- 1Department of Psychiatry and Psychotherapy, Eberhard Karls University of Tübingen, Tübingen, Germany
- 2Werner Reichardt Centre for Integrative Neuroscience, University of Tübingen, Tübingen, Germany
- 3LEAD Research School & Network, University of Tübingen, Tübingen, Germany
- 4Clinical Affective Neuroimaging Laboratory, Magdeburg, Germany
- 5Department for Behavioral Neurology, Leibniz Institute for Neurobiology, Magdeburg, Germany
- 6Max Planck Institute for Biological Cybernetics, Tübingen, Germany
- 7Center for Behavioral Brain Sciences, Magdeburg, Germany
- 8Department of Psychiatry and Psychotherapy, Otto von Guericke University Magdeburg, Magdeburg, Germany
Ketamine is a drug that reduces depressive and elicits schizophrenia-like symptoms in humans. However, it is largely unexplored whether women and men differ with respect to ketamine-action and whether age contributes to drug-effects. In this study we assessed dissociative symptoms via the Clinician Administered Dissociative States Scale (CADSS) in a total of 69 healthy subjects aged between 18 and 30 years (early adulthood) after ketamine or placebo infusion. Dissociative symptoms were generally increased only in the ketamine group post-infusion. Specifically, within the ketamine group, men reported significantly more depersonalization and amnestic symptoms than women. Furthermore, with rising age only men were less affected overall with respect to dissociative symptoms. This suggests a sex-specific protective effect of higher age which may be due to delayed brain maturation in men compared to women. We conclude that it is crucial to include sex and age in studies of drug effects in general and of ketamine-action in specific to tailor more efficient psychiatric treatments.
Clinical Trial Registration: EU Clinical Trials Register (EudraCT), trial number: 2010-023414-31.
Introduction
Ketamine is an N-methyl-D-aspartate (NMDA) receptor antagonist and has been shown to decrease depressive symptoms in humans (Murrough et al., 2013), even for low doses (Xu et al., 2016), leading to rapid acting and long lasting effects. Furthermore, ketamine leads to schizophrenia-like symptoms including positive and negative symptoms and has been used as a psychosis model in both human and animal studies for decades (Krystal et al., 1994; Adler et al., 1998; Newcommer et al., 1999). Additionally, acute ketamine administration induces transient dissociative symptoms, i.e., a kind of experience of detachment from surroundings, body and time (Sleigh et al., 2014). Importantly, ketamine induced dissociative symptoms, especially the degree of depersonalization, can predict the antidepressant response 24 h after ketamine infusion in major depression patients, whereas neither other acute psychotomimetic nor physiological effects can (Luckenbaugh et al., 2014; Niciu et al., 2018).
Despite the long history of ketamine’s use in experimental and clinical medicine, only few studies have addressed the question whether modulatory factors like sex and age may contribute to the effects of ketamine. In animal studies sex-specific effects of repeated ketamine administration have been shown leading to antidepressant effects and enhanced hippocampal synapsin levels in male mice but increased depressive like symptoms and attenuated glutamate and aspartate levels in female mice (Thelen et al., 2016). Other studies reported faster antidepressant effects in female but longer lasting effects in male mice (Franceschelli et al., 2015) and higher sensitivity of female rats to low doses of ketamine (Carrier and Kabbaj, 2013). Furthermore, juvenile males were reported to be less sensitive to antidepressant effects of ketamine in comparison to adult male rats (Parise et al., 2013). Regarding sex-specific effects of ketamine in humans, initial research provides evidence that after drug infusion men show a larger decline of verbal memory than women (Morgan et al., 2006). However, most of the previously published studies did not investigate an effect of sex or the interaction of sex and age on any of the ketamine induced symptoms and physiological alterations, despite its great relevance for uncovering ketamine’s therapeutic potential (Wright and Kabbaj, 2018).
Among many targets, ketamine is primarily an NMDA receptor antagonist and its consequent enhancing effect in the function of another glutamatergic ionotropic receptor, AMPA receptor, is well known (for review see Aleksandrova et al., 2017). The glutamatergic system displays prominent sex differences from the DNA level to physiological behaviors of neurons, potentially contributing to the well-known gap in prevalence rates, symptomatology and treatment success in women and men suffering from mental disorders (for review see Wickens et al., 2018). The little information we have from human studies indicates that women show higher levels of glutamate compared to men, in particular in the striatum and the cerebellum (Zahr et al., 2013) as well as the sensorimotor cortex and the anterior cingulate cortex (Grachev and Apkarian, 2000). Moreover, changes in cerebral glutamate levels across the lifespan have been predominantly reported in adult men, exhibiting a steep decline with age (Sailasuta et al., 2008). Interestingly, serum levels of glutamate increase with older age in adult women, while this is not observed in men (Kouchiwa et al., 2012).
Sex and age effects have been more extensively explored in animal studies, critically pointing to the differential reorganization of the glutamatergic system in the developmental period in females and males especially in the prefrontal cortex (PFC; Spear, 2000). The NR2 subunit of NMDA receptor displays a developmental switch as the NR2B subunits in the PFC play important roles in regulating the maturation of PFC circuits in the transition phase between puberty and early adulthood (Flores-Barrera et al., 2014). Provided that maturation of the PFC is not completed until mid-twenties due to gradual synaptic pruning throughout adolescence and early adulthood (Tsujimoto, 2008; Elston et al., 2010; Kolb et al., 2012), these developmental stages can be called as critical periods (Bale and Epperson, 2017). These critical periods also correspond to the developmental stages, where female and male brains become more and more distinct from each other in many levels (Bale and Epperson, 2017). Men and women differ with respect to brain maturation leading to a 1–2 years earlier peak of gray maturation (Lenroot et al., 2007) as well as reduced cortical gray matter loss during adolescence/early adulthood in women (Sandu et al., 2014). Among other brain regions like the amygdala, hippocampus or hypothalamus, orbital and medial PFC show sexual dimorphisms (Goldstein et al., 2001) and differing maturation processes, e.g., gray matter in frontal cortices becomes thinner earlier in females than males (McEwen and Morrison, 2013). Interestingly, Deakin et al. (2008) showed that dissociative effects of ketamine are associated to activity in ventromedial regions of PFC.
