- 1Department of Urology, Jinshan Hospital, Fudan University, Shanghai, China
- 2Research Center for Clinical Medicine, Jinshan Hospital, Fudan University, Shanghai, China
Prostate cancer is one of the most common malignant tumors that threaten the health of men. It is urgent to explore new molecular targets and develop new drugs for the treatment of prostate cancer. Circular RNAs (circRNAs) are aberrantly expressed in various malignant tumors. The dysregulated circRNAs are involved in the metastasis, tumor growth, drug resistance, and immunosuppression of malignant tumors. The present review systematically summarized publications concerning the biological implications of circRNAs in prostate cancer. The PubMed and Web of Science databases were used to retrieve publications concerning circRNAs and prostate cancer until June 16, 2021. The following keywords were used in the literature search: (circRNA OR circular RNA) AND prostate cancer. 73 publications were enrolled in the present systematic review to summarize the role of circRNAs in prostate cancer. The dysregulated and functional circRNAs were involved in the cell cycle, proliferation, migration, invasion, metastasis, drug resistance and radiosensitivity of prostate cancer. In addition, circRNAs could function through EVs and serve as prognostic and diagnostic biomarkers. Certain circRNAs were correlated with clinicopathological features of prostate cancer. A comprehensive review of the molecular mechanism of the tumorigenesis and progression of prostate cancer may contribute to the development of new therapies of prostate cancer in the future.
Introduction
Prostate cancer (PCa) is one of the most common malignant tumors that threaten the health of men. The American Cancer Society predicted 248,530 new cases and 69,410 deaths in 2021, ranked 1st and 2nd in men respectively (Siegel et al., 2021). Androgen deprivation therapy (ADT) is the first-line treatment for PCa except for surgery. Nevertheless, castration-resistant PCa (CRPC) is still inevitable for PCa patients (Chandrasekar et al., 2015). Although drugs targeting the androgen receptor (AR) pathway significantly improved the survival of CRPC patients, these drugs did not achieve satisfactory efficacy due to the generation of AR variants and the limitation of drug resistance (Attard and Antonarakis, 2016). Therefore, it is urgent to explore novel molecular targets and develop new drugs for the treatment of PCa.
Circular RNAs (circRNAs) are covalently closed RNAs without 3′ or 5′ ends. High-throughput RNA-sequencing (RNA-seq) is the most commonly used method to identify new circRNAs by detecting the spliced reads that cover the back-splicing junctions. Once generated, circRNAs are highly stable due to their circular structure. Therefore, circRNAs in tissues, blood, or urine can be used as promising biomarkers (Wen et al., 2020). The biological functions of most circRNAs are still unclear. However, studies on certain circRNAs in recent years have discovered that circRNAs may sponge microRNAs (miRNAs), bind to RNA-binding proteins and modulate their activity, regulate transcription or alternative splicing, and be translated to produce novel functional peptides (Kristensen et al., 2019; Xiao et al., 2020).
CircRNAs are aberrantly expressed in various malignant tumors, such as renal cell carcinoma (Wang et al., 2020b), breast cancer (Jahani et al., 2020), and PCa (Vo et al., 2019; Chao et al., 2021b). The dysregulated circRNAs can be involved in the metastasis (Shen et al., 2019), tumor growth (Wu et al., 2020), drug resistance (Zhang et al., 2020a), and immunosuppression (Zhang et al., 2020c) of malignant tumors. Studies in patient-derived xenograft mouse models indicated that intratumor injection of small interference RNA (siRNA) targeting oncogenic circRNA might be a promising treatment of gastric cancer (Zhang et al., 2019). Moreover, circRNAs can serve as promising prognostic or diagnostic biomarkers of malignant tumors (Wang et al., 2018; Rajappa et al., 2020).
The present review systematically summarized publications concerning the biological implications of circRNAs in PCa. A comprehensive review of the molecular mechanism of the tumorigenesis and progression of PCa may contribute to the development of new therapies of PCa in the future.
Methods
Search Strategy
The present systematic review was conducted according to the Cochrane guideline. The PubMed and Web of Science databases were used to retrieve publications concerning circRNAs and PCa until June 16, 2021. The following keywords were used in the literature search: (circRNA OR circular RNA) AND prostate cancer. The EndNote X9 (Thompson Reuters, New York, USA) software was used to manage the publications for the present review. In this article, meta-analysis was not performed. The present systematic review was based on previous publications so that ethical consent or approval was not required.
Inclusion and Exclusion Criteria
Two reviewers (Fan Chao and Shiyu Wang) independently evaluated and selected the publications. Any discrepancy was resolved by the supervisor (Gang Chen). Publications that meet any of the following inclusion criteria were included: (i) expression of the circRNA was quantified in PCa; (ii) biological function and/or mechanism of the circRNA was determined; (iii) prognostic or diagnostic value of the circRNA in PCa was evaluated. Publications that meet any of the following exclusion criteria were excluded: (i) studies that did not meet any of the inclusion criteria; (ii) reviews, books, comments, patents, meeting abstracts and retracted articles; (iii) studies that were not related to circRNA or PCa.
Results
Results of the Literature Research
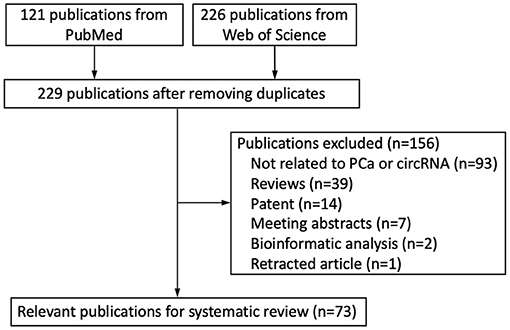
We retrieved 347 publications from the databases mentioned above and 229 publications after the removal of duplications. 156 publications were excluded after reviewing. Finally, 73 publications were enrolled in the present systematic review (Figure 1).
Overview of circRNA
History of circRNA Research
In 1976, Sanger et al. discovered that viroids were single-stranded covalently closed circRNA with a highly base-paired rod-like structure. This is the first report on circRNA (Sanger et al., 1976). In 1979, Hsu et al. identified circRNAs in the cytoplasm of eukaryotic cells using electron microscopy (Hsu and Coca-Prados, 1979). In 1993, circular transcripts of the exons of the genomic DNA were identified. However, these circRNAs have been considered to be yielded by mis-splicing of primary transcripts (Cocquerelle et al., 1993). At the same time, Sry circRNA was discovered in the testis of adult mice and was considered to be the noise of normal splicing (Capel et al., 1993). Due to the lack of 3′ polyadenylated tails, circRNAs cannot be identified by the classical RNA sequencing which detects the linear RNAs with 3′ polyadenylated tails. Novel RNA sequencing methods which detect RNase R treated RNAs and non-polyadenylated RNAs have been used to identified circRNAs in various species including plants (Lu et al., 2015), fruit flies (Westholm et al., 2014), zebrafish (Shen et al., 2017), mice (Memczak et al., 2013), and human (Salzman et al., 2012). In 2013, Memczak et al. analyzed the biological function of circRNA CDR1as and claimed that circRNAs were a large class of transcripts with the regulatory potential of coding sequences (Memczak et al., 2013).
Biogenesis of circRNAs
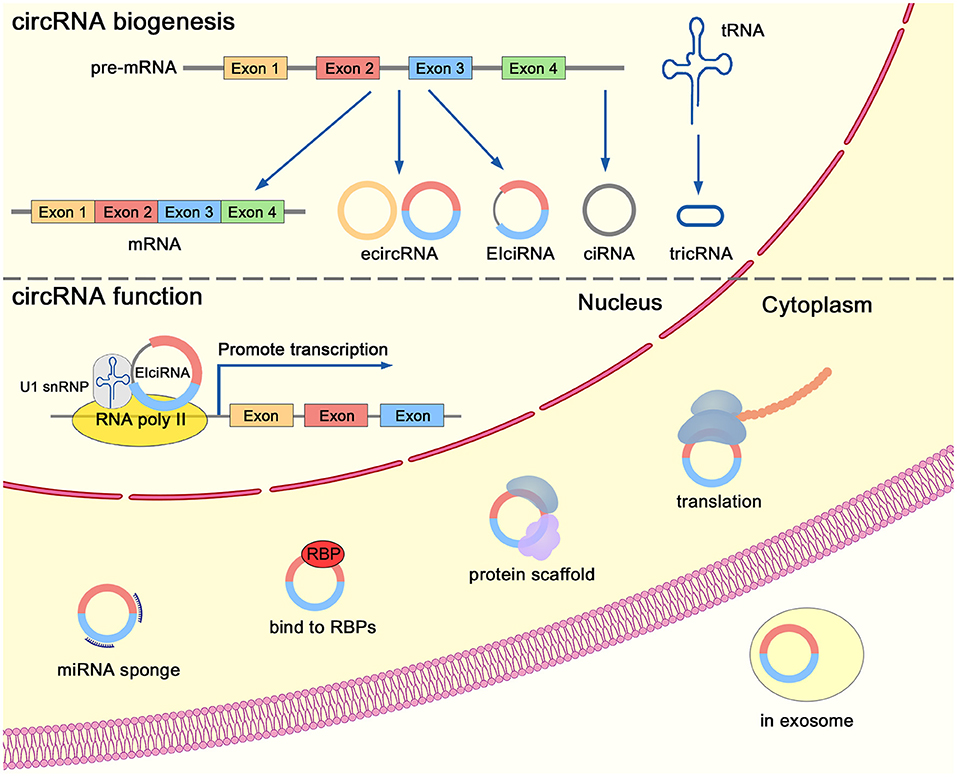
Exonic circRNAs are formed by back-splicing of exons and are located in the cytoplasm (Figure 2). Recent studies have revealed that circRNAs are derived from the back-splicing of primary transcripts (Zhang et al., 2020b). Complementary sequences in the introns can mediate the circularization of exons (Zhang et al., 2014). CircRNAs can be generated through an exon-containing lariat precursor in genes that lack intronic complementary sequences (Barrett et al., 2015). Furthermore, the biogenesis of circRNAs can be regulated by RNA-binding proteins in trans. For instance, Quaking (Conn et al., 2015), FUS (Han et al., 2020), and MBNL1 (Ashwal-Fluss et al., 2014) boost the biogenesis of circRNAs, while ADAR1 (Ivanov et al., 2015) and DHX9 (Aktas et al., 2017) suppress the production of circRNAs. HNRNPL (Fei et al., 2017) is a prostate-specific RNA-binding protein that is involved in the formation of circRNAs through back-splicing. Both HNRNPL and its circRNA clients are clinically relevant and aberrantly expressed in PCa (Fei et al., 2017).
