- 1Department of Virology, Croatian Institute of Public Health, Zagreb, Croatia
- 2School of Medicine, University of Zagreb, Zagreb, Croatia
- 3Poultry Center, Croatian Veterinary Institute, Zagreb, Croatia
- 4Department for Virology, Scientific Veterinary Institute, Novi Sad, Serbia
- 5Veterinary Faculty, University of Ljubljana, Ljubljana, Slovenia
- 6Department of Microbiology and Infectious Diseases With Clinic, Faculty of Veterinary Medicine, University of Zagreb, Zagreb, Croatia
- 7Laboratory for Medical and Veterinary Entomology, Faculty of Agriculture, University of Novi Sad, Novi Sad, Serbia
- 8Center for Microbiology, Institute of Public Health Vojvodina, Novi Sad, Serbia
- 9Medical Faculty, University of Novi Sad, Novi Sad, Serbia
- 10Division of Disinfection, Disinfestation and Pest Control, Andrija Stampar Teaching Institute of Public Health, Zagreb, Croatia
- 11Department of Medicine, Merkur University Hospital, Zagreb, Croatia
- 12OIE Reference Center for West Nile Disease, Istituto Zooprofilattico Sperimentale “G. Caporale”, Teramo, Italy
- 13Laboratory for Diagnostics, Croatian Veterinary Institute, Regional Institute Split, Split, Croatia
The epidemiology of West Nile (WNV) and Usutu virus (USUV) has changed dramatically over the past two decades. Since 1999, there have been regular reports of WNV outbreaks and the virus has expanded its area of circulation in many Southern European countries. After emerging in Italy in 1996, USUV has spread to other countries causing mortality in several bird species. In 2009, USUV seroconversion in horses was reported in Italy. Co-circulation of both viruses was detected in humans, horses and birds. The main vector of WNV and USUV in Europe is Culex pipiens, however, both viruses were found in native Culex mosquito species (Cx. modestus, Cx. perexiguus). Experimental competence to transmit the WNV was also proven for native and invasive mosquitoes of Aedes and Culex genera (Ae. albopictus, Ae. detritus, Cx. torrentium). Recently, Ae. albopictus and Ae. japonicus naturally-infected with USUV were reported. While neuroinvasive human WNV infections are well-documented, USUV infections are sporadically detected. However, there is increasing evidence of a role of USUV in human disease. Seroepidemiological studies showed that USUV circulation is more common than WNV in some endemic regions. Recent data showed that WNV strains detected in humans, horses, birds, and mosquitoes mainly belong to lineage 2. In addition to European USUV lineages, some reports indicate the presence of African USUV lineages as well. The trends in WNV/USUV range and vector expansion are likely to continue in future years. This mini-review provides an update on the epidemiology of WNV and USUV infections in Southern Europe within a multidisciplinary “One Health” context.
Introduction
West Nile virus (WNV) and Usutu virus (USUV) are mosquito-borne flaviviruses characterized by similar clinical manifestations and overlapping geographic distribution, host and vector species. The epidemiology of WNV and USUV has changed dramatically over the past two decades. Since 1996, an increasing number of WNV outbreaks in humans and horses were detected, and the area of its circulation expanded in many Southern European countries (1). After USUV emergence in Austria in 2001, it subsequently spread to neighboring countries causing mortality in several wild bird species, mainly Eurasian blackbirds (2). However, a retrospective analysis of archived bird tissue samples from Italy (Tuscany region) in 1996 identified USUV, indicating a much earlier introduction of this virus into Europe (3).
Since the early 2000s, WNV circulation has been continuously monitored in some European countries with varying number of human and horse cases. USUV infections are reported in birds, while human clinical cases are rarely detected (4–6). However, there is increasing evidence of a role of USUV in human disease. Seroepidemiological studies showed that USUV circulation in humans is more common than WNV in some endemic regions where both viruses circulate (7).
Until the introduction of WNV lineage 2 in 2004 (8), WNV lineage 1 was identified as the cause of human outbreaks in Europe. In the following years, lineage 2 dispersed to the eastern part of Austria and to Southern European countries (9–11). Recent data showed that strains detected in humans, horses, birds, and mosquitoes mainly belong to WNV lineage 2 (6, 12, 13). Although the majority of USUV strains belong to European USUV lineages, some reports indicate the presence of African USUV lineages as well (5).
In 2018, WNV infections in Europe increased dramatically compared to previous transmission seasons. A total of 2,083 human cases and 285 outbreaks among equids were reported with the largest number detected in Italy, Serbia, and Greece (14). USUV was detected in asymptomatic blood donors, birds, and mosquitoes in Italy (15, 16) and in three patients with neuroinvasive disease in Croatia (6).
The Middle East represents an important transit zone for bird migration between Africa and Eurasia and provides valuable information on circulation of WNV/USUV in these regions (17–19). In Israel, serologic evidence and acute WNV infections have been reported in humans, horses, birds, and mosquitoes (20, 21). While strains sequenced from humans mainly belonged to the WNV lineage 1 (22), mosquito surveillance revealed a high genetic diversity of WNV (23). USUV strains detected in mosquitoes belonged to a putative novel lineage Europe 5 (24). WNV/USUV infections were also reported in other countries bordering the Mediterranean Sea (France, Turkey, Cyprus) (5, 25–40). This mini-review provides an update on the epidemiology/molecular epidemiology of WNV and USUV infections in Southern Europe and neighboring countries within a multidisciplinary “One Health” context.
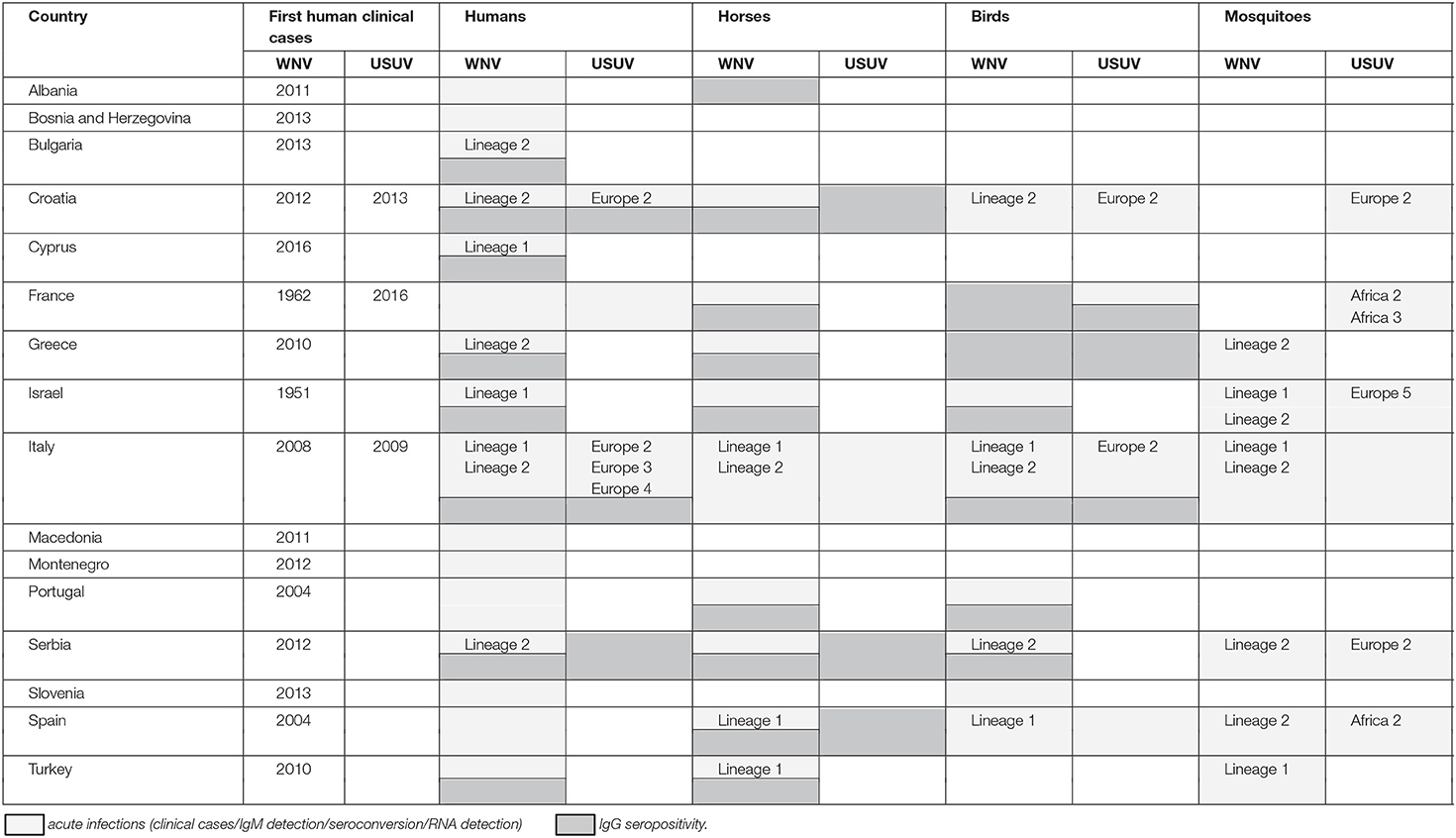
Data on WNV/USUV infections are presented in Table 1 and Figure 1.
