- 1Department of Physiology, Geffen School of Medicine at UCLA, Los Angeles, CA, USA
- 2VA Greater Los Angeles Healthcare System, Los Angeles, CA, USA
- 3Department of Medicine, Geffen School of Medicine at UCLA, Los Angeles, CA, USA
- 4Division of Infectious Diseases, VA Greater Los Angeles Health Care System, Los Angeles, CA, USA
Septins are small GTPases that play a role in several important cellular processes. In this review, we focus on the roles of septins in protein stabilization. Septins may regulate protein stability by: (1) interacting with proteins involved in degradation pathways, (2) regulating the interaction between transmembrane proteins and cytoskeletal proteins, (3) affecting the mobility of transmembrane proteins in lipid bilayers, and (4) modulating the interaction of proteins with their adaptor or signaling proteins. In this context, we discuss the role of septins in protecting four different proteins from degradation. First we consider botulinum neurotoxin serotype A (BoNT/A) and the contribution of septins to its extraordinarily long intracellular persistence. Next, we discuss the role of septins in stabilizing the receptor tyrosine kinases EGFR and ErbB2. Finally, we consider the contribution of septins in protecting hypoxia-inducible factor 1α (HIF-1α) from degradation.
Introduction
Protein turnover is regulated by intracellular degradation pathways, including the ubiquitin-proteasome and lysosome-autophagy systems. Protein ubiquitylation plays a key role in the regulation of protein degradation. It is well-established that polyubiquitin chains, predominantly but not exclusively with Lys48 linkages, target proteins to proteasomal degradation (Peng et al., 2003; Xu et al., 2009; Kaiser et al., 2011; Kim et al., 2011). Ubiquitylation also acts as a signal to endocytosis (Terrell et al., 1998; Mukhopadhyay and Riezman, 2007) or protein sorting of internalized proteins to multi-vesicular bodies followed by lysosomal degradation (Raiborg and Stenmark, 2009; Ren and Hurley, 2010; Stringer and Piper, 2011). In addition, ubiquitylation regulates signaling by modulating protein-protein interactions (Mukhopadhyay and Riezman, 2007; Yau and Rape, 2016), which may affect localization of the protein and hence its susceptibility to degradation.
The half-life of most cellular proteins is typically a few days (~40–69 h) (Varshavsky, 1996). Soluble proteins usually have a relatively short half-life (several hours), while transmembrane proteins tend to survive longer (2–3 days). At the extremes, some proteins, like hypoxia-inducible factor 1α (HIF-1α), last only seconds (Yu et al., 1998), while others, like the light chain of serotype A botulinum neurotoxin, remain intact for several months (Dolly and Aoki, 2006).
Septins are small GTPases that form hetero-oligomeric structures and act as linkers between the plasma membrane and the intracellular cytoskeleton. Through interactions with transmembrane and cytosolic proteins, septins organize segregated membrane microdomains, and membrane-associated protein complexes. The contribution of septins to the regulation of numerous cellular processes, including cell division, protein trafficking, exocytosis, cell migration, and cell proliferation, has been described in several excellent reviews (Hall and Russell, 2012; Mostowy and Cossart, 2012; Dolat et al., 2014; Fung et al., 2014). In this review, we will focus on the roles of septins in the regulation of protein stability.
Septins regulate protein stability by affecting several intracellular processes. First, they interact with components of endocytosis and exocytosis machinery (Beites et al., 1999, 2005; Amin et al., 2008; Maimaitiyiming et al., 2013; Phan et al., 2013; Tokhtaeva et al., 2015; Song et al., 2016). Second, they may regulate the interaction between transmembrane proteins and cytoskeletal proteins (Gilden and Krummel, 2010; Hagiwara et al., 2011; Hall and Russell, 2012; Mostowy and Cossart, 2012; Bridges et al., 2016). Third, by forming diffusion barriers, septins can modulate the mobility of transmembrane proteins in lipid bilayers (Hagiwara et al., 2011; Saarikangas and Barral, 2011; Hall and Russell, 2012; Mostowy and Cossart, 2012; Fung et al., 2014; Bridges et al., 2016). Fourth, septins form scaffolds and thus can modulate the interaction of proteins with their adaptor or signaling proteins (Ihara et al., 2007; Spiliotis and Gladfelter, 2011; Ageta-Ishihara et al., 2013; Ghossoub et al., 2013). All these processes can affect trafficking and sorting of proteins to degradative pathways. Finally, septins interact with proteins involved in degradation pathways, including ubiquitin ligases, and de-ubiquitylating enzymes, thus modulating protein turnover rate (Nakahira et al., 2010; Diesenberg et al., 2015; Marcus et al., 2016).
Septins Contribute to the Remarkable Stability of Botulinum Neurotoxin a Light Chain
Botulinum Neurotoxins and Their Stability
Botulism is a life-threatening illness caused by neurotoxins produced by Clostridium botulinum. Botulinum neurotoxins (BoNTs) are synthesized as a single-chain 150 kDa polypeptide that is later cleaved by proteases for biological activity into heavy (100 kDa) and light (50 kDa) chains. BoNTs bind through the heavy chain to specific receptors on motor neurons followed by receptor-mediated endocytosis and translocation of their light chains into the cytoplasm (Montal, 2010). Once inside the neuron, the light chain acts as a zinc-dependent endoprotease to cleave one of the SNARE (Soluble N-ethylmaleimide-sensitive factor Attachment Protein REceptor) proteins involved in vesicle-membrane fusion. This prevents release of acetylcholine into the synaptic cleft, resulting in neuromuscular paralysis (Dolly and Aoki, 2006; Popoff and Bouvet, 2009; Montal, 2010). As a result, patients with botulism develop severe muscle weakness. The illness may progress to total loss of muscle function, inability to breathe, and death unless supportive care is provided (Arnon et al., 2001).
Of the seven serotypes of BoNT (A–G), human botulism is caused by serotypes A, B, E and rarely by F (Arnon et al., 2001). BoNT/A intoxication lasts surprisingly long: even after 57 weeks following exposure to BoNT/A, humans may still demonstrate 22% muscle paralysis (Eleopra et al., 1998). Current treatment for adult botulism consists of supportive care and passive immunization with heptavalent equine antitoxin (Centers for Disease and Prevention, 2010). Antitoxin lowers death rates and shortens duration of symptoms only if administered within 24 h of disease onset (Arnon et al., 2001; Sobel, 2005). However, antitoxin does not enter neurons and does not reverse paralysis; hence, there is no specific treatment that targets BoNT once it is inside motor neurons.
