- 1Life Sciences College, Gannan Normal University, Ganzhou, Jiangxi, China
- 2Jiangxi Institute of Red Soil and Germplasm Resources/Key Laboratory of Arable Land Improvement and Quality Improvement of Jiangxi Province, Nanchang, Jiangxi, China
Aims: Continuous cropping of peppers not only degrades soil quality but also impairs the normal growth and development of peppers grown in greenhouse production systems in the red soil region of south China. It remains unclear whether the negative effects can be alleviated by applying straw and its carbonization.
Methods: In 2021-2022, a pot experiment was conducted with four treatments: a control group with no organic material added (CK), the addition of biochar (B), straw (S), and a combination of biochar and straw (BS) added to the soil. The changing characteristics of pepper growth and yield, soil quality and rhizosphere soil microbial community were determined.
Results: Results indicated that the application of S and B were beneficial to the formation of high yield of pepper and improved the physicochemical properties, enzyme activities, microbial quantity of continuous cropping soil. Among them, the yield of BS was significantly increased by 144.37% on average. More importantly, BS treatment also demonstrated superior rhizosphere soil microbiome enhancement, with microbial populations increasing by 425.42% (bacteria), 946.68% (fungi), and 232.95% (actinomycetes) compared to control. Bacterial diversity indices showed significant improvement (Chao1 + 92.77%, Shannon +39.37%), accompanied by targeted microbial community restructuring that enriched beneficial Bacteroidota and Verrucomicrobiota while suppressing Proteobacteria and pathogenic Ascomycota. These microbiome modifications correlated strongly with enhanced soil enzyme activities and improved physicochemical properties.
Conclusions: The straw-biochar co-application increased pepper yields by 144.37% by improving soil fertility and microbial diversity through rhizosphere microbiome modulation (reducing pathogens and enhancing nutrient cycling). This integrated approach provides a sustainable strategy for intensive continuous cropping systems.
1 Introduction
Pepper (Capsicum annuum L) is widely favored because it is rich in vitamin C, minerals and multiple nutritional components, and it also helps stimulate appetite and improve digestion (Wu et al., 2023; Duan et al., 2021). In recent years, China’s pepper industry has developed at a high speed, with the planting area stabilized at more than 2.1 million hm2, pepper production was 64 million t, which has become the largest vegetable planting area in China (Zou et al., 2020). With the rapid development of economy and agricultural engineering science and technology, the area of vegetable facility cultivation continues to increase in China, and it has become the most dynamic agricultural industry and an important way to achieve high yield, high quality and efficient production of vegetables in China (Chen et al., 2014). With Jiangxi province as the representative of the southern red soil area is an important production area of China’s facility vegetables, but there were many problems in facility vegetable cultivation, such as a single crop type, high replanting index, planting regionalization and intensive, excessive input of inorganic fertilizers, which led to the deterioration of the quality of the facility vegetable soil, the degradation of soil ecosystems, and the chemosensory autotoxicity effect of facility horticultural crop yield reduction or even extinction. In summary, the quality and safety of the facility crop is a significant decline in the serious constraints on the development of pepper industry sustainability (Chen F, et al., 2023).
Straw was a high-quality renewable organic resource, and crop straw was also increasing in China, with an average annual production of up to 900 million t, accounting for about 30% of the global total, which was often returned to the fields for soil improvement (Bai et al., 2021; Ortiz-Liébana et al., 2022). Among them, China possesses abundant rice straw resources, with an annual production reaching approximately 200 million tons in recent year (Liu et al., 2021). Despite persistent straw burning in agricultural practice, improper management of agricultural and forestry bio-residues can adversely affect soils. When properly managed through field mulching or mechanical incorporation, these residues enhance soil organic matter content, effectively converting waste into valuable resources (Guan et al., 2023). Some related studies have shown that the proportion of unstable carbon is higher than that of biochar when crop residues are returned to the field in the form of black charcoal produced by incineration or direct return of straw to the field, which was not conducive to carbon fixation (Jiang et al., 2016b).
Biochar is a solid, stable, carbon-rich and highly aromatic product obtained by thermal conversion of organic matter under O2-limited conditions (Mao et al., 2022). Biochar has more stabilized carbon than raw materials, and sequestering agroforestry waste in the form of biochar in the soil is more conducive to carbon fixation (Liu et al., 2016). In addition, biochar is characterized by a rich pore structure, a high specific surface area and a high capacity to retain water and nutrients (Wu et al., 2023). The biochar pore structure can provide habitat for soil microorganisms and increase soil microbial activity (Cheng et al., 2019). Biochar is widely used in agriculture as a soil supplement for stabilizing carbon storage and soil fertility and as a medium for the slow release of nutrients (Cheng et al., 2019). It confers many benefits to the soil and the environment (Kocsis et al., 2022), such as the sequestration of atmospheric carbon in the soil and its ability to reduce the leaching of mineral nitrogen and the emission of N2O (Borchard et al., 2019), which is important for sustainable agriculture and the mitigation of global climate change (Gaffar et al., 2021). Significantly, biochar application increases hot pepper (Capsicumannuum L.) production via soil quality improvement (Kebede et al., 2023). Beyond direct soil application, biochar is often co-applied with straw incorporation, where their combination synergistically enhances nutrient retention and crop productivity (Khan et al., 2022).
Microorganisms promote sustainable soil use, enhance soil ecological stability, facilitate nutrient cycling and plant growth and development, and represent an indispensable component of soil ecosystems (Zhang et al., 2021). Soil microbial diversity and soil microbial community structure reflect soil fertility and soil health (Jiao et al., 2022). Different soil microorganisms have different functional and genetic characteristics that regulate soil nutrient effectiveness and elemental cycling (Cairns et al., 2020). Straw returning elevates unclassified bacteria to enhance microbial diversity and nutrient cycling via SOC-nutrient-pH mediation (Guan et al., 2023). The carbon richness of biochar guides the potential impact of carbon mineralization by different soil microbial communities (Chen et al., 2018). The application of 5% (w/w) biochar substantially modified soil microbial composition, stimulating beneficial bacterial groups (Firmicutes, Actinobacteria, and Bacillus) while restructuring fungal populations under continuous cucumber cropping (Li et al., 2024). While both rice straw and biochar increased microbial abundance, only straw improved carbon metabolism and diversity, these results imply that their combined application may provide optimal soil remediation benefits (Zhang et al., 2018). Although biochar and straw amendments have been widely studied in soil microecosystems, their synergistic effects on continuous cropping systems, especially in pepper (Capsicum annuum L.) monoculture, remain unexplored. The deterioration of microecological environment is an important factor limiting the high yield and quality of crop production (Nash et al., 2021). Given the challenges of soil degradation and pathogen buildup in greenhouses, it is necessary to evaluate the role of straw and biochar in shaping soil microbial diversity and abundance in greenhouse pepper systems.
We hypothesized that the combined application of straw and biochar would ameliorate soil microecological degradation in continuous cropping systems by enhancing microbial diversity, improving soil physicochemical properties, and optimizing plant-soil-microbe interactions, thereby promoting pepper growth and yield under continuous cropping system. To test this hypothesis, a pot experiment of continuous pepper was performed with the combined application of rice straw and its carbonization to (1) study the variation characteristics of yield, dry matter growth and plant type of pepper, (2) evaluate the change characteristics of soil physical and chemical properties and soil enzyme activities under the combined application of straw and biochar, (3) studied the regulation effect of straw and its carbonized return on soil microecological environment of continuous cropping. Our results of this study will provide a theoretical guidance for high yield and high quality of pepper continuous cultivation in facilities in south of China.
2 Materials and methods
2.1 Plant materials
The experiment was conducted from March 2021 to December 2022 in the glass greenhouse of the pilot base of the National Navel Orange Engineering and Technology Research Center (Ganzhou, China) at the Baita Campus of Gannan Normal University (114°93′ E, 25°90′ N) (Supplementary Figure S1). The tested acidic red loam soil was collected from 0~30 cm surface layer of local facility vegetable plots. After air-drying, all the rocks and roots were removed, and the soil was sieved through a 2 mm sieve. The basic physical and chemical properties of the soil were as follows: pH 6.26, organic matter 16.21 g·kg-1, total nitrogen 0.33 g·kg-1, alkaline hydrolysable nitrogen 72.33 mg·kg-1, available potassium 135.33 mg·kg-1, and available phosphorus 9.78 mg·kg-1. The pepper variety for test was Seminis 2579 for winter and spring crop in 2021, and thereafter the variety planted was 37–94 Screw Pepper, and the pepper seedlings were provided by the Southern Agricultural Base in Datangbu Town, Xinfeng County, Jiangxi Province, China (114°92′ E, 25°32′ N).
The rice straw used in this study was made from local late rice season straw, dried at 80°C, and then crushed. The Biochar production method is as described by (Chen L, et al., 2023a), the straw used for crushing was taken as carbonized material and fired in a muffle furnace (SX-4–10 type box type resistance furnace; Tianjin Teste Instruments Co, Tianjin, China) at 550°C under the conditions of oxygen limited and high temperature pyrolysis for 2 hours, then passed through a 60-mesh sieve and set aside. The conversion rate of straw-to-straw biochar was 42.0% under these conditions, the carbonation rate data were obtained from indoor experiments, averaged after 30 repetitions.
The physicochemical characteristics of rice straw and its biochar were pH 6.68 and 9.92, organic carbon 340.05 g·kg-1 and 325.82 g·kg-1, total nitrogen 4.61 g·kg-1 and 3.13 g·kg-1, and effective phosphorus 2.33 g·kg-1 and 2.13 g·kg-1.
2.2 Design of experiment
The experiment was conducted as a pot planting trial, with two consecutive crops (winter-spring stubble and autumn-winter stubble) planted each year, and a total of four treatments were set up, namely: CK (no material added), B (1% soil mass of biochar added), S (equal amount of straw added, and 2.4% soil mass of straw added at a carbonization rate of 42.00%), and BS (cumulative application of biochar and straw). Twenty pots of each treatment were planted with one pepper plant each, the pot (height 31 cm; top diameter 28 cm, bottom diameter 22 cm) was filled with 10.8 kg of air-dried soil, and all organic materials were applied in a single soil mix before transplanting of each crop, with a total of four additions in two years. Each bucket was transplanted with one pepper seedling in good growth condition and more consistent growth, and the label of each bucket was fixed and then randomly placed according to a 4×10 layout with 40 cm spacing between buckets, after which all treatments were managed under the same water and fertilizer conditions.
2.3 Growth and development, resistance physiology and yield measurement
2.3.1 The plant type and chlorophyll content determination
Six pots of plants were randomly selected for each treatment, the plant canopy width and height were measured with a ruler, and stem base width was measured with a digital vernier caliper at the end of the experiment, and the leaf length, width and area were determined and analyzed by LA-S plant image analyzer system (Hangzhou Wanshen Detection Technology Co., LTD., China). After 60 d of planting, using 95% ethanol as organic solvent, the absorbance was measured at the wavelengths of 665 nm, 649 nm and 470 nm of UV-Vis spectrophotometer (UV-300, Shanghai Mepida Instrument Co., LTD, China), the contents of leaf pigments (chlorophyll a, chlorophyll b, carotenoids) were calculated.
2.3.2 Resistance physiological related indicators
After 75 d of planting, superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT), malondialdehyde (MDA) and proline content (Pro) of leaf were determined using kits provided by Suzhou Keming Biotechnology Co. Ltd. in China, soluble sugar content was determined by anthrone colorimetry, and soluble protein content was determined by Coomassie Brilliant Blue G-250 staining (Li et al., 2000), the measurements were three replicated for each treatment.
2.3.3 Pepper yield and dry matter production determination
Mature peppers were harvested every 10 d after 90 d of planting until the end of the harvest period, the weight of individual fruits was determined by electronic scales, and the total amount of harvested fruits per plant was counted at the end of the harvest period. The stems, leaves and fruits of the plants were separated, put into the oven at 105°C for 30 min, dried at 75°C until constant weight, and the dry matter accumulation was determined (Wang and Huang, 2015).
2.4 Physicochemical properties and microbial community structure of continuous cropping soils
2.4.1 Methods of soil sample collection
Samples for the determination of soil physicochemical properties and enzyme activity were collected in January and July every year. Multi-point sampling method was used to collect soil samples with topsoil and removed from each pot at a sampling depth of 0~30 cm by a 5 cm diameter soil auger. A total of four small sample points were taken from each pot to make a mixed sample, which was air-dried and removed plant debris, stone gravels, and other debris, then passed through a sieve of 1, 0.15 mm aperture, and then was stored at ambient temperature to be tested.