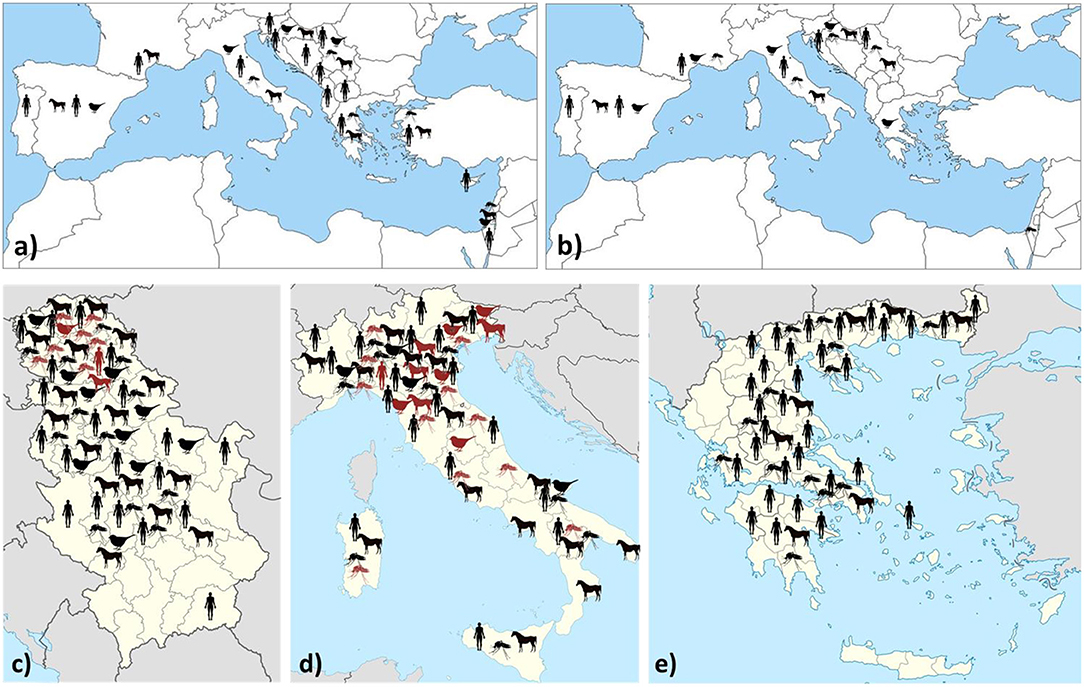
Figure 1. Geographic distribution of WNV (a) and USUV (b) infections/seropositivity in southern Europe and neighboring countries. Regional distribution of WNV (black) and USUV (red) infections (clinical cases/IgM detection/seroconversion/RNA detection) in countries with a high prevalence: Serbia (c), Italy (d), and Greece (e).
Albania
Data on WNV infections in Albania are scarce. To date, only two human cases were reported in 2011 (14). A serological study conducted among horses from 12 districts in Albania showed WNV seropositivity of 22.2%, with significant regional differences (7.7–66.7%). The highest seroprevalence rates were reported in districts near the Mediterranean Sea, whereas low or negative seroprevalence was detected in districts located further inland (41). Data on USUV infection are not available.
Bosnia and Herzegovina
There is only one report, in 2013, of two cases of WNV neuroinvasive disease (WNND) in Bosnia and Herzegovina (detected in the Tuzla and Kladanj region). Three out of nine patients screened for WNV met clinical criteria for WNND, of which two had high serum IgM titres. RT-PCR for WNV RNA was negative. Since the neutralization test was not performed, cases were classified according to the European Union case definition as probable WNV (42). So far, there are no data on USUV infections in Bosnia and Herzegovina.
Bulgaria
In 2015, the first confirmed human case with fatal outcome reported in Bulgaria was caused by a Central/Southern-European lineage 2 WNV (43). Human cases were continuously detected in the following seasons (12). A nationwide study conducted among residents of all 28 districts in Bulgaria revealed a 1.5% WNV seroprevalence with the highest seropositivity (up to 10%) detected in districts near the Danube River (44). USUV infections were not documented in Bulgaria.
Croatia
The first outbreak of human WNND in Croatia was detected in 2012 in eastern counties, thereafter outbreaks (2013, 2017) and sporadic cases (2014–2016) were continuously notified in continental Croatia (4, 45). The largest outbreak was recorded in 2018 with 54 cases of WNND and 7 cases of WNV fever in 10 of the 21 counties (6). During the 2013 WNV outbreak, the first three cases of neuroinvasive USUV disease were detected in Zagreb and surrounding areas (4, 46). Three additional USUV cases were confirmed in the 2018 outbreak, of which one was fatal (6). A serological study conducted from 2010 to 2011 showed WNV antibodies in 3.43% horses and 0.11% cattle with the highest seropositivity in eastern counties bordering Hungary and Serbia (47). Seropositivity and acute asymptomatic WNV infections in horses were detected continuously in subsequent years. Seroprevalence varied greatly by year and region (0–26%) with the highest seropositivity in counties with documented human cases (4, 48). Although passive monitoring of WNV in birds was established in Croatia in 2012, WNV infections were not detected until the summer of 2018, when WNV infection was confirmed serologically in one buzzard presenting with neurological symptoms, and WNV RNA was detected in two dead goshawks from Northwest Croatia. Two USUV seropositive horses were documented in 2011 in Northwest Croatia (49). In 2018, USUV RNA was detected in one dead blackbird in Zagreb County (6). To date, none of the mosquito pools tested were WNV RNA positive. However, USUV-positive mosquito pools were found in 2016 (Aedes albopictus), 2017 (Culex pipiens), and 2018 (Cx. pipiens) in Northwestern counties (6, 50). Sequenced strains from humans, wild birds, and mosquitoes (2018) confirmed circulation of WNV lineage 2 and USUV Europe 2 lineage (6).
Greece
No WNV clinical cases in humans or horses had been reported in Greece prior to the large WNV outbreak in 2010 when 262 human cases with extremely high fatality rates (17%) were reported mostly in Central Macedonia (51). The outbreaks continued in 2011 and 2012 in areas that had not been affected before (52, 53). Thereafter, outbreaks occurred in humans every year except 2015 and 2016 (14, 54). The Greek WNV strains detected during 2010–2018 clustered within the Central/Southern European subclade of lineage 2 (55). In 2018, a novel genetic variant was detected that belonged to the Eastern European subclade of lineage 2 (56). A nationwide WNV seroprevalence study (2013) showed seropositivity of 1.5% in the Greek population (57), whilst WNV antibodies were detected in 4% of horse serum samples (2001–2008) (58). In 2010, WNV infection was reported in 17 horses with neurological symptoms (59). Furthermore, a high WNV seropositivity (18.6%) was reported in cattle (60). In Central Macedonia, pigeon seroprevalence was 54 and 31% at the end of the 2010 and 2011 epidemic seasons, respectively, while one serum was positive for USUV neutralizing antibodies (61). A small-scale entomological study performed in Central Macedonia, at the epicenter of the 2010 WNV outbreak found two positive Cx. pipiens pools. Following this large epidemic, an active mosquito surveillance system was implemented in Greece for a 3-year period (2011–2013). Positive Cx. pipiens pools were detected in different areas of the country and preceded the diagnosed human cases (62). WNV lineage 2 was detected in Cx. pipiens mosquito pools during the 2017 outbreak (63).
