- 1Scottish Association for Marine Science, Oban, Scotland, United Kingdom
- 2Institute of Marine Sciences, University of Dar es Salaam, Dar es Salaam, Tanzania
- 3Centre for Marine Biodiversity, University of Kerala, Thiruvananthapuram, India
- 4Guangdong Provincial Key Laboratory of Marine Biotechnology, Shantou University, Shantou, China
- 5Faculty of Natural Sciences, Institute of Aquaculture, University of Stirling, Stirling, Scotland, United Kingdom
Fed aquaculture accounted for around three-quarters of total aquaculture production in 2022. The most important raw materials in fish feed have traditionally been fishmeal and fish oil. Fishmeal has been the preferred protein source due to its high protein content and excellent amino acid profile. In aquaculture, feeds constitute over 50% of operating costs, with protein being the most expensive dietary source (based on inclusion levels). There is a growing need for alternative feed ingredients in securing the future supply of protein, as the high protein plant feedstuffs currently used are limited due to deficiencies in essential amino acids. Seaweeds have been cited as alternative protein sources for finfish with a quality of protein similar to traditional protein sources. The response of finfish to dietary seaweed is dose-dependent and species-specific, and any new protein source must be assessed on the provision of the most-limiting essential amino acid requirements of the target species. The potential of seaweed as an alternate protein or feed supplement ingredient in aquatic feeds has been well studied suggesting partial substitution of fishmeal up to 15% can be possible in most cases, although it may be higher in some species. While the majority of seaweed species contain insufficient levels of essential amino acids to meet the requirements of most finfish, despite their moderate protein contents, the number and levels of highly bioactive compounds and micro-nutrients in seaweed-derived products could be considered valuable and useful as functional additives for aquafeed fortification.
1 Introduction
There is growing concern about the ability to produce enough nutritious food to feed the global human population, which is predicted to reach 8.5 billion in 2030, 9.7 billion by 2050, and 11.2 billion by 2100 (United Nations (UN), 2015), particularly in a context of climate change, economic and financial uncertainty, and growing competition for natural resources (FAO, 2022). Studies have suggested that the world will need 70 to 100% more food by 2050 and this expansion is expected to increase the demand for agricultural products by around 50% by 2030 (Wheeler and von Braun, 2013). There is tremendous potential for improving food security and nutrition through the strengthening of aquaculture sectors (Bene et al., 2015). Between 2010 and 2020, animal aquaculture grew at 4.2% annually with finfish aquaculture accounting for 66% of aquatic animal production in 2020, falling to 3.7% in the first 3 years of the 2020s decade (FAO, 2024). The percentage of total world fish production (223 million tonnes or mt in 2022) utilised for direct human consumption has increased significantly in recent decades, up from 67% in the 1960s to 89%, or more than 198 mt in 2022 (FAO, 2024). The remaining 25 mt was destined for non-food uses, of which around 80% was reduced to fishmeal (FM) and fish oil (FO), the rest being largely utilised for a variety of purposes including as raw material for direct feeding in aquaculture. The growth of farming fed-aquaculture species has outstripped that of non-fed species and now accounts for just under 80% of the total production volume (up from 45% in 2001) (FAO, 2024). In intensive aquaculture, feeds constitute over 50% of the operating costs and, although the composition of feed is highly variable, protein remains a significant component of the total costs of aquafeeds based on inclusion rates (Pereira et al., 2012). Consequently, there is an urgent need for research towards the production of high nutritional value feed using natural and cheap nutrient sources and, specifically, to find more cost effective or innovative alternatives to FM and FO (Hardy, 2010; FAO, 2012; Ilias et al., 2015). The potential of marine macroalgae (seaweeds) as alternate protein or feed supplement ingredients in aquatic feeds is currently being examined in many parts of the world (Shields and Lupatsch, 2012; Irkin, 2019; Ismail, 2019; Saleh, 2020; Moreira et al., 2022; Mwendwa et al., 2023). Marine macroalgae are divided into 3 groups: red seaweeds (Rhodophyceae) with around 6100 species and crude protein contents generally in the range 18 – 35%, green seaweeds (Chlorophyceae) with approximately 2200 species and crude protein contents ranging generally from 15 – 22%, and brown seaweeds (Phaeophyceae) with around 1800 species and crude protein contents ranging most commonly between 9 – 14%. In addition, seaweeds contain a range of components such as polysaccharides, pigments, and antioxidants, which may have a functional role in fish diets (Shields and Lupatsch, 2012). Thus, seaweeds have been cited as alternative protein sources for finfish for a range of reasons in addition to their nutritional profiles including their wide global distribution, high growth rates, ability to mitigate carbon dioxide (CO2), and independence from terrestrial agricultural resources (Aziz et al., 2013; Angell et al., 2016; Wan et al., 2019). While the above issues are common to both finfish and shrimp farming, this review highlights key aspects of the inclusion of macroalgae within specifically finfish feeds, focussing on major production species, and provides an up to date review of the currently available papers within this rapidly expanding subject area. While it was not possible to discuss all works presented in this review, discussion of key aspects of specific finfish species is provided. This review was structured to provide an overview of the aquafeed industry and the nutritional requirements of major finfish production species (Sections 2 and 3), a description of the nutritional composition of macroalgae with relevance to aquafeeds, and a review of feed trials that have been undertaken (Sections 4 and 5, respectively).
2 Economic cost and demand of finfish feed in aquaculture
While there are year-to-year variations, global FM production over the last decade has been relatively stable averaging around 5 mt annually, with a low of 4.4 mt in 2016 followed two years later by a high of 5.7 mt in 2018 (IFFO (The Marine Ingredient Organisation), 2023). Over this period the overall demand for FM has continued to grow pushing prices to historic highs. In 2023-24, the El Nino Southern Oscillation impacted the anchoveta (Engraulis ringens) fishing seasons in Peru, resulting in lower than average production of FM and FO with consequent impacts on market prices. Previously, it was suggested that the overall demand for FM for use in aquaculture up to 2020 would decrease slightly, albeit the level would still represent a significant fraction of overall FM production (Tacon et al., 2011). In 2010, around 3.7 mt of FM were used in aquaculture feeds, i.e. about 70% of the total global production of 5.3 mt in that year (Tacon et al., 2011). Assuming FM/wet weight ratio of 1:5 and that 20% of FM was produced from fish waste, this would have required about 14.8 mt of fresh fish, or 88% of total reported landings (16.8 mt) (Fox, 2014). In reality in 2022, with the continuing growth of fed aquaculture, 87.8% (4.65 mt) of total global FM production was used in aquaculture, with around 7% and 1% used for pigs and poultry, respectively (IFFO (The Marine Ingredient Organisation), 2023). The equivalent figures for FO in 2022 were that 74.4% (0.95 mt) of global FO production was used in aquaculture, with about 15.6% going to pharma omega-3 (e.g. direct human consumption) and 10% to all other uses (e.g pet foods, cooking oils, biofuels) (IFFO (The Marine Ingredient Organisation), 2023).
Over time the proportion of FM in diets for all reared species has been reduced successfully as terrestrial products, such as soybean and other plant meals and plant protein concentrates, have been used increasingly (Turchini et al., 2010). There has been a similar reduction in the proportion of marine FO used in aquaculture feeds, despite the fact that demand is growing from some sectors. Whilst many important species, such as various carps (Family Cyprinidae) and tilapias (Family Cichlidae), grow well on diets lacking marine FO, many other species currently being cultivated, or being considered for cultivation, do not (Naylor and Burke, 2005). Species such as Atlantic salmon (Salmo salar) and marine finfish such as European seabass (Dicentrarchus labrax) are higher-trophic level predators, and long-chain polyunsaturated fatty acids (LC-PUFA) that are not present in vegetable oil alternatives to FO, are necessary to maintain maximum growth and optimum health in these species (Turchini et al., 2022). Specifically, most marine finfish species lack the enzymes required for elongating and desaturating the shorter-chain C18 PUFA, found in vegetable oils, to LC-PUFA, and as such the latter are essential fatty acids (EFA) for these species (Xie et al., 2021). However, feeds containing low to moderate amounts of FM can be adequate in EFA for some finfish species (Tocher, 2015).
3 Fish diets and feed
In aquaculture, producers commonly rely on formulated feeds to ensure optimal growth, health, and quality of farmed fish. Prepared or artificial diets may be either complete or supplemented. Fish reared in high-density indoor systems or in cages where they cannot forage freely must be provided a complete diet. Most fish farmers use complete diets containing all the required protein (18 - 50%), lipid (10 - 25%), carbohydrate (15 - 20%), ash (< 8.5%), phosphorus (< 1.5%), moisture (<10%), and trace amounts of vitamins and minerals (Craig and Helfrich, 2002). In terms of energy density, proteins, lipids, and carbohydrates have average caloric values of 5.65, 9.45, and 4.15 kilocalories per gram (kcal/g), respectively (Montgomery and Gerking, 1980). Supplemental diets are intended to support natural food (insects, algae, small fish) normally available to fish in ponds or outdoor raceways and do not necessarily contain a full complement of vitamins or minerals, and are used to help fortify the naturally available diet with extra protein, carbohydrate and/or lipid. Good nutrition in animal production systems is essential, particularly from an economic point of view, and fish require a high quality, nutritionally complete, and balanced diet to grow rapidly and remain healthy, particularly when reared at high densities (Craig and Helfrich, 2002). Protein for fish is mostly provided through compound diets (Boland et al., 2013) and FM and FO are important raw materials used in the fish feed industry. Traditionally, FM was the favoured protein source due to its high protein content, excellent amino acid profile, lack of anti-nutrients, and high palatability and digestibility (Anastasiou and Nengas, 2005). The use of FM and FO as raw materials for feeds is largely to ensure that key nutrients, particularly essential amino acids and fatty acids, are nutritionally available in sufficient amounts for the finfish species being cultivated (Slaski and Franklin, 2011). However, there has always existed the fear that long-term sustainability of aquaculture may be threatened by its overdependence on FM and FO (FAO, 2002). As stated above, in intensive aquaculture, feeding finfish constitutes over 50% of the operating costs, with protein being the most expensive dietary ingredient (Pereira et al., 2012). To offset the high price of feed as demand increases, the amount of FM and FO used in compound feeds has taken a downward trend (FAO, 2016) and they are being used more selectively as strategic ingredients at lower concentrations and for specific stages of production, particularly hatchery, broodstock and finishing diets (Güroy et al., 2007).
3.1 Essential amino acids
In a dietary sense, it is not the protein per se but rather the amino acids within the protein that are important. It is those essential amino acids (EAA) that cannot be synthesised by livestock that are critical in a diet and define the quality of a protein source (Boland et al., 2013). There is substantial variation in EAA requirements not only between fish species, but between different life stages. A requirement for 10 amino acids has been demonstrated in fish species (Angell et al., 2016) (Table 1). For most EAA, dietary deficiency is indicated by a reduction in weight gain, however, in some fish species, a deficiency of methionine or tryptophan can lead to disease as these amino acids are not only incorporated into proteins, but also used for the synthesis of other essential compounds (National Research Council (NRC), 1993). Salmonids, including rainbow trout (Oncorhynchus mykiss), Atlantic salmon and lake trout (Salvelinus namaycus) have been shown to suffer from cataracts when given a diet deficient in methionine (Poston and Rumsey, 1983). Cataracts can also occur as a consequence of tryptophan deficiency in rainbow trout (Walton et al., 1984). The author stated that this is probably due to alterations in the protein pattern of the lens tissue, similar to that observed in Atlantic salmon given a methionine-deficient diet. Tryptophan deficiency can also lead to lateral curvature of the vertebral column, known as scoliosis, although in some fish species such as chum salmon (Oncorhynchus keta), scoliosis may be reversed by restoring tryptophan to normal concentrations in the diet.
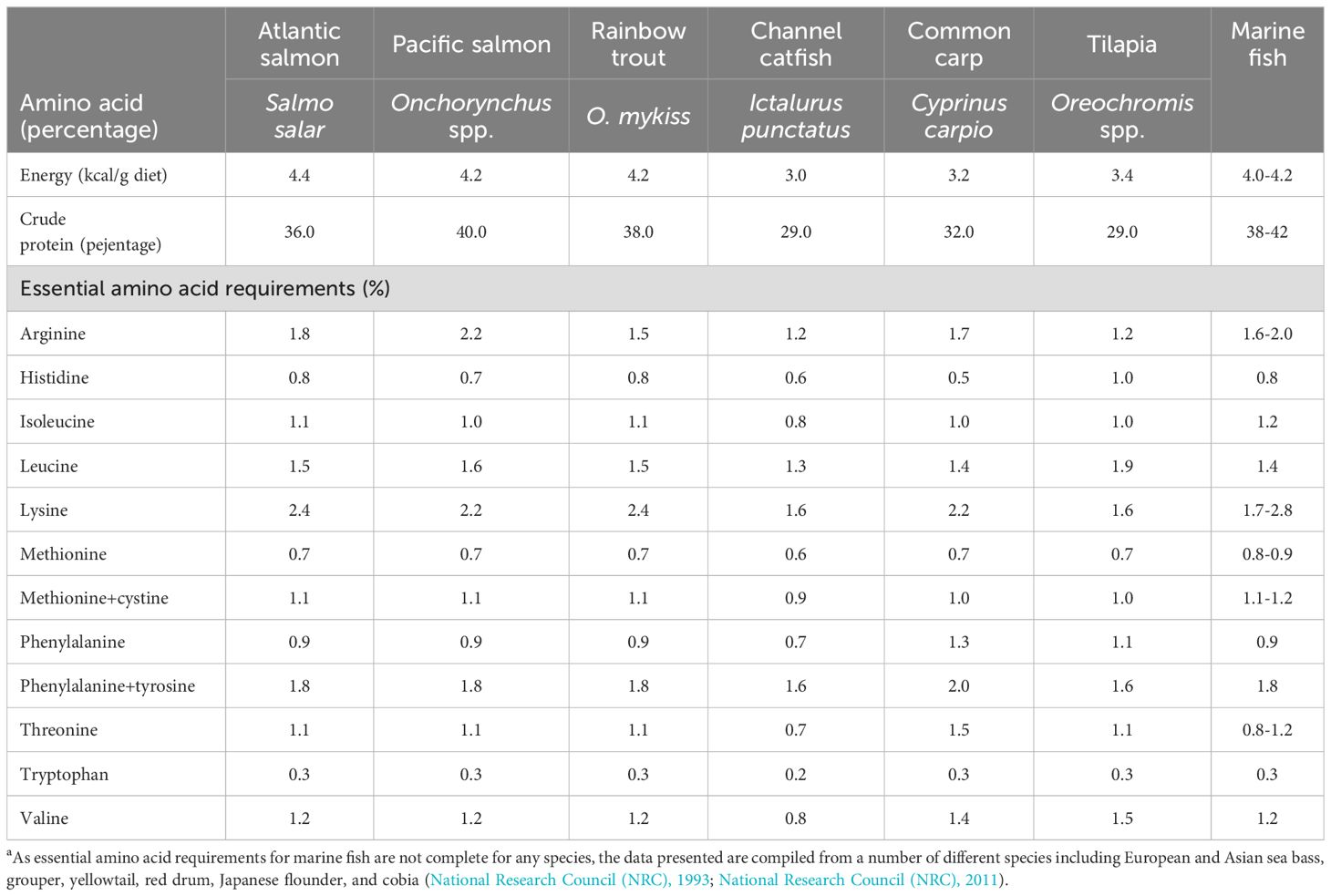
Table 1. Typical energy and crude protein (N x 6.25) contents of commercial feeds and essential amino acid (EAA) requirements of salmonid, freshwater and marine fish (National Research Council (NRC), 1993; National Research Council (NRC), 2011).
3.2 Essential fatty acids
Lipids are a broad group of naturally occurring molecules including fats and oils and are indispensable in vertebrate diets as they supply crucial nutrients, the essential fatty acids (EFA). Like all vertebrates, fish cannot synthesise either linoleic acid (18:2n-6) or alpha-linolenic acid (18:3n-3) and, therefore, one or both of these fatty acids must be supplied in the diet, depending on specific EFA requirements. There appears to be a major difference between freshwater and stenohaline (intolerant to wide fluctuations in water salinity) marine finfish (Owen et al., 1975). In general, EFA requirements in freshwater finfish can be satisfied by either dietary 18:2n-6 or 18:3n-3, or both, whereas dietary EFA requirements in stenohaline marine finfish can only be satisfied by the n-3 LC-PUFA, eicosapentaenoic acid (EPA, 20:5n-3), and/or docosahexaenoic acid (DHA, 22:6n-3). Among freshwater species, channel catfish (Ictalurus punctatus), coho salmon (Oncorhynchus kisutch), and rainbow trout require 18:3n-3, chum salmon and common carp (Cyprinus carpio) may require both 18:3n-3 and 18:2n-6, whereas, Nile tilapia (Oreochromis niloticus) and redbelly tilapia (Coptodon zillii) appear to require mainly 18:2n-6 for maximum growth and feed efficiency (Murata and Nakazoe, 2001). Modern aquaculture, particularly salmonid farming, is geared towards optimising feed conversion. This means the use of high-energy, high lipid diets, with reduced protein levels and, whilst economically successfully, these practices are not always acceptable to the consumer. It is widely accepted that wild fish contain lower lipid levels than intensively farmed fish (Rasmussen, 2001), however, higher lipid levels can be unappealing to consumers and are often problematic in freezing and smoking practices (Güroy et al., 2010).
3.3 Carbohydrates
Carbohydrates (starches and sugars) are the most economical and inexpensive sources of energy in fish feeds. Although finfish do not require carbohydrates in their diet, incorporation in feeds has advantages such as binding activity during feed manufacturing, and their protein and lipid sparing action that can reduce feed costs (Keshavanath et al., 2002; Trushenski et al., 2006). In fish, carbohydrates are stored as glycogen that can be mobilised to satisfy energy demands and, although no dietary requirement has been demonstrated in fish, if carbohydrates are not provided in the diet then other compounds, such as protein and lipids, are catabolised for energy and for the synthesis of various biologically important compounds (Craig and Helfrich, 2002). However, the nutritional value of carbohydrates in fish varies so, although many finfish species exhibit appropriate gut morphology and possess abundant intestinal microflora, there is considerable variation between species in their ability to effectively and/or efficiently digest and process carbohydrates (Stickney and Shumway, 1974; National Research Council (NRC), 1993). Cold water carnivorous fish species lack the appropriate enzymatic suite necessary for digestion of carbohydrates and, because of this, dietary inclusion generally no higher than 20% is recommended. In contrast, warm water omnivorous and herbivorous finfish species such as channel catfish, tilapia and common carp adapt well to diets containing up to 40% dietary carbohydrate (Stone, 2003).
3.4 Terrestrial plant proteins and oils
As stated above, feeds represent over 50% of operating costs in intensive aquaculture, with protein being the most expensive dietary component (Glencross et al., 2007; Turchini et al., 2022). Considerable efforts have been made over the past 2-3 decades to find alternatives to FM as protein sources, with particular emphasis on terrestrial plants such as legumes, grains and oilseeds (Borquez et al., 2010) including soybean, peas, rapeseed (Canola) and lupin that are important agricultural crops (Valente et al., 2006). Provided EAA requirements are met, replacement of up to 98% of FM by different terrestrial plant protein sources from soybean, wheat, corn, and rapeseed have shown to be generally acceptable, with no major adverse consequences, albeit these feeds were often supplemented with higher amounts of FO (Kaushik et al., 2004). Limitations to the use of plant protein sources in fish diets have been attributed to nutritional profiles that do not fully match fish requirements (Francis et al., 2001; Geurden et al., 2005), including deficiencies in certain EAA, contents of anti-nutritional compounds, and palatability issues (Hardy, 1996; Drew et al., 2007). The food security of plant proteins is also a growing concern due to increasing population and growth in consumption (Tacon and Metian, 2008; Godfray et al., 2010), limited resources of arable land and freshwater (Pretty, 2008) and, more recently, competition with biofuels (Nigam and Singh, 2011). As a consequence, there is a critical need for other alternatives to FM in securing the future supply of protein (and lipids) for finfish aquaculture (Boland et al., 2013).
4 Nutritional value of seaweed
Fish feed manufacturers are constantly searching for new and innovative alternatives to FM and FO for the production of feeds that are both cost-effective and of high nutritional value (Hardy, 2010; FAO, 2012; Ilias et al., 2015). The potential of marine macroalgae (seaweeds) as alternate protein sources and/or nutritional supplements in aquafeeds is currently the subject of intense research (Wan et al., 2019). As mentioned above, seaweeds have many characteristics that make them attractive as potential feed ingredients including their global distribution and high growth rates that make them widely available, and their environmental and ecological benefits such as their ability to mitigate carbon dioxide (Aziz et al., 2013), and independence from terrestrial agricultural resources (Angell et al., 2016). Seaweeds are classified as red algae (Rhodophyta), brown algae (Phaeophyta) or green algae (Chlorophyta), depending on their chemical composition and evolutionary history (Dawczynski et al., 2007), and many can be suitable for a wide range of applications in the food industry both as animal feed and human food (Garcia-Vaquero and Hayes, 2016; Costa et al., 2021; Løvdal and Skipnes, 2022; González-Meza et al., 2023). Due to their unique character, seaweeds are able to generate a wide range of secondary metabolites not found in other organisms (Ferraces-Casais et al., 2012) and are a natural source of compounds (proteins, lipids, and polysaccharides) with biological activity and with the potential to be used as functional ingredients in food/feed (Lopes et al., 2020; Guo et al., 2022; O’Brien et al., 2022). The term “functional food” is used to describe foods and food components that have been demonstrated to provide specific health benefits beyond basic nutrition (Holdt and Kraan, 2011). While seaweeds contain proteins, lipids (omega-3 LC-PUFA), carbohydrates (polysaccharides), vitamins, minerals, pigments, polyphenols, and fibres, the types and abundance of these compounds vary between seaweed species (Dawczynski et al., 2007; Mansilla and Avila, 2011) (Tables 2–5). Seaweeds also possess anti-diabetic, anti-allergic, anti-coagulant, anti-cancer, anti-inflammatory, antioxidant, and immune-protective compounds, as well as anti-bacterial, anti-viral, and anti-fungal properties (Misurcova et al., 2012; Ferreira et al., 2021).
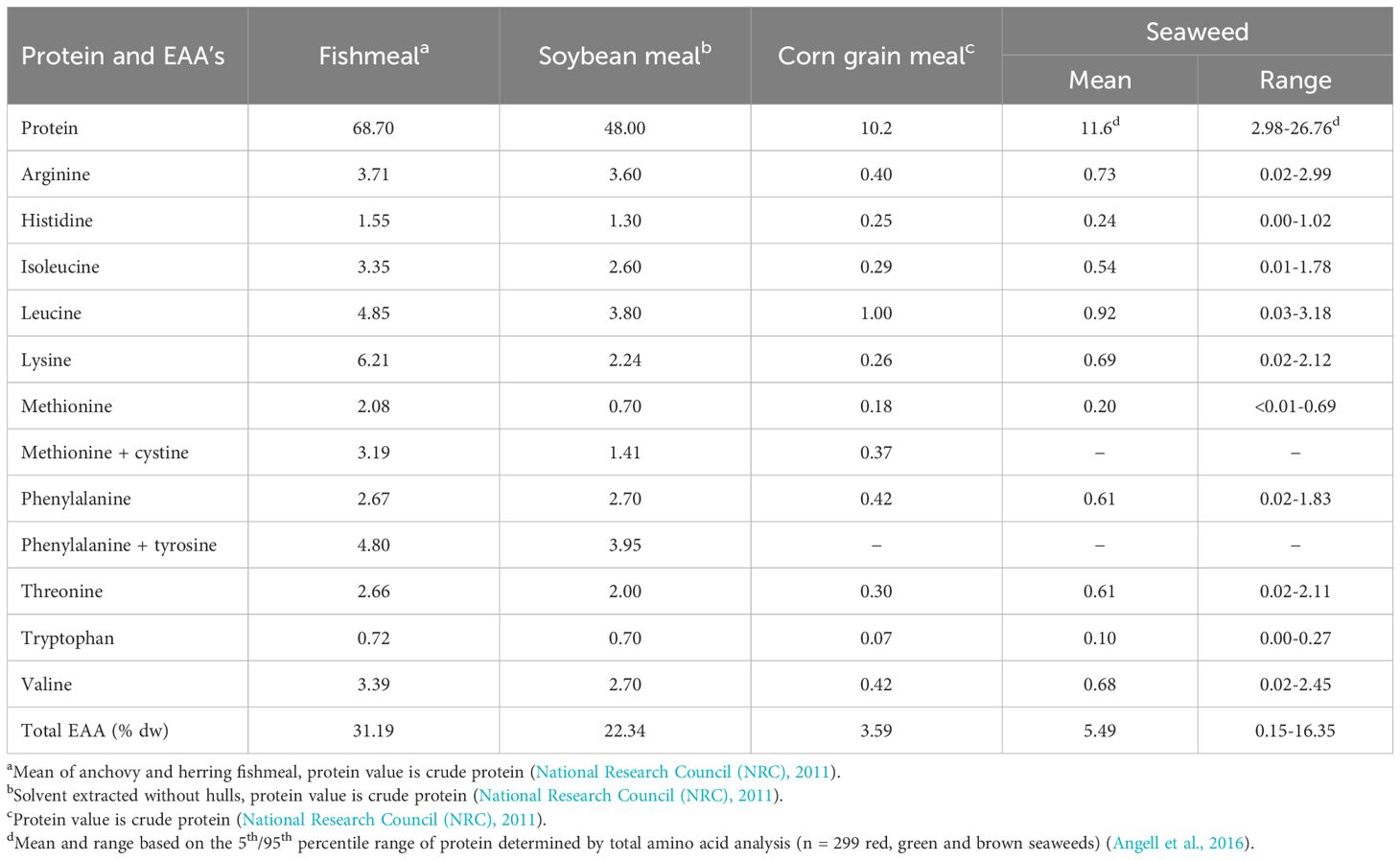
Table 5. The concentration of protein and essential amino acids (% dry weight) of traditional protein sources, other feed ingredients, and seaweed.
4.1 Protein and amino acids
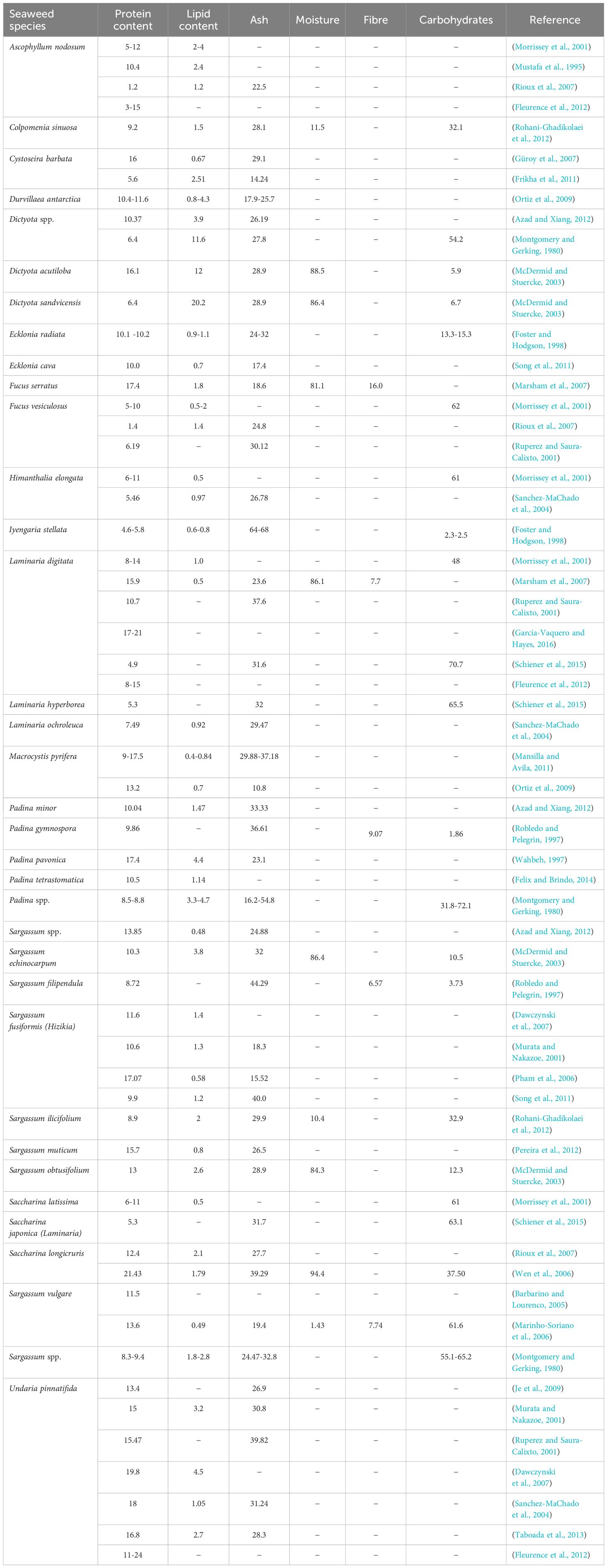
In both humans and animals, protein is required for growth, health, reproduction, and optimal performance (Boland et al., 2013). The protein content of seaweeds varies substantially more between species within the taxonomic groups (red, green or brown seaweed), than between the taxonomic groups themselves (Dawczynski et al., 2007). Red and green varieties of seaweed can be protein-rich, depending on environmental growth conditions, location, maturity, and season (Michalak et al., 2009; Holdt and Kraan, 2011). Red seaweeds contain some of the highest levels of protein, including up to 47% on a dry-weight basis for nori or laver (Porphyra tenera), 35% for dulse (Palmaria palmata) and 33% for Gracilaria spp. (Table 2). These levels are comparable to those found in high-protein plants such as legumes including soybean in which protein represents 35% of the dry mass (Burtin, 2003). Green seaweeds, such as Ulva spp., can show protein levels of up to 35% dry weight (Table 3). Protein content is generally lowest in brown seaweeds with the highest protein content of 24% noted in wakame (Undaria pinnatifida) (Table 4). Seasonal factors can affect the protein content in the fronds of seaweed species. For example, Saccharina spp., Laminaria spp. and winged kelp (Alaria esculenta) were shown to have highest protein levels during the period February to May, with the younger parts of the fronds of Saccharina spp. and Laminaria spp. being considerably richer in protein than the older parts (Fleurence et al., 2012). It was reported that a seasonal variation of protein content was clearly observed in Palmaria palmata with highest values of 21.9% occurring during the winter-spring period and lower levels of 11.9% during the summer-early autumn period (Galland-Irmouli et al., 1999).
