- 1Department of Microbiology, Babcock University, Ilishan Remo, Nigeria
- 2Department of Agrobiotechnology (IFA–Tulln), Institute of Bioanalytics and Agro-Metabolomics, University of Natural Resources and Life Sciences Vienna (BOKU), Tulln, Austria
- 3Westerdijk Fungal Biodiversity Institute, Uppsalalaan, Netherlands
- 4Institute for Global Food Security, School of Biological Sciences, Queen’s University Belfast, Belfast, United Kingdom
Low moisture content ready-to-eat foods vended in Nigerian markets could be pre-packaged or packaged at point of sale. These foods are widely and frequently consumed across Nigeria as quick foods. Despite their importance in the daily diets of Nigerians, a comprehensive study on the diversity of fungi, fungal metabolite production potential, and mycotoxin contamination in the foods has not yet been reported. Therefore, this study assessed the diversity of fungi in 70 samples of low moisture content ready-to-eat foods [cheese balls, garri (cassava-based), granola (a mix of cereals and nuts) and popcorn] in Nigeria by applying a polyphasic approach including morphological examination, genera/species-specific gene marker sequencing and secondary metabolite profiling of fungal cultures. Additionally, mycotoxin levels in the foods were determined by LC–MS/MS. Fungal strains (n = 148) were recovered only from garri. Molecular analysis of 107 representative isolates revealed 27 species belonging to 12 genera: Acremonium, Allophoma, Aspergillus, Cladosporium, Fusarium, Microdochium, Penicillium, Sarocladium, Talaromyces, and Tolypocladium in the Ascomycota, and Fomitopsis and Trametes in the Basidiomycota. To the best of our knowledge Allophoma, Fomitopsis, Microdochium, Tolypocladium, and Trametes are reported in African food for the first time. A total of 21 uncommon metabolites were found in cultures of the following species: andrastin A and sporogen AO1 in Aspergillus flavus; paspalin in A. brunneoviolaceus; lecanoic acid and rugulusovin in A. sydowii; sclerotin A in P. citrinum and Talaromyces siamensis; barceloneic acid, festuclavine, fumigaclavine, isochromophilons (IV, VI, and IX), ochrephilone, sclerotioramin, and sclerotiorin in P. sclerotium; epoxyagroclavine, infectopyron, methylorsellinic acid and trichodermamide C in P. steckii; moniliformin and sporogen AO1 in P. copticola; and aminodimethyloctadecanol in Tolypocladium. Twenty-four mycotoxins in addition to other 73 fungal and plant toxins were quantified in the foods. In garri, cheeseballs, popcorn and granola were 1, 6, 12, and 23 mycotoxins detected, respectively. Deoxynivalenol, fumonisins, moniliformin, aflatoxins and citrinin contaminated 37, 31, 31, 20, and 14% of all food samples, respectively. Overall, citrinin had the highest mean concentration of 1481 μg/kg in the foods, suggesting high citrinin exposures in the Nigerian populace. Fungal and mycotoxin contamination of the foods depend on pre-food and post-food processing practices.
Introduction
Cereal and tuber crops (e.g., cassava, maize, groundnut, sorghum and wheat) contribute substantially to food security in sub-Saharan Africa (SSA) (Oguntoyinbo and Narbad, 2012; FAOSTAT, 2017; Tadesse et al., 2018). In Nigeria, these crops are produced in large quantities (FAOSTAT, 2017). However, significant portions are often lost to post-harvest catastrophe mainly due to lack of adequate handling and storage infrastructure (Bankole et al., 2006). As such, the crops are commonly processed into non-perishable forms, such as cheese balls, garri, granola and popcorn. These foods are considered as ready-to-eat (RTE), constituting additional diversity of foods for both the rural and urban populace.
Cheese balls are orange-colored, soft wafer-like snacks processed by local industries in Nigeria and made from corn grits and natural cheese solids. They are consumed mostly by children under age five. Garri is a dry, farinated, granular starchy food spontaneously produced from the fermentation and roasting (dry frying) of cassava (Okafor, 1977; Figure 1). It is a household food in many parts of West Africa, and can be consumed directly in the granulated form, or mixed with either cold or hot water. Cassava processing into garri mostly happens at household level and involves peeling, washing and grating cassava tubers, sack-packaging of the derived slurry, dewatering and fermentation of the packaged slurry for 2–4 days, sieving of the dewatered fermented substrate, and dry frying of sievate at approximately 100°C for 15–20 min (Okafor, 1977; Omonigho and Ikenebomeh, 2002). Granola is a baked, crunchy breakfast food made from mixed cereals and nuts and consumed by many households in Nigeria; Figure 1. It is produced mainly at household level but is also commercially available. The ingredients for granola principally include maize, groundnut, wheat, and honey or sugar. In some cases, bananas and coconut are added as supplements. Typically, granola processing includes mixing all ground raw ingredients in appropriate proportions and with water to form thick granules prior to baking in a pre-heated oven at about 180°C for approximately 1 h. Popcorn is a snack produced from popping dry popcorn maize mixed with honey or sugar for 5–10 min. This snack is usually consumed by all ages including children.

Figure 1. Pictorial representation of dried ready-to-eat foods (A) garri; (B) popcorn; (C): granola; (D): cheese balls vended in markets (E) market scene) in Ogun State, Nigeria.
The safety of the aforementioned RTE foods may, however, be threatened by the presence of diverse fungi and their toxic metabolites (Makinde et al., 2020). However, the fungal and mycotoxicological safety of these foods, except for garri and other cassava products (Ogiehor et al., 2007; Manjula et al., 2009; Ediage et al., 2011; Thomas et al., 2012, 2017; Makun et al., 2013; Sulyok et al., 2015; Abass et al., 2017; Chilaka et al., 2018), were not previously studied in Nigeria and other African countries. This creates a gap in the fungal and mycotoxin surveillance database for commonly consumed foods in Africa. Previous studies on the fungal contamination of garri in Nigeria (Ogiehor et al., 2007; Thomas et al., 2012, 2017) applied either the conventional identification method or a molecular method using 18S ribosomal RNA gene sequences for identification. Both methods have several known limitations such as low precision and misleading taxonomy (Houbraken et al., 2011, 2012; Samson et al., 2014, 2019; Rico-Munoz et al., 2018). Therefore, the need to conduct a more robust fungal profiling and taxonomic studies on the foods is required. Consequently, this study aimed at a comprehensive assessment of the diversity of fungi and mycotoxin profiles in low moisture content RTE foods commonly vended in markets and widely consumed in Nigeria by applying robust polyphasic fungal taxonomic approaches and LC–MS/MS-based analysis of the foods and fungal cultures.
Materials and Methods
RTE Food Sampling
A total of 70 dried RTE food samples consisting of cheese balls (n = 10), garri (n = 23), granola (n = 18) and popcorn (n = 19) were randomly purchased from markets in Ogun State, Nigeria, between January and March 2018. Cheese balls, granola and popcorn were purchased as pre-packed foods in sealed polyethylene packs, while garri samples were purchased unpackaged from open vessels (basins and bags). Each sample of cheese balls and popcorn weighed approximately 200 g, whereas garri and granola samples weighed 1 kg each. Food samples were comminuted into fine powder in an electric blender (MX-AC400, Panasonic, India) and each sample was batched into three equal parts: batch A for moisture analysis, batch B for mycological analysis, and batch C for mycotoxin analysis. The batch A and B samples were stored at 4°C prior to analysis within 48 h, while batch C samples were frozen at −20°C prior to multi-mycotoxin analysis.
Moisture Content Analysis of the Dried RTE Foods
The food samples were subjected to moisture content analysis by the oven-drying to constant weight method (AOAC, 1990). Heating temperature was set at 105°C for 1–3 h in a UNISCOPE hot air oven (SM9053, Surgifriend Medicals, England) (Commission of the European Communities, 2000) and readings were taken for triplicate samples per food sample.
Mycological Analysis of Dried RTE Foods
Isolation and Enumeration of Fungi
The dilution plating technique as described by Samson et al. (1995) was applied for the isolation and enumeration of fungi present in the food samples. Each comminuted sample (10 g) was diluted in sterile distilled water (90 mL) and homogenized for 2 min. Exactly 100 μL of the homogenized mixture was surface-plated out in duplicate on Dichloran 18% Glycerol (DG18) agar. Incubation of all inoculated plates was performed at 25°C for 3 to 5 days. Thereafter, fungal colonies were counted and are reported below as colony forming units per gram (CFU/g) of analyzed food sample. All distinct colonies on the DG18 agar plates were transferred to freshly prepared plates of malt extract agar (MEA; Oxoid, United Kingdom). The purified cultures were retained at 4°C on MEA slants overlaid with sterile distilled water in 4 mL vials.
Polyphasic Characterization of Fungal Isolates
A polyphasic approach consisting of morphological examination, molecular typing and secondary metabolite profiling of fungal cultures was adopted for elucidating the diversity of fungi in the RTE foods. All fungal isolates obtained from the food samples were cultivated on MEA, assessed for macroscopic and microscopic characters, and compared with descriptions in appropriate keys (Frisvad and Samson, 2004; Leslie and Summerell, 2006; Pitt and Hocking, 2009; Samson et al., 2011, 2019). Isolates were clustered into phenotypic groups and representatives of each group were selected for further identification using a sequence-based approach. The representative isolates were grown on MEA for 3 to 5 d prior to DNA extraction. A part of the β-tubulin (BenA) and/or calmodulin (CaM) gene was amplified and sequenced for the isolates belonging to Aspergillus, Penicillium and Talaromyces as previously described (Houbraken et al., 2011, 2012; Samson et al., 2019). For other isolates, the ITS regions, a part of the translation elongation factor 1 alpha (TEF-1α) and/or the RNA polymerase II subunit (RPB2) gene were amplified and sequenced (Groenewald et al., 2005, 2013; Chen et al., 2015, 2017). Species identities were confirmed by comparing the generated sequences with sequences housed in the NCBI database and the internal curated database of the Westerdijk Fungal Biodiversity Institute (WI). All the identified isolates were deposited in the working culture collection of WI (“DTO culture collection”) and newly generated sequences are deposited in GenBank (Supplementary Table S1).
For secondary metabolite profiling, fungal cultures were cultivated on appropriate sets of media, depending on fungal genera/species, for 7 and 14 d at 25°C. The media used were Czapek yeast autolysate (CYA) agar, yeast extract sucrose (YES) agar, MEA and oatmeal (OA) agar (Yilmaz et al., 2014; Samson et al., 2019). Secondary metabolites were extracted from the cultures according to the agar plug extraction method of Filtenborg et al. (1983) with modifications from Smedsgaard (1997). Extraction of agar plugs was performed in ethylacetate/dichloromethane/methanol (3:2:1, v/v/v) containing 1% formic acid. Extracts were dried to the air prior to LC–MS/MS metabolite profiling (see section below).
Multi-Metabolite Analysis of Agar Plug Extracts and RTE Foods
A robust dilute and shoot LC–MS/MS method described by Sulyok et al. (2020) was applied in the determination of more than 300 microbial metabolites including mycotoxins in the extracts from fungal cultures and from the food samples. The dried extracts from fungal cultures were first dissolved in 1 mL (ratio 1:1, v/v) of extraction solvent (acetonitrile/water/acetic acid 79:20:1, v/v/v) and then diluted with acetonitrile/water/acetic acid 20:79:1, v/v/v prior to injection into the LC–MS/MS instrument. For the extraction of food samples, 5 g of homogenized food was taken and mixed with 20 mL of extraction solvent in a 50 mL polypropylene tube (Sarstedt, Nümbrecht, Germany). Apparent recovery of the method was determined by spiking a multi-analyte stock solution into 0.25 g of the food samples. Spiked samples were retained overnight in order to reach equilibrium, and thereafter they were extracted with 1 mL of extraction solvent. Extraction of food samples lasted for 90 min on a GFL 3017 rotary shaker (GFL, Burgwedel, Germany). Extracts were then diluted with acetonitrile/water/acetic acid 20:79:1 (v/v/v) in a 1:1 (v/v) ratio prior to injection into the LC–MS/MS instrument (Sulyok et al., 2007).
