- 1Department of Cardiothoracic Surgery, Stanford University School of Medicine, Stanford, CA, United States
- 2Stanford Cardiovascular Institute, Stanford University School of Medicine, Stanford, CA, United States
- 3Department of Neurology and Neurological Sciences, Stanford University School of Medicine, Stanford, CA, United States
Establishing an appropriate disease model that mimics the complexities of human cardiovascular disease is critical for evaluating the clinical efficacy and translation success. The multifaceted and complex nature of human ischemic heart disease is difficult to recapitulate in animal models. This difficulty is often compounded by the methodological biases introduced in animal studies. Considerable variations across animal species, modifications made in surgical procedures, and inadequate randomization, sample size calculation, blinding, and heterogeneity of animal models used often produce preclinical cardiovascular research that looks promising but is irreproducible and not translatable. Moreover, many published papers are not transparent enough for other investigators to verify the feasibility of the studies and the therapeutics’ efficacy. Unfortunately, successful translation of these innovative therapies in such a closed and biased research is difficult. This review discusses some challenges in current preclinical myocardial infarction research, focusing on the following three major inhibitors for its successful translation: Inappropriate disease model, frequent modifications to surgical procedures, and insufficient reporting transparency.
Introduction
Cardiovascular diseases (CVDs) are devastating health problems worldwide; they accounted for 18.6 million deaths globally in 2019, which amounted to an increase of 17.1% since 2010 (Virani et al., 2021). Myocardial ischemia is the most prevalent cause of death within the spectrum of cardiovascular illnesses. Myocardial ischemia occurs when blood flow to the myocardium is obstructed by a partial or complete blockage of the coronary artery due to plaque buildup (atherosclerosis). Coronary artery narrowing and plaque rupture causes insufficient oxygen delivery to the myocardium, causing myocardial infarction (MI). The American Heart Association estimates that a new MI case is diagnosed every 40 s in the United States (Virani et al., 2021). Over the past several decades, the pathophysiological mechanisms driving these cardiovascular complications have extensively been studied in animal models, resulting in the development of numerous interventional and pharmacological treatments (Nicolini and Gherli, 2009).
Various therapeutic strategies have been proposed to mitigate the risk of myocardial infarction with cardioprotective effects in preclinical studies, but only a few have shown positive clinical study results (Bolli et al., 2004; Kloner, 2013). Ischemic remote, pre-, per-, or post-conditioning (i.e., a series of alternating intervals of brief ischemia and reperfusion) and pharmacological manipulation have been extensively studied over the last 30 years to treat acute myocardial infarction with many positive conclusions and discoveries of many pharmacological targets in preclinical settings (Heusch, 2015). However, most of the clinical outcomes remain mixed or statistically underpowered (Heusch, 2013; Kloner, 2013; Hausenloy and Yellon, 2016; Giustino and Dangas, 2017). For example, reperfusion therapy, often coupled with the administration of adjunctive therapies, has shown to reduce infarct size in animal models of acute myocardial infarction (AMI) and improve left ventricular function; however, it has failed to show similar effects in human AMI patients, potentially due to significant discrepancies between different preclinical animal models and clinical situations (Cannon, 2005; Dirksen et al., 2007; Miura and Miki, 2008; Trankle et al., 2016).
Several cardiac repair strategies have been recently developed with promising preclinical results but also with little translational success. One strategy is the direct injection of cells or biomimetic scaffolds made of polymers with cells, growth factors, or cytokines (Ungerleider and Christman, 2014). However, the grafted cells directly injected through a needle into the myocardium easily aggregate and undergo necrosis, and they are poorly localized on the myocardium of interest, thus limiting the efficacy of the therapy (Menasché, 2018). The tissue engineering using biomaterial scaffolds is limited due to their questionable immuno- or bio-compatibility and bio-functionality (Christman and Lee, 2006; Guo et al., 2020). As an alternative, scaffold-free stem cell sheet treatment has been developed with increased cell engraftment and survival on the host myocardium and promising therapeutic effects in animal studies (Shudo et al., 2011, 2013, 2014), but there are not yet many clinical studies to date (Miyagawa et al., 2017).
Despite the disagreement over the optimal cell type, cell counts, cell delivery methods, and unknown therapeutic mechanisms, stem cell therapies seem to demonstrate some degree of therapeutic improvements in terms of reduced ischemic injury size or improved left ventricular function in MI animal models in preclinical studies (Laflamme et al., 2007; Wang et al., 2009; Wolf et al., 2009; Shudo et al., 2011; Lu et al., 2012; Okura et al., 2012; Li et al., 2013; Chong et al., 2014; Zhao et al., 2014; Alestalo et al., 2015; Haller et al., 2015; Suzuki et al., 2016; Kim et al., 2017; Sharp et al., 2017; Lim et al., 2018; Crisostomo et al., 2019; Ishida et al., 2019; Romagnuolo et al., 2019; Sun et al., 2020). Nevertheless, the promising results of many preclinical studies on cell therapies have not been successfully replicated in randomized clinical trials (Janssens et al., 2006; Lunde et al., 2006; Penicka et al., 2007; Makkar et al., 2012; Perin et al., 2012; Gao et al., 2013; Quyyumi et al., 2017; Wollert et al., 2017). According to the review of articles on PubMed (preclinical) and ClinicalTrials.Gov (clinical research), no regenerative medicine was commercialized between 2008 and 2014, and only about 50 cell therapies and eight gene therapies moved onto the clinical phase, although there had been approximately 800 preclinical studies per year (Ungerleider and Christman, 2014). The frequent failure to translate the cardio-protective and regenerative therapeutics from the bench to the bedside has been attributed to the large gap between animal models and humans and inadequate preclinical study design (Bolli et al., 2004; Kloner and Rezkalla, 2004; Downey and Cohen, 2009; Hausenloy et al., 2010; Ludman et al., 2010; Heusch, 2017). There is a growing concern over the safety and efficacy of regenerative therapeutics, which many researchers have determined to be due to low internal and external validities in preclinical animal research (Ioannidis, 2005, 2016; Bracken, 2009; van der Worp et al., 2010; Hooijmans and Ritskes-Hoitinga, 2013; Steele et al., 2017; Pound and Ritskes-Hoitinga, 2018; Voelkl et al., 2018; Lüscher, 2019; Ferreira et al., 2020). This review addresses the issues prevalent in preclinical MI research, which hinder the successful therapeutic translation of promising treatment strategies. The review proceeds by discussing (1) the obstacles in building a representative animal model for MI studies, (2) factors limiting the scientific rigor in the MI study design, and (3) suggestions for improving the relevance of preclinical MI studies.
Review
Suitability of Animal Models for Human MI
A major hurdle in clinical translation from bench to bedside for MI therapies is the difficulty in creating a representative disease model. Modeling MI induced heart failure (HF) that resembles human cardiac conditions is challenging because human MI develops as a result of the interplay of many causes over time and is often complicated by comorbidity and polypharmacy (Pound and Ritskes-Hoitinga, 2018). A wide range of comorbid health conditions, such as epilepsy, smoking, alcoholism, cancer, diabetes, and rheumatoid arthritis, are known to remarkably affect MI fatality (Quintana et al., 2018). The incidence of HF caused by MI is often age- and gender-biased, with higher rates in men than women and in the elderly than young adults (Savarese and Lund, 2017; Virani et al., 2021). Specific racial and ethnic populations, especially minority groups, are at a considerable risk of developing MI, which may lead to death (Graham, 2015, 2016; Virani et al., 2021). However, many animal studies have failed to reflect the heterogeneity observed in the patients with MI. The animal models currently used in the laboratory settings tend to be relatively homogeneous, young, and healthy, with no genetic predisposition or underlying medical conditions (van der Worp et al., 2010; Pound and Ritskes-Hoitinga, 2018). Many preclinical studies induce MI through direct ligation of coronary artery, which does not represent the natural pathophysiology of atherosclerosis that develops over life time in humans (Getz and Reardon, 2012; Gao et al., 2016; Lee et al., 2017). Different species are used to recapitulate the pathogenesis of MI with its own advantages and disadvantages. Small animal models (rodents) are widely used in MI studies for their practical benefits, such as small body size, easy pre-/post-care, low maintenance cost, shorter generation time, and well-defined genetics. However, small animals have limitations in that their anatomy and cardiac kinetics are fundamentally different from those of humans. For example, rodent hearts function at very high heart rates (HRs), with their resting HR being more than five times higher than in humans. Their small body and organ sizes and short lifespan require expression of different genes related to action potential properties and contractile kinetics in ventricular cardiomyocytes (CMs) (Locher et al., 2009; Milani-Nejad and Janssen, 2014). For example, their ventricular CMs predominately express fast α-myosin heavy chain (MHC) (>94–100%), whereas human LV cardiomyocytes (CMs) predominately expresses slow β-MHC (>90–95%), thus resulting in differential cardiac contractile and kinetic responses to cardiac dysfunction (Milani-Nejad and Janssen, 2014). These differences in cardiac parameters may lead to different results of cell therapy experiments across different animal models. For example, Laflamme et al. (2007) observed frequent arrhythmias in non-human primates and pigs following transplantation of embryonic stem cell-derived cardiomyocytes, but not in rats, possibly because rats’ high heart rate could mask arrhythmias (Chong et al., 2014; Romagnuolo et al., 2019).
Small animals’ body and organ sizes make it even more challenging to mimic the natural pathophysiology of human atherosclerosis and thus MI. The gradual occlusion of the coronary artery can be established in animal models by using interventional operation using various materials, such as Ameroid Constrictors (Shudo et al., 2011; Potz et al., 2018; Ishida et al., 2019). However, small animals’ heart is too small to correctly identify each vasculature, which is tricky to occlude using these materials. The most feasible way to induce MI in small animals is the permanent ligation of the coronary artery using a suture loop, but the etiology is different from that naturally occurring MI in humans in this case. Even though there have been attempts to model atherosclerosis in transgenic or high fat-fed rodents, rodents rarely develop atherosclerosis in coronary arteries but readily in the aortic root probably due to their rapid heart rate and blood flow and often in the absence of complications seen in human MI patients such as thrombosis (Getz and Reardon, 2012; Gao et al., 2016; Lee et al., 2017).
