- 1Department of Anesthesiology, School of Medicine, Shanghai Children’s Hospital, Shanghai Jiao Tong University, Shanghai, China
- 2Department of Anesthesiology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Department of SICU, School of Medicine, Shanghai Children’s Hospital, Shanghai Jiao Tong University, Shanghai, China
Background: Pheochromocytoma and paraganglioma (PPGL) pose significant perioperative challenges in pediatric populations due to catecholamine-driven hemodynamic instability. This study systematically evaluates perioperative management strategies—including preoperative optimization, intraoperative protocols, and postoperative monitoring—to establish evidence-based guidance for improving outcomes in pediatric PPGL surgery.
Methods: A single-center retrospective cohort study was conducted at a tertiary specialty hospital from January 2014 to October 2023. Clinical data from eight pediatric PPGL patients undergoing surgical resection were analyzed alongside a synthesis of contemporary literature and consensus guidelines.
Results: All eight patients received multimodal antihypertensive therapy (phenoxybenzamine, propranolol, and/or calcium channel blockers) for preoperative blood pressure control, achieving normotensive thresholds (<130/80 mmHg). Intraoperative hemodynamic stability was maintained through invasive arterial monitoring and targeted fluid resuscitation, with no hypertensive crises or arrhythmias reported. Postoperatively, normalized urinary vanillylmandelic acid (VMA) levels and blood pressure confirmed biochemical remission. During a median follow-up of 4.5 years (range 7 months–7 years), no instances of tumor recurrence or metastasis were identified.
Conclusions: Protocolized perioperative care incorporating α-adrenergic blockade, real-time hemodynamic monitoring, and comprehensive biochemical surveillance ensures safe tumor resection and mitigates surgical risks in pediatric PPGL. These findings underscore the importance of multidisciplinary coordination and long-term follow-up to optimize outcomes in this rare pediatric cohort.
1 Introduction
Pheochromocytoma (PCC) is a rare neuroendocrine tumor that originates in the adrenal medulla, while paraganglioma (PGL) arises from the extra-adrenal sympathetic nervous system (1). Together, these tumors are known as pheochromocytoma and paraganglioma (PPGL). These tumors are characterized by the excessive production of catecholamines, including dopamine, epinephrine, and norepinephrine, which result in clinical manifestations such as hypertension, tachycardia, and hypermetabolism (2, 3). Such alterations in the body's physiology can lead to life-threatening complications, including cardiomyopathy, stroke, renal failure, and vascular damage.
In pediatric populations, PPGL presents a particularly challenging perioperative management condition due to the heightened risk of hemodynamic instability. Effective perioperative management strategies aimed at controlling blood pressure and stabilizing cardiovascular function are critical in reducing intraoperative and postoperative complications. By optimizing preoperative blood pressure, controlling intraoperative fluctuations, and ensuring appropriate postoperative monitoring, surgical outcomes can be significantly improved. This paper retrospectively reviews the clinical data of pediatric PPGL patients treated at a specialty hospital in recent years, and also examines the current literature on perioperative management to provide evidence-based recommendations for optimizing care in this rare and high-risk group.
2 Methods
2.1 Clinical data
Clinical data were retrospectively collected from pediatric patients diagnosed with PPGL at a tertiary specialty hospital. The collected data included the patients' age, gender, weight, tumor location, highest blood pressure during the course of the illness, morning blood pressure on the day of surgery, 24 h urine vanillylmandelic acid (VMA) levels, blood concentrations of methoxyepinephrine and methoxynorepinephrine, preoperative medication regimens, rehydration status, and American Society of Anesthesiologists (ASA) classification.
2.2 Perioperative antihypertensive management
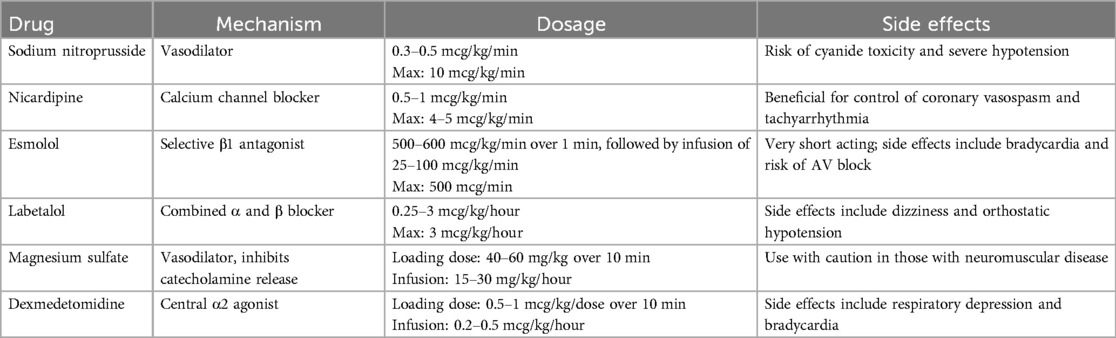
In our institution, perioperative management of PPGL begins once the diagnosis is confirmed. We integrate the child's medical history, clinical symptoms, laboratory tests, and imaging studies to develop a personalized antihypertensive plan. Initially, we start with phenoxybenzamine (0.2–0.25 mg/kg/day) and adjust the dosage based on blood pressure response, with a maximum dose of 2–4 mg/kg/day or 60 mg/day. In cases where the heart rate increases by more than 30% above the normal range for age during blood pressure reduction, β-blockers such as short-acting propranolol or long-acting metoprolol may be introduced to further control blood pressure. To mitigate the effects of catecholamine-induced vasoconstriction and prevent hypovolemia, a high-sodium diet and increased fluid intake are recommended during the perioperative period. If blood pressure remains inadequately controlled, calcium channel blockers can be added to the regimen, and dexmedetomidine hydrochloride may be used to alleviate anxiety during hospitalization (4–6). The specific recommended dosages and potential adverse effects of the preoperative antihypertensive medications are summarized in Table 1.
