- 1V.A. Greater Los Angeles Healthcare System, Los Angeles, CA, United States
- 2Department of Neurology, University of California, Los Angeles, Los Angeles, CA, United States
- 3Departments of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Los Angeles, CA, United States
- 4David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, United States
Background: Empathy deficits are a widely recognized symptom in the behavioral variant frontotemporal dementia (bvFTD), and although several reviews have examined cognitive empathy deficits, there are no meta-analytic studies on affective empathy deficits.
Objective: Identify salience of affective empathy in bvFTD.
Method: A thorough review of affective empathy found 139 possible studies, but only 10 studies included measures of affective empathy and met standardized criteria.
Results: BvFTD patients demonstrated a modest impairment compared to controls across all tasks (d = 0.98). Empathic concern as measured by the interpersonal reactivity index was particularly effected (d = 1.12).
Conclusions: This study provides evidence for an increased commitment to observing affective empathy in bvFTD and capturing its role in the disorder.
Introduction
The behavioral variant of frontotemporal dementia (bvFTD) is neurodegenerative disorder that preys upon the social centers of the frontal and temporal lobes. Early in the progression, individuals with bvFTD demonstrate marked socioemotional behavioral disturbances including lack of insight, emotional blunting, and social disinhibition (1). One of the most problematic social changes is their loss of empathy as it deeply impacts their relationships (2). The loss of empathy is one of the five behavioral criteria in the International Consensus Criteria for the diagnosis of bvFTD (1). However, the concept of empathy itself is complicated and continues to be poorly defined in studies of bvFTD.
Empathy can be broadly defined as identifying with other's feeling states (3). More precisely it is an awareness of inhabiting an affect state corresponding to an affect state of another through observing or imagining that other's state (4, 5). This involves multiple affective experiences and includes emotional contagion or “affect sharing” in addition to affective perspective-taking, an extension of mentalizing (6). Thus, empathy is frequently broken down into affective and cognitive components, primarily: affect sharing and mentalizing (7). Given this characterization, we might have better insight into the type of empathy deficits that bvFTD patients demonstrate.
Several recent reviews and meta-analytic studies highlight the role of mentalizing, the basis of cognitive empathy, in bvFTD. Mentalizing or “Theory of Mind” (ToM) involves apprehending the thoughts (cognitive ToM) or feelings (affective ToM) of others. Lesion studies have indicated that ventromedial frontal lesions result in deficits in ToM and cognitive empathy (8). These deficits have been used to contrast bvFTD from Alzheimer's disease (AD). Bora et al. (9) reviewed 30 studies finding ToM deficits in bvFTD patients compared AD patients particularly in recognizing social faux pas. Clearly there is utility in examining cognitive empathy or mentalizing in this population. In a recent review by Henry et al. (10) of studies totalling 312 patients with bvFTD, they found significant difficulty with mentalizing tasks among these patients. More germane to this study, they found that emotion recognition played a salient role in studies despite not capturing affect sharing itself.
Whereas several robust reviews of mentalizing help to illuminate the cognitive impact on empathy, there are relatively few studies examining emotional empathy or affect sharing. Studies can evaluate emotional empathy by gauging aspects of affective empathy or the presence of visceral reactions to others affective states (7). For example, several studies of bvFTD patients indicate a greater level of emotional blunting and callous interactions with loved-ones (11–13).
Deficits in emotional or affective empathy most prominently arises from disturbances in the medial frontal cortex and the anterior insula (8); however, deficits and may also arise from disease affecting bilateral amygdala (14), precentral gyrus (15) orbitofrontal cortex (16), inferior parietal lobule, brainstem, and thalamus (17). Given that bvFTD has early and prominent medial frontal (including anterior cingulate) and anterior insula degeneration, these patients may have a pronounced impairment inn affective empathy. Additionally, affective empathy involves functional connectivity among the ventral anterior insula, orbitofrontal cortex, amygdala, and perigenual anterior cingulate (18). The white matter tracts such as the right uncinate fasciculus lesions may also be problematic in bvFTD empathy (19). Measures of affective empathy frequently come in the form of self or caregiver inventories. A common measure used for empathy is the Interpersonal Reactivity Index (20, 21). The subscale empathic concern assesses “other-oriented” feelings e.g., one's affective reaction to another's emotions. Previous literature have identified lower levels of empathic concern in bvFTD patients when rated by their caregivers (22–24); however, this is typically denied on self-reports (22). In one study (25) indicated that a reduced capacity for empathic concern in bvFTD is associated with relate decreases in left orbitofrontal cortex, left inferior frontal gyrus, left insular cortex, and the bilateral mid-cingulate gyrus.
