Abstract
Over the last 25 years, cloned animals have been produced by transferring somatic cell nuclei into enucleated oocytes (SCNT) in more than 20 mammalian species. Among domestic animals, pigs are likely the leading species in the number of clones produced by SCNT. The greater interest in pig cloning has two main reasons, its relevance for food production and as its use as a suitable model in biomedical applications. Recognized progress in animal cloning has been attained over time, but the overall efficiency of SCNT in pigs remains very low, based on the rate of healthy, live born piglets following embryo transfer. Accumulating evidence from studies in mice and other species indicate that new strategies for promoting chromatin and epigenetic reprogramming may represent the beginning of a new era for pig cloning.
Introduction
The transfer of somatic cell nuclei to enucleated oocytes has proved that differentiated cells can be reverted to a totipotent state (Wilmut et al., 1997), and has helped to attain a better understanding of cell differentiation and reprogramming through chromatin and epigenetic mechanisms (Matoba and Zhang, 2018; de Macedo et al., 2021). The SCNT technology has also been applied to clone animals for different purposes, including the preservation of endangered species, replication of companion and production animals, and creation of transgenic and gene-edited animals for agricultural and biomedical purposes (Bordignon, 2019). Pigs are important for both food production and biomedical research (Gutierrez et al., 2015). Thus, pigs with unique traits and genomes of agricultural and biomedical importance have been created by SCNT, nonetheless, with low and variable efficiency. In addition of ameliorating technical aspects (e.g., oocyte maturation, enucleation, activation, embryo culture, cell fusion, cell cycle coordination), researchers have identified some roadblock mechanisms for cell reprogramming and tested new approaches to facilitate chromatin and epigenetic remodeling in SCNT embryos (Figure 1). These approaches may not only facilitate the erasure of repressive epigenetic marks that are incompatible with cell reprogramming but may also enhance the acquisition or reestablishment of specific marks required for normal embryo development and success of SCNT. This article highlights the recent progress in understanding and improving SCNT efficiency in pigs.
FIGURE 1
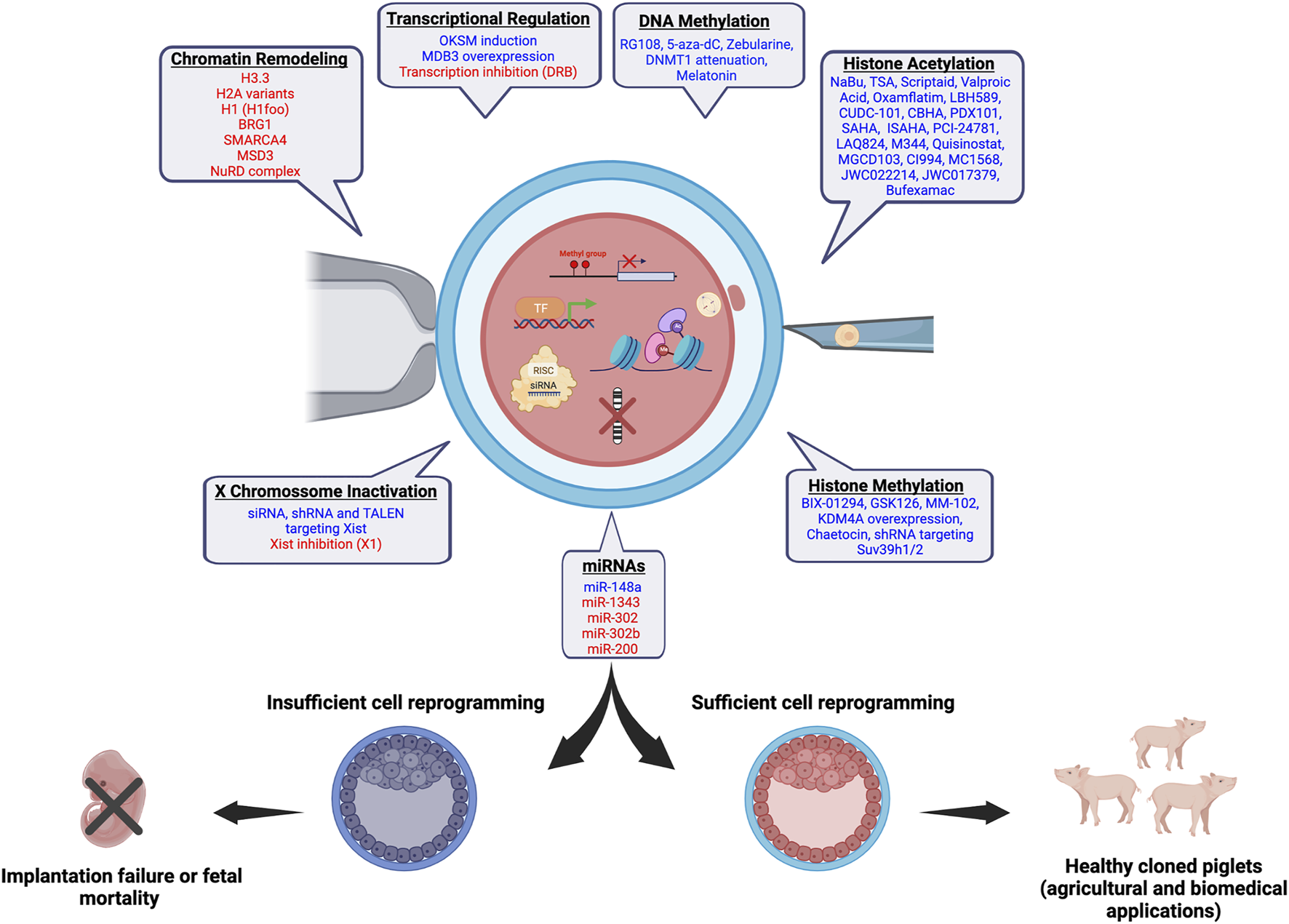
Chromatin and epigenetic reprogramming impacting SCNT efficiency in pigs. In blue, chromatin/epigenetic modifiers that have been previously shown to play a role on development of pig SCNT embryos and cloning efficiency are listed. In red, modifiers that are believed to have a beneficial effect on the development of pig SCNT embryos based on studies in other species are listed. The figure was created with BioRender.com.
Chromatin Remodeling
Successful development of SCNT embryos depends on the reestablishment of cell totipotency, which relies on changes in chromatin structure, epigenetic marks, and transcriptional profile. Nucleosomes’ positioning and occupancy affect chromatin function by either facilitating or restricting DNA access to transcription factors (TFs) and other regulators, which impacts both the reacquisition of cell totipotency following SCNT and cloning efficiency (Matoba and Zhang, 2018). Nucleosome occupancy seems to be rapidly, but likely insufficiently, reprogrammed in SCNT embryos. In pig SCNT embryos, nucleosome occupancy 10 h after nuclear transfer was lower in promoter sequences but higher in coding sequences, compared to nuclear donor cells, indicating a reprogramming process (Tao et al., 2017). Chromatin accessibility in mouse SCNT embryos was reprogrammed to a similar state of fertilized zygotes within 12 h, except for reprogramming resistant regions enriched for histone three lysine nine trimethylation (H3K9me3) (Djekidel et al., 2018).
The incorporation of maternally-derived histones facilitates chromatin remodeling in SCNT embryos. The histone H3 is replaced by the maternally-derived H3.3 in bovine (Wang et al., 2020) and mouse (Wen et al., 2014a) SCNT embryos. This switch is involved in the activation of pluripotency genes such as the Octamer-binding transcript factor (Oct-4 or Pou5f1) and Nanog homeobox (Nanog) (Wen et al., 2014b), and reduction of epigenetic barriers for cell reprogramming, such as H3K27me3 and H3K9me3 (Wen et al., 2014b; Wang et al., 2020). In murine SCNT embryos, H2A and H2A.Z were replaced by the H2A.X variant (Nashun et al., 2011). H2A.X participates in the DNA damage response, and has been negatively correlated with embryo quality (Bohrer et al., 2013). The macro H2A variant, which is involved in the repression of pluripotency genes and considered a barrier for cell reprogramming (Gaspar-Maia et al., 2013), was also stripped from the chromatin after SCNT in mice (Chang et al., 2010). In addition, somatic variants of the linker histone H1 are reprogrammed after nuclear transfer (Bordignon et al., 1999), and are replaced by the oocyte-specific variant H1foo in SCNT embryos (Teranishi et al., 2004; Yun et al., 2012). Although H1foo′s role in chromatin reprogramming in SCNT embryos remains poorly understood, induction of H1foo expression along with the pluripotency factors Oct-4, SRY-Box Transcription Factor 2 (Sox2) and Kruppel Like Factor 4 (Klf4) improved the efficiency of mouse induced pluripotent cells (iPSCs) production (Kunitomi et al., 2016).