Metabolites were screened on a QTrap 5500 LC–MS/MS System (Applied Biosystem, Foster City, CA, United States) equipped with TurboIonSpray electrospray ionisation (ESI) source and a 1290 Series HPLC System (Agilent, Waldbronn, Germany). Chromatographic separation was performed at 25°C on a Gemini® C18–column, 150 × 4.6 mm i.d., 5 μm particle size, equipped with a C18 4 × 3 mm i.d. security guard cartridge (Phenomenex, Torrance, CA, United States). The chromatographic method, chromatographic and mass spectrometric parameters are as described by Sulyok et al. (2020). ESI-MS/MS was conducted in the time-scheduled multiple reaction monitoring (MRM) mode both in positive and negative polarities in two separate chromatographic runs per sample by scanning two fragmentation reactions per analyte. The MRM detection window of each analyte was set to its expected retention time ± 27 s and ± 48 s in the positive and the negative modes, respectively. The identified positive analytes were confirmed by the acquisition of two MRMs per analyte (with the exception of moniliformin (MON), which exhibited only one fragment ion). This yielded 4.0 identification points according to European Commission decision 2002/657 (European Commission [EC], 2002). Additionally, the LC retention time and the intensity ratio of the two MRM transitions were in agreement with the related values of an authentic standard within 0.1 min and 30% respectively. The accuracy of the method for food analysis was verified by participation in inter-laboratory comparison studies organized by BIPEA (Gennevilliers, France). Presently, 94% of the more than 1100 results submitted for different types of grains, nuts, dried fruits and baby food were in the satisfactory range of z-score between −2 and 2.
Data Analysis
Data analysis was performed using IBM Statistical Package for SPSS 21.0 (SPSS® Inc., IL, United States). Means for data on moisture content were calculated and tested for significance by One-way ANOVA (α = 0.05).
Results and Discussion
Moisture Content of Dried RTE Foods
The moisture levels of foods are critical for fungal contamination and mycotoxin accumulation (Magan et al., 2010). Low moisture contents (range: 2.80–10.6%; mean: 7.73 ± 2.03) were recorded in the foods analyzed in this study. The mean moisture levels of granola (7.47 ± 1.56; range: 5.60–10.6%), garri (7.90 ± 1.23; range: 2.80–9.00%) and popcorn (9.54 ± 1.13; range: 6.80–10.6%) were significantly (p < 0.05) higher than the mean level recorded for cheese balls (4.37 ± 1.05; range: 3.28–5.98%).
Fungal Occurrence (Load and Incidence) in Dried RTE Foods and Their Implications
Of all the food types examined in this study, only garri (unpackaged food from busy open markets; Figure 1) yielded fungal propagules (Figure 2). The food types (cheese balls, granola and popcorn) without viable fungal propagules were purchased in pre-packaged forms. This data indicate that the processing steps probably reduced the fungal levels in the food and these levels remained low due to packaging, therefore avoiding recontamination. The fungal contamination of the garri samples may be attributed to post-food production practices, because it is highly unlikely that the fungal propagules can survive the frying step at approximately 100°C for 15–20 min. To be precise, exposure of garri in the unpackaged form to the busy open markets, where human activities including constant and high human traffic and motorist influx occur for more than 10 h on a daily basis, will result in massive food contamination by diverse fungi. Thus, these open markets and the foods being sold therein (e.g., garri) become hot spots of transmission of pathogenic and/or toxigenic strains to new environments. Therefore, packaging after the frying step might limit fungal contamination and prevent the dissemination/dispersal of harmful strains (Guynot et al., 2003). Future research may investigate the impact of packaging (diverse materials and duration) of garri on fungal contamination. No visible fungal growth was observed on the investigated garri samples. Alternative to packaging, challenge test studies with relevant fungal strains (incl. xerophilic fungi) could be performed to study the impact of fungal contaminants on the shelf-life of garri.
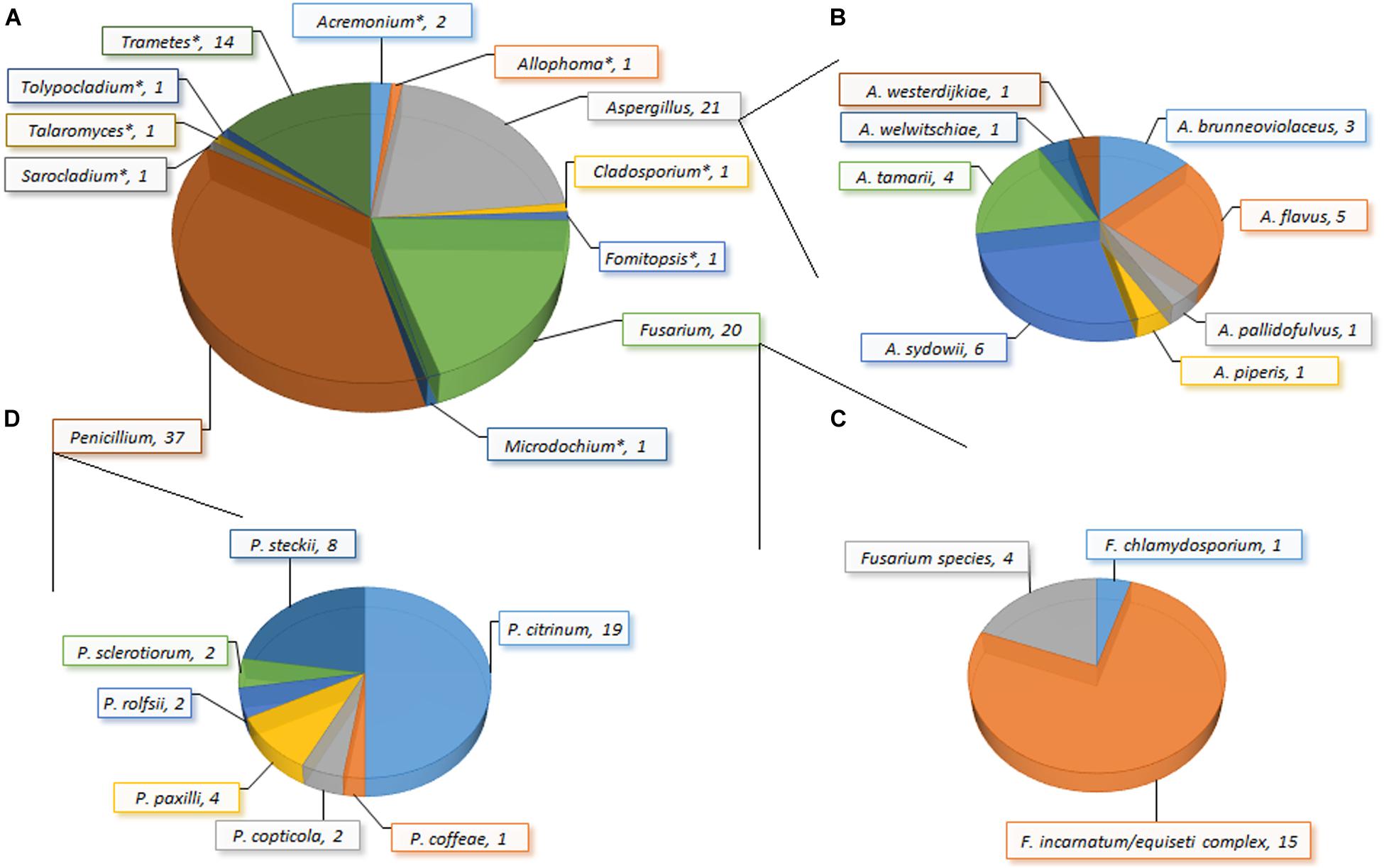
Figure 2. Incidence of fungi (A): genera; (B) species within Aspergillus; (C) species within Fusarium; (D) species within Penicillium) in garri (farinated cassava) sold in markets in Ogun State, Nigeria. *Only one species of the genus was recovered: Acremonium charticola, Allophoma species, Cladosporium tenuissimum, Fomitopsis meliae/durescens, Microdochium lycopodinum, Sarocladium strictum, Talaromyces siamensis, Tolypocladium species, and Trametes polyzona.
The load of fungal propagules in the garri samples ranged from 200 to 2,500 (mean: 712 ± 621) CFU/g, and 148 isolates were recovered from 83% of the samples (Figure 2). The recovered fungi comprised 27 species belonging to 12 genera clustered into two taxonomic divisions (Ascomycota and Basidiomycota) based on a comprehensive taxonomic approach. Acremonium, Allophoma, Aspergillus, Cladosporium, Fusarium, Microdochium, Penicillium, Sarocladium, Talaromyces, and Tolypocladium constituted the Ascomycota, while Fomitopsis and Trametes represented the Basidiomycota. Here, we present to the best of our knowledge the first report of Allophoma, Fomitopsis, Microdochium, Tolypocladium, and Trametes in food in Africa (Chen et al., 2017). Several of these fungal species (e.g., Allophoma oligotrophica and M. lycopodinum) together with species belonging to Aspergillus, Penicillium and Talaromyces are frequent in outdoor and household/indoor air samples from different continents (Visagie et al., 2014a; Hernández-Restrepo et al., 2016; Chen et al., 2017). These airborne fungi, though commonly saprophytic, have been associated with human infections. For example, Aspergillus sydowii causes aspergillosis, keratomycosis and onychomycosis (Hoog, 2000), while A. brunneoviolaceus, A. pallidofulvus and the filamentous basidiomycete (Trametes polyzona) were previously implicated in human pulmonary (fungal lung) infection (e.g., invasive and disseminated aspergillosis) (Masih et al., 2016; Gauthier et al., 2017).
In addition, Acremonium, Cladosporium and Sarocladium were previously reported in cereals (maize and rice) and cocoa powder beverages in Nigeria (Makun et al., 2011; Egbuta et al., 2015). Cladosporium has also been reported in fruits from Nigeria, chili and Musa species (banana and plantains) from different countries (Bensch et al., 2018). Acremonium and Sarocladium are two phenotypically-related genera and a comprehensive phylogenetic study led to the re-allocation of some hypocrealean Acremonium (including the clinically important species A. strictum) to Sarocladium (Summerbell et al., 2011; Giraldo et al., 2015). The two members of the Basidiomycota recorded in the present study are wood inhabiting fungi. Fomitopsis meliae/durescens is a notable brown rot fungus commonly reported in the Americas, Asia and the United Kingdom (Han et al., 2016), while Trametes polyzona possesses ligninolytic capabilities (Lueangjaroenkit et al., 2018). These wood-related fungi may have accessed the garri samples during the improper storage of the food; it is a common practice for local food vendors to store their foods in improperly closed bags/containers/basins after the day’s sale together with woods collected from the farms for making fire.
