- 1School of Biological Science and Medical Engineering, Beihang University, Beijing, China
- 2School of Clinical Medicine, Sun Yat-sen University, Shenzhen, Guangdong, China
- 3Department of Breast and Thyroid Surgery of Changchun Tumor Hospital, Changchun, Jijlin, China
- 4Department of Vascular Surgery, Qianwei Hospital of Jilin Province, Changchun, Jilin, China
- 5Department of Cardiovascular Surgery of the Second Hospital of Jilin University, Changchun, Jilin, China
Arteriovenous fistula (AVF) stenosis is a complex pathological process caused by venous intimal hyperplasia, and its development is influenced by factors such as surgical injury, hemodynamic changes, inflammatory responses, and cellular proliferation and migration. Exosomes are critical mediators of intercellular communication and carry biomolecules (e.g., deoxyribonucleic acid, ribonucleic acid [RNA], and proteins) that can regulate cell functions and impact inflammatory responses, endothelial cell proliferation, and vascular smooth muscle cell migration. Studies have shown that molecules such as microRNAs within exosomes play significant roles in vascular stenosis-related diseases and can function as potential therapeutic tools and biomarkers for disease diagnosis. In addition, exosomes can serve as drug carriers with good biocompatibility and targeting capabilities, providing new avenues for the diagnosis and treatment of AVF stenosis. This article reviews the application of exosomes in AVF stenosis.
1 Introduction
Arteriovenous fistulas (AVFs) are the preferred vascular access for hemodialysis in patients with end-stage renal disease (ESRD). However, their long-term patency is often compromised by venous stenosis, primarily driven by neointimal hyperplasia, endothelial dysfunction, and vascular remodeling (Roy-Chaudhury et al., 2003). Traditional treatments such as percutaneous transluminal angioplasty (PTA) are limited by high restenosis rates and lack of long-term efficacy (Lee and Roy-Chaudhury, 2009).
In recent years, exosomes—nanosized extracellular vesicles released by most cell types—have attracted growing interest due to their ability to mediate intercellular communication via their cargo, including miRNAs, proteins, and lipids (Kalluri and LeBleu, 2020). A growing body of research indicates that exosomes regulate key cellular events implicated in AVF failure, such as vascular smooth muscle cell (VSMC) phenotypic switching, endothelial-to-mesenchymal transition (EndoMT), and chronic inflammation (Yanez-Mo et al., 2015).
This review aims to summarize the current knowledge on the biological functions of exosomes and their involvement in AVF stenosis, with a focus on their potential as diagnostic biomarkers and therapeutic delivery systems.
2 Exosomes: overview and biomedical relevance
Exosomes are nanosized extracellular vesicles (30–150 nm) secreted by most cells, playing critical roles in intercellular communication. They are formed via the endosomal pathway, in which multivesicular bodies (MVBs) release exosomes into the extracellular environment (Ludwig and Giebel, 2012). These vesicles carry various bioactive molecules such as proteins, lipids, DNA, and RNAs (including miRNAs, lncRNAs, and circRNAs), enabling them to influence recipient cell behavior (Robbin et al., 2014). Exosomes can cross biological barriers and deliver their cargo to recipient cells through endocytosis, membrane fusion, or receptor-ligand interactions.
Beyond their cargo composition, an essential property of exosomes lies in their intrinsic targeting ability. Exosomes exhibit selective tropism toward recipient cells depending on their membrane surface molecules, such as integrins, tetraspanins (CD9, CD63, CD81), and adhesion molecules. These molecules facilitate the interaction with specific receptors on target cells—including endothelial cells (ECs), vascular smooth muscle cells (VSMCs), macrophages, and fibroblasts—thereby determining tissue-specific delivery. Upon reaching the target, exosomes are internalized via multiple pathways, including clathrin-mediated endocytosis, macropinocytosis, and lipid raft–mediated fusion. This targeted delivery system enables exosomes to transfer functional RNA and protein payloads with high specificity and biocompatibility, making them promising candidates for vascular therapy and precision diagnostics (Ka et al., 2020; Yanez-Mo et al., 2015).
2.1 Biogenesis and target cell uptake
Exosome biogenesis involves invagination of the plasma membrane to form early sorting endosomes, which mature into late endosomes and MVBs (Ka et al., 2020). The sorting of cargo into intraluminal vesicles occurs through both ESCRT-dependent and independent mechanisms (van Niel et al., 2018). MVBs then fuse with the plasma membrane to release exosomes. Once secreted, exosomes interact with recipient cells via endocytosis, receptor-mediated uptake, or direct membrane fusion. These processes are regulated by proteins such as Rab GTPases, tetraspanins, and various cytoskeletal elements (Thery et al., 2018). The biogenesis, release, and interactions with target cells of exosomes are showed in Figure 1.
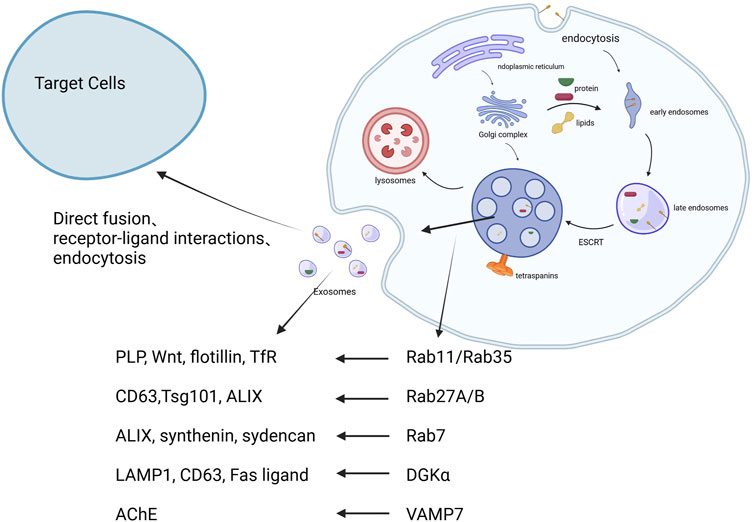
Figure 1. The biogenesis, release, and interactions with target cells of exosomes. 1. Early endosomes are formed through endocytosis. 2. These develop into multivesicular bodies (MVBs), regulated by ESCRT and tetraspanins. 3. MVBs fuse with lysosomes or the plasma membrane, releasing exosomes. 4. Exosomes interact with recipient cells via endocytosis, receptor-ligand interaction, or fusion Created in BioRender.com.
2.2 The composition of exosomes
Exosomes contain a lipid bilayer that protects their internal cargo. They are enriched with proteins such as CD9, CD63, and TSG101; lipids such as ceramide and cholesterol; and nucleic acids including miRNAs, lncRNAs, and circRNAs (Skog et al., 2008; Figure 2). These molecules mediate their functional effects. For example, miR-21, miR-146a, and miR-145 have been implicated in inflammation and vascular remodeling. lncRNAs and circRNAs contribute to transcriptional and post-transcriptional regulation (Valadi et al., 2007; Jiang N. et al., 2019).
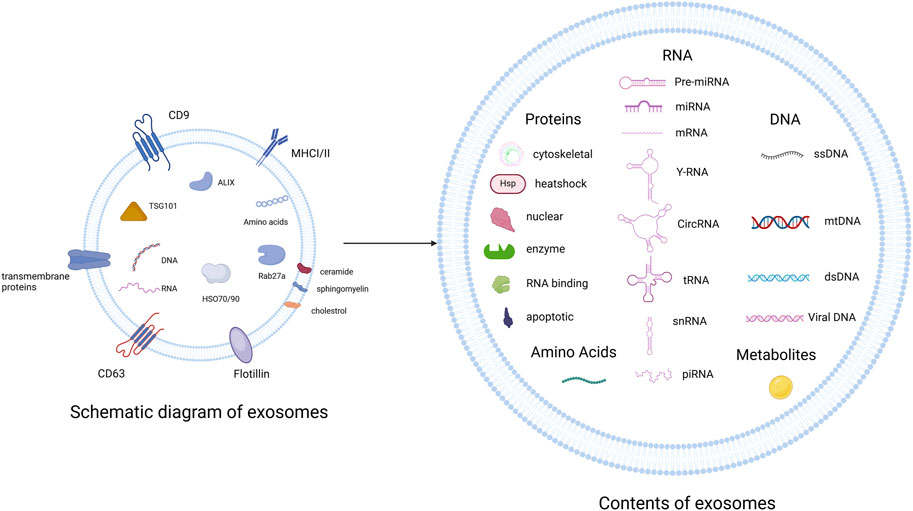
Figure 2. Exosomes are enriched with transmembrane proteins (CD9, CD63), MHC class I/II, TSG101/ALIX (ESCRT), HSP70/90, and various lipids (ceramide, sphingomyelin). Their cargo includes proteins, RNA species, DNA, and metabolites Created in BioRender.com.
2.2.1 Proteins
The proteins within exosomes exhibit diverse functions and participate in various cellular processes such as cell adhesion, membrane fusion, signal transduction, and fundamental metabolism (He et al., 2018). Regardless of the cell type that secretes them, exosomes are rich in proteins and are highly stable owing to the protection offered by their bilayer lipid membranes. Flotillin-1, a lipid raft scaffold protein, is a nonspecific exosomal protein, involved in membrane fusion and transport (Kalluri and LeBleu, 2020). Rab GTPases control membrane and vesicle budding by recruiting effector proteins that play crucial roles in membrane fusion (Zhan et al., 2023). Heat shock proteins, such as the 70-kDa heat shock protein, can also be encapsulated in exosomes and primarily function in protein folding, refolding, and maintenance of protein homeostasis (Stenmark, 2009). Exosomes also contain major histocompatibility complex class I and II proteins, suggesting their potential for development as cancer vaccines owing to this characteristic. Exosomes derived from bodily fluids, such as blood, urine, and saliva, can be used as diagnostic biomarkers and prognostic indicators for cancer (Rosenzweig et al., 2019). A study employing highly rigorous isolation methods discovered multiple integrins and tetraspanins in exosomes that assisted in cell targeting and adhesion; these included CD9, CD63, CD81, and CD82 (Lyu et al., 2024). In contrast to the high abundance of these proteins, organelle-specific proteins from the Golgi apparatus and mitochondria are present in lower quantities in exosomes. Although different exosomal subpopulations may have varying protein profiles owing to environmental factors and different stimuli, there is no single marker that can be used to specifically identify exosomes (Pluchino and Smith, 2019; Yanez-Mo et al., 2015; Tauro et al., 2013).
2.2.2 Lipids
Lipids are integral components of the exosomal membrane and contribute to the structural stability and fluidity of exosomes. They ensure the integrity of exosomes in body fluids and play crucial roles in exosome release. Owing to the presence of lipids, exosomes can fuse with the recipient cell membrane or be internalized by recipient cells, delivering their bioactive molecules to the target cells. The lipid composition of exosomes is not entirely identical to that of parent cells. Exosomes commonly contain lipids such as cholesterol, glycosphingolipids, sphingomyelin, ceramide, and phosphatidylinositol (Pallet et al., 2013; Skotland et al., 2019). The lipid composition of exosomes is similar to that of lipid rafts, exhibiting a higher lipid order and detergent resistance.
Some lipids in exosomes, which have not received much attention previously, also possess specific functions. For instance, ether lipids are involved in cell signaling and membrane transport (Skotland et al., 2020), whereas phosphatidylinositol phosphates participate in signal transduction and recruit proteins with specific recognition sites on the membrane (Dorninger et al., 2017). Phosphatidylserine (PS) and sphingomyelin on the exosomal membrane can bind to cell membrane receptors to initiate signaling pathways. For example, PS binds to phagocytic receptors, promotes exosome uptake, and influences immune responses (Nascimbeni et al., 2017). Lipids in circulating exosomes, particularly sphingosine and lysophosphatidylcholine, are closely associated with the development of hepatocellular carcinoma and can serve as potential biomarkers for the early detection of this disease.
2.2.3 Nucleic acids
Nucleic acids are indispensable components of exosomes, playing a vital role as intermediaries in intercellular communication, and hold promise as diagnostic and prognostic biomarkers (Record et al., 2014). Exosomes contain various types of RNA, which are incorporated through specific sorting mechanisms, and even include some RNAs that are absent in parent cells. This allows cells to transfer RNA to other cells and tissues, thereby exerting functional effects (Kim et al., 2017). Additionally, some mRNAs are preferentially present in EVs and have been shown to translate into proteins at target locations using fluorescence methods (Skog et al., 2008). Another study demonstrated that miRNAs produced by EBV-infected cells can be transferred to uninfected cells to exert functional effects (Pegtel and Gould, 2019), confirming the functional transfer capability of exosomes and their nucleic acid content.
Exosomes encompass specific RNA subgroups rich in miRNAs, lncRNAs, and circRNAs. miRNAs have been extensively studied as endogenous non-coding nucleotides that post-transcriptionally regulate gene expression. Certain proteins recognize specific binding motifs of miRNAs and load them into exosomes (Skog et al., 2008). The miRNA profile of exosomes determines their cellular origin and influences the specificity of target organ uptake. miRNAs are associated with tumors and numerous diseases. For instance, miR-21-5p and miR-125-3p are linked to the pathogenesis of diabetic kidney disease, participating in cellular fibrosis and extracellular matrix accumulation (Pegtel et al., 2010). MiR-210 is related to TIMP-1 expression in lung adenocarcinoma cells, promoting tube formation activity in human umbilical vein endothelial cells (HUVECs) and accelerating lung adenocarcinoma metastasis (Villarroya-Beltri et al., 2013). miR-877 is involved in the induction of EBV-related tumors, while miR-21 and miR-29a bind to toll-like receptors (TLRs) in immune cells, triggering pro-metastatic inflammatory responses that lead to tumor growth and metastasis (Assmann et al., 2018). Furthermore, exosomal miRNAs may serve as markers to study degenerative conditions in humans. A study using microarray analysis to investigate the age-dependent differential expression of miRNAs found that although some highly abundant miRNAs did not exhibit differential expression, stratified analysis identified certain differentially expressed miRNAs (Cui et al., 2015). Another study screened and detected several miRNAs in saliva samples collected from subjects of different ages and found that salivary exosomal miRNAs provided a simple and effective means of measuring and assessing the aging process (Fabbri et al., 2012).
lncRNA are RNA molecules longer than 200 nucleotides that, despite not encoding proteins, play crucial roles in regulating gene expression. The structural characteristics of lncRNAs allow them to form various secondary and tertiary structures such as stem loops, pseudoknots, and triple helices. These structural features enable lncRNAs to interact with multiple molecules, including DNA, RNA, and proteins, thereby regulating gene expression and cellular functions (Tietje et al., 2014). LncRNAs possess specific domains that can fold into complex three-dimensional shapes. For example, Lnc-Nr6a1 mediates the assembly of the glycolysis complex by acting as a scaffold molecule for glycolytic enzymes, utilizing its structural domains (Machida et al., 2015). LncRNAs perform numerous functions in various biological processes. They regulate gene transcription and translation by interacting with transcription factors, chromatin-modifying enzymes, and ribosomes. Ye et al. discovered that the lncRNA KCNQ1OT1 interacts with the IκBα protein, reducing its phosphorylation, promoting IκBα ubiquitination, and sequestering miR-221, thereby increasing IκBα levels. Through this mechanism, KCNQ1OT1 inhibits inflammation and proliferation of vascular smooth muscle cells (VSMCs), reducing intimal hyperplasia (IH) (Zhang et al., 2024). lncRNAs can also act as signaling molecules, decoys, or guide molecules in various biological processes. Fan et al. identified lncRNA-XLOC_098131 as a novel lncRNA with immunoregulatory functions that acts as a competitive endogenous RNA to regulate toll-like receptor signaling pathways and immune functions (Polo-Generelo et al., 2022). Furthermore, lncRNAs play a critical role in cell differentiation. Qin et al. found that lncRNAs are involved in the regulation of muscle and adipose tissue differentiation via various molecular mechanisms in the nucleus and cytoplasm (Ye et al., 2020).
