- 1College of Agricultural Engineering, Guangdong Meizhou Vocational and Technical College, Meizhou, China
- 2Meizhou Engineering Research Center for Veterinary Medicine and Natural Medicine, Guangdong Meizhou Vocational and Technical College, Meizhou, China
- 3State Key Laboratory of Animal Biotech Breeding, Institute of Animal Science, Chinese Academy of Agricultural Sciences, Beijing, China
Although studies have investigated Solanum nigrum L. (SNL) in mice, its effects on broilers remain unclear. This study examined how dietary SNL influences growth performance, antioxidant capacity, ileal transcriptome, and gut microbiota in broilers. A total of 200 one-day-old healthy Wuhua yellow-feathered chickens were randomly divided into four groups of five replicates (10 birds each). The groups received: a basal diet (CON), a basal diet with 500 mg/kg amoxicillin (AMO), a basal diet with 1000 mg/kg SNL grass meal (0.1% SNL), and a basal diet with 2000 mg/kg SNL grass meal (0.2% SNL). The experiment lasted 35 days. SNL supplementation modestly improved feed efficiency and jejunal villus height (p = 0.019). It also altered cecal microbiota by increasing Bacteroidetes, Bacteroides, and Faecalibacterium, while decreasing Firmicutes and Oscillibacter. Ileal transcriptomics identified multiple differentially expressed genes (DEGs) across comparisons, which were enriched in intestinal immune network pathways for IgA production. Correlation analysis linked cecal microbiota changes to ileal gene expression. In conclusion, SNL exhibits the potential as an alternative to antibiotics in chickens, and this study provides empirical support for its broader adoption in poultry industry.
1 Introduction
Antibiotics play a pivotal role in safeguarding animal health and improving feed conversion efficiency owing to their antimicrobial activity, which inhibits or eliminates pathogenic microorganisms (Fernández Miyakawa et al., 2024). However, the overuse and misuse of antibiotics have contributed to a marked rise in antibiotic-resistant bacterial strains, posing a substantial public health threat (Abd El-Ghany, 2020; Obianwuna et al., 2024; Oladeji et al., 2025). In light of growing concerns over environmental sustainability and food safety, the development of antibiotic alternatives has become a major focus of scientific research. Among these alternatives, Chinese herbal medicines have garnered significant interest as cost-effective feed additives, particularly due to their demonstrated benefits in enhancing intestinal health in broiler chickens (Xu et al., 2022; Wang et al., 2024a), along with their antioxidative, immunomodulatory, and growth-promoting properties (Wang et al., 2021; Xu et al., 2022; Li et al., 2023).
Solanum nigrum L. (SNL), a dicotyledonous weed belonging to the Solanaceae family, is a spontaneous plant commonly found in wastelands, along fences and roadsides, and as an agricultural weed throughout China. SNL exhibits a range of pharmacological effects, including antimicrobial, anticancer, antioxidant, anti-inflammatory, antimetastatic, antiproliferative, and antitumor activities (Sivaraj et al., 2020; Bhuvaneshwari et al., 2022), attributable to its rich and diverse phytochemical composition. Its primary bioactive constituents include alkaloids, flavonoids, steroids, glycoproteins, tannins, polysaccharides, and polyphenols, with notable compounds such as caffeic acid, catechin, esculin, protocatechuic acid, rutin, and epicatechin (Zeeshan et al., 2023). To date, numerous studies have explored the effects of SNL in murine models. For instance, one study indicated that extracts from SNL berries can modulate the gut microbiota by reducing the Firmicutes-to-Bacteroidetes ratio and restoring beneficial microbial populations, while concurrently mitigating oxidative stress in mice (Wang et al., 2024b). Another study demonstrated that SNL exerts anti-inflammatory effects by inhibiting NF-κB nuclear translocation and downregulating the expression of iNOS, COX-2, IL-1β, and IL-6 in a concentration-dependent manner (Deng et al., 2023). Given these pharmacological characteristics and the presence of medicinally active secondary metabolites, SNL holds promising potential as an antibiotic alternative in poultry production.
However, to the best of our knowledge, no studies have specifically examined the effects of SNL supplementation in broiler chickens. To bridge this gap, the current study employed integrated microbiome and transcriptome analyses to evaluate how SNL influences intestinal health and production performance in broilers. By uncovering the mechanistic basis of its probiotic activity, this research seeks to establish a scientific foundation for the resource-efficient use of SNL and its application as an eco-friendly feed additive that supports animal health in antibiotic-free poultry production systems.
2 Materials and methods
2.1 Ethics statement
The animal study protocol was approved by the Ethics Committee of Guangdong Meizhou Vocational and Technical College (protocol code: GDMZVTC-2023–003 and date of approval: 2023.02.17).
2.2 Experimental design and diets
A total of 200 one-day-old healthy Wuhua yellow-feathered chickens with similar initial body weight (BW; 31.5±0.09g) were obtained from a commercial hatchery and randomly divided into 4 groups, with 5 replicate floor pens per group and 10 birds per pen. The four groups were: group A (CON group, fed the basal diet as control), group B (AMO group, fed the basal diet+500mg/kg amoxicillin), group C (SNL group, fed the basal diet+1000mg/kg Solanum nigrum L. grass meal), group D (SNL group, fed the basal diet+2000mg/kg Solanum nigrum L. grass meal), respectively. The basal diets were formulated referring to Chinese Nutrient Requirements of Yellow broilers (Ministry of Agriculture and Rural Affairs of the People’s Republic of China, 2020).
The experiment lasted for 35 days, consisted of a 7 days pre-trial period and a 28 days experimental period. Birds were housed and fed in disease-free pens (200 cm × 200 cm × 60 cm) and had free access to feed and water. During the first week, the indoor temperature was maintained at approximately 33°C under continuous artificial lighting. From day 8 to day 35, the temperature was gradually reduced by 2°C per week until it stabilized within the range of 26–27°C. Body weight was measured on a weekly basis, and feed intake was recorded daily.
2.3 Growth performance and sample collection
On the 36th day, after an eight-hour fasting period, measurements were made of the body weight (BW) and the amount of feed remaining in each replicate. Afterwards, the growth performance parameters for each group were computed, which included the mean average daily feed intake (ADFI), mean average daily gain (ADG), and the feed conversion ratio (F/G). F/G = feed intake (F)/weight gain (G).
Then, five birds per group (1 bird per pen, 20 birds in total) were randomly selected for sample collection, including blood samples, jejunum tissue, ileum tissue and cecal digesta. The samples were cryopreserved at -80°C after undergoing the necessary processing for subsequent analysis.
2.4 Determination of serum oxidative stress related indices
The serum total antioxidant capacity (T-AOC), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) activities and the level of malondialdehyde (MDA) were performed by appropriate assay kits (Nanjing Jiancheng Biotechnology Institute, Nanjing, China) using the builder’s standard method (Bai et al., 2021).
2.5 Intestinal morphology
The jejunum tissue samples (1-2cm) were fixed with 4% paraformaldehyde and prepared on tissue slides (Tang et al., 2022). The tissues were embedded in paraffin blocks, cut into 4-µm slices, and stained with hematoxylin and eosin (H&E). Then, the slices were dried and sealed for follow-up observation and analysis (the villus height (VH) and crypt depth (CD)) through optical microscopy.
2.6 16s rRNA gene sequencing analysis
2.6.1 DNA extraction and amplification
Total genomic DNA of cecal contents was extracted using MagPure Soil DNA KF Kit (Magan) following the manufacture’ s instructions. DNA concentration and integrity were measured with NanoDrop 2000 (Thermo Fisher Scientific, USA) and agarose gel electrophoresis. Extracted DNA was stored at -20°C until further processing. The extracted DNA was used as template for PCR amplification of bacterial 16S rRNA genes with the barcoded primers and Takara Ex Taq (Takara). For bacterial diversity analysis, V3-V4 variable regions of 16S rRNA genes was amplified with universal primers 343F (5’ - TACGGRAGGCAGCAG-3’) and 798R (5’-AGGGTATCTAATCCT-3’) (Nossa et al., 2010).
2.6.2 Library construction and sequencing
The Amplicon quality was visualized using agarose gel electrophoresis. The PCR products were purified with AMPure XP beads (Agencourt) and amplified for another round of PCR. After purified with the AMPure XP beads again, the final amplicon was quantified using Qubit dsDNA Assay Kit (Thermo Fisher Scientific,USA). The concentrations were then adjusted for sequencing. Sequencing was performed on an Illumina NovaSeq 6000 with 250 bp paired-end reads. (Illumina Inc., San Diego, CA; OE Biotech Company; Shanghai, China).
2.6.3 Bioinformatic analysis
Raw sequencing data, in FASTQ format, were preprocessed using Cutadapt software to detect and cut off the adapter. After trimming, quality control of the adapter-removed paired-end reads, including low quality sequences removal, denoised, merged and chimera reads removal, was conducted by using DADA2 plugin (Callahan et al., 2016) with the default parameters of QIIME2 (Bolyen et al., 2019) (2020.11). At last, the software output the representative reads and the amplicon sequence variant (ASV) abundance table. The representative read of each ASV was selected using QIIME2 package. All representative reads were annotated and blasted against Silva database (Version 138) using q2-feature-classifier with the default parameters.
QIIME2 software was used for alpha and beta diversity analysis. Then the R package was used to analyze the significant differences between different groups using ANOVA/Kruskal Wallis statistical test. The linear discriminant analysis effect size (LEfSe) method was used to compare the taxonomy abundance spectrum.
2.7 Transcriptome sequencing analysis
2.7.1 RNA isolation and library preparation
Total RNA was extracted from ileum tissue using the TRIzol reagent (Invitrogen, CA, USA) according to the manufacture’s protocol. RNA purity and quantification were evaluated using the NanoDrop 2000 spectrophotometer (Thermo Scientific, USA). RNA integrity was assessed using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Then the libraries were constructed using VAHTS Universal V6 RNA-seq Library Prep Kit according to manufacture’s instructions.
2.7.2 RNA sequencing and differentially expressed genes analysis
RNA Sequencing was facilitated in collaboration with OE Biotech Co., Ltd. (Shanghai, China). The libraries were sequenced on an Ilumina Novaseq 6000 platform and 150 bp paired-end reads were generated (the sequencing depth/volume is 6 G). Raw reads of fastq format were firstly processed using fastp (Chen et al., 2018) and the low quality reads were removed to obtain the clean reads. Then the clean reads were mapped to the chicken reference genome using HISAT2 (Kim et al., 2015). FPKM (Roberts et al., 2011) of each gene was calculated and the read counts of each gene were obtained by HTSeq-count4. PCA analysis were performed using R (v 3.2.0) to evaluate the biological duplication of samples.
