- 1Department of Oral and Maxillofacial Surgery, Hospital of Stomatology, Jilin University, Changchun, China
- 2Laboratory Animal Center, College of Animal Science, Jilin University, Changchun, China
- 3School of Pharmacy, Changchun University of Chinese Medicine, Changchun, China
- 4Jilin Provincial Key Laboratory of Tooth Development and Bone Remodeling, Hospital of Stomatology, Jilin University, Changchun, China
Long non-coding RNA (lncRNA) plays a significant role in the pathogenesis of many human malignant tumors, including oral cancer. LncRNA can act as a gene regulator in a variety of cancers. It regulates the growth of malignant cells via many cellular signal pathways such as the PI3K (phosphoinositide 3-kinase)/AKT (α-serine/threonine-protein kinase) pathway. In this review, we have analyzed the role of lncRNAs, such as lncRNA X inactive specific transcript (XIST), in oral cancer, including its effects on the proliferation, apoptosis, invasion, migration, and resistance to chemotherapy of oral cancer. We have also focused on the role of lncRNA XIST as the core of X chromosome inactivation. Here, we provide a brief overview of the role of many kinds of lncRNAs, including XIST, which provides a theoretical basis for the study of the role of XIST in oral cancer. Our review may provide a new direction for the study of the occurrence, development, and prognosis of oral cancer and provide a new target for its treatment.
Introduction
Oral cancer is the 11th most common carcinoma around the globe that has attracted global attention (D'Souza and Addepalli, 2018). There were 377,713 new cases and 177,757 new deaths of oral cancer in 2020 (Sung et al., 2021). Most patients are diagnosed with oral cancer at an advanced stage (Bagan et al., 2010). It is characterized by a poor prognosis and a high rate of lymphatic metastasis (Okura et al., 2009; Noguti et al., 2012). Surgical resection in combination with radiotherapy and chemotherapy is currently the main treatment for oral cancer. However, this treatment regimen has not helped in significantly improving the five-year survival rate (Alkhadar et al., 2021). There is mounting evidence that has demonstrated that lncRNAs play a crucial role related to the survival rate in oral cancer (Zhang et al., 2018a; Momen-Heravi and Bala, 2018; Gao et al., 2020; Chen et al., 2021). Moreover, the expression pattern of lncRNAs plays a role in the diagnosis and therapy of oral cancer (Momen-Heravi and Bala, 2018). LncRNA MALAT1 can be used as a biomarker and therapeutic target for oral squamous cell carcinoma (OSCC) (Zhou et al., 2015).
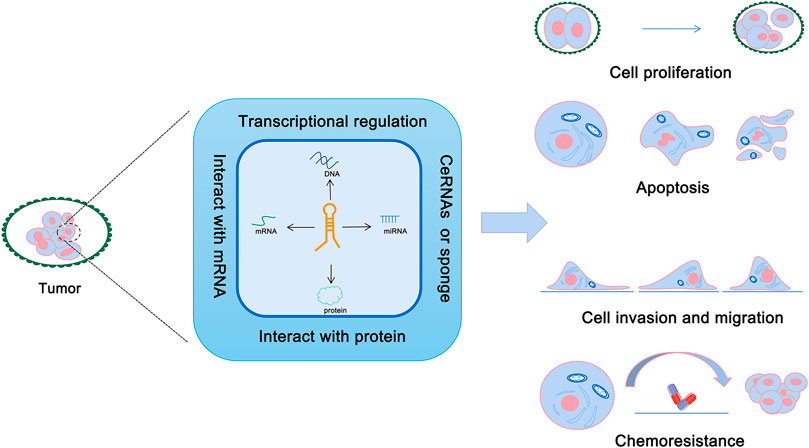
With the in-depth study of the human genome, it has been found that most of the genome can be transcribed into RNA, yet only 1%–2% of the protein-coding genes encode proteins involved in various cellular life activities (Beermann et al., 2016). Compare to the coding RNAs, non-coding RNAs were initially considered to be the “junk DNA,” which were produced during the process of gene transcription (Doolittle, 2013). However, it has been found that non-coding RNA plays a critical regulatory role in gene expression and other biological processes (Anastasiadou et al., 2018). Non-coding RNAs include microRNAs, circRNAs, intron RNAs, and long non-coding RNAs (Morris and Mattick, 2014). Long non-coding RNAs (lncRNAs) are defined as any non-protein-coding RNA >200 bp in length (Morris and Mattick, 2014; Qian et al., 2019). LncRNAs help to protect the integrity of the genome and regulate gene expression by interacting with DNA, RNA, and proteins (Mercer et al., 2009; Chen, 2016). Moreover, lncRNA can play multiple roles in pathogenesis of tumors via different mechanisms (Figure 1). A recent study has shown that lncRNA XIST is one of the lncRNAs that play a key role in the pathogenesis of OSCC (Tao et al., 2021). Results from this study demonstrated that XIST inhibited apoptosis, and promoted cell proliferation, invasion, and migration to promote the growth of oral cancer (Tao et al., 2021). The findings of this study provide a new direction for the study of XIST in oral cancer.