In recent years, circRNAs have become a significant research focus. CircRNAs are non-coding RNAs with a circular structure that lack a polyadenylated tail at the 3′end and a cap structure at the 5′end. This unique structure renders them resistant to exonucleases and provides high stability and abundant presence. Consequently, circRNAs degrade slowly and have been detected in blood, urine, the liver, and the pancreas, making them excellent biomarkers (Fan et al., 2019). circRNAs are formed by back-splicing when the spliceosome assembles on long exons, catalyzed by exon definition complexes (Yang et al., 2018). They can serve as templates for proteins and peptides. During translation, they directly bind to initiation factors. In addition, circRNAs often act as sponges for miRNAs or proteins and participate in the regulation of gene transcription. CircRNAs have been implicated in the pathophysiology of various tumors (Li et al., 2015), influencing glycolysis, lipid metabolism, and other energy metabolism processes by regulating transport proteins, transcription factors, and signaling pathways. These processes play crucial roles in tumor development (Li et al., 2019). CircRNAs also regulate the epithelial–mesenchymal transition process, contributing to tumor proliferation and metastasis. In addition to oncology, circRNAs are involved in other diseases, such as osteoarthritis, cardiovascular diseases, and degenerative conditions (Tang et al., 2022).
2.3 Target cell uptake
After exosomes are secreted, they are taken up by target cells through several distinct mechanisms. Endocytosis is the primary method of exosome uptake and is mediated by various substances including lipid rafts, clathrin, and caveolin. For example, exosomes derived from Schwann cells enter target cells via lipid raft-mediated endocytosis and regulate macrophage polarization via the SOCS3/STAT3 pathway to alleviate inflammatory responses (Yu et al., 2019). Additionally, the primary mechanism for exosome-like vesicle uptake from Opisthorchis felineus by human cholangiocytes is clathrin-mediated endocytosis, highlighting the significance of different endocytic pathways in various cell types (Wang et al., 2019). Myriam et al. reported a unique pathway for exosome uptake in hypoxic environments. They discovered that under hypoxic conditions, heparan sulfate proteoglycan-dependent endocytosis promotes exosome uptake, leading to lipid droplet formation in glioma cells (Ren et al., 2023). Exosomes are preferentially taken up by homologous cells through receptor-ligand interactions, making them highly likely to be enclosed by lysosomes. Shabirul et al. designed exosomes to express CD19 chimeric antigen receptors, enabling them to target CD19-positive leukemia B cells through receptor-ligand interactions and induce cell death without affecting CD19-negative cells (Pakharukova et al., 2023). Membrane fusion is a common mechanism for heterologous cell uptake. Exosomal contents are delivered to the target cell when the exosomal membrane fuses with the plasma membrane, and the exosomal contents are then delivered into the target cell (Cerezo-Magana et al., 2021). Exosomes secreted by dendritic cells can fuse with target cells, creating fusion pores that release their contents (Haque et al., 2021). The uptake of exosomes by target cells is influenced by the specific surface proteins of both the exosomes and target cells, as well as the external environment. Plasma proteins attached to the surfaces of exosomes can alter their functional properties, provide new functionalities, and affect their uptake by target cells (Montecalvo et al., 2012). Lower pH levels in the environment significantly affect EV secretion and increase EV uptake (Chernomordik and Kozlov, 2008).
2.4 Isolation and identification methods
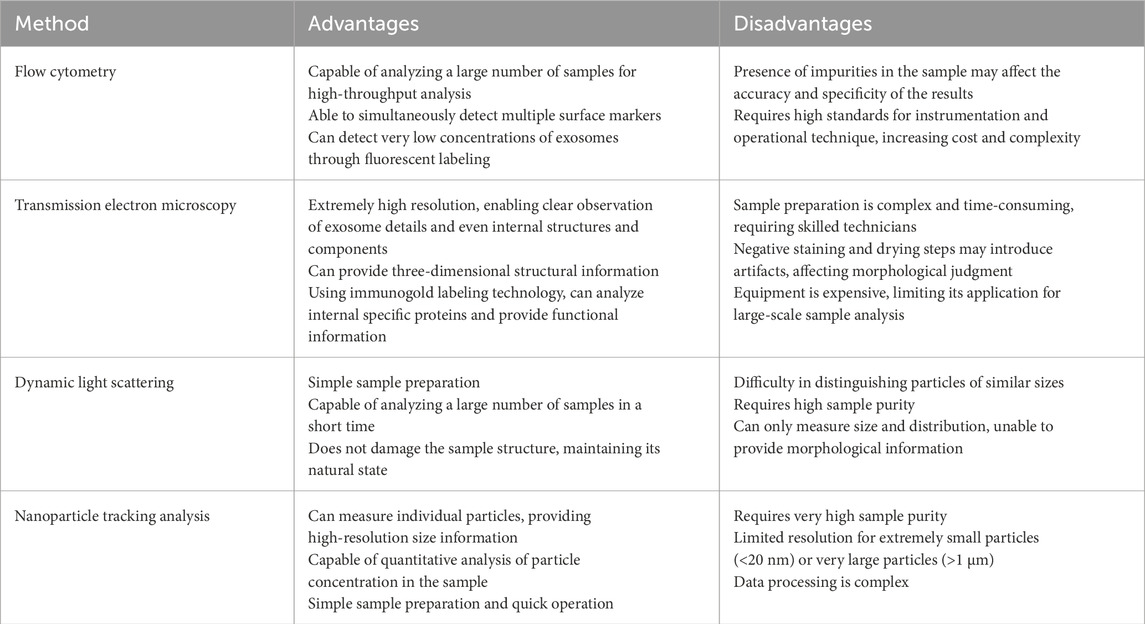
Common exosome isolation techniques include ultracentrifugation (Figure 3), PEG precipitation, and size-exclusion chromatography (Konoshenko et al., 2018). Immunoaffinity capture and commercial kits like ExoQuick are also used. Characterization methods include nanoparticle tracking analysis (NTA), transmission electron microscopy (TEM), and flow cytometry (Table 1). While each technique has advantages and drawbacks, combining approaches improves yield and purity (Zhang et al., 2016). Detailed reviews on methodology are available for further reference (Mateescu et al., 2017).
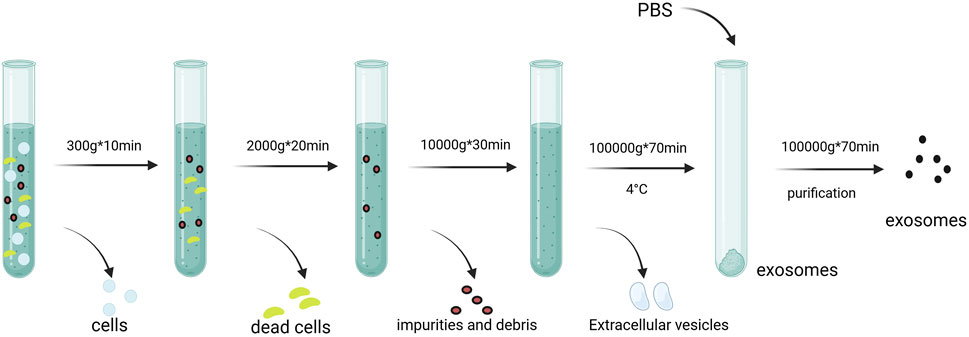
Figure 3. Isolation and extraction of exosomes. Centrigugal speeds of 300*g, 2000*g, and 10,000*g are used to remove impurities and debris. Exosomes are finally obtained by 100,000*g speed Created in BioRender.com.
2.5 Storage of exosomes
Exosomes are a focal point of research in the fields of medicine and biomedicine, and optimizing their preservation is a prerequisite for further study. Research indicates that the storage methods of exosomes significantly impact their concentration, physical state, and biological functionality (Jeyaram and Jay, 2017). Therefore, optimizing storage conditions is crucial for the reliability of fundamental research results on exosomes, the efficacy of clinical applications, and the quality and safety of commercialized products. Currently, storage at −80°C is a widely adopted method. Stefano et al. systematically evaluated eight different strategies, including simple −80°C freezing and the addition of seven different protective agents, as well as the effects of freeze-thaw cycles on exosomes. The results indicated a negative correlation between storage time and the concentration and purity of exosomes, with particle size and variability changing over time. They recommended that, in most cases, exosomes should be sourced from fresh samples. For strict storage requirements, biological samples may be stored short-term, while storing isolated exosomes is not advised (Gelibter et al., 2022). Yuan et al. proposed that storage at 4°C can be utilized for short-term preservation of exosomes. However, for long-term retention of functionality and extended shelf life, freeze-drying presents a promising method (Yuan et al., 2021). André et al. found that storing exosomes in phosphate-buffered saline (PBS) leads to a significant decrease in recovery rates, particularly for pure samples. They applied a novel buffer, which is a PBS formulation supplemented with human albumin and trehalose (PBS-HAT), and discovered that it significantly enhances both the short-term and long-term preservation of exosomes, especially under −80°C conditions (Gorgens et al., 2022). Further research is essential to explore optimal methods for the long-term or short-term preservation of exosomes while maintaining their inherent properties and functionalities.
2.6 Functions of exosomes
Almost all human cells secrete exosomes that fuse with target cells to transfer functional molecules or activate receptors or effectors. Exosomes released through various pathways can act as signaling messengers, regulating the behavior of nearby, distant, or even originating cells. For instance, hepatocyte-derived exosomes mediate increased synthesis of sphingosine-1-phosphate by transferring neutral ceramidase and sphingosine kinase 2, thereby promoting hepatocyte proliferation and regeneration following ischemia-reperfusion injury or partial hepatectomy (Wu and He, 2023). Exosomes released by ECs under oxidative stress conditions can enhance EC proliferation and migration and transport miR-92a-3p to VSMCs, promoting their proliferation and migration (Guo and Zhang, 2023). Using a rat model, Qu et al. demonstrated that exosomes derived from human umbilical cord mesenchymal stem cells (hucMSCs) enhanced endothelial function, inhibited neointimal hyperplasia in vein grafts, and accelerated reendothelialization (Nojima et al., 2016). These exosomes promoted EC proliferation and migration, thereby contributing to vascular repair and regeneration.
Exosomes play crucial roles in cellular immune responses. Certain exosomes secreted by macrophages can induce proinflammatory reactions. For instance, exosomes containing glycopeptidolipids from Mycoplasma gallisepticum-infected macrophages can stimulate resting cells to exert proinflammatory responses (Yao et al., 2022). Under stress conditions, heat shock proteins are embedded in the plasma membrane and then released into the environment, inducing tumor necrosis factor alpha (TNF-α) release and activating macrophages (Qu et al., 2020). Neutrophil-derived exosomes transfer miR-30d-5p, inducing M1 macrophage polarization and activating the nuclear factor kappa B (NF-κB) signaling pathway, leading to an inflammatory response in macrophages (Bhatnagar and Schorey, 2007). Zhang et al. studied the effects of exosomes derived from Treponema pallidum-stimulated dendritic cells on EC inflammation. These exosomes activated the LR4/MyD88/NF-κB signaling pathway, increasing the expression of interleukin (IL)-1β, IL-6, and TNF-α in ECs, thereby promoting inflammation. Plasma exosomes from patients with acute myocardial infarction can induce NF-κB signaling activation, contributing to proinflammatory immune responses and EC injury (Vega et al., 2008). Moreover, exosomes can modulate the tumor microenvironment and enhance or reduce the responsiveness of cancer cells to therapies. They transport non-coding RNAs and regulate molecular signaling pathways such as PTEN and PI3K/Akt, promoting chronic inflammation, immune evasion, and tumor progression (Hazrati et al., 2022).
Exosomes play a significant role in neuronal signal transmission, promoting neuronal survival and synaptic assembly. They can cross the blood–brain barrier and are involved in myelination. Exosomes are associated with the pathological processes of neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, amyotrophic lateral sclerosis, and Huntington’s disease (Giannotta et al., 2013). Aging is regulated by the hypothalamus. A study involving mice revealed that exosomes secreted by normal hypothalamic stem cells can slow aging, suggesting that the hypothalamus partially controls this process through the release of exosomes (Gong et al., 2023).
3 Arteriovenous fistula stenosis
The increasing aging population and prevalence of chronic diseases have led to an increased incidence of end-stage renal disease. Well-functioning hemodialysis access is crucial for the survival of patients with kidney disease. An autogenous arteriovenous fistula (AVF) is the first clinical choice for dialysis access; however, dysfunction of the dialysis pathway often leads to repeated surgeries and even patient mortality. Venous IH is the primary cause of dialysis access failure. The factors contributing to IH are categorized as upstream and downstream events. Upstream events include endothelial injury caused by surgical trauma and hemodynamic changes, whereas downstream events include oxidative stress response, inflammatory response, cell proliferation, and migration (Cheng et al., 2023). (Figure 4.) IH is a complex pathophysiological process involving numerous cells and signaling pathways. For instance, endothelial cell injury leads to a weakened barrier function of the vascular wall, the release of growth factors such as PDGF, and the secretion of pro-inflammatory factors that activate inflammatory pathways. Ultimately, this results in the proliferation and migration of smooth muscle cells. These smooth muscle cells migrate from the media to the intima and proliferate, forming a thickened intimal layer, which consequently causes vascular narrowing. Therefore, a detailed understanding of the migratory and transformed cellular components and molecular pathways involved is essential for developing targeted therapies in the future.