Chromatin relaxation, which is induced by remodeling complexes such as the transcription activator Brahma-related gene 1 (BRG1 or SMARCA4), a member of the SWItch/sucrose nonfermentable (SWI/SNF) complex, represents another important feature for attaining cell totipotency and normal embryo development (Hansis et al., 2004; Egli and Eggan, 2010; Glanzner et al., 2017). The methyl-CpG-binding domain protein 3 (MBD3), a core component of the nucleosome remodeling and deacetylation (NuRD) complex, is also essential for embryo development (Hendrich et al., 2001). The NuRD complex regulates nucleosome occupancy and recruitment of TFs (Bornelov et al., 2018). Lower MBD3 expression was detected in pig SCNT embryos compared to control embryos, and its overexpression in SCNT embryos improved blastocyst formation and total cell number (Wang et al., 2019) (Table 1).
TABLE 1
| Chromatin/Epigenetic target | Modifier | Concentration/Duration | Target cell | Main outcomesa | References |
|---|---|---|---|---|---|
| Transcriptional regulation | OKSM ectopic expression | After OKSM induction iPSc-like cells were used for SCNT | Fibroblasts, Mesenchymal cells | Increased blastocyst rates and embryo cell numbers | Song et al. (2014b); Kim et al. (2019) |
| DNA methylation/Chromatin function | MBD3 overexpression | 10 ng/μl (∼10 pL/embryo) of the pcDNA3.1 plasmid expressing pig MBD3 | Embryos | Increased blastocyst rates (from 7.4 to 20.3%) and embryo cell numbers, decreased DNA methylation, and increased NANOG, OCT-4 and LINE1 expression | Wang et al. (2019) |
| DNA methylation | RG108 | 5 nM-20 µM/1–48 hb | Nuclear donor cells (fibroblasts) | Increased blastocyst rates, and expression of DNA and histone methylation regulators | Diao et al., (2013); Zhai et al. (2018) |
| DNA methylation | Zebularine | 5 nM/1–24 hb | Embryos and nuclear donor cells (fibroblasts) | Increased blastocyst rates, lowered global DNA methylation, and improved expression of epigenetic regulators and pluripotency genes | Diao et al. (2013); Taweechaipaisankul et al. (2019b) |
| DNA methylation | 5-aza-dC | 5 nM–0.5 µM/1–96 hb | Embryos and nuclear donor cells (fibroblasts) | Increased blastocyst rates and embryo cell numbers, improved DNA methylation levels and expression of epigenetic regulators and pluripotency genes | Diao et al. (2013); Huan et al. (2013); Kumar et al. (2013); Huan et al. (2015a); Huan et al. (2015b) |
| DNA methylation | siRNA targeting DNMT1 | 50 nM in cells and 20 µM in embryos | Embryos and nuclear donor cells (fibroblasts) | Increased blastocyst rates, improved DNA methylation patterns, and increased expression of OCT-4, THY1 and pluripotency genes | Huan et al., (2015b); Song et al., (2017) |
| DNA methylation | Melatonin | 100 nM/During IVC | Embryos | Increased blastocyst rates (from ∼20 to ∼31%) and embryo cell numbers, reduced apoptosis, improved DNA methylation of OCT-4, H19, IGF2 and THY1, and upregulated expression of EGA and pluripotency genes | Qu et al. (2020) |
| Histone acetylation | Sodium Butyrate (NaBu) | 1 mM/4–12 hb for embryos 1 mM/96 h for cells | Embryos or nuclear donor cells (fibroblasts)b | Increased blastocyst rates and embryo cell numbers, regulated DNMTs mRNA levels in embryos. In donor cells: increased apoptosis, affected cell cycle, and deregulated gene expression (2 mM or higher) | Mohana Kumar et al. (2007); Das et al. (2010); Liu et al. (2012); Diao et al. (2013); Kumar et al. (2013) |
| Histone acetylation | Trichostatin A (TSA) | 5–50 nM/10–24 hb | Embryos or nuclear donor cells (fibroblasts)b | Increased blastocyst rates and embryo cell numbers, upregulated pluripotency and developmental related genes, decreased DNMT1 mRNA and DNA methylation, reduce apoptosis, produced pregnancies to term and improved cloning efficiency | Zhang et al. (2007); Li et al. (2008); Cervera et al. (2009); Yamanaka et al. (2009); Martinez-Diaz et al. (2010); Zhao et al. (2010); Chawalit et al. (2012); Cong et al. (2013); Diao et al, (2013); Luo et al. (2015); Jeong et al. (2021) |
| Histone acetylation | Scriptaid | 500 nM/12–15 h for embryos | Embryos or nuclear donor cells (fibroblasts)b | Increased blastocyst rates and embryo cell numbers, reduced embryo apoptosis, DNA methylation and DNMT1 expression, upregulated pluripotency and developmental related genes, increased miR-152 expression, improved the derivation of ES-like cells, produced pregnancies to term and improved cloning efficiency | Zhao et al. (2009); Zhao et al. (2010); Diao et al. (2013); Liang et al. (2015); Whitworth et al. (2015); Lee et al. (2016); Rissi et al. (2016); Zhang et al. (2017b); No et al. (2018); Ongaratto et al. (2020) |
| Histone acetylation | Valproic Acid | 1–8 mM/14–24 hb | Embryos | Increased blastocyst rates and embryo cell numbers, increased OCT-4 mRNA, produced pregnancies to term and improved cloning efficiency | Miyoshi et al. (2010); Huang et al. (2011); Kim et al. (2011); Miyoshi et al. (2016); Sun et al. (2017) |
| Histone acetylation | Oxamflatin | 150 nM or 1 μM/9 or 14–16 hb | Embryos | Increased blastocyst rates and embryo cell numbers, decreased mRNA levels of DNMT1 and DNA methylation, upregulated OCT-4 mRNA, produced pregnancies (not to term) | Park et al. (2012b); Hou et al. (2014); Mao et al. (2015) |
| Histone acetylation | LBH589 | 50 nM/24 h | Embryos | Increased blastocyst rates (from 10.1 to 32.5%) and embryo cell numbers, produced pregnancies (not to term) | Jin et al. (2013) |
| Histone acetylation | CUDC-101 | 1 μM/24 h | Embryos | Increased blastocyst rates (from 9.5 to 19%), produced pregnancies (not to term) | Jin et al. (2014a) |
| Histone acetylation | CBHA | 2 μM/24 h | Embryos | Increased blastocyst rates (from 12.7 to 26.5%), and mRNA levels of OCT-4, CDX2 and IGF2, produce pregnancies to term, but did not improve cloning efficiency | Song et al. (2014a) |
| Histone acetylation | PDX101 | 0.5 μM/24 h | Embryos | Increased blastocyst rates (from 10.6 to 25.7%) and embryo cell numbers, produced pregnancies (not to term) | Jin et al. (2015) |
| Histone acetylation | SAHA | 7.5–10 μM/6–16 hb for embryos 1.5–6 μM/24–72 hb for cells | Embryos or nuclear donor cells (fibroblasts)b | Embryo treatment: increased blastocyst rates, upregulated lysosome, steroid biosynthesis and glycosaminoglycan degradation, downregulated KEGG pathways, modulated OCT-4 and HDAC1 mRNA levels, and produced pregnancies to term. Cell treatment: increased blastocyst rates, and improved gene expression in SCNT embryos | Whitworth et al. (2015); Sun et al. (2017); Kim et al. (2018); Sun et al. (2020) |
| Histone acetylation | ISAHA | 1 μM/14–16 h | Embryos | Increased embryo cell numbers, upregulated some lysosome, steroid biosynthesis and terpenoid backbone biosynthesis, produced 75% pregnancy rates with development to term | Whitworth et al. (2015) |
| Histone acetylation | PCI-24781 | 0.5 nM/6 h | Embryos | Increased blastocyst rates (from 10.2 to 25.2%), decreased apoptosis, produced pregnancies (not to term) | Jin et al. (2016) |
| Histone acetylation | LAQ824 | 100 nM/24 h | Embryos | Increased blastocyst rates (from 14.2 to 29.9%), embryo cell numbers, decreased DNA methylation, improved mRNA levels of developmental genes | Jin et al. (2017a) |