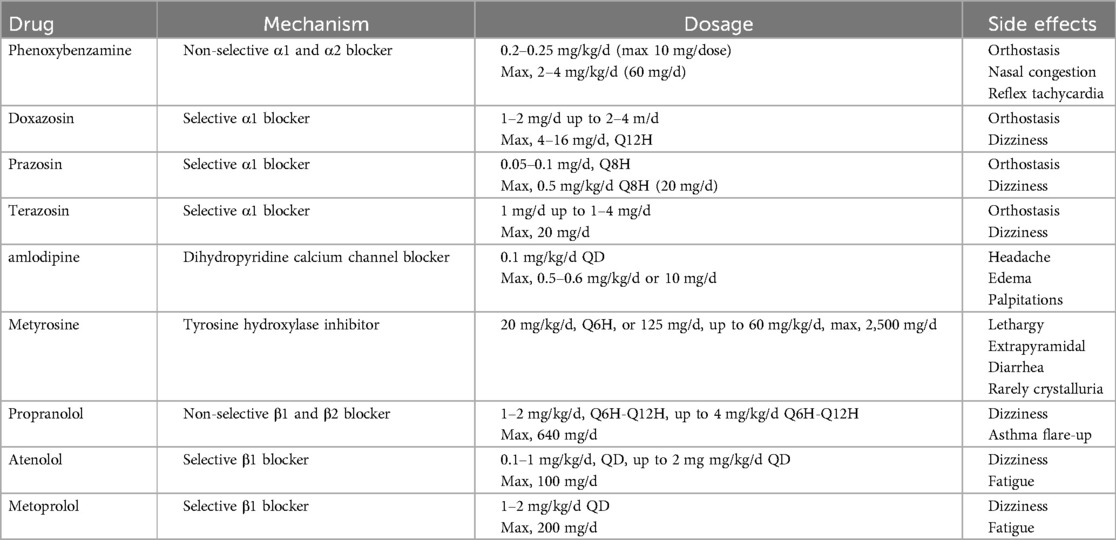
Table 1. Commonly used medications for preoperative management of hypertension in PPGL (9, 22, 28, 32).
As there are currently no specific guidelines or expert consensus on PPGL management in children, preoperative preparation standards are still based on adult protocols published by Roizen (7). These standards include: ① maintaining blood pressure at ≤160/90 mmHg 24 h prior to surgery, with permissive orthostatic hypotension defined as >80/45 mmHg; ② ensuring blood volume recovery, indicated by decreased hematocrit, weight gain, warm extremities, and improved microcirculation; ③ improvement in hypermetabolism syndrome and abnormal glucose metabolism; and ④ no ST-T changes in ECG for at least one week, with no more than one premature ventricular contraction every five minutes (2, 8).
2.3 Intraoperative anesthetic and hemodynamic management
Once preoperative preparation is complete, general anesthesia is performed with tracheal intubation and nerve block, using sevoflurane to maintain a MAC value of 1.5–2.0. Invasive arterial access is established for real-time blood pressure monitoring, and the internal jugular vein is cannulated for central venous pressure (CVP) monitoring and infusion of vasoactive drugs. Two peripheral veins are also prepared for fluid resuscitation. During the procedure, dexmedetomidine hydrochloride, phentolamine mesylate, remifentanil hydrochloride, and propofol are administered to maintain blood pressure. In cases of hypertension, phentolamine and esmolol are continuously infused to control blood pressure. Following ligation of the tumor veins, antihypertensive drug infusions are discontinued, and colloids are administered to maintain volume status. In the event of hypotension, epinephrine or norepinephrine is infused based on preoperative laboratory results, and arterial blood is drawn for blood glucose monitoring. Methylprednisolone infusion may also be employed as necessary. The recommended drug regimens and dosages during the procedure are summarized in Table 2. Postoperatively, routine monitoring of blood pressure, blood glucose, and hormone levels is conducted. Glucocorticoids or mineralocorticoids are supplemented as indicated, and urinary vanillylmandelic acid (VMA) levels are re-evaluated.
