- 1Department of Veterinary Sciences, Università degli studi di Messina, Messina, Italy
- 2Department of Animal Sciences and Division of Nutritional Sciences, University of Illinois, Urbana, IL, United States
- 3Department of Animal Production, College of Food and Agriculture Sciences, King Saud University, Riyadh, Saudi Arabia
- 4Faculty of Agriculture Natural Resource and Environment, Naresuan University, Phitsanulok, Thailand
- 5Zinpro Corporation, Eden Prairie, MN, United States
Requirement for Cobalt and folic acid (FOA) in late-pregnant dairy cows is unknown, but dietary supply of one or both could impact activity of one-carbon metabolism. Holstein cows were fed the same basal diet supplemented with Cobalt glucoheptonate (CoPro, n =16) or a slow-release Cobalt polysaccharide (CoPectin, n = 14) for the last 30 days prepartum to assess impacts on calf growth and skeletal muscle metabolism. Cobalt treatments delivered 1 ppm Cobalt/kg DM and both diets supplied 50 mg ruminally-available FOA/day. Calves were weighed at birth and growth performance recorded weekly through 9-weeks of age. Prior to weaning (day 42), calves (n = 7 and 8 for CoPro and CoPectin group, respectively) were subjected to biopsy of semitendinosus muscle for Western blotting and targeted metabolomics using LC-MS-MS. Although birth measures of development did not differ (P > 0.05), calves born from CoPectin cows had greater hip width (HW) at weeks 8–9 (Diet×Time, P = 0.03). Overall, withers height (WH) tended (84.6 vs. 82.4 ± 0.9 cm; P = 0.10) to be greater in CoPectin than CoPro calves. Metabolomic profiling revealed greater concentrations of betaine (5.11 ± 0.36 × 106 vs. 4.12 ± 0.36 × 106 AUC; P = 0.04) and S-adenosylmethionine (3.87 ± 0.42 × 106 vs. 2.61 ± 0.42 × 106 AUC; P = 0.02), with tendencies for greater cystathionine (1.02 ± 0.10 × 106 vs. 0.71 ± 0.10 × 106 AUC; P = 0.06) and choline (8.04 ± 1.15 × 106 vs. 5.83 ± 1.15 × 106 AUC; P = 0.10) in CoPectin compared with CoPro calves. Protein abundance (relative to GAPDH) of INSR (1.34 ± 0.07 vs. 1.12 ± 0.05; P = 0.05), p-AKT (1.22 ± 0.08 vs. 1.01 ± 0.06; P = 0.05), and p-AKT : AKT ratio (1.37 ± 0.09 vs. 1.00 ± 0.07; P = 0.001) were greater, whereas total 4EBP1 (0.81 ± 0.06 vs. 1.03 ± 0.05; P = 0.03) and MRF4 (0.75 ± 0.05 vs. 0.96 ± 0.07; P = 0.04) were lower in CoPectin calves. These results suggest that the slow-release cobalt source (CoPectin) enhanced maternal cobalt utilization and fetal one-carbon metabolism, leading to greater activation of the insulin–AKT–mTOR pathway in calf skeletal muscle. Further research could help determine the degree to which slow-release Cobalt alters ruminal synthesis of vitamin B12 and its impact on the physiology of the neonatal calf.
1 Introduction
Fetal programming represents the process through which offspring performance is shaped by a wide range of maternal and environmental factors including nutritional deficits or excesses, disease states, environmental conditions (e.g., heat stress), and exposure to toxicant and/or drugs, all of which are relevant in dairy cattle (Cattaneo et al., 2022; Lopreiato et al., 2023b; Ouellet et al., 2020). In this context, the nutritional management of cows during late-gestation plays a particularly important role in determining postnatal growth, metabolism, and productivity (Barcelos et al., 2022; Christofaro Fernandes et al., 2023; Polizel et al., 2021; Schalch Junior et al., 2022). Several studies have pointed out that nutritional status of the dam during late-pregnancy influences fetal life (Alharthi et al., 2019; Hulbert and Moisá, 2016; Jacometo et al., 2018; Lopes et al., 2021). Several metabolic functions such as growth, development, reproduction, and immunity require trace elements. Besides the strategy to feed trace minerals in order to enhance their status in cattle (Spears and Weiss, 2008), some data indicated that feeding trace minerals complexed with organic compounds such as amino acids especially in late-gestation could represent an alternative to further optimize postnatal offspring performance (Marques et al., 2016). For example, there is evidence that maternal diets induce transcriptomic changes in a stage-dependent manner in the liver of pigs (Oster et al., 2016) and dairy calves (Palombo et al., 2021), pointing to molecular routes that contribute to the adaptation of the organism.
Trace minerals are essential for fetal development (Hostetler et al., 2003), since the fetus depends completely on the dam for proper supply of these elements (Marques et al., 2016). Inadequate supply from the dam might impair fetal development and postnatal performance. Among micronutrients, the minerals Zn, Cu, Mn, and Co are required for proper development of the fetal nervous, reproductive, and immune systems (Hostetler et al., 2003; Pepper and Black, 2011). Cobalt (Co) and folic acid (FOA) play complementary roles in vitamin B12 metabolism. Cobalt is an essential component for ruminal synthesis of vitamin B12 by microorganisms, as it constitutes the central atom of the cobalamin molecule. Folic acid, on the other hand, is a key factor in the one-carbon metabolism pathway, where vitamin B12 functions as a cofactor. Thus, adequate folic acid availability enhances the metabolic utilization of vitamin B12 synthesized in the rumen (Duplessis et al., 2022; Girard and Duplessis, 2023; Tiffany et al., 2006, 2003).
It was established several years ago that young ruminant diets deficient in Co content might result in a lower vitamin B12 (cobalamin) biosynthesis in the rumen (Stangl et al., 1999). This in turn leads to lower post-ruminal supply and average daily gain (ADG), decreased plasma and liver vitamin B12, elevated plasma methylmalonic acid (MMA), and homocysteine (Stangl et al., 1999). At the tissue level, the decrease in ruminally-derived vitamin B12 can decrease methionine synthase and methylmalonyl-CoA mutase activities (Kennedy et al., 1990). Until the ruminal environment develops a microbiota population, at least for vitamin B12, newborn calves rely on placental and colostral transfer to cover its requirements. Duplessis and Girard (2019) demonstrated that supplementing cows with biotin, folic acid, and vitamin B12 in late-gestation effectively boosts the concentrations of these vitamins in both colostrum and the newborn calf's plasma. Thus, there is growing recognition that dietary supply of micronutrients including Co and the methyl donors such as methionine, choline, and/or FOA, all ensure benefits to both dams and offspring in terms of production and health (Coleman et al., 2020, 2021; Lopes et al., 2021; Lopreiato et al., 2023a; Zenobi et al., 2018).
Numerous studies have linked maternal micronutrient supplementation with positive responses in energy metabolism, inflammation, antioxidant status, and birth weight of the offspring (Alharthi et al., 2019; Duplessis and Girard, 2019; Jacometo et al., 2016). Micronutrient supplementation, including Zn, Se, and Co has also led to improvements in reproductive performance, lamb production, and health indices (Aliarabi et al., 2019; Hatfield et al., 1995; Rock et al., 2001). Furthermore, maternal supplementation of Zn, Se, and Co via slow-release ruminal bolus during late-pregnancy enhanced mineral status in ewes and their offspring until weaning, resulting in higher lamb body weight at weaning (Aliarabi et al., 2019).
In a previously studied cohort of calves reported by Lopes et al. (2021), which underwent the same experimental conditions as those presented in the current study, we observed that maternal supplementation of FOA, Co sources varying in ruminal release (e.g., Co glucoheptonate, Co pectin), and rumen-protected methionine influenced specific plasma biomarkers of inflammation, and molecular responses associated with inflammatory mechanisms during the neonatal period. For example, there is evidence that maternal diets can induce transcriptomic and metabolic reprogramming in offspring tissues, pointing to molecular routes that contribute to postnatal adaptation. In ruminants, maternal plane of nutrition or trace mineral supply during late-gestation altered hepatic and muscle transcriptome profiles (Polizel et al., 2025) and modified metabolomic pathways associated with energy metabolism, amino acid turnover, and one-carbon metabolism (Elolimy et al., 2019; Shang et al., 2024). These findings highlight the complexity of nutrient-mediated fetal programming and support the hypothesis that trace mineral bioavailability during gestation can influence the metabolic phenotype of newborn calves.
We reported evidence for maternal effects of organic trace mineral supplementation on calf circulating immune cell microRNA profiles (Jacometo et al., 2015), but we are unaware of similar studies focused on offspring growth and muscle metabolism. Although several studies have evaluated prepartum cobalt supplementation in ruminants, most have focused on maternal serum or ruminal parameters (e.g., vitamin B12 concentration or ruminal fermentation), whereas the potential programming effects on offspring tissues have received far less attention. In particular, the molecular mechanisms linking maternal trace mineral nutrition to the development of neonatal muscle remain poorly characterized. Furthermore, potential differences in the bioavailability and physiological effects among cobalt chemical forms are still unclear. Slow-release or organic cobalt sources may provide a more stable cobalt supply to rumen microbes, sustaining vitamin B12 synthesis and methyl-donor production in the dam, which could in turn influence fetal one-carbon metabolism and muscle development.