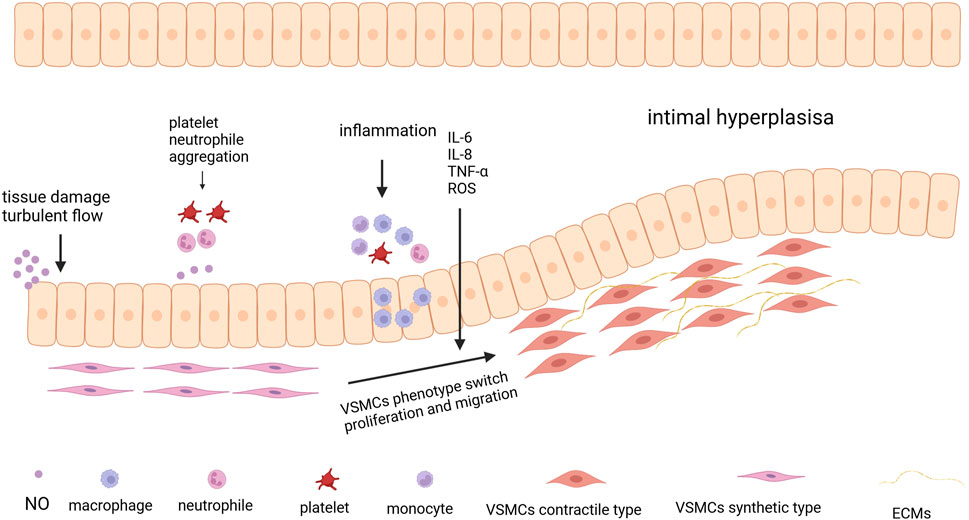
Figure 4. The process of Intimal Hyperplasia. The process begins with tissue damage and turbulent blood flow, which can injure the endothelial cells lining the blood vessel. Following endothelial injury, platelets and neutrophils aggregate at the site of damage. Then the aggregation of immune cells, such as monocytes and macrophages, leads to the release of pro-inflammatory cytokines like IL-6, IL-8, TNF-α, and reactive oxygen species (ROS) exacerbating the inflammatory response. Under the influence of inflammatory signals, VSMCs undergo a phenotypic switch from a contractile type to a synthetic type Created in BioRender.com.
3.1 Tissue injury
Surgical procedures and dialysis punctures can cause tissue injury, and EC damage is particularly significant. Under physiological conditions, ECs form a continuous endothelium that lines the inner walls of blood vessels. This endothelium not only facilitates the metabolic exchange of plasma and tissue fluid but also secretes various bioactive substances that maintain vascular tone, regulate blood pressure, and balance antiinflammatory and proinflammatory responses. Compromised integrity of the endothelium leads to the invasion of inflammatory cells, platelet aggregation, and thrombosis (Galardi et al., 2023). Oxidative stress is a crucial mechanism underlying endothelial damage. During oxidative stress, the balance between oxidation and antioxidation is disrupted owing to the excessive production of reactive oxygen species (ROS). Hypoxia and substances, such as angiotensin II, can induce ROS production, leading to endothelial tissue damage (Lee and Roy-Chaudhury, 2009). ROS reduces the bioavailability of nitric oxide (NO) through redox reactions, causing vascular dysfunction. NO plays a crucial role in the cardiovascular system by relaxing vascular smooth muscle, promoting vasodilation, and enhancing blood flow. Additionally, it possesses anti-inflammatory properties, supports endothelial cell function, and inhibits platelet aggregation. Zhu et al. designed a patch that covalently binds a nitrite compound to a biodegradable polymer for localized NO release. Their study, conducted in animal models, demonstrated that the released NO improves cardiac function, aids in cardiac tissue repair, and alleviates cardiac remodeling and fibrosis (Zhu et al., 2021). In another study, researchers developed a precise system for delivering nitric oxide (NO) to tissues, which demonstrated the critical role of NO in vascular regulation and tissue repair in both rat hind limb ischemia models and mouse acute kidney injury models (Hou et al., 2019). Furthermore, ROS can trigger endothelial inflammation, disrupt mitochondrial function, and lead to abnormal energy metabolism, exacerbating EC damage (Wettschureck et al., 2019). ROS can induce EC apoptosis by increasing the expression of p38MAPK and caspases and by inhibiting the expression of Bcl-2 while promoting the expression of Bax and Fas. It can also induce apoptosis in VSMCs (Shaw et al., 2014). ROS have been recognized as a significant target in ischemia-reperfusion injury. Li et al. developed a ROS-sensitive hydrogel for the delivery of basic fibroblast growth factor (bFGF) aimed at repairing damaged cardiomyocytes and enhancing angiogenesis (Zhenhua Li et al., 2021). The NLRP3 inflammasome activation plays a significant role in endothelial dysfunction (Shaito et al., 2022). The NLRP3 inflammasome can activate and promote the maturation of IL-1β and IL-18 and mediate the formation of the pore protein, N-GSDMD, leading to cell swelling, rupture, and death (Higashi, 2022). It can also disrupt tight junction proteins between ECs, thereby increasing vascular permeability. As an upstream signal of NLRP3, ROS initiates and activates the NLRP3 inflammasome, thereby triggering pyroptosis.
3.2 Hemodynamic changes
Blood flow in vessels can be categorized into laminar and turbulent flows based on different flow patterns, which are closely related to the vascular configuration. In smooth and straight sections of the lumen, blood flow is typically laminar, with a Reynolds number (Re) of less than 2000. Conversely, in irregular sections, including the inner sides of curved vessels and upstream stenotic regions, the flow becomes unstable and disturbed, manifesting as a turbulent flow with an Re greater than 2000. The original vascular anatomy is altered following AVF creation, resulting in different angles between the arteries and veins. This alters the type of shear stress acting on the endothelium, shifting it from laminar shear stress to low oscillatory shear stress (Bai B. et al., 2020). The EC membrane contains numerous receptors that detect and transmit mechanical stimuli, including receptor tyrosine kinases, G protein-coupled receptors, and various ion channels (Zhang Y. et al., 2019). This mechanosensory capability helps differentiate blood flow characteristics and make physiological adjustments.
Under laminar shear stress, the expression of the cyclin-dependent kinase inhibitor p21 increases, slowing EC DNA synthesis and inhibiting proliferation. Laminar shear stress can suppress apoptosis induced by serum starvation, oxidative stress, and TNFα, potentially through increased expression of nitric oxide synthase and superoxide dismutase (Pfenniger et al., 2015). NO produced by ECs can elevate cGMP and PKG levels, reducing Ca2+ concentration and promoting vasodilation (He et al., 2022). Under physiological conditions, laminar shear stress also reduces the expression of the adhesion molecules, vascular cell adhesion molecule one and intercellular adhesion molecule 1. This in turn decreases leukocyte and platelet aggregation, reduces ROS levels, activates anticoagulant genes, and inhibits VSMC proliferation and migration. These actions help maintain EC vitality and functional integrity (Rennier and Ji, 2013; Cirino et al., 2017). In contrast, under low shear stress, cell proliferation is accelerated, ROS levels are increased, and IL-6 and IL-8 secretion is significantly increased, activating neutrophils and promoting their adhesion to ECs, thereby fostering inflammation and apoptosis.
3.3 Inflammation
Inflammation is a key factor in IH development. The inflammatory response involves various miRNAs that target and regulate multiple proteins to promote inflammation; these include miR-125, miR-155, and miR-125. Following tissue injury, leukocytes aggregate at the injury site under the influence of chemokines (Zheng et al., 2013). Macrophages and neutrophils release a plethora of inflammatory cytokines such as interleukins, C-reactive protein, and TNF-α during the inflammatory response. These pro-inflammatory cytokines, in conjunction with growth factors, further promote the aggregation of inflammatory cells and initiate the phenotypic transformation of VSMCs, leading to their proliferation and migration (Wang et al., 2014). Chemokines that promote leukocyte aggregation, such as platelet-derived growth factor (PDGF) and tumor growth factor-β, are secreted by VSMCs and activated by ECs. Local inflammation also triggers vascular wall remodeling and neointimal formation, leading to vascular intima stenosis. Yi et al. discovered that Nik-related kinase expression in VSMCs affects IH and vascular inflammation by regulating the expression of matrix metalloproteinases and inflammatory cytokines/chemokines (Charo and Taubman, 2004). The epigenetic factor, PCAF, is also involved in regulating vascular inflammation by promoting NF-κB-mediated inflammation (Carracedo et al., 2019). Further, reduced PCAF levels significantly decreased IH, the intima/media ratio, and luminal stenosis. Xu et al. revealed that Sox10 is a key regulator of vascular inflammation (Lu et al., 2020). It activates the PI3K/AKT signaling pathway and drives the transdifferentiation of VSMCs into macrophages through lactylation and phosphorylation modifications, thereby promoting pyroptosis and exacerbating the inflammatory response.
3.4 Endothelial cells
Upon stimulation, ECs contract, leading to the disruption of adhesive junctions. Adhesion between ECs is primarily maintained by vascular endothelial cadherin (VE-cadherin), which interacts with cytoskeletal molecules to regulate EC adhesion, vascular regeneration, and migration (de Jong et al., 2017). VE-cadherin connects with β-catenin, plakoglobin, and p120-catenin to stabilize EC junctions (Xu et al., 2023). The barrier function of ECs is dynamic and maintained by adhesive junctions. Endothelial permeability increases under the influence of autocrine substances such as vascular endothelial growth factor (VEGF) and thrombin. This dynamic change during vascular injury promotes the accumulation of inflammatory mediators, leukocyte migration, and Ca2+ elevation and activates Ca2+-driven proteins such as myosin light chain kinase and membrane-associated protein A2. These processes promote actin contraction and the degradation of endothelial cadherin, leading to EC detachment and barrier dysfunction (Giannotta et al., 2013; Oas et al., 2013). Additionally, talin-dependent activation of EC β1-integrin weakens adhesion and reduces tyrosine phosphorylation of the VE-cadherin-catenin complex, and VEGF activation of FAK kinase opens endothelial junction structures (Dalal et al., 2020). Collectively, these processes contribute to the loss of endothelial function.
Endothelial–mesenchymal transition (EndoMT) refers to a pathological process in which endothelial cells lose their specific markers (e.g., CD31 and VE-cadherin) and acquire a mesenchymal phenotype characterized by the expression of α-SMA, vimentin, and other mesenchymal proteins. This transition is commonly induced by inflammatory cytokines, oxidative stress, or transforming growth factor-beta (TGF-β), and has been shown to play a pivotal role in vascular remodeling and neointimal hyperplasia, particularly following vascular injury (Qian et al., 2025). Emerging studies have indicated that exosomes also play critical roles in modulating EndoMT. Exosomes derived from diseased or inflamed tissues may promote EndoMT, whereas those from mesenchymal stem cells or other regenerative sources can inhibit EndoMT and preserve endothelial identity, offering potential therapeutic avenues for limiting intimal hyperplasia in AVF (Sishuai et al., 2024). Additionally, under oxidative stress conditions, exosomes derived from adipose-derived stem cells (ADSC-Exo) have been shown to alleviate hydrogen peroxide-induced EndoMT in human umbilical vein endothelial cells (HUVECs) by inhibiting the miR-486-3p/Sirt6/Smad signaling axis, suggesting their antifibrotic potential in endothelial dysfunction (Li et al., 2024).
During neointimal formation following vascular injury, ECs play a crucial role, with some cells undergoing competitive selection to become tip cells that rearrange dynamically to form new blood vessels (Pulous et al., 2019). In this process, the interaction between the Notch and YAP/TAZ signaling pathways regulate the selection and phenotype of tip cells (Chen et al., 2012). Chen et al. found that the transcription factor Foxp1 in ECs can regulate VSMC proliferation and migration by targeting the matrix metalloproteinase (MMP)-9 and Cdkn1b signaling pathways (Blanco and Gerhardt, 2013). When Foxp1 is absent, VSMC proliferation and migration increase, while endothelial cell proliferation decreases.
3.5 Vascular smooth muscle cells
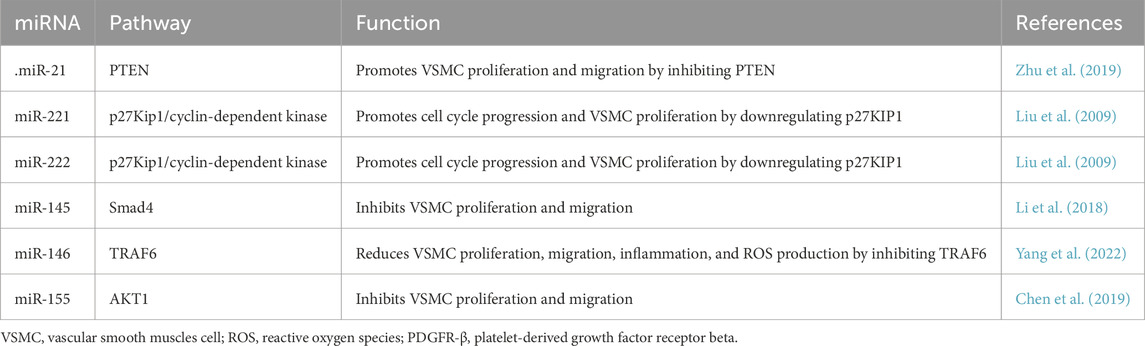
VSMCs are abundant in the vasculature and primarily comprise the medial layer of blood vessels. The active contraction and passive recoil of elastin fibers contribute to the elasticity of the blood vessels, thereby regulating blood pressure and flow distribution. VSMCs exhibit high plasticity, phenotypic switching capabilities, and proliferative potential, which enable adaptive responses to environmental changes. They are regulated by many miRNAs (Table 2). Under normal physiological conditions, VSMCs express many contractile proteins such as myosin heavy chain 11, SM22α, and α smooth muscle actin, which confer a contractile phenotype (Guo et al., 2024). Their contraction activity is regulated by calcium ions, and calmodulin activates myosin light chain phosphorylation, leading to myofibril contraction. However, under various pathological conditions, VSMCs can switch from a contractile phenotype to a synthetic phenotype, aiding in injury repair. In the synthetic phenotype, the cells change from a spindle shape to an irregular shape, with a significant reduction in contractile markers. Their proliferation and migration abilities are enhanced, and they secrete large amounts of extracellular matrix, including collagen, elastin, and matrix metalloproteinases, in response to injury (Chen et al., 2022; Chakraborty et al., 2021). Phenotypic switching of VSMCs is typically regulated by inflammatory mediators, growth factors, transcription factors, and noncoding RNAs. For instance, growth factors can stimulate VSMC dedifferentiation through the MAPK pathway, whereas transcription factors such as KLF4 can regulate phenotypic switching by modulating epithelial adhesion complexes and actin cytoskeleton dynamics (Lynch et al., 2016; Jain et al., 2020). Synthetic VSMCs migrate from the medial layer to the intima, forming a new intimal layer that causes venous neointimal hyperplasia.