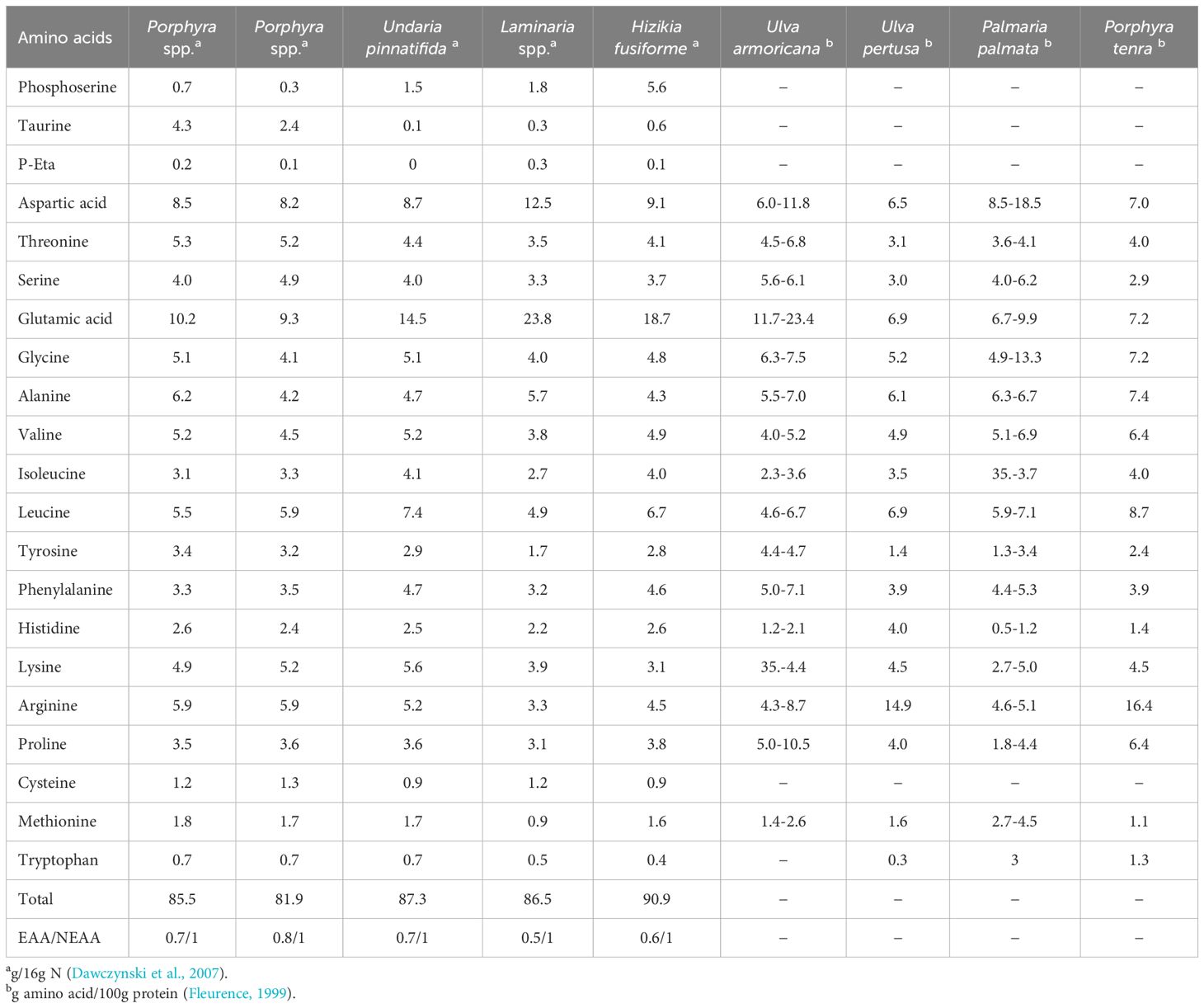
Seaweeds have a relatively high quality of protein as defined by the proportion of essential (EAA) to total amino acids (Boland et al., 2013). The quality of protein of many seaweed species is similar to, if not better than, protein sources traditionally used in aquatic feeds (National Research Council (NRC), 2011). More than 75% of seaweeds have higher proportions of total EAA than FM and 50% have higher proportions than soybean meal (Angell et al., 2016). The concentrations of EAA, specifically those that are often limiting in the diets of mono-gastric livestock such as finfish (i.e. lysine and methionine), determine how much of a protein source can be utilised in feeds. Lysine is rarely limiting in seaweeds and, although threonine is also rarely limiting in seaweeds for any livestock, it is often supplemented in commercial diets. The concentrations of protein and EAA (% of dry weight) of seaweeds in comparison to FM other common feed ingredients are presented in Table 5.
In addition to variation in protein level throughout the year, seasonal fluctuations in amino acid profiles have also been reported in a number of macroalgae with the concentration of each amino acid varying between species (Galland-Irmouli et al., 1999) (Table 6). Individual macroalgal species have been shown to contain high levels of specific amino acids e.g. oarweed (Laminaria digitata) is rich in alanine, Undaria pinnatifida is rich in alanine, glycine, arginine, leucine, valine, and lysine and also contains significant levels of methionine. Palmaria palmata has a high content of glycine and is also a good source of methionine, threonine, and leucine. Porphyra spp., Irish moss (Chondrus crispus) and sea lettuce (Ulva pertusa) are all rich in arginine, whereas the sea lettuce Ulva armoricana is known to contain high levels of proline (Harnedy and FitzGerald, 2013; Garcia-Vaquero and Hayes, 2016). Porphyra spp. and Undaria spp. appear to be interesting potential sources of food protein with respect to their high protein levels and their amino acid composition (Dawczynski et al., 2007).
Thus, the true value of seaweeds as a protein source must be assessed on a species by species basis, based on the provision of the limiting EAA. The nutritional value of proteins is referred to as the “amino acid score”, defined as the smallest ratio of any of the 10 EAAs, and sets the limiting EAA and this ratio determines how much protein or total amino acids can be efficiently utilised by livestock (Angell et al., 2016). In general, the amino acid scores of the proteins in cereals and other plants are lower than that of the proteins of animal origin. However, the amino acid scores of the proteins of marine algae range from 60 to 100, values generally higher than those of the proteins in cereal and vegetables. For instance, the amino acid scores of proteins in Porphyra spp. and Undaria spp. are 91 and 100, respectively, similar to beef, sardine, and milk that have amino acid scores of around 100 (Murata and Nakazoe, 2001).
4.2 Lipids
Lipids are a broad group of naturally occurring molecules that includes fatty acids, oils, fats, waxes, sterols, phospholipids, fat-soluble vitamins (such as vitamins A, D, E, and K), and mono-, di-, and triacylglycerols (Holdt and Kraan, 2011). Major biological functions of lipids include energy storage, structural components of cell membranes, signalling, and metabolic regulation and, in the diet, they supply the essential fatty acids (EFA). Glycolipids, neutral lipids, and phospholipids are considered the main forms of lipid available in marine algae (Garcia-Vaquero and Hayes, 2016), although the reported levels and their importance varies between studies. Total lipid levels are species-specific and can range from less than 1% up to 20% of dry matter depending on season, environment, age, and growth stage of seaweed species (Nomura et al., 2013). Some species contain higher levels, such as the green seaweed Ulva rigida at 12% and the brown seaweed Dictyota spp., ranging from 12 to 20% (Tables 2–4). In Saccharina spp., Laminaria spp. and Alaria esculenta, the highest lipid contents of fronds were generally found in winter, whereas total lipids of Fucus spp. were most abundant in summer, with highest levels recorded in August (Kim et al., 1996). It has been reported that tropical species have significantly less total lipid than cold water species (Sanchez-MaChado et al., 2004).
Marine lipids generally contain a wider range of fatty acids than their terrestrial counterparts and can contain substantial amounts of monounsaturated and polyunsaturated fatty acids (PUFA) (Narayan et al., 2006). The PUFAs include both omega-3 (n-3) and omega-6 (n-6) fatty acids and have been regarded as functional healthy compounds of human food with attention particularly focused on the n-3 LC-PUFA, EPA and DHA (Nomura et al., 2013) that are major components of marine lipids. Cold water aquatic species generally contain larger quantities of LC-PUFA including EPA and DHA, and seaweed can accumulate PUFA when environmental temperature is lower (Khotimchenko, 2005). As with other biochemical components, fatty acid compositions can vary between seaweed species (Table 7), and have been shown to be affected by many factors such as light, salinity, mineral ions, heavy metals (Cu, Cd and Pb), pollution, herbicides, infection, habitat, and environmental factors (Kim et al., 1996; Denis et al., 2010). Recent work has also indicated that marine macroalgae may contain bioactive lipids, as lipid extracts from different brown, green, and red seaweeds can have putative antioxidant, anti-inflammatory, and antiproliferative properties that, again, could make certain seaweeds useful as functional feeds (Lopes et al., 2020).
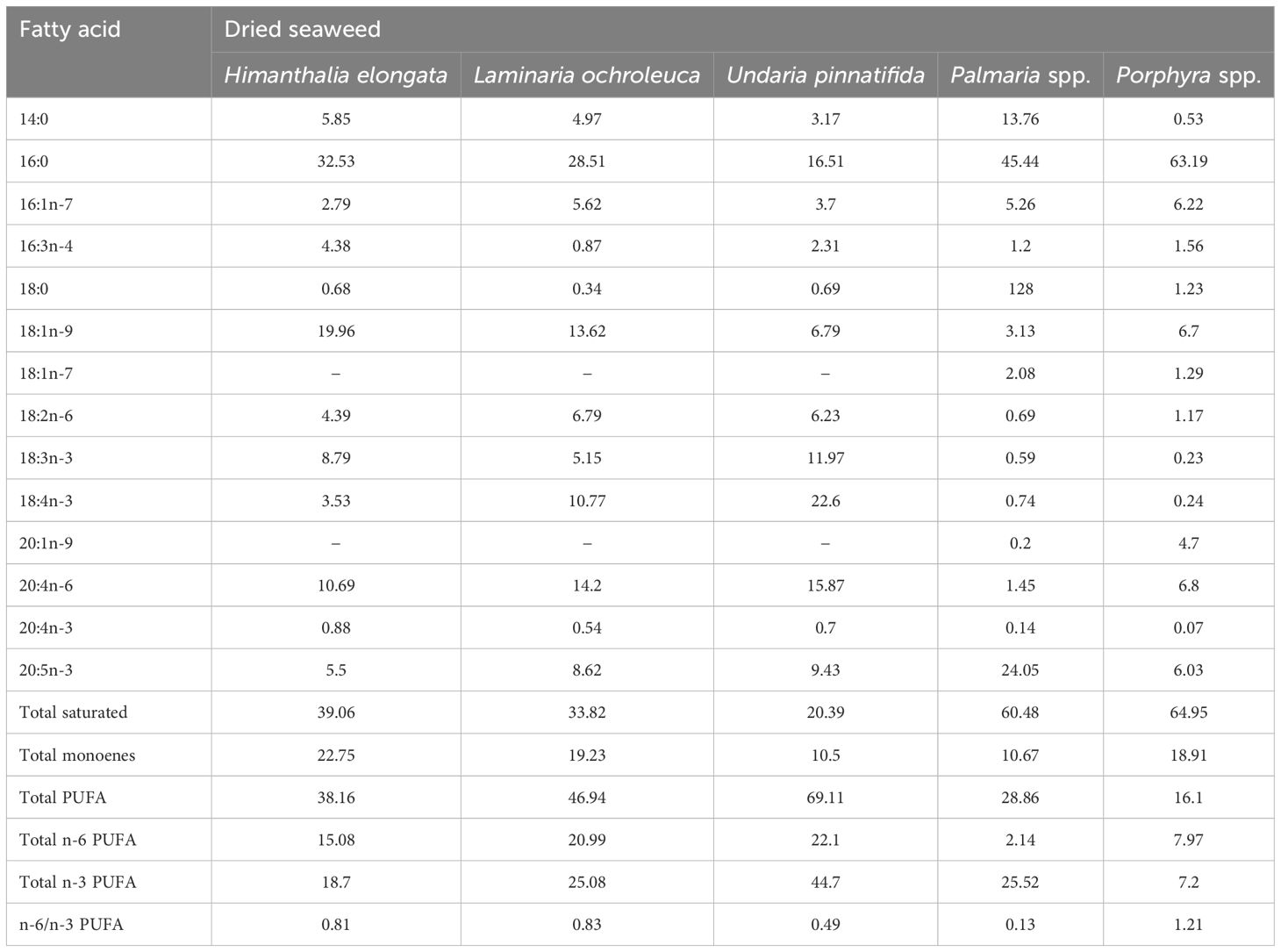
Table 7. Relative fatty acid composition of various seaweed species (% of total fatty acid content) (Sanchez-MaChado et al., 2004).
4.3 Polysaccharides and fibre
Polysaccharides are polymers of simple sugars (monosaccharides) linked together by glycosidic bonds with an enormous variety of structures (Laurienzo, 2010). The functions of polysaccharides in living systems are both structural, due to their role in the construction of cell walls in plants, and metabolic due to their roles in energy metabolism and storage in all organisms. Depending on their specific compositions, chemical structures, and physiochemical properties, algal polysaccharides have been shown to exhibit a wide range of activities in a variety of biological systems (Jiang et al., 2010). Seaweeds contain large amounts of polysaccharides, notably structural (cell wall) polysaccharides, mycopolysaccharides, and storage polysaccharides whose composition can differ significantly from terrestrial plants (Murata and Nakazoe, 2001) (Table 8). Cell wall polysaccharides consist mainly of cellulose, hemicelluloses, and neutral polysaccharides and have numerous commercial applications such as stabilisers, thickeners, and emulsifiers in food, beverages, and feeds (Tseng, 2001).
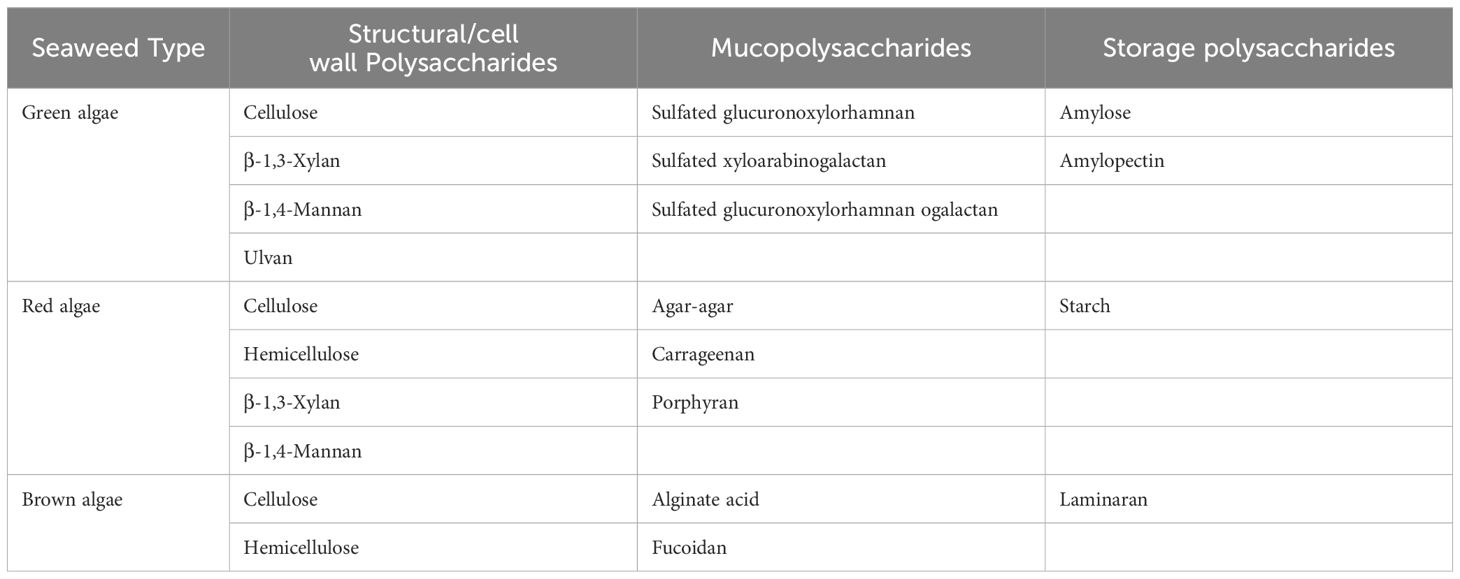
Table 8. Polysaccharides of marine algae (Murata and Nakazoe, 2001).
Polysaccharides can be anti-nutritional factors limiting the digestibility of protein fractions (Horie et al., 1995). When considering human intestinal bacteria, most seaweed polysaccharides (agars, carrageenans, ulvans, and fucoidans) are not digested and, therefore, can be regarded as dietary fibres (Burtin, 2003). These complex polysaccharides are highly diverse in their structure and composition and this is reflected in a great variation in their functional properties and the way they are utilised by gut flora, and subsequent impacts on the physiology of the animal (FAO, 2012). Dietary fibres found in marine algae are classified into two types, i.e. insoluble, such as cellulose, mannans, and xylan, and water-soluble such as agars, alginic acid, furonan, laminaran, and porphyran. Green algae contain cellulose, sulphated galactans (ulvans), sulphated polysaccharides, and xylans, brown seaweeds contain alginic acid, fucoidan, laminarin, and sargassan, and red seaweeds contain agars, carrageenans, xylans, floridean starch, sulphated galactan, and porphyrin (Table 9). Total polysaccharide concentrations found in seaweeds range from 4% to 76% of dry weight with some of the highest contents found in species such as the brown alga Ascophyllum nodosum, Porphyra spp., Palmaria palmata and Ulva spp (Holdt and Kraan, 2011)., although seaweed polysaccharides are species-specific and exhibit seasonal variation. While polysaccharide antinutritional factors found in seaweed can interfere with the bioavailability and/or digestibility of proteins, studies have shown that treatment of algae by enzymatic digestion with appropriate polysaccharidases can significantly improve the digestibility of proteins from red seaweed such as Palmaria palmata (Marrion et al., 2003). It was also suggested that digestibility could also be enhanced by treating raw seaweed with enzymes and acids to produce an acceptable silage-type product for pelleting (Davies et al., 1997).
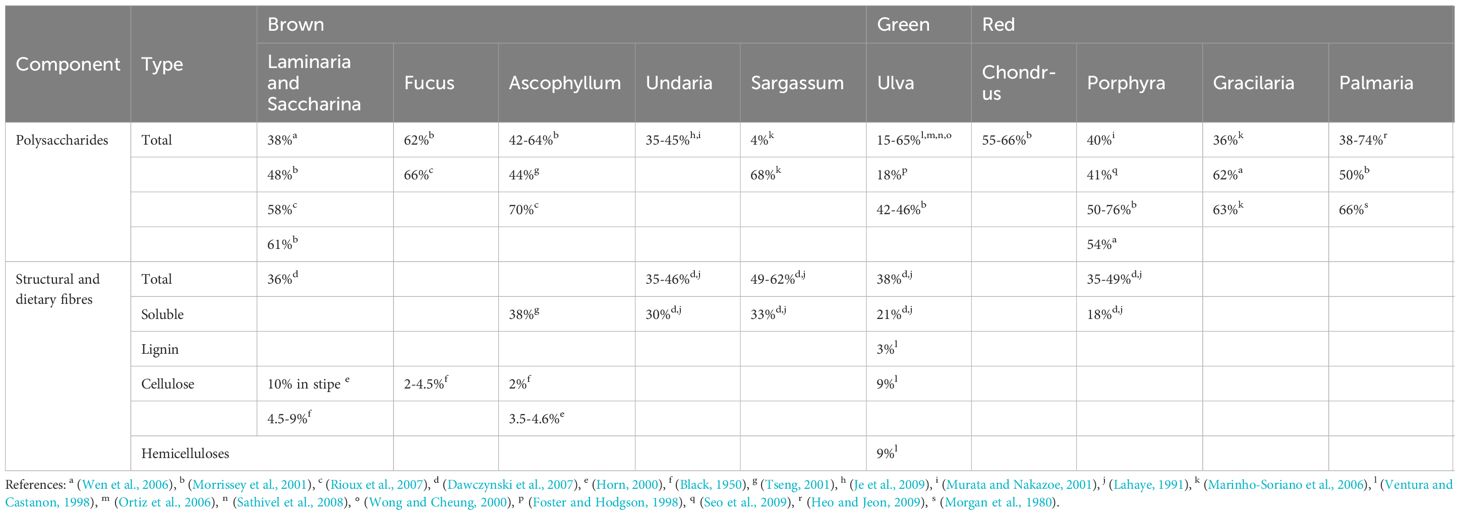
Table 9. Contents of total polysaccharides and structural and dietary fibres (% dry weight) in seaweed species in Northwest Europe (Holdt and Kraan, 2011).
4.4 Vitamins and minerals
Seaweeds can be particularly rich sources of both water-soluble (mainly B group vitamins and vitamin C) and fat-soluble (A, D, E, and K) vitamins (Watanabe et al., 2002; McDermid and Stuercke, 2003; Hamid et al., 2015). There is a high degree of inter-species variation but, in general, red seaweeds are particularly rich in vitamins A, C, and B9 (folic acid). Seaweeds are noted for being rare sources of non-animal derived vitamin B12 and, in addition, brown seaweeds are a good source of vitamin E (Watanabe et al., 1999; Kumar et al., 2008).
Minerals are inorganic elements and maintain their chemical structure meaning that minerals in soil and water enter the body via consumption of plants, fish, animals, and fluids. The mineral contents of seaweeds, determined by the dry ash method, are generally much higher than those of most terrestrial plants other than spinach (Sanchez-MaChado et al., 2004) and, therefore, are used as feed and food supplements (Dawczynski et al., 2007). However, mineral contents of seaweeds vary according to species, geographical location, season, wave exposure, oceanic residence time, and method of processing (Cofrades et al., 2010). Most macroalgal species are a rich source of minerals and contain higher amounts of both macro-minerals (8 – 17 g/100 g dry weight) including sodium, potassium, calcium, and magnesium, and trace elements (5 – 15 mg/100 g dry weight) including iron, zinc, manganese, and copper, than those reported for edible land plants (Ruperez, 2002; Holdt and Kraan, 2011).
Calcium has been shown to accumulate at much higher levels in seaweeds than in terrestrial foodstuffs. For example, Ulva species have been found to contain up to 3.25 g of calcium per kg of dry weight (MacArtain et al., 2007). Iron and copper are also present at high levels in seaweeds, higher than those found in red meat and spinach. In many parts of the developing world and some parts of Europe, iodine deficiency in humans is still a problem. Iodine is an essential trace element required for the synthesis of thyroid hormones in the body. Insufficient iodine intake leads to reduced production of thyroid hormones resulting in iodine deficiency disorders (IDD) that can result in goitre, hypothyroidism, reproductive failure, and childhood mortality (He et al., 2002). The uptake of dietary iodine by humans and animals leads to the formation of the thyroid hormone and, therefore, the concentration of iodine in macroalgae is one of the most studied research areas (Rey-Crespo et al., 2014). Brown seaweed, especially the species Laminaria digitata, is rich in iodine (up to 10 g per kg of dry weight). There is high variability in the levels of iodine in seaweeds reported in the literature, ranging from 5 mg/kg in Porphyra spp. to 5-10 g/kg in different Laminaria species (Holdt and Kraan, 2011). Like all essential minerals, when in excess, iodine content can also be harmful and in France the final concentration in Laminaria products must be below 500 mg per kg of dry weight (Fleurence et al., 2012).
4.5 Pigments
Seaweeds contain carotenoids, the most widespread pigments in nature, present in all algae, higher plants, and many photosynthetic bacteria, and the main pigments in many aquatic animals (Wilke et al., 2015). Carotenoids represent photosynthetic pigments in the red, orange or yellow wavelengths (Holdt and Kraan, 2011). Green seaweed species contain β-carotene, lutein, violaxanthin, neoxanthin, and zeaxanthin, whilst red seaweed species contain mainly α-and β-carotene, lutein, and zeaxanthin, while violaxanthin and fucoxanthin are present in brown seaweed species (Haugan and Liaaen-Jensen, 1994). The β-carotene content in seaweed ranges from 36 to 4,500 mg/kg dry weight, with Porphyra spp. having the highest contents at up to 4,500 mg/kg. Palmaria palmata has the second highest content at 456 mg/kg, however, carotenes in Palmaria show seasonal variations, with highest content in summer at 420 mg/kg and lowest in winter at 37 mg/kg (Soler-Vila et al., 2009). The red protein pigment, R-phycoerythrin, is found in red seaweeds and is often present at high levels (12% of dry weight), and its content follows changes in protein content and subject to marked seasonal variation (Denis et al., 2010; National Research Council (NRC), 2011). It is used in Asia as a food colourant (Fleurence et al., 2012). Gracilaria species are also rich in carotenoids, including zeaxanthin, β-carotene, fucoxanthin, lutein, and antheraxanthin (Ortiz et al., 2009), and it was reported that dietary inclusion of zeaxanthin-rich Gracilaria vermiculophylla in aquafeeds could be a cost-effective alternative to the use of expensive synthetic carotenoids (Araujo et al., 2016). Carotenoids contribute to muscle and skin pigmentation in salmonids, which is an important quality parameter for consumers (Colihueque, 2010). Salmonids depend entirely on dietary supplements to achieve their natural pigmentation as they cannot synthesise carotenoids (Barbosa et al., 1999) and so astaxanthin and canthaxanthin are commonly used in the feeds for farmed salmonids. Astaxanthin is preferable to canthaxanthin because it produces nature-identical pigmentation and is more efficiently deposited. Colouration of flesh in salmonids using seaweeds such as Porphyra spp. as a natural pigment source may enhance the potential of seaweed inclusion in finfish feed and could perhaps replace or reduce artificial colourants currently used by the industry (Nickell and Bromage, 1998).
4.6 Binders
Binders are used in fish feed to improve stability in water and increase pellet firmness. The most widely used binders are sodium and calcium bentonites, lignosulfonates, hemicellulose, carboxymethylcellulose, guar gum, and alginate. Most binders are long-chain macromolecules considered to be inert with limited or no nutritional value (National Research Council (NRC), 1993). Binders also improve the efficiency of the feed manufacturing process and reduce feed wastage. During pelleting, binders are used to reduce the frictional force of the feed mixture through the pellet dies thereby increasing output. In order to utilise some of the by-products from fishing, which represent valuable resources of high-quality protein and energy to the industry, a method for preparing wet feed using a binder made from the brown seaweed Ascophyllum nodosum was developed by Gabrielsen & Austreng (Gabrielsen and Austreng, 1998). The results suggested that jellified wet feed was superior to dry feed as it remained water-stable for 24 h, with very little nutrient leaching and that the wet alginate feed sunk 80% slower than dry feed. A significantly higher level of lysozyme in fish fed the wet feed implied an immuno-stimulating potential effect of alginate. The authors suggested that a switch to this technology may increase profit margins without jeopardising the quality or quantity of fish production. The positive effect of using seaweed and alginates as binders on the immune status of finfish such as Atlantic salmon and gilthead seabream (Sparus aurata) has been well known for many years (Gabrielsen and Austreng, 1998) and the use of alginate as a binder in fish feed was common practice in the pioneering days of Norwegian fish farming (Storebakken and Austreng, 1987). The use of seaweed as binding agents in pelleted feed for striped snakehead (Channa striatus) fry was shown to have good water stability and to improve growth rate and feed efficiency (Hashim and Saat, 1992).
4.7 Bioactive and immuno-stimulatory properties of seaweed
Fish welfare and tolerance to stress are increasingly important issues in the aquaculture industry (Norambuena et al., 2015). As it is important to minimise the use of anti-bacterial drugs in fish farming because of the risk of developing environmental drug resistance, an alternative approach could be to introduce naturally occurring immunostimulating agents. The immune system is classified into innate (non-specific) and adaptive (specific), and enhancement of the immune system appears to be a promising method of preventing fish diseases (Sakthivel et al., 2015). The non-specific immune system of fish is the first line of defence against invading pathogens and is more important for fish than for mammals (Jian and Wu, 2003). Seaweeds contain several immunologically active substances including polysaccharides such as sodium alginate and carrageenan that have been reported previously to modify the activity of some components of the immune system and increase protection against certain diseases (Harikrishnan et al., 2011a; Harikrishnan et al., 2011b). In addition, marine macroalgae can contain sulphated polysaccharides that are increasingly appreciated as highly bioactive molecules with, among other activities, apparent immunostimulatory properties that could prove beneficial if seaweeds are supplemented in relatively small amounts in functional feeds (Abdel-Latif et al., 2022; Bakky et al., 2022; Bahnamiri et al., 2023).
As mentioned above, while the use of alginate in fish feed as a binder was common practice in the pioneering days of Norwegian fish farming when the use of wet or moist feed was common (Storebakken and Austreng, 1987), the potential beneficial effects of seaweed-derived polysaccharides in fish have also been known for more than a quarter of a century. For instance, the positive effect of Ulva meal or wet feed including alginate, on the immune status of fish such as gilthead seabream or Atlantic salmon was first reported 25 years ago (Gabrielsen and Austreng, 1998). Administration by injection of carrageenan from the red seaweed Chondrus crispus and sodium alginate from the brown seaweed Undaria pinnatifida increased macrophage phagocytic activity and resistance against bacterial infections in common carp (Cyprinus carpio) (Fujiki et al., 1997a). Similarly, administration of carrageenan from C. crispus and sodium alginate from the brown seaweed Macrocystis pyritera by injection increased resistance to bacterial infections in grouper (Epinephelus coioides) (Cheng et al., 2007). Sodium alginate was found to enhance migration of head kidney phagocytes to the peritoneal cavity, increase phagocytic activity and, hence, to enhance the non-specific defence system of the common carp (Fujiki and Yano, 1997). Intraperitoneal injection of Ergosan, an algal extract containing alginic acid, was also observed to increase the non-specific defence response of striped snakehead (Miles et al., 2001), rainbow trout (Peddie et al., 2002), and European seabass (Bagni et al., 2005). Other studies suggested that dietary supplementation with hijiki (Sargassum fusiforme) and kajime (Ecklonia cava) appeared to enhance the non-specific immune response of juvenile olive flounder (Paralichthys olivaceus) (Kim and Lee, 2008; Kim et al., 2014). Laminaran (aka laminarin) and β-glucan obtained from the brown alga Laminaria hyperborea was shown to have immunomodulatory effects on Atlantic salmon macrophages (Dalmo and Seljelid, 1995). Macrophage activation is important in the host non-specific defence against microbial infections, thus Laminaran has the potential to serve as an immunomodulator in fish immune systems. The positive results obtained in these, and other studies, stimulated considerable interest that has resulted in a substantial body of research investigating the possible effects of polysaccharide extracts from seaweeds as bioactive ingredients in feeds for finfish. Some representative examples of such studies are presented in Table 10, and further studies that included investigation of seaweed extracts including polysaccharides are presented in subsequent Tables that focus on fish species groups.