Differential expression analysis was performed using the DESeq2 (Love et al., 2014). Q value < 0.05 and foldchange > 2 was set as the threshold for significantly DEGs. Based on the hypergeometric distribution, Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes(KEGG) pathway enrichment analysis of DEGs were performed to screen the significant enriched term using R (v 3.2.0), respectively. R (v 3.2.0) was used to draw the column diagram and bubble diagram of the significant enrichment term.
2.8 Comprehensive analysis of the cecal microbiome and ileal transcriptome
To explore the potential relationship between the cecal microbiome and its hosts, the Spearman correlation coefficient was employed to assess the correlation between differentially abundant microbiota and DEGs. P-value < 0.05 is considered indicative of significant relationships. The correlation heatmap of cecal microbiota and DEGs was constructed using the R package. To decrease the risk of false positives, correction for multiple testing is also applied.
2.9 Statistical analysis
Quantitative data were analyzed using IBM SPSS 22.0 software (SPSS Inc., USA) and shown as the mean±SEM. Shapiro - Wilk test was used to test the normality of data. For the analysis of growth performance data, in addition to considering the grouping factors, initial body weight was incorporated into the analysis model as a covariate. For intestinal morphology data and the serum induces, only the grouping information was taken into account. Among different treatments, comparisons of means were performed using ANOVA followed by the least significant difference (LSD) post hoc test. Significance was established at p ≤ 0.05, and 0.05 < P < 0.10 was considered a trend.
3 Results
3.1 Growth performance
Group C was excluded from further analysis due to its growth performance showing no substantial deviation from that of the control group (Group A, CON). Consequently, only Group A (CON), Group B (AMO), and Group D (SNL) were included in the subsequent statistical analyses.
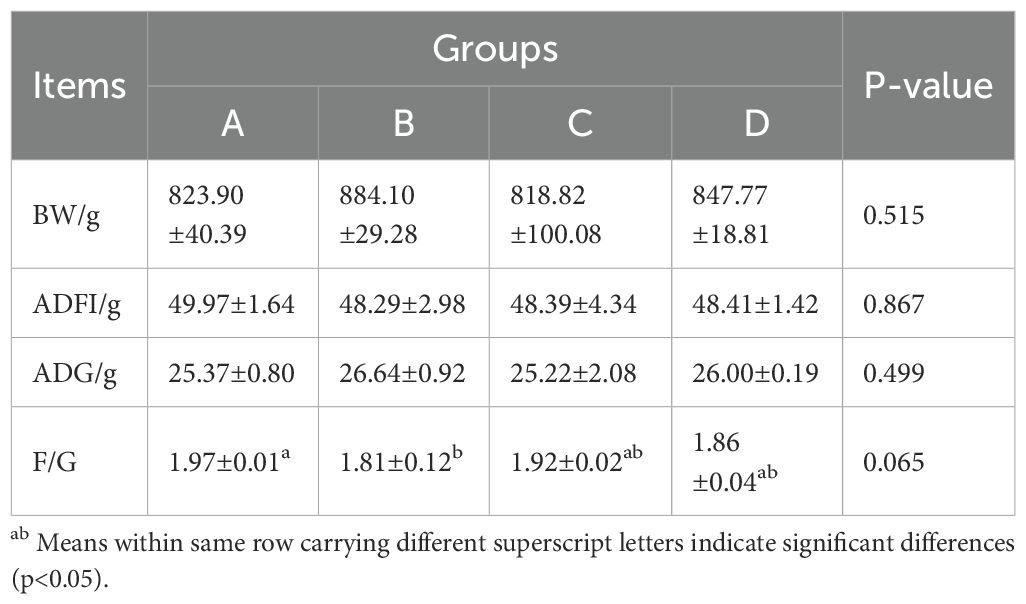
Table 1 presents the effects of dietary AMO and SNL supplementation on the growth performance of Wuhua yellow-feathered broilers. No statistically significant differences in growth performance were observed among the three groups during the overall experimental period (P > 0.05). Although the differences did not reach statistical significance, certain numerical trends were noted in BW, ADFI, ADG and F/G across the groups. Both the AMO and SNL groups exhibited numerically higher BW and ADG, as well as lower ADFI and F/G, compared to the CON group. Notably, the SNL group demonstrated a lower F/G (1.86±0.04) than the CON group (1.97±0.01), while its F/G was comparable to that of the AMO group (1.81±0.12).
3.2 MDA and antioxidant enzyme activity changes
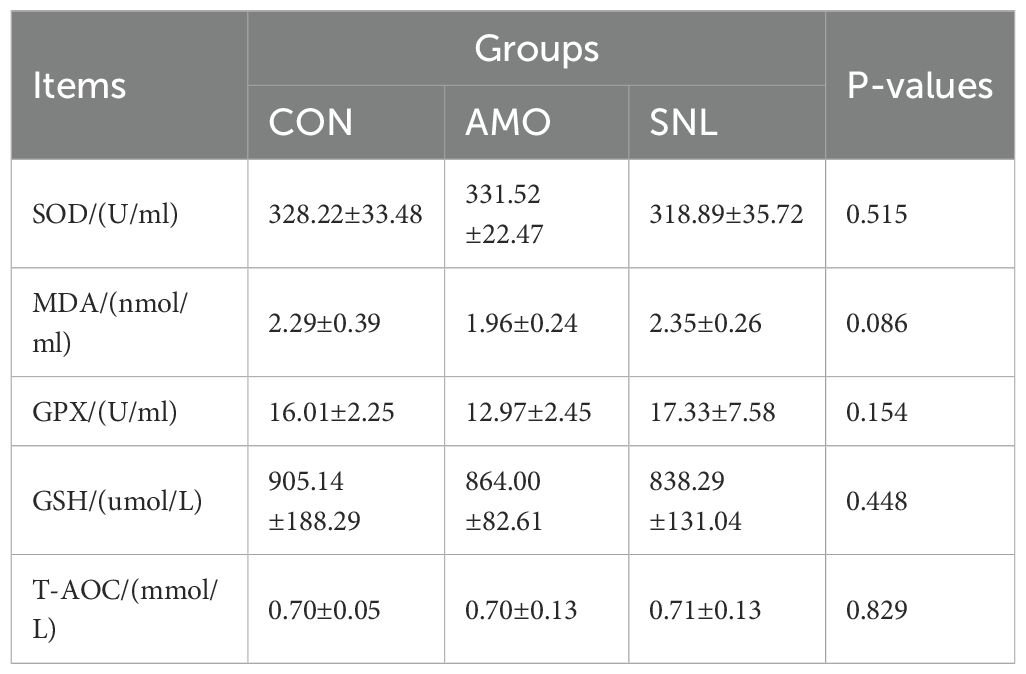
The effects of AMO and SNL supplementation on serum antioxidant parameters in Wuhua yellow-feathered broilers are shown in Table 2. No statistically significant differences (p > 0.05) were observed in the levels of SOD, GPX, T-AOC, GSH, or MDA among the treatment groups. However, compared with other dietary treatments, the SNL-supplemented group exhibited a notable increasing trend in both GPX activity and T-AOC.
3.3 Jejunum morphological change
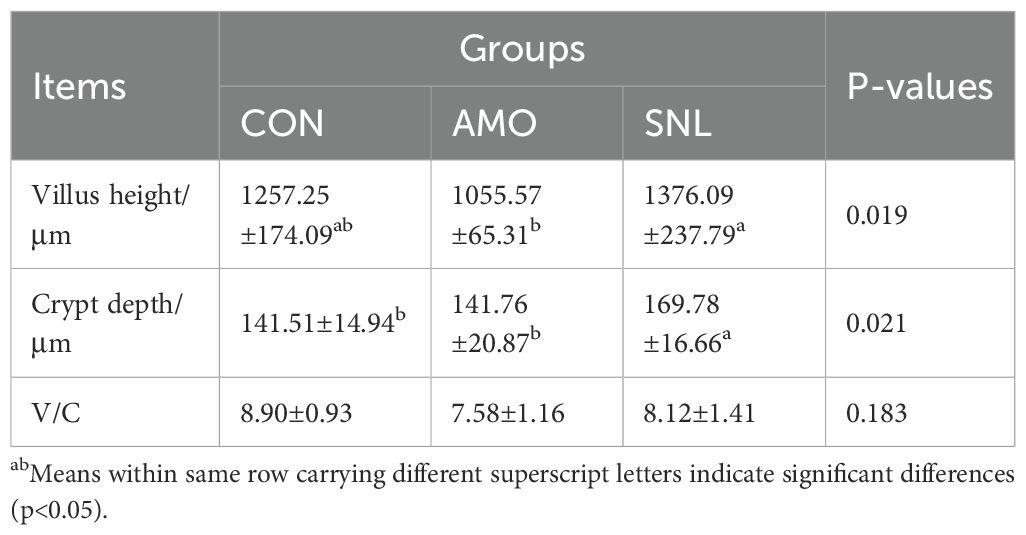
The jejunal tissues were subjected to H&E staining to evaluate the effects of different experimental treatments on intestinal morphology (Figure 1 and Table 3). Significantly longer VH (about 9.5%, p = 0.019) was observed in the SNL-supplemented group compared to the AMO-treated group, with a notable increasing trend relative to the control group. Consequently, the V/C ratio was significantly improved in the SNL treatment group compared to the AMO-supplemented group, while no significant differences were detected between the CON and SNL treatment groups.
