- 1Jiangsu Key Laboratory for Microbes and Functional Genomics, Jiangsu Engineering and Technology Research Center for Industrialization of Microbial Resources, College of Life Science, Nanjing Normal University, Nanjing, China
- 2Jiangsu Key Laboratory for Food Quality and Safety-State Key Laboratory Cultivation Base, Ministry of Science and Technology, Key Laboratory for Agro-Product Safety Risk Evaluation (Nanjing), Ministry of Agriculture and Rural Affairs, Collaborative Innovation Center for Modern Grain Circulation and Safety, Institute of Food Safety and Nutrition, Jiangsu Academy of Agricultural Sciences, Nanjing, China
- 3Department of Agricultural Biotechnology, Seoul National University, Seoul, South Korea
- 4School of Food and Biological Engineering, Jiangsu University, Zhenjiang, China
Deoxynivalenol (DON), a common mycotoxin of type B trichothecene, is produced mainly by several Fusarium species. DON causes great losses in farming and poses severe safety risks to human and animal health. Thus, DON contamination in cereals and DON toxicity are of worldwide concern. In this study, we screened the bacterial consortium C20, which efficiently degraded almost 70 μg ml−1 DON within 5 days. The bacterial consortium also had the ability to degrade 15-acetyl-DON, 3-acetyl-DON, and T-2 toxin. The bacterial consortium C20 was able to degrade DON under a wide range of pH and temperature conditions. The optimal temperature and pH for DON degradation were 30°C and pH 8.0, respectively. The bacterial consortium C20 comprised of different bacterial genera, and several strains were found to significantly increase when cultured in Mineral Medium with 100 μg ml−1 DON based on the analysis of the sequences of the hypervariable V3-V4 region of the 16S rRNA gene. 3-keto-DON was confirmed as a degradation product of DON by liquid chromatography/time-of-flight/mass spectrometry (LC-TOF-MS) and nuclear magnetic resonance (NMR) analyses. The results indicated that the bacterial consortium C20 is a potential candidate for the biodegradation of DON in a safe and environmentally friendly manner.
Introduction
Mycotoxins have adverse effects on grain quality, resulting in economic losses, besides the threat to human and animal health. The main groups of mycotoxins in world-wide concern are aflatoxin, ochratoxin, patulin, fumonisin, trichothecene, deoxynivalenol, and zearalenone due to their toxicity and their contamination of cereals (James and Zikankuba, 2018). Among these mycotoxins, trichothecenes are mainly produced by a range of fungi, including Fusarium, Myrothecium, Trichoderma, and Stachybotrys (Pessu et al., 2011). Trichothecenes not only cause growth impairment and immune dysfunction at low doses but also contribute to diarrhea, emesis, leukocytosis, hemorrhage, endotoxemia, and death at higher doses (Ueno, 1983). Deoxynivalenol (DON), also known as vomitoxin, produced by Fusarium graminearum and Fusarium culmorum, is one of the most important type B-trichothecene mycotoxins (Sobrova et al., 2010). It causes acute toxicity, vomiting, anorexia, growth retardation, reproductive and teratogenic effects, skin disorders, diarrhea, carcinogenesis, and even immune dysregulation (Pestka, 2010; Sobrova et al., 2010). DON is a frequent contaminant of grain cereals, such as wheat, maize, and barley, which can be also detected in the foods of many countries (Thuvander et al., 2001; Rasmussen et al., 2003; Leblanc et al., 2005; Schollenberger et al., 2005; Castillo et al., 2008; Sugiyama et al., 2009; Alkadri et al., 2014; Stanciu et al., 2016; Shi et al., 2018). Since DON is commonly detected in grains and is chemically stable, it represents a permanent health risk to humans and domestic animals. Therefore, the development of efficient decontamination strategies is essential to DON in grains and agricultural products.
Several strategies have been developed to control DON contamination. Firstly, cultural practices may help to combat plant diseases and fungal growth, such as the multi-field rotation of crops, the selection of seeds that are resistant to Fusarium head blight, and the early harvest of crops (Awad et al., 2010). The suitable storage of grains after harvest can also help to avoid increased DON contamination (Shapira et al., 2004). Physical methods, such as damaged grain removal, sieving, dehulling, radiation, and adsorbent use, were successful in detoxifying DON in cereal crops (Leibetseder, 2006). A wide variety of chemical methods have also been found to be effective against DON, including heating, alkaline hydrolysis, and oxidation (Young et al., 1986; He et al., 2010).
Compared with physical and chemical detoxification, microbial biotransformation attracted considerable interests due to high degradation efficiency, the metrics of mild reaction condition, sustainable and eco-friendly process (Awad et al., 2010). Several studies have demonstrated that DON could be metabolized to lower toxicity compounds performed by several microorganisms. For instance, DON was found to be converted into 3-keto-DON under aerobic conditions by the Agrobacterium-Rhizobium strain E3–39, isolated from soil samples (Shima et al., 1997). Zhou et al. revealed that two metabolites of DON during degradation by Barpee were identified as stereoisomers of DON and 3-keto-DON (Zhou et al., 2008). In addition, microbial consortia have shown the potential to degrade DON. One mixed culture was able to convert DON into two chromatographically separable metabolites (Andrea et al., 2004). Chicken intestinal microbes were also capable of degrading DON by de-epoxidation (Young et al., 2007).
Compared with single bacterium and anaerobic microbes, bacterial consortia with ability of DON degradation under aerobic conditions are rarely reported. Moreover, the DON degradation conditions of these anaerobic bacteria were still limited. In the present study, we isolated a novel bacterial consortium C20 from the wheat field in Jiangsu Province, China, which can stably and efficiently degrade DON into 3-keto-DON under a wide range of pH and temperature with aerobic condition. Further, the performance of biodegradation of other trichothecene mycotoxins was also evaluated.
Materials and Methods
Samples
A total of 174 samples from different locations, including soil, rice, corn, wheat, rice panicles and fresh corn leaves, were collected during 2016–2017 in Jiangsu Province, China (Table 1). Notably, soil samples were randomly collected from the upper layers (0–30 cm) of different crop fields, such as rice, soybean, corn, and winter wheat fields.
Culture Media and Mycotoxins
Minimal medium (MM) was used as a culture medium for the enrichment and isolation of DON-degrading strains. The composition of MM (pH 7.0) was described by Wang et al. (2019b). Ingredients were purchased from Sigma-Aldrich (Oakville, Ontario, Canada), Guanghua Sci-Tech Co., Led. (Shantou, Guangdong, China), XILONG SCIENTIFIC (Shantou, Guangdong, China), and Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Toxin standards for DON, HT-2, T-2, 3-acetyldeoxynivalenol (3-ADON), and 15-acetyldeoxynivalenol (15-ADON) were purchased from Romer Labs (Erber Campus, Getzersdorf, Austria).
Selection of Deoxynivaleno-Degrading Microbes
Five-gram aliquots of each sample from the 174 field samples were smashed by a pulverizer (JYL-c020E, Joyoung, China), dispersed in 50 ml distilled water, and cultured with shaking at 180 rpm for 30 min. About 500 μl of the suspension was immediately transferred to 4.5 ml MM broth media with DON (10 μg ml−1) as the sole carbon source. Tebuconazole and Carbendazim (1 or 10 μg ml−1) were added in the cultures to inhibit fungal growth, respectively. The cultures were grown at 30°C, 180 rpm. To exclude physical adsorption, samples autoclaved at 121°C for 20 min were set as negative controls. The extent of DON biotransformation was determined by high-performance liquid chromatography (HPLC). Then, the cultures with the ability of DON degradation were further inoculated on the MM broth containing 10 μg ml−1 DON at intervals of 7 days. To investigate DON degradation activity, the cultures were used to degrade 100 μg ml−1 DON. MM with DON (100 μg ml−1) was set as negative control. The bacterial growth was measured by optical density. The changes in DON concentration and OD 600 values were monitored every day.
