- 1Department of Conservative Dentistry and Periodontology, University Clinic of Dentistry, Medical University of Vienna, Vienna, Austria
- 2Austrian Cluster for Tissue Regeneration, Vienna, Austria
- 3Department of Endodontics, Faculty of Dentistry, Tabriz University of Medical Sciences, Tabriz, Iran
Sclerostin (Sost) and dickkopf (Dkk)-1 are inhibitors of the Wnt signaling pathway that plays a role in regenerative processes. Hypoxia-based strategies are used for regenerative approaches, but the influence of hypoxia on Sost and Dkk-1 production in a pro-inflammatory environment is unclear. The aim of this study was to assess if pro-inflammatory molecules have an influence on Sost and Dkk-1 production in dental pulp cells (DPC) under normoxia and hypoxia. Human DPC were treated with interleukin (IL)-1β, tumor necrosis factor (TNF)α or transforming growth factor (TGF)β, with L-mimosine (L-MIM) or hypoxia or a combination. Sost and Dkk-1 mRNA and protein levels were measured with qPCR and western blot, respectively. TNFα, TGFβ, L-MIM, or combined treatment did not modulate Sost and Dkk-1. IL-1β downregulated Sost at the mRNA level. Hypoxia alone and together with inflammatory markers downregulated Dkk-1 at the mRNA level. Sost and Dkk-1 protein production was below the detection limit. In conclusion, there is a differential effect of hypoxia and IL-1β on the mRNA production of Sost and Dkk-1. Pro-inflammatory molecules do not further modulate the effects of L-MIM or hypoxia on Sost and Dkk-1 production in DPC.
Introduction
The Wnt signaling pathway regulates regenerative processes in various tissues, including oral tissues (Seo et al., 2012). Sclerostin (Sost) and dickkopf (Dkk)-1 are the main inhibitors of the Wnt signaling pathway. With that, they are important regulators of the signaling activity. In dentistry, Sost plays a role in tooth development where it is produced by odontoblasts (Naka and Yokose, 2011), decelerates reparative dentinogenesis and contributes to dental pulp volume (Collignon et al., 2017), influences bone and cementum phenotypes (Kuchler et al., 2014) and is associated with senescence in dental pulp cells (DPC) (Ou et al., 2018). For periodontitis treatment, a monoclonal antibody against Sost has already been evaluated in a pre-clinical study (Taut et al., 2013). Based on the current knowledge, Sost is also considered an interesting target for therapy in endodontics. Dkk-1 is a contributor to dentin formation and mineralization (Han et al., 2011), it might play a role in root resorption (Zhu et al., 2013) and it is possibly connected to the inflammatory response and bone resorption in periapical lesions (Zhang et al., 2014). Hence, also Dkk-1 could be of interest as target in regenerative endodontic approaches.
Hypoxia-based strategies aim to improve angiogenesis in regenerative approaches. One approach is based on the idea to pre-condition cells with hypoxic conditions or hypoxia mimetic agents “to train” them for the hypoxic region of a defect where they are supposed to support regeneration (Janjić et al., 2018b). Diverse effects of hypoxia on Sost and Dkk-1 were discussed (Genetos et al., 2010; Chen et al., 2013; Guo et al., 2014). In human DPC, the hypoxia mimetic agent L-mimosine (L-MIM) downregulated SOST and hypoxia downregulated DKK-1 mRNA production, but this effect could not be reproduced at protein levels, where SOST and DKK-1 were only produced marginally or not at all (Janjić et al., 2018a). Interleukin (IL)-1 (Weng et al., 2012; Ruscitti et al., 2015), tumor necrosis factor (TNF)α (Korkosz et al., 2014; Sebastian and Loots, 2017) and transforming growth factor (TGF)β (Loots et al., 2012; Al Shareef et al., 2018), markers of inflammation, are able to increase levels of Sost and Dkk-1.
Thus, we hypothesized that basal levels of Sost and Dkk-1 can be elevated with inflammatory markers, such that basal levels of Sost and Dkk-1 as well as effects of treatment with hypoxia mimetic agents or hypoxia would be detectable. The aim of the study is to test if pro-inflammatory molecules alone or together with hypoxic conditions have an impact on Sost and Dkk-1 production in human DPC. This knowledge will help to evaluate if Sost and Dkk-1 should be considered as pharmacological targets under inflammatory or hypoxic conditions, e.g. after dental trauma, when regenerative endodontic therapy is indicated.