While they cleave the same SNARE protein (SNAP-25), BoNT/A causes much longer duration of paralysis than BoNT/E (Keller et al., 1999; Adler et al., 2001; Bajohrs et al., 2004). In cultured spinal cord neurons, the proteolytic activity of BoNT/A, and BoNT/E persists for more than 80 days and <1 day, respectively (Keller et al., 1999). When expressed in cultured neuronal cells, the light chain of BoNT/A (LCA) survives significantly longer than the light chain of BoNT/E (LCE) due to less efficient degradation (Fernández-Salas et al., 2004; Tsai et al., 2010; Vagin et al., 2014). However, LCA is cleaved by proteases in vitro as efficiently as LCE (Gimenez and DasGupta, 1993; Beecher and DasGupta, 1997; Prabakaran et al., 2001). Thus, intrinsic resistance to intracellular proteases does not explain LCA persistence.
Septins Protect Botulinum Toxin Light Chain a from Intracellular Degradation
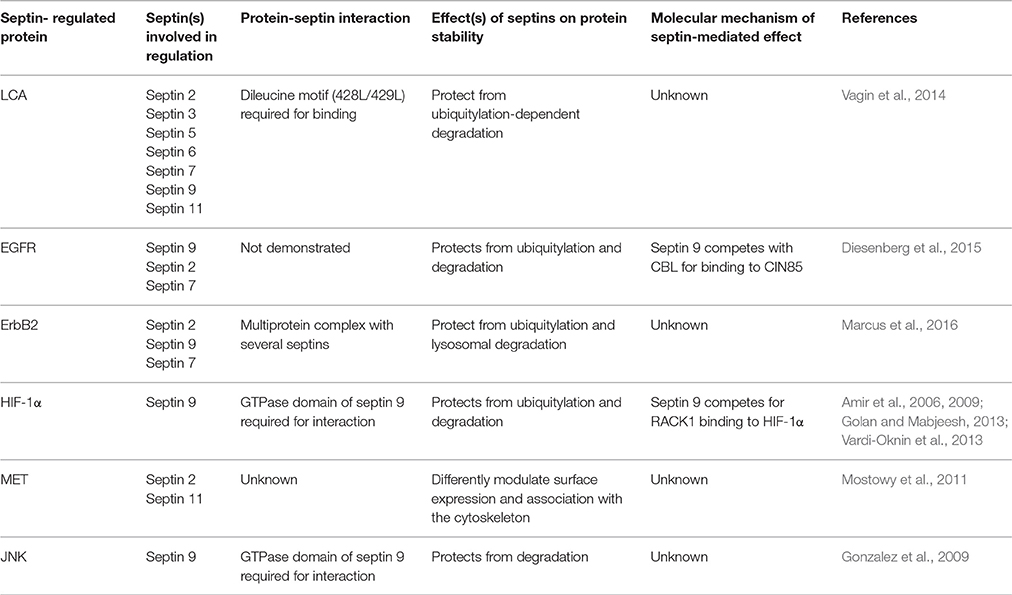
Even though LCA does not possess a transmembrane domain, it is localized in clusters almost exclusively at the plasma membrane (Vagin et al., 2014). In contrast, LCE remains in the cytoplasm, where its half-life corresponds to that of typical cytoplasmic proteins (Foran et al., 2003; Fernández-Salas et al., 2004). A double mutation, L428A/L429A, in a dileucine-containing motif (EFYKLL), which is present in LCA but not in LCE, decreases association of LCA with the plasma membrane (Fernández-Salas et al., 2004), prevents its clustered distribution, and shortens the half-life of LCA in cultured neuronal cells (Vagin et al., 2014) and shortens neuroparalytic effects of BoNT/A in mice (Wang et al., 2011). Furthermore, fusion of LCE with BoNT/A stabilizes LCE, while L428A/L429A mutation in the LCA portion of this chimera reverts LCE back into a short-lived protein (Wang et al., 2011). Mass spectrometry analysis identified septins as proteins that interact with LCA but not with the L428A/L429A LCA mutant (Table 1) (Vagin et al., 2014). Septins co-localize with LCA in plasma membrane-associated clusters, and the L428A/L429A mutation decreases this co-localization and accelerates ubiquitylation-dependent degradation of LCA (Vagin et al., 2014). Similarly, impairment of septin oligomerization with forchlorfenuron (FCF), decreases LCA clustering, and increases LCA degradation (Vagin et al., 2014). Therefore, the dileucine-mediated formation of membrane-attached LCA- and septin-containing complexes is crucial for the long-lasting stabilization of LCA and LCA-related neuroparalytic activity. The involvement of septins in LCA stabilization is consistent with the reports on enrichment of septins in the presynaptic sites in neurons (Kinoshita et al., 2000; Xue et al., 2004; Yang et al., 2010; Tsang et al., 2011) and interaction of septins with SNARE proteins (Beites et al., 1999; Dent et al., 2002; Ihara et al., 2007; Ito et al., 2009; Wasik et al., 2012; Tokhtaeva et al., 2015).
Septins Stabilize Receptor Tyrosine Kinases
Persistent Signaling of Receptor Tyrosine Kinases
EGFR family receptor tyrosine kinases (ErbB1 or EGFR, ErbB2, ErbB3, ErbB4) trigger signaling cascades that regulate critical cellular processes, such as growth, differentiation, proliferation, adhesion, survival, and migration (Blume-Jensen and Hunter, 2001). One of the primary mechanisms that regulates the duration of downstream signaling after activation is the removal of these receptors from the membrane by endocytosis followed by trafficking back to the cell surface or to lysosomes for degradation (Wiley and Burke, 2001; Sorkin and Goh, 2009). Over-expression and aberrant degradation of ErbB receptors lead to enhanced or continuous signaling, promoting malignant transformation (Sangwan and Park, 2006; Lemmon and Schlessinger, 2010; Tebbutt et al., 2013; Arteaga and Engelman, 2014).
Endocytosis and degradative sorting of EGFR are regulated by the ubiquitin ligase CBL (Levkowitz et al., 1996; Schmidt and Dikic, 2005). EGFR stimulation by ligand binding triggers the recruitment of CBL, which induces multi-mono- and K63-polyubiquitylation of the receptor (Mosesson et al., 2003; Huang et al., 2006, 2013). Whether ubiquitylation is required for endocytosis is still a point of controversy. However, it is generally agreed that CBL-mediated receptor ubiquitylation targets internalized receptors for lysosomal degradation, while non-ubiquitylated receptors are recycled back to the membrane. CBL-interacting protein of 85 kDa (CIN85) mediates the interaction between CBL and EGFR and hence is involved in the downregulation of EGFR (Soubeyran et al., 2002; Haglund et al., 2003).
In contrast to EGFR, ErbB2 does not have a known ligand (Garrett et al., 2003) and is thought to be resistant to endocytic down-regulation (Wang et al., 1999; Haslekås et al., 2005; Roepstorff et al., 2008; Sorkin and Goh, 2008). Ubiquitylation is important for ErbB2 degradation (Vuong et al., 2013), but the involvement of CBL is not clear (Levkowitz et al., 1996; Klapper et al., 2000). Heat shock protein 90 (HSP90) is required for stable expression of ErbB2 at the plasma membrane, and disruption of ErbB2 association with HSP90 allows for recruitment of HSP70 and ubiquitin ligases CHIP and/or Cullin-5, leading to ErbB2 ubiquitylation (Xu et al., 2002; Ehrlich et al., 2009), internalization and lysosomal degradation (Tikhomirov and Carpenter, 2000; Austin et al., 2004; Lerdrup et al., 2006). Proteasomal activity is required for endocytosis and lysosomal targeting of ErbB2 but is not directly involved in ErbB2 proteolysis (Lerdrup et al., 2006, 2007; Roepstorff et al., 2008).