We hypothesized that, compared with an inorganic cobalt source, the slow-release cobalt pectin complex (CoPectin) would provide a more consistent cobalt release and higher vitamin B12 availability, thereby enhancing methyl-donor intermediates (e.g., betaine and S-adenosylmethionine) and activating the insulin–AKT–mTOR signaling pathway in the skeletal muscle of newborn calves. Thus, the aim of the current study was to use a subset of calves from the study of Lopes et al. (2021) to investigate the influence of feeding Co sources to Holstein cows in late-pregnancy on the abundance of proteins in the mTOR and insulin signaling pathway as well as intermediates of one-carbon metabolism in skeletal muscle of neonatal calves.
2 Materials and methods
2.1 Animal ethics
Calves used in the present study were a subset from the experiment reported recently by Lopes et al. (2021) which was approved by the Institutional Animal Care and Use Committee (protocol no. 17168) of the University of Illinois, Urbana-Champaign. A total of 72 multiparous Holstein cows were used in the original study.
2.2 Experimental design and management
In the original study, a total of 72 multiparous Holstein cows were utilized. Details of the experimental design, diet composition, and nutrient profiles were reported by Lopreiato et al. (2023a). Briefly, from -30 days from calving until delivery (close-up period), cows were housed in a sand-bedded freestall barn with an individual Calan gate feeding system (American Calan, Northwood, NH, USA). They were fed a total mixed ration (TMR) daily at 0600 h (1.37 Mcal/kg of dry matter, 14.6% crude protein; Table 1). Weekly samples of the TMR were collected and frozen at –20 °C before making a composite that was analyzed for DM (method 942.05; AOAC, 2006), crude protein (method 990.03; AOAC, 2006), acid detergent fiber (method 973.18; AOAC, 1997), neutral detergent fiber (Van Soest et al., 1991), starch (Bach Knudsen, 1997; YSI 2950D-1 Biochemistry Analyzer, YSI Inc., Yellow Springs, OH), crude fat (method Am 5-04; AOCS, 2013), Ca, P, Mg, K, Na, Cl, and S (CEM-Dairy One digestion method; analysis in Thermo iCAP Pro XP Inductively Coupled Plasma Radial Spectrometer, Waltham, MA, USA) using wet chemistry methods at a commercial laboratory (Dairy One Cooperative Inc., Ithaca, NY, USA).
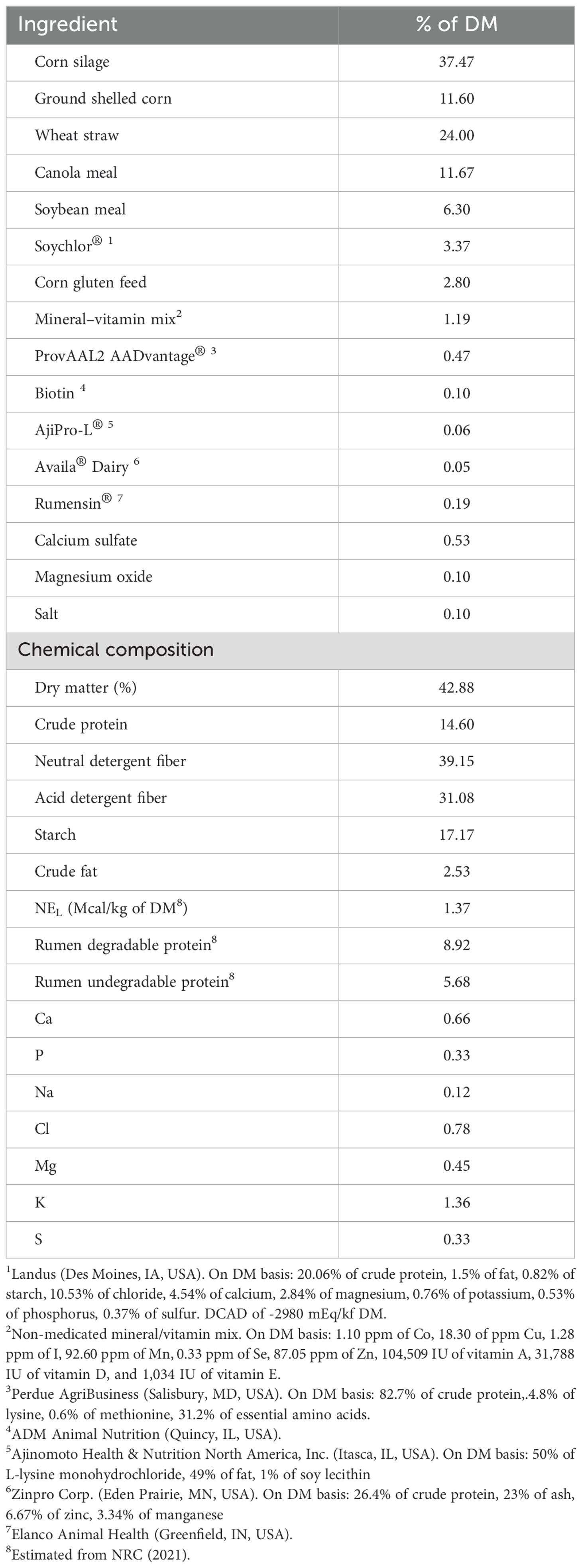
Table 1. Ingredient and chemical composition of the total mixed ration fed from -30 days from parturition until parturition.
This larger study involved two experimental sources of Co with different rates of ruminal release of the mineral fed in combination with the same experimental ruminally-available folic acid (FOA) source, and one of the Co sources fed with FOA plus rumen-protected methionine (RPM; Smartamine M from Adisseo, Alpharetta, GA). The level of FOA supplemented from -30 to 30 days in milk (DIM) was 50 mg, and the Co treatments provided 1 mg of Co/kg of dry matter (CoPro or CoPectin from Zinpro Corp.). The cobalt inclusion level exceeded the NRC (2001) requirement to ensure adequate cobalt supply during the late-gestation period when feed intake typically declines and microbial vitamin B12 synthesis may be limited. All supplements were administered by top-dressing onto the TMR. For the data in the present study, 16 calves were used in the CoPro group and 14 in the CoPectin group. Some of these were also included in the study by Lopes et al. (2021) dealing with mRNA abundance of immune markers in whole blood. The management of the calves was described by Lopes et al. (2021). Briefly, a digital scale was used to weigh calves at birth and 3.8 L first-milking fresh colostrum from their respective dams provided. Subsequently, calves were housed in individual outdoor hutches bedded with straw. Milk replacer (Advance Excelerate, Milk Specialties; 28.5% crude protein, 15% fat) was fed at 0600 and 1800 h daily until 35 d of age. Then, calves were only fed once daily until weaning at 42 d of age. In detail, calves received 4.54 L of milk replacer (0.59 kg of milk replacer in 3.95 L of water) from 1 to 10 d of age, 5.90 L (0.77 kg of milk replacer in 5.13 L of water) from 11 to 20 d of age, 7.26 L (0.94 kg of milk replacer in 6.32 L of water) from 21 to 35 d of age, and 3.63 L (0.47 kg of milk replacer in 3.16 L of water) in a single feeding from 36 to 42 d of age. During the whole period, calves had ad-libitum access to textured starter (Ampli-Calf Starter 20, 19.9% crude protein and 13.5% neutral detergent fiber, Purina Animal Nutrition). None of these calves experience an episode of diarrhea or other clinical diseases.
2.3 Growth measurements
Growth measurements (n = 16 in CoPro and 14 in CoPectin) were conducted weekly during the first 9 weeks of age by using a scale, wherein two individuals measured body weight, body length, hip height, wither height, and hip width for each calf. All calves included in the present study remained clinically healthy throughout the experiment. Procedures were exactly as described in our previous publications (Jacometo et al., 2015; Alharthi et al., 2018).
2.4 Muscle biopsy
Semitendinosus biopsies were harvested from a total of 5 heifers and 3 bulls in the CoPro group and 4 heifers and 3 bulls in the CoPectin group at 42 d of age before weaning. Briefly, an area of approximately 10 × 10 cm over the tuber ischia was closely clipped and scrubbed thoroughly with betadine and 70% ethanol. Local anesthesia was achieved with 2% lidocaine (5 mL s.c.; NexGen Animal Health, Weatherford, TX, USA). A 2-cm incision was made using a sterile scalpel blade. Approximately 200 mg of tissue was harvested using a biopsy needle (Bard MAGNUM; 12 gauge × 16 cm; C. R. Bard, Inc., Murray Hill, NJ) from the right hind leg. Once the biopsies were completed, a sterile gauze was used to apply pressure in order to stop any external bleeding and the blood on the surrounding area was cleansed with sterile saline. Afterward, the incision was closed with 3 surgical staples and an antimicrobial ointment was then applied to the incision site. Calves were monitored for 7 days post biopsy for swelling or infection of the incision site, and any staples that remained removed followed by application of iodine ointment.