Materials and Methods
Cell Culture
Human DPC were isolated from the dental pulp tissue of teeth that were extracted for orthodontic reasons and did not show any signs of inflammation. A detailed description of DPC isolation has already been published (Müller et al., 2015). Patients gave oral and written consent to their donation. Processing and use of patient material has been approved by the Ethical committee of the Medical University of Vienna, Vienna, Austria (#631/2007). DPC were cultured in α-minimal essential medium (αMEM medium; Gibco, Invitrogen Corporation, Carlsbad, CA, USA) including fetal bovine serum (FBS; Gibco) and antibiotics (penicillin, streptomycin and amphotericin; Gibco) at 37°C, 5% CO2 and 95% atmospheric moisture. DPC are adherent cells and appear spindle-shaped, corresponding to characteristic fibroblast morphology (Figure 1A). It has been reported that human DPC express surface markers for mesenchymal stem cells, thereby fulfilling the minimal criteria for mesenchymal stem cells (Rosowski et al., 2019).
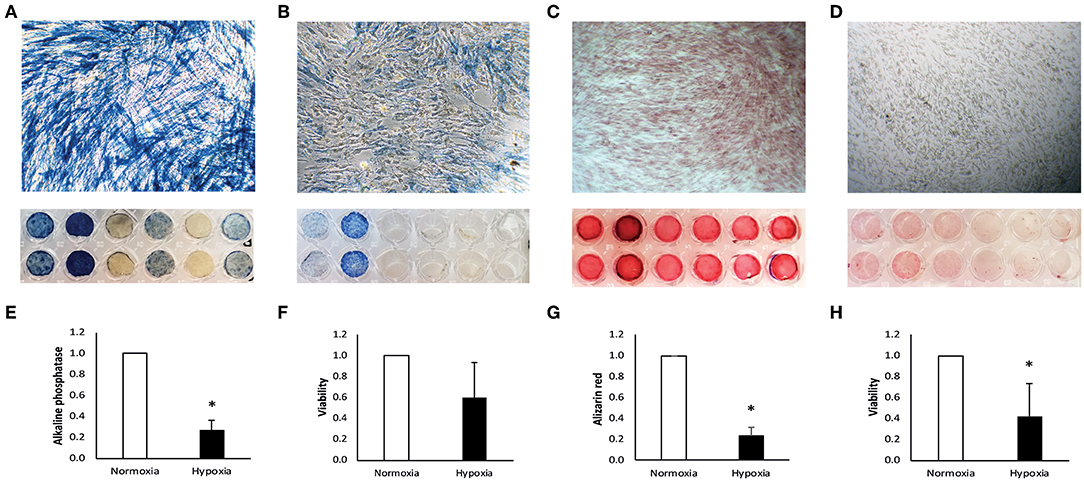
Figure 1. Characterization of human dental pulp cells (DPC). DPC under normoxia (A) and hypoxia (B) were stained for alkaline phosphatase. Staining was quantified and is displayed normalized to the normoxic control (E). Metabolic activity under normoxia and hypoxia was measured in parallel after 7 days (F). After 14 days, DPC were stained with alizarin red for calcium deposition under normoxia (C) and hypoxia (D) and quantified normalized to the normoxic control (G). Metabolic activity was measured at the same time under normoxia and hypoxia (H). Bars represent mean + standard deviation. N = 6. *p < 0.05 relative to normoxic control.
For experiments, 50,000 DPC/cm2 in αMEM medium, supplemented with FBS and antibiotics were seeded into cell culture plates. DPC in passages 2–5 were used, harvested from 6 different donors (N = 6) at 80% confluency. Human DPC have an approximate doubling time of 1 day (Rosowski et al., 2019).