Besides, small animals’ cardiac anatomy and physiology make it challenging to visualize and quantify the spatial distribution of blood flow and assess microvascular histomorphology following MI (Krueger et al., 2013; Liu et al., 2020). To overcome these technical difficulties, some new imaging technologies have been developed to improve spatial resolution, such as the Imaging Cryomicrotome (Krueger et al., 2013), micro-PET/CT hybrid systems (Gargiulo et al., 2012), and magnetic resonance (MR) tagging (Epstein et al., 2002; Thomas et al., 2004). Researchers must consider these fundamental differences in anatomy and cardiac kinetics across species when interpreting the animal study results as they give rise to different phenotypes between humans with genetic predispositions and transgenic animal models that recapitulate the diseases (Riehle and Bauersachs, 2019). Consideration of available options for post-operative evaluation must be made when choosing an animal model as well. Large animals, such as swine and sheep, which are anatomically and physiologically closer to the humans, are used to minimize these phenotypic differences between humans and animal models. In MI research, it is essential to correctly identify the perfusion and coronary collateral circulation systems in the animal of choice, as the variations in these structures across animals can significantly affect the early and progressive response to ischemia (Harken et al., 1981; Hill and Iaizzo, 2009). In this regard, swine and ovine models are preferred to smaller animals, such as rodents and canines, as their coronary arterial structure and scant collateral arteries resemble those of humans, which allows for the creation of predictable infarct size at a preferred location in the myocardium (Dixon and Spinale, 2009; Nguyen and Wu, 2015). Moreover, swine, sheep, and human myocardia share high degrees of similarities in cardiac kinetics (Milani-Nejad and Janssen, 2014) and healing characteristics following injury (Lelovas et al., 2014). A domestic sheep is ideal in size for clinical imaging modalities (such as MRI and CT) and medical devices (such as pacemakers and stents) designed for the humans (Ribitsch et al., 2020).
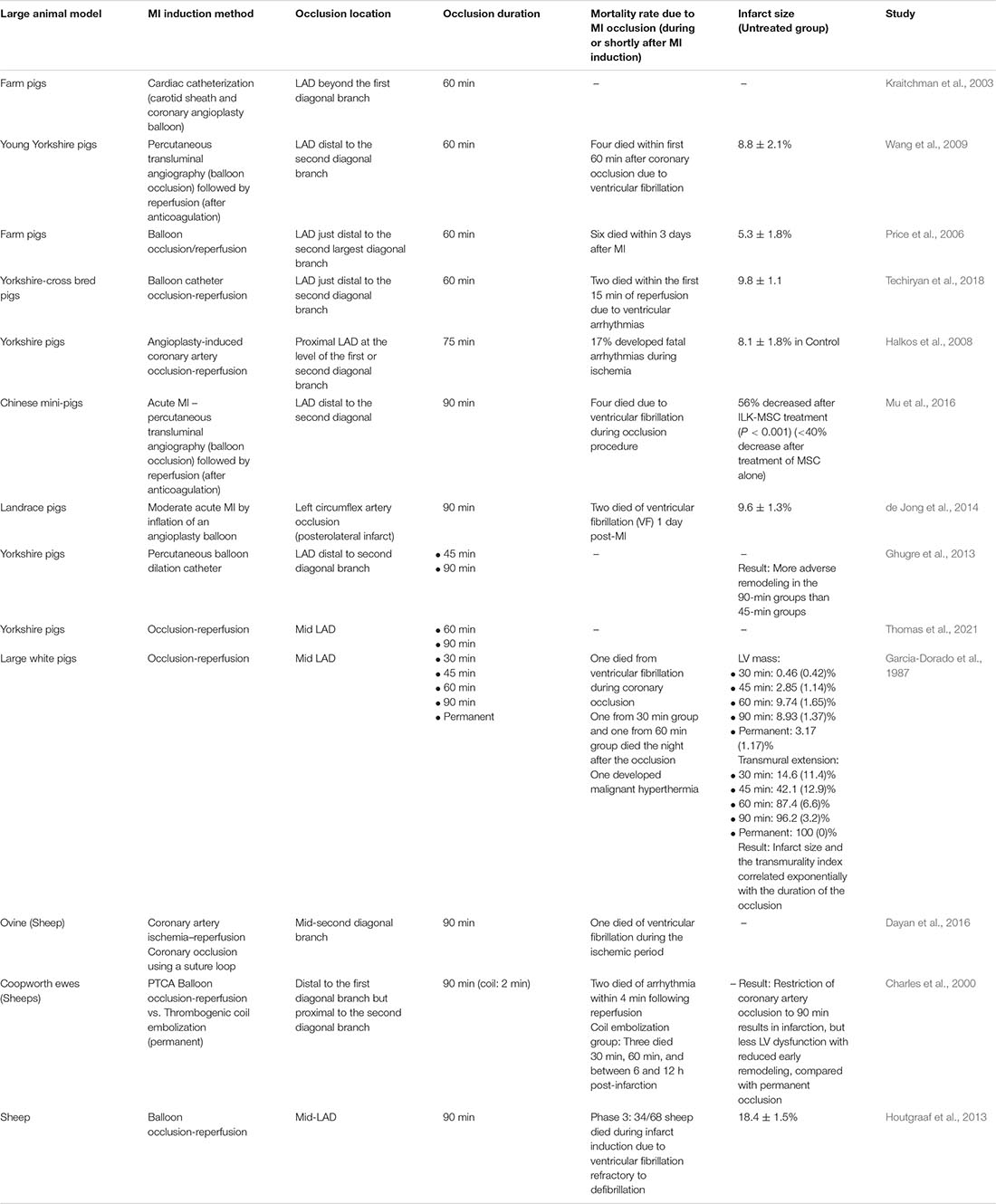
However, there are several disadvantages of using large animal models, which can eventually limit the reproducibility of the research. Some of the factors that discourage their use in research are the high cost required for performing the experiments, housing/maintenance and care, and lower acceptance as model animals by society (Freedman et al., 2015; Camacho et al., 2016; Spannbauer et al., 2019). The public’s growing concern about the welfare of research animals, especially companion animals such as dogs and cats, has led to more stringent laws, policies, and guidelines, limiting their prevalent use in research (National Research Council (Us) Committee on Scientific and Humane Issues in the Use of Random Source Dogs and Cats in Research, 2009). Additionally, swine, especially the Yorkshire pigs, dramatically gain weight in adulthood, which complicates long-term follow-up and makes it an unsuitable model for chronic IHF studies (Schuleri et al., 2008; Tohyama and Kobayashi, 2019). Anesthetized swine of MI models often display high mortality rates due to fatal arrhythmia, such as ventricular fibrillation, during or shortly after the coronary artery occlusion or ischemia (Halkos et al., 2008; Lim et al., 2018), which may introduce sample size bias and confound experimental results. Table 1 shows a comparative analysis of different animal models commonly used in MI studies.
No single animal model can sufficiently answer every question raised in the field of cardiovascular research. Different species as animal models for MI studies may vary in size, anatomical structure, and genetic and phenotypic expression, and have their own advantages and disadvantages. Because of the heterogeneity and multimorbidity observed in patients with MI, animal models in the preclinical studies are considered by some as too remote to be applicable in translational efforts. Some researchers emphasize the use of human-based research methods, such as the use of human-induced pluripotent stem cells (iPSCs), cardiac organoids, and cardiovascular “organs-on-chips” (Ribas et al., 2016; Pound and Ritskes-Hoitinga, 2018; Richards et al., 2020). However, it is undeniable that there is no adequate substitute for animal models that allow us to systematically examine how the entire body systems respond to a disease. The ideal approach to preclinical studies would be to use multiple, complementary animal models, and human-based models to utilize the advantages of strengths of each model and take preventive measures to minimize bias in the experimental design and data interpretation.
Seeing What We Want to See: Biased Experiments in Animal Studies Decelerate Reliable Clinical Translations in MI Studies
A rapid technological advancement has dramatically improved our understanding of human heart diseases and therapeutic development; however, the translation of these findings has not been keeping up with this trend (Ioannidis, 2005, 2016). This review argues that two primary sources of this slow translation are: (1) the lack of transparency in experimental design and data assessment and (2) excessive variation in the protocols for animal surgeries; both of these factors may have resulted from the inherent technical difficulties in dealing with large animal models. Compared to small animals, a higher degree of financial, husbandry, and technical obstacles exist in large animal studies, which often limit the study scale and lead to self-justified modifications in the surgical protocols along with lack of internal validity. Studies involving large animal models are expensive and technically demanding as they require advanced surgical and anesthetic techniques and materials. However, most published papers do not report the precise and detailed protocols or visual representations needed for other researchers to reproduce the same animal model or verify the surgical procedure and experimental results. The difficulty in finding a verifiable open reference leads to poor experimental designs and varied animal survival rates; this introduces sampling bias, which is especially detrimental for small-scale studies involving large animal models.