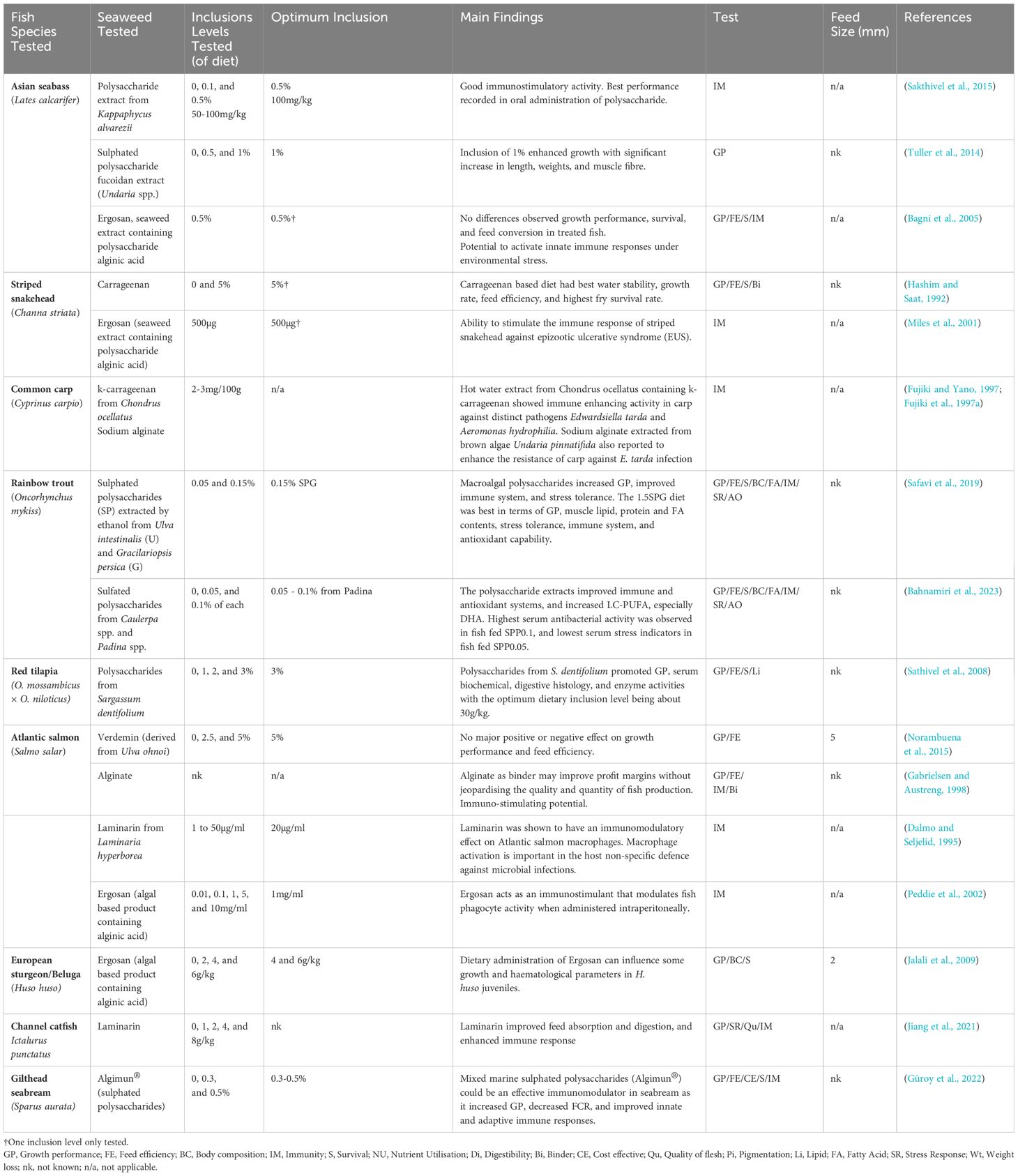
Table 10. Summary of feeding trials incorporating seaweed polysaccharides in diets as a functional feed ingredient (typically below 5% inclusion rate).
Polysaccharides extracted from brown algae including sodium alginate and the algal extracts Ergosan and laminarin have been among the most intensively studied. While these polysaccharides are non-sulphated, the polysaccharides extracted from red algae including the various carrageenan types are sulphated. In recent years, extracts of sulphated polysaccharide have received increased attention. Ethanol extracts of sulphated polysaccharides from the red alga Gracilariopsis persica and the green alga Ulva intestinalis were tested successfully in rainbow trout with the G. persica extract shown to be more effective in improving the immune system and stress tolerance (Safavi et al., 2019). Similarly, sulphated polysaccharides extracted by ethanol from brown algae Padina spp. and green algae Caulerpa spp. were tested in rainbow trout and, while both gave some benefits to the immune system, the Padina extract showed highest serum antibacterial activity and lowest serum stress indicators (Bahnamiri et al., 2023). Recently, Algimun®, a commercial mix of extracts of sulphated polysaccharides from red algae that enhance gut barrier function and green algae that modulate immune functions, was tested in gilthead seabream and shown to be an effective immunomodulator, enhancing innate and adaptive immune responses (Güroy et al., 2022). A number of studies have looked at the immunostimulatory effects of polysaccharides isolated from seaweed on Asian seabass (Lates calcarifer) as disease is recognised as a constraint on the industry. Thus, Sakthivel et al. (Sakthivel et al., 2015) looked at the effect of the red seaweed Kappaphycus alvarezii on Asian seabass and its resistance against Vibrio parahaemolyticus infection, and showed that the polysaccharide extract incorporated into the diet enhanced lysozyme activity. The dietary influence of the sulphated polysaccharide fucoidan, isolated from the brown algae Undaria pinnatifida on the growth of juvenile seabass, was investigated and found to have growth promoting effects (Tuller et al., 2014).
5 Feed trials
5.1 General considerations regarding the utilisation of marine macroalgae in feeds for finfish
5.1.1 Dietary requirements of herbivorous, omnivorous and carnivorous fish
Finfish nutrition is limited by several constraints associated with the aquatic environment and the adaptations acquired to inhabit it. Finfish have evolved to exploit virtually every conceivable niche, feeding strategy, trophic level and habitat (Trushenski et al., 2006). Protein and lipid requirements are usually higher for carnivorous fish and lower for herbivorous and omnivorous fish. Generally, protein requirements for smaller fish are higher with protein requirements decreasing as fish increase in size (Craig and Helfrich, 2002). Carnivorous fish species are very efficient at using dietary protein and lipid for energy, but less efficient at using dietary carbohydrates although simple carbohydrates such as glucose and dextrose are more bioavailable than complex carbohydrates. Carnivorous fish fed complex carbohydrate diets exhibit prolonged hyperglycemia similar to diabetic mammals, followed by hepatic degeneration from glycogen accumulation. Herbivorous and omnivorous finfish experience similar hyperglycemic effects following ingestion of digestible carbohydrates, although the duration is much shorter because of greater clearance rates (Stone et al., 2003).
5.1.2 Incorporation of macroalgae in feeds
As detailed above, seaweeds have been recognised as sources of dietary protein (Valente et al., 2006; Dantagnan et al., 2009; Ragaza et al., 2015; Araujo et al., 2016), amino acids and fatty acids (Wahbeh, 1997; Soler-Vila et al., 2009), vitamins and minerals (Ruperez, 2002; Watanabe et al., 2002; McDermid and Stuercke, 2003), colouring agents, and binders (Gabrielsen and Austreng, 1998; Soler-Vila et al., 2009; Asino et al., 2011; Mansilla and Avila, 2011), as well as other biologically active phytochemicals (Mustafa et al., 1995; Nakagawa, 1997). Consequently, a large number of studies have been conducted on a variety of finfish species to evaluate seaweeds as major feed ingredients often as an alternative to dietary FM (Wahbeh, 1997; Wan et al., 2019), but also as valuable sources of bioactive compounds (Holdt and Kraan, 2011) with the potential to be used as feed additives. Various seaweed species have been evaluated with the most commonly tested species including the red algae Gracilaria spp., Palmaria palmata, Porphyra/Pyropia spp., Kappaphycus alvarezii, the brown algae Ascophyllum nodosum, Macrocystis pyrifera, Laminaria digitata, Sargassum spp., Hizikia fusiformis, Undaria pinnatifida, and the green algae Ulva spp. and Enteromorpha spp. The majority of these studies suggested that small amounts of seaweed may be used at up to 15% as partial substitutes for dietary FM with no adverse effects, and with promising impacts including enhancement of growth performance, feed efficiency, body composition, survival and disease resistance (Wassef et al., 2001; Wassef et al., 2005; Valente et al., 2006; Dantagnan et al., 2009; Ergun et al., 2009; Güroy et al., 2010; Ragaza et al., 2015; Valente et al., 2015; Wan et al., 2016). Higher inclusion levels of 20% to 50% have also been tested using seaweed species including Ulva spp., Enteromorpha intestinalis, Kappaphycus alvarezii, Gracilaria spp., Porphyra spp., and Sargassum spp. with variable results.
Despite the many studies and various seaweed species investigated, some results appear contradictory and show that the responses of fish to dietary seaweed inclusion are dose-dependent and species-specific in terms of both seaweed and finfish species, and that nutritional value and digestibility differ among seaweed species (Mustafa et al., 1995; Pereira et al., 2012). However, in addition, the variations likely reflect the fact that seaweeds have been used in feeds in different ways. Seaweeds can be included in fish diets as fresh seaweed as complete feed in certain herbivorous/omnivorous fish species, as dried meals prepared from whole seaweeds and incorporated into pelleted feed formulations, or as a range of extracts, which may or may not be concentrates of specific bioactive compounds, that can be supplemented to feeds. Therefore, the success of dietary inclusion of seaweed in finfish diets depends not only on the fish species and the seaweed species, but also on the form in which the seaweed is delivered and whether it is supplemented as freshly harvested seaweed, dry meals, or extracts (Tacon et al., 1990). Prepared feeds can simplify feed management and eliminate potential pathogens, but complete information regarding nutrient requirements is required to prevent ineffective or detrimental diets. Prepared feeds can also have issues around palatability. When manufacturing prepared feeds, consideration must also be given to water stability, size, and density (floating or sinking) of pellets (Güroy et al., 2007). While nutritional content is paramount, processing, manufacturing, storage, and mode of delivery are also critical factors in finfish nutrition (Trushenski et al., 2006).
Supplementary Tables 1–4 summarise the key aspects and results of a considerable number of the studies that have investigated the impacts of seaweeds and seaweed-derived products and extracts on finfish, with the data set grouped systematically based on whether the fish species are classified as marine or freshwater, and inhabiting cold- or warm-water. As such, the Tables are largely stand-alone and, therefore, the accompanying text does not aim to further describe comprehensively all the studies, but simply to provide some discussion of the key aspects or points of the research on a per fish species basis.
5.2 Marine finfish
5.2.1 Cold water species
See Supplementary Table 1 for a list of studies presented in the following sections.
5.2.1.1 Atlantic salmon (Salmo salar, Linnaeus, 1758)
The Atlantic salmon is an anadromous species, living in freshwater as juveniles, but migrating to sea as adults before returning to rivers to spawn. The worldwide production of farmed Atlantic salmon in 2022 exceeded 2.86 mt (FAO, 2024) with the major markets being in Japan, the European Union, and North America. Salmonids are unable to synthesise carotenoids and farmed Atlantic salmon diets are supplemented mostly with astaxanthin and canthaxanthin to produce colouration similar to that of wild fish. Moroney et al. (Moroney et al., 2014) examined the effect of the red seaweed Palmaria palmata at 5, 10 and 15% inclusion levels in farmed Atlantic salmon diets, on the quality of fillets. P. palmata contains a variety of fat-soluble carotenoids, including lutein, α- and β-carotene, and chlorophyll a/b (Barbosa et al., 1999). Results showed that the dietary addition of P. palmata had no effect on pH, lipid oxidation, and microbiological status and that, at 5% inclusion, P. palmata may offer enhancement of overall acceptability without negatively impacting texture, odour, and oxidation flavour. The authors stated that dietary P. palmata enhanced fillets with a yellow/orange colour and may prove to be a natural pigment alternative to canthaxanthin in Atlantic salmon feeds. A similar study carried out by Wan et al. (Wan et al., 2016) using P. palmata found that inclusions of 5 – 15% may have beneficial effects on liver health of Atlantic salmon and that 5% inclusion also had positive effects on body lipid content. The author stated that the use of P. palmata meal could serve as a functional component in formulated Atlantic salmon diets.
Studies have also been carried out to look at the potential of using commercially available seaweed products such as Verdemin, a dry algae meal derived from the green seaweed Ulva ohnoi. This product was assessed at 2.5 and 5% inclusion levels in Atlantic salmon diets to look at effects on fish performance, feed efficiency, lipid metabolism, and final product quality (Norambuena et al., 2015). Results suggested that although Verdemin had limited potential for inclusion in feed with respect to overall performance, an increase in n-3 LC-PUFA content was found in the whole body of fish fed the algae product. Another proprietary blend of seaweeds (OceanFeed™) was assessed at 15% inclusion in Atlantic salmon diets with the results indicating that inclusion of seaweed may change the lipid profile of Atlantic salmon in a favourable way (Wilke et al., 2015). Other experiments carried out using OceanFeed™ suggested that Atlantic salmon fed this blend of several, unspecified species of seaweed appeared healthier, more active, and with improved flavour and texture (Kraan and Mair, 2010), and had enhanced immune response (Palstra et al., 2018). In addition, dietary inclusion of up to 10% AquaArom, a product from Laminaria spp., increased feed intake and growth, and improved plasma antioxidant capacity in Atlantic salmon (Kamunde et al., 2019).
In order to utilise some of the by-products from fishing which represent valuable resources of high-quality protein and energy to the industry, a method for preparing wet feed using a binder made from the brown seaweed Ascophyllum nodosum was developed (Gabrielsen and Austreng, 1998). The results showed that jellified wet feed was superior to dry feed as it remained water-stable for 24 h, with very little nutrient leaching and that the wet alginate feed sunk 80% slower than dry feed. The authors stated that a significantly higher level of lysozyme in the fish fed the wet feed implied a potential immuno-stimulating effect of alginate and that a switch to this technology may increase profit margins without jeopardising the quality or quantity of fish production. Extracts from the brown seaweed Laminaria hyperborea were shown to have an immunomodulatory (i.e. immune system modification in terms of enhancing phagocytic and bactericidal activity) effect on Atlantic salmon head kidney macrophages (Dalmo and Seljelid, 1995). Macrophage activation is important in the host non-specific defence against microbial infections and, therefore, laminarin may have the potential to serve as an immunomodulator in fish systems (Peddie and Secombes, 2002). The potential of seaweeds or seaweed extracts as immunomodulators has been further investigated in Atlantic salmon fed Pyropia columbina and Gracilaria chilensis with, in particular, 10% G. chilensis increasing antiviral activity (Lozano et al., 2016), while cryo-concentrates of these red seaweeds were shown to modulate Mx antiviral protein in white blood cells (Lozano et al., 2019). Recently, dietary supplementation with 1.2% of a methanolic extract of the red seaweed Asparagopsis taxiformis stimulated innate immune response and modulated gut microbiota in Atlantic salmon (Thépot et al., 2022).
5.2.1.2 Atlantic cod (Gadus morhua, Linnaeus, 1758)
Atlantic cod inhabit cold temperate waters and have a wide distribution on both sides of the Atlantic Ocean. The farming of Atlantic cod has a long history starting in Norway in the 1880s with the artificial rearing of cod. This work was the basis for the development of modern cod aquaculture (Rosenlund and Skretting, 2006) although it is still a small industry with around 6000 tonnes produced in Norway in 2023 (Fish Farmer, 2023). Terrestrial plant proteins such as soybean products have been included in the diets of several finfish species (Drew et al., 2007; Goda et al., 2007; Hansen et al., 2007; Borquez et al., 2010). However, in carnivorous species such as cod their inclusion may be limited because of amino acid and fatty acid imbalances and the presence of complex carbohydrates and antinutritional factors (Angell et al., 2016). The partial replacement of FM with the red seaweed Porphyra umbilicalis (nori) was investigated in diets for Atlantic cod (Walker et al., 2009). Results indicated that replacing 30% FM with P. umbilicalis had no adverse effects on growth or feed conversion and could provide a suitable FM replacement in diets for juvenile cod. Recently, supplementing diets for juvenile cod with 10% of the sea lettuce Ulva rigida had no negative impacts on growth or intestinal microbiota whereas 10% dietary inclusion of egg wrack Ascophyllum nodosum reduced growth and altered gut morphology and microbiome (Keating et al., 2021).
5.2.2 Warm water species
See Supplementary Table 2 for a list of studies presented in the following sections.
5.2.2.1 Milkfish (Chanos chanos, Forsskål, 1775)
Milkfish, the only living species in the family Chanidae, is native to the tropical and parts of the Northern sub-tropical Indo-Pacific, and has been used for centuries as a food fish in Southeast Asia and Oceania. Milkfish is one of the most important aquaculture species in Asia and is a successful industry in the Philippines, Taiwan and Indonesia with smaller scale production in some Pacific islands despite the fact that, until recently, farming was based largely on the capture of wild fry (FAO, 1997). Nursery operations in milkfish producing countries vary according to established cultural practices and the fish are on-grown in ponds, pens, and cages. The farming of milkfish has grown rapidly to a very large industry and, in 2022, total global production reached almost 1.2 mt (FAO, 2024).
Traditionally feeding practices for milkfish production consisted of natural food or a combination of phytoplankton and green seaweeds e.g. Enteromorpha intestinalis, Cladophora spp., and Chaetomorpha linum. Commercial feeds for milkfish were developed in the 1980s and became almost exclusively used, and were further developed in the 1990’s for cage and pen culture both in marine and inland waters. Feeds are now manufactured commercially in the form of starter, grower, and finisher diets. Seaweeds of the green and brown strain Kappaphycus alvarezii, red seaweed Gracillaria gigas and brown seaweed Sargassum spp. were examined as sources of carbohydrate and for their effects on growth, survival, digestibility, and chemical composition of juvenile milkfish (Aslamyah et al., 2016). The seaweeds were fermented using bacteria and yeast and supplemented at a level of 20% in feed. Sargassum spp. inclusion showed highest growth rate while inclusion of the green strain of K. alvarezii resulted in the highest survival rate and digestibility of carbohydrates and protein. The growth rate, survival rate, and feed digestibility showed excellent results with all types of seaweed tested. The authors stated that the addition of seaweed as a source of carbohydrate and binder in feed showed positive responses in terms of growth performance of juvenile milkfish.
5.2.2.2 Family Mugilidae (mullets)
Mullets of the Family Mugilidae family are found worldwide in the coastal waters of the tropical and subtropical zones of all seas and some species are also found in freshwater. Mugilid mullets are now the third most farmed marine finfish with production totalling over 291,000 tonnes in 2020 (FAO, 2022). The flathead grey mullet Mugil cephalus (Linnaeus, 1758) is a very important aquaculture species in the Mediterranean especially Egypt, Italy, and Tunisa, and in Southeast Asia, Taiwan, Japan, and Hawaii. Full-scale commercial production of flathead grey mullet is not yet common and most of the fry used in commercial aquaculture are collected from the wild, especially in the Eastern and Southern Mediterranean, Saudi Arabia and Gulf States, and Southeast Asia (Saleh, 2008). In well-fertilised ponds, grey mullet fry and fingerlings can find enough food for growth, but supplemental feed may also be added. Mullets are an important component in the energy flow through ecosystems due to their low position in the food chain and, for the same reason, mullet readily accept supplemental feeds such as by-products of plant origin (Larson and Shanks, 1996).
A study was conducted to evaluate algae meal as an inexpensive and locally available feed ingredient in a supplementary diet for flathead grey mullet (Wassef et al., 2001). The green alga Ulva lactuca at 10, 15, 20 and 25% inclusion levels was tested with results suggesting an optimum level of 20% for best growth performance and nutrient utilisation. In addition to growth enhancement, 20% inclusion resulted in improved muscle firmness and quality, as shown from the examination of fish muscle ultrastructure by electron microscopy at the end of feeding trial. A further study tested a mixture of Ulva spp. and the microalga Nannochloropsis oculata at inclusion levels of 21, 28, 35 and 42% on growth and feed utilisation (El-Dahhar et al., 2014). The authors recommended that best weight gain in mullet fingerlings was obtained at 21% inclusion of mixed Ulva spp. and microalgae. An inclusion level of 20–21% was higher than for most other seaweed species tested in finfish diets, however, mullet have thick-walled gizzard-like segments in their stomach along with a long gastrointestinal tract that enables them to feed on detritus. More recently, inclusion of the red seaweed Gracilaria arcuata at low concentrations (1.0–1.5% of diet) improved growth performance and lowered serum glucose and lipid concentrations in M. cephalus (Akbary et al., 2020).
5.2.2.3 Gilthead seabream (Sparus aurata, Linnaeus, 1758)
Gilthead seabream are common throughout the Mediterranean and are also found along Eastern Atlantic coasts, from the UK to the Canary Islands. Gilthead seabream are one of the most important and successfully farmed Mediterranean fish species. In coastal lagoons, seabream are generally reared with mullet, European seabass, and European eels and fed either under intensive systems with commercial pellets or under semi-extensive systems where the available natural food is supplemented with additional feed. Total global production of farmed gilthead seabream was approximately 344,400 tons in 2022 (Zoli et al., 2023; FAO, 2024), with major production in Turkey and the European Union (EU) where Greece is the largest producer followed by Spain (Pauly and Froese, 2025; Zoli et al., 2023).
A feeding trial was carried out to evaluate the red seaweed Pterocladia capillacea and the green seaweed Ulva lactuca as feed additives in gilthead seabream diets at 5, 10 and 15% inclusion levels (Wassef et al., 2005). The results showed that dietary supplementation of P. capillacea meal at 10% significantly improved feed intake, palatability, and protein intake with survival rate of fish highest among all the treatments. A diet of 10% P. capillacea also resulted in significantly higher percentage weight gain and daily weight gain among the levels tested, however increasing levels to 15% resulted in inferior growth performance and feed utilisation efficiency. In contrast, dietary U. lactuca meal supplementation at 5% produced significantly higher percentage weight gain, daily weight gain and survival rate. Lipids are one of the important nutrients for carnivorous finfish and dietary lipids are a source of energy, EFA and fat-soluble vitamins for finfish (Nakagawa, 1997), and optimal dietary lipid levels are important for growth and quality of the final product. Another study investigated the effects of Ulva rigida meal on growth performance, feed utilisation, and body composition of gilthead seabream at different levels of dietary lipid and showed that a low level (4%) of Ulva rigida could be used in diets without causing any adverse effects (Emre et al., 2013). Addition of an equal mix of Ulva rigida, Gracilaria gracilis and Fucus vesiculosus at a total incorporation rate of 5% of diet was reported to have anti-genotoxic properties in gilthead seabream as it reduced oxidative damage to DNA (Pereira et al., 2019) and showed genoprotection against formalin, oxytetracycline, and cyclophosphamide (Marques et al., 2020). On its own, G. gracilis at 5% of diet increased growth and feed efficiency, and improved bacterial resistance in S. aurata (Passos et al., 2021a). Recently, a multispecies mix of the brown seaweeds, wracks, including Lobophora spp., Dictyota spp., Asparagopsis taxiformis, Cymopolia spp., Hypnea spp., Laurencia spp., and Stypocaulon spp. was explored in feeds for gilthead seabream. The multispecies mix at 7% of diet did not compromise growth or survival, and had no adverse effects on biochemical composition, lipid and fatty acid profiles, or antioxidant status (Galindo et al., 2023). Similarly, channelled wrack Pelvetia caniculata could be added at up to 10% of the diet without negatively affecting biochemical composition or fatty acid profile of S. auratus (Antunes et al., 2023).
Iodine is an essential trace element and an integral component of thyroid hormones, with multiple biological functions, and marine fish are a rich source of iodine in the human diet. Iodine deficiency is still a major problem in some parts of the world, and this may be improved by eating fish fed seaweed with high iodine content as it has been shown that iodine carries over from plant to fish to humans (Schmid et al., 2003). Fish easily absorb iodine from the diet through the digestive tract, but can also take it up from the surrounding water via the gills (Hunn and Fromm, 1966). Seaweed species of the genus Laminaria are the strongest iodine accumulators among all living systems (Holdt and Kraan, 2011). A study was undertaken to test the efficacy of various dietary iodine supplement forms, including the iodine-rich brown algae Laminaria digitata at 10% inclusion level, on the growth performance of gilthead seabream and the nutritional value of the fillets (Ribeiro et al., 2015). Results indicated dietary incorporation of L. digitata as the most efficient form in which to fortify seabream fillets with iodine and resulted in a 6.5-fold increase (0.84 mg/kg) in fillet iodine content over levels found in fish fed the control treatment. The authors stated that dietary inclusion of L. digitata was an effective and natural strategy to increase iodine content in gilthead seabream fillets, with no negative effects on growth performance, feed conversion, or nutrient utilisation (Ribeiro et al., 2015). Thus, it may be that iodine-rich species of seaweed, particularly L. digitata, could be useful supplements to increase dietary iodine levels in fish feeds.
Intensive aquaculture practices involve rearing fish at high densities, exposing fish to environmentally stressful conditions such as sub-optimal dissolved O2 levels (hypoxia). All aerobic organisms rely on the presence of O2 to obtain energy and low environmental O2 represents a major physiological challenge (Barbour and Turner, 2014). A study evaluated the role of 5% dietary supplementation of heat-treated red seaweed Gracilaria vermiculophylla and green seaweed Ulva lactuca on the metabolic profile and antioxidant capacity in juvenile gilthead seabream, during and after an acute hypoxic event (Magnoni et al., 2017). Results suggested that the antioxidant properties of heat-treated seaweeds may have a protective role against oxidative stress in fish with practical implications for the aquaculture industry since susceptibility of fish to biotic and abiotic stressors may be a restrictive factor in fish production in intensive aquaculture conditions.
5.2.2.4 Large yellow croaker (Larimichthys crocus, Richardson, 1846)
The large yellow croaker is a carnivorous marine species that inhabits coastal waters and estuaries. It is found in the Northwest Pacific Ocean from central Vietnam to South Korea and Japan, Yellow and East China Seas. Large yellow croaker is an economically important cultured marine fish species in China because of its high nutritive value and good flavour. It is cultured in sea cages with trash fish the main food source but, at times of shortage in fishery resources, trash fish may not meet the demand from the expanding culture of this species and, therefore, commercial feeds are needed that will produce maximum growth of juvenile large yellow croaker in culture while ensuring good water quality. Global aquaculture production of this species was approximately 254,000 tonnes in 2020 (FAO, 2022). A study was carried out to determine the effects of supplementation of the green seaweed Enteromorpha prolifera at inclusion levels 5, 10 and 15% on growth performance, survival, and body composition of large yellow croaker (Asino et al., 2011). The results indicated that although the feed efficiency ratio and protein retention was best at 5%, inclusion, up to 15% was feasible without affecting growth and still maintained high survival rate. The authors stated that E. prolifera has emerged as a cheap and highly abundant alternative ingredient for croaker diets.
5.2.2.5 European seabass (Dicentrarchus labrax, Linnaeus, 1758)
The European seabass is a carnivorous marine fish that shows demersal behaviour and is tolerant of a wide range of temperatures (5-28°C) and salinities (3‰ to full-strength sea water) making it able to frequent coastal inshore waters, estuaries, and brackish water (Pawson et al., 2007). The European seabass was the first non-salmonid marine species to be commercially cultured in Europe and, at present, is an important commercial fish widely cultured in Mediterranean areas. Turkey, Greece, Egypt, Spain, and Croatia are the biggest producers (shown in descending order), with world production of farmed European sea bass in excess of 293,000 tonnes in 2022 (FAO, 2024).
Although a hardy species, European seabass are subject to a wide range of diseases under rearing conditions, which can have important effects on commercial production. Stress is considered an important factor co-responsible for disease outbreaks (Mourente et al., 2005). Evaluation of the suitability of the red seaweed Pterocladia capillacea and the green seaweed Ulva lactuca in seabass diets found that both species could potentially be used as an additional feed component for enhancement of seabass fry performance, nutrient composition, and stress resistance, especially when subjected to transportation from hatchery to weaning ponds/tanks (Wassef et al., 2013). The findings suggested ideal inclusion of dietary P. capillacea and U. lactuca at 10% and 5%, respectively.
A study was carried out to evaluate inclusion of the red seaweeds Gracilaria bursa-pastoris and Gracilaria cornea and the green seaweed Ulva rigida as dietary ingredients on the performance, nutrient utilisation, and body composition of European seabass juveniles (Valente et al., 2006). The results suggested that G. bursa-pastoris and U. rigida could be included up to 10% as no negative effects on growth performance, nutrient utilisation, or body composition were observed, but that G. cornea inclusion should be limited to 5%. Further studies examined the use of seaweed-supplemented diets to improve growth performance and health by testing the hypothesis that enhancing the innate immune and antioxidant responses using seaweeds can alter fish metabolism and, therefore, growth in European seabass (Peixoto et al., 2016b; Peixoto et al., 2016a). Results revealed that supplementation of Gracilaria spp. at 7.5% and a mix of Gracilaria spp., Fucus spp., and Ulva spp. at 2.5% each, had no effect on fish growth and metabolism. However, evidence of altered innate immune and antioxidant responses in fish fed the seaweed supplemented diets was observed.
Further studies with Gracilaria spp. in D. labrax showed that an aqueous extract at 5% of diet increased resistance to pathogen infection and up-regulated immune and antioxidant pathways without compromising growth (Peixoto et al., 2019). Similarly, dietary inclusion of 2.5% Gracilaria gracilis as a dried meal increased plasma lysozyme and intestinal acid goblet cells, enhanced immune response, and improved antioxidation responses (Passos et al., 2021b). Another study reported that G. gracilis at 8% could partially replace FM in diets for D. labrax with no negative effects on growth or feed efficiency, although lower digestibility values for protein and energy suggested processing of the algae may be required (Batista et al., 2020). In a subsequent study, the same group showed that 8% inclusion reduced gut microbial diversity, which may be interpreted as a negative impact, but that G. gracilis also promoted bacteria capable of outcompeting pathogens (Ferreira et al., 2022). The improvement of nutritional value of macroalgae by pre-treatment was tested in D. labrax in a study where the green seaweed U. rigida was subjected to various processing methods with solid-state fermentation shown to improve feed efficiency without negatively impacting growth (Fernandes et al., 2022).
A further study in European seabass investigated the immunomodulatory activity of sodium alginate using the proprietary blend Ergosan, derived from the brown alga Laminaria digitata (Bagni et al., 2005). The authors stated that using the blend increased lysozyme activity with potential to activate innate immunity in seabass particularly under conditions of immunodepression related to environmental stress.
5.2.2.6 Epinephelus spp. (groupers)
Groupers are widespread in warm and temperate waters of all the seas and oceans of the planet (Pierre et al., 2008). Culture of groupers is widespread in Asia and the Pacific, and they are important species for mariculture due to their fast growth, popular taste, and high nutritional and economic value (Harikrishnan et al., 2011a). In 2020, global production of Epinephelus spp. of groupers was over 226,000 tonnes (FAO, 2022). As groupers, like all farmed fish, are prone to diseases, particularly viral diseases and parasitic infestations (Fujiki et al., 1997b), prevention of disease and maintenance of fish health have been of concern. Therefore, so far, studies on seaweed supplementation to groupers have tended to use extracts and generally low levels of supplementation with a focus on fish health as well as growth and feed efficiency.