Cell Treatment
DPC were treated 1 day after seeding for 24 h with the inflammatory markers IL-1β at 10 ng/ml (PeproTech Austria, Vienna, Vienna, Austria), TNFα at 10 ng/ml (PeproTech Austria) or TGFβ at 10 ng/ml (isoform TGFβ1; PeproTech Austria) in serum-free αMEM medium (Gibco) with penicillin and streptomycin (Gibco). The time frame of cell treatment was chosen to correspond to a biologically and clinically relevant situation where hypoxic conditions and inflammation occur due to e.g., trauma and normally should undergo regenerative therapy. Hypoxic conditions were mimicked with the hypoxia mimetic agent L-MIM at 1 mM or hypoxia at < 1% O2 was induced using the BD GasPak EZ Pouch system (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) as it matches the biologically relevant range (Agata et al., 2008, 2013). The procedure was previously described in detail (Janjić et al., 2018a). DPC were additionally treated in combination with inflammatory markers and L-MIM or hypoxia, respectively. Untreated DPC under normoxia were used as control. All treatment solutions were prepared in serum-free αMEM medium with antibiotics. The choice of IL-1β (Weng et al., 2012), TNFα (Paula-Silva et al., 2009), TGFβ (Loots et al., 2012) and L-MIM (Janjić et al., 2018a) concentrations was based on previous publications.
Alkaline Phosphatase Assay
DPC were cultured in αMEM medium containing 50 mmol/L L-ascorbic acid and 10 mmol/L β-glycerophosphate (Sigma-Aldrich) under normoxic and hypoxic conditions for 7 days. Hypoxia was induced as described above. Results on alkaline phosphatase production in DPC upon treatment with L-MIM were already published (Müller et al., 2015). A medium change was performed on day 5. For histochemical staining for alkaline phosphatase, DPC were fixed after 7 days with neutral buffered formaldehyde for 5 min at room temperature. A solution of Naphthol AS-TR phosphate disodium salt and Fast Blue BB Salt (Sigma-Aldrich) was added to fixed cells for 30 min at 37°C. Images of stained cells were taken under a light microscope at 100-fold magnification. A photometric measurement of the color change was performed at 405 nm.
Alizarin Red Assay
DPC were cultured under same conditions as described for the alkaline phosphatase assay. Fixation with formaldehyde was performed after 14 days of culture, followed by staining with a 0.5 % solution of alizarin red (Sigma-Aldrich). Light microscopic images of the staining were taken at 100-fold magnification and color change was quantified at 450 nm with a photometer.
Metabolic Activity Assay
A resazurin-based toxicity assay (Merck, Darmstadt, Germany) was performed as described by the manufacturer to evaluate metabolic activity of DPC cultured in αMEM medium containing 50 mmol/L L-ascorbic acid and 10 mmol/L β-glycerophosphate (Sigma-Aldrich) under normoxic and hypoxic conditions for 7 and 14 days. Fluorescence was measured in a photometer at an excitation wavelength of 540/34 nm and an emission wavelength of 600/40 nm.
RNA Isolation and cDNA Synthesis
RNA was isolated from DPC 24 h after treatment with the RNeasy Plus Mini Kit (Qiagen, Hilden, NW, Germany) according to the protocol given by the manufacturer. The quality of isolated RNA was determined by the 260 nm/280 nm ratio and with a mean value of 2.036 ± 0.053 considered as pure material. RNA concentrations were measured photometrically and diluted to 1 μg for cDNA synthesis with a High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA, USA).
qPCR
Produced cDNA was used to measure mRNA levels with TaqMan assays of SOST (Hs00228830_m1; Thermo Fisher Scientific, Waltham, MA, USA) and DKK-1 (Hs00183740_m1; Thermo Fisher Scientific) by qPCR. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Hs02758991_g1; Thermo Fisher Scientific) was used as reference gene. GAPDH showed a more stable mRNA expression in treated and untreated DPC than beta-actin and 18S rRNA, therefore it was chosen as reference gene. Data were analyzed with the 2−ΔΔCT method.
Western Blot
Total protein from DPC was extracted 24 h after treatment using Laemmli Sample Buffer (Bio-Rad Laboratories GmbH, Vienna, Austria). Protein on nitrocellulose membranes were incubated with primary antibodies against SOST (AF1406; R&D Systems, Inc., Minneapolis, MN, USA) and DKK-1 (MA5-32229; Thermo Fisher Scientific). GAPDH (MA5-15738; Thermo Fisher Scientific) was used as reference protein. Chemiluminescence was detected in a ChemiDoc MP System (Bio-Rad Laboratories GmbH).