In view of these findings, we expected sex differences with respect to dissociative symptoms after single ketamine infusion in women and men during early adulthood when brain maturation is still ongoing. This study was designed to compare effects of a ketamine infusion in healthy young women and men using the Clinician-Administered Dissociative States Scale (CADSS) assessing dissociative symptoms, i.e., derealization, depersonalization, and amnestic effects. To do so, we matched women and men for age and restricted the age range from 18 to 30 years.
Materials and Methods
Participants
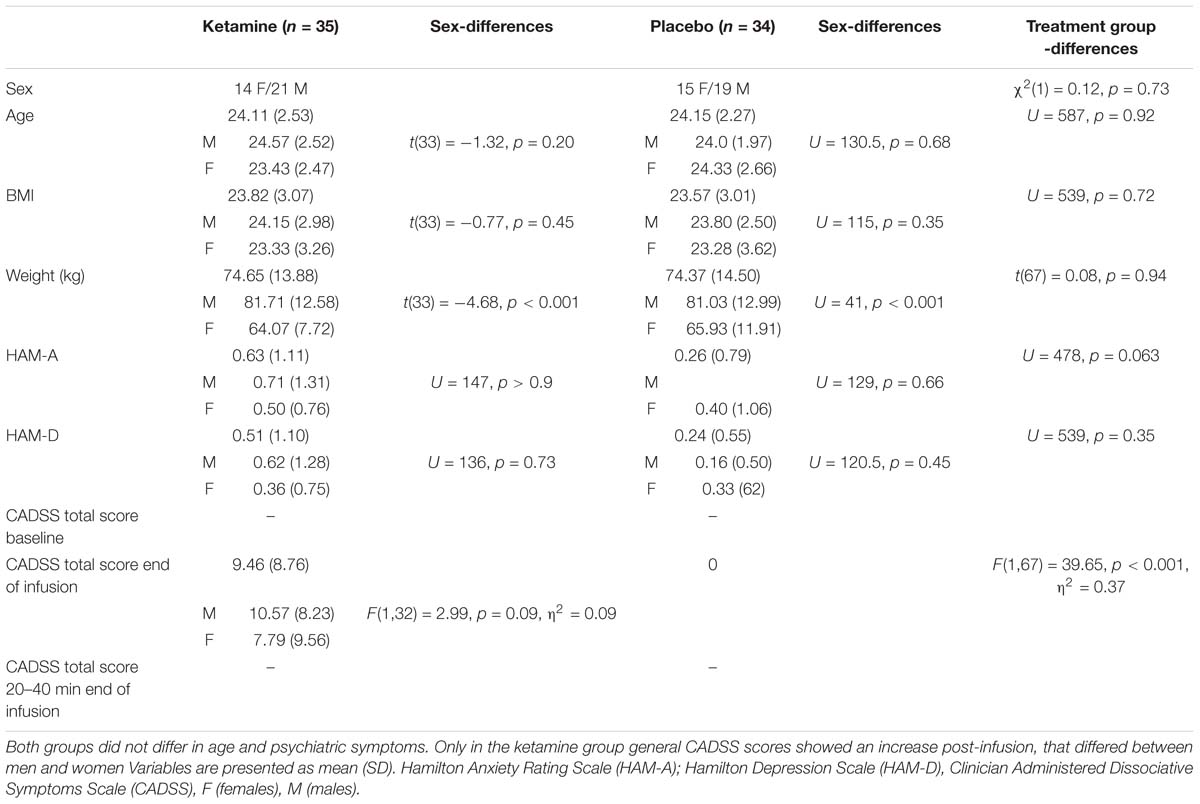
The study was part of a randomized, double-blind, placebo-controlled trial (EudraCT number: 2010-023414-31). Participants were recruited by public advertisement. Participants were screened for MR compatibility and completed extensive medical examination to assure healthy physical status. The German version of Mini-International Neuropsychiatric Interview (MINI; Sheehan et al., 1998) was used to exclude DSM–IV psychiatric disorders. Participants were additionally screened for general psychiatric (BPRS; Overall and Gorham, 1960) depressive (HAM-D; Hamilton, 1960) and anxiety related symptoms (HAM-A; Hamilton, 1959). Moreover, participants were free of current substance use or abuse (excluding smoking) and did not take any medication (excluding contraception pills). Seventeen subjects were excluded during screening process. The age range was set to 18–30 years. Thus, 29 healthy female and 40 male participants were recruited and randomly assigned to receive either a racemic ketamine or a placebo (saline) infusion. Study investigators, research coordinators, attending care teams and subjects were blind to treatment allocation. Fourteen women (mean age = 23.43 years; SD = 2.47) and 21 men (mean age = 24.57; SD = 2.51) received ketamine, whereas 15 women (mean age = 24.33 years; SD = 2.66) and 19 men (mean age = 24.00 years; SD = 1.97) received a placebo infusion. The Ethics Committee of the Medical Faculty of the University of Magdeburg approved the experimental protocol of the study and the study was conducted in accordance with the Declaration of Helsinki (World Medical Association, 2002). Participants provided written informed consent prior to participation and received financial compensation for their participation.
Procedure
First participants completed a baseline assessment of the CADSS (Bremner et al., 1998) which assesses dissociative symptoms divided into depersonalization, derealization, and amnestic symptoms. Afterward, 50 ml of either 0.9% saline (NaCl 0.9%; Berlin-Chemie AG, Berlin, Germany) or 0.5 mg/kg body weight of ketamine +/− racemate (Ketamine-ratiopharm®500 mg/10 ml; Ratiopharm GmbH, Ulm, Germany) were infused continuously over 40 min via an infusion pump (Injectomat 2000; Fresenius Kabi GmbH, Langenhagen, Germany). Immediately after the end of infusion, participants completed the CADSS the second time and again 20–40 min after the end of infusion. During infusion, participants were monitored for cardiovascular response every 5 min, and again 20 as well as 60 min after infusion (Liebe et al., 2017). To ensure the safety of participants, they were additionally asked about their general condition after the end of infusion.
Statistical Analysis
Statistical analyses were conducted via SPSS 23 (IBM). First independent samples t- or U-tests compared the placebo and ketamine group with respect to demographical variables. Further women and men in the ketamine group were also compared for the same variables.
To assess whether placebo and ketamine group differed for baseline or post-infusion dissociative symptoms, a 2 × 2 independent samples analysis of variance (ANOVA) was conducted including the within-subject-factor Time (baseline, post-infusion) and the between-subject-factor Treatment (placebo, ketamine).
