- 1Physical Therapy, Arkansas State University, Jonesboro, AR, United States
- 2Physical Therapy, University of Tennessee Health Science Center, Memphis, TN, United States
- 3Silverberry Genomics, San Francisco, CA, United States
Physical activity—a lifestyle factor that is associated with immune function, neuroprotection, and energy metabolism—modulates the cellular and molecular processes in the brain that are vital for emotional and cognitive health, collective mechanisms that can go awry in depression. Physical activity optimizes the stress response, neurotransmitter level and function (e.g., serotonergic, noradrenergic, dopaminergic, and glutamatergic), myokine production (e.g., interleukin-6), transcription factor levels and correlates [e.g., peroxisome proliferator-activated receptor C coactivator-1α [PGC-1α], mitochondrial density, nitric oxide pathway activity, Ca2+ signaling, reactive oxygen specie production, and AMP-activated protein kinase [AMPK] activity], kynurenine metabolites, glucose regulation, astrocytic health, and growth factors (e.g., brain-derived neurotrophic factor). Dysregulation of these interrelated processes can effectuate depression, a chronic mental illness that affects millions of individuals worldwide. Although the biogenic amine model has provided some clinical utility in understanding chronic depression, a need remains to better understand the interrelated mechanisms that contribute to immune dysfunction and the means by which various therapeutics mitigate them. Fortunately, convergent evidence suggests that physical activity improves emotional and cognitive function in persons with depression, particularly in those with comorbid inflammation. Accordingly, the aims of this review are to (1) underscore the link between inflammatory correlates and depression, (2) explicate immuno-neuroendocrine foundations, (3) elucidate evidence of neurotransmitter and cytokine crosstalk in depressive pathobiology, (4) determine the immunomodulatory effects of physical activity in depression, (5) examine protocols used to effectuate the positive effects of physical activity in depression, and (6) highlight implications for clinicians and scientists. It is our contention that a deeper understanding of the mechanisms by which inflammation contributes to the pathobiology of depression will translate to novel and more effective treatments, particularly by identifying relevant patient populations that can benefit from immune-based therapies within the context of personalized medicine.
Introduction
Depression is a pervasive health problem that includes emotional, psychomotor, cognitive, and biorhythmic disturbances (Kessler et al., 2005), symptoms that are associated with a 20-fold increase in the risk of suicide (Lépine and Briley, 2011). Current estimates suggest that close to 300 million persons are affected worldwide (Ferrari et al., 2013a), making depression the leading cause of disability as measured by disability-adjusted life years (Reddy, 2010). In addition to the incredible personal toll, the direct and indirect costs of treating depression are staggering. Spending on depression-related costs is $83.1 billion annually in the United States (Greenberg et al., 2003).
Initial progress toward understanding the pathobiology of depression was made following the serendipitous discovery that amine modulation effectuated disturbances in mood (Ghasemi et al., 2017), a finding that suggested that depression was caused by deficits in monoamine function. Accordingly, the majority of therapies for treating depression were derived to target the monoaminergic system. Notwithstanding, extant therapeutics exert a slow pace of action (3–5 weeks), have extensive side effects, and fail to provide full symptom relief in a significant proportion of persons treated (Paul and Skolnick, 2003; Trivedi, 2006). These limitations implicated other factors in depression and prompted stakeholders to diversify their search for mechanisms, biomarkers, and treatments.
Among the alternative mechanisms and therapeutics that have garnered increased attention are immune mechanisms that promote the body's natural response to protect against injury, infection, and emotional stress. The brain regulates central and peripheral immune processes via modulation of neurotransmitters (e.g., serotonergic, noradrenergic, dopaminergic, and glutamatergic), endocrine hormones, and cytokines (nonstructural proteins that are secreted by distinct cell populations and that exert variable effects at multiple levels of the central nervous system, e.g., neuroendocrine, autonomic, and behavioral) (Besedovsky and del Rey, 1992; Niciu et al., 2014; Ghasemi et al., 2017), processes that can go awry in the case of depression.
Indeed, a bevy of research indicates a link between inflammation and depression. Preclinical study demonstrates that stress paradigms (e.g., chronic unpredictable stress, learned helplessness, social defeat, and social isolation) induce pro-inflammatory cytokines centrally and peripherally (Steptoe et al., 2001; Bartolomucci et al., 2003; Grippo et al., 2005; Chourbaji et al., 2006; Audet et al., 2011; Gómez-Lázaro et al., 2011; Moller et al., 2013), changes that correlate with depressive-like symptoms (Kenis and Maes, 2002; Tuglu et al., 2003; Basterzi et al., 2005; Tsao et al., 2006; Dantzer et al., 2008; Miller et al., 2009; Elgarf et al., 2014; Lu et al., 2017) but can be mitigated following antidepressant administration (Dantzer et al., 2008; Guo et al., 2009; Miller et al., 2009; Elgarf et al., 2014; Lu et al., 2017). Others have shown that genetically modified rodents with impaired pro-inflammatory immune signaling fail to exhibit depressive-like behaviors that are induced in wild-type mice following chronic mild stress (Goshen et al., 2008; Brüning et al., 2015). Agents that induce inflammation (e.g., recombinant cytokines) in humans recapitulate symptoms of depression, effects that are circumvented with selective serotonin reuptake inhibitor (SSRI) administration (Hauser et al., 2002). In persons with autoimmune or inflammatory disorders, tumor necrosis factor (TNF) antagonists and certain nonsteroidal anti-inflammatory agents (Brunello et al., 2006; Müller et al., 2006; Tyring et al., 2006; Krishnan et al., 2007; Soczynska et al., 2009; Menter et al., 2010; Fond et al., 2014; Köhler et al., 2014; Abbott et al., 2015) exert antidepressant effects. In the general population, clinical evidence suggests an increased tendency for pro-inflammatory markers in persons with depression (increased interleukin [IL]-6, TNF-α, and CRP) relative to controls (Laske et al., 2008; Steiner et al., 2012), a trend that normalizes following response to antidepressants (Myint et al., 2005). Others have shown that administration of anti-inflammatory agents in combination with antidepressants accelerates and enhances treatment response in a subset of persons with depression (Mendlewicz et al., 2006; Müller et al., 2006; Nery et al., 2008; Akhondzadeh et al., 2009; Abbasi et al., 2012; Raison et al., 2013). Certain polymorphisms in genes associated with inflammation are associated with the risk for the development of mood disorders and treatment response (Bufalino et al., 2013; Michopoulos et al., 2015). Together, these findings implicate inflammation in a subset of persons with depression who likely exhibit unique variations in pathobiology and clinical presentation.
Fascinatingly, parallel evidence demonstrates that a lack of physical activity (PA) promotes the accumulation of visceral fat, adipose infiltration by proinflammatory immune cells, persistent low-grade inflammation (Ouchi et al., 2011), and, thereby, an increased risk for depression (Leonard, 2007). Conversely, adequate levels of persistent PA exert positive immunomodulatory (Hamer and Steptoe, 2007; Walsh et al., 2011) and antidepressant effects (Cooney et al., 2013; Schuch et al., 2016), even in persons who did not remit with conventional antidepressant treatment (Trivedi et al., 2011). Some reports suggest that PA outcomes compare favorably to antidepressant and cognitive behavioral therapy in mild to moderate depression (Mead et al., 2009). The therapeutic effect of long-term PA on depression likely includes the optimization of neurotransmitter level and function, hormone regulation, muscle-derived protein (e.g., peroxisome proliferator-activated receptor C coactivator-1α [Pgc-1α] and IL-6), and neurotrophic factors (Phillips, 2017a). Within contracting skeletal muscles, PA elicits intermittent elevations of IL-6 (Pedersen et al., 2001; Pedersen and Fischer, 2007), which then induces the synthesis of IL-10 and inhibits the release of TNF-α (Schindler et al., 1990; Apostolopoulos et al., 2014; Silverman and Deuster, 2014). Upon release into the local and systemic circulation, IL-10 promotes an anti-inflammatory milieu in the periphery. In the long-term, PA appears to lower levels of proinflammatory cytokines by altering visceral fat mass (de Lemos et al., 2012; Sell et al., 2012) and Toll-like receptors (TLRs) (Lambert et al., 1985; Francaux, 2009; Gleeson et al., 2011; Sell et al., 2012; Drummond et al., 2013), changes that may be particularly beneficial to persons with comorbid depression and metabolic disorders given that activation of TLRs contribute to the development of insulin resistance (Francaux, 2009; Liang et al., 2013). Consideration of its inexpensive low-risk profile and ease of implementation (Barbour et al., 2007) has increasingly led to the suggestion that PA can be deployed as a therapeutic strategy to reduce the degree of depressive symptoms (Cooney et al., 2013; Pemberton and Fuller Tyszkiewicz, 2016) in mild to moderate depression (Carek et al., 2011; Stanton and Reaburn, 2014; Kvam et al., 2016; Schuch et al., 2016) in all age groups (Abu-Omar et al., 2004; Motl et al., 2004).
Because it is important that clinicians and scientists understand the means by which PA can exert immunomodulatory effects in depression from both a self- and patient-education perspective, the aims of this review are to (1) underscore the link between inflammatory correlates and depression, (2) explicate immuno-neuroendocrine foundations, (3) elucidate evidence of monoaminergic and cytokine crosstalk in depressive pathobiology, (4) articulate the immunomodulatory mechanisms and pathways that confer the benefits of PA in depression, (5) examine protocols used to effectuate the benefits of PA in depression, and (6) highlight implications for clinicians and scientists. It is our contention that a deeper understanding of the mechanisms by which inflammation contributes to the pathobiology of depression will translate to novel and more effective treatments, particularly by identifying relevant patient populations that can benefit from immune-based therapies within the context of personalized medicine.
Immuno-Neuroendocrine Foundations
Within the context of homeostatic challenge, stressors initiate behavioral and immune responses so as to favor vigilance and protection against injury and immune challenge in lieu of explorative activities. The hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system coordinate these activities following activation by endogenous or exogenous stressors (Tsagarakis et al., 1989; Besedovsky and Rey, 2007). Specifically, exposure to psychological and physiological stressors activates the paraventricular nucleus of the hypothalamus. Activation of the paraventricular nucleus results in the release of corticotropin-releasing hormone (CRH), which then stimulates the release of adrenocorticotropic hormone (ACTH) from the pituitary. This, in turn, induces cortisol and catecholamine secretion from the adrenals (Figure 1). Initially, the HPA and sympathetic nervous system function to increase cortisol and catecholamine release during challenge to coordinate the fight-or-flight response. These stress hormones inhibit excess production of proinflammatory cytokines (e.g., IL-12, TNF-α, and interferon [IFN]-γ) in healthy individuals with optimal regulatory capacity, while simultaneously increasing production of anti-inflammatory cytokines (e.g., IL-10 and IL-4) (Elenkov and Chrousos, 1999). Concomitantly, cortisol exerts inhibitory effects upon the hypothalamus and pituitary (Crosby and Bains, 2012) through medial prefrontal cortex (mPFC) receptors (Hill et al., 2011a,b) and reduces stress-induced over excitability of the amygdala (Gray et al., 2015) in conditions of health. Temporal and sequential regulation of the immune response is paramount as failure in negative feedback effectuates persistent hypersecretion of proinflammatory cytokines, which can then induce central neuroinflammation (Leonard, 2001; Lacy and Stow, 2011) via active transport mechanisms at the circumventricular organs, binding to blood vessel receptors, or by retrograde transport by the vagus nerve (Maier and Watkins, 2003). By accessing the brain via the aforementioned mechanisms, systemic inflammation can activate resident microglia in the brain (McCusker and Kelley, 2013) following peripheral immune challenge (D'Mello et al., 2009) and promote depression in vulnerable individuals.
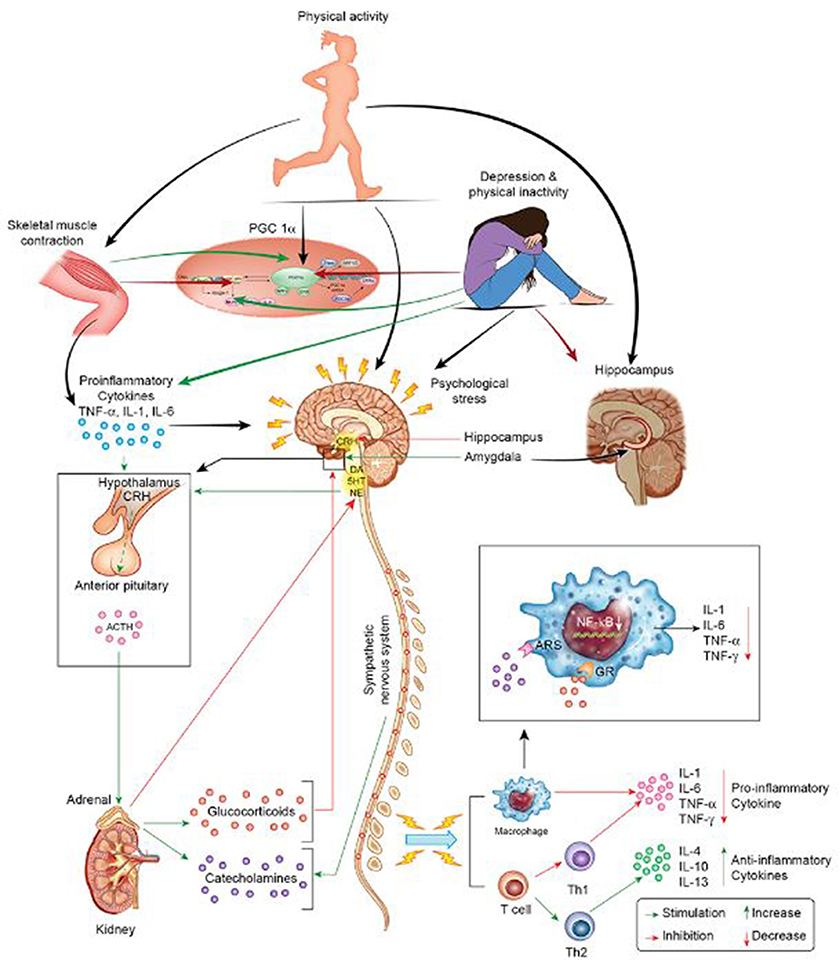
Figure 1. Stress, inflammation, and depression. The HPA and sympathetic nervous systems regulate the response to stressors, i.e., cytokines, psychological stress, and PA. The systemic response to stress is initiated via CRH secretion by the hypothalamus. CRH stimulates the pituitary to secrete ACTH into systemic circulation. In turn, ACTH secretion stimulates the adrenals to release catecholamines and glucocorticoids, factors that collectively induce pro- or anti-inflammatory cytokine release. Negative feedback mechanisms limit the process of inflammation in times of health. Conversely, persistent stress leads to dysregulation of the HPA with resultant endocrine disturbances in states of disease, e.g., depression. Stress-related disturbances in neuroendocrine hormones are problematic as they disrupt immune modulation and lead to a pro-inflammatory state. By acting as an intermittent stressor, PA exerts its' central and peripheral neuroprotective effects via several avenues. During PA, muscle contractions induce the release of myokines. These factors increase the expression of PGC-1α and decrease the expression of pro-inflammatory cytokines at the molecular level. Moreover, PA directly modulates neurotransmitter level and function (e.g., noradrenergic function), which is important promoting a pro- or anti-inflammatory milieu. Finally, PA increases hippocampal neurotrophic factor levels (e.g., BDNF) to promote hippocampal health and, thereby, promotes stress hormone regulation (e.g., cortisol regulation).
Undoubtedly, persistent systemic inflammation alters the function and expression of glucocorticoid receptors in the HPA axis, changes that impair negative feedback mechanisms (Karanth et al., 1997) at the level of the hypothalamus and anterior pituitary (Besedovsky and Rey, 2007). Chronic stress results in lower diurnal cortisol secretion as well as a blunted stress response (Peeters et al., 2003), which is problematic for immune modulation given the anti-inflammatory characteristics of catecholamines (e.g., noradrenaline (Bergmann et al., 1999) and cortisol (Cupps and Fauci, 1982). Whereas basal levels of noradrenaline promote an anti-inflammatory milieu (Bergmann et al., 1999), depletion of noradrenaline promotes a proinflammatory milieu, an effect that can be blocked by the beta adrenergic receptor agonist isoproterenol (Madrigal et al., 2005). Additionally, disruption of the catecholamine response is deleterious to the brain given that noradrenaline modulates the neuroprotective effects of astrocytes via trophic factor release (Junker et al., 2002). Much evidence clearly demonstrates that HPA dysregulation and prolonged inflammation contribute to depressive pathobiology (Pariante and Miller, 2001).
Thus, on the one hand the immune system defends against endogenous and exogenous stressors. On the other hand, it acts as a regulatory system that is in continual communication with the nervous and the endocrine systems via reciprocal communication mediated by cytokines, hormones, and neurotransmitters (Besedovsky and Rey, 2007). Imbalances among these mediators induce chronic disease conditions such as depression. Some persons with depression exhibit activated cell-mediated immunity with a T helper (Th)1-style response and elevated levels of IFN-γ (Maes et al., 1990; Maes, 2011), whereas others exhibit a distinct subtype wherein a Th2-style response predominates (Fornaro et al., 2013), possibly reflective of different stressor profiles, disease points, or genetic contributions.
At the molecular level, pro-inflammatory cytokine interactions with their cognate receptors initiate signaling events that promote a feedforward inflammatory process if left unchecked. IkB proteins typically sequester inactive transcription factors in the cytoplasm in unstimulated cells. Yet in states of low-grade inflammation, persistent receptor stimulation by cytokines and TLR agonists triggers intracellular signaling events that activate IkB kinase (IKK) activity and induce the dissociation of the IkB protein complex and, in turn, promote IkB degradation. The resultant release of nuclear factor κB (NFkB) dimers (e.g., p50/p60) permits their translocation to the nucleus and binding to cognate DNA sites that then regulates transcription of inflammatory genes and antioxidant defense (Baldwin, 1996).
Within the context of metabolic disorders, it has become increasingly evident that the presence of persistent pro-inflammatory cytokines plays a vital role in certain depressive phenotypes. The global prevalence of depression is approximately 5% in the general population; and yet, this figure approximates 20% or more in persons with obesity, diabetes, and coronary artery disease (Ferrari et al., 2013b), subsets of the population that may be particularly resistant to conventional antidepressant therapy (Raison et al., 2013; Felger et al., 2016; Haroon et al., 2016). The low-grade inflammation that results in these persons derives in part from macrophages and T-cells infiltration of adipocytes in white adipose tissue, liver, and skeletal muscle. This infiltration elicits a state of persistent secretion of proinflammatory cytokines, including TNF-α, IL-1, and IL-6 and a reduction in anti-inflammatory cytokines (e.g., adiponectin) (Hotamisligil, 2006; Kanda et al., 2006; Pedersen, 2009; Ouchi et al., 2011). The proinflammatory cytokines IL-1, IL-6, and TNF-α are thought to play a primal role in the neurotransmitter and neuroendocrine changes that occur in depression given their central role in sickness behavior. Undoubtedly, the presence of pro-inflammatory cytokines markedly affects neurotransmission within regulatory brain circuits related to emotions and induces hormonal changes commensurate with those observed following stress (Gadek-Michalska et al., 2013).