Statistics
Data are displayed as mean + standard deviation. The mean was calculated from results of six different donors (N = 6). The Kruskal-Wallis test and the Mann-Whitney test were used for statistical analysis and calculated with IBM SPSS Statistics Version 23 (IBM Corporation, Armonk, NY, USA). Statistical significance was determined as p < 0.05.
Results
Alkaline Phosphatase Assay
From six alkaline phosphatase DPC donors two donors did not respond under normoxia (Figure 1A) and only two stained positive for alkaline phosphatase under hypoxia (Figure 1B). Intensity of alkaline phosphatase staining was donor-dependent. Alkaline phosphatase production was significantly reduced under hypoxia, relative to the normoxic control (Figure 1E).
Alizarin Red Assay
Positive staining for calcium deposition in alizarin red staining was found in all DPC donors under normoxia (Figure 1C), but only weak or no staining was found in DPC under hypoxia (Figure 1D). Calcium deposition was significantly reduced under hypoxic conditions, relative to normoxic conditions (Figure 1G).
Metabolic Activity Assay
After incubation in αMEM medium containing 50 mmol/L L-ascorbic acid and 10 mmol/L β-glycerophosphate (Sigma-Aldrich) under normoxia and hypoxia, DPC under hypoxic conditions show a trend for reduced metabolic activity after 7 days (Figure 1F) and significant reduction after 14 days (Figure 1H).
Sclerostin mRNA Levels
SOST mRNA levels significantly decreased in DPC treated with IL-1β. L-MIM and hypoxia did not significantly modulate SOST. A combined treatment of IL-1β and L-MIM or hypoxia did not have any significant effect on SOST production. There was no significant difference in SOST production in DPC treated with L-MIM or hypoxia alone compared to combined treatment with IL-1β (Figure 2A).
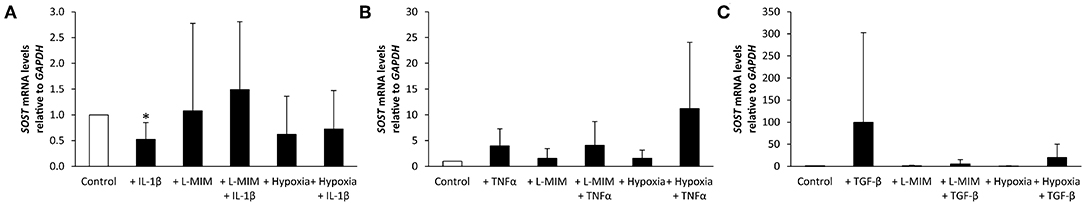
Figure 2. Sclerostin (SOST) mRNA levels in human dental pulp cells (DPC), normalized to the reference gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and relative to the normoxic control (white bar). DPC were treated with different inflammatory markers [(A) interleukin-1beta (IL-1β), (B) tumor necrosis factor alpha (TNFα), (C) transforming growth factor beta (TGFβ)], a hypoxia mimetic agent [L-mimosine (L-MIM)], hypoxia, or their combinations (black bars). Bars represent mean + standard deviation. N = 6. *p < 0.05 relative to control.
SOST mRNA levels showed a trend to increase upon treatment with TNFα alone or combined with L-MIM or hypoxia, but this trend did not reach the level of significance. L-MIM and hypoxia did not show any significant modulation on SOST production. There was no significant difference in SOST production in DPC treated with L-MIM or hypoxia alone compared to combined treatment with TNFα (Figure 2B).
SOST mRNA levels showed a trend to increase upon treatment with TGFβ. L-MIM and hypoxia alone or in combination with TGFβ did not show any significant modulation on SOST production. There was no significant difference in SOST production in DPC treated with L-MIM or hypoxia alone compared to combined treatment with TGFβ (Figure 2C).
Dickkopf-1 mRNA Levels
DKK-1 mRNA levels in DPC were not modulated when treated with IL-1β, L-MIM, or the combination of both. In contrast, DPC treated with hypoxia alone or hypoxia and IL-1β led to a significant downregulation of DKK-1. There was no significant difference in DKK-1 production in DPC treated with L-MIM or hypoxia alone compared to combined treatment with IL-1β (Figure 3A).