To investigate dissociative symptoms in the ketamine group, a multivariate analysis of covariance (MANCOVA) with sex (women, men) as fixed factor and the three CADSS-subscales (scores after ketamine infusion) as dependent variables was conducted to assess sex differences across all subscales. Test statistics are reported according to Pillai’s Trace. Furthermore, a univariate ANCOVA was computed to assess sex differences for the total score. To adjust for potential differences on the total dose depending on body weight, this variable was added as a covariate to both tests. In each case, the statistical threshold was set to α = 0.05. P-values between 0.05 and 0.09 were labeled trend-significant.
Correlation Analyses
To assess whether age was associated to symptom manifestation for the total score or one of the three subscales within the ketamine group, partial correlations controlling for weight were conducted separately for women and men. To address the question whether correlation coefficients were different for women and men, Fisher’s z tests were computed. Differences in correlation scores were corrected for multiple comparisons, with an effective threshold of p < 0.0125.
Results
Demographics
Independent samples t-tests confirmed that the placebo and ketamine group did not differ significantly in demographic variables or psychiatric symptoms. Also, women and men in both groups did not differ in all parameters except weight (see Table 1).
General Effects of Ketamine Infusion
First, a 2 × 2 ANOVA detected main effects of Treatment, F(1,67) = 39.65, p < 0.001, η2 = 0.37, and Time, F(1,67) = 39.65, p < 0.001, η2 = 0.37, and a significant interaction of Treatment × Time, F(1,67) = 39.65, p < 0.001, η2 = 0.37, indicating enhanced dissociative symptoms only in the ketamine group immediately post-infusion.
Sex-Specific Effects in the Ketamine Group Depending on CADSS Subscale
Second, sex- and subscale-specific effects of ketamine were investigated. The MANCOVA including weight as covariate showed a significant effect of sex [F(3,30) = 3.60, p = 0.025, η2 = 0.27]. Looking at subscales individually, depersonalization [F(1,32) = 7.38, p = 0.011, η2 = 0.19] and amnesia [F(1,32) = 5.09, p = 0.031, η2 = 0.14] showed significantly higher scores in men, which was not the case for derealization [F(1,32) = 0.93, p = 0.34, η2 = 0.03]. Univariate ANCOVA showed a marginal effect on the total score [F(1,32) = 2.99, p = 0.09, η2 = 0.09] (see Figure 1).
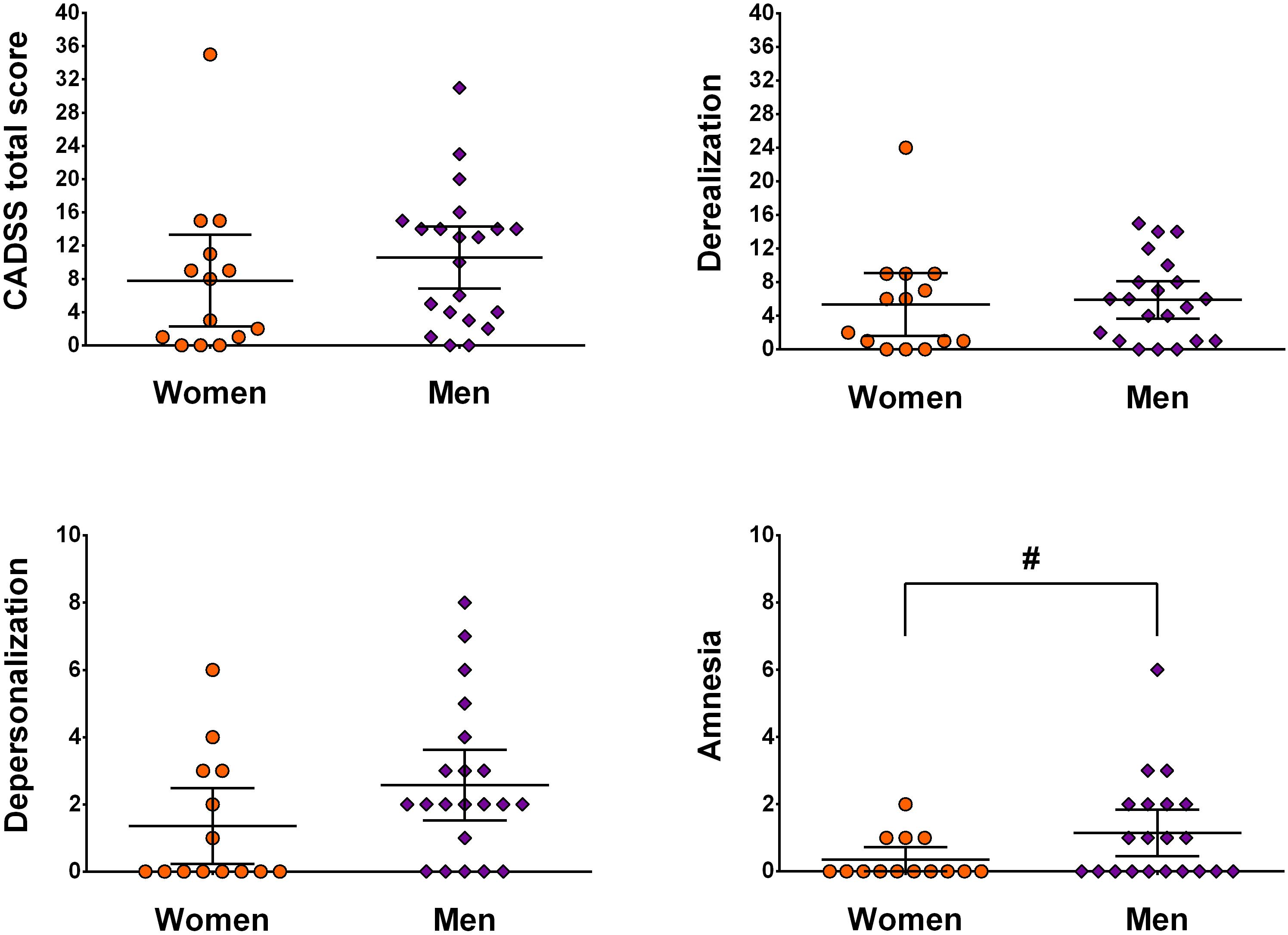
Figure 1. Illustration of sex- and subscale-specific effects of ketamine. Data is presented as mean ± 95 CI and is not corrected for weight. # indicates p < 0.05.