The role of lncRNA in oral cancer
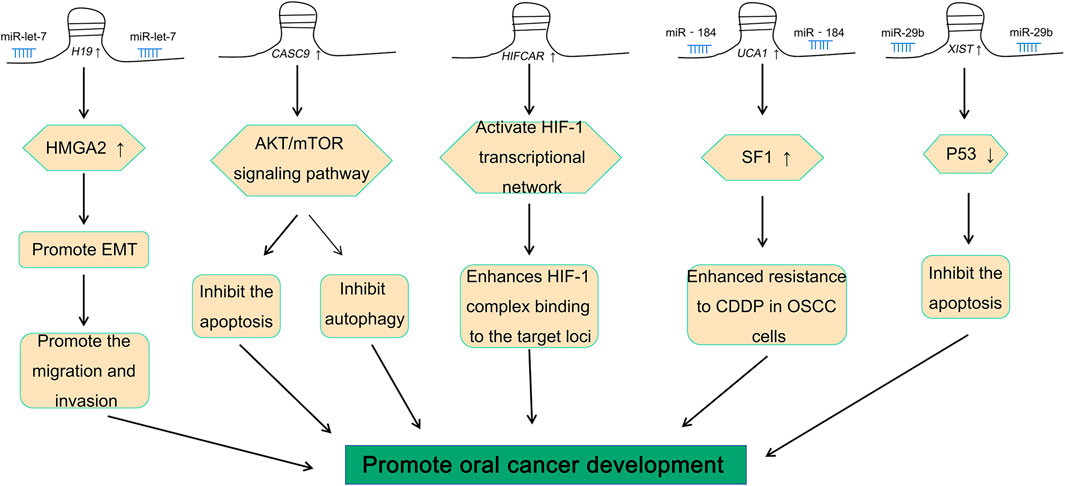
Studies have shown that there is a variety of abnormal expression patterns of lncRNAs in oral cancer, and they have a critical role in the pathogenesis of cancer (Momen-Heravi and Bala, 2018). Different lncRNAs have a variety of different effects on oral cancer (Fang et al., 2020; Ghafouri-Fard et al., 2020; Sur et al., 2020). H19, the first lncRNA to be discovered, is considered to be closely related to embryonic development and tumorigenesis (Raveh et al., 2015; Zhang et al., 2017; Wang et al., 2019a; Kou et al., 2019; Zhang et al., 2020). H19 is abnormally expressed in oral cancer and plays a role as a “sponge molecule” (Kou et al., 2019). It regulates the expression of the high mobility group A2 (HMGA2) protein through miRNA let-7 to promote the migration and invasion of tongue squamous cell carcinoma (TSCC) (Kou et al., 2019). In addition to H19, there are many lncRNAs that aggravate the development of oral cancer by promoting the migration and invasion of cancer cells, such as lncRNA FGD5-AS1 (Liu et al., 2020a), lncRNA MYOSLID (Xiong et al., 2019), and lncRNA HOTAIR (Tao et al., 2020). In addition, lncRNAs such as lncRNA CASC9 can also promote the growth of cancerous cells by suppressing autophagy-mediated cell apoptosis in oral cancer (Yang et al., 2019). It has been reported previously that lncRNA CASC9 plays a vital role in the pathogenesis of OSCC and inhibits apoptosis and autophagy via the AKT (Protein kinase B)/mTOR (mammalian target of rapamycin) signal pathway (Yang et al., 2019). LncRNA HIFCAR can be used as a co-activating factor of hypoxia-inducible factor HIF-1α for regulating the hypoxia signal pathway in oral cancer (Shih et al., 2017). It also plays a role in the pathogenesis of oral cancer, thereby suggesting that it could be a new therapeutic target for the treatment of oral cancer (Shih et al., 2017). It has also been reported that lncRNA HAS2-AS1 was abnormally expressed in oral cancer, which was closely related to the anoxic state of oral cancer (Zhu et al., 2017).
Resistance to chemotherapeutic drugs is a major challenge in the treatment of cancer (Cui et al., 2018; Bukowski et al., 2020). One of the key research focus areas in oncology research is to identify the mechanisms by which resistance is developed by the cancer cells to chemotherapeutic drugs (Li et al., 2020a). Studies have found that lncRNA UCA1 was highly expressed in cisplatin-resistant OSCC (Fang et al., 2017). There is also mounting evidence that lncRNA UCA1 can promote the proliferation of OSCC cells and inhibit the sensitivity of OSCC cells to cisplatin (Fang et al., 2017). Meanwhile, the aforementioned results suggested that lncRNA UCA1 also can regulate the growth of OSCC via miR-184 (Fang et al., 2017). This suggested that an interaction between lncRNA and miRNA might be able to regulate the pathogenesis of oral cancer (Zhou et al., 2019).
XIST is an lncRNA located in the XIST gene, which plays a key role in dose compensation of the X chromosome and embryonic development (Sahakyan et al., 2018). As a key regulator of chromosome dose compensation, XIST can achieve dose compensation by randomly inactivating the X chromosome (Payer and Lee, 2008; Chen and Yen, 2019). XIST may recruit chromatin modification enzymes to participate in the regulation of chromatin structure, thereby leading to changes in the expression of oncogenic or anti-oncogene genes regulated by XIST (Cerase et al., 2015).
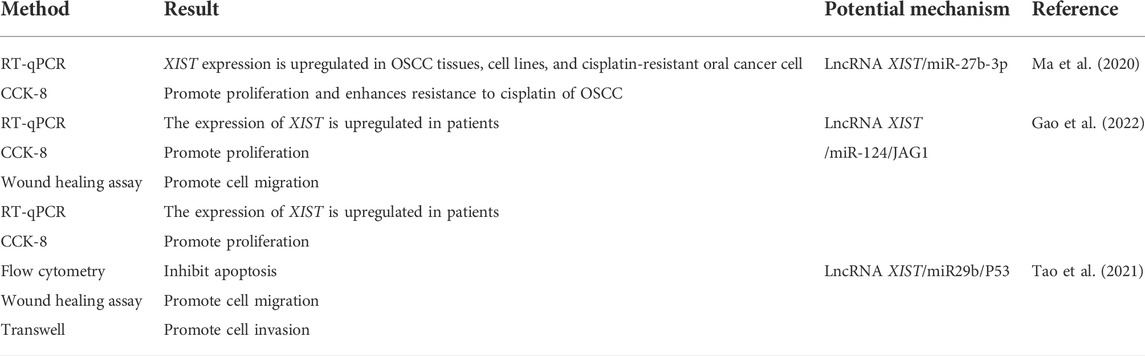
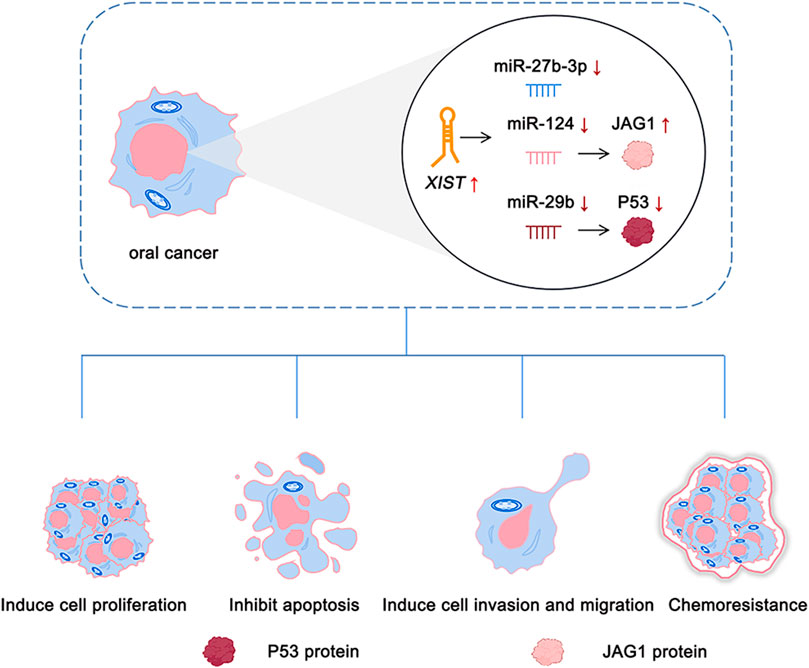
There is an abnormal expression of lncRNA XIST in a variety of cancers, such as thyroid cancer (Liu et al., 2018), colorectal cancer (Chen and Shen, 2020), breast cancer (Soudyab et al., 2016), and oral cancer (Tao et al., 2021). A previous study by our group demonstrated that there was an abnormal expression of lncRNA XIST in TSCC (Tao et al., 2021). Knocking down the expression of XIST significantly inhibited the proliferation, migration, and invasion of TSCC and induced their apoptosis (Tao et al., 2021). The role of XIST in oral cancer has been investigated, and the potential mechanism has been elaborated (Table 1 and Figure 2). In addition, possible methods, such as RNA-seq and fluorescence in situ hybridization (FISH) assay, can be performed to determine the expression of XIST to provide a potential implication in oral cancer (Shiura and Abe, 2019; Tao et al., 2021; Wang et al., 2021). This suggested that many lncRNAs, especially XIST, are abnormally expressed in a variety of cancers, including oral cancer, and play a key role in their pathogenesis via the regulation of different pathways (Figure 3).