After two years of continuous cropping, the rhizosphere soil of peppers was collected by shaking the roots (Liang et al., 2021). That is, the soil with complete root system was selected from 0~30 cm soil layer of potting soil, and then gently shaken off, the soil that was tightly attached to the root surface and not easy to be shaken off was the rhizosphere soil, and the residual soil from root hairs were collected using a brush and thoroughly mixed (Supplementary Figure S2). Then transported to the laboratory on ice with a sterile sealing bag, the soil samples were passed through the 2 mm sieve, and the plant residue was removed, a part of the rhizosphere soil sample was stored in the -80°C for the determination of the composition of the microbial community, and a part was stored in the 4°C for the determination of the number of the rhizosphere soil microorganisms (Chen et al., 2018).
2.4.2 Methods for determination of soil physicochemical properties, enzyme activity and microbial quantity
The soil pH and the content of alkaline hydrolysable nitrogen, organic matter, available phosphorus, available potassium, and total nitrogen were determined as described by Bao (2000). The activities of urease, catalase, sucrase, acid phosphatase, amylase and polyphenol oxidase in the soil were assayed as described by Wang et al. (2018b). The soil microbial population was determined as described by Xu and Zhen (1986).
2.4.3 Determination of rhizosphere soil microbial community composition
The extraction of total microbial DNA was completed by Suzhou Jinweizhi Biotechnology Co., Ltd (Suzhou, China) using the HiPure Soil DNA Kit. Qubit ® dsDNA HS Assay Kit was used to detect DNA concentration and complete the construction and sequencing of the second-generation sequencing library. PCR primers amplify two highly variable regions, V3 and V4, on the 16S rDNA of prokaryotes. Use contains “CCTACGGRRBGCASCAGKVRVGAAT” sequence primers and contains “GGACTACNVGGGTWTCTAATCC” sequence upstream of downstream primer amplification V3 and V4 area. The target fragment of PCR product library was detected by 1.5% agarose gel electrophoresis at 600bp. The amplification conditions are shown in Supplementary Table S1. PCR primers were used to amplify eukaryotic ITS rDNA using upstream primers containing the GTGAATCATCGARTC sequence and downstream primers containing the TCCTCCGCTTATTGATGAT sequence. The PCR product library in ITS was detected by 1.5% agarose gel electrophoresis with the target fragment of 400 bp. The amplification conditions are shown in Supplementary Table S2. The library concentration was detected by Tecan, Infinite 200 Pro, and sequenced according to Illumina MiSeq/Novaseq (Illumina, San Diego, CA, USA) instruction manual. After quality filtering, the chimeric sequences were removed, and the operational taxonomic unit (OTU) clustering was performed at 97% similarity, followed by species annotation with Silva and UNITE databases. Then, using the RDP classifier (Ribosomal Database Program) Bayesian algorithm and SILVA and UNITE databases, representative sequences of 97% similar OTUs were subjected to taxonomic analysis, and the community composition of each sample was statistically analyzed at different taxonomic levels.
2.5 Data processing and analysis
Prior to statistical analysis, all datasets were tested for normality (Shapiro-Wilk test) and homogeneity of variance (Levene’s test). Data meeting parametric assumptions were analyzed using one-way ANOVA followed by LSD post-hoc tests for multiple comparisons. Non-normal data were appropriately transformed or analyzed using non-parametric alternatives. All data are presented as the mean ± standard deviation of three biological replicates which were statistically analyzed and processed using SPSS 22.0 software (IBM Research, New York, NY, USA) and Microsoft Excel 2019. R language software (3.3.1) and Origin 2023b were used to draw the chart. Microbial community analysis included: LEfSe (1.0) was used to estimate the relative abundance difference, and QIIME (1.91) software was used to calculate the alpha diversity indices, The STAMP (v2.1.3) software was used to compare significant differences in bacterial abundance at different taxonomic levels.
3 Results
3.1 Pepper yield and dry matter production
After two years of continuous cropping, the yield and fruit length of pepper decreased significantly, and the yield of the fourth crop decreased by 49.05% compared with the first three crops (Table 1). The addition of organic materials was beneficial to increase the yield, single fruit weight and fruit shape index of pepper. Among them, compared with CK treatment, the B, S and BS treatments increased the pepper yield by 29.25%, 117.72% and 144.37% on average, respectively, and S and BS treatment had a significant increase effect. The combined application of straw and biochar was significantly beneficial to the increase of pepper yield.
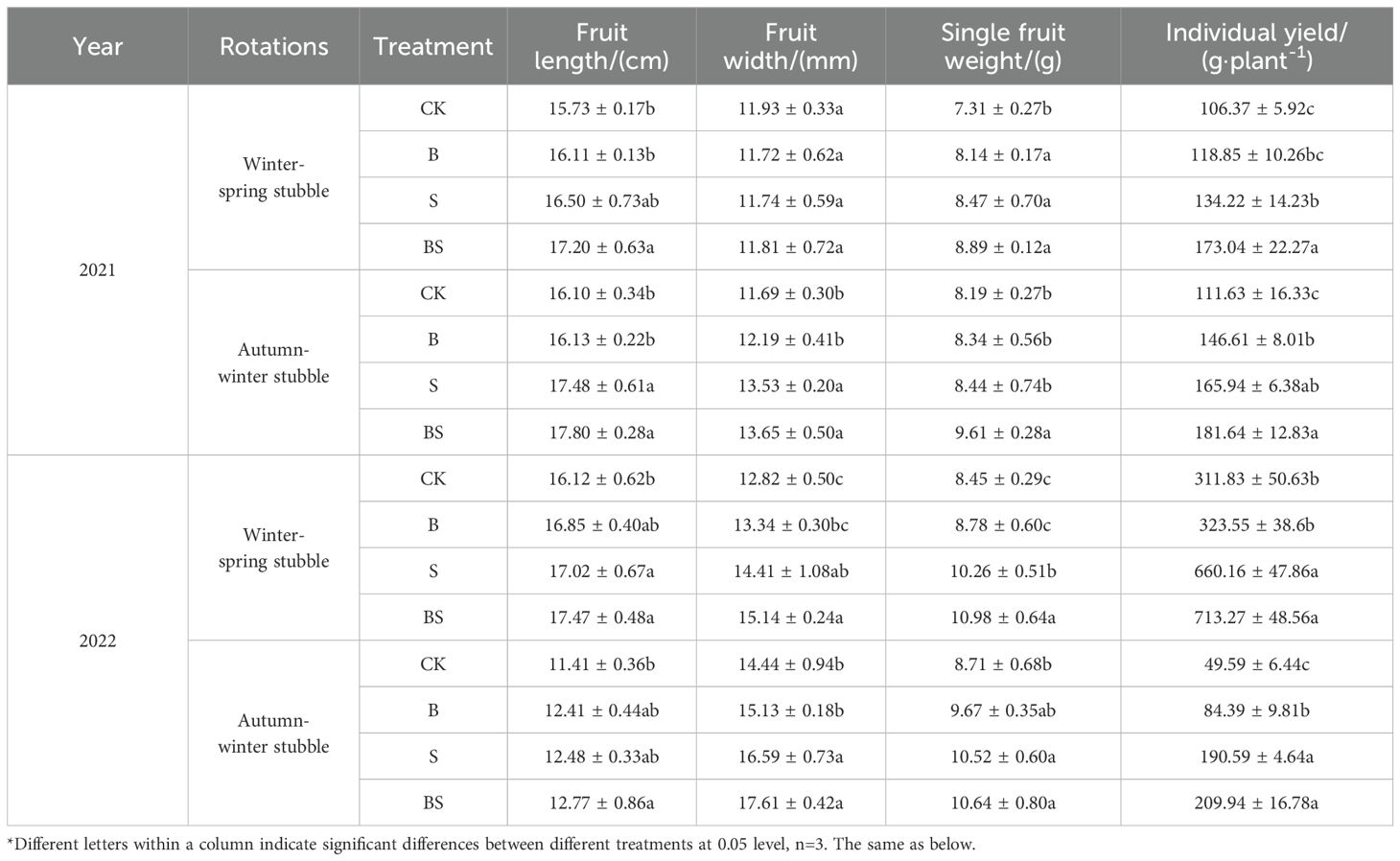
Table 1. Effects of straw and biochar application on yield and appearance quality of pepper in facility continuous cropping.
The dry matter weight of whole plant and each organs showed an increasing and then decreasing trend with the extension of continuous cropping years and reached the highest value in the winter-spring stubble in 2022 (Table 2). Compared with CK treatment, the total dry matter weight of B, S and BS treatments were increased by 8.36%, 48.31% and 73.66% on average, respectively. There is a significant difference between straw addition treatments (S and BS treatment) and CK treatment. Similarly, the dry matter weight of root, stem and leaf was significantly higher than that of CK treatment.
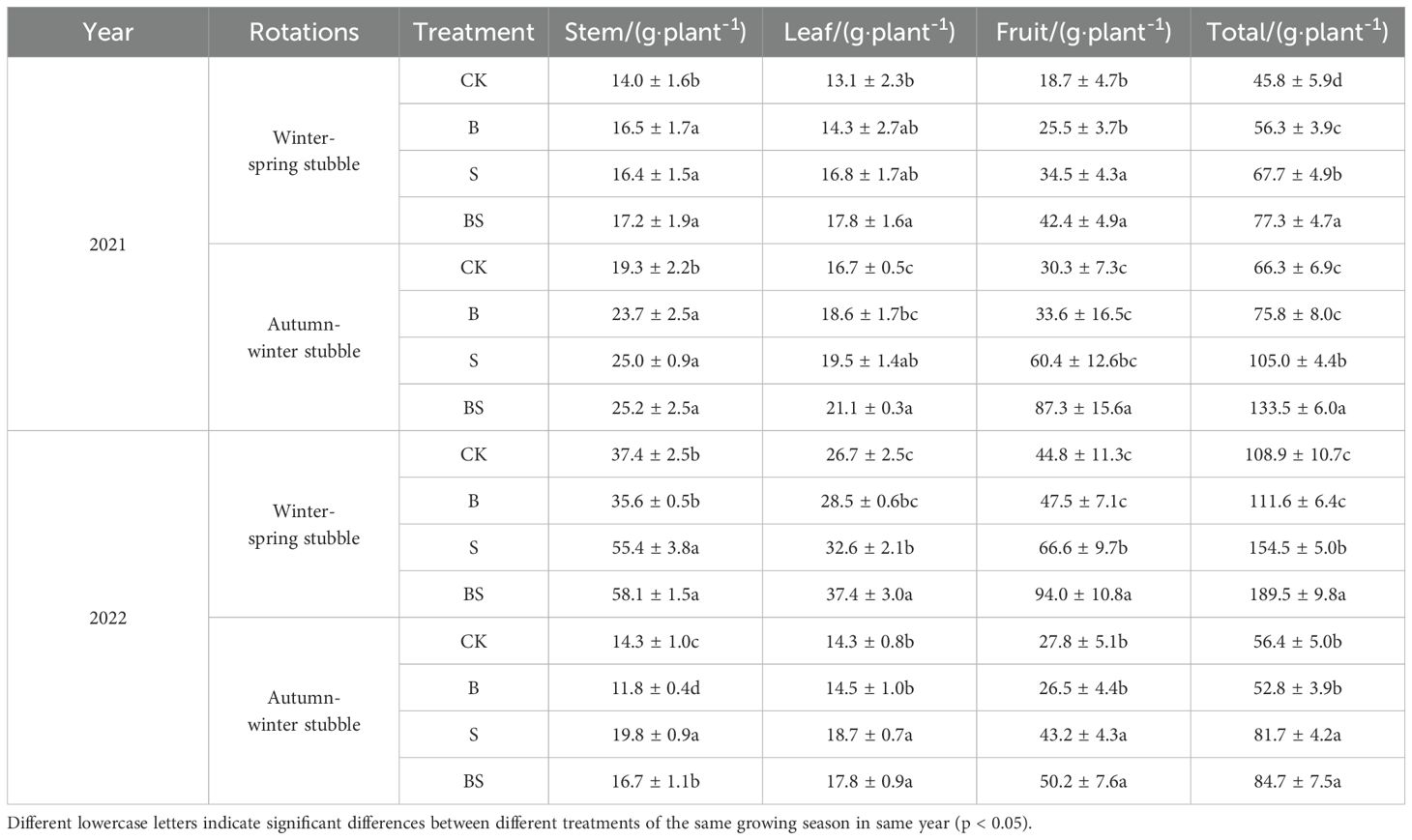
Table 2. Effects of straw and biochar application on dry matter production of pepper in facility continuous cropping.