Septins Protect Receptor Tyrosine Kinases from Ubiquitylation, Endocytosis and Degradation
CIN85 is involved in down-regulation of EGFR by tethering CBL to the endocytic machinery in an EGF-dependent manner (Dikic, 2002). A number of studies have suggested that CIN85 participates in both the initial step of EGFR internalization (Soubeyran et al., 2002) and also in receptor trafficking and degradation (de Melker et al., 2001; Kowanetz et al., 2004; Schroeder et al., 2012). Knockdown of CIN85 results in a decrease in EGFR ubiquitylation (Rønning et al., 2011), while prevention of CIN85 phosphorylation affects efficient sorting and degradation of EGFR but has no effect on receptor endocytosis (Schroeder et al., 2012). A recent study has identified CIN85 as an interacting partner of septin 9 (Table 1) (Diesenberg et al., 2015). This interaction depends on the presence of a conserved ProArg motif in the N-terminus of septin 9 and mediates the formation of a multiprotein complex of CIN85 with septin 9 and other septins. In vitro binding studies suggest that septin 9 competes with CBL for binding to CIN85 (Diesenberg et al., 2015). Stimulation of EGFR with EGF in Hela cells results in the recruitment of the CIN85-septin complex to the plasma membrane. Depletion of septin 9 increases the degree of EGFR ubiquitylation and accelerates its degradation. Taken together, these data suggest that septin 9 negatively regulates EGFR degradation by preventing the association of the ubiquitin ligase CBL with CIN85, resulting in reduced EGFR ubiquitylation, and degradation (Diesenberg et al., 2015).
Another recent study has identified septins as interacting partners and regulators of the persistent expression of ErbB2 (Marcus et al., 2016). Several septins, including septin 2, septin 7, and septin 9, co-localize and interact with ErbB2 at the plasma membrane in gastric cancer cells. Inhibition of septin filament assembly-disassembly with FCF: (1) decreases association of septins with ErbB2, (2) reduces plasma-membrane localization of septins, (3) increases the amount of septin-free ErbB2, (4) induces ubiquitylation of ErbB2, and (5) accelerates its lysosomal degradation. A similar increase in ErbB2 degradation is observed in septin 2-depleted cells. These results imply that normally organized septin filaments protect ErbB2 from ubiquitylation, endocytosis, and lysosomal degradation.
This protective effect of septins is not related to the regulation of ErbB2 interaction with its chaperone HSP90 because the FCF-induced ubiquitylation and degradation of ErbB2 is not altered by geldanamycin (Marcus et al., 2016). This inhibitor down-regulates ErbB2 by disrupting the ErbB2-HSP90 interaction (Tikhomirov and Carpenter, 2000; Lerdrup et al., 2006, 2007). Therefore, distinct and complementary effects of FCF, and geldanamycin present a potential for augmented targeting of ErbB2 persistence in cancer.
FCF-induced ubiquitylation of ErbB2 is unlikely to be due to septin 9-mediated regulation of CBL as has been reported for EGFR (Diesenberg et al., 2015), because ErbB2 does not interact with CIN85 and FCF does not affect levels of EGFR (Marcus et al., 2016). The involvement of other ubiquitin ligases identified as septin interacting proteins (Nakahira et al., 2010; Tokhtaeva et al., 2015) in septin-regulated ErbB2 ubiquitylation has not been evaluated. Alternatively, septins may recruit de-ubiquitylating enzymes to ErbB2, resulting in a lower steady state level of ErbB2 ubiquitylation. In particular, ErbB2 interacts with a de-ubiquitylating enzyme USP9x (Marx et al., 2010; Marcus et al., 2016) that has been shown to protect ErbB2 from bortezomib-induced lysosomal degradation (Marx et al., 2010). In addition, septins play critical roles in endocytosis and exocytosis (Beites et al., 1999, 2005; Maimaitiyiming et al., 2013; Phan et al., 2013; Tokhtaeva et al., 2015; Song et al., 2016), and disruption of septin dynamics with FCF may induce ErbB2 internalization or delay its recycling to the plasma membrane.
Septins Stabilize Hypoxia-Inducible Factor 1α
Role of HIF-1α in Cell Responses to Hypoxia
HIF-1 is a transcription factor and key regulator of cellular responses to changes in oxygen concentration that allow cell adaptation and survival under hypoxic conditions (Semenza, 2014). HIF-1 is composed of the oxygen-regulated subunit, HIF-1α, and the constitutively expressed HIF-1β subunit. HIF-1α is constantly produced and degraded under normoxic conditions due to oxygen-dependent hydroxylation, which promotes binding of von Hippel-Lindau protein (VHL), leading to ubiquitylation, and proteasomal degradation of HIF-1α. Under hypoxic conditions, HIF-1α is not hydroxylated, does not interact with VHL, translocates to the nucleus, and binds to hypoxia-response elements in target genes. The expression of over 70 genes is known to be activated at the transcriptional level by HIF-1 (Semenza, 2014). HIF-1α rapidly accumulates under hypoxic conditions and is degraded upon reoxygenation with a half-life of under 1 min (Yu et al., 1998). Another level of post-translational regulation does not depend on oxygen, hydroxylation, or VHL but requires the interaction between HIF-1α and RACK1 (receptor of activated protein C kinase). RACK1 competes with HSP90 for binding to HIF-1α and promotes the proteasome-dependent degradation of HIF-1α (Liu et al., 2007; Liu and Semenza, 2007). Activation of the HIF system has been observed in carcinogenesis and numerous cancers (Semenza, 2012, 2016a,b; Hubbi and Semenza, 2015). Increased levels of HIF-1 activity are often associated with increased tumor aggressiveness, therapeutic resistance, and mortality.
Septin 9 Protects HIF-1α from Degradation
A search for HIF-1α-interacting proteins in human prostate cancer cells identified septin 9 (Sept9_v1 isoform) (Amir et al., 2006). Over-expression of septin 9 decreases ubiquitylation and degradation of HIF-1α and activates HIF-1α-dependent transcriptome (Amir et al., 2006). Co-immunoprecipitation experiments show that septin 9 competes with RACK1 for binding to HIF-1α (Amir et al., 2009). Inhibition of HSP90 induces RACK1-dependent HIF-1α degradation, and the rate of this degradation is significantly lower in cells over-expressing septin 9 (Amir et al., 2009). Taken together, these results implicate septin 9 in oxygen-independent stabilization of HIF-1α. As long as HIF-1α is bound to HSP90 or septin 9, it is protected from RACK1-mediated degradation via the proteasome. In the presence of HSP90 inhibitors, the HIF-1α-HSP90 association is disrupted, leading to a competition between RACK1, which promotes HIF-1α degradation, and septin 9, which confers HIF-1α stabilization (Amir et al., 2009). In support of this HIF-1α-stabilizing role of septin 9, disruption of normal assembly-disassembly of septin oligomers with FCF induces degradation of HIF-1α, and inhibition in HIF-1α transcriptional activity in various cancer cell types (Vardi-Oknin et al., 2013).
