- Department of Anesthesiology and Perioperative Medicine, University of California Los Angeles, Los Angeles, CA, United States
Perioperative Neurocognitive disorders, including delirium and long-term cognitive dysfunction following surgery, are an ever-increasing focus of investigation for anesthesiologists and researchers. The desire to bring patients safely through the perioperative period with an absolute minimum impact of the anesthetic, surgery, and post-operative period on the patient's functional status has brought a high level of scrutiny to entities that most impact patients. Perioperative neurocognitive disorders (PND) have the potential to vastly alter patient functional status after surgery and anesthesiologists are often the first physician asked about the effects this may have on the patient during the informed consent process. While the underlying mechanisms of PND are not well understood, more tools are being brought to bear with non-invasive imaging shedding light on the pathophysiology of PND. In this narrative mini-review, we discuss the current investigation into PND with a focus on non-invasive imaging and preventative strategies that are being employed to better protect patients.
Introduction
“Is this surgery or anesthetic going to harm my family member”s brain? Will my family member lose some of their cognitive function after surgery?” These are questions that many anesthesiologists are faced with during the informed consent process. With the many advances in the fields of anesthesiology and surgery, patients are more focused on returning to their baseline or an improved level of function, not just survival through the immediate perioperative period. There are over 300 million surgical procedures performed worldwide every year with over 100 million more needed (1) and as the surgical population ages, perioperative neurocognitive disorders (PND) are significantly impact patient outcomes. Patients with poor neurocognitive function are likely to have experienced post operative delirium (POD), delayed cognitive recovery, increased time requiring mechanical ventilation, increased ICU length of stay, increased hospital stay, dramatically increased healthcare costs, and higher risk of dementia (2–5).
In this mini-review we focus on the non-invasive imaging modalities of MRI and PET imaging. MRI imaging is likely the most promising modality to be used in future studies as it is non-invasive, exposes patients to no ionizing radiation exposure, and is frequently ordered on patients across the healthcare spectrum. PET imaging, while including exposure to some ionizing radiation, provides additional metabolic information not easily obtained elsewhere and is briefly discussed as well. We utilized a systematic approach to identifying the studies discussed utilizing PubMed database searches with the following keywords: “MRI,” “Imaging,” “Perioperative Neurocognitive Disorder,” “Cognitive Dysfunction,” and “Delirium.” Given the mini-review format, we focused on work with good methodological quality, clearly defined cases and controls, and a focus on specifically investigating POD/PND in their scope.
Nomenclature
Given the severe impacts that PND can have on patient outcomes, significant work has been done to better describe and classify them (6). The most recent consensus PND terminology describes cognitive impairment in the perioperative period: preoperative neurocognitive disorders in the period leading up to surgery, delirium occurring up to 7 days after surgery, cognitive impairment up to 30 days post-operatively (termed delayed neurocognitive recovery), and cognitive impairment up to 12 months post-operatively (post-operative neurocognitive disorders) (Figure 1). The utilization of standardized nomenclature will better support the investigation of specific pathology seen in different time frames of the continuum of disease that is PND.
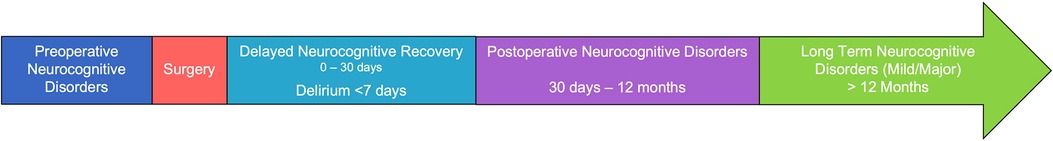
Figure 1. Timeline and nomenclature for perioperative neurocognitive disorders (PND) classification.
Susceptibility and precipitating factors
PND likely stem from a multifaceted interaction between a patient's underlying comorbid susceptibilities and factors related to their surgical course (7, 8). Multiple risk factors have been proposed, including aging, however, the exact mechanisms underlying the development of PND remain poorly understood (9–15). Advanced age (16–18) and prior cognitive impairment (18–20) remain the most highly correlated risk factors with PND, but additional factors such as educational level, cardiopulmonary disease, and malnutrition appear to play significant roles as well. One crucial area which almost certainly plays an important role in the development of PND is that of surgical-stress induced neuro-inflammatory processes (7, 21–28). Many risk factors have been identified and both surgery and anesthesia can increase neuro-inflammation leading to serious exacerbation of underlying preoperative neuro-inflammation (21, 23).
