was performed at 10 m water depth in the fore reef section (29° 27′ 31″ N, 34° 58′ 26″ E). Two extensive fieldwork campaigns were performed in 2013: one during the mixed period (January–April; 12 weeks), and one during the stratified period (September–November; 13 weeks). The studied reef consists of a shallow reef flat (<1 m water depth) surrounded by a carbonate sediment belt at ca. 5 m water depth and a coral dominated middle-fore reef facing the open sea [see Cardini et al. (2016) for a visual description of the site].
Light intensity, water temperature, chlorophyll a (Chl a), dissolved organic carbon (DOC), particulate organic carbon (POC), particulate nitrogen (PN), and inorganic nutrients, i.e., NH4+, PO43-, NO2- and NO3-, were monitored during each period (at 10 m water depth). Light intensity measurements recorded by data loggers in lux units were converted to photosynthetically active radiation (PAR) by a conversion factor calculated from a simultaneous minute-by-minute measurement of lux and PAR (08:00–14:00 on 1 day, n = 353) using a HOBO pendant logger and a LI-COR LI192SA underwater quantum sensor: lux = PAR x 52.0, R2 = 0.83. This value is comparable to the conversion factor given by Valiela (1984): 51.2. PAR values measured per minute were summed for each weekly water-sampling day: values in μmol quanta m-2 s-1 were recalculated to mol quanta m-2 d-1. Water temperature, measured per minute on the water-sampling day, was averaged over the 24 h period. Water samples were collected from 10 m water depth on a weekly basis (during both campaigns) in clean high density poly-ethylene (HDPE) containers (volume: 5 L) using SCUBA. All samples were collected between 08:00 and 10:00 within a 10 min timeframe. Sampling was performed 1 m above the seafloor without disturbing the benthos. Chl a and NH4+ were measured fluorometrically while remaining parameters (i.e., POC, PN, PO43-, NO2-, and NO3-) were measured photometrically as detailed by Bednarz et al. (2015b). In addition, DOC was measured according to Cardini et al. (2015) using a Shimadzu TOC-VCPH total organic carbon analyser. For temperature, PAR, nutrient (DIN and phosphate) and chl a data along the entire depth gradient, see Bednarz et al. (2018).
Quantification of Primary Production
All incubations for primary production and N2 fixation rates were performed in a flow through mesocosm (∼4000 l h-1 exchange rate) with water from the reef at 10 m sampling depth to ensure in situ conditions (temperature). Light intensity was adjusted to 10 m depth values by using black netting.
Two water subsamples were taken per HDPE container, one for net photosynthesis (Pn) and one for dark respiration (R) measurement. Weekly incubations were performed in individual 1 L closed cell respirometric glass chambers (n = 6) under constant stirring (600 rpm). Chambers for R measurements were placed in bags made of dense opaque plastic (volume: 10 L) for incubation in the dark. Pn incubations were performed from 10:00 until sunset (ca. 17:00–18:00; depending on the period), while R incubations ran for 24 h to get measurable rates and to account for diurnal fluctuations. Pn and R-values were obtained by using a conductivity- and temperature-corrected O2 optode sensor (MultiLine® IDS 3430, WTW, accuracy: ± 0.5% of measured value) and calculated by subtracting O2 start from end concentrations. Subsequently, values obtained for Pn and R were corrected for incubation duration and chamber volume, and recalculated to molar equivalents resulting in hourly O2 measurements in μmol O2 L-1 h-1. R is presented here as a positive value. Estimates of gross photosynthesis (Pg) were calculated as Pg = Pn + R. As R is averaged over 24 h, it represents a value assuming a constant hourly rate. Since R is susceptible to daily fluctuations, with possible lower R during darkness, Pg-values are possibly underestimated. However, R-values measured here also includes the heterotrophic component of the community adding to Pg.
To assess the contribution of planktonic C to the water column and C budget of the ecosystem, Pn and R rates were recalculated to metabolic C production per day by the following equations, assuming photosynthetic and respiratory quotients of 1.4 and 1.1, respectively (McKinnon et al., 2013). Gross primary production (GPP) = (Pn + R) × h of daylight (using the average hourly rate of R), calculates the daily fixation of C by autotrophs; community respiration (CR) = R × 24 h, calculates daily respiration of the entire community, i.e., auto- and heterotrophs; net community production (NCP) = GPP–CR, calculates whether the system is net autotrophic or heterotrophic over the 24 h. Values presented as μmol C L-1 d-1. Finally, daily contribution of planktonic C to total organic C (TOC = DOC + POC) was calculated by dividing GPP, NCP, or CR with TOC, and multiplying by 100 to obtain percentages.
Quantification of N2 Fixation
Planktonic N2 fixation rates were measured during the mixed and stratified periods using a modified acetylene (C2H2) reduction technique (Capone, 1993; Wilson et al., 2012). Weekly incubations were performed in 1 L chambers (n = 8) containing 800 mL seawater and a 200 mL headspace both being 10% C2H2-enriched under constant stirring (600 rpm). Control incubations were performed without C2H2 addition to measure biological ethylene (C2H4) production, as well as with sterile filtered seawater (0.2 μm) to measure inorganic C2H4 production from C2H2. The incubations lasted for 24 h, and 1 mL gas samples were extracted from a port in the lid at 0, 4, and 24 h with a gastight syringe and analyzed for C2H4 concentration using a customized reducing compound photometer (Peak Laboratories, Mountain View, CA, United States, detection limit = 100 ppb). C2H4 measurements were recalculated to nmol C2H4 in the whole chamber water volume. It was determined in pilot experiments that the C2H2 concentration equilibrated between headspace and incubation water in the first 4 h, the C2H4 production over 4 to 24 h were therefore used for calculations. Changes in C2H4 concentration over time were corrected for incubation duration and volume of water in the chamber, resulting in measurements of nmol C2H4 L-1 h-1. Due to the ongoing debate concerning conversion factors, C2H4 evolution rates measured here are used as a proxy for estimated N2 fixation rates and are thus not converted to actual nitrogen fixed (Wilson et al., 2012). Only for estimating the contribution of N2 fixation to the N requirement of primary production, C2H4 rates were converted to N m-3 d-1 using the conservative theoretical C2H4:N2 conversion ratio of 4:1 (Mulholland et al., 2004).
Statistical Analyses
The values of replicate measurements for environmental parameters, primary production and N2 fixation for each week were averaged prior to statistical analyses. All statistics were performed in Sigmaplot 12.0 for Windows (Systat software). Environmental parameters, primary production and N2 fixation during the two periods were tested for normality with the Shapiro–Wilk test. Comparisons between the two periods were performed with independent samples T-tests if data were normally distributed and with Mann–Whitney U-tests if data lacked normality.
In addition, relationships between N2 fixation rates, Pn, Pg, and R rates per period and across both periods with environmental water parameters were determined via linear regression. Differences were deemed significant at p < 0.05. All values are given as mean ± SE.
Results
Environmental Variables
Mean weekly measurements of environmental parameters were variable over time (Figure 1). Daily PAR increased from January to April (i.e., mixed period) from 3.45 ± 0.26 to 5.76 ± 0.11 mol quanta m-2 d-1, and decreased again from September to November (i.e., stratified period) from 6.72 ± 0.37 to 4.25 ± 0.38 mol quanta m-2 d-1 (Figure 1A). However, between periods no significant differences were found for PAR (Table 1). Temperature was stable throughout the mixed period (22.1–22.9°C), but increased to a maximum of 27.5°C in early September followed by a decrease to 24.7°C at the end of November. Inorganic nutrient and Chl a concentrations were all significantly lower during the stratified than during the mixed period (Figure 1B and Table 1, all p < 0.002), while DOC was significantly higher during the stratified period than during the mixed period (Figure 1C and Table 1). POC concentrations showed no significant differences between periods, while PN was significantly lower in the stratified period (Figure 1C), causing a significantly higher POC:PN ratio (Table 1). The DIN:PO43- ratio was not significantly different between periods (Table 1).