As mentioned previously, laminarin is an abundant polysaccharide in brown algae that is found mainly in the fronds of Laminaria spp. and can reach up to 32% of dry weight, although its abundance varies with season and habitat (Rioux et al., 2007). A study was conducted to evaluate the effects of laminarin on growth performance, and immunological and biochemical parameters in orange-spotted grouper Epinephelus coioides (Hamilton, 1822) (Yin et al., 2014). In the trial, grouper were fed a basal diet supplemented with 0.5, 1.0 and 1.5% levels of laminarin. Feed intake was found to be significantly higher at 0.5% inclusion and the results showed that laminarin significantly improved growth rate and feed efficiency and can enhance the immune response. Similar results were found in another trial studying the effects of dietary administration of sodium alginate from the brown seaweed Lessonia nigrescens on growth and resistance of orange spotted grouper fingerlings to Streptococcus spp. and iridovirus (Yeh et al., 2008). Results showed that the addition of sodium alginate at 1% and 2% significantly increased the survival rate of grouper suggesting that sodium alginate administration may enhance fish resistance to bacterial and viral pathogens.
A study on orange-spotted grouper examined the immunostimulatory effects of sodium alginate, from the brown seaweed Macrocystis pyritera and iota (i)-carrageenan from the red seaweed Chondrus crispus and resistance against Vibrio alginolyticus, a viral disease common in the intensive culture of grouper (Cheng et al., 2007). Fish were injected intraperitoneally with sodium alginate and i-carrageenan at 10, 20, and 30 mg/kg and results showed that fish that received sodium alginate at 20 mg/kg or i-carrageenan at 30 mg/kg showed enhanced non-specific immune response and resistance to V. alginolyticus infection. A further study examined survival, growth, and innate cellular and humoral responses in brown-marbled grouper Epinephelus fuscoguttatus (Forsskål, 1775) with similar results to the authors’ previous work (Cheng et al., 2008). In the latter study, fish were fed diets containing the polysaccharides sodium alginate and kappa (k)-carrageenan at 5, 10 and 20 g/kg. Results showed that survival of fish fed a diet containing sodium alginate at 10 g/kg or less, or k-carrageenan at 5 g/kg was significantly higher than fed fish the control diet and suggested enhancement of the non-specific immune response and resistance to V. alginolyticus infection. Dietary sodium alginate administered at 1.0 g/kg was also found to enhance immunity and resistance against Streptococcus spp. and iridovirus in brown marbled grouper, E. fuscoguttatus (Chiu et al., 2008). Similar results were found in a study on kelp (or longtooth) grouper Epinephelus bruneus (Bloch, 1793), which showed that dietary administration of sodium alginate at 1.0 or 2.0 g/kg can enhance innate immunity and disease resistance against Streptococcus iniae (Harikrishnan et al., 2011b). These promising results obtained in the above studies suggested that further research into dietary inclusion of higher levels of some seaweeds in groupers is warranted.
5.2.2.7 Golden pompano (Trachinotus ovatus, Linnaeus, 1758)
Golden pompano belongs to genus Trachinotus within the carangidae family and is widely farmed in China because of its rapid growth, taste, and tolerance to environmental changes, with total production being 160,000 tonnes in 2020 (FAO, 2022). In a recent study, the brown seaweeds Saccharina japonica and Undaria pinnatifida, and the red seaweeds Gracilaria lemaneiformis and Porphyra haitanensis were tested as feed supplements in T. ovatus at 2% of diet (Xie and Niu, 2022). There were no differences in feed efficiency or survival between the macroalgae, but growth performance was best with P. haitanensis and U. pinnatifida, and P. haitanensis also improved antioxidant status.
5.2.2.8 Asian seabass (Lates calcarifer, Bloch, 1790)
The Asian seabass (Lates calcarifer), also known as barramundi in Australia, is a catadromous fish that lives in freshwater and enters salt water to spawn. It has a wide physiological tolerance for salinity and inhabits a wide variety of marine, brackish, and freshwater habitats including coastal waters, estuaries, streams, and lakes (Rimmer and Russell, 1998). Aquaculture of this species commenced in the 1970s in Thailand, and rapidly spread throughout much of Southeast Asia with production totalling almost 106,000 tonnes in 2020 (FAO, 2022).
The red seaweeds Kappaphycus alvarezii and Eucheuma denticulatum and the brown seaweed Sargassum polycystum at 5% inclusion level were evaluated in feeds for juvenile Asian seabass (Shapawi and Zamry, 2016). The study indicated that all three species had high potential to be alternative ingredients in juvenile seabass diets without deleterious effects on growth performance, feed utilisation, survival, and body composition, as well as serving as a feed binder. In terms of feed utilisation and body composition, S. polycystum showed better results, whereas K. alvarezii demonstrated a good water stability profile. The authors further recommended S. polycystum as a seaweed to be included in fish diets, particularly due to its natural availability in coastal waters in the area of study. The results were consistent with a previous study that had suggested that the red seaweed K. alvarezii had great potential as an ingredient in fish feed based simply on its nutritional content and availability (Shapawi et al., 2015). Dietary inclusion of the red seaweed Gracilaria pulvinata (3%) and the brown seaweed Lobophora variegata (5%) both had positive effects on growth in Asian sea bass (Udayasoundari et al., 2016; Morshedi et al., 2018).
5.2.2.9 Red seabream (Pagrus major, Temminck & Schlegel, 1843)
Red seabream, also known as red porgy, is a marine carnivorous species inhabiting depth ranges from 300 to 700 m in the Northwest Pacific, but is also found in the Eastern Atlantic and Western Mediterranean. It is a particularly important food fish species in Japan, where it has been farmed for over 50 years with production peaking at almost 88,000 tons in 1999 and, although production declined in the 2000s, the level has stabilised at around 60,000 tonnes annually since 2014 (Kato, 2023). An early study examined the merits of seaweed as an effective source of protein in red seabream diets and stated that supplementation with 5% of the green seaweed Ulva pertusa regulated lipid accumulation and mobilisation (Nakagawa and Kasahara, 1986). Although the exact mechanism was not known, the assumption was that seaweed supplementation enhanced physiological activities and accordingly lipid metabolism improved. A later study also investigated the effects of U. pertusa at 5% inclusion level in red seabream diets, along with the red seaweed Porphyra yezoensis and the brown seaweed Ascophyllum nodosum (Mustafa et al., 1995). Results showed that seaweed increased weight gain of seabream and tended to increase feed efficiency and muscle protein deposition. Feeding P. yezoensis showed the most pronounced effects on growth and energy accumulation, followed by A. nodosum and U. pertusa. A similar trial found that replacing FM with 5% A. nodosum increased body weight and muscle protein in red seabream, although 10% led to lower growth and feed efficiency (Nakagawa et al., 1997). The results were consistent with an earlier study that examined dietary supplements of the brown seaweeds Undaria pinnatifida and A. nodosum at 5 and 10% inclusion levels (Yone et al., 1986). Results showed best growth rate, feed efficiency, and muscle lipid content at 5% U. pinnatifida, followed by 5% A. nodosum, 10% U. pinnatifida, the control diet, and finally 10% A. nodosum. Results suggested the practical efficacy of using algae at low level inclusion as a feed additive for effective nutrient utilisation in cultured seabream.
Seaweeds contain a wide variety of complex polysaccharides in their rigid cell walls that can disturb digestion when included in fish diets and removal of this rigid cell wall may ensure the successful availability of all nutrients. Spheroplasts are plant cells from which the cell wall has been removed, and their isolation requires three kinds of enzymes capable of degrading constituent polysaccharides (Araki et al., 1994). Spheroplasts may be suitable material as a feed ingredient as removal of the cell walls improves digestion and absorption. Positive results were found with spheroplasts in diets of red seabream, with growth performance, survival and nutrient retention significantly higher in fish fed spheroplasts of Porphyra spp (Kalla et al., 2008). More recently, dietary inclusion of the red seaweed Gracilaria lemaneiformis at up to 3% improved growth performance and reduced liver lipid deposition in P. major (Xuan et al., 2019).
5.2.2.10 Olive flounder (Paralichthys olivaceus, Temminck & Schlegel, 1846)
The olive or Japanese flounder, as it is sometime known, is a carnivorous marine species distributed in the Western Pacific Ocean from Japan to the South China Sea. In aquaculture, the olive flounder is an economically important fish species in China, Japan, and Korea (Kim et al., 2014). Global aquaculture production amounted to approximately 47,000 tonnes in 2020 (Kim et al., 2023). In some countries, there has been extensive use of a wide range of antimicrobial compounds to treat infectious bacterial diseases in aquaculture and, consequently, the occurrence of antibiotic-resistant bacteria associated with fish diseases has become a serious problem. Streptococcus iniae has emerged as a leading fish pathogen in aquaculture operations worldwide, with infections reported in at least 27 species of cultured or wild fish around the world (Zhang et al., 2017). A number of feed trials have examined the effect of seaweeds as dietary supplements on growth performance, immune response and resistance to disease in olive flounder. One such experiment incorporating the brown seaweed Hizikia fusiformis (Sargassum fusiforme) found that a 6% inclusion had a positive effect on the growth performance, enhanced the non-specific immune response, and improved resistance to Streptococcus iniae (Pham et al., 2006). Further studies on the brown seaweeds Sargassum fusiformis and Ecklonia cava investigated antioxidant activity and immune responses, and found that low level inclusions of 4-6% appeared to enhance the non-specific immune response in juvenile olive flounder (Kim and Lee, 2008; Kim et al., 2014).
Another study looked at the effects of 3, 6 and 9% supplementation levels of the red seaweed Eucheuma denticulatum on growth performance, carcass composition, and blood chemistry of juvenile olive flounder (Ragaza et al., 2015). Fish showed highest growth performance and feed utilisation efficiency at 3% inclusion of E. denticulatum, while higher supplementation levels of 6% and 9% resulted in a progressive decline in growth performance and feed efficiency. No indication of feed rejection by the fish during the trial was noted and supplementation of seaweed did not alter carcass composition of the fish. Results suggested that E. denticulatum at low levels can be efficiently utilised by olive flounder and can promote optimum growth and feed utilisation.
5.2.2.11 Siganus species (rabbitfish and spinefoots)
The family Siganidae, with 28 species in the genus Siganus, are widely distributed in the Indo-Pacific region (Pauly and Froese, 2025). Each Siganus species can have a plethora of common names that generally include “rabbitfish” and “spinefoot”, sometimes interchangeably. The names used below are based on the most commonly used and/or those used by the authors. Siganus species are popular food fish and currently obtained largely from capture fisheries. However, the high demand in Southeast Asia, and consequent overfishing and decline in wild populations, has prompted research into Siganus spp. as new candidate species for aquaculture. In addition, Siganus species can attain large size, maintain high reproduction in captivity, withstand overcrowding, tolerate low dissolved oxygen levels, and have high market acceptability. Siganus species are cultivated in many different systems such as tanks, sea pens, and floating cages as well as in brackish water ponds (Li et al., 2008).
Although they can be fed formulated feed or trash fish, Siganus species have been studied particularly in relation to dietary seaweeds as they are herbivores, in the wild grazing mainly on seaweeds. The use of fresh live red seaweeds Gracilaria lichenoides and Eucheuma cottonii against formulated diets e.g. commercial pellet, crumble, and frozen fish, were tested in the diets of white-spotted rabbitfish Siganus canaliculatus (Park, 1797) (Tacon et al., 1990). Seaweeds were fed by placing a known weight of living algal biomass (sufficient for 5 to 7 days) in a submersible plastic feeding basket at the bottom of the cage. In contrast to the formulated diets, the live seaweeds G. lichenoides and E. cottonii displayed very poor feed efficiency. Best growth and feed efficiency was observed for S. canaliculatus fed a dry in-house crumble containing 31% crude protein. Recently, testing the preference of S. canaliculatus for different macroalgae showed that the order of consumption was Padina crassa > Gracilaria lichenoides > Ulva reticulata, associated with protein and lipid contents, and inversely to carbohydrate contents, and that co-feeding increased consumption (Latuconsina et al., 2023). An earlier study had shown that feeding whole Enteromorpha prolifera and Gracilaria lemaneiformis to S. canaliculatus inhibited stomach protease activity, but this may be compensated by increased intenstinal length and mucosal folds (Xie et al., 2018).
Dried green seaweed Enteromorpha spp. at inclusion levels of 10%, 20%, and 30% was investigated as a dietary ingredient for S. canaliculatus (Yousif et al., 2004). Results showed that growth performance and feed utilisation efficiency decreased with increasing seaweed inclusion, although carcass protein was not affected by the different treatments, while lipid content was observed to increase in the group of fish supplemented with fresh Enteromorpha spp. Studies on the stomach contents and food preferences in siganids revealed that, among the many different algal species and vascular plants eaten, there was a high presence and preference for Enteromorpha spp. Bryan (1975) stated that this preference was not directly related to the calorific value of the algae, but was related to the thin and crispy texture of the thalli. Another study examined the use of G. lemaneiformis as a low-cost feed for S. canaliculatus (Xu et al., 2011). Firstly, as a partial replacement protein to reduce FM inclusion, and secondly, as a source of carbohydrate to reduce the amount of supplemental starch in formulated feeds and minimise competition with human food sources. Reduced growth and feed utilisation were observed in fish fed G. lemaneiformis compared with the FM group, possibly due to the lower levels of some EAA such as methionine or tyrosine and the relatively high inclusion (33%) of G. Lemaneiformis. The authors stated that, despite the relatively slower growth and lower feed utilisation efficiency obtained, analysis of the biochemical composition of G. lemaneiformis, along with the immunity and digestibility in fish fed the G. lemaneiformis diet, indicated that incorporation in the diet for S. canaliculatus was feasible. Addition of NSP-degrading enzymes to diets containing 12% of either G. lemaneiformis, Ulva prolifera or U. pertusa mitigated negative effects on growth, and enhanced innate immune capability and disease resistance in S. canaliculatus (Xie et al., 2019).
A study was carried out to develop an appropriate feeding protocol for brackish water pond culture of orange-spotted spinefoot Siganus guttatus (Bloch, 1787) (Rabia, 2016). To evaluate growth and survival, fish were fed three different diets: a commercial feed; the filamentous green seaweed Chaetomorpha linum: or a diet consisting of both the commercial feed and Chaetomorpha linum. The commercial feed used had a proximate composition of 28% crude protein, 6% crude lipid, 6% fibre, 12% ash, and 12% moisture. Orange spotted spinefoot fed the commercial diet only and the combination diet of commercial + C. linum showed significantly higher growth rate at 0.44 g day-1 and 0.43 g day-1 respectively. The results verified that co-feeding with filamentous algae was feasible in terms of growth, which, in turn, may reduce feed costs as algae can be simultaneously grown in empty ponds. Supplementing a pelleted diet with 25–50% fresh Ulva lactuca gave similar growth rates to fish fed only the commercial diet and, despite low growth, fish fed 100% Ulva produced a brighter body colour (Sulaeman et al., 2022). Replacing a pellet diet for marbled spinefoot Siganus rivulatus (Forsskål & Niebuhr, 1775) with fresh Ulva fasciata or Enteromorpha flaxusa resulted in reduced growth with 100% replacement, but 50% replacement had positive effects on growth with E. flaxusa > U. fasciata (Abdel-Aziz and Ragab, 2017). Inclusion of Pavina pavonica at up to 10% in diets for S. rivulatus improved growth and feed efficiency, and immune responses against P. anguilliseptica bacteria (Monier et al., 2022). The impact of 11 seaweed species from within Chlorophyta, Phaeophyta and Rhodophyta on immune responses when incorporated at 3% in diets was assessed in mottled rabbitfish Siganus fuscescens (Houttuyn, 1782) (Thépot et al., 2021a). Asparagopsis taxiformis and Dictyota intermedia boosted humoral and cellular innate immune defences including increased haemolytic activity, while Ulva fasciata gave positive effects on fish innate immune responses.
5.2.2.12 Senegal sole (Solea senegalensis, Kaup, 1858)
Senegal sole is a demersal marine flatfish and a member of the Soleidae family of true soles that traditionally inhabited the Eastern Atlantic from south of the UK and Ireland to the Canary Islands, but is now also found throughout the Mediterranean. While farming started around 50 years ago in Portugal and has expanded to other Mediterranean countries, especially Spain, aquaculture of Senegal sole is generally extensive and limited by poor reproduction of captive breeders. The inclusion of the green seaweed Ulva rigida and the brown seaweed Undaria pinnatifida was tested at 10% of diet in Senegal sole and it was found that U. rigida could replace plant meals without major effects on growth or nutrient utilisation, whereas U. pinnatifida impaired growth (Moutinho et al., 2018). Subsequently, a series of studies investigating dietary inclusion of 5% Ulva ohnoi showed that, while it had modest and conflicting effects on growth (Vizcaíno et al., 2019; Sáez et al., 2020), it had generally positive effects on intestinal morphology, function, and microbiota (Vizcaíno et al., 2019; Cerezo et al., 2022), and immune response and resistance to pathogens (Fumanal et al., 2020; Cerezo et al., 2022), as well as lowering muscle lipid while favouring retention of EPA and DHA, and improving fillet texture and colour (Sáez et al., 2020).
5.2.2.13 Black seabream (Acanthopagrus schlegelii, Bleeker, 1854)
The black seabream, or black porgy, is a marine omnivorous fish, widely distributed in the coastal waters of the South China Sea and around Japan and Korea. It is a popular food fish and this has impacted stocks, and fisheries have declined resulting in several stock enhancement programmes in China, Japan, and Korea, and it is currently being actively studied as a new candidate species for aquaculture in China (Li et al., 2022). One study looked at the feasibility of using the red seaweed Gracilaria lemaneiformis as a feed ingredient in juvenile black seabream diets (Xuan et al., 2013). Growth performance, carcass composition, activities of digestive enzymes, and enzymes associated with amino acid metabolism and transaminases activities were assessed using 5, 10, 15, and 20% inclusion levels of G. lemaneiformis. Growth performance in terms of weight gain did not decrease with inclusion levels up to 15% but, at 20% inclusion, significantly poorer growth performance of the fish was observed. All diets were accepted by the fish indicating that seaweed inclusion levels had no adverse effect on palatability. The large-scale cultivation of G. lemaneiformis has been encouraged in Chinese coastal waters to meet the demands of the agar and abalone culture industry, which could make its practical use feasible. An early study investigated the inclusion of 5% Ulva spp. as a feed additive in black seabream diets and suggested that dietary algae accelerated the assimilation of ascorbic acid (vitamin C) in fish and improved the physiological conditions relating to vitamin C nutrition (Nakagawa, 1997). A later feed trial investigated the effects of spheroplasts produced from Porphyra spp. as a feed additive in diets for black seabream (Khan et al., 2008). Experimental diets with inclusion levels of 1%, 3%, and 5% were formulated to determine the optimum incorporation level for best growth, carcass composition, and utilisation. Results revealed that growth performance (weight gain and specific growth rate) remained at the same level in all the dietary groups tested, nevertheless survival, nutrient utilisation, and retention (feed efficiency, protein efficiency ratio, protein retention rate, and lipid retention rate) were significantly higher in fish fed 3% spheroplasts.
5.2.2.14 White spotted snapper (Lutjanus stellatus, Akazaki, 1983)
The white-spotted snapper is a marine carnivore widely cultured in the temperate West Pacific ranging from southern Japan to Taiwan and the area of Hong Kong, due to its high economic value (Akazaki, 1983). A study assessed the feasibility of using the green seaweed Ulva lactuca, the red seaweed Gracilaria lemaneiformis, and the brown seaweed Sargassum horneri as feed ingredients and partial replacement for FM in diets for juvenile white spotted snapper based on growth performance, body composition, and enzyme activities (Zhu et al., 2016). The results concluded that incorporation of U. lactuca at 5% had beneficial effects on growth performance, but supplementation at higher levels of 10, 15 and 20% reduced fish performance and physiological status. Further studies based on a quadratic regression model of weight gain suggested maximum incorporation of G. lemaneiformis and S. horneri at 16.4 and 15%, respectively (Zhu et al., 2017). All diets were readily accepted by the fish and the authors concluded that the results provided important information regarding the potential application of these seaweed species as valuable alternative feed sources for fish culture and for reducing the use of FM.
5.2.2.15 Thick-lipped mullet (Chelon labrosus, Risso, 1827)
Thick-lipped mullet live in the northeastern Atlantic Ocean from Iceland to Senegal and Cape Verde, including the Mediterranean Sea and the southwestern Black Sea. It is considered to have interesting aquaculture potential due to its capacity to adapt to a wide range of salinities, including freshwater, and as a candidate for re-stocking waters (Khemis et al., 2013; García-Márquez et al., 2021). An early study in thick-lipped grey mullet carried out experiments to effectively replace 9% and 18% of dietary FM with the red seaweed Porphyra purpurea (Davies et al., 1997). Results revealed that the use of P. purpurea in the diet had limitations as increasing levels of seaweed resulted in reduced growth, although, the authors stated that its use as a partial substitute for dietary FM may prove to be cost effective.
5.2.2.16 Barred knifejaw, (Oplegnathus fasciatus, Temminck & Schlegel, 1844)
Parrotfish are a group of about 95 species traditionally regarded as family Scaridae, but now often considered a subfamily Scarinae of the wrasses (Westneat and Alfaro, 2005). In 1967, the Aquaculture Research Institute in Japan became the first in the world to hatchery produce Oplegnathus fasciatus (or Scaradon fasciatus) that is regarded as a type of parrotfish but is also known as barred knifejaw, striped beakfish or rock bream, and is one of the emerging aquaculture species in Asian countries and its high commercial value makes it a promising aquaculture species (Kim et al., 2009). There have been few feed trials on the use of seaweeds as partial replacement for FM in barred knifejaw diets. However, a trial was carried out to determine the effects of dietary supplementation of the brown seaweeds Hizikia fusiformis and Ecklonia cava showed that both species of seaweed could enhance the innate immune responses of O. fasciatus during their growth stage (Song et al., 2011).
5.2.2.17 Dusky kob/Japanese meagre (Argyrosomus japonicus, Linnaeus, 1758)
Argyrosomus japonicus is a member of the Sciaenidae family inhabiting coastal waters in the Indo-Pacific region around Africa, India, Australia, China, and Japan. As A. japonicus is a fast-growing species with high consumer demand, it is currently a new candidate species for mariculture. Dusky kob fingerlings were fed diets containing 0 - 20% meal produced from unspecified Ulva species of green seaweed as partial replacement for FM (Madibana et al., 2017; Madibana et al., 2020). Although dietary Ulva generally reduced growth and feed efficiency, it could be incorporated at 5% without major negative effects on physiology or fillet fatty acid composition.
5.3 Freshwater finfish
5.3.1 Cold water species
See Supplementary Table 3 for a list of studies presented in the following sections.
5.3.1.1 Rainbow trout (Oncorhynchus mykiss, Walbaum, 1792)
Rainbow trout are native to the cold-water rivers and lakes of the Pacific coasts of North America and Asia. Since 1874 it has been introduced to waters on all continents, except Antarctica, for recreational angling and aquaculture purposes. The production of rainbow trout has increased since the 1950s, especially in Europe and more recently in Chile (Pauly and Froese, 2025). This is primarily due to increased inland production in countries such as France, Italy, Denmark, Germany, and Spain to supply domestic markets, and mariculture in cages in Norway and Chile for the export market (D’Agaro et al., 2022). Chile is currently the largest producer, and other major producing countries include Norway, France, Italy, Spain, Denmark, USA, Germany, Iran, and the UK. Total worldwide production in 2022 was approximately 769,000 tonnes in freshwater, with an additional 232,000 tonnes produced in sea cages (FAO, 2024).
Rainbow trout are aggressive, opportunistic feeders eating a variety of aquatic and terrestrial invertebrates and small fishes depending on age and size of fish, size of food item, and habitat occupied (Refstie et al., 2000). Supplementation of rainbow trout diets with Integrated Multitrophic Aquaculture (IMTA)-cultivated red seaweed Gracilaria vermiculophylla was tested at 5% and 10% to assess impacts on growth performance, flesh quality traits, body composition, and iodine content (Valente et al., 2015). Inclusion appeared possible up to 5% and the iodine content of the flesh doubled, but higher inclusion levels resulted in significantly smaller fish. A longer feeding trial may result in higher iodine content levels in fillets as a significant increase of flesh iodine was reported in seabream fed the brown seaweed Laminaria digitata, but only after a feeding period of 118 days (Ribeiro et al., 2015). It may be that the magnitude of increase in iodine content is dependent, not only on the seaweed species used, but also on duration of the trial. A further study on IMTA G. vermiculophylla tested inclusion levels of 5 and 10% to investigate effects on growth and feed efficiency, carotenoid concentration in skin and muscle, immunological parameters, and intestinal morphology (Araujo et al., 2016). Results indicated that protein intake was similar among the groups tested, but 10% inclusion resulted in lowest protein retention. The authors concluded that inclusion of IMTA G. vermiculophylla at up to 5% enhanced the innate immune response without compromising growth performance or nutrient utilisation, but a higher level of 10% affected gut morphology and impaired growth. However, the higher level significantly increased skin carotenoid content. Dietary inclusion of G. pygmaea at 9% had no detrimental effects on growth, antioxidant status, and gut morphology of rainbow trout (Sotoudeh and Mardani, 2018). Similarly, inclusion of 5% of the red seaweeds Gracilariopsis persica and Hypnea flagelliformis and the brown seaweed Sargassum boveanum in diets for rainbow trout had no adverse effects on growth, health, or flesh quality, and may improve immune and antioxidant responses (Vazirzadeh et al., 2020; Vazirzadeh et al., 2022).
Protein digestibility of G. vermiculophylla, Ulva spp., Sargassum muticum, and Porphyra dioica was investigated at inclusion levels of 30% in rainbow trout diets (Pereira et al., 2012). G. vermiculophylla, Ulva spp. and P. dioica were produced in land-based IMTA systems while S. muticum was wild harvested. The IMTA production method resulted in increased protein content of seaweed when compared to wild specimens and to wild-harvested S. muticum. Protein composition of the tested seaweeds reached 43.1% in P. dioica, followed by 33% and 35% in G. vermiculophylla and Ulva spp., respectively. The lipid content of these algae was low and ranged between 1.4% and 1.5%. S. muticum had the lowest protein and lipid contents at 15.7% and 0.8%, respectively. In general, the results showed that the protein digestibility of Porphyra dioica, G. vermiculophylla and Ulva spp., in rainbow trout were high (76–88%) and within the range of values reported previously for plant ingredients in trout (Borquez et al., 2010). Another study reported that the final body weights of rainbow trout fed the red seaweed Porphyra dioica at 5 and 10% inclusion levels showed no significant differences, while at 15% a significant depression in final weight from the control was recorded (Soler-Vila et al., 2009). The noted positive effect on pigmentation of the flesh from the addition of P. dioica may be of considerable interest to the organic salmonid farming industry. A further study reported that dietary supplementation of U. lactuca and Enteromorpha linza at 10% resulted in poorer growth and feed utilisation of rainbow trout when compared to those of the control group (Yildirim et al., 2009).
Dietary inclusion levels of 1.5%, 3%, and 6% of the brown seaweed Macrocystis pyrifera were evaluated to test the effects on body fatty acid composition in rainbow trout (Dantagnan et al., 2009). The findings showed inclusion of 3% and 6% elevated flesh levels of LC-PUFA, particularly EPA and DHA, after 124 days of feeding. Composition of the final product is particularly important in aquaculture given that fish consumption has been recommended as a good source of nutrients and, therefore, inclusion of M. pyrifera may represent a way to increase the nutritional value of farmed freshwater fish as a source of essential n-3 LC-PUFA for humans. Similarly, dietary supplementation with 1–2% Saccharina latissima had no detrimental effect on the growth of rainbow trout, but had a lipid-lowering effect without compromising fillet EPA and DHA levels (Ferreira et al., 2020). An earlier study evaluated the use of M. pyrifera as a dietary supplement for intensive rainbow trout culture and showed that inclusion of the seaweed did not improve levels of protein or lipids in diets, but that the contribution of minerals was significant with 6% inclusion (Mansilla and Avila, 2011). The percentage of PUFA in the muscle, mainly EPA, DHA and linoleic acid, increased at both 3% and 6% inclusion levels. Studies have shown that seaweed and seaweed-derived bioactive extracts affected gut morphology such as villi length and thickness, and induced changes in the digestion and absorption of nutrients in rainbow trout (Araujo et al., 2016).
Starvation prior to slaughter is common practice in farmed trout in order to empty the gastrointestinal tract and prevent fish spoilage. Starvation in salmonids is also practiced in order to hold back stocks to regulate supply in accordance with demand and also to reduce excess lipid levels in fish (Zhang et al., 2007). Guroy et al. (Güroy et al., 2010) looked at the effects of dietary inclusion of Ulva rigida at 5% and 10% on the weight loss and body composition of rainbow trout during periods of starvation. The authors stated that the addition of dietary U. rigida can reduce weight loss by enhancing mobilisation of lipid reserves in rainbow trout and it may be that low level dietary supplement of seaweed in aquafeeds can have economic advantages in terms of reducing weight loss in fish when subjected to a short-term fasting period as used prior to harvest. The stimulatory properties of the algal-based product, Ergosan, on localised and systemic innate immune responses was investigated in rainbow trout following intraperitoneal administration (Peddie et al., 2002). The authors concluded that Ergosan, which is composed of 0.002% unspecified plant extract, 1% alginic acid from Laminaria digitata, and 98.998% algal-based carrier, acted as an immunostimulant and modulated fish phagocyte activity when administered. The phagocytic process is an important mechanism for the destruction of extra-cellular bacterial pathogens in fish (Hong et al., 2003).
5.3.1.2 Arctic charr (Salvelinus alpinus, Linnaeus, 1758)
Arctic charr (or char), a salmonid fish species with circumpolar distribution and both landlocked and diadromous populations, is the northernmost distributed and cold-adapted freshwater fish. It is an important species in intensive aquaculture being a schooling fish tolerant of high-density conditions. The global production of Arctic charr in 2019 was around 8500 tonnes, with the main producers located in Scandinavia and Canada (Helgadóttir et al., 2021).
Schmid et al. (Schmid et al., 2003) investigated whether iodine supply in humans may be improved by consuming freshwater fish fed seaweed with high iodine content. To test the theory, Laminaria digitata, which contains large amounts of iodine, 4 g per kg of dry matter, was supplemented at 0.8% in the diet of Arctic charr. The author reported that the use of dietary L. digitata induced a 4-fold increase in iodine content of the fillet of charr with no negative influence on meat quality parameters such as pH, colour, and firmness of the flesh, and humans eating the fish had elevated levels of iodine.