2.6 Western blotting
The Western blotting protocol employed in our studies utilized a "two-step probing" approach, without stripping the blot. Briefly, Semitendinosus muscle samples (50 mg) were homogenized in 1 mL of protein extraction reagent (catalog no. 78510; Thermo Fisher Scientific) supplemented with 1 µL of Halt™ protease and phosphatase inhibitor cocktail (100x, catalog no. 78442; Thermo Fisher Scientific). Total protein concentration was quantified using a Nano-Drop ND-1000 (Thermo Fisher Scientific). Protein samples (40 µg) were denatured by heating at 37 °C for 5 min. Denatured samples were loaded onto 4–20% Mini-PROTEAN® TGX™ Precast Protein Gels (Bio-Rad Laboratories) and electrophoresed at 180 V for 10 min followed by 120 V for 50 to 60 min. A polyvinylidene fluoride membrane (catalog number 1620261; Bio-Rad) was activated with methanol for 1 min. Proteins were transferred onto polyvinylidene fluoride membranes (catalog number 1620261; Bio-Rad) using a Trans-Blot SD Semi-Dry Electrophoretic Transfer Cell (Bio-Rad). Membranes were activated with methanol and blocked for 90 min at room temperature in 1x Tris-buffered saline (1xTBST) with 5% nonfat milk. Membranes were then incubated overnight at 4 °C with primary antibodies targeting specific proteins, including mTOR, phosphorylated (p)-mTOR, eIF2alpha, 4EBP1, p-4EBP1, S6K1, p-S6K1, TSC2, ERK, INSR, PI3K, AKT, p-AKT, MRF4, SLC2A4, PPARGC1alpha, SLC1A3, SLC38A1, SLC1A5, and BCKDK, along with the internal control anti-GAPDH (catalog no. 2118S; Cell Signaling Technology; catalogue numbers and dilution ratios are included in Supplementary Table 1). Although we did not perform validations for any of these antibodies, per the manufacturers, each are polyclonal and some are reported to crossreact with bovine samples (AKT, p-AKT, GAPDH, ERK1/2). Furthermore, we have used these antibodies in several previous publications dealing with different types of bovine tissues (Coleman et al., 2022a, b). After washing 5 times for 2 min with 1x TBST, membranes were incubated with secondary antibody (HRP conjugated anti-rabbit IgG, catalog no. 7074S; Cell Signaling Technology) for 1 h at room temperature. Following another wash step, membranes were exposed to ECL reagent (catalog no. 170-5060; Bio-Rad) for 3 min in the dark, and images were captured using the ChemiDOC MP Imaging System (Bio-Rad). Band intensities were quantified using Image Lab software (version 3.0, Bio-Rad). Specific target protein band densities were normalized to GAPDH density values, which served as an internal control with stable expression across tissues and variation in intensity of 10% or less.
2.8 Targeted metabolomics
Metabolites were extracted from 50–80 mg of tissue using a solution of 4 mL/g of cold methanol and 0.85 mL/g of cold water, following the method outlined in our previous publication (Vailati-Riboni et al., 2020). Following centrifugation at 4 °C for 10 min at 12,000 × g, the resulting supernatant was collected and divided into two aliquots. The first aliquot was utilized to determine the protein concentration using the Bradford assay (no. 500–0205, Bio-Rad). The second aliquot was submitted to the Metabolomics unit at the Roy J. Carver Biotechnology Center (University of Illinois, Urbana) for targeted analyses [liquid chromatography (LC)–MS-MS] employing commercial standards (Sigma-Aldrich, St. Louis, MO, USA) previously measured by our group in bovine liver. The instrumentation, run settings, and data acquisition details remained consistent with those reported previously (Vailati-Riboni et al., 2020). Before statistical analysis, the peak area under the curve (AUC) was normalized by the protein concentration of each sample. Notably, the following metabolites were undetectable: N-methyl-glycine, 5-methyltetrahydrofolic acid, succinyl-CoA, acetyl-CoA, vitamin B12, NADPH, NADH, trimethyl-lysine, homocysteine, vitamin B6, cysteinesulfinic acid, nicotinic acid, ATP, GTP, fructose-1,6 bisphosphate, and folic acid.
2.9 Statistical analysis
The statistical analysis was conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, United States). Repeated measures analysis of body measurements and starter intake was performed using the MIXED procedure according to the following general model:
Where the dependent, continuous variable; the overall mean; is the random effect of calf within maternal diet (CoPro or CoPectin); the fixed effects in the model include maternal diet ( = CoPro or CoPectin), Time (), and interactions. is the residual error. The AR (1) covariance structure was used. Cow DMI prior to parturition was analyzed using a similar model including diet (CoPro or CoPectin), time, and their interaction. Metabolomics and protein abundance data of skeletal muscle were analyzed using the MIXED procedure according to the following model:
Where the dependent, continuous variable; the overall mean; is the random effect of calf within maternal diet (CoPro or CoPectin); the fixed effect in the model included maternal diet ( = CoPro or CoPectin). is the residual error. The normality and homoscedasticity of model residuals were assessed using the PROC UNIVARIATE procedure in SAS, with the NORMAL option and associated diagnostic plots. All means were compared using the PDIFF statement of SAS, and Tukey-Kramer's post hoc adjustment was applied. It was determined that all data were normally distributed and did not require log transformation. Significance was determined at P ≤ 0.05 whereas tendencies were declared at P ≤ 0.10.
3 Results
Feed intake by the pregnant cows during the close-up period did not differ due to source of Co (P = 0.98; Table 2). In terms of postnatal growth, only hip width had an interaction (Diet × Time, P = 0.01; Figure 1) over the 9 wk of age because of a greater response in CoPectin calves at 8 and 9 wk of age. All other growth measurements were not affected by the maternal treatment (P > 0.05; Table 2, Figure 2).
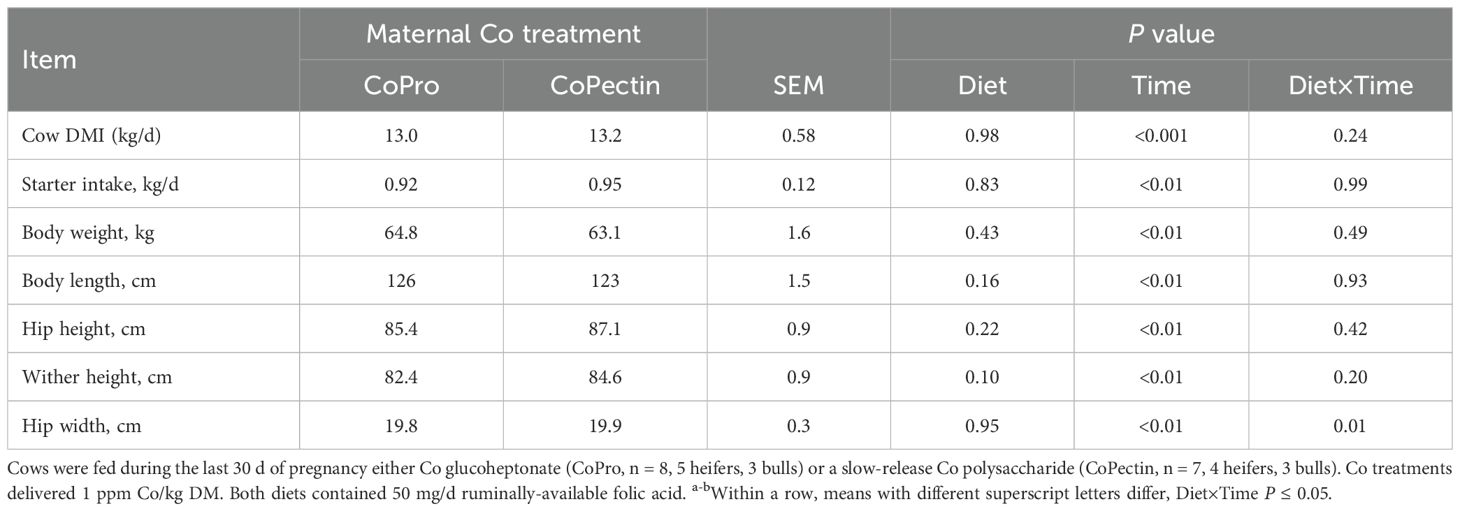
Table 2. Cow dry matter intake (DMI) during the last 30 days prepartum (close-up period) and growth performance of calves during the first 9-weeks of age.