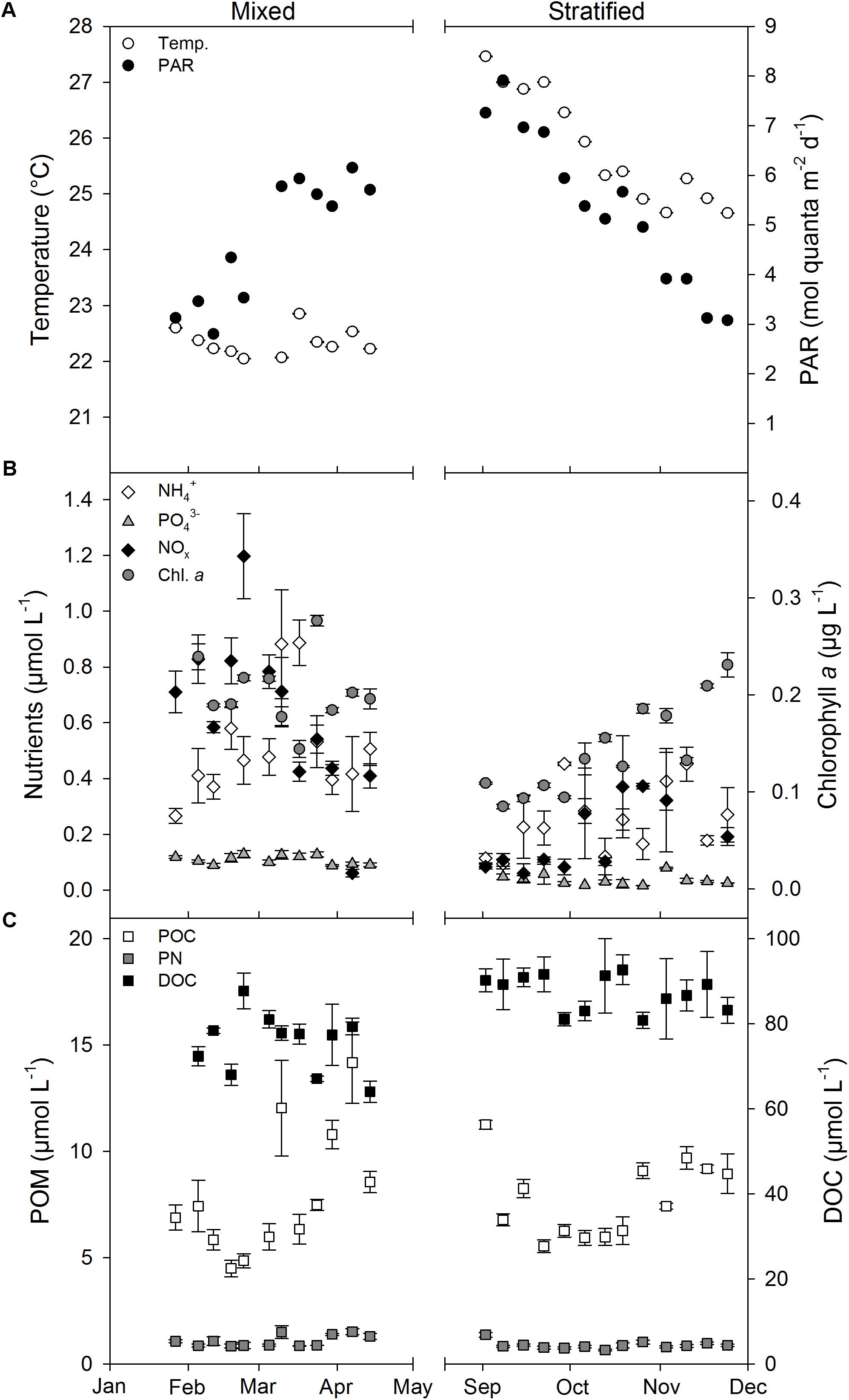
FIGURE 1. Mean weekly environmental water parameters measured at 10 m water depth (1 m over coral reef benthos) during the mixed and stratified periods. (A) Temperature and PAR. (B) Nutrients and chlorophyll a. (C) POM and DOC. Values given as mean ± SE. Temp, water temperature; PAR, photosynthetically active radiation measured at 10 m water depth; NOx, NO2- + NO3-; POC, particulate organic carbon; PN, particulate nitrogen; POM, particulate organic matter (POC and PN); DOC, dissolved organic carbon.
Primary Production
Mean water column Pn was always negative (-0.54–-0.09 μmol O2 L-1 h-1), except on 18 February and 3 March (0.00 and 0.02 μmol O2 L-1 h-1, respectively), and significantly lower during the stratified period than the mixed period (Figure 2 and Table 2). R ranged from 0.15 to 0.46 μmol O2 L-1 h-1 and was not significantly different between periods. Estimates of Pg were higher for the mixed period compared to the stratified period with averages of 0.09 and 0.02 μmol O2 L-1 h-1, respectively. However, these estimates were not significantly different (Table 2). GPP and CR was relatively stable and not significantly different between periods (Table 2 and Figure 3). Mean water column TOC was 83.51 and 95.10 μmol L-1 d-1 for the mixed and stratified periods, respectively (Table 1). The contribution of net daily planktonic C (NCP) to TOC was comparable during the mixed and stratified periods, 7.25 ± 0.95, and 7.73 ± 0.49% d-1, respectively (Figure 3).
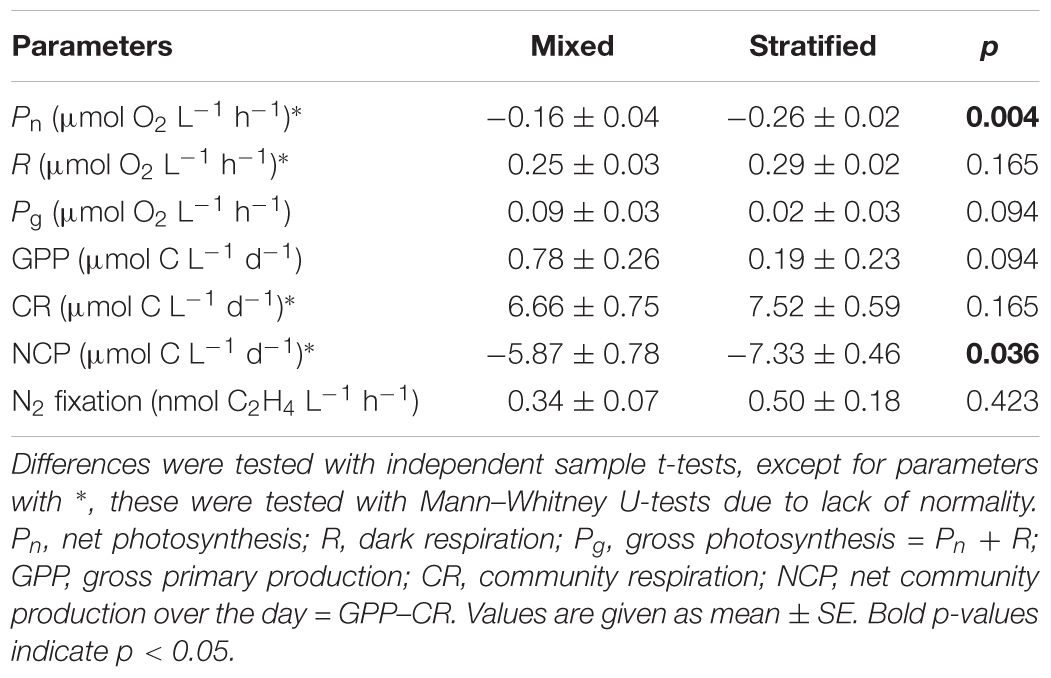
TABLE 2. Primary production (Pn, R and Pg, GPP, CR and NCP) and N2 fixation in the mixed and stratified periods.
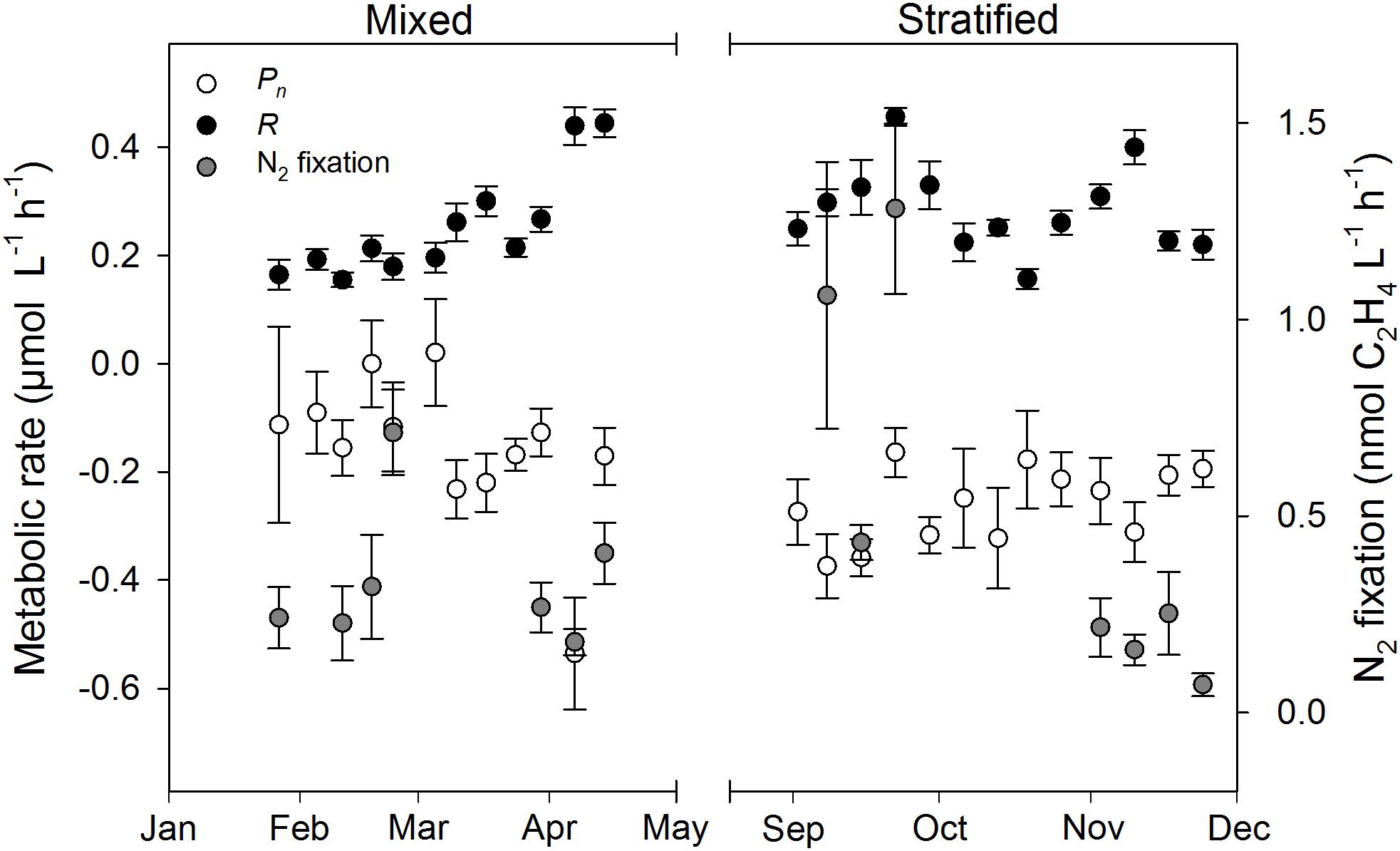
FIGURE 2. Planktonic primary production and N2 fixation rates measured weekly at 10 m water depth (1 m above the benthos) during the mixed and stratified periods. Net photosynthesis (Pn), dark respiration (R). Values given as mean ± SE.