| Histone acetylation | M344 | 5 μM/6 h | Embryos | Increased blastocyst rates (from 10.9 to 25.1%), reduced apoptosis, produced pregnancies to term | Jin et al. (2017b) |
| Histone acetylation | Quisinostat | 10–100 nM/24 hb | Embryos | Increased blastocyst rates, decreased DNA methylation, improved mRNA levels of pluripotency/developmental genes, produced pregnancies to term | Jin et al. (2017c); Taweechaipaisankul et al. (2019a) |
| Histone acetylation | MGCD0103 | 0.2 μM/6 h | Embryos | Increased blastocyst rates (from 10.5 to 21.2%), produced pregnancies (not to term) | Jin et al. (2017d) |
| Histone acetylation | CI994 | 10 μM/24 h | Embryos | Increased blastocyst rates (from 11.4 to 21.9%), decreased apoptosis, increased OCT-4 and SOX2 expression | Jin et al. (2018) |
| Histone acetylation | MC1568 | 10 μM/12 h | Embryos | Increased blastocyst rates (from 20.6 to 33.2%) and embryo cell numbers, increased mRNA levels of OCT-4, SOX2, NANOG and CDX2 | Wang et al. (2018) |
| Histone acetylation | JWC022214 | 2 μM/22–24 h | Embryos | Increased blastocyst rates (from 36.9 to 59.7%) | Siriboon et al. (2018) |
| Histone acetylation | JWC017379 | 2–4 μM/22–24 h | Embryos | Increased blastocyst rates (from 50 to 79.4%) and primary outgrowths of SCNT-derived ES-like cells | Siriboon et al. (2018) |
| Histone acetylation | Bufexamac | 20 μM/oocyte IVM | Host oocytes during maturation | Increased blastocyst rates (from 16.3 to 25%) and mRNA levels of OCT-4 and CDX2 | Sun et al. (2021) |
| Histone acetylation/DNA methylation | Combination of RG108 and Scriptaid | 200 µM RG108 and 100 nM Scriptaid/17–19 h | Embryo | Increased blastocyst rates (from 19.1 to 29.3%) and embryo cell numbers, rescued methylation patterns of H19 and XIST, increased NANOG expression | Xu et al. (2013) |
| Histone acetylation/DNA methylation | Combination of 5-aza-dC and TSA | 50 nM–0.025 μmol/L (TSA); 2.5 nM–0.01 μmol/L (5-aza-dC)/1–24 h (TSA); 1–72 h (5-aza-dC)b | Nuclear donor cells (fibroblasts) | Increased blastocyst rate, decreased DNA methylation levels | Diao et al. (2013); Ning et al. (2013) |
| Histone methylation | BIX-01294 (G9A inhibitor) | 50 nM/14–16 h | Embryos | Increased blastocysts rates (from 16.4 to 23.2%), modulated mRNA levels of pluripotency and epigenetic related genes, produced pregnancies to term and improved cloning efficiency | Huang et al. (2016) |
| Histone methylation | GSK126 (EZH2 inhibitor) | 0.75 µM/48 h in cells and 0.1 µM/24 h in embryos | Embryos and nuclear donor cells (fibroblasts) | Increased blastocysts rates (from 20.2 to 31.3%) | Xie et al. (2016) |
| Histone methylation | MM-102 (H3K4 HMT inhibitor) | 75 µM/72 h | Embryos | Increased blastocysts rates (from 8.9 to 25.7%) and embryo cell numbers, modulated DNA methylation, and mRNA levels of pluripotency and epigenetic related genes | Zhang et al. (2018b) |
| Histone methylation | KDM4A overexpression | In vitro synthetized mRNA coding KDM4A (500 ng/μL) injected (10 pL) 5 h post-activation | Embryos | Increased blastocysts rates (from 20.9 to 32.2%) and embryo cell numbers | Weng et al. (2020) |
| Histone methylation | Chaetocin | 0.5–10 nM/6 or 24 h or during 6 h in the 4-cell stageb | Embryos | Increased blastocysts rates, hatching rates and embryo cell numbers, upregulated pluripotency genes, downregulated DNMTs, reduced DNA methylation and apoptosis, and regulated developmental related genes | Jeong et al. (2020); Weng et al. (2020); Jeong et al. (2021) |
| Histone methylation | shRNA targeting SUV39H1/2 | MOI-600 of a lentivirus containing shRNA targeting SUV39H1/2. SCNT performed after 72 h post-incubation | Nuclear donor cells (fibroblasts) | Increased blastocyst rates (from 15.5 to 29.9%) and embryo cell numbers. In the donor cells: modulated mRNA levels of cell cycle-related genes and epigenetic modifiers | Cao et al. (2021) |
| Histone methylation/Histone acetylation | BIX-01294/Scriptaid | 25 nM BIX-01294 + 250 nM Scriptaid/14–16 h | Embryos | Increased blastocysts rates (from 14 to 23.7%) | Huang et al. (2016) |
| Histone methylation/Histone acetylation | BIX-01294/TSA | 1 µM BIX-01294 + 50 nM TSA/24 h | Embryos | Increased blastocysts rates (from ∼20 to ∼44%) compared to control but not to TSA alone, decreased global DNA methylation in the trophectoderm | Cao et al. (2017) |
| Histone methylation/Histone acetylation | Chaetocin/TSA | 0.5 nM Chaetocin +50 nM TSA/24 h | Embryos | Increased blastocyst rates (from ∼21 to ∼34%), hatching rates, and embryo cell numbers, reduced apoptosis and DNA methylation, modulated EGA and imprinting related genes | Jeong et al. (2021) |
| miRNA | miR-148a overexpression | MOI-50 of a lentivirus containing miRNA-148a sequence were transfected in cells/SCNT was performed with puromycin selected cells | Nuclear donor cells (fibroblasts) | Increased blastocysts rates (from 14.3 to 20.8%), hatching rates, and embryo cell numbers, reduced DNA methylation, increased H3K9 acetylation levels in 2-cell embryos, increased OCT-4 and NANOG mRNA. | Wang et al. (2017) |
| X chromosome inactivation | siRNA targeting XIST | 10 pL of 5 μM; injected 6–7 h post-activation/Effective KD until 16 cell-stage | Embryos | Increased embryo cell numbers, produced pregnancies to term and improved cloning efficiency | Zeng et al. (2016) |
| X chromosome inactivation | TALEN targeting XIST | 20 μg of DNA template and 10 μg of XIST TALEN electroporated into the porcine fetal fibroblasts | Nuclear donor cells (fibroblasts) | Increased blastocyst rates (from 25.4 to 36.4%) and embryo cell numbers, produced pregnancies to term and improved cloning efficiency | Ruan et al. (2018) |
| X chromosome inactivation | shRNA targeting XIST | 10 pL of 5 ng/μL plasmid; injected into the cytoplasm of each blastomere of 2 cell-embryo | Embryos | Increased blastocyst rates (from 15.5 to 28.7%) and embryo cell numbers, increased expression of X-linked genes | Yang et al. (2019) |
Impact of different modifiers of chromatin or epigenetic marks on development of SCNT embryos and pig cloning efficiency.
Main outcomes considering results observed in all the publications. The same outcomes are not necessarily observed by all researchers or reported in all publications.
Different concentrations, periods of incubation or target cells (nuclear donor cells or embryos) have been used in the different publications.
Transcriptional Regulation
Proper regulation of TFs is crucial for reprograming cell totipotency and successful development of SCNT embryos (Table 1). Overexpression of the iPSC inducing factors OCT-4, SOX2, KLF4 and c-MYC (OSKM) in porcine cells prior to nuclear transfer improved blastocyst rates and quality of SCNT embryos (Song Z. et al., 2014; Kim et al., 2019). The co-expression of OKSM and the estrogen-related receptor B (ESRRB), which is abundantly expressed in pig embryos (Yu et al., 2021), improved iPSCs production by regulating pluripotency factors (Shi et al., 2020). In addition, pig iPSCs overexpressing ESRRB showed higher potential for trophectoderm differentiation when injected into 8-cell stage embryos (Yu et al., 2021).