Other measures of affective empathy involve direct behavioral tasks or observations. For example, the Picture Viewing Paradigms (26) attempts to capture affect sharing by having participants view an object or scene then report their level of distress or emotionality in response to the task. In another example, Oliver et al. (27), observed that bvFTD patients demonstrated lower levels of shared emotional experience, diminished arousal and more positive valence when viewing negative social scenarios. Finally, tasks with psychophysiological measures have been limited. One notable example demonstrates that bvFTD patients tend to have lower blood pressure than controls when viewing a video of a man completing a disgusting act (28). Across these studies, patients with bvFTD exhibit marked deficits sharing affective states of various stimuli.
We sought to summarize and evaluate the existing studies on affective empathy in bvFTD. The literature on affect sharing and empathic concern in bvFTD is reviewed. This quantitative review provides important point estimates that may clarify the magnitude of affective empathy deficits in bvFTD. Additionally, it can lead to recommendations for further investigation.
Methods
Literature Search
We conducted a systematic review of the literature by searching the following databases: PubMed, Psych INFO, Web of Science, and Google-Scholar. The search consisted of the following terms: “bvFTD,” “bvFTD,” “FTD,” “empathy,” “experiencing sharing,” “affective empathy,” “prosocial concern,” “empathic concern,” “empathic motivation,” “IRI.” The literature search began March 3, 2017 and concluded June 11, 2017.
Inclusion Criteria
We chose studies based upon the following criteria: (1) the use of an accepted international consensus criteria for bvFTD (1, 29); (2) the presence of a non-bvFTD comparison group; (3) the presence of statistics necessary for the calculation of effect size, and (4) the use of a measure of affective empathy with a primary focus on emotion sharing. Multifactor empathy measures that incorporated cognitive theory of mind or perspective taking were excluded.
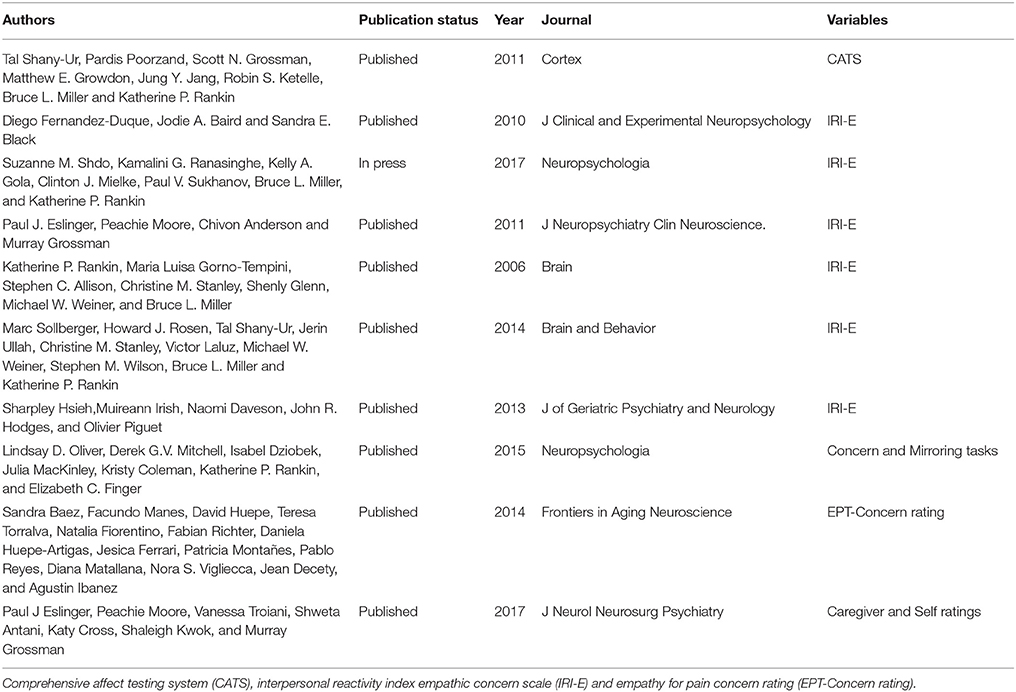
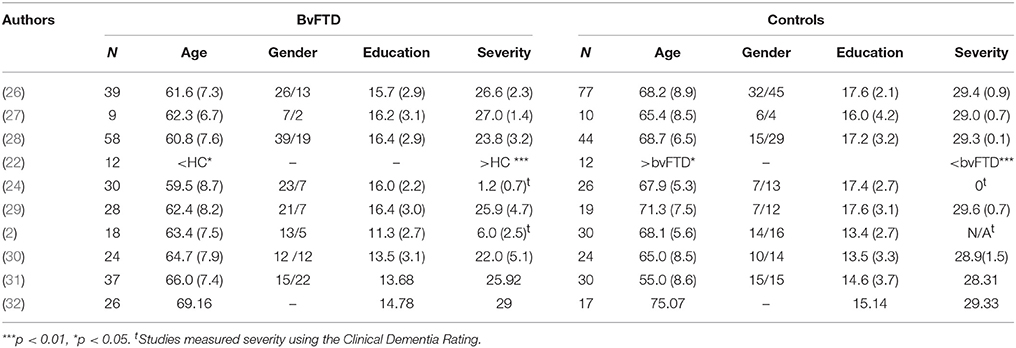
As see in Figure 1, the initial search yielded 139 studies across the databases. Only 25 studies met the first three criteria: (1) the use of an accepted international consensus criteria for bvFTD; (2) the presence of a non-bvFTD comparison group; and (3) the presence of statistics necessary for the calculation of effect size. Of those 25 only 10 included a measure of affective empathy with appropriate statistics present. Of those studies not selected empathy was characterized by emotional recognition or cognitive empathy task such as Reading the Mind in the Eyes Task (31–41). The characteristics of the included studies are included on Table 1 and the demographic information (Table 2).