3.2 Plant type and chlorophyll content
All plant type indices of pepper showed the trend of increasing and then decreasing with the increase of continuous cropping years and reached the highest value in the winter-spring stubble in 2022 (Supplementary Table S3). Continuous cropping to 2022 autumn-winter stubble, S and BS treatments were significantly higher in plant height, leaf length, number of leaves and canopy width compared with the CK. Among them, and S and BS treatments significantly increased in leaf area of pepper per plant by 33.32% and 54.65% compared with CK treatment, respectively (Supplementary Figure S3). In addition, the addition of straw and biochar had no significant effects on SPAD and chlorophyll content of continuous cropping pepper leaves (Supplementary Tables S3, S5).
3.3 Resistance physiology
MDA content of pepper leaves were reduced by both straw and biochar supplementation, but the difference was not significant (Supplementary Figure S4). The addition of straw and biochar can improve the antioxidant enzyme activity of pepper leaves, and compared with CK treatment, the SOD, POD and CAT enzyme activities of BS treatment increased significantly by 56.62%, 16.54% and 37.04%, respectively (Figure 1). Compared with the CK treatment, the osmotic regulator that Pro content was significantly increased by 37.04% under the cooperative application of straw and biochar, but the difference of soluble sugar and soluble protein content was not significant (Supplementary Figure S4).
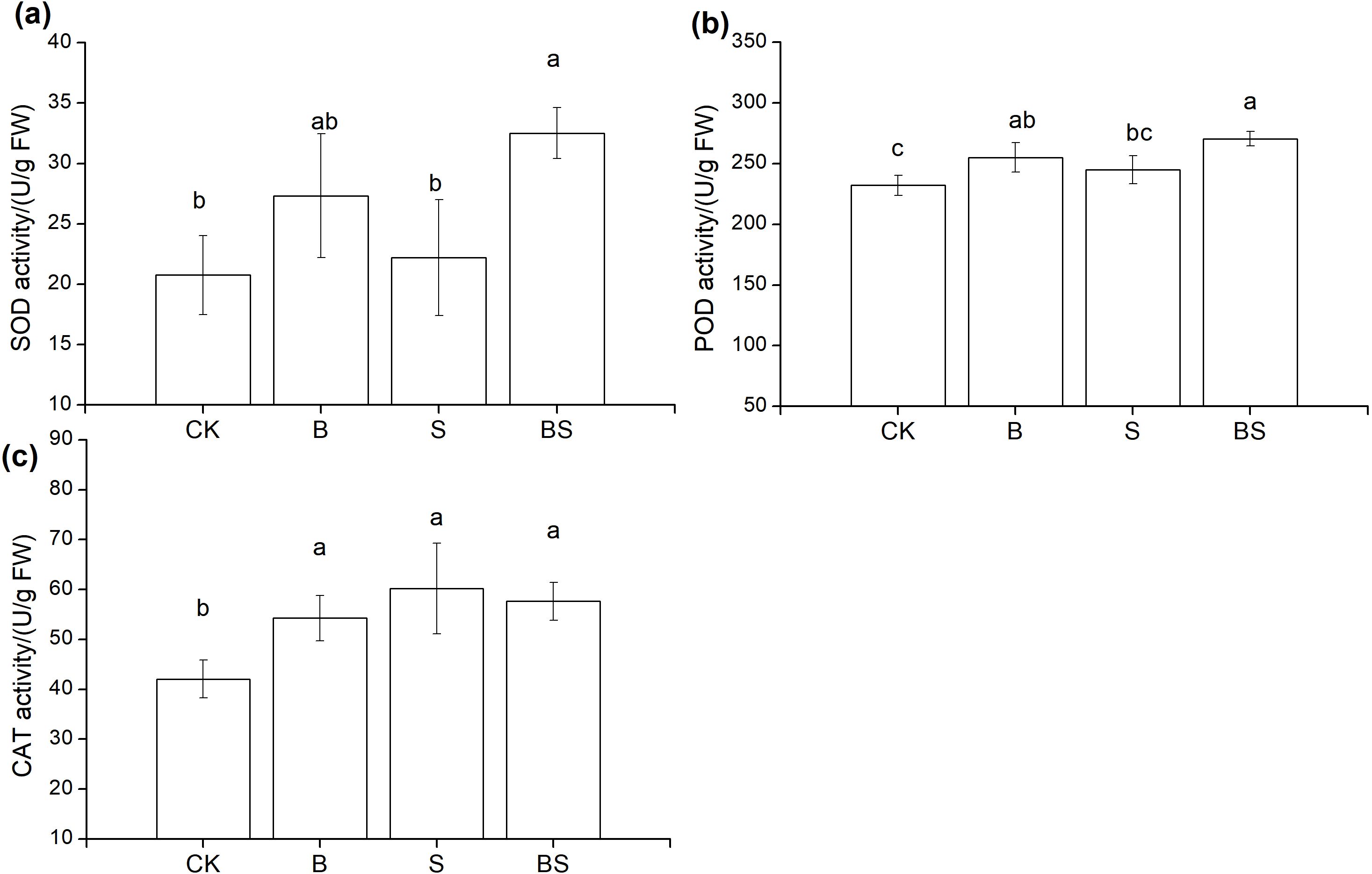
Figure 1. Effects of straw and biochar application on pepper leaf antioxidant enzyme activity after two years of continuous cropping. SOD: Superoxide Dismutase; POD: Peroxidase; CAT: Catalase. (a, b, and c) represent the changes in SOD, POD, and CAT activities in pepper leaves under different treatments, respectively. Different lowercase letters indicate significant differences between different treatments (p < 0.05)
The cumulative contribution rate of PCA analysis axis 1 (49.4%) and axis 2 (17.9%) was 67.3%, SOD and POD are positively correlated with stem diameter and leaf width, while CAT is positively correlated with height, leaf length, number of leaves and canopy width (Supplementary Figure S5). There are differences in the distribution of sample points between each treatment and CK, indicating that straw and biochar treatments have a significant impact on the combination of antioxidant enzyme activity in pepper leaves and plant morphological indicators.
3.4 Soil physicochemical properties
With the extension of continuous cropping years, soil pH and available potassium content showed a downward trend, The content of total nitrogen, alkali hydrolyzed nitrogen and available phosphorus showed an upward trend (Table 3). Interesting, the content of organic matter of CK and B treatment increased first and then decreased with the continuous cropping period delayed, while that of straw treatment (S and BS) continued to increase. After two years of continuous cropping, soil pH and nutrient content (except available phosphorus) were significantly increased by the addition of straw and biochar compared with the CK, and the synergistic application of the two was more effective.
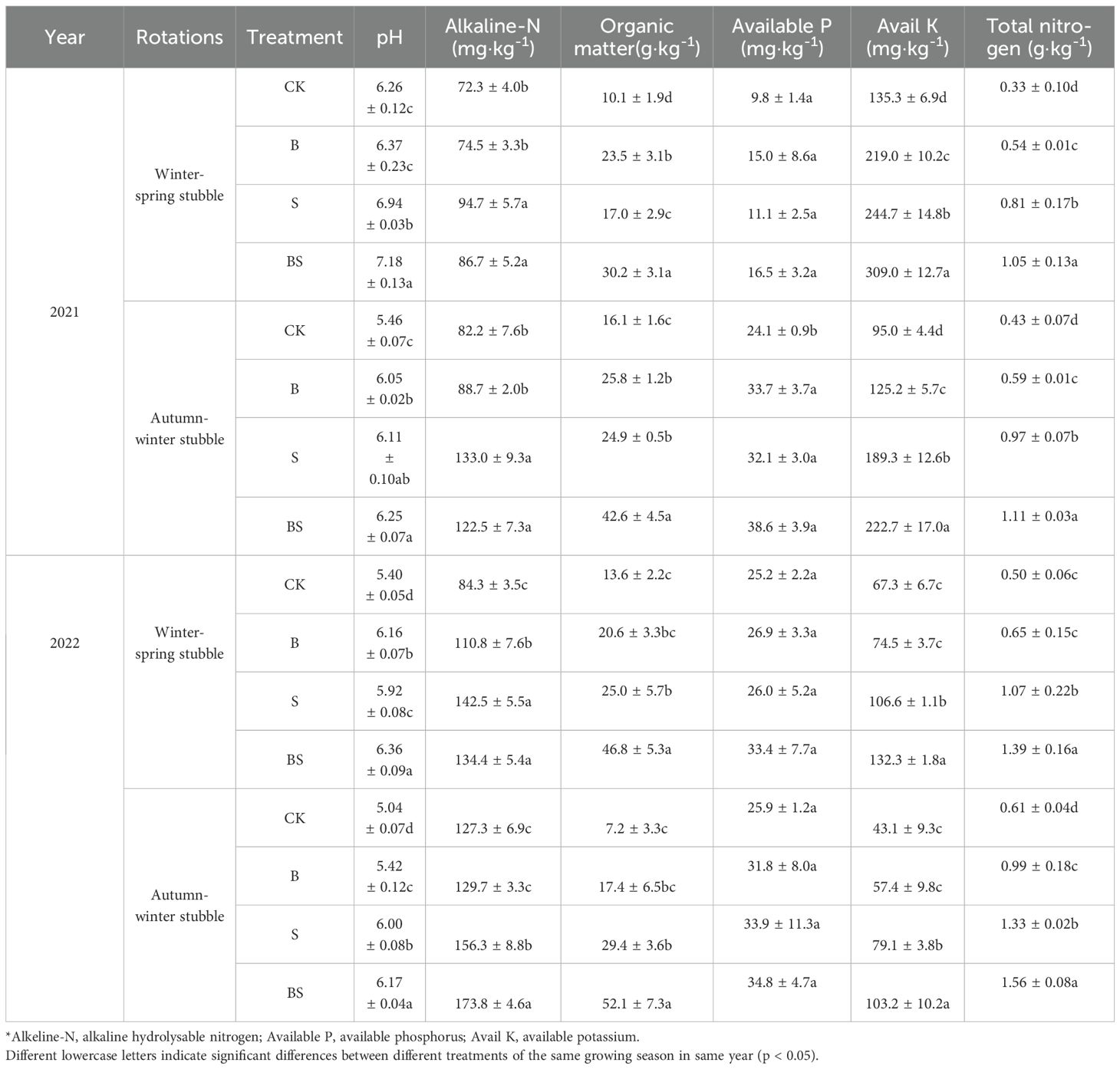
Table 3. Effects of straw and biochar application on soil physicochemical properties of pepper in facility continuous cropping.
3.5 Soil microbial quantity and enzyme activity
The addition of all organic materials such as straw and biochar could promote the increase of soil microbial quantity, especially the improvement effect of S and BS treatments reached a significant level compared with the CK, among which the combined application of straw and biochar had the best effect (Table 4). Compared with CK treatment, the number of bacteria, fungi and actinomycetes in BS treatment increased significantly by 425.42%, 946.68% and 232.95%, respectively.
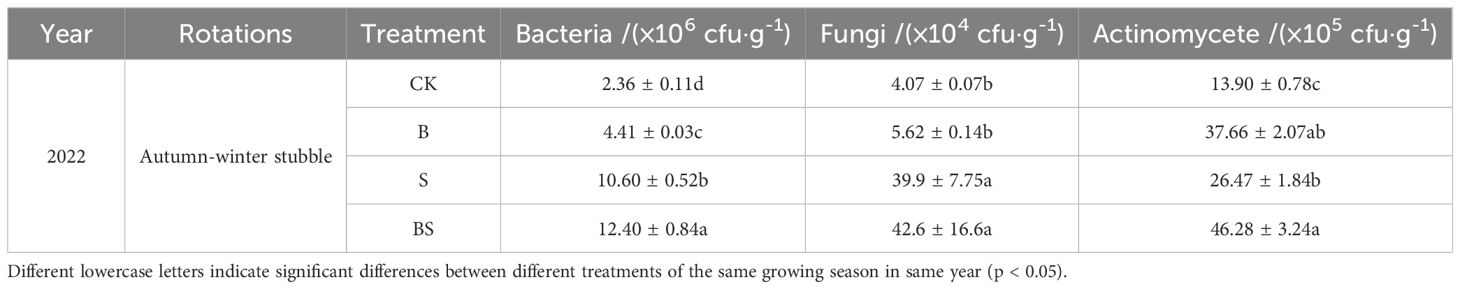
Table 4. Effects of straw and biochar application on rhizosphere soil microbial quantity of pepper in facility continuous cropping.
Compared with CK treatment, the activities of urease, catalase, sucrase and amylase in the soil were improved by adding organic materials, and the above indices of S and BS treatments were significantly higher than CK treatment except urease after two years of continuous cropping (Supplementary Table S5). However, the activity of soil polyphenol oxidase was decreased by the organic material addition. After two years of continuous cropping, soil acid phosphatase and polyphenol oxidase in BS treatment were significantly decreased by 34.90% and 61.47% compared with CK treatment, respectively.