3 Results
3.1 General clinical data of the patient
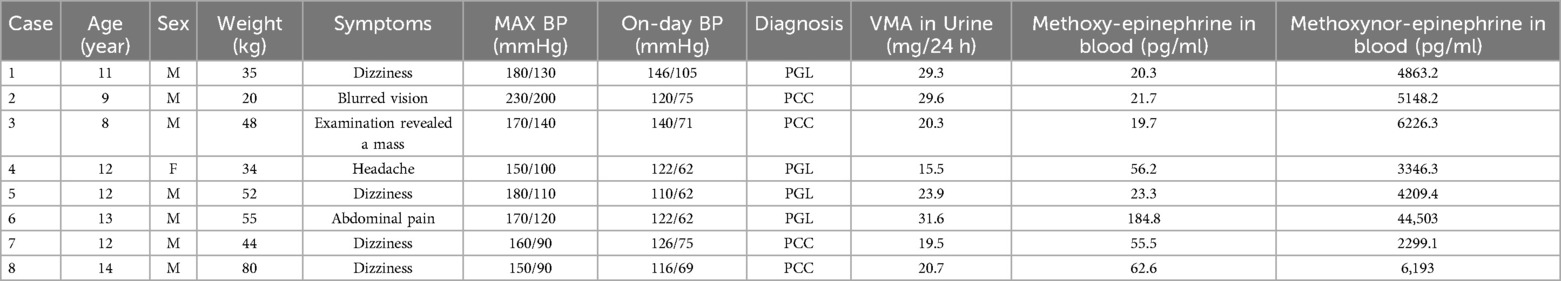
Clinical data were collected from eight pediatric patients (Table 3), Cases 1, 4, 5, and 6 were diagnosed with PGL, while Cases 2, 3, 7, and 8 were identified as PCC. The patients' ages ranged from 8 to 14 years, and their symptoms included dizziness, headache, blurred vision, and abdominal pain. Blood pressure measurements varied significantly, with the highest preoperative systolic/diastolic readings ranging from 150/90 mmHg to 230/200 mmHg. Urinary VMA levels ranged from 15.5 to 31.6 mg/24 h, and blood methoxyepinephrine and methoxynorepinephrine levels also varied widely across the cases. Cases 2 and 7 refer to the same patient (9), diagnosed with PCC, presented with the highest levels of catecholamine metabolites in the blood.
3.2 Postoperative results and follow-up
Case 1: A pediatric patient was diagnosed with multiple PGL, located on the bladder roof and left iliac vessel wall, in conjunction with an abdominal aortic malformation (Figure 1). The patient was treated with oral phenoxybenzamine to control blood pressure, reaching the maximum dose of 60 mg/day, and propranolol was added to manage heart rate. Blood pressure, heart rate, and hematocrit were optimized to within surgical thresholds before proceeding with surgery. During the operation, the bladder tumor was successfully resected, and intraoperative exploration revealed that the tumor on the left iliac vessel wall did not affect the circulation, so a biopsy was performed instead of resection. Postoperative pathology confirmed that the bladder tumor was a secretory paraganglioma, while the tumor on the iliac vessel wall was non-secretory. The patient has been followed up regularly for 7 years, with no evidence of recurrence or metastasis.
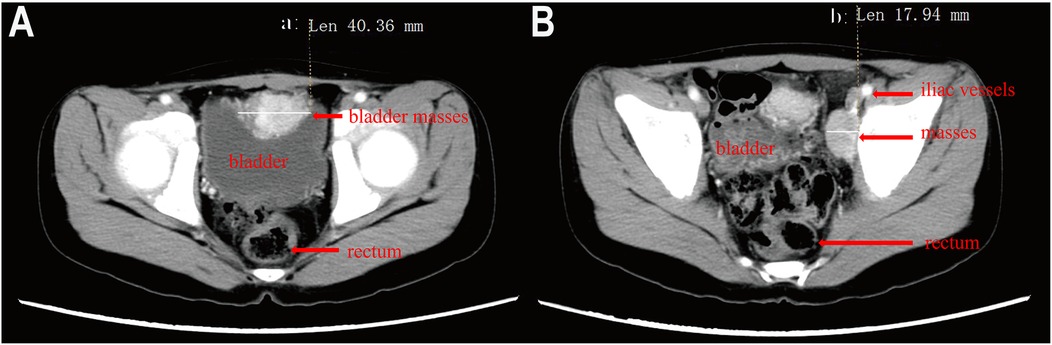
Figure 1. Enhanced pelvic CT of a child with pheochromocytoma. (A) Mass at the top of the bladder, (B) Mass on the wall of the left iliac vessels.).