Aspergillus, Fusarium and Penicillium were predominantly present and multiple species were recovered; all other genera were represented by single species (Figure 2). Eight Aspergillus species covering four sections were recovered in this study: Circumdati (A. pallidofulvus; Visagie et al., 2014c), Flavi (A. flavus and A. tamarii; Frisvad et al., 2019), Nidulantes (A. sydowii; Chen et al., 2016), and Nigri (A. brunneoviolaceus, A. piperis, A. welwitschiae and A. westerdijkiae; Samson et al., 2007; Perrone et al., 2011; Varga et al., 2011). The Fusarium isolates comprised two species (F. chlamydosporum and F. incarnatum/equiseti species complex; O’Donnell et al., 2013; Villani et al., 2019) and one unresolvable species reported here as Fusarium sp. The Penicillia were clustered into four sections: Charlesia (P. coffeae; Houbraken and Samson, 2011; Visagie et al., 2014c), Citrina (P. citrinum, P. copticola, P. paxilli, and P. steckii; Houbraken and Samson, 2011; Houbraken et al., 2011; Visagie et al., 2014b), Lanata-Divaricata (P. rolfsii; Houbraken and Samson, 2011), and Sclerotiora (P. sclerotiorum; Houbraken and Samson, 2011; Rivera and Seifert, 2011; Visagie et al., 2014b). Overall, Penicillium (incidence: 37.4%) was the predominant genus and P. citrinum (incidence: 18.7%) was the most frequently isolated species in the garri samples.
Proper characterization of fungal species is a fundamental step to understand the role of fungi in relation to food spoilage and/or possible mycotoxin production. The array of fungal species recovered from the garri samples in this study is more comprehensive and diverse than the previously reported spectra of species in several foods in Nigeria including garri (Ogiehor et al., 2007; Thomas et al., 2012, 2017), cereals (Makun et al., 2011; Egbuta et al., 2015), and nuts (Ezekiel et al., 2013, 2016; Oyedele et al., 2017) as well as in fermented foods vended in Nigerian and South African markets (Adekoya et al., 2017, 2018). The disparities in fungal species in the present study compared to the aforementioned literature may be mainly attributed to the adoption of a more robust fungal identification technique in our study. We applied specific molecular typing markers (e.g., BenA, CaM, ITS, RPB2, and/or TEF-1α) which have been shown to have high taxonomic resolving power at species (or genus) level (Groenewald et al., 2005, 2013; Houbraken et al., 2011, 2012; Chen et al., 2015, 2017; Frisvad et al., 2019; Samson et al., 2019). The sole application of ITS or 18S rRNA gene sequencing for identification of important foodborne species (e.g., Aspergillus, Fusarium, Penicillium, and Talaromyces) will lead to misidentifications and incorrect associations will be made between species on one side and produced metabolites and ecology (food) on the other.
Secondary Metabolites in Fungal Cultures
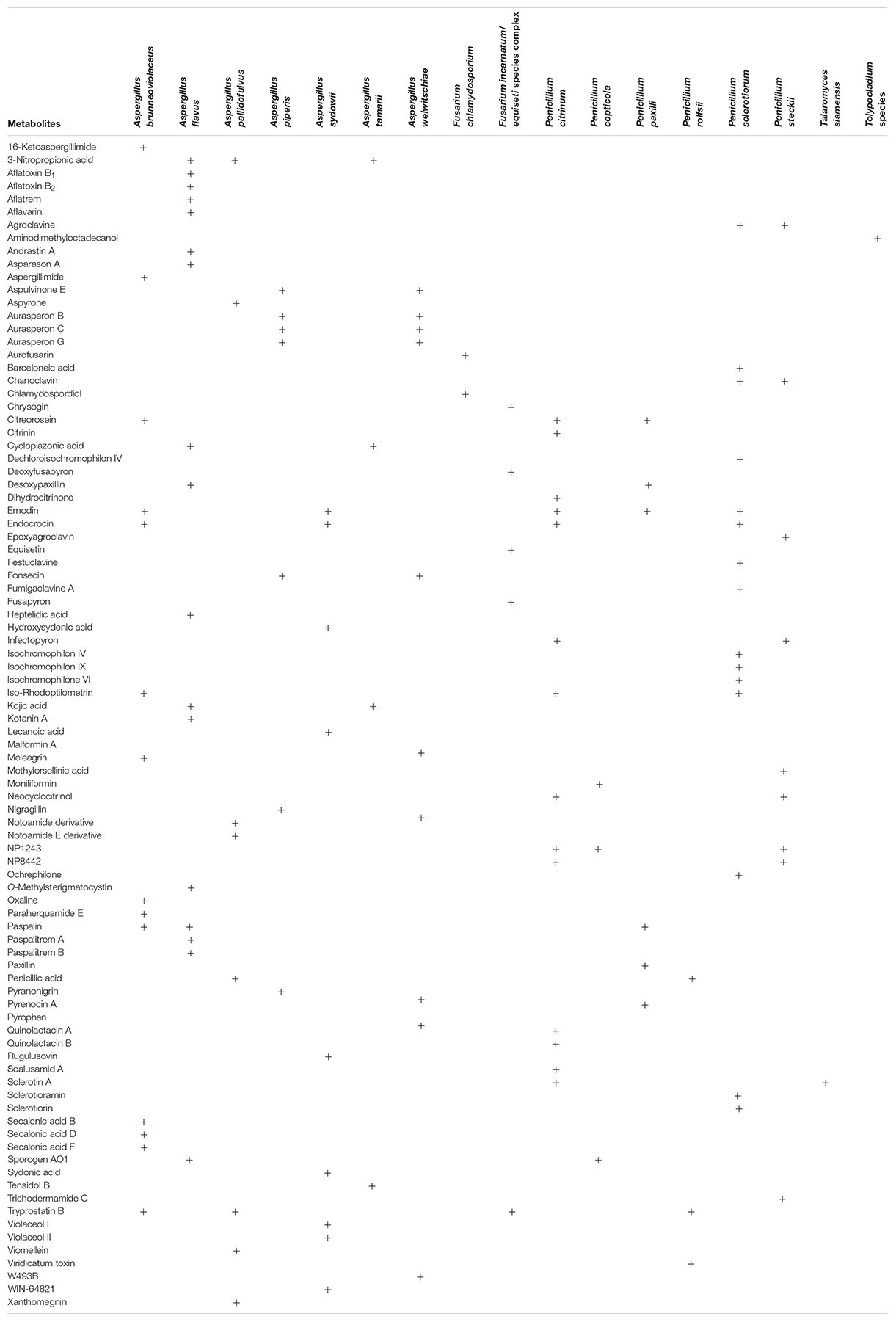
LC-MS/MS-based chemotaxonomy, which utilizes fingerprints of secondary metabolites excreted in fungal cultures, is a valuable complementary approach during the characterization and industrial exploitation of fungi for human benefits (Frisvad and Samson, 2004; Frisvad et al., 2007, 2018; Samson et al., 2019). Strains of 20 out of the 27 fungal species identified in this study were examined for secondary metabolite production in mycological media. Cultures of strains of 17 of the examined species (excluding Acremonium charticola, Penicillium coffeae and Sarocladium strictum) contained diverse metabolites as shown in Table 1.
Metabolites From Aspergillus
Secondary metabolite production is often consistent within a species but can be highly variable within members of closely related species as well as within a section (Frisvad et al., 2007; Samson et al., 2007). Here, the metabolite patterns of three members of the section Nigri (A. brunneoviolaceus, A. piperis, and A. welwitschiae) were investigated (Table 1). Aspergillus brunneoviolaceus (syn. A. fijiensis) produced aspergillimides, emodin, meleagrin, oxaline, paraherquamide E and secalonic acids B, D, and F. Calbistrin C, pre-aurantiamin, neoxaline and okaramins (Varga et al., 2011; Vesth et al., 2018) were not detected in cultures of this species while asperparalines are not included in the target list of compounds in the LC-MS/MS method applied in the present study. Aspergillus piperis and A. welwitschiae produced distinct arrays of metabolites. Aspergillus welwitschiae produced malformin A, tensidol B and trace amounts of pyrophen which were not found in cultures of A. piperis. These metabolites from A. welwitschiae (syn. A. awamori) agree with the literature and indicate that this species shares similar metabolite profiles with A. niger (Samson et al., 2007; Nielsen et al., 2009; Perrone et al., 2011; Hong et al., 2013; Lamboni et al., 2016). The metabolites produced by A. piperis isolated from the Nigerian garri samples were also consistent with previous reports for naphtho-γ-pyrones (aurasperones) and pyranonigrin A (Samson et al., 2007), except for aflavinins that were not detected in cultures in the present study because the compounds are also not included in the LC-MS/MS method list of compounds. Additionally, aspulvinone E recently found in cultures of A. niger isolated during cocoa beans processing in Nigeria (Akinfala et al., 2020) and other notable metabolites (fonsecin and nigragillin) of the section Nigri (Samson et al., 2007; Akinfala et al., 2020) were also found in the A. piperis cultures in our present study. The chemical profile data obtained here reiterates the fact that the identification of species within section Nigri (black Aspergilli) is complicated, requiring great skill and the application of a polyphasic approach involving the right set of genetic markers (Samson et al., 2007; Nielsen et al., 2009; Perrone et al., 2011; Lamboni et al., 2016).
Four A. flavus strains were considered for metabolite profiling; three strains were aflatoxigenic while the DTO 481-G5 strain was non-aflatoxigenic but produced cyclopiazonic acid and kojic acid. The aflatoxigenic strains produced the B aflatoxins together with other known metabolites including 3-nitropropionic acid, aflatrem, aflavarins, asparason A cyclopiazonic acid, desoxypaxillin, kojic acid, kotanin A, paspalin and paspalitrems; Frisvad et al., 2019; Uka et al., 2019). We also found heptelidic acid in a culture of one aflatoxigenic A. flavus strain; this corroborates the findings of a previous study from our group wherein we reported this metabolite in both mycelia and culture media extracts of aflatoxigenic A. flavus (Kovač et al., 2020). The metabolites uncommon to A. flavus that were detected in this study include sporogen AO1 produced by all the four strains and andrastin A found in culture of one aflatoxigenic strain (Supplementary Figure S1). Sporogen AO1 has been documented in members of the Aspergillus section Flavi –A. luteovirescens and A. oryzae, which is the domesticated form of A. flavus.
Several metabolites including aspyrone, notamide, penicillic acid, viomellein and xanthomegnin were found in the A. pallidofulvus culture and these were consistent with literature (Visagie et al., 2014c). However, some metabolites were shared across different genera and sections. For example, penicillic acid produced by A. pallidofulvus cultures was also present in cultures of Penicillium rolfsii, whilst tryprostatin B previously reported in A. fumigatus (Cui et al., 1996) was found in A. brunneoviolaceus, A. pallidofulvus, P. citrinum and P. rolfsii. Additionally, emodin and endocrocin occurred in some species within Aspergillus sections Nigri and Nidulantes and sections Citrina and Sclerotiora of Penicillium (Table 1). Paspalin, a metabolite of species within the A. flavus clade (Frisvad et al., 2019), is reported for the first time in one strain of A. brunneoviolaceus (Supplementary Figure S1). Paspalin was also found in cultures of P. paxilli, which is in accordance with a report associating this metabolite as a stable intermediate compound in the biosynthetic pathway of paxillin in P. paxilli (Saikia et al., 2007). For A. sydowii, the sydonic acids, violaceols (I and II), WIN-64821 and several other compounds not included in the list of compounds in our LC-MS/MS method were so far documented (Chen et al., 2016; Samson et al., 2019). But here, we present the unique occurrence of lecanoic acid and rugulusovin in addition to the aforementioned compounds in the cultures of this species (Supplementary Figure S1). These compounds could represent unique fingerprints for this species since they were not yet reported in any member of the section Nidulantes (Chen et al., 2016; Samson et al., 2019).