Regulatory mechanism of XIST and its expression pattern in cancer
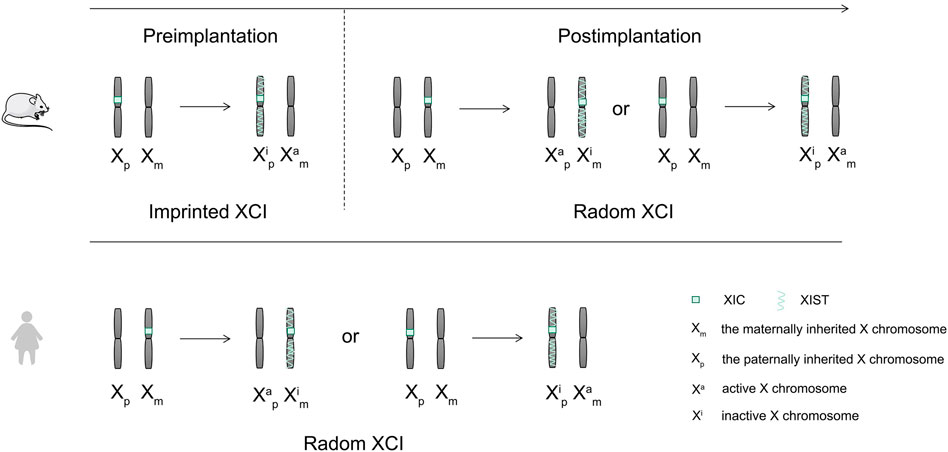
LncRNA XIST is the earliest discovered lncRNA (Loda and Heard, 2019). It mainly plays a role in regulating X chromosome inactivation in cells and can silence most genes on the inactivated X chromosome (Cerase et al., 2015). There are about 1,000 genes present on the X chromosome, which can cause a variety of diseases such as cancer and hemophilia (Lee and Bartolomei, 2013). Therefore, the role of XIST in X chromosome inactivation is important in mammals (Figure 4).
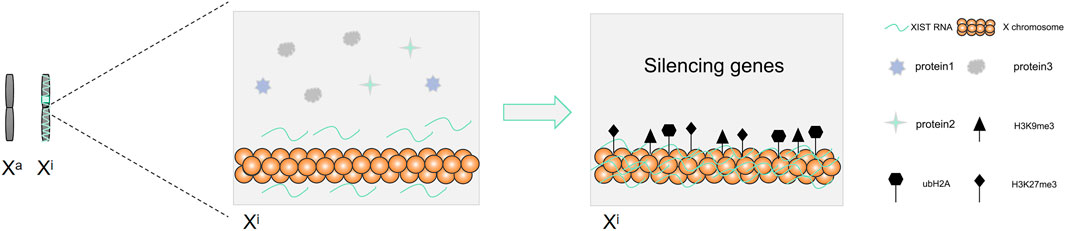
There are two X chromosomes that are present in the somatic cells of female mammals, while there is only one X chromosome present in the male mammalian cells (Wang et al., 2016). To maintain the balance of gene expression between female and male mammals in embryo development, female mammals achieve X chromosome dose compensation via X chromosome inactivity (Sahakyan et al., 2018). X chromosome inactivation (XCI) needs the activation of the X chromosome inactivation center (Dixon-McDougall and Brown, 2016). The X chromosome inactivation center (XIC) is located in the region from 100 to 500 kb on the X chromosome (Dixon-McDougall and Brown, 2016). The core of XIC is long non-coding RNA XIST, which is only expressed in the female inactive X chromosome (Gendrel and Heard, 2014). XCI includes two forms, the random XCI and the imprinted XCI (Sahakyan et al., 2018). Two forms of imprinted XCI and random XCI occur during the embryonic development of mice (Sahakyan et al., 2018). The imprinted XCI first occurs in the female mouse pre-embryonic stage, silencing the patrilineal X chromosome (Sahakyan et al., 2018; Chen and Yen, 2019). Subsequently, following blastocyst formation, the imprinted XCI remains unchanged in trophoblast ectoderm cells, while the cells in blastocyst ectoderm reactivate the paternal X chromosome, and random XCI occurred before and after implantation (Sahakyan et al., 2018). However, the dose compensation of the X chromosome is realized by random XCI in human (Sahakyan et al., 2018). XIST wraps around the X chromosome on which it sits, recruits heterochromatin factors, and silences gene expression (Figure 5). The expression of XIST only plays a role in the initiation of X chromosome inactivation. There is a need for other supporting factors to maintain the inactivation of the X chromosome (Fatica and Bozzoni, 2014).
During the process of X chromosome inactivation (XCI), the entire X chromosome is permanently silenced and transformed into Barr bodies (Dixon-McDougall and Brown, 2016). Studies have found that the expression of histone markers on the chromosome increased, such as H3K27me3, H3K9me3, and ubH2A, once XIST was enriched on the X chromosome (Mermoud et al., 2002; Plath et al., 2003; Fang et al., 2004; Brinkman et al., 2006; Dixon-McDougall and Brown, 2016). Meanwhile, the corresponding gene expression in the histone-rich region was silenced (Mermoud et al., 2002; Plath et al., 2003; Fang et al., 2004; Brinkman et al., 2006; Dixon-McDougall and Brown, 2016). XIST can recruit and bind Polycomb-repressive complex 2 (PRC2) to the transcriptional site of XIST gene, cause histone modification in this region, and mediate X chromosome inactivation (Wutz, 2011). This suggested that histone modification plays a key role in XCI. High DNA methylation also plays a role in stabilizing the inhibitory state of silent genes, thereby suggesting that DNA methylation plays an important role in maintaining the stability of XCI (Dixon-McDougall and Brown, 2016).
The link between XCI and cancer was proposed more than 50 years ago (Pageau et al., 2007). They found that there was a lack of Barr bodies in some breast cancer cells, thereby suggesting that the inactivation of the X chromosome may be related to cancer (Pageau et al., 2007). As a specific expression of lncRNA in female cells, a large number of studies have shown that XIST is closely related to the high incidence of cancer in women, such as breast cancer (Schouten et al., 2016), cervical cancer (Zhu et al., 2018), thyroid cancer (Liu et al., 2018), and ovarian cancer (Zuo et al., 2019). XIST acts as an oncogene to promote the development of cancer via multiple pathways. The abnormal expression of XIST has been observed in human oral cancer, and it has been found that XIST plays a major role in the pathogenesis of oral cancer (Tao et al., 2021). The aforementioned results suggested that the abnormal expression of XIST may lead to the abnormality of X chromosome inactivation, lead to a gene mutation on it, and then promote the occurrence of many diseases including cancer. These results suggested that XIST and XCI have a close connection with cancers including oral cancer.