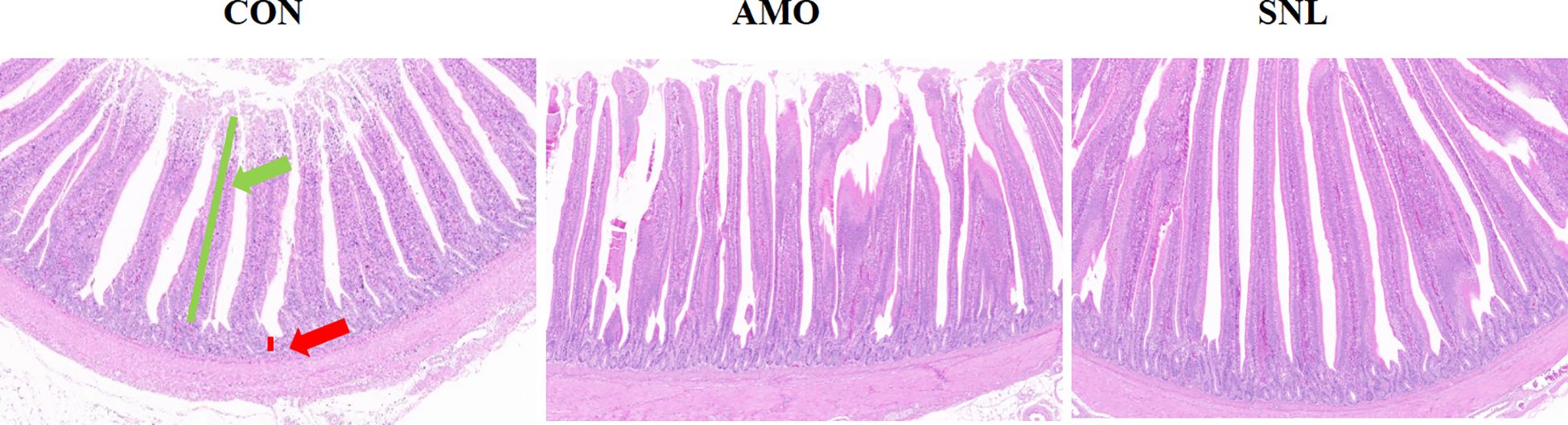
Figure 1. Photomicrograph of the jejunum: hematoxylin and eosin stained. Pictures were observed at 100× magnification. The green arrow indicates intestinal villi and the red arrow indicates crypts.
3.4 Caecal microbiota analysis
3.4.1 Analysis of the caecal microbiota diversity
The Illumina MiSeq high-throughput sequencing platform was employed to characterize the cecal microbial communities across different experimental groups. The raw data ranges from 78,484 to 81,729 reads across the samples, as detailed in Supplementary Table S1. Following stringent quality filtering and the removal of chimeric sequences, the number of processed reads per sample averaged at 52,990 (Supplementary Table S1). A total of 1,084 ASVs were yielded with confirmed domain-level taxonomic assignments, ranging from 281 to 338, from 98 to 381, from 214 to 284 in CON, AMO, SNL group, respectively. Based on a 99% species similarity threshold, the α-diversity indices at the ASV level for each group are presented in Figure 2A. No statistically significant differences were observed in either the Chao1 (richness) or Simpson (evenness) indices among the three treatments, suggesting comparable community diversities. However, the AMO group exhibited a significantly higher Shannon index compared to the SNL-supplemented group, indicating greater microbial richness in the AMO-treated caecal microbiota.
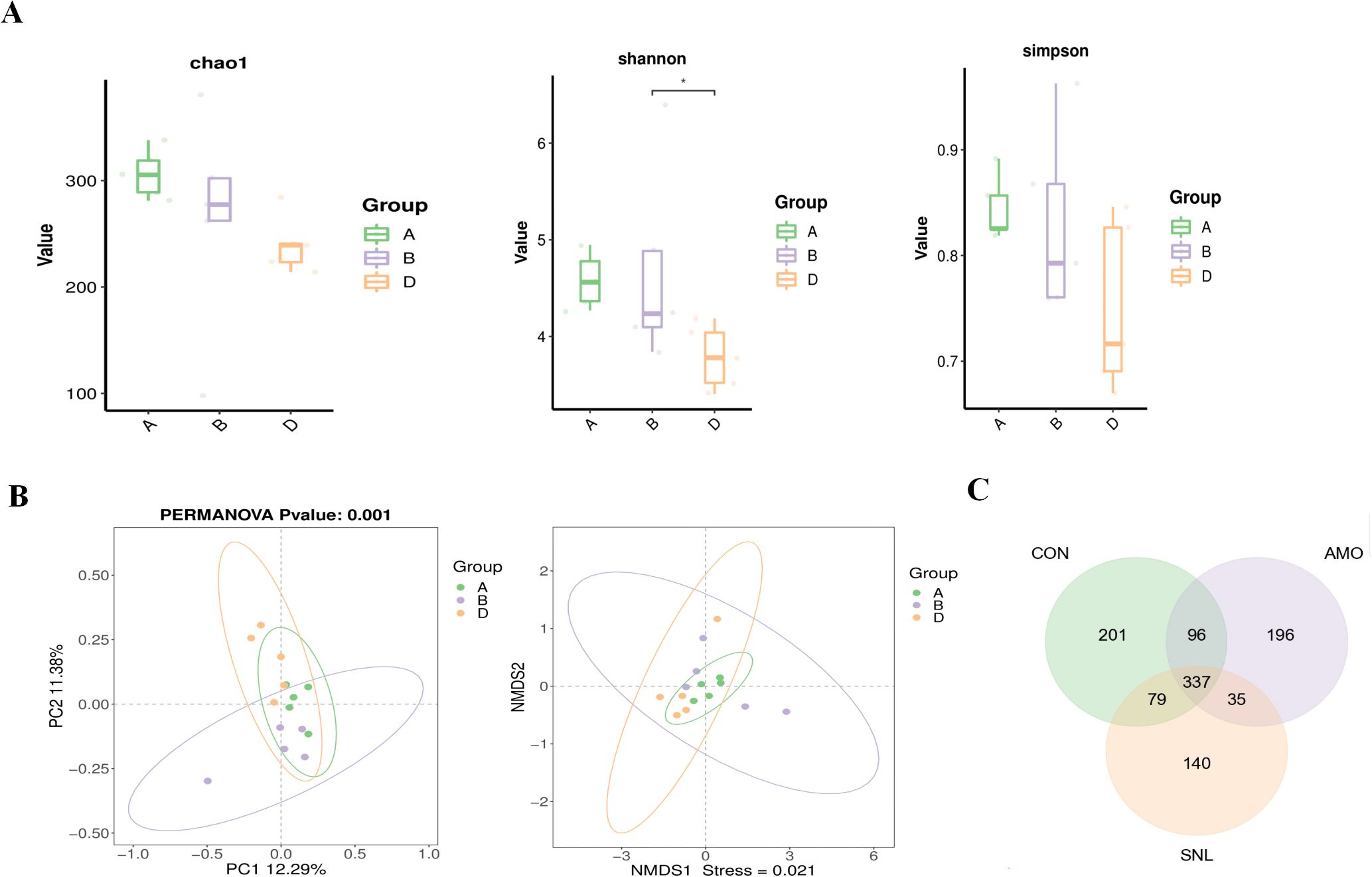
Figure 2. Caecal microbiota diversity analysis. CON (Group A), AMO (Group B), SNL(Group D). (A) The α-diversity of the microbial communities (Chao1, Shannon and Simpson) a, bar with the asterisk (*) level suggests the degree of significant difference, (* p < 0.05); (B) b-diversity metrics: Principal component analysis (PCA) and non-metric multidimensional scaling (NMDS); (C) Venn diagram.
For β-diversity analysis, multivariate statistical approaches—including PCA and non-metric multidimensional scaling (NMDS)—were applied to assess compositional similarities among the treatment groups. The PCA and NMDS plots revealed distinct clustering patterns (at the ASV level) of caecal microbial communities across treatments. While the CON and AMO groups displayed partial overlap, the SNL group formed a separate cluster, distinctly differentiated from the other groups (Figure 2B).
A Venn diagram illustrated the distribution of unique and shared intestinal ASVs among the cecal microbiota of different groups. Among the identified ASVs, 337 were common to all groups (core microbiota), with 201 ASVs unique to the control group, 196 ASVs exclusive to the AMO group, and 140 ASVs specific to the SNL group (Figure 2C).
3.4.2 Composition and structure analysis of the cecal microbiota
Figures 3A, B illustrate the relative abundance of gut microbiota in each group at the phylum and genus levels, respectively. At the phylum level, the cecal microbiota of all groups were predominantly composed of 10 major bacterial phyla, with Bacteroidetes and Firmicutes being the two most abundant (Supplementary Table S2). Compared with the CON and AMO groups, the SNL group exhibited a significant increase in the relative abundance of Bacteroidetes, whereas the relative abundances of Proteobacteria and Firmicutes decreased. Notably, the SNL group displayed the highest relative abundance of Bacteroidetes and the lowest abundances of Firmicutes and Deferribacterota. At the genus level, Bacteroides, Clostridia_UCG-014, Faecalibacterium, [Eubacterium]_coprostanoligenes_group, and [Ruminococcus]_torques_group were the predominant taxa in the ceca of broilers across all groups (Supplementary Table S3). The SNL treatment significantly promoted the growth of Bacteroides, Faecalibacterium, and [Ruminococcus]_torques_group, while markedly suppressing the abundance of Oscillibacter relative to other groups.