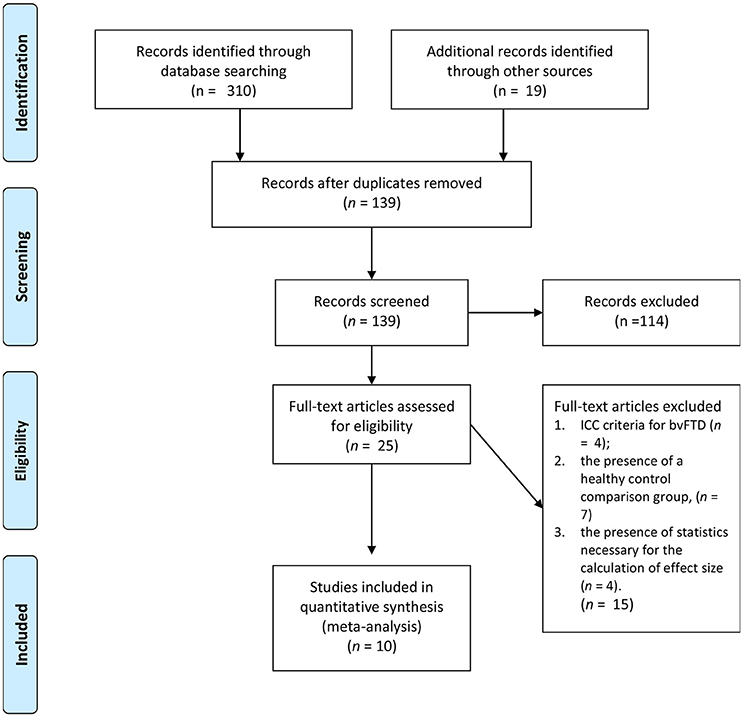
Figure 1. Literature selection process (30).
Outcomes
The primary objective of this review is to assess the impact of affective empathy in bvFTD. We include studies reporting the following outcomes. We examined affect matching evidenced by comprehensive affect testing system [CATS; (48)] and mirroring tasks (27). We also examined empathic concern ratings as evidenced by the interpersonal reactivity index empathic concern scale [IRI-E; (20)] and empathy for pain concern rating [EPT-Concern rating (49)]. We also explored outcomes for self and caregiver ratings as well as behavioral tasks.
Statistical Analysis
The authors combined the findings from the identified studies using the MetaEasy MS 1.04 Statistical package. Effect sizes were taken from pre-treatment measures in studies involving a repeated measure design.
Given the high likelihood of heterogeneity among the studies, the summary effect and 95% confidence intervals emerged from a random effects approach assuming both random and systematic error vary within the study's effect sizes using the DerSimonian-Laird (DL) approach (50). As such the Cochrane Q statistic tested heterogeneity and the I2 assessed the variation around the mean effect (51). Publication bias was assessed using Orwin's fail safe N, Egger's regression intercept.