Neurotransmitter and Cytokine Interactions in Depression
Heretofore, we have established how depression is associated with peripheral and central inflammation and, thereby, how anti-inflammatory agents mitigate symptoms. Now we review evidence that inflammatory cytokines alter the level and function of key neurotransmitters that are relevant to depression neurobiology. We also explicate how cytokines activate the kynurenine pathway, lower tryptophan levels, and produce metabolites that modulate dopamine and glutamate function. We then shown how anti-inflammatory therapeutics (pharmacotherapy and PA) can optimize monoamine neurotransmitter level and function by modulating the synthesis, metabolism, and release of serotonin, noradrenaline, dopamine, and glutamate (Phillips, 2017a). Via these mechanisms, inflammation and therapeutics that mitigate depression may dramatically alter its pathobiology by directly impinging on the levels and function of key neurotransmitters that regulate depression circuit function and integrity and, ultimately, the affected individual's emotional and cognitive health.
Serotonergic Interactions
The majority of central serotonin-synthesizing neurons within the brain derive from the raphe nuclei, which are located near the midline of the brainstem (Das et al., 2014). The raphe sends more than 500,000 terminals to the cortical and limbic system (Cowen, 1991). This immense number of connections enable the raphe to modulate mood (Canli and Lesch, 2007), appetite (Blundell, 1984), arousal (Dubovsky, 1994), impulsivity (Dubovsky, 1994), aggression (Passamonti et al., 2012), and the sleep-wake cycle (Monti, 2011). Serotonin is synthesized from dietary tryptophan and translocated to the central nervous system, a rate-limiting step in serotonin synthesis. The fact that the serotonergic dorsal raphe is juxtaposed to the cerebral aqueduct suggests a vulnerability to inflammation. Indeed, evidence demonstrates that proinflammatory cytokines alter the functional status of the serotonergic system in the raphe and beyond in a manner similar to that seen in depression, providing a mechanistic explanation for the serotonergic abnormalities and associated symptoms seen in persons with depression.
Direct evidence that cytokines contribute to serotonergic dysfunction in depression pathobiology derives from data from electrophysiological, neurochemical, genetic, behavioral models, as well as from translational investigations. Intracellular recordings performed in a guinea-pig brain stem slice preparation demonstrated that IL-1β decreased spontaneous firing rates of serotonergic neurons by 50%, an effect that was reversible with washout (Manfridi et al., 2003). A parallel investigation in rodents showed that IL-1β inhibited the firing of dorsal raphe serotonergic neurons by enhancement of GABA-ergic inhibitory tone (Brambilla et al., 2007). These electrophysiological findings are congruent with the idea that IL-1 promotes non-rapid eye movement sleep by inhibiting the spontaneous firing of wake-active serotonergic neurons in the dorsal raphe nucleus (Brambilla et al., 2007). By corollary, the latter findings suggest that IL-1 alterations may contribute to alterations in arousal in depression, particularly to disturbances in stage III and IV sleep (Jones et al., 1987). Other work demonstrates that peripheral immune challenge alters the release and metabolism of central serotonin across brain regions (Dunn, 1992; Palazzolo and Quadri, 1992; Cho et al., 1999), changes that alter transporter activity. Serotonin transporters are responsible for transporting serotonin from the synaptic cleft to the presynaptic neurons to terminate signaling. As a high-affinity transporter, serotonin transporters maintain low extracellular serotonin levels in the synapse to prevent overstimulation of receptors and ensure responsiveness. Within this context, it has been determined that chronic exposure to proinflammatory cytokines alters serotonin transporter activity in a regionally specific manner and, thereby, modulates serotonin levels in the nerve terminal (Haase and Brown, 2015). Some preclinical evidence shows that stress-induced activation of the 5-HT2a serotonin receptor decreased hippocampal brain-derived neurotrophic factor (BDNF) levels (Vaidya et al., 1999).
Fortunately, several lines of work suggest that chronic PA modulates the serotonergic system to mitigate depressive symptoms in persons with inflammation. Translational studies demonstrate that plasma-free tryptophan is increased following PA (Davis et al., 1992; Melancon et al., 2012) by catecholamine-induced elevations in lipolysis and non-esterified fatty acids that displace albumin-bound tryptophan (Horowitz and Klein, 2000). Elevations in free tryptophan levels in the periphery increase tryptophan availability to the brain and, in turn, enhance serotonin synthesis (Chaouloff et al., 1986).
It seems logical that serotonin levels are more readily maintained with chronic PA given that it reduces proinflammatory markers (e.g., IFN-γ and TNF-α) and increases anti-inflammatory markers (e.g., IL-6 and IL-10) (Petersen and Pedersen, 1985; Smith et al., 1999; Panagiotakos et al., 2005; Kohut et al., 2006; Liu et al., 2013). For example, persons with depression exhibit a decrease in the pro-inflammatory cytokine TNF-α after submax exercise along with an increase in anti-inflammatory IL-4 (Hallberg et al., 2010). Within adipose tissue, PA limits proinflammatory cytokine secretion and inhibits macrophage infiltration phenotypic switching (from pro- to anti-inflammatory) of macrophages (Kawanishi et al., 2010). Within immune cells and skeletal muscles, PA decreases TLR4 and TLR2 expression (Gleeson, 1985; Lambert et al., 1985; Francaux, 2009), changes that decrease the inflammatory capacity of leukocytes and may alter whole-body chronic inflammation (Gleeson et al., 2006). Studies of human peripheral blood following PA demonstrate a reduction in circulating proinflammatory monocytes (Timmerman et al., 2008) and an increase in circulating regulatory T cells (Yeh et al., 2006).
Undoubtedly, the ability of PA to bias the immune system toward an anti-inflammatory state is significant for the serotonergic system because this state downregulates IDO activity and shifts the ratio of kynurenine metabolites toward neuroprotective kynurenic acid and away from neurotoxic quinolinic acid (Ito et al., 2003; Kiank et al., 2010). Moreover, the reduction of pro-inflammatory cytokines by PA reduces serotonin uptake by transporters to increase serotonin in the nerve terminal (Mössner et al., 2001). Finally, the ability of PA to optimize levels of tryptophan and serotonin exerts positive effects on BDNF and neurogenesis in the prefrontal cortex and hippocampus, underscoring the interrelationship between these two signaling systems (Mattson et al., 2004; Ernst et al., 2006; Esch and Stefano, 2010).
Noradrenergic Interactions
Noradrenergic neurons are a vital component of the central “stress circuitry” that induces “fight or flight” behavior, fear, and anger. Noradrenergic synthesizing neurons are primarily located in the locus coeruleus (LC), a brainstem structure within close proximity to the fourth ventricle (Phillips et al., 2016). The LC sends extensive projections to a number of brain regions including the thalamus, frontal and entorhinal cortices, basal lateral amygdala, and hippocampus (Loughlin et al., 1986). The LC's vast arborization and divergence of collaterals permit widespread synaptic and extrasynaptic release of neurotransmitters and neuropeptides throughout the central neuraxis onto neuronal and non-neuronal cells (Fornai et al., 2007, 2011), enabling the LC to exert a significant modulatory effect on behavior and HPA axis secretion. The latter occurs via dense noradrenergic projections from the LC to corticotropin-releasing hormones in the paraventricular nucleus of the hypothalamus, enabling psychological stress and immune challenges to activate the hypothalamus and trigger glucocorticoid release (Gadek-Michalska et al., 2013). Robust evidence demonstrates that proinflammatory cytokines alter the functional status of the noradrenergic system in the LC and beyond, providing a mechanistic explanation for the noradrenergic abnormalities and associated symptoms seen in persons with depression.
Direct evidence that cytokines can contribute to noradrenergic dysfunction derives from electrophysiological, neurochemical, genetic, behavioral models, and from translational studies. Microinjection of IL-1 into the LC region increased firing activity of LC neurons in the rat brain, an effect that was blocked by an IL-1 antagonist. Intraperitoneal injection of a low dose of lipopolysaccharide increased LC firing activity, an effect that lasted 3 weeks after injection (Borsody and Weiss, 2002). IL-2 and IFN-α administration altered LC electrical activity (De Sarro et al., 1990; Nisticò and De Sarro, 1991; Nisticò, 1993). The ability of inflammation to increase LC activity is significant because a common characteristic of effective antidepressants is their ability to decrease LC neuronal activity. Moreover, LC activity is highly correlated with arousal: higher rates of neuronal firing occur during the awake state, whereas complete LC neuronal inhibition occurs during rapid eye movement sleep (Hobson et al., 1974; Aston-Jones and Bloom, 1981), suggesting that alterations in firing patterns in depression may contribute to the sleep disturbances seen in depression. Juxtaposed with the electrophysiological studies are neurochemical investigations that showed that a common response to cytokine release involved an increased noradrenaline metabolism in multiple brain regions (Dunn et al., 1989), but with a preferential activation of the ventral component of the system, which derived from the nucleus tractus solitarius and LC (Dunn, 1988). Lipopolysaccharides IL-1, IL-2, and IFN-α potently activate the noradrenergic system across brain regions (De Sarro et al., 1990; Dunn, 1992; Smagin et al., 1996), a change that is not surprising because sustained stress increases noradrenergic requirements. Pharmacological manipulations that increase noradrenergic action and duration at the synapse (e.g., noradrenergic reuptake inhibitors) elevate mood and attention, mitigating the effects of stress-mediated noradrenergic depletion.
Outside the brain, noradrenaline can modulate autonomic sympathetic postganglionic fibers via primary and secondary lymphoid organs (bone marrow and thymus versus spleen and lymph nodes, respectively) during immune challenge. These actions are accomplished via direct activation of β2-adrenergic receptors that are present on Th1 cells, but not on Th 2 cells (Sanders et al., 1997). Purportedly, the anti-inflammatory effects of β2-adrenergic receptors activation stem from the inhibition of Th1 pro-inflammatory cytokines (e.g., IFN-γ, IL-12, TNF-α) or stimulation of Th2 anti-inflammatory cytokines (IL-10, IL-6, or TGF-β) (van der Poll et al., 1996; Elenkov et al., 2000). It has been shown that noradrenaline suppresses IL-12 production in a dose-dependent fashion and at physiological concentrations, whereas it dose-dependently increases the production of IL-10, effects that are blocked completely by propranolol, a β-adrenergic receptor antagonist (Elenkov et al., 1996). These findings suggest that immune balance is regulated via peripheral end-effectors of the stress system and that chronic dysregulation may bias the system toward a pro-inflammatory status (Elenkov et al., 1996).
Several lines of evidence suggest that PA modulates the noradrenergic system directly and indirectly to mitigate depressive symptoms in persons with inflammation. Within minutes, PA activates the sympathetic nervous system in an activity-dependent manner to modulate the secretion of the adrenal hormone adrenaline. Similarly, within minutes PA activates release of ACTH from the hypothalamus. These intermittent hormonal responses are vital for an anti-inflammatory milieu because intermittent elevations in adrenaline (Bergmann et al., 1999) and cortisol exert anti-inflammatory effects (Cupps and Fauci, 1982). Most studies of PA have reported a higher adrenaline response post exercise in endurance trained persons as compared to untrained controls (Zouhal et al., 2008). Other work has shown that running elevates cortisol levels in saliva and plasma in healthy persons (Duclos et al., 1998; Labsy et al., 2013) when performed at 60% of VO2max (maximum capacity of oxygen uptake) (Labsy et al., 2013). The intermittent nature of PA also contributes to a proportional increase in inactivation of the active steroid (cortisol) into the inert steroid (cortisone). The ability of PA to optimize catecholamine and cortisol levels is paramount in persons with comorbid depression and inflammation because these hormones modulate immune function and yet may be blunted as a consequence of chronic stress.
Within the brain, PA induces the neuronal adaptation that is requisite for mitigating stressful stimuli in varied ways across brain regions. Preclinical study demonstrates that 6 weeks of wheel running reduces LC firing following stress (Greenwood et al., 2003; Greenwood and Fleshner, 2008). Underlying these adaptive effects (Greenwood and Fleshner, 2008) is the upregulation of galanin in the LC, which induces a hyperpolarization of noradrenergic neurons and, thereby, inhibits excessive noradrenaline release (Seutin et al., 1989; Pieribone et al., 1995; Reiss et al., 2009; Murray et al., 2010) in some brain regions. The latter changes are essential for reducing noradrenaline levels in the amygdala to limit anxiety behavior (Sciolino and Holmes, 2012). Recapitulating these findings in humans, it has been shown that galanin increases in plasma after acute episodes of PA (Legakis et al., 2000). Conversely, long-term PA increases noradrenaline levels in the hippocampus to improve cognitive outcomes (Sarbadhikari and Saha, 2006), a finding that may have important implications for microglia and astrocytes in this brain region. The maintenance of basal levels of noradrenaline is important for inhibiting the release of the proinflammatory cytokines by microglia (Feinstein et al., 2002; Mori et al., 2002) and stimulating astrocytes to release trophic factors (e.g., BDNF) for neuroprotection (Junker et al., 2002). Prospective randomized controlled trials have demonstrated that hippocampal volumes increase following long-term aerobic PA (i.e., 1–2 years) (Erickson et al., 2011; Rosano et al., 2017).
Dopaminergic Interactions
The majority of dopaminergic neurons are found in the ventral tegmental area (VTA) of the midbrain, the substantia nigra pars compacta, and the arcuate nucleus of hypothalamus. The dopaminergic neurons of these areas project to different brain structures through the mesocortical (with neurons originating in VTA and transporting dopamine to the amygdala, hippocampus, septum, and prefrontal cortex), mesolimbic (with neurons originating in the VTA and transporting dopamine to the nucleus accumbens through the amygdala and hippocampus), and nigrostriatal pathways (with neurons originating in the substantia nigra and transporting dopamine to the hippocampus and dorsal striatum that is comprised of the caudate nucleus and putamen) (Prasad and Pasterkamp, 2009). The diverse origins and ramifications of these pathways explain the varied effects produced by dopaminergic activation (Cho et al., 2006). Whereas optimal signaling in the mesolimbic pathway induces feelings of enjoyment and reinforcement following exposure to pleasurable stimuli (e.g., food, sex, and drugs) and associated contexts (Maas et al., 1997), optimal signaling in the mesocortical is vital for concentration and working memory. In the nigrostriatal system, signaling modulates motoric (planning and execution) and cognitive responses. In contrast, decrements in dopaminergic neurotransmission can effectuate symptoms of impaired ability to experience pleasure (anhedonia), motivation, executive function, and motricity in persons with depression (Nestler and Carlezon, 2006; Tye et al., 2013), a cluster of symptoms that traditional SSRIs often fail to assuage (Dunlop and Nemeroff, 2007; Trivedi et al., 2008). Knowledge that proinflammatory cytokines alter the functional status of the dopaminergic system in a similar manner to that seen in depression (reduces ventral striatal activity to reward cues) suggests a mechanistic explanation for their co-occurrence of inflammation in a distinct subset of persons who are clinically depressed.
Direct evidence that cytokines induce dopaminergic dysfunction derives from data from neurochemical, behavioral, electrophysiological, genetic, and human clinical studies. For instance, it has been shown that both peripheral and central administration of inflammatory agents alter dopamine levels in the brain (Miller et al., 2009), particularly in the striatum (Kamata et al., 2000; Mauriño et al., 2010). Interestingly, some evidence suggests cytokine-specific alterations across brain regions: IL-1 and IL-2 administration increased dopamine turnover in the prefrontal cortex, whereas IL-6 increased turnover in the hippocampus and prefrontal cortex (Zalcman et al., 1994). The effects of cytokine challenge may also be concentration and time dependent. Low concentration of IL-2 administered to mesencephalic cell cultures increased dopamine release, whereas higher concentrations had no effect (Alonso et al., 1993). A parallel in vivo micro dialysis study showed that acute treatment of monkeys with IFN-α increased dopamine release in the striatum, whereas chronic treatment with IFN-α decreased dopamine release. Notably, the decreased dopamine that occurred in the striatum after chronic treatment was correlated with reduced effort-based sucrose consumption (Felger et al., 2013b), an effect that was mitigated by levodopa administration via reverse in vivo microdialysis, suggesting that inflammatory cytokines reduce the availability of dopamine precursors without affecting end-product synthesis or vesicular packaging or release (Felger et al., 2015). Other work demonstrated that immune challenge effectuated decreased intracranial self-stimulation of lateral hypothalamus (Borowski et al., 1998). One of the structures affected by intracranial self-stimulation is the medial forebrain bundle which contains ascending dopaminergic projections from the VTA to the nucleus accumbens (mesolimbic pathway) and passes through the lateral hypothalamus (You et al., 2001; Nestler and Carlezon, 2006). Under basal conditions, the dopaminergic neurons in the VTA area oscillate between low-frequency regular action potentials (tonic activity) and bursts of action potentials (phasic activity patterns) (Schultz, 2002). Transient increases in phasic firing are thought to occur with exposure to unexpected rewards or aversive stimuli, thereby encoding a “reward prediction error” and reinforcing certain behaviors (Tsai et al., 2009). Notably, inflammatory stimuli decrease rodent responding for rewarding electrical stimulation in the lateral hypothalamus (Anisman et al., 1998; Borowski et al., 1998), a change that likely reflects anhedonia secondary to a loss of reward function. Translating this work to humans, it has been shown that volunteers exposed to low-dose polysaccharide exhibited reduced ventral striatal activity to monetary reward cues, a change that correlated with increased depressive symptoms (Eisenberger et al., 2010). It appears that there is a rate of long burst-like spike activity that is requisite for VTA dopaminergic neurons to release sufficient levels of dopamine to promote feelings of reward (Yadid and Friedman, 2008), a phenomenon that may be deleteriously altered in depression and inflammation. Indeed, administration of desipramine to Flinders-sensitive line rats (an animal model of depression) increased the rate of long-bursting, high-spike activity in the VTA in a manner similar to that seen in controls (Yadid and Friedman, 2008). Additionally, integrated behavioral, pharmacological, optogenetic, and electrophysiological methods used by Tye et al. (2013) to assess freely moving rodents showed that inhibition or excitation of dopaminergic neurons in the VTA immediately and bi-directionally modulated (induced or relieved) depressive-like symptoms effectuated by chronic mild stress.
One of the implicated mechanisms by which inflammation can alter dopaminergic signaling is via modulation of tetrahydrobiopterin (BH4), a cofactor that is essential for the degradation of amino acid phenylalanine and biosynthesis of dopamine. Chronic immune challenge correlates with reduced dopamine synthesis (Neurauter et al., 2008). In fact, patients treated with IFN-α demonstrate reduced BH4 function in the brain (Felger et al., 2013a). By corollary, persons subjected to dietary depletion of dopamine (via a tryptophan-depleting beverage) exhibited blunted activation of the ventral striatum during reward anticipation activities (Bjork et al., 2014), recapitulating the effects of immune challenge (Eisenberger et al., 2010). Further attesting to the effects of inflammation and dopaminergic function is recent work in a subgroup of clinically depressed individuals that showed that infliximab (a highly selective TNF-α antagonist) effectuated a strong antidepressant effect (with greatest effects seen in area of motivation), but only in patients with elevated CRP at baseline (Raison et al., 2013).