Another source of bias is the flexibility in surgical procedures for creating MI in animals. For example, the most common method to induce acute MI is the permanent or catheter-assisted temporary coronary artery occlusion with the left anterior descending coronary artery (LAD) as the primary target vasculature. The mortality rate from LAD occlusion is relatively high, especially for large animals, as they are at a considerable risk of developing ventricular fibrillation following MI (de Jong et al., 2014; Mu et al., 2016; Lim et al., 2018). To avoid this occurrence, the left circumflex artery (LCx) is often used as an alternative target at the cost of inducing a smaller infarct at a different location (Hirano et al., 2017; Cremer et al., 2019). The substantial inconsistency in the occlusion site along these two coronary arteries further complicates the MI studies. Some segments of the LAD and LCx commonly targeted for occlusion are as follows and can be found in Figure 1:
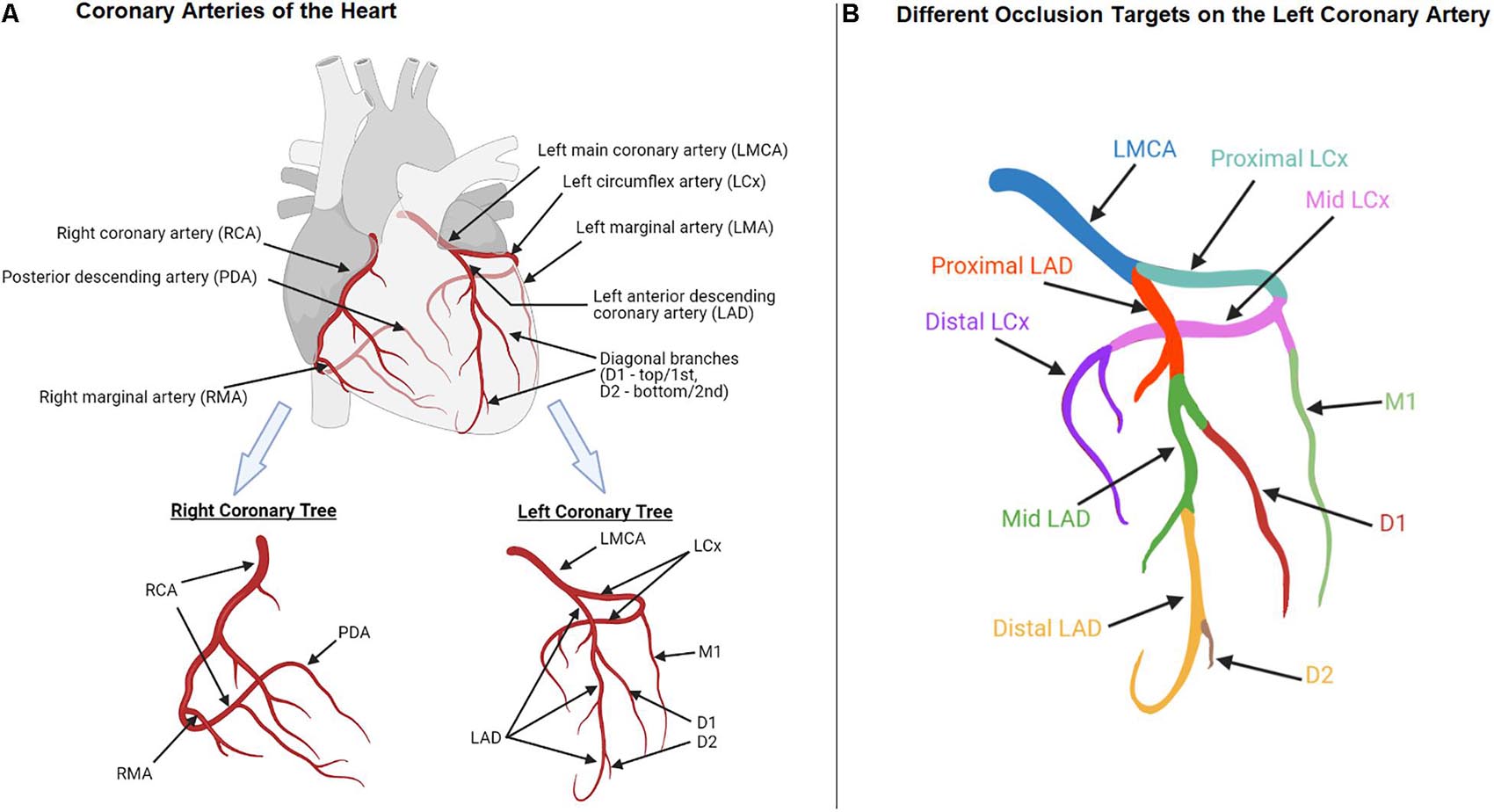
Figure 1. Anatomy of coronary arteries of the heart (A) Left and right coronary trees; (B) Different occlusion targets (indicated in different colors) on the left coronary artery.
• The LAD “distal to” the 1st diagonal branch (Kraitchman et al., 2003; Okura et al., 2012; Li et al., 2013; Sharp et al., 2017).
• The “Mid”-LAD “just beyond” the 1st diagonal branch (Lim et al., 2018).
• The “Mid-left” LAD “distal to” the 1st diagonal branch (de Jong et al., 2014).
• The LAD “beyond” the 1st diagonal branch (Wolf et al., 2009).
• The LAD “distal to” the 2nd diagonal branch (Rabbani et al., 2008; Wang et al., 2009; Mu et al., 2016; Rabbani et al., 2017).
• The “proximal” LCx (Timmers et al., 2011; Wang B. et al., 2017).
• The 1st “marginal” branch of the LCx (Gálvez-Montón et al., 2014).
Several other studies have not specified the exact location of occluded segments of the LAD (Wolf et al., 2009; Crisostomo et al., 2019) or LCx (van der Velden et al., 2004; Charles et al., 2020). Without appropriate visual representation, this inconsistent and vague language, such as “beyond” and “mid,” leaves room for arbitrary interpretation and changes in the surgical procedures, potentially leading to varied experimental outcomes. One study determined the site(s) and number of ligatures based on the visual inspection of the LAD and LCx branches in each ovine, in order to produce a consistent anterolateral infarct size across different animals (Locatelli et al., 2011) while many studies have not reported the infarct size (Kraitchman et al., 2003; Zhao et al., 2014; Alestalo et al., 2015; Haller et al., 2015; Kim et al., 2017; Lim et al., 2018; Ishida et al., 2019).
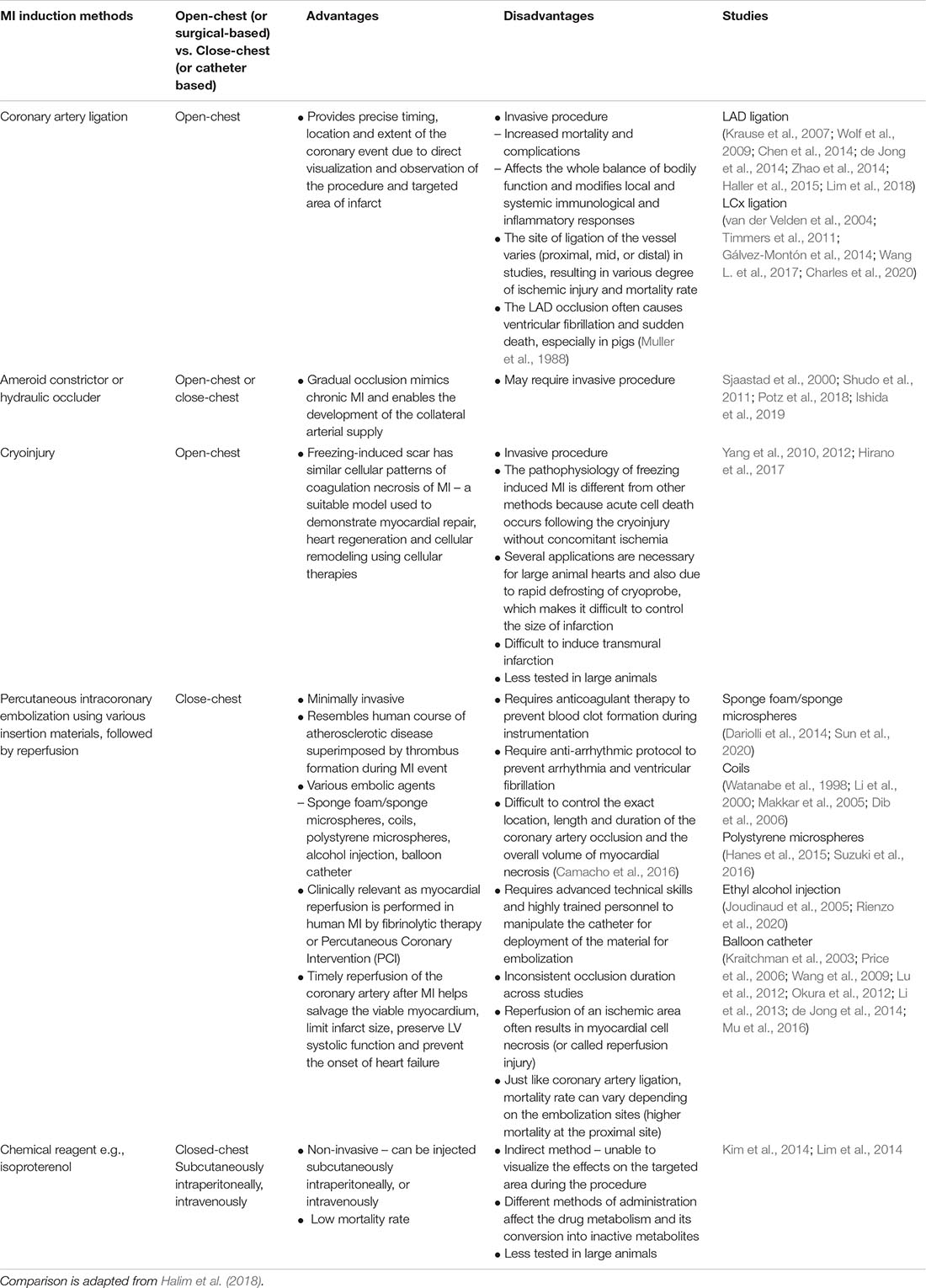
Additionally, different surgical procedures for inducing MI often create various degrees of ischemia via different pathogenic mechanisms thus generating different MI models (Table 2). For example, catheter-based occlusion is often used as a non-invasive way to induce MI, but there is a significant variation in the occlusion sites and durations followed by reperfusion across different studies (Table 3). Some studies using pig models have demonstrated that the longer occlusion duration resulted in bigger infarct sizes and more severe left ventricular dysfunction (Garcia-Dorado et al., 1987; Ghugre et al., 2013; Thomas et al., 2021). However, besides the occlusion site and duration, this inconsistent infarct size and ventricular remodeling were likely to be affected by the subsequent reperfusion. Myocardial reperfusion using thrombolytic therapy or primary percutaneous coronary intervention is a treatment option for human MI patients. However, it is known that the reperfusion of myocytes irreversibly injured by ischemia following coronary occlusion may accelerate the necrotic process, a phenomenon called “myocardial ischemia-reperfusion injury.” This could consequently affect the infarct size and lead to adverse cardiac remodeling (Braunwald and Kloner, 1985; Yellon and Hausenloy, 2007; Hausenloy and Yellon, 2013; Acharya, 2020). All these situational specifics of a surgical procedure as part of MI preclinical study design (for example, method, site, and duration of coronary artery occlusion, and presence and duration of reperfusion following occlusion) potentially limit the generalizability and reproducibility of scientific results and likely contribute to the failure of subsequent clinical trials.
Potential of Human-Based Models as an Alternative for Animal Models?
Whether small or large, animal models cannot fully recapitulate human CVD phenotypes, thus requiring new forms of human-based experimentation. The tissue engineering community has been developing in vitro and in silico CVD models for more physiologically and clinically relevant readouts of CVDs (Savoji et al., 2019). Human organs-on-chips are 3D microfluidic cell culture devices that mimic the physical and mechanical microenvironment of key organ systems and provide dynamic vascular perfusion in vitro, which is difficult to achieve in 2D cell culture (Ingber, 2018). This burgeoning biomimetic system can incorporate patient-specific cell models, allowing the study of pathophysiology and pharmacological responses unique to each patient (Ingber, 2020; Wu et al., 2020).