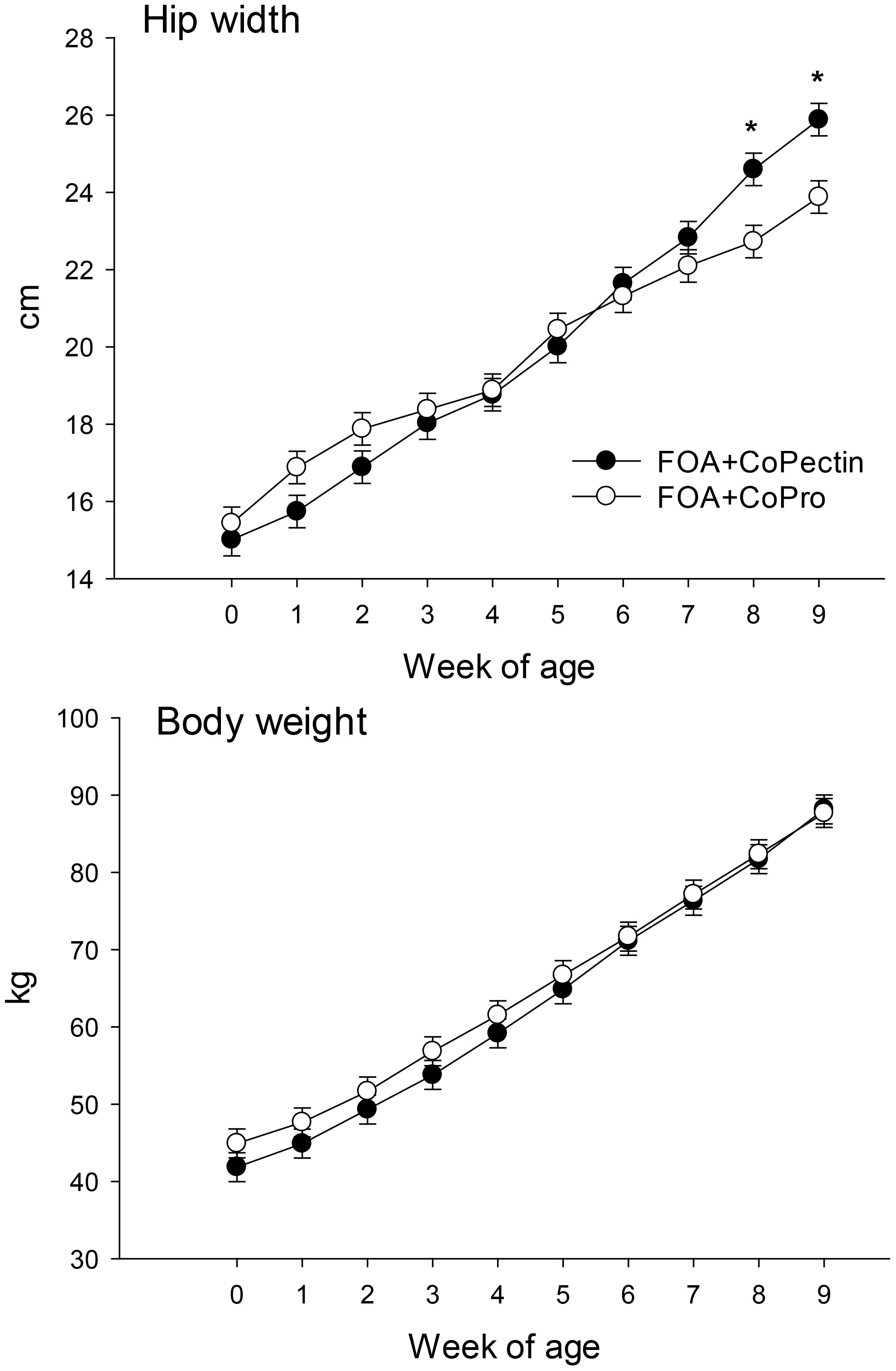
Figure 1. Hip width and body weight of calves born to cows fed during the last 30 d of pregnancy either Co glucoheptonate (CoPro, n = 8, 5 heifers, 3 bulls) or a slow-release Co polysaccharide (CoPectin, n = 7, 4 heifers, 3 bulls). Co treatments delivered 1 ppm Co/kg DM. Both diets contained 50 mg/d ruminally-available folic acid. Calves were biopsied prior to weaning at 6 wk of age. Co treatments delivered 1 ppm Co/kg DM. Asterisks in Figure denote significant differences at P ≤ 0.05.
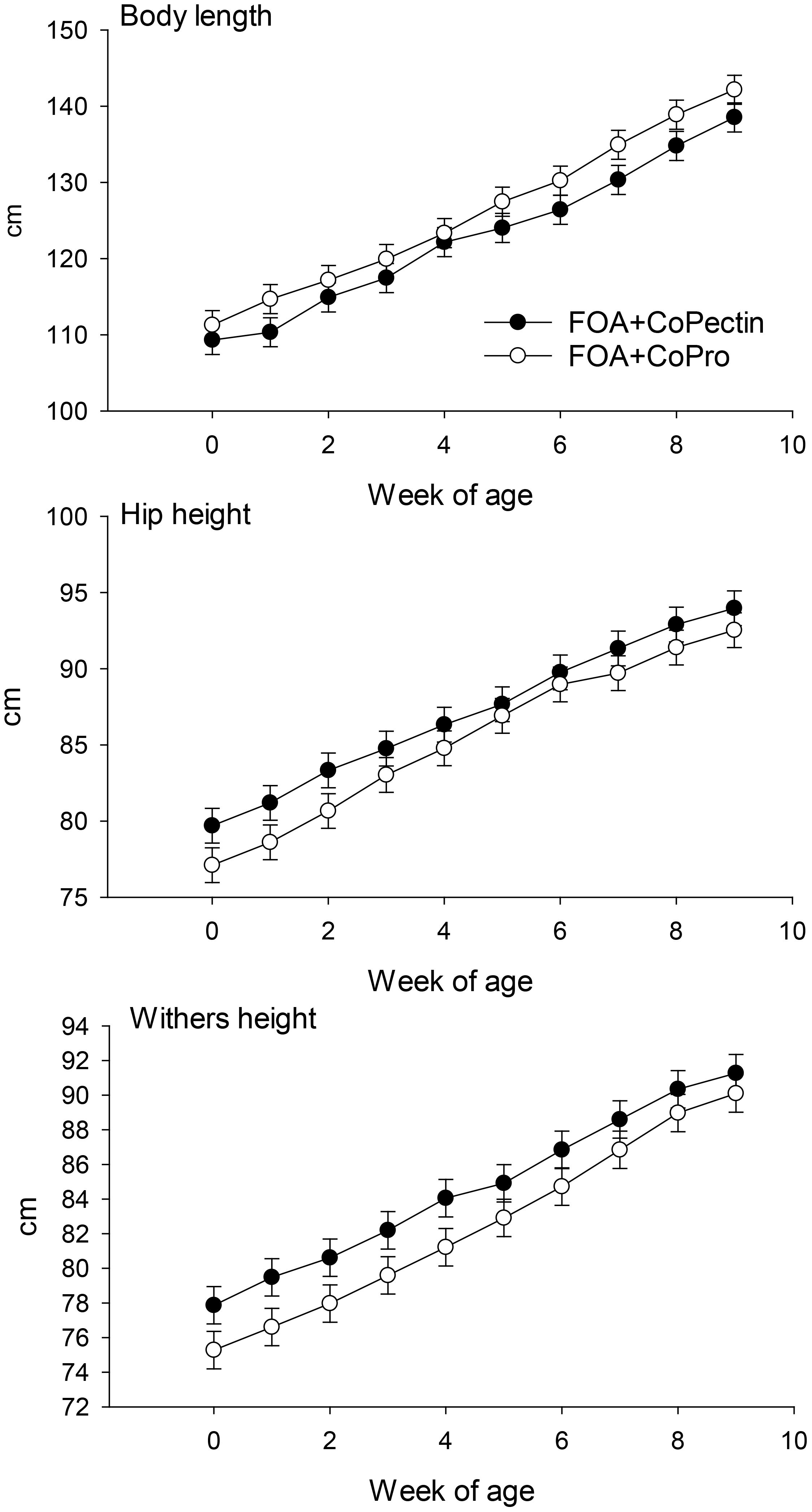
Figure 2. Body length, hip height, and withers height Hip width and body weight of calves born to cows fed during the last 30 d of pregnancy either Co glucoheptonate (CoPro, n = 8, 5 heifers, 3 bulls) or a slow-release Co polysaccharide (CoPectin, n = 7, 4 heifers, 3 bulls). Co treatments delivered 1 ppm Co/kg DM. Both diets contained 50 mg/d ruminally-available folic acid. Calves were biopsied prior to weaning at 6 wk of age. Co treatments delivered 1 ppm Co/kg DM. Asterisks in Figure denote significant differences at P ≤ 0.05.
Calves fed to cows supplemented with CoPectin also had alterations of certain metabolites within the one-carbon metabolism pathway (Table 3). Compared with CoPro calves, there was a greater concentration of betaine (P = 0.04), S-5'-adenosyl-methionine (P = 0.02), and a tendency for greater concentration of cystathionine (P = 0.06) and choline (P = 0.10) in CoPectin calves.