Interestingly, most studies suggest that PA increases dopamine levels in several brain regions (Brown et al., 1979; de Castro and Duncan, 1985; Dishman, 1997; Meeusen et al., 1997; Soares et al., 1999). These effects putatively stem from the ability of PA to alter metabolism (de Castro and Duncan, 1985; Chaouloff et al., 1986) via modulation of calcium levels (Goffer et al., 2013; Morris and Berk, 2015) and calcium/calmodulin-dependent activation of tyrosine hydroxylase (Greenwood et al., 2011) and mitigate BH4 depletion by inhibiting iNOS induction (Kitagami et al., 2003). Greenwood et al. (2011) demonstrated that young adult male Fischer rats that participated in voluntary wheel—running for 6 weeks exhibited a conditioned place preference for the wheel as well as increased ΔFosB/FosB immunoreactivity in the nucleus accumbens, increased tyrosine hydroxylase mRNA levels in the VTA, and compensatory down-regulation of D2 receptor mRNA in the nucleus accumbens; these findings suggest that (1) long-term voluntary PA is rewarding and alters gene transcription in mesolimbic reward neurocircuitry (Greenwood et al., 2011), (2) post-exercise increases in serum Ca2+ may activate tyrosine hydroxylase enzyme and dopamine synthesis, and (3) PA may reverse inflammation-induced disruptions in dopaminergic transmission in the nucleus accumbens and ventral striatum in models of depression. Another study showed that wheel running increases tyrosine hydroxylase mRNA in the LC (Droste et al., 2006) and substantia nigra (Foley and Fleshner, 2008). Receptor-binding studies suggest that 9 weeks of voluntary PA induced hypersensitivity to dopamine release (Gilliam et al., 1984; MacRae et al., 1987). In line with the aforementioned, it has been suggested that voluntary wheel running alters behavior because the activity is intrinsically rewarding and affects neuroplasticity in the mesolimbic reward pathway (Greenwood et al., 2011). Thereby, PA could serve as a feed-forward mechanism and further increase PA, a phenomenon that would reduce inflammation and metabolic disease in the long- term (Waters et al., 2008).
Glutamatergic Interactions
Excitatory glutamatergic neurotransmission provides a basis for communication in the forebrain, cortex, hippocampus, caudate nuclei, thalamic nuclei, and cerebellar nuclei (Paul and Skolnick, 2003). Once released into the synaptic cleft, glutamate acts upon pre- and post-synaptic ionotropic N-methyl-d-aspartate (NMDA) glutamate receptors (NMDARs) in brain regions that modulate monoaminergic activity, emotionality, learning, and behavior (Ghasemi et al., 2014, 2017). NMDARs are tetrameric structures comprised of 7 subunits, including an obligatory GluN1 subunit along with various combinations of GluN2 and GluN3 subunits that differ according to anatomical distribution, developmental profile, and functional activity. Multiple binding sites exist on NMDARs, including those for glycine (D-serine), Mg2+, and other polyamines.
Some evidence suggests that the fronto-limbic glial alterations that occur in depression (Rajkowska and Miguel-Hidalgo, 2007) and comorbid inflammation are the result of an imbalance between the quinolinic acid and kynurenic acid arms of the pathway. Cytokine activation of the kynurenine pathway induces the breakdown of kynurenine into either quinolinic acid or kynurenic acid. The two end- products have diametrically opposing functions that can contribute to neurodegenerative or neuroprotective processes in the brain. Quinolinic acid is a neurotoxic endogenous NMDA receptor agonist, whereas kynurenic acid is a neuroprotective endogenous NMDA receptor antagonist. Within the brain, quinolinic acid is exclusively produced in microglial cells, intimating that microglial activation by proinflammatory cytokines may bias quinolinic acid production and facilitate NMDA agonism (Myint et al., 2007; McNally et al., 2008) along with astrocytic activation and apoptosis (Guillemin et al., 2005). The loss of astrocytes is particularly problematic because they uptake synaptic glutamate to prevent neuronal excitotoxicity, provide metabolic support to neurons via lactate production, produce neuroprotective mediators, and defend against oxidative stress. Quinolinic acid agonism of extrasynaptic NMDARs also inhibits the activity of cAMP response element binding (CREB) protein to block induction of BDNF gene expression (Hardingham et al., 2002). Interestingly, the NMDA antagonist ketamine decreased lipopolysaccharide-induced TNF-α production in astrocyte and microglial cultures (Shibakawa et al., 2005). In the hippocampus, ketamine down-regulated pro-inflammatory cytokines (Wang et al., 2015), an effect that might reduce depression-related hippocampal atrophy and preserve HPA axis feedback (Chen and Guillemin, 2009; Leonard and Maes, 2012). Memantine, another NMDA antagonist, has been shown to mitigate lipopolysaccharide-induced neuroinflammation and restore behaviorally- induced gene expression and spatial learning in the rat (Rosi et al., 2006). Together, these studies suggest that inflammation-induced astrocytic pathology may play an important role in depression via the production of pathogenic substances and loss of normal function. By corollary, strategies that normalize astrocytic function may improve neuronal health and decrease microglial activation.
Notably, evidence suggests that PA positively modulates the glutamatergic system in states of depression and inflammation. PA increases glutamate turnover and prevents excitotoxicity (Jia et al., 2009; Herbst and Holloway, 2016) by improving calcium regulation (Sutoo and Akiyama, 1996). PA also exerts a neuroprotective effect on the brain by modulating glial function. Recently it was shown that rodents exposed to long-term PA (5 days per week × 4 weeks) demonstrate increased BDNF synthesis and release in the dentate gyrus along with altered orientation and morphology of astrocytes (Fahimi et al., 2017). The latter findings suggest the antidepressant effects of aerobic PA may stem in part from (1) PA-induced changes in astrocytic projection length and density that enhance glutamate clearance from the synapse to mitigate glutamate excitotoxicity and (2) astrocytic production of neuroprotective mediators. Others have shown that PA induces an increase in astrocyte cell body area and number of contacts between astrocytic endfeet and blood vessels in the hippocampus, medial prefrontal cortex, and orbitofrontal cortex (Brockett et al., 2015). Since astrocytic endfeet express glucose transporters (Iadecola and Nedergaard, 2007), it seems plausible that PA-induced upregulation of astrocytic endfeet contact with blood vessels may serve as a means to respond to intense energy demand (van Hall et al., 2009), an adaptation that may be particularly important during inflammation and depression. Finally, it has been shown that PA reduced age-related microglial proliferation rate in aged mice 1.5-fold as well as the number of activated microglia 1.8-fold, suggesting that PA reduced inflammatory molecules that stimulate microglia proliferation (Kohman et al., 2012).
At the Nexus of Antidepressant Efficacy and PA: PGC-1α
The nuanced relationship between PA and immune function is complex and incompletely understood, particularly in depression. On the one hand, extreme PA results in inflammation and immunosuppression. On the other hand, moderate PA promotes an anti-inflammatory environment (Gleeson, 1985). At the nexus of PA and immune interactions is a regulator of adaptation: the muscle-derived protein peroxisome proliferator-activated receptor C coactivator-1α (PGC-1α). PA upregulates skeletal expression of PGC-1α (Irrcher et al., 2003; Russell et al., 2003), which is important because this factor controls pro-inflammatory gene expression in muscle partly via inhibition of the NF-κB pathway. The NF-κB pathway contributes to cytokine production and cell survival (Eisele et al., 2013). A reification of the aforementioned concept can be seen in persons with comorbid depression and Type-2 diabetes.
Persons with Type-2 diabetes exhibit persistently elevated basal levels of inflammatory cytokines, which contribute to a state of insulin resistance (Hotamisligil, 2006). Low-grade inflammation putatively results when macrophages inundate white adipose tissue, liver, and skeletal muscle and elicit the persistent secretion of several types of “adipokines,” including the proinflammatory cytokines TNF-α, IL-1, IL-6, and monocyte chemoattractant protein-1 (MCP1) (Skurk et al., 2007). Interestingly, muscle tissue levels of TNF-α and IL-6 negatively correlate with PGC-1α levels in healthy and glucose-intolerant models (Handschin et al., 2007). Therefore, it seems plausible that the reciprocal regulation of PGC-1α and NF-κB is the molecular pivot in skeletal muscle that determines the balance between the trained anti-inflammatory environment endemic to conditions of health and the atrophic pro-inflammatory conditions endemic to states of disease (Figure 2). To better understand this pivot, a closer examination of IL-6 becomes warranted.
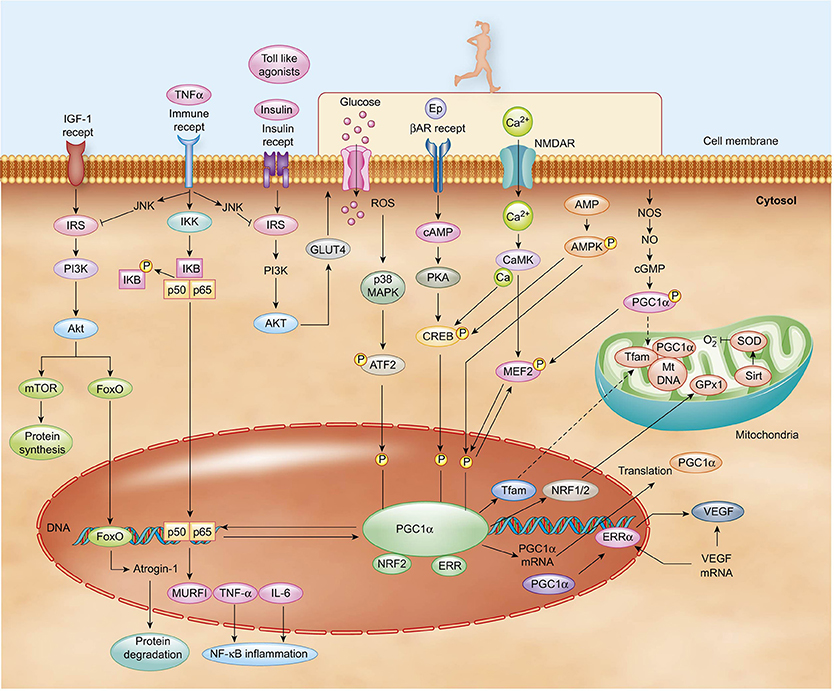
Figure 2. PA induces the upregulation of PGC-1α expression via multiple signaling pathways. Included among the pathway inputs are contributions from β-adrenergic receptor signaling, Ca2+, AMPK, ROS, and NO. Cytosolic PGC-1α protein translocates to the nucleus and mitochondria once activated. Various transcription factors can modulate metabolic processes, including MEF2, FoxO, ATF, and CREB. In turn, the factors are impinged upon by a multiplicity of signaling pathways. For instance, PA and cytokines activate p38 MAPK, which then induces the activation of MEF2 and ATF2. Insulin activates AKT, which then inhibits FoxO. PGC-1α and NFkB family p60 subunits reciprocally modulate one another to regulate inflammatory pathways.
IL-6 is produced in skeletal muscle and adipose tissue, with adipose tissue contributing 10% to 35% of the body's basal circulating IL-6 level, a percentage that increases alongside rising body fat composition (Mohamed-Ali et al., 1997; Fried et al., 1998; Pedersen and Febbraio, 2012). Chronically elevated baseline IL-6 plasma levels are associated with obesity, insulin resistance, and Type-2 diabetes (Kern et al., 2001; Duncan et al., 2003; Dandona et al., 2004). Obesity-related elevations in IL-6 appear to help fuel the process of low-grade inflammation that accompanies obesity in a feedback response designed to offset energy excess. Bolstering the latter notion is evidence that PA increases IL-6 mRNA expression (Ostrowski et al., 1998; Starkie et al., 2001) in a manner that is contraction and duration dependent (Steensberg et al., 2000), suggesting that IL-6 signals the liver to increase glucose output to regulate blood glucose concentration during times of energy need (Steensberg et al., 2000). Other work shows that IL-6 can increase up to 100-fold with prolonged PA (Fischer, 2006), a trend that was attenuated with carbohydrate ingestion during PA (Nehlsen-Cannarella et al., 1985; Starkie et al., 2001) and pre-exercise glycogen depletion (50%) (Steensberg et al., 2001). Others showed that cytokines can induce white fat browning in peripheral tissue to promote energy expenditure (Lee et al., 2013; Petruzzelli et al., 2014). Furthermore, the induction of cytokine release by NFκB p65 in fat tissue induces energy expenditure in mice (Tang et al., 2010; Jiao et al., 2012). Notably, the chronic IL-6 and TNF-α secretion that results from obesity induces suppressor of cytokine signaling proteins (SOCS) 1 and 2. The net effect is a decrease in insulin-induced activation of insulin receptor substrate (IRS) with a reduction in the metabolic effects of insulin (Tanti et al., 2012) and failed skeletal muscle regeneration and atrophy (Coletti et al., 2005) via mechanisms that likely involve upregulation of TLRs (Lambert et al., 1985; Francaux, 2009; Gleeson et al., 2011; Drummond et al., 2013). With time, the hyperinsulinaemic response results in a decline in secretory capacity of β-cells that are responsible for insulin secretion. Conversely, administration of salicylate or blocking of IKK kinase reversed obesity and diet-induced insulin resistance (Gao et al., 2003; de Alvaro et al., 2004). Similarly, exercise improves insulin sensitivity and glucose uptake in muscle (Wojtaszewski et al., 2000, 2003; Sakamoto et al., 2004). Implicated mechanisms include increased phosphorylation of insulin receptor substrate (IRS) (Weigert et al., 2006).
Paradoxically, endurance-trained athletes exhibit increased levels of intramuscular triglycerides and yet are highly insulin sensitive (Goodpaster et al., 2001). Yet unlike sedentary individuals with comorbid depression and Type-2 diabetes, endurance-trained athletes appear to exhibit a higher mitochondrial density and mitochondrial enzyme capacity, which enhances oxidative phosphorylation and reduces the degree of insulin-sensitizing metabolic byproducts (Attie and Kendziorski, 2003; Mootha et al., 2003; Patti et al., 2003; Tarnopolsky et al., 2007). Moreover, the exercise-induced IL-6 profile in athletes differs from that with chronic inflammation. Whereas IL-6 is released from contracting muscle fibers following flux in Ca2+ and glycogen in exercising athletes (Pedersen, 2009), it appears to be primarily elicited from TLRs in persons with inflammation. Together, the aforementioned evidence suggests that an acute elevation in IL-6 exemplifies an attempt to mitigate energy crises during times of deprivation or excess, but that chronic inflammation promotes sickness behaviors, muscle wasting, and insulin resistance. Undoubtedly, the maintenance of metabolic equilibrium during inflammation involves PGC-1α, the master regulator of energy expenditure and mitochondrial biogenesis.
PGC-1α co-localizes to mitochondria-rich tissues, including skeletal muscle, liver, and brain. Transgenic studies of PGC-1α in rodents suggest the factor modulates local and systemic inflammation, including levels of TNF-α and IL-6 (Handschin and Spiegelman, 2008; Handschin, 2009; Arnold et al., 2011). The ability of PGC-1α to respond to changing metabolic needs during inflammation stems from its ability to selectively bind transcription factors, particularly peroxisome proliferator-activated receptor (PPAR)γ (Puigserver and Spiegelman, 2003), PPARα (Vega et al., 2000), estrogen-related α (ERRα) (Huss et al., 2002), forkhead box O (FoxO) (Puigserver et al., 2003), hepatocyte nuclear factor 4α (HNF4α) (Yoon et al., 2001), and nuclear respiratory factors (NRFs) (Wu et al., 1999). These coregulators affect biological responses that enable cells modulate mitochondrial biogenesis, cellular respiration rates, and energy substrate uptake and utilization—changes that are particularly important for contractile and metabolic adaptations in skeletal muscle (Puigserver and Spiegelman, 2003; Wende et al., 2007; Scarpulla, 2008). For example, PGC-1α coactivation of NRF-1,2 elicits the expression of nuclear-encoded mitochondrial proteins and mitochondrial transcription factor A (Tfam) to stimulate mitochondrial DNA replication and transcription (Kelly et al., 2003; Puigserver and Spiegelman, 2003; Lin et al., 2005). Cell culture studies of myoblasts show that overexpression PGC-1α effectuates an upregulation in respiratory subunit mRNAs, cytochrome c oxidase subunit 4 (COXIV) protein levels, and steady-state levels of mitochondrial DNA (Wu et al., 1999) in an adaptation to facilitate increased oxygen utilization. PGC-1α activity is regulated after PA via translational modifications that include phosphorylation (Jäger et al., 2007), deacetylation (Cantó et al., 2009), and sumoylation (Rytinki and Palvimo, 2008), changes that enhance expression of target genes and PGC-1α itself. Several pathways appear to contribute to these exercise-related modifications, including Ca2+/calmodulin, AMPK, p38/MAPK, and nitric oxide (NO) pathways.
PGC-1α activity is partially regulated by muscle-induced Ca2+ changes and their downstream signaling pathways. PA induces Ca2+ signaling via calmodulin-dependent protein kinase IV (CaMKIV) and calcineurin A, changes that activate myocyte enhancer factor (MEF) 2 (which is important for glucose transport) and impinge upon PGC-1α transcription (Handschin et al., 2003). Interestingly, transgenic mice overexpressing calcineurin A in skeletal muscle exhibit increased slow twitch myofibers, glucose transporter type 4 (GLUT4), mitochondrial enzymes, and PGC-1α (Naya et al., 2000; Ryder et al., 2003), suggesting that PGC-1α may have an insulin-sensitizing role. Corroborating the latter notion are studies showing a negative correlation between muscle PGC-1α levels and mitochondrial activity in insulin resistance and diabetes (Attie and Kendziorski, 2003; Mootha et al., 2003; Patti et al., 2003). In an alternate signaling path, CaMKIV activates cAMP response element (CRE) binding (CREB) protein to augment PGC-1α transcription in various tissues (Herzig et al., 2001; Wu et al., 2002; Handschin et al., 2003) and, in an autoregulatory manner, activates MEF2C and MEF2D (Michael et al., 2001; Lin et al., 2005). Another factor that upregulates PGC-1α during PA is p38MAPK via activation of transcription factor 2 (ATF2) (Cao et al., 2004). Also, aerobic PA-induced Ca2+ release upregulates PGC-1α activity and initiates its translocation to the nucleus, where it interacts with LRP130 to inhibit transcriptional activity of FoxO, which suppresses muscle protein degradation and atrophy (Vechetti-Junior et al., 2016). Via these mechanisms, aerobic PA and its induction of PGC-1α influences muscle fiber type composition, modulates GLUT4 gene expression (Michael et al., 2001), and promotes protein synthesis in muscle cells.
PA also generates reactive oxygen species (ROS), which induces inflammatory cytokine production in skeletal muscle (Ji, 2008), an effect that can be mitigated by the upregulation of mitochondrial ROS-detoxifying enzymes via PGC-1α (St-Pierre et al., 2003; Valle et al., 2005). Deficits in PGC-1α secondary to disuse may promote an inflammatory state that attenuates early benefits of exercise, particularly in those with comorbid depression and chronic inflammation. Yet restoration of PA effectuates a reduction in the ubiquitin-proteasome actions of atrogin-1 and Murf-1, proteins that are involved in atrophy under catabolic conditions (Dupont-Versteegden et al., 1985; Haddad et al., 1985; Okamoto et al., 2011; Suetta et al., 2012), via upregulation of PGC-1α, a metabolic change that promotes muscle recovery by inhibiting the FoxO pathway, possibly by involvement of LRP130 (Vechetti-Junior et al., 2016).
PA also induces changes in AMP-activated protein kinase (AMPK), an energy sensor that becomes active when the AMP/ATP ratio is high (Jørgensen et al., 2005; Pedersen and Febbraio, 2012). Activated AMPK enhances mitochondrial biogenesis and function, for which PGC-1α plays an essential role in activation (Jäger et al., 2007). The up-regulation of PGC-1α putatively occurs following direct phosphorylation by activated AMPK (Jäger et al., 2007). Then, activated PGC-1α may exert significant impact on mitochondrial signal transduction by up-regulating the expression of ERRα, nuclear respiratory factor (NRF)-1, and NRF-2 (Ye et al., 2016), which is important for antioxidant defense (Asghar et al., 2007). Other work suggests that PGC-1α is requisite for the upregulation of skeletal muscle VEGF expression, an effect that is AMPK- mediated (Leick et al., 2009). In addition to its importance in muscle physiology, the AMPK pathway may be particularly important for central neurons that possess small energy reserves (Ronnett and Aja, 2008) as suggested by concomitant AMPK activation in the rodent hippocampus and antidepressant-like effects following ketamine administration (Xu et al., 2013). Via these complex pathways, PGC-1α mediates many known beneficial effects of PA in skeletal muscle physiology and immune function.