XIST acts as a sponge molecule
The “competitive endogenous RNA” (ceRNA) hypothesis was proposed in 2011 (Salmena et al., 2011). It was believed that the ceRNA mechanism formed a large-scale regulatory network in transcriptome and plays an important role in diseases such as cancer (Salmena et al., 2011). Major elements such as microRNAs (miRNAs), protein-coding genes, and lncRNAs are included in the mechanism of ceRNAs (Salmena et al., 2011). LncRNA can further regulate the expression of mRNA via competitive binding with microRNAs in a variety of cancers. Studies conducted in breast cancer have shown that lncRNA BCRT1 acts as a molecular sponge of miR-1303, negatively regulates the expression of miR-1303, and upregulates the expression of PTBP3 via exocrine, which, in turn, promotes the development of breast cancer (Liang et al., 2020). In colorectal cancer, miR-181a-5p can be used as a target for lncRNA CRNDE to competitively combine with lncRNA CRNDE for promoting the growth of colorectal cancer (Han et al., 2017). XIST also can act as a sponge molecule for miRNA in cells. For instance, XIST upregulates Fus (fused in sarcoma) via competitive binding with miR-200a, which in turn acts as a ceRNA in the development of cervical cancer (Zhu et al., 2018). XIST and MET protein compete for the combination of miR-34a, which in turn leads to the progression of thyroid cancer (Liu et al., 2018). The aforementioned data indicate that XIST regulates the expression of target mRNA and target genes. A key role is played by lncRNA XIST in X chromosome inactivation. It also acts as a sponge molecule of microRNAs to participate in the ceRNA regulatory network in the development of oral cancer. Previous research by our group suggested that XIST is abnormally expressed in TSCC and acts as a sponge molecule to compete with miR-29b to stimulate the growth of TSCC (Tao et al., 2021).
XIST regulates the expression of miRNAs via the ceRNA mechanism and downstream signaling pathway to impact the growth of cells. Studies have found that XIST can act as the sponge molecule of miR-34a to participate in ceRNAs in thyroid carcinoma cells and further inhibit the growth of thyroid cancer cells via its downstream signal pathway MET (hepatocyte growth factor receptor)-PI3K (phosphoinositide 3-kinase)-AKT (α-serine/threonine-protein kinase) signal pathway (Liu et al., 2018). Abnormal glucose metabolism is very important for the progression of cancer. It has been previously demonstrated that lncRNA XIST can control the growth and glucose metabolism in glioblastoma cells via the insulin receptor substrate 1 (IRS1)/the phosphoinositide 3-kinase (PI3K)/protein kinase B(Akt) pathway (Cheng et al., 2020). To summarize, studying the mechanism and signal pathway of XIST in other cancer cells may provide a theoretical foundation for the future study of XIST in oral cancer. XIST, as a molecular sponge, plays important role in oral cancer by regulating miRNA and competitively binding with target mRNA.
The role of XIST in other cancers
As mentioned previously, a link between the X chromosome and cancer has been found (Pageau et al., 2007). XIST, which is the core of XCI, plays a key role in many cancers (Zhang et al., 2018b; Liu et al., 2019; Li et al., 2020b; Liu et al., 2020b). High expression of XIST can be used to identify BRCA1-like breast cancer and has a worse prognosis than BRCA1-like breast cancer patients with low expression of XIST (Schouten et al., 2016). XIST can also upregulate the expression of the Polycomb group protein RING1 mRNA via competitive binding with miR-744 and further promote the occurrence and development of non-small cell lung cancer (NSCLC) via the Wnt (Wingless/Integrated)/β-catenin signal pathway (Wang et al., 2019b).
The tumor microenvironment plays a key role in the occurrence and development of cancer (Chanmee et al., 2014; Zhao et al., 2021). Previous studies have reported that tumor-associated macrophages (TAMs) in the tumor microenvironment are closely related to tumor proliferation, migration, and angiogenesis (Zhao et al., 2021). XIST can regulate the polarization of tumor-associated macrophages through miR-101-3p and then promote the proliferation and migration of breast and ovarian cancer cells (Zhao et al., 2021). It also promotes the development of colon cancer via the miR-34a-mediated Wnt/β-catenin signaling pathway (Sun et al., 2018). Thus, the expression of XIST is upregulated in a variety of cancers that promotes the occurrence and development of cancer via different mechanisms. To summarize, XIST is abnormally expressed in a variety of cancers, including oral cancer. It can affect the occurrence and development of cancer by inhibiting apoptosis and promoting proliferation, invasion, and migration of cancer cells.
Discussion
Oral cancer is a common malignant tumor of the oral and maxillofacial region. It has a surgical injury and a high fatality rate. Multiple factors are involved in the pathogenesis of oral cancer (Gharat et al., 2016). External factors including smoking (Huang et al., 2019), drinking (Varoni et al., 2015), and chewing betel nut (Su et al., 2020), and internal factors like gene mutation (Ali et al., 2017), the human papillomavirus (HPV) (Jiang and Dong, 2017), and abnormal gene expression also play a major role in the development of oral cancer. As a class of gene regulatory factors, lncRNA plays an important role in oral cancer cells. It can be used as a gene target in the study of oral cancer gene therapy. The expression pattern of lncRNA XIST is particularly important in oral carcinoma. XIST binds competitively with miRNA and affects apoptosis, proliferation, cycle, and migration and also influences other related genes of cancer cells, such as cyclinD1 (Wang et al., 2019b), p53 (Hu et al., 2019), and E-cadherin (Shi et al., 2020).
Some epigenetic modifications are closely related to a variety of human diseases including cancer (Villanueva et al., 2015). XIST is regulated by some epigenetic modifications, such as DNA methylation and M6A modification. For example, the expression of M6A “writer” protein methyltransferase-like 14 (METTL14) is downregulated in colorectal cancer, and it has been found that XIST is downregulated by METTL14 in a YTHDF2-dependent way, which is responsible for inhibiting the growth of colorectal cancer (Yang et al., 2020).
Studies found that targeted regulation of the expression of XIST in oral cancer, and some other cancers can inhibit the occurrence and development of tumors (Li et al., 2020b; Shi et al., 2020; Tao et al., 2021). The expression of lncRNA XIST is increased in non-small cell lung cancer (Wang et al., 2019b). A decrease in the expression level of XIST in lung cancer cells significantly reduced their proliferation and invasion (Wang et al., 2019b). In glioblastoma cells, knocking down the expression of XIST also significantly reduced the glucose uptake of glioblastoma cells and helped to inhibit tumor growth (Cheng et al., 2020). Similarly, studies have shown that knocking down the expression level of XIST can significantly inhibit the proliferation and promote the apoptosis of oral cancer cells (Tao et al., 2021). The abnormal expression of XIST was shown in oral cancer. Moreover, knockdown expression of XIST has the ability to induce apoptosis and inhibit cell growth, migration, and invasion (Ma et al., 2020; Tao et al., 2021; Gao et al., 2022). Moreover, TCGA (http://ualcan.path.uab.edu/analysis.html) database showed that XIST was closely associated with the rainfall of HNSCC, and patients with higher XIST expression levels had a worse prognosis.