5.3.1.3 European or Beluga sturgeon (Huso huso, Linnaeus, 1758)
Sturgeon is the common name for the 27 species of fish belonging to the family Acipenseridae. Sturgeons are native to subtropical, temperate, and sub-Arctic rivers, lakes, and coastlines of Eurasia and North America (Pauly and Froese, 2025). The European or Beluga sturgeon (Huso huso) is found primarily in the Caspian and Black Sea basins, and occasionally in the Adriatic Sea. The species is heavily fished for the female’s valuable roe (caviar) and they are particularly vulnerable to overexploitation and other threats, including pollution and habitat fragmentation. Growth performance, body composition, survival, and haematological changes were determined in European sturgeon juveniles fed diets supplemented with Ergosan, the seaweed extract containing the polysaccharide alginic acid, at 0, 2.0, 4.0, and 6.0 g kg_1 inclusion levels (Jalali et al., 2009). Alginates affect oxygen transference through the lymphocyte cell membrane, metabolic activity, stimulate peritoneal leucocytes and improve disease resistance in fish (Fujiki and Yano, 1997), and the study concluded that dietary administration of Ergosan influenced some growth and haematological parameters in sturgeon juveniles.
5.3.1.4 Persian sturgeon (Acipenser persicus, Borodin, 1897)
Persian sturgeon are also found in the Caspian and Black Seas and migrate up the Volga, Kura, Araks, and Ural Rivers to spawn. Like Beluga sturgeon, Persian sturgeon have been subjected to excessive fishing for both roe and flesh, and are currently listed as critically endangered. Dietary inclusion of 5–10% of the red seaweed G. persica had no negative effects on growth or health of Persian sturgeon, and increased carotenoid levels in fillet, which may beneficially effect flesh colour (Adel et al., 2021). In addition, dietary G. persica enhanced blood biochemical indices, antioxidative capacity, and mucus immune responses.
5.3.2 Warm water species
See Supplementary Table 4 for a list of studies presented in the following sections.
5.3.2.1 Grass carp (Ctenopharyngodon Idella, Valenciennes, 1844)
Grass carp Ctenopharyngodon Idella are a large, rapidly growing, herbivorous freshwater teleost in the Cyprinid family. It is native to the Pacific Far East, with its range stretching from Vietnam north to the Russian border. Of all farmed finfish species, grass carp is produced in the greatest volume and world production in 2020 reached almost 5.8 mt (FAO, 2022). Brown seaweeds, wracks, have been investigated recently in feeds for grass carp (Galindo et al., 2022). Inclusion of a multispecies wrack mix including Lobophora spp., Dictyota spp., Asparagopsis taxiformis, Cymopolia spp., Hypnea spp., Laurencia spp., and Stypocaulon spp. at 7% of diet showed no negative effects on growth, digestive capacity and biochemical composition, and also increased liver catalase activity. The above Phaeophycea-rich multispecies wrack mix, and a monospecific wrack (95% Lobophora spp.), both at 7% inclusion, also lowered fat deposition.
5.3.2.2 Nile tilapia (Oreochromis niloticus, Linnaeus, 1758)
The farming of Nile tilapia (Oreochromis niloticus), and other cichlid species, is the most widespread type of aquaculture in the world with farmed tilapia production statistics having been recorded for 135 countries and territories on all continents (FAO, 2014). In 2020, a total of over 4.5 mt of farmed tilapia were produced with 98% produced in inland/freshwater systems, and around 108,000 tonnes produced in coastal aquaculture systems (FAO, 2022). There have been a number of studies on the partial substitution of FM by seaweeds in Nile tilapia diets using red, brown and green seaweeds and inclusion levels ranging from 5 to 30%, with results suggesting a positive effect on growth performance, survival, body composition, feed efficiency, digestion, immunity, and reduction in production costs.
Several studies used the green algae Ulva spp. as a supplement in Nile tilapia diets. Two studies showed that when Ulva rigida was included at 5% of diet, growth performance, feed efficiency, nutrient utilisation, and body composition were improved (Ergun et al., 2009; Khalafalla and El-Hais, 2015). Previous work had reported that dietary inclusion of U. rigida meal could be considered a useful dietary ingredient for Nile tilapia if supplemented at the correct level, and that inclusion up to 10% had no negative effects on growth performance, feed utilisation, and body composition (Güroy et al., 2007), and similar results were reported with 10% inclusion of Ulva spp (Marinho et al., 2013; Silva et al., 2014). Other studies reported that a level of 15% could improve growth performance without any adverse effects on feed efficiency or survival rates (El-Tawil, 2010), and that U. rigida could be included up to 20% in tilapia diets, with no detrimental effects (Azaza et al., 2008). Ulva spp. are rich in vitamin C and it has been suggested that this suppresses lipid peroxidation (oxidative degradation of lipids) and improves lipid metabolism (Garcia-Casal et al., 2007), which may result in changes in nutrient retention and fish carcass composition (Nakagawa, 1997; Nakagawa et al., 2000).
Other green seaweed species have been tested for inclusion in Nile tilapia diets. For example, growth and protein utilisation efficiencies increased with 5% inclusion of the green algae Hydrodictyon reticulatum, but growth was reduced with higher dietary inclusion levels (Appler, 1985). However, the benefit of using this species of seaweed is that it can be produced at little cost as H. reticulatum is a by-product growing in treated effluents. The author stated that, as a sole seaweed meal, the protein level of 26% may be too low to be a major protein source for small tilapia with 30–50% dietary protein requirements. However, this protein level may provide the required protein for fish above 35 g with lower 20–25% protein requirements and, therefore, H. reticulatum may prove to be a useful cheap source of protein, particularly for larger fish. Enteromorpha intestinalis was trialled at 15% inclusion with no adverse effects on growth, nutrient utilisation, or body composition (Aquino et al., 2014), and the highest growth and reduction in production costs was observed at 20% inclusion of E. intestinalis (Siddik et al., 2015). It was also reported that 12.6% inclusion of Rhizoclonium riparium var. implexum could replace the equivalent of 45% soybean meal in Nile tilapia diets, resulting in significantly higher final average body weight, protein efficiency ratio, and better feed conversion ratio (Cabanero et al., 2016).
The brown seaweeds Cystoseira barbata at up to 15% inclusion (Güroy et al., 2007) and Sargassum spp. at 5% inclusion (Yangthong et al., 2014) were supplemented to Nile tilapia diets with no negative effects on growth performance, feed utilisation, and body composition, and were found to improve carcass quality. The red algae Porphyra yezoensis was trialled at 15 and 30% in Nile tilapia diets with results indicating that the lower level (15%) improved growth and feed utilisation (Stadtlander et al., 2013). The effect of 10% inclusion of Porphyra dioica and Gracilaria vermiculophylla as alternative protein sources was also evaluated (Silva et al., 2014). While 10% inclusion of P. dioica showed no negative consequences on growth performance or body composition, 10% inclusion of G. vermiculophylla had a negative effect on palatability, and reduced fish feed intake and growth performance. The nutrient digestibility of four seaweeds species P. dioica, G. vermiculophylla, Ulva spp., and Sargassum muticum at 30% supplementation were investigated in juvenile tilapia and rainbow trout diets with overall results suggesting that trout seemed to better digest Gracilaria, whereas tilapia did better with Ulva spp. and Sargassum spp (Pereira et al., 2012). Other studies have also tested red seaweeds with mixed results. Addition of G. arcuata to feeds at 7, 14, and 21% of diet reduced growth and feed efficiency at all levels, although tilapia fed 7% were least affected (Younis et al., 2018). However, a positive benefit was that carcass lipid was reduced as inclusion level increased. In contrast, inclusion of Halymenia floresii at 10, 20, 30, and 40% of diet improved growth, feed efficiency, and haematological parameters as the level of inclusion of the macroalgae increased (Raja et al., 2022). In another study in tilapia, a liquid seaweed extract (True AlgaMax, TAM) prepared from the red seaweeds Jania rubens and Pterocladia capillace along with the green algae Ulva lactuca, was supplemented to feeds at 0.5, 1.0. 1.5, and 2% of the diet (Ashour et al., 2020). Increasing levels of TAM up to 2% of the diet improved growth and feed efficiency, and also enhanced non-specific immunity of O. niloticus challenged with Aeromanas hydrophila.
5.3.2.3 Common carp (Cyprinus carpio, Linnaeus, 1758)
Common carp belong to the family Cyprinidae, which is considered the largest family of freshwater fish (Rahman, 2015). Carp are the most cultivated species throughout the world and are particularly popular in Asia and Europe. Global farmed production was approximately 4.24 mt in 2020 (FAO, 2022). Artificial feed-based intensive monoculture production is carried out in irrigation reservoirs, ponds, cages, and tanks, or recirculation systems (Pauly and Froese, 2025). As an omnivore, the common carp can effectively utilise feed that includes plant material and, therefore, a study investigated the efficiency of Ulva rigida as a feed ingredient on growth, feed intake, nutrient utilisation, and body composition at inclusion levels of 5, 10, 15, and 20% (Diler et al., 2007). Best growth performance was achieved at 5% supplementation with the poorest recorded at 20% leading the authors to suggest that dietary inclusion of U. Rigida at 5 to 15% could replace wheat meal in carp diets.
5.3.2.4 Indian major carps (Catla catla, Labeo rohita, and Cirrhinus mrigala, Hamilton, 1822)
Indian major carp occur throughout northern and central India, Bangladesh, Nepal, Myanmar, and Pakistan and have been introduced in many other countries, including Sri Lanka, the former USSR, Japan, China, the Philippines, Malaysia, Nepal, and some African countries (Jena, 2006a; Jena, 2006b). Amongst the group of cyprinids known as the Indian major carps the three species, catla (Catla catla), rohu (Labeo rohita) and, to a lesser extent, mrigal (Cirrhinus mrigala), are of greatest economic importance. In 2020, the global production of catla and rohu reached 3.54 and 2.49 mt, respectively (FAO, 2022).
As fish depend on innate immunity for protection against disease, the effect of dietary supplementation of carrageenan from red seaweed on the innate immune response was examined to determine the effect on growth, haematology, biochemistry, and innate immunity in the Indian major carp, L. rohita (Kumar et al., 2014). The findings indicated that a diet containing i-carrageenan at 10 g/kg played an important role in enhancing overall health status and innate immunity in L. rohita. Another study investigated the production of a cost effective, cheap, and efficient feed for improved carp growth using various seaweeds (Kotnala et al., 2010). The growth performance of C. catla was assessed using formulated feeds consisting of the green seaweed Chlorodesmis fastigiata and the brown seaweeds Padina tetrastomatica and Stoechospermum marginatum at 10% inclusion level. Increased growth was noted in fish fed all seaweed supplemented diets, with maximum growth rate in terms of weight gain found with the C. fastigiata diet. A similar growth trend was noted for P. tetrastomatica, but growth was relatively low for S. marginatum. The results demonstrated that C. fastigiata and P. tetrastomatica could be used in commercial formulated feed to improve growth of Indian major carp fingerlings. Recently, it was shown that marine red seaweed Halymenia dilatate could replace 50% of dietary FM (5% of diet) in feeds for rohu and improve growth, body composition, and digestive enzymes (Manikandan et al., 2022).
5.3.2.5 Striped catfish (Pangasianodon hypophthalmus, Sauvage, 1878)
The striped catfish (or iridescent shark catfish) is an omnivorous freshwater fish and a member of the Pangasilidae family native to the rivers of Southeast Asia. Commercially, P. hypothalamus, is marketed, along with Pangasius bocourti, as “Pangasius” or other common names including swai, basa, panga, and river cobbler. Striped catfish is the most heavily farmed Pangasalid species, with the main farming area being the Mekong Delta in Southern Vietnam, with total world production exceeding 2.5 mt in 2020 (FAO, 2022). While macroalgae have not been extensively studied in Pangasius, the effects of a mixture of Ulva lactuca, Jania rubens, and Pterocladia capillacea, added to pelleted feed at inclusion rates of 1, 2, and 3%, was investigated (Abdelhamid et al., 2021). Supplementation of the seaweed mixture extract had beneficial effects on growth performance, enhanced antioxidant status, and improved innate immune biomarkers, and the authors concluded that inclusion in feeds at around 2.5% could increase immune-competency and disease resistance (Abdelhamid et al., 2021).
5.3.2.6 African catfish (Clarias gariepinus, Burchell, 1822)
The North African catfish, generally referred to as African catfish, inhabits lakes, streams, rivers, swamps, and floodplains, many of which are subject to seasonal drying. The African catfish is a major warm water aquaculture species in Africa, Asia, and more recently, Europe and Latin America (Pouomogne, 2010). Global production of all Clarius spp. of catfish totalled almost 1.25 mt in 2020 (FAO, 2024).
Two studies evaluated the impacts of the red and green seaweeds, Gracilaria arcuata and Ulva lactuca, respectively, as dietary ingredients to partially replace FM, on the growth performance, feed utilisation, and body composition of African catfish (Abdel-Wahab et al., 2016; Al-Asgah et al., 2016). Inclusions levels of 10, 20 and 30% were tested and the results revealed that African catfish fed diets supplemented with the macroalgae at 20% and 30% exhibited poor growth and feed utilisation, but that catfish could accept up to 10% dietary inclusion of either seaweed with no adverse effects on growth performance and feed efficiency. Carnivorous fish such as African catfish digest high animal protein diets efficiently but less so plant protein diets (Degani and Revach, 1991), which may explain the poor growth at high inclusion levels.
5.3.2.7 Striped snakehead (Channa striata, Bloch, 1793)
The freshwater striped snakehead Channa striata, also known as striped murrel, from the family Channidae, is distributed across Asia and Africa and has a wide range of habitats including rivers, streams, swamps, ponds, canals, lakes, and rice fields (Song et al., 2013). The snakehead is a valuable food fish of economic importance and is cultured commercially in Thailand, Taiwan, and the Philippines, with global farmed production reaching almost 20,000 tonnes in 2016 (FAO, 2018). Snakehead fish are carnivorous and normally fed on trash fish with the addition of rice bran as the fish grow. The use of seaweed as binding agents in pelleted feed for snakehead fry and their effects on growth has been tested (Hashim and Saat, 1992). Four locally sourced seaweeds, Ulva spp., Sargassum spp., Polycavernosa spp., and Gracilaria spp., as well as carrageenan from red seaweed, were evaluated at 5% inclusion level. Overall results showed the carrageenan-based diet had best water stability, best growth rate and feed efficiency as well as the highest fry survival rate. Among the seaweeds tested, the Ulva meal diet had the best water stability and gave the highest relative growth rate and feed efficiency value, while the lowest fry survival was observed with the Sargassum meal diet.
As mentioned above, a clear response in the innate immune system was reported in rainbow trout following intraperitoneal administration of the seaweed extract Ergosan (Peddie et al., 2002). Similarly, an increase in the non-specific defence response was reported in striped snakehead injected intraperitoneally with 500 µg of Ergosan, when investigating immunostimulation against epizootic ulcerative syndrome (Miles et al., 2001). Epizootic ulcerative syndrome (EUS), also known as mycotic granulomatosis or red spot disease, caused by the water mould Aphanomyces invadans is a disease of international significance (Boys et al., 2012). It is a disease of fresh and brackish water and one of the most destructive diseases affecting freshwater aquaculture in the Asia-Pacific region and Australia. Outbreaks are frequently precipitated by adverse environmental conditions, and infection usually occurs during the winter months when the host immune system is suppressed. The development and use of vaccines appears remote (Pauly and Froese, 2025) and, therefore, using seaweed extracts, mainly polysaccharides, can modify the activity of some components of the immune system and increase protection against certain diseases.
5.3.2.8 Zebrafish (Danio rerio, Hamilton, 1822)
While not a food species, zebrafish is included in this section as a very important model species for investigating physiological, biochemical, and molecular mechanisms in teleosts and vertebrate species in general. A series of studies have been performed recently to investigate the supplementation of red (Gracilaria gracilis and Halopithys incurva) and green macroalgae (Ulva intestinalis) to zebrafish feeds at 0.25, 0.50, and 1.0% of the diet with a focus on effects on antioxidant and immune functions (Hoseinifar et al., 2018; Hoseinifar et al., 2022; Rouhani et al., 2022). Supplementation of G. gracilis up to 1% up-regulated mucosal immune and antioxidant enzymes without affecting growth (Hoseinifar et al., 2018). Similarly, inclusion of H. incurva increased antioxidant enzyme activities and decreased malondialdehyde in serum and mucus, and all immune-related genes measured were upregulated in fish fed 0.5% H. incurva (Hoseinifar et al., 2022). Dietary inclusion of U. intestinalis also upregulated immune-related genes including lysozyme and Interleukin 1 beta (IL-1β), and antioxidant-related genes such as superoxide dismutase and catalase and, in addition, upregulated growth-related genes including growth hormone and insulin-like growth factor-1 (Rouhani et al., 2022). Trials have also been conducted using the brown macroalga Dictyota dichotoma that demonstrated dietary inclusion at 1% led to significantly higher skin mucus immune parameters and significant up-regulation of immune related genes compared to fish fed a diet without seaweed (Mahmoudi et al., 2022).
6 Discussion
At the Second International Conference on Nutrition (ICN2), held in Rome in November 2014, world leaders renewed their commitments to eradicating malnutrition and transforming food systems to make nutritious diets available to all the world’s citizens. It confirmed the importance of fish as a source of nutrition and health for many communities that depend on their protein and essential micronutrients. It also stressed the unique window of opportunity that fisheries and aquaculture have towards achieving healthy diets and that, with this greater awareness of the sector’s important role in nutrition, comes greater responsibility for how resources are managed (FAO, 2016). Feed is widely regarded as becoming a major constraint to the growth of aquaculture production in many developing countries. In 2022, fed aquaculture produced 69 mt, representing 73.1% of world production of all aquaculture produced animal species (FAO, 2024). Although production of non-fed species can be more beneficial in terms of food security and the environment, growth in production has been faster for fed species than for non-fed species, leading to a proportional greater demand for feed ingredients (FAO, 2024). Increasing demand for terrestrial plant proteins is a growing concern due to increasing population, along with increasing consumption (Tacon and Metian, 2008; Godfray et al., 2010), and limited resources of arable land and freshwater (Pretty, 2008), and there is now a critical role for alternative crops in securing the future supply of protein (Boland et al., 2013). Seaweeds have the potential to be used as feed additives or supplements due to their nutritional value (Hashim and Saat, 1992; Michalak et al., 2009), and various seaweed species have been evaluated for incorporation into aquafeeds. Studies have shown that small amounts of seaweed may be used as a partial substitute for dietary FM up to 15% for various finfish species. Seaweed species include, among others, the red macroalgae Gracilaria spp., Palmaria palmata, Porphyra/Pyropia spp., and Kappaphycus alvarezii, the brown algae Ascophyllum nodosum, Macrocystis pyrifera, Laminaria digitata, Sargassum spp. (Hizikia fusiformis), and Undaria pinnatifida, and the green algae Ulva spp., Enteromorpha spp., and Hydrodictyon reticulatum. Higher inclusions have also been tested in finfish diets with favourable results. These include 20% inclusion of Ulva spp. in striped mullet and Indian major carp, 20% inclusion of Kappaphycus alvarezii in milkfish, 20% Enteromorpha intestinalis, 30% Ulva spp., and 30% Gracilaria vermiculophylla in Nile tilapia, 30% Gracilaria spp. and Porphyra spp. in rainbow trout, and 30% Porphyra spp. in Atlantic cod. Despite the many studies and various seaweed species investigated some results appear contradictory and show that the response of fish to dietary seaweed inclusion is dose-dependent and species-specific, and that nutritional composition and digestibility differ between seaweed species depending on location and season of harvest (Mustafa et al., 1995; Pereira et al., 2012).
Seaweeds are regarded not only as important sources of nutrients, but also as valuable sources of bioactive compounds (Holdt and Kraan, 2011). Thus seaweeds have been recognised as sources of dietary protein (Valente et al., 2006; Dantagnan et al., 2009; Ragaza et al., 2015; Araujo et al., 2016), amino acids (Wahbeh, 1997; Soler-Vila et al., 2009), vitamins and minerals (Ruperez, 2002; Watanabe et al., 2002; McDermid and Stuercke, 2003), pigments (Soler-Vila et al., 2009; Asino et al., 2011; Mansilla and Avila, 2011), and other biologically active phytochemicals (Mustafa et al., 1995; Nakagawa, 1997). A number of authors have reported that supplementing fish feed with seaweed meals has resulted in enhanced growth rates and diet utilisation, and improved flesh quality and disease resistance (Wassef et al., 2001; Wassef et al., 2005; Valente et al., 2006; Dantagnan et al., 2009; Ergun et al., 2009; Güroy et al., 2010; Ragaza et al., 2015; Wan et al., 2019). Further studies have indicated favourable use as a binder (Penaflorida and Golez, 1996). Maintenance of health benefits, taste, and other quality characteristics are important to processor and consumers preferences. In order to meet the consumer preference for red colouration in salmon flesh, synthetic keto-carotenoid pigments such as astaxanthin or canthaxanthin are often used as pigments in pelleted feed. Public health concerns prompted the European Commission to reduce the permitted level of such pigments to 25 mg/kg from the previous maximum level of 80 mg/kg (Nickell and Bromage, 1998). Colouration of flesh in salmonids using seaweeds such as Gracilaria vermiculophylla (Araujo et al., 2016) and Palmaria palmata (Moroney et al., 2014) as natural pigment sources may enhance the potential of seaweed inclusion in fish feed and could perhaps replace or reduce artificial pigments currently used by the industry. Pigmentation from dietary carotenoids is considered a vital aspect of commercial feed formulation and fish management.
Seaweeds have been cited as appropriate alternative protein sources for finfish (Aziz et al., 2013) with protein quality similar to traditional protein sources, i.e. marine FM. However, on a whole biomass basis, seaweeds contain insufficient concentration of protein, specifically insufficient EAA, to meet the requirements of most finfish. The main limitation for the use of seaweed protein in fish feed is the concentration of EAA on a whole basis, not the quality of the total amino acids or protein. In short, the large proportion of non-amino acid material, e.g. indigestible carbohydrates, fibre, and ash, dilutes the high-quality protein of seaweed. Ash contents are made up of external and internal salts and can constitute between 20-50% of dry weight (Angell et al., 2016). The processing method for soybeans provides a model for the production of concentrated protein products that may be used for seaweed species. After extraction of lipids, residual soybean biomass (soybean meal) is further processed to concentrate protein by the removal of non-protein components or by the direct extraction of proteins to form soy protein concentrate (SPC) (Chiesa and Gnansounou, 2011). For most seaweeds, the aim would be to concentrate protein by a factor of 75-300% to provide a protein source comparable to SPC.
6.1 Key points
● Studies have shown that small amounts of seaweeds up to 15% may be used as partial substitutes for dietary FM. Seaweeds contain proteins, lipids, vitamins, minerals, pigments, polysaccharides, and dietary fibres, although the types and abundance of these compounds vary within and between seaweed species (Dawczynski et al., 2007; Mansilla and Avila, 2011) and, therefore, the true value of seaweeds as feed resources has to be assessed on a species by species basis.
● The composition of seaweeds varies considerably, depending upon environmental conditions, location, life-cycle stage, and season of harvest and it may be that optimising culture conditions for selected seaweed species could produce improved nutritional components for future use in feeds for farmed finfish. The fact that there are large variations in the main nutritional components of seaweeds such as proteins, lipids, fatty acids, and ash suggest it is impossible to rule out the future use of seaweeds as major sources of components for aquaculture feeds (Slaski and Franklin, 2011).
● Analysis of the quality and concentration of protein is critical in determining the nutritional value of seaweed biomass if it is to be used in its whole form as an ingredient. Any new protein source requires to be assessed for the provision of the most-limiting essential amino acid on a whole weight basis relative to the requirements of the targeted fish species (Fleurence, 1999; Harnedy and FitzGerald, 2013).
● The response of fish to dietary seaweed inclusion is dose-dependent and species-specific (Mustafa et al., 1995; Pereira et al., 2012). Protein and lipid requirements are usually lower for herbivorous and omnivorous fish than for carnivorous fish species, and are higher for fish reared in high-density, recirculating aquaculture systems than in low-density pond aquaculture systems (Craig and Helfrich, 2002). Carnivorous fish species are very efficient at using dietary protein and lipid for energy, but less efficient at using dietary carbohydrates.
● Lipids, especially those rich in the n-3 LC-PUFA, DHA and EPA, are the most globally limiting commodity for farmed finfish feeds (Naylor et al., 2009). Although most freshwater and diadromous fish species require generally less LC-PUFA in their diet than carnivorous marine fish (Glencross et al., 2007), the major marine resource bottleneck for aquaculture is the finite and limited availability of n-3 LC-PUFA found in marine lipids (Olsen, 2011). There is a pressing issue in terms of feed sustainability to find alternative, cost effective, sources of marine oils, and EPA and DHA in particular.
● As the fish farming industry is now increasingly replacing EPA and DHA-rich FO with plant-based oils, part of the health benefits that are associated with a high fish intake such as protection against cardiovascular diseases, could be lost (Diler et al., 2007; Ergun et al., 2009). Seaweed supplementation may alter the fatty acid profile of fish in a manner that results in health benefits to the consumer (Wilke et al., 2015). The n-3 LC-PUFA, EPA and DHA, are essential dietary components for many fish and humans alike, and whether they are sourced from marine animals or from seaweed is relatively unimportant if the cost is competitive (Slaski and Franklin, 2011).
● The use of IMTA-produced seaweeds to partially replace FM in aquafeeds is advantageous mainly due to reduced operational costs and increased protein content (Marinho et al., 2013; Silva et al., 2014). The extraction and/or concentration of protein from seaweeds will be an important goal in their development as alternative sources of dietary protein for finfish (Angell et al., 2016).
● While the potential of using algae as a feedstuff depends on the costs involved in production and harvesting (Xie et al., 2019), the low-level inclusion of marine macroalgae in aquafeeds may have economic advantages. Fish farming relies on safe supplies of feeds, and the entire finfish aquaculture industry depends on the development and documentation of a wider range of sustainable raw materials for future cost-efficient feed production (Rosenlund and Skretting, 2006). Seaweed products would need to be price competitive with vegetable proteins if viewed as sources of protein. Seaweed products will have to have value as a source of micronutrients and/or value-added factors in order to find a niche, particularly in salmonid feeds. Therefore, the use of seaweed in finfish diets may satisfy the increased consumer demand for natural, health-promoting products (Slaski and Franklin, 2011).
● Prevention of fish disease through the stimulation of non-specific immune response by natural compounds is a potential solution for development of sustainable antibiotic-free aquaculture (Sakthivel et al., 2015). Seaweeds are rich in bioactive compounds and they produce a great variety of secondary metabolites with broad spectrum biological activities (Thépot et al., 2021b). The use of natural compounds to improve the non-specific immune response of fish has been considered to be a feasible solution to developing sustainable and antibiotic-free aquaculture systems (Kim and Lee, 2008).
In the future we will need to conserve as much terrestrial plant material as possible for direct human nutrition and to increasingly rely on mariculture for the provision of animal proteins for human food (Olsen and Hasan, 2012). The current challenge facing the aquaculture industry is to identify economically viable and environmentally friendly alternatives to FM and FO on which many aquafeeds are presently and largely based (Olsen, 2011). Plant feedstuffs must provide nutritious diets for efficient growth of finfish species and produce high-quality fish flesh to confer human health benefits in a cost-effective manner with minimal environmental impact (Gatlin et al., 2007). Terrestrial plant proteins, particularly soybean products, have been included in the diets of several finfish species because of the abundance and relatively low cost of such products. However, their inclusion may be limited, particularly in carnivorous species, because of amino acid and fatty acid imbalances and the presence of complex carbohydrates and antinutritional factors (Gatlin et al., 2007).
Despite moderate protein content, seaweeds may have great potential in the animal feed sector as a functional ingredient product incorporated in feed to convey a benefit above and beyond fulfilling the basic nutritional needs. Within a broad concept of sustainable, chemical-free, and organic farming, which is highly appealing to consumers, seaweeds stand out as a potential source of natural mineral additives to replace inorganic mineral salts that are most commonly used in the animal feed industry (Shields and Lupatsch, 2012). The use of seaweed as part of a dietary fortification strategy based on macroalgae of farmed fish shows great potential in delivering tailored fish with health valuable trace minerals to consumers (Ribeiro et al., 2015). Because of the potentially highly bioactive compounds, seaweed-derived products could be considered as highly valuable and useful “micro-ingredients” for aquafeed fortification, when used at a low inclusion level (Norambuena et al., 2015; Thépot et al., 2021b).
Given the economic importance of feed, nutritionists need to develop nutritionally balanced diets using commonly available raw ingredients. Once there are reliable data on the nutrient and energy requirements of the target species, then specific feeds can be formulated and a feeding regime established (Shields and Lupatsch, 2012). As protein is the most expensive part of fish feed, it is important to accurately determine the protein requirements for each species and age/size of cultured fish (Craig and Helfrich, 2002). To be a viable alternative feedstuff to FM in aquafeeds, a candidate ingredient must possess certain nutritional characteristics, such as low levels of fibre, starch, especially non-soluble carbohydrates and anti-nutrients, plus have a relatively high protein content, favourable amino acid profile, high nutrient digestibility and reasonable palatability. Furthermore, it must possess wide availability, competitive price, plus ease of handling, shipping, and storage (Gatlin et al., 2007). Dietary content is paramount, but manufacturing, processing, and mode of delivery are also critical factors in finfish nutrition.
7 Conclusion
Inclusion of seaweeds and seaweed products in feeds as sources either of macronutrients such as protein and energy as alternative feed ingredients, or of bioactive compounds as feed additives, can have beneficial effects in many species of finfish. Specifically, dietary seaweed can support and/or improve growth, feed efficiency and biochemical composition, have favourable health impacts including immunostimulatory effects and resistance to pathogens, increased antioxidant status, and improved intestinal function, and low-level inclusion could have economic advantages. However, there is substantial variability in the results obtained from fish feeding trials, which is likely due to differences in both the feeds tested and the fish species studied. The biochemical composition of seaweeds varies depending, not only on species, but also on environmental conditions, location, life-cycle, and season and this, along with differences in how seaweed products are processed post-harvest and added to the feeds, has contributed to the variability observed. In addition, the response of fish to dietary seaweed inclusion is both highly dose-dependent and species-specific with, in general, herbivorous and omnivorous species more able to tolerate higher seaweed inclusion than carnivorous species based upon fundamental physiological differences in digestion and metabolism albeit variation can also be observed within feeding behaviour groups. Overall, the use of seaweed-derived products in feeds for finfish can be beneficial and their evaluation warrants further study. In particular, studies to optimise culture conditions for selected seaweed species to improve nutritional composition and produce more consistent products would be highly useful. In addition, considering the current limitations in supply and the increasing demand for the n-3 (omega-3) LC-PUFA, EPA and DHA, more studies in future should also investigate the potential of different seaweeds to supply these essential nutrients.