Additionally linking PGC-1α with depression is recent groundbreaking preclinical work that demonstrated that exercise-induced augmentation of PGC-1α directly influenced mood by altering the kynurenine pathway via immune-dependent mechanisms (Agudelo et al., 2014). Initially this work proved onerous because PGC-1α is expressed in a variety of systems throughout the body making it difficult to disentangle whether the effects of PA originated from central or peripheral mechanisms. To tackle the problem, Agudelo and colleagues used mice that were genetically modified to produce excessive levels of PGC-1α in type-II skeletal muscle fibers and exposed them to chronic stress in an attempt to induce depressive-like symptoms (Agudelo et al., 2014). They found that mice overexpressing PGC-1α were far more resistant to depressive symptoms in comparison to mice with normal levels of PGC-1α. The researchers then attempted to induce depressive-like symptoms in mice that were genetically engineered to produce lower levels of PGC-1α in their skeletal muscles. This time, after a significant amount of stress, the low PGC-1α mice appeared to “lose hope,” as evidenced by their decreased survival efforts during forced swimming (an indicator of depression), behaviors that were inflammation-dependent (Agudelo et al., 2014; Phillips and Salehi, 2016). Importantly, PGC-1α overexpression effectuated an increased production of kynurenine aminotransferase (KAT), an enzyme that converts kynurenine into kynurenic acid, a substance that cannot pass from the blood to the brain. The conversion of kynurenine into kynurenic acid has tremendous translational potential given that high levels of kynurenine are found in persons with mental illness and rodents administered kynurenine display depressive behavior. Fascinatingly, recent human studies show that aerobic PA increases skeletal muscle KAT levels and, thereby, shifts kynurenine metabolism in the periphery toward kynurenic acid (Schlittler et al., 2016). Altogether, these results suggest that PA induces the release of “hope molecules” from the skeletal muscles of rodents to influence mood disorder symptoms.
To date, much of the aforementioned work has not been extended to large-scale patient populations with comorbid depression and inflammation. Nevertheless, the work provides a strong theoretical basis for the idea that PA can modulate PGC-1α, increase mitochondrial density, alter muscle fiber type, mitigate inflammation, and reduce depressive symptoms, particularly in persons with comorbid depression and diabetes. Undoubtedly, the ability of PA to optimize insulin control would exert significant peripheral and central effects. Acute aerobic PA significantly increases muscle glucose uptake via insulin-dependent mechanisms for 1 h after cessation, and increased glucose uptake persists 12–48 h following prolonged activity via insulin-independent mechanisms (Magkos et al., 2008). Also, improvements in insulin signaling may persist for 24 h when the intensity is increased to near-maximal effort intermittently during trainings of shorter duration (20 min) (Manders et al., 2010; Gillen et al., 2012), with some evidence suggesting that those with the highest baseline insulin resistance yield the greatest effects early in disease progression (Dubé et al., 2012). High-intensity interval training robustly enhances skeletal muscle oxidative capacity and insulin sensitivity in adults with Type-2 diabetes (Cochran et al., 2014; Jelleyman et al., 2015). Similarly, resistance training enhances insulin action (Bacchi et al., 2013), and some evidence suggests that a combination of endurance and resistance exercise effectuates greater improvements (Sigal et al., 2007). Improved insulin sensitivity is paramount as insulin signaling regulates mitochondrial function, energy homeostasis, circuit structure and function (via transmitter receptor trafficking), and plasticity (via alterations in synapse density) (Chiu et al., 2008), effects that may be particularly important in the aging hippocampus (Zhao et al., 2008; De Felice et al., 2009) given its importance for HPA regulation.
So, the question arises as to whether there is currently enough evidence to support the deployment of PA to positively influence depressive symptoms in clinical populations. To answer this important question, Cooney and colleagues conducted a meta-analysis of randomized trials that were published up to March 2013 in which exercise (defined according to American College of Sports Medicine criteria) was compared to standard treatment, no treatment or a placebo treatment, pharmacological treatment, psychological treatment, or other active treatment in adults (aged 18 and over) with depression (Cooney et al., 2013). Thirty-nine studies with a total of 2,326 participants were included in the review. The authors reported that aerobic exercise produced effects comparable to treatment by either antidepressants or psychotherapy. Another meta-analytic study by Silveira and colleagues demonstrated that aerobic PA moderately reduced the signs of depression, with populations over 60 years of age and those with mild depression deriving the greatest response (Silveira et al., 2013). Notwithstanding, there is currently little evidence to indicate which modality of PA is optimal (aerobic, strengthening, flexibility, or combinations). Stanton and Reaburn tried to determine optimal parameters for using PA to treat depression (e.g., frequency, intensity, duration, and type of exercise). All five randomized controlled studies meeting inclusion criteria were aerobic in nature (walking on treadmill or outdoors, cycling on a stationary bike, or training on an elliptical machine) (Stanton and Reaburn, 2014). Positive evidence was found that aerobic PA of moderate intensity, undertaken 3 times weekly, was effective in treating depression, with the ultimate recommendation for duration being a minimum of 9 weeks (Stanton and Reaburn, 2014).
Given evidence that it may be more difficult for persons with comorbid depression and inflammation to benefit from conventional antidepressants, it seems likely that associating pharmacological and nonpharmacological interventions that reduce inflammation may enhance treatment response in persons with comorbid depression and inflammation. Bolstering this notion is evidence that inflammatory cytokines may cancel mechanisms requisite for antidepressant efficacy by increasing monoamine transporter activity, reducing monoamine precursors, reducing enzyme cofactors necessary for monoamine synthesis, activating NF-κB, and reducing glutamate transporters. Fornaro et al. (2013) reported that non-responders to duloxetine exhibited increased levels of proinflammatory cytokine levels in comparison to early-responders. Yoshimura et al. (2009) showed that antidepressant efficacy was contingent upon the restoration of pro- and anti-inflammatory balance and lowering of baseline IL-6 levels (Yoshimura et al., 2009). Post-hoc analysis of clinical trial results by Raison et al. (2013) demonstrated that persons with treatment-resistant depression and high baseline CRP (>5 mg/L) exhibited a higher rate of treatment response (62 vs. 33%) when administered infliximab as compared to a placebo-treated group. Conversely, persons with low CRP (<5 mg/L) levels who were administered placebo experienced a greater reduction in depressive symptoms in comparison to those administered infliximab, a finding that argues against administration of anti-inflammatory agents in cases of depression without apparent inflammation (Raison et al., 2013).
So the question arises as to what can be expected when combining antidepressants with PA: an enhanced effect, a lower antidepressant dose, a higher rate of responders, a decreased rate of relapse, or a reduction in the delay of action? To answer this question, Carneiro et al. (2015) administered pharmacotherapy plus 16 weeks of supervised structured aerobic exercise training program to women with clinical depression in a randomized clinical trial, finding that aerobic exercise was an effective adjuvant to pharmacological therapy (Carneiro et al., 2015). Helgadóttir et al. (2017) assessed outcomes for four interventions: treatment as usual, light intensity exercise, moderate intensity exercise, and vigorous exercise; while all groups experienced decrements in depressive symptoms, persons in light exercise group reported greater symptom relief at 12-month follow-up (Helgadóttir et al., 2017). Siqueira et al. (2016) reported that a 4-week (4x/week) add-on aerobic exercise program significantly decreased the need to render higher doses of sertraline as compared to sertraline monotherapy (Siqueira et al., 2016). Kerling et al. (2015) demonstrated that treatment response was more frequent in persons assigned to an add-on exercise group in comparison to treatment as usual (Kerling et al., 2015). Babyak et al. (2000) assessed the effects of a 4-month course of aerobic exercise, sertraline therapy, or a combination of exercise and sertraline in persons with depression, finding that remitted persons in the exercise group exhibited significantly lower relapse rates than subjects in the medication group (Babyak et al., 2000). Preclinical work suggests that both voluntary PA and antidepressant therapies induce changes in neuroplasticity substrates in a similar time course (Russo-Neustadt et al., 1999), although it remains to be determined whether PA-induced reductions in inflammation could alter the time course in those with comorbid depression and high basal levels of inflammation.
The precise activity parameters that need to be deployed to mitigate depression and comorbid inflammation need to be determined in future work. Some translational work suggests that moderate PA may be an optimal intensity of PA for the promotion of mental health by decreasing TNF-α (Paolucci et al., 2018). Clearly, PA prescriptions are needed that take into account the basal levels of inflammation and response to stress (neuroendocrine and immune status) during intervention. The end goal would be to deploy various forms of PA to intermittently stimulate the immune response so that the levels of pro-inflammatory mediators and stress hormones are optimized. Doing so may require different modalities, depending upon personal factors.
Conclusions and Future Directions
Undoubtedly, psychiatric illness is defined by a constellation of different symptoms that can be influenced by multiple neural processes and circuits. The heterogeneity of presentations complicates the precise targeting of dysfunction and, by corollary, therapeutics that target those impairments. This conundrum is patently apparent in the management of depression, wherein a growing body of work suggests that a specific subtype of depression with comorbid chronic inflammation exists. Further research that aims to characterize the relationship between inflammation and depression is warranted as it may yield novel treatments for this subgroup that has been shown to be resistant to conventional antidepressant pharmacotherapy. Biomarker identification efforts will be enhanced by methods that triangulate protein and genetic analysis with neuroimaging and behavioral analyses. Ultimately, these studies should be used to identify the immune, endocrine, and neurotransmitter responses in depressive subtypes so that optimal treatments, both pharmacological and nonpharmacological, can be identified and tailored to select patient populations. Clearly, large-scale, multiple-site clinical investigations that study the relationship between PA and depression are needed. Longitudinal studies will be required to evaluate the short- and long-term benefits of combination therapies. The effects of PGC-1α on phenotypic traits—such as adiposity, lean mass, and fasting glucose—and the way that they could be modulated by genetic background (ethnicity) are not precisely understood. Studies that disentangle the relationship between PA, cognitive engagement, diet, social factors, and stress are desperately needed to determine the independent and additive protective effects that each factor exerts (Phillips et al., 2014, 2015; Phillips, 2017b,c), particularly how these factors affect cognitive and emotional function at the synaptic and circuit level (Das et al., 2015). Finally, strategies for overcoming the core symptoms of depression and comorbid health problems are needed so that PA prescriptions can be personalized and adherence maximized. Personalized prescriptions are particularly germane to the topic of depression as the condition is associated with increased morbidity and mortality (Kiecolt-Glaser and Glaser, 2002), and activation of the inflammatory response in persons with depression may engender different treatment responses to various activity regimens. These studies are vital to work at the frontiers of neuroscience that seeks to enable novel application of PA to health and disease and provide a personalized approach to intervention.
Author Contributions
CP developed the concept for the study. CP and AF were responsible for manuscript writing and editing.
Conflict of Interest Statement
AF reports a consulting relationship with Silverberry Genomix, but acknowledges the company did not have a role in this paper.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ACTH, adrenocorticotropic hormone; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; BH4, tetrahydrobiopterin; BDNF, brain-derived neurotrophic factor; CRH, corticotropin-releasing hormone; CREB, cAMP response element-binding protein; CRP, c-reactive protein; ERRα, estrogen-related α; FoxO, forkhead box O; GLUT4, glucose transporter type 4; HPA, hypothalamic–pituitary–adrenal; IDO, indolamine 2, 3-dioxygenase; IFN, interferon; IL, interleukin; IRS, insulin receptor substrate; KAT, kynurenine aminotransferase; KMO, kynurenine 3-monooxygenase; LC, locus coeruleus; MAPK, mitogen-activated protein kinase; MEF, myocyte enhancer factor; MHPG, 3-methoxy-4-hydroxypheny-glycol; NFkB, nuclear factor κB; NRF, nuclear respiratory factor; NMDA, N-methyl-D-aspartate; NO, nitric oxide; SOCS, suppressor of cytokine signaling proteins; PA, physical activity; Pgc-1α, peroxisome proliferator-activated receptor C coactivator-1α; SSRI, selective serotonin reuptake inhibitor; Th, T helper; TNF, tumor necrosis factor; TDO, tryptophan 2, 3-dioxygenase; TLRs, Toll-like receptors; VTA, ventral tegmental area.
References
Abbasi, S. H., Hosseini, F., Modabbernia, A., Ashrafi, M., and Akhondzadeh, S. (2012). Effect of celecoxib add-on treatment on symptoms and serum IL-6 concentrations in patients with major depressive disorder: randomized double-blind placebo-controlled study. J. Affect. Disord. 141, 308–314. doi: 10.1016/j.jad.2012.03.033
Abbott, R., Whear, R., Nikolaou, V., Bethel, A., Coon, J. T., Stein, K., et al. (2015). Tumour necrosis factor-alpha inhibitor therapy in chronic physical illness: A systematic review and meta-analysis of the effect on depression and anxiety. J. Psychosom. Res. 79, 175–184. doi: 10.1016/j.jpsychores.2015.04.008
Abu-Omar, K., Rutten, A., and Lehtinen, V. (2004). Mental health and physical activity in the European Union Soz Praventivmed 49, 301–309. doi: 10.1007/s00038-004-3109-8
Agudelo, L. Z., Femenia, T., Orhan, F., Porsmyr-Palmertz, M., Goiny, M., Martinez-Redondo, V., et al. (2014). Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 159, 33–45. doi: 10.1016/j.cell.2014.07.051
Akhondzadeh, S., Jafari, S., Raisi, F., Nasehi, A. A., Ghoreishi, A., Salehi, B., et al. (2009). Clinical trial of adjunctive celecoxib treatment in patients with major depression: a double blind and placebo controlled trial. Depress Anxiety 26, 607–611. doi: 10.1002/da.20589
Alonso, R., Chaudieu, I., Diorio, J., Krishnamurthy, A., Quirion, R., and Boksa, P. (1993). Interleukin-2 modulates evoked release of [3H]dopamine in rat cultured mesencephalic cells. J. Neurochem. 61, 1284–1290. doi: 10.1111/j.1471-4159.1993.tb13620.x
Anisman, H., Kokkinidis, L., Borowski, T., and Merali, Z. (1998). Differential effects of interleukin (IL)-1beta, IL-2 and IL-6 on responding for rewarding lateral hypothalamic stimulation. Brain Res. 779, 177–187. doi: 10.1016/S0006-8993(97)01114-1
Apostolopoulos, V., Borkoles, E., Polman, R., and Stojanovska, L. (2014). Physical and immunological aspects of exercise in chronic diseases. Immunotherapy 6, 1145–1157. doi: 10.2217/imt.14.76
Arnold, A. S., Egger, A., and Handschin, C. (2011). PGC-1alpha and myokines in the aging muscle-a mini-review. Gerontology 57, 37–43. doi: 10.1159/000281883
Asghar, M., George, L., and Lokhandwala, M. F. (2007). Exercise decreases oxidative stress and inflammation and restores renal dopamine D1 receptor function in old rats. Am. J. Physiol. Renal Physiol. 293, F914–F919. doi: 10.1152/ajprenal.00272.2007
Aston-Jones, G., and Bloom, F. E. (1981). Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci. 1, 876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981
Attie, A. D., and Kendziorski, C. M. (2003). PGC-1alpha at the crossroads of type 2 diabetes. Nat. Genet. 34, 244–245. doi: 10.1038/ng0703-244
Audet, M. C., Jacobson-Pick, S., Wann, B. P., and Anisman, H. (2011). Social defeat promotes specific cytokine variations within the prefrontal cortex upon subsequent aggressive or endotoxin challenges. Brain Behav. Immun. 25, 1197–1205. doi: 10.1016/j.bbi.2011.03.010
Babyak, M., Blumenthal, J. A., Herman, S., Khatri, P., Doraiswamy, M., Moore, K., et al. (2000). Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom. Med. 62, 633–638. doi: 10.1097/00006842-200009000-00006
Bacchi, E., Negri, C., Targher, G., Faccioli, N., Lanza, M., Zoppini, G., et al. (2013). Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 randomized trial). Hepatology 58, 1287–1295. doi: 10.1002/hep.26393
Baldwin, A. S. Jr. (1996). The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 14, 649–683. doi: 10.1146/annurev.immunol.14.1.649
Barbour, K. A., Edenfield, T. M., and Blumenthal, J. A. (2007). Exercise as a treatment for depression and other psychiatric disorders: a review. J. Cardiopulm. Rehabil. Prev. 27, 359–367. doi: 10.1097/01.HCR.0000300262.69645.95
Bartolomucci, A., Palanza, P., Sacerdote, P., Ceresini, G., Chirieleison, A., Panerai, A. E., et al. (2003). Individual housing induces altered immuno-endocrine responses to psychological stress in male mice. Psychoneuroendocrinology 28, 540–558. doi: 10.1016/S0306-4530(02)00039-2
Basterzi, A. D., Aydemir, C., Kisa, C., Aksaray, S., Tuzer, V., Yazici, K., et al. (2005). IL-6 levels decrease with SSRI treatment in patients with major depression. Hum. Psychopharmacol. 20, 473–476. doi: 10.1002/hup.717
Bergmann, M., Gornikiewicz, A., Sautner, T., Waldmann, E., Weber, T., Mittlbock, M., et al. (1999). Attenuation of catecholamine-induced immunosuppression in whole blood from patients with sepsis. Shock 12, 421–427. doi: 10.1097/00024382-199912000-00002
Besedovsky, H. O., and del Rey, A. (1992). Immune-neuroendocrine circuits: integrative role of cytokines. Front. Neuroendocrinol. 13, 61–94.