Concluding Remarks
Septins stabilize integral membrane proteins (EGFR and ErbB2), membrane-associated proteins (LCA), and cytosolic/nuclear proteins (HIF-1α) by attenuating their ubiquitylation and degradation. Septin 9 also interacts with and stabilizes another cytosolic/nuclear protein, c-Jun-N-terminal kinase (JNK) (Gonzalez et al., 2009), but whether septin 9 affects the degree of JNK ubiquitylation has not been investigated. Septin 2 and septin 11 regulate surface expression of MET receptor tyrosine kinase by modulating its mobility in the lipid bilayer and its linkage to the underlying cytoskeleton (Mostowy et al., 2011). However, it is not clear if septins affect MET degradation. Only some of the molecular events underlying the protective effects of septins against protein ubiquitylation and degradation are understood (Table 1), and further studies are required to determine the detailed mechanisms of septin dependent protein stabilization.
Understanding the mechanism by which septins contribute to intracellular stability of LCA would provide valuable insights for treating BoNT intoxication. On the other hand, the remarkable stability of LCA is fundamental in the success of BoNT/A for long-term treatment of several disorders as well as cosmetic therapies (Bhidayasiri and Truong, 2005; Chancellor et al., 2013; Esquenazi et al., 2013; Hallett et al., 2013; Naumann et al., 2013; Jost et al., 2015; Choi et al., 2016), and a super-stable LCA would be useful therapeutically.
A better understanding of septin contribution to the abnormal persistence of EGFR and ErbB2 in cancer cells will provide a potential treatment target for aggressive malignancies. Receptor-targeted therapies, such as monoclonal antibodies (e.g., trastuzumab), and tyrosine kinase inhibitors (e.g., gefitinib), are effective against several types of malignancies, but tumors may develop resistance to these agents due to compensatory mechanisms (Hynes and Lane, 2005; Takeuchi and Ito, 2011; Arteaga and Engelman, 2014), emphasizing the evolving need to develop new synergistic treatment strategies.
Knowledge of the pathways of septin contribution in oxygen-independent stabilization of HIF-1α would provide a better understanding of the reasons for HIF-1α over-expression in various cancers even in aerobic conditions, which correlates with poor prognosis, making HIF-1α an important target for cancer therapy. A better understanding of septin-mediated stabilization of HIF-1α opens the way to new therapeutic approaches to target “normoxic” tumor cells (Semenza, 2012; Burroughs et al., 2013; Warfel and El-Deiry, 2014).
Author Contributions
OV and DB wrote, revised, and approved the final version of the manuscript.
Funding
Supported by National Heart, Lung, and Blood Institute (NHLBI) grant R01HL113350 (OV).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Drs. George Sachs and Laura Dada for critical reading of the manuscript and helpful suggestions.
References
Adler, M., Keller, J. E., Sheridan, R. E., and Deshpande, S. S. (2001). Persistence of botulinum neurotoxin A demonstrated by sequential administration of serotypes A and E in rat EDL muscle. Toxicon 39, 233–243. doi: 10.1016/S0041-0101(00)00120-3
Ageta-Ishihara, N., Miyata, T., Ohshima, C., Watanabe, M., Sato, Y., Hamamura, Y., et al. (2013). Septins promote dendrite and axon development by negatively regulating microtubule stability via HDAC6-mediated deacetylation. Nat. Commun. 4:2532. doi: 10.1038/ncomms3532
Amin, N. D., Zheng, Y. L., Kesavapany, S., Kanungo, J., Guszczynski, T., Sihag, R. K., et al. (2008). Cyclin-dependent kinase 5 phosphorylation of human septin SEPT5 (hCDCrel-1) modulates exocytosis. J. Neurosci. 28, 3631–3643. doi: 10.1523/JNEUROSCI.0453-08.2008
Amir, S., Wang, R., Matzkin, H., Simons, J. W., and Mabjeesh, N. J. (2006). MSF-A interacts with hypoxia-inducible factor-1alpha and augments hypoxia-inducible factor transcriptional activation to affect tumorigenicity and angiogenesis. Cancer Res. 66, 856–866. doi: 10.1158/0008-5472.CAN-05-2738
Amir, S., Wang, R., Simons, J. W., and Mabjeesh, N. J. (2009). SEPT9_v1 up-regulates hypoxia-inducible factor 1 by preventing its RACK1-mediated degradation. J. Biol. Chem. 284, 11142–11151. doi: 10.1074/jbc.M808348200
Arnon, S. S., Schechter, R., Inglesby, T. V., Henderson, D. A., Bartlett, J. G., Ascher, M. S., et al. (2001). Botulinum toxin as a biological weapon: medical and public health management. JAMA 285, 1059–1070. doi: 10.1001/jama.285.8.1059
Arteaga, C. L., and Engelman, J. A. (2014). ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell 25, 282–303. doi: 10.1016/j.ccr.2014.02.025
Austin, C. D., De Mazière, A. M., Pisacane, P. I., van Dijk, S. M., Eigenbrot, C., Sliwkowski, M. X., et al. (2004). Endocytosis and sorting of ErbB2 and the site of action of cancer therapeutics trastuzumab and geldanamycin. Mol. Biol. Cell 15, 5268–5282. doi: 10.1091/mbc.E04-07-0591
Bajohrs, M., Rickman, C., Binz, T., and Davletov, B. (2004). A molecular basis underlying differences in the toxicity of botulinum serotypes A and E. EMBO Rep. 5, 1090–1095. doi: 10.1038/sj.embor.7400278
Beecher, D. J., and DasGupta, B. R. (1997). Botulinum neurotoxin type A: limited proteolysis by endoproteinase Glu-C and alpha-chymotrypsin enhanced following reduction; identification of the cleaved sites and fragments. J. Protein Chem. 16, 701–712. doi: 10.1023/A:1026358504860
Beites, C. L., Campbell, K. A., and Trimble, W. S. (2005). The septin Sept5/CDCrel-1 competes with alpha-SNAP for binding to the SNARE complex. Biochem. J. 385(Pt 2), 347–353. doi: 10.1042/BJ20041090
Beites, C. L., Xie, H., Bowser, R., and Trimble, W. S. (1999). The septin CDCrel-1 binds syntaxin and inhibits exocytosis. Nat. Neurosci. 2, 434–439. doi: 10.1038/8100
Bhidayasiri, R., and Truong, D. D. (2005). Expanding use of botulinum toxin. J. Neurol. Sci. 235, 1–9. doi: 10.1016/j.jns.2005.04.017
Blume-Jensen, P., and Hunter, T. (2001). Oncogenic kinase signalling. Nature 411, 355–365. doi: 10.1038/35077225
Bridges, A. A., Jentzsch, M. S., Oakes, P. W., Occhipinti, P., and Gladfelter, A. S. (2016). Micron-scale plasma membrane curvature is recognized by the septin cytoskeleton. J. Cell Biol. 213, 23–32. doi: 10.1083/jcb.201512029
Burroughs, S. K., Kaluz, S., Wang, D., Wang, K., Van Meir, E. G., and Wang, B. (2013). Hypoxia inducible factor pathway inhibitors as anticancer therapeutics. Future Med. Chem. 5, 553–572. doi: 10.4155/fmc.13.17
Centers for Disease Prevention (2010). Investigational heptavalent botulinum antitoxin (HBAT) to replace licensed botulinum antitoxin AB and investigational botulinum antitoxin E. MMWR Morb. Mortal. Wkly. Rep. 59:299. Available online at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5910a4.htm
Chancellor, M. B., Elovic, E., Esquenazi, A., Naumann, M., Segal, K. R., Schiavo, G., et al. (2013). Evidence-based review and assessment of botulinum neurotoxin for the treatment of urologic conditions. Toxicon 67, 129–140. doi: 10.1016/j.toxicon.2013.01.020
Choi, Y.-J., Lee, W.-J., Lee, H.-J., Lee, K.-W., Kim, H.-J., and Hu, K.-S. (2016). Effective botulinum toxin injection guide for treatment of temporal headache. Toxins (Basel). 8:265. doi: 10.3390/toxins8090265
de Melker, A. A., van der Horst, G., Calafat, J., Jansen, H., and Borst, J. (2001). c-Cbl ubiquitinates the EGF receptor at the plasma membrane and remains receptor associated throughout the endocytic route. J. Cell Sci. 114(Pt 11), 2167–2178.