Italy
A multi-species national surveillance plan was implemented by the Italian government in 2002, following the first WNV outbreak (64). In the following years, the program has been adapted according to the WNV new epidemiological scenarios and a National Integrated Plan Monitoring USUV and WNV is in place since 2016. Human WNV outbreaks were continuously notified since 2008 (14). WNV lineage 1 was responsible for reported WNND cases in 2010. In 2011–2012, lineage 1 and 2 co-circulated with a higher proportion of lineage 1 and from 2013 to 2016 lineage 2 was most prevalent (64–66). From 2017, only lineage 2 has been detected. Since 2008, WNV infections were continuously detected also in horses and birds (65). WNV lineage 2 was confirmed in wild birds (2014) (11, 67) and horses with fatal WNV neuroinvasive infection (68). The presence of USUV in humans was serologically confirmed for the first time in four blood donors in 2009 (69). A survey conducted retrospectively on cerebrospinal fluid (CSF) and serum samples in Modena (2008–2011) found USUV RNA in 1.1% CSF samples, while WNV RNA was not detected. USUV antibody levels were significantly higher (6.57%) compared to that of WNV (2.96%) indicating that USUV infection is not a sporadic event in humans in Italy (70). A very high USUV seroprevalence was found in forestry workers (18.1%) (71). Neuroinvasive USUV infection was detected for the first time in Italy in two immunocompromised patients in 2009 (72, 73). During 2017–2018, USUV Europe 2, 3, and 4 lineages were confirmed in blood donations in the Lazio region (15). USUV neutralizing antibodies were detected in horses with a higher seropositivity in 2008 (89.2%) compared to 2009 (7.8%). In the same study, USUV RNA was found in blackbirds and magpies (Emilia Romagna and Veneto). Additionally, USUV neutralizing antibodies were detected in rock pigeon, blackbird and, magpie (74). Culex pipiens is the mosquito species most involved in the WNV and USUV circulation in Italy, although other species would also support the spread of both viruses during winter months (75). An entomologic investigation conducted in 2013 showed WNV RNA in 1.9% and USUV in 2.6% mosquito pools. Each virus was detected mainly in Cx. pipiens pools; however some pools tested positive for both viruses. The majority of the WNV strains detected in mosquitoes belonged to WNV lineage 2, while WNV lineage 1 which predominantly circulated in 2008–2012, was still detected at low levels until 2016 (64, 76). Sequence analysis of the first known isolate of USUV to cause human encephalitis indicated European USUV lineage (77). The whole genome sequences of USUV strains isolated from mosquitoes and wild birds in Northern Italy (2010–2014) showed Europe 2 and Europe 4 lineages, respectively (78).
Portugal
In the summer of 2004, two human cases of WNV infection were reported in Portugal in tourists (Ria Formosa, Algarve) (79). Shortly after this report, a WNV monitoring program was established. The presence of WNV was assessed by serological surveys in horses (2004–2010), wild birds, and birds from zoological parks. Detection of WNV antibodies in horses and birds in all the years covered by the study as well as the presence of WNV IgM antibodies in horses with neurological signs supported the evidence of WNV circulation in Portugal (80). However, no human clinical cases were reported in the country from 2005 to 2010. In 2010, another WNV human case was identified (81). Following a 5-year period of apparent absence, WNV re-emerged in southern Portugal in 2015 when WNND was diagnosed in a man from the Algarve region (82). There are no data on USUV infections in Portugal.
Serbia
The serological surveys (2005–2010) revealed the presence of WNV IgG antibodies in 3.99% human serum samples in Vojvodina Province (northern Serbia), with varying yearly rates (1.97–6.04%) (83). The first outbreak of human WNND was detected in 2012 (Belgrade and Vojvodina) with 69 reported and 41 clinically and laboratory confirmed cases (14, 84). Each year thereafter, seasonal outbreaks were observed. In 2013, 303 cases were reported from 18/25 districts in Serbia; 202 WNND cases were confirmed with lethality of 11.6%. From 41 to 76 WNND cases were documented annually between 2014 and 2017. The largest epidemic so far was recorded in 2018 with 415 WNND cases detected in 13/25 districts (14). A serological study conducted in 2009–2010 showed for the first time WNV neutralizing antibodies in 12% of horses from Vojvodina (85). Seropositivity and asymptomatic WNV infections in horses were detected continuously in the subsequent years; 28.6% seroprevalence from 2010 to 2011 and 49.23% in 2012 in Vojvodina (86, 87). In 2014, seroconversion was detected in 2.57% horses from the whole country. In addition, in 2015, 2017, and 2018, acute WNV infections were detected in 0.53, 0.41, and 1.47% of horses tested, respectively (88–90). WNV antibodies were detected in 7.6% wild birds sampled from 2011 to 2012 in Vojvodina, of which 133 birds were found dead. Virus presence was confirmed in 9.87% of tissue samples and in the blood sample of one bird (91). From 2014 onwards, WNV was detected in wild birds, with the highest percentage of positive samples being observed in 2018 when WNV was detected in 11.61% of tissues and 6.56% of pharyngeal swabs (88–90). All WNV isolates belonged to WNV lineage 2 (88, 91). Additionally, WNV antibodies were identified in 15.4% farm pigs, 17.6% wild boars, and 18.7% roe deer sampled from 2011 to 2012 (Vojvodina) (92). WNV was detected in mosquitoes for the first time in 2010 in the city of Novi Sad. At that time, 3/841 mosquito pools were positive by WNV specific RT-PCR. The pools originated from 66 localities in 29 settlements in Vojvodina (60). More than 9% of the mosquito pools examined during 2012–2013 were positive for WNV (93). Thereafter, country-wide surveys detected WNV in 2.31, 2.09, and 2.51% of Cx. pipiens during 2014, 2015, and 2017, respectively, whereas 12.21% of Cx. pipiens tested positive in 2018. Other naturally-infected mosquito species were Ae. vexans and Culiseta annulata (83). All isolates from mosquitoes (2010–2018) were shown to belong to WNV lineage 2 (83, 88–92, 94–96). USUV neutralizing antibodies were detected in 1/349 horses (2009-2010), and 4/318 wild boars (2011–2012) in Vojvodina (85, 92). Additionally, USUV RNA was confirmed in 0.4% of Cx. pipiens pools from Vojvodina in 2014 (74). In 2015, USUV was detected in 0.93% of Cx. pipiens pools while USUV antibodies were detected in 5% of human serum samples from South Bačka District (Vojvodina) (97). Moreover, in 2017, USUV RNA was detected in 2.75% of Cx. pipiens mosquitoes collected in Vojvodina. Two isolates were typed as USUV Europe 2 lineage (98). Thus, far, USUV has not yet been detected in wild birds in Serbia.
Slovenia
Only four human cases of WNV infections were reported in Slovenia so far. The first case was confirmed in 2013 in a patient with meningitis. Thereafter, in 2018, three cases of WNND were confirmed by detection of IgM antibodies in the CSF samples (99). In 2009, 481/1912 (25.2%) clinically healthy horses were positive for WNV IgG antibodies (100). From 2003 until 2009, serum samples from 34 species of songbirds were tested with WNV seroprevalence of 4.7% (101). Furthermore, from 2010 to 2012, wild bird carcasses were collected through passive monitoring of wild bird mortality and tested for WNV RNA, however, there was no evidence of wild bird mortality due to WNV (102). As well, there was no evidence of USUV circulation in Slovenia.
Spain
The first human clinical case of WNV infection was diagnosed in 2004 in a patient with aseptic meningitis visiting Southwestern Spain (103). Autochthonous cases were subsequently notified in 2010, 2016, and 2018 transmission seasons (14, 104). WNV cases in horses caused by WNV lineage 1 were continuously reported since 2010 (105). A seroprevalence study conducted in equine populations of Mallorca Island (2011–2012) showed seropositivity rates to WNV and USUV of 6.4 and 1.2%, respectively (106). Another study conducted among horses from central Spain (2011–2013) found WNV seroprevalence of 1.35% (107). WNV lineage 1 was isolated from diseased and dead golden eagles in 2007 (108). In 2011, a survey conducted in waterfowl used as decoys in Andalusia showed that the frequency of seropositive decoys ranged 1.5–3.1% for WNV and 4.4–5.9% for USUV (109). Additionally, WNV and USUV antibodies were found in 23 and 10% of hunted wild red-legged partridges and pheasants from Cádiz (2011–2012) (110). In 2012, USUV was confirmed in two song thrushes (111). WNV and USUV antibodies were also detected in wild birds (2013) (112) as well as pigeons and zoo birds from Cordoba (2013–2014) (113). Moreover, USUV Africa 2 lineage in 2006 (Northeastern Spain) and 2009 (Southern Spain) as well as WNV lineage 1 in 2008 (Southern Spain) were detected in mosquitoes (114, 115).