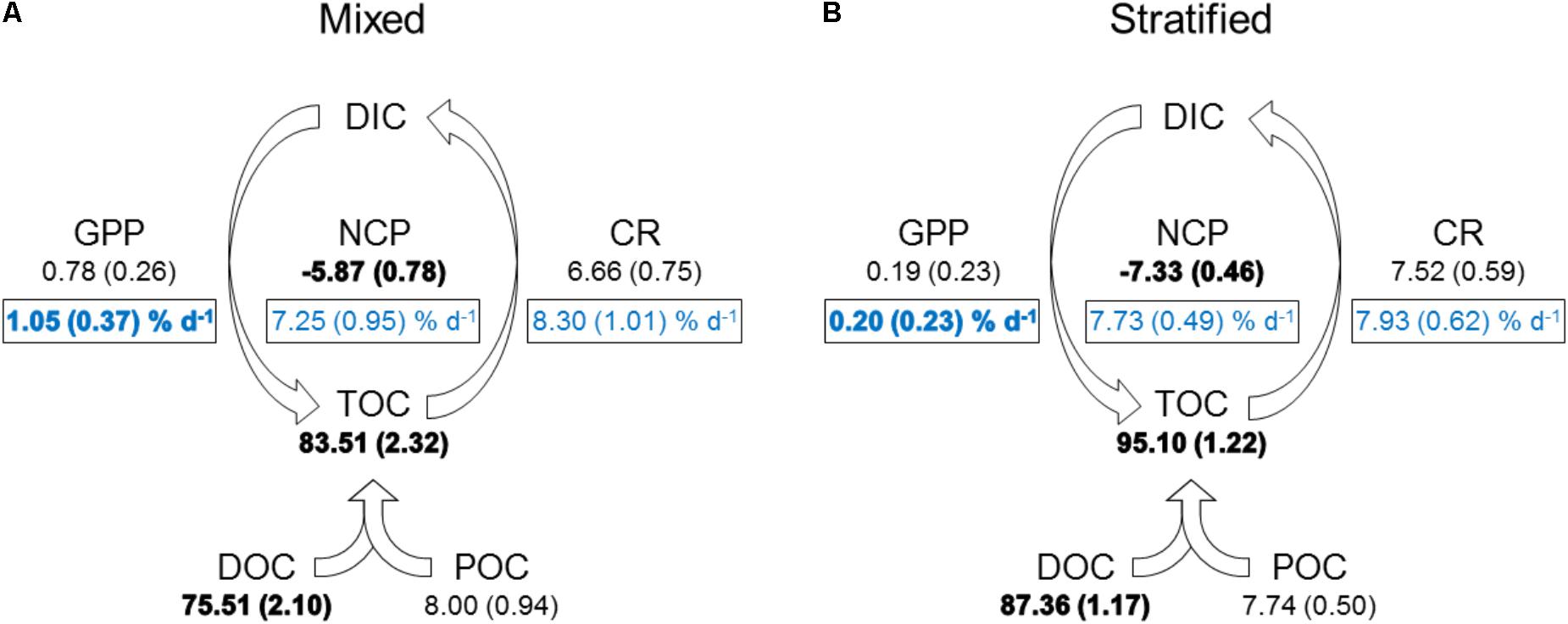
FIGURE 3. Mean daily planktonic carbon balance in mixed (A) and stratified (B) periods. GPP, gross primary production; CR, community respiration; NCP, net community production (GPP–CR). Values given as mean (SE). GPP, CR, NCP in μmol C L-1 d-1. DOC, dissolved organic carbon; TOC, total organic carbon [POC (particulate organic carbon) + DOC], TOC, DOC, and POC in μmol C L-1; DIC, dissolved inorganic carbon. Boxed light blue text constitutes the percentage of planktonic C contributed daily to TOC [mean (SE)]. Bold indicates a significant difference between periods (p < 0.05).
N2 Fixation Activity
Weekly mean N2 fixation ranged from 0.18 to 0.71 and 0.07 to 1.28 nmol C2H4 L-1 h-1 in the mixed and stratified periods, respectively (Figure 2). Although mean N2 fixation on 8 and 22 September, during stratification, were substantially higher than during the mixed period, overall there was no significant difference (Table 2).
Relationship Analyses
Positive relationships were revealed for Pn and availability of N (NOx and/or DIN) during the mixed and stratified period and when both periods were combined (Table 3). Additionally, negative relationships were revealed for Pn with temperature and PAR. R revealed a negative relationship with NOx during the mixed period and when both periods are combined (Table 3). No relationships were found for Pg with any environmental parameter (Table 3). Positive relationships for N2 fixation and N availability were found for the mixed period while for the stratified period positive relationships were established with both temperature and PAR. A positive relationship was established for PAR when periods were combined (Table 3). Additionally, no relationships were found between any of the primary production parameters and N2 fixation (not shown in Table 3).
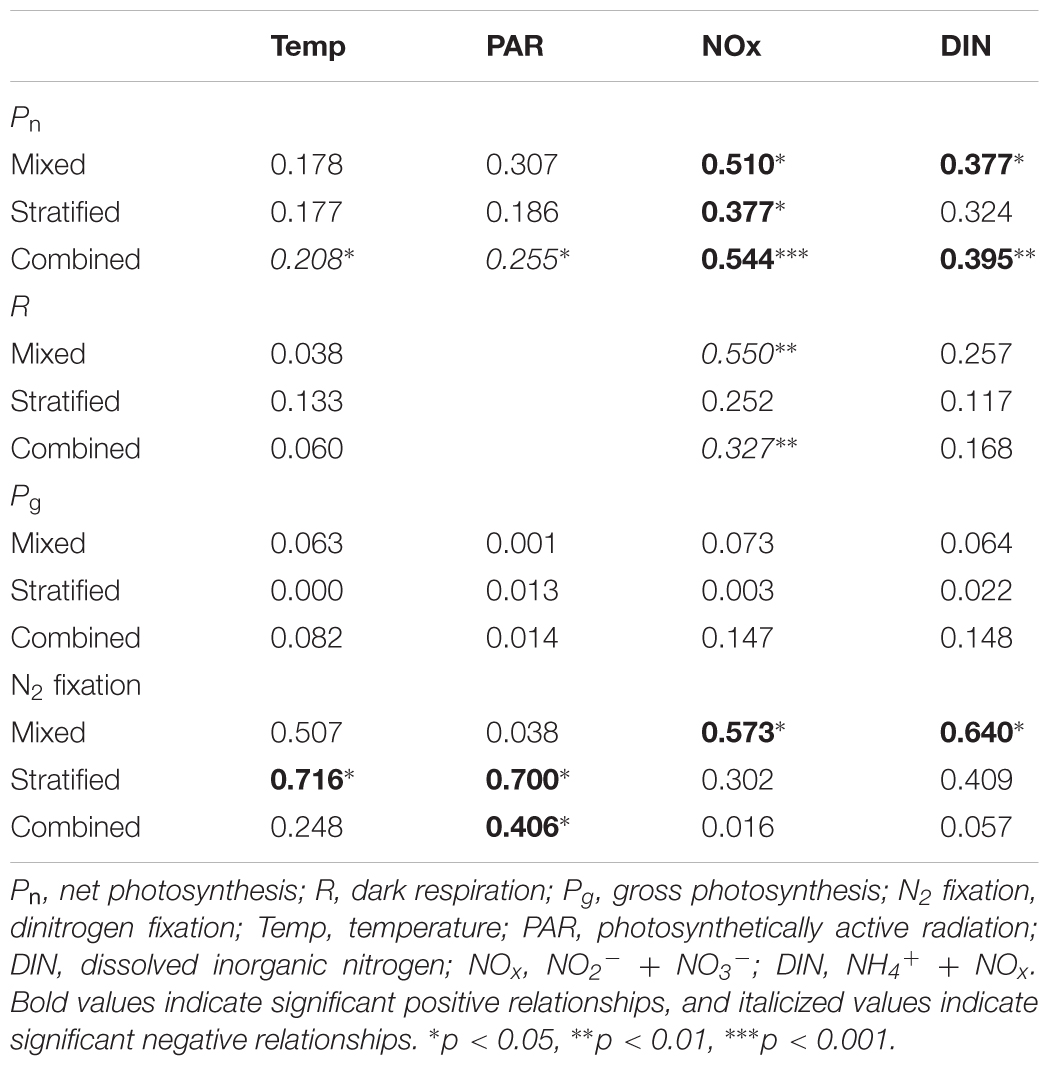
TABLE 3. Linear regression analysis (r2-values) examining the relationships between the physiological parameters.
DISCUSSION
Studies relating planktonic N2 fixation and primary production in the highly seasonal Gulf of Aqaba in the northern Red Sea are, to our knowledge, only related to open water settings (Rahav et al., 2015). Rahav et al. (2015) highlighted the need for both spatial and temporal sampling of planktonic communities in the Gulf of Aqaba to disentangle the complex dynamics of these communities. In this study, water samples taken 1 m above the reef benthos were used to measure planktonic primary production and N2 fixation rates on a weekly basis during both the mixed and the stratified period. Findings revealed significantly higher primary production (Pn) during the mixed period (Table 2), while planktonic N2 fixation rates remained stable between both periods (Table 2). Similar to open water, higher net heterotrophic activity was measured during the stratified compared to the mixed period, but the open water column remained net autotrophic. Moreover, Rahav et al. (2015) found a potential coupling between pelagic primary production and N2 fixation only during the mixed period, while linear regression analyses supported no such relationship here. However, results obtained here do suggest that N2 fixation, as estimated by C2H4 evolution, has the potential to maintain stable pelagic GPP throughout the year by contributing ∼6 times more bioavailable N for GPP during the N depleted stratified period compared to the mixed period.