As previously reported, Case 2 had a complex clinical presentation. Comprehensive clinical details of this patient were provided in our previously published article in Frontiers in Pediatrics (9). The child presented with bilateral adrenal masses, with the left tumor measuring approximately 40 mm and the right tumor 12 mm in diameter (Figure 2A). Pheochromocytoma was diagnosed based on the patient's medical history, blood tests, and imaging studies. Initial treatment involved beta-blockers, phentolamine, and phenoxybenzamine to control blood pressure. During the perioperative period, the patient developed multiple complications, including fever, diarrhea, pulmonary infiltrates, and hepatic dysfunction characterized by elevated transaminases (ALT 134 U/L, AST 233 U/L), hypoalbuminemia (22 g/L), and electrolyte imbalances (Na+ 129 mmol/L, K+ 3.2 mmol/L, Cl− 90 mmol/L, Mg2+ 0.57 mmol/L). Coagulation abnormalities were noted, with a prolonged PT (25.8 s) and aPTT (45.7 s). The patient subsequently developed polyuria and left femoral vein thrombosis, with worsening electrolyte disturbances secondary to persistent polyuria. An emergency resection of the right adrenal tumor was performed, which was confirmed as pheochromocytoma by pathological examination. During perioperative management, the patient developed diabetes insipidus, evidenced by low urine osmotic pressure and low blood osmotic pressure, with brain and pituitary MRIs showing no abnormalities. Multidisciplinary consultation led to addressing the metabolic disruption may cause by adrenal gland space occupation. Despite mineralocorticoid supplementation, the patient's condition worsened, prompting emergency resection of the adrenal tumor. The operation was performed with colloids and crystalloid fluids, and no further episodes of diabetes insipidus occurred. Oral mineralocorticoid therapy was continued for three months postoperatively. Three years later, follow-up imaging revealed enlargement of the left adrenal tumor (Figure 2B). Genetic testing identified a heterozygous mutation in the VHL gene, confirming a diagnosis of Von Hippel-Lindau (VHL) syndrome. To achieve adequate blood pressure control, the patient received a combination of phenoxybenzamine (initiated at 10 mg and titrated to 20 mg every 12 h), amlodipine (5 mg once daily), and metoprolol (25 mg once daily). In addition, dietary sodium intake was increased over approximately three weeks to facilitate plasma volume expansion. The left adrenal tumor was resected laparoscopically. One year post-surgery, the patient's blood pressure had returned to near-normal levels.
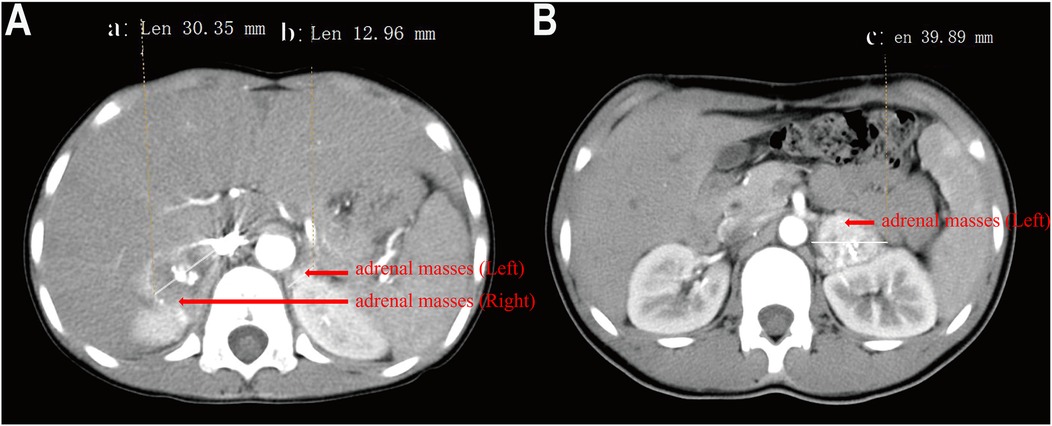
Figure 2. Contrast-enhanced CT image of a child with pheochromocytoma. (A) bilateral adrenal masses, with the left 40 mm and the right 12 mm at the first hospitalization, (B) The left adrenal mass measures 39 mm in diameter).
Case 3 (10) involved a pediatric patient who was initially found to have hypertension and a right adrenal mass, and was subsequently diagnosed with pheochromocytoma. Following standardized perioperative management, the patient underwent a robotic-assisted laparoscopic adrenal tumor resection. Intraoperative blood pressure fluctuations were minimal, and the procedure was uneventful. Postoperatively, the patient was transferred to the SICU for continued monitoring. During follow-up, urinary VMA levels, as well as catecholamine metabolites in blood and urine, were all within normal limits. Blood pressure returned to normal, and abdominal ultrasound revealed no evidence of tumor recurrence.