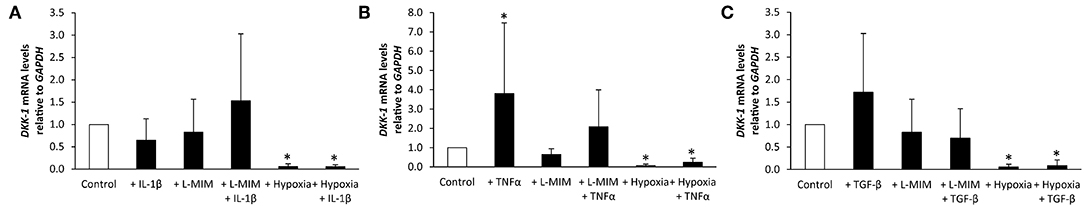
Figure 3. Dickkopf-1 (DKK-1) mRNA levels in human dental pulp cells (DPC), normalized to the reference gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and relative to the normoxic control (white bar). DPC were treated with different inflammatory markers [(A) interleukin-1beta (IL-1β), (B) tumor necrosis factor alpha (TNFα), (C) transforming growth factor beta (TGFβ)], a hypoxia mimetic agent [L-mimosine (L-MIM)], hypoxia or their combinations (black bars). Bars represent mean + standard deviation. N = 6. *p < 0.05 relative to control.
DKK-1 mRNA levels in DPC significantly increased upon treatment with TNFα. L-MIM and the combination of L-MIM and TNFα did not modulate DKK-1. Treatment with hypoxia alone or hypoxia and TNFα significantly decreased DKK-1 production. There is no significant difference in DKK-1 production in DPC treated with L-MIM or hypoxia alone compared to combined treatment with TNFα (Figure 3B).
DKK-1 mRNA levels in DPC was not modulated when treated with TGFβ, L-MIM or the combination of both. DPC treated with hypoxia alone or hypoxia and TGFβ led to a significant downregulation of DKK-1. There was no significant difference in DKK-1 production in DPC treated with L-MIM or hypoxia alone compared to combined treatment with TGFβ (Figure 3C).
Sclerostin and Dickkopf-1 Protein Levels
No bands could be visualized for SOST or DKK-1 with western blot analysis in any of the DPC samples, including untreated controls (Supplementary Figure 1). Detection of GAPDH bands confirmed functionality of the method.
Discussion
The current study aimed to raise production of Sost and Dkk-1 in human DPC with the inflammatory markers IL-1β, TNFα, and TGFβ, such that assessment of basal levels of Sost and Dkk-1 would be enabled as well as studying effects of hypoxic conditions on the production of Sost and Dkk-1.
A previous study revealed that basal levels of Sost and Dkk-1 proteins are hardly detectable in human DPC (Janjić et al., 2018a). Also in the current study, measurement of SOST and DKK-1 remains a major challenge. Current literature reports that pro-inflammatory markers, including IL-1β, TNFα and TGFβ (Loots et al., 2012; Weng et al., 2012; Korkosz et al., 2014; Sebastian and Loots, 2017; Al Shareef et al., 2018), can increase Sost and Dkk-1 in bone and cartilage by transcriptional control. This finding could not be reproduced in our study with DPC. Treatment with IL-1β even led to a contrary effect, i.e. shows downregulation of SOST and DKK-1. This is in line with another publication in fibroblast-like synoviocytes (Yoshida et al., 2018). Thus, pro-inflammatory molecules might have potential to enhance as well as to downregulate Sost and Dkk-1, depending on the cell type. Future studies will have to evaluate, if other oral cell types behave different from DPC in Wnt signaling inhibitor responses under inflammatory conditions. Currently there are hints that periodontitis in gingival tissues increases Sost and Dkk-1 expression (Napimoga et al., 2014).
Tissue mineralization is a characteristic function of bone tissue (Orimo, 2010), but also DPC show mineralization capacity by alkaline phosphatase production and calcium deposition (Müller et al., 2015). Our results confirmed this under normoxia, but under hypoxia mineralization capacity seems to be strongly suppressed. Parallel metabolic activity assays confirmed that an increasing percentage of DPC under hypoxic conditions for 7 and 14 days is metabolically inactive, thus, do not contribute to mineralization.