Sex- and Subscale Specific Effects in Connection With Participant Age
Next, we assessed whether participant age was associated with symptom manifestation in CADSS total and sub-scores, separately for men and women. In men, significant negative correlations between age and CADSS scores were observed (for depersonalization on a trend-level), which was not the case for women (see Figure 2 and Table 2).
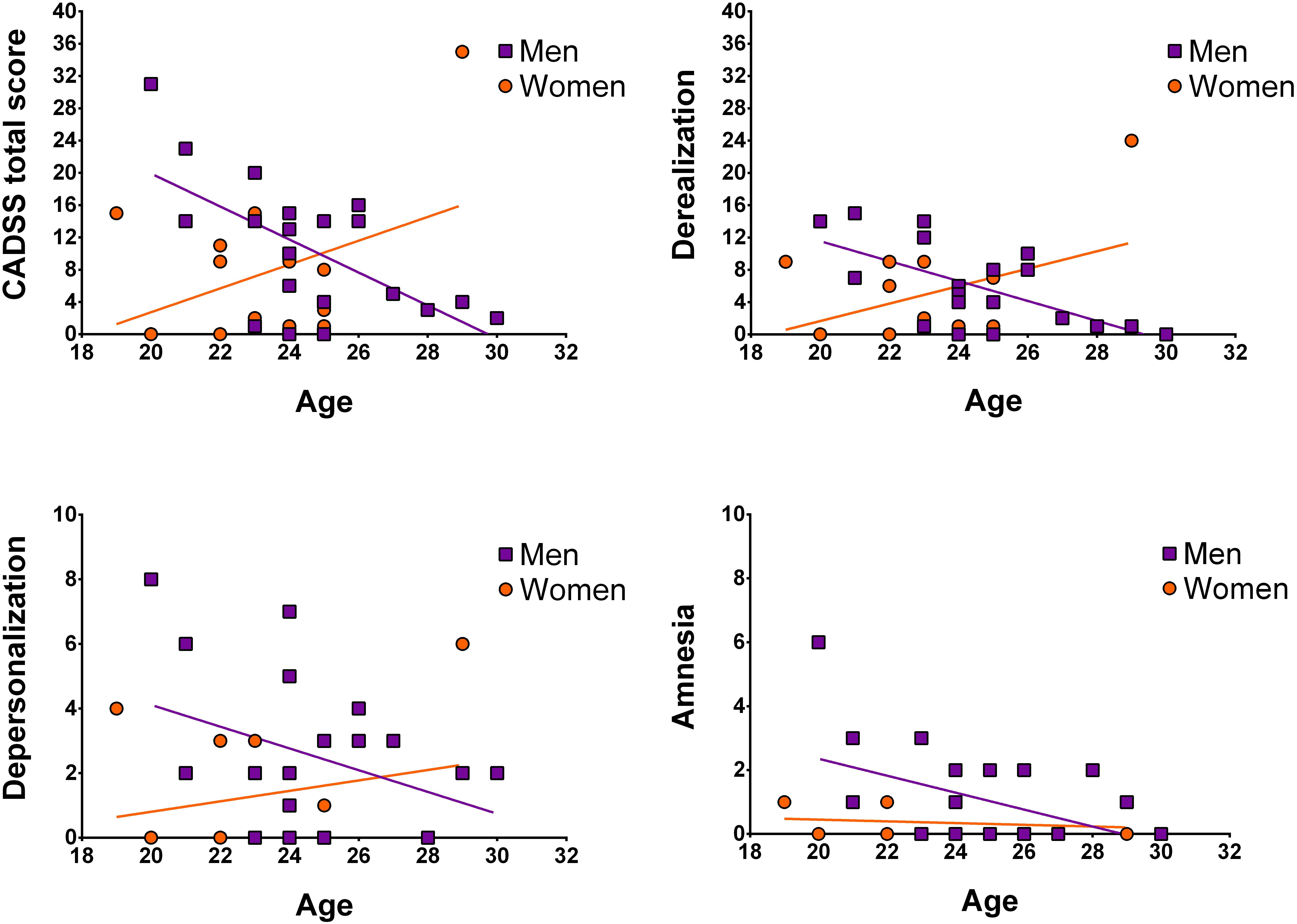
Figure 2. Illustration of correlations between age and CADSS scores separately for women and men. Data points are not corrected for weight.
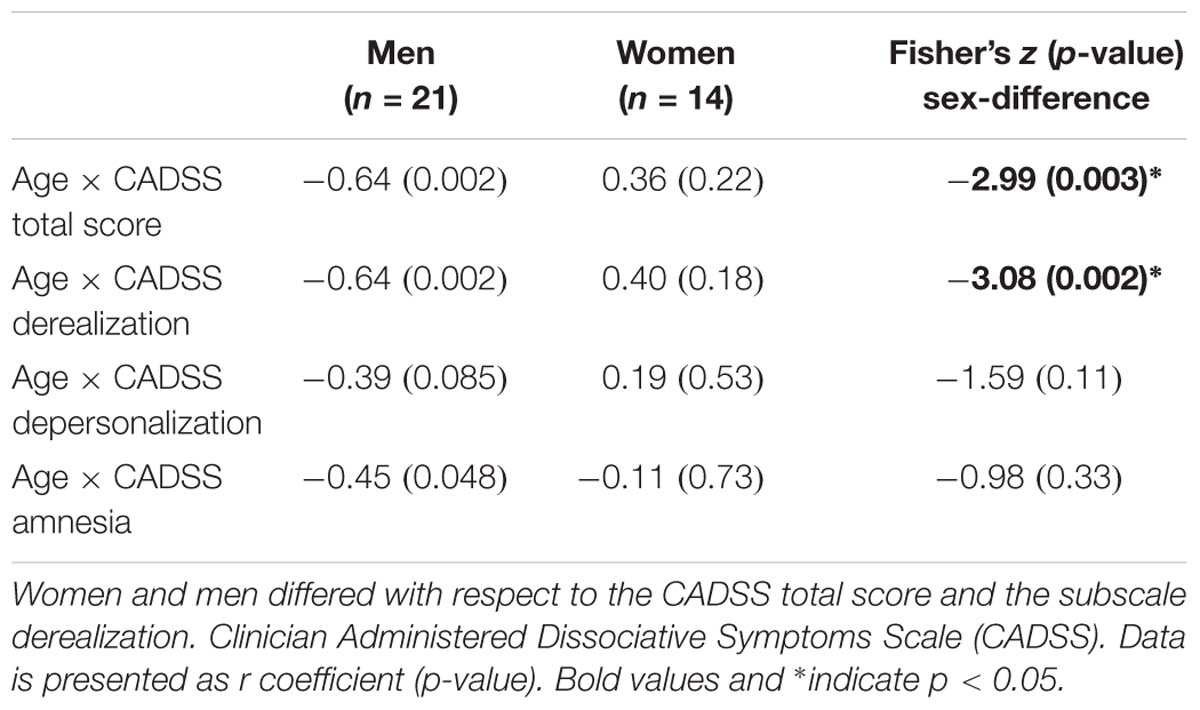
Table 2. Correlation analyses testing sex-specific associations between Age and CADSS scores immediately after infusion.