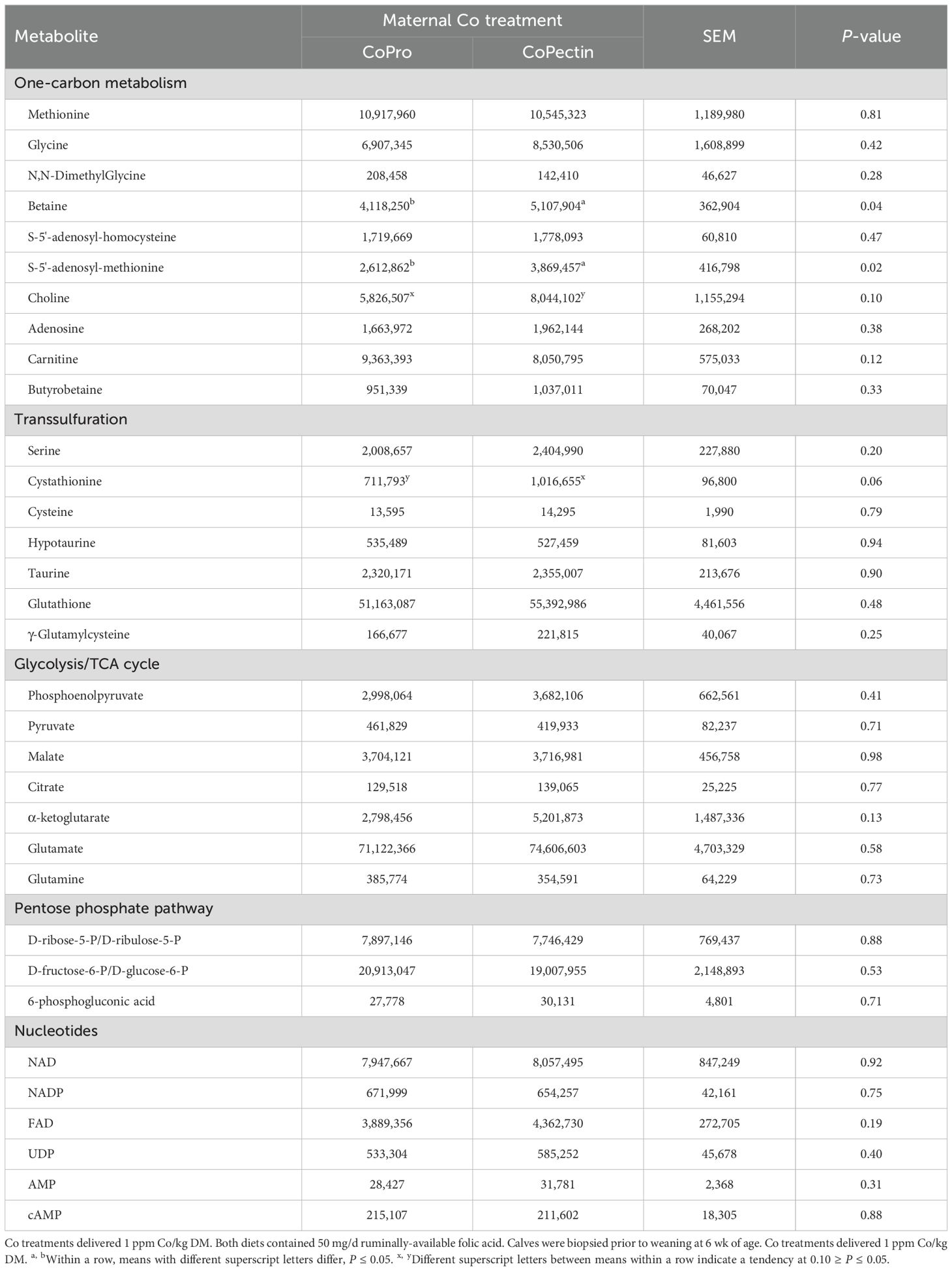
Table 3. Metabolite concentrations (peak AUC) in semitendinosus muscle from calves born to cows fed during the last 30 d of pregnancy either Co glucoheptonate (CoPro, n = 8, 5 heifers, 3 bulls) or a slow-release Co polysaccharide (CoPectin, n = 7, 4 heifers, 3 bulls).
Results of the Western blotting analysis (Table 4) revealed that, compared with calves born to cows fed CoPro, calves born to cows fed CoPectin had greater abundance of INSR (P = 0.05), p-AKT (P = 0.05), and p-AKT/AKT ratio (P = 0.001), which is used as a proxy of AKT activity. In contrast, these calves had lower abundance of total 4EBP1 (P = 0.03) and MRF4 (P = 0.04).
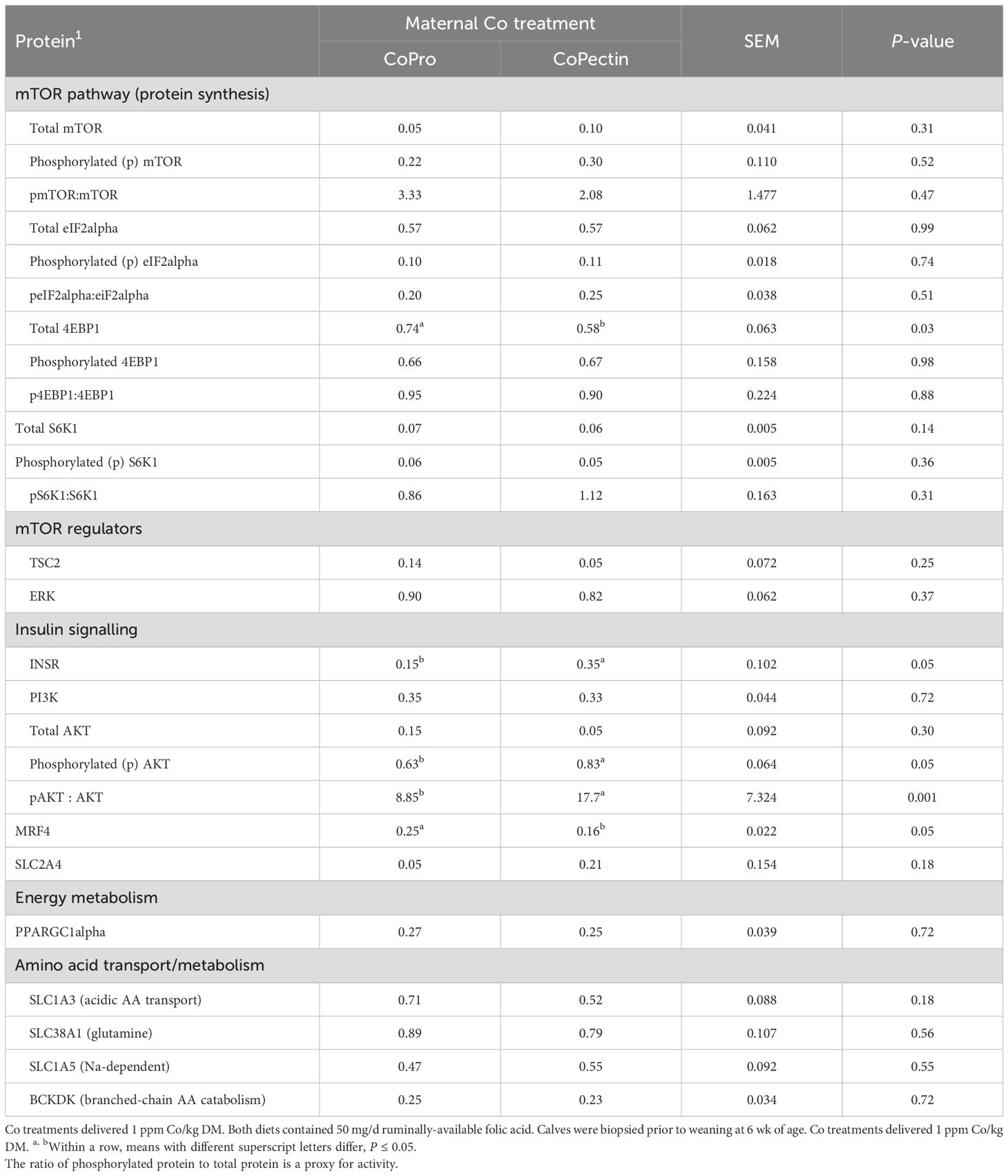
Table 4. Abundance of proteins (relative to GAPDH) in semitendinosus muscle from calves born to cows fed during the last 30 d of pregnancy either Co glucoheptonate (CoPro, n = 8, 5 heifers, 3 bulls) or a slow-release Co polysaccharide (CoPectin, n = 7, 4 heifers, 3 bulls).
4 Discussion
The present study demonstrates that the chemical form of cobalt supplemented to the dam during late gestation can modulate the postnatal muscle molecular response of calves. Because cobalt is a structural component of vitamin B12, its bioavailability directly influences one-carbon metabolism and the generation of methyl donors such as S-adenosylmethionine (SAM). The higher concentrations of SAM and betaine observed in calves from CoPectin-fed cows suggest an enhanced methylation potential that may favor activation of the insulin–AKT–mTOR signalling pathway.
Wither height represents the best parameter for evaluating skeletal growth of heifers/calves because this measure is not affected by body condition and is correlated with body weight (Heinrichs et al., 1992). In one of our previous studies, a greater wither height in dairy calves born to cows fed an amino acid-complexed mixture of Zn, Mn, and Cu, and Co glucoheptonate compared with sulfate sources was suggestive of enhanced bone growth (Jacometo et al., 2015). Among the potential mechanisms that could affect bone development in utero, processes such as endochondrial ossification and cartilage formation require trace minerals (Chen et al., 2012) and recent data suggested a direct role of Co on angiogenesis and bone formation in rats (Zheng et al., 2024). Although no differences in dam DMI were achieved during the last period of pregnancy, similar to our previous study (Jacometo et al., 2015), the differences in the bioavailability of Co from CoPro versus CoPectin could have had an impact on its supply to the developing calf.
Both CoPro and CoPectin are rumen-available sources of Co (Zinpro Corp.), but the speed at which Co releases differs between sources because pectin is more resistant to microbial action than glucoheptonate. In fact, pectin itself can induce changes in the ruminal microbiota (Liu et al., 2015; Poulsen et al., 2012), which in turn can alter the assimilation of Co for the synthesis of vitamin B12 (McDowell, 2000). Dietary Co content positively influences ruminal vitamin B12 synthesis (Nutrient Requirements of Dairy Cattle, 2021). Thus, although either source results in a gradual release of Co in the rumen, feeding CoPectin would be expected to enhance Co availability to bacteria and potentially the animal.