Sample Preparation and High-Performance Liquid Chromatography Analyses
DON was extracted from fermentation broth with an equal volume of ethyl acetate for three times. After dried under an N2 stream, it was re-dissolved in methanol and quantified by HPLC (Waters 2695, Milford, USA) equipped with a PDA (photo-diode array) detector at a wavelength of 218 nm and an Atlantis T3 column (octadecylsilyl, ODS; 4.6 mm × 150 mm, 3 μm; Waters), as described previously (Wang et al., 2019b). The mobile phase was methanol/water (20/40, v/v) with a flow rate of 0.6 ml min−1 at 35°C. The identification of mycotoxin was achieved by comparing the retention times while using DON as a standard. DON and degradation products were quantified by measuring peak areas and comparing with MS calibration curves. All data are presented as mean ± standard deviation of three replicates. Degradation percentage of DON was calculated as follows:
where Dr represents the degradation percentage, and Ct and Cck are the DON concentrations in the experimental treatment and the control groups, respectively (Wang et al., 2019b).
Analysis of Bacterial Population Diversity
To analyze the bacterial population diversity and its change at high concentrations of DON, the enriched microbial consortium C20 was isolated from environmental samples and was added to MM to degrade DON, with DON concentrations of 10 and 100 μg ml−1. The bacteria were incubated in media with different concentrations of DON at 30°C for 72 h. Genomic DNA of the enriched microbial cultures was extracted and purified with Gentra Puregene Yeast/Bacteria Kit (Qiagen Inc., Mississauga, Ontario, Canada). The hypervariable V3-V4 region of the 16S rRNA gene was amplified by PCR with primers 341F (CCTACGGGNGGCWGCAG) and 805R (GACTACHVGGGTATCTAATCC) (Ahad et al., 2017) and sequenced by Sangon Biotech Co. (Shanghai, China). Further, the 16S rRNA (V3-V4 region) gene sequences were compared with the ribosomal database project (RDP)1. All treatments were conducted in triplicates.
Factors Influencing Deoxynivaleno Degradation
To screen for optimal degrading conditions of DON by C20, the degradation rates of DON were determined at the different conditions including temperature, pH, and bacterial concentrations. An aliquot (200 μl) of each microbial culture was added to 1.8 ml of MM supplemented with 10 μg ml−1 DON. The effect of incubation temperature (20, 25, 30, 35, and 40°C), medium pH (4.0, 5.0, 6.0, 7.0, 8.0, and 9.0), and inoculation size (1, 3, 5, 10, 15, and 20%) on biodegradation of DON were investigated. All treatments were performed by three independent experiments with three replicates. The optimal degradation conditions of temperature, pH, and inoculation amount were determined by the degradation rates of DON.
Type A- and B-Trichothecene Mycotoxins Degradation
To examine the ability of C20 to transform type A- and B-trichothecene mycotoxins, which included two type A-trichothecene mycotoxins (HT-2 toxin and T-2 toxin) and three type B-trichothecene mycotoxins (DON, 3-ADON, and 15-ADON), 10 μg ml−1 of each mycotoxin was added to MM in 2 ml microcentrifuge tubes. Each tube was inoculated with 200 μl of C20, which was cultured in MM with 10 μg ml−1 DON for 72 h. Three replicate cultures were evaluated for each trichothecene mycotoxin, and the negative controls lacked C20. All cultures were incubated at 30°C and 180 rpm in a shaking incubator for 72 h, extracted three times with ethyl acetate, as described, re-dissolved in methanol and analyzed by liquid chromatography with tandem mass spectrometry (LC-MS/MS).
The LC-MS/MS system was equipped with a Shimadzu 20 AD XR (Kyoto, Japan) coupled with an AB Sciex QTRAP 3500 mass spectrometer (Foster City, CA, USA) and an XDB-C18 analytical column (2.1 mm × 150 mm, 3.5-μm bead diameter; Agilent) maintained at 30°C. Nitrogen was used as the drying gas. The capillary voltage was 4 kV, the nebulizer pressure was 30 psi, and the drying gas temperature was 300°C. The gradient elution conditions were methanol/water (5/95, v/v) to methanol/water (90/10, v/v) in 10 min and were held at 90% methanol for 3 min, the flow rate was set at 0.2 ml min−1 (Wang et al., 2019b). Analyst 1.6.1 software was used for data acquisition and processing.
Identification of Deoxynivaleno Degradation Products
To analyze the transformation of DON, C20 was added to broth containing 10 μg ml−1 DON and incubated at 30°C on a rotary shaker (180 rpm) for 72 h. Culture broth containing C20 alone was used as a control. Samples were prepared as described above and detected by HPLC. The mobile phase was methanol/water (50:50, v/v) with the flow rate of 0.8 ml min−1.
To investigate the structure of DON degradation products, C20 was inoculated into MM medium with 100 μg ml−1 DON and cultured for 72 h at 30°C with shaking at 180 rpm. The DON degradation products were extracted three times with ethyl acetate. The organic phase was pooled, concentrated and applied to a Waters 1525 Prep-HPLC system (Milford, MA, USA) equipped with a UV detector. Each 20 μl extract was injected onto an XBridge™ Prep C18 column (19 mm × 100 mm, 5 μm, Waters) with methanol/water (50:50, v/v) at a flow rate of 2 ml min−1. DON and its degradation products were detected at a wavelength of 218 nm at 35°C. The resulting degradation products were dried under an N2 stream and re-dissolved in CDCl3 for nuclear magnetic resonance (NMR) analysis.
The 1H NMR spectra of the biodegradation products were measured by a Bruker DRX-600 spectrometer operated at 600 MHz. The chemical shift (δ) was recorded in parts per million (ppm) relative to the solvent signals [δ(H) 7.26], and the coupling constant (J) was measured in Hz.
Data Analysis
Data were organized using Microsoft Excel 2010. All values are expressed as the mean of three replicates ± standard deviation. The mean differences among treatments were considered significantly at p < 0.05 and were evaluated with Tukey’s-honestly significant difference (HSD) test (Ahad et al., 2017).