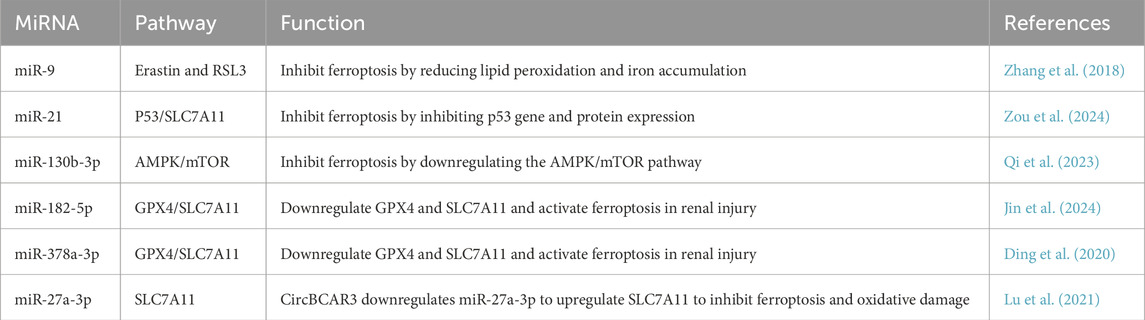
3.6 Ferroptosis
Ferroptosis, a research hotspot in recent years, is a form of programmed cell death that is dependent on iron and lipid peroxidation. It is primarily regulated by three major mechanisms—iron metabolism, lipid metabolism, and the glutathione peroxidase 4 (GPX4) pathway activation. Other pathways involved include FSP1-CoQ10-NAD(P)H, BH4-DHFR, and P53 signaling (Yoshida et al., 2012). (Figure 5.) Ferroptosis is closely associated with intimal vascular stenosis. Using differentially expressed gene analysis, Zhang et al. identified 34 and 31 significantly differentially expressed ferroptosis-related genes at 2 days and 14 days after carotid artery ligation in mice, respectively (Han et al., 2021). Exposure to ZnO nanoparticles (ZnONP) induces endothelial dysfunction via ferroptosis. When ZnONP exposure triggers vascular inflammation, ferroptosis markers increase, and the inhibition of ferroptosis reduces vascular damage (Zhang et al., 2021). Chen et al. found that the phospholipid oxidation product, PGPC, increases ferrous ion content and peroxide production, inducing EC ferroptosis via the CD36 receptor, thereby impairing endothelial function by increasing FABP3 expression (Zhang L. et al., 2022). Luo et al. discovered that ferroptosis is closely related to EC death, activating the p53-xCT-GSH axis and causing endothelial dysfunction (Qin et al., 2021). Zhang et al. found that BaP/BPDE exposure upregulates free iron, accelerates lipid peroxidation, and reduces GPX4 protein levels, thereby inducing ferroptosis, inhibiting EC function, and affecting angiogenesis (Chen et al., 2024).
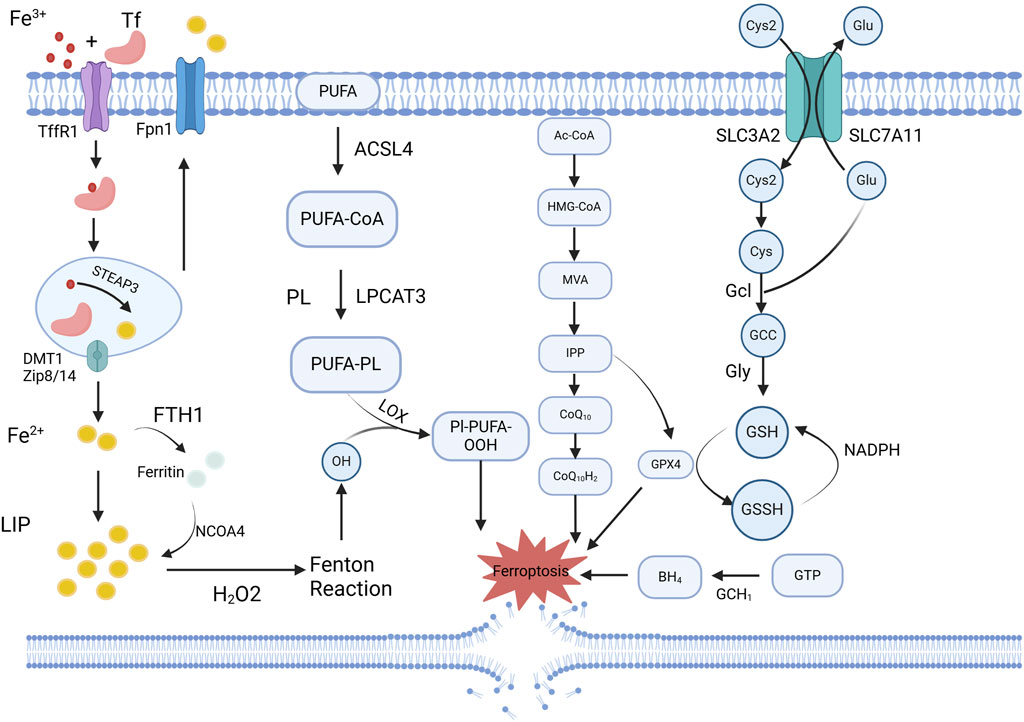
Figure 5. Schematic overview of ferroptosis-related mechanisms regulated by exosomal miRNAs. Ferroptosis is characterized by iron accumulation, lipid peroxidation, and impaired antioxidant defense, particularly through downregulation of GPX4 and system Xc− (SLC7A11). Exosomal miRNAs, such as miR-27a-3p and miR-125b, modulate these pathways by targeting key regulators like SLC7A11, Keap1/Nrf2, or GPX4, thereby influencing ferroptotic sensitivity in vascular cells. Created in BioRender.com.
Ferroptosis also induces VSMC phenotypic switching. In vivo activation of ferroptosis by RSL3 promotes VSMC phenotypic switching and exacerbates IH. A previous study showed that the ferroptosis inhibitor, Fer-1, counteracted the effects of RSL3 by reducing IH (Luo et al., 2021). Sun et al. discovered that ferroptosis promotes VSMC aging and vascular aging (Zhang et al., 2023). Activation of ferroptosis signaling led to a decrease in NAD + levels and drove VSMC aging via NCOA4-mediated ferritinophagy. Using a specific inhibitor of lysine-specific demethylase 1 has been found to reduce intracellular iron levels and decrease VSMC ferroptosis (Zhang S. et al., 2022). Targeted inhibition of the endogenous histone acetyltransferase P300 activates the hypoxia-inducible factor 1-alpha (HIF1-α)/HMOX1(Heme Oxygenase 1) axis, promoting CD and IKE-induced VSMC ferroptosis, thus contributing to vascular stenosis-related diseases. Ferroptosis is closely related to inflammation, which influences vascular intimal stenosis by modulating inflammation-related pathways. Chen et al. concluded that ferroptosis regulate the activity of five inflammatory signaling pathways—JAK-STAT, NF-κB, inflammasome, cGAS-STING, and MAPK, thereby affecting cellular functions (Sun et al., 2024).
4 Role of exosomes in arteriovenous fistula stenosis
Exosomes play a significant role in the progression of AVF stenosis by affecting EC function, inflammatory responses, and proliferation and migration of VSMCs (Figure 6). One of the causes of AVF stenosis is EC damage and dysfunction, which are responsible for regulating inflammation, vascular tone, and permeability. The damaged ECs are replaced by proliferating resident ECs, followed by endothelial apoptosis and necrosis. Exosomes are released when ECs are subjected to shear stress and injury, affecting NO release and altering vascular tone, further compromising endothelial function (He et al., 2024). Endothelial progenitor cells (EPCs) are a type of stem cells that serve as precursors to ECs, with strong growth and differentiation potential. Exosomes derived from EPCs contain various factors that regulate vascular function, promote neovascularization, and repair endothelial dysfunction (Chen et al., 2023; Lovren and Verma, 2013). For example, EPC-derived exosomes are enriched in angiogenic microRNAs such as miR-126, which enhances endothelial proliferation and migration via the PI3K/Akt pathway. Moreover, these exosomes may carry angiogenic proteins such as VEGF, promoting endothelial cell survival and tube formation, thereby facilitating vascular repair and new vessel formation (Jansen et al., 2012; Zhang et al., 2015). EPCs and ECs can influence phenotypic switching of VSMCs, thereby contributing to AVF stenosis. A recent study found that exosomes from EPCs adhere to damaged vascular areas, inhibit post-injury neointimal formation, reduce inflammation levels, and enhance endothelial function by antagonizing apoptosis via the Bcl2/Bax/Caspase-3 signaling pathway (Xing et al., 2020). Exosomes secreted by ECs contain miR-195, which can regulate VSMC proliferation, as well as proteins (e.g., PDGF-BB) that affect VSMCs (Bai S. et al., 2020; Tan et al., 2024). Another study demonstrated that EC-derived EVs also participate in regulating monocyte activation, thereby influencing vascular inflammation (Gu et al., 2017). Under the influence of angiotensin-converting enzyme 2, EPC-derived exosomes enhance mitochondrial function and regulate VSMC phenotype, thereby protecting against EC injury (Wang et al., 2020a).
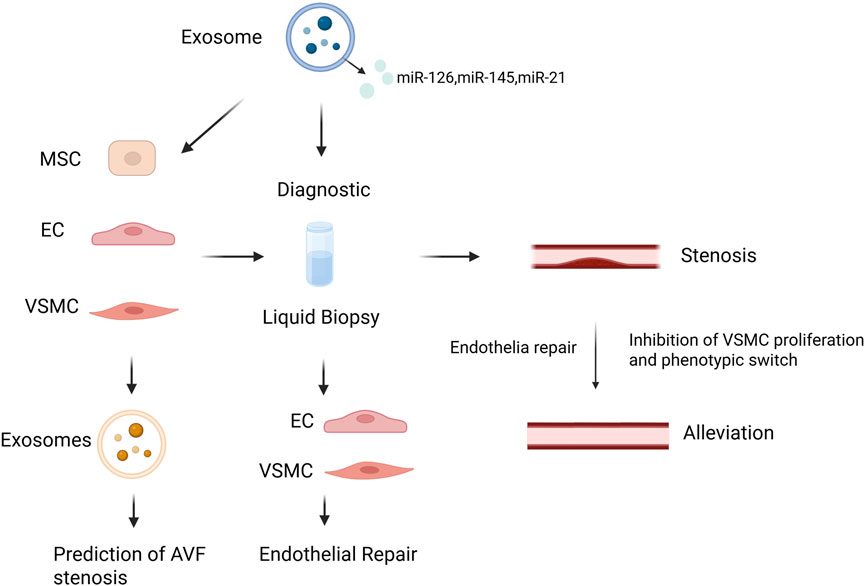
Figure 6. Schematic illustration of exosome-based diagnostic and therapeutic strategies in AVF stenosis. Created in BioRender.com.
Inflammation is a crucial pathological process leading to vascular stenosis, and exosomes play a significant role in this process. Exosomal miRNAs can regulate inflammatory responses. The expression profiles of exosomal miRNAs in patients with asymptomatic carotid stenosis are associated with post-endarterectomy inflammation and major adverse cardiovascular events (Togliatto et al., 2018). Proteins within exosomes such as MFGE8 promote the polarization of antiinflammatory M2b macrophages and reduce collagen deposition and inflammation. Animal experiments have shown that MFGE8 prevents esophageal stenosis following endoscopic submucosal dissection (Wang et al., 2020b). Exosomes derived from MSCs have significant potential in reducing inflammation and promoting vascular healing. Adipose-derived MSCs overexpressing stanniocalcin-1 can inhibit NLRP3 inflammasome-mediated inflammation and promote reendothelialization in mechanically injured carotid arteries after endarterectomy (Sardu et al., 2021). Another study established a rat model of renal artery stenosis-induced kidney injury and found that adipose-derived stem cell exosomes reduced the expression of the hypoxia marker, HIF1-α and stabilized blood pressure, indicating their role in mitigating inflammation and vascular damage (Lai et al., 2024). Yang et al. discovered that EC-derived exosomes reduced neointimal hyperplasia resulting from carotid artery injury in rats by inhibiting the ROS-NLRP3 inflammasome and reducing VSMC phenotypic switching (Liu et al., 2022).
Exosomes have been found to influence the behavior of VSMCs and play significant roles in various pathophysiological processes. In atherosclerotic diseases, exosomes regulate the proliferation and migration of VSMCs via the circ-100696/miR-503-5p/PAPPA axis, thereby promoting the progression of atherosclerosis (Ishiy et al., 2020). Additionally, exosomes secreted by VSMCs in patients with diabetes enhance endothelial activation and inflammatory polarization of macrophages through miR-221/222, thereby promoting vascular inflammation and accelerating atherosclerotic plaque formation (Yang et al., 2023). A study verified whether miR-339-3p affects angiotensin II type-1 receptor autoantibody-induced vascular inflammation using an animal model. The results revealed that miR-339-3p in exosomes promotes inflammatory responses by upregulating the NFATc3 protein. This demonstrates that miRNAs in exosomes can modulate inflammatory responses in VSMCs and contribute to vascular stenosis (Liu et al., 2023). Furthermore, studies related to carotid artery stenosis have shown that lncRNA PCA3 inhibits the proliferation, migration, and invasion of VSMCs by negatively regulating the miR-124-3p/ITGB1 axis, providing valuable therapeutic targets for vascular stenosis-related diseases (Yu et al., 2023). Many other miRNAs are involved in the proliferation, migration, and phenotypic switch (Table 3).
5 Clinical applications of exosomes in arteriovenous fistula stenosis
Exosomes have shown significant potential for the treatment of vascular stenosis-related diseases. Traditionally, stem cell therapies have operated primarily through paracrine mechanisms, leading to the development of cell-free stem cell therapies that focus on the application of exosomes. As mediators of intercellular communication, exosomes carry various bioactive molecules including proteins, lipids, and RNA. This capability enables them to play crucial roles in promoting tissue repair, regulating immune responses, and facilitating cellular signaling.
5.1 Diagnostic application
Exosomes, found in various body fluids, can reflect the state of their parent cells. Their significant advantages include the capability for rapid detection and noninvasive collection of body fluids. Notably, miRNAs within exosomes offer superior sensitivity and specificity compared to circulating miRNAs in blood, as they can be purified to ensure high sensitivity and specificity. A study utilizing microarray analysis compared miRNA expression profiles between stenotic AVF sites and control veins. The results showed that hsa-miR-214-3p, hsa-miR-374B-3p, and hsa-miR30e-3p were upregulated, whereas hsa-miR-4708-3p, hsa-miR-371b-5p, and hsa-miR-3960 were downregulated in primary AVF (Li et al., 2023). These findings provide valuable guidance for the use of exosomes as biomarkers to detect AVF stenosis. Zhao et al. induced VSMC proliferation using angiotensin II and observed that the expressions of KLF5 and cyclin D1 also increased with increased VSMC proliferation, along with miR-146a upregulation (Li and Chen, 2023). They used salvianolic acid B to downregulate miR-146a expression, thereby inhibiting neointimal hyperplasia. miR-146a has been proposed as a biomarker for inflammation and fibrosis (Lv et al., 2016). A study on patients with periodontitis revealed that serum exosomal miR-146a levels were significantly elevated and exhibited a positive correlation with inflammatory factor levels and disease severity. Inflammation is critical in acute coronary syndrome. Serum miR-146a levels are significantly higher in patients with this condition than in healthy controls. miR-21 is also crucial for vascular intimal stenosis. Overexpression studies have shown that miR-21 downregulation reduces intimal formation in rat carotid arteries after angioplasty. miR-21 can inhibit VSMC proliferation and promote apoptosis. Clinically, samples from stenotic AVF sites show high miR-21 expression, which is predominantly localized in the intimal region where fibroblasts are situated (Zhao et al., 2019). Exosomes containing miR-155 induce EC inflammation, leading to endothelial dysfunction. They increase the production of IL-6 and TNF-α and exert their effects through SHIP1 and SOCS1 (Liao et al., 2023).