From a molecular and physiological standpoint, the muscle protein abundance data also help understand the effect of maternal supplementation of Co sources on postnatal calf growth. In tissues such as the liver, it is well-established in non-ruminants and ruminants (Coleman et al., 2021) that the activity of methionine synthase (MTR; a key component of the folic and Met cycles) requires vitamin B12. This enzyme catalyzes the transfer of a methyl group from 5-methyltetrahydrofolate (5-MTH) to homocysteine to generate methionine, and vitamin B12 (cobalamin) acts as a cofactor in this reaction (MTR is a vitamin B12-dependent enzyme) (Krupenko, 2020). We recently demonstrated that the Holstein bovine fetal skeletal muscle at the last third of gestation expresses MTR and other enzymes with key roles in one-carbon metabolism (Aboragah et al., 2023). Thus, Co supplementation over currently recommended dosages [doubled from 0.11 to 0.20 mg/kg of dry matter in the latest Nutrient Requirements of Dairy Cattle, 8th edition (2021)] and during the last period of pregnancy (the most critical period where voluntary DMI is reduced) could further improve the production of methyl groups via the activity of methionine adenosyl transferase 1A (MAT1A) within the Met cycle. This idea is supported by recent data we generated demonstrating that MAT1A protein is highly-abundant in the bovine fetal (Aboragah et al., 2023) and adult cow skeletal muscle (Thanh et al., 2023).
The greater abundance of p-AKT (and in turn the greater p-AKT/AKT ratio) and INSR in CoPectin calves underscored the likely existence of improved skeletal muscle insulin sensitivity, a response that would be advantageous for postnatal calf growth (Hammon et al., 2012). To fully meet its requirements for glucose immediately after birth, the neonatal calf must activate glycogenolysis and especially gluconeogenesis to maintain normal glycemia (Baldwin et al., 2007). Hence, at equal amount of lactose provided by milk or milk replacer, the faster the calf acquires the ability to generate energy endogenously the better it can deal with the extrauterine demands of the body. As calves grow and receive increasing amounts of solid feed, the development of a ruminal microbial population ensures that propionate becomes the predominant gluconeogenic substrate (Donkin and Armentano, 1995).
At the cellular level, at least in non-ruminants, a greater abundance of p-AKT positively regulates a wide variety of functions including cell proliferation, survival, metabolism, and angiogenesis (Nader, 2005). Activity of p-AKT also feeds into the mTOR signaling pathway which controls the assembly of the eukaryotic translation initiation factor 4F (eIF4E) complex that responds to extracellular signals such as growth factors, nutrients, and cytokines (Powell et al., 2012).
Another potential mechanistic framework that links a greater availability of vitamin B12 with the enhanced insulin signalling [through greater protein abundance of the Insulin Receptor (INSR)] is the intermediate metabolite S-adenosyl methionine (SAM). Based on present results, greater production of the methionine-cycle product SAM could lead to an enhanced activation of AKT-mTOR signalling. In fact, research with non-ruminant models has indicated that products of the Met cycle can activate mTOR. The activation of mTOR via SAM occurs indirectly as a result of SAM binding to the protein SAMTOR, which is an inhibitor of mTOR complex 1 (MTORC1), and the subsequent inhibition of the association of SAMTOR and GATOR1, allowing MTORC1 to be activated (Gu et al., 2017). Upon activation of mTORC1, this kinase phosphorylates S6K and inhibits it, resulting in an increase of insulin sensitivity and upregulation of IRS1 and INSR (Guo 2016).
The observed differences in muscle metabolites are suggestive of alterations in the Met cycle and the transsulfuration pathway in relation to the availability of Co during the last stages of pregnancy, where cows face physiological challenges that might impinge on their voluntary DMI. Ensuring a slow release of Co in the rumen would lead to increased availability to ruminal microbes and formation of vitamin B12, which in turn would be available for uptake at the intestinal level at a sustained rate. If such a mechanism holds, then the supply of vitamin B12 to the liver and other extrahepatic tissues of the fetus would increase.
Along with the presence of intermediate metabolites, protein abundance for components of one-carbon metabolism in skeletal muscle allow for greater understanding of the long-term biological impacts on the calf. Thus, together with metabolite data discussed above, the lower protein abundance of MRF4 in muscle of CoPectin calves supports the idea of enhanced growth. At least in non-ruminants, this protein binds DNA and exerts control over transcription of genes associated with muscle cell differentiation and maintenance of postnatal muscle mass (Moretti et al., 2016). Knockout mouse studies demonstrated that inhibition of MRF4 induces muscle protein synthesis via activation of muscle-specific genes (Moretti et al., 2016), thus, along with the greater abundance of the insulin receptor INSR and p-AKT : AKT and the lower total 4EBP1, the semitendinosus muscle in CoPectin calves seemed to have been programmed for more efficient growth.
In the context of the present study, we speculate that besides its direct positive effect on semitendinosus muscle growth, other muscle types including those of the hip region (e.g., iliopsoas and sartorius) and their interactions with surrounding osteoblasts could help explain the tendency for greater wither height and the greater hip width over time. Mechanistically, although not measured in the present study, it is well-known in non-ruminants that skeletal muscle and bone anabolism are tightly coupled during growth such that growth factors produced by muscle could impact growth of the surrounding osteoblasts (Hamrick et al., 2010). Thus, despite the lack of published data with dairy calves, the present data are indicative of a synergistic effect of prenatal nutrition on skeletal muscle development through the pre-weaning stage.
A limitation of the present study is that muscle samples were collected only once, at 42 days of age, which prevents assessment of temporal changes in molecular and metabolic profiles. In addition, only one muscle (semitendinosus) was analysed; other tissues could respond differently to maternal cobalt supplementation. These aspects limit the generalization of the current findings. Future studies should include multiple time points and tissues to better capture the dynamic effects of cobalt source and the persistence of these programming mechanisms.
5 Conclusions
Trace minerals, especially the ones that are important drivers of key metabolic reactions (also known as cofactors), can be essential for adequate growth of the fetus, which depends completely on the dam for supply of these elements. Compared with cobalt glucoheptonate, cobalt pectin supplementation led to higher S-adenosylmethionine and betaine concentrations, along with greater INSR and phosphorylated AKT abundance, suggesting that maternal cobalt source affects the activity of one-carbon metabolism and insulin signaling in calf skeletal muscle. Although growth performance parameters were largely unaffected, the combined metabolite and protein data indicate that maternal cobalt source may shape early-life muscle metabolic programming. Because muscle tissue was evaluated only once and no functional or anatomical growth measures were performed, these results should be interpreted as molecular-level adaptations rather than physiological outcomes. Future studies should investigate how different cobalt sources modulate vitamin B12 synthesis, one-carbon metabolism, and downstream anabolic signaling across multiple tissues and developmental stages to clarify their long-term implications for calf growth and health.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the Institutional Animal Care and Use Committee (protocol no. 17168) of the University of Illinois, Urbana-Champaign. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
VL: Investigation, Writing – original draft. CJ: Data curation, Formal analysis, Methodology, Writing – original draft. AA: Data curation, Formal analysis, Investigation, Writing – review & editing. TI: Investigation, Writing – review & editing. FA: Investigation, Writing – original draft. MS: Resources, Writing – review & editing. JL: Conceptualization, Funding acquisition, Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Zinpro Corporation provided financial support to cover costs of animal use, data collection, and sample analyses. The cobalt pectin and folic acid products used in this study are products in development at Zinpro Corporation, and it is the intent of Zinpro Corporation to eventually commercialize the cobalt pectin product and a folic acid product.
Acknowledgments
We thank Perdue AgriBusiness for the donation of ProvAAL2 AADvantage during the course of the experiment. We also thank ADM Animal Nutrition for the donation of Soychlor® during the course of the experiment. The cobalt pectin and folic acid products used in this study are products in development at Zinpro Corporation, and it is the intent of Zinpro Corporation to eventually commercialize the cobalt pectin product and a folic acid product.
Conflict of interest
Author MS is employed by the company Zinpro Corporation.
The authors declare that this study received funding from Zinpro Corporation. The funder had the following involvement in the study: study design.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2025.1686755/full#supplementary-material
References
Aboragah A. A., Sherlock D. N., Wichasit N., Mauck J., and Loor J. J. (2023). Intermediate metabolites and molecular correlates of one‑carbon and nutrient metabolism differ in tissues from Holstein fetuses. Research in Veterinary Science 164, 104988. doi: 10.1016/j.rvsc.2023.104988
Alharthi A. S., Batistel F., Abdelmegeid M. K., Lascano G., Parys C., Helmbrecht A., et al. (2018). Maternal supply of methionine during late-pregnancy enhances rate of Holstein calf development in utero and postnatal growth to a greater extent than colostrum source. J. Anim. Sci. Biotechnol. 9, 83. doi: 10.1186/s40104-018-0298-1
Alharthi A. S., Lopreiato V., Dai H., Bucktrout R., Abdelmegeid M., Batistel F., et al. (2019). Short communication: Supply of methionine during late pregnancy enhances whole-blood innate immune response of Holstein calves partly through changes in mRNA abundance in polymorphonuclear leukocytes. J. Dairy Sci. 102, 10599–10605. doi: 10.3168/jds.2018-15676
Aliarabi H., Fadayifar A., Alimohamady R., and Dezfoulian A. H. (2019). The effect of maternal supplementation of zinc, selenium, and cobalt as slow-release ruminal bolus in late pregnancy on some blood metabolites and performance of ewes and their lambs. Biol. Trace Element Res. 187, 403–410. doi: 10.1007/s12011-018-1409-8
AOAC (1997). Association of Official Analytical Chemists International Official Methods of Analysis. AOAC: Arlington.