Results
Isolation of Deoxynivaleno-Degrading Bacteria
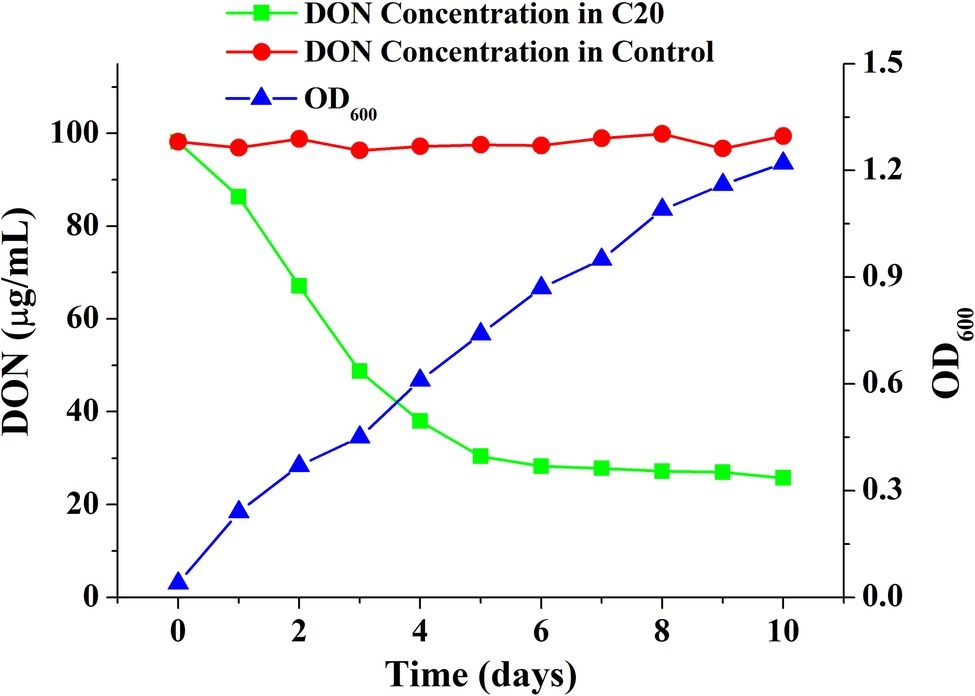
To screen for the microbes that degraded DON, DON was used as the sole carbon source for the enrichment and isolation of the mixed culture. The mixed microbial culture C20, isolated from a soil sample from Suqian, degraded 74.29 μg ml−1 DON after incubation at 30°C for 10 days. DON was degraded rapidly within 5 days of incubation and was continuously degraded after incubation for 10 days. The biomass increased continuously with the amount of DON decreased (Figure 1). Furthermore, no difference was found between the samples with and without fungal inhibitors. And the DON concentration in the autoclaved sample was not reduced (Supplementary Figure S5). Importantly, the enriched bacterial consortium C20 could efficiently degrade DON, even after more than 100 subcultures in broth (Supplementary Figure S3).
Bacterial Diversity Analysis of the Microbial Consortium C20
To examine mainly bacteria involved in DON degradation, the microbial diversity dynamics of C20 with different concentrations of DON was defined through analyzing the sequences of the 16S rRNA genes, as described in methods. The results showed that the enriched bacterial consortium C20 mainly consisted of the genera Hyphomicrobium (69.54%), followed by Acidovorax (12.09%), unknown microbes (7.04%), Prosthecomicrobium (2.71%), Pseudomonas (2.68%), and Methylophilus (2.31%) in MM with 10 μg ml−1 DON (Figure 2A). In contrast, the culture in MM with 100 μg ml−1 DON contained a heterogeneous community composed of Methylophilus (35.48%), followed by Hyphomicrobium (27.71%), Ancylobacter (14.73%), Pseudomonas (4.87%), Prosthecomicrobium (4.6%), unknown microbes (2.39%), Taonella (2.33%), Bosea (2.33%), and others (Figure 2B). Noticeably, as the DON concentration increased to 100 μg ml−1, there was a marked increase in Methylophilus, Ancylobacter, Pseudomonas, Prosthecomicrobium, Taonella, Bosea, Terrimonasin, and Devosia in C20.
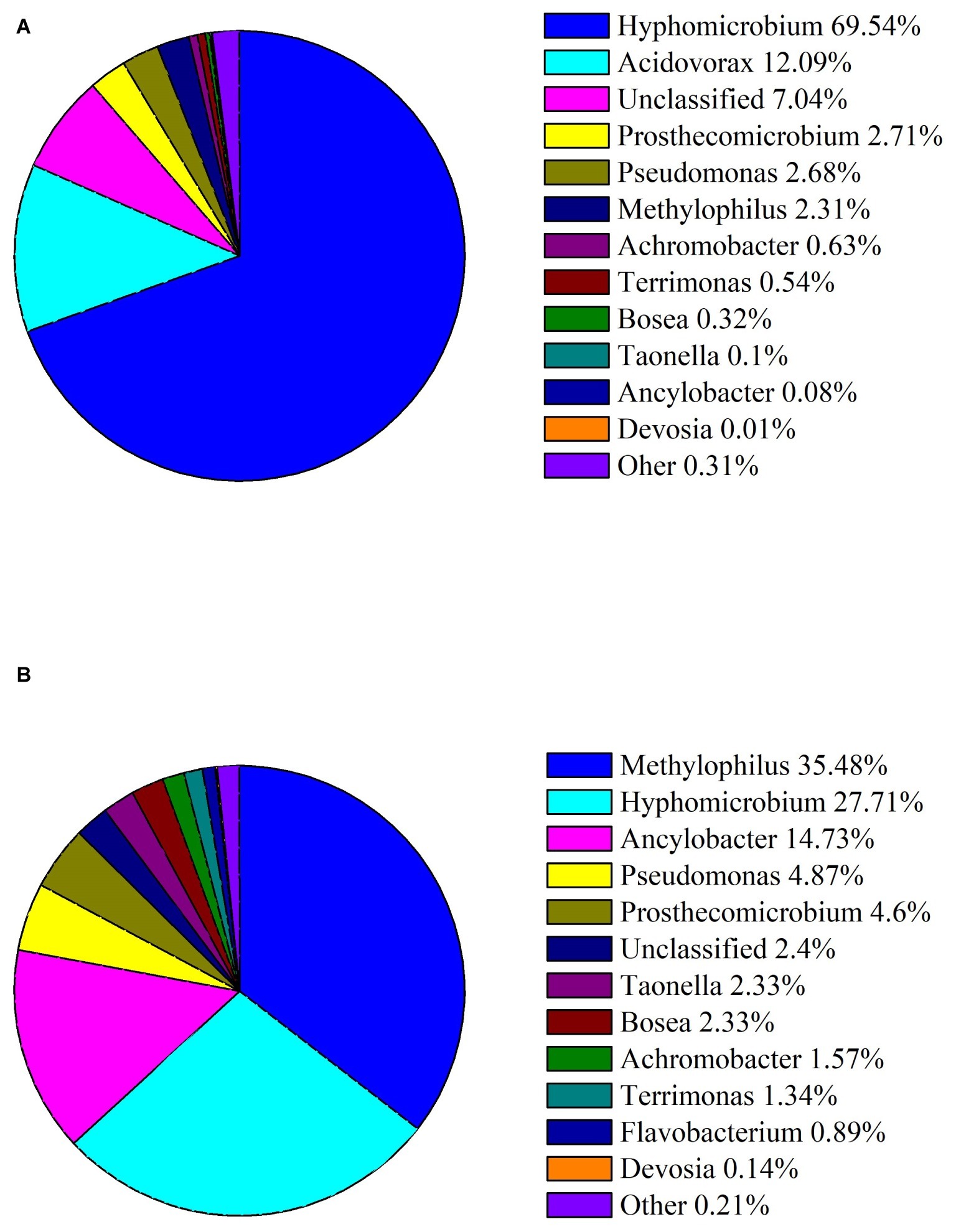
Figure 2. Abundances of bacterial genera in the enriched culture C20. (A) Relative abundance of bacterial genera in C20 in MM with 10 μg ml−1 DON. (B) Relative abundance of bacterial genera in C20 in MM with 100 μg ml−1 DON. The different colors and areas represent different bacterial genera and their relative abundance present in C20.