Some drugs can inhibit the expression of XIST and induce cell apoptosis. Platycodon grandiflorum saponin D (PD) is a saponin extracted from Platycodon grandiflorum (Chen et al., 2020). Studies have demonstrated that PD reduced the expression of XIST and inhibited the growth of bladder cancer cells (Chen et al., 2020). Research carried out by our group has shown that cucurbitacin B effectively inhibited the proliferation of oral cancer and significantly reduced the expression of XIST in oral cancer cells (Tao et al., 2021). XIST can regulate the progression of oral cancer via multiple mechanisms. This suggested that XIST can potentially be an important target for the diagnosis and treatment of oral cancer.
Conclusion
Many different lncRNAs have been found in the human genome, which are involved in various biological processes of cells. They are important regulatory factors in these biological processes. XIST is an important initiator of X chromosome inactivation and a gene regulator like other lncRNAs in many cancers, including oral cancer. An abnormal expression of XIST is seen in a variety of cancers, including oral cancer. XIST promotes proliferation, invasion, and migration, and inhibits apoptosis. Moreover, XIST can increase the drug resistance of cancer cells; this may seriously increase the difficulty of chemotherapy treatment for oral cancer. This indicated that the abnormal expression of XIST is closely related to the occurrence, development, and treatment of oral cancer. Therefore, XIST can be a potential new target for the treatment of oral cancer.
Author contributions
HL and DW wrote the manuscript. HL, SK, MH, LC, PL, YL, YJ, and WL collected the references and prepared figures. All authors reviewed the manuscript.
Funding
This work was supported by the Fundamental Research Funds for the Central Universities (Grant Nos. 2019JCKT-70 and 2020JCXK-45), the Jilin Province Department of Finance (Grant No. JCSZ2019378-8), the Jilin Scientific and Technological Development Program (Grant Nos. 20210101010JC, 20200801077GH, and 20210204013YY), and the Science and Technology Research Project of Jilin Provincial Department of Education (Grant No. JJKH20210995KJ). The Changchun Scientific and Technological Development Program (Grant No. 21ZY26). The Jilin Province, Department of Finance (Grant No. jcsz202189313), the Jilin Scientific and Technological Development Program (Grant No. 81602377).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ali, J., Sabiha, B., Jan, H. U., Haider, S. A., Khan, A. A., Ali, S. S., et al. (2017). Genetic etiology of oral cancer. Oral Oncol. 70, 23–28. doi:10.1016/j.oraloncology.2017.05.004
Alkhadar, H., Macluskey, M., White, S., Ellis, I., and Gardner, A. (2021). Comparison of machine learning algorithms for the prediction of five-year survival in oral squamous cell carcinoma. J. Oral Pathol. Med. 50, 378–384. doi:10.1111/jop.13135
Anastasiadou, E., Jacob, L. S., and Slack, F. J. (2018). Non-coding RNA networks in cancer. Nat. Rev. Cancer 18, 5–18. doi:10.1038/nrc.2017.99
Bagan, J., Sarrion, G., and Jimenez, Y. (2010). Oral cancer: clinical features. Oral Oncol. 46, 414–417. doi:10.1016/j.oraloncology.2010.03.009
Beermann, J., Piccoli, M. T., Viereck, J., and Thum, T. (2016). Non-coding RNAs in development and disease: Background, mechanisms, and therapeutic approaches. Physiol. Rev. 96, 1297–1325. doi:10.1152/physrev.00041.2015
Brinkman, A. B., Roelofsen, T., Pennings, S. W., Martens, J. H., Jenuwein, T., Stunnenberg, H. G., et al. (2006). Histone modification patterns associated with the human X chromosome. EMBO Rep. 7, 628–634. doi:10.1038/sj.embor.7400686
Bukowski, K., Kciuk, M., and Kontek, R. (2020). Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 21, E3233. doi:10.3390/ijms21093233
Cerase, A., Pintacuda, G., Tattermusch, A., and Avner, P. (2015). Xist localization and function: new insights from multiple levels. Genome Biol. 16, 166. doi:10.1186/s13059-015-0733-y
Chanmee, T., Ontong, P., Konno, K., and Itano, N. (2014). Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 6, 1670–1690. doi:10.3390/cancers6031670
Chen, D., Chen, T., Guo, Y., Wang, C., Dong, L., Lu, C., et al. (2020). Platycodin D (PD) regulates LncRNA-XIST/miR-335 axis to slow down bladder cancer progression in vitro and in vivo. Exp. Cell Res. 396, 112281. doi:10.1016/j.yexcr.2020.112281
Chen, L. L. (2016). Linking long noncoding RNA localization and function. Trends biochem. Sci. 41, 761–772. doi:10.1016/j.tibs.2016.07.003
Chen, S., and Shen, X. (2020). Long noncoding RNAs: functions and mechanisms in colon cancer. Mol. Cancer 19, 167. doi:10.1186/s12943-020-01287-2
Chen, S., Yang, M., Wang, C., Ouyang, Y., Chen, X., Bai, J., et al. (2021). Forkhead box D1 promotes EMT and chemoresistance by upregulating lncRNA CYTOR in oral squamous cell carcinoma. Cancer Lett. 503, 43–53. doi:10.1016/j.canlet.2020.11.046
Chen, Y. K., and Yen, Y. (2019). The ambivalent role of lncRNA xist in Carcinogenesis. Stem Cell Rev. Rep. 15, 314–323. doi:10.1007/s12015-019-9871-z
Cheng, Z., Luo, C., and Guo, Z. (2020). LncRNA-XIST/microRNA-126 sponge mediates cell proliferation and glucose metabolism through the IRS1/PI3K/Akt pathway in glioma. J. Cell. Biochem. 121, 2170–2183. doi:10.1002/jcb.29440
Cui, Q., Wang, J. Q., Assaraf, Y. G., Ren, L., Gupta, P., Wei, L., et al. (2018). Modulating ROS to overcome multidrug resistance in cancer. Drug resist. updat. 41, 1–25. doi:10.1016/j.drup.2018.11.001
D'Souza, S., and Addepalli, V. (2018). Preventive measures in oral cancer: An overview. Biomed. Pharmacother. 107, 72–80. doi:10.1016/j.biopha.2018.07.114
Dixon-McDougall, T., and Brown, C. (2016). The making of a Barr body: the mosaic of factors that eXIST on the mammalian inactive X chromosome. Biochem. Cell Biol. 94, 56–70. doi:10.1139/bcb-2015-0016
Doolittle, W. F. (2013). Is junk DNA bunk? A critique of encode. Proc. Natl. Acad. Sci. U. S. A. 110, 5294–5300. doi:10.1073/pnas.1221376110
Fang, J., Chen, T., Chadwick, B., Li, E., and Zhang, Y. (2004). Ring1b-mediated H2A ubiquitination associates with inactive X chromosomes and is involved in initiation of X inactivation. J. Biol. Chem. 279, 52812–52815. doi:10.1074/jbc.C400493200