AOAC (2006). Official Methods of Analysis Association of Official Analytical Chemists: Washington DC.
AOCS (2013). Official methods and recommended practices of the American Oil Chemists' Society. Champaign, IL: AOCS Press.
(2021). Nutrient Requirements of Dairy Cattle (Washington, D.C: National Academies Press). doi: 10.17226/25806
Baldwin R. L., McLeod K. R., McNamara J. P., Elsasser T. H., and Baumann R. G. (2007). Influence of abomasal carbohydrates on subcutaneous, omental, and mesenteric adipose lipogenic and lipolytic rates in growing beef steers. J. Anim. Sci. 85, 2271–2282. doi: 10.2527/jas.2006-588
Barcelos S. D. S., Nascimento K. B., Silva T. E. D., Mezzomo R., Alves K. S., De Souza Duarte M., et al. (2022). The effects of prenatal diet on calf performance and perspectives for fetal programming studies: A meta-analytical investigation. Animals 12, 2145. doi: 10.3390/ani12162145
Cattaneo L., Laporta J., and Dahl G. E. (2022). Programming effects of late gestation heat stress in dairy cattle. Reprod. Fertil Dev. 35, 106–117. doi: 10.1071/RD22209
Chen H., Miller S., Lane R., and Moyer-Mileur L. (2012). Intrauterine growth restriction decreases endochondral ossification and bone strength in female rats. Am. J. Perinatology 30, 261–266. doi: 10.1055/s-0032-1323588
Christofaro Fernandes A., Beline M., Polizel G. H. G., Cavalcante Cracco R., Ferreira Dias E. F., Furlan É., et al. (2023). Fetal programming and its effects on meat quality of nellore bulls. Veterinary Sci. 10, 672. doi: 10.3390/vetsci10120672
Coleman D. N., Alharthi A. S., Liang Y., Lopes M. G., Lopreiato V., Vailati-Riboni M., et al. (2021). Multifaceted role of one-carbon metabolism on immunometabolic control and growth during pregnancy, lactation and the neonatal period in dairy cattle. J. Anim. Sci. Biotechnol. 12, 27. doi: 10.1186/s40104-021-00547-5
Coleman D., Lopreiato V., Alharthi A. S., and Loor J. J. (2020). Amino acids and the regulation of oxidative stress and immune function in dairy cattle Amino acids and the regulation of oxidative stress and immune function in dairy cattle. J Anim Sci. 98, S175–S193. doi: 10.1093/jas/skaa138
Coleman D. N., Vailati-Riboni M., Pate R. T., Aboragah A., Luchini D., Cardoso F. C., et al. (2022a). Increased supply of methionine during a heat-stress challenge in lactating holstein cows alters mammary tissue mTOR signaling and its response to lipopolysaccharide. J Anim Sci 100, skac175. doi: 10.1093/jas/skac175
Coleman D. N., Totakul P., Onjai-Uea N., Aboragah A., Jiang Q., Vailati-Riboni M., et al. (2022b). Rumen-protected methionine during heat stress alters mTOR, insulin signaling, and 1-carbon metabolism protein abundance in liver, and whole-blood transsulfuration pathway genes in Holstein cows. J Dairy Sci. 105, 7787–7804. doi: 10.3168/jds.2021-21379
Donkin S. S. and Armentano L. E. (1995). Insulin and glucagon regulation of gluconeogenesis in preruminating and ruminating bovine. J. Anim. Sci. 73, 546–551. doi: 10.2527/1995.732546x
Duplessis M. and Girard C. L. (2019). Effect of maternal biotin, folic acid, and vitamin B12 supplementation before parturition on colostral and Holstein calf plasma concentrations in those vitamins. Anim. Feed Sci. Technol. 256, 114241. doi: 10.1016/j.anifeedsci.2019.114241
Duplessis M., Lapierre H., Sauerwein H., and Girard C. L. (2022). Combined biotin, folic acid, and vitamin B12 supplementation given during the transition period to dairy cows: Part I. Effects on lactation performance, energy and protein metabolism, and hormones. J. Dairy Sci. 105, 7079–7096. doi: 10.3168/jds.2021-21677
Elolimy A., Alharthi A., Zeineldin M., Parys C., Helmbrecht A., and Loor J. J. (2019). Supply of methionine during late-pregnancy alters fecal microbiota and Metabolome in neonatal dairy calves without changes in daily feed intake. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.02159
Girard C. L. and Duplessis M. (2023). Review: State of the knowledge on the importance of folates and cobalamin for dairy cow metabolism. Animal 17, 100834. doi: 10.1016/j.animal.2023.100834
Gu X., Orozco J. M., Saxton R. A., Condon K. J., Liu G. Y., Krawczyk P. A., et al. (2017). SAMTOR is an S-adenosylmethionine sensor for the mTORC1 pathway. Science 358, 813–818. doi: 10.1126/science.aao3265
Guo Y., Tang C. Y., Man X. F., Tang H. N., Tang J., Wang F., et al. (2016). Insulin receptor substrate-1 time-dependently regulates bone formation by controlling collagen Iα2 expression via miR-342. FASEB J. 30, 4214–4226. doi: 10.1096/fj.201600445RR
Hammon H. M., Steinhoff-Wagner J., Schönhusen U., Metges C. C., and Blum J. W. (2012). Energy metabolism in the newborn farm animal with emphasis on the calf: endocrine changes and responses to milk-born and systemic hormones. Domest. Anim. Endocrinol. 43, 171–185. doi: 10.1016/j.domaniend.2012.02.005
Hamrick M. W., McNeil P. L., and Patterson S. L. (2010). Role of muscle-derived growth factors in bone formation. J. Musculoskeletal Neuronal Interact. 10, 64–70.
Hatfield P. G., Snowder G. D., Head W. A., Glimp H. A., Stobart R. H., and Besser T. (1995). Production by ewes rearing single or twin lambs: effects of dietary crude protein percentage and supplemental zinc methionine1. J. Anim. Sci. 73, 1227–1238. doi: 10.2527/1995.7351227x
Heinrichs A. J., Rogers G. W., and Cooper J. B. (1992). Predicting body weight and wither height in holstein heifers using body measurements. J. Dairy Sci. 75, 3576–3581. doi: 10.3168/jds.S0022-0302(92)78134-X
Hostetler C. E., Kincaid R. L., and Mirando M. A. (2003). The role of essential trace elements in embryonic and fetal development in livestock. Veterinary J. 166, 125–139. doi: 10.1016/S1090-0233(02)00310-6
Hulbert L. E. and Moisá S. J. (2016). Stress, immunity, and the management of calves. J. Dairy Sci. 99, 3199–3216. doi: 10.3168/jds.2015-10198
Jacometo C. B., Alharthi A. S., Zhou Z., Luchini D., and Loor J. J. (2018). Maternal supply of methionine during late pregnancy is associated with changes in immune function and abundance of microRNA and mRNA in Holstein calf polymorphonuclear leukocytes. J. Dairy Sci. 101, 8146–8158. doi: 10.3168/jds.2018-14428
Jacometo C. B., Osorio J. S., Socha M., Corrêa M. N., Piccioli-Cappelli F., Trevisi E., et al. (2015). Maternal consumption of organic trace minerals alters calf systemic and neutrophil mRNA and microRNA indicators of inflammation and oxidative stress. J Dairy Sci 98, 7717–7729. doi: 10.3168/jds.2015-9359
Jacometo C. B., Zhou Z., Luchini D., Trevisi E., Corrêa M. N., and Loor J. J. (2016). Maternal rumen-protected methionine supplementation and its effect on blood and liver biomarkers of energy metabolism, inflammation, and oxidative stress in neonatal Holstein calves. J. Dairy Sci. 99, 6753–6763. doi: 10.3168/jds.2016-11018
Kennedy D. G., Cannavan A., Molloy A., O’ harte F., Taylor S. M., Kennedy S., et al. (1990). Methylmalonyl-CoA mutase ( EC 5.4.99.2) and methionine synthetase (EC 2.1.1.13) in the tissues of cobalt–vitamin B 12 deficient sheep. Br. J. Nutr. 64, 721–732. doi: 10.1079/BJN19900074
Knudsen K. E. B. (1997). Carbohydrate and lignin contents of plant materials used in animal feeding. Animal Feed Science and Technology 67, 319–338. doi: 10.1016/S0377-8401(97)00009-6
Krupenko N. I. (2020). “Folate and vitamins B6 and B12,” in Principles of nutrigenetics and nutrigenomics (Cambridge, Massachusetts: Academic Press), 295–302. doi: 10.1016/B978-0-12-804572-5.00039-2
Liu J., Pu Y.-Y., Xie Q., Wang J.-K., and Liu J.-X. (2015). Pectin induces an in vitro rumen microbial population shift attributed to the pectinolytic treponema group. Curr. Microbiol. 70, 67–74. doi: 10.1007/s00284-014-0672-y