3.6 Analysis of microbial diversity in pepper rhizosphere
With the application of organic matter, the Chao1 and Shannon indices of the bacteria were significantly higher in the B, S and BS treatments than in the CK treatment, which were significantly 58.59% and 27.09%, 96.14% and 41.46%, and 92.77% and 39.37% higher than in the CK treatment, respectively (Figures 2a, b). It indicated that straw and its carbonization additions can increase the α-diversity of soil bacterial communities, with the S treatment having a more significant effect. There were differences in the α diversity of fungal communities applied with organic materials, but the differences were not significant (Figures 2c, d). The fungal Chao1 was higher in the B, S and BS treatments than in the CK treatment, but the Shannon was lower in all of them. The Simpson’s index of microorganisms and the fungal cover index were higher in the CK, B, S and BS treatments, indicating that the results of the experiment were highly reliable (Supplementary Table S6). The abundance of bacterial species with added organic material was greater than that of CK in all cases, with the S treatment being the largest (Supplementary Figure S6).
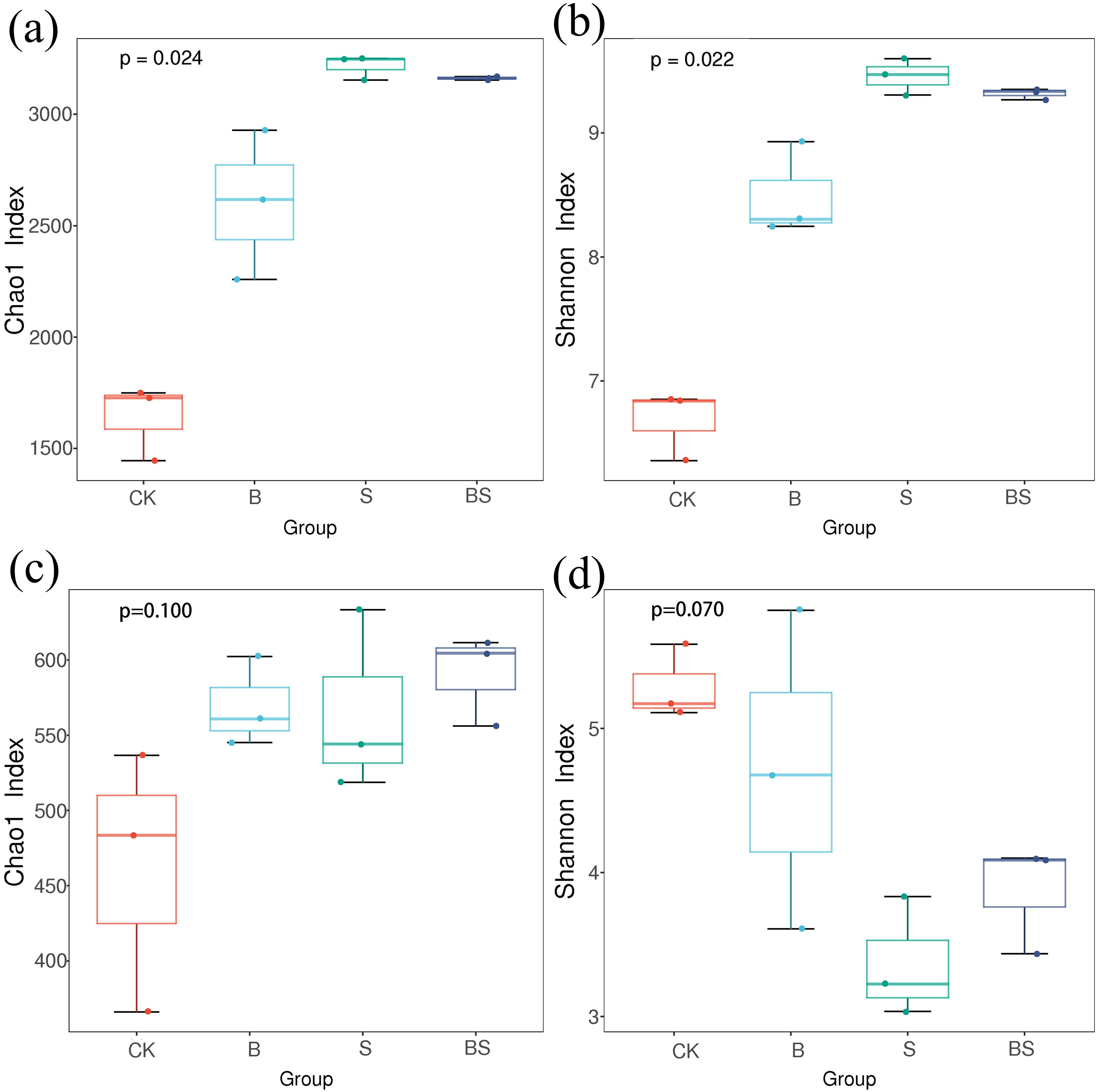
Figure 2. Boxplots of the alpha diversity of bacteria and fungi in the rhizosphere soil under straw and biochar application after two years of pepper continuous cropping. (a, b) represent Chao 1 and Shannon index of bacteria in the rhizosphere soil of different treatments, respectively. (c, d) represent Chao 1 and Shannon index of fungi in the rhizosphere soil of different treatments, respectively.
The cumulative contribution rate of PCoA analysis axis 1 (61.55%) and axis 2 (18.52%) was 80.07%. The Stress value of NMDS analysis was < 0.001, which was representative and could well reflect the impact of different treatments on the functional differences of species (Figures 3a, b). In conclusion, different treatments had significant effects on bacterial community structure and functional composition, both of which showed that B treatment and CK treatment had a higher degree of dispersion at axis 2, indicating that there were significant differences in bacterial community structure and functional composition between B treatment and CK treatment. The dispersion degree of S, BS treatment and CK treatment was higher at axis 2, indicating that the bacterial community structure and functional composition were highly similar between S and BS treatment, but significantly different from CK treatment. In PCoA analysis and NMDS analysis of fungal community structure, the three replicates of S and BS treatment were basically clustered together, indicating that the fungal community structure of the two treatments had good replication (Figures 3c, d). The Stress value of NMDS analysis was less than 0.054, indicating that different treatments had no significant effect on the functional difference of species.
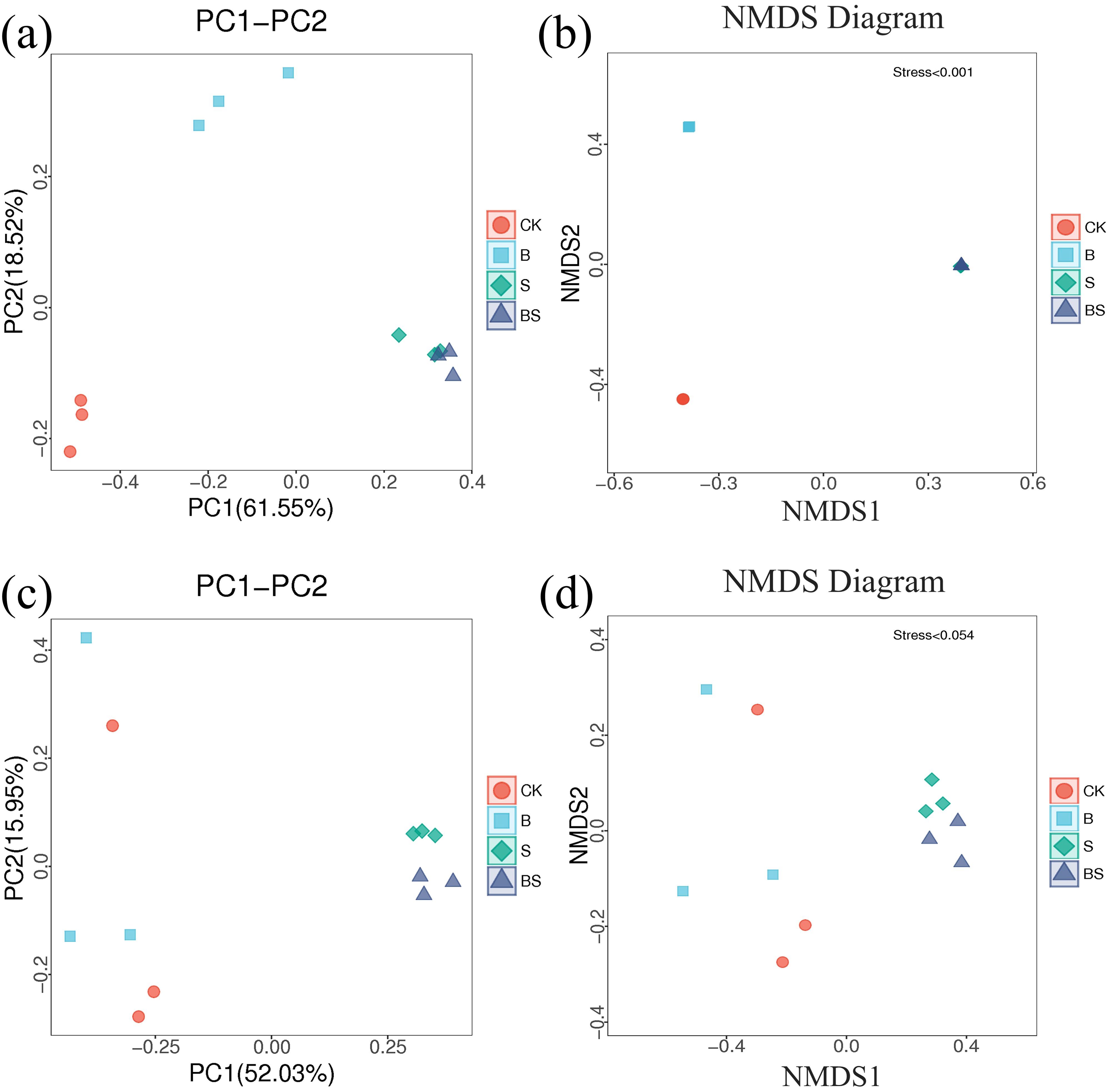
Figure 3. PCoA and NMDS were analyzed for bacteria and fungi in the rhizosphere soil under straw and biochar application after two years of pepper continuous cropping. (a) PCoA analysis of bacterial community structure. (b) NMDS analysis of bacterial community structure. (c) PCoA analysis of fungal community structure. (d) NMDS analysis of fungal community structure.
3.7 Analysis of microbial community structure in pepper rhizosphere
CK, B, S, and BS treatments resulted in an average of 54,810, 67,965, 63,999, and 62,389 valid sequences per bacterial sample, respectively; On average, each fungal sample contains 65,838, 75,280, 93,486, and 87,156 valid sequences (Supplementary Table S7). By drawing a Venn diagram of soil microorganisms, a total of 5,143 bacterial OTUs and 1,272 fungal OTUs were obtained (Figures 4a, b). In addition, CK, B, S, and BS treatments obtained 1,984, 3,261, 3,775, and 3,642 bacterial OTUs, respectively, with a total of 1,210 OTUs in the four samples; CK, B, S, and BS treatments resulted in 721, 802, 672, and 729 fungal OTUs, with a total of 331 OTUs in the four samples.

Figure 4. Venn diagrams of bacterial and fungal OTUs in the rhizosphere soil under straw and biochar application after two years of pepper continuous cropping. (a) Venn diagram of bacterial OTUs. (b) Venn diagram of fungal OTUs.
The OTUs of the different treated soil samples were annotated with species classification to obtain the community composition of each sample. Bacterial communities with relative abundance greater than 10% were defined as dominant zones, and bacterial phyla with relative abundance greater than 1% and less than 10% were defined as subdominant zones. The system of dominant and subdominant phyla is referred to as the dominant phylum. B, S and BS compared with CK treatment showed the number of species at the bacterial species level is S>BS>B>CK and at the fungal species level is B>BS>CK>S (Supplementary Table S8). At the level of soil bacterial phylum, the relative abundance of Proteobacteria and Bacteroidota varied significantly. The relative abundance of Proteobacteria in B, S, and BS treatments was significantly lower than that in CK treatment (P <0.05), while the relative abundance of Bacteroidota was significantly higher than that in CK treatment (P <0.05) (Figure 5a). Compared with the CK, all of the applied organic matter treatments increased Myxococcota as a subdominant phylum, with the S and BS treatments also increasing Verrucomicrobiota as a subdominant phylum. The relative abundance of Ascomycota was more variable, with the relative abundance of Ascomycota in the B, S and BS treatments being significantly lower than that in the CK treatment (P<0.05) (Figure 5b). Compared with CK, the relative abundance of Chytridiomycota was significantly increased in the B treatment, and the relative abundance of Basidiomycota was significantly increased in the S and BS treatments. The S treatment had a decrease in Glomeromycota as a subdominant phylum compared with CK, and the BS treatment had an increase in Mortierellomycota as a subdominant phylum compared with CK.