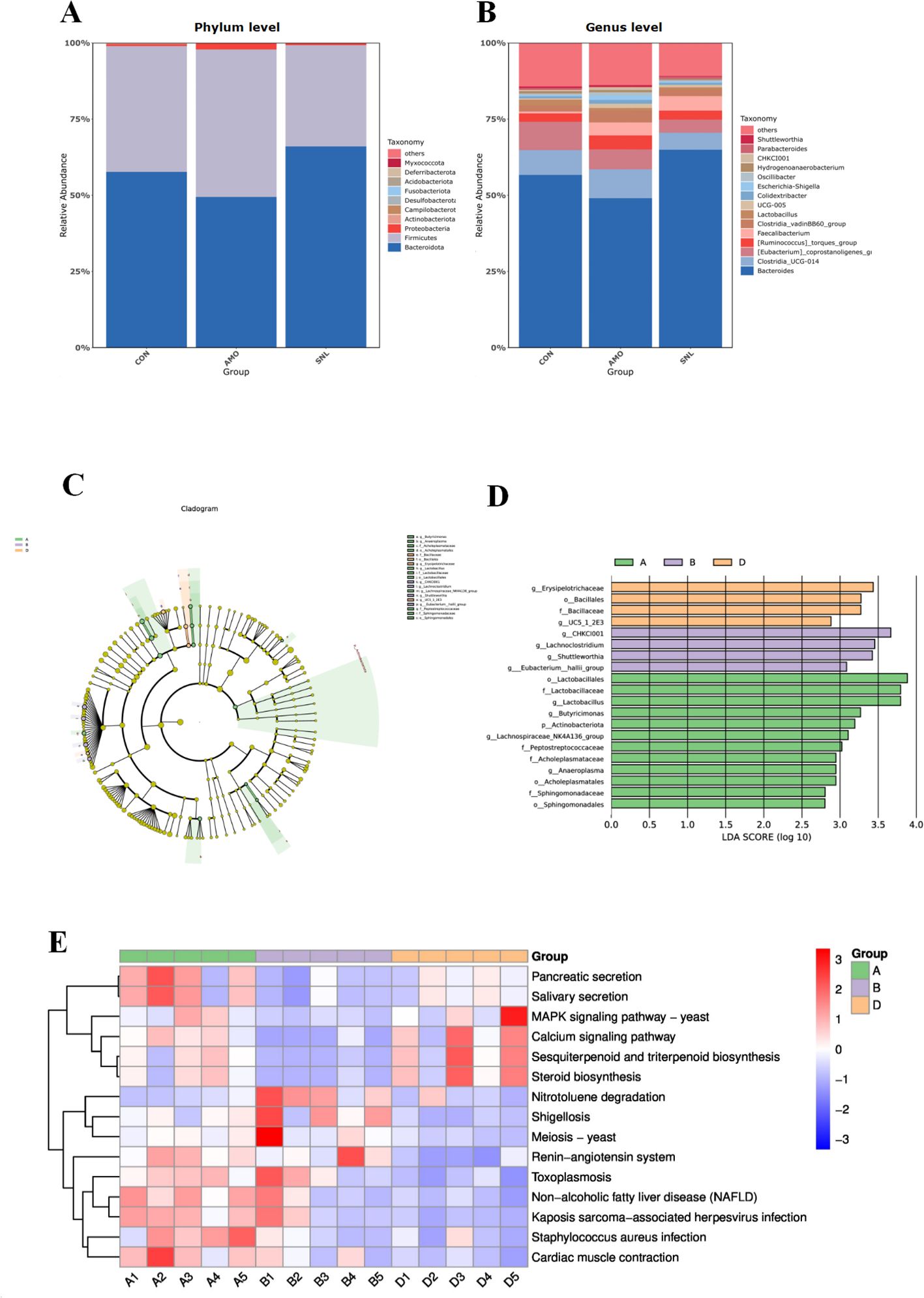
Figure 3. Microbiota analysis in cecum chyme. CON (Group A), AMO (Group B), SNL (Group D). (A) Community column diagram at the phylum level. (B) Community column diagram at the genus level. (C, D) Multilevel species differences from the phylum to genus level in different groups determined using the linear discriminant analysis (LDA) effect size (LEfSe) algorithm. (E) Difference in the metabolic functions of the cecal microbiota.
3.4.3 Analysis of cecal microbiota composition and metabolic function changes
The LEfSe revealed the dominant microbial taxa among different experimental groups. As illustrated in Figures 3C, D, the SNL, AMO, and CON groups exhibited 3, 4, and 7 significantly different taxonomic units at various classification levels (LDA score >3), respectively. Specifically, the predominant bacterial phylotypes in the SNL group comprised Erysipelotrichaceae, Bacillales, and Bacillaceae. In the AMO group, the dominant taxa included CHKCI001, Lachnoclostridium, Shuttleworthia, and Eubacterium:hallii_group. Meanwhile, the CON group was characterized by the predominance of Lactobacillales, Lactobacillaceae, Lactobacillus, Butyricimonas, Actinobacteriota, Lachnospiraceae_NK4A136_group, and Peptostreptococcaceae.
Significant intergroup differences in KEGG functional pathways, as predicted by PICRUSt-based metagenomic analysis, are presented in Figure 3E. Comparative analysis demonstrated that the SNL dietary intervention elicited elevated heatmap scores for functional gene clusters associated with the MAPK signaling pathway – yeast, steroid biosynthesis, and sesquiterpenoid/triterpenoid biosynthesis, relative to both the AMO and CON groups. Furthermore, microbial gene functions linked to metabolic pathways—including pancreatic secretion and salivary secretion—were significantly upregulated in the SNL group compared to the AMO group, whereas pathways related to Staphylococcus aureus infection, shigellosis, and nitrotoluene degradation exhibited downregulation.
3.5 Transcriptome analysis of intestinal tissue
To elucidate the probiotic mechanisms of SNL, we conducted ileal transcriptomic profiling using RNA sequencing (RNA-seq). As depicted in Figure 4A, differential expression analysis (FDR < 0.05 and |log2FC| > 1) identified 174 DEGs (100 upregulated and 74 downregulated) in the CON vs. AMO comparison, 135 DEGs (76 upregulated and 59 downregulated) in the CON vs. SNL comparison, and 219 DEGs (88 upregulated and 131 downregulated) in the AMO vs. SNL comparison.
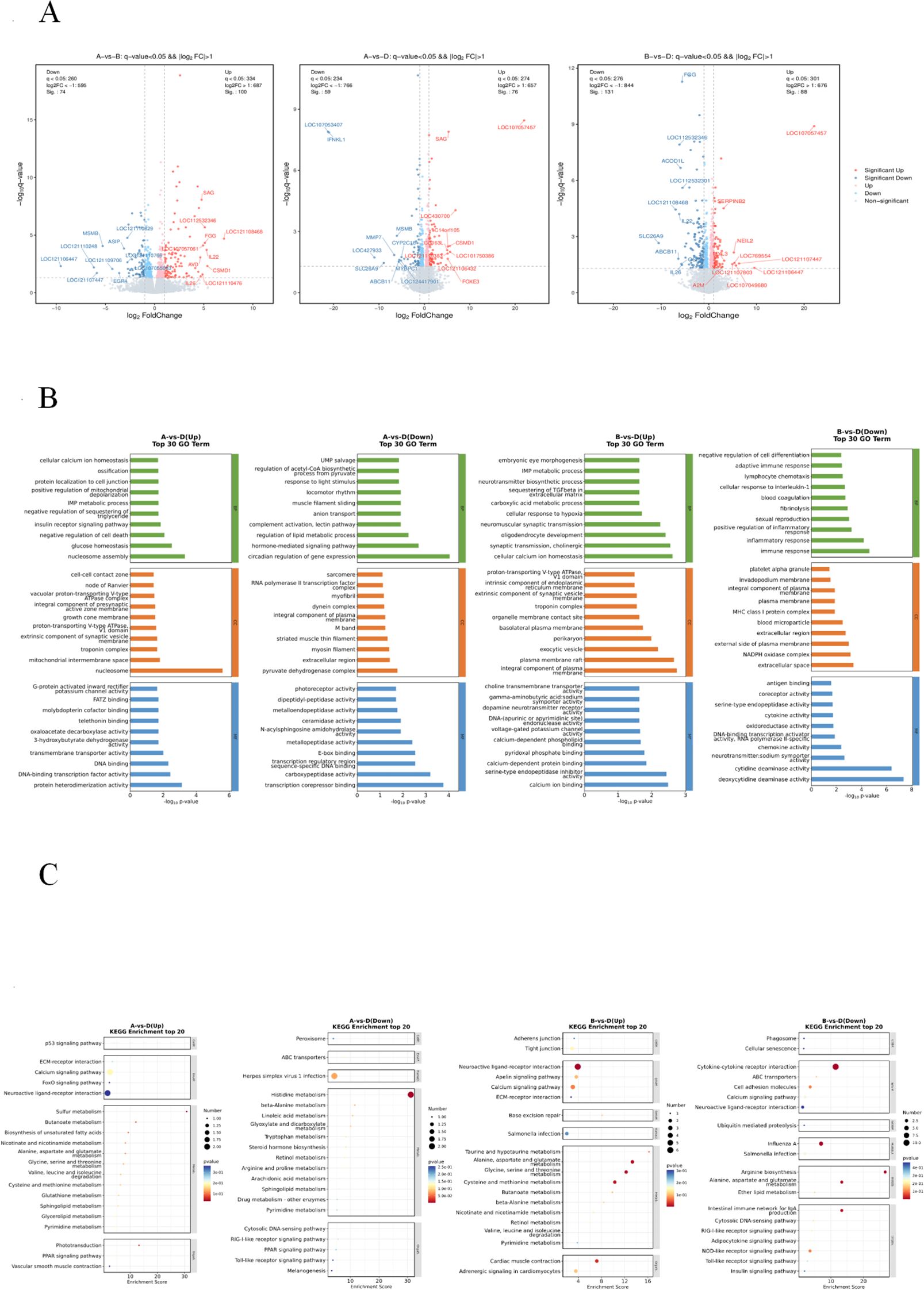
Figure 4. Transcriptome sequencing analysis of chicken ileum tissues in three treatments, CON (Group A), AMO (Group B), SNL (Group D). (A) Volcano plot of DEGs between CON and AMO groups, CON and SNL groups, AMO and SNL groups, respectively. Red, blue, light red and light blue dots represent significant up-regulated, significant down-regulated, up-regulated and down-regulated transcripts, respectively, while gray dots indicate genes with insignificant differences. (B) Venn diagram for the DEGs. (B) GO term analysis of DEGs between CON and SNL groups, AMO and SNL groups, respective-ly (Top 30). (C) KEGG pathway analysis of DEGs between CON and SNL groups, AMO and SNL groups, respectively (Top 20). The size of the circle represents the enriched num-ber of DEGs and color represents significance.
To further investigate the functional implications of these DEGs, we performed GO and KEGG pathway enrichment analyses. The GO analysis of upregulated DEGs in CON vs. SNL chickens suggested potential involvement in glucose homeostasis, insulin receptor signaling, oxaloacetate decarboxylase activity, and various enzymatic processes. KEGG enrichment highlighted pathways including the peroxisome proliferator-activated receptor (PPAR) signaling pathway, FoxO signaling pathway, butanoate metabolism, sphingolipid metabolism, and other amino acid metabolic processes. Conversely, downregulated DEGs in CON vs SNL chickens were predominantly enriched in hormone-mediated signaling, lipid metabolic regulation, complement activation (lectin pathway), and carboxypeptidase activity, with KEGG analysis emphasizing the PPAR signaling pathway, Toll-like receptor signaling, RIG-I-like receptor signaling, peroxisome-related functions, and additional amino acid metabolism.
In the AMO vs SNL comparison, GO analysis indicated that upregulated DEGs may participate in TGF-β sequestration within the extracellular matrix and cellular calcium ion homeostasis. KEGG enrichment identified pathways related to Salmonella infection, neuroactive ligand-receptor interactions, adrenergic signaling in cardiomyocytes, and cardiac muscle contraction. Meanwhile, downregulated DEGs were primarily associated with immune and inflammatory responses (including positive regulation of inflammation, cellular responses to IL-1, and adaptive immunity), oxidoreductase activity, and various enzymatic processes. KEGG analysis further revealed enrichment in Salmonella infection, neuroactive ligand-receptor interactions, the intestinal immune network for IgA production, RIG-I-like receptor signaling, adipocytokine signaling, NOD-like receptor signaling, Toll-like receptor signaling, insulin signaling, and additional amino acid metabolism (Figures 4B, C).