Factors Influencing Deoxynivaleno Degradation
The effects of the temperature, pH, and inoculum on DON degradation by C20 were investigated (Figure 3). DON degradation activity was examined at various temperatures from 20 to 40°C in the enriched cultures (Figure 3A). The highest degradation rate occurred at 30°C and decreased only slightly at 25 and 35°C. Importantly, approximately 50% of DON degradation was observed even at 40°C, indicating that the consortium C20 can effectively degrade DON over a wide range of temperatures (25–35°C). Moreover, the effects of pH on DON degradation was detected under six different pH levels conditions. The degradation rate of DON increased markedly from pH 4.0 to 8.0. The maximum DON degradation rate was observed at pH 8.0, and the degradation for 10 μg ml−1 DON at pH 9.0 was reduced to 70.08% (Figure 3B). The inoculum size significantly affected the degradation of DON. The degradation rates of 10 μg ml−1 DON after 72 h of incubation increased from 30 to 85% when the inoculum size was raised from 1 to 10% (Figure 3C).
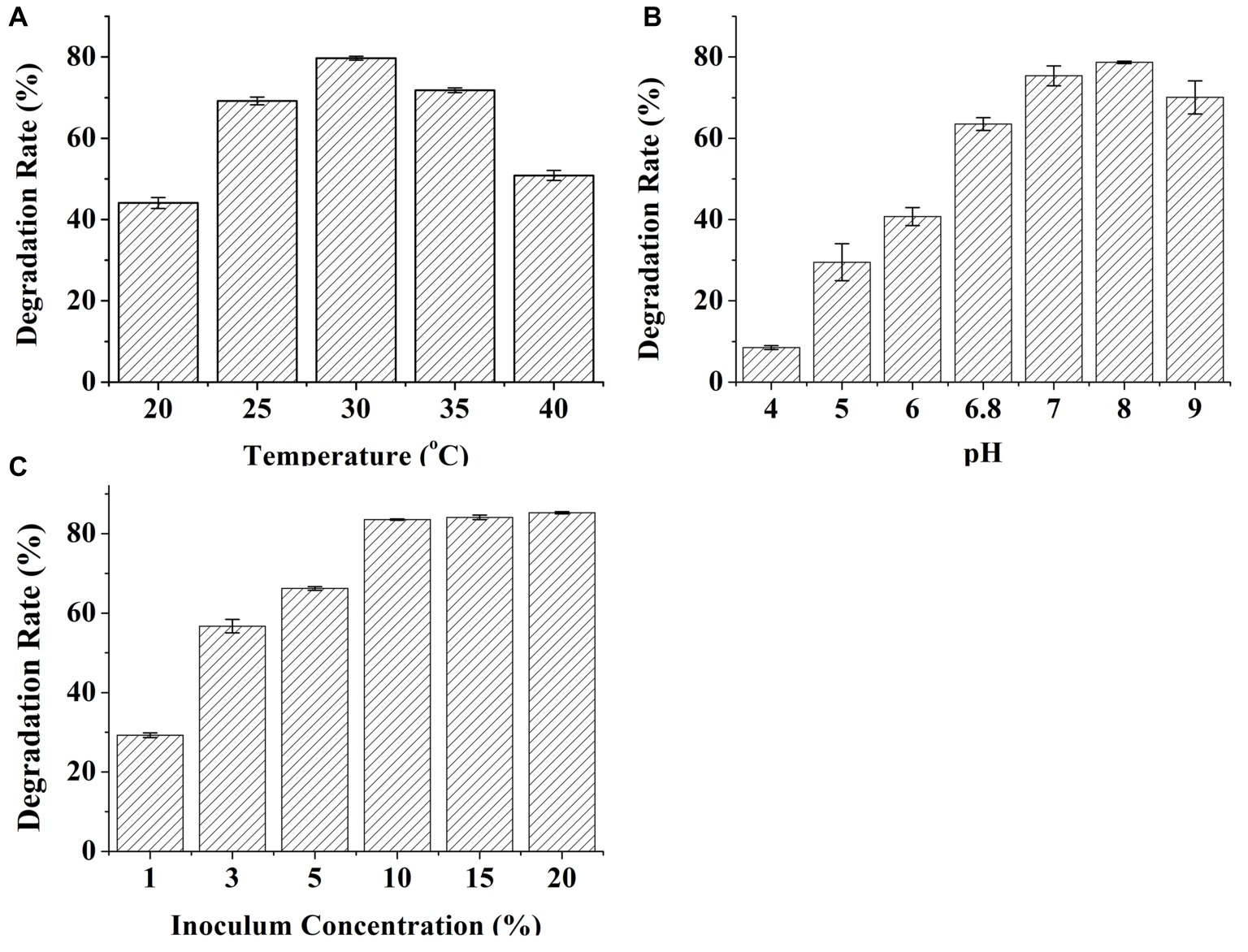
Figure 3. Effects of temperature, pH, and inoculum concentrations on the degradation of DON by C20. (A) The effect of incubation temperature on the degradation of DON. (B) The effect of pH on the degradation rate of DON. (C) The effect of inoculum concentrations on the degradation of DON. Results are the mean of five replicate observations, and bars shown are ± standard errors of the means.
Degradation of Type A- and B-Trichothecene Mycotoxins by Consortium C20
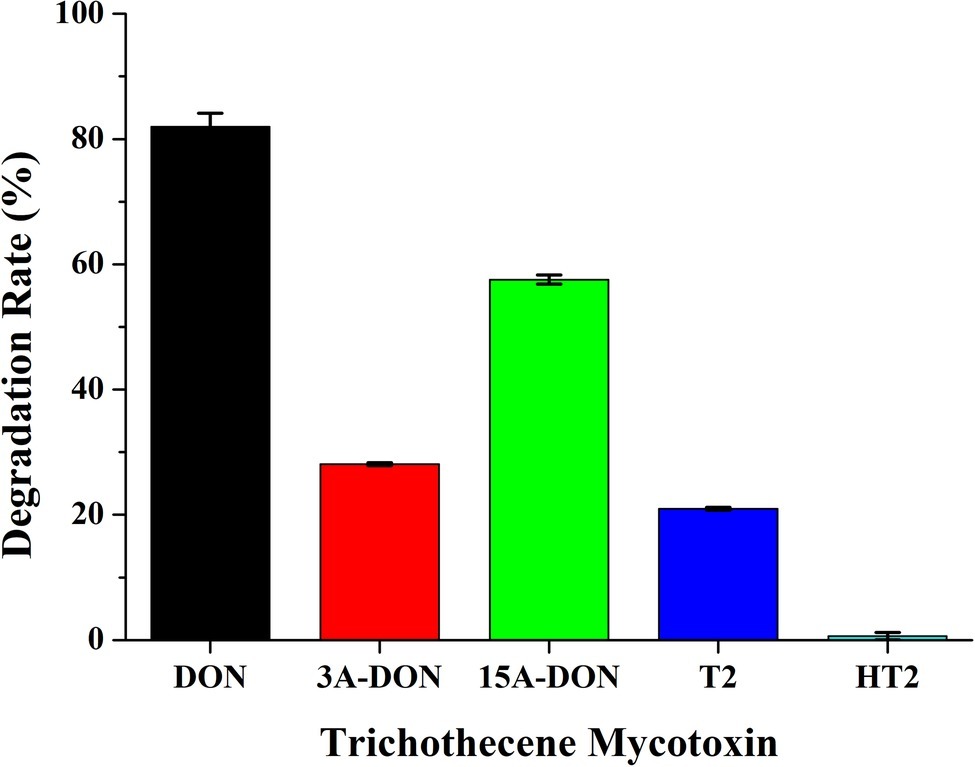
To investigate the degradation of other trichothecene mycotoxins by C20, the consortium C20 was incubated with T-2, HT-2, 3-ADON, and 15-ADON for 72 h. The results demonstrated that the consortium C20 degraded 58% of 15-ADON, 28% of 3-ADON, and 21% of T-2. However, the degradation of HT-2 was not observed (Figure 4). 15-ADON and 3-ADON are the main acetylated derivatives of DON, and the degradation of 15-ADON and 3-ADON indicates that C20 possesses the ability to degrade acetylated DON derivatives (Payros et al., 2016).