Lopes M. G., Alharthi A. S., Lopreiato V., Abdel-Hamied E., Liang Y., Coleman D. N., et al. (2021). Maternal supplementation with cobalt sources, folic acid, and rumen-protected methionine and its effects on molecular and functional correlates of the immune system in neonatal Holstein calves. J. Dairy Sci. 104, 9340–9354. doi: 10.3168/jds.2020-19674
Lopreiato V., Alharthi A. S., Liang Y., Elolimy A. A., Bucktrout R., Socha M. T., et al. (2023a). Influence of cobalt source, folic acid, and rumen-protected methionine on performance, metabolism, and liver tissue one-carbon metabolism biomarkers in peripartal holstein cows. Animals 13, 2107. doi: 10.3390/ani13132107
Lopreiato V., Minuti A., Trevisi E., Piccione G., Ferronato G., Loor J. J., et al. (2023b). Maternal treatment with pegbovigrastim influences growth performance and immune-metabolic status of calves during the pre-weaning period. Res. Veterinary Sci. 158, 151–163. doi: 10.1016/j.rvsc.2023.03.019
Marques R. S., Cooke R. F., Rodrigues M. C., Cappellozza B. I., Mills R. R., Larson C. K., et al. (2016). Effects of organic or inorganic cobalt, copper, manganese, and zinc supplementation to late-gestating beef cows on productive and physiological responses of the offspring1. J. Anim. Sci. 94, 1215–1226. doi: 10.2527/jas.2015-0036
McDowell L. R. (2000). Vitamins in Animal and Human Nutrition (Iowa State University Press: Wiley). doi: 10.1002/9780470376911
Moretti I., Ciciliot S., Dyar K. A., Abraham R., Murgia M., Agatea L., et al. (2016). MRF4 negatively regulates adult skeletal muscle growth by repressing MEF2 activity. Nat. Commun. 7, 12397. doi: 10.1038/ncomms12397
Nader G. A. (2005). Molecular determinants of skeletal muscle mass: getting the “AKT” together. Int. J. Biochem. Cell Biol. 37, 1985–1996. doi: 10.1016/j.biocel.2005.02.026
Oster M., Nuchchanart W., Trakooljul N., Muráni E., Zeyner A., Wirthgen E., et al. (2016). Methylating micronutrient supplementation during pregnancy influences foetal hepatic gene expression and IGF signalling and increases foetal weight. Eur. J. Nutr. 55, 1717–1727. doi: 10.1007/s00394-015-0990-2
Ouellet V., Laporta J., and Dahl G. E. (2020). Late gestation heat stress in dairy cows: Effects on dam and daughter. Theriogenology 150, 471–479. doi: 10.1016/j.theriogenology.2020.03.011
Palombo V., Alharthi A., Batistel F., Parys C., Guyader J., Trevisi E., et al. (2021). Unique adaptations in neonatal hepatic transcriptome, nutrient signaling, and one-carbon metabolism in response to feeding ethyl cellulose rumen-protected methionine during late-gestation in Holstein cows. BMC Genomics 22, 280. doi: 10.1186/s12864-021-07538-w
Pepper M. R. and Black M. M. (2011). B12 in fetal development. Semin. Cell Dev. Biol. 22, 619–623. doi: 10.1016/j.semcdb.2011.05.005
Polizel G. H. G., De Francisco Strefezzi R., Cracco R. C., Fernandes A. C., Zuca C. B., Castellar H. H., et al. (2021). Effects of different maternal nutrition approaches on weight gain and on adipose and muscle tissue development of young bulls in the rearing phase. Trop. Anim. Health Prod 53, 536. doi: 10.1007/s11250-021-02982-y
Polizel G. H. G., Santos M. E. P. D., Cesar A. S. M., Diniz W. J. S., Ramírez-Zamudio G. D., Fantinato-Neto P., et al. (2025). Late gestation maternal nutrition has a stronger impact on offspring liver transcriptome than full-gestation supplementation in beef cattle. Veterinary Sci. 12, 406. doi: 10.3390/vetsci12050406
Poulsen M., Jensen B. B., and Engberg R. M. (2012). The effect of pectin, corn and wheat starch, inulin and pH on in vitro production of methane, short chain fatty acids and on the microbial community composition in rumen fluid. Anaerobe 18, 83–90. doi: 10.1016/j.anaerobe.2011.12.009
Powell J. D., Pollizzi K. N., Heikamp E. B., and Horton M. R. (2012). Regulation of immune responses by mTOR. Annu. Rev. Immunol. 30, 39–68. doi: 10.1146/annurev-immunol-020711-075024
Rock M. J., Kincaid R. L., and Carstens G. E. (2001). Effects of prenatal source and level of dietary selenium on passive immunity and thermometabolism of newborn lambs. Small Ruminant Res. 40, 129–138. doi: 10.1016/S0921-4488(01)00167-5
Schalch Junior F. J., Polizel G. H. G., Cançado F. A. C. Q., Fernandes A. C., Mortari I., Pires P. R. L., et al. (2022). Prenatal supplementation in beef cattle and its effects on plasma metabolome of dams and calves. Metabolites 12, 347. doi: 10.3390/metabo12040347
Shang K., Guan J., An T., Zhao H., Bai Q., Li H., et al. (2024). Effects of perinatal nutrition supplementation and early weaning on serum biochemistry, metabolomics, and reproduction in yaks. Front. Vet. Sci. 11. doi: 10.3389/fvets.2024.1443856
Spears J. W. and Weiss W. P. (2008). Role of antioxidants and trace elements in health and immunity of transition dairy cows. Veterinary J. 176, 70–76. doi: 10.1016/j.tvjl.2007.12.015
Stangl G. I., Schwarz F. J., and Kirchgessner M. (1999). Moderate long-term cobalt-deficiency affects liver, brain and erythrocyte lipids and lipoproteins of cattle. Nutr. Res. 19, 415–427. doi: 10.1016/S0271-5317(99)00010-X
Thanh L. P., Wichasit N., Li Y., Batistel F., Tartrakoon W., Parys C., et al(2023). Alterations in skeletal muscle abundance of protein turnover, stress, and antioxidant proteins during the periparturient period in dairy cows fed ethyl-cellulose rumen-protected methionine. Journal of Dairy Science 106, 5127–5145. doi: 10.3168/jds.2022-23187
Tiffany M. E., Fellner V., and Spears J. W. (2006). Influence of cobalt concentration on vitamin B12 production and fermentation of mixed ruminal microorganisms grown in continuous culture flow-through fermentors1. J. Anim. Sci. 84, 635–640. doi: 10.2527/2006.843635x
Tiffany M. E., Spears J. W., Xi L., and Horton J. (2003). Influence of dietary cobalt source and concentration on performance, vitamin B12 status, and ruminal and plasma metabolites in growing and finishing steers1,2. J. Anim. Sci. 81, 3151–3159. doi: 10.2527/2003.81123151x
Vailati-Riboni M., Crookenden M., Kay J. K., Meier S., Mitchell M. D., Heiser A., et al. (2020). Hepatic one-carbon metabolism enzyme activities and intermediate metabolites are altered by prepartum body condition score and plane of nutrition in grazing Holstein dairy cows. J. Dairy Sci. 103, 2662–2676. doi: 10.3168/jds.2019-16798
Van Soest P. J., Robertson J. B., and Lewis B. A. (1991). Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. Journal of Dairy Science 74, 3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
Zenobi M. G., Gardinal R., Zuniga J. E., Dias A. L. G., Nelson C. D., Driver J. P., et al. (2018). Effects of supplementation with ruminally protected choline on performance of multiparous Holstein cows did not depend upon prepartum caloric intake. J. Dairy Sci. 101, 1088–1110. doi: 10.3168/jds.2017-13327
Keywords: nutritional programming, maternal cobalt supplementation, muscle metabolism, metabolomics, dairy calf
Citation: Lopreiato V, Jacometo CB, Alharthi AA, Incharoen T, Arfuso F, Socha MT and Loor JJ (2025) Prepartal dietary cobalt source alters holstein calf semitendinosus muscle abundance of mTOR and insulin signaling proteins and intermediates of one-carbon metabolism. Front. Anim. Sci. 6:1686755. doi: 10.3389/fanim.2025.1686755
Received: 15 August 2025; Accepted: 03 November 2025;
Published: 03 December 2025.
Edited by:
Ravikanthreddy Poonooru, University of Missouri, United StatesReviewed by:
Yuwen Dong, University of Pennsylvania, United StatesGuilherme Henrique Gebim Polizel, University of São Paulo, Brazil
Copyright © 2025 Lopreiato, Jacometo, Alharthi, Incharoen, Arfuso, Socha and Loor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan J. Loor, amxvb3JAaWxsaW5vaXMuZWR1
 Vincenzo Lopreiato
Vincenzo Lopreiato Carolina B. Jacometo2
Carolina B. Jacometo2 Abdulrahman A. Alharthi
Abdulrahman A. Alharthi Tossaporn Incharoen
Tossaporn Incharoen Francesca Arfuso
Francesca Arfuso Juan J. Loor
Juan J. Loor