Results
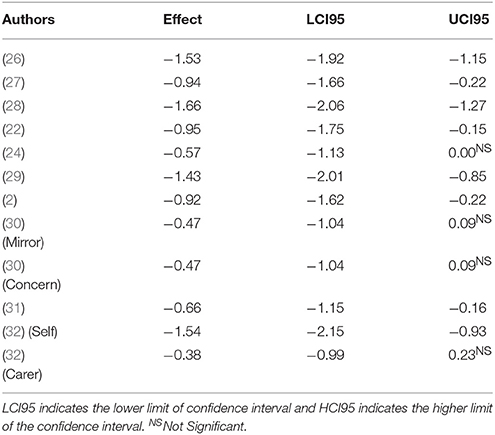
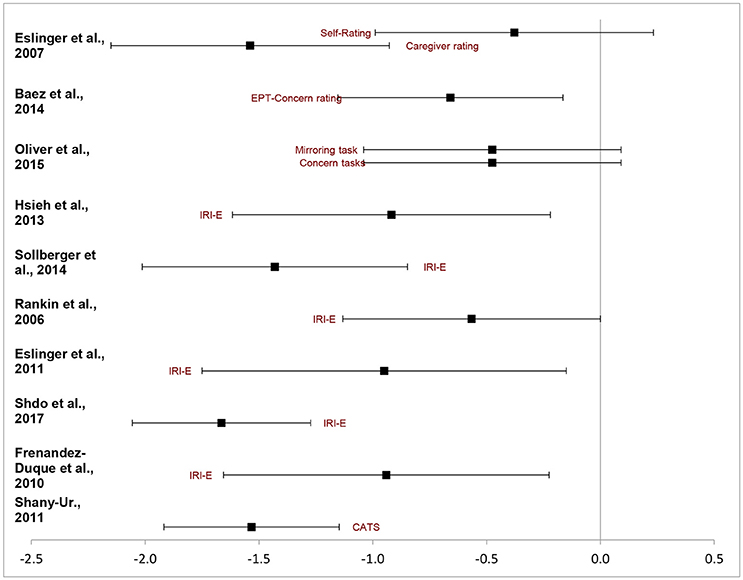
Table 3 presents a study-by-study chart of the effect sizes on emotional empathy task in bvFTD relative to others. First an overall weighted mean effect size was calculated. A negative effect indicated the bvFTD group performed at a reduced ability compared to the reference group whereas a positive one indicated the reverse. The overall random effect DL model indicated a moderate effect size, d = 0.98 95%CI (−1.25, −0.71). Thus collapsed across all studies bvFTD patients are impaired in measures of emotional empathy compared to other non FTLD groups. Figure 2 depicts the ranges of the individual studies effect sizes.
However, the analysis yielded significant heterogeneity amongst studies, Q = 30.72, pq = 0.001. The I2 estimate indicated at 64 percent difference in random and systemic error between the studies. This could be attributable to the types of measures used and the variability within bvFTD behavioral presentations. Few clear subdivisions could emerge. When examining solely the effect size of the IRI-Empathic Concern scale a relatively similar pattern immerged. The five studies identified had an overall effect of dDL = 1.12, 95%CI (−1.46, −0.08). However it too had a significant level of heterogeneity, Q = 12.33, pq = 0.031. In each of these studies bvFTD patients had a more difficult time with emotional concern than their peers. On the other ratings listed, there was a strong effect for caregiver rated measures of empathy, Z = −1.54, 95%CI (−2.17 to −0.91) but no effect for self-rated empathy, Z = −0.38, 95%CI (−1.01, −0.91).
In terms of their performance on task-based measures of emotional empathy, the results were relatively mixed. On Oliver et al.'s (27) study in which participants view a video and provided a response to indicate their emotional concern and emotional there was a trend toward significance, 95%CI (−1.06, 0.11). However, the Shany-Ur affect matching test there was a strong deficit in the bvFTD group (z = −1.53, 95%CI (−1.92, −1.14).
Discussion
The present meta-analytic study illuminates the magnitude of affective empathy deficits in bvFTD in the current published literature. This review examined the effects of 10 studies that depict impairments in affective empathy among patients with bvFTD. Given the magnitude of the effect size generated, it is likely that affective empathy plays a large role in the socioemotional alterations that characterize this disease. However, in addition to the paucity of studies, the overall heterogeneity issues across the samples indicate problems with measurement. Despite this, this meta-analysis indicates that the source of the impaired empathy in bvFTD extends beyond deficits in mentalizing to include significant primary deficits in affective empathy.
A lack of affective empathy is a central feature of bvFTD. This disorder is associated with neuropathology in areas of the brain that mediate affective empathy (52). These areas include medial frontal regions such as the ventromedial prefrontal cortex and anterior cingulate gyrus, the anterior insula, and associated areas such as the amygdale and the right anterior temporal lobe as well as corresponding neural networks according to Seeley et al. (53). It is not surprising that one of the main criteria and presentations of bvFTD is with impairments in expressions of empathy and sympathy toward other (54). These behaviors include a spectrum from simple lack of responsiveness to the concerns of loved ones to frank antisocial behaviors leading to trouble with society and the law (55). This meta-analysis supports this pathological and clinical profile of bvFTD.