Cases 4–8: In these patients, tumors were incidentally discovered during routine imaging for hypertension or unrelated symptoms. Preoperative management included phenoxybenzamine and propranolol for blood pressure control, along with increased daily oral fluid intake. Dexmedetomidine hydrochloride was used to reduce preoperative anxiety. Intraoperatively, blood pressure remained stable, with minimal fluctuations and no hypertensive crises. Following tumor resection, norepinephrine was administered as needed to maintain blood pressure but was discontinued by the end of the operation. No additional hormone supplementation was required postoperatively. During follow-up periods ranging from 4 months to 1 year, all patients demonstrated satisfactory blood pressure control, with no evidence of tumor recurrence or metastasis. Postoperative urinary vanillylmandelic acid (VMA) levels for all patients returned to normal ranges, indicating effective biochemical remission.
4 Discussion
PPGL is a rare neuroendocrine tumor with an annual incidence of approximately 1 in 300,000, with 20% of cases diagnosed in children and adolescents. Among these diagnoses, PCC accounts for about 80%–85%, while PGL represents around 15%. The prevalence of PPGL is estimated to be between 0.2% and 0.6% in adults with hypertension, rising to 1.7% in the pediatric population with hypertension (11). The clinical manifestations and perioperative management of PPGL in children are similar to those in adults (8). Recent pediatric studies indicate that up to 40% of PPGL cases are associated with germline mutations (e.g., VHL, SDHB), and malignancy rates exceed 20% in children, underscoring the need for genetic screening (1, 4, 12). In contrast, about 66% of pediatric cases are PGL, 24% are bilateral, 80% have a family history or present with symptoms, and 50% exhibit metastases. Given these statistics, diligent follow-up is particularly important for children with PPGL (13, 14). Common symptoms of PPGL include headaches, palpitations, and sweating, characterized by paroxysmal hypertension and hypovolemia. Most children require blood pressure control and fluid management prior to surgery. Guidelines recommend long-term follow-up for PPGL patients, particularly those with hereditary tumors, including regular monitoring of blood catecholamine levels and genetic testing to assess the risk of recurrence or metastasis (3, 8, 15, 16). Case 2, which is associated with a VHL gene mutation, had a tumor primarily secreting norepinephrine. The tumor was located bilaterally in the adrenal glands, and postoperative follow-up revealed progressive enlargement, consistent with previous reports (17).
Cases 1 and 2 received low-dose phenoxybenzamine for blood pressure control, while cases 3 to 8 were administered slightly higher doses. Postoperative hospital stays were significantly shorter for these later cases; however, due to the small sample size, no statistical analysis was performed. A small retrospective study indicated that among patients with PPGL undergoing surgical treatment, the incidence of intraoperative hemodynamic instability and postoperative hypotension was 69.3% and 31.6%, respectively (18). Effective perioperative management can reduce the occurrence of adverse events during surgery. The duration of α-receptor blocker administration was identified as the sole predictive factor for intraoperative hemodynamic instability, while elevated preoperative urinary metanephrine levels and heart rates were predictive of postoperative hypotension (18). Conversely, some studies suggest that α-receptor blockers may contribute to orthostatic hypotension and intraoperative or postoperative hypotension, raising safety concerns. A dual-center retrospective study found no significant differences in postoperative complications between groups receiving and not receiving α-receptor blockers; however, the former had a lower likelihood of requiring ICU admission (0% vs. 73.5%) and shorter hospital stays (19).
Nevertheless, multiple guidelines continue to recommend phenoxybenzamine as the first-line perioperative agent for PPGL (19). A multicenter randomized controlled trial compared adverse events associated with phenoxybenzamine and doxazosin during perioperative management of PPGL. The total duration of intraoperative blood pressure exceeding predetermined target levels did not differ significantly between those receiving preoperative phenoxybenzamine and doxazosin; however, phenoxybenzamine was found to be more effective in preventing intraoperative hemodynamic instability (20). Additionally, phenoxybenzamine reduced preoperative preparation time compared to doxazosin (18.3 vs. 38.8 days), but no significant differences were observed in hemodynamic fluctuations during surgery or in the incidence of postoperative hypotension (21). A retrospective analysis of 105 PPGL patients indicated that the use of calcium channel blockers alone could not prevent intraoperative hemodynamic fluctuations; however, their perioperative use was associated with a reduced risk of mortality (6). It is recommended that calcium channel blockers be considered for patients with normal or mildly elevated blood pressure, particularly those who experience severe side effects from α-receptor blockers (22). The perioperative administration of amlodipine effectively replaces prazosin in preventing intraoperative hemodynamic instability in pheochromocytoma cases. The frequency and duration of hypertension were higher in the prazosin group compared to the amlodipine group, while there were no significant differences in the occurrence and duration of hypotension between the two groups (23).