Environmental Parameters in Mixed and Stratified Period
Photosynthetically active radiation, water temperature, and inorganic nutrient concentrations during the mixed and stratified periods were comparable to previous research at the study site (Rasheed et al., 2002; Naumann et al., 2010). The average mixed period DIN:PO43- ratio (10.42) was lower than the Redfield ratio for N:P [16:1; Redfield (1958)], suggesting N as the limiting nutrient. The average N:P ratio in the stratified period was similar to the Redfield N:P ratio, indicating that inorganic N and P availability in that period was on average balanced. However, ratios between 31 and 33 found in three non-consecutive weeks in October and November suggest that there were times when P may have been limiting. Previous studies have also found that both N and P are limiting factors for primary production in the northern Red Sea (Stihl et al., 2001; Mackey et al., 2007; Suggett et al., 2009). Chl a decreased significantly from the mixed to the stratified period, coinciding with declining inorganic nutrients and increasing water temperature and PAR. A strong negative relationship with PAR was found especially during the stratified period (r2 = 0.776, p < 0.001). Indeed, higher PAR often causes a reduction in Chl a in phytoplankton (Laabir et al., 2011), but this response is specific per species (Kulk et al., 2011). Furthermore, Gittings et al. (2018) found a strong, near perfect, negative correlation between Chl a and sea surface temperature in the northern Red Sea. This is also confirmed by our data as a strong negative relationship was found between Chl a and temperature when both periods were combined (r2 = 0.597, p < 0.001). Average POC:PN ratios in the mixed and stratified period were higher than Redfield proportions (106:16 = 6.625), 7.16 and 8.77, respectively, suggesting that the POM in the water column was impoverished in N throughout the year, particularly so during the stratified period when PN concentrations were significantly reduced. The dominant source of POM in coral reef-surrounding waters can be mucus released by hard corals (Johannes, 1967; Naumann et al., 2012a). Mucus POC and PN release by the dominant hard corals in the studied reef is constant over the year (Naumann et al., 2010). However, the average POC:PN ratio of coral mucus (12 ± 1; Naumann et al., 2010), is far higher than the periodic ratios found for our water samples, indicating that a large fraction of water column PN originated from another source subject to differences in environmental conditions. This is confirmed by Hadas et al. (2009), who found that the majority of water column PN in a Gulf of Aqaba reef consisted of or was produced by pelagic prokaryotes. In addition, DOC measured in our water samples was significantly higher in the stratified period (87.36 μmol L-1) compared to the mixed period (75.51 μmol L-1). DOC may be more abundantly released by benthic coral reef algae than by co-occurring hard corals (Haas et al., 2013; Mueller et al., 2014). DOC release by turf algae and the algal genus Peyssonnelia in the studied reef is indeed higher during the stratified than during the mixed period (Haas et al., 2010). A positive relation between DOC release and temperature is common in marine macrophytes (e.g., Maher and Eyre, 2010). Thus, increased benthic release could explain the increased water column DOC concentration found during the stratified period.
Planktonic Primary Production and N2 Fixation
The planktonic GPP rates measured in the mixed and stratified periods fall within the range previously recorded for the Gulf of Aqaba (Levanon-Spanier et al., 1979) and rates measured for other reef-surrounding waters worldwide (Table 4). However, compared to open surface waters of the Gulf of Aqaba, GPP found here was three- to fourfold higher during the mixed period and four- to ninefold higher during the stratified period (Rahav et al., 2015). NCP was always negative, indicating that the planktonic community as a whole acted net heterotrophically, particularly during the stratified period (Cardini et al., 2016). Planktonic communities in waters surrounding coral reefs are often net heterotrophic and are likely fueled by a steady supply of organic matter released from the reef benthos (Haas et al., 2013; Naumann et al., 2012b). Even though PAR and temperature were both negatively related with Pn, the strong positive relationship of Pn with both DIN and/or NOx within each period and combined indicated that inorganic N, rather than PAR or temperature, was the strongest environmental parameter that limited Pn in these oligotrophic waters.
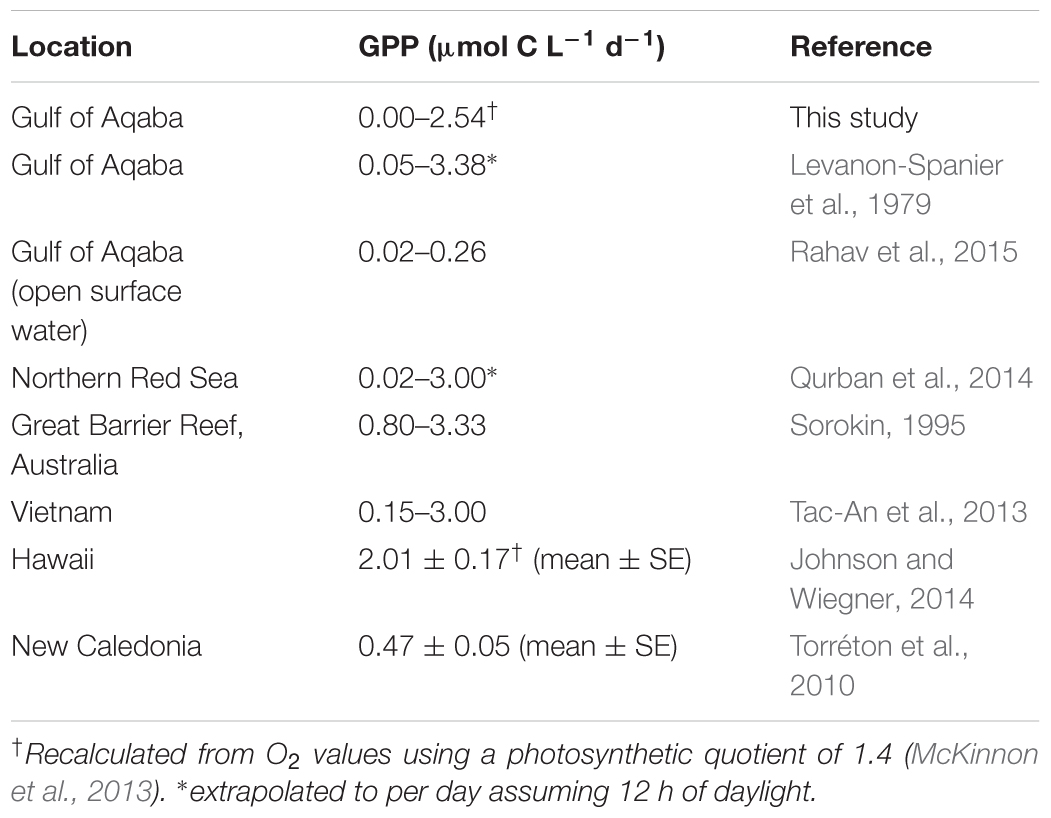
TABLE 4. Comparison of daily gross primary production (GPP) rates from this study and literature references.
Dinitrogen fixation rates measured in this study were within the range found for planktonic communities from different locations worldwide (Table 5). Our maximum rates appear high compared to most literature values, but this is mainly due to two high values measured in September (1.06 and 1.28 nmol C2H4 L-1 h-1). The other weekly mean values in the present study average 0.29 ± 0.05 nmol C2H4 L-1 h-1, which falls within the range of most literature values (0.004–0.46 nmol C2H4 L-1 h-1). These values, like the values in this study, were found in the upper water column up to 10 m depth, while higher values (∼2.75 and 0.87 nmol C2H4 L-1 h-1) were measured at depths >25 m (Wilson et al., 2012; Wu et al., 2018). Measured values from the tropical Atlantic Ocean [up to 1.04 C2H4 L-1 h-1 (Großkopf et al., 2012), assuming the conservative theoretical C2H4:N2 conversion ratio of 4:1 (Mulholland et al., 2004)], indicate that high rates, such as those found in September, are possible under comparably oligotrophic conditions. Moreover, the high September N2 fixation rates coincided with the lowest DIN concentrations of all sampling occasions (0.20–0.29 μmol L-1). N2 fixation is energy-costly and many diazotrophs can increase their N2 fixation in times of inorganic/organic N scarcity (Mulholland et al., 2001). However, this potential negative relationship could not be evidenced with linear regression. On the contrary, a positive relationship was found during the mixed period while no relationship was found during the stratified period. A large part of the DIN measured during this study consisted of NOx. NO3- in particular has a lower inhibitory effect on nitrogenase (the enzyme responsible for N2 fixation), compared to NH4+, depending on, e.g., light or PO43- availability (Dekaezemacker and Bonnet, 2011; Knapp, 2012; Garcia and Hutchins, 2014). Thus, certain abiotic factors may mitigate the nitrogenase inhibiting effects of NO3-. Alternatively, ambient DIN concentrations may not be high enough to inhibit nitrogenase (Mulholland et al., 2001). During the stratified period, when temperature was highest and the highest PAR values were measured, N2 fixation rates coincided with these parameters. However, when combined, PAR was the environmental parameter that best explained N2 fixation rates found throughout the year suggesting a prominent role for photoautotrophic diazotrophs. This coincides with relationships found for other organisms and substrates (Bednarz et al., 2015b; Tilstra et al., 2017).