The impact of OCT-4 on development of SCNT embryos has been investigated in multiple different species. In pigs, expression of OCT-4 and OCT-4 related genes, such as Calcium binding and coiled-coil domain 2 (NDP52l1), and Developmental pluripotency associated 2, three and 5 (DPPA2,3,5), was lower in SCNT than control embryos (Lee et al., 2006). The Double homeobox (Dux) transcription factor and its upstream regulators Dppa2 and four have been identified as major regulators of embryonic genome activation (EGA) (Eckersley-Maslin et al., 2019). Overexpression of Dux in mouse SCNT embryos improved development and normalized EGA transcripts, comparable to levels of control embryos produced by fertilization (Yang et al., 2020). Despite of these relevant findings in other species, the impact of manipulating EGA regulators has not been studied in porcine SCNT embryos.
Proper reprogramming to totipotency in SCNT embryos can be hampered by both lack of gene activation and failure to inactivate the transcriptional memory of somatic nuclei following nuclear transfer (Ng and Gurdon, 2005; Matoba et al., 2014). The transcriptional memory of somatic cells was more efficiently reprogrammed in SCNT embryos that cleaved early and produced higher blastocyst rates than late cleaving embryos (Liu et al., 2020). In addition, attenuation of the transcriptional memory for 15 h after nuclear transfer, using the inhibitor of RNA polymerase II, 5,6-Dichlorobenzimidazole 1-β-d-ribofuranoside (DRB), improved gene expression in bovine SCNT embryos and increased blastocyst cell numbers (Rissi et al., 2018). However, more studies are needed to determine the impact of transcriptional inhibition following SCNT on cloning efficiency in pigs.
DNA Methylation
DNA methylation is usually associated with transcriptional silencing. Substantial DNA demethylation occurs during early embryo development but remethylation happens at later stages (Reik et al., 2001; Ivanova et al., 2020). In SCNT embryos, timely DNA demethylation and remethylation should occur to enable proper gene expression and cell reprogramming. However, donor cells are usually highly methylated and demethylation seems to be incomplete in SCNT embryos (Cao et al., 2020). In mice, several genes important for early development, including Dppa2/4, Oocyte-specific homeobox 6 (Obox6) and TEA domain transcription factor 4 (Tead4), failed to activate in SCNT embryos due to abnormal DNA methylation (Cao et al., 2020). In pigs, 4-cell SCNT embryos presented higher DNA methylation levels and expression of the DNA methyl transferase 1 (DNMT1) than control embryos (Deshmukh et al., 2011; Song et al., 2017). Attenuation of DNMT1 in nuclear donor cells 36 h prior to nuclear transfer decreased DNA methylation levels of OCT-4, NANOG and SOX2, increased their expression at EGA and blastocyst stages, and improved blastocyst rates of pig SCNT embryos (Song et al., 2017). A similar effect was observed by attenuating DNMT1 expression after nuclear transfer, which normalized the methylation status of OCT-4 and the Thy-1 cell surface antigen (THY1), a fibroblast marker, promoting OCT-4 activation and THY1 silencing in pig SCNT embryos (Huan et al., 2015b). Overexpression of MBD3 in pig SCNT embryos corrected methylation levels of NANOG, OCT-4 and long interspersed nuclear elements (LINEs), and increased blastocyst rates and quality (Wang et al., 2019).
Treatment of nuclear donor cells with 5-aza-2′-deoxycytidine (5-aza-dC), which incorporates onto DNA during replication leading to hypomethylation by inhibiting Dnmt1 action (Stresemann and Lyko, 2008), has been another strategy tested to improve SCNT efficiency. This approach increased the development and quality of pig SCNT embryos (Diao et al., 2013; Kumar et al., 2013), and enhanced transcript levels of DNMT1, two and 3 (Kumar et al., 2013; Huan et al., 2015b). Treatment of pig SCNT embryos with 5-aza-dC for 24 h after nuclear transfer increased embryo development, which was associated with a decrease in methylation levels of NANOG and an increase in the expression of NANOG and SOX2 (Huan et al., 2015a). Treatment with other inhibitors of DNA methyltransferases, including Zeburaline and RG108, also increased development of pig SCNT embryos (Zhai et al., 2018; Taweechaipaisankul et al., 2019b). A combined treatment of RG108 with Scriptaid, a histone deacetylase inhibitor, rescued defective methylation patters in the imprinted gene H19/insulin-like growth factor 2 (IGF2), and increased development of pig SCNT embryos (Xu et al., 2013). Moreover, melatonin was shown to favor DNA methylation reprogramming, and expression of imprinted, pluripotency and EGA related genes in pig SCNT embryos (Qu et al., 2020) (Table 1). Overexpression of the Ten-eleven translocation 3 (TET3) enhanced SCNT efficiency in bovine (Zhang et al., 2020), and goats (Han et al., 2018), however, this has not been investigated in pigs yet.
Histone Acetylation
Pig oocytes have high levels of acetylated histones H3 and H4, especially at the germinal vesicle (GV) stage (Endo et al., 2005). Histone acetylation levels are controlled by acetyltransferases (HAT) and deacetylases (HDAC) enzymes (Endo et al., 2011). Pig oocytes express several HDACs, which regulate histone acetylation levels during oocyte maturation (Endo et al., 2008). The acetylation status of the lysine nine in the histone 3 (H3K9ac) has been proposed as a biomarker for embryo outcome, since it can be altered by culture conditions and it affects EGA and embryo development (Rollo et al., 2017). Normal maturation of pig oocytes is affected by HDACs inhibition (Jin Y.-X. et al., 2014; Huang et al., 2021).
There is consensus, based on studies conducted in different species, that development of SCNT embryos is improved by treatment with HDAC inhibitors (HDACi). Several HDACi molecules were shown to enhance pig SCNT efficiency and cell reprogramming. This includes PDX101 (Belinosat), LBH589 (Panobinostat), CI994, CUDC-101, MGCD0103, MC1568, JWC022214 (HDACi-14), JWC017379 (HDACi-79), PCI-24781, m-carboxycin- namic acid bishydroxamide (CBHA), Suberoylanilide hydroxamic acid (SAHA) 4-iodo-SAHA (ISAHA), Quisinostat, Bufexamac, M344, LAQ824, Oxamflatin, Sodium butyrate (NaBu), Valproic acid, and the more commonly used Scriptaid, and Trichostatin A (TSA) (Table 1). HDACi have also been associated with other molecules, including DNA methylation modifying agents (Table 1).
Although HDACi treatment has become an important component of SCNT protocols, the mechanism by which it promotes cell reprogramming has not been fully elucidated. In addition of enabling a more permissive chromatin state and improving gene expression by increasing acetylation levels, HDCAi treatment may also facilitate DNA damage repair in embryos (Bohrer et al., 2014; Wang et al., 2015). There is evidence that the effect of HDACi treatment in SCNT embryos is influenced by the differentiation state of the nuclear donor cell (Kishigami et al., 2006; Martinez-Diaz et al., 2010), and also by cell cycle interactions between the host cytoplast and the nuclear donor cell at the time of nuclear transfer (Rissi et al., 2016).
Histone Methylation
Histone methylation is crucial for cell reprograming and normal embryo development by controlling important embryo features such as EGA, cell differentiation and DNA damage response (Dahl et al., 2016; Qin et al., 2016; Glanzner et al., 2017; Glanzner et al., 2018; Jambhekar et al., 2019; Rissi et al., 2019; Glanzner et al., 2020). In the context of cell reprogramming in SCNT embryos, interest in histone methylation has gained more emphasis after an increase of 3.4-fold in blastocyst rates (88 vs. 26%) and 8.7-fold development to term (8.7 vs. 1%) was obtained by expressing the demethylase Kdm4d in mouse SCNT embryos (Matoba et al., 2014). In addition, Kdm4d expression in human SCNT embryos improved the establishment of SCNT-derived embryonic stem cell cultures (Chung et al., 2015). The impact of manipulating the expression of specific demethylases on development of SCNT embryos has been studied in several species including sheep (Zhang Y. et al., 2018), cattle (Liu et al., 2018; Zhou et al., 2019), and swine (Rissi et al., 2019; Glanzner et al., 2020). Among the histone methylation markers studied, H3K9me3 and H3K27me3 have been identified as the most important barriers of SCNT success (Matoba et al., 2014; Xie et al., 2016). In pigs, SCNT efficiency increased by attenuating H3K9me3 levels, either by suppressing specific methyltransferases (SUV39H1/2, G9A) (Huang et al., 2016; Jeong et al., 2020; Weng et al., 2020; Cao et al., 2021), or by expressing the demethylase KDM4A (Weng et al., 2020). Similarly, the inhibition of the H3K27 methyltransferase EZH2 increased pig SCNT efficiency, while the inhibition of the H3K27 demethylase UTX (also known as KDM6A) decreased the efficiency (Xie et al., 2016). There is also evidence supporting an important role for H3K4 methylation, which is normally associated with an accessible chromatin state and transcriptional activity, in the regulation of SCNT embryos (Hormanseder et al., 2017). In pigs, depletion of H3K4 methylation increased blastocyst rates and improved gene expression patterns in SCNT embryos (Zhang Z. et al., 2018).