Both rating scales and behavioral tasks find deficits in affective empathy. The tasks-based studies demonstrate clear problems with bvFTD patients' capacity to connect emotively with the world around them. These studies should be further replicated as they may produce insight to the individual behaviors within affective empathy, such as the lack of reciprocity in communication and the disconcerting prolongation of eye gaze (56). These compliment the robust inventories that speak to the day to day loss of affect connection which is a significant problem for family members of bvFTD (57).
The most frequent task used in the analysis was the IRI Empathic Concern scale. These studies indicated that caregivers generally feel a lack of warmth and connectedness to bvFTD patients. Although this caregiver assessment of empathic concern is only a proxy measure of affect sharing, it does indicate that bvFTD patients fail to convey affective empathy to those who know them. Given that an important evolutionary function of empathy is for prosocial connection (58), bvFTD patients fail to connect with the emotional experience of those they care about. Only the Rankin et al. (24) study failed to reach a significant effect size, which may be attributable to the use of older clinical criteria for bvFTD which has less specific socioemotional elements (29).
The task-based assessments yielded variable results. In the Shany-Ur study (42), the bvFTD patients had difficulty targeting the nonverbal aspects of affect sharing, and in the Oliver et al. study (27), the bvFTD patients did not show a significant effect. These studies differed in the required attention to nonverbal language processes and self-insight. Previous studies have shown that bvFTD patients have difficulty expressing their feeling states and lack the insight to know how well they are connecting to various social prompts (52, 59). They may instead report overlearned or social normed responses to various social situations (60). In other words, in scenarios with an easily detectable emotional prompt, like the Oliver et al study, they may respond typically, whereas when more subtly is involved in detecting emotionality from nonverbal aspects, as in the Shany-Ur study, they may disclose deficits in affect sharing.
This meta-analysis discloses several other findings from this research. One major takeaway is the paucity of studies using affective empathy as a core variable. Another finding is that most studies use a proxy measure such as a caregiver report. However, it is clear that direct task-based measures can be very informative in examining affective empathy in bvFTD. In particular, psychophysiological investigations of affective empathy can yield a more direct assessment of affective empathy among patients with bvFTD (12). Further connections to the basic sciences may help those studying early-onset dementias develop new paradigms for assessing socioemotional issues within this population.
As any study, this meta-analytic review is not without its limitations. Although many studies look at various aspects of empathy in bvFTD patients, there were surprisingly few that met all criteria. This is in large part due to the heterogeneity in research studies in this field. Often, exploring empathy within bvFTD patients is a secondary function of larger studies and uses crude measures to undertake such a task. More robust studies should be done to explore this as behavioral features are prominent in the diagnosis of this syndrome. Additionally, the use of a healthy control group excludes the typical comparisons of other dementia syndromes. Again, this was due to the low number of quality studies that involved multiple types of dementia patients. Future studies would do well to explore this more detail.
In conclusion, this review supports the presence of primary deficits in affective empathy among patients with bvFTD. Empathic concern, in particular, is a widely studied and broadly declined function in these patients. Future studies using task-based measures coupled with psychophysiological assessments and neuroimaging analysis would help further clarify this relationship and the brain-behavior mechanisms involved.