High-mobility group box 1 (HMGB1) present in exosomes, and they play a pivotal role in neointimal hyperplasia. HMGB1 promotes inflammatory responses through damage-associated molecular patterns and activates immune cells, leading to the generation of IL-1β (Kilari et al., 2019). HMGB1 and IL-1β together can upregulate MMP-1, MMP-3, and MMP-9 expression, which exacerbate vascular wall degradation and remodeling (Jiang K. et al., 2019). Following vascular injury, VSMCs migrate from the intima to the site of injury. During this process, HMGB1 interacts with receptors for advanced glycation end products and TLRs, thereby promoting VSMC proliferation and migration (Yang and Tracey, 2010). This nuclear protein, which circulates via EVs, can be used as a biomarker, offering significant insights into vascular pathology.
5.2 Therapeutic applications
Currently, many nanometer-scale particles are used as platforms for the delivery of therapeutic drugs. However, artificially prepared carriers often face issues such as immune rejection and inability to penetrate specific biological barriers. In contrast, exosomes exhibit excellent biocompatibility and possess membrane structures on their surfaces, making them ideal drug carriers. In addition, proteins, nucleic acids, and other drug molecules encapsulated within exosomes demonstrate greater stability. Exosomes can be artificially engineered to encapsulate various types of drugs and are designed with surface antigens that target specific cells or tissues. Common antiproliferative drugs, such as paclitaxel, can be delivered in a targeted manner using exosomes. Exosomes contain a plethora of proangiogenic and antiinflammatory factors that promote angiogenesis and mitigate inflammatory responses following vascular injury. Exosome-releasing stents have been developed to gradually release exosomes fixed to the stent surface, thereby promoting EC regeneration and vascular wall repair. These stents significantly enhance EC migration and proliferation while reducing smooth muscle cell proliferation in animal models. This technology effectively reduces the restenosis rate after stent implantation and accelerates vascular healing (Wang and Khalil, 2018). Researchers have initiated mesenchymal stem cells release with paclitaxel, reporting that the released microvesicles exhibit strong antiproliferative activity (Wang and Khalil, 2018; Inoue et al., 2007).
The therapeutic applications of exosomes encapsulating miRNAs, lncRNAs, and circRNAs have been extensively studied. VSMC-derived exosomes are rich in miR-145, which regulates VSMC function by inhibiting autophagy, thereby reducing VSMC proliferation and migration (Hu et al., 2021). Animal studies on miR-145 transduction have demonstrated its role in phenotypic modulation, converting proliferative VSMCs into a contractile state. This conversion alleviates neointimal hyperplasia and has shown promising results in the treatment of venous graft diseases (Wang et al., 2020a). Zhao et al. loaded an miR-145-5p agomir (miR-145) into injectable and in situ self-assembling RAD hydrogels, allowing for the slow and controlled release of miR-145. This method reduced smooth muscle cell migration, promoted the contractile phenotype, and facilitated EC regeneration. Studies using rat vascular injury models revealed that miR-451 upregulation improved intimal thickening by mitigating VSMC damage caused by PDGF-BB and inhibited VSMC proliferation and migration (Nishio et al., 2020). Researchers using the Shexiang Baoxin pill to influence miR-451 expression and regulate PDGF-BB found that miR-451 can also regulate VSMC phenotypic transformation and promote apoptosis to reduce neointimal hyperplasia after vascular injury (Zhang W. et al., 2019). In another study using a mouse tissue-engineered vascular graft model, miR-451 expression was found to be downregulated in graft stenosis (Li et al., 2022). miR-451 downregulation leads to acute proliferation of macrophages and VSMCs, highlighting a direct regulatory relationship between miR-451 and macrophage migration inhibitory factor, suggesting miR-451 as a potential therapeutic target for intimal stenosis. Exosomes derived from hucMSCs are rich in miR-148a, which targets the 3′untranslated region to inhibit SERPINE1, reducing VSMC phenotypic transformation and migration (Hibino et al., 2016). SERPINE1, also known as plasminogen activator inhibitor-1, is a critical factor contributing to IH.
LncRNAs have emerged as significant targets for intervention in neointimal hyperplasia. Silencing lncRNA-p21 induces proliferation while inhibiting the apoptosis of VSMCs and macrophages (Zhang X. et al., 2022). Liang et al. found that the lncRNA Xist functions in VSMCs by interacting with miR-29b-3p and inducing arterial smooth muscle cell apoptosis via the miR-29b-3p/Eln pathway (Wu et al., 2014). Song et al. demonstrated that overexpression of lncRNA SENCR regulates the miR206/myocardin axis, thereby inhibiting VSMC proliferation, migration, and phenotypic transformation (Liang et al., 2021). Cheng et al. identified that the lncRNA LLNC00281 negatively regulates ANXA2 and inhibits VSMC proliferation, migration, and dedifferentiation through the NF-κBp65 signaling pathway (Song et al., 2022). Another study found that the lncRNA RP11-531A24.3 also negatively regulates ANXA2 (Verma Nagendra and Arora, 2025). Similarly, circRNAs have been implicated in IH and are considered potential therapeutic targets. Fu et al. found that circMAPK1 regulates VSMC proliferation and migration via the miR22-3p/MECP2 axis (Cheng et al., 2022). Lin et al. reported that circ_0021155 expression increases ox-LDL-induced VSMC proliferation and migration (Wu et al., 2021). This circRNA promotes TRPM7 expression while inhibiting the expression of α-smooth muscle actin and calmodulin, thereby enhancing smooth muscle cell proliferation and migration.
Exosomes influence neointimal hyperplasia by interfering with ferroptosis. Various miRNAs within exosomes regulate iron and ROS metabolism during ferroptosis, thereby affecting cellular redox states (Lin et al., 2022). Understanding the mechanisms by which miRNAs regulate ferroptosis is crucial for diagnosing and treating IH. Exosomes derived from human umbilical cord blood stem cells can inhibit ferroptosis by suppressing DMT1 expression via miR-23a-3p (Zong et al., 2022). Jia et al. found that exosomes from endothelial progenitor cells upregulate miR-30e-5p in HUVECs, activating the AMPK pathway. miR-30e-5p directly targets and inhibits SP1, thereby preventing EC ferroptosis (Song et al., 2021). Additionally, Li et al. demonstrated that exosomes from endothelial progenitor cells transfer miR-199a-3p to ECs, reducing ROS production and iron accumulation, ultimately inhibiting EC ferroptosis and death (Xia et al., 2022).
However, the use of exosomes as drug carriers has several limitations. First, exosomes derived from stem cells are limited by the manufacturing methods, making large-scale, high-yield production challenging (Li et al., 2021). Second, the loading capacity of exosomes is relatively low, and they are easily captured by non-target organs, thereby reducing their effective dosage. Thus, surface modification of exosomes is crucial to enhance their targeting and efficacy (Pascucci et al., 2014; Colao et al., 2018).
5.3 Challenges and limitations in clinical translation
Despite the promising roles of exosomes in regulating vascular remodeling, inflammation, and cell proliferation in AVF stenosis, several critical challenges hinder their clinical translation. First, the heterogeneity of exosome populations derived from different cell types and biofluids complicates standardization and quality control. Secondly, current isolation and purification techniques, such as ultracentrifugation and precipitation, often yield preparations with impurities or low reproducibility, limiting their scalability for clinical-grade production. Moreover, the targeting efficiency and in vivo biodistribution of exogenously administered exosomes remain suboptimal, raising concerns about their delivery to pathological sites. In addition, the mechanisms underlying exosome-mediated signaling are not fully elucidated, especially in the context of AVF-specific microenvironments. Regulatory hurdles, lack of large-scale clinical trials, and insufficient understanding of long-term safety further complicate the path to clinical application. Therefore, while exosomes hold great promise, their use in treating AVF stenosis remains at a preclinical stage and requires further technological, mechanistic, and translational advancements (Verma Nagendra and Arora, 2025).
6 Conclusion
Exosomes have demonstrated significant potential in the diagnosis and treatment of AVF stenosis. As noninvasive biomarkers, exosomes can be used to detect AVF stenosis at an early stage. They also regulate VSMC function by carrying specific miRNAs and proteins, thereby mitigating inflammatory responses and preventing stenosis progression. Furthermore, exosome-based therapeutic approaches offer an accurate targeted treatment strategy, potentially enhancing therapeutic efficacy and reducing adverse effects. This study reviewed the biogenesis, isolation, and identification of exosomes and their potential applications in AVF stenosis. Despite the growing body of evidence supporting the potential of exosomes in regulating AVF pathophysiology, significant challenges remain before their clinical translation. The lack of standardized isolation protocols, variability in exosomal cargo, and limited in vivo validation hinder progress toward therapeutic implementation. In our view, future research should prioritize the development of engineered exosomes with targeted delivery capabilities, integration with biomaterials such as hydrogels for sustained release, and validation using large-scale clinical cohorts. Furthermore, establishing diagnostic panels using exosome-derived biomarkers specific to AVF stenosis could significantly improve early detection and personalized treatment strategies. Addressing these challenges through multidisciplinary approaches will be crucial to unlocking the full clinical utility of exosomes in AVF management.
Author contributions
YC: Data curation, Formal Analysis, Investigation, Software, Validation, Writing – original draft, Writing – review and editing. FG: Data curation, Formal Analysis, Investigation, Software, Writing – original draft. DC: Data curation, Formal Analysis, Investigation, Software, Writing – original draft. LL: Data curation, Formal Analysis, Investigation, Supervision, Validation, Writing – original draft. XJ: Writing – original draft. WW: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review and editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the project of the National Natural Science Foundation, China (Grant no. 81970399). Basic Department of Jilin Provincial Science and Technology Department, China (Grant no. 20190901008JC). Jilin Province Health Science and Technology Capability Enhancement Project, China (Grant no. 2021JC043). Jilin Province Science and Technology Development Plan Project, China (Grant no. 212553HJ010288477).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Assmann, T. S., Recamonde-Mendoza, M., de Souza, B. M., Bauer, A. C., and Crispim, D. (2018). MicroRNAs and diabetic kidney disease: systematic review and bioinformatic analysis. Mol. Cell Endocrinol. 477, 90–102. doi:10.1016/j.mce.2018.06.005
Bai, B., Yang, Y., Wang, Q., Li, M., Tian, C., Liu, Y., et al. (2020a). NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 11 (9), 776. doi:10.1038/s41419-020-02985-x
Bai, S., Yin, Q., Dong, T., Dai, F., Qin, Y., Ye, L., et al. (2020b). Endothelial progenitor cell-derived exosomes ameliorate endothelial dysfunction in a mouse model of diabetes. Biomed. Pharmacother. 131, 110756. doi:10.1016/j.biopha.2020.110756
Bhatnagar, S., and Schorey, J. S. (2007). Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory. J. Biol. Chem. 282 (35), 25779–25789. doi:10.1074/jbc.M702277200
Blanco, R., and Gerhardt, H. (2013). VEGF and notch in tip and stalk cell selection. Cold Spring Harb. Perspect. Med. 3 (1), a006569. doi:10.1101/cshperspect.a006569
Carracedo, M., Artiach, G., Arnardottir, H., and Bäck, M. (2019). The resolution of inflammation through omega-3 fatty acids in atherosclerosis, intimal hyperplasia, and vascular calcification. Semin. Immunopathol. 41 (6), 757–766. doi:10.1007/s00281-019-00767-y
Cerezo-Magana, M., Christianson, H. C., van Kuppevelt, T. H., Forsberg-Nilsson, K., and Belting, M. (2021). Hypoxic induction of exosome uptake through proteoglycan-dependent endocytosis fuels the lipid droplet phenotype in Glioma. Mol. Cancer Res. 19 (3), 528–540. doi:10.1158/1541-7786.MCR-20-0560
Chakraborty, R., Chatterjee, P., Dave, J. M., Ostriker, A. C., Greif, D. M., Rzucidlo, E. M., et al. (2021). Targeting smooth muscle cell phenotypic switching in vascular disease. JVS Vasc. Sci. 2, 79–94. doi:10.1016/j.jvssci.2021.04.001