Identification of Deoxynivaleno Degradation Products
The metabolic products of DON were identified by using HPLC, LC-TOF-MS, and NMR. HPLC analysis showed that DON was transformed to another metabolite during degradation by the enriched bacterial consortium C20 (Compound P, Figure 5). LC-TOF-MS results (Supplementary Figure S1) showed that the molecular ion of the metabolite was at m/z 295.1151 ([M + H]+, C15H19O6+; calcd. 295.1182), which indicated a di-dehydrogenation of the DON molecule. The 1H-NMR spectrum for compound P (Supplementary Figure S2) was 6.55 (dd, J = 6.0, 1.5), 4.91 (d, J = 1.3), 4.57 (d, J = 6.0), 3.91 (d, J = 11.6), 3.81 (d, J = 1.5), 3.75 (d, J = 11.6), 3.52 (s), 3.36 (d, J = 4.2), 3.23 (d, J = 4.2), 3.13 (d, J = 19.5), 2.29 (d, J = 19.5), 1.90 (3H, s), and 1.34 (3H, s). The chemical formula and the 1H-NMR signals of compound P were identified as 3-keto-DON (Hassan et al., 2017; Wang et al., 2019b).
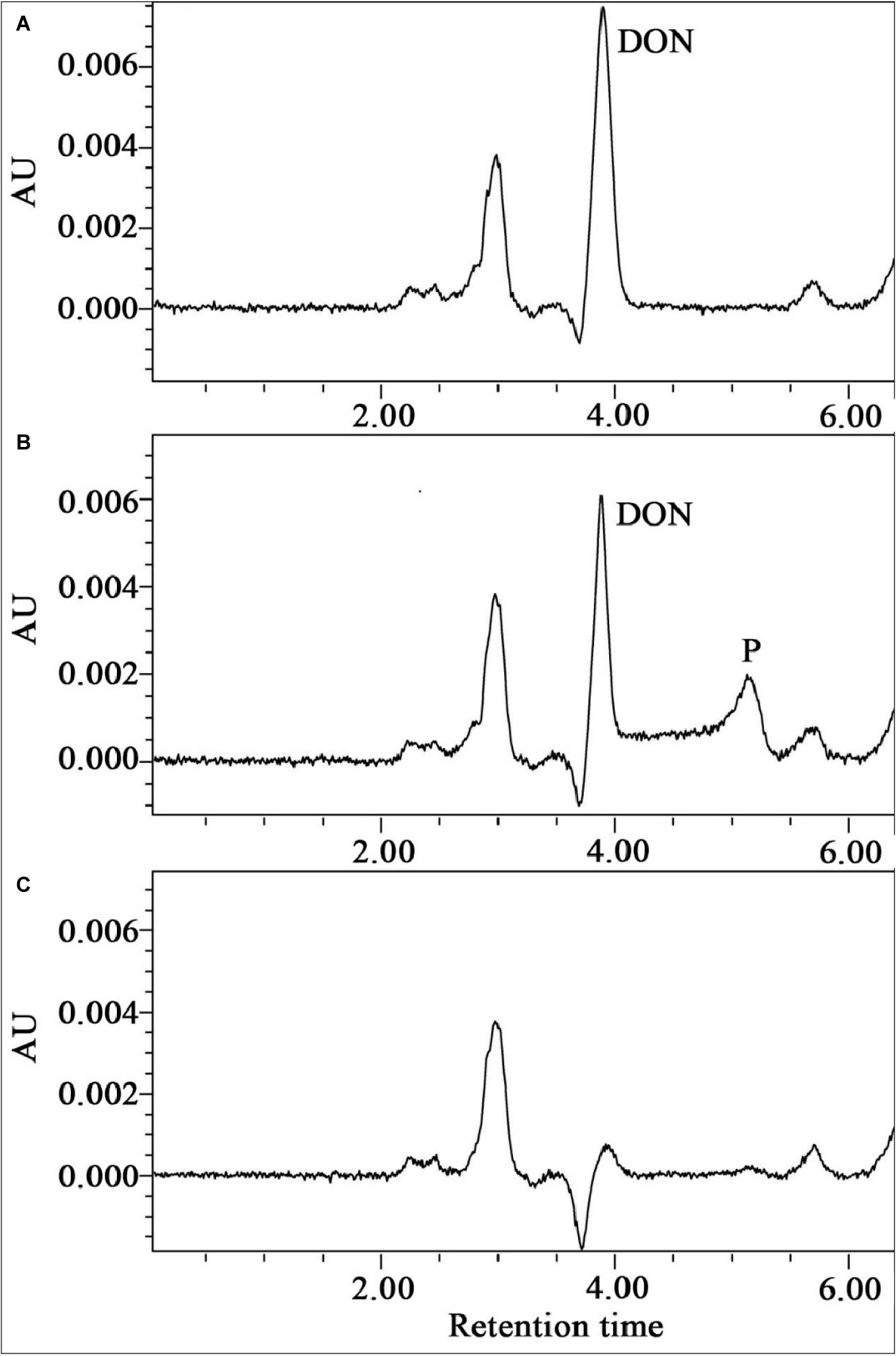
Figure 5. The HPLC spectra of the metabolism of DON by the enriched culture C20. (A) Substrate control broth containing the substrate DON alone. (B) Broth containing DON and C20. (C) Culture control broth containing C20 alone.
Discussion
DON is one of the most commonly detected contaminants of crops in worldwide, such as maize, wheat, and rice (Lee and Ryu, 2015). The widespread occurrence of this mycotoxin, coupled with the detriments to human health and animal productivity resulting from its consumption, has promoted intensive efforts to identify detoxification measures, including microbial biotransformation (Pestka, 2010; Sobrova et al., 2010; Kris et al., 2014). Although several single microbial strains for the degradation of DON were isolated, there are still many limitations to application for agricultural fields because of the diversity and complexity of crop cultivation and storage environments (Brenner et al., 2008). Fortunately, microbial consortia provide a promising application for mycotoxin decontamination owing to their special advantages in the degradation of complex compounds. Microbial consortia combine multiple metabolic capacities of different species, improving the ability and efficiency of complex substrates biotransformation process, as well as the tolerance of complex environments (Shong et al., 2012). To date, microbial consortia has been widely used for a variety of important processes, such as the treatment of wastewater (Daims et al., 2006), the biosynthesis of compounds for medicine and industry (Shong et al., 2012), the biodegradation of bispyribac-sodium and phenanthrene (Janbandhu and Fulekar, 2011; Ahmad et al., 2018), and the production of hydrogen (Zhang et al., 2017), due to their high adaptability and broad substrate spectra (Kleerebezem and Van Loosdrecht, 2007). However, few attempts have been made to degrade DON by a microbial consortium. In this study, the highly enriched, stable and effective microbial consortium C20 was found to be able to degrade DON, resulting in a significant reduction in DON (Figure 1). In addition, tebuconazole and carbendazim have no effects on the degradation of DON by the enriched culture, indicating that the degradation of DON is owing to bacteria. Furthermore, there was no change in DON concentration in the autoclaved sample, suggesting that the soil sample cannot detoxify DON by physical adsorption (Supplementary Figure S5).