Dent, J., Kato, K., Peng, X. R., Martinez, C., Cattaneo, M., Poujol, C., et al. (2002). A prototypic platelet septin and its participation in secretion. Proc. Natl. Acad. Sci. U.S.A. 99, 3064–3069. doi: 10.1073/pnas.052715199
Diesenberg, K., Beerbaum, M., Fink, U., Schmieder, P., and Krauss, M. (2015). SEPT9 negatively regulates ubiquitin-dependent downregulation of EGFR. J. Cell Sci. 128, 397–407. doi: 10.1242/jcs.162206
Dikic, I. (2002). CIN85/CMS family of adaptor molecules. FEBS Lett. 529, 110–115. doi: 10.1016/S0014-5793(02)03188-5
Dolat, L., Hu, Q., and Spiliotis, E. T. (2014). Septin functions in organ system physiology and pathology. Biol. Chem. 395, 123–141. doi: 10.1515/hsz-2013-0233
Dolly, J. O., and Aoki, K. R. (2006). The structure and mode of action of different botulinum toxins. Eur. J. Neurol. 13(Suppl. 4), 1–9. doi: 10.1111/j.1468-1331.2006.01648.x
Ehrlich, E. S., Wang, T., Luo, K., Xiao, Z., Niewiadomska, A. M., Martinez, T., et al. (2009). Regulation of Hsp90 client proteins by a Cullin5-RING E3 ubiquitin ligase. Proc. Natl. Acad. Sci. U.S.A. 106, 20330–20335. doi: 10.1073/pnas.0810571106
Eleopra, R., Tugnoli, V., Rossetto, O., De Grandis, D., and Montecucco, C. (1998). Different time courses of recovery after poisoning with botulinum neurotoxin serotypes A and E in humans. Neurosci. Lett. 256, 135–138. doi: 10.1016/S0304-3940(98)00775-7
Esquenazi, A., Albanese, A., Chancellor, M. B., Elovic, E., Segal, K. R., Simpson, D. M., et al. (2013). Evidence-based review and assessment of botulinum neurotoxin for the treatment of adult spasticity in the upper motor neuron syndrome. Toxicon 67, 115–128. doi: 10.1016/j.toxicon.2012.11.025
Fernández-Salas, E., Steward, L. E., Ho, H., Garay, P. E., Sun, S. W., Gilmore, M. A., et al. (2004). Plasma membrane localization signals in the light chain of botulinum neurotoxin. Proc. Natl. Acad. Sci. U.S.A. 101, 3208–3213. doi: 10.1073/pnas.0400229101
Foran, P. G., Mohammed, N., Lisk, G. O., Nagwaney, S., Lawrence, G. W., Johnson, E., et al. (2003). Evaluation of the therapeutic usefulness of botulinum neurotoxin B, C1, E, and F compared with the long lasting type A. Basis for distinct durations of inhibition of exocytosis in central neurons. J. Biol. Chem. 278, 1363–1371. doi: 10.1074/jbc.M209821200
Fung, K. Y., Dai, L., and Trimble, W. S. (2014). Cell and molecular biology of septins. Int. Rev. Cell Mol. Biol. 310, 289–339. doi: 10.1016/B978-0-12-800180-6.00007-4
Garrett, T. P., McKern, N. M., Lou, M., Elleman, T. C., Adams, T. E., Lovrecz, G. O., et al. (2003). The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol. Cell 11, 495–505. doi: 10.1016/S1097-2765(03)00048-0
Ghossoub, R., Hu, Q., Failler, M., Rouyez, M. C., Spitzbarth, B., Mostowy, S., et al. (2013). Septins 2, 7 and 9 and MAP4 colocalize along the axoneme in the primary cilium and control ciliary length. J. Cell Sci. 126(Pt 12), 2583–2594. doi: 10.1242/jcs.111377
Gilden, J., and Krummel, M. F. (2010). Control of cortical rigidity by the cytoskeleton: emerging roles for septins. Cytoskeleton (Hoboken). 67, 477–486. doi: 10.1002/cm.20461
Gimenez, J. A., and DasGupta, B. R. (1993). Botulinum type A neurotoxin digested with pepsin yields 132, 97, 72, 45, 42, and 18 kD fragments. J. Protein Chem. 12, 351–363. doi: 10.1007/BF01028197
Golan, M., and Mabjeesh, N. J. (2013). SEPT9_i1 is required for the association between HIF-1α and importin-α to promote efficient nuclear translocation. Cell Cycle 12, 2297–2308. doi: 10.4161/cc.25379
Gonzalez, M. E., Makarova, O., Peterson, E. A., Privette, L. M., and Petty, E. M. (2009). Up-regulation of SEPT9_v1 stabilizes c-Jun-N-terminal kinase and contributes to its pro-proliferative activity in mammary epithelial cells. Cell. Signal. 21, 477–487. doi: 10.1016/j.cellsig.2008.11.007
Hagiwara, A., Tanaka, Y., Hikawa, R., Morone, N., Kusumi, A., Kimura, H., et al. (2011). Submembranous septins as relatively stable components of actin-based membrane skeleton. Cytoskeleton (Hoboken). 68, 512–525. doi: 10.1002/cm.20528
Haglund, K., Sigismund, S., Polo, S., Szymkiewicz, I., Di Fiore, P. P., and Dikic, I. (2003). Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat. Cell Biol. 5, 461–466. doi: 10.1038/ncb983
Hall, P. A., and Russell, S. E. (2012). Mammalian septins: dynamic heteromers with roles in cellular morphogenesis and compartmentalization. J. Pathol. 226, 287–299. doi: 10.1002/path.3024