Author Contributions
AC participated in manuscript preparation, literature review, analysis, and formatting. MM participated in manuscript preparation, literature review, and analysis.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain (2011) 134:2456–77. doi: 10.1093/brain/awr179
2. Hsieh S, Irish M, Daveson N, Hodges JR, Piguet O. When one loses empathy: its effect on carers of patients with dementia. J Geriatr Psychiatry Neurol. (2013) 26:174–84. doi: 10.1177/0891988713495448
3. Preston SD, De Waal FB. Empathy: its ultimate and proximate bases. Behav Brain Sci. (2002) 25:1–20. doi: 10.1017/S0140525X02000018
4. De Vignemont F, Singer T. The empathic brain: how, when and why? Trends Cogn Sci. (2006) 10:435–41. doi: 10.1016/j.tics.2006.08.008
5. Eisenberg N, Fabes RA. Empathy: conceptualization, measurement, and relation to prosocial behavior. Motiv Emot. (1990) 14:131–49. doi: 10.1007/BF00991640
6. Hillis AE. Inability to empathize: brain lesions that disrupt sharing and understanding another's emotions. Brain (2014) 137:981–97. doi: 10.1093/brain/awt317
7. Zaki J, Ochsner KN. The neuroscience of empathy: progress, pitfalls and promise. Nat Neurosci. (2012) 15:675–80. doi: 10.1038/nn.3085
8. Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain (2009) 132:617–27. doi: 10.1093/brain/awn279
9. Bora E, Walterfang M, Velakoulis D. Theory of mind in behavioural-variant frontotemporal dementia and Alzheimer's disease: a meta-analysis. J Neurol Neurosurg Psychiatry (2015) 86:714–9. doi: 10.1136/jnnp-2014-309445
10. Henry JD, Phillips LH, Von Hippel C. A meta-analytic review of theory of mind difficulties in behavioural-variant frontotemporal dementia. Neuropsychologia (2014) 56:53–62. doi: 10.1016/j.neuropsychologia.2013.12.024
11. Joshi A, Barsuglia JP, Mather MJ, Jimenez EE, Shapira J, Mendez MF. Evaluation of emotional blunting in behavioral variant frontotemporal dementia compared to Alzheimer's disease. Demen Geriatr Cogn Dis. (2014) 38:79–88. doi: 10.1159/000357838
12. Joshi A, Mendez MF, Kaiser N, Jimenez E, Mather M, Shapira JS. Skin conductance levels may reflect emotional blunting in behavioral variant frontotemporal dementia. J Neuropsychiatry Clin Neurosci. (2014) 26:227–32. doi: 10.1176/appi.neuropsych.12110332
13. Lough S, Gregory C, Hodges JR. Dissociation of social cognition and executive function in frontal variant frontotemporal dementia. Neurocase (2001) 7:123–30. doi: 10.1093/neucas/7.2.123
14. Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci. (2010) 30:4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010
15. Hooker CI, Verosky SC, Germine LT, Knight RT, D'Esposito M. Neural activity during social signal perception correlates with self-reported empathy. Brain Res. (2010) 1308:100–13. doi: 10.1016/j.brainres.2009.10.006
16. Hynes CA, Baird AA, Grafton ST. Differential role of the orbital frontal lobe in emotional versus cognitive perspective-taking. Neuropsychologia (2006) 44:374–83. doi: 10.1016/j.neuropsychologia.2005.06.011
17. Nummenmaa L, Hirvonen J, Parkkola R, Hietanen JK. Is emotional contagion special? An fMRI study on neural systems for affective and cognitive empathy. Neuroimage (2008) 43:571–80. doi: 10.1016/j.neuroimage.2008.08.014
18. Cox CL, Uddin LQ, Di Martino A, Castellanos FX, Milham MP, Kelly C. The balance between feeling and knowing: affective and cognitive empathy are reflected in the brain's intrinsic functional dynamics. Soc Cogn Affect Neurosci. (2012) 7:727–37. doi: 10.1093/scan/nsr051
19. Oishi K, Faria AV, Hsu J, Tippett D, Mori S, Hillis AE. Critical role of the right uncinate fasciculus in emotional empathy. Ann Neurol. (2015) 77:68–74. doi: 10.1002/ana.24300
20. Davis MH. (1980). A multidimensional approach to individual differences in empathy. JSAS Catal Select Doc Psychol. 10:685.
21. Davis MH. Measuring individual differences in empathy: evidence for a multidimensional approach. J Person Soc Psychol. (1983) 44:113–26. doi: 10.1037/0022-3514.44.1.113
22. Eslinger PJ, Moore P, Anderson C, Grossman M. Social cognition, executive functioning, and neuroimaging correlates of empathic deficits in frontotemporal dementia. J Neuropsychiatry Clin Neurosci. (2011) 23:74–82. doi: 10.1176/appi.neuropsych.23.1.74
23. Lough S, Kipps CM, Treise C, Watson P, Blair JR, Hodges JR. Social reasoning, emotion and empathy in frontotemporal dementia. Neuropsychologia (2006) 44:950–8. doi: 10.1016/j.neuropsychologia.2005.08.009
24. Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, et al. Structural anatomy of empathy in neurodegenerative disease. Brain (2006) 129:2945–56. doi: 10.1093/brain/awl254