The study indicated that the incidence of PPGL increased by 4.8 times from 1977 to 2015. Among the patients examined, 21.2% (40 out of 189) exhibited the classic triad of headache, sweating, and palpitations, while the proportion of asymptomatic PPGL patients was notably higher at 32.3% (61 out of 189). This suggests that the absence of paroxysmal symptoms does not exclude the possibility of catecholamine-secreting PPGL (24). In asymptomatic PPGL patients with functionally active tumors, catecholamine levels are generally lower compared to symptomatic patients. This is likely due to reduced expression of multiple genes involved in catecholamine synthesis. Therefore, perioperative management should routinely include blood pressure control and fluid management based on the characteristics of functional PPGL. For asymptomatic PPGL patients without secretory function, preoperative treatment decisions should involve multidisciplinary consultation, as there are currently no established guidelines or consensus on this matter (22, 25, 26). Although all PPGL patients underwent perioperative preparation before surgery, the study found that the risk of complications and mortality following elective surgery was significantly lower than that associated with emergency surgery (27).
The goals of perioperative management for PPGL are to normalize blood pressure and heart rate, restore effective circulating blood volume, improve metabolic status, reduce catecholamine storms, and stabilize intraoperative hemodynamics (22, 28). During this period, α-receptor blockers typically restore only about 60% of blood volume in PPGL patients. Consequently, guidelines recommend a high-sodium diet (approximately 5,000 mg per day) and increased fluid intake, with rehydration therapy of 2,500–3,000 ml/m2 of body surface area to minimize intraoperative blood pressure fluctuations (5, 13, 28, 29). In cases 3 to 8, children were instructed to increase fluid intake after starting blood pressure reduction with phenoxybenzamine, supplemented with intravenous crystalloids three days prior to surgery, achieving a total intake of 2,500 ml/m2. In 2007, the Endocrine Society recommended that preoperative control goals for adults include blood pressure below 130/80 mmHg and a heart rate of 60–70 beats/min while sitting, with blood pressure not dropping below 80/45 mmHg and a heart rate of 70–80 beats/min while standing. However, children are not simply small adults; their wide age range results in significant variability in normal blood pressure values across developmental stages. Therefore, individualized, pediatric-specific hemodynamic targets should be established (5, 30). Post-tumor resection, hypotension is a common complication. If systolic blood pressure drops below 80 mmHg for more than 10 min, 500–1,000 ml of colloid and 3–9 mg of ephedrine may be infused. If this is ineffective, epinephrine, norepinephrine, or 10 mg/kg of methylprednisolone can be administered (6).
Future studies should focus on larger cohorts and include genetic screening to better understand the hereditary aspects of PPGL in pediatric patients (28, 31). Surgical resection remains the cornerstone of PPGL treatment (1); however, hemodynamic instability and postoperative hypotension can pose significant risks to patient safety. Accurate and comprehensive recording of minute-by-minute vital sign data during surgery, as well as electronic medical records and medication administration in the intensive care unit, is crucial to mitigate the impact of confounding factors in retrospective analyses. Moreover, it is essential to recognize the potential for this rare tumor in children presenting with hypertension. Pediatric surgeons should collaborate with endocrinologists and anesthesiologists to develop individualized diagnostic and treatment plans, aiming to prevent intraoperative and postoperative complications. Symptom presentation is particularly critical in pediatric patients, as they generally lack the comorbidities commonly observed in adults and exhibit greater physiological compensatory capacity, potentially leading to atypical clinical manifestations. In light of the unique challenges associated with pediatric PPGL, we strongly advocate for the establishment of evidence-based guidelines or expert consensus to guide clinical practice. These resources would enhance the quality and consistency of care provided to affected children.
Therefore, multidisciplinary and individualized approaches should be prioritized for children with PPGL. Given the lack of established guidelines for pediatric PPGL, multicenter collaboration is needed to enhance outcomes for these patients.
5 Conclusions
In conclusion, effective perioperative management and multidisciplinary collaboration are essential for improving outcomes in pediatric PPGL patients. Further research and multicenter studies are needed to establish standardized guidelines for this rare condition.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by Ethics Committee of Shanghai Children's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. The requirement of ethical approval was waived by Ethics Committee of Shanghai Children's Hospital. for the studies involving animals because This study is a retrospective clinical study, and the hematological test results presented in the manuscript were obtained for clinical purposes, with no additional procedures performed on the participants. Informed consent was obtained from the participants. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
SY: Investigation, Writing – original draft. LR: Investigation, Writing – original draft. SW: Data curation, Writing – original draft. GW: Data curation, Writing – original draft. GD: Writing – review & editing, Investigation. YY: Investigation, Writing – original draft. RW: Writing – review & editing, Project administration. RD: Methodology, Project administration, Writing – review & editing. TZ: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding from Special Clinical Research Project of Shanghai Municipal Health Commission (20204Y0470).