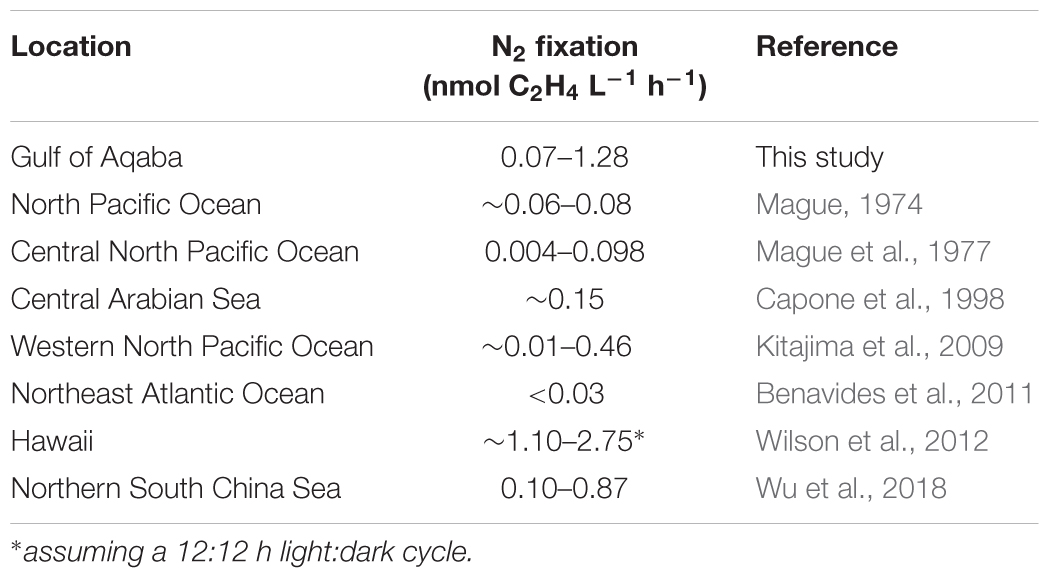
TABLE 5. Comparison of hourly dinitrogen (N2) fixation rates from this study and literature references (given as ranges) measured by acetylene reduction assay.
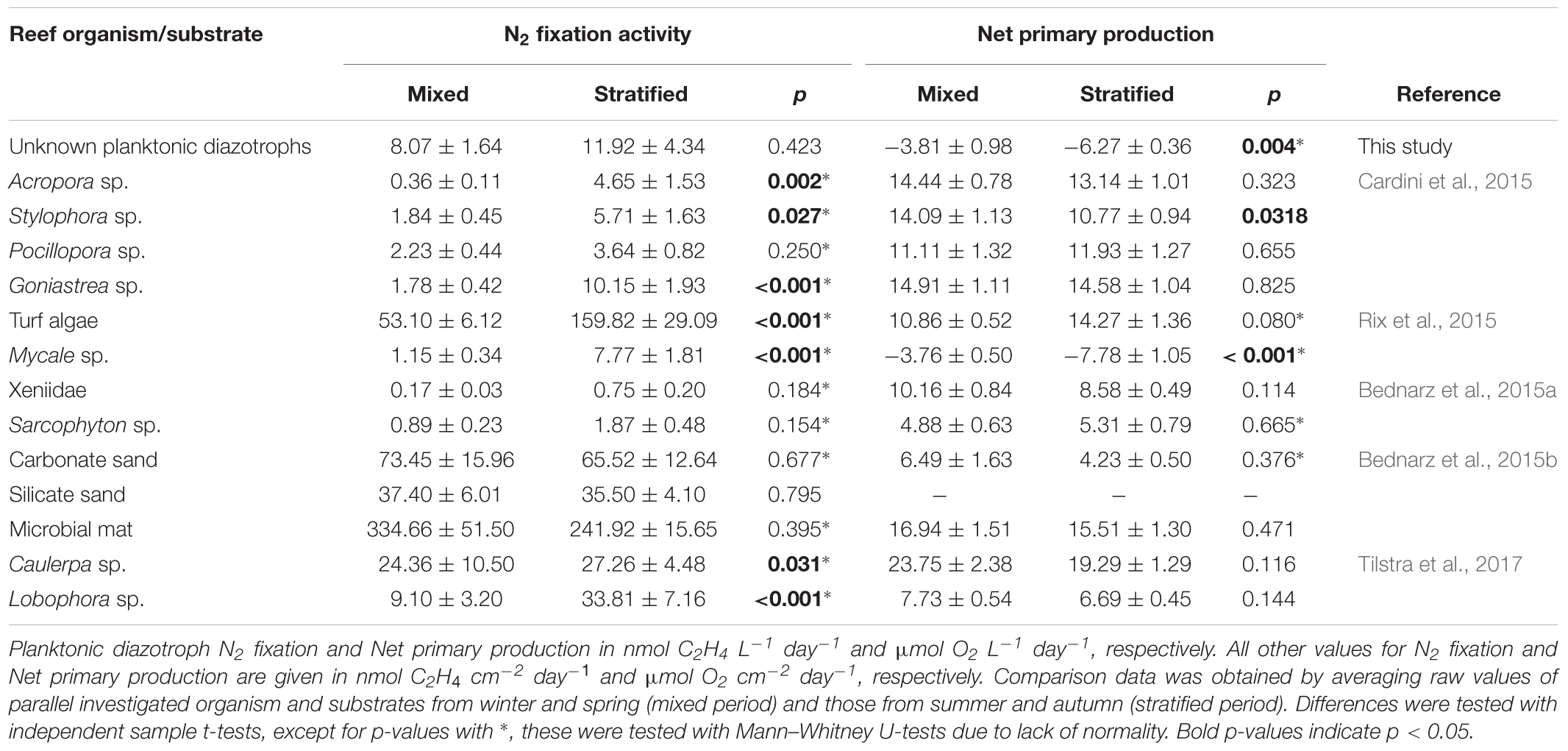
TABLE 6. Comparison of N2 fixation and net primary production associated with different coral reef organisms and substrates from the Gulf of Aqaba.
While GPP remained stable between both periods, the decrease in NCP found in the present study indicates that the planktonic community became more heterotrophic in the stratified period compared to the mixed period. However, this observation is contrasted by linear relationships (Table 3), which show that N2 fixation rates during the stratified period coincided with light availability (indicating autotrophic dominance) rather than DOC availability (not shown in Table 3) despite the higher availability of DOC. The benthos of the study site was primarily dominated by primary producers (see Cardini et al., 2016; van Hoytema et al., 2016), many of which release a significant fraction of their photosynthetically fixed carbon as DOC in the surrounding waters (Haas et al., 2010, 2011; Naumann et al., 2010). Since our water samples were taken 1 m above the benthos, DOC would have been available to be consumed by the bacterioplankton, as previously shown in Haas et al. (2011), potentially causing an increase in heterotrophic abundance and/or activity. While Chl a concentration per planktonic cell could have increased due to increased temperature (Geider, 1987) from the mixed to the stratified period, Chl a concentrations (measured per liter) found here decreased with increasing temperature, lending support that less autotrophs were present in the planktonic community during the stratified period. Moreover, Rahav et al. (2015) showed a shift toward a more heterotrophic diazotroph community from the mixed to the stratified period while Al-Najjar et al. (2007) also showed an increase in Prochlorococcus sp. abundance in the picophytoplankton community. Thus, it is likely that the planktonic community during the stratified period contained heterotrophic diazotrophs with lower N2 fixation rate capacity that was compensated for by higher abundances of Prochlorococcus sp. However, a full assessment of the planktonic community, including the identification of the picophytoplankton community, could shed light into this apparent contradiction. Additionally, the potential increase in Prochlorococcus sp. (Al-Najjar et al., 2007) may explain why a relationship between Pg and PO43- could not be established, despite DIN:PO43- ratios in the stratified period regularly exceeding Redfield (with values up to 33). Foster et al. (2009) also did not detect P limitation of N2 fixation and attributed this to the relatively small size of the N2 fixing microbes in the Gulf of Aqaba, allowing maintenance of N2 fixation at very low P availability. Small cell size theoretically results in increased nutrient uptake affinity due to allometrically higher surface area to volume ratio, which may have allowed Prochlorococcus sp. to maintain photosynthesis under extremely low PO43- availability (Finkel et al., 2010). However, nutrient limitation of planktonic processes is likely more complicated than indicated by the canonical Redfield ratio (Klausmeier et al., 2004).
Assuming the Redfield C:N ratio (6.625), planktonic N2 fixation had, on average, the potential to contribute 3.42% of the N needed for daily GPP during the mixed period. Remarkably, the average potential contribution during stratification was substantially higher as N2 fixation generated 20.84% of the potential N demand by GPP. This more than sixfold higher potential contribution indicates that N2 fixation is a substantial source of N for maintaining stable GPP by the autotrophic community as a whole during extremely oligotrophic conditions in the Gulf of Aqaba. Moreover, the percentage contribution calculated in the present study are similar to those under comparable oligotrophic scenarios in other regions (Montoya et al., 2004; White et al., 2007; Wu et al., 2018).
Comparison With Parallel Investigated Organisms
Dinitrogen fixation and net photosynthesis of organisms and substrates were previously examined in a seasonal resolution in parallel with the current study (Bednarz et al., 2015a,b; Cardini et al., 2015; Rix et al., 2015; Tilstra et al., 2017). For comparisons, raw data of each organism or substrate was averaged after combining winter and spring data to represent the mixed period, and summer and autumn to represent the stratified period.
Comparing the pattern of planktonic N2 fixation rates between mixed and stratified periods with those of benthic N2 fixers revealed that, with the exception of Pocillopora sp. and both investigated soft corals (i.e., Xeniidae and Sarcophyton sp.), symbiotic diazotrophs (those with a eukaryotic host) are significantly affected by seasonal mixing and stratification, while planktonic diazotrophs were not (Table 6). The scleractinian coral Pocillopora sp. has recently been shown to have an inflexible microbiome in response to environmental stress (Pogoreutz et al., 2018). This could explain the deviation from the other three scleractinian coral genera as the diazotrophic community could remain relatively stable throughout the year. Furthermore, higher activity of resident diazotrophs is unlikely since abundance and activity of diazotrophs in Red Sea originating Pocillopora sp. have been shown to correlate positively (Pogoreutz et al., 2017). Strikingly, soft corals possessing symbiotic diazotrophs (Bednarz et al., 2015a), also show no differences between both periods (Table 6). Soft corals lack a hard calcium carbonate skeleton and a significant part of a soft corals’ weight can consist of water (∼80%; Al-Sofyani and Niaz, 2007). In this study, planktonic samples were taken 1 m above the reef benthos. Thus, soft corals can potentially take up water holding the same community of planktonic diazotrophs found here by replenishing their water content regularly (Sheppard et al., 2009). Together with its core coral microbiome (Ainsworth et al., 2015), which might be distinct from its surrounding seawater (Bourne et al., 2013), this could potentially alter their total diazotrophic activity to mimic that of the planktonic community described here. While a similar case could be made for the sponge Mycale sp., research has shown that between 72 and 93% of plankton is grazed and metabolized resulting in 74% of total daily C intake (Pile et al., 1996), while in corals, up to 95% of daily C is translocated from their photosynthetic endosymbiont Symbiodinium (Muscatine, 1990) suggesting a low need for grazing the diazotrophs that are taken up with the surrounding water.