25. Dermody N, Wong S, Ahmed R, Piguet O, Hodges JR, Irish M. Uncovering the neural bases of cognitive and affective empathy deficits in Alzheimer's disease and the behavioral-variant of frontotemporal dementia. J Alzheimers Dis. (2016) 53:801–16. doi: 10.3233/JAD-160175
26. Westbury HR, Neumann DL. Empathy-related responses to moving film stimuli depicting human and non-human animal targets in negative circumstances. Biol Psychol. (2008) 78:66–74. doi: 10.1016/j.biopsycho.2007.12.009
27. Oliver LD, Mitchell DG, Dziobek I, MacKinley J, Coleman K, Rankin KP, et al. Parsing cognitive and emotional empathy deficits for negative and positive stimuli in frontotemporal dementia. Neuropsychologia (2015) 67:14–26. doi: 10.1016/j.neuropsychologia.2014.11.022
28. Eckart JA, Sturm VE, Miller BL, Levenson RW. Diminished disgust reactivity in behavioral variant frontotemporal dementia. Neuropsychologia (2012) 50:786–90. doi: 10.1016/j.neuropsychologia.2012.01.012
29. Neary D, Snowden J, Mann D. Frontotemporal dementia. Lancet Neurol. (2005) 4:771–80. doi: 10.1016/S1474-4422(05)70223-4
30. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the pRISMA statement. PLoS Med (2009) 6:e1000097. doi: 10.1371/journal.pmed1000097
31. Bertoux M, Delavest M, de Souza LC, Funkiewiez A, Lépine J.-P., Fossati P, et al. Social cognition and emotional assessment differentiates frontotemporal dementia from depression. J Neurol Neurosurg Psychiatry (2012) 83:411–6. doi: 10.1136/jnnp-2011-301849
32. Bertoux M, Volle E, De Souza L, Funkiewiez A, Dubois B, Habert M. Neural correlates of the mini-SEA (Social cognition and Emotional Assessment) in behavioral variant frontotemporal dementia. Brain Imaging Behav. (2014) 8:1–6. doi: 10.1007/s11682-013-9261-0
33. Caminiti SP, Canessa N, Cerami C, Dodich A, Crespi C, Iannaccone S, et al. Affective mentalizing and brain activity at rest in the behavioral variant of frontotemporal dementia. NeuroImage Clin. (2015) 9:484–497. doi: 10.1016/j.nicl.2015.08.012
34. Couto B, Manes F, Montañés P, Matallana D, Reyes P, Velasquez M, et al. Structural neuroimaging of social cognition in progressive non-fluent aphasia and behavioral variant of frontotemporal dementia. Front Hum Neurosci. (2013) 7:467. doi: 10.3389/fnhum.2013.00467
35. Downey LE, Mahoney CJ, Buckley AH, Golden HL, Henley SM, Schmitz N, et al. White matter tract signatures of impaired social cognition in frontotemporal lobar degeneration. NeuroImage Clin. (2015) 8:640–51. doi: 10.1016/j.nicl.2015.06.005
36. Freedman M, Binns MA, Black SE, Murphy C, Stuss DT. Theory of mind and recognition of facial emotion in dementia: challenge to current concepts. Alzheimer Dis Assoc Disord. (2013) 27:56–61. doi: 10.1097/WAD.0b013e31824ea5db
37. Gleichgerrcht E, Ibáñez A, Roca M, Torralva T, Manes F. Decision-making cognition in neurodegenerative diseases. Nat Rev Neurol. (2010) 6:611–23. doi: 10.1038/nrneurol.2010.148
38. Gregory C, Lough S, Stone V, Erzinclioglu S, Martin L, Baron-Cohen S, et al. Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer's disease: theoretical and practical implications. Brain (2002) 125:752–64. doi: 10.1093/brain/awf079
39. Kamminga J, Kumfor F, Burrell JR, Piguet O, Hodges JR, Irish M. Differentiating between right-lateralised semantic dementia and behavioural-variant frontotemporal dementia: an examination of clinical characteristics and emotion processing. J Neurol Neurosurg Psychiatry (2014) 86:1082–8. doi: 10.1136/jnnp-2014-309120
40. Kumfor F, Irish M, Leyton C, Miller L, Lah S, Devenney E, et al. Tracking the progression of social cognition in neurodegenerative disorders. J Neurol Neurosurg Psychiatry (2014) 85:1076–83. doi: 10.1136/jnnp-2013-307098
41. Sedeño L, Couto B, García-Cordero I, Melloni M, Baez S, Sepúlveda JPM, et al. Brain network organization and social executive performance in frontotemporal dementia. J Int Neuropsychol Soc. (2016) 22:250–62. doi: 10.1017/S1355617715000703
42. Shany-Ur T, Poorzand P, Grossman SN, Growdon ME, Jang JY, Ketelle RS, et al. Comprehension of insincere communication in neurodegenerative disease: lies, sarcasm, and theory of mind. Cortex (2011) 48:1329–41. doi: 10.1016/j.cortex.2011.08.003
43. Fernandez-Duque D, Hodges SD, Baird JA, Black SE. Empathy in frontotemporal dementia and Alzheimer's disease. J Clin Exp Neuropsychol. (2010) 32:289–98. doi: 10.1080/13803390903002191
44. Shdo SM, Ranasinghe KG, Gola KA, Mielke CJ, Sukhanov PV, Miller BL, et al. Deconstructing empathy: neuroanatomical dissociations between affect sharing and prosocial motivation using a patient lesion model. Neuropsychologia (2017). doi: 10.1016/j.neuropsychologia.2017.02.010. [Epub ahead of print].