The association of different epigenetic modifiers has been tested to improve pig SCNT efficiency. For example, increasing histone acetylation along with decreasing H3K9me3 levels (Cao et al., 2017; Jeong et al., 2021) or decreasing DNA methylation (Huang et al., 2016; Cao et al., 2017), improved gene expression patterns in SCNT embryos and pig cloning efficiency (Table 1).
Micro RNAs
Micro RNAs (miRNAs) have important roles on cell reprogramming and regulation of normal embryo development (Blakaj and Lin, 2008; Pauli et al., 2011; O'Brien et al., 2018). miRNAs are small RNA sequences (∼22/23 nucleotides) that regulate gene functions by pairing to target mRNAs and inducing their degradation or repressing translation (Bartel, 2004; O'Brien et al., 2018). There is evidence that miRNAs are required for normal oocyte growth and maturation, and early embryogenesis in several species, including pigs (Prather et al., 2009; Kaczmarek et al., 2020). For example, deletion of the RNase III endonuclease DICER, which is important for the biogenesis of miRNAs, impaired mouse embryo development due to a decrease in mature miRNAs (Tang et al., 2007). During early embryo development, miRNAs regulate the silencing of transcripts that are no longer necessary for development, modulate chromatin rearrangements, and promote cell pluripotency (Tang et al., 2007; Prather et al., 2009; Pauli et al., 2011). They also contribute to the regulation of embryo implantation and embryo-maternal communication (Kaczmarek et al., 2020). Examples of miRNAs identified to have important roles on embryo gene silencing, cell pluripotency and cell differentiation include miR-430, miR-125, miR-145 and let-7 (Blakaj and Lin, 2008; Bartel, 2009; Pauli et al., 2011).
Studies in pigs revealed that miRNAs, including miR-1343, miR-302, miR-302b and miR-200, are involved in the acquisition and maintenance of cell pluripotency by regulating the expression of TFs, such as SOX2 and OCT-4 (Ma et al., 2014; Qiao et al., 2019; Xie et al., 2019). However, the impact of miRNAs during cell reprogramming and development of pig SCNT embryos has not been extensively explored. In mice, miR-125b was identified as a crucial factor for cell reprogramming in SCNT embryos by regulating the expression of methyltransferases (e.g., Suv39h1) that control H3K9me levels and chromatin accessibility (Zhang J. et al., 2017). In pigs, overexpression of miR-148a in nuclear donor cells downregulated DNMT1 expression and increased blastocyst rates and total cell numbers in SCNT embryos (Wang et al., 2017) (Table 1). Treatment of pig SCNT embryos with the HDACi Scriptaid attenuated DNMT1 expression and H3K9me3 levels, as well as increased miR-152 expression, suggesting a link between miRNAs, DNMT1, and histone methylation and acetylation (Liang et al., 2015). However, more studies are required to dissect the regulation and cross talk between miRNAs and epigenetic regulation in pig SCNT embryos.
X Chromosome Inactivation
Long non-coding RNAs (lncRNAs) are known to regulate several biological processes such as chromatin function, signaling pathways, and mRNA stability (Statello et al., 2021). The X-inactive specific transcript gene produces a lncRNA (Xist), which is a major regulator of X chromosome inactivation (XCI) (Penny et al., 1996). During normal embryo development, XCI is required for compensation of X-linked genes between males and females. In mouse embryos, inactivation of the paternally-derived X chromosome starts at the early cleavage stages of development (Okamoto et al., 2011). In pig embryos, XCI is random and only observed in the late epiblast stage, as evidenced by the reduction of biallelic expression of X-linked genes and increase in H3K27me3 levels (Ramos-Ibeas et al., 2019).
Proper reprogramming of the X chromosome seems a critical component of SCNT efficiency (Table 1). Downregulation of X-linked genes due to the ectopic expression of Xist was detected in mouse SCNT embryos (Matoba et al., 2011). Similarly, XIST and X-linked genes were aberrantly expressed in pig SCNT embryos (Park C.-H. et al., 2012; Mao et al., 2015), and SCNT fetuses having abnormal development (Yuan et al., 2014; Ruan et al., 2018). Inactivation of XIST in nuclear donor cells or its attenuation in SCNT embryos improved blastocyst rates and cloning efficiency in mice and pigs (Matoba et al., 2011; Zeng et al., 2016; Ruan et al., 2018; Yang et al., 2019). A recent study demonstrated that the small molecule X1 can target a specific motif of Xist and blocks initiation of XCI (Aguilar et al., 2022). This may represent a new alternative for preventing early inaction of X chromosome in SCNT embryos, however, more studies are needed to evaluate efficiency and toxicity in pig embryos.
Concluding Remarks and Future Perspectives
Recent progress in xenotransplantation of pig organs, along with the evolution of technologies for editing the pig genome has expanded interest in the production of cloned pigs by SCNT. Since it was proven that SCNT can make somatic cells regain totipotency, researchers in the field have been attempting to increase the efficiency of this reproductive method, but the progress has been modest. While in vitro development to the blastocyst stage of SCNT embryos is often similar to fertilized embryos, development to term and rate of alive cloned piglets remain low, confirming that not all chromatin functions regulating development have been reset properly. Cumulating evidence, mainly from mouse studies, pointed out that epigenetic marks, such as DNA and histone methylation, as well as histone acetylation, transcription factors, and non-coding RNAs, can all affect cell reprogramming and SCNT efficiency. Moreover, efforts to modulate these factors in SCNT embryos to mimic fertilized embryos by using molecules, or either attenuating or overexpressing genes, has shown encouraging results that improved not only the blastocyst rate and quality, but also development to term of cloned animals (Figure 1). In addition, some attempts taken to modulate multiple factors have further ameliorated mouse cloning efficiency, suggesting this route could be explored to improve SCNT protocols in other species, including pigs. For example, inhibition of HDAC and transcription along with either attenuation or expression of specific modulators of histones or DNA methylation may likely improve porcine SCNT efficiency. It is worth highlighting however, that there are fundamental differences between species in the regulation of early development, including the timing of EGA and first cell lineage specification, which should be taken in consideration when translating findings from one species to another.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization, WGG, MPM, KG, and VB; writing: original draft preparation and figures, WGG, MPM, and KG; review and editing, WGG and VB. All authors have read and agreed to the content of the manuscript.