Particulate nitrogen of all investigated organisms and substrates remained relatively stable with the exception of Stylophora sp. and Mycale sp. The latter due to high respiration rates measured during summer and autumn (Rix et al., 2015). In general, the pattern of Pn of the planktonic (free living) diazotrophs differed from the benthic (symbiotic) diazotrophs (Table 6).
Concluding Remarks
Primary production in the water column directly overlying a coral reef in the Gulf of Aqaba appears to be primarily regulated by inorganic N availability, driven by mixing conditions. While inorganic N concentrations declined due to stratification, daily contribution of planktonic GPP to TOC declined significantly while GPP itself remained relatively stable. Compared to open water GPP (Rahav et al., 2015), GPP measured here was between three- and ninefold higher suggesting potential benthic coupling. NCP was significantly more heterotrophic during stratification. However, the daily contribution of NCP to TOC was similar between the two periods due to increased DOC concentrations. The maintenance of biological activity in the water column due to increased DOC availability in times of reduced GPP highlights the importance of the microbial loop in planktonic trophodynamics in these waters (Azam and Malfatti, 2007; Nelson et al., 2011).
In addition to the change in the C budget, there were indications of a potential shift in the N2 fixation community toward higher heterotrophic activity, similar to findings of Rahav et al. (2015). Causes for this shift could be the decline in inorganic nutrients as well as the increased DOC concentration providing a competitive advantage to heterotrophic diazotrophs (Suggett et al., 2009). This increase in DOC could be attributable to increased release by benthic turf- and macroalgae (Haas et al., 2013; Mueller et al., 2014). Algal-derived organic matter may also promote a more heterotrophic planktonic community than organic matter released by hard corals (Haas et al., 2011; Nelson et al., 2013). During stratification, when GPP is strongly nutrient-limited, N2 fixation shows the potential to contribute a substantial fraction of the N needed, by compensating for low DIN, to maintain stable GPP in the water column.
Dinitrogen fixation was maintained at comparable rates in both periods. Moreover, the results obtained here strongly suggest that N2 fixation is an important source of N to planktonic primary production. In addition, DOC appears to play an important role in the dynamics of planktonic C and N production/consumption. Further investigation into DOC dynamics through coral reefs is warranted to unravel its effect on energy and nutrient cycles in coral reefs and their surrounding waters. Finally, the findings presented here may be applied to lower latitude coral reefs where the more stable environmental conditions make the disentanglement of driving environmental parameters more complicated.
Author Contributions
AT analyzed data, wrote the manuscript, prepared the figures and tables, made revisions, and in charge of submission. NH wrote part of the manuscript, reviewed drafts of the manuscript, and designed and performed the experiments. UC, VB, LR, and MN reviewed drafts of the manuscript and designed and performed the experiments. FA-H contributed reagents, materials, and analysis tools, and designed the experiments. CW reviewed drafts of the manuscript and designed the experiments.
Funding
This work was funded through German Research Foundation (DFG) grants Wi 2677/6-1 and Wi 2677/9-1 to CW. VB was funded by a stipend of “Evangelisches Studienwerk Villigst e.V.”
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank S. Basuoni and S. Helber for assistance in the field, as well as C. Staschok, M. Birkicht, and D. Dasbach for field work preparation and sample analyses. This paper is an adapted version previously published in the Ph.D. thesis of van Hoytema (2015).
References
Adey, W. H. (1998). Coral reefs: algal structured and mediated ecosystems in shallow, turbulent, alkaline waters. J. Phycol. 34, 393–406. doi: 10.1046/j.1529-8817.1998.340393.x
Ainsworth, T. D., Krause, L., Bridge, T., Torda, G., Raina, J.-B., Zakrzewski, M., et al. (2015). The coral core microbiome identifies rare bacterial taxa as ubiquitous endosymbionts. ISME J. 9, 2261–2274. doi: 10.1038/ismej.2015.39
Al-Najjar, T., Badran, M. I., Richter, C., Meyerhoefer, M., and Sommer, U. (2007). Seasonal dynamics of phytoplankton in the Gulf of Aqaba, Red Sea. Hydrobiologia 579, 69–83. doi: 10.1007/s10750-006-0365-z
Al-Sofyani, A. A., and Niaz, G. R. (2007). A comparative study of the components of the hard coral Seriatopora hystrix and the soft coral Xenia umbellata along the Jeddah coast, Saudi Arabia. Rev. Biol. Mar. Oceanogr. 42, 207–219. doi: 10.4067/S0718-19572007000300001
Azam, F., and Malfatti, F. (2007). Microbial structuring of marine ecosystems. Nat. Rev. Microbiol. 5, 782–791. doi: 10.1038/nrmicro1747
Bednarz, V. N., Cardini, U., Van Hoytema, N., Al-Rshaidat, M. M. D., and Wild, C. (2015a). Seasonal variation in dinitrogen fixation and oxygen fluxes associated with two dominant zooxanthellate soft corals from the northern Red Sea. Mar. Ecol. Prog. Ser. 519, 141–152. doi: 10.3354/meps11091
Bednarz, V. N., Naumann, M. S., Cardini, U., van Hoytema, N., Rix, L., Al-rshaidat, M. M. D., et al. (2018). Contrasting seasonal responses in dinitrogen fixation between shallow and deep-water colonies of the model coral Stylophora pistillata in the northern Red Sea. PLoS One 13:e0199022. doi: 10.1371/journal.pone.0199022
Bednarz, V. N., van Hoytema, N., Cardini, U., Naumann, M., Al-Rshaidat, M., and Wild, C. (2015b). Dinitrogen fixation and primary productivity by carbonate and silicate reef sand communities of the Northern Red Sea. Mar. Ecol. Prog. Ser. 527, 47–57. doi: 10.3354/meps11224
Benavides, M., Agawin, N., Arístegui, J., Ferriol, P., and Stal, L. (2011). Nitrogen fixation by Trichodesmium and small diazotrophs in the subtropical northeast Atlantic. Aquat. Microb. Ecol. 65, 43–53. doi: 10.3354/ame01534
Biton, E., and Gildor, H. (2011a). Stepwise seasonal restratification and the evolution of salinity minimum in the Gulf of Aqaba (Gulf of Eilat). J. Geophys. Res. Ocean. 116, 1–7. doi: 10.1029/2011JC007106
Biton, E., and Gildor, H. (2011b). The coupling between exchange flux through a strait and dynamics in a small convectively driven marginal sea: the gulf of Aqaba (Gulf of Eilat). J. Geophys. Res. Ocean. 116, 1–17. doi: 10.1029/2011JC006944
Biton, E., and Gildor, H. (2011c). The general circulation of the Gulf of Aqaba (Gulf of Eilat) revisited: the interplay between the exchange flow through the straits of tiran and surface fluxes. J. Geophys. Res. Oceans 116, 1–15. doi: 10.1029/2010JC006860
Bourne, D. G., Dennis, P. G., Uthicke, S., Soo, R. M., Tyson, G. W., and Webster, N. (2013). Coral reef invertebrate microbiomes correlate with the presence of photosymbionts. ISME J. 7, 1452–1458. doi: 10.1038/ismej.2012.172
Capone, D. G. (1993). “Determination of nitrogenase activity in aquatic samples using the acetylene reduction procedure,” in Handbook of Methods in Aquatic Microbial Ecology, eds P. F. Kemp, J. J. Cole, B. F. Sherr, and E. B. Sherr (London: CRC Press), 800.
Capone, D., Zehr, J., Paerl, H., Bergman, B., and Carpenter, E. (1997). Trichodesmium, a globallly significant marine cyanobacterium. Science 276, 1221–1229.
Capone, D. G., Subramaniam, A., Montoya, J. P., Voss, M., Humborg, C., Johansen, A. M., et al. (1998). An extensive bloom of the N2-fixing Cyanobacterium Trichodesmium erythraeum in the central Arabian Sea. Mar. Ecol. Prog. Ser. 172, 281–292. doi: 10.3354/meps172281
Cardini, U., Bednarz, V., van Hoytema, N., Rovere, A., Naumann, M., Al Rshaidat, M., et al. (2016). Budget of primary production and dinitrogen fixation in a highly seasonal Red Sea coral reef. Ecosystems 19, 771–785. doi: 10.1007/s10021-016-9966-1
Cardini, U., Bednarz, V. N., Naumann, M. S., van Hoytema, N., Rix, L., Foster, R. A., et al. (2015). Functional significance of dinitrogen fixation in sustaining coral productivity under oligotrophic conditions. Proc. R. Soc. B Biol. Sci. 282:20152257. doi: 10.1098/rspb.2015.2257
Carlson, D. F., Fredj, E., and Gildor, H. (2014). The annual cycle of vertical mixing and restratification in the Northern Gulf of Eilat/Aqaba (Red Sea) based on high temporal and vertical resolution observations. Deep Res. Part I Oceanogr. Res. Pap. 84, 1–17. doi: 10.1016/j.dsr.2013.10.004
Chipman, L., Huettel, M., and Laschet, M. (2012). Effect of benthic-pelagic coupling on dissolved organic carbon concentrations in permeable sediments and water column in the northeastern Gulf of Mexico. Cont. Shelf Res. 45, 116–125. doi: 10.1016/j.csr.2012.06.010
de Goeij, J. M., van Oevelen, D., Vermeij, M. J. A., Osinga, R., Middelburg, J. J., de Goeij, A. F. P. M., et al. (2013). Surviving in a marine desert: the sponge loop retains resources within coral reefs. Science 342, 108–110. doi: 10.1126/science.1241981
Dekaezemacker, J., and Bonnet, S. (2011). Sensitivity of N2 fixation to combined nitrogen forms (NO3- and NH4-) in two strains of the marine diazotroph Crocosphaera watsonii (Cyanobacteria). Mar. Ecol. Prog. Ser. 438, 33–46. doi: 10.3354/meps09297
D’Elia, C. F., and Wiebe, W. J. (1990). “Biogeochemical nutrient cycles in coral-reef ecosystems,” in Coral Reefs, ed. Z. Dubinsky (Amsterdam: Elsevier), 49–74.