Category and Nuclear Export of circRNAs
The splicing and circularization of transcripts lead to the generation of various types of circRNAs: exonic circRNAs (ecircRNAs), exon-intron circRNAs (EIciRNAs), intronic circRNAs (ciRNAs), and tRNA intronic circRNAs (tricRNAs) (Figure 2). EcircRNAs are the most investigated class of circRNAs. Although back-splicing of transcripts occurs in the nucleus, a majority of exonic circRNAs were located in the cytoplasm (Chen, 2020). A recent study indicated that DDX39A and DDX39B was involved in the nuclear export of long (> 1,300 nucleotides) and short (< 400 nucleotides) circRNAs, respectively (Huang et al., 2018).
Biological Functions of circRNAs
Recent studies have uncovered the biological activity of functional circRNAs (Figure 2). CircRNAs are reported to act as microRNA (miRNA) sponges, to serve as decoys for proteins, to modulate gene expression, to act as scaffolds of proteins, and to function as templates for translation (Chen, 2020). For instance, circRNA CDR1as contains more than 70 sponging targets for miRNA miR-7. Although CDR1as is resistant to the miRNA-mediated degradation of target RNAs, it strongly inhibits the activity of miR-7 and thereby increases the level of other miR-7 targets (Hansen et al., 2013). In addition, CDR1as can suppress the metastasis of melanoma through modulating the activity of its interactor, IGF2BP3 (Hanniford et al., 2020). CircSMARCA5 interacts with its host gene through regulating transcription of partial exons (Xu et al., 2020c). CircMALAT1 can serve as a brake to retard PAX5 mRNA translation in ribosomes (Chen et al., 2020b). Circ-Ccnb1 acts as a protein scaffold of Ccnb1 and Cdk1, resulting in the dissociation of cyclin B1-Cdk1 complex and cell death (Fang et al., 2019). Several studies have reported that circRNAs could encode peptides. For instance, circFNDC3B encodes a novel protein and inhibits tumor progression in colon cancer (Pan et al., 2020).
The role of circRNAs in PCa
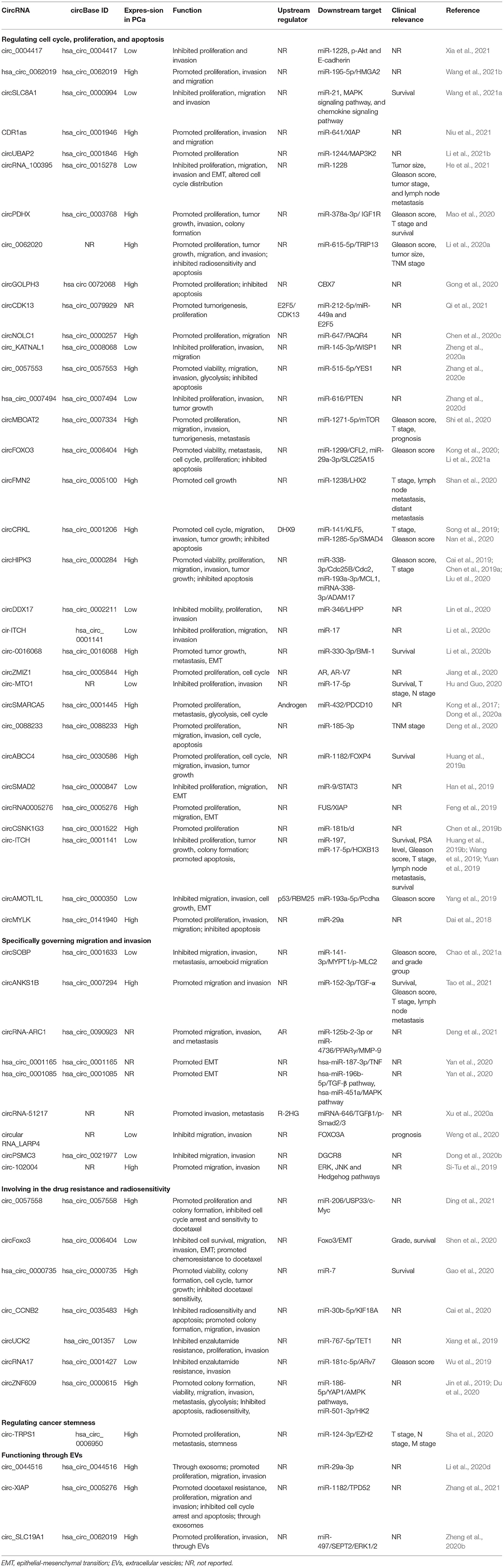
The functions and downstream targets of the dysregulated circRNAs in PCa are listed in Table 1. The dysregulated and functional circRNAs are involved in the cell cycle, proliferation, migration, invasion, metastasis, drug resistance and radiosensitivity of PCa (Figure 3). Some circRNAs can serve as prognostic and diagnostic biomarkers. Here, we summarized the role of circRNAs in PCa.
Regulating Cell Cycle, Proliferation, and Apoptosis
The most basic characteristics of cancer cells involve their long-lasting and long-term proliferation ability. Normal tissues can precisely control the generation and release of growth-promoting signals, which govern the start and progress of the cell proliferation-differentiation cycle. This maintains the balance of cell number, thereby ensuring the structure and function of normal tissues. These signals in cancer cells are dysregulated, thereby they can sustain the proliferation of cancer cells (Hanahan and Weinberg, 2011). In addition, tumor cells have evolved a series of mechanisms that limit or allow them to avoid apoptosis. Under pathological conditions, especially in cancer, cells lose the ability to undergo cell death induced by apoptosis, leading to uncontrolled proliferation. There are several mechanisms by which cells escape programmed cell death, one of which is the expression of anti-apoptotic molecules (Mohammad et al., 2015).
Current studies have revealed that circRNAs were involved in the proliferation, cell cycle, and apoptosis of PCa cells. For instance, Shan et al. reported that knockdown of circFMN2 suppressed tumor growth of PCa in vivo and inhibited proliferation of PCa cells by inducing cell-cycle arrest and apoptosis through regulating miR-1238/LHX2 axis (Shan et al., 2020). Mao et al. reported that circPDHX was associated with Gleason score, pathological T stage and overall survival of PCa, promoted cell proliferation in vitro and tumor growth in vivo (Mao et al., 2020). Liu et al. suggested that circHIPK3 expression was upregulated in PCa and promoted G2/M transition by sponging miR-338-3p (Liu et al., 2020). Deng et al. claimed that the suppression of circ_0088233 reduced cell proliferation and induced G1 phase arrest and apoptosis through targeting hsa-miR-185-3p (Deng et al., 2020). Zhang et al. indicated that the knockdown of circ_0057553 could inhibit cell viability and facilitate apoptosis (Zhang et al., 2020e). Huang et al. suggested that knockdown of circABCC4 inhibited tumor growth in vivo and cell-cycle progression in vitro by targeting miR-1182-FOXP4 regulatory axis (Huang et al., 2019a).
Governing Migration and Invasion
The mechanism of cancer metastasis was largely an enigma until 2000. The multiple steps of invasion and metastasis have been schematized as a series of independent steps, commonly termed as the invasion-metastasis cascade (Fidler, 2003; Talmadge and Fidler, 2010). This description depicted a series of cellular biological changes. Invasion and metastasis of cancer cells start with local invasion, then intravasation of the cancer cells allows them to infiltrate the surrounding blood vessels and lymph vessels. Subsequently, cancer cells are transported through the lymphatic or blood system, followed by the extravasation which helps cancer cells to escape from the vessels into the parenchyma of the distal tissues. Escaped cancer cells grow into small nodules, which are termed micrometastases. The last step, colonization, refers to the growth of micrometastases into metastatic tumors. Research on invasion and metastasis capabilities has been greatly accelerated in recent years.