However, an organ-on-a-chip is still limited in that although it can capture distinct functional units of organ systems separately (e.g., heart vs. liver), it cannot link each unit via vascular channels (e.g., the hepatic portal system). “Multi” organ-on-a-chip device may allow combining several cellular models in a single chip; however, certain technical difficulties, such as selecting a co-culture medium required for incorporating multiple cell lineages and ensuring the correct sizing of each organ, need to be resolved (Bovard and Sandoz, 2020). However, this innovative in vitro model is still distant from a complete replacement of animal studies because they cannot mimic the complex nervous and immune systems of humans. Thus, investigators, particularly those concerned with cognition, behavior, immune responses, and pain management, still require animal studies to systematically monitor disease progression and develop corresponding therapeutic interventions. Animal studies have been misinterpreted as poor predictors of clinical study outcomes. This may be true merely because animals and humans are inherently different, and the human body and pathogenesis of CVDs and other diseases are far too complicated to be replicated in other models. However, this inherent difficulty should not be used as an excuse to adopt a less rigorous but more convenient experimental design and data interpretation. Although new technological advances will allow us to adopt more disease-representative models, the clinical study outcomes will still largely depend on scientific rigor.
Discussion
Despite increasing knowledge about the etiologies of MI and relevant therapeutic strategies, the translational gap between basic science and clinical research is widening. Lack of experimental rigor and quality in preclinical research has been accused as the main cause of slow translation of “promising” preclinical results, and various issues regarding reproducibility have been raised across different biomedical and social science fields (Pound et al., 2004; Begley and Ioannidis, 2015).
In section “Suitability of Animal Models for Human MI,” we discussed the importance of choosing a representative animal model in preclinical studies and considering the differences between different animal species and humans when interpreting experimental data. Some researchers believe that the limited opportunities to carry out studies based on large animal models prevent them from testing their hypothesis more rigorously and openly, justifying adjustments in an experimental design and biased interpretations of study outcomes. Yet, the discordance between animal-based preclinical and human-based clinical studies is often attributed for the failures of clinical trials for cardiovascular and other disease therapies (Pound et al., 2004; Perel et al., 2007). Some human-based preclinical models have been proposed as a complementary platform to overcome the limitations of using an animal model. However, they will not replace animal models entirely soon as discussed in section “Potential of Human-Based Models as an Alternative for Animal Models?” The difficulty of establishing the optimal animal model prompts a periodic systematic review or meta-analysis of animal studies (Sandercock and Roberts, 2002; Pound et al., 2004; Hooijmans and Ritskes-Hoitinga, 2013). However, a systematic review of studies with poor methodological quality is likely to produce additional animal studies of similarly poor quality. Instead, the preclinical, animal study quality must be scrutinized at the original study design process and journals’ review process at the time of submission.
In section “Seeing What We Want to See: Biased Experiments in Animal Studies Decelerate Reliable Clinical Translations in MI Studies,” we reviewed how the lack of standardized protocols and transparency in preclinical MI studies involving animal experiments could allow investigators too much flexibility in their study design and data assessment, depriving “promising” preclinical research results reproducibility and translational power. Investigators often adopt a disease model that is remote from what they intend to model and tend to report the desired results that are harmonious with their hypothesis alone (Baker, 2016). A standardized experimental method is critical for ensuring reproducibility, but the lack of overall methodological rigor in preclinical cardiovascular studies is prevalent, delaying the translational process; this issue has called for a set of improved reporting standards, more strict funding policies, and better instructions for peer reviewer (Hooijmans et al., 2010; Hirst and Altman, 2012; Henderson et al., 2013; Anon, 2013; Principles and Guidelines for Reporting Preclinical Research and National Institutes of Health (NIH), 2021).
Four elements of methodological quality of preclinical research that critically determine its translational power are randomization, sample size calculation, blinding, and heterogeneity of animals used (i.e., strains, ages, and sexes) (Henderson et al., 2013). A recently added critical element of heterogeneity of animal models is environmental factors, which suggests the benefit of multi-laboratory experiments (Richter et al., 2009; Voelkl et al., 2018). Ramirez et al. (2017) found that randomization was reported only in 21.8%, blinding in 32.7%, and sample size estimation in 2.3% of all preclinical cardiovascular studies published in five leading cardiovascular journals between July 2006 and June 2016 (Ramirez et al., 2017). Similar or worse results are found in the review of thirty-one systematic reviews of animal studies on treatments for various diseases (Hirst et al., 2014; van Luijk et al., 2014). Additionally, the quality of these study design elements has not improved in all disease-specific studies, except for stroke research (Hirst et al., 2014). From 1997 to 2007, the number of cardiovascular papers and journals increased by 56.9 and 75.2%, respectively, yet 46% of original papers published in cardiovascular journals in the same period were poorly cited (with < = 5 citations in the 5 years following publication); however, 44% of cardiovascular journals had more than three-fourths of the journal’s content poorly cited at 5 years (Ranasinghe et al., 2015). Interestingly, studies that employed randomization, blinding, or sample size estimation were equally cited in numbers as those that did not; however, studies that included both males and females were less frequently cited, suggesting that methodological rigor might have been overlooked by cardiovascular researchers (Ramirez et al., 2017). This suggests the need for strict enforcement of a comprehensive guideline and requirements by journals and funding institutions to ensure the rigor of animal studies and publication to the level of human-involving, clinical studies, which consequently promotes reproducibility and animal welfare (Hooijmans et al., 2010; Carbone and Austin, 2016).
It is almost always impossible to control every aspect of a scientific experiment and to perfectly mimic human pathophysiology in a disease model. Consequently, any experimental data are biased, and it is a matter of how biased they are and whether researchers are aware of and report those biases correctly. Additionally, the failure to reproduce or conflicting data is not always a vice but could be a valuable resource that potentially enriches biomedical research (Daugherty et al., 2016). However, in translational medicine, reproducibility is the ultimate goal, and this review article emphasizes there is much room for improvements in preclinical study design and animal models for MI research. Methodological rigors such as sample randomization, consistent surgical procedures, blind analyses, and greater sample statistical power are essential in animal models of human CVDs or other diseases. Along with following the correct procedures during research, transparent reporting of experimental protocols and results is equally essential to improve reproducibility, effectiveness, predictability, and safety of the clinical studies.
Considering the economic and emotional cost of a clinical trial and the exponentially growing number of published articles, it may be much more cost-effective from the standpoint of the entire population to maintain rigor and quality in the preclinical study level with good practice and additional cost than to see a series of “promising” preclinical study continuously failing in clinical trials (Freedman et al., 2015). However, probably most trained researchers may be well aware of these prerequisites of successful translation mentioned above. The root cause of the imbalance between the translational crisis and exponentially growing research in the cardiovascular field might be the competition for grants and positions (Baker, 2016). In this case, more opportunities for quality training and mentorship within research communities as well as a clear publication or funding guideline by journals and funding institutions are proposed (Begley and Ioannidis, 2015).
Yet, probably most trained researchers may be well aware of these prerequisites of successful translation. The root cause of the imbalance between the translational crisis and exponentially growing research in cardiovascular field might be the competition for grants and positions (Baker, 2016). In this case, more opportunities for quality training and mentorship within research communities in addition to a clear publication or funding guideline by journals and funding institutions are proposed (Begley and Ioannidis, 2015).
Author Contributions
HSy, HSe, and YS: conceptualization, writing—review and editing, and visualization. HSy and HSe: methodology, investigation, and writing—original draft preparation. HSy and YS: resources and project administration. YS: supervision and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Stanford Cardiovascular Institute (CVI) 2020 Seed Grant funded from Staford CVI and Gootter Foundation (YS).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alestalo, K., Korpi, R., Mäkelä, J., Lehtonen, S., Mäkelä, T., Yannopoulos, F., et al. (2015). High number of transplanted stem cells improves myocardial recovery after AMI in a porcine model. Scand. Cardiovasc. J. 49, 82–94. doi: 10.3109/14017431.2015.1018311
Baker, M. (2016). 1,500 scientists lift the lid on reproducibility. Nature 533, 452–454. doi: 10.1038/533452a
Begley, C. G., and Ioannidis, J. P. A. (2015). Reproducibility in science: improving the standard for basic and preclinical research. Circ. Res. 116, 116–126. doi: 10.1161/circresaha.114.303819
Blair, E. (1961). Anatomy of the ventricular coronary arteries in the dog. Circ. Res. 9, 333–341. doi: 10.1161/01.res.9.2.333
Bode, G., Clausing, P., Gervais, F., Loegsted, J., Luft, J., Nogues, V., et al. (2010). The utility of the minipig as an animal model in regulatory toxicology. J. Pharmacol. Toxicol. Methods 62, 196–220. doi: 10.1016/j.vascn.2010.05.009
Bolli, R., Becker, L., Gross, G., Mentzer, R., Balshaw, D., Lathrop, D. A., et al. (2004). Myocardial protection at a crossroads: the need for translation into clinical therapy. Circ. Res. 95, 125–134. doi: 10.1161/01.res.0000137171.97172.d7
Bovard, D., and Sandoz, A. (2020). How to build your multiorgan-on-a-chip system: a case study. Organ-on-a-chip. Amsterdam: Elsevier, 463–506.
Bracken, M. B. (2009). Why animal studies are often poor predictors of human reactions to exposure. J. R. Soc. Med. 102, 120–122. doi: 10.1258/jrsm.2008.08k033
Braunwald, E., and Kloner, R. A. (1985). Myocardial reperfusion: a double-edged sword? J. Clin. Invest. 76, 1713–1719. doi: 10.1172/jci112160
Camacho, P., Fan, H., Liu, Z., and He, J.-Q. (2016). Large mammalian animal models of heart disease. J. Cardiovasc. Dev. Dis. 3:4.
Cannon, R. O. (2005). Mechanisms, management and future directions for reperfusion injury after acute myocardial infarction. Nat. Clin. Pract. Cardiovasc. Med. 2, 88–94. doi: 10.1038/ncpcardio0096
Carbone, L., and Austin, J. (2016). Pain and laboratory animals: publication practices for better data reproducibility and better animal welfare. PLoS One 11:e0155001. doi: 10.1371/journal.pone.0155001
Charles, C. J., Elliott, J. M., Nicholls, M. G., Rademaker, M. T., and Richards, M. (2000). Myocardial infarction with and without reperfusion in sheep: early cardiac and neurohumoral changes. Clin. Sci. 98, 703–711. doi: 10.1042/cs19990266
Charles, C. J., Li, R. R., Yeung, T., Mazlan, S. M. I., Lai, R. C., de Kleijn, D. P. V., et al. (2020). systemic mesenchymal stem cell-derived exosomes reduce myocardial infarct size: characterization with mri in a porcine model. Front. Cardiovasc. Med. 7:601990. doi: 10.3389/fcvm.2020.601990
Chen, C.-H., Chang, M.-Y., Wang, S.-S., and Hsieh, P. C. H. (2014). Injection of autologous bone marrow cells in hyaluronan hydrogel improves cardiac performance after infarction in pigs. Am. J. Physiol. Heart Circ. Physiol. 306, H1078–H1086.