Charo, I. F., and Taubman, M. B. (2004). Chemokines in the pathogenesis of vascular disease. Circ. Res. 95 (9), 858–866. doi:10.1161/01.RES.0000146672.10582.17
Chen, X. L., Nam, J. O., Jean, C., Lawson, C., Walsh, C. T., Goka, E., et al. (2012). VEGF-induced vascular permeability is mediated by FAK. Dev. Cell 22 (1), 146–157. doi:10.1016/j.devcel.2011.11.002
Chen, L., Zheng, S. Y., Yang, C. Q., Ma, B. M., and Jiang, D. (2019). MiR-155-5p inhibits the proliferation and migration of VSMCs and HUVECs in atherosclerosis by targeting AKT1. Eur. Rev. Med. Pharmacol. Sci. 23 (5), 2223–2233. doi:10.26355/eurrev_201903_17270
Chen, X., Xu, J., Bao, W., Li, H., Wu, W., and Liu, J. (2022). Endothelial Foxp1 regulates Neointimal Hyperplasia via matrix Metalloproteinase-9/Cyclin dependent kinase inhibitor 1B signal pathway. J. Am. Heart Assoc. 11 (15), e026378. doi:10.1161/JAHA.122.026378
Chen, Y., Fang, Z. M., Yi, X., Wei, X., and Jiang, D. S. (2023). The interaction between ferroptosis and inflammatory signaling pathways. Cell Death Dis. 14 (3), 205. doi:10.1038/s41419-023-05716-0
Chen, S., Gao, J. J., Liu, Y. J., Mo, Z. W., Wu, F. Y., Hu, Z. J., et al. (2024). The oxidized phospholipid PGPC impairs endothelial function by promoting endothelial cell ferroptosis via FABP3. J. Lipid Res. 65 (2), 100499. doi:10.1016/j.jlr.2024.100499
Cheng, L., Wang, H., Maboh, R., Mao, G., Wu, X., and Chen, H. (2022). LncRNA LINC00281/Annexin A2 regulates vascular smooth muscle cell phenotype switching via the nuclear Factor-Kappa B signaling pathway. J. Cardiovasc. Transl. Res. 15 (5), 971–984. doi:10.1007/s12265-022-10242-z
Cheng, W. X., Wei, S. B., Zhou, Y., Shao, Y., and Li, M. Y. (2023). Exosomes: potential diagnostic markers and drug carriers for adenomyosis. Front. Pharmacol. 14, 1216149. doi:10.3389/fphar.2023.1216149
Chernomordik, L. V., and Kozlov, M. M. (2008). Mechanics of membrane fusion. Nat. Struct. Mol. Biol. 15 (7), 675–683. doi:10.1038/nsmb.1455
Cirino, G., Vellecco, V., and Bucci, M. (2017). Nitric oxide and hydrogen sulfide: the gasotransmitter paradigm of the vascular system. Br. J. Pharmacol. 174 (22), 4021–4031. doi:10.1111/bph.13815
Colao, I. L., Corteling, R., Bracewell, D., and Wall, I. (2018). Manufacturing exosomes: a promising therapeutic platform. Trends Mol. Med. 24 (3), 242–256. doi:10.1016/j.molmed.2018.01.006
Cui, H., Seubert, B., Stahl, E., Dietz, H., Reuning, U., Moreno-Leon, L., et al. (2015). Tissue inhibitor of metalloproteinases-1 induces a pro-tumourigenic increase of miR-210 in lung adenocarcinoma cells and their exosomes. Oncogene 34 (28), 3640–3650. doi:10.1038/onc.2014.300
Dalal, P. J., Muller, W. A., and Sullivan, D. P. (2020). Endothelial cell calcium signaling during barrier function and inflammation. Am. J. Pathol. 190 (3), 535–542. doi:10.1016/j.ajpath.2019.11.004
de Jong, R., Ewing, M. M., de Vries, M. R., Karper, J. C., Bastiaansen, A. J. N. M., Peters, H. A. B., et al. (2017). The epigenetic factor PCAF regulates vascular inflammation and is essential for intimal hyperplasia development. PLoS One 12 (10), e0185820. doi:10.1371/journal.pone.0185820
Ding, C., Ding, X., Zheng, J., Wang, B., Li, Y., Xiang, H., et al. (2020). miR-182-5p and miR-378a-3p regulate ferroptosis in I/R-induced renal injury. Cell Death Dis. 11 (10), 929. doi:10.1038/s41419-020-03135-z
Dorninger, F., Forss-Petter, S., and Berger, J. (2017). From peroxisomal disorders to common neurodegenerative diseases - the role of ether phospholipids in the nervous system. FEBS Lett. 591 (18), 2761–2788. doi:10.1002/1873-3468.12788
Fabbri, M., Paone, A., Calore, F., Galli, R., Gaudio, E., Santhanam, R., et al. (2012). MicroRNAs bind to toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. U. S. A. 109 (31), E2110–E2116. doi:10.1073/pnas.1209414109
Fan, H., Lv, Z., Gan, L., Ning, C., Li, Z., and Yang, M. (2019). A novel lncRNA regulates the toll-like receptor signaling pathway and related immune Function by Stabilizing FOS mRNA as a competitive endogenous RNA. Front. Immunol. 10, 838. doi:10.3389/fimmu.2019.00838
Galardi, A., De Bethlen, A., Di Paolo, V., Lampis, S., Mastronuzzi, A., and Di Giannatale, A. (2023). Recent advancements on the use of exosomes as drug carriers for the treatment of glioblastoma. Life (Basel) 13 (4), 964. doi:10.3390/life13040964
Gelibter, S., Marostica, G., Mandelli, A., Siciliani, S., Podini, P., Finardi, A., et al. (2022). The impact of storage on extracellular vesicles: a systematic study. J. Extracell. Vesicles 11 (2), e12162. doi:10.1002/jev2.12162
Giannotta, M., Trani, M., and Dejana, E. (2013). VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev. Cell 26 (5), 441–454. doi:10.1016/j.devcel.2013.08.020
Gong, Z., Wen, M., Zhang, W., Yu, L., Huang, C., Xu, Y., et al. (2023). Plasma exosomes induce inflammatory immune response in patients with acute myocardial infarction. Arch. Physiol. Biochem. 129 (5), 1168–1176. doi:10.1080/13813455.2021.1912102
Gorgens, A., Corso, G., Hagey, D. W., Jawad Wiklander, R., Gustafsson, M. O., Felldin, U., et al. (2022). Identification of storage conditions stabilizing extracellular vesicles preparations. J. Extracell. Vesicles 11 (6), e12238. doi:10.1002/jev2.12238
Gu, J., Zhang, H., Ji, B., Jiang, H., Zhao, T., Jiang, R., et al. (2017). Vesicle miR-195 derived from endothelial cells inhibits expression of serotonin transporter in vessel smooth muscle cells. Sci. Rep. 7, 43546. doi:10.1038/srep43546
Guo, M., and Zhang, J. (2023). Metabolomic analysis of bone-derived exosomes in osteonecrosis of the femoral head based on UPLC-MS/MS. Metabolomics 19 (4), 34. doi:10.1007/s11306-023-01986-z
Guo, Y., Zhang, S., Wang, D., Heng, B. C., and Deng, X. (2024). Role of cell rearrangement and related signaling pathways in the dynamic process of tip cell selection. Cell Commun. Signal 22 (1), 24. doi:10.1186/s12964-023-01364-1
Han, J. H., Park, H. S., Lee, D. H., Jo, J. H., Heo, K. S., and Myung, C. S. (2021). Regulation of autophagy by controlling Erk1/2 and mTOR for platelet-derived growth factor-BB-mediated vascular smooth muscle cell phenotype shift. Life Sci. 267, 118978. doi:10.1016/j.lfs.2020.118978
Haque, S., Vaiselbuh, S. R., Acharyya, R., Mukherjee, S., Kathirvel, M., and Patra, C. R. (2021). Biosynthesized silver nanoparticles for cancer therapy and in vivo bioimaging. Cancers (Basel) 13 (6), 6114. doi:10.3390/cancers13236114
Hazrati, A., Soudi, S., Malekpour, K., Mahmoudi, M., Rahimi, A., Hashemi, S. M., et al. (2022). Immune cells-derived exosomes function as a double-edged sword: role in disease progression and their therapeutic applications. Biomark. Res. 10 (1), 30. doi:10.1186/s40364-022-00374-4
He, C., Zheng, S., Luo, Y., and Wang, B. (2018). Exosome theranostics: biology and translational medicine. Theranostics 8 (1), 237–255. doi:10.7150/thno.21945
He, L., Zhang, C. L., Chen, Q., Wang, L., and Huang, Y. (2022). Endothelial shear stress signal transduction and atherogenesis: from mechanisms to therapeutics. Pharmacol. Ther. 235, 108152. doi:10.1016/j.pharmthera.2022.108152
He, Y., Wang, X., Chen, S., Luo, H., Huo, B., Guo, X., et al. (2024). SP2509 functions as a novel ferroptosis inhibitor by reducing intracellular iron level in vascular smooth muscle cells. Free Radic. Biol. Med. 219, 49–63. doi:10.1016/j.freeradbiomed.2024.04.220
Hibino, N., Best, C. A., Engle, A., Ghimbovschi, S., Knoblach, S., Nath, D. S., et al. (2016). Novel Association of miR-451 with the incidence of TEVG stenosis in a murine model. Tissue Eng. Part A 22 (1-2), 75–82. doi:10.1089/ten.TEA.2014.0664
Higashi, Y. (2022). Roles of oxidative stress and inflammation in vascular endothelial dysfunction-related disease. Antioxidants (Basel) 11 (10), 1958. doi:10.3390/antiox11101958
Hou, J., Pan, Y., Zhu, D., Fan, Y., Feng, G., Wei, Y., et al. (2019). Targeted delivery of nitric oxide via a 'bump-and-hole'-based enzyme-prodrug pair. Nat. Chem. Biol. 15 (2), 151–160. doi:10.1038/s41589-018-0190-5
Hu, S., Li, Z., Shen, D., Zhu, D., Huang, K., Su, T., et al. (2021). Exosome-eluting stents for vascular healing after ischaemic injury. Nat. Biomed. Eng. 5 (10), 1174–1188. doi:10.1038/s41551-021-00705-0
Inoue, K., Kawahara, K. i., Biswas, K. K., Ando, K., Mitsudo, K., Nobuyoshi, M., et al. (2007). HMGB1 expression by activated vascular smooth muscle cells in advanced human atherosclerosis plaques. Cardiovasc. Pathol. 16 (3), 136–143. doi:10.1016/j.carpath.2006.11.006
Ishiy, C., Ormanji, M. S., Maquigussa, E., Ribeiro, R. S., da Silva Novaes, A., and Boim, M. A. (2020). Comparison of the effects of mesenchymal stem cells with their extracellular vesicles on the treatment of kidney damage induced by chronic renal artery stenosis. Stem Cells Int. 2020, 8814574. doi:10.1155/2020/8814574
Jain, M., Dhanesha, N., Doddapattar, P., Chorawala, M. R., Nayak, M. K., Cornelissen, A., et al. (2020). Smooth muscle cell-specific fibronectin-EDA mediates phenotypic switching and neointimal hyperplasia. J. Clin. Invest 130 (1), 295–314. doi:10.1172/JCI124708
Jansen, F., Yang, X., Hoyer, F. F., Paul, K., Heiermann, N., Becher, M. U., et al. (2012). Endothelial microparticle uptake in target cells is annexin I/phosphatidylserine receptor dependent and prevents apoptosis. Arterioscler. Thromb. Vasc. Biol. 32 (8), 1925–1935. doi:10.1161/ATVBAHA.112.253229
Jeyaram, A., and Jay, S. M. (2017). Preservation and storage stability of extracellular vesicles for therapeutic applications. AAPS J. 20 (1), 1. doi:10.1208/s12248-017-0160-y
Jiang, N., Pan, J., Fang, S., Zhou, C., Han, Y., Chen, J., et al. (2019a). Liquid biopsy: circulating exosomal long noncoding RNAs in cancer. Clin. Chim. Acta 495, 331–337. doi:10.1016/j.cca.2019.04.082
Jiang, K., Yang, J., Guo, S., Zhao, G., Wu, H., and Deng, G. (2019b). Peripheral circulating exosome-mediated delivery of miR-155 as a novel mechanism for acute lung inflammation. Mol. Ther. 27 (10), 1758–1771. doi:10.1016/j.ymthe.2019.07.003
Jin, L., Yu, X., Zhou, X., Li, G., Li, W., He, Y., et al. (2024). The miR-182-5p/GPX4 pathway contributes to sevoflurane-induced ototoxicity via ferroptosis. Int. J. Mol. Sci. 25 (12), 6774. doi:10.3390/ijms25126774
Kalluri, R., and LeBleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. Science 367 (6478), eaau6977.
Kalluri, R., and LeBleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. Science 367 (6478), eaau6977. doi:10.1126/science.aau6977
Kilari, S., Cai, C., Zhao, C., Sharma, A., Chernogubova, E., Simeon, M., et al. (2019). The role of MicroRNA-21 in venous neointimal hyperplasia: implications for targeting miR-21 for VNH treatment. Mol. Ther. 27 (9), 1681–1693. doi:10.1016/j.ymthe.2019.06.011
Kim, K. M., Abdelmohsen, K., Mustapic, M., Kapogiannis, D., and Gorospe, M. (2017). RNA in extracellular vesicles. Wiley Interdiscip. Rev. RNA 8 (4), e1413. doi:10.1002/wrna.1413
Konoshenko, M. Y., Lekchnov, E. A., Vlassov, A. V., and Laktionov, P. P. (2018). Isolation of extracellular vesicles: general methodologies and latest trends. Biomed. Res. Int. 2018, 8545347. doi:10.1155/2018/8545347
Lai, H., Yip, H. C., Gong, Y., Chan, K. F., Leung, K. K. C., Chan, M. S., et al. (2024). MFGE8 in exosomes derived from mesenchymal stem cells prevents esophageal stricture after endoscopic submucosal dissection in pigs. J. Nanobiotechnology 22 (1), 143. doi:10.1186/s12951-024-02429-0
Lee, T., and Roy-Chaudhury, P. (2009). Advances and new frontiers in the pathophysiology of venous neointimal hyperplasia and dialysis access stenosis. Adv. Chronic Kidney Dis. 16 (5), 329–338. doi:10.1053/j.ackd.2009.06.009
Li, G., and Chen, Q. (2023). lncRNA PCA3 suppressed carotid artery stenosis and vascular smooth muscle cell function via negatively modulating the miR-124-3p/ITGB1 axis. Clin. Appl. Thromb. Hemost. 29, 10760296231190383. doi:10.1177/10760296231190383
Li, Y., Zheng, Q., Bao, C., Li, S., Guo, W., Zhao, J., et al. (2015). Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 25 (8), 981–984. doi:10.1038/cr.2015.82
Li, L., Mao, D., Li, C., and Li, M. (2018). miR-145-5p inhibits vascular smooth muscle cells (VSMCs) proliferation and migration by dysregulating the transforming growth Factor-b signaling Cascade. Med. Sci. Monit. 24, 4894–4904. doi:10.12659/MSM.910986
Li, X., Liu, S., Zhang, L., Issaian, A., Hill, R. C., Espinosa, S., et al. (2019). A unified mechanism for intron and exon definition and back-splicing. Nature 573 (7774), 375–380. doi:10.1038/s41586-019-1523-6
Li, L., Wang, H., Zhang, J., Chen, X., Zhang, Z., and Li, Q. (2021). Effect of endothelial progenitor cell-derived extracellular vesicles on endothelial cell ferroptosis and atherosclerotic vascular endothelial injury. Cell Death Discov. 7 (1), 235. doi:10.1038/s41420-021-00610-0
Li, Y. P., Qiang, T. T., Wang, K. Y., and Wang, X. L. (2022). Shexiang Baoxin pill regulates intimal hyperplasia, migration, and apoptosis after platelet-derived growth Factor-BB-Stimulation of vascular smooth muscle cells via miR-451. Chin. J. Integr. Med. 28 (9), 785–793. doi:10.1007/s11655-022-2891-6
Li, Y., Long, Y., Zhi, X., Hao, H., Wang, X., Liu, H., et al. (2023). miR-339-3p promotes AT1-AA-induced vascular inflammation by upregulating NFATc3 protein expression in vascular smooth muscle cells. Acta Biochim. Biophys. Sin. (Shanghai) 55 (2), 295–303. doi:10.3724/abbs.2023009