Neuroinflammation
Large amounts of evidence have been produced demonstrating that surgical stress leads to a systemic inflammatory response which produces host of downstream effects, including neuroinflammation, blood brain barrier (BBB) disruption (26), and neuroimmune responses including microglia activation (7, 29). High hippocampal levels of interleukin (IL)1β, TNFα, and IL6 are associated with cognitive decline after surgery in rodents (29, 30). In human patients undergoing surgery, a strong neuro-inflammatory response leads to detectable inflammatory markers found in the cerebrospinal fluid (CSF) and blood (IL-1β, IL1ra, IL2, IL6, IL8, IL10) within 12 h of surgery (23, 31–34). These pro-inflammatory cytokines lead to the disruption of the BBB integrity and further induces neuro-inflammation (26, 35–37). Through a permeable BBB, bone marrow-derived macrophages (BM-DM) are attracted into the brain parenchyma by trauma-induced expression of chemokine MCP-1 (29, 35). Within the hippocampus and other areas, BM-DM synthesize and release a variety of pro-inflammatory cytokines that interfere with brain processes required for cognition, including learning and memory (35). In this setting, there is a transient yet profound downregulation of the human brain immune system, measured as a decrease in glial activity within the early postoperative period. These studies provide insights into the possible molecular underpinnings of PND and there are ongoing efforts to correlate them with specific cognitive domain changes and tissue changes in the brain.
Imaging techniques
While excellent ongoing work is occurring in mouse models of PND and biomarker studies, these methods require invasive access which is challenging when applied to patients. This is especially true when this access is needed for obtaining CSF, serum, or brain tissue sampling to investigate the underlying pathophysiology. However, one of the most exciting areas of investigation has been utilizing non-invasive imaging technology to shed light on the pathophysiology of PND. To date there are a paucity of studies, however, the potential is vast for meaningful insights these studies may provide. Magnetic resonance imaging (MRI) based diffusion tensor imaging (DTI) can be used to examine regional neuro-inflammatory-induced brain tissue changes (38–42). Using DTI, several quantitative indices, including mean diffusivity (MD), can be calculated and used to evaluate brain tissue injury (43, 44). MD of water within tissue is influenced by the presence of tissue barriers (45), and extra-cellular/extra-axonal fluid. In acute neuro-inflammation and tissue changes, neuronal and axonal swelling increase tissue barriers, reduce extracellular volume, and escalate cytotoxic edema; all factors contributing to reduced MD values (38, 40, 46, 47). Other methods including relative relaxivity (RR), BBB disruption, intracranial volume (ICV), white matter hyperintensity (WMH), hippocampal volume, cerebral blood flow mapping (CBF), cortical thickness, and overall gray matter volume (GMV) changes can be correlated with incidence of delirium or PND in the 7 day to 12 month postoperative period and beyond.
Imaging in postoperative delirium
Despite the challenges of imaging patients with POD, multiple groups have endeavored to correlate some of these imaging techniques with POD. These groups have utilized data from the Successful Aging after Elective Surgery (SAGES) study which collected large amounts of clinical data from patients 70 and older with daily assessments during hospitalization including biomarker, neuroimaging, and cognitive reserve markers. Saczynski and colleagues utilized this mixed surgical population (TKA/THA and colectomy) which had MRI scans performed on a subset of the patients, however, they were not able to discover a correlation between cortical atrophy and POD with multiple additional analysis of this image data set by other groups also failing to find correlations except for WMH (48). Cavallari and colleagues utilized preoperative DTI imaging in their 2016 study which interestingly revealed that decreased fractional anisotropy and increased axial, mean, and radial diffusivity in the basal forebrain, thalamus, cerebellum, and hippocampus were associated with delirium incidence and severity (49). Their follow-up study showed that in the case of DTI measurements done one year after surgery, POD was associated with a decrease in fractional anisotropy, and an increase in MD in the frontal, parietal, and temporal lobes (50). They also found that cognitive dysfunction was correlated with a decrease in FA and increase in MD in the white matter of parietal, posterior temporal, and occipital lobes. Hshieh and colleagues also utilize the SAGES data set to assess cerebral blood flow (CBF) but did not show a significant association between global CBF and POD but did show a correlation with test results from trails B and digit symbol substitution test (51). Racine and colleagues utilized cortical thickness in brain regions that have been associated with Alzheimer's disease but did not find an association with the risk of delirium but that in specifically the superior parietal region, cortical thickness was smaller in patients with POD (52).
Root and colleagues performed a separate case-control study utilizing patients undergoing pneumonectomy/lobectomy for NSCLC and showed that delirium was associated with a higher degree of white matter hyperintensity compared to age and sex matched controls without delirium. However, there was no association between cortical atrophy and delirium which is consistent with prior studies. Fislage and colleagues utilized the BioCog study to perform a prospective observational cohort investigation associating preoperative thalamic MD values with POD (53). They were able to demonstrate that MD changes in bilateral hemispheres, pulvinar nuclei, mediodorsal nuclei, and the left anterior nucleus were associated with POD (53). These studies appear to show that overall cortical atrophy and CBF may not be a correlate of POD, however, specific brain regions are likely implicated and further work tying the MD changes and WMH in areas such as the thalamus and hippocampus are likely to be helpful.