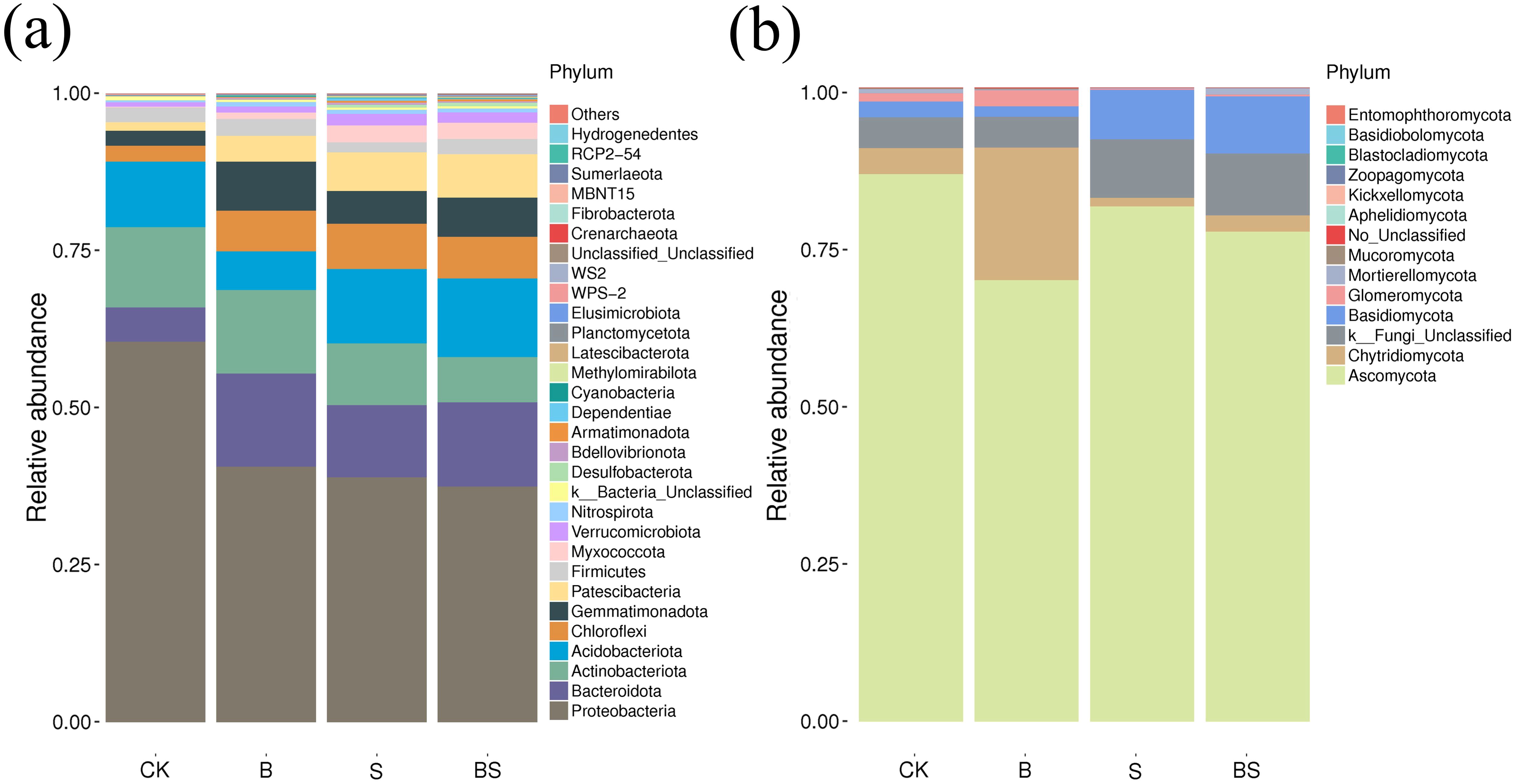
Figure 5. Relative abundance distribution of the rhizosphere soil microbial community at phylum level under straw and biochar application after two years of pepper continuous cropping. (a) Bar plot of the bacterial community at the phylum level. (b) Bar plot of the fungal community at the phylum level. The relative abundance in each sample was calculated on the basis of the percentage of the total effective sequences, which were classified using the RDP classifier. ‘Other’ represents the sum of the relative abundances of phylum level except the top 30 relative abundances.
Blue, purple, and white colors represent the relative abundance of soil microbial communities from high to low, respectively (Figure 6a). At the level of soil bacterial community compendium, all four treatments had dominant species as Gammaproteobacteria, Alphaproteobacteria, Bacteroidia and Actinobacteria with relative abundance above 5%. Gammaproteobacteria was the highest, followed by Alphaproteobacteria. At the level of soil fungal community phyla, the dominant species was Sordariomycetes in all the four treatments with 59.69%, 46.07%, 79.13% and 72.95% in CK, B, S and BS treatments, respectively (Figure 6b). Among them, the S and BS treatments were significantly higher by 32.56% and 22.21%, and the B treatment was significantly lower by 22.81% compared with the CK treatment. Eurotiomycetes were significantly lower in B, S and BS treatments than in CK treatment by 40.41%, 96.06% and 92.39%, respectively.
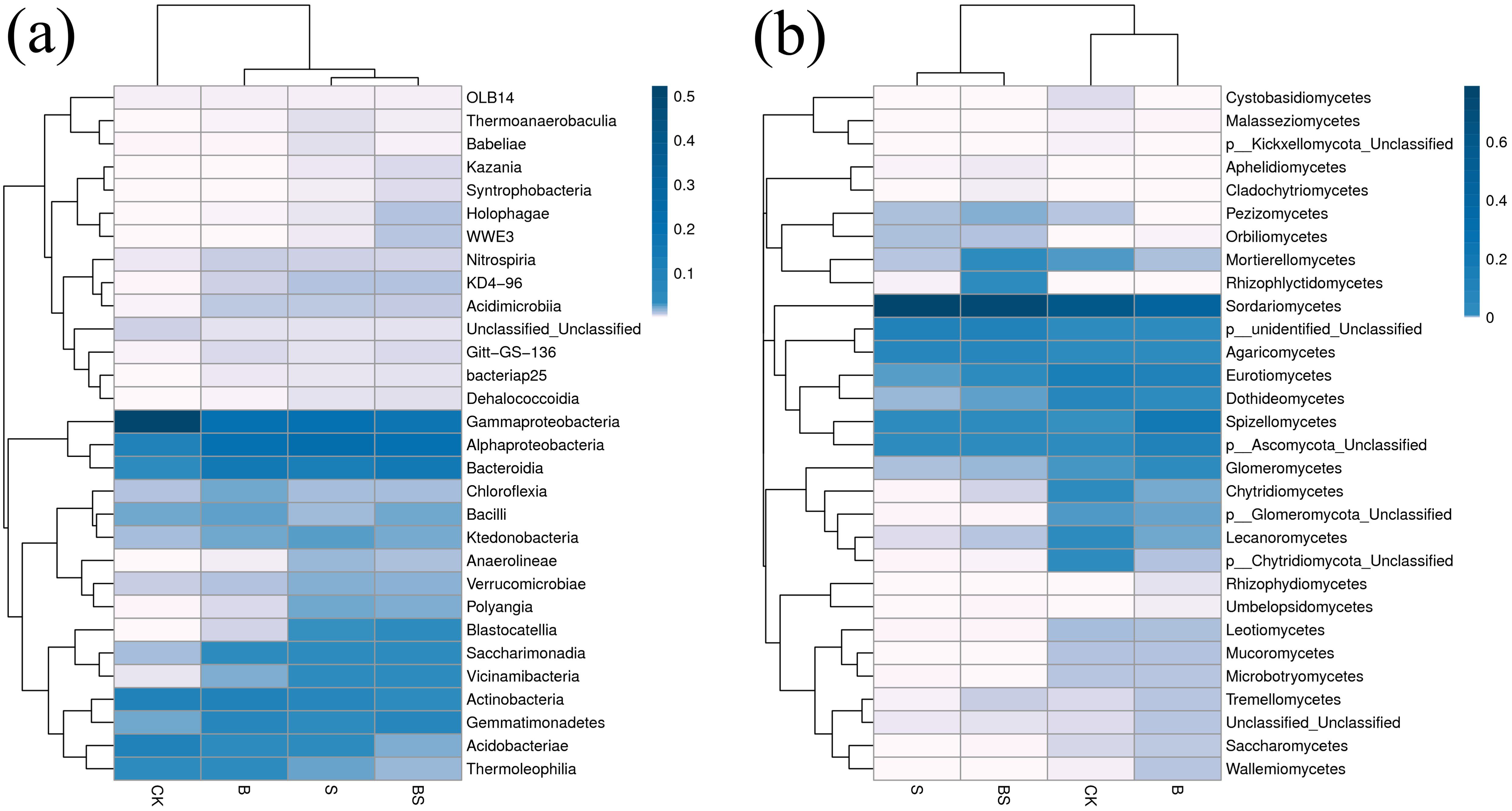
Figure 6. Heatmaps of bacterial and fungal communities at the class level in the rhizosphere soil under straw and biochar application after two years of pepper continuous cropping. (a) Heatmap of the bacterial community at the class level. (b) Heatmap of the fungal community at the class level. The relative abundance in each sample was calculated on the basis of the percentage of the total effective sequences, which were classified using the RDP classifier.
For the results of the Anosim analysis, the rank values obtained based on the ordering of the distance values between two samples are between for between groups and within for within groups. An R-value close to 1 indicates that between-group differences are greater than within-group differences, and a P-value = 0.001 indicates that between-group differences reached the level of significance (Supplementary Figure S7). The genera with significant differences in abundance across treatments were further elaborated by LEfSe (LDA effect size) analysis. The CK, B, S, and BS treatments had 21, 18, 20, and 26 significantly different bacteriophage genera, respectively, with the CK treatment having the highest LDA score for f_Rhodanobacteraceae (Figure 7a). The CK, B, S and BS treatments had 21, 8, 4 and 17 significantly different fungal genera, respectively, with o_Sordariales in the BS treatment having the highest LDA score (Figure 7b). For the relative abundance of dominant taxa of the bacterial community, the CK treatment had the highest relative abundance of Chujaibacter, which was significantly reduced by the addition of straw and biochar treatments (Supplementary Figure S8A). In addition, the relative abundance of fungal Myceliophthora was significantly increased compared with CK in the S and BS treatments, with the highest relative abundance in the S treatment and a significant decrease in the B treatment (Supplementary Figure S8B).

Figure 7. Differential analysis of the rhizosphere soil bacterial (a) and fungal (b) species under straw and biochar application after two years of pepper continuous cropping. The circled figure shows the species clustering evolutionary tree, the red background area and the green background area indicate the different subgroups, the red nodes in the branches indicate the microbial taxa that played an important role in the B treatment, the green nodes indicate the microbial taxa that played an important role in the BS treatment, and the yellow nodes indicate the microbial taxa that did not play an important role in either of the two groups.
3.8 Correlation analysis of rhizosphere microbial community composition and soil environmental factors of pepper
The correlation analysis showed that soil physicochemical properties (pH, organic matter, total nitrogen, alkali-hydrolyzed nitrogen and available potassium) were significantly positively correlated with OTUs (OTU27, OTU13, OTU10, OTU12, OTU5, OTU14, OTU59, OTU32 and OTU18) in bacterial community composition (Figure 8a), except alkali-hydrolyzed nitrogen was no significant correlation with OTU27. at the same time, the correlation analysis between soil physicochemical properties (pH, organic matter, total nitrogen, and available potassium) were significantly negatively correlated in OTU (OTU10, OTU13, OTU16, OTU15, OTU21, OTU8, and OTU14) in fungal community composition, except for pH was not significantly correlated with OTU10 (Figure 8b).