Chong, J. J. H., Yang, X., Don, C. W., Minami, E., Liu, Y.-W., Weyers, J. J., et al. (2014). Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510, 273–277.
Christman, K. L., and Lee, R. J. (2006). Biomaterials for the treatment of myocardial infarction. J. Am. Coll Cardiol. 48, 907–913. doi: 10.1016/j.jacc.2006.06.005
Cremer, S., Schloss, M. J., Vinegoni, C., Zhang, S., Rohde, D., Feruglio, P. F., et al. (2019). A mouse model of recurrent myocardial infarction reports diminished emergency hematopoiesis and cardiac inflammation. BioRxiv 2019:4.
Crisostomo, V., Baez, C., Abad, J. L., Sanchez, B., Alvarez, V., Rosado, R., et al. (2019). Dose-dependent improvement of cardiac function in a swine model of acute myocardial infarction after intracoronary administration of allogeneic heart-derived cells. Stem Cell Res. Ther. 10:152.
Dariolli, R., Takimura, C. K., Campos, C. A., Lemos, P. A., and Krieger, J. E. (2014). Development of a closed-artery catheter-based myocardial infarction in pigs using sponge and lidocaine hydrochloride infusion to prevent irreversible ventricular fibrillation. Physiol. Rep. 2:8.
Daugherty, A., Hegele, R. A., Mackman, N., Rader, D. J., Schmidt, A. M., and Weber, C. (2016). Complying with the national institutes of health guidelines and principles for rigor and reproducibility: refutations. Arterioscler. Thromb. Vasc. Biol. 36, 1303–1304. doi: 10.1161/atvbaha.116.307906
Dayan, V., Sotelo, V., Delfina, V., Delgado, N., Rodriguez, C., Suanes, C., et al. (2016). Human mesenchymal stromal cells improve cardiac perfusion in an ovine immunocompetent animal model. J. Invest. Surg. 29, 218–225. doi: 10.3109/08941939.2015.1128997
de Jong, R., van Hout, G. P. J., Houtgraaf, J. H., Kazemi, K., Wallrapp, C., Lewis, A., et al. (2014). Intracoronary infusion of encapsulated glucagon-like peptide-1-eluting mesenchymal stem cells preserves left ventricular function in a porcine model of acute myocardial infarction. Circ. Cardiovasc. Interv. 7, 673–683. doi: 10.1161/circinterventions.114.001580
Dib, N., Diethrich, E. B., Campbell, A., Gahremanpour, A., McGarry, M., and Opie, S. R. A. (2006). percutaneous swine model of myocardial infarction. J. Pharmacol. Toxicol. Methods 53, 256–263.
Dirksen, M. T., Laarman, G. J., Simoons, M. L., and Duncker, D. J. G. M. (2007). Reperfusion injury in humans: a review of clinical trials on reperfusion injury inhibitory strategies. Cardiovasc. Res. 74, 343–355. doi: 10.1016/j.cardiores.2007.01.014
Dixon, J. A., and Spinale, F. G. (2009). Large animal models of heart failure: a critical link in the translation of basic science to clinical practice. Circ. Heart Fail. 2, 262–271. doi: 10.1161/circheartfailure.108.814459
Downey, J. M., and Cohen, M. V. (2009). Why do we still not have cardioprotective drugs? Circ. J. 73, 1171–1177. doi: 10.1253/circj.cj-09-0338
Epstein, F. H., Yang, Z., Gilson, W. D., Berr, S. S., Kramer, C. M., and French, B. A. M. R. (2002). tagging early after myocardial infarction in mice demonstrates contractile dysfunction in adjacent and remote regions. Magn. Reson. Med. 48, 399–403. doi: 10.1002/mrm.10210
Ferreira, G. S., Veening-Griffioen, D. H., Boon, W. P. C., Moors, E. H. M., and van Meer, P. J. K. (2020). Levelling the translational gap for animal to human efficacy data. Animals 10:7.
Freedman, L. P., Cockburn, I. M., and Simcoe, T. S. (2015). The economics of reproducibility in preclinical research. PLoS Biol. 13:e1002165. doi: 10.1371/journal.pbio.1002626
Gálvez-Montón, C., Prat-Vidal, C., Díaz-Güemes, I., Crisóstomo, V., Soler-Botija, C., Roura, S., et al. (2014). Comparison of two preclinical myocardial infarct models: coronary coil deployment versus surgical ligation. J. Transl. Med. 12:137. doi: 10.1186/1479-5876-12-137
Gandolfi, F., Vanelli, A., Pennarossa, G., Rahaman, M., Acocella, F., and Brevini, T. A. L. (2011). Large animal models for cardiac stem cell therapies. Theriogenology 75, 1416–1425. doi: 10.1016/j.theriogenology.2011.01.026
Gao, L. R., Pei, X. T., Ding, Q. A., Chen, Y., Zhang, N. K., Chen, H. Y., et al. (2013). A critical challenge: dosage-related efficacy and acute complication intracoronary injection of autologous bone marrow mesenchymal stem cells in acute myocardial infarction. Int. J. Cardiol. 168, 3191–3199. doi: 10.1016/j.ijcard.2013.04.112
Gao, M., Xin, G., Qiu, X., Wang, Y., and Liu, G. (2016). Establishment of a rat model with diet-induced coronary atherosclerosis. J. Biomed. Res. 31, 47–55.
Garcia-Dorado, D., Théroux, P., Elizaga, J., Galiñanes, M., Solares, J., Riesgo, M., et al. (1987). Myocardial reperfusion in the pig heart model: infarct size and duration of coronary occlusion. Cardiovasc. Res. 21, 537–544. doi: 10.1093/cvr/21.7.537
Gargiulo, S., Greco, A., Gramanzini, M., Petretta, M. P., Ferro, A., Larobina, M., et al. (2012). PET/CT imaging in mouse models of myocardial ischemia. J. Biomed. Biotechnol. 2012:541872.
Getz, G. S., and Reardon, C. A. (2012). Animal models of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 32, 1104–1115.
Ghugre, N. R., Pop, M., Barry, J., Connelly, K. A., and Wright, G. A. (2013). Quantitative magnetic resonance imaging can distinguish remodeling mechanisms after acute myocardial infarction based on the severity of ischemic insult. Magn. Reson. Med. 70, 1095–1105. doi: 10.1002/mrm.24531
Giustino, G., and Dangas, G. D. (2017). Ischemia-reperfusion injury and ischemic post-conditioning in acute myocardial infarction: Lost in translation. Catheter. Cardiovasc. Interv. 90, 1068–1069. doi: 10.1002/ccd.27436
Graham, G. (2015). Disparities in cardiovascular disease risk in the United States. Curr. Cardiol. Rev. 11, 238–245. doi: 10.2174/1573403x11666141122220003
Graham, G. (2016). Racial and ethnic differences in acute coronary syndrome and myocardial infarction within the united states: from demographics to outcomes. Clin. Cardiol. 39, 299–306. doi: 10.1002/clc.22524
Guo, R., Morimatsu, M., Feng, T., Lan, F., Chang, D., Wan, F., et al. (2020). Stem cell-derived cell sheet transplantation for heart tissue repair in myocardial infarction. Stem Cell Res. Ther. 11:19.
Halim, S. A. S. A., Ghafar, N. A., Jubri, Z., and Das, S. (2018). Induction of myocardial infarction in experimental animals: A review. JCDR 2018:12221.
Halkos, M. E., Zhao, Z.-Q., Kerendi, F., Wang, N.-P., Jiang, R., Schmarkey, L. S., et al. (2008). Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic Res. Cardiol. 103, 525–536. doi: 10.1007/s00395-008-0741-0
Haller, C., Sobolewska, B., Schibilsky, D., Avci-Adali, M., Schlensak, C., Wendel, H.-P., et al. (2015). One-staged aptamer-based isolation and application of endothelial progenitor cells in a porcine myocardial infarction model. Nucleic Acid Ther. 25, 20–26. doi: 10.1089/nat.2014.0499
Hanes, D. W., Wong, M. L., Jenny Chang, C. W., Humphrey, S., Grayson, J. K., Boyd, W. D., et al. (2015). Embolization of the first diagonal branch of the left anterior descending coronary artery as a porcine model of chronic trans-mural myocardial infarction. J. Transl. Med. 13:187.
Harken, A. H., Simson, M. B., Haselgrove, J., Wetstein, L., Harden, W. R., and Barlow, C. H. (1981). Early ischemia after complete coronary ligation in the rabbit, dog, pig, and monkey. Am. J. Physiol. 241, H202–H210.