The characterization of DON degradation in this study showed the activity of the microbial culture at a wide range of pH (6.0–9.0) and temperatures (25–35°C) compared with previously studied microbial cultures (Guan et al., 2009; Wang et al., 2019b). The microbial consortium C20 might degrade DON in complex and diverse environments. The optimal pH (8.0) and temperature (30°C) of the microbial consortium C20 for DON degradation are similar to that of most arable plants. It has been suggested that microbial consortia have potential applications for the detoxification of DON in field crops (Karlovsky, 1999; Islam et al., 2012).
The functional and stability of microbial consortium are usually modulated through interactions among microorganisms. These interactions can be indirect, such as exchanging different metabolites and information signals (Wintermute and Silver, 2010). For example, Bacillus cereus A1 and Brevundimonas naejangsanensis B1 mutually cooperate to enhance hydrogen production and starch utilization. In this consortium, strain A1 produced lactate as carbon source for strain B1 to cell growth and hydrogen production. In return, formate produced by strain B1 as an electron shuttle for strain A1 to generate hydrogen (Wang et al., 2019a). In direct interactions, microorganisms exchange electrons or deliver macromolecules such as DNA and proteins by direct interspecies contact. Rotaru et al. reported that besides H2 or formate as the electron donor for interspecies electron transfer, direct electrical connections was also found in the production of methane between Geobacter metallireducens and Methanosaeta harundinacea (Rotaru et al., 2014).
As the sole carbon, DON provided more substrate to the microbial consortium C20 in MM with 100 μg ml−1 DON than in the original culture. The increasement of DON might enhance the proportion of DON-degraders during the consecutive enrichment, resulting in a change in the community composition (Ahad et al., 2017). Strains belonging to Methylophilus, Ancylobacter, Pseudomonas, Prosthecomicrobium, Bosea, Taonella and Devosia were significantly increased (p < 0.05, one-way ANOVA) in C20 after enrichment with 100 μg ml−1 DON. These results suggest that the changes in these bacterial populations might be related to the microbial degradation of DON. It was demonstrated that many bacteria isolated for the degradation of DON were identified as Devosia strains (Sato et al., 2012; Onyango et al., 2014; Yin et al., 2016; Zhao et al., 2016; Hassan et al., 2017). Moreover, DON was transformed by these bacteria to 3-epi-DON, 3-keto-DON, or other products. Therefore, the Devosia strains in C20 have great potential for DON degradation.
The 3-OH group in DON, as well as the epoxide group on the trichothecene backbone, is responsible for its toxicity. When DON was epimerized or degraded, its interaction with ribosome will be changed, leading to an absence of MAP kinase activation and toxicity reduction. It was reported that the intestinal toxicity of DON would be decreased when it was converted into depoxy-DON or 3-epi-DON by microbial transformation (Pierron et al., 2016). In this study, 3-keto-DON was found to the main degradation product of DON analyzed by HPLC, LC-TOF-MS and NMR. It is all known that the oxidation of DON to 3-keto-DON plays a vital role in the detoxification of DON-contaminated grains (Shima et al., 1997). In addition, the 3-keto-DON showed a reduction of more than 90% in immunosuppressive toxicity compared with that of DON (He et al., 2015).
A DON degrading bacterial strain was isolated from the consortium C20, but the strain did not maintain its DON degradation activity after a few generations of culture in broth medium (Supplementary Figure S4). It is still challenging to isolated DON-degrader through selective enrichment procedures because the degradation reaction involves the removal of an oxygen molecule (Islam et al., 2012; Yoshizawa et al., 2014), which does not supply any nutrients for bacteria (Horvath, 1972; Fedorak and Grbić Galić, 1991) and prevents the utilization of DON as the sole carbon or nitrogen source for the selective isolation of degrader from the consortium C20 (Ahad et al., 2017).
The enriched culture C20 had the ability to degrade 15-ADON, 3-ADON and T-2, as well as DON in this study. Previous studies demonstrated that 15-ADON is higher toxicity than DON or 3-ADON (Pinton et al., 2012; Kadota et al., 2013). Moreover, both DON and 15-ADON are frequently found in wheat and other cereals (Han et al., 2014; Dong et al., 2016). Thus, the microbial consortium C20 has great potential to degrade DON and 15-ADON in agricultural products.
In conclusion, the enriched bacterial consortium C20, which can efficiently degrade DON and its derivatives 15-ADON and 3-ADON through the combined strengths of individual organisms, was isolated from environmental samples in this study. C20 is comprised of different bacterial genera, with Methylophilus, Ancylobacter, and Devosia significantly increasing in cultures with high concentrations of DON. Importantly, about 70 μg ml−1 DON could be degraded by consortium C20 within 5 days under a wide range of pH and temperature conditions. And the final degradation product was identified as 3-keto-DON with less toxicity. These results suggest that the C20 microbial consortium provides a new potential method for DON degradation. Extending DON degradation capabilities from single bacteria to microbial consortia represents a new frontier in DON microbial biotransformation. Although stable community behavior remains a great challenge in this area, it still has great potential to be used to degrade DON in food/feedstuffs and to improve food/feed quality and safety.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
YaW performed experiments and wrote the paper. GW, YuW, and Y-WL designed and performed experiments. JX, JS, and YD designed experiments, supervised the students, and wrote the paper. All authors have approved the final version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (31872914, U1604234), Jiangsu Provincial Natural Science Foundation (BK20160595), Jiangsu Agriculture Science and Technology Innovation Fund (CX(17)1003), The Special Fund for Risk Assessment of China (GJFP2019002), and International Science and Technology Cooperation Program of China (2016YFE0112900).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.02964/full#supplementary-material
Footnotes
References
Ahad, R., Zhou, T., Lepp, D., and Pauls, K. P. (2017). Microbial detoxification of eleven food and feed contaminating trichothecene mycotoxins. BMC Biotechnol. 17:30. doi: 10.1186/s12896-017-0352-7
Ahmad, F., Anwar, S., Firdous, S., Da-Chuan, Y., and Iqbal, S. (2018). Biodegradation of bispyribac sodium by a novel bacterial consortium BDAM: optimization of degradation conditions using response surface methodology. J. Hazard. Mater. 349, 272–281. doi: 10.1016/j.jhazmat.2017.12.065
Alkadri, D., Rubert, J., Prodi, A., Pisi, A., Mañes, J., and Soler, C. (2014). Natural co-occurrence of mycotoxins in wheat grains from Italy and Syria. Food Chem. 157, 111–118. doi: 10.1016/j.foodchem.2014.01.052
Andrea, V. L., Bernhard, V., Margit, S., and Petr, K. (2004). Microbial detoxification of mycotoxin deoxynivalenol. J. Basic Microbiol. 44, 147–156. doi: 10.1002/jobm.200310353