Epithelial-mesenchymal transition (EMT) plays an important role in the metastasis of malignant tumors (Huber et al., 2005). CircRNAs are involved in the EMT program. Yan et al. identified EMT-related circRNAs through RNA-seq in interferon-γ (IFN-γ) induced EMT cells and found that hsa_circ_0001165 and hsa_circ_0001085 were EMT-related circRNAs (Yan et al., 2020). Hsa_circ_0001165 and hsa_circ_0001085 played a regulatory role in the EMT of PCa. Feng et al. reported that circ0005276 promoted cell proliferation and EMT through interacting with FUS (Feng et al., 2019). Han et al. found a decrement of circSMAD2 in PCa tissues. Restoration of circSMAD2 could inhibit the impaired EMT process through miR-9 inhibition (Han et al., 2019). Yang et al. claimed that p53 regulated EMT through circAMOTL1L/miR-193a-5p/Pcdha regulatory axis (Yang et al., 2019). Li et al. suggested that circ-0016068 promoted EMT of PCa cells by regulating the miR-330-3p/BMI-1 axis (Li et al., 2020b). Shen et al. reported that circFoxo3 inhibited PCa cell migration and invasion through regulating Foxo3 and EMT (Shen et al., 2020).
In addition to EMT, circRNAs can govern the metastasis of PCa through other pathways. Xu et al. found that circRNA-51217 could sponge miRNA-646, which could induce TGFβ1/p-Smad2/3 signaling pathway and promote PCa cell invasion (Xu et al., 2020a). Weng et al. suggested that circular RNA_LARP4 could inhibit cell migration and invasion through upregulating FOXO3A (Weng et al., 2020). Si-Tu et al. reported that circ-102004 played an oncogenic role by promoting migration and invasion of PCa cells (Si-Tu et al., 2019). Overexpression of circ-102004 alters ERK, JNK, and Hedgehog pathways. Chao et al. found that circSOBP inhibited amoeboid migration and metastasis of PCa cells through miR-141-3p/MYPT1/p-MLC2 axis (Chao et al., 2021a).
Involving in the Drug Resistance and Radiosensitivity
In 1940, Charles Huggins reported that castration of men with PCa induced dramatic symptomatic improvements and resulted in the regression of cancer sites (Huggins and Hodges, 1941). Since then, ADT has been a mainstay therapy for advanced PCa, including medical castration, surgical castration, and inhibitors for androgen biosynthesis (Feng and He, 2019). Given psychological and aesthetic concerns, medical castration has been more adopted instead of surgical castration in the treatment for PCa. However, progression to castration-resistant PCa (CRPC) is inevitable after ADT. Novel second-generation antiandrogens have been developed to achieve better blockade for androgen (Tran et al., 2009), including darolutamide, apalutamide, and enzalutamide. The second-generation antiandrogens have successfully prolonged the survival of PCa patients. Unfortunately, the prolonged survival is temporary. The CRPC can be resistant to the latest antiandrogens.
CircRNAs are involved in the drug resistance of various cancers (Xu et al., 2020b). Recent studies have investigated the role of circRNAs in the CRPC. Cao et al. (2019) identified 13 circRNAs derived from the AR gene through RNA-seq of 47 metastatic CRPC samples, cell models, and RNase R RNA-seq of patient-derived xenografts (PDXs). Expression of the four most abundant circRNAs are upregulated during the castration-resistant progression of PDXs and can be detected in the plasma of patients with PCa. These AR-derived circRNAs might serve as biomarkers for CRPC. Greene et al. (2019) uncovered that circRNAs were more often downregulated in enzalutamide-resistant PCa cells using a high-throughput circRNA microarray. Hsa_circ_0004870 was one of the downregulated circRNAs which might mediate the development of enzalutamide resistance in PCa. Wu et al. (2019) revealed the low expression of circRNA17 in CRPC C4-2 enzalutamide-resistant cell lines compared to the parental sensitive cells. This work suggested that circRNA17 could govern the enzalutamide sensitivity and cell invasion through the miR-181c-5p/ARv7 axis. Xiang et al. (2019) identified that circUCK2 was also downregulated in enzalutamide-resistant cells. Targeting these circRNAs might help develop new therapies for the treatment of CRPC.
Docetaxel is the first chemotherapeutic agent which was proved to prolong the survival of patients with metastatic CRPC (Sartor and de Bono, 2018). Results of the STAMPEDE trial advocated the upfront use of docetaxel for patients with metastatic hormone-naive PCa (Clarke et al., 2019). Recent studies have revealed that circRNAs were involved in the docetaxel sensitivity of PCa. Shen et al. (2020) suggested that reduction circFoxo3 boosted chemoresistance to docetaxel of PCa. Depletion of circFoxo3 using siRNAs promoted chemoresistance to docetaxel in mice with xenografts, while the delivery of circFoxo3 prolonged the survival of tumor-bearing mice and enhanced the sensitivity to docetaxel. Gao et al. (2020) uncovered that hsa_circ_0000735 was upregulated in docetaxel-resistant PCa tissues and was correlated with worse overall survival. Knockdown of hsa_circ_0000735 boosted sensitivity to docetaxel of PCa cells and inhibited viability in vivo. In addition, suppressing hsa_circ_0000735 promoted docetaxel sensitivity and suppressed tumor growth in vivo. Zhang et al. reported that exosomal circ-XIAP promoted docetaxel resistance in PCa by altering miR-1182/TPD52 axis (Zhang et al., 2021).
External radiotherapy is a radical treatment for low-risk PCa, which has been considered to achieve the same effect as surgical treatment. Resistance to radiotherapy is an obstacle for the treatment of PCa. Du et al. (2020) reported that circ-ZNF609 suppressed the radiosensitivity of PCa. Knockdown of circ-ZNF609 inhibited the radioresistance, viability and promoted apoptosis through regulating glycolysis. Silencing circ-ZNF609 enhanced the sensitivity to radiotherapy in vivo. Cai et al. (2020) focused on the effect of circ_CCNB2 on radiosensitivity of PCa. Depletion of circ_CCNB2 facilitated the radiosensitivity of irradiation-resistant PCa cells in vitro and in vivo through suppressing autophagy via miR-30b-5p/KIF18A axis.
Regulating Cancer Stemness
Cancer is characterized by infinite proliferation of the malignant cells with different morphology and functions. There are currently two models that explain this cellular diversity in tumors. The first model that explain the initiation and development of cancer postulates that each sequential accumulation of mutations promotes the loss of specific tissue characteristics, until the occurrence of dedifferentiation and the regression into a more primitive phenotype. In this model, every cancer cell has a similar tumorigenic potential. The second mode is the cancer stem cell (CSC) hypothesis (Aguilar-Gallardo and Simon, 2013; Pattabiraman and Weinberg, 2014). The CSCs refer to cells in tumors that have the ability to self-renew and produce heterogeneous cancer cells. These cells with stemness are responsible for producing various offspring of highly proliferative cells that form the bulk of the tumors.
So far, only one study reported the role of circRNA in regulating the stemness of PCa cells. Sha et al. (2020) indicated that circ-TRPS1 was upregulated in high-grade PCa tissues and was associated with aggressive PCa phenotypes. Knockdown of circ-TRPS1 inhibited proliferation and metastasis of PCa through miR-124-3p/EZH2 axis-mediated stemness.
Functioning Through Extracellular Vesicles (EVs)
There are two types of EVs based on their size and origin: exosomes and microvesicles (van Niel et al., 2018). Exosomes ranged in size from 50 to 100 nm are derived from intraluminal vesicles (ILVs) during the formation of multivesicular endosomes (MVEs), in which ILVs are formed by the inward budding of an endosomal membrane of MVEs and then secreted upon the fusion of MVE membrane and the cell membrane. Microvesicles ranged in size from 50 to 1,000 nm in diameter are also called oncosomes due to their role in cellular communication in cancer (Al-Nedawi et al., 2008). Microvesicles are directly generated from the outward budding followed by fission of the cell membrane. Subsequently, microvesicles are released into the extracellular space.
EVs were usually isolated from body fluids or cell culture media using special kits or ultra-high-speed centrifugation, followed by identification using an electron microscope. CircRNAs in blood or EVs can be served as biomarkers or functional factors (Hu et al., 2020; Wen et al., 2020). CircSLC19A (Zheng et al., 2020b) was increased in both PCa cells and their EVs. EVs with high circSLC19A could be taken up by PCa cells and boosted cell proliferation and invasion. Exosomal circSLC19A promoted proliferation and invasion of cells through the miR-497/septin 2 axis. Li et al. (2020d) identified the existence of circ_0044516 in exosomes using circRNA microarray. Circ_0044516 was upregulated in the exosomes of PCa patients and cell lines. However, this study did not investigate whether exosomal circRNAs could be taken up by cells and function. Zhang et al. discovered that circ-XIAP was up-regulated in exosomes from docetaxel-resistant cell lines and could be transmitted via exosomes (Zhang et al., 2021). Adding exosomes from docetaxel-resistant cells increased circ-XIAP level in prostate cancer cell lines, suggesting that exosomes containing circ-XIAP could be absorbed.