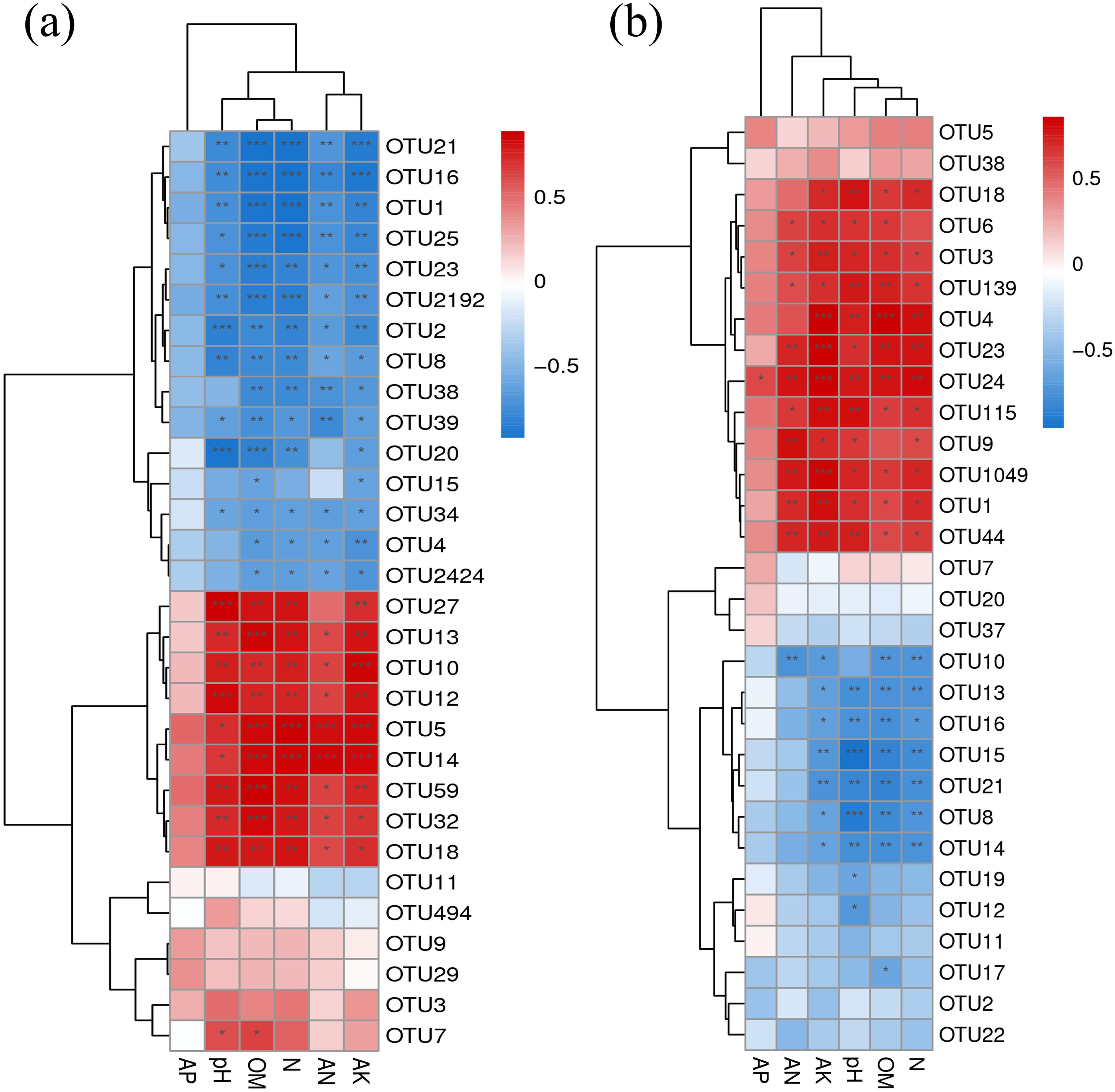
Figure 8. Correlation analysis between the composition of rhizosphere microbial community and soil physicochemical properties under continuous cultivation of pepper cropping. (a) rhizosphere bacteria; (b) rhizosphere fungi. Soil physicochemical properties are labeled horizontally at the bottom of the graph, and OTUs are labeled vertically on the right side of the graph. AP: available phosphorus, OM: organic matter, N: total nitrogen, AN: Alkali hydrolyzed nitrogen, AK: available K. The value corresponding to each square in the heat map presents the Spearman correlation coefficient r [-1~1] between OTUs and soil physicochemical properties, with r >0 being a positive correlation, and r <0 being a negative correlation. Significance test P values less than 0.001 are labeled ***, P values between 0.01 and 0.001 are labeled **, and P values between 0.01 and 0.05 are labeled *.
Through Pearson analysis, it was found that Ace, Chao1, Shannon and Simpson indices of soil bacteria were significantly positively correlated with soil physicochemical properties (pH, alkali-hydrolyzed nitrogen, organic matter, available potassium and total nitrogen), except Simpson indices and alkali-hydrolyzed nitrogen were not significantly correlated (Figure 9a). Ace indices of soil fungi were significantly positively correlated with pH, alkali-hydrolytic nitrogen and total nitrogen, Chao1 indices was significantly positively correlated with alkali-hydrolytic nitrogen and total nitrogen, Shannon indices was significantly negatively correlated with pH and total nitrogen, and Simpson indices was not significantly correlated with soil physicochemical properties (Figure 9b). In addition, the correlation analysis showed that changes in soil enzyme activity have a significant impact on soil bacterial diversity, among which changes in soil urease, catalase and sucrase are important environmental factors affecting the richness and diversity of soil microbial communities (Supplementary Figure S9).

Figure 9. Pearson correlation analysis of soil microbial community diversity and soil environmental factors under continuous cultivation of pepper cropping. (a) rhizosphere bacteria; (b) rhizosphere fungi. AP: available phosphorus, OM: organic matter, N: total nitrogen, AN: Alkali hydrolyzed nitrogen, AK: available K. Significance test P values less than 0.001 are labeled ***, P values between 0.01 and 0.001 are labeled **, and P values between 0.01 and 0.05 are labeled *.
4 Discussion
4.1 Effects of straw and its carbonization addition on pepper growth and yield in continuous cropping facilities
Straw and straw biochar are rich in carbon, nitrogen, phosphorus, potassium and medium and trace elements, which are conducive to fertilizing soil and promoting plant nutrient absorption, so as to achieve the goal of improving crop yield and quality (Poormansour et al., 2019). Our study showed that both S and BS treatments significantly improved the yield, dry matter production capacity, plant growth and stress resistance of continuous cropping pepper, particularly the combined application of straw and biochar had the best yield increase effect, effectively alleviating the yield decline caused by continuous cropping. This was consistent with the results of previous studies, Khan et al. (2022) and Liu et al. (2022) believe that the combined application of straw and straw biochar was more effective in improving crop yield in maize planting system and rice-wheat rotation system. This may be related to the following three reasons, firstly, a small amount of allelopathic substances and mineral element are released after straw decomposition treatment, which are decomposed and utilized by microorganisms to effectively improve the growth environment of roots, thus promoting the uptake of nutrients by roots and accelerating the growth and development of crops (Gao et al., 2020). Secondly, biochar is a kind of insoluble, stable, highly aromatic and nitrogen-rich solid, which can improve soil microbial activity and enzyme activity markedly less than straw. However, the high adsorption capacity of biochar also provides rich mineral elements for the growth and development of plants (Liu et al., 2022). Thirdly, as a carbon-rich alkaline substance, biochar can increase the content of soil organic matter, improve soil structure, promote the utilization of water and fertilizer by crop roots, and provide “source” supply for above-ground growth of crops, thus providing favorable external conditions (Kocsis et al., 2022). Therefore, the combined application of straw and biochar has the best effect, the growth and development of continuous cropping pepper was effectively promoted by the soil management strategy of synergistic return of the two, and improved the harm caused by continuous cropping.
4.2 Effect of straw and its carbonization additions on soil physicochemical properties
The evaluation of soil fertility depends on the physical, chemical, and biological properties of soil (Xu et al., 2022). Reasonable addition of organic materials can not only reduce non-point source pollution and prevent soil compaction, but also improve soil physicochemical properties (Wang, 2018a). Our results showed that continuous cropping led to a significant decrease in soil pH, resulting in different degrees of soil acidification, and the application of straw and biochar can adjust soil acid-alkalinity and significantly increase pH value, among which BS treatment had the best effect. This aligns with previous studies, particularly the well-documented role of biochar in improving soil quality by increasing pH (Chen L et al., 2022; Kebede et al., 2023). The results of this study showed that the application of biochar and straw significantly increased the contents of organic matter, available potassium, available phosphorus and total nitrogen, with the synergistic application of the two having the best effect, consistent with findings from Xia et al. (2022) and (He et al., 2017). The increase in soil organic carbon may be attributed to straw returning, which promotes macroaggregate formation, enhances exogenous carbon protection, and stimulates microbial activity, facilitating carbon accumulation (Liao et al., 2024). Simultaneously, biochar application induces a negative priming effect on soil organic carbon decomposition, regulates microbial carbon use efficiency, and promote the accumulation and sequestration of soil carbon (Almagro et al., 2013). Since the carbon and nitrogen sources in straw and biochar are efficiently utilized by soil microorganisms, microbial activity is stimulated. Our study showed that the number of soil microorganisms increased significantly under the combined application of straw and biochar, thus promoting the mineralization and decomposition of soil organic carbon and the fixation of nitrogen, and effectively increasing the content of multiple nutrients in soil (Liang et al., 2014). Therefore, straw and biochar incorporation not only increase soil nutrient content but also contributes to carbon sequestration and emission reduction. In contrast, continuous monocropping leads to inefficient microbial nutrient uptake and unsustainable nutrient supply.
In addition, the soil matrix (physical, chemical, and biological properties) critically influences the short-, medium-, and long-term effects of biochar. Our study demonstrated that biochar application (B and BS treatments) significantly improved soil physicochemical properties, thereby enhancing overall soil quality within a two-year period, which is consistent with previous results (Carvalho et al., 2016; Song et al., 2019). Even after 10 years of application, biochar-amended soils still showed significantly higher organic carbon content and pH compared to controls (Idbella et al., 2024). These long-term effects can be attributed to multiple mechanisms: (1) biochar’s stabilization of non-labile C that gradually becomes microbially available, (2) its protective effect on plant-derived organic inputs that enhances SOC persistence (Ventura et al., 2015), and (3) the formation of nutrient-rich organic coatings during biochar aging that further stabilize labile C (Hagemann et al., 2017). The sustained increase in soil pH is mainly due to biochar’s inherent alkalinity and its slow-release properties over time. These findings collectively demonstrate how soil properties interact with biochar characteristics to produce time-dependent effects on soil quality.
4.3 Regulation of rhizosphere soil microecological environment by straw and its carbonization additions
Soil microorganisms play an important role in maintaining soil health, participating in nutrient cycling, biodegradation and improving soil fertility (Jan et al., 2020). Soil microbial diversity, community structure and community microecological network are important indicators to reflect soil nutrient cycling and farmland environment stability (Ren et al., 2022). Innerebner et al. (2006) pointed out that adding organic materials did not change soil microbial diversity. On the contrary, our results showed that long-term application of straw and biochar could improve the diversity of rhizosphere soil bacteria, indicating that the two kinds of organic materials had positive effects on the diversity of soil bacteria, which was consistent with the results of Chen et al. (2023) and Xun et al. (2016). Among them, the effect of S treatment was the best in α diversity of bacterial. Wang et al. (2020) revealed that the increase of soil diversity index was related to the reproduction of Acidobacteriota, while S and BS treatments significantly increased the relative abundance of Acidobacteriota compared with the CK. Simultaneously, S treatment had more bacterial OTUs and more species at the bacterial species level than other treatments. Our study also showed that Ace, Chao1, Shannon and Simpson indices of rhizosphere soil bacteria were significantly correlated with soil pH, OM, AK and N, and in particular, pH, total N and bacterial diversity index were significantly different. However, the addition of straw and biochar increased the rhizosphere soil fungal Chao1 indices but decreased the Shannon indices with no significant difference, which indicated that the two organic materials did not change soil fungal diversity. In addition, our results showed that fungal Shannon indices was significantly negatively correlated with pH and total nitrogen. Chen et al. (2023) also found that the soil pH was an important factor affecting fungal diversity.
Microbial community composition serves as a critical indicator of soil quality. In our study, the top four dominant bacterial phyla were Proteobacteria, Bacteroidota, Actinobacteriota and Acidobacteriota, a distribution pattern consistent with previous reports (Chen L, et al., 2023b; Kolton et al., 2011). Notably, the addition of organic amendments would alter this microbial community structure (Jiang et al., 2016a). Specifically, our results showed a significant decrease in the relative abundance of Proteobacteria following straw and biochar amendments. Since this phylum contains many documented plant pathogens (Gao et al., 2020), the observed result suggests that organic inputs can effectively suppress soil pathogenic bacterial populations in continuous cropping systems. Beyond pathogen suppression, our results revealed fundamental shifts in microbial life strategies. Microorganisms can be classified as eutrophic species, which thrive in nutrient-rich environments and preferentially utilize labile carbon, or oligotrophic species, which dominate in low-nutrient conditions and metabolize recalcitrant organic matter (Ma et al., 2022; Demoling et al., 2007). we observed: (1) increased the relative abundance of Bacteroidota following straw and biochar amendments, a eutrophic bacterium involved in organic matter decomposition and enhances C/N cycling, its positive correlation with organic matter availability (Fierer et al., 2012); and (2) decreased the relative abundance of Actinobacteriota under BS treatments compare to the CK, an bacteria oligotrophic phylum that dominates in low-nutrient environments (Kim et al., 2015), showing an inverse relationship with soil nutrient availability (Gong et al., 2022).