Finkel, Z. V., Beardall, J., Flynn, K. J., Quigg, A., Rees, T. A. V., and Raven, J. A. (2010). Phytoplankton in a changing world: cell size and elemental stoichiometry. J. Plankton Res. 32, 119–137. doi: 10.1093/plankt/fbp098
Foster, R. A., Kuypers, M. M. M., Vagner, T., Paerl, R. W., Musat, N., and Zehr, J. P. (2011). Nitrogen fixation and transfer in open ocean diatom-cyanobacterial symbioses. ISME J. 5, 1484–1493. doi: 10.1038/ismej.2011.26
Foster, R. A., Paytan, A., and Zehr, J. P. (2009). Seasonality of N2 fixation and nifH gene diversity in the Gulf of Aqaba (Red Sea). Limnol. Oceanogr. 54, 219–233. doi: 10.4319/lo.2009.54.1.0219
Furnas, M., Alongi, D., McKinnon, D., Trott, L., and Skuza, M. (2011). Regional-scale nitrogen and phosphorus budgets for the northern (14°S) and central (17°S) Great Barrier Reef shelf ecosystem. Cont. Shelf Res. 31, 1967–1990. doi: 10.1016/j.csr.2011.09.007
Garcia, N. S., and Hutchins, D. A. (2014). Light-limited growth rate modulates nitrate inhibition of dinitrogen fixation in the marine unicellular cyanobacterium Crocosphaera watsonii. PLoS One 9:e114465. doi: 10.1371/journal.pone.0114465
Geider, R. J. (1987). Light and temperature dependence of the carbon to chlorophyll a ratio in microalgae and cyanobacteria: implications for physiology and growth of phytoplankton. New Phytol. 106, 1–34. doi: 10.1111/j.1469-8137.1987.tb04788.x
Gittings, J. A., Raitsos, D. E., Krokos, G., and Hoteit, I. (2018). Impacts of warming on phytoplankton abundance and phenology in a typical tropical marine ecosystem. Sci. Rep. 8, 1–12. doi: 10.1038/s41598-018-20560-5
Großkopf, T., Mohr, W., Baustian, T., Schunck, H., Gill, D., Kuypers, M. M. M., et al. (2012). Doubling of marine dinitrogen-fixation rates based on direct measurements. Nature 488, 361–364. doi: 10.1038/nature11338
Haas, A. F., Naumann, M. S., Struck, U., Mayr, C., el-Zibdah, M., and Wild, C. (2010). Organic matter release by coral reef associated benthic algae in the Northern Red Sea. J. Exp. Mar. Bio. Ecol. 389, 53–60. doi: 10.1016/j.jembe.2010.03.018
Haas, A. F., Nelson, C. E., Kelly, L. W., Carlson, C. A., Rohwer, F., Leichter, J. J., et al. (2011). Effects of coral reef benthic primary producers on dissolved organic carbon and microbial activity. PLoS One 6:e27973. doi: 10.1371/journal.pone.0027973
Haas, A. F., Nelson, C. E., Rohwer, F., Wegley-Kelly, L., Quistad, S. D., Carlson, C. A., et al. (2013). Influence of coral and algal exudates on microbially mediated reef metabolism. PeerJ 1:e108. doi: 10.7717/peerj.108
Hadas, E., Shpigel, M., and Ilan, M. (2009). Particulate organic matter as a food source for a coral reef sponge. J. Exp. Biol. 212, 3643–3650. doi: 10.1242/jeb.027953
Hatcher, B. G. (1997). Coral reef ecosystems: how much greater is the whole than the sum of the parts? Coral Reefs 16, S77–S91. doi: 10.1007/s003380050244
Johannes, R. E. (1967). Ecology of organic aggregates in the vicinity of a coral reef. Limnol. Oceanogr. 12, 189–195. doi: 10.4319/lo.1967.12.2.0189
Johnson, E. E., and Wiegner, T. N. (2014). Surface water metabolism potential in groundwater-fed coastal waters of Hawaii Island, USA. Estuaries Coasts 37, 712–723. doi: 10.1007/s12237-013-9708-y
Karl, D., Michaels, A., Bergman, B., Capone, D., Carpenter, E., Letelier, R., et al. (2002). Dinitrogen fixation in the world’s oceans. Biogeochemistry 5, 47–98.
Kitajima, S., Furuya, K., Hashihama, F., Takeda, S., and Kanda, J. (2009). Latitudinal distribution of diazotrophs and their nitrogen fixation in the tropical and subtropical western North Pacific. Limnol. Oceanogr. 54, 537–547. doi: 10.4319/lo.2009.54.2.0537
Klausmeier, C. A., Litchman, E., Daufresne, T., and Levin, S. A. (2004). Optimal nitrogen-to-phosphorous stoichiometry of phytoplankton. Nature 429, 171–174.
Knapp, A. N. (2012). The sensitivity of marine N2 fixation to dissolved inorganic nitrogen. Front. Microbiol. 3:374. doi: 10.3389/fmicb.2012.00374
Kulk, G., van de Poll, W. H., Visser, R. J. W., and Buma, A. G. J. (2011). Distinct differences in photoacclimation potential between prokaryotic and eukaryotic oceanic phytoplankton. J. Exp. Mar. Bio. Ecol 398, 63–72. doi: 10.1016/j.jembe.2010.12.011
Laabir, M., Jauzein, C., Genovesi, B., Masseret, E., Grzebyk, D., Cecchi, P., et al. (2011). Influence of temperature, salinity and irradiance on the growth and cell yield of the harmful red tide dinoflagellate Alexandrium catenella colonizing Mediterranean waters. J. Plankton Res. 33, 1550–1563. doi: 10.1093/plankt/fbr050
Levanon-Spanier, I., Padan, E., and Reiss, Z. (1979). Primary production in a desert-enclosed sea- the Gulf of Elat (Aqaba), Red Sea. Deep Sea Res. Part A Oceanogr. Res. Pap. 26, 673–685. doi: 10.1016/0198-0149(79)90040-2
Mackey, K. R. M., Labiosa, R. G., Calhoun, M., Street, J. H., Post, A. F., and Paytan, A. (2007). Phosphorus availability, phytoplankton community dynamics, and taxon-specific phosphorus status in the Gulf of Aqaba, Red Sea. Limnol. Oceanogr. 52, 873–885. doi: 10.4319/lo.2007.52.2.0873
Mague, T. H. (1974). Nitrogen fixation in the North Pacific Ocean. Mar. Biol. 24, 109–119. doi: 10.1007/BF00389344
Mague, T. H., Mague, F. C., and Holm-Hansen, O. (1977). Physiology and chemical composition of nitrogen-fixing phytoplankton in the central North Pacific Ocean. Mar. Biol. 41, 213–227. doi: 10.1007/BF00394908
Maher, D. T., and Eyre, B. D. (2010). Benthic fluxes of dissolved organic carbon in three temperate Australian estuaries: implications for global estimates of benthic DOC fluxes. J. Geophys. Res. Biogeosciences 115:G04039. doi: 10.1029/2010JG001433
McKinnon, A. D., Logan, M., Castine, S. A., and Duggan, S. (2013). Pelagic metabolism in the waters of the great barrier reef. Limnol. Oceanogr. 58, 1227–1242. doi: 10.4319/lo.2013.58.4.1227
Montoya, J. P., Holl, C. M., Zehr, J. P., Hansen, A., Villareal, T. A., and Capone, D. G. (2004). High rates of N2 fixation by unicellular diazotrophs in the oligotrophic Pacific Ocean. Nature 430, 1027–1031. doi: 10.1038/nature02744.1
Mueller, B., van der Zande, R., van Leent, P., Meesters, E., Vermeij, M., and van Duyl, F. (2014). Effect of light availability on dissolved organic carbon release by Caribbean reef algae and corals. Bull. Mar. Sci. 90, 875–893. doi: 10.5343/bms.2013.1062
Mulholland, M. R., Bronk, D. A., and Capone, D. G. (2004). Dinitrogen fixation and release of ammonium and dissolved organic nitrogen by Trichodesmium IMS101. Aquat. Microb. Ecol. 37, 85–94. doi: 10.3354/ame037085
Mulholland, M. R., Ohki, K., and Capone, D. G. (2001). Nutrient controls on nitrogen uptake and metabolism by natural populations and cultures of Trichodesmium (Cyanobacteria). J. Phycol. 37, 1001–1009. doi: 10.1046/j.1529-8817.2001.00080.x
Muscatine, L. (1990). “The role of symbiotic algae in carbon and energy flux in reef corals,” in Coral Reefs, ed. Z. Dubinsky (Amsterdam: Elsevier),75–87.