To further validate whether men and women differed for any of the above correlations, Fisher’s Z-tests were conducted. For derealization, z = −3.08, p = 0.002, and the CADSS total score, z = −2.99, p = 0.003, significant sex-differences were detected indicating that in men symptom manifestation decreased more strongly with age than in women. Correlation coefficients of men and women did not differ significantly for depersonalization z = −1.59, p = 0.11 and amnesia, z = −0.98, p = 0.33 (see Table 2).
Discussion
The present study investigated whether dissociative symptoms as induced by the anti-depressive drug ketamine differ as a function of sex and age. In general, a sub-anesthetic dose of ketamine led to profound dissociate symptoms affecting women and men, though men showed significantly stronger symptom manifestation regarding depersonalization and amnesia than women. Furthermore, taking into account our participants age, in men dissociative symptoms in total and derealization in specific decreased with rising age while this association was not observed in women.
Surprisingly, the effects of sex and age on ketamine’s actions have not been broadly examined in humans, although prevalence rates and symptomatology of mental disorders associated with the glutamate system and ketamine-action, e.g., depression, significantly differ between women and men (Whiteford et al., 2013; Strong and Kabbaj, 2018; Wickens et al., 2018; Wright and Kabbaj, 2018). Although animal studies point to a variation in sensitivity to antidepressant and addictive effects of ketamine depending on age and sex (Carrier and Kabbaj, 2013; Parise et al., 2013; Franceschelli et al., 2015; Zanos et al., 2016; Schoepfer et al., 2017; Strong et al., 2017), human studies rarely report sex or age effects (Cho et al., 2005; Niciu et al., 2014). In humans, the reported differences between women and men focused on metabolites and hepatic clearance of ketamine (Saland et al., 2017), biomarkers (Colic et al., 2019) or side effects (Liebe et al., 2017). Sigtermans et al. (2009) reported that S-Ketamine is metabolized faster in female subjects and the effect of ketamine is greater on cardiac output and heat pain. Another study which used racemic ketamine, as also used in the current study indicated a sex-specific metabolism of ketamine in depressed and bipolar patients (Zarate et al., 2012). Additionally, a previous meta-analysis reported a significant association between effect sizes of ketamine response at later time points, i.e., 7 days post-infusion, and percentage males, but the number of included studies that contributed data was quite low (Coyle and Laws, 2015). Reviewing the relevant literature, Wright and Kabbaj (2018) stressed that most of the clinical studies lack the information about sensitivity to the effects of ketamine because generally one particular dose of ketamine is administered instead of an application of a dose-response regime like in animal studies. Indeed, Morgan et al. (2006) showed that men are more sensitive to verbal and subjective memory disturbances induced by intravenous ketamine infusions, of which doses ranged between 0.5 and 1.3 mg/kg. Likewise, in the current study male participants reported higher subjective memory disturbances measured by CADSS supporting earlier findings by Morgan et al. The single dose regime that was applied in the current study might have hindered the clear sex effect insensitivity to the amnesic effect of ketamine. More studies using a wider range of doses would be beneficial to understand both the role of sex and age in effects of ketamine.
Concerning age effects, dissociative effects of ketamine were negatively associated with age only in male participants. Early adulthood is a critical development stage that engenders vulnerability for a variety of mental disorders for women and men (Paksarian et al., 2016). Plenty of previously reported findings indicate that the effects of ketamine show variation across participants according to the basal status of associated circuits (Lahti et al., 2001; Corlett et al., 2006; Morgan et al., 2008). Regarding clinical populations, reports on geriatric patients are scarce but seem to be similar to generally observed effects (although see Szymkowicz et al., 2014), but the samples were very small or case-control studies (Iglewicz et al., 2015; George et al., 2017; Medeiros da Frota Ribeiro and Riva-Posse, 2017). Regarding depressed adolescents and young adults, studies investigating antidepressant effects of ketamine are virtually non-existent.
This study is limited in that we specifically focused on young adults, thus included only data from participants younger than 30 years. However, to fully test our assumption of an age-specific decline of dissociative symptoms in men, future studies should include a broader age range, informing about age- and sex-specific effects across different developmental stages (e.g., from puberty to menopause and further) as changes across the lifespan have been reported for cerebral glutamate levels in men (Sailasuta et al., 2008) and serum levels in women (Kouchiwa et al., 2012). Moreover, a modulatory role of estradiol has been shown in animal studies addressing glutamate transmission (Smejkalova and Woolley, 2010). Regarding ketamine, sex differences in ketamine pharmacokinetics in rats have been reported, however, the impact of circulating hormone levels was negligible (Saland and Kabbaj, 2018). Another limitation to be addressed is the lack of measurements of ketamine and its active metabolites in the blood. Evidence indicates that the metabolism of both racemic and S-ketamine differ between men and women (Sigtermans et al., 2009; Zarate et al., 2012). A common limitation of placebo-controlled ketamine studies is the reliability of blinding. Ketamine induces symptoms that are evident mostly to the participants and also to the involved scientists. For this reason, studies are conducted with active placebos like midazolam (Wilkinson et al., 2019), which result in their own caveats.