MRI in delayed neurocognitive recovery and neurocognitive disorders after surgery
Several groups have focused on neurocognitive impairment in the period after 7 days postoperatively to beyond one year. This would include delayed neurocognitive recovery to major/minor long term neurocognitive disorders. Chen and colleagues performed a cross-sectional study in abdominal surgical patients and found hippocampal volume loss was more highly present in the patient population with cognitive impairment after surgery (54). This is in contrast to a study performed by Price and colleagues in which a total knee arthroplasty patient population (case-control study) exhibited cognitive impairment >20% in the first 3 months and 9% at 1 year correlated with 2 week MRI scans which ultimately showed no difference in hippocampal volume (55). They did demonstrate an increase in volumes of leukoaraiosis and lacunae which they correlated with impairment in memory at 3 weeks and 1 year post-operatively and correlated total brain volume reduction with a decline in executive function (55). Sato and colleagues utilized a prospective study in breast surgery patients with baseline and post-op day 5 MRI scans coupled with a cognition battery of tests to study associations between the changes in GMV and changes to patient cognition scores (56). They correlated a reduction in thalamic volume with cognitive decline as they defined it at 5 days post operatively. Vandiver et al. performed a retrospective analysis of the UCLA lung transplant database to identify patients who received MRI scan pre and post-operatively but were not diagnosed with CVA and showed that these patients have high levels of cognitive impairment associated with decreased gray matter volumes in the frontal, prefrontal, parietal, temporal, anterior cingulate, putamen, and cerebella cortices (57). Studying a different mechanism entirely, Lascola and colleagues utilized delayed contrast extravasation subtraction to determine BBB permeability and found that nearly half of patients had increases in post-operative BBB permeability with regional variations in permeability associated with lower cognitive performance suggesting that this mechanism may also play a role in PND (58).
Functional MRI and connectivity in PND
Additional groups have investigated cognition in the perioperative period, utilizing functional MRI to assess functional connectivity. Browndyke and colleagues assessed a small number of patients undergoing cardiac surgery vs. non-surgical controls and showed significant differences in resting-state functional connectivity (RSFC) and global cognitive change suggesting that RSFC may be tied to cognitive changes 6 weeks after cardiac surgery and could be used as a diagnostic marker of PND (59). Nir and colleagues utilized a similar technique in patients that only underwent anesthesia (and not surgery) to assess RSFC trajectory in the post-anesthesia recovery period (60). They showed a transient global reduction in anticorrelated activity after emergence from anesthesia which did return to baseline by the following day which may suggest that without the additional inflammatory insult of surgical stress that the anesthetics may contribute to but might not be adequate to induce PND. However, they did not perform cognitive testing in these patients making definitive conclusion difficult to draw. Yang and colleagues have also utilized RSFC focused fMRI to assess functional connectivity density (FCD) in patients in the first 7 days after surgery. They were able to correlate changes in FCD with the results of a battery of cognitive tests. They demonstrated post-operative declines in FCD present in the supplementary motor area (SMA) were correlated with declines in cognition measured by Warrington's recognition memory test (60).
PET imaging in PND
Additional imaging technologies such as positron emission tomography (PET) have also been utilized in the investigation of PND. Forsberg and colleagues utilized [11C]PBR28 brain PET imaging to evaluate brain immune activation in the perioperative period in patients undergoing abdominal surgery. They were able to show a global downregulation of gray matter at 3–4 days postoperatively (compared to baseline) correlated with changes in the Stroop Color-Word Test performance which suggests likely post-surgical impairment of cognitive function (21). These types of changes may also be seen in other neuroinflammatory conditions such as multiple sclerosis as well as general immune activation in the brain such as the microglial activation of the brain after LPS administration.
Brain region changes and cognition
Tying specific brain areas to cognitive impairment is complex and there is significant interplay between regions that mediate cognitive function. However, multiple brain areas, including the prefrontal cortex, caudate, and hippocampus have been implicated in cognition. Injury in the prefrontal cortex, which receives input and reciprocal connections to limbic sites, has adverse implications in planning complex cognitive behavior, personality expression, decision making, and moderating social behavior (61). Procedural and associative learning are linked to the caudate nuclei, which are an integral part of the cortico-basal ganglia-thalamic loop and are well recognized for involvement in cognition (62–64). The hippocampus has extensive connections with the prefrontal cortex, including direct reciprocal connections between the medial prefrontal cortex and the medial temporal lobe, unidirectional projections from the hippocampus to the ventral medial prefrontal cortex, and bidirectional connections from the subiculum and neocortical medial temporal regions (65). The fornix is constituted from fibers of the hippocampus and projects to the mammillary bodies, which serve vital roles in learning and memory (66). Although the role of particular brain sites in PNCD is unclear, the studies discussed above appear to strongly suggest that compromised global gray matter volume, hippocampal volume reduction, white matter hyperintensity changes, and changes in resting-state functional connectivity are present in the postoperative period in patients that develop cognitive deficits. Additional brain regions showed impaired perfusion in patients with delirium, including the inferior frontal, parietal, caudate, thalami, and occipital sites that improved after delirium resolution (53, 67).