Hausenloy, D. J., and Yellon, D. M. (2013). Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J. Clin. Invest. 123, 92–100. doi: 10.1172/jci62874
Hausenloy, D. J., and Yellon, D. M. (2016). Ischaemic conditioning and reperfusion injury. Nat. Rev. Cardiol. 13, 193–209. doi: 10.1038/nrcardio.2016.5
Hausenloy, D. J., Baxter, G., Bell, R., Bøtker, H. E., Davidson, S. M., Downey, J., et al. (2010). Translating novel strategies for cardioprotection: the Hatter Workshop Recommendations. Basic Res. Cardiol. 105, 677–686. doi: 10.1007/s00395-010-0121-4
Hearse, D. (2000). The elusive coypu: the importance of collateral flow and the search for an alternative to the dog. Cardiovasc. Res. 45, 215–219. doi: 10.1016/s0008-6363(99)00331-4
Henderson, V. C., Kimmelman, J., Fergusson, D., Grimshaw, J. M., and Hackam, D. G. (2013). Threats to validity in the design and conduct of preclinical efficacy studies: a systematic review of guidelines for in vivo animal experiments. PLoS Med. 10:e1001489. doi: 10.1371/journal.pmed.1001489
Heusch, G. (2013). Cardioprotection: chances and challenges of its translation to the clinic. Lancet 381, 166–175. doi: 10.1016/s0140-6736(12)60916-7
Heusch, G. (2015). Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ. Res. 116, 674–699. doi: 10.1161/circresaha.116.305348
Heusch, G. (2017). Critical issues for the translation of cardioprotection. Circ. Res. 120, 1477–1486. doi: 10.1161/circresaha.117.310820
Hill, A. J., and Iaizzo, P. A. (2009). “Comparative Cardiac Anatomy,” in Handbook of cardiac anatomy, physiology, and devices, ed. P. A. Iaizzo (Totowa, NJ: Humana Press), 87–108. doi: 10.1007/978-1-60327-372-5_6
Hirano, A., Fujita, J., Kanazawa, H., Kawaguchi, S., Handa, N., Yamada, Y., et al. (2017). Cryoinjury-induced acute myocardial infarction model and ameroid constrictor-induced ischemic heart disease model in adult micro-mini pigs for preclinical studies. Transl. Med. Commun. 2:1. doi: 10.1155/2014/571076
Hirst, A., and Altman, D. G. (2012). Are peer reviewers encouraged to use reporting guidelines? A survey of 116 health research journals. PLoS One 7:e35621. doi: 10.1371/journal.pone.0035621.g001
Hirst, J. A., Howick, J., Aronson, J. K., Roberts, N., Perera, R., Koshiaris, C., et al. (2014). The need for randomization in animal trials: an overview of systematic reviews. PLoS One 9:e98856. doi: 10.1371/journal.pone.0098856
Hooijmans, C. R., and Ritskes-Hoitinga, M. (2013). Progress in using systematic reviews of animal studies to improve translational research. PLoS Med. 10:e1001482. doi: 10.1371/journal.pmed.1001482
Hooijmans, C. R., Leenaars, M., and Ritskes-Hoitinga, M. A. (2010). gold standard publication checklist to improve the quality of animal studies, to fully integrate the Three Rs, and to make systematic reviews more feasible. Altern. Lab. Anim. 38, 167–182. doi: 10.1177/026119291003800208
Houtgraaf, J. H., de Jong, R., Kazemi, K., de Groot, D., van der Spoel, T. I. G., Arslan, F., et al. (2013). Intracoronary infusion of allogeneic mesenchymal precursor cells directly after experimental acute myocardial infarction reduces infarct size, abrogates adverse remodeling, and improves cardiac function. Circ. Res. 113, 153–166. doi: 10.1161/circresaha.112.300730
Ingber, D. E. (2020). Is it time for reviewer 3 to request human organ chip experiments instead of animal validation studies? Adv. Sci. 7:2002030. doi: 10.1002/advs.202002030
Ioannidis, J. P. A. (2005). Why most published research findings are false. PLoS Med. 2:e124. doi: 10.1371/journal.pmed.0020124
Ioannidis, J. P. A. (2016). Why most clinical research is not useful. PLoS Med. 13:e1002049. doi: 10.1371/journal.pmed.1002049
Ishida, M., Miyagawa, S., Saito, A., Fukushima, S., Harada, A., Ito, E., et al. (2019). Transplantation of Human-induced Pluripotent Stem Cell-derived Cardiomyocytes Is Superior to Somatic Stem Cell Therapy for Restoring Cardiac Function and Oxygen Consumption in a Porcine Model of Myocardial Infarction. Transplantation 103, 291–298. doi: 10.1097/tp.0000000000002384
Janssens, S., Dubois, C., Bogaert, J., Theunissen, K., Deroose, C., Desmet, W., et al. (2006). Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet 367, 113–121. doi: 10.1016/s0140-6736(05)67861-0
Joudinaud, T. M., Kegel, C. L., Gabster, A. A., Sanz, M. L., MacDonald, A., Propp, D., et al. (2005). An experimental method for the percutaneous induction of a posterolateral infarct and functional ischemic mitral regurgitation. J. Heart Valve Dis. 14, 460–466.
Kamimura, R., Suzuki, S., Nozaki, S., Sakamoto, H., Maruno, H., and Kawaida, H. (1996). Branching patterns in coronary artery and ischemic areas induced by coronary arterial occlusion in the CLAWN miniature pig. Exp. Anim. 45, 149–153. doi: 10.1538/expanim.45.149
Khan, M. A. (1984). Minipig: advantages and disadvantages as a model in toxicity testing. J. Am. Coll Toxicol. 3, 337–342. doi: 10.3109/10915818409104396
Kim, J.-H., Chung, H.-S., Antonisamy, P., Lee, S. R., and Bae, H. (2014). Cardioprotective effect of rhizomes of Acorus gramineus against isoproterenol-induced cardiac damage in pigs. Cardiovasc. Toxicol. 14, 183–192. doi: 10.1007/s12012-014-9243-5
Kim, M. C., Kim, Y. S., Kang, W. S., Lee, K. H., Cho, M., Hong, M. H., et al. (2017). Intramyocardial injection of stem cells in pig myocardial infarction model: the first trial in korea. J. Korean. Med. Sci. 32, 1708–1712. doi: 10.3346/jkms.2017.32.10.1708
Kloner, R. A. (2013). Current state of clinical translation of cardioprotective agents for acute myocardial infarction. Circ. Res. 113, 451–463. doi: 10.1161/circresaha.112.300627
Kloner, R. A., and Rezkalla, S. H. (2004). Cardiac protection during acute myocardial infarction: where do we stand in 2004? J. Am. Coll. Cardiol. 44, 276–286. doi: 10.1016/j.jacc.2004.03.068
Kraitchman, D. L., Heldman, A. W., Atalar, E., Amado, L. C., Martin, B. J., Pittenger, M. F., et al. (2003). In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation 107, 2290–2293. doi: 10.1161/01.cir.0000070931.62772.4e
Krause, U., Harter, C., Seckinger, A., Wolf, D., Reinhard, A., Bea, F., et al. (2007). Intravenous delivery of autologous mesenchymal stem cells limits infarct size and improves left ventricular function in the infarcted porcine heart. Stem Cells Dev. 16, 31–37. doi: 10.1089/scd.2006.0089
Krueger, M. A., Huke, S. S., and Glenny, R. W. (2013). Visualizing regional myocardial blood flow in the mouse. Circ. Res. 112, e88–e97.
Kumar, D., Hacker, T. A., Buck, J., Whitesell, L. F., Kaji, E. H., Douglas, P. S., et al. (2005). Distinct mouse coronary anatomy and myocardial infarction consequent to ligation. Coron. Artery Dis. 16, 41–44. doi: 10.1097/00019501-200502000-00008
Laflamme, M. A., Chen, K. Y., Naumova, A. V., Muskheli, V., Fugate, J. A., Dupras, S. K., et al. (2007). Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 25, 1015–1024. doi: 10.1038/nbt1327
Lee, Y. T., Lin, H. Y., Chan, Y. W. F., Li, K. H. C., To, O. T. L., Yan, B. P., et al. (2017). Mouse models of atherosclerosis: a historical perspective and recent advances. Lipids Health Dis. 16:12.
Lelovas, P. P., Kostomitsopoulos, N. G., and Xanthos, T. T. A. (2014). comparative anatomic and physiologic overview of the porcine heart. J. Am. Assoc. Lab. Anim. Sci. 53, 432–438.
Li, R. K., Weisel, R. D., Mickle, D. A., Jia, Z. Q., Kim, E. J., Sakai, T., et al. (2000). Autologous porcine heart cell transplantation improved heart function after a myocardial infarction. J. Thorac. Cardiovasc. Surg. 119, 62–68. doi: 10.1016/s0022-5223(00)70218-2
Li, X., Zhang, F., Song, G., Gu, W., Chen, M., Yang, B., et al. (2013). Intramyocardial injection of pig pluripotent stem cells improves left ventricular function and perfusion: A study in a porcine model of acute myocardial infarction. PLoS One. 8:e66688. doi: 10.1371/journal.pone.0066688
Lim, K. H., Cho, J. Y., Kim, B., Bae, B.-S., and Kim, J.-H. (2014). Red ginseng (Panax ginseng) decreases isoproterenol-induced cardiac injury via antioxidant properties in porcine. J. Med. Food. 17, 111–118. doi: 10.1089/jmf.2013.2768
Lim, M., Wang, W., Liang, L., Han, Z.-B., Li, Z., Geng, J., et al. (2018). Intravenous injection of allogeneic umbilical cord-derived multipotent mesenchymal stromal cells reduces the infarct area and ameliorates cardiac function in a porcine model of acute myocardial infarction. Stem Cell Res. Ther. 9:129.
Liu, X., Wang, Y., Tang, M., Liu, Y., Hu, L., and Gu, Y. (2020). Three-dimensional visualization of coronary microvasculature in rats with myocardial infarction. Microvasc. Res. 130:103990. doi: 10.1016/j.mvr.2020.103990
Locatelli, P., Olea, F. D., Mendiz, O., Salmo, F., Fazzi, L., Hnatiuk, A., et al. (2011). An ovine model of postinfarction dilated cardiomyopathy in animals with highly variable coronary anatomy. ILAR J. 52, E16–E21.
Locher, M. R., Razumova, M. V., Stelzer, J. E., Norman, H. S., Patel, J. R., and Moss, R. L. (2009). Determination of rate constants for turnover of myosin isoforms in rat myocardium: implications for in vivo contractile kinetics. Am. J. Physiol. Heart Circ. Physiol. 297, H247–H256.
Lu, M., Zhao, S., Liu, Q., Jiang, S., Song, P., Qian, H., et al. (2012). Transplantation with autologous mesenchymal stem cells after acute myocardial infarction evaluated by magnetic resonance imaging: an experimental study. J. Thorac. Imaging 27, 125–135. doi: 10.1097/rti.0b013e31820446fa
Ludman, A. J., Yellon, D. M., and Hausenloy, D. J. (2010). Cardiac preconditioning for ischaemia: lost in translation. Dis. Model Mech. 3, 35–38. doi: 10.1242/dmm.003855
Lunde, K., Solheim, S., Aakhus, S., Arnesen, H., Abdelnoor, M., Egeland, T., et al. (2006). Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N. Engl. J. Med. 355, 1199–1209.
Makkar, R. R., Price, M. J., Lill, M., Frantzen, M., Takizawa, K., Kleisli, T., et al. (2005). Intramyocardial injection of allogenic bone marrow-derived mesenchymal stem cells without immunosuppression preserves cardiac function in a porcine model of myocardial infarction. J. Cardiovasc. Pharmacol. Ther. 10, 225–233. doi: 10.1177/107424840501000403
Makkar, R. R., Smith, R. R., Cheng, K., Malliaras, K., Thomson, L. E., Berman, D., et al. (2012). Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 379, 895–904. doi: 10.1016/s0140-6736(12)60195-0
Maxwell, M. P., Hearse, D. J., and Yellon, D. M. (1987). Species variation in the coronary collateral circulation during regional myocardial ischaemia: a critical determinant of the rate of evolution and extent of myocardial infarction. Cardiovasc. Res. 21, 737–746. doi: 10.1093/cvr/21.10.737
Menasché, P. (2018). Cell therapy trials for heart regeneration - lessons learned and future directions. Nat. Rev. Cardiol. 15, 659–671. doi: 10.1038/s41569-018-0013-0
Milani-Nejad, N., and Janssen, P. M. L. (2014). Small and large animal models in cardiac contraction research: advantages and disadvantages. Pharmacol. Ther. 141, 235–249. doi: 10.1016/j.pharmthera.2013.10.007
Miura, T., and Miki, T. (2008). Limitation of myocardial infarct size in the clinical setting: current status and challenges in translating animal experiments into clinical therapy. Basic Res. Cardiol. 103, 501–513. doi: 10.1007/s00395-008-0743-y
Miyagawa, S., Domae, K., Yoshikawa, Y., Fukushima, S., Nakamura, T., Saito, A., et al. (2017). Phase I Clinical Trial of Autologous Stem Cell-Sheet Transplantation Therapy for Treating Cardiomyopathy. J Am. Heart Assoc. 6:4.