3.6 Correlation analysis of host transcriptome and cecal microbiome
Subsequently, we investigated the potential correlations between the microbiome and transcriptome by calculating Spearman correlation coefficients in CON, AMO, and SNL chickens, respectively. Based on the results of 16S rRNA amplicon sequencing, the top 30 most abundant bacterial genera were selected for analysis. The relationships between differentially abundant bacterial genera and key DEGs were visualized as heatmaps (Figures 5A-C, Supplementary Table S4-S6). Based on P-value, our analysis revealed that Oscillibacter exhibited a significant positive correlation with MAP3K7CL and PON1. In contrast, MAP3K7CL displayed a significant negative correlation with Bacteroides, which in turn showed a significant negative correlation with PRLHRL.
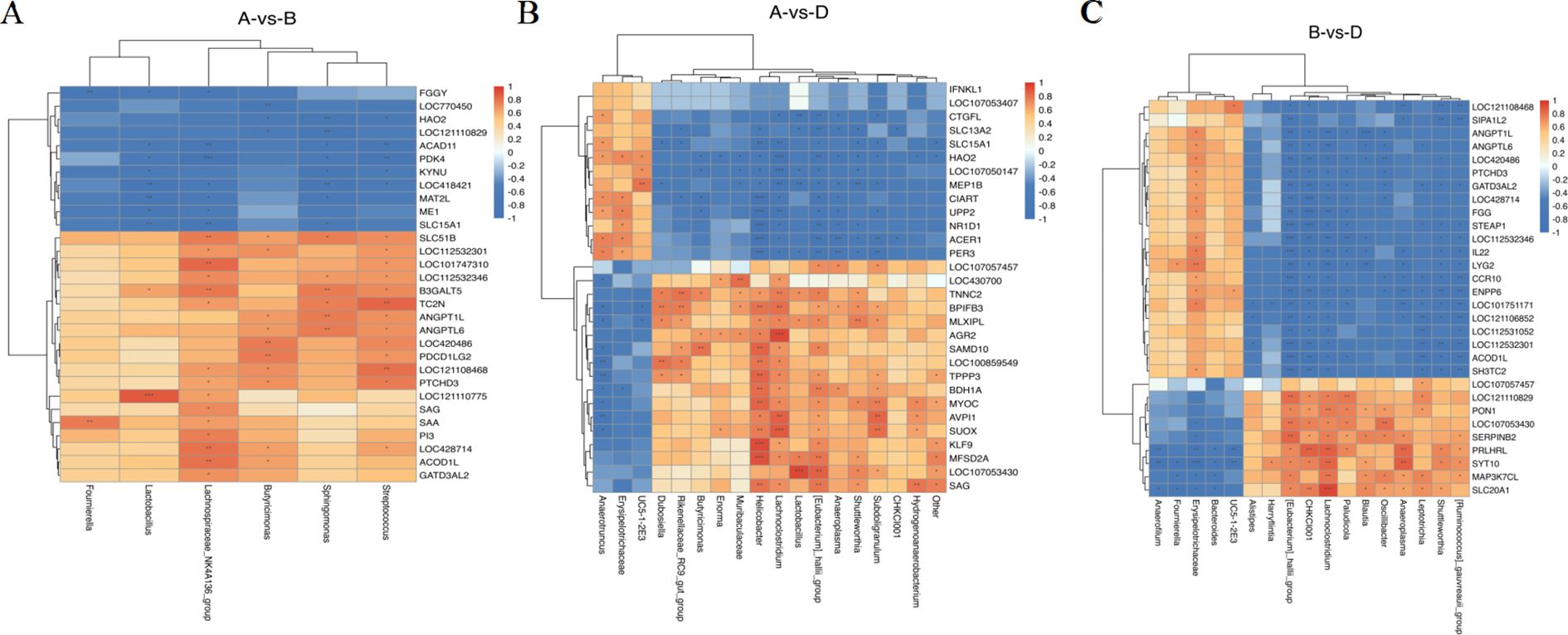
Figure 5. Integrated analysis of the microbiome and transcriptome in the CON vs AMO (A), CON vs SNL (B) and AMO vs SNL (C). CON (Group A), AMO (Group B), SNL(Group D). Heatmap of Spearman’s correlation coefficients between differential microbiota and dif-ferential expressed mRNAs. Each row represents a differentially expressed mRNAs and each column represents a differentially microbiota. Orange-red and blue represent the positive and negative correlations, respectively. The darker the color, the higher the corre-lation was. Correlation significance, *:P < 0.05, **:P < 0.01, ***:P < 0.001.
4 Discussion
Historically, the use of antibiotics in the management of microbial infections and intestinal regulation has been a standard practice. However, due to the adverse environmental and food chain impacts of antibiotics, there is an urgent need for antibiotic alternatives in poultry production. Accumulating evidence suggests that phytogenic feed additives can modulate gut microbiota composition and activity, thereby conferring beneficial physiological effects on the host (Abdelli et al., 2021). While numerous studies have investigated the probiotic properties of Chinese herbal medicines in broilers, knowledge regarding the effects of SNL in avian species remains limited.
The present study aimed to evaluate the effects of SNL supplementation on broiler growth performance, intestinal barrier function, antioxidant status, cecal microbiota, and ileal gene expression. Compared to the CON group, SNL supplementation at 0.2% improved, although not significantly, body weight gain (BW, ADG) while reducing the F/G and ADFI, indicating enhanced nutrient utilization. No significant differences in growth performance or feed efficiency were observed between the AMO and SNL groups, suggesting that SNL is comparably effective to AMO in supporting broiler development. We hypothesize that SNL contains bioactive compounds, including polysaccharides and saponins, which may enhance digestive and absorptive efficiency, improve nutrient utilization, reduce feed wastage, and optimize dietary efficiency (Yao et al., 2020; Anzoom et al., 2023).
Although the growth performance is not significantly improved, the numeral trends demonstrate potential economic benefits from a production standpoint. In poultry industry, the cost of feed accounts for about 70% of the total costs, which means even a small improvement in feeding efficiency can significantly reduce production costs. Given that SNL is typically a byproduct, its utilization as a feed additive presents a cost-effective strategy for poultry farming without compromising growth performance, while also yielding high-quality broilers.
The antioxidant capacity of the organism plays a crucial role in suppressing free radical generation, preventing free radical chain reactions, and mitigating oxidative damage to the host. Enhanced antioxidant capacity is positively correlated with optimal health status and improved poultry production performance (Yang et al., 2016). Key antioxidant enzymes, including SOD for scavenging superoxide radicals (O2−), GPX and CAT for decomposing hydrogen peroxide (H2O2), are recognized as essential defense mechanisms against free radical-induced damage (Shang et al., 2023). The overall antioxidant status is comprehensively assessed through T-AOC, while MDA, a terminal product of lipid peroxidation induced by free radicals, serves as a reliable biomarker for oxidative stress intensity (Liu et al., 2021).
Our experimental results demonstrated no statistically significant differences in the levels of SOD, GPX, T-AOC, GSH, or MDA among the treatment groups. This is likely associated with the added concentration. Here, we added 0.2% SNL grass meal to the basal diet, the concentration of the bioactive constituents is relatively lower compared to other studies. A recent research indicated that dietary supplementation with SNL berries extract (200 mg/kg BW/d) can potentiate the body’s antioxidant defense system via modulation of NRF2-related protein expression (Wang et al., 2024b). Such effects are likely attributable to the bioactive constituents in SNL—namely polysaccharides, saponins, and flavonoids—which have been demonstrated to exert potent antioxidant properties (Guediri, 2021; Anzoom et al., 2023).
The intestine serves as the primary site for animals to absorb exogenous nutrients, with its fundamental structural components comprising crypts and villi. Intestinal villi extend the mucosal surface area, thereby enhancing nutrient absorption (Kai, 2021). Consequently, VH, CD, and their V/C have long been recognized as critical indicators for assessing intestinal physiological function and tissue integrity (Zhao et al., 2023). Generally, a longer villus height, higher V/C ratio, and shallower crypt depth reflect a superior structural and functional state of the intestinal epithelium, correlating with enhanced digestive and absorptive capacity as well as improved disease resistance (Banday et al., 2024). In the present study, dietary supplementation with 0.2% SNL elicited a marked trend toward increased jejunal VH in chickens compared to the CON group, although no statistically significant difference was observed in the V/C ratio. Notably, when compared to the AMO group, the 0.2% SNL treatment significantly elevated jejunal VH and improved the V/C ratio. These results indicate that incorporating an optimal dosage of SNL into the diet can enhance intestinal morphology and preserve structural integrity in chickens, thereby promoting intestinal health in yellow-feathered broilers. The mechanism underlying this difference is needed further investigation, and one possible reason for this may be the consequence of the alternation of the cecal microbiota. The observed improvements in VH and the consequent expansion of the small intestinal absorptive surface area likely underlie the enhanced growth performance observed following SNL supplementation.
The intestinal microbiota plays a pivotal role in enhancing nutrient assimilation, conferring disease resistance, and maintaining intestinal homeostasis (Śliżewska et al., 2020; Martin-Gallausiaux et al., 2021). In the present investigation, cecal microbiota analysis in broilers demonstrated that multiple α-diversity metrics, as well as the observed number of ASVs, were significantly reduced in the SNL group relative to both the CON and AMO groups. These findings indicate that dietary supplementation with SNL reduces both microbial species richness and diversity, implying a disruption in microbial evenness and potential dysbiosis (Zhang et al., 2025). The decrease in microbial species richness and diversity may be attributed to the antimicrobial characteristics mentioned before. This outcome contrasts with previous studies on other Chinese herbal preparations containing similar bioactive compounds (Che et al., 2024; Huang et al., 2024). Although the addition of SNL reduced intestinal microbial diversity, when combined with other results of this study, no significant impairment of intestinal health was observed. This observation was further supported by the metabolic function analysis of the cecal microbiota, which revealed that, compared to both the CON and AMO groups, the SNL group exhibited enrichment in metabolic pathways associated with reduced Staphylococcus aureus infection and shigellosis (Figure 3E). Besides, we observed SNL supplementation may potentiate the chicken’s innate resistance to Salmonella infections from the transcriptome data in the present study. However, further investigation is still needed to clarify the reasons behind the decrease in α-diversity observed in the SNL group and to evaluate the impact on gut health.