Metabolites From Penicillium and Talaromyces
Diverse secondary metabolite profiles were detected in the cultures of the Penicillia examined in this study (Table 1). As expected, the P. citrinum strains biosynthesized citrinin, dihydrocitrinone and quinolactins (A and B) (Houbraken et al., 2011). These strains from garri appeared to be low citrinin producers (range: 3,146–3,739; mean: 3,474 μg/kg) compared to the strains from cocoa processing in Nigeria which secreted mean citrinin levels exceeding 350,000 μg/kg (Akinfala et al., 2020). Additionally, scalusamid A, which was recently reported in cultures of P. citrinum obtained from cocoa processing in Nigeria (Akinfala et al., 2020), were detected here together with two metabolites tentatively named NP1243 and NP8442. Few metabolites were shared among species and across sections. For instance, neocyclocitrinol was produced by P. citrinum and P. steckii, while sclerotin A (an antimicrobial compound; Curtin and Reilly, 1940) was also found for the first time in cultures of P. citrinum and Talaromyces siamensis (Supplementary Figure S1). Sclerotin A was the only metabolite in cultures of T. siamensis and was not previously reported in Penicillium section Citrina or in Talaromyces (Houbraken et al., 2011; Yilmaz et al., 2014) but is known from Penicillium sclerotiorum (Rivera and Seifert, 2011). Penicillium steckii (section Citrina) and P. sclerotiorum (section Sclerotiora) also shared the production of two clavine alkaloids (agroclavine and chanoclavine). However, barceloneic acid, festuclavine, fumigaclavine, isochromophilons (IV, VI and IX), ochrephilone, sclerotioramin and sclerotiorin were additional compounds in P. sclerotiorum cultures, and P. steckii produced epoxyagroclavine, infectopyron, methylorsellinic acid and trichodermamide C (Supplementary Figure S1). These aforementioned compounds were never reported yet in any of the two species (Houbraken et al., 2011; Rivera and Seifert, 2011). It is noteworthy to mention that infectopyron is usually produced by Alternaria species (Escrivá et al., 2017). Thus, its presence in all cultures of P. citrinum and P. steckii in the present study (Supplementary Figure S1) corroborates its recent detection in cultures of P. citrinum isolated from cocoa processing (Akinfala et al., 2020), and confirms production in section Citrina.
All the cultures of P. paxilli contained paxillin, desoxypaxillin and pyrenocin A as expected (Houbraken et al., 2011), while moniliformin and sporogen AO1 were detected for the first time in all the strains of P. copticola (Supplementary Figure S1 and Table 1). Moniliformin, a Fusarium mycotoxin, was first reported in Penicillium (P. melanoconidium of the section Fasciculata) associated with cereals in 2016 (Hallas-Møller et al., 2016). The occurrence of moniliformin in Penicillia from two sections (Citrina and Fasciculata) suggests that gene cluster responsible for the biosynthesis of this mycotoxin has a common ancestor; however, this hypothesis needs to be further investigated by whole genome sequencing of several Penicillia. Penicillic acid and viridicatum toxin were the metabolites in the culture of P. rolfsii.
Metabolites From Fusarium and Tolypocladium
The metabolites from F. chlamydosporum were aurofusarin, chlamydospordiol and W493B (Table 1). Contrary to expectations (Shier and Abbas, 1992; Solfrizzo et al., 1994; Munkvold, 2016), chlamydosporol was not found in the cultures of this species in our present study. For the F. incarnatum/equiseti species complex, chrysogin, deoxyfusapyron, fusapyron and equisetin were detected. All metabolites obtained are common to the genus Fusarium (Nesic et al., 2013; Broda et al., 2016; Beccari et al., 2018; Mastanjević et al., 2018). The examined Tolypocladium species produced only aminodimethyloctadecanol, which is reported for the first time in this species (Supplementary Figure S1). This metabolite is a sphingosine analog previously reported in only Fusarium species and which plays a role in apple rot (Uhlig et al., 2005; Sørensen et al., 2009).
Fungal Secondary Metabolites and Plant Toxins in RTE Foods
The 70 food samples were contaminated with 95 fungal secondary metabolites and two plant toxins (cyanogenic glycosides) as quantified by the LC-MS/MS method (Table 2). Among the fungal metabolites were 24 mycotoxins commonly found in foods. The mean mycotoxin levels together with mean levels of the two cyanogenic glycosides computed for all food samples are given in Figure 3. In addition, the incidence and contamination levels of mycotoxins and cyanogenic glycosides in the food types are highlighted in Tables 3, 4, while the distribution of other fungal secondary metabolites in the food types are presented in Supplementary Table S2. At least 70% of all the food samples contained one fungal metabolite and cyanogenic glycoside. Overall, 1, 6, 12, and 23 mycotoxins were found in garri, cheeseballs, popcorn and granola. The spectrum of metabolites, including mycotoxins, found in the cereal-based and mixed grain/nut RTE foods agree with previous reports of diverse mycotoxins in processed foods of cereal, nuts or mixed cereal and nut origin (Ediage et al., 2011; Ezekiel et al., 2012, 2019; Kayode et al., 2013; Abia et al., 2017; Ojuri et al., 2018).
Table 2. LC-MS/MS method performance characteristics for 97 metabolites in dried ready-to-eat foods in Nigeria.
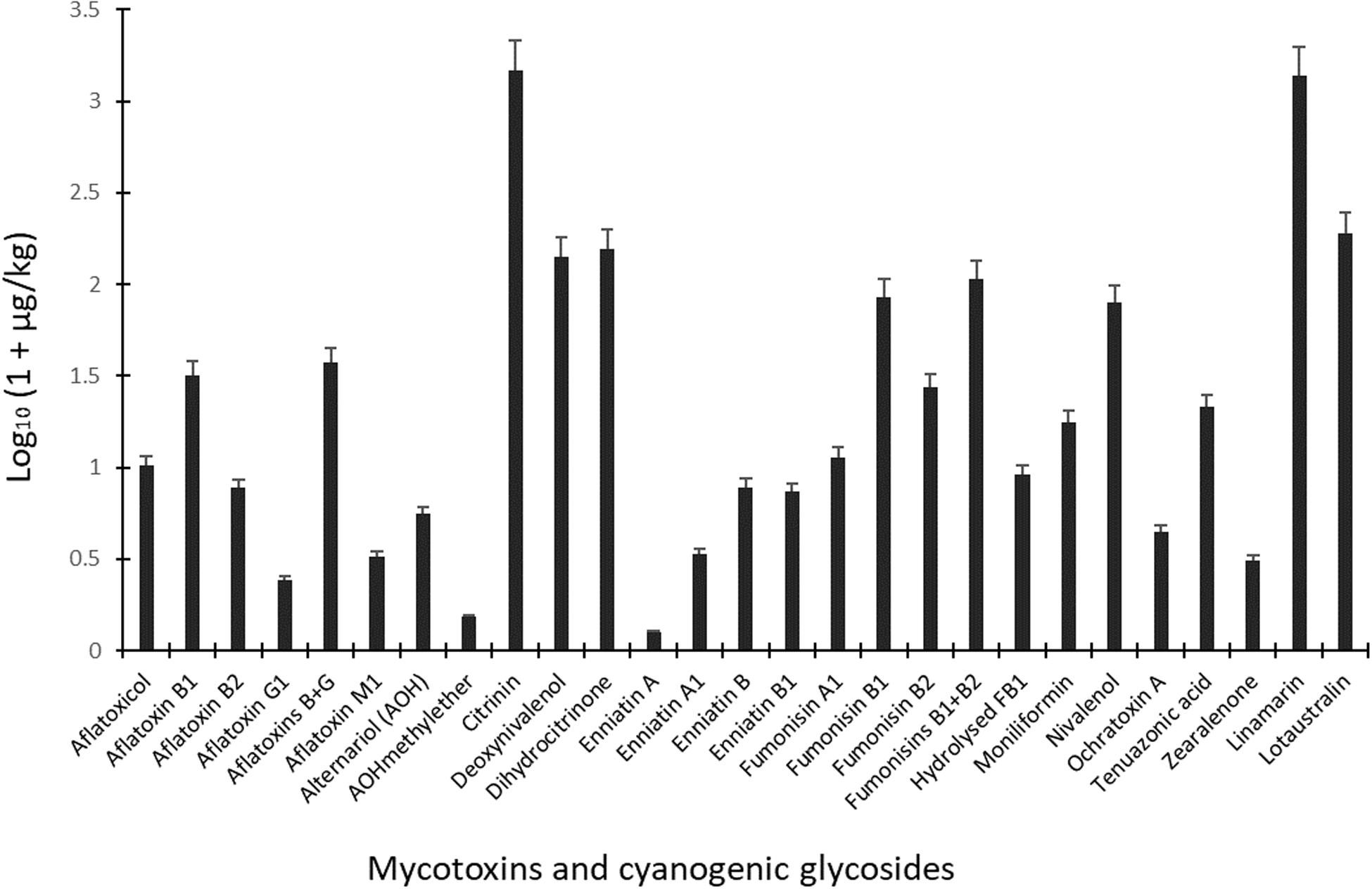
Figure 3. Mean levels of mycotoxins and cyanogenic glycosides in dried ready-to-eat foods vended in markets in Ogun State, Nigeria. Ochratoxin B was not included in the graph due to occurrence in only one sample.
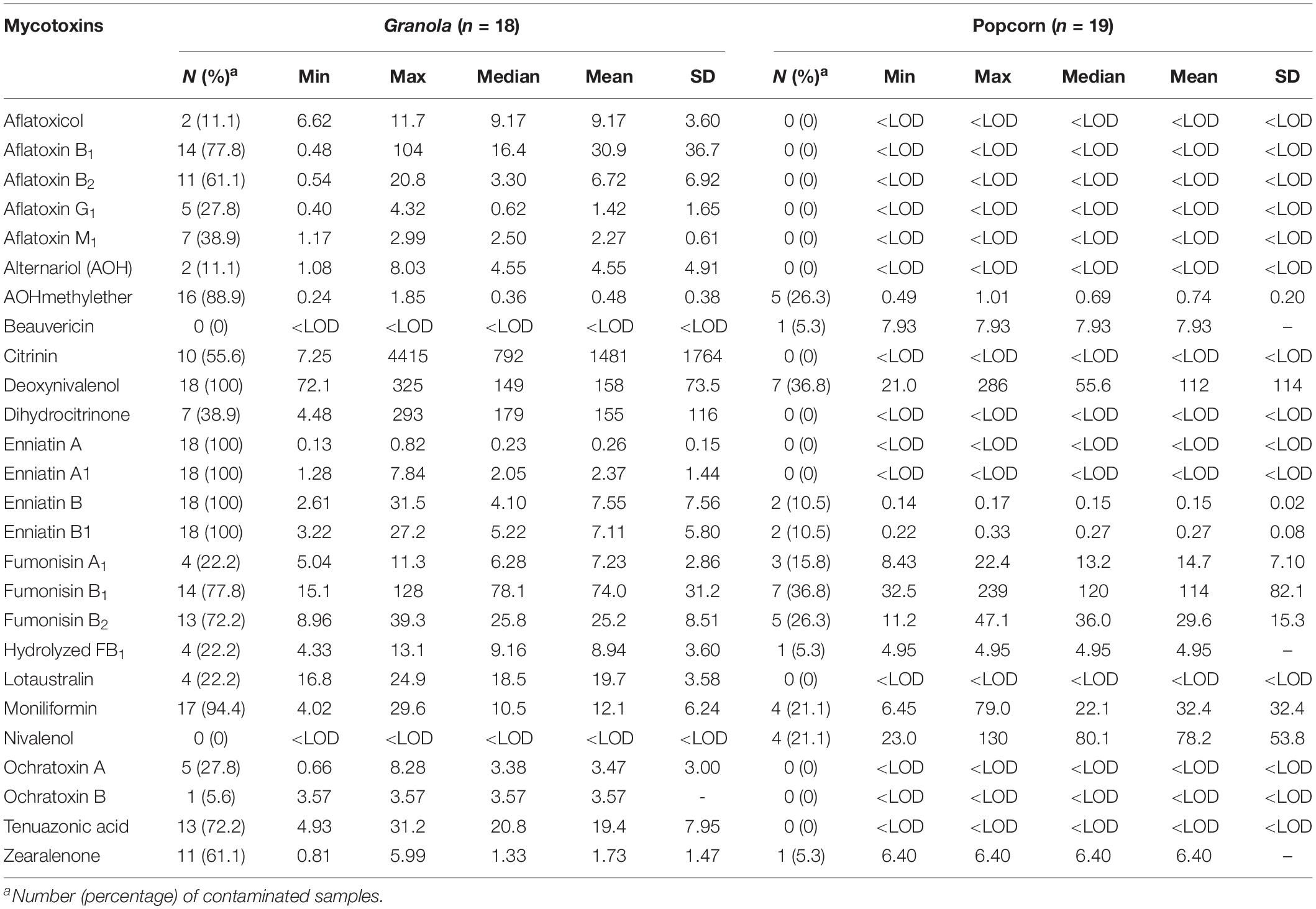
Table 3. Occurrence of 26 mycotoxins and cyanogenic glucosides in dried cereal-based ready-to-eat foods from Ogun state, Nigeria.