Funding
Research in our laboratory is funded by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canada Foundation for Innovation (CFI), le Consortium de Recherche et Innovations en Bioprocédés Industriels au Québec (CRIBIQ), and Mitacs. KG was supported by a scholarship from the Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES). MPM is supported by a scholarship from the Fonds de recherche du Québec—Nature et technologie (FRQNT). KG and MPM received financial support from the NSERC—Collaborative Research and Training Experience Program—Genome Editing for Food Security and Environmental Sustainability (GEFSES).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Aguilar R. Spencer K. B. Kesner B. Rizvi N. F. Badmalia M. D. Mrozowich T. et al (2022). Targeting Xist with Compounds that Disrupt RNA Structure and X Inactivation. Nature604, 160–166. 10.1038/s41586-022-04537-z
2
Bartel D. P. (2004). MicroRNAs. Cell.116 (2), 281–297. 10.1016/s0092-8674(04)00045-5
3
Bartel D. P. (2009). MicroRNAs: Target Recognition and Regulatory Functions. Cell.136 (2), 215–233. 10.1016/j.cell.2009.01.002
4
Blakaj A. Lin H. (2008). Piecing Together the Mosaic of Early Mammalian Development through microRNAs. J. Biol. Chem.283 (15), 9505–9508. 10.1074/jbc.R800002200
5
Bohrer R. C. Che L. Gonçalves P. B. D. Duggavathi R. Bordignon V. (2013). Phosphorylated Histone H2A.X in Porcine Embryos Produced by IVF and Somatic Cell Nuclear Transfer. Reproduction146 (4), 325–333. 10.1530/rep-13-0271
6
Bohrer R. C. Duggavathi R. Bordignon V. (2014). Inhibition of Histone Deacetylases Enhances DNA Damage Repair in SCNT Embryos. Cell. Cycle13 (13), 2138–2148. 10.4161/cc.29215
7
Bordignon V. (2019). “Animal Cloning - State of the Art and Applications,” in Comprehensive Biotechnology. Editor Moo-YoungM.. 3th edn (Netherlands: Elsevier), 450–465. 10.1016/b978-0-444-64046-8.00266-4
8
Bordignon V. Clarke H. J. Smith L. C. (1999). Developmentally Regulated Loss and Reappearance of Immunoreactive Somatic Histone H1 on Chromatin of Bovine Morula-Stage Nuclei Following Transplantation into Oocytes1. Biol. Reprod.61 (1), 22–30. 10.1095/biolreprod61.1.22
9
Bornelöv S. Reynolds N. Xenophontos M. Gharbi S. Johnstone E. Floyd R. et al (2018). The Nucleosome Remodeling and Deacetylation Complex Modulates Chromatin Structure at Sites of Active Transcription to Fine-Tune Gene Expression. Mol. Cell.71 (1), 56–72. e54. 10.1016/j.molcel.2018.06.003
10
Cao L. Dai X. Huang S. Shen K. Shi D. Li X. (2021). Inhibition of Suv39h1/2 Expression Improves the Early Development of Debao Porcine Somatic Cell Nuclear Transfer Embryos. Reprod. Dom. Anim.56 (7), 992–1003. 10.1111/rda.13942
11
Cao P. Li H. Zuo Y. Nashun B. (2020). Characterization of DNA Methylation Patterns and Mining of Epigenetic Markers during Genomic Reprogramming in SCNT Embryos. Front. Cell. Dev. Biol.8, 570107. 10.3389/fcell.2020.570107
12
Cao Z. Hong R. Ding B. Zuo X. Li H. Ding J. et al (2017). TSA and BIX-01294 Induced Normal DNA and Histone Methylation and Increased Protein Expression in Porcine Somatic Cell Nuclear Transfer Embryos. PLoS One12 (1), e0169092. 10.1371/journal.pone.0169092
13
Cervera R. P. Martí-Gutiérrez N. Escorihuela E. Moreno R. Stojkovic M. (2009). Trichostatin A Affects Histone Acetylation and Gene Expression in Porcine Somatic Cell Nucleus Transfer Embryos. Theriogenology72 (8), 1097–1110. 10.1016/j.theriogenology.2009.06.030
14
Chang C.-C. Gao S. Sung L.-Y. Corry G. N. Ma Y. Nagy Z. P. et al (2010). Rapid Elimination of the Histone Variant MacroH2A from Somatic Cell Heterochromatin after Nuclear Transfer. Cell. Reprogr.12 (1), 43–53. 10.1089/cell.2009.0043
15
Chawalit S. Nguyen N. T. Tseng J.-K. Lo N.-W. Tu C.-F. Ju J.-C. (2012). Trichostatin A and Ascorbic Acid Assist in the Development of Porcine Handmade Cloned Embryos via Different Physiologic Pathways. Reprod. Sci.19 (9), 976–986. 10.1177/1933719112440049
16
Chung Y. G. Matoba S. Liu Y. Eum J. H. Lu F. Jiang W. et al (2015). Histone Demethylase Expression Enhances Human Somatic Cell Nuclear Transfer Efficiency and Promotes Derivation of Pluripotent Stem Cells. Cell. Stem Cell.17 (6), 758–766. 10.1016/j.stem.2015.10.001
17
Cong P. Zhu K. Ji Q. Zhao H. Chen Y. (2013). Effects of Trichostatin A on Histone Acetylation and Methylation Characteristics in Early Porcine Embryos after Somatic Cell Nuclear Transfer. Animal Sci. J.84 (9), 639–649. 10.1111/asj.12059
18
Dahl J. A. Jung I. Aanes H. Greggains G. D. Manaf A. Lerdrup M. et al (2016). Broad Histone H3K4me3 Domains in Mouse Oocytes Modulate Maternal-To-Zygotic Transition. Nature537 (7621), 548–552. 10.1038/nature19360
19
Das Z. C. Gupta M. K. Uhm S. J. Lee H. T. (2010). Increasing Histone Acetylation of Cloned Embryos, but Not Donor Cells, by Sodium Butyrate Improves TheirIn VitroDevelopment in Pigs. Cell. Reprogr.12 (1), 95–104. 10.1089/cell.2009.0068
20
de Macedo M. P. Glanzner W. G. Gutierrez K. Bordignon V. (2021). Chromatin Role in Early Programming of Embryos. Anim. Front.11 (6), 57–65. 10.1093/af/vfab054
21
Deshmukh R. S. Østrup O. Østrup E. Vejlsted M. Niemann H. Lucas-Hahn A. et al (2011). DNA Methylation in Porcine Preimplantation Embryos Developed In Vivo and Produced by In Vitro Fertilization, Parthenogenetic Activation and Somatic Cell Nuclear Transfer. Epigenetics6 (2), 177–187. 10.4161/epi.6.2.13519
22
Diao Y.-F. Naruse K.-J. Han R.-X. Li X.-X. Oqani R. K. Lin T. et al (2013). Treatment of Fetal Fibroblasts with DNA Methylation Inhibitors And/or Histone Deacetylase Inhibitors Improves the Development of Porcine Nuclear Transfer-Derived Embryos. Animal Reproduction Sci.141 (3-4), 164–171. 10.1016/j.anireprosci.2013.08.008
23
Djekidel M. N. Inoue A. Matoba S. Suzuki T. Zhang C. Lu F. et al (2018). Reprogramming of Chromatin Accessibility in Somatic Cell Nuclear Transfer Is DNA Replication Independent. Cell. Rep.23 (7), 1939–1947. 10.1016/j.celrep.2018.04.036
24
Eckersley-Maslin M. Alda-Catalinas C. Blotenburg M. Kreibich E. Krueger C. Reik W. (2019). Dppa2 and Dppa4 Directly Regulate the Dux-Driven Zygotic Transcriptional Program. Genes. Dev.33 (3-4), 194–208. 10.1101/gad.321174.118
25
Egli D. Eggan K. (2010). Recipient Cell Nuclear Factors Are Required for Reprogramming by Nuclear Transfer. Development137 (12), 1953–1963. 10.1242/dev.046151
26
Endo T. Imai A. Shimaoka T. Kano K. Naito K. (2011). Histone Exchange Activity and its Correlation with Histone Acetylation Status in Porcine Oocytes. Reproduction141 (4), 397–405. 10.1530/REP-10-0164
27