Fang, X., Tang, Z., Zhang, H., and Quan, H. (2020). Long non-coding RNA DNM3OS/miR-204-5p/HIP1 axis modulates oral cancer cell viability and migration. J. Oral Pathol. Med. 49, 865–875. doi:10.1111/jop.13047
Fang, Z., Zhao, J., Xie, W., Sun, Q., Wang, H., Qiao, B., et al. (2017). LncRNA UCA1 promotes proliferation and cisplatin resistance of oral squamous cell carcinoma by sunppressing miR-184 expression. Cancer Med. 6, 2897–2908. doi:10.1002/cam4.1253
Fatica, A., and Bozzoni, I. (2014). Long non-coding RNAs: new players in cell differentiation and development. Nat. Rev. Genet. 15, 7–21. doi:10.1038/nrg3606
Gao, L., Wang, S., Meng, J., and Sun, Y. (2020). LncRNA LUADT1 promotes oral squamous cell carcinoma cell proliferation by regulating miR-34a/GAS1 Axis. Cancer Manag. Res. 12, 3401–3407. doi:10.2147/CMAR.S238830
Gao, S., Yu, J., Shan, Z., Zuo, L., Huang, W., Gan, L., et al. (2022). IncRNA XIST targets miR-124/JAG1 via CeRNA mechanism to facilitate the migration and proliferation of tongue squamous cell carcinoma. Clin. Lab. 68, 210325. doi:10.7754/Clin.Lab.2021.210325
Gendrel, A. V., and Heard, E. (2014). Noncoding RNAs and epigenetic mechanisms during X-chromosome inactivation. Annu. Rev. Cell Dev. Biol. 30, 561–580. doi:10.1146/annurev-cellbio-101512-122415
Ghafouri-Fard, S., Esmaeili, M., and Taheri, M. (2020). H19 lncRNA: Roles in tumorigenesis. Biomed. Pharmacother. 123, 109774. doi:10.1016/j.biopha.2019.109774
Gharat, S. A., Momin, M., and Bhavsar, C. (2016). Oral squamous cell carcinoma: Current treatment strategies and nanotechnology-based approaches for prevention and therapy. Crit. Rev. Ther. Drug Carr. Syst. 33, 363–400. doi:10.1615/CritRevTherDrugCarrierSyst.2016016272
Han, P., Li, J. W., Zhang, B. M., Lv, J. C., Li, Y. M., Gu, X. Y., et al. (2017). The lncRNA CRNDE promotes colorectal cancer cell proliferation and chemoresistance via miR-181a-5p-mediated regulation of Wnt/β-catenin signaling. Mol. Cancer 16, 9. doi:10.1186/s12943-017-0583-1
Hu, B., Shi, G., Li, Q., Li, W., and Zhou, H. (2019). Long noncoding RNA XIST participates in bladder cancer by downregulating p53 via binding to TET1. J. Cell. Biochem. 120, 6330–6338. doi:10.1002/jcb.27920
Huang, C., Wang, L., Song, H., and Wu, C. (2019). Interactive effects of AURKA polymorphisms with smoking on the susceptibility of oral cancer. Artif. Cells Nanomed. Biotechnol. 47, 2333–2337. doi:10.1080/21691401.2019.1601101
Jiang, S., and Dong, Y. (2017). Human papillomavirus and oral squamous cell carcinoma: A review of HPV-positive oral squamous cell carcinoma and possible strategies for future. Curr. Probl. Cancer 41, 323–327. doi:10.1016/j.currproblcancer.2017.02.006
Kou, N., Liu, S., Li, X., Li, W., Zhong, W., Gui, L., et al. (2019). H19 facilitates tongue squamous cell carcinoma migration and invasion via sponging miR-let-7. Oncol. Res. 27, 173–182. doi:10.3727/096504018X15202945197589
Lee, J. T., and Bartolomei, M. S. (2013). X-inactivation, imprinting, and long noncoding RNAs in health and disease. Cell 152, 1308–1323. doi:10.1016/j.cell.2013.02.016
Li, B., Jiang, J., Assaraf, Y. G., Xiao, H., Chen, Z. S., Huang, C., et al. (2020). Surmounting cancer drug resistance: New insights from the perspective of N(6)-methyladenosine RNA modification. Drug resist. updat. 53, 100720. doi:10.1016/j.drup.2020.100720
Li, P., Wang, L., Li, P., Hu, F., Cao, Y., Tang, D., et al. (2020). Silencing lncRNA XIST exhibits antiproliferative and proapoptotic effects on gastric cancer cells by up-regulating microRNA-132 and down-regulating PXN. Aging (Albany NY) 13, 14469–14481. doi:10.18632/aging.103635
Liang, Y., Song, X., Li, Y., Chen, B., Zhao, W., Wang, L., et al. (2020). LncRNA BCRT1 promotes breast cancer progression by targeting miR-1303/PTBP3 axis. Mol. Cancer 19, 85. doi:10.1186/s12943-020-01206-5
Liu, H., Deng, H., Zhao, Y., Li, C., and Liang, Y. (2018). LncRNA XIST/miR-34a axis modulates the cell proliferation and tumor growth of thyroid cancer through MET-PI3K-AKT signaling. J. Exp. Clin. Cancer Res. 37, 279. doi:10.1186/s13046-018-0950-9
Liu, J., Yao, L., Zhang, M., Jiang, J., Yang, M., Wang, Y., et al. (2019). Downregulation of LncRNA-XIST inhibited development of non-small cell lung cancer by activating miR-335/SOD2/ROS signal pathway mediated pyroptotic cell death. Aging (Albany NY) 11, 7830–7846. doi:10.18632/aging.102291
Liu, L., Zhan, Y., Huang, Y., and Huang, L. (2020). LncRNA FGD5-AS1 can be predicted as therapeutic target in oral cancer. J. Oral Pathol. Med. 49, 243–252. doi:10.1111/jop.12989
Liu, P. J., Pan, Y. H., Wang, D. W., and You, D. (2020). Long noncoding RNA XIST promotes cell proliferation of pancreatic cancer through miR137 and Notch1 pathway. Eur. Rev. Med. Pharmacol. Sci. 24, 12161–12170. doi:10.26355/eurrev_202012_24005
Loda, A., and Heard, E. (2019). Xist RNA in action: Past, present, and future. PLoS Genet. 15, e1008333. doi:10.1371/journal.pgen.1008333
Ma, S. Q., Wang, Y. C., Li, Y., Li, X. Y., Yang, J., Sheng, Y. M., et al. (2020). LncRNA XIST promotes proliferation and cisplatin resistance of oral squamous cell carcinoma by downregulating miR-27b-3p. J. Biol. Regul. Homeost. Agents 34, 1993–2001. doi:10.23812/20-222-A
Mercer, T. R., Dinger, M. E., and Mattick, J. S. (2009). Long non-coding RNAs: insights into functions. Nat. Rev. Genet. 10, 155–159. doi:10.1038/nrg2521
Mermoud, J. E., Popova, B., Peters, A. H., Jenuwein, T., and Brockdorff, N. (2002). Histone H3 lysine 9 methylation occurs rapidly at the onset of random X chromosome inactivation. Curr. Biol. 12, 247–251. doi:10.1016/s0960-9822(02)00660-7
Momen-Heravi, F., and Bala, S. (2018). Emerging role of non-coding RNA in oral cancer. Cell. Signal. 42, 134–143. doi:10.1016/j.cellsig.2017.10.009
Morris, K. V., and Mattick, J. S. (2014). The rise of regulatory RNA. Nat. Rev. Genet. 15, 423–437. doi:10.1038/nrg3722
Noguti, J., De Moura, C. F., De Jesus, G. P., Da Silva, V. H., Hossaka, T. A., Oshima, C. T., et al. (2012). Metastasis from oral cancer: an overview. Cancer Genomics Proteomics 9, 329–335.