Conclusions and Future Perspectives
An integrated “One Health” surveillance of mosquitoes and birds in several countries has proven to be useful for early detection of WNV/USUV circulation and identification of enzootic areas (48, 61, 116). Virus detection in mosquitoes and birds preceded human and horse cases (48, 62). In addition, a high seroprevalence in sentinel animals was detected in areas with documented human cases (4, 22, 48, 61).
There are still many challenges in the epidemiology of WNV/USUV. In addition to birds, which are well-known reservoirs, WNV and USUV antibodies were found in different animal species. WNV and USUV neutralizing antibodies were documented in red deer in Spain (117). WNV seropositive dogs were detected in Italy, Spain, and Corsica Island (118), while USUV antibodies were found in hunting dogs in Italy (119). Moreover, WNV and USUV antibodies were found in gray squirrels in Italy, broadening the host range for these viruses (120). WNV neutralizing antibodies were also found in two wild rodents (Apodemus flavicollis) captured in forested areas of Italy (121). However, the short-term and low-level viremia makes it unlikely that these animal species play a role in the WNV transmission cycle. Culex pipiens mosquitoes appear to be a major vector for WNV and USUV transmission in Europe, but Cx. modestus and Cx. perexiguus play an important role in marshlands of some southern countries (115). However, detection of WNV and USUV in different field-collected native (Ae. vexans) and invasive (Ae. albopictus, Ae. japonicus) mosquito species indicates their possible role in promoting the overwintering of these viruses (75, 83, 122–125). Additionally, a recently published study identified Cx. torrentium as a highly competent vector for WNV in Central and Northern Europe (126).
While several licensed veterinary WNV vaccines are currently available, there is no WNV or USUV vaccine for humans (127, 128). Since data from Europe indicate that both WNV and USUV appear to be expanding their geographical ranges and the trends indicate that this spread is likely to continue in future years, the development of an effective vaccine is urgently needed to protect at-risk populations from neurological complications.
Author Contributions
TV-C and TP made contributions to conception and design of the study, involved in data collection, and drafting the manuscript. VSa, LB, ITa, IH-C, MB, AK, AM, VSt, PD-K, LR, FM, and EL were involved in data collection and drafting the manuscript. ITo, DP, and GS revised the manuscript critically. All authors read and approved the final manuscript.
Funding
This work was supported by the Croatian Science Foundation, Project No. IP 2016-06-7456: Prevalence and molecular epidemiology of emerging and re-emerging neuroinvasive arboviral infections in Croatia; CRONEUROARBO (to TV-C), by bilateral project funded by Croatian Ministry of Science and Education and Serbian Ministry of Education, Science and Technological Development: Optimization of diagnosis and surveillance of emerging and re-emerging viral vector-borne zoonoses (to LB and TP), and by Project No. TR31084 funded by Serbian Ministry of Education, Science and Technological Development.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Zannoli S, Sambri V. West Nile virus and Usutu virus co-circulation in Europe: epidemiology and implications. Microorganisms. (2019) 7:E184. doi: 10.3390/microorganisms7070184
2. Weissenböck H, Kolodziejek J, Url A, Lussy H, Rebel-Bauder B, Nowotny N. Emergence of Usutu virus, an African mosquito-borne flavivirus of the Japanese encephalitis virus group, central Europe. Emerg Infect Dis. (2002) 8:652–6. doi: 10.3201/eid0807.020094
3. Weissenböck H, Bakonyi T, Rossi G, Mani P, Nowotny N. Usutu virus, Italy, 1996. Emerg Infect Dis. (2013) 19:274–7. doi: 10.3201/eid1902.121191
4. Vilibic-Cavlek T, Kaic B, Barbic L, Pem-Novosel I, Slavic-Vrzic V, Lesnikar V, et al. First evidence of simultaneous occurrence of West Nile virus and Usutu virus neuroinvasive disease in humans in Croatia during the 2013 outbreak. Infection. (2014) 42:689–95. doi: 10.1007/s15010-014-0625-1
5. Simonin Y, Sillam O, Carles MJ, Gutierrez S, Gil P, Constant O, et al. Human Usutu virus infection with atypical neurologic presentation, Montpellier, France, 2016. Emerg Infect Dis. (2018) 24:875–8. doi: 10.3201/eid2405.171122
6. Vilibic-Cavlek T, Savic V, Sabadi D, Peric L, Barbic L, Klobucar A, et al. Prevalence and molecular epidemiology of West Nile and Usutu virus infections in Croatia in the “One health” context, 2018. Transbound Emerg Dis. (2019) 66:1946–57. doi: 10.1111/tbed.13225
7. Faggioni G, De Santis R, Pomponi A, Grottola A, Serpini GF, Meacci M, et al. Prevalence of Usutu and West Nile virus antibodies in human sera, Modena, Italy, 2012. J Med Virol. (2018) 90:1666–8. doi: 10.1002/jmv.25230
8. Bakonyi T, Ivanics E, Erdélyi K, Ursu K, Ferenczi E, Weissenböck H, et al. Lineage 1 and 2 strains of encephalitic West Nile virus, central Europe. Emerg Infect Dis. (2006) 12:618–23. doi: 10.3201/eid1204.051379
9. Hernández-Triana LM, Jeffries CL, Mansfield KL, Carnell G, Fooks AR, Johnson N. Emergence of West Nile virus lineage 2 in Europe: a review on the introduction and spread of a mosquito-borne disease. Front Public Health. (2014) 2:271. doi: 10.3389/fpubh.2014.00271
10. Ravagnan S, Montarsi F, Cazzin S, Porcellato E, Russo F, Palei M, et al. First report outside Eastern Europe of West Nile virus lineage 2 related to the Volgograd 2007 strain, northeastern Italy, 2014. Parasit Vectors. (2015) 8:418. doi: 10.1186/s13071-015-1031-y
11. Savini G, Capelli G, Monaco F, Polci A, Russo F, Di Gennaro A, et al. Evidence of West Nile virus lineage 2 circulation in Northern Italy. Vet Microbiol. (2012) 158:267–73. doi: 10.1016/j.vetmic.2012.02.018
12. Kurolt IC, Krajinović V, Topić A, Kuzman I, Baršić B, Markotić A. First molecular analysis of West Nile virus during the 2013 outbreak in Croatia. Virus Res. (2014) 189:63–6. doi: 10.1016/j.virusres.2014.04.017
13. Savini G, Puggioni G, Di Gennaro A, Di Francesco G, Rocchigiani AM, Polci A, et al. West Nile virus lineage 2 in Sardinian wild birds in 2012: a further threat to public health. Epidemiol Infect. (2013) 141:2313–6. doi: 10.1017/S0950268812003147
14. European Centre for Disease Prevention and Control (ECDC). Historical Data by Year - West Nile Fever Seasonal Surveillance. (2018). Available online at: https://ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/historical
15. Carletti F, Colavita F, Rovida F, Percivalle E, Baldanti F, Ricci I, et al. Expanding Usutu virus circulation in Italy: detection in the Lazio region, central Italy, 2017 to 2018. Euro Surveill. (2019) 24:1800649. doi: 10.2807/1560-7917.ES.2019.24.3.1800649
16. IZSAM. 2018 West Nile disease in Italy. Epidemiological Bulletin. (2018). Available online at: https://westnile.izs.it
17. Lustig Y, Kaufman Z, Mannasse B, Koren R, Katz-Likvornik S, Orshan L, et al. West Nile virus outbreak in Israel in 2015: phylogenetic and geographic characterization in humans and mosquitoes. Clin Microbiol Infect. (2017) 23:986–93. doi: 10.1016/j.cmi.2017.04.023