In summary, male participants in our study reported stronger depersonalization and amnestic symptoms following ketamine infusion. Interestingly, this effect was potentiated by age, i.e., the younger the age the stronger the symptoms. Thus, our findings suggest a sex-specific protective effect of age, which may be due to progressed brain maturation in women compared to men. We conclude that it is crucial to include sex and age in studies of drug effects in general and of ketamine-action in specific to tailor more efficient psychiatric treatment strategies.
Data Availability
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
Participants provided written informed consent prior to participation, received financial compensation for their participation, and the Ethics Committee of the Medical Faculty of the University of Magdeburg approved the experimental protocol of the study.
Author Contributions
BD, JH, and ZS analyzed the parts of the data and wrote the first draft. LC and ML collected the data, contributed to the data analyses, and corrected the manuscript. MW designed the study, supervised the data collection and analyses, and corrected the manuscript.
Funding
BD was supported by the DFG (DE2319/6-1) and the CIN (EXC307). This study was supported by the German Research Foundation (SFB 779/A06 for MW and DFG WA 2673/4-1 for MW), the Center for Behavioral Brain Sciences (CBBS NN05 for MW), and Leibniz Association (Pakt für Forschung und Innovation for MW). LC received a scholarship from the German Research Foundation (SFB 779, 2013–2016).
Conflict of Interest Statement
MW received research support from HEEL and Janssen Pharmaceutical Research for a clinical trial on ketamine in patients with major depression which were not investigated in this manuscript. Other authors declare no conflict of interest.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Claus Tempelmann and Renate Blobel (Department of Neurology) for data acquisition; Dr. Melanie Weigel (Department of Ophthalmology) and Dr. Conrad Friedrich Genz (Department of Cardiology) for clinical screening; and Dr. Julia Noack and Linda Frolik Endrulat (Clinical Affective Neuroimaging Laboratory) for trial management. We would also like to acknowledge the help of all the participants in this study. The article processing charge was funded by the DFG and the Eberhard Karls University Tbingen in the funding program Open Access Publishing.
References
Adler, C. M., Goldberg, T. E., Malhotra, A. K., Pickar, D., and Breier, A. (1998). Effects of ketamine on thought disorder, working memory, and semantic memory in healthy volunteers. Biol. Psychiatry 43, 811–816. doi: 10.1016/s0006-3223(97)00556-8
Aleksandrova, L. R., Phillips, A. G., and Wang, Y. T. (2017). Antidepressant effects of ketamine and the roles of AMPA glutamate receptors and other mechanisms beyond NMDA receptor antagonism. J. Psychiatry Neurosci. 42, 222–229. doi: 10.1503/jpn.160175
Bale, T. L., and Epperson, C. N. (2017). Sex as a biological variable: who. what, when, why, and how. Neuropsychopharmacology 42, 386–396. doi: 10.1038/npp.2016.215
Bremner, J. D., Krystal, J. H., Putnam, F. W., Southwick, S. M., Marmar, C., Charney, D. S., et al. (1998). Measurement of dissociative states with the clinician-administered dissociative states scale (CADSS). J. Trauma Stress 11, 125–136. doi: 10.1023/A:1024465317902
Carrier, N., and Kabbaj, M. (2013). Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology 70, 27–34. doi: 10.1016/j.neuropharm.2012.12.009
Cho, H. S., D’Souza, D. C., Gueorguieva, R., Perry, E. B., Madonick, S., Karper, L. P., et al. (2005). Absence of behavioral sensitization in healthy human subjects following repeated exposure to ketamine. Psychopharmacology 179, 136–143. doi: 10.1007/s00213-004-2066-5
Colic, L., McDonnell, C., Li, M., Woelfer, M., Liebe, T., Kretzschmar, M., et al. (2019). Neuronal glutamatergic changes and peripheral markers of cytoskeleton dynamics change synchronically 24 h after sub-anaesthetic dose of ketamine in healthy subjects. Behav. Brain Res. 359, 312–319. doi: 10.1016/j.bbr.2018.10.021
Corlett, P. R., Honey, G. D., Aitken, M. R., Dickinson, A., Shanks, D. R., Absalom, A. R., et al. (2006). Frontal responses during learning predict vulnerability to the psychotogenic effects of ketamine: linking cognition, brain activity, and psychosis. Arch. Gen. Psychiatry 63, 611–621. doi: 10.1001/archpsyc.63.6.611
Coyle, C. M., and Laws, K. R. (2015). The use of ketamine as an antidepressant: a systematic review and meta-analysis. Hum. Psychopharm. Clin. Exp. 30, 152–163. doi: 10.1002/hup.2475
Deakin, J. F., Lees, J., McKie, S., Hallak, J. E., Williams, S. R., and Dursun, S. M. (2008). Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch. Gen. Psychiatry 65, 154–164. doi: 10.1001/archgenpsychiatry.2007.37
Elston, G. N., Oga, T., Okamoto, T., and Fujita, I. (2010). Spinogenesis and pruning from early visual onset to adulthood: an intracellular injection study of layer III pyramidal cells in the ventral visual cortical pathway of the macaque monkey. Cereb. Cortex 20, 1398–1408. doi: 10.1093/cercor/bhp203
Flores-Barrera, E., Thomases, D. R., Heng, L. J., Cass, D. K., Caballero, A., and Tseng, K. Y. (2014). Late adolescent expression of GluN2B transmission in the prefrontal cortex is input-specific and requires postsynaptic protein kinase A and D1 dopamine receptor signaling. Biol. Psychiatry 75, 508–516. doi: 10.1016/j.biopsych.2013.07.033
Franceschelli, A., Sens, J., Herchick, S., Thelen, C., and Pitychoutis, P. M. (2015). Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naive and “depressed” mice exposed to chronic mild stress. Neuroscience 290, 49–60. doi: 10.1016/j.neuroscience.2015.01.008
George, D., Galvez, V., Martin, D., Kumar, D., Leyden, J., Hadzi-Pavlovic, D., et al. (2017). Pilot randomized controlled trial of titrated subcutaneous ketamine in older patients with treatment-resistant depression. Am. J. Geriatr. Psychiatry 25, 1199–1209. doi: 10.1016/j.jagp.2017.06.007
Goldstein, J. M., Seidman, L. J., Horton, N. J., Makris, N., Kennedy, D. N., Caviness, V. S., et al. (2001). Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb. Cortex 11, 490–497. doi: 10.1093/cercor/11.6.490
Grachev, I. D., and Apkarian, A. V. (2000). Chemical heterogeneity of the living human brain: a proton MR spectroscopy study on the effects of sex, age, and brain region. Neuroimage 11(5 Pt 1), 554–563. doi: 10.1006/nimg.2000.0557
Hamilton, M. (1959). The assessment of anxiety states by rating. Br. J. Med. Psychol. 32, 50–55. doi: 10.1111/j.2044-8341.1959.tb00467
Iglewicz, A., Morrison, K., Nelesen, R. A., Zhan, T., Iglewicz, B., Fairman, N., et al. (2015). Ketamine for the treatment of depression in patients receiving hospice care: a retrospective medical record review of thirty-one cases. Psychosomatics 56, 329–337. doi: 10.1016/j.psym.2014.05.005
Kolb, B., Mychasiuk, R., Muhammad, A., Li, Y., Frost, D., and Gibb, R. (2012). Experience and the developing prefrontal cortex. Proc. Natl. Acad. Sci. U.S.A. 109, 17186–17193. doi: 10.1073/pnas.1121251109
Kouchiwa, T., Wada, K., Uchiyama, M., Kasezawa, N., Niisato, M., Murakami, H., et al. (2012). Age-related changes in serum amino acids concentrations in healthy individuals. Clin. Chem. Lab. Med. 50, 861–870. doi: 10.1515/cclm-2011-0846
Krystal, J. H., Karper, L. P., Seibyl, J. P., Freeman, G. K., Delaney, R., and Charney, D. S. (1994). Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry 51, 199–214.