Gene and protein expression was studied for a shorter period. A treatment duration of 24 h was chosen, because after dental trauma inflammation and hypoxia usually would not be left untreated for longer than 24 h. It is possible that the translation process of SOST and DKK-1 takes more time, which has to be evaluated in further experiments. Even though mRNA levels of SOST and DKK-1 were quantifiable, protein production was not detectable by western blot analysis (Supplementary Figure 1) or enzyme-linked immunosorbent assays (unpublished data), although our previous study was successful in detection of low levels of SOST and DKK-1 (Janjić et al., 2018a). The current study differs in culture conditions from the previous study where cell culture medium contained serum. For this study, only serum-free medium was used to avoid cross-activity with pro-inflammatory molecules. This difference in culture conditions might influence gene expression behavior of the cells. For both studies primary cells from human donors were used, a possibility of patient-specific reactivity has to be considered. In both cases protein expression was hardly detectable or not at all. This suggests that in the dental pulp transcriptional regulation of Sost and Dkk-1 might play a more important role than protein expression, at least under tested conditions. Underlying mechanisms have to be clarified in further experiments.
Taken together, our results show that basal levels of Sost and Dkk-1 in human DPC are very low and often not even detectable. Thus, it would be of interest for experimental purposes to find out if any molecule can stimulate Sost and Dkk-1 production and which function they have in the dental pulp. For clinical purposes, it might be of interest to target Sost and Dkk-1 at a transcriptional level rather than to target protein expression. The hypoxia mimetic agent L-MIM does not seem to have a pronounced effect on SOST and DKK-1 production, while hypoxia significantly downregulates them. Since effects of hypoxia mimetic agents and hypoxia are based on different molecular mechanisms it would be interesting to find out which pathway regulates the decrease of DKK-1 upon hypoxia treatment. Our previous study gives a hint that hypoxia-inducible factor (HIF) is one component that participates in this mechanism (Janjić et al., 2018a). Our data show that seen effects are independent of the influence of the inflammatory markers IL-1β, TNFα and TGFβ, which do not seem to have a pronounced effect on SOST and DKK-1 in DPC. Finally, if modulations in this study were detected, they were detected at mRNA level only. Therefore, the biological function of the two Wnt signaling inhibitors Sost and Dkk-1 is questionable in the dental pulp, since the molecules would exert their biological activity as proteins.
Conclusions
Based on our studies on Sost and Dkk-1 in human DPC it can be concluded that hypoxia significantly downregulates DKK-1 mRNA levels, independent of pro-inflammatory markers. Overall, the protein levels of Sost and Dkk-1 are so low that a biological function of the here observed downregulation is unlikely. Future research will have to assess what exact role Dkk-1 plays in the dental pulp and further how DKK-1 can be targeted with hypoxia-based approaches for regenerative endodontic strategies.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
We used an established protocol for cell isolation. When informed and written consent was given extracted third molars were used to prepare fibroblasts from the dental pulp. The protocol was approved by the Ethics committee of the Medical University of Vienna (631/2007).
Author Contributions
KJ and MS were involved in study design, literature research, data analysis, writing, and submitting the manuscript. AM was involved in the study design, study, and manuscript preparation. HA was involved in study design, data analysis, writing, and submission of the manuscript.
Funding
This study was funded by the European Society of Endodontology (ESE) research grant 2015 from the ESE.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2019.00430/full#supplementary-material
Supplementary Figure 1. Sclerostin (SOST) and Dickkopf-1 (DKK-1) protein levels in human dental pulp cells (DPC), compared to the reference protein glyceraldehyde-3-phosphate dehydrogenase (GAPDH). DPC were treated with interleukin-1beta (A; 2), tumor necrosis factor alpha (B; 2), and transforming growth factor beta (C; 2). Additionally, treatment was combined with L-mimosine (A–C; 4) or hypoxia (A–C; 6). Untreated DPC (A–C; 1), L-mimosine (A–C; 3), and hypoxia (A–C, 5) were added as control.