18. Lustig Y, Kaufman Z, Mendelson E, Orshan L, Anis E, Glazer Y, et al. Spatial distribution of West Nile virus in humans and mosquitoes in Israel, 2000-2014. Int J Infect Dis. (2017) 64:20–6. doi: 10.1016/j.ijid.2017.08.011
19. Salama M, Amitai Z, Lustig Y, Mor Z, Weiberger M, Chowers M, et al. Outbreak of West Nile Virus disease in Israel (2015): a retrospective analysis of notified cases. Travel Med Infect Dis. (2019) 28:41–5. doi: 10.1016/j.tmaid.2018.07.008
20. Azmi K, Tirosh-Levy S, Manasrah M, Mizrahi R, Nasereddin A, Al-Jawabreh A, et al. West Nile virus: seroprevalence in animals in Palestine and Israel. Vector Borne Zoonotic Dis. (2017) 17:558–66. doi: 10.1089/vbz.2016.2090
21. Bassal R, Shohat T, Kaufman Z, Mannasse B, Shinar E, Amichay D, et al. The seroprevalence of West Nile virus in Israel: a nationwide cross sectional study. PLoS ONE. (2017) 12:e0u179774. doi: 10.1371/journal.pone.0179774
22. Lustig Y, Gosinov R, Zuckerman N, Glazer Y, Orshan L, Sofer D, et al. Epidemiologic and phylogenetic analysis of the 2018 West Nile virus (WNV) outbreak in Israel demonstrates human infection of WNV lineage I. Euro Surveill. (2019) 24:1800662. doi: 10.2807/1560-7917.ES.2019.24.1.1800662
23. Lustig Y, Hindiyeh M, Orshan L, Weiss L, Koren R, Katz-Likvornik S, et al. Mosquito surveillance for 15 years reveals high genetic diversity among West Nile viruses in Israel. J Infect Dis. (2016) 213:1107–14. doi: 10.1093/infdis/jiv556
24. Mannasse B, Mendelson E, Orshan L, Mor O, Shalom U, Yeger T, et al. Usutu virus RNA in mosquitoes, Israel, 2014-2015. Emerg Infect Dis. (2017) 23:1699–702e. doi: 10.3201/eid2310.171017
25. Del Giudice P, Schuffenecker I, Vandenbos F, Counillon E, Zellet H. Human West Nile virus, France. Emerg Infect Dis. (2004) 10:1885–6. doi: 10.3201/eid1010.031021
26. Vittecoq M, Lecollinet S, Jourdain E, Thomas F, Blanchon T, Arnal A, et al. Recent circulation of West Nile virus and potentially other closely related flaviviruses in Southern France. Vector Borne Zoonotic Dis. (2013) 13:610–3. doi: 10.1089/vbz.2012.1166
27. Cazeau G, Leblond A, Sala C, Froustey M, Beck C, Lecollinet S, et al. Utility of examining fallen stock data to monitor health-related events in equids: application to an outbreak of West Nile Virus in France in 2015. Transbound Emerg Dis. (2019) 66:1417–9. doi: 10.1111/tbed.13150
28. Bahuon C, Marcillaud-Pitel C, Bournez L, Leblond A, Beck C, Hars J, et al. West Nile virus epizootics in the Camargue (France) in 2015 and reinforcement of surveillance and control networks. Rev Sci Tech Off Int Epiz. (2016) 35:81–6. doi: 10.20506/rst.35.3.2571
29. Lecollinet S, Blanchard Y, Manson C, Lowenski S, Laloy E, Quenault H, et al. Dual Emergence of Usutu virus in common blackbirds, Eastern France, 2015. Emerg Infect Dis. (2016) 22:2225. doi: 10.3201/eid2212.161272
30. Eiden M, Gil P, Ziegler U, Rakotoarivony I, Marie A, Frances B, et al. Emergence of two Usutu virus lineages in Culex pipiens mosquitoes in the Camargue, France, 2015. Infect Genet Evol. (2018) 61:151–4. doi: 10.1016/j.meegid.2018.03.020
31. Balança G, Gaidet N, Savini G, Vollot B, Foucart A, Reiter P, et al. Low West Nile virus circulation in wild birds in an area of recurring outbreaks in Southern France. Vector Borne Zoonotic Dis. (2009) 9:737–41. doi: 10.1089/vbz.2008.0147
32. Ozkul A, Yildirim Y, Pinar D, Akcali A, Yilmaz V, Colak D Serological evidence of West Nile virus (WNV) in mammalian species in Turkey. Epidemiol Infect. (2006) 134:826–9. doi: 10.1017/S0950268805005492
33. Ergunay K, Bakonyi T, Nowotny N, Ozkul A. Close relationship between West Nile virus from Turkey and lineage 1 strain from Central African Republic. Emerg Infect Dis. (2015) 21:352–5. doi: 10.3201/eid2102.141135
34. Ergünay K, Litzba N, Brinkmann A, Günay F, Sarikaya Y, Kar S, et al. Co-circulation of West Nile virus and distinct insect-specific flaviviruses in Turkey. Parasit Vectors. (2017) 10:149. doi: 10.1186/s13071-017-2087-7
35. Akiner MM, Öztürk M, Başer AB, Günay F, Hacioglu S, Brinkmann A, et al. Arboviral screening of invasive Aedes species in northeastern Turkey: West Nile virus circulation and detection of insect-only viruses. PLoS Negl Trop Dis. (2019) 13:e0007334. doi: 10.1371/journal.pntd.0007334
36. Yilmaz H, Barut K, Karakullukcu A, Kasapcopur O, Kocazeybek B, Altan E, et al. Serological evidence of tick-borne encephalitis and West Nile virus infections among children with arthritis in Turkey. Vector Borne Zoonotic Dis. (2019) 19:446–9. doi: 10.1089/vbz.2018.2349
37. Öncü C, Brinkmann A, Günay F, Kar S, Öter K, Sarikaya Y, et al. West Nile virus, Anopheles flavivirus, a novel flavivirus as well as Merida-like rhabdovirus Turkey in field-collected mosquitoes from Thrace and Anatolia. Infect Genet Evol. (2018) 57:36–45. doi: 10.1016/j.meegid.2017.11.003
38. Paphitou NI, Tourvas A, Floridou D, Richter J, Tryfonos C, Christodoulou C. The first human case of neuroinvasive West Nile virus infection identified in Cyprus. J Infect Public Health. (2017) 10:891–3. doi: 10.1016/j.jiph.2017.02.003
39. Richter J, Tryfonos C, Tourvas A, Floridou D, Paphitou NI, Christodoulou C. Complete Genome sequence of West Nile virus (WNV) from the first human case of neuroinvasive WNV infection in Cyprus. Genome Announc. (2017) 5:e01110–17. doi: 10.1128/genomeA.01110-17
40. Billioud G, Tryfonos C, Richter J. The prevalence of antibodies against sandfly fever viruses and West Nile virus in Cyprus. J Arthropod Borne Dis. (2019) 13:116–25. doi: 10.18502/jad.v13i1.938
41. Berxholi K, Ziegler U, Rexhepi A, Schmidt K, Mertens M, Korro K, et al. (2013) Indigenous West Nile virus infections in horses in Albania. Transbound Emerg Dis. 60(Suppl. 2):45–50. doi: 10.1111/tbed.12141
42. Ahmetagić S, Petković J, Hukić M, Smriko-Nuhanović A, Piljić D. Human West Nile virus infection in Bosnia and Herzegovina. Med Glas. (2015) 12:47–51.
43. Baymakova M, Trifonova I, Panayotova E, Dakova S, Pacenti M, Barzon L, et al. Fatal case of West Nile neuroinvasive disease in Bulgaria. Emerg Infect Dis. (2016) 22:2203–4. doi: 10.3201/eid2212.151968
44. Christova I, Panayotova E, Tchakarova S, Taseva E, Trifonova I, Gladnishka T. A nationwide seroprevalence screening for West Nile virus and Tick-borne encephalitis virus in the population of Bulgaria. J Med Virol. (2017) 89:1875–8. doi: 10.1002/jmv.24855
45. Pem-Novosel I, Vilibic-Cavlek T, Gjenero-Margan I, Pandak N, Peric L, Barbic L, et al. First outbreak of West Nile virus neuroinvasive disease in humans, Croatia, 2012. Vector Borne Zoonotic Dis. (2014) 14:82–4. doi: 10.1089/vbz.2012.1295
46. Santini M, Vilibic-Cavlek T, Barsic B, Barbic L, Savic V, Stevanovic V, et al. First cases of human Usutu virus neuroinvasive infection in Croatia, August-September 2013: clinical and laboratory features. J Neurovirol. (2015) 21:92–7. doi: 10.1007/s13365-014-0300-4
47. Barbić L, Listeš E, Katić S, Stevanović V, Madić J, Starešina V, et al. Spreading of West Nile virus infection in Croatia. Vet Microbiol. (2012) 159:504–8. doi: 10.1016/j.vetmic.2012.04.038
48. Savić V, Barbić Lj, Vilibić-Cavlek T, Balenović M, Stevanović V, Listeš E, et al. Chickens and horses as sentinels for early warning system in prevention of human West Nile virus infections in Croatia. Slov Vet Res. (2016) 53(Suppl 17):292–4.