Hallett, M., Albanese, A., Dressler, D., Segal, K. R., Simpson, D. M., Truong, D., et al. (2013). Evidence-based review and assessment of botulinum neurotoxin for the treatment of movement disorders. Toxicon 67, 94–114. doi: 10.1016/j.toxicon.2012.12.004
Haslekås, C., Breen, K., Pedersen, K. W., Johannessen, L. E., Stang, E., and Madshus, I. H. (2005). The inhibitory effect of ErbB2 on epidermal growth factor-induced formation of clathrin-coated pits correlates with retention of epidermal growth factor receptor-ErbB2 oligomeric complexes at the plasma membrane. Mol. Biol. Cell 16, 5832–5842. doi: 10.1091/mbc.E05-05-0456
Huang, F., Kirkpatrick, D., Jiang, X., Gygi, S., and Sorkin, A. (2006). Differential regulation of EGF receptor internalization and degradation by multiubiquitination within the kinase domain. Mol. Cell 21, 737–748. doi: 10.1016/j.molcel.2006.02.018
Huang, F., Zeng, X., Kim, W., Balasubramani, M., Fortian, A., Gygi, S. P., et al. (2013). Lysine 63-linked polyubiquitination is required for EGF receptor degradation. Proc. Natl. Acad. Sci. U.S.A. 110, 15722–15727. doi: 10.1073/pnas.1308014110
Hubbi, M. E., and Semenza, G. L. (2015). Regulation of cell proliferation by hypoxia-inducible factors. Am. J. Physiol. Cell Physiol. 309, C775–C782. doi: 10.1152/ajpcell.00279.2015
Hynes, N. E., and Lane, H. A. (2005). ERBB receptors and cancer: the complexity of targeted inhibitors. Nat. Rev. Cancer 5, 341–354. doi: 10.1038/nrc1609
Ihara, M., Yamasaki, N., Hagiwara, A., Tanigaki, A., Kitano, A., Hikawa, R., et al. (2007). Sept4, a component of presynaptic scaffold and Lewy bodies, is required for the suppression of alpha-synuclein neurotoxicity. Neuron 53, 519–533. doi: 10.1016/j.neuron.2007.01.019
Ito, H., Atsuzawa, K., Morishita, R., Usuda, N., Sudo, K., Iwamoto, I., et al. (2009). Sept8 controls the binding of vesicle-associated membrane protein 2 to synaptophysin. J. Neurochem. 108, 867–880. doi: 10.1111/j.1471-4159.2008.05849.x
Jost, W. H., Benecke, R., Hauschke, D., Jankovic, J., Kanovský, P., Roggenkämper, P., et al. (2015). Clinical and pharmacological properties of incobotulinumtoxinA and its use in neurological disorders. Drug Des. Dev. Ther. 9, 1913–1926. doi: 10.2147/DDDT.S79193
Kaiser, S. E., Riley, B. E., Shaler, T. A., Trevino, R. S., Becker, C. H., Schulman, H., et al. (2011). Protein standard absolute quantification (PSAQ) method for the measurement of cellular ubiquitin pools. Nat. Methods 8, 691–696. doi: 10.1038/nmeth.1649
Keller, J. E., Neale, E. A., Oyler, G., and Adler, M. (1999). Persistence of botulinum neurotoxin action in cultured spinal cord cells. FEBS Lett. 456, 137–142. doi: 10.1016/S0014-5793(99)00948-5
Kim, W., Bennett, E. J., Huttlin, E. L., Guo, A., Li, J., Possemato, A., et al. (2011). Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol. Cell 44, 325–340. doi: 10.1016/j.molcel.2011.08.025
Kinoshita, A., Noda, M., and Kinoshita, M. (2000). Differential localization of septins in the mouse brain. J. Comp. Neurol. 428, 223–239. doi: 10.1002/1096-9861(20001211)428:2<223::AID-CNE3>3.0.CO;2-M
Klapper, L. N., Waterman, H., Sela, M., and Yarden, Y. (2000). Tumor-inhibitory antibodies to HER-2/ErbB-2 may act by recruiting c-Cbl and enhancing ubiquitination of HER-2. Cancer Res. 60, 3384–3388.
Kowanetz, K., Husnjak, K., Höller, D., Kowanetz, M., Soubeyran, P., Hirsch, D., et al. (2004). CIN85 associates with multiple effectors controlling intracellular trafficking of epidermal growth factor receptors. Mol. Biol. Cell 15, 3155–3166. doi: 10.1091/mbc.E03-09-0683
Lemmon, M. A., and Schlessinger, J. (2010). Cell signaling by receptor tyrosine kinases. Cell 141, 1117–1134. doi: 10.1016/j.cell.2010.06.011
Lerdrup, M., Bruun, S., Grandal, M. V., Roepstorff, K., Kristensen, M. M., Hommelgaard, A. M., et al. (2007). Endocytic down-regulation of ErbB2 is stimulated by cleavage of its C-terminus. Mol. Biol. Cell 18, 3656–3666. doi: 10.1091/mbc.E07-01-0025
Lerdrup, M., Hommelgaard, A. M., Grandal, M., and van Deurs, B. (2006). Geldanamycin stimulates internalization of ErbB2 in a proteasome-dependent way. J. Cell Sci. 119(Pt 1), 85–95. doi: 10.1242/jcs.02707
Levkowitz, G., Klapper, L. N., Tzahar, E., Freywald, A., Sela, M., and Yarden, Y. (1996). Coupling of the c-Cbl protooncogene product to ErbB-1/EGF-receptor but not to other ErbB proteins. Oncogene 12, 1117–1125.