Serving as Diagnostic and Prognostic Biomarkers
The characteristics of circRNAs make them promising biomarkers. Firstly, circRNAs are specifically expressed in various tissues and body fluids. CircRNAs can be enriched in exosomes and released from their original tissues into various body fluids, including plasma, saliva, and urine. Moreover, they can be released from dead or dying cells with the rupture of cell membranes. Secondly, the covalently closed structure and resistance to RNase have endowed circRNAs with high stability. The half-life of circRNAs is about 2.5 times longer than linear RNAs in cells and 6.3 times longer in exosomes (Jeck et al., 2013; Li et al., 2015; Enuka et al., 2016). Thirdly, circRNAs can be easily measured using RNA-seq or quantitative polymerase chain reaction (qPCR). The exact copy number and mutant of circRNAs can be detected. This is an advantage when comparing with protein biomarkers which are quantified by antigen-antibody interaction. A current meta-analysis suggested that CDR1as was a reliable prognostic and diagnostic biomarker for solid tumors by summarizing 26 studies (Zou et al., 2020). CircRNAs can serve as remarkable biomarkers in colorectal cancer (Yuan et al., 2020), lung cancer (Yang et al., 2020), gastric cancer (Chen et al., 2020a), and glioma (Ding et al., 2020).
Current studies indicated that circRNAs were correlated with clinicopathological features of PCa. The expression levels of circMBOAT2 (Shi et al., 2020), circFoxo3 (Shen et al., 2020), circCRKL (Song et al., 2019; Nan et al., 2020), and circHIPK3 (Cai et al., 2019; Liu et al., 2020) were associated with the histological grade of PCa. The expression levels of circFoxo3 (Shen et al., 2020), circ-0016068 (Li et al., 2020b), circ-MTO1 (Hu and Guo, 2020), hsa_circ_0000735 (Gao et al., 2020), circ-ITCH (Wang et al., 2019), and circABCC4 (Huang et al., 2019a) were associated with the survival of patients with PCa, suggesting that circRNAs had the potential to predict the prognosis of PCa patients. Wang et al. established an eight-circRNA prognosis model to predict the biochemical recurrence of PCa. This prognosis model based on circRNAs was better than the clinical indexes (Wang et al., 2020a).
Discussion
CircRNAs has attracted more and more attention from the scientific community, and their involvement in the molecular regulation in cells has been continuously revealed. Similar to protein and lncRNAs, circRNAs can regulate the malignant behavior of cancer cells through cell signaling pathways. Although a large number of circRNAs have been discovered and even included in the databases, the biological implications of most circRNAs in eukaryotic cells remained unclear. In recent years, a large number of studies have systematically identified circRNAs and their functions. Targeting circRNAs has great potential in the field of cancer intervention, diagnosis and treatment. In the present review, we systematically summarized the functional circRNAs in PCa. Various circRNAs governed the proliferation, apoptosis, migration, invasion, drug resistance, and radiosensitivity in PCa. In addition, circRNAs could function through EVs and serve as prognostic and diagnostic biomarkers.
The limitations and pitfalls of the studies on circRNAs should not be ignored. The studies concerning circRNAs are in the preliminary stage. At the same time, the application of circRNAs in clinical practice remained up in the air. Current studies reported the dysregulation of circRNAs in PCa. These circRNAs that were clinically significantly dysregulated could serve as remarkable biomarkers. A prognosis model based on multiple circRNAs has been established to predict the biochemical recurrence of PCa (Wang et al., 2020a). However, circRNA biomarkers were not used in clinical practice due to the lack of further research. Subsequent clinical researches should be carried out to verify the sensitivity and specificity of circRNA biomarkers according to larger cohorts.
Although circRNAs are more stable than linear RNAs, they are still not as good as proteins when serving as biomarkers. The expression abundance of circRNAs in cells is low and is lower in body fluids, so it is very difficult to quantitatively detect them using traditional methods. Thus, it is urgent to develop new detecting techniques in the future. In addition, detecting techniques and bioinformatic analyses should be standardized to collect reliable data. When these technical obstacles are solved in the future, circRNAs will play an important role in the field of biomarkers and are expected to replace existing markers.
Although biological functions of certain circRNAs have been revealed, the application based on the functional circRNAs in the treatment of PCa is poorly studied. The precise delivery of functional circRNAs to the cancer cells, as well as the overexpression or suppression of functional circRNA in the cancer cells, are the current difficulty in the clinical use of circRNAs. The delivery can be achieved using nanoparticles or viral vectors; however, their specificity is not satisfactory. Since circRNAs are functional in human cells, non-selective application to the human body may cause a variety of side effects, so accurate delivery is very important. Recombinant adeno-associated virus (rAAV) vectors can be used in gene therapy of cancer due to its advantages including tissue specificity, long-term transgene expression, and low immunogenicity (Luo et al., 2015; Santiago-Ortiz and Schaffer, 2016). Sun et al. developed a prostate-specific rAAV that inhibited tumor growth of PCa through gene silencing (Sun et al., 2010). Ai et al. investigated the transgene efficiency of various serotypes of rAAVs and discovered that rAAV6.2 and rAAV7 outperformed other serotypes in the whole prostate (Ai et al., 2016). Therefore, the delivery of circRNAs or their siRNAs through prostate-specific rAAVs could be used as novel gene therapy for PCa in the future.
Although a number of studies have reported the biological functions of circRNAs in prostate cancer, it is uncertain which ones are prostate-specific due to the lack of experimental verification. Further research should focus on discovering the prostate-specific circRNAs. Targeting prostate-specific circRNA and developing new therapeutic targets are of great significance.
Researchers have reported the dysregulation of circRNAs and their biological function as well as the mechanism. Nevertheless, the reason why circRNAs were dysregulated was poorly studied. A previous study reported that the expression level of circRNA was positively correlated with its host gene (Chao et al., 2021a). Since circRNA and mRNA derived from the same gene are encoded by the same genomic sequence, they may be regulated by common factors. If a certain circRNA has the same biological functions as the protein encoded by its host gene, targeting their upstream regulatory mechanism may achieve a better effect. Nevertheless, Yu et al. found that the expression trend of circRNA was opposite to its host gene, and might be governed by the RNA-binding proteins that mediated the cyclization of circRNAs (Yu et al., 2018). Targeting the upstream regulators of circRNAs is expected to fundamentally block or promote their biological functions, thereby exerting a therapeutic effect on human diseases. Hence, the regulators of circRNAs should be systematically determined in the future.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