Lahti, A. C., Weiler, M. A., Tamara Michaelidis, B. A., Parwani, A., and Tamminga, C. A. (2001). Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology 25, 455–467. doi: 10.1016/S0893-133X(01)00243-3
Lenroot, R. K., Gogtay, N., Greenstein, D. K., Wells, E. M., Wallace, G. L., Clasen, L. S., et al. (2007). Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 36, 1065–1073. doi: 10.1016/j.neuroimage.2007.03.053
Liebe, T., Li, S., Lord, A., Colic, L., Krause, A. L., Batra, A., et al. (2017). Factors influencing the cardiovascular response to subanesthetic ketamine: a randomized. placebo-controlled trial. Int. J. Neuropsychopharmacol. 20, 909–918. doi: 10.1093/ijnp/pyx055
Luckenbaugh, D. A., Niciu, M. J., Ionescu, D. F., Nolan, N. M., Richards, E. M., Brutsche, N. E., et al. (2014). Do the dissociative side effects of ketamine mediate its antidepressant effects? J. Affect. Disord. 159, 56–61. doi: 10.1016/j.jad.2014.02.017
McEwen, B. S., and Morrison, J. H. (2013). The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 79, 16–29. doi: 10.1016/j.neuron.2013.06.028
Medeiros da Frota Ribeiro, C., and Riva-Posse, P. (2017). Use of ketamine in elderly patients with treatment-resistant depression. Curr. Psychiatry Rep. 19:107. doi: 10.1007/s11920-017-0855-x
Morgan, C. J., Perry, E. B., Cho, H. S., Krystal, J. H., and D’Souza, D. C. (2006). Greater vulnerability to the amnestic effects of ketamine in males. Psychopharmacology 187, 405–414. doi: 10.1007/s00213-006-0409-0
Morgan, C. J., Rees, H., and Curran, H. V. (2008). Attentional bias to incentive stimuli in frequent ketamine users. Psychol. Med. 38, 1331–1340. doi: 10.1017/S0033291707002450
Murrough, J. W., Iosifescu, D. V., Chang, L. C., Al Jurdi, R. K., Green, C. E., Perez, A. M., et al. (2013). Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am. J. Psychiatry 170, 1134–1142. doi: 10.1176/appi.ajp.2013.13030392
Newcommer, J. W., Farber, N. B., Jevtovic-Todorovic, V., Selke, G., Melson, A. K., Hershey, T. et al. (1999). Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology 20, 106–118. doi: 10.1016/S0893-133X(98)00067-0
Niciu, M. J., Luckenbaugh, D. A., Ionescu, D. F., Richards, E. M., Vande Voort, J. L., Ballard, D. D. et al. (2014). Ketamine’s antidepressant efficacy is extended for at least four weeks in subjects with a family history of an alcohol use disorder. Int. J. Neuropsychopharmacol. 18:pyu039. doi: 10.1093/ijnp/pyu039
Niciu, M. J., Shovestul, B. J., Jaso, B. A., Farmer, C., Luckenbaugh, D. A., Brutsche, N. E., et al. (2018). Features of dissociation differentially predict antidepressant response to ketamine in treatment-resistant depression. J. Affect. Disord. 232, 310–315. doi: 10.1016/j.jad.2018.02.049
Overall, J. E., and Gorham, D. R. (1960). Factor space D2 analysis applied to the study of changes in schizophrenic symptomatology during chemotherapy. J. Clin. Exp. Psychopathol. Q. Rev. Psychiatry Neurol. 21,187–195.
Paksarian, D., Cui, L., Angst, J., Ajdacic-Gross, V., Rossler, W., and Merikangas, K. R. (2016). Latent trajectories of common mental health disorder risk across 3 decades of adulthood in a population-based cohort. JAMA Psychiatry 73, 1023–1031. doi: 10.1001/jamapsychiatry.2016.1921
Parise, E. M., Alcantara, L. F., Warren, B. L., Wright, K. N., Hadad, R., Sial, O. K., et al. (2013). Repeated ketamine exposure induces an enduring resilient phenotype in adolescent and adult rats. Biol. Psychiatry 74, 750–759. doi: 10.1016/j.biopsych.2013.04.027
Sailasuta, N., Ernst, T., and Chang, L. (2008). Regional variations and the effects of age and gender on glutamate concentrations in the human brain. Magn. Reson. Imaging 26, 667–675. doi: 10.1016/j.mri.2007.06.007
Saland, S. K., Duclot, F., and Kabbaj, M. (2017). Integrative analysis of sex differences in the rapid antidepressant effects of ketamine in preclinical models for individualized clinical outcomes. Curr. Opin. Behav. Sci. 14, 19–26. doi: 10.1016/j.cobeha.2016.11.002
Saland, S. K., and Kabbaj, M. (2018). Sex differences in the pharmacokinetics of low-dose ketamine in plasma and brain of male and female Rats. J. Pharmacol. Exp. Ther. 367, 393–404. doi: 10.1124/jpet.118.251652
Sandu, A. L., Izard, E., Specht, K., Beneventi, H., Lundervold, A., and Ystad, M. (2014). Post-adolescent developmental changes in cortical complexity. Behav. Brain Funct. 10:44. doi: 10.1186/1744-9081-10-44
Schoepfer, K. J., Strong, C. E., Saland, S. K., Wright, K. N., and Kabbaj, M. (2017). Sex- and dose-dependent abuse liability of repeated subanesthetic ketamine in rats. Physiol. Behav. 203, 60–69. doi: 10.1016/j.physbeh.2017.10.021
Sheehan, D. V., Lecrubier, Y., Sheehan, K. H., Amorim, P., Janavs, J., Weiller, E. et al. (1998). The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59, 22–33.