Endo T. Kano K. Naito K. (2008). Nuclear Histone Deacetylases Are Not Required for Global Histone Deacetylation during Meiotic Maturation in Porcine Oocytes1. Biol. Reprod.78 (6), 1073–1080. 10.1095/biolreprod.107.067397
28
Endo T. Naito K. Aoki F. Kume S. Tojo H. (2005). Changes in Histone Modifications during In Vitro Maturation of Porcine Oocytes. Mol. Reprod. Dev.71 (1), 123–128. 10.1002/mrd.20288
29
Gaspar-Maia A. Qadeer Z. A. Hasson D. Ratnakumar K. Adrian Leu N. Leroy G. et al (2013). MacroH2A Histone Variants Act as a Barrier upon Reprogramming towards Pluripotency. Nat. Commun.4, 1565. 10.1038/ncomms2582
30
Glanzner W. G. Gutierrez K. Rissi V. B. de Macedo M. P. Lopez R. Currin L. et al (2020). Histone Lysine Demethylases KDM5B and KDM5C Modulate Genome Activation and Stability in Porcine Embryos. Front. Cell. Dev. Biol.8, 151. 10.3389/fcell.2020.00151
31
Glanzner W. G. Rissi V. B. de Macedo M. P. Mujica L. K. S. Gutierrez K. Bridi A. et al (2018). Histone 3 Lysine 4, 9, and 27 Demethylases Expression Profile in Fertilized and Cloned Bovine and Porcine Embryos†. Biol. Reprod.98 (6), 742–751. 10.1093/biolre/ioy054
32
Glanzner W. G. Wachter A. Coutinho A. R. S. Albornoz M. S. Duggavathi R. GonÇAlves P. B. D. et al (2017). Altered Expression of BRG1 and Histone Demethylases, and Aberrant H3K4 Methylation in Less Developmentally Competent Embryos at the Time of Embryonic Genome Activation. Mol. Reprod. Dev.84 (1), 19–29. 10.1002/mrd.22762
33
Gutierrez K. Dicks N. Glanzner W. G. Agellon L. B. Bordignon V. (2015). Efficacy of the Porcine Species in Biomedical Research. Front. Genet.6, 293. 10.3389/fgene.2015.00293
34
Han C. Deng R. Mao T. Luo Y. Wei B. Meng P. et al (2018). Overexpression of Tet3 in Donor Cells Enhances Goat Somatic Cell Nuclear Transfer Efficiency. FEBS J.285 (14), 2708–2723. 10.1111/febs.14515
35
Hansis C. Barreto G. Maltry N. Niehrs C. (2004). Nuclear Reprogramming of Human Somatic Cells by xenopus Egg Extract Requires BRG1. Curr. Biol.14 (16), 1475–1480. 10.1016/j.cub.2004.08.031
36
Hendrich B. Guy J. Ramsahoye B. Wilson V. A. Bird A. (2001). Closely Related Proteins MBD2 and MBD3 Play Distinctive but Interacting Roles in Mouse Development. Genes. Dev.15 (6), 710–723. 10.1101/gad.194101
37
Hörmanseder E. Simeone A. Allen G. E. Bradshaw C. R. Figlmüller M. Gurdon J. et al (2017). H3K4 Methylation-dependent Memory of Somatic Cell Identity Inhibits Reprogramming and Development of Nuclear Transfer Embryos. Cell. Stem Cell.21 (1), 135–143. e136. 10.1016/j.stem.2017.03.003
38
Hou L. Ma F. Yang J. Riaz H. Wang Y. Wu W. et al (2014). Effects of Histone Deacetylase Inhibitor Oxamflatin onIn VitroPorcine Somatic Cell Nuclear Transfer Embryos. Cell. Reprogr.16 (4), 253–265. 10.1089/cell.2013.0058
39
Huan Y. J. Zhu J. Xie B. T. Wang J. Y. Liu S. C. Zhou Y. et al (2013). Treating Cloned Embryos, but Not Donor Cells, with 5-Aza-2'-Deoxycytidine Enhances the Developmental Competence of Porcine Cloned Embryos. J. Reproduction Dev.59 (5), 442–449. 10.1262/jrd.2013-026
40
Huan Y. Wang H. Wu Z. Zhang J. Zhu J. Liu Z. et al (2015a). Epigenetic Modification of Cloned Embryos ImprovesNanogReprogramming in Pigs. Cell. Reprogr.17 (3), 191–198. 10.1089/cell.2014.0103
41
Huan Y. Wu Z. Zhang J. Zhu J. Liu Z. Song X. (2015b). Epigenetic Modification Agents Improve Gene-specific Methylation Reprogramming in Porcine Cloned Embryos. PLoS One10 (6), e0129803. 10.1371/journal.pone.0129803
42
Huang J. Zhang H. Yao J. Qin G. Wang F. Wang X. et al (2016). BIX-01294 Increases Pig Cloning Efficiency by Improving Epigenetic Reprogramming of Somatic Cell Nuclei. Reproduction151 (1), 39–49. 10.1530/REP-15-0460
43
Huang R. Sui L. Fu C. Zhai Y. Dai X. Zhang S. et al (2021). HDAC11 Inhibition Disrupts Porcine Oocyte Meiosis via Regulating α-tubulin Acetylation and Histone Modifications. Aging13 (6), 8849–8864. 10.18632/aging.202697
44
Huang Y. Tang X. Xie W. Zhou Y. Li D. Yao C. et al (2011). Histone Deacetylase Inhibitor Significantly Improved the Cloning Efficiency of Porcine Somatic Cell Nuclear Transfer Embryos. Cell. Reprogr.13 (6), 513–520. 10.1089/cell.2011.0032
45
Ivanova E. Canovas S. Garcia-Martínez S. Romar R. Lopes J. S. Rizos D. et al (2020). DNA Methylation Changes during Preimplantation Development Reveal Inter-species Differences and Reprogramming Events at Imprinted Genes. Clin. Epigenet12 (1), 64. 10.1186/s13148-020-00857-x
46
Jambhekar A. Dhall A. Shi Y. (2019). Roles and Regulation of Histone Methylation in Animal Development. Nat. Rev. Mol. Cell. Biol.20 (10), 625–641. 10.1038/s41580-019-0151-1
47
Jeong P.-S. Sim B.-W. Park S.-H. Kim M. J. Kang H.-G. Nanjidsuren T. et al (2020). Chaetocin Improves Pig Cloning Efficiency by Enhancing Epigenetic Reprogramming and Autophagic Activity. Ijms21 (14), 4836. 10.3390/ijms21144836
48
Jeong P.-S. Yang H.-J. Park S.-H. Gwon M. A. Joo Y. E. Kim M. J. et al (2021). Combined Chaetocin/Trichostatin A Treatment Improves the Epigenetic Modification and Developmental Competence of Porcine Somatic Cell Nuclear Transfer Embryos. Front. Cell. Dev. Biol.9, 709574. 10.3389/fcell.2021.709574
49
Jin J.-X. Kang J.-D. Li S. Jin L. Zhu H.-Y. Guo Q. et al (2015). PXD101 Significantly Improves Nuclear Reprogramming and the In Vitro Developmental Competence of Porcine SCNT Embryos. Biochem. Biophysical Res. Commun.456 (1), 156–161. 10.1016/j.bbrc.2014.11.051
50
Jin J.-X. Lee S. Taweechaipaisankul A. Kim G. A. Lee B. C. (2017a). The HDAC Inhibitor LAQ824 Enhances Epigenetic Reprogramming and In Vitro Development of Porcine SCNT Embryos. Cell. Physiol. Biochem.41 (3), 1255–1266. 10.1159/000464389
51
Jin J.-X. Li S. Gao Q.-S. Hong Y. Jin L. Zhu H.-Y. et al (2013). Significant Improvement of Pig Cloning Efficiency by Treatment with LBH589 after Somatic Cell Nuclear Transfer. Theriogenology80 (6), 630–635. 10.1016/j.theriogenology.2013.06.006
52
Jin J.-X. Li S. Hong Y. Jin L. Zhu H.-Y. Guo Q. et al (2014a). CUDC-101, a Histone Deacetylase Inhibitor, Improves the In Vitro and In Vivo Developmental Competence of Somatic Cell Nuclear Transfer Pig Embryos. Theriogenology81 (4), 572–578. 10.1016/j.theriogenology.2013.11.011
53
Jin L. Guo Q. Zhang G.-L. Xing X.-X. Xuan M.-F. Luo Q.-R. et al (2018). The Histone Deacetylase Inhibitor, CI994, Improves Nuclear Reprogramming and In Vitro Developmental Potential of Cloned Pig Embryos. Cell. Reprogr.20 (3), 205–213. 10.1089/cell.2018.0001
54
Jin L. Guo Q. Zhu H.-Y. Xing X.-X. Zhang G.-L. Xuan M.-F. et al (2017b). Histone Deacetylase Inhibitor M344 Significantly Improves Nuclear Reprogramming, Blastocyst Quality, and In Vitro Developmental Capacity of Cloned Pig Embryos. J. Anim. Sci.95 (3), 1388–1395. 10.2527/jas.2016.1240