FC performed the literature search, analyzed the data, made the schematic diagram, and wrote the manuscript. SW performed the literature search and the review on the overview of circRNA. CZ performed the review on the role of circRNA in PCa. DH determined the inclusion and exclusion criteria. GX revised the manuscript. GC conceived the idea and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Science Foundation of Shanghai (No. 18ZR1405800) and the Project for Key Medical Specialty Construction in Jinshan District (6th Period, Type A) (No. JSZK2019A03) to GC.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aguilar-Gallardo, C., and Simon, C. (2013). Cells, stem cells, and cancer stem cells. Semin. Reprod. Med. 31, 5–13. doi: 10.1055/s-0032-1331792
Ai, J., Wang, D., Wei, Q., Li, H., and Gao, G. (2016). Adeno-associated virus serotype vectors efficiently transduce normal prostate tissue and prostate cancer cells. Eur. Urol. 69, 179–181. doi: 10.1016/j.eururo.2015.10.019
Aktas, T., Avsar Ilik, I., Maticzka, D., Bhardwaj, V., Pessoa Rodrigues, C., Mittler, G., et al. (2017). DHX9 suppresses RNA processing defects originating from the Alu invasion of the human genome. Nature 544, 115–119. doi: 10.1038/nature21715
Al-Nedawi, K., Meehan, B., Micallef, J., Lhotak, V., May, L., Guha, A., et al. (2008). Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 10, 619–624. doi: 10.1038/ncb1725
Ashwal-Fluss, R., Meyer, M., Pamudurti, N. R., Ivanov, A., Bartok, O., Hanan, M., et al. (2014). circRNA biogenesis competes with pre-mRNA splicing. Mol. Cell 56, 55–66. doi: 10.1016/j.molcel.2014.08.019
Attard, G., and Antonarakis, E. S. (2016). Prostate cancer: AR aberrations and resistance to abiraterone or enzalutamide. Nat. Rev. Urol. 13, 697–698. doi: 10.1038/nrurol.2016.212
Barrett, S. P., Wang, P. L., and Salzman, J. (2015). Circular RNA biogenesis can proceed through an exon-containing lariat precursor. Elife 4:e07540. doi: 10.7554/eLife.07540.025
Cai, C., Zhi, Y., Wang, K., Zhang, P., Ji, Z., Xie, C., et al. (2019). CircHIPK3 overexpression accelerates the proliferation and invasion of prostate cancer cells through regulating miRNA-338-3p. Onco. Targets. Ther. 12, 3363–3372. doi: 10.2147/OTT.S196931
Cai, F., Li, J., Zhang, J., and Huang, S. (2020). Knockdown of Circ_CCNB2 sensitizes prostate cancer to radiation through repressing autophagy by the miR-30b-5p/KIF18A axis. Cancer Biother. Radiopharm. doi: 10.1089/cbr.2019.3538
Cao, S., Ma, T., Ungerleider, N., Roberts, C., Kobelski, M., Jin, L., et al. (2019). Circular RNAs add diversity to androgen receptor isoform repertoire in castration-resistant prostate cancer. Oncogene 38, 7060–7072. doi: 10.1038/s41388-019-0947-7
Capel, B., Swain, A., Nicolis, S., Hacker, A., Walter, M., Koopman, P., et al. (1993). Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell 73, 1019–1030. doi: 10.1016/0092-8674(93)90279-Y
Chandrasekar, T., Yang, J. C., Gao, A. C., and Evans, C. P. (2015). Mechanisms of resistance in castration-resistant prostate cancer (CRPC). Transl. Androl. Urol. 4, 365–380. doi: 10.3978/j.issn.2223-4683.2015.05.02
Chao, F., Song, Z., Wang, S., Ma, Z., Zhuo, Z., Meng, T., et al. (2021a). Novel circular RNA circSOBP governs amoeboid migration through the regulation of the miR-141-3p/MYPT1/p-MLC2 axis in prostate cancer. Clin. Transl. Med. 11:e360. doi: 10.1002/ctm2.360
Chao, F., Wang, S., Zhang, C., Han, D., Ma, Z., and Chen, G. (2021b). Circular RNA circSMARCA5 is a prognostic biomarker in patients with malignant tumor: a meta-analysis. BMC Cancer 21:600. doi: 10.1186/s12885-021-08316-3
Chen, D., Lu, X., Yang, F., and Xing, N. (2019a). Circular RNA circHIPK3 promotes cell proliferation and invasion of prostate cancer by sponging miR-193a-3p and regulating MCL1 expression. Cancer Manag. Res. 11, 1415–1423. doi: 10.2147/CMAR.S190669
Chen, H., Liang, C., Wang, X., Liu, Y., Yang, Z., Shen, M., et al. (2020a). The prognostic value of circRNAs for gastric cancer: A systematic review and meta-analysis. Cancer Med. 9, 9096–9106. doi: 10.1002/cam4.3497
Chen, L., Kong, R., Wu, C., Wang, S., Liu, Z., Liu, S., et al. (2020b). Circ-MALAT1 functions as both an mRNA translation brake and a microRNA sponge to promote self-renewal of hepatocellular cancer stem cells. Adv. Sci. 7:1900949. doi: 10.1002/advs.201900949
Chen, L. L. (2020). The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol. 21, 475–490. doi: 10.1038/s41580-020-0243-y
Chen, S., Huang, V., Xu, X., Livingstone, J., Soares, F., Jeon, J., et al. (2019b). Widespread and Functional RNA Circularization in Localized Prostate Cancer. Cell 176, 831–843.e822. doi: 10.1016/j.cell.2019.01.025
Chen, W., Cen, S., Zhou, X., Yang, T., Wu, K., Zou, L., et al. (2020c). Circular RNA CircNOLC1, upregulated by NF-KappaB, promotes the progression of prostate cancer via miR-647/PAQR4 axis. Front. Cell Dev. Biol. 8, 624764. doi: 10.3389/fcell.2020.624764
Clarke, N. W., Ali, A., Ingleby, F. C., Hoyle, A., Amos, C. L., Attard, G., et al. (2019). Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results from the STAMPEDE trial. Ann. Oncol. 30, 1992–2003. doi: 10.1093/annonc/mdz396
Cocquerelle, C., Mascrez, B., Hetuin, D., and Bailleul, B. (1993). Mis-splicing yields circular RNA molecules. FASEB J. 7, 155–160. doi: 10.1096/fasebj.7.1.7678559
Conn, S. J., Pillman, K. A., Toubia, J., Conn, V. M., Salmanidis, M., Phillips, C. A., et al. (2015). The RNA binding protein quaking regulates formation of circRNAs. Cell 160, 1125–1134. doi: 10.1016/j.cell.2015.02.014
Dai, Y., Li, D., Chen, X., Tan, X., Gu, J., Chen, M., et al. (2018). Circular RNA myosin light chain kinase (MYLK) promotes prostate cancer progression through modulating Mir-29a expression. Med. Sci. Monit. 24, 3462–3471. doi: 10.12659/MSM.908009
Deng, G., Wang, R., Sun, Y., Huang, C. P., Yeh, S., You, B., et al. (2021). Targeting androgen receptor (AR) with antiandrogen Enzalutamide increases prostate cancer cell invasion yet decreases bladder cancer cell invasion via differentially altering the AR/circRNA-ARC1/miR-125b-2-3p or miR-4736/PPARγ/MMP-9 signals. Cell Death Differ. 28, 2145–2159. doi: 10.1038/s41418-021-00743-w
Deng, Z. H., Yu, G. S., Deng, K. L., Feng, Z. H., Huang, Q., Pan, B., et al. (2020). Hsa_circ_0088233 Alleviates proliferation, migration, and invasion of prostate cancer by targeting hsa-miR-185-3p. Front. Cell Dev. Biol. 8:528155. doi: 10.3389/fcell.2020.528155
Ding, T., Zhu, Y., Jin, H., Zhang, P., Guo, J., and Zheng, J. (2021). Circular RNA circ_0057558 controls prostate cancer cell proliferation through regulating miR-206/USP33/c-Myc axis. Front. Cell Dev. Biol. 9:644397. doi: 10.3389/fcell.2021.644397
Ding, X., Yang, L., Geng, X., Zou, Y., Wang, Z., Li, Y., et al. (2020). CircRNAs as potential biomarkers for the clinicopathology and prognosis of glioma patients: a meta-analysis. BMC Cancer 20:1005. doi: 10.1186/s12885-020-07446-4
Dong, C., Fan, B., Ren, Z., Liu, B., and Wang, Y. (2020a). CircSMARCA5 facilitates the progression of prostate cancer through miR-432/PDCD10 axis. Cancer Biother. Radiopharm. 36, 70–83. doi: 10.1089/cbr.2019.3490
Dong, J. S., Wu, B., and Chen, X. H. (2020b). Circ PSMC3 inhibits prostate cancer cell proliferation by downregulating DGCR8. Eur. Rev. Med. Pharmacol. Sci. 24, 2264–2270. doi: 10.26355/eurrev_202003_20492
Du, S., Zhang, P., Ren, W., Yang, F., and Du, C. (2020). Circ-ZNF609 accelerates the radioresistance of prostate cancer cells by promoting the glycolytic metabolism through miR-501-3p/HK2 axis. Cancer Manag. Res. 12, 7487–7499. doi: 10.2147/CMAR.S257441
Enuka, Y., Lauriola, M., Feldman, M. E., Sas-Chen, A., Ulitsky, I., and Yarden, Y. (2016). Circular RNAs are long-lived and display only minimal early alterations in response to a growth factor. Nucleic Acids Res. 44, 1370–1383. doi: 10.1093/nar/gkv1367