Acknowledgments
We would like to express our sincere gratitude to our colleagues in the Department of Anesthesiology at Shanghai Children's Hospital—Dr. Jiang Yan, Fu Yuezhen and Zhang Saiji—for their invaluable technical assistance. We also extend our heartfelt thanks to Shi Minhua, from the hospital's Information Technology Department, for their dedicated support in maintaining the surgical anesthesia system.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Passman JE, Wachtel H. Management of pheochromocytomas and paragangliomas. Surg Clin North Am. (2024) 104(4):863–81. doi: 10.1016/j.suc.2024.02.014
2. Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. (2005) 366(9486):665–75. doi: 10.1016/S0140-6736(05)67139-5
3. Fischer A, Del Rivero J, Wang K, Nölting S, Jimenez C. Systemic therapy for patients with metastatic pheochromocytoma and paraganglioma. Best Pract Res Clin Endocrinol Metab. (2025) 39(1):101977. doi: 10.1016/j.beem.2025.101977
4. Saavedra TJ, Nati-Castillo HA, Valderrama Cometa LA, Rivera-Martinez WA, Asprilla J, Castano-Giraldo CM, et al. Pheochromocytoma: an updated scoping review from clinical presentation to management and treatment. Front Endocrinol. (2024) 15:1433582. doi: 10.3389/fendo.2024.1433582
5. Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. (2007) 92(11):4069–79. doi: 10.1210/jc.2007-1720
6. Lebuffe G, Dosseh ED, Tek G, Tytgat H, Moreno S, Tavernier B, et al. The effect of calcium channel blockers on outcome following the surgical treatment of phaeochromocytomas and paragangliomas. Anaesthesia. (2005) 60(5):439–44. doi: 10.1111/j.1365-2044.2005.04156.x
7. Roizen MF, Hunt TK, Beaupre PN, Kremer P, Firmin R, Chang CN, et al. The effect of alpha-adrenergic blockade on cardiac performance and tissue oxygen delivery during excision of pheochromocytoma. Surgery. (1983) 94(6):941–5.6648809
8. Naranjo J, Dodd S, Martin YN. Perioperative management of pheochromocytoma. J Cardiothorac Vasc Anesth. (2017) 31(4):1427–39. doi: 10.1053/j.jvca.2017.02.023
9. Yu S, Zou T, Wei S, Yu Y, Ding G. Comprehensive case report and literature review on perioperative management of multiple pheochromocytoma in a pediatric patient. Front Pediatr. (2025) 13:1439186. doi: 10.3389/fped.2025.1439186
10. Chen Y, Zhou L, Wang Y, Xie H. Robot-assisted laparoscopic adrenalectomy for children with adrenal pheochromocytoma:one case re-port with a literature review. J Clin Pediatr Surg. (2021) 20(8):731–6. doi: 10.3969/j.issn.1671-6353.2021.08.006
11. Jain A, Baracco R, Kapur G. Pheochromocytoma and paraganglioma-an update on diagnosis, evaluation, and management. Pediatr Nephrol. (2020) 35(4):581–94. doi: 10.1007/s00467-018-4181-2
12. Stachowicz-Stencel T, Pasikowska N, Synakiewicz A. Pheochromocytoma and paraganglioma in children and adolescents. Acta Biochim Pol. (2023) 70(3):487–93. doi: 10.18388/abp.2020_6955
13. Bholah R, Bunchman TE. Review of pediatric pheochromocytoma and paraganglioma. Front Pediatr. (2017) 5:155. doi: 10.3389/fped.2017.00155
14. Peard L, Cost NG, Saltzman AF. Pediatric pheochromocytoma: current status of diagnostic imaging and treatment procedures. Curr Opin Urol. (2019) 29(5):493–9. doi: 10.1097/MOU.0000000000000650
15. Ishizaki F, Taguchi T, Murata M, Hoshino S, Toba T, Takeda K, et al. Long-term outcomes and prognostic factors of metastatic or recurrent pheochromocytoma and paraganglioma: a 20-year review in a single institution. Sci Rep. (2024) 14(1):26456. doi: 10.1038/s41598-024-75354-9
16. Huang BL, Liu Q, Teng YY, Peng SQ, Liu Z, Li ML, et al. Global trends and current status in pheochromocytoma: a bibliometric analysis of publications in the last 20 years. Front Endocrinol. (2023) 14:1167796. doi: 10.3389/fendo.2023.1167796
17. Li T, Cui Y, Zhou Y, Zhou T, Chen S, Lu L, et al. Pheochromocytoma in von Hippel-Lindau disease: clinical features and comparison with sporadic pheochromocytoma. Clin Endocrinol. (2025) 102(3):355–61. doi: 10.1111/cen.15190