According to that analysis of the relative abundance of fungi at phylum level, the relative abundance of Ascomycota in rhizosphere soil was significant, which may be related to the continuous cropping system resulted in an increase in the relative abundance of Ascomycota (Gao et al., 2020). Compared with the CK, the abundance of Ascomycota was significantly reduced under straw and biochar application, the addition of organic materials could effectively reduce the adverse effects caused by continuous cropping, which was inconsistent with previous findings (Ren et al., 2021). Simultaneously, the abundance of Basidiomycota was significantly increased in S and BS treatments, which was similar to the study by (Ren et al., 2022). It might be because the application of organic matter increased the soil carbon pool and its turnover rate, regulated the soil nutrient content, and then affected the activities of some microorganisms. In addition, we also found the contents of alkali hydrolyzed nitrogen, available potassium, organic matter and total nitrogen were significantly negatively correlated with Ascomycota and positively correlated with Basidiomycota, which was consistent with the results of Xu et al. (2022).
Beneficial rhizosphere microorganisms mitigate pathogen damage through antimicrobial production and plant immune system activation. Our findings demonstrate that key soil properties, including pH, organic matter, total nitrogen, alkali-hydrolyzable nitrogen, and available potassium, exhibit significant positive correlations with beneficial bacterial taxa (OTU13, OTU10, OTU5, and OTU32) (Figure 8a, Supplementary Table S9). These microorganisms are known to promote plant growth, enhance stress tolerance, and improve crop yiled under abiotic stresses (Li et al., 2022). Similarly, these soil parameters were positively associated with the fungal OTU18 (Figure 8b, Supplementary Table S10), a key rhizosphere fungus with potential biocontrol capabilities against plant pathogens (Chen J et al., 2022). In this study, the addition of straw and biochar significantly increased soil pH and nutrient content. Therefore, the application of both could improve the stress resistance and disease resistance of continuous cropping capsicum by regulating related microorganisms. Alternatively, rhizosphere microorganisms contribute to the cycling of soil substances through their own metabolism, which in turn affects plant growth and development.
4.4 Link between microbial-properties soil-plant under straw and biochar application
Our findings support our hypothesis that the combined application of straw and biochar ameliorates soil microecological degradation in continuous cropping systems by enhancing microbial diversity, improving soil physicochemical properties, and optimizing plant-soil-microbe interactions. Specifically, straw and biochar not only increased soil pH, organic matter, and nutrient availability (nitrogen, phosphorus, and potassium) but also significantly enriched the abundance and diversity of soil microorganisms, particularly beneficial bacteria and fungi. This shift in microbial composition likely contributed to improved soil fertility and plant performance, as evidenced by the significant increases in pepper yield, dry matter weight, and canopy growth (plant height, leaf area, and canopy width). These results align with previous studies demonstrating that soil microbial communities play a pivotal role in soil-root interactions that influence crop productivity (Lambers et al., 2009; Xiao et al., 2019). The observed feedback between microbial community restructuring and plant growth underscores the importance of microbial-mediated processes in overcoming continuous cropping obstacles. Furthermore, our results demonstrate that organic amendments facilitate the transfer of rhizosphere microbial communities, which in turn regulate key soil biochemical processes (e.g., nutrient cycling and organic matter decomposition) in response to changes in soil properties (e.g., pH, organic carbon, and aggregate stability) (Bai et al., 2020; Gao et al., 2021).
In addition, while this study demonstrates the potential benefits of biochar and straw amendments in alleviating continuous cropping obstacles, several limitations must be acknowledged. The relatively short duration (2 years), controlled pot conditions using sieved soil, and absence of field validation may lead to over-optimistic interpretations of the amendments’ efficacy. The observed improvements in soil properties and crop performance, though statistically significant, should be cautiously extrapolated to field conditions where factors like soil heterogeneity, climate variability, and management practices may substantially modify amendment effects. Future research should prioritize long-term field trials to verify these findings under real agricultural systems.
5 Conclusions
Our pot-based study demonstrated that after two years of continuous cropping (fourth season), pepper yield decreased by 49.05% on average compared to the first three seasons, while soil quality and plant performance were improved by the addition of organic materials. Key findings include: (i) compared to the control, pepper yields showed significant average increases of 117.72% (straw treatment) and 144.37% (straw+biochar treatment); (ii) soil physicochemical and biological properties (pH, nutrients, microbial abundance, and enzyme activity) were improved by the combined application of straw and biochar; and (iii) organic amendments altered bacterial/fungal community structure and increased diversity, particularly benefiting soil bacteria. Thus, based on our controlled-environment results, we recommend for sustainable yield improvement in continuous pepper cropping systems, integrated biochar and straw amendments are recommended to harness their synergistic benefits. While this study revealed biochar’s potential in pot conditions, its field performance may vary with soil-climate interactions. Future work should establish optimal application protocols through multi-site field trials and long-term monitoring, enabling tailored biochar use in diverse farming systems.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
CC: Conceptualization, Writing – original draft, Writing – review & editing. ZR: Investigation, Writing – review & editing. WW: Investigation, Writing – review & editing. XL: Investigation, Writing – review & editing. YL: Investigation, Writing – original draft. HL: Investigation, Writing – original draft. YY: Investigation, Writing – original draft. BZ: Investigation, Writing – review & editing. ZG: Investigation, Writing – review & editing. FY: Funding acquisition, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Science and Technology Research Project of Jiangxi Provincial Department of Education (GJJ2401107), the Key Project of Technology Research and Development of Jiangxi Province Take-and-lead Program (20223BBF61008), and the Key Research and Development Project of Ganzhou city, Jiangxi Province of China (GZ2024ZDY032).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fagro.2025.1604073/full#supplementary-material
References
Almagro M., Querejeta J. I., Boix-Fayos C., and Martínez-Mena M. (2013). Links between vegetation patterns, soil C and N pools and respiration rate under three different land uses in a dry Mediterranean ecosystem. J. Soil Sediment 13, 641–653. doi: 10.1007/s11368-012-0643-5
Bai Y. C., Chang Y. Y., Hussain M., Lu B., Zhang J. P., Song X. B., et al. (2020). Soil chemical and microbiological properties are changed by long-term chemical fertilizers that limit ecosystem functioning. Microorganisms 8, 694. doi: 10.3390/microorganisms8050694
Bai J., Li Y., Zhang J., Xu F., Bo Q., Wang Z., et al. (2021). Straw returning and one-time application of a mixture of controlled release and solid granular urea to reduce carbon footprint of plastic film mulching spring maize. J. Clean Prod 280, 124478. doi: 10.1016/j.jclepro.2020.124478
Bao S. D. (2000). Soil Agrochemical Analysis. 3rd Edition (Beijing, China: China Agriculture Press).
Borchard N., Schirrmann M., Cayuela M. L., Kammann C., Wrage-Mönnig N., Estavillo J. M., et al. (2019). Biochar, soil and land-use interactions that reduce nitrate leaching and N2O emissions: A meta-analysis. Sci. Total Environ. 651, 2356–2364. doi: 10.1016/j.scitotenv.2018.10.060
Cairns S., Robertson I., Sigmund G., and Street-Perrott A. (2020). The removal of lead, copper, zinc and cadmium from aqueous solution by biochar and amended biochars. Environ. Sci. Pollut. R 27, 21702–21715. doi: 10.1007/s11356-020-08706-3
Carvalho M. T. M., Madari B. E., Bastiaans L., van Oort P. A. J., Leal W. G. O., Heinemann A. B., et al. (2016). Properties of a clay soil from 1.5 to 3.5 years after biochar application and the impact on rice yield. Geoderma 276, 7–18. doi: 10.1016/j.geoderma.2016.04.013
Chen L., Guo L., Xiong Q., Liao P., Deng X., Pan X., et al. (2023a). Biochar-mediated Cd accumulation in rice grains through altering chemical forms, subcellular distribution, and physiological characteristics. Biochar 5, 48. doi: 10.1007/s42773-023-00248-4
Chen J., He J., Zhang Y., Huang J., Chen Z., Zeng W., et al. (2022). Effects of tobacco plant residue return on rhizosphere soil microbial community. Ann. Microbiol 72, 42. doi: 10.1186/s13213-022-01699-z
Chen Y., Huang B., Hu W., Weindorf D. C., Liu X., and Niedermann S. (2014). Assessing the risks of trace elements in environmental materials under selected greenhouse vegetable production systems of China. Sci. Total Environ. 470-471, 1140–1150. doi: 10.1016/j.scitotenv.2013.10.095
Chen H., Ma J., Wei J., Gong X., Yu X., Guo H., et al. (2018). Biochar increases plant growth and alters microbial communities via regulating the moisture and temperature of green roof substrates. Sci. Total Environ. 635, 333–342. doi: 10.1016/j.scitotenv.2018.04.127
Chen L., Sun S., Yao B., Peng Y., Gao C., Qin T., et al. (2022). Effects of straw return and straw biochar on soil properties and crop growth: A review. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.986763
Chen L., Sun S., Zhou Y., Zhang B., Peng Y., Zhuo Y., et al. (2023b). Straw and straw biochar differently affect fractions of soil organic carbon and microorganisms in farmland soil under different water regimes. Environ. Technol. inno 32, 103412. doi: 10.1016/j.eti.2023.103412
Chen F., Xie Y., Jia Q., Li S., Li S., Shen N., et al. (2023). Effects of the continuous cropping and soilborne diseases of panax ginseng C. A. meyer on rhizosphere soil physicochemical properties, enzyme activities, and microbial communities. Agron 13, 210. doi: 10.3390/agronomy13010210
Cheng J., Lee X., Tang Y., and Zhang Q. (2019). Long-term effects of biochar amendment on rhizosphere and bulk soil microbial communities in a karst region, southwest China. Appl. Soil Ecol. 140, 126–134. doi: 10.1016/j.apsoil.2019.04.017
Demoling F., Figueroa D., and Bååth E. (2007). Comparison of factors limiting bacterial growth in different soils. Soil Biol. Biochem. 39, 2485–2495. doi: 10.1016/j.soilbio.2007.05.002
Duan Y., Gao S., and Hanson B. (2021). Effects of biochar and fertilizer sources on nitrogen uptake by chilli pepper plants under Mediterranean climate. Soil Use Manage 38, 714–728. doi: 10.1111/sum.12759
Fierer N., Lauber C. L., Ramirez K. S., Zaneveld J., Bradford M. A., and Knight R. (2012). Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 6, 1007–1017. doi: 10.1038/ismej.2011.159
Gaffar S., Riquelme C., and Jayachandran K. (2021). Investigating the effects of twelve biochars on the growth of Capsicum annuum ‘Jalapeno’ pepper, microbial population and enzyme activities in soil. J. Hortic. 8, 296.