Fang, L., Du, W. W., Awan, F. M., Dong, J., and Yang, B. B. (2019). The circular RNA circ-Ccnb1 dissociates Ccnb1/Cdk1 complex suppressing cell invasion and tumorigenesis. Cancer Lett. 459, 216–226. doi: 10.1016/j.canlet.2019.05.036
Fei, T., Chen, Y., Xiao, T., Li, W., Cato, L., Zhang, P., et al. (2017). Genome-wide CRISPR screen identifies HNRNPL as a prostate cancer dependency regulating RNA splicing. Proc. Natl. Acad. Sci. U.S.A. 114, e5207–e5215. doi: 10.1073/pnas.1617467114
Feng, Q., and He, B. (2019). Androgen receptor signaling in the development of castration-resistant prostate cancer. Front. Oncol. 9:858. doi: 10.3389/fonc.2019.00858
Feng, Y., Yang, Y., Zhao, X., Fan, Y., Zhou, L., Rong, J., et al. (2019). Circular RNA circ0005276 promotes the proliferation and migration of prostate cancer cells by interacting with FUS to transcriptionally activate XIAP. Cell Death Dis. 10:792. doi: 10.1038/s41419-019-2028-9
Fidler, I. J. (2003). The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat. Rev. Cancer 3, 453–458. doi: 10.1038/nrc1098
Gao, Y., Liu, J., Huan, J., and Che, F. (2020). Downregulation of circular RNA hsa_circ_0000735 boosts prostate cancer sensitivity to docetaxel via sponging miR-7. Cancer Cell Int. 20:334. doi: 10.1186/s12935-020-01421-6
Gong, L., Tang, Y., Jiang, L., Tang, W., and Luo, S. (2020). Regulation of circGOLPH3 and its binding protein CBX7 on the proliferation and apoptosis of prostate cancer cells. Biosci. Rep. 40:BSR20200936. doi: 10.1042/BSR20200936
Greene, J., Baird, A. M., Casey, O., Brady, L., Blackshields, G., Lim, M., et al. (2019). Circular RNAs are differentially expressed in prostate cancer and are potentially associated with resistance to enzalutamide. Sci. Rep. 9:10739. doi: 10.1038/s41598-019-47189-2
Han, K., Wang, F. W., Cao, C. H., Ling, H., Chen, J. W., Chen, R. X., et al. (2020). CircLONP2 enhances colorectal carcinoma invasion and metastasis through modulating the maturation and exosomal dissemination of microRNA-17. Mol. Cancer 19:60. doi: 10.1186/s12943-020-01184-8
Han, N., Ding, L., Wei, X., Fan, L., and Yu, L. (2019). circSMAD2 governs migration and epithelial-mesenchymal transition by inhibiting microRNA-9. J. Cell. Biochem. 1–10. doi: 10.1002/jcb.29638
Hanahan, D., and Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell 144, 646–674. doi: 10.1016/j.cell.2011.02.013
Hanniford, D., Ulloa-Morales, A., Karz, A., Berzoti-Coelho, M. G., Moubarak, R. S., Sanchez-Sendra, B., et al. (2020). Epigenetic silencing of CDR1as drives IGF2BP3-mediated melanoma invasion and metastasis. Cancer Cell 37, 55–70e15. doi: 10.1016/j.ccell.2019.12.007
Hansen, T. B., Jensen, T. I., Clausen, B. H., Bramsen, J. B., Finsen, B., Damgaard, C. K., et al. (2013). Natural RNA circles function as efficient microRNA sponges. Nature 495, 384–388. doi: 10.1038/nature11993
He, H., Li, J., Luo, M., and Wei, Q. (2021). Inhibitory role of circRNA_100395 in the proliferation and metastasis of prostate cancer cells. J. Int. Med. Res. 49:300060521992215. doi: 10.1177/0300060521992215
Hsu, M. T., and Coca-Prados, M. (1979). Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature 280, 339–340. doi: 10.1038/280339a0
Hu, W., Liu, C., Bi, Z. Y., Zhou, Q., Zhang, H., Li, L. L., et al. (2020). Comprehensive landscape of extracellular vesicle-derived RNAs in cancer initiation, progression, metastasis and cancer immunology. Mol. Cancer 19:102. doi: 10.1186/s12943-020-01199-1
Hu, Y., and Guo, B. (2020). Circ-MTO1 correlates with favorable prognosis and inhibits cell proliferation, invasion as well as miR-17-5p expression in prostate cancer. J. Clin. Lab. Anal. 34:e23086. doi: 10.1002/jcla.23086
Huang, C., Deng, H., Wang, Y., Jiang, H., Xu, R., Zhu, X., et al. (2019a). Circular RNA circABCC4 as the ceRNA of miR-1182 facilitates prostate cancer progression by promoting FOXP4 expression. J. Cell. Mol. Med. 23, 6112–6119. doi: 10.1111/jcmm.14477
Huang, C., Liang, D., Tatomer, D. C., and Wilusz, J. E. (2018). A length-dependent evolutionarily conserved pathway controls nuclear export of circular RNAs. Genes Dev. 32, 639–644. doi: 10.1101/gad.314856.118
Huang, E., Chen, X., and Yuan, Y. (2019b). Downregulated circular RNA itchy E3 ubiquitin protein ligase correlates with advanced pathologic T stage, high lymph node metastasis risk and poor survivals in prostate cancer patients. Cancer Biomark. 26, 41–50. doi: 10.3233/CBM-182111
Huber, M. A., Kraut, N., and Beug, H. (2005). Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 17, 548–558. doi: 10.1016/j.ceb.2005.08.001
Huggins, C., and Hodges, C. V. (1941). I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1, 948–951.
Ivanov, A., Memczak, S., Wyler, E., Torti, F., Porath, H. T., Orejuela, M. R., et al. (2015). Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep. 10, 170–177. doi: 10.1016/j.celrep.2014.12.019
Jahani, S., Nazeri, E., Majidzadeh, A. K., Jahani, M., and Esmaeili, R. (2020). Circular RNA; a new biomarker for breast cancer: A systematic review. J. Cell. Physiol. 235, 5501–5510. doi: 10.1002/jcp.29558
Jeck, W. R., Sorrentino, J. A., Wang, K., Slevin, M. K., Burd, C. E., Liu, J., et al. (2013). Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 19, 141–157. doi: 10.1261/rna.035667.112
Jiang, H., Lv, D. J., Song, X. L., Wang, C., Yu, Y. Z., and Zhao, S. C. (2020). Upregulated circZMIZ1 promotes the proliferation of prostate cancer cells and is a valuable marker in plasma. Neoplasma 67, 68–77. doi: 10.4149/neo_2019_190213N116
Jin, C., Zhao, W., Zhang, Z., and Liu, W. (2019). Silencing circular RNA circZNF609 restrains growth, migration and invasion by up-regulating microRNA-186-5p in prostate cancer. Artif. Cells Nanomed. Biotechnol. 47, 3350–3358. doi: 10.1080/21691401.2019.1648281
Kong, Z., Wan, X., Lu, Y., Zhang, Y., Huang, Y., Xu, Y., et al. (2020). Circular RNA circFOXO3 promotes prostate cancer progression through sponging miR-29a-3p. J. Cell. Mol. Med. 24, 799–813. doi: 10.1111/jcmm.14791
Kong, Z., Wan, X., Zhang, Y., Zhang, P., Zhang, Y., Zhang, X., et al. (2017). Androgen-responsive circular RNA circSMARCA5 is up-regulated and promotes cell proliferation in prostate cancer. Biochem. Biophys. Res. Commun. 493, 1217–1223. doi: 10.1016/j.bbrc.2017.07.162
Kristensen, L. S., Andersen, M. S., Stagsted, L. V. W., Ebbesen, K. K., Hansen, T. B., and Kjems, J. (2019). The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 20, 675–691. doi: 10.1038/s41576-019-0158-7
Li, H., Zhi, Y., Ma, C., Shen, Q., Sun, F., and Cai, C. (2020a). Circ_0062020 knockdown strengthens the radiosensitivity of prostate cancer cells. Cancer Manag. Res. 12, 11701–11712. doi: 10.2147/CMAR.S273826
Li, P., Wang, Z., Li, S., and Wang, L. (2021a). Circ_0006404 accelerates prostate cancer progression through regulating miR-1299/CFL2 signaling. Oncol. Targets. Ther. 14, 83–95. doi: 10.2147/OTT.S277831
Li, Q., Wang, W., Zhang, M., Sun, W., Shi, W., and Li, F. (2020b). Circular RNA circ-0016068 promotes the growth, migration, and invasion of prostate cancer cells by regulating the miR-330-3p/BMI-1 axis as a competing endogenous RNA. Front. Cell Dev. Biol. 8:827. doi: 10.3389/fcell.2020.00827
Li, S., Yu, C., Zhang, Y., Liu, J., Jia, Y., Sun, F., et al. (2020c). Circular RNA cir-ITCH Is a Potential Therapeutic Target for the Treatment of Castration-Resistant Prostate Cancer. Biomed Res. Int. 2020:7586521. doi: 10.1155/2020/7586521
Li, T., Sun, X., and Chen, L. (2020d). Exosome circ_0044516 promotes prostate cancer cell proliferation and metastasis as a potential biomarker. J. Cell. Biochem. 121, 2118–2126. doi: 10.1002/jcb.28239