Furthermore, PCA and NMDS revealed distinct compositional variations among the microbial communities of the three experimental groups. At the phylum level, consistent with extensive research on the gut microbiota of healthy broilers, Bacteroidetes and Firmicutes emerged as the predominant microbial phyla in our study (Bai et al., 2023). Notably, we observed a significant elevation in the relative abundance of both the phylum Bacteroidetes and its constituent genus Bacteroides in the SNL group, concomitant with significant reductions in the phyla Firmicutes, Proteobacteria, and Deferribacterota. Additionally, a marked decrease in the Firmicutes-to-Bacteroidetes (F/B) ratio was evident.
Bacteroidetes plays an indispensable role in complex carbohydrate degradation and propionate production, while the genus Bacteroides not only furnishes essential nutrients to other microbial populations but also contributes to host protection against intestinal pathogens through polysaccharide degradation and butyrate salt formation (Chen et al., 2019; Zafar and Saier, 2021). As the dominant gut microbiota in healthy individuals, an elevated F/B ratio is widely recognized as an indicator of intestinal microbiome dysbiosis (Xia et al., 2022). Proteobacteria has been identified as a potential diagnostic biomarker for malnutrition and disease susceptibility (Chen et al., 2021), while Deferribacterota exhibits an association with obesity (Walker et al., 2014). At the genus level, the cecal microbiota of broilers across all three experimental groups was predominantly composed of Bacteroides, Clostridia_UCG-014, Faecalibacterium, [Eubacterium]_coprostanoligenes_group, and [Ruminococcus]_torques_group. Among these, Bacteroides—a genus well-established for its role in complex carbohydrate degradation and short-chain fatty acid (SCFA) production (Yan et al., 2023a)—was the most abundant taxon in the SNL group. This genus has been positively correlated with weight gain and improved growth performance in poultry (Chang et al., 2016), suggesting that its elevated abundance in the SNL group may contribute to the observed higher ADG and lower F/G.
As a key butyrate-producing bacterium, Faecalibacterium plays a pivotal role in gut health evaluation (Li et al., 2019). Notably, Faecalibacterium prausnitzii, a prominent butyrate-producing species, exhibits a positive association with feed conversion efficiency (Kameyama and Itoh, 2014; Ye et al., 2019). The SNL-supplemented diet not only significantly enhanced the abundance of Faecalibacterium but also increased its proportional representation within the microbial community, which may explain the improved F/G ratio in the SNL group.
Furthermore, the cecal abundance of [Ruminococcus]_torques_group was higher in the SNL group compared to the COM group. Given that reduced abundance of Ruminococcaceae has been linked to inflammatory bowel disease (IBD) in humans (Joossens et al., 2011), this elevation may confer a protective effect. Conversely, the decreased abundance of Oscillibacter in the SNL group is likely beneficial, as this genus has been associated with impaired gut barrier function and intestinal inflammation (Li et al., 2021). Collectively, the dietary inclusion of SNL exerted favorable modulatory effects on the cecal microbiota of broilers, characterized by an enrichment of beneficial bacterial taxa and a reduction in potentially detrimental ones. These microbial shifts likely underpin the observed enhancements in growth performance and feed efficiency. The SNL-supplemented diet was demonstrated to suppress the proliferation of Gram-negative and potentially pathogenic bacterial populations while concurrently enhancing the growth of Gram-positive bacteria. These observations are congruent with the documented structural remodeling of the cecal microbial community induced by dietary SNL intervention in the present investigation. Furthermore, metabolic pathway analysis revealed that SNL supplementation significantly attenuated the pathogenic potential associated with Staphylococcus aureus infections, shigellosis, and nitrotoluene biodegradation processes. This protective effect is partially attributable to the inherent anti-inflammatory bioactivity of SNL (Xiang et al., 2018; Deng et al., 2023).
The substantial number of DEGs suggests that dietary supplementation with SNL exerted a measurable influence on the ileal transcriptome of chickens. Comparative analysis of the AMO and SNL groups revealed that the top 30 significantly enriched GO terms and top 20 KEGG pathways in both groups prominently featured the Salmonella infection and neuroactive ligand-receptor interaction pathways. Notably, the Salmonella infection pathway warrants particular attention, given that Salmonella constitutes a primary poultry pathogen, contributing to considerable economic losses and posing significant public health risks. The observed upregulation of genes within this pathway implies that SNL supplementation may potentiate the chicken’s innate resistance to Salmonella infections, thereby potentially diminishing the reliance on antibiotics in poultry production (Zhao et al., 2022). Similarly, the neuroactive ligand-receptor interaction pathway—which plays an indispensable role in cellular communication and signal transduction—was also significantly enriched. This enrichment suggests that SNL may modulate diverse physiological processes, including immune regulation and stress responses, ultimately contributing to enhanced poultry health (Han et al., 2022; Qiu et al., 2025). Transcriptomic analyses further indicated that SNL may confer health benefits by regulating multiple immune-associated pathways, notably the RIG-I-like receptor signaling pathway, NOD-like receptor signaling pathway, Toll-like receptor signaling pathway, and adipocytokine signaling pathway, with particular emphasis on the intestinal immune network for IgA production. As a pivotal immunoglobulin in mucosal immunity, IgA constitutes the first line of defense against gastrointestinal pathogens (Mantis et al., 2011). The enrichment of DEGs within this pathway implies that SNL supplementation may strengthen mucosal immune barriers, consequently reducing the incidence of gastrointestinal infections in chickens (Pabst, 2012; Jiao et al., 2023). Additionally, the RIG-I-like receptor signaling pathway, which mediates the detection of viral RNA and orchestrates antiviral responses, was also enriched. This finding suggests that the SNL-enriched diet may augment the chicken’s antiviral defenses, providing protection against viral infections (Zhao et al., 2022). Furthermore, the identification of DEGs linked to the insulin signaling pathway highlights the potential role of SNL in modulating metabolic processes, including glucose homeostasis and energy utilization (He et al., 2024). This is particularly pertinent in poultry production, where metabolic efficiency critically influences growth performance and feed conversion rates (Zhang et al., 2023; Guo et al., 2024). Comparative analysis of the CON and SNL groups demonstrated that DEGs were predominantly enriched in the PPAR signaling pathway, lipid metabolic process regulation, and other immune-related pathways, including the RIG-I-like receptor signaling pathway and Toll-like receptor signaling pathway. The PPAR signaling pathway governs multiple biological functions, encompassing energy metabolism, inflammation, cellular differentiation, and lipid homeostasis (Contreras et al., 2013; Fuior et al., 2023; Kim et al., 2023). The observed improvement in production performance within the SNL group is, at least in part, attributable to the functional modulation of the PPAR signaling pathway.
To investigate the interactions between the gut microbiota and host transcriptome following dietary supplementation with AMO and SNL in chickens, this study employed Spearman correlation analysis to examine the associations between transcriptomic and metagenomic profiles. Notably, chickens fed with SNL exhibited a significant increase in cecal Bacteroides abundance, which demonstrated a significant negative correlation with the gene MAP3K7CL (based on P-value).
MAP3K7CL is implicated in the activation of NF-κB and MAPK signaling pathways, both of which are central to inflammatory and immune responses (Ling et al., 2022). The downregulation of MAP3K7CL in response to elevated Bacteroides levels suggests that these bacteria may modulate host inflammatory pathways, potentially through the production of short-chain fatty acids (SCFAs) or other microbial metabolites (Yan et al., 2023b, 2023a). This observation aligns with previous studies demonstrating that certain Bacteroides species, such as B. vulgatus and B. fragilis, can attenuate inflammation by regulating cytokine production and immune cell differentiation (Liu et al., 2022). Additionally, MAP3K7CL exhibited a positive correlation with the abundance of Oscillibacter (based on P-value). In hybrid yellow catfish subjected to transport stress, MAP3K7CL was significantly downregulated, indicating its involvement in immune signaling pathways, particularly those mediated by Toll-like and Nod-like receptors (Zheng et al., 2021). Furthermore, reduced MAP3K7CL expression has been observed in Chinese patients with non-small cell lung cancer, suggesting that leukocyte-derived MAP3K7CL may contribute to disease pathogenesis by modulating inflammatory processes, mitochondrial reactive oxygen species signaling, and the Wnt pathway (Niu et al., 2021).
The decreased abundance of Oscillibacter in SNL-fed chickens may indicate a diet-induced shift in gut microbiota composition that could mitigate inflammation and improve metabolic outcomes. This hypothesis is further supported by the positive correlation between Oscillibacter and PON1, a gene encoding paraoxonase 1—an enzyme with anti-inflammatory and antioxidant properties that protects against oxidative stress and lipid peroxidation (Xue et al., 2022).
PRLHRL, a gene associated with prolactin signaling, may also influence metabolic and immune responses, although its specific role in poultry remains incompletely understood. The enrichment of Bacteroides in the ceca of SNL-fed chickens suggests that microbial metabolites may modulate host gene expression, thereby influencing immune and metabolic pathways (Fan et al., 2023). For example, SCFAs such as butyrate have been shown to regulate gene expression by inhibiting histone deacetylases and activating G protein-coupled receptors, which may indirectly affect PRLHRL expression (Cheng et al., 2022).
5 Conclusions
The results of the current study indicate that supplementation of broiler diets with 0.2% SNL might modestly improve growth performance. In addition, SNL increases jejunal villus height, alters microbial diversity or richness and consequently modulates host gene expression in broilers. Due to the relatively small sample size and the other limitations in the present study, further investigations are warranted to clarify the potential of SNL as an alternative to antibiotics in chickens.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/sra/PRJNA1311735.
Ethics statement
The animal study was approved by the Ethics Committee of Guangdong Meizhou Vocational and Technical College. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MZ: Writing – original draft, Methodology, Data curation, Conceptualization. YL: Writing – original draft, Software, Visualization, Conceptualization. MC: Writing – original draft, Validation. ML: Validation, Writing – original draft. RH: Validation, Writing – original draft. WX: Writing – original draft, Formal Analysis. XL: Writing – original draft, Investigation. YX: Resources, Writing – original draft. CJ: Project administration, Supervision, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by Youth Innovation Talents Program for Regular Institutions of Higher Education in Guangdong Province, grant number 2022KQNCX305 and the APC was funded by The Doctor studio projects of Guangdong Meizhou Vocational and Technical College, grant number DSGDMZVTC-JCJ-2021.
Acknowledgments
We would like to thank OE Biotech Co., Ltd. (Shanghai, China) for the technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2025.1698465/full#supplementary-material
Supplementary Table 1 | The statistics of the sequencing data.
Supplementary Table 2 | Mean of microbial community relative abundance at the phylum level in the cecum.
Supplementary Table 3 | Mean of microbial community relative abundance at the genus level in the cecum.