Liu, Y. V., Baek, J. H., Zhang, H., Diez, R., Cole, R. N., and Semenza, G. L. (2007). RACK1 competes with HSP90 for binding to HIF-1alpha and is required for O(2)-independent and HSP90 inhibitor-induced degradation of HIF-1alpha. Mol. Cell 25, 207–217. doi: 10.1016/j.molcel.2007.01.001
Liu, Y. V., and Semenza, G. L. (2007). RACK1 vs. HSP90: competition for HIF-1 alpha degradation vs. stabilization. Cell Cycle 6, 656–659. doi: 10.4161/cc.6.6.3981
Maimaitiyiming, M., Kobayashi, Y., Kumanogoh, H., Nakamura, S., Morita, M., and Maekawa, S. (2013). Identification of dynamin as a septin-binding protein. Neurosci. Lett. 534, 322–326. doi: 10.1016/j.neulet.2012.12.002
Marcus, E. A., Tokhtaeva, E., Turdikulova, S., Capri, J., Whitelegge, J. P., Scott, D. R., et al. (2016). Septin oligomerization regulates persistent expression of ErbB2/HER2 in gastric cancer cells. Biochem. J. 473, 1703–1718. doi: 10.1042/BCJ20160203
Marx, C., Held, J. M., Gibson, B. W., and Benz, C. C. (2010). ErbB2 trafficking and degradation associated with K48 and K63 polyubiquitination. Cancer Res. 70, 3709–3717. doi: 10.1158/0008-5472.CAN-09-3768
Montal, M. (2010). Botulinum neurotoxin: a marvel of protein design. Annu. Rev. Biochem. 79, 591–617. doi: 10.1146/annurev.biochem.051908.125345
Mosesson, Y., Shtiegman, K., Katz, M., Zwang, Y., Vereb, G., Szollosi, J., et al. (2003). Endocytosis of receptor tyrosine kinases is driven by monoubiquitylation, not polyubiquitylation. J. Biol. Chem. 278, 21323–21326. doi: 10.1074/jbc.C300096200
Mostowy, S., and Cossart, P. (2012). Septins: the fourth component of the cytoskeleton. Nat. Rev. Mol. Cell Biol. 13, 183–194. doi: 10.1038/nrm3284
Mostowy, S., Janel, S., Forestier, C., Roduit, C., Kasas, S., Pizarro-Cerdá, J., et al. (2011). A role for septins in the interaction between the Listeria monocytogenes INVASION PROTEIN InlB and the Met receptor. Biophys. J. 100, 1949–1959. doi: 10.1016/j.bpj.2011.02.040
Mukhopadhyay, D., and Riezman, H. (2007). Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science 315, 201–205. doi: 10.1126/science.1127085
Nakahira, M., Macedo, J. N., Seraphim, T. V., Cavalcante, N., Souza, T. A., Damalio, J. C., et al. (2010). A draft of the human septin interactome. PLoS ONE 5:e13799. doi: 10.1371/journal.pone.0013799
Naumann, M., Dressler, D., Hallett, M., Jankovic, J., Schiavo, G., Segal, K. R., et al. (2013). Evidence-based review and assessment of botulinum neurotoxin for the treatment of secretory disorders. Toxicon 67, 141–152. doi: 10.1016/j.toxicon.2012.10.020
Peng, J., Schwartz, D., Elias, J. E., Thoreen, C. C., Cheng, D., Marsischky, G., et al. (2003). A proteomics approach to understanding protein ubiquitination. Nat. Biotechnol. 21, 921–926. doi: 10.1038/nbt849
Phan, Q. T., Eng, D. K., Mostowy, S., Park, H., Cossart, P., and Filler, S. G. (2013). Role of endothelial cell septin 7 in the endocytosis of Candida albicans. MBio 4, e00542–e00513. doi: 10.1128/mBio.00542-13
Popoff, M. R., and Bouvet, P. (2009). Clostridial toxins. Future Microbiol. 4, 1021–1064. doi: 10.2217/fmb.09.72
Prabakaran, S., Tepp, W., and DasGupta, B. R. (2001). Botulinum neurotoxin types B and E: purification, limited proteolysis by endoproteinase Glu-C and pepsin, and comparison of their identified cleaved sites relative to the three-dimensional structure of type A neurotoxin. Toxicon 39, 1515–1531. doi: 10.1016/S0041-0101(01)00124-6
Raiborg, C., and Stenmark, H. (2009). The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 458, 445–452. doi: 10.1038/nature07961
Ren, X., and Hurley, J. H. (2010). VHS domains of ESCRT-0 cooperate in high-avidity binding to polyubiquitinated cargo. EMBO J. 29, 1045–1054. doi: 10.1038/emboj.2010.6
Roepstorff, K., Grøvdal, L., Grandal, M., Lerdrup, M., and van Deurs, B. (2008). Endocytic downregulation of ErbB receptors: mechanisms and relevance in cancer. Histochem. Cell Biol. 129, 563–578. doi: 10.1007/s00418-008-0401-3
Rønning, S. B., Pedersen, N. M., Madshus, I. H., and Stang, E. (2011). CIN85 regulates ubiquitination and degradative endosomal sorting of the EGF receptor. Exp. Cell Res. 317, 1804–1816. doi: 10.1016/j.yexcr.2011.05.016
Saarikangas, J., and Barral, Y. (2011). The emerging functions of septins in metazoans. EMBO Rep. 12, 1118–1126. doi: 10.1038/embor.2011.193
Sangwan, V., and Park, M. (2006). Receptor tyrosine kinases: role in cancer progression. Curr. Oncol. 13, 191–193.
Schmidt, M. H., and Dikic, I. (2005). The Cbl interactome and its functions. Nat. Rev. Mol. Cell Biol. 6, 907–918. doi: 10.1038/nrm1762
Schroeder, B., Srivatsan, S., Shaw, A., Billadeau, D., and McNiven, M. A. (2012). CIN85 phosphorylation is essential for EGFR ubiquitination and sorting into multivesicular bodies. Mol. Biol. Cell 23, 3602–3611. doi: 10.1091/mbc.E11-08-0666
Semenza, G. L. (2012). Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol. Sci. 33, 207–214. doi: 10.1016/j.tips.2012.01.005
Semenza, G. L. (2014). Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu. Rev. Pathol. 9, 47–71. doi: 10.1146/annurev-pathol-012513-104720
Semenza, G. L. (2016a). The hypoxic tumor microenvironment: A driving force for breast cancer progression. Biochim. Biophys. Acta 1863, 382–391. doi: 10.1016/j.bbamcr.2015.05.036
Semenza, G. L. (2016b). Novel strategies for cancer therapy. J. Mol. Med. 94, 119–120. doi: 10.1007/s00109-016-1379-2
Song, K., Russo, G., and Krauss, M. (2016). Septins as modulators of endo-lysosomal membrane traffic. Front. Cell Dev. Biol. 4:124. doi: 10.3389/fcell.2016.00124
Sorkin, A., and Goh, L. K. (2008). Endocytosis and intracellular trafficking of ErbBs. Exp. Cell Res. 314, 3093–3106. doi: 10.1016/j.yexcr.2008.07.029
Sorkin, A., and Goh, L. K. (2009). Endocytosis and intracellular trafficking of ErbBs. Exp. Cell Res. 315, 683–696. doi: 10.1016/j.yexcr.2008.07.029