Addressing PND
As we learn more about the underlying mechanisms of PND, more tools will become available for identification of those most at risk, prevention strategies, and interventions to address PND in the post-operative period. These have mainly focused on preoperative measures starting with risk factor identification, avoidance of medications associated with POD including benzodiazepines and anticholinergics (68), avoidance of long fasting duration (69), and cognitive prehabilitation (70) when possible. Intraoperative interventions such as increased utilization of neuraxial and regional anesthetics, close monitoring of anesthetic depth, use of total intravenous anesthesia (TIVA), and the addition of dexmedetomidine all have an evidential basis suggesting some benefit in high-risk patients and perhaps in the general population as well (71–73). However, the efficacy of these interventions is still controversial for many intraoperative modifications. Other interventions such as avoidance of intraoperative hypotension, hypothermia, hypercarbia, acidosis, and close monitoring of cerebral oxygen oximetry are all intuitively useful for the avoidance of neurologic hypoperfusion, hypoxia, and neuronal injury, but investigation has not shown consistent reduction in PND despite controlling these factors.
A multitude of investigations have been performed looking at ways to both prevent and treat POD and delayed neurocognitive recovery in the immediate post operative period and several strategies appear to be efficacious. Appropriate sleep promotion is key as sleep disturbance is associated with increased risk of cognitive impairment after surgery (74). While specific pharmacologic therapies may be controversial, dexmedetomidine and melatonin do appear to have some benefit. In fact, Zhang and colleagues presented evidence that dexmedetomidine may improve long term neurologic outcomes in patients undergoing non-cardiac surgery up to 3 years later (75). Other preventative post-operative interventions such as avoidance of known delirium associated medications, use of NSAIDS, and early recovery after surgery (ERAS) programs appear to have also improved neurologic outcomes in some studies (68, 76). Pharmacologic treatment of active POD is controversial, and the current recommendations are to treat hyperactive delirium where patient safety is threatened (77). There is evidence that dexmedetomidine may reduce delirium duration (78), but routine use of other agents such as antipsychotic administration for hypoactive and normoactive delirium has not meaningfully reduced delirium in most trials (79).
Summary
Perioperative neurocognitive disorders are both common and high impact disruptions to patient outcomes in the post-operative setting. While we have significantly advanced our understanding of risk factors, likely precipitating factors, and possible prevention strategies, there is much to be done in the understanding of pathophysiologic mechanisms. This review has focused on the current use of imaging technologies to investigate multiple mechanisms implicated in PND and the major findings that have thus far been elucidated. Much more work needs to be done to understand this important entity in clinical care and to tie our improved understanding of PND with prevention and treatment strategies that will be the most effective, ultimately improving patient outcomes and lives.
Author contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Funding
MP is supported by a Seed Grant from the Department of Anesthesiology and Perioperative Medicine at UCLA, Los Angeles, CA.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BBB, blood brain barrier; BM-DM, bone marrow-derived macrophages; CBF, cerebral blood flow; CSF, cerebrospinal fluid; DNC, delayed neurocognitive recovery; DTI, diffusion tensor imaging; ERAS, early recovery after surgery; ICU, fMRI, functional magnetic resonance imaging; GMV, gray matter volume; intensive care unit; ICV, intracranial volume; LTNCD, long term neurocognitive disorders; MD, mean diffusivity; MRI, magnetic resonance imaging; NSAID, non-steroidal anti-inflammatory drug; NSCLC, non-small cell lung cancer; PET, positron emission tomography; PND, perioperative neurocognitive disorders; POD, post operative delirium; RR, relative relaxivity; RSFC, resting-state functional connectivity; SAGES, successful aging after elective surgery; THA, total hip arthroplasty; TKA, total knee arthroplasty; TIVA, total intravenous anesthesia; WMH, white matter hyperintensity.
References
1. Meara JG, Leather AJM, Hagander L, Alkire BC, Alonso N, Ameh EA, et al. Global surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet. (2015) 386(9993):569–624. doi: 10.1016/s0140-6736(15)60160-x
2. Vandiver MS, Vacas S. Interventions to improve perioperative neurologic outcomes. Curr Opin Anaesthesiol. (2020) 33(5):661–7. doi: 10.1097/ACO.0000000000000905
3. Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. (2009) 110(3):548–55. doi: 10.1097/ALN.0b013e318195b569
4. Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, et al. Neurological outcome res grp C. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. (2001) 344(6):395–402. doi: 10.1056/NEJM200102083440601
5. Newman S, Stygall J, Hirani S, Shaefi S, Maze M. Postoperative cognitive dysfunction after noncardiac surgery: a systematic review. Anesthesiology. (2007) 106(3):572–90. doi: 10.1097/00000542-200703000-00023
6. Evered L, Silbert B, Knopman DS, Scott DA, DeKosky ST, Rasmussen LS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Anesth Analg. (2018) 127(5):1189–95. doi: 10.1213/ane.0000000000003634
7. Vacas S, Degos V, Feng X, Maze M. The neuroinflammatory response of postoperative cognitive decline. Br Med Bull. (2013) 106:161–78. doi: 10.1093/bmb/ldt006
8. Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International study of post-operative cognitive dysfunction. Lancet. (1998) 351(9106):857–61. S0140673697073820 9525362
9. Abildstrom H, Rasmussen LS, Rentowl P, Hanning CD, Rasmussen H, Kristensen PA, et al. Cognitive dysfunction 1–2 years after non-cardiac surgery in the elderly. ISPOCD group. International study of post-operative cognitive dysfunction. Acta Anaesthesiol Scand. (2000) 44(10):1246–51. doi: 10.1034/j.1399-6576.2000.441010.x
10. Altay S, Gurdogan M, Kaya C, Kardas F, Zeybey U, Cakir B, et al. The etiology and age-related properties of patients with delirium in coronary intensive care unit and its effects on inhospital and follow up prognosis. Ideggyogy Sz. (2020) 73(05–06):189–97. doi: 10.18071/ISZ.73.0189
11. Dyer CB, Ashton CM, Teasdale TA. Postoperative delirium. A review of 80 primary data-collection studies. Arch Intern Med. (1995) 155(5):461–5. doi: 10.1001/archinte.1995.00430050035004
12. Hood R, Budd A, Sorond FA, Hogue CW. Peri-operative neurological complications. Anaesthesia. (2018) 73(Suppl 1):67–75. doi: 10.1111/anae.14142
13. Lemstra AW, Kalisvaart KJ, Vreeswijk R, van Gool WA, Eikelenboom P. Pre-operative inflammatory markers and the risk of postoperative delirium in elderly patients. Int J Geriatr Psychiatry. (2008) 23(9):943–8. doi: 10.1002/gps.2015
14. O'Keeffe ST, Ni Chonchubhair A. Postoperative delirium in the elderly. Br J Anaesth. (1994) 73(5):673–87. doi: 10.1093/bja/73.5.673
15. Whitlock EL, Vannucci A, Avidan MS. Postoperative delirium. Minerva Anestesiol. (2011) 77(4):448–56. PMCID: PMC3615670.21483389
16. Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. (2008) 108(1):18–30. doi: 10.1097/01.anes.0000296071.19434.1e
17. Chen Y, Qin J. Modified frailty index independently predicts postoperative delirium and delayed neurocognitive recovery after elective total joint arthroplasty. J Arthroplasty. (2021) 36(2):449–53. doi: 10.1016/j.arth.2020.07.074
18. Kang SY, Seo SW, Kim JY. Comprehensive risk factor evaluation of postoperative delirium following major surgery: clinical data warehouse analysis. Neurol Sci. (2019) 40(4):793–800. doi: 10.1007/s10072-019-3730-1
19. Bekker A, Lee C, de Santi S, Pirraglia E, Zaslavsky A, Farber S, et al. Does mild cognitive impairment increase the risk of developing postoperative cognitive dysfunction? Am J Surg. (2010) 199(6):782–8. doi: 10.1016/j.amjsurg.2009.07.042
20. Silbert B, Evered L, Scott DA, McMahon S, Choong P, Ames D, et al. Preexisting cognitive impairment is associated with postoperative cognitive dysfunction after hip joint replacement surgery. Anesthesiology. (2015) 122(6):1224–34. doi: 10.1097/aln.0000000000000671
21. Forsberg A, Cervenka S, Jonsson Fagerlund M, Rasmussen LS, Zetterberg H, Erlandsson Harris H, et al. The immune response of the human brain to abdominal surgery. Ann Neurol. (2017) 81(4):572–82. doi: 10.1002/ana.24909
22. Cao Y, Li Z, Ma L, Ni C, Li L, Yang N, et al. Isoflurane-induced postoperative cognitive dysfunction is mediated by hypoxia-inducible factor-1α-dependent neuroinflammation in aged rats. Mol Med Rep. (2018) 17(6):7730–6. doi: 10.3892/mmr.2018.8850
23. Hirsch J, Vacas S, Terrando N, Yuan M, Sands LP, Kramer J, et al. Perioperative cerebrospinal fluid and plasma inflammatory markers after orthopedic surgery. J Neuroinflammation. (2016) 13(1):211. doi: 10.1186/s12974-016-0681-9
24. Kalman J, Juhasz A, Bogats G, Babik B, Rimanoczy A, Janka Z, et al. Elevated levels of inflammatory biomarkers in the cerebrospinal fluid after coronary artery bypass surgery are predictors of cognitive decline. Neurochem Int. (2006) 48(3):177–80. doi: 10.1016/j.neuint.2005.10.007
25. Peng L, Xu L, Ouyang W. Role of peripheral inflammatory markers in postoperative cognitive dysfunction (POCD): a meta-analysis. PLoS One. (2013) 8(11):e79624. doi: 10.1371/journal.pone.0079624
26. Sharma K, Kalakoti P, Nanda A, Sun H. Chapter 26—blood-brain barrier disruption during neuroinflammation. In: Minagar A, editors. Neuroinflammation (second edition). San Diego, CA: Academic Press (2018). p. 529–39.