Naumann, M. S., Haas, A., Struck, U., Mayr, C., el-Zibdah, M., and Wild, C. (2010). Organic matter release by dominant hermatypic corals of the Northern Red Sea. Coral Reefs 29, 649–659. doi: 10.1007/s00338-010-0612-7
Naumann, M. S., Haas, A. F., Jantzen, C., Iglesias-Prieto, R., and Wild, C. (2012a). “Benthic-pelagic coupling in a Caribbean reef lagoon affected by hurricane “Dolly”,” in Proceedings of the 12th International Coral Reef Symposium, Cairns.
Naumann, M. S., Richter, C., Mott, C., El-Zibdah, M., Manasrah, R., and Wild, C. (2012b). Budget of coral-derived organic carbon in a fringing coral reef of the Gulf of Aqaba, Red Sea. J. Mar. Syst. 105–108, 20–29. doi: 10.1016/j.jmarsys.2012.05.007
Nelson, C. E., Alldredge, A. L., McCliment, E. A., Amaral-Zettler, L. A., and Carlson, C. A. (2011). Depleted dissolved organic carbon and distinct bacterial communities in the water column of a rapid-flushing coral reef ecosystem. ISME J. 5, 1374–1387. doi: 10.1038/ismej.2011.12
Nelson, C. E., Goldberg, S. J., Wegley Kelly, L., Haas, A. F., Smith, J. E., Rohwer, F., et al. (2013). Coral and macroalgal exudates vary in neutral sugar composition and differentially enrich reef bacterioplankton lineages. ISME J. 7, 962–979. doi: 10.1038/ismej.2012.161
Pile, A. J., Patterson, M. R., and Witman, J. D. (1996). In situ grazing on plankton < 10 μm by the boreal sponge Mycale lingua. Mar. Ecol. Prog. Ser. 141, 95–102. doi: 10.3354/meps141095
Pogoreutz, C., Rädecker, N., Cárdenas, A., Gärdes, A., Wild, C., and Voolstra, C. R. (2017). Nitrogen fixation aligns with nifH abundance and expression in two coral trophic functional groups. Front. Microbiol. 8:1187. doi: 10.3389/fmicb.2017.01187
Pogoreutz, C., Rädecker, N., Cárdenas, A., Gärdes, A., Wild, C., and Voolstra, C. R. (2018). Dominance of Endozoicomonas bacteria throughout coral bleaching and mortality suggests structural inflexibility of the Pocillopora verrucosa microbiome. Ecol. Evol. 8, 2240–2252. doi: 10.1002/ece3.3830
Qurban, M. A., Balala, A. C., Kumar, S., Bhavya, P. S., and Wafar, M. (2014). Primary production in the northern Red Sea. J. Mar. Syst. 132, 75–82. doi: 10.1016/j.jmarsys.2014.01.006
Rahav, E., Herut, B., Mulholland, M. R., Belkin, N., Elifantz, H., and Berman-Frank, I. (2015). Heterotrophic and autotrophic contribution to dinitrogen fixation in the Gulf of Aqaba. Mar. Ecol. Prog. Ser. 522, 67–77. doi: 10.3354/meps11143
Rasheed, M., Al-Trabeen, K., and Badran, M. (2012). Long-term water quality monitoring at an industrial site on the Northern Gulf of Aqaba, Red Sea. Mediterr. Mar. Sci. 13, 250–258.
Rasheed, M., Badran, M. I., Richter, C., and Huettel, M. (2002). Effect of reef framework and bottom sediment on nutrient enrichment in a coral reef of the Gulf of Aqaba. Red Sea. Mar. Ecol. Ser. 239, 277–285. doi: 10.3354/meps239277
Redfield, A. (1958). The biological control of chemical factors in the environment. Am. Sci. 46, 205–221.
Rix, L., Bednarz, V. N., Cardini, U., Van Hoytema, N., Al-Horani, F. A., Wild, C., et al. (2015). Seasonality in dinitrogen fixation and primary productivity by coral reef framework substrates from the northern Red Sea. Mar. Ecol. Prog. Ser. 533, 79–92. doi: 10.3354/meps11383
Sheppard, C. R. C., Davy, S. K., and Pilling, G. M. (2009). The Biology of Coral Reefs. Oxford: Oxford University Press.
Shiozaki, T., Bombar, D., Riemann, L., Sato, M., Hashihama, F., Kodama, T., et al. (2018). Linkage between dinitrogen fixation and primary production in the oligotrophic South Pacific Ocean. Global Biogeochem. Cycles 32, 1028–1044. doi: 10.1029/2017GB005869
Silverman, J., Lazar, B., and Erez, J. (2007). Community metabolism of a coral reef exposed to naturally varying dissolved inorganic nutrient loads. Biogeochemistry 84, 67–82. doi: 10.1007/s10533-007-9075-5
Sorokin, Y. I. (1995). Role of plankton in the turnover of organic matter on the Great Barrier Reef, Australia. Hydrobiologia 308, 35–44. doi: 10.1007/BF00037785
Stihl, A., Sommer, U., and Post, A. F. (2001). Alkaline phosphatase activities among populations of the colony-forming diazotrophic cyanobacterium Trichodesmium spp. (Cyanobacteria) in the red sea. J. Phycol. 37, 310–317. doi: 10.1046/j.1529-8817.2001.037002310.x
Suggett, D. J., Stambler, N., Prášil, O., Kolber, Z., Quigg, A., Vázquez-Dominguez, E., et al. (2009). Nitrogen and phosphorus limitation of oceanic microbial growth during spring in the Gulf of Aqaba. Aquat. Microb. Ecol. 56, 227–239. doi: 10.3354/ame01357
Tac-An, N., Minh-Thu, P., Cherbadji, I. I., Propp, M. V., Odintsov, V. S., and Propp, L. H. (2013). Primary production of coral ecosystems in the Vietnamese coastal and adjacent marine waters. Deep Res. Part II Top. Stud. Oceanogr. 96, 56–64. doi: 10.1016/j.dsr2.2013.05.020
Tilstra, A., Bednarz, V. N., Cardini, U., Van Hoytema, N., Al-Rshaidat, M. M. D., and Wild, C. (2017). Seasonality affects dinitrogen fixation associated with two common macroalgae from a coral reef in the northern Red Sea. Mar. Ecol. Prog. Ser. 575, 69–80. doi: 10.3354/meps12206
Torréton, J. P., Rochelle-Newall, E., Pringault, O., Jacquet, S., Faure, V., and Briand, E. (2010). Variability of primary and bacterial production in a coral reef lagoon (New Caledonia). Mar. Pollut. Bull. 61, 335–348. doi: 10.1016/j.marpolbul.2010.06.019
van Hoytema, N. (2015). The Importance of Carbon Dioxide and Dinitrogen Fixation For Seasonal Coral Reef Metabolism: From Organism to Ecosystem Functioning. Bremen: University of Bremen.
van Hoytema, N., Bednarz, V. N., Cardini, U., Naumann, M. S., Al-Horani, F. A., and Wild, C. (2016). The influence of seasonality on benthic primary production in a Red Sea coral reef. Mar. Biol. 163, 1–14. doi: 10.1007/s00227-015-2787-5
White, A. E., Prahl, F. G., Letelier, R. M., and Popp, B. N. (2007). Summer surface waters in the Gulf of California: Prime habitat for biological N2 fixation. Global Biogeochem. Cycles 21, 1–11. doi: 10.1029/2006GB002779
Wild, C., Huettel, M., Klueter, A., and Kremb, S. G. (2004). Coral mucus functions as an energy carrier and particle trap in the reef ecosystem. Nature 428, 66–70. doi: 10.1038/nature02333.1
Wild, C., Naumann, M. S., Haas, A., Struck, U., Mayer, F. W., Rasheed, M. Y., et al. (2009). Coral sand O2 uptake and pelagic-benthic coupling in a subtropical fringing reef, Aqaba, Red Sea. Aquat. Biol. 6, 133–142. doi: 10.3354/ab00181
Wilson, S. T., Böttjer, D., Church, M. J., and Karl, D. M. (2012). Comparative assessment of nitrogen fixation methodologies, conducted in the oligotrophic north pacific ocean. Appl. Environ. Microbiol. 78, 6516–6523. doi: 10.1128/AEM.01146-12
Wu, C., Fu, F. X., Sun, J., Thangaraj, S., and Pujari, L. (2018). Nitrogen fixation by Trichodesmium and unicellular diazotrophs in the northern South China Sea and the Kuroshio in summer. Sci. Rep. 8, 1–12. doi: 10.1038/s41598-018-20743-0
Keywords: plankton, primary production, dinitrogen fixation, Gulf of Aqaba, diazotrophs
Citation: Tilstra A, van Hoytema N, Cardini U, Bednarz VN, Rix L, Naumann MS, Al-Horani FA and Wild C (2018) Effects of Water Column Mixing and Stratification on Planktonic Primary Production and Dinitrogen Fixation on a Northern Red Sea Coral Reef. Front. Microbiol. 9:2351. doi: 10.3389/fmicb.2018.02351
Received: 23 May 2018; Accepted: 12 September 2018;
Published: 01 October 2018.
Edited by:
Pia H. Moisander, University of Massachusetts Dartmouth, United StatesReviewed by:
Amy Apprill, Woods Hole Oceanographic Institution, United StatesEmilio Maranon, University of Vigo, Spain
Copyright © 2018 Tilstra, van Hoytema, Cardini, Bednarz, Rix, Naumann, Al-Horani and Wild. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arjen Tilstra, dGlsc3RyYUB1bmktYnJlbWVuLmRl
†These authors have contributed equally to this work
‡Senior author
 Arjen Tilstra
Arjen Tilstra Nanne van Hoytema2†
Nanne van Hoytema2† Ulisse Cardini
Ulisse Cardini Vanessa N. Bednarz
Vanessa N. Bednarz Laura Rix
Laura Rix Malik S. Naumann
Malik S. Naumann Fuad A. Al-Horani
Fuad A. Al-Horani Christian Wild
Christian Wild