Author contributions
AH: Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. GT: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. FM: Investigation, Writing – original draft, Writing – review & editing. KP: Writing – original draft, Writing – review & editing. DT: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Department of Biotechnology (DBT), Government of India, New Delhi, India (Dy. No. 102/IFD/SAN/4678/2015-2016, dated 28.3.2016) and the Biotechnology and Biological Science Research Council (BBSRC) Newton Fund Global Research Partnership Project (BB/N005031/1).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/faquc.2025.1570842/full#supplementary-material
References
Abdel-Aziz M. F. A. and Ragab M. A. (2017). Effect of use fresh macro algae (seaweed) Ulva fasciata and Enteromorpha flaxusa with or without artificial feed on growth performance and feed utilization of rabbitfish (Siganus rivulatus) fry. J. Aquacult. Res. Dev. 8, 482. doi: 10.4172/2155-9546.1000482
Abdelhamid A. F., Ayoub H. F., El-Gawad E. A. A., Abdelghany M. F., and Abdel-Tawwab M. (2021). Potential effects of dietary seaweeds mixture on the growth performance, antioxidant status, immunity response, and resistance of striped catfish (Pangasianodon hypophthalmus) against Aeromonas hydrophila infection. Fish. Shellfish. Immunol. 119, 76–83. doi: 10.1016/j.fsi.2021.09.043
Abdel-Latif H. M. R., Dawood M. A. O., Alagawany M., Faggio C., Nowosad J., and Kucharczyk D. (2022). Health benefits and potential applications of fucoidan (FCD) extracted from brown seaweeds in aquaculture: An updated review. Fish. Shellfish. Immunol. 122, 115–130. doi: 10.1016/j.fsi.2022.01.039
Abdel-Wahab A. A., Younis E. M. I., and Al-Asgah N. A. (2016). Potential use of green macroalgae Ulva lactuca as a feed supplement in diets on growth performance, feed utilization and body composition of the African catfish, Clarias gariepinus. Saudi. J. Biol. Sci. 23, 404–409. doi: 10.1016/j.sjbs.2015.11.010
Adel M., Omidi A. H., Dawood M. A. O., Karimi B., and Shekarabi S. P. H. (2021). Dietary Gracilaria persica mediated the growth performance, fillet colouration, and immune response of Persian sturgeon (Acipenser persicus). Aquaculture 530, 735950. doi: 10.1016/j.aquaculture.2020.735950
Aguilera-Morales M., Casas-Valdez M., Carrillo-Dominguez S., Gonzalez-Acosta B., and Perez-Gil F. (2005). Chemical composition and microbiological assays of marine algae Enteromorpha spp. as a potential food source. J. Fd. Comp. Anal. 18, 79–88. doi: 10.1016/j.jfca.2003.12.012
Akazaki M. (1983). New lutjanid fish, Lutjanus stellatus, from southern Japan and a related species, L. rivulatus (Cuvier). Jap. J. Ichthyol. 29, 365–373.
Akbary P., Sohrab Zaei Z., and Aminikhoei Z. (2020). Role of dietary inclusion of Gracillaria arcuata extract on growth performance and biochemical responses in grey mullet, Mugil cephalus (Linnaeus, 1758). Iranian. J. Fish. Sci. 19, 401–409. doi: 10.22092/ijfs.2019.119601
Al-Asgah N. A., Younis E. M., Abdel-Warith A. W. A., and Shamlol F. S. (2016). Evaluation of red seaweed Gracilaria arcuata as dietary ingredient in African catfish, Clarias gariepinus. Saudi. J. Biol. Sci. 23, 205–210. doi: 10.1016/j.sjbs.2015.11.006
Anastasiou S. and Nengas I. (2005). “A general review on the use of alternative protein sources in diets for Mediterranean fish,” in Mediterranean Fish Nutrition, vol. 63 . Eds. Montero D., Basurco B., Nengas I., Alexis M., and Izquierdo M. (CIHEAM (Cahiers Options Méditerranéennes, Zaragosa, Spain), 121–126.
Angell A. R., Angell S. F., de Nys R., and Paul N. A. (2016). Seaweed as a protein source for mono-gastric livestock. Trends Fd. Sci. Technol. 54, 74–84. doi: 10.1016/j.tifs.2016.05.014
Antunes M., Neves M., Pires D., Passos R., do Carmo B., Tchobanov C. F., et al. (2023). Proximate composition and fatty acid profile of gilthead seabream (Sparus aurata) fed with Pelvetia caniculata-supplemented diets: An insight towards the valorization of seaweed biomass. Foods 12, 1810. doi: 10.3390/foods12091810
Appler H. N. (1985). Evaluation of Hydrodictyon reticulatum as protein source in feeds for Oreochromis (Tilapia) niloticus and Tilapia zillii. J. Fish. Biol. 27, 327–334. doi: 10.1111/j.1095-8649.1985.tb04034.x
Aquino J. I. L., Serrano A. E. Jr., and Corre V. L. Jr. (2014). Dried Enteromorpha intestinalis could partially replace soybean meal in the diet of juvenile Oreochromis niloticus. Anim. Biol. Anim. Husbandry. Internat. J. Bioflux. Soc. 6, 95–101.
Araki T., Hayakawa M., Tamaru Y., Yoshimatsu K., and Morishita T. (1994). Isolation and regeneration of haploid protoplasts from Bangia atropurpurea (Rhodophyta) with marine bacterial enzymes. J. Phycol. 30, 1040–1046. doi: 10.1111/j.0022-3646.1994.01040.x
Araujo M., Rema P., Sousa-Pinto I., Cunha L. M., Peixoto M. J., and Pires M. A. (2016). Dietary inclusion of IMTA-cultivated Gracilaria vermiculophylla in rainbow trout (Oncorhynchus mykiss) diets: effects on growth, intestinal morphology, tissue pigmentation and immunological response. J. Appl. Phycol. 28, 679–689. doi: 10.1007/s10811-015-0591-8
Ashour M., Mabrouk M. M., Ayoub H. F., El-Feky M. M. M. M., Zaki S. Z., Hoseinifar S. H., et al. (2020). Effect of dietary seaweed extract supplementation on growth, feed utilization, hematological indices, and non-specific immunity of Nile tilapia, Oreochromis niloticus challenged with Aeromonas hydrophila. J. Appl. Phycol. 32, 3467–3479. doi: 10.1007/s10811-020-02178-1
Asino H., Ai Q., and Mai K. (2011). Evaluation of Enteromorpha prolifera as a feed component in large yellow croaker (Pseudosciaena crocea, Richardson, 1846) diets. Aquacult. Res. 42, 525–533. doi: 10.1111/j.1365-2109.2010.02648.x
Aslamyah S., Karim Y., and Badraeni A. M. (2016). Seaweed as a source of carbohydrates in the feed of milk fish (Chanos chanos Forsskål). Internat. J. PharmTech. Res. 9, 64–67.
(2023). Fish Farmer. Available online at: https://www.fishfarmermagazine.com/2023/09/06/Norways-farmed-cod-exports-on-the-rise/ (Accessed 10 January 2024).
Azad S. A. and Xiang T. Z. (2012). Suitability of seaweed meal incorporated with Rhodovulum sp. bacterium as feed supplement for finfish larvae. Borneo. Sci. 30, 57–63.
Azaza M. S., Mensi F., Ksouri J., Dhraief M. N., Brini B., and Abdelmouleh (2008). Growth of Nile tilapia (Oreochromis niloticus L.) fed with diets containing graded levels of green algae ulva meal (Ulva rigida) reared in geothermal waters of southern Tunisia. J. Appl. Ichthyol. 24, 202–207. doi: 10.1111/j.1439-0426.2007.01017.x
Aziz M., Oda T., and Kashiwagi T. (2013). Enhanced high energy efficient steam drying of algae. Appl. Energy 109, 163–170. doi: 10.1016/j.apenergy.2013.04.004
Bagni M., Romano N., Finoia M. G., Abelli L., Scapigliati G., and Tiscar P. G. (2005). Short- and long-term effects of a dietary yeast beta-glucan (Macrogard) and alginic acid (Ergosan) preparation on immune response in sea bass (Dicentrarchus labrax). Fish. Shellfish. Immunol. 18, 311–325. doi: 10.1016/j.fsi.2004.08.003
Bahnamiri A., Abedian Kenari A., Babaei S., Banavreh A., and Soltanian S. (2023). Dietary sulfated polysaccharides extracted from Caulerpa sp. and Padina sp. modulated physiological performance, antibacterial activity and ammonia challenge test in juvenile rainbow trout (Oncorhynchus mykiss). J. Anim. Physiol. Anim. Nutr. (Berl). 108, 324–337. doi: 10.1111/jpn.13894
Bakky M. A. H., Tran N. T., Zhang Y., and Li S. (2022). Utilization of marine macroalgae-derived sulphated polysaccharides as dynamic nutraceutical components in the feed of aquatic animals: A review. Aquacult. Res. 53, 5787–5808. doi: 10.1111/are.v53.17
Banerjee K., Ghosh R., Homechaudhuri S., and Mitra A. (2009). Biochemical composition of marine macroalgae from Gangetic delta at the apex of Bay of Bengal. Afr. J. Basic. Appl. Sci. 1, 96–104.
Barbarino E. and Lourenco S. O. (2005). An evaluation of methods for extraction and quantification of protein from marine macro- and microalgae. J. Appl. Phycol. 17, 447–460. doi: 10.1007/s10811-005-1641-4
Barbosa M. J., Morais R., and Choubert G. (1999). Effect of carotenoid source and dietary lipid content on blood astaxanthin concentration in rainbow trout (Oncorhynchus mykiss). Aquaculture 176, 331–341. doi: 10.1016/S0044-8486(99)00115-5
Barbour J. A. and Turner N. (2014). Mitochondrial stress signaling promotes cellular adaptations. Internat. J. Cell Biol. 2014, 156020. doi: 10.1155/2014/156020
Batista S., Pereira R., Oliveira B., Baião L. F., Jessen F., Tulli F., et al. (2020). Exploring the potential of seaweed Gracilaria gracilis and microalga Nannochloropsis oceanica, single or blended, as natural dietary ingredients for European seabass Dicentrarchus labrax. J. Appl. Phycol. 32, 2041–2059. doi: 10.1007/s10811-020-02118-z
Bene C., Barange M., Subasinghe R., Pinstrup-Andersen P., Merion G., and Hemre G. I. (2015). Feeding 9 billion by 2050 – Putting fish back on the menu. Fd. Secur. 7, 261–274. doi: 10.1007/s12571-015-0427-z
Black W. A. P. (1950). The seasonal variation in the cellulose content of the common Scottish Laminariaceae and Fucaceae. J. Mar. Biol. Assoc. UK. 29, 379–387. doi: 10.1017/S0025315400055429
Boland M. J., Rae A. N., Vereijken J. M., Meuwissen M. P. M., Fischer A. R. H., and van Boekel M. A. J. S. (2013). The future supply of animal-derived protein for human consumption. Trends Fd. Sci. Technol. 54, 74–84. doi: 10.1016/j.tifs.2012.07.002
Borquez A., Serrano E., Dantagnan P., Carrasco J., and Hernandez A. (2010). Feeding high inclusion of whole grain white lupin (Lupinus albus) to rainbow trout (Oncorhynchus mykiss): effects on growth, nutrient digestibility, liver and intestine histology and muscle fatty acid composition. Aquacult. Res. 42, 1067–1078. doi: 10.1111/j.1365-2109.2010.02690.x
Boys C. A., Rowland S. J., Gabor M., Gabor L., Marsh I. B., and Hum S. (2012). Emergence of epizootic ulcerative syndrome in native fish of the Murray-Darling river system, Australia: Hosts, distribution and possible vectors. PloS One 7, 1–10. doi: 10.1371/journal.pone.0035568
Bryan P. G. (1975). Food habits, functional digestive morphology, and assimilation efficiency of the rabbitfish Siganus spinus (Pisces, Siganidae) on Guam. Pac. Sci. 29, 269–277.
Burtin P. (2003). Nutritional value of seaweeds. Electron. J. Environ. Agricult. Fd. Chem. 2, 498–503.
Cabanero P. C., Tumbokon B. L. M., and Serrano A. E. Jr. (2016). Nutritional evaluation of Rhizoclonium riparium var implexum meal to replace soybean in the diet of Nile tilapia fry. Israeli. J. Aquacult. – Bamidgeh. 59, 1–8. doi: 10.46989/001c.20818
Cerezo I. M., Fumanal M., Tapia-Paniagua S. T., Bautista R., Anguís V., Fernández-Díaz C., et al. (2022). Solea Senegalensis bacterial intestinal microbiota is affected by low dietary inclusion of Ulva ohnoi. Front. Microbiol. 12, 801744. doi: 10.3389/fmicb.2021.801744
Cheng A. C., Chen Y. Y., and Chen J. C. (2008). Dietary administration of sodium alginate and k-carrageenan enhances the innate immune response of brown-marbled grouper Epinephelus fuscoguttatus and its resistance against Vibrio alginolyticus. Vet. Immunol. Immunopathol. 121, 206–215. doi: 10.1016/j.vetimm.2007.09.011
Cheng A. C., Tu C. W., Chen Y. Y., Nan F. H., and Chen J. C. (2007). The immunostimulatory effects of sodium alginate and iota-carrageenan on orange-spotted grouper Epinephelus coioides and its resistance against Vibrio alginolyticus. Fish. Shellfish. Immunol. 22, 197–205. doi: 10.1016/j.fsi.2006.04.009
Chiesa S. and Gnansounou E. (2011). Protein extraction from biomass in a bioethanol refinery – Possible dietary applications: Use as animal feed and potential extension to human consumption. Bioresour. Technol. 102, 427–436. doi: 10.1016/j.biortech.2010.07.125
Chiu S. T., Tsai R. T., Hsu J. P., Liu C. H., and Cheng W. (2008). Dietary sodium alginate administration to enhance the non-specific immune responses, and disease resistance of the juvenile grouper Epinephelus fuscoguttatus. Aquaculture 277, 66–72. doi: 10.1016/j.aquaculture.2008.01.032
Cofrades S., Lopez-Lopez I., Bravo L., Ruiz-Capillas C., Bastida S., and Larrea M. T. (2010). Nutritional and antioxidant properties of different brown and red Spanish edible seaweeds. Fd. Sci. Technol. Internat. 16, 361–370. doi: 10.1177/1082013210367049
Colihueque N. (2010). Genetics of salmonid skin pigmentation: clues and prospects for improving the external appearance of farmed salmonids. Rev. Fish. Biol. Fish. 20, 71–86. doi: 10.1007/s11160-009-9121-6
Costa M., Cardoso C., Afonso C., Bandarra N. M., and Prates J. A. M. (2021). Current knowledge and future perspectives of the use of seaweeds for livestock production and meat quality: a systematic review. J. Anim. Physiol. Anim. Nutr. (Berl). 105, 1075–1102. doi: 10.1111/jpn.v105.6
Craig S. and Helfrich L. A. (2002). “Understanding fish nutrition, feeds and feeding,” in Virginia Cooperative Extension Publication 420-256 (Virginia State University, Petersburg).
D’Agaro E., Gibertoni P., and Esposito S. (2022). Recent trends and economic aspects in the rainbow trout (Oncorhynchus mykiss) sector. Appl. Sci. 12, 8773. doi: 10.3390/app12178773
Dalmo R. A. and Seljelid R. (1995). The immunomodulatory effect of LPS, laminaran and sulphated laminaran [β(1,3)-D-glucan] on Atlantic salmon, Salmo salar L., macrophages in vitro. J. Fish. Dis. 18, 175–185. doi: 10.1111/j.1365-2761.1995.tb00275.x
Dantagnan P., Hernandez A., Borquez A., and Mansilla A. (2009). Inclusion of macroalgae meal (Macrocystis pyrifera) as feed ingredient for rainbow trout (Oncorhynchus mykiss): effect on flesh fatty acid composition. Aquacult. Res. 41, 87–94. doi: 10.1111/j.1365-2109.2009.02308.x
Davies S. J., Brown M. T., and Camilleri M. (1997). Preliminary assessment of the seaweed Porphyra purpurea in artificial diets for thick-lipped grey mullet (Chelon labrosus). Aquaculture 152, 249–258. doi: 10.1016/S0044-8486(96)01513-X
Dawczynski C., Schubert R., and Jahreis G. (2007). Amino acids, fatty acids, and dietary fibre in edible seaweed products. Fd. Chem. 103, 891–899. doi: 10.1016/j.foodchem.2006.09.041
Degani G. and Revach A. (1991). Digestive capabilities of three commensal fish species: carp, Cyprinus carpio L., tilapia, Oreochromis aureus x O. niloticus and African catfish, Clarias gariepinus (Burchell 1822). Aquacult. Fish. Manage. 22, 397–403. doi: 10.1111/j.1365-2109.1991.tb00753.x
Denis C., Morancais M., Li M., Deniaud E., Gaudin P., and Wielgosz-Collin G. (2010). Study of the chemical composition of edible red macroalgae Grateloupia turuturu from Brittany (France). Fd. Chem. 119, 913–917. doi: 10.1016/j.foodchem.2009.07.047
Diler I., Tekinay A. A., Guroy D., Guroy B. K., and Soyuturk M. (2007). Effects of Ulva rigida on the growth, feed intake and body composition of common carp, Cyprinus carpio L. J. Biol. Sci. 7, 305–308. doi: 10.3923/jbs.2007.305.308
Drew M. D., Borgeson T. L., and Thiessen D. L. (2007). A review of processing of feed ingredients to enhance diet digestibility in finfish. Anim. Feed. Sci. Technol. 138, 118–136. doi: 10.1016/j.anifeedsci.2007.06.019
El-Dahhar A. A., Salama M. E., Moustafa Y. T., and Elmorshedy E. M. (2014). Effect of using equal mixture of seaweeds and marine algae in striped mullet (Mugil Cephalus) larval diets on growth performance and feed utilization. J. Arabian. Aquacult. Soc 9, 145–160. doi: 10.12816/0026642
El-Tawil N. E. (2010). Effects of green seaweeds (Ulva sp.) as feed supplements in red tilapia (Oreochromis sp.) diet on growth performance, feed utilization and body composition. J. Arabian. Aquacult. Soc 5, 179–194.
Emre Y., Ergun S., Kurtoglu A., Gury B., and Guroy D. (2013). Effects of Ulva meal on growth performance of gilthead seabream (Sparus aurata) at different levels of dietary lipid. Turkish. J. Fish. Aquat. Sci. 13, 841–846. doi: 10.4194/1303-2712-v13_5_08
Ergun S., Soyuturk M., Guroy B., Guroy D., and Merrifield D. (2009). Influence of Ulva meal on growth, feed utilization, and body composition of juvenile Nile tilapia (Oreochromis niloticus) at two levels of dietary lipid. Aquacult. Internat. 17, 355–361. doi: 10.1007/s10499-008-9207-5
FAO (1997). Aquaculture Production Statistics, 1986-1995. FAO Fisheries Circular No. 815 (Rome, Italy: Food and Agriculture Organisation of the United Nations), 195 pp.
FAO (2002). The State of the World Fisheries and Aquaculture (Rome. Italy: Food and Agriculture Organisation of the United Nations), 150 pp.
FAO (2012). The State of Food Security in the World 2012, Technical Note 18 pp. Methodology to estimate the prevalence of undernourishment (Rome, Italy: Food and Agriculture Organisation of the United Nations).
FAO (2014). The State of World Fisheries and Aquaculture. Opportunities and Challenges (Rome, Italy: Food and Agriculture Organisation of the United Nations), 221 pp.
FAO (2016). Food and Agriculture – Key to achieving the 2030 Agenda for Sustainable Development (Rome, Italy: Food and Agriculture Organisation of the United Nations), 31 pp.
FAO (2018). The State of World Fisheries and Aquaculture. Meeting the Sustainable Development Goals (Rome, Italy: Food and Agriculture Organisation of the United Nations), 227 pp.
FAO (2022). The State of World Fisheries and Aquaculture. Towards Blue Transformation (Rome, Italy: Food and Agriculture Organisation of the United Nations), 236 pp.
FAO (2024). The State of World Fisheries and Aquaculture. Blue Transformation in Action (Rome, Italy: Food and Agriculture Organisation of the United Nations), 264 pp.
Felix N. and Brindo R. A. (2014). Effects of raw and fermented seaweed, Padina tetrastomatica on the growth and food conversion of giant freshwater prawn Macrobrachium rosenbergii. Int. J. Fish. Aquat. Stud. 1, 108–113.
Fernandes H., Martins N., Vieira L., Salgado J. M., Castro C., Oliva-Teles A., et al. (2022). Pre-treatment of Ulva rigida improves its nutritional value for European seabass (Dicentrarchus labrax) juveniles. Algal. Res. 66, 102803. doi: 10.1016/j.algal.2022.102803
Ferraces-Casais P., Lage-Yusty M. A., Rodriguez-Bernaldo de Quiros A., and Lopez-Hernandez J. (2012). Evaluation of bioactive compounds in fresh edible seaweeds. Fd. Anal. Meth. 5, 828–834. doi: 10.1007/s12161-011-9321-2
Ferreira M., Abdelhafiz Y., Abreu H., Silva J., Valente L. M. P., and Kiron V. (2022). Gracilaria gracilis and Nannochloropsis oceanica, singly or in combination, in diets alter the intestinal microbiota of European seabass (Dicentrarchus labrax). Front. Mar. Sci. 9, 1001942. doi: 10.3389/fmars.2022.1001942
Ferreira M., Larsen B. K., Granby K., Cunha S. C., Monteiro C., Fernandes J. O., et al. (2020). Diets supplemented with Saccharina latissima influence the expression of genes related to lipid metabolism and oxidative stress modulating rainbow trout (Oncorhynchus mykiss) fillet composition. Fd. Chem. Toxicol. 140, 111332. doi: 10.1016/j.fct.2020.111332
Ferreira M., Teixeira C., Abreu H., Silva J., Costas B., Kiron V., et al. (2021). Nutritional value, antimicrobial and antioxidant activities of micro- and macroalgae, single or blended, unravel their potential use for aquafeeds. J. Appl. Phycol. 33, 3507–3518. doi: 10.1007/s10811-021-02549-2
Fleurence J. (1999). Seaweed proteins: biochemical, nutritional aspects and potential uses. Trends Fd. Sci. Technol. 10, 25–28. doi: 10.1016/S0924-2244(99)00015-1
Fleurence J., Morancais M., Dumay J., Decottignies P., Turpin V., and Munier M. (2012). What are the prospects for using seaweed in human nutrition and for marine animals raised through aquaculture? Trends Fd. Sci. Technol. 27, 57–61. doi: 10.1016/j.tifs.2012.03.004
Foster G. G. and Hodgson A. N. (1998). Consumption and apparent dry matter digestibility of six intertidal macroalgae by Turbo sarmaticus (Mollusca: Vetigastropoda: Turbinidae). Aquaculture 167, 211–227. doi: 10.1016/S0044-8486(98)00315-9
Fox C. J. (2014). Issues around fisheries for small pelagic fish. SAMS Internal Report No. 284 (Oban, Scotland: Scottish Association for Marine Science), 260 pp.
Francis G., Makkar H. P. S., and Becker K. (2001). Antinutritional factors present in plant derived alternate fish feed ingredients and their effects in fish. Aquaculture 199, 197–227. doi: 10.1016/S0044-8486(01)00526-9
Frikha F., Kammoun M., Hammami N., Mchirgui R. A., Belbahri L., and Gargouri Y. (2011). Chemical composition and some biological activities of marine algae collected in Tunisia. Cienc. Marinas. 37, 113–124. doi: 10.7773/cm.v37i2.1712
Fujiki K., Shin D. H., Nakao M., and Yano T. (1997a). Protective effect of carrageenan against bacterial infections in carp Cyprinus carpio. J. Faculty. Agricult. 42, 113–119. doi: 10.5109/24198
Fujiki K., Shin D. H., Nakao M., and Yano T. (1997b). Effects of k-carrageenan on the non-specific defense system of carp Cyprinus carpio. Fish. Sci. 63, 934–938. doi: 10.2331/fishsci.63.934
Fujiki K. and Yano T. (1997). Effects of sodium alginate on the non-specific defence system of the common carp (Cyprinus carpio L.). Fish. Shellfish. Immunol. 7, 417–427. doi: 10.1006/fsim.1997.0095
Fumanal M., Di Zeo D. E., Anguís V., Fernández-Diaz C., Alarcón F. J., Piñera R., et al. (2020). Inclusion of dietary Ulva ohnoi 5% modulates Solea Senegalensis immune response during Photobacterium damselae subsp. piscicida infection. Fish. Shellfish. Immunol. 100, 186–197. doi: 10.1016/j.fsi.2020.03.007
Gabrielsen B. O. and Austreng E. (1998). Growth, product quality and immune status of Atlantic salmon, Salmo salar L., fed wet feed with alginate. Aquacult. Res. 29, 397–401. doi: 10.1046/j.1365-2109.1998.00215.x
Galindo A., Perez J. A., Martín V., Acosta N. G., Reis D. B., Jimenez I. A., et al. (2023). Effect of feed supplementation with seaweed wracks on performance, muscle lipid composition, antioxidant status, digestive enzyme activities, and plasma biochemistry of gilthead seabream (Sparus aurata) juveniles. Aquacult. Rep. 31, 101673. doi: 10.1016/j.aqrep.2023.101673
Galindo A., Rodríguez C., Reis D. B., Marrero M., Acosta N. G., Barreto M. C., et al. (2022). Valorization of seaweed wracks: Inclusion as additive in diets for grass carp (Ctenopharyngodon idella). Aquacult. Nutr. 2022, 6992682. doi: 10.1155/2022/6992682
Galland-Irmouli A. V., Fleurence J., Lamghari R., Lucon M., Rouxel C., and Barbaroux O. (1999). Nutritional value of proteins from edible seaweed Palmaria palmata (Dulse). J. Nutr. Biochem. 10, 353–359. doi: 10.1016/S0955-2863(99)00014-5
Garcia-Casal M. N., Pereira A. C., Leets I., Ramirez J., and Quiroga M. F. (2007). High iron content and bioavailability in humans from four species of marine algae. J. Nutr. 137, 2691–2695. doi: 10.1093/jn/137.12.2691
García-Márquez J., Galafat A., Alarcón F. J., Figueroa F. L., Martínez-Manzanares E., Arijo S., et al. (2021). Cultivated and wild Juvenile thick-lipped grey mullet, Chelon labrosus: A comparison from a nutritional point of view. Anim. (Basel). 11, 2112. doi: 10.3390/ani11072112
Garcia-Vaquero M. and Hayes M. (2016). Red and green macroalgae for fish and animal feed and human functional food development. Fd. Rev. Internat. 32, 15–45. doi: 10.1080/87559129.2015.1041184
Gatlin D. M., Barrows F. T., Brown P., Dabrowski K., Gaylord T. G., and Hardy R. W. (2007). Expanding the utilization of sustainable plant products in aquafeeds: a review. Aquacult. Res. 38, 551–579. doi: 10.1111/j.1365-2109.2007.01704.x
Geurden I., Cuvier A., Gondouin E., Olsen R. E., Ruohonen K., and Kaushik S. (2005). Rainbow trout can discriminate between feeds with different oil sources. Physiol. Behav. 85, 107–114. doi: 10.1016/j.physbeh.2005.03.010
Glencross B. D., Booth M., and Allan G. L. (2007). A feed is only as good as its ingredients – a review of ingredient evaluation strategies for aquaculture feeds. Aquacult. Nutr. 13, 17–34. doi: 10.1111/j.1365-2095.2007.00450.x
Goda A. M., El-Haroun E. R., and Chowdhury M. A. K. (2007). Effect of totally or partially replacing fish meal by alternative protein sources on growth of African catfish Clarias gariepinus (Burchell, 1822) reared in concrete tanks. Aquacult. Res. 38, 279–287. doi: 10.1111/j.1365-2109.2007.01663.x
Godfray H. C. J., Beddington J. R., Crute I. R., Haddad L., Lawrence D., and Muir J. F. (2010). Food security: The challenge of feeding 9 billion people. Science 327, 812–818. doi: 10.1126/science.1185383
González-Meza G. M., Elizondo-Luevano J. H., Cuellar-Bermudez S. P., Sosa-Hernández J. E., Iqbal H. M. N., Melchor-Martínez E. M., et al. (2023). New perspective for macroalgae-based animal feeding in the context of challenging sustainable food production. Plants 12, 3609. doi: 10.3390/plants12203609
Guo J., Qi M., Chen H., Zhou C., Ruan R., Yan X., et al. (2022). Macroalgae-derived multifunctional bioactive substances: The potential applications for food and pharmaceuticals. Foods 11, 3455. doi: 10.3390/foods11213455
Güroy B. K., Cirik S., Güroy D., Sanver F., and Tekinay A. A. (2007). Effects of Ulva rigida and Cystoseira barbata meals as a feed additive on growth performance, feed utilization, and body composition of Nile tilapia, Oreochromis niloticus. J. Vet. Anim. Sci. 31, 91–97.
Güroy D., Güroy B., Bilen S., Terzi E., Kenanoğlu O. N., García-Suárez M., et al. (2022). Effects of dietary marine sulphated polysaccharides (Algimun®) on growth performance, immune responses and disease resistance of juvenile gilthead seabream (Sparus aurata) to Photobacterium damselae subsp. piscicida. Fish. Shellfish. Immunol. 127, 1139–1147. doi: 10.1016/j.fsi.2022.07.054
Güroy D., Güroy B., Merrifield D. L., Ergun S., Tekinay A. A., and Yigit M. (2010). Effect of dietary Ulva and Spirulina on weight loss and body composition of rainbow trout, Oncorhynchus mykiss (Walbaum), during a starvation period. Anim. Physiol. Anim. Nutr. 95, 320–327. doi: 10.1111/j.1439-0396.2010.01057.x
Hamid N., Ma Q., Boulom S., Liu T., Zheng Z., Balbas J., et al. (2015). “Seaweed minor constituents,” in Seaweed Sustainability. Eds. Tiwari B. K. and Troy D. J. (Amsterdam, Netherlands: Academic Press), 193–242.