Awad, W. A., Ghareeb, K., Böhm, J., and Zentek, J. (2010). Decontamination and detoxification strategies for the Fusarium mycotoxin deoxynivalenol in animal feed and the effectiveness of microbial biodegradation. Food Addit. Contam. 27, 510–520. doi: 10.1080/19440040903571747
Brenner, K., You, L., and Arnold, F. H. (2008). Engineering microbial consortia: a new frontier in synthetic biology. Trends Biotechnol. 26, 483–489. doi: 10.1016/j.tibtech.2008.05.004
Castillo, M., Montes, R., Navarro, A., Segarra, R., Cuesta, G., and Hernández, E. (2008). Occurrence of deoxynivalenol and nivalenol in Spanish corn-based food products. J. Food Compos. Anal. 21, 423–427. doi: 10.1016/j.jfca.2008.03.009
Daims, H., Taylor, M. W., and Wagner, M. (2006). Wastewater treatment: a model system for microbial ecology. Trends Biotechnol. 24, 483–489. doi: 10.1016/j.tibtech.2006.09.002
Dong, F., Qiu, J., Xu, J., Yu, M., Wang, S., Sun, Y., et al. (2016). Effect of environmental factors on Fusarium population and associated trichothecenes in wheat grain grown in Jiangsu province, China. Int. J. Food Microbiol. 230, 58–63. doi: 10.1016/j.ijfoodmicro.2016.04.020
Fedorak, P. M., and Grbić Galić, D. (1991). Aerobic microbial cometabolism of benzothiophene and 3-methylbenzothiophene. Appl. Environ. Microbiol. 57, 932–940. doi: 10.1002/bit.260370814
Guan, S., He, J. W., Young, J. C., Zhu, H. H., Li, X. Z., Ji, C., et al. (2009). Transformation of trichothecene mycotoxins by microorganisms from fish digesta. Aquaculture 290, 290–295. doi: 10.1016/j.aquaculture.2009.02.037
Han, Z., Nie, D., Ediage, E. N., Yang, X., Wang, J., Chen, B., et al. (2014). Cumulative health risk assessment of co-occurring mycotoxins of deoxynivalenol and its acetyl derivatives in wheat and maize: case study, Shanghai, China. Food Chem. Toxicol. 74, 334–342. doi: 10.1016/j.fct.2014.10.018
Hassan, Y. I., He, J. W., Perilla, N., Tang, K., Karlovsky, P., and Zhou, T. (2017). The enzymatic epimerization of deoxynivalenol by Devosia mutans proceeds through the formation of 3-keto-DON intermediate. Sci. Rep. 7:6929. doi: 10.1038/s41598-017-07319-0
He, J. W., Bondy, G. S., Zhou, T., Caldwell, D., Boland, G. J., and Scott, P. M. (2015). Toxicology of 3-epi-deoxynivalenol, a deoxynivalenol-transformation product by Devosia mutans 17-2-E-8. Food Chem. Toxicol. 84, 250–259. doi: 10.1016/j.fct.2015.09.003
He, J., Zhou, T., Young, J. C., Boland, G. J., and Scott, P. M. (2010). Chemical and biological transformations for detoxification of trichothecene mycotoxins in human and animal food chains: a review. Trends Food Sci. Technol. 21, 67–76. doi: 10.1016/j.tifs.2009.08.002
Horvath, R. S. (1972). Microbial co-metabolism and the degradation of organic compounds in nature. Bacteriol. Rev. 36, 146–155.
Islam, R., Goodwin, P. H., and Pauls, K. P. (2012). Aerobic and anaerobic de-epoxydation of mycotoxin deoxynivalenol by bacteria originating from agricultural soil. World J. Microbiol. Biotechnol. 28, 7–13. doi: 10.1007/s11274-011-0785-4
James, A., and Zikankuba, V. L. (2018). Mycotoxins contamination in maize alarms food safety in sub-Sahara Africa. Food Control 90, 372–381. doi: 10.1016/j.foodcont.2018.03.018
Janbandhu, A., and Fulekar, M. H. (2011). Biodegradation of phenanthrene using adapted microbial consortium isolated from petrochemical contaminated environment. J. Hazard. Mater. 187, 333–340. doi: 10.1016/j.jhazmat.2011.01.034
Kadota, T., Furusawa, H., Hirano, S., Tajima, O., Kamata, Y., and Sugitakonishi, Y. (2013). Comparative study of deoxynivalenol, 3-acetyldeoxynivalenol, and 15-acetyldeoxynivalenol on intestinal transport and IL-8 secretion in the human cell line Caco-2. Toxicol. In Vitro 27, 1888–1895. doi: 10.1016/j.tiv.2013.06.003
Karlovsky, P. (1999). Biological detoxification of fungal toxins and its use in plant breeding, feed and food production. Nat. Toxins 7, 1–23. doi: 10.1002/(SICI)1522-7189(199902)7:1<1::AID-NT37>3.0.CO;2-9
Kleerebezem, R., and Van Loosdrecht, M. C. (2007). Mixed culture biotechnology for bioenergy production. Curr. Opin. Biotechnol. 18, 207–212. doi: 10.1016/j.copbio.2007.05.001
Kris, A., Adriaan, V., Monica, H., and Geert, H. (2014). Deoxynivalenol: a major player in the multifaceted response of fusariumto its environment. Toxins 6, 1–19. doi: 10.3390/toxins6010001
Leblanc, J. C., Tard, A., Volatier, J. L., and Verger, P. (2005). Estimated dietary exposure to principal food mycotoxins from the first French Total diet study. Food Addit. Contam. 22, 652–672. doi: 10.1080/02652030500159938
Lee, H. J., and Ryu, D. (2015). Advances in mycotoxin research: public health perspectives. J. Food Sci. 80, T2970–T2983. doi: 10.1111/1750-3841.13156
Leibetseder, J. (2006). Decontamination and detoxification of mycotoxins. Biol. Grow. Anim. 4, 439–465. doi: 10.1016/S1877-1823(09)70102-X
Onyango, M., Wang, Y., Nickel, O., Zhao, C., Zhang, X., Hartke, A., et al. (2014). First genome sequence of potential mycotoxin-degrading bacterium Devosia nanyangense DDB001. Genome A 2, e00922–e00914. doi: 10.1128/genomea.00922-14
Payros, D., Alassane-Kpembi, I., Pierron, A., Loiseau, N., Pinton, P., and Oswald, I. P. (2016). Toxicology of deoxynivalenol and its acetylated and modified forms. Arch. Toxicol. 90, 2931–2957. doi: 10.1007/s00204-016-1826-4
Pessu, P. O., Agoda, S., Isong, I. U., Adekalu, O. A., Echendu, M. A., and Falade, T. C. (2011). Fungi and mycotoxins in stored foods. Afr. J. Microbiol. Res. 5, 4373–4382. doi: 10.5897/AJMR11.487
Pestka, J. J. (2010). Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 84, 663–679. doi: 10.1007/s00204-010-0579-8
Pierron, A., Mimoun, S., Murate, L. S., Loiseau, N., Lippi, Y., Bracarense, A. P. F. L., et al. (2016). Microbial biotransformation of DON: molecular basis for reduced toxicity. Sci. Rep. 6:29105. doi: 10.1038/srep29105
Pinton, P., Tsybulskyy, D., Lucioli, J., Laffitte, J., Callu, P., Lyazhri, F., et al. (2012). Toxicity of deoxynivalenol and its acetylated derivatives on the intestine: differential effects on morphology, barrier function, tight junction proteins, and mitogen-activated protein kinases. Toxicol. Sci. 130, 180–190. doi: 10.1093/toxsci/kfs239
Rasmussen, P. H., Ghorbani, F., and Berg, T. (2003). Deoxynivalenol and other Fusarium toxins in wheat and rye flours on the Danish market. Food Addit. Contam. 20, 396–404. doi: 10.1080/0265203031000082495
Rotaru, A. E., Shrestha, P. M., Liu, F., Shrestha, M., Shrestha, D., Embree, M., et al. (2014). A new model for electron flow during anaerobic digestion: direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ. Sci. 7, 408–415. doi: 10.1039/C3EE42189A
Sato, I., Ito, M., Ishizaka, M., Ikunaga, Y., Sato, Y., Yoshida, S., et al. (2012). Thirteen novel deoxynivalenol-degrading bacteria are classified within two genera with distinct degradation mechanisms. FEMS Microbiol. Lett. 327, 110–117. doi: 10.1111/j.1574-6968.2011.02461.x
Schollenberger, M., Drochner, W., Rüfle, M., Suchy, S., Terryjara, H., and Muller, H. M. (2005). Trichothecene toxins in different groups of conventional and organic bread of the German market. J. Food Compos. Anal. 18, 69–78. doi: 10.1016/j.jfca.2003.10.009
Shapira, R., Paster, N., Magan, N., and Olsen, M. (2004). “Control of mycotoxins in storage and techniques for their decontamination” in Mycotoxins in Food: Detection and Control. eds. N. Magan and M. Olsen (Cambridge, UK: Woodhead Publishing), 190–223.