49. Barbic L, Vilibic-Cavlek T, Listes E, Stevanovic V, Gjenero-Margan I, Ljubin-Sternak S, et al. Demonstration of Usutu virus antibodies in horses, Croatia. Vector Borne Zoonotic Dis. (2013) 13:772–4. doi: 10.1089/vbz.2012.1236
50. Klobucar A, Benic N, Krajcar D, Kosanovic-Licina ML, Tesic V, Merdic E, et al. An overview of mosquitoes and emerging arboviral infections in the Zagreb area, Croatia. J Infect Dev Ctries. (2016) 10:1286–93. doi: 10.3855/jidc.7988
51. Papa A, Danis K, Baka A, Bakas A, Dougas G, Lytras T, et al. Ongoing outbreak of West Nile virus infections in humans in Greece, July-August 2010. Euro Surveill. (2010) 15:19644. doi: 10.2807/ese.15.34.19644-en
52. Danis K, Papa A, Papanikolaou E, Dougas G, Terzaki I, Baka A, et al. Ongoing outbreak of West Nile virus infection in humans, Greece, July to August 2011. Euro Surveill. (2011) 16:19951.
53. Papa A. West Nile virus infections in humans-focus on Greece. J Clin Virol. (2013) 58:351–3. doi: 10.1016/j.jcv.2013.02.020
54. Mavrouli M, Vrioni G, Kapsimali V, Tsiamis C, Mavroulis S, Pervanidou D, et al. Reemergence of West Nile Virus Infections in Southern Greece, 2017. Am J Trop Med Hyg. (2019) 100:420–6. doi: 10.4269/ajtmh.18-0339
55. Barzon L, Papa A, Lavezzo E, Franchin E, Pacenti M, Sinigaglia A, et al. Phylogenetic characterization of Central/Southern European lineage 2 West Nile virus: analysis of human outbreaks in Italy and Greece, 2013–2014. Clin Microbiol Infect. (2015) 21:1122.e1–10. doi: 10.1016/j.cmi.2015.07.018
56. Papa A, Papadopoulou E, Chatzixanthouliou C, Glouftsios P, Pappa S, Pervanidou D, et al. Emergence of West Nile virus lineage 2 belonging to the Eastern European subclade, Greece. Arch Virol. (2019) 164:1673–5. doi: 10.1007/s00705-019-04243-8
57. Hadjichristodoulou C, Pournaras S, Mavrouli M, Marka A, Tserkezou P, Baka A, et al. West Nile virus seroprevalence in the Greek population in 2013: a nationwide cross-sectional survey. PLoS ONE. (2015) 10:e0143803. doi: 10.1371/journal.pone.0143803
58. Mangana-Vougiouka O, Boutsini S, Ntousi D, Patakakis M, Orfanou E, Zafiropoulou K, et al. Epizootiological investigation of the most important infectious equine diseases in Greece. Rev Sci Tech. (2013) 32:755–87. doi: 10.20506/rst.32.2.2217
59. Bouzalas IG, Diakakis N, Chaintoutis SC, Brellou GD, Papanastassopoulou M, Danis K, et al. Emergence of equine West Nile encephalitis in Central Macedonia, Greece, 2010. Transbound Emerg Dis. (2016) 63:e219–27. doi: 10.1111/tbed.12334
60. Giadinis N, Katsoulos P, Chochlakis D, Tselentis Y, Ntais P, Lafi S, et al. Serological investigation for West Nile virus, Anaplasma ovis and Leishmania infantum in Greek cattle. Vet Ital. (2015) 51:205–9. doi: 10.12834/VetIt.174.523.4
61. Chaintoutis SC, Dovas CI, Papanastassopoulou M, Gewehr S, Danis K, Beck C, et al. Evaluation of a West Nile virus surveillance and early warning system in Greece, based on domestic pigeons. Comp Immunol Microbiol Infect Dis. (2014) 37:131–41. doi: 10.1016/j.cimid.2014.01.004
62. Patsoula E, Vakali A, Balatsos G, Pervanidou D, Beleri S, Tegos N, et al. West Nile virus circulation in mosquitoes in Greece (2010–2013). Biomed Res Int. (2016) 2016:2450682. doi: 10.1155/2016/2450682
63. Mavridis K, Fotakis EA, Kioulos I, Mpellou S, Konstantas S, Varela E, et al. Detection of West Nile virus - lineage 2 in Culex pipiens mosquitoes, associated with disease outbreak in Greece, 2017. Acta Trop. (2018) 182:64–8. doi: 10.1016/j.actatropica.2018.02.024
64. West Nile Disease. (2018). Available online at: http://sorveglianza.izs.it/emergenze/west_nile/emergenze_en.html
65. Rizzo C, Napoli C, Venturi G, Pupella S, Lombardini L, Calistri P, et al. West Nile virus transmission: results from the integrated surveillance system in Italy, 2008 to 2015. Euro Surveill. (2016) 21:30340. doi: 10.2807/1560-7917.ES.2016.21.37.30340
66. Magurano F, Remoli ME, Baggieri M, Fortuna C, Marchi A, Fiorentini C, et al. Circulation of West Nile virus lineage 1 and 2 during an outbreak in Italy. Clin Microbiol Infect. (2012) 18:E545–7. doi: 10.1111/1469-0691.12018
67. Chiari M, Prosperi A, Faccin F, Avisani D, Cerioli M, Zanoni M, et al. West Nile virus surveillance in the Lombardy Region, Northern Italy. Transbound Emerg Dis. (2015) 62:343–9. doi: 10.1111/tbed.12375
68. Calistri P, Monaco F, Savini G, Guercio A, Purpari G, Vicari D, et al. Further spread of West Nile virus in Italy. Vet Ital. (2010) 46:467–74.
69. Gaibani P, Pierro A, Alicino R, Rossini G, Cavrini F, Landini MP, et al. Detection of Usutu-virus-specific IgG in blood donors from northern Italy. Vector-Borne Zoonotic Dis. (2012) 12:431–3. doi: 10.1089/vbz.2011.0813
70. Grottola A, Marcacci M, Tagliazucchi S, Gennari W, Di Gennaro A, Orsini M, et al. Usutu virus infections in humans: a retrospective analysis in the municipality of Modena, Italy. Clin Microbiol Infect. (2017) 23:33–7. doi: 10.1016/j.cmi.2016.09.019
71. Percivalle E, Sassera D, Rovida F, Isernia P, Fabbi M, Baldanti F, et al. Usutu virus antibodies in blood donors and healthy forestry workers in the Lombardy region, Northern Italy. Vector Borne Zoonotic Dis. (2017) 17:658–61. doi: 10.1089/vbz.2017.2126
72. Pecorari M, Longo G, Gennari W, Grottola A, Sabbatini A, Tagliazucchi S, et al. First human case of Usutu virus neuroinvasive infection, Italy, August-September 2009. Euro Surveill. (2009) 14:19446. doi: 10.2807/ese.14.50.19446-en
73. Cavrini F, Gaibani P, Longo G, Pierro AM, Rossini G, Bonilauri P, et al. Usutu virus infection in a patient who underwent orthotropic liver transplantation, Italy, August-September 2009. Euro Surveill. (2009) 14:19448. doi: 10.2807/ese.14.50.19448-en
74. Savini G, Monaco F, Terregino C, Di Gennaro A, Bano L, Pinoni C, et al. Usutu virus in Italy: an emergence or a silent infection? Vet Microbiol. (2011) 151:264–74. doi: 10.1016/j.vetmic.2011.03.036
75. Mancini G, Montarsi F, Calzolari M, Capelli G, Dottori M, Ravagnan S, et al. Mosquito species involved in the circulation of West Nile and Usutu viruses in Italy. Vet Ital. (2017) 53:97–110. doi: 10.12834/VetIt.114.933.4764.2
76. Calzolari M, Pautasso A, Montarsi F, Albieri A, Bellini R, Bonilauri P, et al. West Nile virus surveillance in 2013 via mosquito screening in Northern Italy and the influence of weather on virus circulation. PLoS ONE. (2015) 10:e0140915. doi: 10.1371/journal.pone.0140915
77. Gaibani P, Cavrini F, Gould EA, Rossini G, Pierro A, Landini MP, et al. Comparative genomic and phylogenetic analysis of the first Usutu virus isolate from a human patient presenting with neurological symptoms. PLoS ONE. (2013) 8:e64761. doi: 10.1371/journal.pone.0064761
78. Calzolari M, Chiapponi C, Bonilauri P, Lelli D, Baioni L, Barbieri I, et al. Co-circulation of two Usutu virus strains in Northern Italy between 2009 and 2014. Infect Genet Evol. (2017) 51:255–62. doi: 10.1016/j.meegid.2017.03.022
79. Almeida AP, Freitas F, Novo MT, Sousa CA, Rodrigues JC, Alves R, et al. Mosquito surveys and West Nile virus screening in two different areas of Southern Portugal, 2004-2007. Vector Borne Zoonotic Dis. (2010) 10:673–680. doi: 10.1089/vbz.2009.0245
80. Barros SC, Ramos F, Fagulha T, Duarte M, Henriques M, Luís T, et al. Serological evidence of West Nile virus circulation in Portugal. Vet Microbiol. (2011) 152:407–10. doi: 10.1016/j.vetmic.2011.05.013
81. Alves MJ, Poças JM, Luz T, Amaro F, Zé-Zé L, Osório HC. West Nile virus infection in Portugal: considerations about a clinical case with febrile syndrome and rash. Rev. Port Doenças Infec. (2012) 8:46–51.