Morrissey, P. J., Murphy, K. R., Daley, J. M., Schofield, L., Turan, N. N., Arunachalam, K., et al. (2017). A novel method of standardized myocardial infarction in aged rabbits. Am. J. Physiol. Heart Circ. Physiol. 312, H959–H967.
Mu, D., Zhang, X.-L., Xie, J., Yuan, H.-H., Wang, K., Huang, W., et al. (2016). Intracoronary Transplantation of Mesenchymal Stem Cells with Overexpressed Integrin-Linked Kinase Improves Cardiac Function in Porcine Myocardial Infarction. Sci. Rep. 6:19155.
Muller, C. A., Opie, L. H., Hamm, C. W., Peisach, M., Pineda, C. A., and Thandroyen, F. T. (1988). Verapamil and tiapamil in prevention of ventricular fibrillation in pigs with coronary ligation. Comparative effects on left ventricular function. Circulation 78, 227–232. doi: 10.1161/01.cir.78.1.227
National Research Council (Us) Committee on Scientific and Humane Issues in the Use of Random Source Dogs and Cats in Research (2009). Use of Dogs and Cats in Research: Public Perception and Evolution of Laws and Guidelines - Scientific and Humane Issues in the Use of Random Source Dogs and Cats in Research - NCBI Bookshelf. Washington, DC: National Research Council.
Nguyen, P. K., and Wu, J. C. (2015). Large animal models of ischemic cardiomyopathy: are they enough to bridge the translational gap? J. Nucl. Cardiol. 22, 666–672. doi: 10.1007/s12350-015-0078-7
Nicolini, F., and Gherli, T. (2009). Alternatives to transplantation in the surgical therapy for heart failure. Eur. J. Cardiothorac. Surg. 35, 214–228. doi: 10.1016/j.ejcts.2008.11.003
Anon, J. (2013). Announcement: Reducing our irreproducibility. Nature 496, 398–398. doi: 10.1038/496398a
Nunoya, T., Shibuya, K., Saitoh, T., Yazawa, H., Nakamura, K., Baba, Y., et al. (2007). Use of Miniature Pig for Biomedical Research, with Reference to Toxicologic Studies. J. Toxicol. Pathol. 20, 125–132. doi: 10.1293/tox.20.125
Okura, H., Saga, A., Soeda, M., Miyagawa, S., Sawa, Y., Daimon, T., et al. (2012). Intracoronary artery transplantation of cardiomyoblast-like cells from human adipose tissue-derived multi-lineage progenitor cells improve left ventricular dysfunction and survival in a swine model of chronic myocardial infarction. Biochem. Biophys. Res. Commun. 425, 859–865. doi: 10.1016/j.bbrc.2012.08.004
Penicka, M., Horak, J., Kobylka, P., Pytlik, R., Kozak, T., Belohlavek, O., et al. (2007). Intracoronary injection of autologous bone marrow-derived mononuclear cells in patients with large anterior acute myocardial infarction: a prematurely terminated randomized study. J. Am. Coll. Cardiol. 49, 2373–2374. doi: 10.1016/j.jacc.2007.04.009
Perel, P., Roberts, I., Sena, E., Wheble, P., Briscoe, C., Sandercock, P., et al. (2007). Comparison of treatment effects between animal experiments and clinical trials: systematic review. BMJ 334:197. doi: 10.1136/bmj.39048.407928.be
Perin, E. C., Willerson, J. T., Pepine, C. J., Henry, T. D., Ellis, S. G., Zhao, D. X. M., et al. (2012). Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA 307, 1717–1726.
Podesser, B., Wollenek, G., Seitelberger, R., Siegel, H., Wolner, E., Firbas, W., et al. (1997). Epicardial branches of the coronary arteries and their distribution in the rabbit heart: The rabbit heart as a model of regional ischemia. Anatomical. Record 1997:1.
Potz, B. A., Scrimgeour, L. A., Pavlov, V. I., Sodha, N. R., Abid, M. R., and Sellke, F. W. (2018). Extracellular vesicle injection improves myocardial function and increases angiogenesis in a swine model of chronic ischemia. J. Am. Heart Assoc. 7:12.
Pound, P., and Ritskes-Hoitinga, M. (2018). Is it possible to overcome issues of external validity in preclinical animal research? Why most animal models are bound to fail. J. Transl. Med. 16:304.
Pound, P., Ebrahim, S., Sandercock, P., Bracken, M. B., and Roberts, I. (2004). Reviewing Animal Trials Systematically (RATS) Group. Where is the evidence that animal research benefits humans? BMJ 328, 514–517. doi: 10.1136/bmj.328.7438.514
Price, M. J., Chou, C.-C., Frantzen, M., Miyamoto, T., Kar, S., Lee, S., et al. (2006). Intravenous mesenchymal stem cell therapy early after reperfused acute myocardial infarction improves left ventricular function and alters electrophysiologic properties. Int. J. Cardiol. 111, 231–239. doi: 10.1016/j.ijcard.2005.07.036
Principles and Guidelines for Reporting Preclinical Research and National Institutes of Health (NIH) (2021). Available online at: https://www.nih.gov/research-training/rigor-reproducibility/principles-guidelines-reporting-preclinical-research (acceessed date 28, March 2021)
Quintana, H. K., Janszky, I., Kanar, A., Gigante, B., Druid, H., Ahlbom, A., et al. (2018). Comorbidities in relation to fatality of first myocardial infarction. Cardiovasc. Pathol. 32, 32–37. doi: 10.1016/j.carpath.2017.11.002
Quyyumi, A. A., Vasquez, A., Kereiakes, D. J., Klapholz, M., Schaer, G. L., Abdel-Latif, A., et al. (2017). PreSERVE-AMI: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial of Intracoronary Administration of Autologous CD34+ Cells in Patients With Left Ventricular Dysfunction Post STEMI. Circ. Res. 120, 324–331. doi: 10.1161/circresaha.115.308165
Rabbani, S., Ahmadi, H., Fayazzadeh, E., Sahebjam, M., Boroumand, M. A., Sotudeh, M., et al. (2008). Development of an ovine model of myocardial infarction. ANZ J. Surg. 78, 78–81. doi: 10.1111/j.1445-2197.2007.04359.x
Rabbani, S., Soleimani, M., Sahebjam, M., Imani, M., Nassiri, S. M., Atashi, A., et al. (2017). Effects of endothelial and mesenchymal stem cells on improving myocardial function in a sheep animal model. J. Tehran. Heart Cent. 12, 65–71.
Ramirez, F. D., Motazedian, P., Jung, R. G., Di Santo, P., MacDonald, Z. D., Moreland, R., et al. (2017). Methodological rigor in preclinical cardiovascular studies: targets to enhance reproducibility and promote research translation. Circ. Res. 120, 1916–1926. doi: 10.1161/circresaha.117.310628
Ranasinghe, I., Shojaee, A., Bikdeli, B., Gupta, A., Chen, R., Ross, J. S., et al. (2015). Poorly cited articles in peer-reviewed cardiovascular journals from 1997 to 2007: analysis of 5-year citation rates. Circulation 131, 1755–1762. doi: 10.1161/circulationaha.114.015080
Ribas, J., Sadeghi, H., Manbachi, A., Leijten, J., Brinegar, K., Zhang, Y. S., et al. (2016). Cardiovascular Organ-on-a-Chip Platforms for Drug Discovery and Development. Appl. Vitro Toxicol. 2, 82–96. doi: 10.1089/aivt.2016.0002
Ribitsch, I., Baptista, P. M., Lange-Consiglio, A., Melotti, L., Patruno, M., Jenner, F., et al. (2020). Large animal models in regenerative medicine and tissue engineering: to do or not to do. Front. Bioeng. Biotechnol. 8:972. doi: 10.3389/fbioe.2020.00972
Richards, D. J., Li, Y., Kerr, C. M., Yao, J., Beeson, G. C., Coyle, R. C., et al. (2020). Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat. Biomed. Eng. 4, 446–462. doi: 10.1038/s41551-020-0539-4
Richter, S. H., Garner, J. P., and Würbel, H. (2009). Environmental standardization: cure or cause of poor reproducibility in animal experiments? Nat. Methods 6, 257–261. doi: 10.1038/nmeth.1312
Riehle, C., and Bauersachs, J. (2019). Small animal models of heart failure. Cardiovasc. Res. 115, 1838–1849. doi: 10.1093/cvr/cvz161
Rienzo, M., Imbault, J., El Boustani, Y., Beurton, A., Carlos Sampedrano, C., Pasdois, P., et al. (2020). A total closed chest sheep model of cardiogenic shock by percutaneous intracoronary ethanol injection. Sci. Rep. 10:12417.
Romagnuolo, R., Masoudpour, H., Porta-Sánchez, A., Qiang, B., Barry, J., Laskary, A., et al. (2019). Human Embryonic Stem Cell-Derived Cardiomyocytes Regenerate the Infarcted Pig Heart but Induce Ventricular Tachyarrhythmias. Stem Cell Rep. 12, 967–981. doi: 10.1016/j.stemcr.2019.04.005
Sandercock, P., and Roberts, I. (2002). Systematic reviews of animal experiments. Lancet 360:586. doi: 10.1016/s0140-6736(02)09812-4
Savarese, G., and Lund, L. H. (2017). Global public health burden of heart failure. Card Fail Rev. 3, 7–11.
Savoji, H., Mohammadi, M. H., Rafatian, N., Toroghi, M. K., Wang, E. Y., Zhao, Y., et al. (2019). Cardiovascular disease models: A game changing paradigm in drug discovery and screening. Biomaterials 198, 3–26. doi: 10.1016/j.biomaterials.2018.09.036
Schuleri, K. H., Boyle, A. J., Centola, M., Amado, L. C., Evers, R., Zimmet, J. M., et al. (2008). The adult Göttingen minipig as a model for chronic heart failure after myocardial infarction: focus on cardiovascular imaging and regenerative therapies. Comp. Med. 58, 568–579.