18. Kim JH, Lee HC, Kim SJ, Yoon SB, Kong SH, Yu HW, et al. Perioperative hemodynamic instability in pheochromocytoma and sympathetic paraganglioma patients. Sci Rep. (2021) 11(1):18574. doi: 10.1038/s41598-021-97964-3
19. Buscemi S, Di Buono G, D'Andrea R, Ricci C, Alberici L, Querci L, et al. Perioperative management of pheochromocytoma: from a dogmatic to a tailored approach. J Clin Med. (2021) 10(16):3759. doi: 10.3390/jcm10163759
20. Buitenwerf E, Osinga TE, Timmers H, Lenders JWM, Feelders RA, Eekhoff EMW, et al. Efficacy of alpha-blockers on hemodynamic control during pheochromocytoma resection: a randomized controlled trial. J Clin Endocrinol Metab. (2020) 105(7):2381–91. doi: 10.1210/clinem/dgz188
21. Malec K, Miskiewicz P, Witkowska A, Krajewska E, Toutounchi S, Galazka Z, et al. Comparison of phenoxybenzamine and doxazosin in perioperative management of patients with pheochromocytoma. Kardiol Pol. (2017) 75(11):1192–8. doi: 10.5603/KP.a2017.0147
22. Fang F, Ding L, He Q, Liu M. Preoperative management of pheochromocytoma and paraganglioma. Front Endocrinol. (2020) 11:586795. doi: 10.3389/fendo.2020.586795
23. Jaiswal SK, Memon SS, Lila A, Sarathi V, Goroshi M, Garg R, et al. Preoperative amlodipine is efficacious in preventing intraoperative HDI in pheochromocytoma: pilot RCT. J Clin Endocrinol Metab. (2021) 106(8):e2907–18. doi: 10.1210/clinem/dgab231
24. Ebbehoj A, Stochholm K, Jacobsen SF, Trolle C, Jepsen P, Robaczyk MG, et al. Incidence and clinical presentation of pheochromocytoma and sympathetic paraganglioma: a population-based study. J Clin Endocrinol Metab. (2021) 106(5):e2251–61. doi: 10.1210/clinem/dgaa965
25. Haissaguerre M, Courel M, Caron P, Denost S, Dubessy C, Gosse P, et al. Normotensive incidentally discovered pheochromocytomas display specific biochemical, cellular, and molecular characteristics. J Clin Endocrinol Metab. (2013) 98(11):4346–54. doi: 10.1210/jc.2013-1844
26. Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2014) 99(6):1915–42. doi: 10.1210/jc.2014-1498
27. Scholten A, Cisco RM, Vriens MR, Cohen JK, Mitmaker EJ, Liu C, et al. Pheochromocytoma crisis is not a surgical emergency. J Clin Endocrinol Metab. (2013) 98(2):581–91. doi: 10.1210/jc.2012-3020
28. Ambarsari CG, Nadhifah N, Lestari HI. Perioperative blood pressure management recommendations in pediatric pheochromocytoma: a 10-year narrative review. Kidney Blood Press Res. (2025) 50(1):61–82. doi: 10.1159/000542897
29. Ardicli B, User IR, Ciftci AO, Akyuz C, Kutluk MT, Gonc N, et al. Approach to pheochromocytoma and paraganglioma in children and adolescents: a retrospective clinical study from a tertiary care center. J Pediatr Urol. (2021) 17(3):400.e1–e7. doi: 10.1016/j.jpurol.2021.01.043
30. Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. (2017) 140(3):e20171904. doi: 10.1542/peds.2017-1904
31. Liu Z, Ma J, Jimenez C, Zhang M. Pheochromocytoma: a clinicopathologic and molecular study of 390 cases from a single center. Am J Surg Pathol. (2021) 45(9):1155–65. doi: 10.1097/PAS.0000000000001768
Keywords: pheochromocytoma, paraganglioma, pediatric endocrinology, perioperative management, hypertension
Citation: Yu S, Ren L, Wei S, Wang G, Ding G, Yu Y, Wei R, Dong R and Zou T (2025) Multidisciplinary perioperative management of pediatric pheochromocytoma/paraganglioma: a retrospective cohort study with long-term outcomes. Front. Anesthesiol. 4:1572804. doi: 10.3389/fanes.2025.1572804
Received: 7 February 2025; Accepted: 27 June 2025;
Published: 25 July 2025.
Edited by:
Thomas Schricker, McGill University, CanadaReviewed by:
Alessandro Crocoli, Bambino Gesù Children's Hospital (IRCCS), ItalyTheodoros Aslanidis, Agios Pavlos General Hospital, Greece
Copyright: © 2025 Yu, Ren, Wei, Wang, Ding, Yu, Wei, Dong and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Wei, d2VpckBzaGNoaWxkcmVuLmNvbS5jbg==; Rong Dong, c2FsbHk5MTMyQDE2My5jb20=; Tianxiao Zou, em91dGlhbnhpYW9Ac2hjaGlsZHJlbi5jb20uY24=
†These authors have contributed equally to this work
 Shenghua Yu
Shenghua Yu Lulu Ren3,†
Lulu Ren3,† Sisi Wei
Sisi Wei Yani Yu
Yani Yu Rong Wei
Rong Wei