Li, X., Azhati, B., Wang, W., Rexiati, M., Xing, C., and Wang, Y. (2021b). Circular RNA UBAP2 promotes the proliferation of prostate cancer cells via the miR-1244/MAP3K2 axis. Oncol. Lett. 21:486. doi: 10.3892/ol.2021.12747
Li, Y., Zheng, Q., Bao, C., Li, S., Guo, W., Zhao, J., et al. (2015). Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 25, 981–984. doi: 10.1038/cr.2015.82
Lin, Q., Cai, J., and Wang, Q. Q. (2020). The significance of circular RNA DDX17 in prostate cancer. Biomed Res. Int. 2020:1878431. doi: 10.1155/2020/1878431
Liu, F., Fan, Y., Ou, L., Li, T., Fan, J., Duan, L., et al. (2020). CircHIPK3 facilitates the G2/M transition in prostate cancer cells by sponging miR-338-3p. Onco. Targets. Ther. 13, 4545–4558. doi: 10.2147/OTT.S242482
Lu, T., Cui, L., Zhou, Y., Zhu, C., Fan, D., Gong, H., et al. (2015). Transcriptome-wide investigation of circular RNAs in rice. RNA 21, 2076–2087. doi: 10.1261/rna.052282.115
Luo, J., Luo, Y., Sun, J., Zhou, Y., Zhang, Y., and Yang, X. (2015). Adeno-associated virus-mediated cancer gene therapy: current status. Cancer Lett. 356(2 Pt B), 347–356. doi: 10.1016/j.canlet.2014.10.045
Mao, Y., Li, W., Hua, B., Gu, X., Pan, W., Chen, Q., et al. (2020). Circular RNA_PDHX promotes the proliferation and invasion of prostate cancer by sponging MiR-378a-3p. Front. Cell Dev. Biol. 8:602707. doi: 10.3389/fcell.2020.602707
Memczak, S., Jens, M., Elefsinioti, A., Torti, F., Krueger, J., Rybak, A., et al. (2013). Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495, 333–338. doi: 10.1038/nature11928
Mohammad, R. M., Muqbil, I., Lowe, L., Yedjou, C., Hsu, H. Y., Lin, L. T., et al. (2015). Broad targeting of resistance to apoptosis in cancer. Semin. Cancer Biol. 35 Suppl, S78–S103. doi: 10.1016/j.semcancer.2015.03.001
Nan, C., Wang, Y., Yang, S., and Chen, Y. (2020). circCRKL suppresses the progression of prostate cancer cells by regulating the miR-141/KLF5 axis. Pathol. Res. Pract. 216:153182. doi: 10.1016/j.prp.2020.153182
Niu, Y., He, J. H., Zhang, Y., Li, K., and Xing, C. (2021). Effect of the circCDR1as/miR-641/XIAP regulatory axis on the proliferation and invasion of the prostate cancer PC-3 cell line. Oncol. Lett. 21:469. doi: 10.3892/ol.2021.12730
Pan, Z., Cai, J., Lin, J., Zhou, H., Peng, J., Liang, J., et al. (2020). A novel protein encoded by circFNDC3B inhibits tumor progression and EMT through regulating Snail in colon cancer. Mol. Cancer 19:71. doi: 10.1186/s12943-020-01179-5
Pattabiraman, D. R., and Weinberg, R. A. (2014). Tackling the cancer stem cells—what challenges do they pose? Nat. Rev. Drug Discov. 13, 497–512. doi: 10.1038/nrd4253
Qi, J. C., Yang, Z., Lin, T., Ma, L., Wang, Y. X., Zhang, Y., et al. (2021). CDK13 upregulation-induced formation of the positive feedback loop among circCDK13, miR-212-5p/miR-449a and E2F5 contributes to prostate carcinogenesis. J. Exp. Clin. Cancer Res. 40:2. doi: 10.1186/s13046-020-01814-5
Rajappa, A., Banerjee, S., Sharma, V., and Khandelia, P. (2020). Circular RNAs: emerging role in cancer diagnostics and therapeutics. Front. Mol Biosci. 7, 577938. doi: 10.3389/fmolb.2020.577938
Salzman, J., Gawad, C., Wang, P. L., Lacayo, N., and Brown, P. O. (2012). Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS ONE 7:e30733. doi: 10.1371/journal.pone.0030733
Sanger, H. L., Klotz, G., Riesner, D., Gross, H. J., and Kleinschmidt, A. K. (1976). Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc. Natl. Acad. Sci. U.S.A. 73, 3852–3856. doi: 10.1073/pnas.73.11.3852
Santiago-Ortiz, J. L., and Schaffer, D. V. (2016). Adeno-associated virus (AAV) vectors in cancer gene therapy. J. Control. Release 240, 287–301. doi: 10.1016/j.jconrel.2016.01.001
Sartor, O., and de Bono, J. S. (2018). Metastatic prostate cancer. N. Engl. J. Med. 378, 645–657. doi: 10.1056/NEJMra1701695
Sha, J., Xia, L., Han, Q., Chi, C., Zhu, Y., Pan, J., et al. (2020). Downregulation of circ-TRPS1 suppressed prostatic cancer prognoses by regulating miR-124-3p/EZH2 axis-mediated stemness. Am. J. Cancer Res. 10, 4372–4385. doi: 10.21203/rs.3.rs-48783/v1
Shan, G., Shao, B., Liu, Q., Zeng, Y., Fu, C., Chen, A., et al. (2020). circFMN2 sponges miR-1238 to promote the expression of LIM-homeobox gene 2 in prostate cancer cells. Mol. Ther. Nucleic Acids 21, 133–146. doi: 10.1016/j.omtn.2020.05.008
Shen, B., Wang, Z., Li, Z., Song, H., and Ding, X. (2019). Circular RNAs: an emerging landscape in tumor metastasis. Am. J. Cancer Res. 9, 630–643.
Shen, Y., Guo, X., and Wang, W. (2017). Identification and characterization of circular RNAs in zebrafish. FEBS Lett. 591, 213–220. doi: 10.1002/1873-3468.12500
Shen, Z., Zhou, L., Zhang, C., and Xu, J. (2020). Reduction of circular RNA Foxo3 promotes prostate cancer progression and chemoresistance to docetaxel. Cancer Lett. 468, 88–101. doi: 10.1016/j.canlet.2019.10.006
Shi, J., Liu, C., Chen, C., Guo, K., Tang, Z., Luo, Y., et al. (2020). Circular RNA circMBOAT2 promotes prostate cancer progression via a miR-1271-5p/mTOR axis. Aging 12, 13255–13280. doi: 10.18632/aging.103432
Siegel, R. L., Miller, K. D., Fuchs, H. E., and Jemal, A. (2021). Cancer statistics, 2021. CA Cancer J. Clin. 71, 7–33. doi: 10.3322/caac.21654
Si-Tu, J., Cai, Y., Feng, T., Yang, D., Yuan, S., Yang, X., et al. (2019). Upregulated circular RNA circ-102004 that promotes cell proliferation in prostate cancer. Int. J. Biol. Macromol. 122, 1235–1243. doi: 10.1016/j.ijbiomac.2018.09.076
Song, Z., Zhuo, Z., Ma, Z., Hou, C., Chen, G., and Xu, G. (2019). Hsa_Circ_0001206 is downregulated and inhibits cell proliferation, migration and invasion in prostate cancer. Artif. Cells Nanomed. Biotechnol. 47, 2449–2464. doi: 10.1080/21691401.2019.1626866
Sun, A., Tang, J., Terranova, P. F., Zhang, X., Thrasher, J. B., and Li, B. (2010). Adeno-associated virus-delivered short hairpin-structured RNA for androgen receptor gene silencing induces tumor eradication of prostate cancer xenografts in nude mice: a preclinical study. Int. J. Cancer 126, 764–774. doi: 10.1002/ijc.24778
Talmadge, J. E., and Fidler, I. J. (2010). AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 70, 5649–5669. doi: 10.1158/0008-5472.CAN-10-1040
Tao, L. J., Pan, X. Y., Wang, J. W., Zhang, L., Tao, L. S., and Liang, C. Z. (2021). Circular RNA circANKS1B acts as a sponge for miR-152-3p and promotes prostate cancer progression by upregulating TGF-α expression. Prostate 81, 271–278. doi: 10.1002/pros.24102
Tran, C., Ouk, S., Clegg, N. J., Chen, Y., Watson, P. A., Arora, V., et al. (2009). Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324, 787–790. doi: 10.1126/science.1168175
van Niel, G., D'Angelo, G., and Raposo, G. (2018). Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213–228. doi: 10.1038/nrm.2017.125
Vo, J. N., Cieslik, M., Zhang, Y., Shukla, S., Xiao, L., Zhang, Y., et al. (2019). The Landscape of Circular RNA in Cancer. Cell 176, 869–881.e813. doi: 10.1016/j.cell.2018.12.021
Wang, D., Yan, S., Wang, L., Li, Y., and Qiao, B. (2021a). circSLC8A1 Acts as a Tumor Suppressor in Prostate Cancer via Sponging miR-21. Biomed Res. Int. 2021:6614591. doi: 10.1155/2021/6614591
Wang, M., Yang, Y., Xu, J., Bai, W., Ren, X., and Wu, H. (2018). CircRNAs as biomarkers of cancer: a meta-analysis. BMC Cancer 18:303. doi: 10.1186/s12885-018-4213-0
Wang, P., Zhang, L., Yin, S., Xu, Y., Tai, S., Zhang, L. I., et al. (2021b). hsa_circ_0062019 promotes the proliferation, migration, and invasion of prostate cancer cells via the miR-195-5p/HMGA2 axis. Acta Biochim. Biophys. Sin. 53, 815–822. doi: 10.1093/abbs/gmab058
Wang, S., Su, W., Zhong, C., Yang, T., Chen, W., Chen, G., et al. (2020a). An eight-CircRNA assessment model for predicting biochemical recurrence in prostate cancer. Front. Cell Dev. Biol. 8:599494. doi: 10.3389/fcell.2020.599494