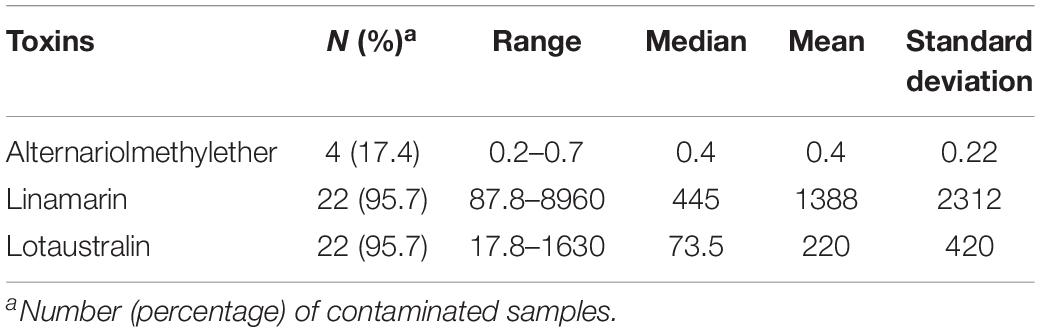
Table 4. Mycotoxin and cyanogenic glucoside levels in garri (farinated cassava; n = 23) samples from Ogun state, Nigeria.
Due to the occurrence of mycotoxins [deoxynivalenol (20.4 μg/kg), fumonisin B1 (25.9 μg/kg), HT-2 toxin (21.6 μg/kg), moniliformin (32.6 μg/kg), T-2 toxin (20.3 μg/kg) and tenuazonic acid (32.5 μg/kg)] in only one sample of cheese balls, the data for this food is not presented in the tables. However, in view of the cocktail of toxic fungal metabolites found in cheese balls and the fact that this food is consumed mostly by pre-school and early school-aged children (under age 5), more surveillance studies are required to understand the extent of contamination in this food and possible co-exposure patterns (Ojuri et al., 2019). Aflatoxins contaminated 20% of all food samples at mean total B and G aflatoxin levels of 36.6 μg/kg (range: 0.48–118 μg/kg). Citrinin and its hydroxylated metabolite (dihydrocitrinone) were quantified in 14 and 10% of the food samples and at high mean concentrations of 1,481 μg/kg (range: 7.25–4,415 μg/kg) and 155 μg/kg (range: 4.48–293 μg/kg), respectively (Figure 3). Amongst all mycotoxins quantified in the food samples, citrinin had the highest mean level. Citrinin is a nephrotoxin presently classed as group 3 compound (International Agency for Research on Cancer [IARC], 1993) because of insufficient evidence for carcinogenicity. In addition, legal requirement or regulation is unavailable for this food in the instance of surveillance activities; thus, limited dietary exposure data abound (Ali and Degen, 2019). However, the European Food Safety Authority (EFSA) has set a 0.2 μg/kg bw/day “level of no concern for nephrotoxicity” in humans based on recent data available and given uncertainties (European Food Safety Authority [EFSA], 2012; Ali and Degen, 2019). The mean citrinin and dihydrocitrinone concentrations in the commonly consumed foods analyzed in the present study are remarkably high, suggesting high citrinin exposures in the Nigerian populace. This view is supported by recent biomonitoring data in Nigeria where citrinin and dihydrocitrinone were found in human urines (Šarkanj et al., 2018) and estimations presented high citrinin intake and exposure exceeding the set preliminary tolerable daily intake value by EFSA (Ali and Degen, 2019). More surveillance and human biomonitoring studies are required to understand the extent of citrinin contamination and dietary exposure as well as the patterns of its co-exposures with other chemical contaminants in Nigeria.
With respect to mycotoxins in individual food types, aflatoxins, citrinin, dihydrocitrinone, enniatins A and A1, and ochratoxins A and B were mycotoxins found only in granola in addition to other mycotoxins also found in the other foods (Table 3). Additionally, fumonisins (mean FB1 + FB2: 106 μg/kg; range: 15.1–286 μg/kg) were quantified in 31% of all 70 food samples (Figure 3), occurring only in 1, 14, and 7 samples of cheese balls, granola (mean FB1 + FB2: 97.5 μg/kg) and popcorn (mean FB1 + FB2: 135 μg/kg), respectively (Table 3). Deoxynivalenol (mean: 140 μg/kg; range: 20.4–325 μg/kg) and moniliformin (mean: 16.7 μg/kg; range: 4.02–78.9 μg/kg) also contaminated at least 30% of all food samples and occurred in all foods except garri. The occurrence of mycotoxins in cheese balls, granola and popcorn were not previously reported in Africa. However, the raw ingredients used in making these foods have been reported to contain mycotoxins similar to those reported in the finished foods (Warth et al., 2012; Abia et al., 2013; Adetunji et al., 2014; Matumba et al., 2015; Egbontan et al., 2017; Oyedele et al., 2017). Nonetheless, dos Santos et al. (2015) reported aflatoxins in 60% of 60 samples of granola in Teresina (Brazil), while Moreira et al. (2016) did not find any aflatoxins in three granola samples in northeastern regions of Brazil. In addition, aflatoxins were either not detected or were found together with ochratoxin A in 7 to 33% of popcorn samples in southern Pennsylvania (United States) or in popcorn imported into Spain from the United States, respectively (Alborch et al., 2012; Gourama, 2015). Thus, these commercial foods as well as other RTE foods or snacks highly consumed by children in Nigeria require routine monitoring. In Nigeria, only aflatoxins are regulated in foods at present and at a limit of 4 μg/kg. Even though aflatoxin was not detected in the other RTE food samples examined in this study, this toxin was detected in granola which serves as breakfast cereal in many homes. The detection of several mycotoxins regulated in the European Union, coupled with the documented toxicological effects of these mycotoxins in addition to their potential combinatory effects with the non-regulated toxins (Ostry, 2009; Klarić et al., 2013; Lee et al., 2015; Rychlik et al., 2016; Vejdovszky et al., 2016, 2017a,b; European Food Safety Authority [EFSA], 2018), and the routine high consumption of mixtures of all the examined foods together with other high risk foods in households, especially among children, necessitate a need for the urgent revision of regulations on industrially-processed foods consumed by children in Nigeria. The revision should prioritize the inclusion of other mycotoxins beyond aflatoxins in the regulations as well as setting of reasonable limits.
Linamarin and lotaustralin (two cyanogenic glycosides) were the major compounds in 96% of the 23 garri samples (Table 4), at mean concentrations of 1388 and 220 μg/kg, respectively. Concentrations of the two cyanogenic glycosides in the garri samples were obviously below the regulations for the toxic cyanides in food (Food and Agricultural Organization of the United Nations [FAO] and World Health Organization [WHO], 1992). Similar compliant levels of cyanogenic glycosides were also reported in cassava products including garri in Nigeria (Abass et al., 2017) but negates the high levels documented in processed cassava samples from Tanzania (Sulyok et al., 2015), obviously due to differences in cyanide contents of cassava varieties and/or differences in adopted processing methods in the two countries.
Alternariolmethylether was the only mycotoxin found in 4 garri samples, albeit at low concentrations (mean: 0.4 μg/kg; range: 0.2–0.7 μg/kg; Table 4) that may not obviously constitute a threat to the safety of the food. The non-detection of major mycotoxins in the garri samples we analyzed negates previous reports on the presence of aflatoxins, deoxynivalenol, fumonisins, moniliformin, T-2 toxin and zearalenone in cassava products including garri from various African countries including Nigeria (Essono et al., 2009; Sulyok et al., 2015; Abass et al., 2017; Chilaka et al., 2018). Since mycotoxins are uncommon in fresh tubers (e.g., cassava and yam) and stored tuber products could contain mycotoxins as reported in the literature, the disparity in our results from those that reported mycotoxins in cassava products may be attributed to two major factors. These include: possible low moisture content (range: 2.80–9.00%) resulting from adequate dry frying of the garri samples and short storage time (less than 2 weeks) of the samples analyzed in the present study compared to the other reports wherein these details were not provided. However, when the levels of the toxins in garri from all the studies are compared, garri may be regarded as a safe food in terms of mycotoxin contamination due to the low concentrations (within safe limits for processed foods) in the samples. However, this high carbohydrate/energy food may constitute a bigger health risk in the present unpackaged form in which it is vended. Unpackaged foods, as seen in the case of garri, could harbor fungal propagules from the air. Thus, these foods may serve as vehicle for the transmission of the propagules into environments with individuals that are susceptible for fungal infections (Hoog, 2000; Visagie et al., 2014c; Masih et al., 2016; Gauthier et al., 2017). Additionally, unpackaged garri contaminated with fungal propagules and stored for days in consumer household stores could serve as vehicle for fungal spore dispersal into other foods co-stored within the households and even to household/indoor air. Furthermore, in accordance with previous reports and findings from our present study, it is obvious that further mycotoxin research of cassava products including the exported ones (e.g., garri, cassava balls and chips to Europe) is required.
Conclusion
Low moisture ready-to-eat foods have been shown in this study to harbor diverse fungal species and genera, including potential industrial workhorses, mushrooms, human pathogens and notable mycotoxin producers. We have also presented a rich diversity in the secondary metabolite profiles from several fungal species. Several of the presented metabolites were not previously reported in the screened species and several of them may serve as markers for species chemotaxonomy. In addition, the mycotoxin contamination profiles and levels in the foods were examined and found to be of potential public health concern, especially the mixed cereal food (granola). Furthermore, this study has revealed that pre-food production practices (e.g., crop cultivation, harvesting, cleaning, drying and storage) were responsible for mycotoxin levels in the RTE foods, whilst post-production food handling (e.g., food packaging vs non-packaging) influenced the fungal diversity in the RTE foods, though none of the foods were visibly molded. Consequently, a set of simple interventions to curb fungal and mycotoxin exposure through these foods is hereby proposed: (a) ensure proper grain cultivation, timely harvesting, safe grain cleaning, drying to safe moisture levels, and storage of grains under safe/non-damp conditions; (b) careful hand sorting of grains for production of granola or other foods to remove physically damaged, insect infested, discolored and moldy grains; and (c) application of simple polyethylene pre-packaging to all low moisture foods retailed in local markets in Nigeria.
Data Availability Statement
The datasets generated for this study can be found in the GenBank, Please see Supplementary Table S1 for sequence details.