Abbreviations
BW, body weight; ADG, average daily gain; ADFI, average daily feed intake; F/G, feed-to-gain ratio; DEG, differentially expressed gene; SNL, Solanum nigrum L; T-AOC, total antioxidant capacity; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; H&E, hematoxylin and eosin; VH, villus height; CD, crypt depth; ASV, amplicon sequence variant; PCA, principal component analysis; NMDS, non-metric multidimensional scaling.
References
Abd El-Ghany W. A. (2020). Paraprobiotics and postbiotics: Contemporary and promising natural antibiotics alternatives and their applications in the poultry field. Open Vet. J. 10, 323–330. doi: 10.4314/ovj.v10i3.11
Abdelli N., Solà-Oriol D., and Pérez J. F. (2021). Phytogenic feed additives in poultry: achievements, prospective and challenges. Anim. (Basel) 11, 3471. doi: 10.3390/ani11123471
Anzoom S., Tahsin, Md. R., Kabir S., and Amran, Md. S. (2023). A comprehensive review on black nightshade (Solanum nigrum): chemical constituents, pharmacological activities and its role in COVID-19 treatment. J. Asiatic Soc. Bangladesh Sci. 49, 237–263. doi: 10.3329/JASBS.V49I2.70771
Bai M., Liu H., Wang S., Shu Q., Xu K., Zhou J., et al. (2021). Dietary moutan cortex radicis improves serum antioxidant capacity and intestinal immunity and alters colonic microbiota in weaned piglets. Front. Nutr. 8, 679129. doi: 10.3389/fnut.2021.679129
Bai M., Liu H., Zhang Y., Wang S., Shao Y., Xiong X., et al. (2023). Peppermint extract improves egg production and quality, increases antioxidant capacity, and alters cecal microbiota in late-phase laying hens. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1252785
Banday M. T., Wani M. A., Alqhtani H. A., Bin-Jumah M., Rudayni H. A., Allam A. A., et al. (2024). Malva sylvestris leaf powder as a feed additive affects the performance, carcass traits, meat quality attributes, serum antioxidants, stress physiology, intestinal bacterial counts, and gut morphology of broiler chicken. Front. Physiol. 15. doi: 10.3389/fphys.2024.1462018
Bhuvaneshwari C., Ambiga S., Ashokkumar P. K., and Ramasubbu P. R. (2022). Antioxidant and anticancer activities of Solanum nigrum Linn leaves. J. Curr. Opin. Crop Sci. 3, 79–89. Available online at: https://api.semanticscholar.org/CorpusID:268847780 (Accessed June 28, 2022).
Bolyen E., Rideout J. R., Dillon M. R., Bokulich N. A., Abnet C. C., Al-Ghalith G. A., et al. (2019). Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857. doi: 10.1038/s41587-019-0209-9
Callahan B. J., McMurdie P. J., Rosen M. J., Han A. W., Johnson A. J. A., and Holmes S. P. (2016). DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583. doi: 10.1038/nmeth.3869
Chang C. L. T., Chung C.-Y., Kuo C.-H., Kuo T.-F., Yang C.-W., and Yang W.-C. (2016). Beneficial effect of bidens pilosa on body weight gain, food conversion ratio, gut bacteria and coccidiosis in chickens. PloS One 11, e0146141. doi: 10.1371/journal.pone.0146141
Che Y., Li L., Kong M., Geng Y., Wang D., Li B., et al. (2024). Dietary supplementation of Astragalus flavonoids regulates intestinal immunology and the gut microbiota to improve growth performance and intestinal health in weaned piglets. Front. Immunol. 15. doi: 10.3389/fimmu.2024.1459342
Chen L., Wang Z., Wang P., Yu X., Ding H., Wang Z., et al. (2021). Effect of long-term and short-term imbalanced zn manipulation on gut microbiota and screening for microbial markers sensitive to zinc status. Microbiol. Spectr. 9, e0048321. doi: 10.1128/Spectrum.00483-21
Chen J., Yu B., Chen D., Zheng P., Luo Y., Huang Z., et al. (2019). Changes of porcine gut microbiota in response to dietary chlorogenic acid supplementation. Appl. Microbiol. Biotechnol. 103, 8157–8168. doi: 10.1007/s00253-019-10025-8
Chen S., Zhou Y., Chen Y., and Gu J. (2018). fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890. doi: 10.1093/bioinformatics/bty560
Cheng J., Hu J., Geng F., and Nie S. (2022). Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health. Food Sci. Hum. Wellness 11, 1101–1110. doi: 10.1016/j.fshw.2022.04.002
Contreras A. V., Torres N., and Tovar A. R. (2013). PPAR-α as a key nutritional and environmental sensor for metabolic adaptation. Adv. Nutr. 4, 439–452. doi: 10.3945/an.113.003798
Deng J., Wang L., Jin Q., Zeng J., Xu J., He X., et al. (2023). Anti-inflammatory steroids from the stems of Solanum nigrum L. Phytochemistry 210, 113667. doi: 10.1016/j.phytochem.2023.113667
Fan Y., Ju T., Bhardwaj T., Korver D. R., and Willing B. P. (2023). Week-old chicks with high bacteroides abundance have increased short-chain fatty acids and reduced markers of gut inflammation. Microbiol. Spectr. 11, e0361622. doi: 10.1128/spectrum.03616-22
Fernández Miyakawa M. E., Casanova N. A., and Kogut M. H. (2024). How did antibiotic growth promoters increase growth and feed efficiency in poultry? Poultry Sci. 103, 103278. doi: 10.1016/j.psj.2023.103278
Fuior E. V., Zvintzou E., Filippatos T., Giannatou K., Mparnia V., Simionescu M., et al. (2023). Peroxisome proliferator-activated receptor α in lipoprotein metabolism and atherosclerotic cardiovascular disease. Biomedicines 11, 2696. doi: 10.3390/biomedicines11102696
Guediri I. (2021). Total phenolic contents and determination of Antioxidant activity by DPPH, FRAP, and cyclic voltammetry of the fruit of Solanum nigrum (black nightshade) growing in the south of Algeria. Asian J. Res. Chem. 14. doi: 10.5958/0974-4150.2021.00008.0
Guo R., Huang K., Yu K., Xue F., Liang Y., Yang X., et al. (2024). Effects of high-protein feeds on growth, free amino acid metabolism and protein metabolism-related genes in larvae and juveniles of rice flower carp (Procypris merus). Comp. Biochem. Physiol. Part D Genomics Proteomics 52, 101345. doi: 10.1016/j.cbd.2024.101345
Han H., Yang S., Li J., Zhao J., Wei H., Ha S., et al. (2022). Intersex goats show different gene expression levels in the hypothalamus and pituitary compared with non-intersex goats based on RNA-Seq. Vet. Med. Sci. 8, 367–376. doi: 10.1002/vms3.672
He Z., Li X., Zhang X., Ouyang Q., Hu J., Hu S., et al. (2024). Effects of rearing systems (cage versus floor) on the microbial composition and transcriptome of goose ileum. Front. Vet. Sci. 11. doi: 10.3389/fvets.2024.1394290
Huang X., Jiang F., Chen X., and Xian Y. (2024). Plant-derived polysaccharides benefit weaned piglets by regulating intestinal microbiota: A review. J. Agric. Food Chem. 72, 28225–28245. doi: 10.1021/acs.jafc.4c08816
Jiao X., Guo Z., yao, Sun J., Bi C., Qian A., et al. (2023). Transcriptome analysis reveals the mechanism of the effect of perfluorocaproic acid exposure on brain injury in Carassius auratus. Aquat. Toxicol. 263, 106709. doi: 10.1016/j.aquatox.2023.106709
Joossens M., Huys G., Cnockaert M., De Preter V., Verbeke K., Rutgeerts P., et al. (2011). Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut 60, 631–637. doi: 10.1136/gut.2010.223263
Kai Y. (2021). Intestinal villus structure contributes to even shedding of epithelial cells. Biophys. J. 120, 699–710. doi: 10.1016/j.bpj.2021.01.003
Kameyama K. and Itoh K. (2014). Intestinal colonization by a Lachnospiraceae bacterium contributes to the development of diabetes in obese mice. Microbes Environ. 29, 427–430. doi: 10.1264/jsme2.ME14054
Kim D., Langmead B., and Salzberg S. L. (2015). HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360. doi: 10.1038/nmeth.3317
Kim I. S., Silwal P., and Jo E.-K. (2023). Peroxisome proliferator-activated receptor-targeted therapies: challenges upon infectious diseases. Cells 12, 650. doi: 10.3390/cells12040650
Li Y., Du X., Pian H., Fan X., Zhang Y., Wang T., et al. (2023). Effects of dietary supplement with licorice and rutin mixture on production performance, egg quality, antioxidant capacity, and gut microbiota in quails (Turnix tanki). Poult Sci. 102, 103038. doi: 10.1016/j.psj.2023.103038
Li H., Li H., Xie P., Li Z., Yin Y., Blachier F., et al. (2019). Dietary supplementation with fermented Mao-tai lees beneficially affects gut microbiota structure and function in pigs. AMB Express 9, 26. doi: 10.1186/s13568-019-0747-z
Li Y., Liu M., Liu H., Sui X., Liu Y., Wei X., et al. (2021). The anti-inflammatory effect and mucosal barrier protection of clostridium butyricum RH2 in ceftriaxone-induced intestinal dysbacteriosis. Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.647048
Ling N., Zhang X., Forsythe S., Zhang D., Shen Y., Zhang J., et al. (2022). Bacteroides fragilis ameliorates Cronobacter malonaticus lipopolysaccharide-induced pathological injury through modulation of the intestinal microbiota. Front. Immunol. 13. doi: 10.3389/fimmu.2022.931871
Liu S. J., Wang J., He T. F., Liu H. S., and Piao X. S. (2021). Effects of natural capsicum extract on growth performance, nutrient utilization, antioxidant status, immune function, and meat quality in broilers. Poultry Sci. 100, 101301. doi: 10.1016/j.psj.2021.101301
Liu L., Xu M., Lan R., Hu D., Li X., Qiao L., et al. (2022). Bacteroides vulgatus attenuates experimental mice colitis through modulating gut microbiota and immune responses. Front. Immunol. 13. doi: 10.3389/fimmu.2022.1036196
Love M. I., Huber W., and Anders S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550. doi: 10.1186/s13059-014-0550-8
Mantis N. J., Rol N., and Corthésy B. (2011). Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 4, 603–611. doi: 10.1038/mi.2011.41
Martin-Gallausiaux C., Marinelli L., Blottière H. M., Larraufie P., and Lapaque N. (2021). SCFA: mechanisms and functional importance in the gut. Proc. Nutr. Soc. 80, 37–49. doi: 10.1017/S0029665120006916
Ministry of Agriculture and Rural Affairs of the People’s Republic of China. (2020). Nutrient requirements of yellow chickens. Agricultural Industry Standards of the People's Republic of China. NY/T 3645-2020.