27. Shih R-H, Wang C-Y, Yang C-M. NF-kappaB Signaling pathways in neurological inflammation: a Mini review. Front Mol Neurosci. (2015) 8:77. doi: 10.3389/fnmol.2015.00077
28. Su X, Feng X, Terrando N, Yan Y, Chawla A, Koch LG, et al. Dysfunction of inflammation-resolving pathways is associated with exaggerated postoperative cognitive decline in a rat model of metabolic syndrome. Mol Med. (2013) 18:1481–90. doi: 10.2119/molmed.2012.00351
29. Vacas S, Degos V, Tracey KJ, Maze M. High-mobility group box 1 protein initiates postoperative cognitive decline by engaging bone marrow-derived macrophages. Anesthesiology. (2014) 120(5):1160–7. doi: 10.1097/ALN.0000000000000045
30. Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, et al. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol. (2010) 68(3):360–8. doi: 10.1002/ana.22082
31. Buvanendran A, Kroin JS, Berger RA, Hallab NJ, Saha C, Negrescu C, et al. Upregulation of prostaglandin E2 and interleukins in the central nervous system and peripheral tissue during and after surgery in humans. Anesthesiology. (2006) 104(3):403–10. doi: 10.1097/00000542-200603000-00005
32. Linstedt U, Meyer O, Kropp P, Berkau A, Tapp E, Zenz M. Serum concentration of S-100 protein in assessment of cognitive dysfunction after general anesthesia in different types of surgery. Acta Anaesthesiol Scand. (2002) 46(4):384–9. doi: 10.1034/j.1399-6576.2002.460409.x
33. Rasmussen LS, Christiansen M, Rasmussen H, Kristensen PA, Moller JT. Do blood concentrations of neurone specific enolase and S-100 beta protein reflect cognitive dysfunction after abdominal surgery? ISPOCD group. Br J Anaesth. (2000) 84(2):242–4. doi: 10.1093/oxfordjournals.bja.a013410
34. Ali MS, Harmer M, Vaughan R. Serum S100 protein as a marker of cerebral damage during cardiac surgery. Br J Anaesth. (2000) 85(2):287–98. doi: 10.1093/bja/85.2.287
35. Degos V, Vacas S, Han Z, van Rooijen N, Gressens P, Su H, et al. Depletion of bone marrow-derived macrophages perturbs the innate immune response to surgery and reduces postoperative memory dysfunction. Anesthesiology. (2013) 118(3):527–36. doi: 10.1097/ALN.0b013e3182834d94
36. Rodrigues MC, Sanberg PR, Cruz LE, Garbuzova-Davis S. The innate and adaptive immunological aspects in neurodegenerative diseases. J Neuroimmunol. (2014) 269(1–2):1–8. doi: 10.1016/j.jneuroim.2013.09.020
37. Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in toll-like receptor-mediated neuronal injury. Glia. (2010) 58(3):253–63. doi: 10.1002/glia.20928
38. Ahlhelm F, Schneider G, Backens M, Reith W, Hagen T. Time course of the apparent diffusion coefficient after cerebral infarction. Eur Radiol. (2002) 12(9):2322–9. doi: 10.1007/s00330-001-1291-0
39. Benveniste H, Hedlund LW, Johnson GA. Mechanism of detection of acute cerebral ischemia in rats by diffusion-weighted magnetic resonance microscopy. Stroke. (1992) 23(5):746–54. doi: 10.1161/01.STR.23.5.746
40. Matsumoto K, Lo EH, Pierce AR, Wei H, Garrido L, Kowall NW. Role of vasogenic edema and tissue cavitation in ischemic evolution on diffusion-weighted imaging: comparison with multiparameter MR and immunohistochemistry. AJNR Am J Neuroradiol. (1995) 16(5):1107–15. PMCID: PMC8337787.7639135
41. Pierpaoli C, Alger JR, Righini A, Mattiello J, Dickerson R, Des Pres D, et al. High temporal resolution diffusion MRI of global cerebral ischemia and reperfusion. J Cereb Blood Flow Metab. (1996) 16(5):892–905. doi: 10.1097/00004647-199609000-00013
42. Pierpaoli C, Righini A, Linfante I, Tao-Cheng JH, Alger JR, Di Chiro G. Histopathologic correlates of abnormal water diffusion in cerebral ischemia: diffusion-weighted MR imaging and light and electron microscopic study. Radiology. (1993) 189(2):439–48. doi: 10.1148/radiology.189.2.8210373
43. Basser PJ, Pierpaoli C. A simplified method to measure the diffusion tensor from seven MR images. Magn Reson Med. (1998) 39(6):928–34. doi: 10.1002/mrm.1910390610
44. Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. (2001) 13(4):534–46. doi: 10.1002/jmri.1076
45. Le Bihan D, Moonen CT, van Zijl PC, Pekar J, DesPres D. Measuring random microscopic motion of water in tissues with MR imaging: a cat brain study. J Comput Assist Tomogr. (1991) 15(1):19–25. doi: 10.1097/00004728-199101000-00002