Author Contributions
CE conceived the study. CE, JH, KA, MS, OO, and RK designed the study. BK, CE, JH, MS, and OO performed the experiments in Austria, Nigeria, and Netherlands. CE, JH, and MS analyzed the data. JH and RK supervised the overall study. CE, KA, and OO drafted the manuscript. All authors reviewed and approved the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Amber Elkamash for her support during the molecular analysis of fungi.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.00615/full#supplementary-material
References
Abass, A. B., Awoyale, W., Sulyok, M., and Alamu, E. O. (2017). Occurrence of regulated mycotoxin and other microbial metabolites in dried cassava products from Nigeria. Toxins 9:207. doi: 10.3390/toxins9070207
Abia, W. A., Warth, B., Ezekiel, C. N., Sarkanj, B., Turner, P. C., Marko, D., et al. (2017). Uncommon toxic microbial metabolite patterns in traditionally home-processed maize dish (fufu) consumed in rural Cameroon. Food Chem. Toxicol. 107, 10–19. doi: 10.1016/j.fct.2017.06.011
Abia, W. A., Warth, B., Sulyok, M., Krska, R., Tchana, A. N., Njobeh, P. B., et al. (2013). Determination of multi-mycotoxin occurrence in cereals, nuts and their products in Cameroon by liquid chromatography tandem mass spectrometry (LC-MS/MS). Food Control 31, 438–453. doi: 10.1016/j.foodcont.2012.10.006
Adekoya, I., Obadina, A., Phoku, J., De Boevre, M., De Saeger, S., and Njobeh, P. (2018). Fungal and mycotoxin contamination of fermented foods from selected South African markets. Food Control 90, 295–303. doi: 10.1016/j.foodcont.2018.02.040
Adekoya, I., Obadina, A., Phoku, J., Nwinyi, O., and Njobeh, P. (2017). Contamination of fermented foods in Nigeria with fungi. LWT Food Sci. Technol. 86, 76–84. doi: 10.1016/j.lwt.2017.07.044
Adetunji, M. C., Atanda, O. O., Ezekiel, C. N., Sulyok, M., Warth, B., Beltrán, E., et al. (2014). Fungal and bacterial metabolites of stored maize (Zea mays L.) from five agro-ecological zones of Nigeria. Mycotoxin Res. 30, 89–102. doi: 10.1007/s12550-014-0194-2
Akinfala, T. O., Houbraken, J., Sulyok, M., Adedeji, A. R., Odebode, A. C., Krska, R., et al. (2020). Moulds and their secondary metabolites associated with the fermentation and storage of two cocoa bean hybrids in Nigeria. Int. J. Food Microbiol. 316:108490. doi: 10.1016/j.ijfoodmicro.2019.108490
Alborch, L., Bragulat, M. R., Castellá, G., Abarca, M. L., and Cabañes, F. J. (2012). Mycobiota and mycotoxin contamination of maize flours and popcorn kernels for human consumption commercialized in Spain. Food Microbiol. 32, 97–103. doi: 10.1016/j.fm.2012.04.014
Ali, N., and Degen, G. H. (2019). Citrinin biomarkers: a review of recent data and application to human exposure assessment. Arch. Toxicol. 93, 3057–3066. doi: 10.1007/s00204-019-02570-y
AOAC, (1990). Official Methods of Analysis, 15th Edn. Arlington, VA: Association of Official Analytical Chemists.
Bankole, S., Schollenburger, M., and Drochner, W. (2006). Mycotoxins in food systems in sub Saharan Africa: a review. Mycotoxin Res. 22, 163–169. doi: 10.1007/BF02959270
Beccari, G., Colasante, V., Tini, F., Senatore, M. T., Prodi, A., Sulyok, M. L., et al. (2018). Causal agents of Fusarium head blight of durum wheat (Triticum durum Desf.) in central Italy and their in vitro biosynthesis of secondary metabolites. Food Microbiol. 70, 17–27. doi: 10.1016/j.fm.2017.08.016
Bensch, K., Groenewald, J. Z., Meijer, M., Dijksterhuis, J., Jurjeviæ, Ž, Andersen, B., et al. (2018). Cladosporium species in indoor environments. Stud. Mycol. 89, 177–301. doi: 10.1016/j.simyco.2018.03.002
Broda, L., Klapec, T., Sulyok, M., Krska, R., and Sarkanj, B. (2016). “Occurrence of Fusarium mycotoxins in Croatian cereals harvested in 2015,” in Proceedings of the Abstracts of Lectures and Posters, World Mycotoxin Forum Meets IUPAC 2016/, ed. H. B. Bastiaanse (Winnipeg, MB: IUPAC).
Chen, A. J., Frisvad, J. C., Sun, B. D., Varga, J., Kocsube, S., Dijksterhuis, J., et al. (2016). Aspergillus section Nidulantes (formerly Emericella): polyphasic taxonomy, chemistry and biology. Stud. Mycol. 84, 1–118. doi: 10.1016/j.simyco.2016.10.001
Chen, Q., Hou, L. W., Duan, W. J., Crous, P. W., and Cai, L. (2017). Didymellaceae revisited. Stud. Mycol. 87, 105–159. doi: 10.1016/j.simyco.2017.06.002
Chen, Q., Jiang, J. R., Zhang, G. Z., Cai, L., and Crous, P. W. (2015). Resolving the Phoma enigma. Stud. Mycol. 82, 137–217. doi: 10.1016/j.simyco.2015.10.003
Chilaka, C. A., De Boevre, M., Atanda, O. O., and De Saeger, S. (2018). Prevalence of Fusarium mycotoxins in cassava and yam products from some selected Nigerian markets. Food Control 84, 226–231. doi: 10.1016/j.foodcont.2017.08.005
Commission of the European Communities, (2000). Commission regulation (EC) No 824/2000 of 19 April 2000 establishing procedures for the taking-over of cereals by intervention agencies and laying down methods of analysis for determining the quality of cereals. Off. J. Eur. Commun. L100, 31–50.
Cui, C. B., Kakeya, H., and Osada, H. (1996). Novel mammalian cell cycle inhibitors, tryprostatins A, B and other diketopiperazines produced by Aspergillus fumigatus II physico-chemical properties and structures. J. Antibiot. 49, 534–540. doi: 10.7164/antibiotics.49.534
Curtin, T. P., and Reilly, J. (1940). Sclerotiorine, C20H20O5Cl, a chlorine-containing metabolic product of Penicillium sclerotiorum van Beyma. Biochem. J. 34, 1418–1421. doi: 10.1042/bj0341418
dos Santos, M. R. R., Cardoso Filho, F. D. C., Calvet, R. M., Andrade, R. R., Pires, R. M. C., Periera, M. M. G., et al. (2015). Aflatoxin B1 in commercial granolas. Afr. J. Microbiol. Res. 9, 2391–2395. doi: 10.5897/AJMR2015.7633
Ediage, E. N., Di Mavungu, J. D., Monbaliu, S., Van Peteghem, C., and De Saeger, S. A. (2011). A validated mulitanalyte LC-MS/MS method for quantification of 25 mycotoxins in cassava flour, peanut cake and maize samples. J. Agric. Food Chem. 59, 5173–5180. doi: 10.1021/jf2009364
Egbontan, A. O., Afolabi, C. G., Kehinde, I. A., Enikuomehin, O. A., Ezekiel, C. N., Sulyok, M., et al. (2017). A mini-survey of moulds and mycotoxins in locally grown and imported wheat grains in Nigeria. Mycotoxin Res. 33, 59–64. doi: 10.1007/s12550-016-0264-8
Egbuta, M. A., Mwanza, M., Njobeh, P. B., Phoku, J. Z., Chilaka, C. A., and Dutton, M. F. (2015). Isolation of filamentous fungi species contaminating some Nigerian food commodities. J. Food Res. 4:38. doi: 10.5539/jfr.v4n1p38
Escrivá, L., Oueslati, S., Font, G., and Manyes, L. (2017). Alternaria mycotoxins in food and feed: an overview. J. Food Qual. 2017:1569748. doi: 10.1155/2017/1569748
Essono, G., Ayodele, M., Akoa, A., Foko, J., Filtenborg, O., and Olembo, S. (2009). Aflatoxin-producing Aspergillus spp. and aflatoxin levels in stored cassava chips as affected by processing practices. Food Control 20, 648–654. doi: 10.1016/j.foodcont.2008.09.018
European Commission [EC], (2002). Commission decision 2002/657 of 12 august 2002 implementing council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Union L221, 8–36.
European Food Safety Authority [EFSA], (2012). Scientific opinion on the risks for public and animal health related to the presence of citrinin in food and feed. EFSA J. 10:2605. doi: 10.2903/j.efsa.2012.2605
European Food Safety Authority [EFSA], (2018). Draft Guidance on Harmonised Methodologies for Human Health, Animal Health and Ecological Risk Assessment of Combined Exposure to Multiple Chemicals. For Public Consultation. EFSA Scientific Committee. Available online at: https://www.efsa.europa.eu/sites/default/files/consultation/consultation/180626-1-ax1.pdf (accessed July 18, 2019).
Ezekiel, C. N., Sulyok, M., Babalola, D. A., Warth, B., Ezekiel, V. C., and Krska, R. (2013). Incidence and consumer awareness of toxigenic Aspergillus section Flavi and aflatoxin B1 in peanut cake from Nigeria. Food Control 30, 596–601. doi: 10.1016/j.foodcont.2012.07.048
Ezekiel, C. N., Sulyok, M., Ogara, I. M., Abia, W. A., Warth, B., Šarkanj, B., et al. (2019). Mycotoxins in uncooked and plate-ready household food from rural northern Nigeria. Food Chem. Toxicol. 128, 171–179. doi: 10.1016/j.fct.2019.04.002
Ezekiel, C. N., Sulyok, M., Somorin, Y., Odutayo, F. I., Nwabekee, S. U., Balogun, A. T., et al. (2016). Mould and mycotoxin exposure assessment of melon and bush mango seeds, two common soup thickeners consumed in Nigeria. Int. J. Food Microbiol. 237, 83–91. doi: 10.1016/j.ijfoodmicro.2016.08.019
Ezekiel, C. N., Sulyok, M., Warth, B., Odebode, A. C., and Krska, R. (2012). Natural occurrence of mycotoxins in peanut cake from Nigeria. Food Control 27, 338–342. doi: 10.1016/j.foodcont.2012.04.010
FAOSTAT (2017). FAOSTAT Homepage. Available online at: http://www.fao.org/faostat/en/#data/QC/visualize (accessed September 27, 2019).
Filtenborg, O., Frisvad, J. C., and Svendsen, J. A. (1983). Simple screening method for moulds producing intracellular mycotoxins in pure cultures. Appl. Environ. Microbiol. 45, 581–585. doi: 10.1128/aem.45.2.581-585.1983
Food, and Agricultural Organization of the United Nations [FAO], and World Health Organization [WHO], (1992). Codex Alimetarius Commission. Codex Standard for Edible Cassava flour. African Regional Standard. Eighth Session of the Codex Committee aon Cereals, Pulses and Legumes, CX/CPL 92/9 June 1992. Rome: FAO Food Standards Program.
Frisvad, J. C., Hubka, V., Ezekiel, C. N., Hong, S.-B., Novakova, A., Chen, A. J., et al. (2019). Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Stud. Mycol. 93, 1–63. doi: 10.1016/j.simyco.2018.06.001
Frisvad, J. C., Larsen, T. O., de Vries, R., Meijer, M., Houbraken, J., Cabañes, F. J., et al. (2007). Secondary metabolite profiling, growth profiles and other tools for species recognition and important Aspergillus mycotoxins. Stud. Mycol. 59, 31–37. doi: 10.3114/sim.2007.59.04
Frisvad, J. C., Møller, L. L. H., Larsen, T. O., Kumar, R., and Arnau, J. (2018). Safety of the fungal workhorses of industrial biotechnology: update on the mycotoxin and secondary metabolite potential of Aspergillus niger, Aspergillus oryzae, and Trichoderma reesei. Appl. Microbiol. Biotechnol. 102, 9481–9515. doi: 10.1007/s00253-018-9354-1
Frisvad, J. C., and Samson, R. A. (2004). Polyphasic taxonomy of Penicillium subgenus Penicillium. A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Stud. Mycol. 49, 1–174.