Besedovsky, H. O., and Rey, A. D. (2007). Physiology of psychoneuroimmunology: a personal view. Brain Behav. Immun. 21, 34–44. doi: 10.1016/j.bbi.2006.09.008
Bjork, J. M., Grant, S. J., Chen, G., and Hommer, D. W. (2014). Dietary tyrosine/phenylalanine depletion effects on behavioral and brain signatures of human motivational processing. Neuropsychopharmacology 39, 595–604. doi: 10.1038/npp.2013.232
Blundell, J. E. (1984). Serotonin and appetite. Neuropharmacology 23, 1537–1551. doi: 10.1016/0028-3908(84)90098-4
Borowski, T., Kokkinidis, L., Merali, Z., and Anisman, H. (1998). Lipopolysaccharide, central in vivo biogenic amine variations, and anhedonia. Neuroreport 9, 3797–3802. doi: 10.1097/00001756-199812010-00006
Borsody, M. K., and Weiss, J. M. (2002). Peripheral endotoxin causes long-lasting changes in locus coeruleus activity via IL-1 in the brain. Acta Neuropsychiatr. 14, 303–321. doi: 10.1034/j.1601-5215.2002.140605.x
Brambilla, D., Franciosi, S., Opp, M. R., and Imeri, L. (2007). Interleukin-1 inhibits firing of serotonergic neurons in the dorsal raphe nucleus and enhances GABAergic inhibitory post-synaptic potentials. Eur. J. Neurosci. 26, 1862–1869. doi: 10.1111/j.1460-9568.2007.05796.x
Brockett, A. T., LaMarca, E. A., and Gould, E. (2015). Physical exercise enhances cognitive flexibility as well as astrocytic and synaptic markers in the medial prefrontal cortex. PLoS ONE 10:e0124859. doi: 10.1371/journal.pone.0124859
Brown, B. S., Payne, T., Kim, C., Moore, G., Krebs, P., and Martin, W. (1979). Chronic response of rat brain norepinephrine and serotonin levels to endurance training. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 46, 19–23. doi: 10.1152/jappl.1979.46.1.19
Brunello, N., Alboni, S., Capone, G., Benatti, C., Blom, J. M., Tascedda, F., et al. (2006). Acetylsalicylic acid accelerates the antidepressant effect of fluoxetine in the chronic escape deficit model of depression. Int. Clin. Psychopharmacol. 21, 219–225. doi: 10.1097/00004850-200607000-00004
Brüning, C. A., Martini, F., Soares, S. M., Savegnago, L., Sampaio, T. B., and Nogueira, C. W. (2015). Depressive-like behavior induced by tumor necrosis factor-alpha is attenuated by m-trifluoromethyl-diphenyl diselenide in mice. J. Psychiatr. Res. 66–67, 75–83. doi: 10.1016/j.jpsychires.2015.04.019
Bufalino, C., Hepgul, N., Aguglia, E., and Pariante, C. M. (2013). The role of immune genes in the association between depression and inflammation: a review of recent clinical studies. Brain Behav. Immun. 31, 31–47. doi: 10.1016/j.bbi.2012.04.009
Canli, T., and Lesch, K. P. (2007). Long story short: the serotonin transporter in emotion regulation and social cognition. Nat. Neurosci. 10, 1103–1109. doi: 10.1038/nn1964
Cantó, C., Gerhart-Hines, Z., Feige, J. N., Lagouge, M., Noriega, L., Milne, J. C., et al. (2009). AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458, 1056–1060. doi: 10.1038/nature07813
Cao, W., Daniel, K. W., Robidoux, J., Puigserver, P., Medvedev, A. V., Bai, X., et al. (2004). p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol. Cell Biol. 24, 3057–3067. doi: 10.1128/MCB.24.7.3057-3067.2004
Carek, P. J., Laibstain, S. E., and Carek, S. M. (2011). Exercise for the treatment of depression and anxiety. Int. J. Psychiatry Med. 41, 15–28. doi: 10.2190/PM.41.1.c
Carneiro, L. S., Fonseca, A. M., Vieira-Coelho, M. A., Mota, M. P., and Vasconcelos-Raposo, J. (2015). Effects of structured exercise and pharmacotherapy vs. pharmacotherapy for adults with depressive symptoms: a randomized clinical trial. J. Psychiatr. Res. 71, 48–55. doi: 10.1016/j.jpsychires.2015.09.007
Chaouloff, F., Laude, D., Guezennec, Y., and Elghozi, J. L. (1986). Motor activity increases tryptophan, 5-hydroxyindoleacetic acid, and homovanillic acid in ventricular cerebrospinal fluid of the conscious rat. J. Neurochem. 46, 1313–1316. doi: 10.1111/j.1471-4159.1986.tb00656.x
Chen, Y., and Guillemin, G. J. (2009). Kynurenine pathway metabolites in humans: disease and healthy states. Int. J. Tryptophan. Res. 2, 1–19. doi: 10.4137/IJTR.S2097
Chiu, S. L., Chen, C. M., and Cline, H. T. (2008). Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron 58, 708–719. doi: 10.1016/j.neuron.2008.04.014
Cho, B. P., Song, D. Y., Sugama, S., Shin, D. H., Shimizu, Y., Kim, S. S., et al. (2006). Pathological dynamics of activated microglia following medial forebrain bundle transection. Glia 53, 92–102. doi: 10.1002/glia.20265
Cho, L., Tsunoda, M., and Sharma, R. P. (1999). Effects of endotoxin and tumor necrosis factor alpha on regional brain neurotransmitters in mice. Nat. Toxins 7, 187–95. doi: 10.1002/1522-7189(200009/10)7:5<187::AID-NT58>3.0.CO;2-1
Chourbaji, S., Urani, A., Inta, I., Sanchis-Segura, C., Brandwein, C., Zink, M., et al. (2006). IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiol. Dis. 23, 587–594. doi: 10.1016/j.nbd.2006.05.001
Cochran, A. J., Percival, M. E., Tricarico, S., Little, J. P., Cermak, N., Gillen, J. B., et al. (2014). Intermittent and continuous high-intensity exercise training induce similar acute but different chronic muscle adaptations. Exp. Physiol. 99, 782–791. doi: 10.1113/expphysiol.2013.077453
Coletti, D., Moresi, V., Adamo, S., Molinaro, M., and Sassoon, D. (2005). Tumor necrosis factor-alpha gene transfer induces cachexia and inhibits muscle regeneration. Genesis 43, 120–128. doi: 10.1002/gene.20160
Cooney, G. M., Dwan, K., Greig, C. A., Lawlor, D. A., Rimer, J., Waugh, F. R., et al. (2013). Exercise for depression. Cochrane Database Syst. Rev. CD 004366. doi: 10.1002/14651858.CD004366.pub6
Cowen, P. J. (1991). Serotonin receptor subtypes: implications for psychopharmacology. Br. J. Psychiatry 7–14.
Crosby, K. M., and Bains, J. S. (2012). The intricate link between glucocorticoids and endocannabinoids at stress-relevant synapses in the hypothalamus. Neuroscience 204, 31–37. doi: 10.1016/j.neuroscience.2011.11.049
Cupps, T. R., and Fauci, A. S. (1982). Corticosteroid-mediated immunoregulation in man. Immunol. Rev. 65, 133–155. doi: 10.1111/j.1600-065X.1982.tb00431.x
Dandona, P., Aljada, A., and Bandyopadhyay, A. (2004). Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 25, 4–7. doi: 10.1016/j.it.2003.10.013
Dantzer, R., O'Connor, J. C., Freund, G. G., Johnson, R. W., and Kelley, K. W. (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–56. doi: 10.1038/nrn2297
Das, D., Phillips, C., Hsieh, W., Sumanth, K., Dang, V., and Salehi, A. (2014). Neurotransmitter-based strategies for the treatment of cognitive dysfunction in Down syndrome. Prog. Neuropsychopharmacol. Biol. Psychiatry 54, 140–148. doi: 10.1016/j.pnpbp.2014.05.004
Das, D., Phillips, C., Lin, B., Mojabi, F., Akif Baktir, M., Dang, V., et al. (2015). Assessment of dendritic arborization in the dentate gyrus of the hippocampal region in mice. J. Vis. Exp. 97:e52371. doi: 10.3791/52371
Davis, J. M., Bailey, S. P., Woods, J. A., Galiano, F. J., Hamilton, M. T., and Bartoli, W. P. (1992). Effects of carbohydrate feedings on plasma free tryptophan and branched-chain amino acids during prolonged cycling. Eur. J. Appl. Physiol. Occup. Physiol. 65, 513–519. doi: 10.1007/BF00602357
de Alvaro, C., Teruel, T., Hernandez, R., and Lorenzo, M. (2004). Tumor necrosis factor alpha produces insulin resistance in skeletal muscle by activation of inhibitor kappaB kinase in a p38 MAPK-dependent manner. J. Biol. Chem. 279, 17070–17078. doi: 10.1074/jbc.M312021200
de Castro, J. M., and Duncan, G. (1985). Operantly conditioned running: effects on brain catecholamine concentrations and receptor densities in the rat. Pharmacol. Biochem. Behav. 23, 495–500. doi: 10.1016/0091-3057(85)90407-1
De Felice, F. G., Vieira, M. N., Bomfim, T. R., Decker, H., Velasco, P. T., Lambert, M. P., et al. (2009). Protection of synapses against Alzheimer's-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc. Natl. Acad. Sci. U.S.A. 106, 1971–1976. doi: 10.1073/pnas.0809158106
de Lemos, E. T., Oliveira, J., Pinheiro, J. P., and Reis, F. (2012). Regular physical exercise as a strategy to improve antioxidant and anti-inflammatory status: benefits in type 2 diabetes mellitus. Oxid. Med. Cell. Longev. 2012:741545. doi: 10.1155/2012/741545
De Sarro, G. B., Masuda, Y., Ascioti, C., Audino, M. G., and Nistico, G. (1990). Behavioural and ECoG spectrum changes induced by intracerebral infusion of interferons and interleukin 2 in rats are antagonized by naloxone. Neuropharmacology 29, 167–179. doi: 10.1016/0028-3908(90)90057-X
Dishman, R. K. (1997). Brain monoamines, exercise, and behavioral stress: animal models. Med. Sci. Sports Exerc. 29, 63–74. doi: 10.1097/00005768-199701000-00010
D'Mello, C., Le, T., and Swain, M. G. (2009). Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J. Neurosci. 29, 2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009
Droste, S. K., Schweizer, M. C., Ulbricht, S., and Reul, J. M. (2006). Long-term voluntary exercise and the mouse hypothalamic-pituitary-adrenocortical axis: impact of concurrent treatment with the antidepressant drug tianeptine. J. Neuroendocrinol. 18, 915–925. doi: 10.1111/j.1365-2826.2006.01489.x
Drummond, M. J., Timmerman, K. L., Markofski, M. M., Walker, D. K., Dickinson, J. M., Jamaluddin, M., et al. (2013). Short-term bed rest increases TLR4 and IL-6 expression in skeletal muscle of older adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305, R216–R223. doi: 10.1152/ajpregu.00072.2013
Dubé, J. J., Allison, K. F., Rousson, V., Goodpaster, B. H., and Amati, F. (2012). Exercise dose and insulin sensitivity: relevance for diabetes prevention. Med. Sci. Sports Exerc. 44, 793–799. doi: 10.1249/MSS.0b013e31823f679f
Dubovsky, S. L. (1994). Beyond the serotonin reuptake inhibitors: rationales for the development of new serotonergic agents. J. Clin. Psychiatry 55(Suppl.), 34–44.
Duclos, M., Corcuff, J. B., Arsac, L., Moreau-Gaudry, F., Rashedi, M., Roger, P., et al. (1998). Corticotroph axis sensitivity after exercise in endurance-trained athletes. Clin. Endocrinol. 48, 493–501. doi: 10.1046/j.1365-2265.1998.00334.x
Duncan, B. B., Schmidt, M. I., Pankow, J. S., Ballantyne, C. M., Couper, D., Vigo, A., et al. (2003). Atherosclerosis risk in communities, low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes 52, 1799–1805. doi: 10.2337/diabetes.52.7.1799
Dunlop, B. W., and Nemeroff, C. B. (2007). The role of dopamine in the pathophysiology of depression. Arch. Gen. Psychiatry 64, 327–337. doi: 10.1001/archpsyc.64.3.327
Dunn, A. J. (1988). Systemic interleukin-1 administration stimulates hypothalamic norepinephrine metabolism parallelling the increased plasma corticosterone. Life Sci. 43, 429–435. doi: 10.1016/0024-3205(88)90522-X
Dunn, A. J. (1992). Endotoxin-induced activation of cerebral catecholamine and serotonin metabolism: comparison with interleukin-1. J. Pharmacol. Exp. Ther. 261, 964–969.
Dunn, A. J., Powell, M. L., Meitin, C., and Small, P. A. Jr. (1989). Virus infection as a stressor: influenza virus elevates plasma concentrations of corticosterone, and brain concentrations of MHPG and tryptophan. Physiol. Behav. 45, 591–594. doi: 10.1016/0031-9384(89)90078-4
Dupont-Versteegden, E. E., Fluckey, J. D., Knox, M., Gaddy, D., and Peterson, C. A. (1985). Effect of flywheel-based resistance exercise on processes contributing to muscle atrophy during unloading in adult rats. J. Appl. Physiol. 101, 202–212.
Eisele, P. S., Salatino, S., Sobek, J., Hottiger, M. O., and Handschin, C. (2013). The peroxisome proliferator-activated receptor gamma coactivator 1alpha/beta (PGC-1) coactivators repress the transcriptional activity of NF-kappaB in skeletal muscle cells. J. Biol. Chem. 288, 2246–2260. doi: 10.1074/jbc.M112.375253
Eisenberger, N. I., Berkman, E. T., Inagaki, T. K., Rameson, L. T., Mashal, N. M., and Irwin, M. R. (2010). Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol. Psychiatry 68, 748–754. doi: 10.1016/j.biopsych.2010.06.010
Elenkov, I. J., and Chrousos, G. P. (1999). Stress hormones Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol. Metab. 10, 359–368. doi: 10.1016/S1043-2760(99)00188-5
Elenkov, I. J., Papanicolaou, D. A., Wilder, R. L., and Chrousos, G. P. (1996). Modulatory effects of glucocorticoids and catecholamines on human interleukin-12 and interleukin-10 production: clinical implications. Proc. Assoc. Am. Physicians 108, 374–381.
Elenkov, I. J., Wilder, R. L., Chrousos, G. P., and Vizi, E. S. (2000). The sympathetic nerve–an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 52, 595–638.
Elgarf, A. S., Aboul-Fotouh, S., Abd-Alkhalek, H. A., El Tabbal, M., Hassan, A. N., Kassim, S. K., et al. (2014). Lipopolysaccharide repeated challenge followed by chronic mild stress protocol introduces a combined model of depression in rats: reversibility by imipramine and pentoxifylline. Pharmacol. Biochem. Behav. 126, 152–162. doi: 10.1016/j.pbb.2014.09.014
Erickson, K. I., Voss, M. W., Prakash, R. S., Basak, C., Szabo, A., Chaddock, L., et al. (2011). Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U.S.A. 108, 3017–3022. doi: 10.1073/pnas.1015950108
Ernst, C., Olson, A. K., Pinel, J. P., Lam, R. W., and Christie, B. R. (2006). Antidepressant effects of exercise: evidence for an adult-neurogenesis hypothesis? J. Psychiatry Neurosci. 31, 84–92.
Esch, T., and Stefano, G. B. (2010). The neurobiology of stress management. Neuro Endocrinol. Lett. 31, 19–39.
Fahimi, A., Baktir, M. A., Moghadam, S., Mojabi, F. S., Sumanth, K., McNerney, M. W., et al. (2017). Physical exercise induces structural alterations in the hippocampal astrocytes: exploring the role of BDNF-TrkB signaling. Brain Struct. Funct. 222, 1797–1808. doi: 10.1007/s00429-016-1308-8
Feinstein, D. L., Heneka, M. T., Gavrilyuk, V., Dello Russo, C., Weinberg, G., and Galea, E. (2002). Noradrenergic regulation of inflammatory gene expression in brain. Neurochem. Int. 41, 357–365. doi: 10.1016/S0197-0186(02)00049-9
Felger, J. C., Hernandez, C. R., and Miller, A. H. (2015). Levodopa reverses cytokine-induced reductions in striatal dopamine release. Int. J. Neuropsychopharmacol. 18:pyu084. doi: 10.1093/ijnp/pyu084
Felger, J. C., Li, L., Marvar, P. J., Woolwine, B. J., Harrison, D. G., Raison, C. L., et al. (2013a). Tyrosine metabolism during interferon-alpha administration: association with fatigue and CSF dopamine concentrations. Brain Behav. Immun. 31, 153–160. doi: 10.1016/j.bbi.2012.10.010
Felger, J. C., Li, Z., Haroon, E., Woolwine, B. J., Jung, M. Y., Hu, X., et al. (2016). Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry 21, 1358–1365. doi: 10.1038/mp.2015.168
Felger, J. C., Mun, J., Kimmel, H. L., Nye, J. A., Drake, D. F., Hernandez, C. R., et al. (2013b). Chronic interferon-alpha decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology 38, 2179–2187. doi: 10.1038/npp.2013.115
Ferrari, A. J., Charlson, F. J., Norman, R. E., Patten, S. B., Freedman, G., Murray, C. J., et al. (2013a). Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 10:e1001547. doi: 10.1371/journal.pmed.1001547
Ferrari, A. J., Somerville, A. J., Baxter, A. J., Norman, R., Patten, S. B., Vos, T., et al. (2013b). Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychol. Med. 43, 471–481. doi: 10.1017/S0033291712001511
Fischer, C. P. (2006). Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc. Immunol. Rev. 12, 6–33.
Foley, T. E., and Fleshner, M. (2008). Neuroplasticity of dopamine circuits after exercise: implications for central fatigue. Neuromolecular Med. 10, 67–80. doi: 10.1007/s12017-008-8032-3
Fond, G., Hamdani, N., Kapczinski, F., Boukouaci, W., Drancourt, N., Dargel, A., et al. (2014). Effectiveness and tolerance of anti-inflammatory drugs' add-on therapy in major mental disorders: a systematic qualitative review. Acta Psychiatr. Scand. 129, 163–179. doi: 10.1111/acps.12211
Fornai, F., di Poggio, A. B., Pellegrini, A., Ruggieri, S., and Paparelli, A. (2007). Noradrenaline in Parkinson's disease: from disease progression to current therapeutics. Curr. Med. Chem. 14, 2330–2334. doi: 10.2174/092986707781745550
Fornai, F., Ruffoli, R., Giorgi, F. S., and Paparelli, A. (2011). The role of locus coeruleus in the antiepileptic activity induced by vagus nerve stimulation. Eur. J. Neurosci. 33, 2169–2178. doi: 10.1111/j.1460-9568.2011.07707.x
Fornaro, M., Rocchi, G., Escelsior, A., Contini, P., and Martino, M. (2013). Might different cytokine trends in depressed patients receiving duloxetine indicate differential biological backgrounds. J. Affect. Disord. 145, 300–307. doi: 10.1016/j.jad.2012.08.007
Francaux, M. (2009). Toll-like receptor signalling induced by endurance exercise. Appl. Physiol. Nutr. Metab. 34, 454–458. doi: 10.1139/H09-036
Fried, S. K., Bunkin, D. A., and Greenberg, A. S. (1998). Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J. Clin. Endocrinol. Metab. 83, 847–850. doi: 10.1210/jc.83.3.847
Gadek-Michalska, A., Tadeusz, J., Rachwalska, P., and Bugajski, J. (2013). Cytokines, prostaglandins and nitric oxide in the regulation of stress-response systems. Pharmacol. Rep. 65, 1655–1662. doi: 10.1016/S1734-1140(13)71527-5
Gao, Z., Zuberi, A., Quon, M. J., Dong, Z., and Ye, J. (2003). Aspirin inhibits serine phosphorylation of insulin receptor substrate 1 in tumor necrosis factor-treated cells through targeting multiple serine kinases. J. Biol. Chem. 278, 24944–24950. doi: 10.1074/jbc.M300423200
Ghasemi, M., Phillips, C., Fahimi, A., McNerney, M. W., and Salehi, A. (2017). Mechanisms of action and clinical efficacy of NMDA receptor modulators in mood disorders. Neurosci. Biobehav. Rev. 80, 555–572. doi: 10.1016/j.neubiorev.2017.07.002
Ghasemi, M., Phillips, C., Trillo, L., De Miguel, Z., Das, D., and Salehi, A. (2014). The role of NMDA receptors in the pathophysiology and treatment of mood disorders. Neurosci. Biobehav. Rev. 47, 336–358. doi: 10.1016/j.neubiorev.2014.08.017
Gillen, J. B., Little, J. P., Punthakee, Z., Tarnopolsky, M. A., Riddell, M. C., and Gibala, M. J. (2012). Acute high-intensity interval exercise reduces the postprandial glucose response and prevalence of hyperglycaemia in patients with type 2 diabetes. Diabetes Obes. Metab. 14, 575–577. doi: 10.1111/j.1463-1326.2012.01564.x
Gilliam, P. E., Spirduso, W. W., Martin, T. P., Walters, T. J., Wilcox, R. E., and Farrar, R. P. (1984). The effects of exercise training on [3H]-spiperone binding in rat striatum. Pharmacol. Biochem. Behav. 20, 863–867. doi: 10.1016/0091-3057(84)90008-X
Gleeson, M. (1985). Immune function in sport and exercise. J. Appl. Physiol. 103, 693–699. doi: 10.1152/japplphysiol.00008.2007
Gleeson, M., Bishop, N. C., Stensel, D. J., Lindley, M. R., Mastana, S. S., and Nimmo, M. A. (2011). The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 11, 607–615. doi: 10.1038/nri3041
Gleeson, M., McFarlin, B., and Flynn, M. (2006). Exercise and toll-like receptors. Exerc. Immunol. Rev. 12, 34–53.