46. Chan KC, Khong PL, Lau HF, Cheung PT, Wu EX. Late measures of microstructural alterations in severe neonatal hypoxic-ischemic encephalopathy by MR diffusion tensor imaging. Int J Dev Neurosci. (2009) 27(6):607–15. doi: 10.1016/j.ijdevneu.2009.05.012
47. Warach S, Mosley M, Sorensen AG, Koroshetz W. Time course of diffusion imaging abnormalities in human stroke. Stroke. (1996) 27(7):1254–6. PMID: 8685940.8685940
48. Saczynski JS, Inouye SK, Kosar C, Tommet D, Marcantonio ER, Fong T, et al. Cognitive and brain reserve and the risk of postoperative delirium in older patients. Lancet Psychiatry. (2014) 1(6):437–43. doi: 10.1016/s2215-0366(14)00009-1
49. Cavallari M, Dai W, Guttmann CR, Meier DS, Ngo LH, Hshieh TT, et al. Neural substrates of vulnerability to postsurgical delirium as revealed by presurgical diffusion MRI. Brain. (2016) 139(Pt 4):1282–94. doi: 10.1093/brain/aww010
50. Cavallari M, Dai W, Guttmann CRG, Meier DS, Ngo LH, Hshieh TT, et al. Longitudinal diffusion changes following postoperative delirium in older people without dementia. Neurology. (2017) 89(10):1020–7. doi: 10.1212/WNL.0000000000004329
51. Hshieh TT, Dai W, Cavallari M, Guttmann CR, Meier DS, Schmitt EM, et al. Cerebral blood flow MRI in the nondemented elderly is not predictive of post-operative delirium but is correlated with cognitive performance. J Cereb Blood Flow Metab. (2017) 37(4):1386–97. doi: 10.1177/0271678X16656014
52. Racine AM, Fong TG, Travison TG, Jones RN, Gou Y, Vasunilashorn SM, et al. Alzheimer's-related cortical atrophy is associated with postoperative delirium severity in persons without dementia. Neurobiol Aging. (2017) 59:55–63. doi: 10.1016/j.neurobiolaging.2017.07.010
53. Fislage M, Winzeck S, Stamatakis E, Correia MM, Preller J, Feinkohl I, et al. Presurgical diffusion metrics of the thalamus and thalamic nuclei in postoperative delirium: a prospective two-centre cohort study in older patients. NeuroImage Clin. (2022) 36:103208. doi: 10.1016/j.nicl.2022.103208
54. Chen MH, Liao Y, Rong PF, Hu R, Lin GX, Ouyang W. Hippocampal volume reduction in elderly patients at risk for postoperative cognitive dysfunction. J Anesth. (2013) 27(4):487–92. doi: 10.1007/s00540-012-1548-6
55. Price CC, Tanner JJ, Schmalfuss I, Garvan CW, Gearen P, Dickey D, et al. A pilot study evaluating presurgery neuroanatomical biomarkers for postoperative cognitive decline after total knee arthroplasty in older adults. Anesthesiology. (2014) 120(3):601–13. doi: 10.1097/aln.0000000000000080
56. Sato C, Sekiguchi A, Kawai M, Kotozaki Y, Nouchi R, Tada H, et al. Postoperative structural brain changes and cognitive dysfunction in patients with breast cancer. PLoS One. (2015) 10(11):e0140655. doi: 10.1371/journal.pone.0140655
57. Vandiver MS, Roy B, Mahmud F, Lavretsky H, Kumar R. Functional comorbidities and brain tissue changes before and after lung transplant in adults. Front Cell Neurosci. (2022) 16:1015568. doi: 10.3389/fncel.2022.1015568
58. Lascola CD, Cotter SF, Klinger RY, Bisanar T, Cooter Wright M, Berger M, et al. Blood-brain barrier permeability and cognitive dysfunction after surgery—a pilot study. J Clin Anesth. (2023) 86:111059. doi: 10.1016/j.jclinane.2023.111059
59. Browndyke JN, Berger M, Harshbarger TB, Smith PJ, White W, Bisanar TL, et al. Resting-state functional connectivity and cognition after Major cardiac surgery in older adults without preoperative cognitive impairment: preliminary findings. J Am Geriatr Soc. (2017) 65(1):e6–e12. doi: 10.1111/jgs.14534
60. Nir T, Jacob Y, Huang KH, Schwartz AE, Brallier JW, Ahn H, et al. Resting-state functional connectivity in early postanaesthesia recovery is characterised by globally reduced anticorrelations. Br J Anaesth. (2020) 125(4):529–38. doi: 10.1016/j.bja.2020.06.058
61. Frith C, Dolan R. The role of the prefrontal cortex in higher cognitive functions. Brain Res Cogn Brain Res. (1996) 5(1–2):175–81. doi: 10.1016/S0926-6410(96)00054-7
62. Manelis A, Reder LM. Procedural learning and associative memory mechanisms contribute to contextual cueing: evidence from fMRI and eye-tracking. Learn Mem. (2012) 19(11):527–34. doi: 10.1101/lm.025973.112
63. Williams ZM, Eskandar EN. Selective enhancement of associative learning by microstimulation of the anterior caudate. Nat Neurosci. (2006) 9(4):562–8. doi: 10.1038/nn1662