Gao J., Lei M., Yang L., Wang P., Tao H., and Huang S. (2021). Reduced row spacing improved yield by optimizing root distribution in maize. Eur. J. Agron. 127, 126291. doi: 10.1016/j.eja.2021.126291
Gao J., Pei H., and Xie H. (2020). Synergistic effects of organic fertilizer and corn straw on microorganisms of pepper continuous cropping soil in China. Bioengineered 11, 1258–1268. doi: 10.1080/21655979.2020.1840753
Gong X., Shi J., Fang L., Fang Y., and Wu F. (2022). Effects of flooding on soil chemical properties and microbial community composition on farmland of continuous cropped pepper. Sci. Agr Sin. 55, 2472–2484. doi: 10.3864/j.issn.0578-1752.2022.12.017
Guan Y., Wu M., Che S., Yuan S., Yang X., Li S., et al. (2023). Effects of continuous straw returning on soil functional microorganisms and microbial communities. J. Microbiol 61, 49–62. doi: 10.1007/s12275-022-00004-6
Hagemann N., Joseph S., Schmidt H. P., Kammann C. I., Harter J., Borch T., et al. (2017). Organic coating on biochar explains its nutrient retention and stimulation of soil fertility. Nat. Commun. 8, 1089. doi: 10.1038/s41467-017-01123-0
He L., Zhong Z., and Yang H. (2017). Effects on soil quality of biochar and straw amendment in conjunction with chemical fertilizers. J. Integ Agr 16, 704–712. doi: 10.1016/S2095-3119(16)61420-X
Idbella M., Baronti S., Giagnoni L., Renella G., Becagli M., Cardelli R., et al. (2024). Long-term effects of biochar on soil chemistry, biochemistry, and microbiota: Results from a 10-year field vineyard experiment. Appl. Soil Ecol. 195, 105217. doi: 10.1016/j.apsoil.2023.105217
Innerebner G., Knapp B., Vasara T., Romantschuk M., and Insam H. (2006). Traceability of ammonia-oxidizing bacteria in compost-treated soils. Soil Biol. Biochem. 38, 1092–1100. doi: 10.1016/j.soilbio.2005.09.008
Jan U., Feiwen R., Masood J., and Chun S. C. (2020). Characterization of soil microorganism from humus and indigenous microorganism amendments. Mycobiology 48, 392–398. doi: 10.1080/12298093.2020.1816154
Jiang X., Haddix M. L., and Cotrufo M. F. (2016b). Interactions between biochar and soil organic carbon decomposition: Effects of nitrogen and low molecular weight carbon compound addition. Soil Biol. Biochem. 100, 92–101. doi: 10.1016/j.soilbio.2016.05.020
Jiang Y., Liang Y., Li C., Wang F., Sui Y., Suvannange N., et al. (2016a). Crop rotations alter bacterial and fungal diversity in paddy soils across East Asia. Soil Biol. Biochem. 95, 250–261. doi: 10.1016/j.soilbio.2016.01.007
Jiao N., Song X., Song R., Yin D., and Deng X. (2022). Diversity and structure of the microbial community in rhizosphere soil of Fritillaria ussuriensis at different health levels. Peer J. 10, e12778. doi: 10.7717/peerj.12778
Kebede T., Berhe D. T., and Zergaw Y. (2023). Effects of biochar and compost application on soil properties and on the growth and yield of hot pepper (Capsicum annuum L.). Appl. Environ. Soil Sci. 2023, 8546135. doi: 10.1155/2023/8546135
Khan I., Iqbal B., Khan A. A., Inamullah, Rehman A., Fayyaz A., et al. (2022). The interactive impact of straw mulch and biochar application positively enhanced the growth indexes of maize (Zea mays L.) crop. Agron. (Basel) 12, 2584. doi: 10.3390/agronomy12102584
Kim Y. S., Lee I. K., and Yun B. S. (2015). Antagonistic effect of streptomyces sp. BS062 against botrytis diseases. Mycobiology 43, 339–342. doi: 10.5941/MYCO.2015.43.3.339
Kocsis T., Ringer M., and Biró B. (2022). Characteristics and applications of biochar in soil–plant systems: A short review of benefits and potential drawbacks. Appl. Sci. 12, 4051. doi: 10.3390/app12084051
Kolton M., Harel Y. M., Pasternak Z., Graber E. R., Elad Y., and Cytryn E. (2011). Impact of biochar application to soil on the root-associated bacterial community structure of fully developed greenhouse pepper plants. Appl. Environ. Microbiol 77, 4924–4930. doi: 10.1128/AEM.00148-11
Lambers H., Mougel C., Jaillard B., and Hinsinger P. (2009). Plant-microbe-soil interactions in the rhizosphere: an evolutionary perspective. Plant Soil 321, 83–115. doi: 10.1007/s11104-009-0042-x
Li H., Lou J., Chen X., Dou Y., Zhang D., and Wei M. (2024). Effects of sweet pepper straw biochar on soil microbial communities and growth of continuously cropped cucumber. Ann. Microbiol 74, 12. doi: 10.1186/s13213-024-01755-w
Li H., Sun Q., and Zhao S. (2000). Principles and techniques of plant physiology and biochemistry experiments (Beijing: Higher Education Press).
Li D., Zhou C., Wu Y., An Q., Zhang J., Fang Y., et al. (2022). Nanoselenium integrates soil-pepper plant homeostasis by recruiting rhizosphere-beneficial microbiomes and allocating signaling molecule levels under Cd stress. J. Hazard Mater 432, 128763. doi: 10.1016/j.jhazmat.2022.128763
Liang F., Li G. T., Lin Q. M., and Zhao X. R. (2014). Crop yield and soil properties in the first 3 years after biochar application to a calcareous soil. J. Integr. Agr 13, 525–532. doi: 10.1016/S2095-3119(13)60708-X
Liang J. P., Xue Z. Q., Yang Z. Y., Chai Z., and Shi Z. Y. (2021). Effects of microbial organic fertilizers on Astragalus membranaceus growth and rhizosphere microbial community. Ann. Microbiol 71, 11. doi: 10.1186/s13213-021-01623-x
Liao Y., Awan M. I., Aamer M., Liu J., Liu J., Hu B., et al. (2024). Evaluating short-term effects of rice straw management on carbon fractions, composition and stability of soil aggregates in an acidic red soil with a vegetable planting history. Heliyon 10, e23724. doi: 10.1016/j.heliyon.2023.e23724
Liu S., Li D., Huang J., Ma C., Wang H., Yu Z., et al. (2021). Temporal and spatial distribution characteristics of rice stalk resources and its potential of synthetic fertilizers substitution returning to farmland in China from 1988 to 2018. Trans. Chin Soc. Agric. Eng 37, 151–161. doi: 10.11975/j.issn.1002-6819.2021.11.017
Liu Y., Li J., Jiao X., Li H., Hu T., Jiang H., et al. (2022). Effects of biochar on water quality and rice productivity under straw returning condition in a rice-wheat rotation region. Sci. total Environ. 819, 152063–152063. doi: 10.1016/j.scitotenv.2021.152063
Liu X., Zheng J., Zhang D., Cheng K., Zhou H., Zhang A., et al. (2016). Biochar has no effect on soil respiration across Chinese agricultural soils. Sci. Total Environ. 554-555, 259–265. doi: 10.1016/j.scitotenv.2016.02.179
Ma L., Li Y., Wei J., Zhou X., Li Z., Li G., et al. (2022). Effects of continuous chemical fertilizer application and straw returning on soil enzyme activity, bacterial community and co-occurrence patterns in a fluvo-aquic soil. J. Plant Nutr. Fert 28, 1353–1363. doi: 10.11674/zwyf.2021621
Mao Q., He B., Ma M., Wang L., Koike T., and Agathokleous E. (2022). Effects of biochar on karst lime soil nutrients, soil microbial communities and physiology of Sichuan pepper plants. Ann. Appl. Biol. 181, 357–366. doi: 10.1111/aab.12787
Nash J. A., Miesel J. R., Bonito G. M., Sakalidis M. L., Ren H., Warnock D., et al. (2021). Biochar restructures plant–soil–microbe relationships in a woody cropping system. Soil Biol. Biochem. 85, 2019–2039. doi: 10.1002/saj2.20334
Ortiz-Liébana N., Zotti M., Barquero M., and González-Andrés F. (2022). An organic fertilizer ‘Doped’ with a Bacillus strain improves melon and pepper yield, modifying the rhizosphere microbiome with negligible changes in the bulk soil microbiome. Agronomy 12, 2620. doi: 10.3390/agronomy12112620
Poormansour S., Razzaghi F., and Sepaskhah A. R. (2019). Wheat straw biochar increases potassium concentration, root density, and yield of faba bean in a sandy loam soil. Commun. Soil Sci. Plant Anal. 50, 1799–1810. doi: 10.1080/00103624.2019.1635145
Ren T., Feng H., Xu C., Xu Q., Fu B., Azwar E., et al. (2022). Exogenous application and interaction of biochar with environmental factors for improving functional diversity of rhizosphere's microbial community and health. Chemosphere 294, 133710. doi: 10.1016/j.chemosphere.2022.133710
Ren T., Gao W., Xu C., Li M., Feng H., Zhang L., et al. (2021). Novel approaches of regulating soil micro-ecological environment based on modified biochar in plastic greenhouse. Environ. Technol. Inno 23, 101740. doi: 10.1016/j.eti.2021.101740
Song D., Xi X., Zheng Q., Liang G., Zhou W., and Wang X. (2019). Soil nutrient and microbial activity responses to two years after maize straw biochar application in a calcareous soil. Ecotoxicol Environ. Saf. 180, 348–356. doi: 10.1016/j.ecoenv.2019.04.073
Ventura M., Alberti G., Viger M., Jenkins J. R., Girardin C., Baronti S., et al. (2015). Biochar mineralization and priming effect on SOM decomposition in two European short rotation coppices. GCB Bioenergy 7, 1150–1160. doi: 10.1111/gcbb.12219
Wang Y. B. (2018a). Experimental techniques for ecological remediation of soil pollution (Beijing, China: Science Press).
Wang X. and Huang J. (2015). Principles and techniques of plant physiology and biochemistry experiments. 3rd edition (Beijing: Higher Education Press).
Wang X., Jia Z., Liang L., Zhao Y., Yang B., Ding R., et al. (2018b). Changes in soil characteristics and maize yield under straw returning system in dryland farming. Field Crop Res. 218, 11–17. doi: 10.1016/j.fcr.2017.12.003
Wang H., Wang S., Wang R., Wang X., and Li J. (2020). Conservation tillage increased soil bacterial diversity and improved soil nutrient status on the Loess Plateau in China. Arch. Agron. Soil Sci. 66, 1509–1519. doi: 10.1080/03650340.2019.1677892
Wu L. D., Lciu Y. T., Lin S. Y., Liao C. S., and Zhong J. X. (2023). Effects of combined application of biochar and chemical fertilizer on yield, quality and fertilizer utilization of pepper. Soil Fert Sci. China 60(8), 144–151. doi: 10.11838/sfsc.1673-6257.22396
Xia H., Riaz M., Liu B., Li Y., El-Desouki Z., and Jiang C. (2022). Over two years study: Peanut biochar promoted potassium availability by mediating the relationship between bacterial community and soil properties. Appl. Soil Ecol. 176, 104485. doi: 10.1016/j.apsoil.2022.104485
Xiao D., Xiao S., Ye Y., Zhang W., He X., and Wang K. (2019). Microbial biomass, metabolic functional diversity, and activity are affected differently by tillage disturbance and maize planting in a typical karst calcareous soil. J. Soil Sediment 19, 809–821. doi: 10.1007/s11368-018-2101-5
Xu C., Li Y., Hu X., Zang Q., Zhuang H., and Huang L. (2022). The Influence of organic and conventional cultivation patterns on physicochemical property, enzyme activity and microbial community characteristics of paddy Soil. Agriculture 12, 121. doi: 10.3390/agriculture12010121
Xu G. H. and Zhen H. Y. (1986). Handbook of Methods for Soil Microbial Analysis (Beijing, China: Agricultural Press).
Xun W., Zhao J., Xue C., Zhang G., Ran W., Wang B., et al. (2016). Significant alteration of soil bacterial communities and organic carbon decomposition by different long-term fertilization management conditions of extremely low-productivity arable soil in South China. Environ. Microbiol 18, 1907–1917. doi: 10.1111/1462-2920.13098
Zhang Y., Liu Y., Zhang G., Guo X., Sun Z., and Li T. (2018). The effects of rice straw and biochar applications on the microbial community in a soil with a history of continuous tomato planting history. Agronomy 8, 65. doi: 10.3390/agronomy8050065
Zhang N., Nunan N., Hirsch P. R., Sun B., Zhou J., and Liang Y. (2021). Theory of microbial coexistence in promoting soil–plant ecosystem health. Biol. Fert Soils 57, 897–911. doi: 10.1007/s00374-021-01586-w
Keywords: straw, biochar, continuous cropping system, soil physicochemical properties, soil microecological environment
Citation: Cheng C, Ren Z, Wang W, Li X, Liao Y, Luo H, Yang Y, Zhu B, Gao Z and Yao F (2025) Synergistic effects of straw and biochar co-application on soil biological restoration and pepper yield enhancement in red soils from China. Front. Agron. 7:1604073. doi: 10.3389/fagro.2025.1604073
Received: 01 April 2025; Accepted: 20 May 2025;
Published: 03 June 2025.
Edited by:
Gazala Nazir, Punjab Agricultural University, IndiaReviewed by:
Amit Anil Shahane, Central Agricultural University, IndiaHermes Pérez Hernández, National Institute of Forestry and Agricultural Research (INIFAP), Mexico
Copyright © 2025 Cheng, Ren, Wang, Li, Liao, Luo, Yang, Zhu, Gao and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fengxian Yao, ZmVuZ3hpYW55YW9AYWxpeXVuLmNvbQ==
 Chen Cheng
Chen Cheng Zhaoyan Ren1
Zhaoyan Ren1 Fengxian Yao
Fengxian Yao