Li, Y., Xiao, Y., Shang, Y., Xu, C., Han, C., Hu, D., et al. (2024). Exosomes derived from adipose tissue-derived stem cells alleviated H(2)O(2)-induced oxidative stress and endothelial-to-mesenchymal transition in human umbilical vein endothelial cells by inhibition of the mir-486-3p/Sirt6/Smad signaling pathway. Cell Biol. Toxicol. 40 (1), 39. doi:10.1007/s10565-024-09881-6
Liang, K., Cui, M., Fu, X., Ma, J., Zhang, K., Zhang, D., et al. (2021). LncRNA Xist induces arterial smooth muscle cell apoptosis in thoracic aortic aneurysm through miR-29b-3p/Eln pathway. Biomed. Pharmacother. 137, 111163. doi:10.1016/j.biopha.2020.111163
Liao, Z., Zheng, R., and Shao, G. (2023). Mechanisms and application strategies of miRNA‑146a regulating inflammation and fibrosis at molecular and cellular levels (Review). Int. J. Mol. Med. 51 (1), 7. doi:10.3892/ijmm.2022.5210
Lin, J., Liu, C., Xu, J., Li, S., Dai, D., Zhang, L., et al. (2022). Circ_0021155 can participate in the phenotypic transformation of human vascular smooth muscle cells via the miR-4459/TRPM7 axis. Biochem. Biophys. Res. Commun. 630, 133–142. doi:10.1016/j.bbrc.2022.08.065
Liu, X., Cheng, Y., Zhang, S., Lin, Y., Yang, J., and Zhang, C. (2009). A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ. Res. 104 (4), 476–487. doi:10.1161/CIRCRESAHA.108.185363
Liu, K., Shi, H., Peng, Z., Wu, X., Li, W., and Lu, X. (2022). Exosomes from adipose mesenchymal stem cells overexpressing Stanniocalcin-1 promote reendothelialization after carotid endarterium mechanical injury. Stem Cell Rev. Rep. 18 (3), 1041–1053. doi:10.1007/s12015-021-10180-4
Liu, J., Zhang, X., Yu, Z., and Zhang, T. (2023). Exosomes promote atherosclerosis progression by regulating Circ_100696/miR-503-5p/PAPPA axis-mediated vascular smooth muscle cells proliferation and migration. Int. Heart J. 64 (5), 918–927. doi:10.1536/ihj.23-089
Lovren, F., and Verma, S. (2013). Evolving role of microparticles in the pathophysiology of endothelial dysfunction. Clin. Chem. 59 (8), 1166–1174. doi:10.1373/clinchem.2012.199711
Lu, Y. J., Jan, Y. J., Ko, B. S., Liang, S. M., Chen, L., Wu, C. C., et al. (2020). Expression of Nik-related kinase in smooth muscle cells attenuates vascular inflammation and intimal hyperplasia. Aging (Albany NY) 12 (8), 7511–7533. doi:10.18632/aging.103104
Lu, X., Kang, N., Ling, X., Pan, M., Du, W., and Gao, S. (2021). MiR-27a-3p promotes non-small cell lung cancer through SLC7A11-Mediated-Ferroptosis. Front. Oncol. 11, 759346. doi:10.3389/fonc.2021.759346
Ludwig, A. K., and Giebel, B. (2012). Exosomes: small vesicles participating in intercellular communication. Int. J. Biochem. Cell Biol. 44 (1), 11–15. doi:10.1016/j.biocel.2011.10.005
Luo, E. F., Li, H-X, Qin, Y-H, Qiao, Y., Yan, G-L, and Yao, Y-Y. (2021). Role of ferroptosis in the process of diabetes-induced endothelial dysfunction. World J. Diabetes 12 (2), 124–137. doi:10.4239/wjd.v12.i2.124
Lv, L., Huang, W., Zhang, J., Shi, Y., and Zhang, L. (2016). Altered microRNA expression in stenoses of native arteriovenous fistulas in hemodialysis patients. J. Vasc. Surg. 63 (4), 1034–1043. doi:10.1016/j.jvs.2014.10.099
Lynch, M., Barallobre-Barreiro, J., Jahangiri, M., and Mayr, M. (2016). Vascular proteomics in metabolic and cardiovascular diseases. J. Intern Med. 280 (4), 325–338. doi:10.1111/joim.12486
Lyu, C., Sun, H., Sun, Z., Liu, Y., and Wang, Q. (2024). Roles of exosomes in immunotherapy for solid cancers. Cell Death Dis. 15 (2), 106. doi:10.1038/s41419-024-06494-z
Machida, T., Tomofuji, T., Ekuni, D., Maruyama, T., Yoneda, T., Kawabata, Y., et al. (2015). MicroRNAs in salivary exosome as potential biomarkers of aging. Int. J. Mol. Sci. 16 (9), 21294–21309. doi:10.3390/ijms160921294
Mateescu, B., Kowal, E. J. K., van Balkom, B. W. M., Bartel, S., Bhattacharyya, S. N., Buzás, E. I., et al. (2017). Obstacles and opportunities in the functional analysis of extracellular vesicle RNA - an ISEV position paper. J. Extracell. Vesicles 6, 1286095. doi:10.1080/20013078.2017.1286095
Montecalvo, A., Larregina, A. T., Shufesky, W. J., Stolz, D. B., Sullivan, M. L. G., Karlsson, J. M., et al. (2012). Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 119 (3), 756–766. doi:10.1182/blood-2011-02-338004
Nascimbeni, A. C., Codogno, P., and Morel, E. (2017). Phosphatidylinositol-3-phosphate in the regulation of autophagy membrane dynamics. FEBS J. 284 (9), 1267–1278. doi:10.1111/febs.13987
Nishio, H., Minatoya, K., and Masumoto, H. (2020). A rabbit venous interposition model mimicking revascularization surgery using vein grafts to assess intimal hyperplasia under arterial blood pressure. J. Vis. Exp. (159). doi:10.3791/60931
Nojima, H., Freeman, C. M., Schuster, R. M., Japtok, L., Kleuser, B., Edwards, M. J., et al. (2016). Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J. Hepatol. 64 (1), 60–68. doi:10.1016/j.jhep.2015.07.030
Oas, R. G., Nanes, B. A., Esimai, C. C., Vincent, P. A., García, A. J., and Kowalczyk, A. P. (2013). p120-catenin and beta-catenin differentially regulate cadherin adhesive function. Mol. Biol. Cell 24 (6), 704–714. doi:10.1091/mbc.E12-06-0471
Pakharukova, M. Y., Savina, E., Ponomarev, D. V., Gubanova, N. V., Zaparina, O., Zakirova, E. G., et al. (2023). Proteomic characterization of Opisthorchis felineus exosome-like vesicles and their uptake by human cholangiocytes. J. Proteomics 283-284, 104927. doi:10.1016/j.jprot.2023.104927
Pallet, N., Sirois, I., Bell, C., Hanafi, L. A., Hamelin, K., Dieudé, M., et al. (2013). A comprehensive characterization of membrane vesicles released by autophagic human endothelial cells. Proteomics 13 (7), 1108–1120. doi:10.1002/pmic.201200531
Pascucci, L., Coccè, V., Bonomi, A., Ami, D., Ceccarelli, P., Ciusani, E., et al. (2014). Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J. Control Release 192, 262–270. doi:10.1016/j.jconrel.2014.07.042
Pegtel, D. M., and Gould, S. J. (2019). Exosomes. Annu. Rev. Biochem. 88, 487–514. doi:10.1146/annurev-biochem-013118-111902
Pegtel, D. M., Cosmopoulos, K., Thorley-Lawson, D. A., van Eijndhoven, M. A. J., Hopmans, E. S., Lindenberg, J. L., et al. (2010). Functional delivery of viral miRNAs via exosomes. Proc. Natl. Acad. Sci. U. S. A. 107 (14), 6328–6333. doi:10.1073/pnas.0914843107
Pfenniger, A., Meens, M. J., Pedrigi, R. M., Foglia, B., Sutter, E., Pelli, G., et al. (2015). Shear stress-induced atherosclerotic plaque composition in ApoE(-/-) mice is modulated by connexin37. Atherosclerosis 243 (1), 1–10. doi:10.1016/j.atherosclerosis.2015.08.029
Pluchino, S., and Smith, J. A. (2019). Explicating exosomes: reclassifying the rising stars of intercellular communication. Cell 177 (2), 225–227. doi:10.1016/j.cell.2019.03.020
Polo-Generelo, S., Torres, B., Guerrero-Martínez, J. A., Camafeita, E., Vázquez, J., Reyes, J. C., et al. (2022). TGF-beta-Upregulated Lnc-Nr6a1 acts as a reservoir of miR-181 and mediates assembly of a glycolytic complex. Noncoding RNA 8 (5), 62. doi:10.3390/ncrna8050062
Pulous, F. E., Grimsley-Myers, C. M., Kansal, S., Kowalczyk, A. P., and Petrich, B. G. (2019). Talin-Dependent integrin activation regulates VE-Cadherin localization and endothelial cell barrier function. Circ. Res. 124 (6), 891–903. doi:10.1161/CIRCRESAHA.118.314560
Qi, Z., Liu, R., Ju, H., Huang, M., Li, Z., Li, W., et al. (2023). microRNA-130b-3p Attenuates septic cardiomyopathy by regulating the AMPK/mTOR signaling pathways and directly targeting ACSL4 against ferroptosis. Int. J. Biol. Sci. 19 (13), 4223–4241. doi:10.7150/ijbs.82287
Qian, C., Dong, G., Yang, C., Zheng, W., Zhong, C., Shen, Q., et al. (2025). Broadening horizons: molecular mechanisms and disease implications of endothelial-to-mesenchymal transition. Cell Commun. Signal 23 (1), 16. doi:10.1186/s12964-025-02028-y
Qin, X., Zhang, J., Wang, B., Xu, G., Yang, X., Zou, Z., et al. (2021). Ferritinophagy is involved in the zinc oxide nanoparticles-induced ferroptosis of vascular endothelial cells. Autophagy 17 (12), 4266–4285. doi:10.1080/15548627.2021.1911016
Qu, Q., Pang, Y., Zhang, C., Liu, L., and Bi, Y. (2020). Exosomes derived from human umbilical cord mesenchymal stem cells inhibit vein graft intimal hyperplasia and accelerate reendothelialization by enhancing endothelial function. Stem Cell Res. Ther. 11 (1), 133. doi:10.1186/s13287-020-01639-1
Record, M., Carayon, K., Poirot, M., and Silvente-Poirot, S. (2014). Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim. Biophys. Acta 1841 (1), 108–120. doi:10.1016/j.bbalip.2013.10.004
Ren, J., Zhu, B., Gu, G., Zhang, W., Li, J., Wang, H., et al. (2023). Schwann cell-derived exosomes containing MFG-E8 modify macrophage/microglial polarization for attenuating inflammation via the SOCS3/STAT3 pathway after spinal cord injury. Cell Death Dis. 14 (1), 70. doi:10.1038/s41419-023-05607-4
Rennier, K., and Ji, J. Y. (2013). Effect of shear stress and substrate on endothelial DAPK expression, caspase activity, and apoptosis. BMC Res. Notes 6, 10. doi:10.1186/1756-0500-6-10
Robbins, P. D., and Morelli, A. E. (2014). Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 14 (3), 195–208. doi:10.1038/nri3622
Rosenzweig, R., Nillegoda, N. B., Mayer, M. P., and Bukau, B. (2019). The Hsp70 chaperone network. Nat. Rev. Mol. Cell Biol. 20 (11), 665–680. doi:10.1038/s41580-019-0133-3
Roy-Chaudhury, P., Kelly, B. S., Zhang, J., Narayana, A., Desai, P., Melham, M., et al. (2003). Hemodialysis vascular access dysfunction: from pathophysiology to novel therapies. Blood Purif. 21 (1), 99–110. doi:10.1159/000067863
Sardu, C., Modugno, P., Castellano, G., Scisciola, L., Barbieri, M., Petrella, L., et al. (2021). Atherosclerotic plaque fissuration and clinical outcomes in pre-diabetics vs. normoglycemics patients affected by asymptomatic significant carotid artery stenosis at 2 years of Follow-Up: role of microRNAs modulation: the ATIMIR Study. Biomedicines 9 (4), 401. doi:10.3390/biomedicines9040401
Shaito, A., Aramouni, K., Assaf, R., Parenti, A., Orekhov, A., Yazbi, A. E., et al. (2022). Oxidative stress-induced endothelial dysfunction in cardiovascular diseases. Front. Biosci. Landmark Ed. 27 (3), 105. doi:10.31083/j.fbl2703105
Shaw, A., Doherty, M. K., Mutch, N. J., MacRury, S. M., and Megson, I. L. (2014). Endothelial cell oxidative stress in diabetes: a key driver of cardiovascular complications Biochem. Soc. Trans. 42 (4), 928–933. doi:10.1042/BST20140113
Sishuai, S., Lingui, G., Pengtao, L., Xinjie, B., and Junji, W. (2024). Advances in regulating endothelial-mesenchymal transformation through exosomes. Stem Cell Res. Ther. 15 (1), 391. doi:10.1186/s13287-024-04010-w
Skog, J., Würdinger, T., van Rijn, S., Meijer, D. H., Gainche, L., Sena-Esteves, M., et al. (2008). Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10 (12), 1470–1476. doi:10.1038/ncb1800
Skotland, T., Hessvik, N. P., Sandvig, K., and Llorente, A. (2019). Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 60 (1), 9–18. doi:10.1194/jlr.R084343
Skotland, T., Sagini, K., Sandvig, K., and Llorente, A. (2020). An emerging focus on lipids in extracellular vesicles. Adv. Drug Deliv. Rev. 159, 308–321. doi:10.1016/j.addr.2020.03.002
Song, Y., Wang, B., Zhu, X., Hu, J., Sun, J., Xuan, J., et al. (2021). Human umbilical cord blood-derived MSCs exosome attenuate myocardial injury by inhibiting ferroptosis in acute myocardial infarction mice. Cell Biol. Toxicol. 37 (1), 51–64. doi:10.1007/s10565-020-09530-8
Song, Y., Wang, T., Mu, C., Gui, W., Deng, Y., and Ma, R. (2022). LncRNA SENCR overexpression attenuated the proliferation, migration and phenotypic switching of vascular smooth muscle cells in aortic dissection via the miR-206/myocardin axis. Nutr. Metab. Cardiovasc. Dis. 32 (6), 1560–1570. doi:10.1016/j.numecd.2022.03.004
Stenmark, H. (2009). Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 10 (8), 513–525. doi:10.1038/nrm2728
Sun, D. Y., Wu, W. B., Wu, J. J., Shi, Y., Xu, J. J., Ouyang, S. X., et al. (2024). Pro-ferroptotic signaling promotes arterial aging via vascular smooth muscle cell senescence. Nat. Commun. 15 (1), 1429. doi:10.1038/s41467-024-45823-w
Tan, W., Li, Y., Ma, L., Fu, X., Long, Q., Yan, F., et al. (2024). Exosomes of endothelial progenitor cells repair injured vascular endothelial cells through the Bcl2/Bax/Caspase-3 pathway. Sci. Rep. 14 (1), 4465. doi:10.1038/s41598-024-55100-x
Tang, X., Deng, Z., Ding, P., Qiang, W., Lu, Y., Gao, S., et al. (2022). A novel protein encoded by circHNRNPU promotes multiple myeloma progression by regulating the bone marrow microenvironment and alternative splicing. J. Exp. Clin. Cancer Res. 41 (1), 85. doi:10.1186/s13046-022-02276-7
Tauro, B. J., Greening, D. W., Mathias, R. A., Mathivanan, S., Ji, H., and Simpson, R. J. (2013). Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol. Cell Proteomics 12 (3), 587–598. doi:10.1074/mcp.M112.021303
Thery, C., Witwer, K. W., Aikawa, E., Alcaraz, M. J., Anderson, J. D., Andriantsitohaina, R., et al. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7 (1), 1535750. doi:10.1080/20013078.2018.1535750