Niu L., Guo W., Song X., Song X., and Xie L. (2021). Tumor-educated leukocytes mRNA as a diagnostic biomarker for non-small cell lung cancer. Thorac. Cancer 12, 737–745. doi: 10.1111/1759-7714.13833
Nossa C. W., Oberdorf W. E., Yang L., Aas J. A., Paster B. J., Desantis T. Z., et al. (2010). Design of 16S rRNA gene primers for 454 pyrosequencing of the human foregut microbiome. World J. Gastroenterol. 16, 4135–4144. doi: 10.3748/wjg.v16.i33.4135
Obianwuna U. E., Chang X., Oleforuh-Okoleh V. U., Onu P. N., Zhang H., Qiu K., et al. (2024). Phytobiotics in poultry: revolutionizing broiler chicken nutrition with plant-derived gut health enhancers. J. Anim. Sci. Biotechnol. 15, 169. doi: 10.1186/s40104-024-01101-9
Oladeji O. M., Mugivhisa L. L., and Olowoyo J. O. (2025). Antibiotic residues in animal products from some African countries and their possible impact on human health. Antibiotics (Basel) 14, 90. doi: 10.3390/antibiotics14010090
Pabst O. (2012). New concepts in the generation and functions of IgA. Nat. Rev. Immunol. 12, 821–832. doi: 10.1038/nri3322
Qiu S., Dong S., Fan J., Wu C., and Qi X. (2025). Effect of high mobility group box 1 pathway inhibition on gene expression in the prefrontal cortex of mice exposed to alcohol. Alcohol 127, 47–53. doi: 10.1016/j.alcohol.2024.10.047
Roberts A., Trapnell C., Donaghey J., Rinn J. L., and Pachter L. (2011). Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 12, R22. doi: 10.1186/gb-2011-12-3-r22
Shang X., Xu W., Zhang Y., Sun Q., Li Z., Geng L., et al. (2023). Transcriptome analysis revealed the mechanism of Luciobarbus capito (L. capito) adapting high salinity: Antioxidant capacity, heat shock proteins, immunity. Mar. pollut. Bull. 192, 115017. doi: 10.1016/j.marpolbul.2023.115017
Sivaraj C., Yamini S., Yahavi A., Kumar R., Arumugam P., and Manimaaran A. (2020). Antioxidant, antimicrobial activities and GCMS analysis of fruit extract of Solanum nigrum L. J. Pharmacognosy Phytochem. 9, 1114–1121.
Śliżewska K., Markowiak-Kopeć P., Żbikowski A., and Szeleszczuk P. (2020). The effect of synbiotic preparations on the intestinal microbiota and her metabolism in broiler chickens. Sci. Rep. 10, 4281. doi: 10.1038/s41598-020-61256-z
Tang S., Xie J., Fang W., Wen X., Yin C., Meng Q., et al. (2022). Chronic heat stress induces the disorder of gut transport and immune function associated with endoplasmic reticulum stress in growing pigs. Anim. Nutr. 11, 228–241. doi: 10.1016/j.aninu.2022.08.008
Walker A., Pfitzner B., Neschen S., Kahle M., Harir M., Lucio M., et al. (2014). Distinct signatures of host-microbial meta-metabolome and gut microbiome in two C57BL/6 strains under high-fat diet. ISME J. 8, 2380–2396. doi: 10.1038/ismej.2014.79
Wang C.-H., Chung K.-T., Su L.-Y., Wu W.-J., Wang P.-H., Lee M.-C., et al. (2024a). Chinese herbal medicines as natural alternative products to antibiotics in weaned piglets through intestinal microbiota regulation. Int. J. Mol. Sci. 25, 11034. doi: 10.3390/ijms252011034
Wang M., Huang H., Wang L., Yang H., He S., Liu F., et al. (2021). Herbal extract mixture modulates intestinal antioxidative capacity and microbiota in weaning piglets. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.706758
Wang X., Sun Z., Wang X., Li M., Zhou B., and Zhang X. (2024b). Solanum nigrum L. berries extract ameliorated the alcoholic liver injury by regulating gut microbiota, lipid metabolism, inflammation, and oxidative stress. Food Res. Int. 188, 114489. doi: 10.1016/j.foodres.2024.114489
Xia T., Zhang Z., Zhao Y., Kang C., Zhang X., Tian Y., et al. (2022). The anti-diabetic activity of polyphenols-rich vinegar extract in mice via regulating gut microbiota and liver inflammation. Food Chem. 393, 133443. doi: 10.1016/j.foodchem.2022.133443
Xiang L., Wang Y., Yi X., and He X. (2018). Anti-inflammatory steroidal glycosides from the berries of Solanum nigrum L. (European black nightshade). Phytochemistry 148, 87–96. doi: 10.1016/j.phytochem.2018.01.019
Xu Q., Cheng M., Jiang R., Zhao X., Zhu J., Liu M., et al. (2022). Effects of dietary supplement with a Chinese herbal mixture on growth performance, antioxidant capacity, and gut microbiota in weaned pigs. Front. Vet. Sci. 9. doi: 10.3389/fvets.2022.971647
Xue C., Lv H., Li Y., Dong N., Wang Y., Zhou J., et al. (2022). Oleanolic acid reshapes the gut microbiota and alters immune-related gene expression of intestinal epithelial cells. J. Sci. Food Agric. 102, 764–773. doi: 10.1002/jsfa.11410
Yan Y., Lei Y., Qu Y., Fan Z., Zhang T., Xu Y., et al. (2023b). Bacteroides uniformis-induced perturbations in colonic microbiota and bile acid levels inhibit TH17 differentiation and ameliorate colitis developments. NPJ Biofilms Microbiomes 9, 56. doi: 10.1038/s41522-023-00420-5
Yan S., Zhu Y., Li L., Qin S., Yuan J., Chang X., et al. (2023a). Alginate oligosaccharide ameliorates azithromycin-induced gut microbiota disorder via Bacteroides acidifaciens-FAHFAs and Bacteroides-TCA cycle axes. Food Funct. 14, 427–444. doi: 10.1039/d2fo02812c
Yang Z., Liu C., Zheng W., Teng X., and Li S. (2016). The functions of antioxidants and heat shock proteins are altered in the immune organs of selenium-deficient broiler chickens. Biol. Trace Elem Res. 169, 341–351. doi: 10.1007/s12011-015-0407-3
Yao H., Wang L., Tang X., Yang Z., Li H., Sun C., et al. (2020). Two novel polysaccharides from Solanum nigrum L. exert potential prebiotic effects in an in vitro fermentation model. Int. J. Biol. Macromolecules 159, 648–658. doi: 10.1016/j.ijbiomac.2020.05.121
Ye G., Zhang L., Wang M., Chen Y., Gu S., Wang K., et al. (2019). The gut microbiota in women suffering from gestational diabetes mellitus with the failure of glycemic control by lifestyle modification. J. Diabetes Res. 2019, 6081248. doi: 10.1155/2019/6081248
Zafar H. and Saier M. H. (2021). Gut Bacteroides species in health and disease. Gut Microbes 13, 1–20. doi: 10.1080/19490976.2020.1848158
Zeeshan A., Amjad W., Amjad W., Faiz S., Faiz S., Noor M., et al. (2023). Medicinal importance of solanum nigrum linn; A review. J. Plant Environ. 5, 181–189. doi: 10.33687/jpe.005.02.4576
Zhang S., Duan Y., Zhong L., Liu H., Wang M., and Chen X. (2023). Using comparative transcriptome analysis to identify molecular response mechanisms to salinity stress in channel catfish (Ictalurus punctatus). Environ. pollut. 333, 121911. doi: 10.1016/j.envpol.2023.121911
Zhang W., Sun Q., Zhang H., Luo T., Zhang Y., Wang F., et al. (2025). Dietary exposure to lambda-cyhalothrin induces intestinal damage in chickens via oxidative stress and gut microbiota dysbiosis. Poultry Sci. 104, 105835. doi: 10.1016/j.psj.2025.105835
Zhao W., Chen Y., Tian Y., Wang Y., Du J., Ye X., et al. (2023). Dietary supplementation with Dendrobium officinale leaves improves growth, antioxidant status, immune function, and gut health in broilers. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1255894
Zhao L., Huang J., Wu S., Li Y., and Pan Y. (2022). Integrative analysis of miRNA and mRNA expression associated with the immune response in the intestine of rainbow trout (Oncorhynchus mykiss) infected with infectious hematopoietic necrosis virus. Fish Shellfish Immunol. 131, 54–66. doi: 10.1016/j.fsi.2022.09.039
Zheng T., Song Z., Qiang J., Tao Y., Zhu H., Ma J., et al. (2021). Transport Stress Induces Skin Innate Immunity Response in Hybrid Yellow Catfish (Tachysurus fulvidraco♀ × P. vachellii♂) Through TLR/NLR Signaling Pathways and Regulation of Mucus Secretion. Front. Immunol. 12. doi: 10.3389/fimmu.2021.740359
Keywords: Solanum nigrum L., growth performance, antioxidant capacity, transcriptome, microbiome, Yellow-feathered chicken
Citation: Zhang M, Li Y, Cai M, Li M, Huang R, Xu W, Liu X, Xiong Y and Jia C (2025) Effects of dietary Solanum nigrum L. supplementation on growth performance, antioxidant capacity, ileal transcriptomic profile, and cecal microbiome in Wuhua yellow-feathered chickens. Front. Anim. Sci. 6:1698465. doi: 10.3389/fanim.2025.1698465
Received: 03 September 2025; Accepted: 22 October 2025;
Published: 06 November 2025.
Edited by:
Teodora Popova, Institute of Animal Sciences, BulgariaReviewed by:
Sohail Ahmad, University of Veterinary and Animal Sciences, PakistanMuzaffer Denli, Dicle University, Türkiye
Muhammad Thohawi Elziyad Purnama, Airlangga University, Indonesia
Copyright © 2025 Zhang, Li, Cai, Li, Huang, Xu, Liu, Xiong and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Congjun Jia, ZGtqY2pAbndhZnUuZWR1LmNu
 Mengling Zhang1,2
Mengling Zhang1,2 Yunlei Li
Yunlei Li Mengkai Cai
Mengkai Cai Congjun Jia
Congjun Jia