Gauthier, A., Jaubert, J., Traversier, N., Lemant, J., Balu, L., Garcia−Hermoso, D., et al. (2017). Trametes polyzona, an emerging filamentous basidiomycete in Réunion Island. Mycoses 60, 412–415. doi: 10.1111/myc.12609
Giraldo, A., Gené, J., Sutton, D. A., Madrid, H., de Hoog, G. S., Cano, J., et al. (2015). Phylogeny of Sarocladium (Hypocreales). Persoonia 34, 10–24. doi: 10.3767/003158515X685364
Gourama, H. (2015). A preliminary mycological evaluation of organic and conventional foods. Food Prot. Trends 35, 385–391.
Groenewald, J. Z., Nakashima, C., Nishikawa, J., Shin, H.-D., Park, J.-H., Jama, A. N., et al. (2013). Species concepts in Cercospora: spotting the weeds among the roses. Stud. Mycol. 75, 115–170. doi: 10.3114/sim0012
Groenewald, M., Groenewald, J. Z., and Crous, P. W. (2005). Distinct species exist within the Cercospora apii morphotype. Phytopathology 95, 951–959. doi: 10.1094/PHYTO-95-0951
Guynot, M. E., Marin, S., Sanchis, V., and Ramos, A. J. (2003). Modified atmosphere packaging for prevention of mold spoilage of bakery products with different pH and water activity levels. J. Food Prot. 66, 1864–1872. doi: 10.4315/0362-028X-66.10.1864
Hallas-Møller, M., Nielsen, K. F., and Frisvad, J. C. (2016). Production of the Fusarium mycotoxin Moniliformin by Penicillium melanoconidium. J. Agric. Food Chem. 64, 4505–4510. doi: 10.1021/acs.jafc.6b00298
Han, M.-L., Chen, Y.-Y., Shen, L.-L., Song, J., Vlasák, J., Dai, Y.-C., et al. (2016). Taxonomy and phylogeny of the brown-rot fungi: fomitopsis and its related genera. Fungal Divers. 80, 343–373. doi: 10.1007/s13225-016-0364-y
Hernández-Restrepo, M., Groenewald, J. Z., and Crous, P. W. (2016). Taxonomic and phylogenetic re-evaluation of Microdochium, Monographella and Idriella. Persoonia 36, 57–82. doi: 10.3767/003158516x688676
Hong, S.-B., Lee, M., Kim, D.-H., Varga, J., Frisvad, J. C., Perrone, G., et al. (2013). Aspergillus luchuensis, An industrially important black Aspergillus in East Asia. PLoS One 8:E63769. doi: 10.1371/Journal.Pone.0063769
Hoog, G. S. (2000). Atlas of Clinical Fungi, 2nd Edn. Utrecht: Centraalbureau voor Schimmelcultures, 505.
Houbraken, J., Frisvad, J. C., and Samson, R. A. (2011). Taxonomy of Penicillium section Citrina. Stud. Mycol. 70, 53–138. doi: 10.3114/sim.2011.70.02
Houbraken, J., Frisvad, J. C., Seifert, K. A., Overy, D. P., Tuthill, D. M., Valdez, J. G., et al. (2012). New penicillin-producing Penicillium species and an overview of section Chrysogena. Persoonia 29, 78–100. doi: 10.3767/003158512X660571
Houbraken, J., and Samson, R. A. (2011). Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Stud. Mycol. 70, 1–51. doi: 10.3114/sim.2011.70.01
International Agency for Research on Cancer [IARC], (1993). IARC Monographs on the Evaluation of Carcinogenic Risks of Chemicals to Humans, Vol 56. Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. Lyon: International Agency for Research on Cancer, 397–444.
Kayode, O. F., Sulyok, M., Fapohunda, S. O., Ezekiel, C. N., Krska, R., and Oguntona, C. R. B. (2013). Mycotoxins and fungal metabolites in groundnut- and -maize based snacks from Nigeria. Food Addit. Contam. Part B Surveill. 6, 294–300. doi: 10.1080/19393210.2013.823626
Klarić, M. S., Rasic, D., and Peraiaca, M. (2013). Deleterious effects of mycotoxin combinations involving ochratoxin A. Toxins 5, 1965–1987. doi: 10.3390/toxins5111965
Kovač, T., Borišev, I., Kovač, M., Lončarić, A., Čačić Kenjerić, F., Djordjevic, A., et al. (2020). Impact of fullerol C60(OH)24 nanoparticles on the production of emerging toxins by Aspergillus flavus. Sci. Rep. 10:725. doi: 10.1038/s41598-020-57706-3
Lamboni, Y., Nielsen, K. F., Linnemann, A. R., Gezgin, Y., Hell, K., Nout, M. J. R., et al. (2016). Diversity in secondary metabolites including mycotoxins from strains of Aspergillus section Nigri isolated from raw cashew nuts from Benin, West Africa. PLoS One 11:e0164310. doi: 10.1371/journal.pone.0164310
Lee, H. B., Patriarca, A., and Magan, N. (2015). Alternaria in food: ecophysiology, mycotoxin production and toxicology. Synapse 43, 93–106. doi: 10.5941/MYCO.2015.43.2.93
Leslie, J. F., and Summerell, B. A. (2006). The Fusarium Laboratory Manual. Ames: Blackwell Publishing.
Lueangjaroenkit, P., Teerapatsakul, C., and Chitradon, L. (2018). Morphological characteristic regulation of ligninolytic enzyme produced by Trametes polyzona. Mycobiology 46, 396–406. doi: 10.1080/12298093.2018.1537586
Magan, N., Aldred, D., Mylona, K., and Lambert, R. J. W. (2010). Limiting mycotoxins in stored wheat. Food Addit. Contam. Part A 27, 644–650. doi: 10.1080/19440040903514523
Makinde, O. M., Ayeni, I., Sulyok, M., Krska, R., Adeleke, R. A., and Ezekiel, C. N. (2020). Microbiological safety of ready−to−eat foods in low−and middle−income countries: a comprehensive 10−year (2009 to 2018) review. Comp. Rev. Food Sci. Food Safety 19, 703–732. doi: 10.1111/1541-4337.12533
Makun, H. A., Adeniran, A. L., Mailafiya, S. C., Ayanda, I. S., Mudashiru, A. T., Ojukwu, U. J., et al. (2013). Natural occurrence of ochratoxin A in some marketed Nigerian foods. Food Control 31, 566–571. doi: 10.1016/j.foodcont.2012.09.043
Makun, H. A., Dutton, M. F., Njobeh, P. B., Phoku, J. Z., and Yah, C. S. (2011). Incidence, phylogeny and mycotoxigenic potentials of fungi isolated from rice in Niger state, Nigeria. J. Food Safety 31, 334–349. doi: 10.1111/j.1745-4565.2011.00305.x
Manjula, K., Hell, K., Fandohan, P., Abass, A., and Bandyopadhyay, R. (2009). Aflatoxin and fumonisin contamination of cassava products and maize grain from markets in Tanzania and republic of the Congo. Toxin 28, 63–69. doi: 10.1080/15569540802462214
Masih, A., Singh, P. K., Kathuria, S., Agarwal, K., Meis, J. F., and Chowdhary, A. (2016). Identification by molecular methods and matrix-assisted laser desorption ionization–time of flight mass spectrometry and antifungal susceptibility profiles of clinically significant rare Aspergillus species in a referral chest hospital in Delhi, India. J. Clin. Microbiol. 54, 2354–2364. doi: 10.1128/JCM.00962-16
Mastanjević, K., Šarkanj, B., Warth, B., Krska, R., Sulyok, M., Mastanjeviæ, K., et al. (2018). Fusarium culmorum multi-toxin screening in malting and brewing by-products. LWT Food Sci. Technol. 98, 642–645. doi: 10.1016/j.lwt.2018.09.047
Matumba, L., Sulyok, M., Monjerezi, M., Biswick, T., and Krska, R. (2015). Fungal metabolites diversity in maize and associated human dietary exposures relate to micro-climatic patterns in Malawi. World Mycotoxin J. 8, 269–282. doi: 10.3920/WMJ2014.1773
Moreira, M. F., Oliveira, T. R., Vieira, I. G. P., Freire, F. C. O., Silva, S. C., Ribeiro, L. M., et al. (2016). Occurrence of fungi and aflatoxins B in nuts and products marketed the Brazilian northeastern regions. Rev. Inst. Adolfo Lutz São Paulo 75:1698.
Munkvold, G. P. (2016). “Fusarium species and their associated mycotoxins,” in Mycotoxigenic Fungi: Methods and Protocols, Methods in Molecular Biology, vol 1542, eds A. Moretti, and A. Susca (New York, NY: Human Press), 51–106. doi: 10.1007/978-1-4939-6707-0_4
Nesic, K., Ivanovic, S., and Nesic, V. (2013). Fusarial toxins: secondary metabolites of Fusarium fungi. Rev. Environ. Contam. Toxicol. 228, 101–120. doi: 10.1007/978-3-319-01619-1_5
Nielsen, K. F., Mogensen, J. M., Johansen, M., Larsen, T. O., and Frisvad, J. C. (2009). Review of secondary metabolites and mycotoxins from the Aspergillus niger group. Anal. Bioanal. Chem. 395, 1225–1242. doi: 10.1007/s00216-009-3081-5
O’Donnell, K., Rooney, A. P., Proctor, R. H., Brown, D. W., McCormick, S. P., Ward, T. J., et al. (2013). Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genet. Biol. 52, 20–31. doi: 10.1016/j.fgb.2012.12.004
Ogiehor, I. S., Ikenebomeh, M. J., and Ekundayo, A. O. (2007). The bioload and aflatoxin content of market garri from some selected states in southern Nigeria: public health significance. Afr. Health Sci. 7, 223–227.