Tietje, A., Maron, K. N., Wei, Y., and Feliciano, D. M. (2014). Cerebrospinal fluid extracellular vesicles undergo age dependent declines and contain known and novel non-coding RNAs. PLoS One 9 (11), e113116. doi:10.1371/journal.pone.0113116
Togliatto, G., Dentelli, P., Rosso, A., Lombardo, G., Gili, M., Gallo, S., et al. (2018). PDGF-BB carried by endothelial cell-derived extracellular vesicles reduces vascular smooth muscle cell apoptosis in diabetes. Diabetes 67 (4), 704–716. doi:10.2337/db17-0371
Valadi, H., Ekström, K., Bossios, A., Sjöstrand, M., Lee, J. J., and Lötvall, J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9 (6), 654–659. doi:10.1038/ncb1596
van Niel, G., D'Angelo, G., and Raposo, G. (2018). Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19 (4), 213–228. doi:10.1038/nrm.2017.125
Vega, V. L., Rodríguez-Silva, M., Frey, T., Gehrmann, M., Diaz, J. C., Steinem, C., et al. (2008). Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J. Immunol. 180 (6), 4299–4307. doi:10.4049/jimmunol.180.6.4299
Verma Nagendra, S. A., and Arora, S. (2025). Navigating the global regulatory landscape for exosome-based therapeutics: challenges, strategies, and future directions. Pharmaceutics 8 (17), 990. doi:10.3390/pharmaceutics17080990
Villarroya-Beltri, C., Gutiérrez-Vázquez, C., Sánchez-Cabo, F., Pérez-Hernández, D., Vázquez, J., Martin-Cofreces, N., et al. (2013). Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 4, 2980. doi:10.1038/ncomms3980
Wang, X., and Khalil, R. A. (2018). Matrix metalloproteinases, vascular remodeling, and vascular disease. Adv. Pharmacol. 81, 241–330. doi:10.1016/bs.apha.2017.08.002
Wang, C., Qin, L., Manes, T. D., Kirkiles-Smith, N. C., Tellides, G., and Pober, J. S. (2014). Rapamycin antagonizes TNF induction of VCAM-1 on endothelial cells by inhibiting mTORC2. J. Exp. Med. 211 (3), 395–404. doi:10.1084/jem.20131125
Wang, Y., Liu, J., Ma, J., Sun, T., Zhou, Q., Wang, W., et al. (2019). Exosomal circRNAs: biogenesis, effect and application in human diseases. Mol. Cancer 18 (1), 116. doi:10.1186/s12943-019-1041-z
Wang, J., Chen, S., and Bihl, J. (2020a). Exosome-Mediated transfer of ACE2 (Angiotensin-Converting enzyme 2) from endothelial progenitor cells promotes survival and function of endothelial cell. Oxid. Med. Cell Longev. 2020, 4213541. doi:10.1155/2020/4213541
Wang, W., Chen, L., Shang, C., Jin, Z., Yao, F., Bai, L., et al. (2020b). miR-145 inhibits the proliferation and migration of vascular smooth muscle cells by regulating autophagy. J. Cell Mol. Med. 24 (12), 6658–6669. doi:10.1111/jcmm.15316
Wettschureck, N., Strilic, B., and Offermanns, S. (2019). Passing the vascular barrier: endothelial signaling processes controlling extravasation. Physiol. Rev. 99 (3), 1467–1525. doi:10.1152/physrev.00037.2018
Wu, W., and He, J. (2023). Unveiling the functional paradigm of exosome-derived long non-coding RNAs (lncRNAs) in cancer: based on a narrative review and systematic review. J. Cancer Res. Clin. Oncol. 149 (16), 15219–15247. doi:10.1007/s00432-023-05273-1
Wu, G., Cai, J., Han, Y., Chen, J., Huang, Z-P, and Chen, C. (2014). LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation 130 (17), 1452–1465. doi:10.1161/CIRCULATIONAHA.114.011675
Wu, Y., Cai, F., Lu, Y., Hu, Y., and Wang, Q. (2021). lncRNA RP11-531A24.3 inhibits the migration and proliferation of vascular smooth muscle cells by downregulating ANXA2 expression. Exp. Ther. Med. 22 (6), 1439. doi:10.3892/etm.2021.10874
Xia, J., Song, X., Meng, J., and Lou, D. (2022). Endothelial progenitor cells-derived exosomes transfer microRNA-30e-5p to regulate Erastin-induced ferroptosis in human umbilical vein endothelial cells via the specificity protein 1/adenosine monophosphate-activated protein kinase axis. Bioengineered 13 (2), 3566–3580. doi:10.1080/21655979.2022.2025519
Xing, Z., Zhao, C., Liu, H., and Fan, Y. (2020). Endothelial Progenitor cell-derived extracellular vesicles: a novel Candidate for regenerative medicine and disease treatment. Adv. Healthc. Mater 9 (12), e2000255. doi:10.1002/adhm.202000255
Xu, X., Zhang, D. D., Kong, P., Gao, Y. K., Huang, X. F., Song, Y., et al. (2023). Sox10 escalates vascular inflammation by mediating vascular smooth muscle cell transdifferentiation and pyroptosis in neointimal hyperplasia. Cell Rep. 42 (8), 112869. doi:10.1016/j.celrep.2023.112869
Yanez-Mo, M., Siljander, P. R. M., Andreu, Z., Zavec, A. B., Borràs, F. E., Buzas, E. I., et al. (2015). Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 4, 27066. doi:10.3402/jev.v4.27066
Yang, H., and Tracey, K. J. (2010). Targeting HMGB1 in inflammation. Biochim. Biophys. Acta 1799 (1-2), 149–156. doi:10.1016/j.bbagrm.2009.11.19
Yang, Q., Wan, Q., Zhang, L., Li, Y., Zhang, P., Li, D., et al. (2018). Analysis of LncRNA expression in cell differentiation. RNA Biol. 15 (3), 413–422. doi:10.1080/15476286.2018.14415
Yang, J., Zhou, X., Lu, J., and Li, M. (2022). miR-146-5p restrains calcification of vascular smooth muscle cells by suppressing TRAF6. Open Med. (Wars) 17 (1), 1515–1527. doi:10.1515/med-2022-0471
Yang, S. Y., Deng, W. W., Zhao, R. Z., Long, X. P., Wang, D. M., Guo, H. H., et al. (2023). Exosomes derived from endothelial cells inhibit neointimal Hyperplasia induced by carotid artery injury in rats via ROS-NLRP3 inflammasome pathway. Bull. Exp. Biol. Med. 174 (6), 762–767. doi:10.1007/s10517-023-05788-0
Yao, J., Cai, L., Chen, Y., Zhang, J., Zhuang, W., Liang, J., et al. (2022). Exosomes: mediators regulating the phenotypic transition of vascular smooth muscle cells in atherosclerosis. Cell Commun. Signal 20 (1), 153. doi:10.1186/s12964-022-00949-6
Ye, B., Wu, Z. H., Tsui, T. Y., Zhang, B. F., Su, X., Qiu, Y. H., et al. (2020). lncRNA KCNQ1OT1 suppresses the inflammation and proliferation of vascular smooth muscle cells through IκBa in intimal hyperplasia. Mol. Ther. Nucleic Acids 20, 62–72. doi:10.1016/j.omtn.2020.01.032
Yoshida, T., Yamashita, M., and Hayashi, M. (2012). Kruppel-like factor 4 contributes to high phosphate-induced phenotypic switching of vascular smooth muscle cells into osteogenic cells. J. Biol. Chem. 287 (31), 25706–25714. doi:10.1074/jbc.M112.361360
Yu, T., Wang, Y., Fan, Y., Fang, N., Wang, T., Xu, T., et al. (2019). CircRNAs in cancer metabolism: a review. J. Hematol. Oncol. 12 (1), 90. doi:10.1186/s13045-019-0776-8
Yu, H., Douglas, H. F., Wathieu, D., Braun, R. A., Edomwande, C., Lightell, D. J., et al. (2023). Diabetes is accompanied by secretion of pro-atherosclerotic exosomes from vascular smooth muscle cells. Cardiovasc. Diabetol. 22 (1), 112. doi:10.1186/s12933-023-01833-4
Yuan, F., Li, Y. M., and Wang, Z. (2021). Preserving extracellular vesicles for biomedical applications: consideration of storage stability before and after isolation. Drug Deliv. 28 (1), 1501–1509. doi:10.1080/10717544.2021.1951896
Zhan, Z., Ye, M., and Jin, X. (2023). The roles of FLOT1 in human diseases (Review). Mol. Med. Rep. 28 (5), 212. doi:10.3892/mmr.2023.13099
Zhang, J., Guan, J., Niu, X., Hu, G., Guo, S., Li, Q., et al. (2015). Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J. Transl. Med. 13, 49. doi:10.1186/s12967-015-0417-0
Zhang, P., He, M., and Zeng, Y. (2016). Ultrasensitive microfluidic analysis of circulating exosomes using a nanostructured graphene oxide/polydopamine coating. Lab. Chip 16 (16), 3033–3042. doi:10.1039/c6lc00279j
Zhang, K., Wu, L., Zhang, P., Luo, M., Du, J., Gao, T., et al. (2018). miR-9 regulates ferroptosis by targeting glutamic-oxaloacetic transaminase GOT1 in melanoma. Mol. Carcinog. 57 (11), 1566–1576. doi:10.1002/mc.22878
Zhang, Y., Chen, Y., Li, P. L., and Li, X. (2019a). Contribution of cathepsin B-dependent Nlrp3 inflammasome activation to nicotine-induced endothelial barrier dysfunction. Eur. J. Pharmacol. 865, 172795. doi:10.1016/j.ejphar.2019.172795
Zhang, W., Liu, D., Han, X., Ren, J., Zhou, P., and Ding, P. (2019b). MicroRNA-451 inhibits vascular smooth muscle cell migration and intimal hyperplasia after vascular injury via Ywhaz/p38 MAPK pathway. Exp. Cell Res. 379 (2), 214–224. doi:10.1016/j.yexcr.2019.03.033
Zhang, J. J., Du, J., Kong, N., Zhang, G. Y., Liu, M. Z., and Liu, C. (2021). Mechanisms and pharmacological applications of ferroptosis: a narrative review. Ann. Transl. Med. 9 (19), 1503. doi:10.21037/atm-21-1595
Zhang, L., Li, W., Shi, B., Zhang, X., and Gong, K. (2022a). Expression profiles and functions of ferroptosis-related genes in intimal hyperplasia induced by carotid artery ligation in mice. Front. Genet. 13, 964458. doi:10.3389/fgene.2022.964458
Zhang, S., Bei, Y., Huang, Y., Huang, Y., Hou, L., Zheng, X. L., et al. (2022b). Induction of ferroptosis promotes vascular smooth muscle cell phenotypic switching and aggravates neointimal hyperplasia in mice. Mol. Med. 28 (1), 121. doi:10.1186/s10020-022-00549-7
Zhang, X., Zhou, Y., Ye, Y., Wu, R., Li, W., Yao, C., et al. (2022c). Human umbilical cord mesenchymal stem cell-derived exosomal microRNA-148a-3p inhibits neointimal hyperplasia by targeting Serpine1. Arch. Biochem. Biophys. 719, 109155. doi:10.1016/j.abb.2022.109155
Zhang, Y., Yang, Y., Chen, W., Mi, C., Xu, X., and Shen, Y. (2023). BaP/BPDE suppressed endothelial cell angiogenesis to induce miscarriage by promoting MARCHF1/GPX4-mediated ferroptosis. Environ. Int. 180, 108237. doi:10.1016/j.envint.2023.108237
Zhang, G., Song, C., Fan, S., Yin, M., Wang, X., Zhang, Y., et al. (2024). LncSEA 2.0: an updated platform for long non-coding RNA related sets and enrichment analysis. Nucleic Acids Res. 52 (D1), D919–D928. doi:10.1093/nar/gkad1008
Zhao, X. S., Zheng, B., Wen, Y., Sun, Y., Wen, J. K., and Zhang, X. H. (2019). Salvianolic acid B inhibits Ang II-induced VSMC proliferation in vitro and intimal hyperplasia in vivo by downregulating miR-146a expression. Phytomedicine 58, 152754. doi:10.1016/j.phymed.2018.11.014
Zheng, C., Azcutia, V., Aikawa, E., Figueiredo, J. L., Croce, K., Sonoki, H., et al. (2013). Statins suppress apolipoprotein CIII-induced vascular endothelial cell activation and monocyte adhesion. Eur. Heart J. 34 (8), 615–624. doi:10.1093/eurheartj/ehs271
Zhenhua Li, D. Z. Q. H., Zhu, D., Hui, Q., Bi, J., Yu, B., Huang, Z., et al. (2021). Injection of ROS-Responsive hydrogel loaded with basic fibroblast growth factor into the pericardial cavity for heart repair. Adv. Funct. Mater. 31 (15), 2004377. doi:10.1002/adfm.202004377
Zhu, J., Liu, B., Wang, Z., Wang, D., Ni, H., Zhang, L., et al. (2019). Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics 9 (23), 6901–6919. doi:10.7150/thno.37357
Zhu, D., Hou, J., Qian, M., Jin, D., Hao, T., Pan, Y., et al. (2021). Nitrate-functionalized patch confers cardioprotection and improves heart repair after myocardial infarction via local nitric oxide delivery. Nat. Commun. 12 (1), 4501. doi:10.1038/s41467-021-24804-3
Zong, Y., Li, W., Xu, R. Y., He, L., Wei, D. H., Wang, Z., et al. (2022). Ferroptosis in AS progression: role of miRNA. Eur. Rev. Med. Pharmacol. Sci. 26 (22), 8425–8436. doi:10.26355/eurrev_202211_30378
Zou, W., Wang, X., Xia, X., Zhang, T., Nie, M., Xiong, J., et al. (2024). Resveratrol protected against the development of endometriosis by promoting ferroptosis through miR-21-3p/p53/SLC7A11 signaling pathway. Biochem. Biophys. Res. Commun. 692, 149338. doi:10.1016/j.bbrc.2023.149338
Glossary
AVF arteriovenous fistula
circRNA circular RNA
DLS dynamic light scattering
DNA deoxyribonucleic acid
EPCs endothelial progenitor cells
ESE early sorting endosome
EVs extracellular vesicles
GPX4 glutathione peroxidase 4
HMGB1 High-mobility group box 1
hucMSCs human umbilical cord mesenchymal stem cells
HUVECs human umbilical vein endothelial cells
IH intimal hyperplasia
IL interleukin
ILVs intracellular vesicles
lncRNA long noncoding RNA
miRNA microRNA
MMP matrix metalloproteinases
mRNA messenger RNA
MSCs mesenchymal stem cells
MVBs multivesicular bodies
NF-κB nuclear factor kappa B
NO nitric oxide
NTA nanoparticle tracking analysis
PDGF platelet-derived growth factor
PEG polyethylene glycol
PS phosphatidylserine
Re Reynolds number
RNA ribonucleic acid
ROS reactive oxygen species
SEC size exclusion chromatography
SM sphingomyelin
TEM transmission electron microscopy
TLRs toll-like receptors
TNF-α tumor necrosis factor alpha
VE-cadherin vascular endothelial cadherin
VEGF vascular endothelial growth factor
VSMC vascular smooth muscle cells
ZnONP ZnO nanoparticles
Keywords: exosomes, arteriovenous fistula (AVF), extracellular vesicles, intimal hyperplasia, endothelial-to-mesenchymal transition (EndoMT), vascular remodeling, chronic kidney disease (CKD)
Citation: Cao Y, Guo F, Chen D, Li L, Jie X and Wang W (2025) Exosomes in arteriovenous fistula stenosis. Front. Cell Dev. Biol. 13:1663973. doi: 10.3389/fcell.2025.1663973
Received: 11 July 2025; Accepted: 01 September 2025;
Published: 19 September 2025.
Edited by:
Gianandrea Pasquinelli, University of Bologna, ItalyReviewed by:
Nagendra Verma, St. Cloud State University, United StatesCarmen Ciavarella, University of Bologna, Italy
Copyright © 2025 Cao, Guo, Chen, Li, Jie and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weitie Wang, MTI1NTY4NDA0M0BxcS5jb20=
 Yushi Cao
Yushi Cao Fangxiao Guo2
Fangxiao Guo2