Soubeyran, P., Kowanetz, K., Szymkiewicz, I., Langdon, W. Y., and Dikic, I. (2002). Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature 416, 183–187. doi: 10.1038/416183a
Spiliotis, E. T., and Gladfelter, A. S. (2011). Spatial guidance of cell asymmetry: septin GTPases show the way. Traffic 13, 195–203. doi: 10.1111/j.1600-0854.2011.01268.x
Stringer, D. K., and Piper, R. C. (2011). A single ubiquitin is sufficient for cargo protein entry into MVBs in the absence of ESCRT ubiquitination. J. Cell Biol. 192, 229–242. doi: 10.1083/jcb.201008121
Takeuchi, K., and Ito, F. (2011). Receptor tyrosine kinases and targeted cancer therapeutics. Biol. Pharm. Bull. 34, 1774–1780. doi: 10.1248/bpb.34.1774
Tebbutt, N., Pedersen, M. W., and Johns, T. G. (2013). Targeting the ERBB family in cancer: couples therapy. Nat. Rev. Cancer 13, 663–673. doi: 10.1038/nrc3559
Terrell, J., Shih, S., Dunn, R., and Hicke, L. (1998). A function for monoubiquitination in the internalization of a G protein-coupled receptor. Mol. Cell 1, 193–202. doi: 10.1016/S1097-2765(00)80020-9
Tikhomirov, O., and Carpenter, G. (2000). Geldanamycin induces ErbB-2 degradation by proteolytic fragmentation. J. Biol. Chem. 275, 26625–26631. doi: 10.1074/jbc.M003114200
Tokhtaeva, E., Capri, J., Marcus, E. A., Whitelegge, J. P., Khuzakhmetova, V., Bukharaeva, E., et al. (2015). Septin dynamics are essential for exocytosis. J. Biol. Chem. 290, 5280–5297. doi: 10.1074/jbc.M114.616201
Tsai, Y. C., Maditz, R., Kuo, C. L., Fishman, P. S., Shoemaker, C. B., Oyler, G. A., et al. (2010). Targeting botulinum neurotoxin persistence by the ubiquitin-proteasome system. Proc. Natl. Acad. Sci. U.S.A. 107, 16554–16559. doi: 10.1073/pnas.1008302107
Tsang, C. W., Estey, M. P., DiCiccio, J. E., Xie, H., Patterson, D., and Trimble, W. S. (2011). Characterization of presynaptic septin complexes in mammalian hippocampal neurons. Biol. Chem. 392, 739–749. doi: 10.1515/bc.2011.077
Vagin, O., Tokhtaeva, E., Garay, P. E., Souda, P., Bassilian, S., Whitelegge, J. P., et al. (2014). Recruitment of septin cytoskeletal proteins by Botulinum toxin A protease determines its remarkable stability. J. Cell Sci. 127, 3294–3308. doi: 10.1242/jcs.146324
Vardi-Oknin, D., Golan, M., and Mabjeesh, N. J. (2013). Forchlorfenuron disrupts SEPT9_i1 filaments and inhibits HIF-1. PLoS ONE 8:e73179. doi: 10.1371/journal.pone.0073179
Varshavsky, A. (1996). The N-end rule: functions, mysteries, uses. Proc. Natl. Acad. Sci. U.S.A. 93, 12142–12149. doi: 10.1073/pnas.93.22.12142
Vuong, T. T., Berger, C., Bertelsen, V., Rødland, M. S., Stang, E., and Madshus, I. H. (2013). Preubiquitinated chimeric ErbB2 is constitutively endocytosed and subsequently degraded in lysosomes. Exp. Cell Res. 319, 32–45. doi: 10.1016/j.yexcr.2012.10.010
Wang, J., Zurawski, T. H., Meng, J., Lawrence, G., Olango, W. M., Finn, D. P., et al. (2011). A dileucine in the protease of botulinum toxin A underlies its long-lived neuroparalysis: transfer of longevity to a novel potential therapeutic. J. Biol. Chem. 286, 6375–6385. doi: 10.1074/jbc.M110.181784
Wang, Z., Zhang, L., Yeung, T. K., and Chen, X. (1999). Endocytosis deficiency of epidermal growth factor (EGF) receptor-ErbB2 heterodimers in response to EGF stimulation. Mol. Biol. Cell 10, 1621–1636. doi: 10.1091/mbc.10.5.1621
Warfel, N. A., and El-Deiry, W. S. (2014). HIF-1 signaling in drug resistance to chemotherapy. Curr. Med. Chem. 21, 3021–3028. doi: 10.2174/0929867321666140414101056
Wasik, A. A., Polianskyte-Prause, Z., Dong, M. Q., Shaw, A. S., Yates, J. R. III Farquhar, M. G., et al. (2012). Septin 7 forms a complex with CD2AP and nephrin and regulates glucose transporter trafficking. Mol. Biol. Cell 23, 3370–3379. doi: 10.1091/mbc.E11-12-1010
Wiley, H. S., and Burke, P. M. (2001). Regulation of receptor tyrosine kinase signaling by endocytic trafficking. Traffic 2, 12–18. doi: 10.1034/j.1600-0854.2001.020103.x
Xu, P., Duong, D. M., Seyfried, N. T., Cheng, D., Xie, Y., Robert, J., et al. (2009). Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell 137, 133–145. doi: 10.1016/j.cell.2009.01.041
Xu, W., Marcu, M., Yuan, X., Mimnaugh, E., Patterson, C., and Neckers, L. (2002). Chaperone-dependent E3 ubiquitin ligase CHIP mediates a degradative pathway for c-ErbB2/Neu. Proc. Natl. Acad. Sci. U.S.A. 99, 12847–12852. doi: 10.1073/pnas.202365899
Xue, J., Tsang, C. W., Gai, W. P., Malladi, C. S., Trimble, W. S., Rostas, J. A., et al. (2004). Septin 3 (G-septin) is a developmentally regulated phosphoprotein enriched in presynaptic nerve terminals. J. Neurochem. 91, 579–590. doi: 10.1111/j.1471-4159.2004.02755.x
Yang, Y. M., Fedchyshyn, M. J., Grande, G., Aitoubah, J., Tsang, C. W., Xie, H., et al. (2010). Septins regulate developmental switching from microdomain to nanodomain coupling of Ca(2+) influx to neurotransmitter release at a central synapse. Neuron 67, 100–115. doi: 10.1016/j.neuron.2010.06.003
Yau, R., and Rape, M. (2016). The increasing complexity of the ubiquitin code. Nat. Cell Biol. 18, 579–586. doi: 10.1038/ncb3358
Keywords: septins, protein stability, botulinum toxins, receptor tyrosine kinases, hypoxia-inducible factor 1α
Citation: Vagin O and Beenhouwer DO (2016) Septins: Regulators of Protein Stability. Front. Cell Dev. Biol. 4:143. doi: 10.3389/fcell.2016.00143
Received: 04 November 2016; Accepted: 02 December 2016;
Published: 20 December 2016.
Edited by:
Manoj B. Menon, Hannover Medical School, GermanyReviewed by:
Michael Krauß, Leibniz-Institut für Molekulare Pharmakologie Berlin, GermanyAnita Baillet, University of Paris-Sud, France
Copyright © 2016 Vagin and Beenhouwer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olga Vagin, b2xnYXZAdWNsYS5lZHU=
 Olga Vagin
Olga Vagin David O. Beenhouwer
David O. Beenhouwer