References
Agata, H., Kagami, H., Watanabe, N., and Ueda, M. (2008). Effect of ischemic culture conditions on the survival and differentiation of porcine dental pulp-derived cells. Differentiation 76, 981–993. doi: 10.1111/j.1432-0436.2008.00282.x
Agata, H., Sumita, Y., Asahina, I., Tojo, A., and Kagami, H. (2013). Ischemic culture of dental pulp-derived cells is a useful model in which to investigate mechanisms of post-ischemic tissue recovery. Histol. Histopathol. 28, 985–991. doi: 10.14670/HH-28.985
Al Shareef, Z., Kardooni, H., Murillo-Garzón, V., Domenici, G., Stylianakis, E., Steel, J. H., et al. (2018). Protective effect of stromal Dickkopf-3 in prostate cancer: opposing roles for TGFBI and ECM-1. Oncogene 37, 5305–5324. doi: 10.1038/s41388-018-0294-0
Chen, D., Li, Y., Zhou, Z., Wu, C., Xing, Y., Zou, X., et al. (2013). HIF-1α inhibits Wnt signaling pathway by activating Sost expression in osteoblasts. PLoS ONE 8:e65940. doi: 10.1371/journal.pone.0065940
Collignon, A. M., Amri, N., Lesieur, J., Sadoine, J., Ribes, S., Menashi, S., et al. (2017). Sclerostin deficiency promotes reparative dentinogenesis. J. Dent. Res. 96, 815–821. doi: 10.1177/0022034517698104
Genetos, D. C., Toupadakis, C. A., Raheja, L. F., Wong, A., Papanicolaou, S. E., Fyhrie, D. P., et al. (2010). Hypoxia decreases sclerostin expression and increases Wnt signaling in osteoblasts. J. Cell. Biochem. 110, 457–467. doi: 10.1002/jcb.22559
Guo, K.-T., Fu, P., Juerchott, K., Motaln, H., Selbig, J., Lah, T., et al. (2014). The expression of Wnt-inhibitor DKK1 (Dickkopf 1) is determined by intercellular crosstalk and hypoxia in human malignant gliomas. J. Cancer Res. Clin. Oncol. 140, 1261–1270. doi: 10.1007/s00432-014-1642-2
Han, X. L., Liu, M., Voisey, A., Ren, Y. S., Kurimoto, P., Gao, T., et al. (2011). Post-natal effect of overexpressed DKK1 on mandibular molar formation. J. Dent. Res. 90, 1312–1317. doi: 10.1177/0022034511421926
Janjić, K., Cvikl, B., Kurzmann, C., Moritz, A., and Agis, H. (2018a). Do hypoxia and L-mimosine modulate sclerostin and dickkopf-1 production in human dental pulp-derived cells? Insights from monolayer, spheroid and tooth slice cultures. BMC Oral Health 18:36. doi: 10.1186/s12903-018-0492-8
Janjić, K., Lilaj, B., Moritz, A., and Agis, H. (2018b). Formation of spheroids by dental pulp cells in the presence of hypoxia and hypoxia mimetic agents. Int Endod. J. 51 (Suppl. 2), e146–e156. doi: 10.1111/iej.12806
Korkosz, M., Gasowski, J., Leszczynski, P., Pawlak-Buś, K., Jeka, S., Siedlar, M., et al. (2014). Effect of tumour necrosis factor-α inhibitor on serum level of dickkopf-1 protein and bone morphogenetic protein-7 in ankylosing spondylitis patients with high disease activity. Scand. J. Rheumatol. 43, 43–48. doi: 10.3109/03009742.2013.805241
Kuchler, U., Schwarze, U. Y., Dobsak, T., Heimel, P., Bosshardt, D. D., Kneissel, M., et al. (2014). Dental and periodontal phenotype in sclerostin knockout mice. Int. J. Oral Sci. 6, 70–76. doi: 10.1038/ijos.2014.12
Loots, G. G., Keller, H., Leupin, O., Murugesh, D., Collette, N. M., and Genetos, D. C. (2012). TGF-β regulates sclerostin expression via the ECR5 enhancer. Bone 50, 663–669. doi: 10.1016/j.bone.2011.11.016
Müller, H.-D., Cvikl, B., Janjić, K., Nürnberger, S., Moritz, A., Gruber, R., et al. (2015). Effects of prolyl hydroxylase inhibitor L-mimosine on dental pulp in the presence of advanced glycation end products. J. Endod. 41, 1852–1861. doi: 10.1016/j.joen.2015.08.002