45. Sollberger M, Rosen HJ, Shany-Ur T, Ullah J, Stanley CM, Laluz V, et al. Neural substrates of socioemotional self-awareness in neurodegenerative disease. Brain Behav. (2014) 4:201–14. doi: 10.1002/brb3.211
46. Baez S, Manes F, Huepe D, Torralva T, Fiorentino N, Richter F, et al. Primary empathy deficits in frontotemporal dementia. Front Aging Neurosci. (2014) 6:262. doi: 10.3389/fnagi.2014.00262
47. Eslinger PJ, Moore P, Troiani V, Antani S, Cross K, Kwok S, et al. Oops! Resolving social dilemmas in frontotemporal dementia. J Neurol Neurosurg Psychiatry (2007) 78:457–60. doi: 10.1136/jnnp.2006.098228
48. Froming K, Levy M, Schaffer S, Ekman P. The Comprehensive Affect Testing System. Psychology Software, Incs. (2006).
49. Decety J, Michalska KJ, Kinzler KD. The contribution of emotion and cognition to moral sensitivity: a neurodevelopmental study. Cereb Cortex (2012) 22:209–20. doi: 10.1093/cercor/bhr111
50. DerSimonian R, Laird N. Meta-analysis in clinical trials. Contr Clin Trials (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
51. Cooper H, Hedges LV, Valentine JC. (2009). The Handbook of Research Synthesis and Meta-Analysis. New York, NY: Russell Sage Foundation.
52. Mendez MF, Shapira JS. Loss of emotional insight in behavioral variant frontotemporal dementia or “frontal anosodiaphoria”. Consc Cogn. (2011) 20:1690–6. doi: 10.1016/j.concog.2011.09.005
53. Seeley WW, Zhou J, Kim EJ. Frontotemporal dementia: what can the behavioral variant teach us about human brain organization? Neuroscientist (2012) 18:373–385. doi: 10.1177/1073858411410354
54. Fong SS, Navarrete CD, Perfecto SE, Carr AR, Jimenez EE, Mendez MF. Behavioral and autonomic reactivity to moral dilemmas in frontotemporal dementia versus Alzheimer's disease. Soc Neurosci. (2016) 12:1–10. doi: 10.1080/17470919.2016.1186111
55. Mendez MF. The unique predisposition to criminal violations in frontotemporal dementia. J Am Acad Psychiatry Law (2010) 38:318–23.
56. Paholpak P, Li-Jung L, Carr DR, Jimenez E, Barrows RJ, Sabodash V, et al. Prolonged visual facial grasp in frontotemporal dementia. J Alzheimers Dis. (2016) 53:327–35. doi: 10.3233/JAD-150864
57. van Vliet D, de Vugt ME, Bakker C, Koopmans RT, Verhey FR. Impact of early onset dementia on caregivers: a review. Int J Geriatr Psychiatry (2010) 25:1091–100. doi: 10.1002/gps.2439
58. De Waal FBM, Suchak M. Prosocial primates: selfish and unselfish motivations. Philosoph Trans R Soc B Biol Sci. (2010) 365:2711–22. doi: 10.1098/rstb.2010.0119
59. Carr AR, Samimi MS, Paholpak P, Jimenez EE, Mendez MF. Emotional quotient in frontotemporal dementia vs. Alzheimer's disease: the role of socioemotional agnosia. Cogn Neuropsychiatry (2017) 22:28–38. doi: 10.1080/13546805.2016.1259612
Keywords: affective empathy, behavioral variant frontotemporal dementia, empathic concern, reactivity index, empathy
Citation: Carr AR and Mendez MF (2018) Affective Empathy in Behavioral Variant Frontotemporal Dementia: A Meta-Analysis. Front. Neurol. 9:417. doi: 10.3389/fneur.2018.00417
Received: 25 February 2018; Accepted: 22 May 2018;
Published: 12 June 2018.
Edited by:
Argye Hillis, Johns Hopkins Medicine, United StatesReviewed by:
Maria Salsone, Consiglio Nazionale Delle Ricerche (CNR), ItalyGuido Gainotti, Università Cattolica del Sacro Cuore, Italy
Copyright © 2018 Carr and Mendez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrew R. Carr, Y29ubmVjdEBkcmV3cmNhcnJwaGQuY29t
 Andrew R. Carr
Andrew R. Carr Mario F. Mendez
Mario F. Mendez