Sharp, T. E., Schena, G. J., Hobby, A. R., Starosta, T., Berretta, R. M., Wallner, M., et al. (2017). Cortical bone stem cell therapy preserves cardiac structure and function after myocardial infarction. Circ. Res. 121, 1263–1278. doi: 10.1161/circresaha.117.311174
Shudo, Y., Cohen, J. E., Macarthur, J. W., Atluri, P., Hsiao, P. F., Yang, E. C., et al. (2013). Spatially oriented, temporally sequential smooth muscle cell-endothelial progenitor cell bi-level cell sheet neovascularizes ischemic myocardium. Circulation 128 (11 Suppl. 1), S59–S68.
Shudo, Y., Miyagawa, S., Fukushima, S., Saito, A., Shimizu, T., Okano, T., et al. (2011). Novel regenerative therapy using cell-sheet covered with omentum flap delivers a huge number of cells in a porcine myocardial infarction model. J. Thorac. Cardiovasc. Surg. 142, 1188–1196. doi: 10.1016/j.jtcvs.2011.07.002
Shudo, Y., Miyagawa, S., Ohkura, H., Fukushima, S., Saito, A., Shiozaki, M., et al. (2014). Addition of mesenchymal stem cells enhances the therapeutic effects of skeletal myoblast cell-sheet transplantation in a rat ischemic cardiomyopathy model. Tissue Eng. Part A. 20, 728–739.
Sjaastad, I., Grund, F., and Ilebekk, A. (2000). Effects on infarct size and on arrhythmias by controlling reflow after myocardial ischaemia in pigs. Acta Physiol. Scand. 169, 195–201. doi: 10.1046/j.1365-201x.2000.00735.x
Spadaro, J., Fishbein, M. C., Hare, C., Pfeffer, M. A., and Maroko, P. R. (1980). Characterization of myocardial infarcts in the rat. Arch. Pathol. Lab. Med. 104, 179–183.
Spannbauer, A., Traxler, D., Zlabinger, K., Gugerell, A., Winkler, J., Mester-Tonczar, J., et al. (2019). Large animal models of heart failure with reduced ejection fraction (hfref). Front. Cardiovasc. Med. 6:117. doi: 10.3389/fcvm.2019.00117
Steele, A. N., MacArthur, J. W., and Woo, Y. J. (2017). Stem cell therapy: healing or hype? why stem cell delivery doesn’t work. Circ. Res. 120, 1868–1870. doi: 10.1161/circresaha.117.310584
Stricker-Krongrad, A., Shoemake, C., Brocksmith, D., Liu, J., Hamlin, R., and Bouchard, G. (2017). Comparative cardiovascular physiology and pathology in selected lineages of minipigs. Toxicol. Res. Appl. 1:239784731769636. doi: 10.1177/2397847317696367
Stubhan, M., Markert, M., Mayer, K., Trautmann, T., Klumpp, A., Henke, J., et al. (2008). Evaluation of cardiovascular and ECG parameters in the normal, freely moving Göttingen Minipig. J. Pharmacol. Toxicol. Methods 57, 202–211. doi: 10.1016/j.vascn.2008.02.001
Sun, S., Jiang, Y., Zhen, Z., Lai, W.-H., Liao, S., and Tse, H.-F. (2020). Establishing a Swine Model of Post-myocardial Infarction Heart Failure for Stem Cell Treatment. J. Vis. Exp. 2020:159.
Suzuki, G., Young, R. F., Leiker, M. M., and Suzuki, T. (2016). Heart-Derived Stem Cells in Miniature Swine with Coronary Microembolization: Novel Ischemic Cardiomyopathy Model to Assess the Efficacy of Cell-Based Therapy. Stem Cells Int. 2016: 6940195.
Tang, Y.-P., Liu, Y., Fan, Y.-J., Zhao, Y.-Y., Feng, J.-Q., and Liu, Y. (2018). To develop a novel animal model of myocardial infarction: A research imperative. Anim. Models Exp. Med. 1, 36–39. doi: 10.1002/ame2.12010
Techiryan, G., Weil, B. R., Palka, B. A., and Canty, J. M. (2018). Effect of intracoronary metformin on myocardial infarct size in swine. Circ. Res. 123, 986–995. doi: 10.1161/circresaha.118.313341
Thomas, D., Ferrari, V. A., Janik, M., Kim, D. H., Pickup, S., Glickson, J. D., et al. (2004). Quantitative assessment of regional myocardial function in a rat model of myocardial infarction using tagged MRI. MAGMA 17, 179–187. doi: 10.1007/s10334-004-0051-y
Thomas, R., Thai, K., Barry, J., Wright, G. A., Strauss, B. H., and Ghugre, N. R. (2021). T2-based area-at-risk and edema are influenced by ischemic duration in acute myocardial infarction. Magn. Reson. Imaging 79, 1–4. doi: 10.1016/j.mri.2021.02.011
Timmers, L., Lim, S. K., Hoefer, I. E., Arslan, F., Lai, R. C., van Oorschot, A. A. M., et al. (2011). Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 6, 206–214.
Tohyama, S., and Kobayashi, E. (2019). Age-Appropriateness of Porcine Models Used for Cell Transplantation. Cell Trans. 28, 224–228.
Trankle, C., Thurber, C. J., Toldo, S., and Abbate, A. (2016). Mitochondrial membrane permeability inhibitors in acute myocardial infarction: still awaiting translation. JACC Basic Transl. Sci. 1, 524–535.
Ungerleider, J. L., and Christman, K. L. (2014). Concise review: injectable biomaterials for the treatment of myocardial infarction and peripheral artery disease: translational challenges and progress. Stem Cells Transl. Med. 3, 1090–1099.
van der Velden, J., Merkus, D., Klarenbeek, B. R., James, A. T., Boontje, N. M., Dekkers, D. H. W., et al. (2004). Alterations in myofilament function contribute to left ventricular dysfunction in pigs early after myocardial infarction. Circ. Res. 95, e85–e95.
van der Worp, H. B., Howells, D. W., Sena, E. S., Porritt, M. J., Rewell, S., O’Collins, V., et al. (2010). Can animal models of disease reliably inform human studies? PLoS Med. 7:e1000245. doi: 10.1371/journal.pmed.1000245
van Luijk, J., Bakker, B., Rovers, M. M., Ritskes-Hoitinga, M., de Vries, R. B. M., and Leenaars, M. (2014). Systematic reviews of animal studies; missing link in translational research? PLoS One 9:e89981. doi: 10.1371/journal.pone.0089981
Virani, S. S., Alonso, A., Aparicio, H. J., Benjamin, E. J., Bittencourt, M. S., Callaway, C. W., et al. (2021). Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 143, e254–e743.
Voelkl, B., Vogt, L., Sena, E. S., and Würbel, H. (2018). Reproducibility of preclinical animal research improves with heterogeneity of study samples. PLoS Biol. 16:e2003693. doi: 10.1371/journal.pbio.2003693
Wang, B., Zhang, L., Cao, H., Yang, J., Wu, M., Ma, Y., et al. (2017). Myoblast transplantation improves cardiac function after myocardial infarction through attenuating inflammatory responses. Oncotarget 8, 68780–68794.
Wang, L., Tao, T., Su, W., Yu, H., Yu, Y., and Qin, J. A. (2017). disease model of diabetic nephropathy in a glomerulus-on-a-chip microdevice. Lab. Chip. 17, 1749–1760.
Wang, X., Jameel, M. N., Li, Q., Mansoor, A., Qiang, X., Swingen, C., et al. (2009). Stem cells for myocardial repair with use of a transarterial catheter. Circulation 120(11 Suppl.), S238–S246.
Watanabe, E., Smith, D. M., Delcarpio, J. B., Sun, J., Smart, F. W., Van Meter, C. H., et al. (1998). Cardiomyocyte transplantation in a porcine myocardial infarction model. Cell Trans. 7, 239–246.
Weaver, M. E., Pantely, G. A., Bristow, J. D., and Ladley, H. D. A. (1986). quantitative study of the anatomy and distribution of coronary arteries in swine in comparison with other animals and man. Cardiovasc. Res. 20, 907–917.
Wolf, D., Reinhard, A., Seckinger, A., Katus, H. A., Kuecherer, H., and Hansen, A. (2009). Dose-dependent effects of intravenous allogeneic mesenchymal stem cells in the infarcted porcine heart. Stem Cells Dev. 18, 321–329.
Wollert, K. C., Meyer, G. P., Müller-Ehmsen, J., Tschöpe, C., Bonarjee, V., Larsen, A. I., et al. (2017). Intracoronary autologous bone marrow cell transfer after myocardial infarction: the BOOST-2 randomised placebo-controlled clinical trial. Eur. Heart J. 38, 2936–2943.
Wu, Q., Liu, J., Wang, X., Feng, L., Wu, J., Zhu, X., et al. (2020). Organ-on-a-chip: recent breakthroughs and future prospects. Biomed. Eng. Online 19:9.
Yang, Y., Gruwel, M. L., Dreessen de Gervai, P., Sun, J., Jilkina, O., and Gussakovsky, E. (2012). MRI study of cryoinjury infarction in pig hearts: i. Effects of intrapericardial delivery of bFGF/VEGF embedded in alginate beads. NMR Biomed. 25, 177–188.
Yang, Y., Sun, J., Gervai, P., Gruwel, M. L., Jilkina, O., Gussakovsky, E., et al. (2010). Characterization of cryoinjury-induced infarction with manganese-and gadolinium-enhanced MRI and optical spectroscopy in pig hearts. Magn. Reson. Imag. 28, 753–766.
Yellon, D. M., and Hausenloy, D. J. (2007). Myocardial reperfusion injury. N. Engl. J. Med. 357, 1121–1135.
Keywords: myocardial infarction, heart failure, large animal models, large animal surgery, preclinical, translational research, review
Citation: Shin H, Shin HH and Shudo Y (2021) Current Status and Limitations of Myocardial Infarction Large Animal Models in Cardiovascular Translational Research. Front. Bioeng. Biotechnol. 9:673683. doi: 10.3389/fbioe.2021.673683
Received: 28 February 2021; Accepted: 06 April 2021;
Published: 29 April 2021.
Edited by:
Vahid Serpooshan, Emory University, United StatesReviewed by:
Richard Jung, University of Ottawa Heart Institute, CanadaNilesh Ghugre, Sunnybrook Research Institute (SRI), Canada
Copyright © 2021 Shin, Shin and Shudo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yasuhiro Shudo, eXNodWRvQHN0YW5mb3JkLmVkdQ==
†These authors have contributed equally to this work
 Hye Sook Shin
Hye Sook Shin Heather Hyeyoon Shin
Heather Hyeyoon Shin Yasuhiro Shudo
Yasuhiro Shudo