Shi, H., Li, S., Bai, Y., Prates, L. L., Lei, Y., and Yu, P. (2018). Mycotoxin contamination of food and feed in China: occurrence, detection techniques, toxicological effects and advances in mitigation technologies. Food Control 91, 202–215. doi: 10.1016/j.foodcont.2018.03.036
Shima, J., Takase, S., Takahashi, Y., Iwai, Y., Fujimoto, H., Yamazaki, M., et al. (1997). Novel detoxification of the trichothecene mycotoxin deoxynivalenol by a soil bacterium isolated by enrichment culture. Appl. Environ. Microbiol. 63, 3825–3830. doi: 10.1089/oli.1.1997.7.523
Shong, J., Diaz, M. R. J., and Collins, C. H. (2012). Towards synthetic microbial consortia for bioprocessing. Curr. Opin. Biotechnol. 23, 798–802. doi: 10.1016/j.copbio.2012.02.001
Sobrova, P., Adam, V., Vasatkova, A., Beklova, M., Zeman, L., and Kizek, R. (2010). Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 3, 94–99. doi: 10.2478/v10102-010-0019-x
Stanciu, O., Juan, C., Miere, D., Loghin, F., and Mañes, J. (2016). Occurrence and co-occurrence of Fusarium mycotoxins in wheat grains and wheat flour from Romania. Food Control 147, 147–155. doi: 10.1016/j.foodcont.2016.07.042
Sugiyama, K. I., Tanaka, H., Kamata, Y., Tanaka, T., and Sugitakonishi, Y. (2009). A reduced rate of deoxynivalenol and nivalenol during bread production from wheat flour in Japan. Mycotoxins 59, 1–6. doi: 10.2520/myco.59.1
Thuvander, A., Möller, T., Barbieri, H. E., Jansson, A., Salomonsson, A. C., and Olsen, M. (2001). Dietary intake of some important mycotoxins by the Swedish population. Food Addit. Contam. 18, 696–706. doi: 10.1080/02652030110047775
Ueno, Y. (ed.) (1983). “Trichothecenes: chemical, biological, and toxicological aspects” in Trichothecenes. Amsterdam: Elsevier Press, 135–146.
Wang, S., Tang, H., Peng, F., Yu, X., Su, H., Xu, P., et al. (2019a). Metabolite-based mutualism enhances hydrogen production in a two-species microbial consortium. Commun. Biol. 2:82. doi: 10.1038/s42003-019-0331-8
Wang, G., Wang, Y., Ji, F., Xu, L., Yu, M., Shi, J., et al. (2019b). Biodegradation of deoxynivalenol and its derivatives by Devosia insulae A16. Food Chem. 276, 436–442. doi: 10.1016/j.foodchem.2018.10.011
Wintermute, E. H., and Silver, P. A. (2010). Dynamics in the mixed microbial concourse. Genes Dev. 24, 2603–2604. doi: 10.1101/gad.1985210
Yin, X., Zhu, Z., Zhou, Y., Ji, F., Yao, Z., Shi, J., et al. (2016). Complete genome sequence of deoxynivalenol-degrading bacterium Devosia sp. strain A16. J. Biotechnol. 218, 21–22. doi: 10.1016/j.jbiotec.2015.11.016
Yoshizawa, T., Takeda, H., and Ohi, T. (2014). Structure of a novel metabolite from deoxynivalenol, a tricho-thecene mycotoxin, in animals. J. Agric. Chem. Soc. Jpn. 47, 2133–2135. doi: 10.1271/bbb1961.47.2133
Young, J. C., Subryan, L. M., Potts, D., Mclaren, M. E., and Gobran, F. H. (1986). Reduction in levels of deoxynivalenol in contaminated wheat by chemical and physical treatment. J. Agric. Food Chem. 34, 461–465. doi: 10.1021/jf00069a021
Young, J. C., Zhou, T., Yu, H., Zhu, H., and Gong, J. (2007). Degradation of trichothecene mycotoxins by chicken intestinal microbes. Food Chem. Toxicol. 45, 136–143. doi: 10.1016/j.fct.2006.07.028
Zhang, Y., Guo, L., and Yang, H. (2017). Enhancement of hydrogen production through a mixed culture of Enterobacter cloacae and Rhodobacter sphaeroides. Energy Fuel 31, 7234–7240. doi: 10.1021/acs.energyfuels.7b01173
Zhao, L., Li, X., Ji, C., Rong, X., Liu, S., Zhang, J., et al. (2016). Protective effect of Devosia sp. ANSB714 on growth performance, serum chemistry, immunity function and residues in kidneys of mice exposed to deoxynivalenol. Food Chem. Toxicol. 92, 143–149. doi: 10.1016/j.fct.2016.03.020
Keywords: deoxynivalenol, bacterial consortium, biodegradation, 3-keto-DON, mycotoxin
Citation: Wang Y, Wang G, Dai Y, Wang Y, Lee Y-W, Shi J and Xu J (2020) Biodegradation of Deoxynivalenol by a Novel Microbial Consortium. Front. Microbiol. 10:2964. doi: 10.3389/fmicb.2019.02964
Edited by:
Carlo Giuseppe Rizzello, University of Bari Aldo Moro, ItalyReviewed by:
Yun Chen, Zhejiang University, ChinaAibo Wu, Shanghai Institutes for Biological Sciences (CAS), China
Copyright © 2020 Wang, Wang, Dai, Wang, Lee, Shi and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yijun Dai, ZGFpeWlqdW5AbmpudS5lZHUuY24=; Jianrong Shi, amlhbnJvbmc2M0AxMjYuY29t; Jianhong Xu, eHVqaWFuaG9uZ25qQDEyNi5jb20=
 Yanxia Wang
Yanxia Wang Gang Wang
Gang Wang Yijun Dai
Yijun Dai Yu Wang1,2
Yu Wang1,2 Yin-Won Lee
Yin-Won Lee