Goffer, Y., Xu, D., Eberle, S. E., D'Amour, J., Lee, M., Tukey, D., et al. (2013). Calcium-permeable AMPA receptors in the nucleus accumbens regulate depression-like behaviors in the chronic neuropathic pain state. J. Neurosci. 33, 19034–19044. doi: 10.1523/JNEUROSCI.2454-13.2013
Gómez-Lázaro, E., Arregi, A., Beitia, G., Vegas, O., Azpiroz, A., and Garmendia, L. (2011). Individual differences in chronically defeated male mice: behavioral, endocrine, immune, and neurotrophic changes as markers of vulnerability to the effects of stress. Stress 14, 537–548. doi: 10.3109/10253890.2011.562939
Goodpaster, B. H., He, J., Watkins, S., and Kelley, D. E. (2001). Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J. Clin. Endocrinol. Metab. 86, 5755–5761. doi: 10.1210/jcem.86.12.8075
Goshen, I., Kreisel, T., Ben-Menachem-Zidon, O., Licht, T., Weidenfeld, J., Ben-Hur, T., et al. (2008). Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol. Psychiatry 13, 717–728. doi: 10.1038/sj.mp.4002055
Gray, J. M., Vecchiarelli, H. A., Morena, M., Lee, T. T., Hermanson, D. J., Kim, A. B., et al. (2015). Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J. Neurosci. 35, 3879–3892. doi: 10.1523/JNEUROSCI.2737-14.2015
Greenberg, P. E., Kessler, R. C., Birnbaum, H. G., Leong, S. A., Lowe, S. W., Berglund, P. A., et al. (2003). The economic burden of depression in the United States: how did it change between 1990 and 2000? J. Clin. Psychiatry 64, 1465–1475. doi: 10.4088/JCP.v64n1211
Greenwood, B. N., and Fleshner, M. (2008). Exercise, learned helplessness, and the stress-resistant brain. Neuromolecular Med. 10, 81–98. doi: 10.1007/s12017-008-8029-y
Greenwood, B. N., Foley, T. E., Le, T. V., Strong, P. V., Loughridge, A. B., Day, H. E., et al. (2011). Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav. Brain Res. 217, 354–362. doi: 10.1016/j.bbr.2010.11.005
Greenwood, B. N., Kennedy, S., Smith, T. P., Campeau, S., Day, H. E., and Fleshner, M. (2003). Voluntary freewheel running selectively modulates catecholamine content in peripheral tissue and c-Fos expression in the central sympathetic circuit following exposure to uncontrollable stress in rats. Neuroscience 120, 269–281. doi: 10.1016/S0306-4522(03)00047-2
Grippo, A. J., Sullivan, N. R., Damjanoska, K. J., Crane, J. W., Carrasco, G. A., Shi, J., et al. (2005). Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology 179, 769–780. doi: 10.1007/s00213-004-2103-4
Guillemin, G. J., Wang, L., and Brew, B. J. (2005). Quinolinic acid selectively induces apoptosis of human astrocytes: potential role in AIDS dementia complex. J. Neuroinflammation 2:16. doi: 10.1186/1742-2094-2-16
Guo, J. Y., Li, C. Y., Ruan, Y. P., Sun, M., Qi, X. L., Zhao, B. S., et al. (2009). Chronic treatment with celecoxib reverses chronic unpredictable stress-induced depressive-like behavior via reducing cyclooxygenase-2 expression in rat brain. Eur. J. Pharmacol. 612, 54–60. doi: 10.1016/j.ejphar.2009.03.076
Haase, J., and Brown, E. (2015). Integrating the monoamine, neurotrophin and cytokine hypotheses of depression–a central role for the serotonin transporter? Pharmacol. Ther. 147, 1–11. doi: 10.1016/j.pharmthera.2014.10.002
Haddad, F., Adams, G. R., Bodell, P. W., and Baldwin, K. M. (1985). Isometric resistance exercise fails to counteract skeletal muscle atrophy processes during the initial stages of unloading. J. Appl. Physiol. 100, 433–441. doi: 10.1152/japplphysiol.01203.2005
Hallberg, L., Janelidze, S., Engstrom, G., Wisen, A. G., Westrin, A., and Brundin, L. (2010). Exercise-induced release of cytokines in patients with major depressive disorder. J. Affect. Disord. 126, 262–267. doi: 10.1016/j.jad.2010.02.133
Hamer, M., and Steptoe, A. (2007). Association between physical fitness, parasympathetic control, and proinflammatory responses to mental stress. Psychosom. Med. 69, 660–666. doi: 10.1097/PSY.0b013e318148c4c0
Handschin, C. (2009). Peroxisome proliferator-activated receptor-gamma coactivator-1alpha in muscle links metabolism to inflammation. Clin. Exp. Pharmacol. Physiol. 36, 1139–1143. doi: 10.1111/j.1440-1681.2009.05275.x
Handschin, C., Choi, C. S., Chin, S., Kim, S., Kawamori, D., Kurpad, A. J., et al. (2007). Abnormal glucose homeostasis in skeletal muscle-specific PGC-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J. Clin. Invest. 117, 3463–3474. doi: 10.1172/JCI31785
Handschin, C., Rhee, J., Lin, J., Tarr, P. T., and Spiegelman, B. M. (2003). An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle. Proc. Natl. Acad. Sci. U.S.A. 100, 7111–7116. doi: 10.1073/pnas.1232352100
Handschin, C., and Spiegelman, B. M. (2008). The role of exercise and PGC1alpha in inflammation and chronic disease. Nature 454, 463–469. doi: 10.1038/nature07206
Hardingham, G. E., Fukunaga, Y., and Bading, H. (2002). Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 5, 405–414. doi: 10.1038/nn835
Haroon, E., Fleischer, C. C., Felger, J. C., Chen, X., Woolwine, B. J., Patel, T., et al. (2016). Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol. Psychiatry 21, 1351–1357. doi: 10.1038/mp.2015.206
Hauser, P., Khosla, J., Aurora, H., Laurin, J., Kling, M. A., Hill, J., et al. (2002). A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol. Psychiatry 7, 942–947. doi: 10.1038/sj.mp.4001119
Helgadóttir, B., Forsell, Y., Hallgren, M., Moller, J., and Ekblom, O. (2017). Long-term effects of exercise at different intensity levels on depression: a randomized controlled trial. Prev. Med. 105, 37–46. doi: 10.1016/j.ypmed.2017.08.008
Herbst, E. A., and Holloway, G. P. (2016). Exercise increases mitochondrial glutamate oxidation in the mouse cerebral cortex. Appl. Physiol. Nutr. Metab. 41, 799–801. doi: 10.1139/apnm-2016-0033
Herzig, S., Long, F., Jhala, U. S., Hedrick, S., Quinn, R., Bauer, A., et al. (2001). CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413, 179–183. doi: 10.1038/35093131
Hill, M. N., Hillard, C. J., and McEwen, B. S. (2011a). Alterations in corticolimbic dendritic morphology and emotional behavior in cannabinoid CB1 receptor-deficient mice parallel the effects of chronic stress. Cereb. Cortex 21, 2056–2064. doi: 10.1093/cercor/bhq280
Hill, M. N., McLaughlin, R. J., Pan, B., Fitzgerald, M. L., Roberts, C. J., Lee, T. T., et al. (2011b). Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J. Neurosci. 31, 10506–10515. doi: 10.1523/JNEUROSCI.0496-11.2011
Hobson, J. A., McCarley, R. W., Freedman, R., and Pivik, R. T. (1974). Time course of discharge rate changes by cat pontine brain stem neurons during sleep cycle. J. Neurophysiol. 37, 1297–1309. doi: 10.1152/jn.1974.37.6.1297
Horowitz, J. F., and Klein, S. (2000). Lipid metabolism during endurance exercise. Am. J. Clin. Nutr. 72, 558S–563S. doi: 10.1093/ajcn/72.2.558S
Hotamisligil, G. S. (2006). Inflammation and metabolic disorders. Nature 444, 860–867. doi: 10.1038/nature05485
Huss, J. M., Kopp, R. P., and Kelly, D. P. (2002). Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and -gamma. Identification of novel leucine-rich interaction motif within PGC-1alpha. J. Biol. Chem. 277, 40265–40274. doi: 10.1074/jbc.M206324200
Iadecola, C., and Nedergaard, M. (2007). Glial regulation of the cerebral microvasculature. Nat. Neurosci. 10, 1369–1376. doi: 10.1038/nn2003
Irrcher, I., Adhihetty, P. J., Joseph, A. M., Ljubicic, V., and Hood, D. A. (2003). Regulation of mitochondrial biogenesis in muscle by endurance exercise. Sports Med. 33, 783–793. doi: 10.2165/00007256-200333110-00001
Ito, Y., Yonekura, R., Maruta, K., Koike, T., Nakagami, Y., Shibata, K., et al. (2003). Tryptophan metabolism was accelerated by exercise in rat. Adv. Exp. Med. Biol. 527, 531–535. doi: 10.1007/978-1-4615-0135-0_61
Jäger, S., Handschin, C., St-Pierre, J., and Spiegelman, B. M. (2007). AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. U.S.A. 104, 12017–12022. doi: 10.1073/pnas.0705070104
Jelleyman, C., Yates, T., O'Donovan, G., Gray, L. J., King, J. A., Khunti, K., et al. (2015). The effects of high-intensity interval training on glucose regulation and insulin resistance: a meta-analysis. Obes. Rev. 16, 942–961. doi: 10.1111/obr.12317
Ji, L. L. (2008). Modulation of skeletal muscle antioxidant defense by exercise: role of redox signaling. Free Radic. Biol. Med. 44, 142–152. doi: 10.1016/j.freeradbiomed.2007.02.031
Jia, J., Hu, Y. S., Wu, Y., Liu, G., Yu, H. X., Zheng, Q. P., et al. (2009). Pre-ischemic treadmill training affects glutamate and gamma aminobutyric acid levels in the striatal dialysate of a rat model of cerebral ischemia. Life Sci. 84, 505–511. doi: 10.1016/j.lfs.2009.01.015
Jiao, P., Feng, B., Ma, J., Nie, Y., Paul, E., Li, Y., et al. (2012). Constitutive activation of IKKbeta in adipose tissue prevents diet-induced obesity in mice. Endocrinology 153, 154–165. doi: 10.1210/en.2011-1346
Jones, D., Gershon, S., Sitaram, N., and Keshavan, M. (1987). Sleep and depression. Psychopathology 20(Suppl. 1), 20–31. doi: 10.1159/000284520
Jørgensen, S. B., Wojtaszewski, J. F., Viollet, B., Andreelli, F., Birk, J. B., Hellsten, Y., et al. (2005). Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J. 19, 1146–1148. doi: 10.1096/fj.04-3144fje
Junker, V., Becker, A., Huhne, R., Zembatov, M., Ravati, A., Culmsee, C., et al. (2002). Stimulation of beta-adrenoceptors activates astrocytes and provides neuroprotection. Eur. J. Pharmacol. 446, 25–36. doi: 10.1016/S0014-2999(02)01814-9
Kamata, M., Higuchi, H., Yoshimoto, M., Yoshida, K., and Shimizu, T. (2000). Effect of single intracerebroventricular injection of alpha-interferon on monoamine concentrations in the rat brain. Eur. Neuropsychopharmacol. 10, 129–132. doi: 10.1016/S0924-977X(99)00067-X
Kanda, H., Tateya, S., Tamori, Y., Kotani, K., Hiasa, K., Kitazawa, R., et al. (2006). MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 116, 1494–1505. doi: 10.1172/JCI26498
Karanth, S., Linthorst, A. C., Stalla, G. K., Barden, N., Holsboer, F., and Reul, J. M. (1997). Hypothalamic-pituitary-adrenocortical axis changes in a transgenic mouse with impaired glucocorticoid receptor function. Endocrinology 138, 3476–3485. doi: 10.1210/endo.138.8.5331
Kawanishi, N., Yano, H., Yokogawa, Y., and Suzuki, K. (2010). Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc. Immunol. Rev. 16, 105–118.
Kelly, A., Vereker, E., Nolan, Y., Brady, M., Barry, C., Loscher, C. E., et al. (2003). Activation of p38 plays a pivotal role in the inhibitory effect of lipopolysaccharide and interleukin-1 beta on long term potentiation in rat dentate gyrus. J. Biol. Chem. 278, 19453–19462. doi: 10.1074/jbc.M301938200
Kenis, G., and Maes, M. (2002). Effects of antidepressants on the production of cytokines. Int. J. Neuropsychopharmacol. 5, 401–412. doi: 10.1017/S1461145702003164
Kerling, A., Tegtbur, U., Gutzlaff, E., Kuck, M., Borchert, L., Ates, Z., et al. (2015). Effects of adjunctive exercise on physiological and psychological parameters in depression: a randomized pilot trial. J. Affect. Disord. 177, 1–6. doi: 10.1016/j.jad.2015.01.006
Kern, P. A., Ranganathan, S., Li, C., Wood, L., and Ranganathan, G. (2001). Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 280, E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745
Kessler, R. C., Chiu, W. T., Demler, O., Merikangas, K. R., and Walters, E. E. (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 617–627. doi: 10.1001/archpsyc.62.6.617
Kiank, C., Zeden, J. P., Drude, S., Domanska, G., Fusch, G., Otten, W., et al. (2010). Psychological stress-induced, IDO1-dependent tryptophan catabolism: implications on immunosuppression in mice and humans. PLoS ONE 5:e11825. doi: 10.1371/journal.pone.0011825
Kiecolt-Glaser, J. K., and Glaser, R. (2002). Depression and immune function: central pathways to morbidity and mortality. J. Psychosom. Res. 53, 873–876. doi: 10.1016/S0022-3999(02)00309-4
Kitagami, T., Yamada, K., Miura, H., Hashimoto, R., Nabeshima, T., and Ohta, T. (2003). Mechanism of systemically injected interferon-alpha impeding monoamine biosynthesis in rats: role of nitric oxide as a signal crossing the blood-brain barrier. Brain Res. 978, 104–114. doi: 10.1016/S0006-8993(03)02776-8
Köhler, O., Benros, M. E., Nordentoft, M., Farkouh, M. E., Iyengar, R. L., Mors, O., et al. (2014). Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry 71, 1381–1391. doi: 10.1001/jamapsychiatry.2014.1611
Kohman, R. A., DeYoung, E. K., Bhattacharya, T. K., Peterson, L. N., and Rhodes, J. S. (2012). Wheel running attenuates microglia proliferation and increases expression of a proneurogenic phenotype in the hippocampus of aged mice. Brain Behav. Immun. 26, 803–810. doi: 10.1016/j.bbi.2011.10.006
Kohut, M. L., McCann, D. A., Russell, D. W., Konopka, D. N., Cunnick, J. E., Franke, W. D., et al. (2006). Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav. Immun. 20, 201–209. doi: 10.1016/j.bbi.2005.12.002
Krishnan, R., Cella, D., Leonardi, C., Papp, K., Gottlieb, A. B., Dunn, M., et al. (2007). Effects of etanercept therapy on fatigue and symptoms of depression in subjects treated for moderate to severe plaque psoriasis for up to 96 weeks. Br. J. Dermatol. 157, 1275–1277. doi: 10.1111/j.1365-2133.2007.08205.x
Kvam, S., Kleppe, C. L., Nordhus, I. H., and Hovland, A. (2016). Exercise as a treatment for depression: a meta-analysis. J. Affect. Disord. 202, 67–86. doi: 10.1016/j.jad.2016.03.063
Labsy, Z., Prieur, F., Le Panse, B., Do, M. C., Gagey, O., Lasne, F., et al. (2013). The diurnal patterns of cortisol and dehydroepiandrosterone in relation to intense aerobic exercise in recreationally trained soccer players. Stress 16, 261–265. doi: 10.3109/10253890.2012.707259
Lacy, P., and Stow, J. L. (2011). Cytokine release from innate immune cells: association with diverse membrane trafficking pathways. Blood 118, 9–18. doi: 10.1182/blood-2010-08-265892
Lambert, C. P., Wright, N. R., Finck, B. N., and Villareal, D. T. (1985). Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J. Appl. Physiol. 105, 473–478.