Hansen A. S. C., Rosenlund G., Karlsen O., Koppe W., and Hemre G. I. (2007). Total replacement of fish meal with plant proteins in diets for Atlantic cod (Gadus morhua L.) I - Effects on growth and protein retention. Aquaculture 272, 599–611. doi: 10.1016/j.aquaculture.2007.08.034
Hardy R. W. (1996). Alternate protein sources for salmon and trout diets. Anim. Feed. Sci. Technol. 59, 71–80. doi: 10.1016/0377-8401(95)00888-8
Hardy R. W. (2010). Utilization of plant proteins in fish diets: effects of global demand and supplies of fishmeal. Aquacult. Res. 41, 770–776. doi: 10.1111/j.1365-2109.2009.02349.x
Harikrishnan R., Balasundaram C., and Heo M. S. (2011a). Impact of plant products on innate and adaptive immune system of cultured finfish and shellfish. Aquaculture 317, 1–15. doi: 10.1016/j.aquaculture.2011.03.039
Harikrishnan R., Kim M. C., Kim J. S., Han Y. J., Jang I. S., and Balasundaram C. (2011b). Immunomodulatory effect of sodium alginate enriched diet in kelp grouper Epinephelus bruneus against Streptococcus iniae. Fish. Shellfish. Immunol. 30, 543–549. doi: 10.1016/j.fsi.2010.11.023
Harnedy P. and FitzGerald R. (2013). In vitro assessment of the cardioprotective, anti-diabetic and antioxidant potential of Palmaria palmata protein hydrolysates. J. Appl. Phycol. 25, 1793–18038. doi: 10.1007/s10811-013-0017-4
Hashim R. and Saat M. A. M. (1992). The utilization of seaweed meals as binding agents in pelleted feeds for snakehead (Channa striatus) fry and their effects on growth. Aquaculture 108, 229–308. doi: 10.1016/0044-8486(92)90114-Z
Haugan J. A. and Liaaen-Jensen S. (1994). Algal carotenoids 54. Carotenoids of brown algae (Phaeophyceae). Biochem. Syst. Ecol. 22, 31–41. doi: 10.1016/0305-1978(94)90112-0
He M. L., Hollwich W., and Rambeck W. A. (2002). Supplementation of algae to the diet of pigs: a new possibility to improve the iodine content in the meat. J. Anim. Physiol. Anim. Nutr. 86, 97–104. doi: 10.1046/j.1439-0396.2002.00363.x
Helgadóttir G., Renssen H., Olk T. R., Oredalen T. J., Haraldsdóttir L., Skúlason S., et al. (2021). Wild and farmed Arctic charr as a tourism product in an era of climate change. Front. Sustain. Fd. Syst. 5, 654117. doi: 10.3389/fsufs.2021
Heo S. J. and Jeon Y. J. (2009). Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B. 65, 101–107. doi: 10.1016/j.jphotobiol.2008.11.011
Holdt S. L. and Kraan S. (2011). Bioactive compounds in seaweed: functional food applications and legislation. J. Appl. Phycol. 23, 543–597. doi: 10.1007/s10811-010-9632-5
Hong S., Peddie S., Campos-Perez J. J., Zou J., and Secombes C. J. (2003). The effect of intraperitoneally administered recombinant IL-1b on immune parameters and resistance to Aeromonas salmonicida in the rainbow trout (Oncorhynchus mykiss). Dev. Comp. Immunol. 27, 801–812. doi: 10.1016/S0145-305X(03)00056-9
Horie Y., Sugase K., and Horie K. (1995). Physiological differences of soluble and insoluble dietary fibre fractions of brown algae and mushrooms in pepsin activity in vitro and protein digestibility. Asia. Pac. J. Clin. Nutr. 4, 251–255.
Horn S. J. (2000). Bioenergy from brown seaweeds (Trondheim, Norway: Department of Biotechnology, Norwegian University of Science and Technology).
Hoseinifar S. H., Fazelan Z., Bayani M., Yousefi M., Van Doan H., and Yazici M. (2022). Dietary red macroalgae (Halopithys incurva) improved systemic and mucosal immune and antioxidant parameters and modulated related gene expression in zebrafish (Danio rerio). Fish. Shellfish. Immunol. 123, 164–171. doi: 10.1016/j.fsi.2022.02.047
Hoseinifar S. H., Yousefi S., Capillo G., Paknejad H., Khalili M., Tabarraei A., et al. (2018). Mucosal immune parameters, immune and antioxidant defence related genes expression and growth performance of zebrafish (Danio rerio) fed on Gracilaria gracilis powder. Fish. Shellfish. Immunol. 83, 232–237. doi: 10.1016/j.fsi.2018.09.046
Hunn J. B. and Fromm P. O. (1966). In vivo uptake of radioiodide by rainbow trout. J. (Water. pollut. Control. Fed). 38, 1981–1985.
IFFO (The Marine Ingredient Organisation) (2023). IFFO Annual Conference, Cape Town, South Africa. Available online at: https://www.iffo.com/iffo-conference-2023-key-takeaways-market-demand-session-25-october2023 (Accessed January 15, 2025).
Ilias N. N., Jamal P., Jaswir I., Sulaiman S., Zainudin Z., and Azmi A. S. (2015). Potentiality of selected seaweed for the production of nutritious fish feed using solid state fermentation. J. Eng. Sci. Technol. 3, 30–40.
Irkin L. C. (2019). The use of macroalgae as a feed supplement in fish diets. Internat. J. Trend. Sci. Res. Dev. 3, 966–969. doi: 10.31142/ijtsrd26538
Ismail M. M. (2019). Review on seaweed as supplement fish feed. Oceanogr. Fish. Open Access J. 11, 5557808. doi: 10.19080/OFOAJ.2019.11.555808
Jalali M. A., Ahmadifar E., Sudagar M., and Takami G. A. (2009). Growth efficiency, body composition, survival and haematological changes in great sturgeon (Huso huso Linnaeus, 1758) juveniles fed diets supplemented with different levels of Ergosan. Aquacult. Res. 40, 804–809. doi: 10.1111/j.1365-2109.2009.02166.x
Je J. Y., Park P. J., Kim E. K., Park J. S., Yoon H. D., and Kim K. R. (2009). Antioxidant activity of enzymatic extracts from the brown seaweed Undaria pinnatifida by electron spin resonance spectroscopy. Fd. Sci. Technol. 42, 874–878. doi: 10.1016/j.lwt.2008.10.012
Jena J. K. (2006a). Cultured Aquatic Species Information Programme. Labeo rohita. FAO Fisheries and Aquaculture Department (Rome, Italy: Food and Agriculture Organisation of the United Nations).
Jena J. K. (2006b). Cultured Aquatic Species Information Programme. Catla catla. FAO Fisheries and Aquaculture Department (Rome, Italy: Food and Agriculture Organisation of the United Nations).
Jian J. and Wu Z. (2003). Effects of traditional Chinese medicine on nonspecific immunity and disease resistance of large yellow croaker, Pseudosciaena crocea (Richardson). Aquaculture 218, 1–9. doi: 10.1016/S0044-8486(02)00192-8
Jiang Z., Okimura T., Yokose T., Yamasaki Y., Yamaguchi K., and Oda T. (2010). Effects of sulfated fucan, ascophyllan, from the brown Alga Ascophyllum nodosum on various cell lines: A comparative study on ascophyllan and fucoidan. J. Biosci. Bioeng. 110, 113–117. doi: 10.1016/j.jbiosc.2010.01.007
Jiang H., Wang M., Zheng Y., Chen F., Fu, Chen X., et al. (2021). Dietary laminarin administration to enhance the immune responses, promote growing and strengthen physique in Ictalurus punctatus. Aquacult. Nutr. 27, 1181–1191. doi: 10.1111/anu.13258
Kalla A., Yoshimatsu T., Araki T., Zhang D., Yamamoto T., and Sakamoto S. (2008). Use of Porphyra spheroplasts as feed additive for red sea bream. Fish. Sci. 74, 104–108. doi: 10.1111/j.1444-2906.2007.01501.x
Kamunde C., Sappal R., and Melegy T. M. (2019). Brown seaweed (AquaArom) supplementation increases food intake and improves growth, antioxidant status and resistance to temperature stress in Atlantic salmon, Salmo salar. PloS One 14, e0219792. doi: 10.1371/journal.pone.0219792
Kato K. (2023). Breeding studies on red sea bream Pagrus major: mass selection to genome editing. Fish. Sci. 89, 103–119. doi: 10.1007/s12562-022-01668-0
Kaushik S. J., Coves D., Duttob G., and Blanca D. (2004). Almost total replacement of fish meal by plant protein sources in the diet of a marine teleost, the European seabass, Dicentrarchus labrax. Aquaculture 230, 391–404. doi: 10.1016/S0044-8486(03)00422-8
Keating C., Bolton-Warberg M., Hinchcliffe J., Davies R., Whelan S., Wan A. H. L., et al. (2021). Temporal changes in the gut microbiota in farmed Atlantic cod (Gadus morhua) outweigh the response to diet supplementation with macroalgae. Anim. Microbiome. 3, 7. doi: 10.1186/s42523-020-00065-1
Keshavanath P., Manjappa K., and Gangadhara B. (2002). Evaluation of carbohydrate rich diets through common carp culture in manured tanks. Aquacult. Nutr. 8, 169–174. doi: 10.1046/j.1365-2095.2002.00202.x
Khalafalla M. M. and El-Hais A. M. A. (2015). Evaluation of seaweeds Ulva rigida and Pterocladia capillaceaas dietary supplements in Nile tilapia fingerlings. J. Aquacult. Res. Dev. 6, 1–5. doi: 10.4172/2155-9546.1000312
Khan M. N. D., Yoshimatsu T., Kalla A., Araki T., and Sakamoto S. (2008). Supplemental effect of Porphyra spheroplasts on the growth and feed utilization of black sea bream. Fish. Sci. 74, 397–404. doi: 10.1111/j.1444-2906.2008.01536.x
Khemis I. B., Gisbert E., Alcaraz C., Zouiten D., Besbes R., and Zouiten A. (2013). Allometric growth patterns and development in larvae and juveniles of thick-lipped grey mullet Chelon labrosus reared in mesocosm conditions. Aquacult. Res. 44, 1872–1888. doi: 10.1111/j.1365-2109.2012.03192.x
Khotimchenko S. V. (2005). Lipids from the marine algae Gracilaria verrucosa. Chemi. Nat. Compounds. 41, 285–288. doi: 10.1007/s10600-005-0130-y
Kim M. K., Dubacq J. P., Thomas J. C., and Giraud G. (1996). Seasonal variations of triacylglycerols and fatty acids in Fucus serratus. Phytochemistry 43, 49–55. doi: 10.1016/0031-9422(96)00243-9
Kim S. S., Galaz G. B., Pham M. A., Jang J. W., Oh D. H., and Yeo I. K. (2009). Effects of dietary supplementation of a Meju, fermented soybean meal, and Aspergillus oryzae for juvenile parrot fish (Oplegnathus fasciatus). Asian-Australasian. J. Anim. Sci. 22, 849–856. doi: 10.5713/ajas.2009.80648
Kim K. W., Kim S. S., Khosravi S., Rahimnejad S., and Lee K. J. (2014). Evaluation of Sargassum fusiforme and Ecklonia cava as dietary additives for olive flounder (Paralichthys olivaceus). Turkish. J. Fish. Aquat. Sci. 14, 321–330. doi: 10.4194/1303-2712-v14_2_03
Kim S. S. and Lee K. J. (2008). Effects of dietary kelp (Ecklonia cava) on growth and innate immunity in juvenile olive flounder Paralichthys olivaceus (Temminck et Schlegel). Aquacult. Res. 39, 1687–1690. doi: 10.1111/j.1365-2109.2008.02046.x
Kim H. J., Park J. S., Kwon S. R., and Park Y. (2023). Determining transcriptomic response of kidneys of olive flounder to viral hemorrhagic septicemia virus infection using next-generation sequencing. Aquaculture 562, 738886. doi: 10.1016/j.aquaculture.2022.738886
Kotnala S., Dhar P., Das P., and Chatterji A. (2010). Growth performance of fingerlings of the Indian major carp, Catla catla (Ham.) fed with feeds supplemented with different seaweeds. Pertanika. J. Sci. Technol. 18, 255–262.
Kumar C. S., Ganesan P., Suresh P. V., and Bhaskar N. (2008). Seaweeds as a source of nutritionally beneficial compounds-a review. J. Fd. Sci. Technol. 45, 1.
Kumar V., Kumar S., Pandey P. K., Raman R. P., Prasad K. P., and Roy S. (2014). Growth and hemato-immunological response to dietary i-carrageenan in Labeo rohita (Hamilton, 1822) juveniles. Israeli. J. Aquacult. Bamidgeh. 66, 971–981. doi: 10.46989/001c.20742
Lahaye M. (1991). Marine algae as sources of fibres: determination of soluble and insoluble dietary fibre contents in some ‘sea vegetables’. J. Sci. Fd. Agric. 54, 587–594. doi: 10.1002/jsfa.2740540410
Larson E. T. and Shanks A. L. (1996). Consumption of marine snow by two species of juvenile mullet and its contribution to their growth. Mar. Ecol. Prog. Ser. 130, 19–28. doi: 10.3354/meps130019
Latuconsina H., Purbiantoro W., and Padang A. (2023). Different marine macroalgae feeding preferences of adult white-spotted rabbitfish (Siganus canaliculatus). AACL. Bioflux. 16, 80–88. doi: 10.1016/j.aquaculture.2022.738117
Laurienzo P. (2010). Marine polysaccharides in pharmaceutical applications: An overview. Mar. Drugs 8, 2435–2465. doi: 10.3390/md8092435
Li Y. Y., Hu C. B., Zheng Y. J., Xia X. A., Xu W. J., and Wang S. Q. (2008). The effects of dietary fatty acids on liver fatty acid composition and Δ6-desaturase expression differ with ambient salinities in Siganus canaliculatus. Comp. Biochem. Physiol. 151, 183–190. doi: 10.1016/j.cbpb.2008.06.013
Li X., Shen Y., Bao Y., Wu Z., Yang B., Jiao L., et al. (2022). Physiological responses and adaptive strategies to acute low-salinity environmental stress of the euryhaline marine fish black seabream (Acanthopagrus schlegelli). Aquaculture 554, 738117. doi: 10.1016/j.aquaculture.2022.738117
Lopes D., Melo T., Rey F., Meneses J., Monteiro F. L., Helguero L. A., et al. (2020). Valuing bioactive lipids from green, red and brown macroalgae from aquaculture, to foster functionality and biotechnological applications. Molecules 25, 3883. doi: 10.3390/molecules25173883
Løvdal T. and Skipnes D. (2022). Assessment of food quality and safety of cultivated macroalgae. Foods 11, 83. doi: 10.3390/foods11010083
Lozano I., Wacyk J. M., Carrasco J., and Cortez-San Martín M. A. (2016). Red macroalgae Pyropia columbina and Gracilaria Chilensis: sustainable feed additive in the Salmo salar diet and the evaluation of potential antiviral activity against infectious salmon anemia virus. J. Appl. Phycol. 28, 1343–1351. doi: 10.1007/s10811-015-0648-8
Lozano I., Wacyk J., Perez C., Carrasco J., and Cortez-San Martín M. A. (2019). Diets enriched in red seaweed (Pyropia columbina and Gracilaria Chilensis) cryo concentrates modulate the immune-relevant gene encoding the Mx antiviral protein in salmon (Salmo salar) white blood cells. J. Appl. Phycol. 31, 1415–1424. doi: 10.1007/s10811-018-1595-y
MacArtain P., Gill C. I. R., Brooks M., Campbell R., and Rowland I. R. (2007). Nutritional value of edible seaweeds. Nutr. Rev. 65, 535–543. doi: 10.1111/j.1753-4887.2007.tb00278.x
Madibana M. J. K., Mlambo V., Lewis B., and Fouche C. (2017). Effect of graded levels of dietary seaweed (Ulva sp.) on growth, hematological and serum biochemical parameters in dusky kob, Argyrosomus japonicus, sciaenidae. Egyptian. J. Aquat. Res. 43, 249–254. doi: 10.1016/j.ejar.2017.09.003
Madibana M. J. K., Mlambo V., Lewis B. R., and Uys L. (2020). Dietary seaweed (Ulva sp.) does not alter fatty acid profiles and concentration in South African juvenile dusky kob (Argyrosomus japonicus, Sciaenidae) fillet. J. Appl. Anim. Res. 48, 7–13. doi: 10.1080/09712119.2020.1715223
Magnoni L. J., Martos-Sitcha J. A., Queiroz A., Calduch-Giner J. A., Gonçalves J. F. M., Rocha C. M. R., et al. (2017). Dietary supplementation of heat-treated Gracilaria and Ulva seaweeds enhanced acute hypoxia tolerance in gilthead sea bream (Sparus aurata). Biol. Open 6, 897–908. doi: 10.1242/bio.024299
Mahmoudi N., Safari R., Shabani A., Hoseinifar S. H., Yazici M., and El-Haroun E. (2022). Can dietary Dictyota dichotoma powder affect performance, serum, and mucus immune parameters, and antioxidant defense in zebrafish (Danio rerio)? Aquacult. Rep. 26, 101279. doi: 10.1016/j.aqrep.2022.101279
Manikandan D. B., Veeran S., Seenivasan S., Sridhar A., Arumugam M., Yangen Z., et al. (2022). Exploration of marine red seaweed as a dietary fish meal replacement and its potentiality on growth, hematological, biochemical, and enzyme activity in freshwater fish Labeo rohita. Trop. Anim. Health Prod. 54, 395. doi: 10.1007/s11250-022-03392-4
Mansilla A. and Avila M. (2011). Using Macrocystis pyrifera (L.) C. Agardh from southern Chile as a source of applied biological compounds. Braz. J. Pharmacogn. 21, 262–267. doi: 10.1590/S0102-695X2011005000072
Marinho G., Nunes C., Sousa-Pinto I., Pereira R., Pema P., and Valente L. M. P. (2013). The IMTA-cultivated Chlorophyta Ulva spp. as a sustainable ingredient in Nile tilapia (Oreochromis niloticus) diets. J. Appl. Phycol. 25, 1359–1367. doi: 10.1007/s10811-012-9965-3
Marinho-Soriano E., Fonseca P. C., Carneiro M. A. A., and Moreira W. S. C. (2006). Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour. Technol. 97, 2402–2406. doi: 10.1016/j.biortech.2005.10.014
Marques A., Marçal R., Pereira V., Pereira P., Mieiro C., Guilherme S., et al. (2020). Macroalgae-enriched diet protects gilthead seabream (Sparus aurata) against erythrocyte population instability and chromosomal damage induced by aqua-medicines. J. Appl. Phycol. 32, 1477–1493. doi: 10.1007/s10811-019-01996-2
Marrion O., Schwertz A., Fleurence J., Gueant J. L., and Villaume C. (2003). Improvement of the digestibility of the proteins of the red alga Palmaria palmata by physical processes and fermentation. Food 45, 339–344. doi: 10.1002/food.200390078
Marsham S., Scott G. W., and Tobin M. L. (2007). Comparison of nutritive chemistry of a range of temperate seaweeds. Fd. Chem. 100, 1331–1336. doi: 10.1016/j.foodchem.2005.11.029
McDermid K. J. and Stuercke B. (2003). Nutritional composition of edible Hawaiian seaweeds. J. Appl. Phycol. 15, 513–524. doi: 10.1023/B:JAPH.0000004345.31686.7f
Mensi F., Jamel K., and Amor E. A. (2005). “Potential use of seaweeds in Nile tilapia (Oreochromis niloticus) diets,” in Mediterranean Fish Nutrition, vol. 63 . Eds. Montero D., Basurco B., Nengas I., Alexis M., and Izquierdo M. (Zaragoz, Spain: CIHEAM (Cahiers Options Méditerranéennes), 151–154.
Michalak I., Chojnacka K., and Glavic P. (2009). The possibilities of the application of feed additives from macroalgae in sustainable mineral animal feeding. Am. J. Appl. Sci. 6, 1458–1466. doi: 10.3844/ajassp.2009.1458.1466
Miles D. J. C., Polchana J., Lilley J. H., Kanchanakhan S., Thompson K. D., and Adams A. (2001). Immunostimulation of striped snakehead Channa striata against epizootic ulcerative syndrome. Aquaculture 195, 1–15. doi: 10.1016/S0044-8486(00)00529-9
Misurcova L., Skrovankova S., Samek D., Ambrozova J., and Machu L. (2012). Health benefits of algal polysaccharides in human nutrition. Adv. Fd. Nutr. Res. 66, 76–132. doi: 10.1016/B978-0-12-394597-6.00003-3
Monier M. N., Abdelrhman A. M., Marzouk S. S., Nashaat M., Eissa H. A., El-Din H. M. E., et al. (2022). The potential role of marine macroalgae Padina pavonica on growth performance, histological status, and resistance of the rabbitfish Siganus rivulatus to Pseudomonas anguilliseptica bacteria. Egyptian. J. Aquat. Biol. Fish. 26, 797–819. doi: 10.21608/ejabf.2022.264571
Montgomery W. L. and Gerking S. D. (1980). Marine macroalgae as foods for fishes: an evaluation of potential food quality. Environ. Biol. Fishes. 5, 143–153. doi: 10.1007/BF02391621
Moreira A., Cruz S., Marques R., and Cartaxana P. (2022). The underexplored potential of green macroalgae in aquaculture. Rev. Aquacult. 14, 5–26. doi: 10.1111/raq.12580
Morgan K. C., Wright J. L. C., and Simpson F. J. (1980). Review of chemical constituents of the red alga Palmaria palmata (Dulse). Econ. Bot. 34, 27–50. doi: 10.1007/BF02859553
Moroney N. C., Wan A. H. L., Soler-Vila A., FitzGerald R. D., Johnson M. P., and Kerry J. P. (2014). Inclusion of Palmaria palmata (red seaweed) in Atlantic salmon diets: effects on the quality, shelf-life parameters and sensory properties of fresh and cooked salmon fillets. J. Sci. Fd. Agri. 95, 897–905. doi: 10.1002/jsfa.2015.95.issue-5
Morrissey J., Kraan S., and Guiry M. D. (2001). A Guide to Commercially Important Seaweeds on the Irish Coast (Dublin Ireland: Irish Sea Fisheries Board).
Morshedi V., Bahabadi M. N., Sotoudeh E., Azodi M., and Hafezieh M. (2018). Nutritional evaluation of Gracilaria pulvinata as partial substitute with fish meal in practical diets of barramundi (Lates calcarifer). J. Appl. Phycol. 30, 619–628. doi: 10.1007/s10811-017-1199-y
Mourente G., Good J. E., and Bell J. G. (2005). Partial substitution of fish oil with rapeseed, linseed and olive oils in diets for European sea bass (Dicentrarchus labrax L.): effects on flesh fatty acid composition, plasma prostaglandins E2 and F2α, immune function and effectiveness of a fish oil finishing diet. Aquacult. Nutr. 11, 25–40. doi: 10.1111/j.1365-2095.2004.00320.x
Moutinho S., Linares F., Rodríguez J. L., Sousa V., and Valente L. M. P. (2018). Inclusion of 10% seaweed meal in diets for juvenile and on-growing life stages of Senegalese sole (Solea Senegalensis). J. Appl. Phycol. 30, 3589–3601. doi: 10.1007/s10811-018-1482-6
Murata M. and Nakazoe J. I. (2001). Production and use of marine algae in Japan. JARQ 35, 281–290. doi: 10.6090/jarq.35.281
Mustafa M. G., Wakamatsu S., Takeda T., Umino T., and Nakagawa H. (1995). Effects of algae meal as feed additive on growth, feed efficiency, and body composition in red sea bream. Fish. Sci. 61, 25–28. doi: 10.2331/fishsci.61.25
Mwendwa R., Wawire M., and Kahenya P. (2023). Potential for use of seaweed as a fish feed ingredient: A review. J. Agri. Sci. 15, 2023. doi: 10.5539/jas.v15n2p96
Nakagawa H. (1997). Effect of dietary algae on improvement of lipid metabolism in fish. BioMed. Pharmacother. 51, 345–348. doi: 10.1016/S0753-3322(97)88053-5
Nakagawa H. and Kasahara S. (1986). Effect of Ulva meal supplement to diet on the lipid metabolism of red sea bream. Bull. Jap. Soc. Sci. Fish. 52, 1887–1893. doi: 10.2331/suisan.52.1887
Nakagawa H., Mustafa M. G., Takil K., Umion T., and Kumai H. (2000). Effect of dietary catechin and Spirulina on vitamin C metabolism in red sea bream. Fish. Sci. 66, 321–326. doi: 10.1046/j.1444-2906.2000.00050.x
Nakagawa H., Umino T., and Tasaka Y. (1997). Usefulness of Ascophyllum meal as a feed additive for red sea bream, Pagrus major. Aquaculture 151, 275–281. doi: 10.1016/S0044-8486(96)01488-3
Narayan B., Miyashita K., and Hosakawa M. (2006). Physiological effects of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) - a review. Fd. Rev. Internat. 22, 291–307. doi: 10.1080/87559120600694622
National Research Council (NRC) (1993). Nutrient Requirements of Fish (Washington, DC: The National Academies Press).
National Research Council (NRC) (2011). Nutrient requirements of Fish and Shrimp (Washington, DC: The National Academies Press).
Naylor R. and Burke M. (2005). Aquaculture and ocean resources: Raising tigers of the sea. Ann. Rev. Environ. Resour. 30, 185–218. doi: 10.1146/annurev.energy.30.081804.121034
Naylor R. L., Hardy R. W., Bureau D. P., Chiu A., Elliott M., and Farrell A. P. (2009). Feeding aquaculture in an era of finite resources. Proc. Natl. Acad. Sci. U.S.A. 106, 15103–15110. doi: 10.1073/pnas.0905235106
Nickell D. C. and Bromage N. R. (1998). The effect of timing and duration of feeding astaxanthin on the development and variation of fillet colour and efficiency of pigmentation in rainbow trout (Oncorhynchus mykiss). Aquaculture 169, 233–246. doi: 10.1016/S0044-8486(98)00385-8
Nigam P. S. and Singh A. (2011). Production of liquid biofuels from renewable resources. Prog. Energy Combustion. Sci. 37, 52–68. doi: 10.1016/j.pecs.2010.01.003
Nomura M., Kamogawa H., Susanto E., Kawagoe C., Yasui H., and Saga N. (2013). Seasonal variations of total lipids, fatty acid composition, and fucoxanthin contents of Sargassum horneri (Turner) and Cystoseira hakodatensis (Yendo) from the northern seashore of Japan. J. Appl. Phycol. 25, 1159–1169. doi: 10.1007/s10811-012-9934-x
Norambuena F., Hermon K., Skrzypczyk V., Emery J. A., Sharon Y., and Beard A. (2015). Algae in fish feed: Performances and fatty acid metabolism in juvenile Atlantic salmon. PloS One 10, 1–17. doi: 10.1371/journal.pone.0124042
O’Brien R., Hayes M., Sheldrake G., Tiwari B., and Walsh P. (2022). Macroalgal proteins: a review. Foods 11, 571. doi: 10.3390/foods11040571
Olsen Y. (2011). Resources for fish feed in future mariculture. Aquacult. Environ. Interact. 1, 187–2011. doi: 10.3354/aei00019
Olsen R. L. and Hasan M. R. (2012). A limited supply of fishmeal: Impact on future increases in global aquaculture production. Trends Fd. Sci. Technol. 27, 120–128. doi: 10.1016/j.tifs.2012.06.003
Ortiz J., Romero N., Robert P., Araya J., Lopez-Hernandez L., and Bozzo C. (2006). Dietary fibre, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea Antarctica. Fd. Chem. 99, 98–104. doi: 10.1016/j.foodchem.2005.07.027
Ortiz J., Uquiche E., Robert P., Araya J., Romero N., and Quitral V. (2009). Functional and nutritional value of the Chilean seaweeds Codium fragile, Gracilaria Chilensis and Macrocystis pyrifera. Eur. J. Lipid Sci. Technol. 111, 320–327. doi: 10.1002/ejlt.200800140
Owen J. M., Adron J. W., Middleton C., and Cowey C. B. (1975). Elongation and desaturation of dietary fatty acids in turbot (Scophthalmus maximus) and rainbow trout (Salmo gairdneri). Lipids 10, 528–531. doi: 10.1007/BF02532354
Palstra A. P., Kals J., Blanco Garcia A., Dirks R. P., and Poelman M. (2018). Immunomodulatory effects of dietary seaweeds in LPS challenged Atlantic salmon Salmo salar as determined by deep RNA sequencing of the head kidney transcriptome. Front. Physiol. 9, 625. doi: 10.3389/fphys.2018.00625
Passos R., Correia A. P., Ferreira I., Pires P., Pires D., Gomes E., et al. (2021a). Effect on health status and pathogen resistance of gilthead seabream (Sparus aurata) fed with diets supplemented with Gracilaria gracilis. Aquaculture 531, 735888. doi: 10.1016/j.aquaculture.2020.735888
Passos R., Correia A. P., Pires D., Pires P., Ferreira I., Simoes M., et al. (2021b). Potential use of macroalgae Gracilaria gracilis in diets for European seabass (Dicentrarchus labrax): Health benefits from a sustainable source. Fish. Shellfish. Immunol. 119, 105–113. doi: 10.1016/j.fsi.2021.09.033
Pauly D. and Froese R. (2018). FishBase (version 2025-05-03). In: Eds. Bánki O., Roskov Y., Döring M., Ower G., D., Robles Hernández R., C., Corredor Plata A., et al. Catalogue of Life (Version 2025-05-13). Amsterdam, Netherlands: Catalogue of Life. doi: 10.48580/dgqdn-37v
Pawson M. G., Kupschus S., and Pickett G. D. (2007). The status of sea bass (Dicentrarchus labrax) stocks around England and Wales, derived using a separable catch-at-age model, and implications for fisheries management. ICES. Mar. Sci. 64, 346–356. doi: 10.1093/icesjms/fsl030
Peddie S. and Secombes C. J. (2002). An Overview of fish immunostimulant research. Fish. Vet. J. 8, 1–31.
Peddie S., Zou J., and Secombes C. J. (2002). Immunostimulation in the rainbow trout (Oncorhynchus mykiss) following intraperitoneal administration of Ergosan. Vet. Immunol. Immunopathol. 86, 101–113. doi: 10.1016/S0165-2427(02)00019-3
Peixoto M. J., Ferraz R., Magnoni L. J., Pereira R., Gonçalves J. F., Calduch-Giner J., et al. (2019). Protective effects of seaweed supplemented diet on antioxidant and immune responses in European seabass (Dicentrarchus labrax) subjected to bacterial infection. Sci. Rep. 9, 16134. doi: 10.1038/s41598-019-52693-6
Peixoto M. J., Salas-Leitón E., Pereira L. F., Queiroz A., Magalhães F., Pereira R., et al. (2016a). Role of dietary seaweed supplementation on growth performance, digestive capacity and immune and stress responsiveness in European seabass (Dicentrarchus labrax). Aquacult. Rep. 3, 189–197. doi: 10.1016/j.aqrep.2016.03.005
Peixoto M. J., Svendsen J. C., Malte H., Pereira L. F., Carvalho P., and Pereira R. (2016b). Diets supplemented with seaweed affect metabolic rate, innate immune, and antioxidant responses, but not individual growth rate in European seabass (Dicentrarchus labrax). J. Appl. Phycol. 28, 2061–2071. doi: 10.1007/s10811-015-0736-9
Penaflorida V. D. and Golez N. V. (1996). Use of seaweed meals from Kappaphycus alvarezii and Gracilaria heteroclada as binders in diets for juvenile shrimp Penaeus monodon. Aquaculture 143, 393–401. doi: 10.1016/0044-8486(96)01282-3
Pereira V., Marques A., Gaivão I., Rego A., Abreu H., Pereira R., et al. (2019). Marine macroalgae as a dietary source of genoprotection in gilthead seabream (Sparus aurata) against endogenous and exogenous challenges. Comp. Biochem. Physiol. Part C.: Toxicol. Pharmacol. 219, 12–24. doi: 10.1016/j.cbpc.2019.01.006
Pereira R., Valente L. M. P., Sousa-Pinto I., and Rema P. (2012). Apparent nutrient digestibility of seaweeds by rainbow trout (Oncorhynchus mykiss) and Nile tilapia (Oreochromis niloticus). Algal. Res. 1, 77–82. doi: 10.1016/j.algal.2012.04.002
Pham M. A., Lee K. J., Lee B. J., Lim S. J., Kim S. S., and Lee Y. D. (2006). Effects of dietary Hizikia fusiformis on growth and immune responses in juvenile olive flounder (Paralichthys olivaceus). Asian-Australasian. J. Anim. Sci. 19, 1769–1775. doi: 10.5713/ajas.2006.1769
Pierre S., Gaillard S., Prevot-D’Alvise N., Aubert J., Rostaing-Capaillon O., and Leung-Tack D. (2008). Grouper aquaculture: Asian success and Mediterranean trials. Aquat. Conserv.: Mar. Freshw. Ecosyst. 18, 297–308. doi: 10.1002/aqc.v18:3
Poston H. A. and Rumsey G. L. (1983). Factors affecting dietary requirement and deficiency signs of L-tryptophan in rainbow trout. J. Nutr. 113, 2568–2577. doi: 10.1093/jn/113.12.2568
Pouomogne V. (2010). Cultured Aquatic Species Information Programme. Clarias gariepinus. FAO Fisheries and Aquaculture Department (Rome, Italy: Food and Agriculture Organisation of the United Nations).