64. Bauer E, Toepper M, Gebhardt H, Gallhofer B, Sammer G. The significance of caudate volume for age-related associative memory decline. Brain Res. (2015) 1622:137–48. doi: 10.1016/j.brainres.2015.06.026
65. Rubin RD, Watson PD, Duff MC, Cohen NJ. The role of the hippocampus in flexible cognition and social behavior. Front Hum Neurosci. (2014) 8:742. doi: 10.3389/fnhum.2014.00742
66. Vann SD, Nelson AJ. The mammillary bodies and memory: more than a hippocampal relay. Prog Brain Res. (2015) 219:163–85. doi: 10.1016/bs.pbr.2015.03.006
67. Brown C, Faigle R, Klinker L, Bahouth M, Max L, LaFlam A, et al. The association of brain MRI characteristics and postoperative delirium in cardiac surgery patients. Clin Ther. (2015) 37(12):2686–99.e9. doi: 10.1016/j.clinthera.2015.10.021
68. Duprey MS, Devlin JW, Griffith JL, Travison TG, Briesacher BA, Jones R, et al. Association between perioperative medication use and postoperative delirium and cognition in older adults undergoing elective noncardiac surgery. Anesth Analg. (2022) 134(6):1154–63. doi: 10.1213/ANE.0000000000005959
69. Radtke FM, Franck M, MacGuill M, Seeling M, Lütz A, Westhoff S, et al. Duration of fluid fasting and choice of analgesic are modifiable factors for early postoperative delirium. Eur J Anaesthesiol. (2010) 27(5):411–6. doi: 10.1097/EJA.0b013e3283335cee
70. Humeidan ML, Reyes JC, Mavarez-Martinez A, Roeth C, Nguyen CM, Sheridan E, et al. Effect of cognitive prehabilitation on the incidence of postoperative delirium among older adults undergoing Major noncardiac surgery: the neurobics randomized clinical trial. JAMA Surg. (2021) 156(2):148–56. doi: 10.1001/jamasurg.2020.4371
71. Jiao XF, Lin XM, Ni XF, Li HL, Zhang C, Yang CS, et al. Volatile anesthetics versus total intravenous anesthesia in patients undergoing coronary artery bypass grafting: an updated meta-analysis and trial sequential analysis of randomized controlled trials. PLoS One. (2019) 14(10):e0224562. doi: 10.1371/journal.pone.0224562
72. Quan C, Chen J, Luo Y, Zhou L, He X, Liao Y, et al. BIS-guided deep anesthesia decreases short-term postoperative cognitive dysfunction and peripheral inflammation in elderly patients undergoing abdominal surgery. Brain Behav. (2019) 9(4):e01238. doi: 10.1002/brb3.1238
73. Yang W, Kong LS, Zhu XX, Wang RX, Liu Y, Chen LR. Effect of dexmedetomidine on postoperative cognitive dysfunction and inflammation in patients after general anaesthesia: a PRISMA-compliant systematic review and meta-analysis. Medicine. (2019) 98(18):e15383. doi: 10.1097/md.0000000000015383
74. Wang X, Hua D, Tang X, Li S, Sun R, Xie Z, et al. The role of perioperative sleep disturbance in postoperative neurocognitive disorders. Nat Sci Sleep. (2021) 13:1395–410. doi: 10.2147/NSS.S320745
75. Zhang DF, Su X, Meng ZT, Li HL, Wang DX, Xue-Ying L, et al. Impact of dexmedetomidine on long-term outcomes after noncardiac surgery in elderly: 3-year follow-up of a randomized controlled trial. Ann Surg. (2019) 270(2):356–63. doi: 10.1097/sla.0000000000002801
76. Mu DL, Zhang DZ, Wang DX, Wang G, Li CJ, Meng ZT, et al. Parecoxib supplementation to morphine analgesia decreases incidence of delirium in elderly patients after hip or knee replacement surgery: a randomized controlled trial. Anesth Analg. (2017) 124(6):1992–2000. doi: 10.1213/ane.0000000000002095
77. Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in older persons: advances in diagnosis and treatment. Jama. (2017) 318(12):1161–74. doi: 10.1001/jama.2017.12067
78. Liu X, Xiong J, Tang Y, Gong CC, Wang DF. Role of dexmedetomidine in the treatment of delirium in critically ill patients: a systematic review and meta-analysis. Minerva Anestesiol. (2021) 87(1):65–76. doi: 10.23736/s0375-9393.20.14492-4
Keywords: delirium, perioperative neurocognitive disorders, cognitive dysfunction, MRI, lung transplanation
Citation: Vandiver MS (2023) Role of non-invasive imaging in perioperative neurocognitive disorders. Front. Anesthesiol. 2:1195175. doi: 10.3389/fanes.2023.1195175
Received: 28 March 2023; Accepted: 27 June 2023;
Published: 12 July 2023.
Edited by:
Rita Bertuetti, ASST Spedali Civili di Brescia, ItalyReviewed by:
Federico Bilotta, Sapienza University of Rome, Italy© 2023 Vandiver. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. Scott Vandiver bXZhbmRpdmVyQG1lZG5ldC51Y2xhLmVkdQ==
 M. Scott Vandiver
M. Scott Vandiver