Naka, T., and Yokose, S. (2011). Spatiotemporal expression of sclerostin in odontoblasts during embryonic mouse tooth morphogenesis. J. Endod. 37, 340–345. doi: 10.1016/j.joen.2010.11.025
Napimoga, M. H., Nametala, C., da Silva, F. L., Miranda, T. S., Bossonaro, J. P., Demasi, A. P. D., et al. (2014). Involvement of the Wnt-β-catenin signalling antagonists, sclerostin and dickkopf-related protein 1, in chronic periodontitis. J. Clin. Periodontol. 41, 550–557. doi: 10.1111/jcpe.12245
Orimo, H. (2010). The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J. Nippon Med. Sch. 77, 4–12. doi: 10.1272/jnms.77.4
Ou, Y., Zhou, Y., Liang, S., and Wang, Y. (2018). Sclerostin promotes human dental pulp cells senescence. PeerJ 6:e5808. doi: 10.7717/peerj.5808
Paula-Silva, F. W. G., Ghosh, A., Silva, L. A., and Kapila, Y. L. (2009). TNF-alpha promotes an odontoblastic phenotype in dental pulp cells. J. Dent. Res. 88, 339–344. doi: 10.1177/0022034509334070
Rosowski, J., Bräunig, J., Amler, A.-K., Strietzel, F. P., Lauster, R., and Rosowski, M. (2019). Emulating the early phases of human tooth development in vitro. Sci. Rep. 9:7057. doi: 10.1038/s41598-019-43468-0
Ruscitti, P., Cipriani, P., Carubbi, F., Liakouli, V., Zazzeroni, F., Di Benedetto, P., et al. (2015). The role of IL-1β in the bone loss during rheumatic diseases. Mediators Inflamm. 2015:782382. doi: 10.1155/2015/782382
Sebastian, A., and Loots, G. G. (2017). Transcriptional control of Sost in bone. Bone 96, 76–84. doi: 10.1016/j.bone.2016.10.009
Seo, M.-S., Hwang, K.-G., Kim, H., and Baek, S.-H. (2012). Analysis of gene expression during odontogenic differentiation of cultured human dental pulp cells. Restor. Dent. Endod. 37, 142–148. doi: 10.5395/rde.2012.37.3.142
Taut, A. D., Jin, Q., Chung, J.-H., Galindo-Moreno, P., Yi, E. S., Sugai, J. V., et al. (2013). Sclerostin antibody stimulates bone regeneration after experimental periodontitis. J. Bone Miner. Res. 28, 2347–2356. doi: 10.1002/jbmr.1984
Weng, L.-H., Ko, J.-Y., Wang, C.-J., Sun, Y.-C., and Wang, F.-S. (2012). Dkk-1 promotes angiogenic responses and cartilage matrix proteinase secretion in synovial fibroblasts from osteoarthritic joints. Arthritis Rheum. 64, 3267–3277. doi: 10.1002/art.34602
Yoshida, Y., Yamasaki, S., Oi, K., Kuranobu, T., Nojima, T., Miyaki, S., et al. (2018). IL-1β enhances wnt signal by inhibiting DKK1. Inflammation 41, 1945–1954. doi: 10.1007/s10753-018-0838-z
Zhang, R., Huang, S., Wang, L., and Peng, B. (2014). Histochemical localization of Dickkopf-1 in induced rat periapical lesions. J. Endod. 40, 1394–1399. doi: 10.1016/j.joen.2014.03.023
Keywords: dental pulp, hypoxia, inflammation, in vitro techniques, regeneration
Citation: Janjić K, Samiei M, Moritz A and Agis H (2019) The Influence of Pro-Inflammatory Factors on Sclerostin and Dickkopf-1 Production in Human Dental Pulp Cells Under Hypoxic Conditions. Front. Bioeng. Biotechnol. 7:430. doi: 10.3389/fbioe.2019.00430
Received: 21 June 2019; Accepted: 02 December 2019;
Published: 17 December 2019.
Edited by:
Martijn van Griensven, Institute for Technology-Inspired Regenerative Medicine (MERLN), Maastricht University, NetherlandsReviewed by:
Kar Wey Yong, University of Calgary, CanadaAbhigyan Satyam, Harvard Medical School, United States
Copyright © 2019 Janjić, Samiei, Moritz and Agis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Klara Janjić, a2xhcmEuamFuamljQG1lZHVuaXdpZW4uYWMuYXQ=
Hermann Agis, aGVybWFubi5hZ2lzQG1lZHVuaXdpZW4uYWMuYXQ=
†These authors have contributed equally to this work
 Klara Janjić
Klara Janjić Mohammad Samiei1,2,3†
Mohammad Samiei1,2,3† Hermann Agis
Hermann Agis