Wang, X., Wang, R., Wu, Z., and Bai, P. (2019). Circular RNA ITCH suppressed prostate cancer progression by increasing HOXB13 expression via spongy miR-17-5p. Cancer Cell Int. 19:328. doi: 10.1186/s12935-019-0994-8
Wang, Y., Zhang, Y., Wang, P., Fu, X., and Lin, W. (2020b). Circular RNAs in renal cell carcinoma: implications for tumorigenesis, diagnosis, and therapy. Mol. Cancer 19:149. doi: 10.1186/s12943-020-01266-7
Wen, G., Zhou, T., and Gu, W. (2020). The potential of using blood circular RNA as liquid biopsy biomarker for human diseases. Protein Cell. 2020, 1–36. doi: 10.1007/s13238-020-00799-3
Weng, X. D., Yan, T., and Liu, C. L. (2020). Circular RNA_LARP4 inhibits cell migration and invasion of prostate cancer by targeting FOXO3A. Eur. Rev. Med. Pharmacol. Sci. 24, 5303–5309. doi: 10.26355/eurrev_202005_21312
Westholm, J. O., Miura, P., Olson, S., Shenker, S., Joseph, B., Sanfilippo, P., et al. (2014). Genome-wide analysis of drosophila circular RNAs reveals their structural and sequence properties and age-dependent neural accumulation. Cell Rep. 9, 1966–1980. doi: 10.1016/j.celrep.2014.10.062
Wu, G., Sun, Y., Xiang, Z., Wang, K., Liu, B., Xiao, G., et al. (2019). Preclinical study using circular RNA 17 and micro RNA 181c-5p to suppress the enzalutamide-resistant prostate cancer progression. Cell Death Dis. 10:37. doi: 10.1038/s41419-018-1048-1
Wu, Y., Zhang, Y., Zheng, X., Dai, F., Lu, Y., Dai, L., et al. (2020). Circular RNA circCORO1C promotes laryngeal squamous cell carcinoma progression by modulating the let-7c-5p/PBX3 axis. Mol. Cancer 19:99. doi: 10.1186/s12943-020-01215-4
Xia, H. Y., Liu, C. D., Liang, W., Huo, X. Y., and Wei, X. W. (2021). Circ_0004417 inhibits the progression of prostate cancer through sponging miR-1228. Eur. Rev. Med. Pharmacol. Sci. 25, 1274–1281. doi: 10.26355/eurrev_202102_24831
Xiang, Z., Xu, C., Wu, G., Liu, B., and Wu, D. (2019). CircRNA-UCK2 increased TET1 inhibits proliferation and invasion of prostate cancer cells via sponge MiRNA-767-5p. Open Med (Wars) 14, 833–842. doi: 10.1515/med-2019-0097
Xiao, M. S., Ai, Y., and Wilusz, J. E. (2020). Biogenesis and functions of circular RNAs come into focus. Trends Cell Biol. 30, 226–240. doi: 10.1016/j.tcb.2019.12.004
Xu, H., Sun, Y., You, B., Huang, C. P., Ye, D., and Chang, C. (2020a). Androgen receptor reverses the oncometabolite R-2-hydroxyglutarate-induced prostate cancer cell invasion via suppressing the circRNA-51217/miRNA-646/TGFβ1/p-Smad2/3 signaling. Cancer Lett. 472, 151–164. doi: 10.1016/j.canlet.2019.12.014
Xu, T., Wang, M., Jiang, L., Ma, L., Wan, L., Chen, Q., et al. (2020b). CircRNAs in anticancer drug resistance: recent advances and future potential. Mol. Cancer 19:127. doi: 10.1186/s12943-020-01240-3
Xu, X., Zhang, J., Tian, Y., Gao, Y., Dong, X., Chen, W., et al. (2020c). CircRNA inhibits DNA damage repair by interacting with host gene. Mol. Cancer 19:128. doi: 10.1186/s12943-020-01246-x
Yan, Z., Xiao, Y., Chen, Y., and Luo, G. (2020). Screening and identification of epithelial-to-mesenchymal transition-related circRNA and miRNA in prostate cancer. Pathol. Res. Pract. 216:152784. doi: 10.1016/j.prp.2019.152784
Yang, X., Tian, W., Wang, S., Ji, X., and Zhou, B. (2020). CircRNAs as promising biomarker in diagnostic and prognostic of lung cancer: an updated meta-analysis. Genomics 113(1 Pt 1), 387–397. doi: 10.1016/j.ygeno.2020.12.013
Yang, Z., Qu, C. B., Zhang, Y., Zhang, W. F., Wang, D. D., Gao, C. C., et al. (2019). Dysregulation of p53-RBM25-mediated circAMOTL1L biogenesis contributes to prostate cancer progression through the circAMOTL1L-miR-193a-5p-Pcdha pathway. Oncogene 38, 2516–2532. doi: 10.1038/s41388-018-0602-8
Yu, J., Xu, Q. G., Wang, Z. G., Yang, Y., Zhang, L., Ma, J. Z., et al. (2018). Circular RNA cSMARCA5 inhibits growth and metastasis in hepatocellular carcinoma. J. Hepatol. 68, 1214–1227. doi: 10.1016/j.jhep.2018.01.012
Yuan, J., Guo, D., Li, X., and Chen, J. (2020). Prognostic and diagnostic value of circRNA expression in colorectal carcinoma: a meta-analysis. BMC Cancer 20:448. doi: 10.1186/s12885-020-06932-z
Yuan, Y., Chen, X., and Huang, E. (2019). Upregulation of circular RNA itchy E3 ubiquitin protein ligase inhibits cell proliferation and promotes cell apoptosis through targeting MiR-197 in prostate cancer. Technol. Cancer Res. Treat. 18:1533033819886867. doi: 10.1177/1533033819886867
Zhang, C., Chen, K., Wei, R., Fan, G., Cai, X., Xu, L., et al. (2020a). The circFASN/miR-33a pathway participates in tacrolimus-induced dysregulation of hepatic triglyceride homeostasis. Signal Transduct. Target Ther. 5:23. doi: 10.1038/s41392-020-0105-2
Zhang, H., Li, M., Zhang, J., Shen, Y., and Gui, Q. (2021). Exosomal circ-XIAP promotes docetaxel resistance in prostate cancer by regulating miR-1182/TPD52 axis. Drug Des. Devel. Ther. 15, 1835–1849. doi: 10.2147/DDDT.S300376
Zhang, P., Zhang, X. O., Jiang, T., Cai, L., Huang, X., Liu, Q., et al. (2020b). Comprehensive identification of alternative back-splicing in human tissue transcriptomes. Nucleic Acids Res. doi: 10.1093/nar/gkaa005
Zhang, P. F., Gao, C., Huang, X. Y., Lu, J. C., Guo, X. J., Shi, G. M., et al. (2020c). Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma. Mol. Cancer 19:110. doi: 10.1186/s12943-020-01222-5
Zhang, S., Zhang, X., Chen, G., Zheng, X., Zhu, X., and Shan, L. (2020d). Hsa_circ_0007494 suppresses prostate cancer progression via miR-616/PTEN axis. Exp. Cell Res. 395:112233. doi: 10.1016/j.yexcr.2020.112233
Zhang, X., Wang, S., Wang, H., Cao, J., Huang, X., Chen, Z., et al. (2019). Circular RNA circNRIP1 acts as a microRNA-149-5p sponge to promote gastric cancer progression via the AKT1/mTOR pathway. Mol. Cancer 18, 20. doi: 10.1186/s12943-018-0935-5
Zhang, X. O., Wang, H. B., Zhang, Y., Lu, X., Chen, L. L., and Yang, L. (2014). Complementary sequence-mediated exon circularization. Cell 159, 134–147. doi: 10.1016/j.cell.2014.09.001
Zhang, Y., Shi, Z., Li, Z., Wang, X., Zheng, P., and Li, H. (2020e). Circ_0057553/miR-515-5p regulates prostate cancer cell proliferation, apoptosis, migration, invasion and aerobic glycolysis by targeting YES1. Onco. Targets. Ther. 13, 11289–11299. doi: 10.2147/OTT.S272294
Zheng, Y., Chen, C. J., Lin, Z. Y., Li, J. X., Liu, J., Lin, F. J., et al. (2020a). Circ_KATNAL1 regulates prostate cancer cell growth and invasiveness through the miR-145-3p/WISP1 pathway. Biochem. Cell Biol. 98, 396–404. doi: 10.1139/bcb-2019-0211
Zheng, Y., Li, J. X., Chen, C. J., Lin, Z. Y., Liu, J. X., and Lin, F. J. (2020b). Extracellular vesicle-derived circ_SLC19A1 promotes prostate cancer cell growth and invasion through the miR-497/septin 2 pathway. Cell Biol. Int. 44, 1037–1045. doi: 10.1002/cbin.11303
Keywords: biomarker, circRNA, metastasis, proliferation, prostate cancer
Citation: Chao F, Wang S, Zhang C, Han D, Xu G and Chen G (2021) The Emerging Role of Circular RNAs in Prostate Cancer: A Systematic Review. Front. Cell Dev. Biol. 9:681163. doi: 10.3389/fcell.2021.681163
Received: 16 March 2021; Accepted: 06 July 2021;
Published: 27 July 2021.
Edited by:
Yubing Zhou, First Affiliated Hospital of Zhengzhou University, ChinaReviewed by:
Thasni Karedath, Weill Cornell Medicine-Qatar, QatarVivek Sharma, Birla Institute of Technology and Science, India
Tianyi Xu, National Genomics Data Center, Beijing Institute of Genomics (CAS), China
Zhigang Tu, Jiangsu University, China
Copyright © 2021 Chao, Wang, Zhang, Han, Xu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Chen, Y2hnYW4zNjVAMTI2LmNvbQ==
 Fan Chao1
Fan Chao1 Guoxiong Xu
Guoxiong Xu Gang Chen
Gang Chen