82. Barros SC, Ramos F, Fagulha T, Duarte M, Henriques AM, Waap H, et al. West Nile virus in horses during the summer and autumn seasons of 2015 and 2016:Portugal. Vet Microbiol. (2017) 212:75–9. doi: 10.1016/j.vetmic.2017.11.008
83. Petrić D, Hrnjaković Cvjetković I, Radovanov J, Cvjetković D, Jerant Patić V, Milošević V, et al. West Nile virus surveillance in humans and mosquitoes and detection of cell fusing agent virus in Vojvodina province (Serbia). HealthMED. (2012) 6:462–8.
84. Popovic N, Milosevic B, Urosevic A, Poluga J, Popovic N, Stevanovic G, et al. Clinical characteristics and functional outcome of patients with West Nile neuroinvasive disease in Serbia. J Neurol. (2014) 261:1104–11. doi: 10.1007/s00415-014-7318-7
85. Lupulović D, Martín-Acebes M, Lazić S, Alonso-Padilla J, Bláquez AB, Escribano-Romero E, et al. First serological evidence of West Nile virus activity in horses in Serbia. Vector Borne Zoonotic Dis. (2011) 11:1303–5. doi: 10.1089/vbz.2010.0249
86. Medić S, van den Hoven R, Petrović T, Lupulović D, Nowotny N. Serological evidence of West Nile virus infection in the horse population of northern Serbia. J Infect Dev Ctries. (2014) 8:914–8. doi: 10.3855/jidc.3885
87. Petrović T, Lazić S, Lupulović D, Lazić G, Bugarski D, Vidanović D, et al. Serological study on WNV presence in horses in Vojvodina after the human outbreak in Serbia in 2012. Arch Biol Sci Belgrade. (2014) 66:473–81. doi: 10.2298/ABS1402473P
88. Petrović T, Šekler M, Petrić D, Lazić S, Debeljak Z, Vidanović D, et al. Methodology and results of integrated WNV surveillance programmes in Serbia. PLoS ONE. (2018) 13:e0195439. doi: 10.1371/journal.pone.0195439
89. Petrović T, Šekler M, Petrić D, Debeljak Z, Vidanović D, Lazić G, et al. Results of WNV monitoring program in Serbia in 2017. In: Book of Abstracts, XX Symposium of Epizootiologists and Epidemiologists. Vrnjačka Banja (2018). p. 92–5.
90. Petrović T, Šekler M, Debeljak Z, Petrić D, Labus T, Vidanović D, et al. Results of the WNV monitoring program in Serbia in 2018. In: Book of Abstracts, XXI Symposium of Epizootiologists and Epidemiologists. Novi Sad (2019). p. 42–5.
91. Petrović T, Blazquez AB, Lupulović D, Lazić G, Escribano-Romero E, Fabijan D, et al. Monitoring West Nile virus (WNV) infection in wild birds in Serbia during 2012: first isolation and characterisation of WNV strains from Serbia. Euro Surveill. (2013) 18:20622. doi: 10.2807/1560-7917.ES2013.18.44.20622
92. Escribano-Romero E, Lupulović D, Merino-Ramos T, Blázquez AB, Lazić G, Lazić S, et al. West Nile virus serosurveillance in pigs, wild boars, and roe deer in Serbia. Vet Microbiol. (2015) 176:365–9. doi: 10.1016/j.vetmic.2015.02.005
93. Petrović T, Šekler M, Petrić D, Vidanović D, Potkonjak A, Hrnjaković Cvjetković I, et al. Flaviviruses at the territory of Serbia - present situation and challenges. Arch Vet Med. (2018) 11:53–70.
94. Petrić D, Petrović T, Hrnjaković Cvjetković I, Zgomba M, Milošević V, Lazić G, et al. West Nile virus 'circulation' in Vojvodina, Serbia: Mosquito, bird, horse and human surveillance. Mol Cell Probes. (2017) 31:28–36. doi: 10.1016/j.mcp.2016.10.011
95. Kemenesi G, Krtinić B, Milankov V, Kutas A, Dallos B, Oldal M, et al. West Nile virus surveillance in mosquitoes, April to October 2013:Vojvodina province, Serbia: implications for the 2014 season. Euro Surveill. (2014) 19:20779. doi: 10.2807/1560-7917.ES2014.19.16.20779
96. Zana B, Kemenesi G, Herczeg R, Dallos B, Oldal M, Marton S, et al. Genomic characterization of West Nile virus strains derived from mosquito samples obtained during 2013 Serbian outbreak. J Vector Borne Dis. (2016) 53:379–83.
97. Kemenesi G, Buzás D, Zana B, Kurucz K, Krtinic B, Kepner A, et al. First genetic characterization of Usutu virus from Culex pipiens mosquitoes Serbia, 2014. Infect Genet Evol. (2018) 63:58–61. doi: 10.1016/j.meegid.2018.05.012
98. Hrnjaković Cvjetković I, Petrovic T, Petric D, Milosevic U, Radovanov J, Kovacevic G, et al. Usutu virus: an emerging flavivirus in Europe. Arch Vet Med. (2017) 10:25–35.
99. Knap N, Korva M, Ivović V, Kalan K, Jelovšek M, Zakotnik S, et al. West Nile infections in Slovenia. In: Vilibić-Cavlek T, Barbić Lj, Savić V, Kaić B, editors. Book of Abstracts. Symposium Diagnosis and Surveillance of West Nile Virus Infections in the “One Health” Context. Zagreb (2019). p. 1.
100. Malovrh T, Krt B. Serological monitoring of West Nile fever in Slovenia in horses. Slov Vet Res. (2011) 48(Suppl 13):300–2.
101. Račnik J, Trilar T, Jelovšek M, Zadravec M, Slavec B, Zorman Rojs O, et al. West Nile virus in birds in Slovenia. In: Madić J, editor. Book of Abstracts. Symposium Epidemiological and Clinical Features of West Nile virus in Croatia and Neighboring Countries. Zagreb (2012). p. 9–10.
102. Račnik J, Slavec B, Zadravec M, Zorman Rojs O. West Nile virus monitoring in wild birds in Slovenia. Rad HAZU Med Sci. (2013) 39:89–93.