Laske, C., Zank, M., Klein, R., Stransky, E., Batra, A., Buchkremer, G., et al. (2008). Autoantibody reactivity in serum of patients with major depression, schizophrenia and healthy controls. Psychiatry Res. 158, 83–86. doi: 10.1016/j.psychres.2006.04.023
Lee, Y. H., Petkova, A. P., and Granneman, J. G. (2013). Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab. 18, 355–367. doi: 10.1016/j.cmet.2013.08.003
Legakis, I. N., Mantzouridis, T., Saramantis, A., Phenekos, C., Tzioras, C., and Mountokalakis, T. (2000). Human galanin secretion is increased upon normal exercise test in middle-age individuals. Endocr. Res. 26, 357–364. doi: 10.3109/07435800009066173
Leick, L., Hellsten, Y., Fentz, J., Lyngby, S. S., Wojtaszewski, J. F., Hidalgo, J., et al. (2009). PGC-1alpha mediates exercise-induced skeletal muscle VEGF expression in mice. Am. J. Physiol. Endocrinol. Metab. 297, E92–E103. doi: 10.1152/ajpendo.00076.2009
Leonard, B. E. (2001). The immune system, depression and the action of antidepressants. Prog. Neuropsychopharmacol. Biol. Psychiatry 25, 767–780. doi: 10.1016/S0278-5846(01)00155-5
Leonard, B. E. (2007). Inflammation, depression and dementia: are they connected? Neurochem. Res. 32, 1749–1756. doi: 10.1007/s11064-007-9385-y
Leonard, B., and Maes, M. (2012). Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci. Biobehav. Rev. 36, 764–785. doi: 10.1016/j.neubiorev.2011.12.005
Lépine, J. P., and Briley, M. (2011). The increasing burden of depression. Neuropsychiatr. Dis. Treat. 7, 3–7. doi: 10.2147/NDT.S19617
Liang, H., Hussey, S. E., Sanchez-Avila, A., Tantiwong, P., and Musi, N. (2013). Effect of lipopolysaccharide on inflammation and insulin action in human muscle. PLoS ONE 8:e63983. doi: 10.1371/journal.pone.0063983
Lin, J., Handschin, C., and Spiegelman, B. M. (2005). Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 1, 361–370. doi: 10.1016/j.cmet.2005.05.004
Liu, W., Sheng, H., Xu, Y., Liu, Y., Lu, J., and Ni, X. (2013). Swimming exercise ameliorates depression-like behavior in chronically stressed rats: relevant to proinflammatory cytokines and IDO activation. Behav. Brain Res. 242, 110–116. doi: 10.1016/j.bbr.2012.12.041
Loughlin, S. E., Foote, S. L., and Grzanna, R. (1986). Efferent projections of nucleus locus coeruleus: morphologic subpopulations have different efferent targets. Neuroscience 18, 307–319. doi: 10.1016/0306-4522(86)90156-9
Lu, Y., Ho, C. S., Liu, X., Chua, A. N., Wang, W., McIntyre, R. S., et al. (2017). Chronic administration of fluoxetine and pro-inflammatory cytokine change in a rat model of depression. PLoS ONE 12:e0186700. doi: 10.1371/journal.pone.0186700
Maas, J. W., Bowden, C. L., Miller, A. L., Javors, M. A., Funderburg, L. G., Berman, N., et al. (1997). Schizophrenia, psychosis, and cerebral spinal fluid homovanillic acid concentrations. Schizophr. Bull. 23, 147–154. doi: 10.1093/schbul/23.1.147
MacRae, P. G., Spirduso, W. W., Cartee, G. D., Farrar, R. P., and Wilcox, R. E. (1987). Endurance training effects on striatal D2 dopamine receptor binding and striatal dopamine metabolite levels. Neurosci. Lett. 79, 138–144. doi: 10.1016/0304-3940(87)90686-0
Madrigal, J. L., Feinstein, D. L., and Dello Russo, C. (2005). Norepinephrine protects cortical neurons against microglial-induced cell death. J. Neurosci. Res. 81, 390–396. doi: 10.1002/jnr.20481
Maes, M. (2011). Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 664–675. doi: 10.1016/j.pnpbp.2010.06.014
Maes, M., Bosmans, E., Suy, E., Vandervorst, C., De Jonckheere, C., and Raus, J. (1990). Immune disturbances during major depression: upregulated expression of interleukin-2 receptors. Neuropsychobiology 24, 115–120. doi: 10.1159/000119472
Magkos, F., Tsekouras, Y., Kavouras, S. A., Mittendorfer, B., and Sidossis, L. S. (2008). Improved insulin sensitivity after a single bout of exercise is curvilinearly related to exercise energy expenditure. Clin. Sci. 114, 59–64. doi: 10.1042/CS20070134
Maier, S. F., and Watkins, L. R. (2003). Immune-to-central nervous system communication and its role in modulating pain and cognition: Implications for cancer and cancer treatment. Brain Behav. Immun. 17(Suppl. 1), S125–S131. doi: 10.1016/S0889-1591(02)00079-X
Manders, R. J., Van Dijk, J. W., and van Loon, L. J. (2010). Low-intensity exercise reduces the prevalence of hyperglycemia in type 2 diabetes. Med. Sci. Sports Exerc. 42, 219–225. doi: 10.1249/MSS.0b013e3181b3b16d
Manfridi, A., Brambilla, D., Bianchi, S., Mariotti, M., Opp, M. R., and Imeri, L. (2003). Interleukin-1beta enhances non-rapid eye movement sleep when microinjected into the dorsal raphe nucleus and inhibits serotonergic neurons in vitro. Eur. J. Neurosci. 18, 1041–1049. doi: 10.1046/j.1460-9568.2003.02836.x
Mattson, M. P., Maudsley, S., and Martin, B. (2004). BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 27, 589–594. doi: 10.1016/j.tins.2004.08.001
Mauriño, R., Machado, A., and Santiago, M. (2010). Effect of in vivo striatal perfusion of lipopolysaccharide on dopamine metabolites. Neurosci. Lett. 475, 121–123. doi: 10.1016/j.neulet.2010.03.050
McCusker, R. H., and Kelley, K. W. (2013). Immune-neural connections: how the immune system's response to infectious agents influences behavior. J. Exp. Biol. 216, 84–98. doi: 10.1242/jeb.073411
McNally, L., Bhagwagar, Z., and Hannestad, J. (2008). Inflammation, glutamate, and glia in depression: a literature review. CNS Spectr. 13, 501–510. doi: 10.1017/S1092852900016734
Mead, G. E., Morley, W., Campbell, P., Greig, C. A., McMurdo, M., and Lawlor, D. A. (2009). Exercise for depression. Cochrane Database Syst. Rev. CD004366. doi: 10.1002/14651858.CD004366.pub4
Meeusen, R., Smolders, I., Sarre, S., de Meirleir, K., Keizer, H., Serneels, M., et al. (1997). Endurance training effects on neurotransmitter release in rat striatum: an in vivo microdialysis study. Acta Physiol. Scand. 159, 335–341. doi: 10.1046/j.1365-201X.1997.00118.x
Melancon, M. O., Lorrain, D., and Dionne, I. J. (2012). Exercise increases tryptophan availability to the brain in older men age 57-70 years. Med. Sci. Sports Exerc. 44, 881–887. doi: 10.1249/MSS.0b013e31823ede8e
Mendlewicz, J., Kriwin, P., Oswald, P., Souery, D., Alboni, S., and Brunello, N. (2006). Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: a pilot open-label study. Int. Clin. Psychopharmacol. 21, 227–231. doi: 10.1097/00004850-200607000-00005
Menter, A., Augustin, M., Signorovitch, J., Yu, A. P., Wu, E. Q., Gupta, S. R., et al. (2010). The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J. Am. Acad. Dermatol. 62, 812–818. doi: 10.1016/j.jaad.2009.07.022
Michael, L. F., Wu, Z., Cheatham, R. B., Puigserver, P., Adelmant, G., Lehman, J. J., et al. (2001). Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc. Natl. Acad. Sci. U.S.A. 98, 3820–3825. doi: 10.1073/pnas.061035098
Michopoulos, V., Rothbaum, A. O., Jovanovic, T., Almli, L. M., Bradley, B., Rothbaum, B. O., et al. (2015). Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am. J. Psychiatry 172, 353–362. doi: 10.1176/appi.ajp.2014.14020263
Miller, A. H., Maletic, V., and Raison, C. L. (2009). Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 65, 732–741. doi: 10.1016/j.biopsych.2008.11.029
Mohamed-Ali, V., Goodrick, S., Rawesh, A., Katz, D. R., Miles, J. M., Yudkin, J. S., et al. (1997). Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J. Clin. Endocrinol. Metab. 82, 4196–4200. doi: 10.1210/jcem.82.12.4450
Moller, M., Du Preez, J. L., Viljoen, F. P., Berk, M., Emsley, R., and Harvey, B. H. (2013). Social isolation rearing induces mitochondrial, immunological, neurochemical and behavioural deficits in rats, and is reversed by clozapine or N-acetyl cysteine. Brain Behav. Immun. 30, 156–167. doi: 10.1016/j.bbi.2012.12.011
Monti, J. M. (2011). Serotonin control of sleep-wake behavior. Sleep Med. Rev. 15, 269–281. doi: 10.1016/j.smrv.2010.11.003
Mootha, V. K., Lindgren, C. M., Eriksson, K. F., Subramanian, A., Sihag, S., Lehar, J., et al. (2003). PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273. doi: 10.1038/ng1180
Mori, K., Ozaki, E., Zhang, B., Yang, L., Yokoyama, A., Takeda, I., et al. (2002). Effects of norepinephrine on rat cultured microglial cells that express alpha1, alpha2, beta1 and beta2 adrenergic receptors. Neuropharmacology 43, 1026–1034. doi: 10.1016/S0028-3908(02)00211-3
Morris, G., and Berk, M. (2015). The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC Med. 13:68. doi: 10.1186/s12916-015-0310-y
Mössner, R., Daniel, S., Schmitt, A., Albert, D., and Lesch, K. P. (2001). Modulation of serotonin transporter function by interleukin-4. Life Sci. 68, 873–880. doi: 10.1016/S0024-3205(00)00992-9
Motl, R. W., Birnbaum, A. S., Kubik, M. Y., and Dishman, R. K. (2004). Naturally occurring changes in physical activity are inversely related to depressive symptoms during early adolescence. Psychosom. Med. 66, 336–342. doi: 10.1097/00006842-200405000-00008
Müller, N., Schwarz, M. J., Dehning, S., Douhe, A., Cerovecki, A., Goldstein-Muller, B., et al. (2006). The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol. Psychiatry 11, 680–684. doi: 10.1038/sj.mp.4001805
Murray, P. S., Groves, J. L., Pettett, B. J., Britton, S. L., Koch, L. G., Dishman, R. K., et al. (2010). Locus coeruleus galanin expression is enhanced after exercise in rats selectively bred for high capacity for aerobic activity. Peptides 31, 2264–2268. doi: 10.1016/j.peptides.2010.09.005
Myint, A. M., Kim, Y. K., Verkerk, R., Scharpe, S., Steinbusch, H., and Leonard, B. (2007). Kynurenine pathway in major depression: evidence of impaired neuroprotection. J. Affect. Disord. 98, 143–151. doi: 10.1016/j.jad.2006.07.013
Myint, A. M., Leonard, B. E., Steinbusch, H. W., and Kim, Y. K. (2005). Th1, Th2, and Th3 cytokine alterations in major depression. J. Affect. Disord. 88, 167–173. doi: 10.1016/j.jad.2005.07.008
Naya, F. J., Mercer, B., Shelton, J., Richardson, J. A., Williams, R. S., and Olson, E. N. (2000). Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J. Biol. Chem. 275, 4545–4548. doi: 10.1074/jbc.275.7.4545
Nehlsen-Cannarella, S. L., Fagoaga, O. R., Nieman, D. C., Henson, D. A., Butterworth, D. E., Schmitt, R. L., et al. (1985). Carbohydrate and the cytokine response to 2.5 h of running. J. Appl. Physiol. 82, 1662–1667. doi: 10.1152/jappl.1997.82.5.1662
Nery, F. G., Monkul, E. S., Hatch, J. P., Fonseca, M., Zunta-Soares, G. B., and Frey, B. N. (2008). Celecoxib as an adjunct in the treatment of depressive or mixed episodes of bipolar disorder: a double-blind, randomized, placebo-controlled study. Hum. Psychopharmacol. 23, 87–94. doi: 10.1002/hup.912
Nestler, E. J., and Carlezon, W. A. Jr. (2006). The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry 59, 1151–1159. doi: 10.1016/j.biopsych.2005.09.018
Neurauter, G., Schrocksnadel, K., Scholl-Burgi, S., Sperner-Unterweger, B., Schubert, C., Ledochowski, M., et al. (2008). Chronic immune stimulation correlates with reduced phenylalanine turnover. Curr. Drug. Metab. 9, 622–627. doi: 10.2174/138920008785821738
Niciu, M. J., Henter, I. D., Sanacora, G., and Zarate, C. A. Jr. (2014). Glial abnormalities in substance use disorders and depression: does shared glutamatergic dysfunction contribute to comorbidity? World J. Biol. Psychiatry 15, 2–16. doi: 10.3109/15622975.2013.829585
Nisticò, G. (1993). Communications among central nervous system, neuroendocrine and immune systems: interleukin-2. Prog. Neurobiol. 40, 463–475. doi: 10.1016/0301-0082(93)90018-N
Nisticò, G., and De Sarro, G. (1991). Behavioral and electrocortical spectrum power effects after microinfusion of lymphokines in several areas of the rat brain. Ann. NY Acad. Sci. 621, 119–134. doi: 10.1111/j.1749-6632.1991.tb16974.x
Okamoto, T., Torii, S., and Machida, S. (2011). Differential gene expression of muscle-specific ubiquitin ligase MAFbx/Atrogin-1 and MuRF1 in response to immobilization-induced atrophy of slow-twitch and fast-twitch muscles. J. Physiol. Sci. 61, 537–546. doi: 10.1007/s12576-011-0175-6
Ostrowski, K., Rohde, T., Zacho, M., Asp, S., and Pedersen, B. K. (1998). Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J. Physiol. 508(Pt 3), 949–953.
Ouchi, N., Parker, J. L., Lugus, J. J., and Walsh, K. (2011). Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 11, 85–97. doi: 10.1038/nri2921
Palazzolo, D. L., and Quadri, S. K. (1992). Interleukin-1 inhibits serotonin release from the hypothalamus in vitro. Life Sci. 51, 1797–1802. doi: 10.1016/0024-3205(92)90050-Y
Panagiotakos, D. B., Pitsavos, C., Chrysohoou, C., Kavouras, S., Stefanadis, C., and Study, A. (2005). The associations between leisure-time physical activity and inflammatory and coagulation markers related to cardiovascular disease: the ATTICA Study. Prev. Med. 40, 432–437. doi: 10.1016/j.ypmed.2004.07.010
Paolucci, E. M., Loukov, D. D., Bowdish, M. E., and Heisz, J. J. (2018). Exercise reduces depression and inflammation but intensity matters. Biol. Psychol. 133, 79–84. doi: 10.1016/j.biopsycho.2018.01.015
Pariante, C. M., and Miller, A. H. (2001). Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol. Psychiatry 49, 391–404. doi: 10.1016/S0006-3223(00)01088-X
Passamonti, L., Crockett, M. J., Apergis-Schoute, A. M., Clark, L., Rowe, J. B., Calder, A. J., et al. (2012). Effects of acute tryptophan depletion on prefrontal-amygdala connectivity while viewing facial signals of aggression. Biol. Psychiatry 71, 36–43. doi: 10.1016/j.biopsych.2011.07.033
Patti, M. E., Butte, A. J., Crunkhorn, S., Cusi, K., Berria, R., Kashyap, S., et al. (2003). Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc. Natl. Acad. Sci. U.S.A. 100, 8466–8471. doi: 10.1073/pnas.1032913100
Paul, I. A., and Skolnick, P. (2003). Glutamate and depression: clinical and preclinical studies. Ann. N.Y. Acad. Sci. 1003, 250–272. doi: 10.1196/annals.1300.016
Pedersen, B. K. (2009). Adolph distinguished lecture: muscle as an endocrine organ, IL-6 and other myokines. J. Appl. Physiol. 107, 1006–1014. doi: 10.1152/japplphysiol.00734.2009
Pedersen, B. K., and Febbraio, M. A. (2012). Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 8, 457–465. doi: 10.1038/nrendo.2012.49
Pedersen, B. K., and Fischer, C. P. (2007). Beneficial health effects of exercise–the role of IL-6 as a myokine. Trends Pharmacol. Sci. 28, 152–156. doi: 10.1016/j.tips.2007.02.002
Pedersen, B. K., Steensberg, A., and Schjerling, P. (2001). Exercise and interleukin-6. Curr. Opin. Hematol. 8, 137–141. doi: 10.1097/00062752-200105000-00002
Peeters, F., Nicholson, N. A., and Berkhof, J. (2003). Cortisol responses to daily events in major depressive disorder. Psychosom. Med. 65, 836–841. doi: 10.1097/01.PSY.0000088594.17747.2E
Pemberton, R., and Fuller Tyszkiewicz, M. D. (2016). Factors contributing to depressive mood states in everyday life: a systematic review. J. Affect. Disord. 200, 103–110. doi: 10.1016/j.jad.2016.04.023
Petersen, A. M., and Pedersen, B. K. (1985). The anti-inflammatory effect of exercise. J. Appl. Physiol. 98, 1154–1162. doi: 10.1152/japplphysiol.00164.2004
Petruzzelli, M., Schweiger, M., Schreiber, R., Campos-Olivas, R., Tsoli, M., Allen, J., et al. (2014). A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 20, 433–447. doi: 10.1016/j.cmet.2014.06.011
Phillips, C. (2017a). Physical activity modulates common neuroplasticity substrates in major, depressive and bipolar disorder. Neural Plast. 2017:7014146. doi: 10.1155/2017/7014146
Phillips, C. (2017b). Brain-derived neurotrophic factor, depression, and physical activity: making the neuroplastic connection. Neural Plast. 2017:7260130. doi: 10.1155/2017/7260130
Phillips, C. (2017c). Lifestyle modulators of neuroplasticity: how physical activity, mental engagement, and diet promote cognitive health during aging. Neural Plast. 2017:3589271. doi: 10.1155/2017/3589271
Phillips, C., Baktir, M. A., Das, D., Lin, B., and Salehi, A. (2015). The link between physical activity and cognitive dysfunction in Alzheimer disease. Phys. Ther. 95, 1046–1060. doi: 10.2522/ptj.20140212
Phillips, C., Baktir, M. A., Srivatsan, M., and Salehi, A. (2014). Neuroprotective effects of physical activity on the brain: a closer look at trophic factor signaling. Front. Cell Neurosci. 8:170. doi: 10.3389/fncel.2014.00170
Phillips, C., Fahimi, A., Das, D., Mojabi, F. S., Ponnusamy, R., and Salehi, A. (2016). Noradrenergic system in down syndrome and Alzheimer's disease a target for therapy. Curr. Alzheimer Res. 13, 68–83. doi: 10.2174/1567205012666150921095924
Phillips, C., and Salehi, A. (2016). A special regenerative rehabilitation and genomics letter: is there a “Hope” molecule? Phys. Ther. 96, 581–583. doi: 10.2522/ptj.2016.96.4.581
Pieribone, V. A., Xu, Z. Q., Zhang, X., Grillner, S., Bartfai, T., and Hokfelt, T. (1995). Galanin induces a hyperpolarization of norepinephrine-containing locus coeruleus neurons in the brainstem slice. Neuroscience 64, 861–874. doi: 10.1016/0306-4522(94)00450-J
Prasad, A. A., and Pasterkamp, R. J. (2009). Axon guidance in the dopamine system. Adv. Exp. Med. Biol. 651, 91–100. doi: 10.1007/978-1-4419-0322-8_9
Puigserver, P., Rhee, J., Donovan, J., Walkey, C. J., Yoon, J. C., Oriente, F., et al. (2003). Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature 423, 550–555. doi: 10.1038/nature01667
Puigserver, P., and Spiegelman, B. M. (2003). Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr. Rev. 24, 78–90. doi: 10.1210/er.2002-0012
Raison, C. L., Rutherford, R. E., Woolwine, B. J., Shuo, C., Schettler, P., Drake, D. F., et al. (2013). A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 70, 31–41. doi: 10.1001/2013.jamapsychiatry.4
Rajkowska, G., and Miguel-Hidalgo, J. J. (2007). Gliogenesis and glial pathology in depression. CNS Neurol. Disord. Drug Targets 6, 219–233. doi: 10.2174/187152707780619326
Reddy, M. S. (2010). Depression: the disorder and the burden. Indian J. Psychol. Med. 32, 1–2. doi: 10.4103/0253-7176.70510
Reiss, J. I., Dishman, R. K., Boyd, H. E., Robinson, J. K., and Holmes, P. V. (2009). Chronic activity wheel running reduces the severity of kainic acid-induced seizures in the rat: possible role of galanin. Brain Res. 1266, 54–63. doi: 10.1016/j.brainres.2009.02.030
Ronnett, G. V., and Aja, S. (2008). AMP-activated protein kinase in the brain. Int. J. Obes. 32(Suppl. 4), S42–S48. doi: 10.1038/ijo.2008.122
Rosano, C., Guralnik, J., Pahor, M., Glynn, N. W., Newman, A. B., Ibrahim, T. S., et al. (2017). Hippocampal response to a 24-Month physical activity intervention in sedentary older adults. Am. J. Geriatr. Psychiatry 25, 209–217. doi: 10.1016/j.jagp.2016.11.007
Rosi, S., Vazdarjanova, A., Ramirez-Amaya, V., Worley, P. F., Barnes, C. A., and Wenk, G. L. (2006). Memantine protects against LPS-induced neuroinflammation, restores behaviorally-induced gene expression and spatial learning in the rat. Neuroscience 142, 1303–1315. doi: 10.1016/j.neuroscience.2006.08.017
Russell, A. P., Feilchenfeldt, J., Schreiber, S., Praz, M., Crettenand, A., Gobelet, C., et al. (2003). Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes 52, 2874–2881. doi: 10.2337/diabetes.52.12.2874
Russo-Neustadt, A., Beard, R. C., and Cotman, C. W. (1999). Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology 21, 679–682. doi: 10.1016/S0893-133X(99)00059-7
Ryder, J. W., Bassel-Duby, R., Olson, E. N., and Zierath, J. R. (2003). Skeletal muscle reprogramming by activation of calcineurin improves insulin action on metabolic pathways. J. Biol. Chem. 278, 44298–44304. doi: 10.1074/jbc.M304510200
Rytinki, M. M., and Palvimo, J. J. (2008). SUMOylation modulates the transcription repressor function of RIP140. J. Biol. Chem. 283, 11586–11595. doi: 10.1074/jbc.M709359200
Sakamoto, K., Arnolds, D. E., Ekberg, I., Thorell, A., and Goodyear, L. J. (2004). Exercise regulates Akt and glycogen synthase kinase-3 activities in human skeletal muscle. Biochem. Biophys. Res. Commun. 319, 419–425. doi: 10.1016/j.bbrc.2004.05.020
Sanders, V. M., Baker, R. A., Ramer-Quinn, D. S., Kasprowicz, D. J., Fuchs, B. A., and Street, N. E. (1997). Differential expression of the beta2-adrenergic receptor by Th1 and Th2 clones: implications for cytokine production and B cell help. J. Immunol. 158, 4200–4210.