Oguntoyinbo, F. A., and Narbad, A. (2012). Molecular characterization of lactic acid bacteria and in situ amylase expression during traditional fermentation of cereal foods. Food Microbiol. 3, 254–262. doi: 10.1016/j.fm.2012.03.004
Ojuri, O. T., Ezekiel, C. N., Eskola, M. K., Sarkanj, B., Babalola, A. D., Sulyok, M., et al. (2019). Mycotoxin co-exposures in infants and young children consuming household- and industrially-processed complementary foods in Nigeria and risk management advice. Food Control 98, 312–322. doi: 10.1016/j.foodcont.2018.11.0249
Ojuri, O. T., Ezekiel, C. N., Sulyok, M., Ezeokoli, O. T., Oyedele, O. A., Ayeni, K. I., et al. (2018). Assessing the mycotoxicological risk from consumption of complementary foods by infants and young children in Nigeria. Food Chem. Toxicol. 121, 37–50. doi: 10.1016/j.fct.2018.08.025
Okafor, N. (1977). Micro-organisms associated with cassava fermentation for garri production. J. Appl. Bacteriol. 42, 279–284. doi: 10.1111/j.1365-2672.1977.tb00693.x
Omonigho, S. E., and Ikenebomeh, M. J. (2002). Microbiological quality assessment of garri with reference to thermonuclease activity. Food Control 13, 535–541. doi: 10.1016/S0956-7135(02)00042-7
Ostry, V. (2009). Alternaria mycotoxins: an overview of chemical characterization, producers, toxicity, analysis and occurrence in food stuffs. World Mycotoxin J. 1, 175–188. doi: 10.3920/WMJ2008.x013
Oyedele, O. A., Ezekiel, C. N., Sulyok, M., Adetunji, M. C., Warth, B., Atanda, O. O., et al. (2017). Mycotoxin risk assessment for consumers of groundnut in domestic markets in Nigeria. Int. J. Food Microbiol. 251, 24–32. doi: 10.1016/j.ijfoodmicro.2017.03.020
Perrone, G., Stea, G., Epifani, F., Varga, J., Frisvad, J. C., and Samson, R. A. (2011). Aspergillus niger contains the cryptic phylogenetic species A. awamori. Fungal Biol. 115, 1138–1150. doi: 10.1016/j.funbio.2011.07.008
Rico-Munoz, E., Samson, R. A., and Houbraken, J. (2018). Mould spoilage of foods and beverages: using the right methodology. Food Microbiol. 81, 51–62. doi: 10.1016/j.fm.2018.03.016
Rivera, K. G., and Seifert, K. A. (2011). A taxonomic and phylogenetic revision of the Penicillium sclerotiorum complex. Stud. Mycol. 70, 139–158. doi: 10.3114/sim.2011.70.03
Rychlik, M., Lepper, H., Weidner, C., and Asam, S. (2016). Risk evaluation of the Alternaria mycotoxin tenuazonic acids in foods for adult and infants and subsequent risk management. Food Control 68, 181–185. doi: 10.1016/j.foodcont.2016.03.035
Saikia, S., Parker, E. J., Koulman, A., and Scott, B. (2007). Defining paxilline biosynthesis in Penicillium paxilli: functional characterization of two cytochrome P450 monooxygenases. J. Biol. Chem. 282, 16829–16837. doi: 10.1074/jbc.M701626200
Samson, R. A., Hoekstra, E. S., Frisvad, J. S., and Filtenborg, O. (1995). “Methods for the detection and isolation of food-borne fungi,” in Introduction to Foodborne Fungi, eds R. A. Samson, E. S. Hoekstra, J. C. Frisvad, and O. Filtenborg (Utrecht: Centraal Bureau voor Schimmel cultures), 235–242.
Samson, R. A., Houbraken, J., Spierenburg, H., Seifert, K. A., Peterson, S. W., Varga, J., et al. (2011). Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud. Mycol. 70, 159–189. doi: 10.3114/sim.2011.70.04
Samson, R. A., Houbraken, J., Thrane, U., Frisvad, J. C., and Andersen, B. (2019). Food and Indoor Fungi. CBS Laboratory Manual Series 2, 2nd Edn. Utrecht: Westerdijk Fungal Biodiversity Institute.
Samson, R. A., Noonim, P., Meijer, M., Houbraken, J., Frisvad, J. C., and Varga, J. (2007). Diagnostic tools to identify black aspergilli. Stud. Mycol. 59, 129–145. doi: 10.3114/sim.2007.59.13
Samson, R. A., Visagie, C. M., Houbraken, J., Hong, S.-B., Hubka, V., Klaassen, C. H. W., et al. (2014). Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 78, 141–173. doi: 10.1016/j.simyco.2014.07.004
Šarkanj, B., Ezekiel, C. N., Turner, P. C., Abia, W. A., Rychlik, M., Krska, R., et al. (2018). Ultra-sensitive, stable isotope assisted quantification of multiple urinary mycotoxin exposure biomarkers. Anal. Chim. Acta 1019, 84–92. doi: 10.1016/j.aca.2018.02.036
Shier, T. W., and Abbas, H. K. (1992). Occurrence of the mycotoxin chlamydosporol in Fusarium species. Toxicon 30, 1295–1298. doi: 10.1016/0041-0101(92)90445-b
Smedsgaard, J. (1997). Micro-scale extraction procedure for standardized screening of fungal metabolite production in cultures. J. Chromatogr. A 760, 264–270. doi: 10.1016/s0021-9673(96)00803-5
Solfrizzo, M., Visconti, A., Savard, M. E., Blackwell, B. A., and Nelson, P. E. (1994). Isolation and characterization of new chlamydosporol related metabolites of Fusarium chlamydosporum and Fusarium tricinctum. Mycopathologia 127, 95–101. doi: 10.1007/bf01103065
Sørensen, J. L., Phipps, R. K., Nielsen, K. F., Schroers, H.-J., Frank, J., and Thrane, U. (2009). Analysis of Fusarium avenaceum metabolites produced during wet apple core rot. J. Agric. Food Chem. 57, 1632–1639. doi: 10.1021/jf802926u
Sulyok, M., Beed, F., Boni, S., Abass, A., Mukunzi, A., and Krska, R. (2015). Quantification of multiple mycotoxins and cyanogenic glucosides in cassava samples from Tanzania and Rwanda by an LC-MS/MS based multi-toxin method. Food Addit. Contam. Part A 32, 488–502. doi: 10.1080/19440049.2014.975752
Sulyok, M., Krska, R., and Schuhmacher, R. (2007). A liquid chromatography/tandem mass spectrometric multi-mycotoxin method for the quantification of 87 analytes and its application to semi-quantitative screening of moldy food samples. Anal. Bioanal. Chem. 389, 1505–1523. doi: 10.1007/s00216-007-1542-2
Sulyok, M., Stadler, D., Steiner, D., and Krska, R. (2020). Validation of an LC-MS/MS-based dilute-and-shoot approach for the quantification of > 500 mycotoxins and other secondary metabolites in food crops: challenges and solutions. Anal. Bioanal. Chem. doi: 10.1007/s00216-020-02489-9
Summerbell, R. C., Gueidan, C., Schroers, H.-J., de Hoog, G. S., Starink, M., Rosete, Y. A., et al. (2011). Acremonium phylogenetic overview and revision of Gliomastix Sarocladium, and Trichothecium. Stud. Mycol. 68, 139–162. doi: 10.3114/sim.2011.68.06
Tadesse, W., Bishaw, Z., and Assefa, S. (2018). Wheat production and breeding in sub-Saharan Africa. Int. J. Climate Change Strateg. Manag. 11, 696–715. doi: 10.1108/ijccsm-02-2018-0015
Thomas, B. T., Effedua, H. I., Davies, A., and Oluwadun, A. (2012). Fungi associated with the deterioration of garri (a traditional fermented cassava product) in Ogun State, Nigeria. Researcher 4, 8–12.
Thomas, B. T., Ogunkanmi, L. A., Iwalokun, B. A., and Agu, G. C. (2017). Molecular characterization and strain typing of fungal contaminants of processed Manihot esculenta Crantz (“garri”) in Ogun State, Nigeria. Ann. Health Res. 3, 112–117.
Uhlig, S., Petersen, D., Flåøyen, A., and Wilkins, A. (2005). 2-Amino-14,16-dimethyloctadecan-3-ol, a new sphingosine analogue toxin in the fungal genus Fusarium. Toxicon 46, 513–522. doi: 10.1016/j.toxicon.2005.06.016
Uka, V., Moore, G. G., Arroyo-Manzanares, N., Nebija, D., De Saeger, S., and Di Mavungu, J. D. (2019). Secondary metabolite dereplication and phylogenetic analysis identify various emerging mycotoxins and reveal the high intra-species diversity in Aspergillus flavus. Front. Microbiol. 10:667. doi: 10.3389/fmicb.2019.00667
Varga, J., Frisvad, J. C., Kocsubé, S., Brankovics, B., Tóth, B., Szigeti, G., et al. (2011). New and revisited species in Aspergillus section Nigri. Stud. Mycol. 69, 1–17. doi: 10.3114/sim.2011.69.01
Vejdovszky, K., Hahn, K., and Braun, D. (2017a). Synergistic estrogenic effects of Fusarium and Alternaria mycotoxins in vitro. Arch. Toxicol. 91:1447. doi: 10.1007/s00204-016-1795-7
Vejdovszky, K., Hahn, K., Warth, B., and Marko, D. (2016). Co-occurring mycoestrogens formed by Fusarium and Alternaria species mediate synergistic estrogenic effects. Toxicol. Lett. 258:S55. doi: 10.1016/j.toxlet.2016.06.1293
Vejdovszky, K., Sack, M., Jarolim, K., Aichinger, G., Somoza, M. M., and Marko, D. (2017b). In vitro combinatory effects of the Alternaria mycotoxins alternariol and altertoxin II and potentially involved miRNAs. Toxicol. Lett. 267, 45–52. doi: 10.1016/j.toxlet.2016.12.011
Vesth, T. C., Nybo, J. L., Theobald, S., Frisvad, J. C., Larsen, T. O., and Nielsen, K. F. (2018). Investigation of inter- and intraspecies variation through genome sequencing of Aspergillus section Nigri. Nat. Genet. 50, 1688–1695. doi: 10.1038/s41588-018-0246-1
Villani, A., Proctor, R. H., Kim, H.-S., Brown, D. W., Logrieco, A. F., Amatulli, M. T., et al. (2019). Variation in secondary metabolite production potential in the Fusarium incarnatum-equiseti species complex revealed by comparative analysis of 13 genomes. BMC Genomics 20:314. doi: 10.1186/s12864-019-5567-7
Visagie, C. M., Hirooka, Y., Tanney, J. B., Whitfield, E., Mwange, K., Meijer, M., et al. (2014a). Aspergillus, Penicillium and Talaromyces isolated from house dust samples collected around the world. Stud. Mycol. 78, 63–139. doi: 10.1016/j.simyco.2014.07.002
Visagie, C. M., Houbraken, J., Frisvad, J. C., Hong, S.-B., Klaassen, C. H. W., Perrone, G., et al. (2014b). Identification and nomenclature of the genus Penicillium. Stud. Mycol. 78, 343–371. doi: 10.1016/j.simyco.2014.09.001
Visagie, C. M., Varga, J., Houbraken, J., Meijer, M., Kocsube, S., Yilmaz, N., et al. (2014c). Ochratoxin production and taxonomy of the yellow aspergilli (Aspergillus section Circumdati). Stud. Mycol. 78, 1–61. doi: 10.1016/j.simyco.2014.07.001
Warth, B., Parich, A., Atehnkeng, J., Bandyopadhyay, R., Schuhmacher, R., Sulyok, M., et al. (2012). Quantitation of mycotoxins in food and feed from Burkina Faso and Mozambique using a modern LC-MS/MS multitoxin method. J. Agric. Food Chem. 60, 9352–9363. doi: 10.1021/jf302003n
Keywords: chemotaxonomy, citrinin, food safety, garri, granola, secondary metabolites
Citation: Ezekiel CN, Oyedele OA, Kraak B, Ayeni KI, Sulyok M, Houbraken J and Krska R (2020) Fungal Diversity and Mycotoxins in Low Moisture Content Ready-To-Eat Foods in Nigeria. Front. Microbiol. 11:615. doi: 10.3389/fmicb.2020.00615
Received: 08 January 2020; Accepted: 19 March 2020;
Published: 09 April 2020.
Edited by:
Anindya Chanda, University of South Carolina, United StatesReviewed by:
Donát Magyar, National Public Health Institute (OKI), HungaryDaniela Jakšić, University of Zagreb, Croatia
Copyright © 2020 Ezekiel, Oyedele, Kraak, Ayeni, Sulyok, Houbraken and Krska. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chibundu N. Ezekiel, Y2hhdWdlekBnbWFpbC5jb20=
 Chibundu N. Ezekiel
Chibundu N. Ezekiel Oluwawapelumi A. Oyedele
Oluwawapelumi A. Oyedele Bart Kraak3
Bart Kraak3 Kolawole I. Ayeni
Kolawole I. Ayeni Michael Sulyok
Michael Sulyok Jos Houbraken
Jos Houbraken