103. Kaptoul D, Viladrich PF, Domingo C, Niubo J, Martinez-Yelamos S, De Ory F, et al. West Nile virus in Spain: report of the first diagnosed case (in Spain) in a human with aseptic meningitis. Scand J Infect Dis. (2007) 39:70–1. doi: 10.1080/00365540600740553
104. López-Ruiz N, Montaño-Remacha MDC, Durán-Pla E, Pérez-Ruiz M, Navarro-Marí JM, Salamanca-Rivera C, et al. West Nile virus outbreak in humans and epidemiological surveillance, west Andalusia, Spain, 2016. Euro Surveill. (2018) 23:17–00261. doi: 10.2807/1560-7917.ES.2018.23.14.17-00261
105. García-Bocanegra I, Belkhiria J, Napp S, Cano-Terriza D, Jiménez-Ruiz S, Martínez-López B. Epidemiology and spatio-temporal analysis of West Nile virus in horses in Spain between 2010 and 2016. Transbound Emerg Dis. (2018) 65:567–77. doi: 10.1111/tbed.12742
106. Vanhomwegen J, Beck C, Desprès P, Figuerola A, García R, Lecollinet S, et al. Circulation of zoonotic arboviruses in equine populations of Mallorca Island (Spain). Vector Borne Zoonotic Dis. (2017) 17:340–6. doi: 10.1089/vbz.2016.2042
107. Abad-Cobo A, Llorente F, Barbero MDC, Cruz-López F, Forés P, Jiménez-Clavero MÁ. Serosurvey reveals exposure to West Nile virus in asymptomatic horse populations in central Spain prior to recent disease foci. Transbound Emerg Dis. (2017) 64:1387–92. doi: 10.1111/tbed.12510
108. Jiménez-Clavero MA, Sotelo E, Fernandez-Pinero J, Llorente F, Blanco JM, Rodriguez-Ramos J, et al. West Nile virus in golden eagles, Spain, 2007. Emerg Infect Dis. (2008) 14:1489–91. doi: 10.3201/eid1409.080190
109. Jurado-Tarifa E, Napp S, Lecollinet S, Arenas A, Beck C, Cerdà-Cuéllar M, et al. Monitoring of West Nile virus, Usutu virus and Meaban virus in waterfowl used as decoys and wild raptors in southern Spain. Comp Immunol Microbiol Infect Dis. (2016) 49:58–64. doi: 10.1016/j.cimid.2016.10.001
110. Llorente F, Pérez-Ramírez E, Fernández-Pinero J, Soriguer R, Figuerola J, Jiménez-Clavero MA. Flaviviruses in game birds, southern Spain, 2011–2012. Emerg Infect Dis. (2013) 19:1023–5. doi: 10.3201/eid1906.130122
111. Höfle U, Gamino V, de Mera IG, Mangold AJ, Ortíz JA, de la Fuente J. Usutu virus in migratory song thrushes, Spain. Emerg Infect Dis. (2013) 19:1173–5. doi: 10.3201/eid1907.130199
112. Ferraguti M, LA Puente JM, Soriguer R, Llorente F, Jiménez-Clavero MÁ, Figuerola J. West Nile virus-neutralizing antibodies in wild birds from southern Spain. Epidemiol Infect. (2016) 144:1907–11. doi: 10.1017/S0950268816000133
113. Cano-Terriza D, Guerra R, Lecollinet S, Cerdà-Cuéllar M, Cabezón O, Almería S, et al. Epidemiological survey of zoonotic pathogens in feral pigeons (Columba livia var. domestica) and sympatric zoo species in Southern Spain. Comp Immunol Microbiol Infect Dis. (2015) 43:22–7. doi: 10.1016/j.cimid.2015.10.003
114. Busquets N, Alba A, Allepuz A, Aranda C, Ignacio Nuñez J. Usutu virus sequences in Culex pipiens (Diptera: Culicidae), Spain. Emerg Infect Dis. (2008) 14:861–3. doi: 10.3201/eid1405.071577
115. Vázquez A, Ruiz S, Herrero L, Moreno J, Molero F, Magallanes A, et al. West Nile and Usutu viruses in mosquitoes in Spain, 2008–2009. Am J Trop Med Hyg. (2011) 85:178–81. doi: 10.4269/ajtmh.2011.11-0042
116. Bellini R, Calzolari M, Mattivi A, Tamba M, Angelini P, Bonilauri P, et al. The experience of West Nile virus integrated surveillance system in the Emilia-Romagna region: five years of implementation, Italy, 2009 to 2013. Euro Surveill. (2014) 19:20953. doi: 10.2807/1560–7917.ES2014.19.44.20953
117. García-Bocanegra I, Paniagua J, Gutiérrez-Guzmán AV, Lecollinet S, Boadella M, Arenas-Montes A, et al. Spatio-temporal trends and risk factors affecting West Nile virus and related flavivirus exposure in Spanish wild ruminants. BMC Vet Res. (2016) 12:249. doi: 10.1186/s12917-016-0876-4
118. Maquart M, Dahmani M, Marié JL, Gravier P, Leparc-Goffart I, Davoust B. First serological evidence of West Nile virus in horses and dogs from Corsica Island, France. Vector Borne Zoonotic Dis. (2017) 17:275–7. doi: 10.1089/vbz.2016.2024
119. Montagnaro S, Piantedosi D, Ciarcia R, Loponte R, Veneziano V, Fusco G, et al. Serological evidence of mosquito-borne flaviviruses circulation in hunting dogs in Campania region, Italy. Vector Borne Zoonotic Dis. (2019) 19:142–7. doi: 10.1089/vbz.2018.2337
120. Romeo C, Lecollinet S, Caballero J, Isla J, Luzzago C, Ferrari N, et al. Are tree squirrels involved in the circulation of flaviviruses in Italy? Transbound Emerg Dis. (2018) 65:1372–6. doi: 10.1111/tbed.12874
121. Cosseddu GM, Sozio G, Valleriani F, Di Gennaro A, Pascucci I, Gavaudan S, et al. Serological survey of hantavirus and flavivirus among wild rodents in central Italy. Vector Borne Zoonotic Dis. (2017) 17:777–9. doi: 10.1089/vbz.2017.2143
122. Fortuna C, Remoli ME, Severini F, Di Luca M, Toma L, Fois F, et al. Evaluation of vector competence for West Nile virus in Italian Stegomyia albopicta (= Aedes albopictus) mosquitoes. Med Vet Entomol. (2015) 29:430–3. doi: 10.1111/mve.12133
123. Blagrove MSC, Sherlock K, Chapman GE, Impoinvil DE, McCall PJ, Medlock J, et al. Evaluation of the vector competence of a native UK mosquito Ochlerotatus detritus Aedes detritus for dengue, chikungunya and West Nile viruses. Parasit Vectors. (2016) 9:1–6. doi: 10.1186/s13071-016-1739-3
124. Puggioli A, Bonilauri P, Calzolari M, Lelli D, Carrieri M, Urbanelli S, et al. Does Aedes albopictus (Diptera: Culicidae) play any role in Usutu virus transmission in Northern Italy? Experimental oral infection and field evidences. Acta Trop. (2017) 172:192–6. doi: 10.1016/j.actatropica.2017.05.006
125. Camp J, Kolodziejek J, Nowotny N. Targeted surveillance reveals native and invasive mosquito species infected with Usutu virus. Parasites Vectors. (2019) 12:46. doi: 10.1186/s13071-019-3316-z
126. Jansen S, Heitmann A, Lühken R, Leggewie M, Helms M, Badusche M, et al. Culex torrentium: a potent vector for the transmission of West Nile virus in Central Europe. Viruses. (2019) 11:E492. doi: 10.3390/v11060492
127. Amanna IJ, Slifka MK. Current trends in West Nile virus vaccine development. Expert Rev Vaccines. (2014) 13:589–608. doi: 10.1586/14760584.2014.906309
Keywords: West Nile virus, Usutu virus, epidemiology, “One Health”, Southern Europe
Citation: Vilibic-Cavlek T, Savic V, Petrovic T, Toplak I, Barbic L, Petric D, Tabain I, Hrnjakovic-Cvjetkovic I, Bogdanic M, Klobucar A, Mrzljak A, Stevanovic V, Dinjar-Kujundzic P, Radmanic L, Monaco F, Listes E and Savini G (2019) Emerging Trends in the Epidemiology of West Nile and Usutu Virus Infections in Southern Europe. Front. Vet. Sci. 6:437. doi: 10.3389/fvets.2019.00437
Received: 28 September 2019; Accepted: 19 November 2019;
Published: 06 December 2019.
Edited by:
Armanda Bastos, University of Pretoria, South AfricaReviewed by:
Irit Davidson, Kimron Veterinary Institute, IsraelAhmed Ali, Beni Suef University, Egypt
Copyright © 2019 Vilibic-Cavlek, Savic, Petrovic, Toplak, Barbic, Petric, Tabain, Hrnjakovic-Cvjetkovic, Bogdanic, Klobucar, Mrzljak, Stevanovic, Dinjar-Kujundzic, Radmanic, Monaco, Listes and Savini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tatjana Vilibic-Cavlek, dGF0amFuYS52aWxpYmljLWNhdmxla0Boemp6Lmhy
 Tatjana Vilibic-Cavlek
Tatjana Vilibic-Cavlek Vladimir Savic3
Vladimir Savic3 Tamas Petrovic
Tamas Petrovic Ljubo Barbic
Ljubo Barbic Ivana Hrnjakovic-Cvjetkovic
Ivana Hrnjakovic-Cvjetkovic Ana Klobucar
Ana Klobucar Federica Monaco
Federica Monaco Giovanni Savini
Giovanni Savini