Pretty J. (2008). Agricultural sustainability: concepts, principles and evidence. Phil. Trans. R. Soc. B-Biol. Sci. 363, 447–465. doi: 10.1098/rstb.2007.2163
Rabia M. D. S. (2016). Growth and yield of orange spotted spinefoot (Siganus guttatus) reared in brackish water pond fed with different diets. Environ. Sci. 4, 31–37. doi: 10.1088/1755-1315/1033/1/012011
Ragaza J. A., Koshio S., Mamauag R. E., Ishikawa M., Yokoyama S., and Villamor S. S. (2015). Dietary supplemental effects of red seaweed Eucheuma denticulatum on growth performance, carcass composition and blood chemistry of juvenile Japanese flounder, Paralichthys olivaceus. Aquacult. Res. 46, 647–657. doi: 10.1111/are.2015.46.issue-3
Rahman M. M. (2015). Role of common carp (Cyprinus carpio) in aquaculture production systems. Front. Life Sci. 8, 399–410. doi: 10.1080/21553769.2015.1045629
Raja E., John Peter Paul J., and Mahesh S. (2022). Growth performance and immune response of Oreochromis niloticus L. with the effect of marine macroalgal fish feed: Halymenia Floresii (Clemente) C.Ag. J. Coast. Life Med. 10, 28–39.
Rasmussen R. S. (2001). Quality of farmed salmonids with emphasis on proximate composition, yield and sensory characteristics. Aquacult. Res. 32, 767–786. doi: 10.1046/j.1365-2109.2001.00617.x
Ratana-arporn P. and Chirapart A. (2006). Nutritional evaluation of tropical green seaweeds Caulerpa lentillifera and Ulva reticulata. Kasetsart. J. (Nat. Sci). 40, 75–83.
Refstie S., Korsoen O. J., Storebakken T., Baeverford G., Lein I., and Roem A. J. (2000). Differing nutritional responses to dietary soybean meal in rainbow trout (Oncorhynchus mykiss) and Atlantic salmon (Salmo salar). Aquaculture 190, 49–63. doi: 10.1016/S0044-8486(00)00382-3
Rey-Crespo F., Lopez-Alonso M., and Miranda M. (2014). The use of seaweed from the Galician coast as a mineral supplement in organic dairy cattle. Animal 8, 580–586. doi: 10.1017/S1751731113002474
Ribeiro A. R., Goncalves A., Colen R., Nunes M. L., Dinis M. T., and Dias J. (2015). Dietary macroalgae is a natural and effective tool to fortify gilthead seabream fillets with iodine: Effects on growth, sensory quality and nutritional value. Aquaculture 437, 51–59. doi: 10.1016/j.aquaculture.2014.11.028
Rimmer M. A. and Russell D. J. (1998). “Aspects of the biology and culture of Lates calcarifer,” in Tropical Mariculture. Ed. De Silva S. S. (San Diego, USA: Academic Press), 449–476.
Rioux L. E., Turgeon S. L., and Beaulieu M. (2007). Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polymers. 69, 530–537. doi: 10.1016/j.carbpol.2007.01.009
Robledo D. and Pelegrin Y. F. (1997). Chemical and mineral compostion of six potentially edible seaweed species of Yucatan. Botanica Marina. 40, 301–306. doi: 10.1515/botm.1997.40.1-6.301
Rohani-Ghadikolaei K., Abdulalian E., and Ng W. K. (2012). Evaluation of the proximate, fatty acid and mineral composition of representative green, brown and red seaweeds from the Persian Gulf of Iran as potential food and feed resources. J. Fd. Sci. Technol. 49, 774–780. doi: 10.1007/s13197-010-0220-0
Rosenlund G. and Skretting M. (2006). Worldwide status and perspective on gadoid culture. ICES. J. Mar. Sci. 63, 194–197. doi: 10.1016/j.icesjms.2005.11.012
Rouhani E., Safari R., Imanpour M. R., Hoseinifar S. H., Yazici M., and El-Haroun E. (2022). Effect of dietary administration of green macroalgae (Ulva intestinalis) on mucosal and systemic immune parameters, antioxidant defence, and related gene expression in zebrafish (Danio rerio). Aquacult. Nutr. 2022, 7693468. doi: 10.1155/2022/7693468
Ruperez P. (2002). Mineral content of edible marine seaweeds. Fd. Chem. 79, 23–26. doi: 10.1016/S0308-8146(02)00171-1
Ruperez P. and Saura-Calixto F. (2001). Dietary fibre and physicochemical properties of edible Spanish seaweeds. Eur. Fd. Res. Technol. 212, 349–354. doi: 10.1007/s002170000264
Sáez M. I., Vizcaíno A., Galafat A., Anguís V., Fernández-Díaz C., Balebona M. C., et al. (2020). Assessment of long-term effects of the macroalgae Ulva ohnoi included in diets on Senegalese sole (Solea Senegalensis) fillet quality. Algal. Res. 47, 101885. doi: 10.1016/j.algal.2020.101885
Safavi S. V., Kenari A. A., Tabarsa M., and Esmaeili N. (2019). Effect of sulfated polysaccharides extracted from marine macroalgae (Ulva intestinalis and Gracilariopsis persica) on growth performance, fatty acid profile, and immune response of rainbow trout (Oncorhynchus mykiss). J. Appl. Phycol. 31, 4021–4035. doi: 10.1007/s10811-019-01902-w
Sakthivel M., Deivasigamani B., Rajasekar T., Kumaran S., and Alagappan K. M. (2015). Immunostimulatory effects of polysaccharide compound from seaweed Kappaphycus alvarezii on Asian seabass (Lates calcarifer) and it’s resistance against Vibrio parahaemolyticus. J. Mar. Biol. Oceanogr. 4, 1–9. doi: 10.4172/2324-8661.1000144
Saleh M. (2008). “Capture-based aquaculture of mullets in Egypt,” in Capture-based aquaculture. Global overview. FAO Fisheries Technical Paper. Eds. Lovatelli A. and Holthus P. F. (Food and Agriculture Organisation of the United Nations, Rome, Italy), 109–126.
Saleh H. H. E. (2020). Review on using of macro algae (seaweeds) in fish nutrition. J. Zool. Res. 2 (02), 6–11. doi: 10.30564/jzr.v2i2.2054
Sanchez-MaChado D. I., Lopez-Cervantes J., Lopez-Hernandez J., and Paseiro-Losada P. (2004). Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Fd. Chem. 85, 439–444. doi: 10.1016/j.foodchem.2003.08.001
Sathivel A., Raghavendran H. R. B., Srinivasan P., and Devaki T. (2008). Anti-peroxidative and anti-hyperlipidemic nature of Ulva lactuca crude polysaccharide on D-galactosamine induced hepatitis in rats. Fd. Chem. Toxicol. 46, 3262–3267. doi: 10.1016/j.fct.2008.07.016
Satpati G. G. and Pal R. (2011). Biochemical composition and lipid characterization of marine green alga Ulva rigida - a nutritional approach. J. Algal. Biomass Util. 2, 10–13.
Schiener P., Black K. D., Stanley M. S., and Green D. H. (2015). The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. J. Appl. Phycol. 27, 363–373. doi: 10.1007/s10811-014-0327-1
Schmid S., Ranz D., He M. L., Burkard S., Lukowicz M. V., and Reiter R. (2003). Marine algae as natural source of iodine in the feeding of freshwater fish - a new possibility to improve iodine supply of man. Rev. Méd. Vét. 154, 645–648.
Seo J. Y., Kim K. D., Shin I. S., Choi K. D., and Lee S. M. (2009). Effects of supplemental dietary wasabi extract, chitosan and Porphyra on growth and body composition of juvenile flounder, Paralichthys olivaceus. Korean. J. Fish. Aquat. Sci. 42, 257–261. doi: 10.5657/kfas.2009.42.3.257
Shapawi R., Safiin N. S. Z., and Senoo S. (2015). Improving dietary red seaweed Kappaphycus alvarezii (Doty) Doty ex. P. Silva meal utilization in Asian seabass Lates calcarifer. J. Appl. Phycol. 27, 1681–1688. doi: 10.1007/s10811-014-0454-8
Shapawi R. and Zamry A. A. (2016). Response of Asian seabass, Lates calcarifer juvenile fed with different seaweed-based diets. J. Appl. Anim. Res. 44, 121–125. doi: 10.1080/09712119.2015.1021805
Shields R. J. and Lupatsch I. (2012). Algae for aquaculture and animal feeds. Technikfolgenabschätzung 21, 23–37. doi: 10.1515/9783110298321.79
Siddik M. A. B., Rahman M. M., Anh N. T. N., Nevejan N., and Bossier P. (2015). Seaweed, Enteromorpha intestinalis, as a diet for Nile tilapia Oreochromis niloticus fry. J. Appl. Aquacult. 27, 113–123. doi: 10.1080/10454438.2015.1008286
Silva D. M., Valente L. M. P., Sousa-Pinto I., Pereira R., Pires M. A., and Seixas F. (2014). Evaluation of IMTA-produced seaweeds (Gracilaria, Porphyra and Ulva) as dietary ingredients in Nile tilapia, Oreochromis niloticus L., juveniles. Effects on growth performance and gut histology. J. Appl. Phycol. 27, 1671–1680. doi: 10.1007/s10811-014-0453-9
Slaski R. J. and Franklin P. T. (2011). A review of the status of the use and potential to use micro and macroalgae as commercially viable raw material sources for aquaculture diets. Report commissioned by SARF (Pitlochry, Scotland: Scottish Aquaculture Research Forum), 94 pp.
Soler-Vila A., Coughlan S., Guiry M. D., and Kraan S. (2009). The red alga Porphyra dioica as a fish-feed ingredient for rainbow trout (Oncorhynchus mykiss): effects on growth, feed efficiency, and carcass composition. J. Appl. Phycol. 21, 617–624. doi: 10.1007/s10811-009-9423-z
Song J. W., Jang J. W., Kim S. S., Oh D. H., Cha J. H., and Lee K. J. (2011). Effect of dietary supplementation with alga (Hizikia fusiformis and Ecklonia cava) on the non-specific immune responses of parrot fish Oplegnathus fasciatus. Korean. J. Fish. Aquat. Sci. 43, 332–338. doi: 10.5657/KFAS.2011.0332
Song L. M., Munian K., Rashid Z. A., and Bhassu S. (2013). Characterisation of Asian snakehead murrel Channa striata (Channidae) in Malaysia: An insight into molecular data and morphological approach. Sci. World J. 2013, 917506. doi: 10.1155/tswj.v2013.1
Sotoudeh E. and Mardani F. (2018). Antioxidant-related parameters, digestive enzyme activity and intestinal morphology in rainbow trout (Oncorhynchus mykiss) fry fed graded levels of red seaweed, Gracilaria pygmaea. Aquacult. Nutr. 24, 777–785. doi: 10.1111/anu.2018.24.issue-2
Stadtlander T., Khalil W. K. B., Focken U., and Becker K. (2013). Effects of low and medium levels of red alga Nori (Porphyra yezoensis Ueda) in the diets on growth, feed utilization and metabolism in intensively fed Nile tilapia, Oreochromis niloticus (L.). Aquacult. Nutr. 19, 64–73. doi: 10.1111/j.1365-2095.2012.00940.x
Stickney R. R. and Shumway S. E. (1974). Occurrence of cellulose activity in the stomachs of fish. J. Fish. Biol. 6, 779–790. doi: 10.1111/j.1095-8649.1974.tb05120.x
Stone D. A. J. (2003). Dietary carbohydrate utilization by fish. Rev. Fish. Sci. 11, 337–369. doi: 10.1080/10641260390260884
Stone D. A. J., Allan G. L., and Anderson A. J. (2003). Carbohydrate utilization by silver perch Bidyanus bidyanus (Mitchell), I. Uptake and clearance of monosaccharides following intraperitoneal injection. Aquacult. Res. 34, 97–108. doi: 10.1046/j.1365-2109.2003.00806.x
Storebakken T. and Austreng E. (1987). Binders in fish feeds II. Effect of different alginates on the digestibility of macronutrients in rainbow trout. Aquaculture 60, 121–131. doi: 10.1016/0044-8486(87)90304-8
Sulaeman H. A., Zainuddin, and Laining A. (2022). Evaluation of seaweed, Ulva lactuca as a fresh diet for nursery stage of golden rabbitfish, Siganus guttatus. IOP. Conf. Ser.: Earth Environ. Sci. 1119, 012057. doi: 10.1088/1755-1315/1119/1/012057
Tabarsa M., Rezaei M., Ramezanpour Z., and Waaland J. R. (2011). Chemical compositions of the marine algae Gracilaria salicornia (Rhodophyta) and Ulva lactuca (Chlorophyta) as a potential food source. J. Sci. Fd. Agricult. 92, 2500–2506. doi: 10.1002/jsfa.v92.12
Taboada C., Millan R., and Miguez I. (2013). Evaluation of marine algae Undaria pinnatifida and Porphyra purpurea as a food supplement: composition, nutritional value and effect of intake on intestinal, hepatic and renal enzyme activities in rats. J. Sci. Fd. Agricult. 93, 1863–1868. doi: 10.1002/jsfa.2013.93.issue-8
Tacon A. G. J., Hasan M. R., and Metian M. (2011). Demand and supply of feed ingredients for farmed fish and crustaceans: trends and prospects. FAO Fisheries and Aquaculture Technical Paper No. 564 (Rome. Italy: Food and Agriculture Organisation of the United Nations), 87 pp.
Tacon A. G. J. and Metian M. (2008). Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture 285, 146–158. doi: 10.1016/j.aquaculture.2008.08.015
Tacon A. G. J., Rausin N., Kadari M., and Cornelis P. (1990). The food and feeding of marine finfish in floating net cages at the National Seafarming Development Centre, Lampung, Indonesia: rabbitfish, Siganus canaliculatus (Park). Aquacult. Fish. Manage. 21, 375–390. doi: 10.1111/j.1365-2109.1990.tb00476.x
Thépot V., Campbell A. H., Paul N. A., and Rimmer M. A. (2021a). Seaweed dietary supplements enhance the innate immune response of the mottled rabbitfish, Siganus fuscescens. Fish. Shellfish. Immunol. 113, 176–184. doi: 10.1016/j.fsi.2021.03.018
Thépot V., Campbell A. H., Rimmer M. A., Jelocnik M., Johnston C., Evans B., et al. (2022). Dietary inclusion of the red seaweed Asparagopsis taxiformis boosts production, stimulates immune response and modulates gut microbiota in Atlantic salmon, Salmo salar. Aquaculture 546, 737286. doi: 10.1016/j.aquaculture.2021.737286
Thépot V., Campbell A. H., Rimmer M. A., and Paul N. A. (2021b). Meta-analysis of the use of seaweeds and their extracts as immunostimulants for fish: a systematic review. Rev. Aquacult. 13, 907–933. doi: 10.1111/raq.12504
Tocher D. R. (2015). Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 449, 94–107. doi: 10.1016/j.aquaculture.2015.01.010
Trushenski J., Kasper C., and Kohler C. (2006). Challenges and opportunities in finfish nutrition. North Am. J. Aquacult. 68, 122–140. doi: 10.1577/A05-006.1
Tseng C. K. (2001). Algal biotechnology industries and research activities in China. J. Appl. Phycol. 13, 375–380. doi: 10.1023/A:1017972812576
Tuller J., De Santis C., and Jerry D. R. (2014). Dietary influence of Fucoidan supplementation on growth of Lates calcarifer (Bloch). Aquacult. Res. 45, 749–754. doi: 10.1111/are.2014.45.issue-4
Turchini G. M., Du Z. Y., Olsen R. E., Francis D., Ringø E., and Tocher D. R. (2022). “The lipids,” in Fish Nutrition, 4th ed. Eds. Hardy R. W. and Kaushik S. J. (London UK: Academic Press), 303–467.
Turchini G. M., Ng W. K., and Tocher D. R. (Eds.) (2010). Fish Oil Replacement and Alternative Lipid Sources in Aquaculture Feeds (Boca Raton: CRC Press).
Udayasoundari D., Jeyanthi S., Santhanam P., Devi A. S., Shyamala V., and Thangaraju N. (2016). Effects of partial replacement of fishmeal with seaweed (Lobophora variegata) meal on the growth and biochemical composition of commercial important fish Asian seabass Lates calcarifer (Bloch, 1790) fingerlings. Internat. J. Mar. Sci. 6, 1–8. doi: 10.5376/ijms.2016.06.0025
United Nations (UN) (2015). “Department of economic and social affairs, population division,” in World Population Prospects: The 2015 Revision, Key Findings and Advance Tables. Working Paper No. ESA/P/WP.241 (United Nations, New York).
Valente L. M. P., Gouveia A., Rema P., Matos J., Gomes E. F., and Pinto I. S. (2006). Evaluation of three seaweeds Gracilaria bursa-pastoris, Ulva rigida and Gracilaria cornea as dietary ingredients in European sea bass (Dicentrarchus labrax) juveniles. Aquaculture 252, 85–91. doi: 10.1016/j.aquaculture.2005.11.052
Valente L. M. P., Rema P., Ferraro V., Pintado M., Sousa-Pinto I., and Cunha L. M. (2015). Iodine enrichment of rainbow trout flesh by dietary supplementation with the red seaweed Gracilaria vermiculophylla. Aquaculture 446, 132–139. doi: 10.1016/j.aquaculture.2015.05.004
Vazirzadeh A., Marhamati A., and Chisti Y. (2022). Seaweed-based diets lead to normal growth, improved fillet color but a down-regulated expression of somatotropic axis genes in rainbow trout (Oncorhynchus mykiss). Aquaculture 554, 738183. doi: 10.1016/j.aquaculture.2022.738183
Vazirzadeh A., Marhamati A., Rabiee R., and Faggio C. (2020). Immunomodulation, antioxidant enhancement and immune genes up-regulation in rainbow trout (Oncorhynchus mykiss) fed on seaweeds included diets. Fish. Shellfish. Immunol. 106, 852–858. doi: 10.1016/j.fsi.2020.08.048
Ventura M. R. and Castanon J. I. R. (1998). The nutritive value of seaweed (Ulva lactuca) for goats. Small. Ruminant. Res. 29, 325–327. doi: 10.1016/S0921-4488(97)00134-X
Vizcaíno A. J., Fumanal M., Sáez M. I., TMartínez T. F., Moriñigo M. A., Fernández-Díaz C., et al. (2019). Evaluation of Ulva ohnoi as functional dietary ingredient in juvenile Senegalese sole (Solea Senegalensis): Effects on the structure and functionality of the intestinal mucosa. Algal. Res. 42, 101608. doi: 10.1016/j.algal.2019.101608
Wahbeh M. I. (1997). Amino acid and fatty acid profiles of four species of macroalgae from Aqaba and their suitability for use in fish diets. Aquaculture 159, 101–109. doi: 10.1016/S0044-8486(97)00183-X
Walker A. B., Fournier H. R., Neefus C. D., Nardi G. C., and Berlinsky D. L. (2009). Partial replacement of fish meal with laver Porphyra spp. in diets for Atlantic cod. North Am. J. Aquacult. 71, 39–45. doi: 10.1577/A07-110.1
Walton M. J., Coloso R. M., Cowey C. B., Adron J. W., and Knox D. (1984). The effects of dietary tryptophan levels on growth and metabolism of rainbow trout (Salmo gairdneri). Br. J. Nutr. 51, 279–287. doi: 10.1079/BJN19840032
Wan A. H. L., Davies S. J., Soler-Vila A., Fitzgerald R., and Johnson M. P. (2019). Macroalgae as a sustainable aquafeed ingredient. Rev. Aquacult. 11, 458–492. doi: 10.1111/raq.2019.11.issue-3
Wan A. H. L., Soler-Vila A., O’Keeffe D., Casburn P., Fitzgerald R., and Johnson M. P. (2016). The inclusion of Palmaria palmata macroalgae in Atlantic salmon (Salmo salar) diets: effects on growth, haematology, immunity and liver function. J. Appl. Phycol. 28, 3091–3100. doi: 10.1007/s10811-016-0821-8
Wassef E. A., El Masry M. H., and Mikhail F. R. (2001). Growth enhancement and muscle structure of striped mullet, Mugil cephalus L., fingerlings by feeding algal meal-based diets. Aquacult. Res. 32, 315–322. doi: 10.1046/j.1355-557x.2001.00043.x
Wassef E. A., El-Sayed A. F. M., Kandeel K. M., and Sakr E. M. (2005). Evaluation of Pterocladia (Rhodophyta) and Ulva (Chlorophyta) meals as additives to gilthead seabream Sparus aurata diets. Egyptian. J. Aquat. Res. 31, 321–332.
Wassef E. A., El-Sayed A. F. M., and Sakr E. M. (2013). Pterocladia (Rhodophyta) and Ulva (Chlorophyta) as feed supplements for European seabass, Dicentrarchus labrax L., fry. J. Appl. Phycol. 25, 1369–1376. doi: 10.1007/s10811-013-9995-5
Watanabe F., Takenaka S., Katsura H., Masumder S. Z., Abe K., Tamura Y., et al. (1999). Dried green and purple lavers (Nori) contain substantial amounts of biologically active vitamin B12 but less of dietary iodine relative to other edible seaweeds. J. Agri. Fd. Chem. 47, 2341–2343. doi: 10.1021/jf981065c
Watanabe F., Takenaka S., Kittaka-Katsura H., Ebara S., and Miyamoto E. (2002). Characterization and bioavailability of vitamin B12-compounds from edible algae. J. Nutr. Sci. Vitaminol. 48, 325–331. doi: 10.3177/jnsv.48.325
Wen X., Peng C., Zhou H., Lin Z., Lin G., and Chen S. (2006). Nutritional composition and assessment of Gracilaria lemaneiformis Bory. J. Integr. Plant Biol. 48, 1047–1053. doi: 10.1111/j.1744-7909.2006.00333.x
Westneat M. W. and Alfaro M. E. (2005). Phylogenetic relationships and evolutionary history of the reef fish family Labridae. Mol. Phylogen. Evol. 36, 370–390. doi: 10.1016/j.ympev.2005.02.001
Wheeler T. and von Braun J. (2013). Climate change impacts on global food security. Science 341, 508–513. doi: 10.1126/science.1239402
Wilke T., Faulkner S., Murphy L., Kealy L., Kraan S., and Brouns F. (2015). Seaweed enrichment of feed supplied to farm-raised Atlantic salmon (Salmo salar) is associated with higher total fatty acid and LC n-3 PUFA concentrations in fish flesh. Eur. J. Sci. Technol. 117, 767–772. doi: 10.1002/ejlt.201400166
Wong K. H. and Cheung P. C. K. (2000). Nutritional evaluation of some subtropical red and green seaweeds Part I - proximate composition, amino acid profiles and some physico-chemical properties. Fd. Chem. 71, 475–482. doi: 10.1016/S0308-8146(00)00175-8
Xie D., Chen C., Dong Y., You C., Wang S., Monroig Ó., et al. (2021). Regulation of long-chain polyunsaturated fatty acid biosynthesis in teleost fish. Prog. Lipid Res. 82, 101095. doi: 10.1016/j.plipres.2021.101095
Xie D., Li X., You C., Wang S., and Li Y. (2019). Supplementation of macroalgae together with non-starch polysaccharide-degrading enzymes in diets enhanced growth performance, innate immune indexes, and disease resistance against Vibrio parahaemolyticus in rabbitfish Siganus canaliculatus. J. Appl. Phycol. 31, 2073–2083. doi: 10.1007/s10811-018-1662-4
Xie J. and Niu J. (2022). Evaluation of four macro-algae on growth performance, anti-oxidant capacity and non-specific immunity in golden pompano (Trachinotus ovatus). Aquaculture 548, 737690. doi: 10.1016/j.aquaculture.2021.737690
Xie D., Xu S., Wu Q., Chen F., Wang S., You C., et al. (2018). Changes of visceral properties and digestive enzymes in the herbivorous marine teleost Siganus canaliculatus fed on different diets. Acta Oceanol. Sin. 37, 85–93. doi: 10.1007/s13131-018-1165-9
Xu S., Zhang L., Wu Q., Liu X., Wang S., and You C. (2011). Evaluation of dried seaweed Gracilaria lemaneiformis as an ingredient in diets for teleost fish Siganus canaliculatus. Aquacult. Internat. 19, 1007–1018. doi: 10.1007/s10499-011-9418-z
Xuan X., Li W., Zhu W., and Wang S. (2019). Effects of different levels of macroalga Gracilaria lemaneiformis on growth performance and feed utilization on the red sea bream, Pagrosomus major. J. Appl. Phycol. 31, 3213–3222. doi: 10.1007/s10811-019-01787-9
Xuan X., Wen X., Li S., Zhu D., and Li Y. (2013). Potential use of macro-algae Gracilaria lemaneiformis in diets for the black sea bream, Acanthopagrus schlegelii, juvenile. Aquaculture 412–413, 167–172. doi: 10.1016/j.aquaculture.2013.07.022
Yangthong M., Oncharoen S., and Sripanomyom J. (2014). Effect of sargassum meal supplementation on growth performance of sex-reversed tilapia (Oreochromis niloticus Linn.). Proc. Kasetsart. Univ. Ann. Conf. 52, 234–241.
Yeh S. P., Chang C. A., Chang C. Y., Liu C. H., and Cheng W. (2008). Dietary sodium alginate administration affects fingerling growth and resistance to Streptococcus sp. and iridovirus, and juvenile non-specific immune responses of the orange-spotted grouper, Epinephelus coioides. Fish. Shellfish. Immunol. 25, 19–27. doi: 10.1016/j.fsi.2007.11.011
Yildirim O., Ergun S., Yaman S., and Turker A. (2009). Effects of two seaweeds (Ulva lactuca and Enteromorpha linza) as a feed additive in diets on growth performance, feed utilization, and body composition of rainbow trout (Oncorhynchus mykiss). J. Fac. Vet. Med. 15, 455–460.
Yin G., Li W., Lin Q., Lin X., Lin J., and Zhu Q. (2014). Dietary administration of laminarin improves the growth performance and immune responses in Epinephelus coioides. Fish. Shellfish. Immunol. 41, 402–406. doi: 10.1016/j.fsi.2014.09.027
Yone Y., Furuichi M., and Urano K. (1986). Effects of dietary wakame Undaria penatifida and Ascophyllum nodosum supplements on growth, feed efficiency, and proximate compositions of liver and muscle of red sea bream. Bull. Jap. Soc. Sci. Fish. 52, 1465–1468. doi: 10.2331/suisan.52.1465
Younis E. M., Al-Quffail A. S., Al-Asgah N. A., Abdel-Warith A. A., and Al-Hafedh Y. S. (2018). Effect of dietary fish meal replacement by red algae, Gracilaria arcuata, on growth performance and body composition of Nile tilapia Oreochromis niloticus. Saudi. J. Biol. Sci. 25, 198–203. doi: 10.1016/j.sjbs.2017.06.012
Yousif O. M., Osman M. F., Anwahi A. R., Zarouni M. A., and Cherian T. (2004). Growth response and carcass composition of rabbitfish, Siganus canaliculatus (Park) fed diets supplemented with dehydrated seaweed, Enteromorpha sp. Emirates. J. Fd. Agricult. 16, 18–26. doi: 10.9755/ejfa.v12i1.5016
Zhang X., Wang G., Sun Z., Wang Y., and Hou J. (2017). Mass production of doubled haploids in Japanese flounder, Paralichthys olivaceus. J. World Aquacult. Soc 49, 420–428. doi: 10.1111/jwas.2018.49.issue-2
Zhang X., Wu T., Cai L., and Zhu Y. (2007). Influence of fasting on muscle composition and antioxidant defenses of market-size Sparus microcephalus. J. Zhejiang. Univ. Sci. B. 8, 906–911. doi: 10.1631/jzus.2007.B0906
Zhu D., Wen X., Li S., Xuan X., and Li Y. (2017). Evaluation of the red alga Gracilaria lemaneiformis and brown alga Sargassum horneri as ingredients in diets for white spotted snapper Lutjanus stellatus Akazaki juveniles. J. Appl. Phycol. 29, 3211–3219. doi: 10.1007/s10811-017-1187-2
Zhu D., Wen X., Xuan X., Li S., and Li Y. (2016). The green alga Ulva lactuca as a potential ingredient in diets for juvenile white spotted snapper Lutjanus stellatus Akazaki. J. Appl. Phycol. 28, 703–711. doi: 10.1007/s10811-015-0545-1
Keywords: diet, feeds, farming, finfish, seaweed
Citation: Hughes AD, Twigg GC, Msuya FE, Padmakumar KP and Tocher DR (2025) The use of macroalgae in feeds for finfish aquaculture. Front. Aquac. 4:1570842. doi: 10.3389/faquc.2025.1570842
Received: 04 February 2025; Accepted: 15 May 2025;
Published: 17 June 2025.
Edited by:
Charles Weirich, NOAA National Sea Grant Office, United StatesReviewed by:
Carmen Guadalupe Paniagua Chavez, Centro de Investigación Científica y de Educación Superior de Ensenada, MexicoMetin Yazici, Iskenderun Technical University, Türkiye
Thomas Mock, Deakin University, Australia
Copyright © 2025 Hughes, Twigg, Msuya, Padmakumar and Tocher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adam D. Hughes, QWRhbS5IdWdoZXNAc2Ftcy5hYy51aw==
 Adam D. Hughes
Adam D. Hughes Gail C. Twigg1
Gail C. Twigg1 Krishna P. Padmakumar
Krishna P. Padmakumar