Okura, M., Aikawa, T., Sawai, N. Y., Iida, S., and Kogo, M. (2009). Decision analysis and treatment threshold in a management for the N0 neck of the oral cavity carcinoma. Oral Oncol. 45, 908–911. doi:10.1016/j.oraloncology.2009.03.013
Pageau, G. J., Hall, L. L., Ganesan, S., Livingston, D. M., and Lawrence, J. B. (2007). The disappearing Barr body in breast and ovarian cancers. Nat. Rev. Cancer 7, 628–633. doi:10.1038/nrc2172
Payer, B., and Lee, J. T. (2008). X chromosome dosage compensation: how mammals keep the balance. Annu. Rev. Genet. 42, 733–772. doi:10.1146/annurev.genet.42.110807.091711
Plath, K., Fang, J., Mlynarczyk-Evans, S. K., Cao, R., Worringer, K. A., Wang, H., et al. (2003). Role of histone H3 lysine 27 methylation in X inactivation. Science 300, 131–135. doi:10.1126/science.1084274
Qian, X., Zhao, J., Yeung, P. Y., Zhang, Q. C., and Kwok, C. K. (2019). Revealing lncRNA structures and interactions by sequencing-based approaches. Trends biochem. Sci. 44, 33–52. doi:10.1016/j.tibs.2018.09.012
Raveh, E., Matouk, I. J., Gilon, M., and Hochberg, A. (2015). The H19 Long non-coding RNA in cancer initiation, progression and metastasis - a proposed unifying theory. Mol. Cancer 14, 184. doi:10.1186/s12943-015-0458-2
Sahakyan, A., Yang, Y., and Plath, K. (2018). The role of xist in X-chromosome dosage compensation. Trends Cell Biol. 28, 999–1013. doi:10.1016/j.tcb.2018.05.005
Salmena, L., Poliseno, L., Tay, Y., Kats, L., and Pandolfi, P. P. (2011). A ceRNA hypothesis: the rosetta stone of a hidden RNA language? Cell 146, 353–358. doi:10.1016/j.cell.2011.07.014
Schouten, P. C., Vollebergh, M. A., Opdam, M., Jonkers, M., Loden, M., Wesseling, J., et al. (2016). High XIST and low 53BP1 expression predict poor outcome after high-dose alkylating chemotherapy in patients with a BRCA1-like breast cancer. Mol. Cancer Ther. 15, 190–198. doi:10.1158/1535-7163.MCT-15-0470
Shi, J., Tan, S., Song, L., Song, L., and Wang, Y. (2020). LncRNA XIST knockdown suppresses the malignancy of human nasopharyngeal carcinoma through XIST/miRNA-148a-3p/ADAM17 pathway in vitro and in vivo. Biomed. Pharmacother. 121, 109620. doi:10.1016/j.biopha.2019.109620
Shih, J. W., Chiang, W. F., Wu, A. T. H., Wu, M. H., Wang, L. Y., Yu, Y. L., et al. (2017). Long noncoding RNA LncHIFCAR/MIR31HG is a HIF-1α co-activator driving oral cancer progression. Nat. Commun. 8, 15874. doi:10.1038/ncomms15874
Shiura, H., and Abe, K. (2019). Xist/Tsix expression dynamics during mouse peri-implantation development revealed by whole-mount 3D RNA-FISH. Sci. Rep. 9, 3637. doi:10.1038/s41598-019-38807-0
Soudyab, M., Iranpour, M., and Ghafouri-Fard, S. (2016). The role of long non-coding RNAs in breast cancer. Arch. Iran. Med. 19, 508–517.