Sarbadhikari, S. N., and Saha, A. K. (2006). Moderate exercise and chronic stress produce counteractive effects on different areas of the brain by acting through various neurotransmitter receptor subtypes: a hypothesis. Theor. Biol. Med. Model. 3:33. doi: 10.1186/1742-4682-3-33
Scarpulla, R. C. (2008). Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 88, 611–638. doi: 10.1152/physrev.00025.2007
Schindler, R., Mancilla, J., Endres, S., Ghorbani, R., Clark, S. C., and Dinarello, C. A. (1990). Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood 75, 40–47.
Schlittler, M., Goiny, M., Agudelo, L. Z., Venckunas, T., Brazaitis, M., Skurvydas, A., et al. (2016). Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am. J. Physiol. Cell. Physiol. 310, C836–C840. doi: 10.1152/ajpcell.00053.2016
Schuch, F. B., Vancampfort, D., Richards, J., Rosenbaum, S., Ward, P. B., and Stubbs, B. (2016). Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J. Psychiatr. Res. 77, 42–51. doi: 10.1016/j.jpsychires.2016.02.023
Schultz, W. (2002). Getting formal with dopamine and reward. Neuron 36, 241–263. doi: 10.1016/S0896-6273(02)00967-4
Sciolino, N. R., and Holmes, P. V. (2012). Exercise offers anxiolytic potential: a role for stress and brain noradrenergic-galaninergic mechanisms. Neurosci. Biobehav. Rev. 36, 1965–1984. doi: 10.1016/j.neubiorev.2012.06.005
Sell, H., Habich, C., and Eckel, J. (2012). Adaptive immunity in obesity and insulin resistance. Nat. Rev. Endocrinol. 8, 709–716. doi: 10.1038/nrendo.2012.114
Seutin, V., Verbanck, P., Massotte, L., and Dresse, A. (1989). Galanin decreases the activity of locus coeruleus neurons in vitro. Eur. J. Pharmacol. 164, 373–376. doi: 10.1016/0014-2999(89)90481-0
Shibakawa, Y. S., Sasaki, Y., Goshima, Y., Echigo, N., Kamiya, Y., Kurahashi, K., et al. (2005). Effects of ketamine and propofol on inflammatory responses of primary glial cell cultures stimulated with lipopolysaccharide. Br. J. Anaesth. 95, 803–810. doi: 10.1093/bja/aei256
Sigal, R. J., Kenny, G. P., Boule, N. G., Wells, G. A., Prud'homme Fortier, M., et al. (2007). Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann. Intern. Med. 147, 357–369. doi: 10.7326/0003-4819-147-6-200709180-00005
Silveira, H., Moraes, H., Oliveira, N., Coutinho, E. S., Laks, J., and Deslandes, A. (2013). Physical exercise and clinically depressed patients: a systematic review and meta-analysis. Neuropsychobiology 67, 61–68. doi: 10.1159/000345160
Silverman, M. N., and Deuster, P. A. (2014). Biological mechanisms underlying the role of physical fitness in health and resilience. Interface Focus 4:20140040. doi: 10.1098/rsfs.2014.0040
Siqueira, C. C., Valiengo, L. L., Carvalho, A. F., Santos-Silva, P. R., Missio, G., de Sousa, R. T., et al. (2016). Antidepressant efficacy of adjunctive aerobic activity and associated biomarkers in major depression: a 4-week, randomized, single-blind, controlled clinical trial. PLoS ONE 11:e0154195. doi: 10.1371/journal.pone.0154195
Skurk, T., Alberti-Huber, C., Herder, C., and Hauner, H. (2007). Relationship between adipocyte size and adipokine expression and secretion. J. Clin. Endocrinol. Metab. 92, 1023–1033. doi: 10.1210/jc.2006-1055
Smagin, G. N., Swiergiel, A. H., and Dunn, A. J. (1996). Peripheral administration of interleukin-1 increases extracellular concentrations of norepinephrine in rat hypothalamus: comparison with plasma corticosterone. Psychoneuroendocrinology 21, 83–93. doi: 10.1016/0306-4530(95)00019-4
Smith, J. K., Dykes, R., Douglas, J. E., Krishnaswamy, G., and Berk, S. (1999). Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischemic heart disease. JAMA 281, 1722–1727. doi: 10.1001/jama.281.18.1722
Soares, J., Holmes, P. V., Renner, K. J., Edwards, G. L., Bunnell, B. N., and Dishman, R. K. (1999). Brain noradrenergic responses to footshock after chronic activity-wheel running. Behav. Neurosci. 113, 558–566. doi: 10.1037/0735-7044.113.3.558
Soczynska, J. K., Kennedy, S. H., Goldstein, B. I., Lachowski, A., Woldeyohannes, H. O., and McIntyre, R. S. (2009). The effect of tumor necrosis factor antagonists on mood and mental health-associated quality of life: novel hypothesis-driven treatments for bipolar depression? Neurotoxicology 30, 497–521. doi: 10.1016/j.neuro.2009.03.004
Stanton, R., and Reaburn, P. (2014). Exercise and the treatment of depression: a review of the exercise program variables. J. Sci. Med. Sport 17, 177–182. doi: 10.1016/j.jsams.2013.03.010
Starkie, R. L., Arkinstall, M. J., Koukoulas, I., Hawley, J. A., and Febbraio, M. A. (2001). Carbohydrate ingestion attenuates the increase in plasma interleukin-6, but not skeletal muscle interleukin-6 mRNA, during exercise in humans. J. Physiol. 533, 585–591. doi: 10.1111/j.1469-7793.2001.0585a.x
Steensberg, A., Febbraio, M. A., Osada, T., Schjerling, P., van Hall, G., Saltin, B., et al. (2001). Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J. Physiol. 537, 633–639. doi: 10.1111/j.1469-7793.2001.00633.x
Steensberg, A., van Hall, G., Osada, T., Sacchetti, M., Saltin, B., and Klarlund Pedersen, B. (2000). Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 529(Pt 1), 237–242. doi: 10.1111/j.1469-7793.2000.00237.x
Steiner, J., Bogerts, B., Sarnyai, Z., Walter, M., Gos, T., Bernstein, H. G., et al. (2012). Bridging the gap between the immune and glutamate hypotheses of schizophrenia and major depression: potential role of glial NMDA receptor modulators and impaired blood-brain barrier integrity. World J. Biol. Psychiatry 13, 482–492. doi: 10.3109/15622975.2011.583941
Steptoe, A., Willemsen, G., Owen, N., Flower, L., and Mohamed-Ali, V. (2001). Acute mental stress elicits delayed increases in circulating inflammatory cytokine levels. Clin. Sci. 101, 185–192. doi: 10.1042/cs1010185
St-Pierre, J., Lin, J., Krauss, S., Tarr, P. T., Yang, R., Newgard, C. B., et al. (2003). Bioenergetic analysis of peroxisome proliferator-activated receptor gamma coactivators 1alpha and 1beta (PGC-1alpha and PGC-1beta) in muscle cells. J. Biol. Chem. 278, 26597–26603. doi: 10.1074/jbc.M301850200
Suetta, C., Frandsen, U., Jensen, L., Jensen, M. M., Jespersen, J. G., Hvid, L. G., et al. (2012). Aging affects the transcriptional regulation of human skeletal muscle disuse atrophy. PLoS ONE 7:e51238. doi: 10.1371/journal.pone.0051238
Sutoo, D. E., and Akiyama, K. (1996). The mechanism by which exercise modifies brain function. Physiol. Behav. 60, 177–181. doi: 10.1016/0031-9384(96)00011-X
Tang, T., Zhang, J., Yin, J., Staszkiewicz, J., Gawronska-Kozak, B., Jung, D. Y., et al. (2010). Uncoupling of inflammation and insulin resistance by NF-kappaB in transgenic mice through elevated energy expenditure. J. Biol. Chem. 285, 4637–4644. doi: 10.1074/jbc.M109.068007
Tanti, J. F., Ceppo, F., Jager, J., and Berthou, F. (2012). Implication of inflammatory signaling pathways in obesity-induced insulin resistance. Front. Endocrinol. 3:181. doi: 10.3389/fendo.2012.00181
Tarnopolsky, M. A., Rennie, C. D., Robertshaw, H. A., Fedak-Tarnopolsky, S. N., Devries, M. C., and Hamadeh, M. J. (2007). Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R1271–R1278. doi: 10.1152/ajpregu.00472.2006
Timmerman, K. L., Flynn, M. G., Coen, P. M., Markofski, M. M., and Pence, B. D. (2008). Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise? J. Leukoc. Biol. 84, 1271–1278. doi: 10.1189/jlb.0408244
Trivedi, M. H. (2006). Major depressive disorder: remission of associated symptoms. J. Clin. Psychiatry 67(Suppl. 6), 27–32.
Trivedi, M. H., Greer, T. L., Church, T. S., Carmody, T. J., Grannemann, B. D., Galper, D. I., et al. (2011). Exercise as an augmentation treatment for nonremitted major depressive disorder: a randomized, parallel dose comparison. J. Clin. Psychiatry 72, 677–684. doi: 10.4088/JCP.10m06743
Trivedi, M. H., Hollander, E., Nutt, D., and Blier, P. (2008). Clinical evidence and potential neurobiological underpinnings of unresolved symptoms of depression. J. Clin. Psychiatry 69, 246–258. doi: 10.4088/JCP.v69n0211
Tsagarakis, S., Gillies, G., Rees, L. H., Besser, M., and Grossman, A. (1989). Interleukin-1 directly stimulates the release of corticotrophin releasing factor from rat hypothalamus. Neuroendocrinology 49, 98–101. doi: 10.1159/000125096
Tsai, H. C., Zhang, F., Adamantidis, A., Stuber, G. D., Bonci, A., de Lecea, L., et al. (2009). Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324, 1080–1084. doi: 10.1126/science.1168878
Tsao, C. W., Lin, Y. S., Chen, C. C., Bai, C. H., and Wu, S. R. (2006). Cytokines and serotonin transporter in patients with major depression. Prog Neuropsychopharmacol. Biol. Psychiatry 30, 899–905. doi: 10.1016/j.pnpbp.2006.01.029
Tuglu, C., Kara, S. H., Caliyurt, O., Vardar, E., and Abay, E. (2003). Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology 170, 429–433. doi: 10.1007/s00213-003-1566-z
Tye, K. M., Mirzabekov, J. J., Warden, M. R., Ferenczi, E. A., Tsai, H. C., Finkelstein, J., et al. (2013). Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493, 537–541. doi: 10.1038/nature11740
Tyring, S., Gottlieb, A., Papp, K., Gordon, K., Leonardi, C., Wang, A., et al. (2006). Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet 367, 29–35. doi: 10.1016/S0140-6736(05)67763-X
Vaidya, V. A., Terwilliger, R. M., and Duman, R. S. (1999). Role of 5-HT2A receptors in the stress-induced down-regulation of brain-derived neurotrophic factor expression in rat hippocampus. Neurosci. Lett. 262, 1–4. doi: 10.1016/S0304-3940(99)00006-3
Valle, I., Alvarez-Barrientos, A., Arza, E., Lamas, S., and Monsalve, M. (2005). PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc. Res. 66, 562–573. doi: 10.1016/j.cardiores.2005.01.026
van der Poll, T., Coyle, S. M., Barbosa, K., Braxton, C. C., and Lowry, S. F. (1996). Epinephrine inhibits tumor necrosis factor-alpha and potentiates interleukin 10 production during human endotoxemia. J. Clin. Invest. 97, 713–719. doi: 10.1172/JCI118469
van Hall, G., Stromstad, M., Rasmussen, P., Jans, O., Zaar, M., Gam, C., et al. (2009). Blood lactate is an important energy source for the human brain. J. Cereb. Blood Flow Metab. 29, 1121–1129. doi: 10.1038/jcbfm.2009.35
Vechetti-Junior, I. J., Bertaglia, R. S., Fernandez, G. J., de Paula, T. G., de Souza, R. W., Moraes, L. N., et al. (2016). Aerobic exercise recovers disuse-induced atrophy through the stimulus of the LRP130/PGC-1alpha complex in aged rats. J. Gerontol. A Biol. Sci. Med. Sci. 71, 601–609. doi: 10.1093/gerona/glv064
Vega, R. B., Huss, J. M., and Kelly, D. P. (2000). The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol. Cell Biol. 20, 1868–1876. doi: 10.1128/MCB.20.5.1868-1876.2000
Walsh, N. P., Gleeson, M., Pyne, D. B., Nieman, D. C., Dhabhar, F. S., Shephard, R. J., et al. (2011). Position statement. Part two: maintaining immune health. Exerc. Immunol. Rev. 17, 64–103.
Wang, N., Yu, H. Y., Shen, X. F., Gao, Z. Q., Yang, C., Yang, J. J., et al. (2015). The rapid antidepressant effect of ketamine in rats is associated with down-regulation of pro-inflammatory cytokines in the hippocampus. Ups J. Med. Sci. 120, 241–248. doi: 10.3109/03009734.2015.1060281
Waters, R. P., Renner, K. J., Pringle, R. B., Summers, C. H., Britton, S. L., Koch, L. G., et al. (2008). Selection for aerobic capacity affects corticosterone, monoamines and wheel-running activity. Physiol. Behav. 93, 1044–1054. doi: 10.1016/j.physbeh.2008.01.013
Weigert, C., Hennige, A. M., Lehmann, R., Brodbeck, K., Baumgartner, F., Schauble, M., et al. (2006). Direct cross-talk of interleukin-6 and insulin signal transduction via insulin receptor substrate-1 in skeletal muscle cells. J. Biol. Chem. 281, 7060–7067. doi: 10.1074/jbc.M509782200
Wende, A. R., Schaeffer, P. J., Parker, G. J., Zechner, C., Han, D. H., Chen, M. M., et al. (2007). A role for the transcriptional coactivator PGC-1alpha in muscle refueling. J. Biol. Chem. 282, 36642–36651. doi: 10.1074/jbc.M707006200
Wojtaszewski, J. F., Hansen, B. F., Gade, K. B., Markuns, J. F., Goodyear, L. J., and Richter, E. A. (2000). Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes 49, 325–331. doi: 10.2337/diabetes.49.3.325
Wojtaszewski, J. F., Jorgensen, S. B., Frosig, C., MacDonald, C., Birk, J. B., and Richter, E. A. (2003). Insulin signalling: effects of prior exercise. Acta Physiol. Scand. 178, 321–328. doi: 10.1046/j.1365-201X.2003.01151.x
Wu, H., Kanatous, S. B., Thurmond, F. A., Gallardo, T., Isotani, E., Bassel-Duby, R., et al. (2002). Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science 296, 349–352. doi: 10.1126/science.1071163
Wu, Z., Puigserver, P., Andersson, U., Zhang, C., Adelmant, G., Mootha, V., et al. (1999). Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98, 115–124. doi: 10.1016/S0092-8674(00)80611-X
Xu, S. X., Zhou, Z. Q., Li, X. M., Ji, M. H., Zhang, G. F., and Yang, J. J. (2013). The activation of adenosine monophosphate-activated protein kinase in rat hippocampus contributes to the rapid antidepressant effect of ketamine. Behav. Brain Res. 253, 305–309. doi: 10.1016/j.bbr.2013.07.032
Yadid, G., and Friedman, A. (2008). Dynamics of the dopaminergic system as a key component to the understanding of depression. Prog. Brain Res. 172, 265–286. doi: 10.1016/S0079-6123(08)00913-8
Ye, Q., Huang, W., Li, D., Si, E., Wang, J., Wang, Y., et al. (2016). Overexpression of PGC-1alpha influences mitochondrial signal transduction of dopaminergic neurons. Mol. Neurobiol. 53, 3756–3770. doi: 10.1007/s12035-015-9299-7
Yeh, S. H., Chuang, H., Lin, L. W., Hsiao, C. Y., and Eng, H. L. (2006). Regular tai chi chuan exercise enhances functional mobility and CD4CD25 regulatory T cells. Br. J. Sports Med. 40, 239–243. doi: 10.1136/bjsm.2005.022095
Yoon, J. C., Puigserver, P., Chen, G., Donovan, J., Wu, Z., Rhee, J., et al. (2001). Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413, 131–138. doi: 10.1038/35093050
Yoshimura, R., Hori, H., Ikenouchi-Sugita, A., Umene-Nakano, W., Ueda, N., and Nakamura, J. (2009). Higher plasma interleukin-6 (IL-6) level is associated with SSRI- or SNRI-refractory depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 722–726. doi: 10.1016/j.pnpbp.2009.03.020
You, Z. B., Chen, Y. Q., and Wise, R. A. (2001). Dopamine and glutamate release in the nucleus accumbens and ventral tegmental area of rat following lateral hypothalamic self-stimulation. Neuroscience 107, 629–639. doi: 10.1016/S0306-4522(01)00379-7
Zalcman, S., Green-Johnson, J. M., Murray, L., Nance, D. M., Dyck, D., Anisman, H., et al. (1994). Cytokine-specific central monoamine alterations induced by interleukin-1,−2 and−6. Brain Res. 643, 40–49. doi: 10.1016/0006-8993(94)90006-X
Zhao, W. Q., De Felice, F. G., Fernandez, S., Chen, H., Lambert, M. P., Quon, M. J., et al. (2008). Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J. 22, 246–260. doi: 10.1096/fj.06-7703com
Keywords: immune, stress, depression, physical activity, neuroprotection, peroxisome proliferator-activated receptor gamma coactivator 1-alpha, growth factors, glutamate
Citation: Phillips C and Fahimi A (2018) Immune and Neuroprotective Effects of Physical Activity on the Brain in Depression. Front. Neurosci. 12:498. doi: 10.3389/fnins.2018.00498
Received: 15 March 2018; Accepted: 03 July 2018;
Published: 26 July 2018.
Edited by:
Abed N. Azab, Ben-Gurion University of the Negev, IsraelReviewed by:
Bruno Pierre Guiard, Université de Toulouse, FranceAnthony John Hannan, Florey Institute of Neuroscience and Mental Health, Australia
Copyright © 2018 Phillips and Fahimi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cristy Phillips, Y3BoaWxsaXBzQGFzdGF0ZS5lZHU=
 Cristy Phillips
Cristy Phillips Atoossa Fahimi
Atoossa Fahimi