Sigtermans, M., Dahan, A., Mooren, R., Bauer, M., Kest, B., Sarton, E., et al. (2009). S(+)-ketamine effect on experimental pain and cardiac outputa population pharmacokinetic-pharmacodynamic modeling study in healthy volunteers. Anesthesiology 111, 892–903. doi: 10.1097/ALN.0b013e3181b437b1
Sleigh, J., Harvey, M., Voss, L., and Denny, B. (2014). Ketamine: more mechanisms of action than just NMDA blockade. Trends Anaesth. Crit. Care 4, 76–81. doi: 10.1016/j.tacc.2014.03.002
Smejkalova, T., and Woolley, C. S. (2010). Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J. Neurosci. 30, 16137–16148. doi: 10.1523/JNEUROSCI.4161-10.2010
Spear, L. P. (2000). The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 24, 417–463. doi: 10.1016/s0149-7634(00)00014-2
Strong, C. E., and Kabbaj, M. (2018). On the safety of repeated ketamine infusions for the treatment of depression: effects of sex and developmental periods. Neurobiol. Stress 9, 166–175. doi: 10.1016/j.ynstr.2018.09.001
Strong, C. E., Schoepfer, K. J., Dossat, A. M., Saland, S. K., Wright, K. N., and Kabbaj, M. (2017). Locomotor sensitization to intermittent ketamine administration is associated with nucleus accumbens plasticity in male and female rats. Neuropharmacology 121, 195–203. doi: 10.1016/j.neuropharm.2017.05.003
Szymkowicz, S. M., Finnegan, N., and Dale, R. M. (2014). Failed response to repeat intravenous ketamine infusions in geriatric patients with major depressive disorder. J. Clin. Psychopharmacol. 34, 285–286. doi: 10.1097/JCP.0000000000000090
Thelen, C., Sens, J., Mauch, J., Pandit, R., and Pitychoutis, P. M. (2016). Repeated ketamine treatment induces sex-specific behavioral and neurochemical effects in mice. Behav. Brain Res. 312, 305–312. doi: 10.1016/j.bbr.2016.06.041
Tsujimoto, S. (2008). The prefrontal cortex: functional neural development during early childhood. Neuroscientist 14, 345–358. doi: 10.1177/1073858408316002
Whiteford, H. A., Degenhardt, L., Rehm, J., Baxter, A. J., Ferrari, A. J., Erskine, H. E., et al. (2013). Global burden of disease attributable to mental and substance use disorders: findings from the global burden of disease study 2010. Lancet 382, 1575–1586. doi: 10.1016/S0140-6736(13)61611-6
Wickens, M. M., Bangasser, D. A., and Briand, L. A. (2018). Sex differences in psychiatric disease: a focus on the glutamate system. Front. Mol. Neurosci. 11:197. doi: 10.3389/fnmol.2018.00197
Wilkinson, S. T., Farmer, C., Ballard, E. D., Mathew, S. J., Grunebaum, M. F., Murrough, J. W., et al. (2019). Impact of midazolam vs. saline on effect size estimates in controlled trials of ketamine as a rapid-acting antidepressant. Neuropsychopharmacology 44, 1233–1238. doi: 10.1038/s41386-019-0317-8
World Medical Association (2002). World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J. Postgrad. Med. 48, 206–208.
Wright, K. N., and Kabbaj, M. (2018). Sex differences in sub-anesthetic ketamine’s antidepressant effects and abuse liability. Curr. Opin. Behav. Sci. 23, 36–41. doi: 10.1016/j.cobeha.2018.02.001
Xu, Y., Hackett, M., Carter, G., Loo, C., Gálvez, V., Glozier, N. et al. (2016). Effects of low-dose and very low-dose ketamine among patients with major depression: a systematic review and meta-analysis. Int. J. Neuropsychopharmacol. 19:pyv124. doi: 10.1093/ijnp/pyv124
Zahr, N. M., Mayer, D., Rohlfing, T., Chanraud, S., Gu, M., Sullivan, E. V., et al. (2013). In vivo glutamate measured with magnetic resonance spectroscopy: behavioral correlates in aging. Neurobiol. Aging 34, 1265–1276. doi: 10.1016/j.neurobiolaging.2012.09.014
Zanos, P., Moaddel, R., Morris, P. J., Georgiou, P., Fischell, J., Elmer, G. I., et al. (2016). NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533, 481–486. doi: 10.1038/nature17998
Keywords: ketamine, dissociation, depersonalization, sex, age, brain maturation
Citation: Derntl B, Hornung J, Sen ZD, Colic L, Li M and Walter M (2019) Interaction of Sex and Age on the Dissociative Effects of Ketamine Action in Young Healthy Participants. Front. Neurosci. 13:616. doi: 10.3389/fnins.2019.00616
Received: 20 February 2019; Accepted: 29 May 2019;
Published: 18 June 2019.
Edited by:
Annie Duchesne, University of Northern British Columbia, CanadaReviewed by:
Jennifer L. Vande Voort, Mayo Clinic, United StatesSiegfried Kasper, Medical University of Vienna, Austria
Copyright © 2019 Derntl, Hornung, Sen, Colic, Li and Walter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. Walter, bWFydGluLndhbHRlckB1bmktdHVlYmluZ2VuLmRl; bWFydGluLndhbHRlckBtZWQub3ZndS5kZQ==
†These authors have contributed equally to this work
 B. Derntl
B. Derntl J. Hornung
J. Hornung Z. D. Sen
Z. D. Sen L. Colic
L. Colic M. Li
M. Li M. Walter
M. Walter