Su, S. Y., Chen, W. T., Chiang, C. J., Yang, Y. W., and Lee, W. C. (2020). Oral cancer incidence rates from 1997 to 2016 among men in Taiwan: Association between birth cohort trends and betel nut consumption. Oral Oncol. 107, 104798. doi:10.1016/j.oraloncology.2020.104798
Sun, N., Zhang, G., and Liu, Y. (2018). Long non-coding RNA XIST sponges miR-34a to promotes colon cancer progression via Wnt/β-catenin signaling pathway. Gene 665, 141–148. doi:10.1016/j.gene.2018.04.014
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN Estimates of incidence and Mortality Worldwide for 36 cancers in 185 Countries. Ca. A Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Sur, S., Nakanishi, H., Steele, R., Zhang, D., Varvares, M. A., Ray, R. B., et al. (2020). Long non-coding RNA ELDR enhances oral cancer growth by promoting ILF3-cyclin E1 signaling. EMBO Rep. 21, e51042. doi:10.15252/embr.202051042
Tao, B., Wang, D., Yang, S., Liu, Y., Wu, H., Li, Z., et al. (2021). Cucurbitacin B inhibits cell proliferation by regulating X-inactive specific transcript expression in tongue cancer. Front. Oncol. 11, 651648. doi:10.3389/fonc.2021.651648
Tao, D., Zhang, Z., Liu, X., Zhang, Z., Fu, Y., Zhang, P., et al. (2020). LncRNA HOTAIR promotes the invasion and metastasis of oral squamous cell carcinoma through metastasis-associated gene 2. Mol. Carcinog. 59, 353–364. doi:10.1002/mc.23159
Varoni, E. M., Lodi, G., and Iriti, M. (2015). Ethanol versus phytochemicals in wine: Oral cancer risk in a light drinking perspective. Int. J. Mol. Sci. 16, 17029–17047. doi:10.3390/ijms160817029
Villanueva, A., Portela, A., Sayols, S., Battiston, C., Hoshida, Y., Mendez-Gonzalez, J., et al. (2015). DNA methylation-based prognosis and epidrivers in hepatocellular carcinoma. Hepatology 61, 1945–1956. doi:10.1002/hep.27732
Wang, F., Shin, J., Shea, J. M., Yu, J., Boskovic, A., Byron, M., et al. (2016). Regulation of X-linked gene expression during early mouse development by Rlim. Elife 5, e19127. doi:10.7554/eLife.19127
Wang, J., Cai, H., Dai, Z., and Wang, G. (2019). Down-regulation of lncRNA XIST inhibits cell proliferation via regulating miR-744/RING1 axis in non-small cell lung cancer. Clin. Sci. 133, 1567–1579. doi:10.1042/CS20190519
Wang, J., Fu, Z., Wang, M., Lu, J., Yang, H., Lu, H., et al. (2021). Knockdown of XIST attenuates Cerebral Ischemia/reperfusion injury through regulation of miR-362/ROCK2 Axis. Neurochem. Res. 46, 2167–2180. doi:10.1007/s11064-021-03354-6
Wang, J., Xie, S., Yang, J., Xiong, H., Jia, Y., Zhou, Y., et al. (2019). The long noncoding RNA H19 promotes tamoxifen resistance in breast cancer via autophagy. J. Hematol. Oncol. 12, 81. doi:10.1186/s13045-019-0747-0
Wutz, A. (2011). Gene silencing in X-chromosome inactivation: advances in understanding facultative heterochromatin formation. Nat. Rev. Genet. 12, 542–553. doi:10.1038/nrg3035
Xiong, H. G., Li, H., Xiao, Y., Yang, Q. C., Yang, L. L., Chen, L., et al. (2019). Long noncoding RNA MYOSLID promotes invasion and metastasis by modulating the partial epithelial-mesenchymal transition program in head and neck squamous cell carcinoma. J. Exp. Clin. Cancer Res. 38, 278. doi:10.1186/s13046-019-1254-4
Yang, X., Zhang, S., He, C., Xue, P., Zhang, L., He, Z., et al. (2020). METTL14 suppresses proliferation and metastasis of colorectal cancer by down-regulating oncogenic long non-coding RNA XIST. Mol. Cancer 19, 46. doi:10.1186/s12943-020-1146-4
Yang, Y., Chen, D., Liu, H., and Yang, K. (2019). Increased expression of lncRNA CASC9 promotes tumor progression by suppressing autophagy-mediated cell apoptosis via the AKT/mTOR pathway in oral squamous cell carcinoma. Cell Death Dis. 10, 41. doi:10.1038/s41419-018-1280-8
Zhang, D. M., Lin, Z. Y., Yang, Z. H., Wang, Y. Y., Wan, D., Zhong, J. L., et al. (2017). IncRNA H19 promotes tongue squamous cell carcinoma progression through β-catenin/GSK3β/EMT signaling via association with EZH2. Am. J. Transl. Res. 9, 3474–3486.
Zhang, Q., Chen, B., Liu, P., and Yang, J. (2018). XIST promotes gastric cancer (GC) progression through TGF-β1 via targeting miR-185.. J. Cell. Biochem. 119, 2787–2796. doi:10.1002/jcb.26447
Zhang, S., Ma, H., Zhang, D., Xie, S., Wang, W., Li, Q., et al. (2018). LncRNA KCNQ1OT1 regulates proliferation and cisplatin resistance in tongue cancer via miR-211-5p mediated Ezrin/Fak/Src signaling. Cell Death Dis. 9, 742. doi:10.1038/s41419-018-0793-5
Zhang, Y., Huang, W., Yuan, Y., Li, J., Wu, J., Yu, J., et al. (2020). Long non-coding RNA H19 promotes colorectal cancer metastasis via binding to hnRNPA2B1. J. Exp. Clin. Cancer Res. 39, 141. doi:10.1186/s13046-020-01619-6
Zhao, Y., Yu, Z., Ma, R., Zhang, Y., Zhao, L., Yan, Y., et al. (2021). lncRNA-Xist/miR-101-3p/KLF6/C/EBPα axis promotes TAM polarization to regulate cancer cell proliferation and migration. Mol. Ther. Nucleic Acids 23, 536–551. doi:10.1016/j.omtn.2020.12.005
Zhou, R. S., Zhang, E. X., Sun, Q. F., Ye, Z. J., Liu, J. W., Zhou, D. H., et al. (2019). Integrated analysis of lncRNA-miRNA-mRNA ceRNA network in squamous cell carcinoma of tongue. BMC Cancer 19, 779. doi:10.1186/s12885-019-5983-8
Zhou, X., Liu, S., Cai, G., Kong, L., Zhang, T., Ren, Y., et al. (2015). Long non coding RNA MALAT1 promotes tumor growth and metastasis by inducing epithelial-mesenchymal transition in oral squamous cell carcinoma. Sci. Rep. 5, 15972. doi:10.1038/srep15972
Zhu, G., Wang, S., Chen, J., Wang, Z., Liang, X., Wang, X., et al. (2017). Long noncoding RNA HAS2-AS1 mediates hypoxia-induced invasiveness of oral squamous cell carcinoma. Mol. Carcinog. 56, 2210–2222. doi:10.1002/mc.22674
Zhu, H., Zheng, T., Yu, J., Zhou, L., and Wang, L. (2018). LncRNA XIST accelerates cervical cancer progression via upregulating Fus through competitively binding with miR-200a. Biomed. Pharmacother. 105, 789–797. doi:10.1016/j.biopha.2018.05.053
Keywords: gene expression, long non-coding RNA, oral cancer, pathogenesis, XIST
Citation: Liu H, Wang D, Kan S, Hao M, Chang L, Lu P, Liu Y, Jin Y and Liu W (2022) The role of lncRNAs and XIST in oral cancer. Front. Cell Dev. Biol. 10:826650. doi: 10.3389/fcell.2022.826650
Received: 01 December 2021; Accepted: 05 July 2022;
Published: 10 August 2022.
Edited by:
Jian-ye Zhang, Guangzhou Medical University, ChinaReviewed by:
A. Thirumal Raj, Sri Venkateswara Dental College, IndiaGokul S, Rutgers, United States
Prashanth Panta, Malla Reddy Institute of Dental Sciences, India
Copyright © 2022 Liu, Wang, Kan, Hao, Chang, Lu, Liu, Jin and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiwei Liu, bGl1d2Vpd0BqbHUuZWR1LmNu
†These authors have contributed equally to this work
 Huimin Liu
Huimin Liu Dongxu Wang
Dongxu Wang Shaoning Kan1
Shaoning Kan1 Ming Hao
Ming Hao Lu Chang
Lu Chang Yangyang Liu
Yangyang Liu Ye Jin
Ye Jin Weiwei Liu
Weiwei Liu