55
Jin L. Guo Q. Zhu H.-Y. Xing X.-X. Zhang G.-L. Xuan M.-F. et al (2017c). Quisinostat Treatment Improves Histone Acetylation and Developmental Competence of Porcine Somatic Cell Nuclear Transfer Embryos. Mol. Reprod. Dev.84 (4), 340–346. 10.1002/mrd.22787
56
Jin L. Zhu H.-Y. Guo Q. Li X.-C. Zhang Y.-C. Cui C.-D. et al (2017d). Effect of Histone Acetylation Modification with MGCD0103, a Histone Deacetylase Inhibitor, on Nuclear Reprogramming and the Developmental Competence of Porcine Somatic Cell Nuclear Transfer Embryos. Theriogenology87, 298–305. 10.1016/j.theriogenology.2016.09.011
57
Jin L. Zhu H.-Y. Guo Q. Li X.-C. Zhang Y.-C. Zhang G.-L. et al (2016). PCI-24781 Can Improve In Vitro and In Vivo Developmental Capacity of Pig Somatic Cell Nuclear Transfer Embryos. Biotechnol. Lett.38 (9), 1433–1441. 10.1007/s10529-016-2141-0
58
Jin Y.-X. Zhao M.-H. Zheng Z. Kwon J.-S. Lee S.-K. Cui X.-S. et al (2014b). Histone Deacetylase Inhibitor Trichostatin A Affects Porcine Oocyte Maturation In Vitro. Reprod. Fertil. Dev.26 (6), 806–816. 10.1071/RD13013
59
Kaczmarek M. M. Najmula J. Guzewska M. M. Przygrodzka E. (2020). MiRNAs in the Peri-Implantation Period: Contribution to Embryo-Maternal Communication in Pigs. Ijms21 (6), 2229. 10.3390/ijms21062229
60
Kim M. J. Oh H. J. Choi Y. B. Lee S. Setyawan E. M. N. Lee S. H. et al (2018). Suberoylanilide Hydroxamic Acid during In Vitro Culture Improves Development of Dog-Pig Interspecies Cloned Embryos but Not Dog Cloned Embryos. J. Reproduction Dev.64 (3), 277–282. 10.1262/jrd.2017-112
61
Kim S.-J. Kwon H.-S. Kwon D.-k. Koo O.-J. Moon J.-H. Park E.-J. et al (2019). Production of Transgenic Porcine Embryos Reconstructed with Induced Pluripotent Stem-like Cells Derived from Porcine Endogenous Factors Using piggyBac System. Cell. Reprogr.21 (1), 26–36. 10.1089/cell.2018.0036
62
Kim Y. J. Ahn K. S. Kim M. Shim H. (2011). Comparison of Potency between Histone Deacetylase Inhibitors Trichostatin A and Valproic Acid on Enhancing In Vitro Development of Porcine Somatic Cell Nuclear Transfer Embryos. Vitro Cell. Dev.Biol.-Animal47 (4), 283–289. 10.1007/s11626-011-9394-7
63
Kishigami S. Mizutani E. Ohta H. Hikichi T. Thuan N. V. Wakayama S. et al (2006). Significant Improvement of Mouse Cloning Technique by Treatment with Trichostatin A after Somatic Nuclear Transfer. Biochem. Biophysical Res. Commun.340 (1), 183–189. 10.1016/j.bbrc.2005.11.164
64
Kumar B. M. Maeng G.-H. Lee Y.-M. Lee J.-H. Jeon B.-G. Ock S.-A. et al (2013). Epigenetic Modification of Fetal Fibroblasts Improves Developmental Competency and Gene Expression in Porcine Cloned Embryos. Vet. Res. Commun.37 (1), 19–28. 10.1007/s11259-012-9542-x
65
Kunitomi A. Yuasa S. Sugiyama F. Saito Y. Seki T. Kusumoto D. et al (2016). H1foo Has a Pivotal Role in Qualifying Induced Pluripotent Stem Cells. Stem Cell. Rep.6 (6), 825–833. 10.1016/j.stemcr.2016.04.015
66
Lee D.-K. Park C.-H. Choi K.-H. Jeong Y.-i. Uh K.-J. Hwang J. Y. et al (2016). Aggregation of Cloned Embryos in Empty Zona Pellucida Improves Derivation Efficiency of Pig ES-like Cells. Zygote24 (6), 909–917. 10.1017/S0967199416000241
67
Lee E. Lee S. H. Kim S. Jeong Y. W. Kim J. H. Koo O. J. et al (2006). Analysis of Nuclear Reprogramming in Cloned Miniature Pig Embryos by Expression of Oct-4 and Oct-4 Related Genes. Biochem. Biophysical Res. Commun.348 (4), 1419–1428. 10.1016/j.bbrc.2006.08.004
68
Li J. Svarcova O. Villemoes K. Kragh P. M. Schmidt M. Bøgh I. B. et al (2008). High In Vitro Development after Somatic Cell Nuclear Transfer and Trichostatin A Treatment of Reconstructed Porcine Embryos. Theriogenology70 (5), 800–808. 10.1016/j.theriogenology.2008.05.046
69
Liang S. Zhao M.-H. Choi J.-w. Kim N.-H. Cui X.-S. (2015). Scriptaid Treatment Decreases DNA Methyltransferase 1 Expression by Induction of MicroRNA-152 Expression in Porcine Somatic Cell Nuclear Transfer Embryos. PLoS One10 (8), e0134567. 10.1371/journal.pone.0134567
70
Liu L. Liu Y. Gao F. Song G. Wen J. Guan J. et al (2012). Embryonic Development and Gene Expression of Porcine SCNTEmbryos Treated with Sodium Butyrate. J. Exp. Zool.318 (3), 224–234. 10.1002/jez.b.22440
71
Liu X. Wang Y. Gao Y. Su J. Zhang J. Xing X. et al (2018). H3K9 Demethylase KDM4E Is an Epigenetic Regulator for Bovine Embryonic Development and a Defective Factor for Nuclear Reprogramming. Development145 (4), dev158261. 10.1242/dev.158261
72
Liu Z. Xiang G. Xu K. Che J. Xu C. Li K. et al (2020). Transcriptome Analyses Reveal Differential Transcriptional Profiles in Early- and Late-Dividing Porcine Somatic Cell Nuclear Transfer Embryos. Genes.11 (12), 1499. 10.3390/genes11121499
73
Luo B. Ju S. Muneri C. W. Rui R. (2015). Effects of Histone Acetylation Status on the Early Development ofIn VitroPorcine Transgenic Cloned Embryos. Cell. Reprogr.17 (1), 41–48. 10.1089/cell.2014.0041
74
Ma K. Song G. An X. Fan A. Tan W. Tang B. et al (2014). miRNAs Promote Generation of Porcine-Induced Pluripotent Stem Cells. Mol. Cell. Biochem.389 (1-2), 209–218. 10.1007/s11010-013-1942-x
75
Mao J. Zhao M.-T. Whitworth K. M. Spate L. D. Walters E. M. O'Gorman C. et al (2015). Oxamflatin Treatment Enhances Cloned Porcine Embryo Development and Nuclear Reprogramming. Cell. Reprogr.17 (1), 28–40. 10.1089/cell.2014.0075
76
Martinez-Diaz M. A. Che L. Albornoz M. Seneda M. M. Collis D. Coutinho A. R. S. et al (2010). Pre- and Postimplantation Development of Swine-Cloned Embryos Derived from Fibroblasts and Bone Marrow Cells after Inhibition of Histone Deacetylases. Cell. Reprogr.12 (1), 85–94. 10.1089/cell.2009.0047
77
Matoba S. Inoue K. Kohda T. Sugimoto M. Mizutani E. Ogonuki N. et al (2011). RNAi-Mediated Knockdown of Xist Can Rescue the Impaired Postimplantation Development of Cloned Mouse Embryos. Proc. Natl. Acad. Sci. U.S.A.108 (51), 20621–20626. 10.1073/pnas.1112664108
78
Matoba S. Liu Y. Lu F. Iwabuchi K. A. Shen L. Inoue A. et al (2014). Embryonic Development Following Somatic Cell Nuclear Transfer Impeded by Persisting Histone Methylation. Cell.159 (4), 884–895. 10.1016/j.cell.2014.09.055
79
Matoba S. Zhang Y. (2018). Somatic Cell Nuclear Transfer Reprogramming: Mechanisms and Applications. Cell. Stem Cell.23 (4), 471–485. 10.1016/j.stem.2018.06.018
80
Miyoshi K. Kawaguchi H. Maeda K. Sato M. Akioka K. Noguchi M. et al (2016). Birth of Cloned Microminipigs Derived from Somatic Cell Nuclear Transfer Embryos that Have Been Transiently Treated with Valproic Acid. Cell. Reprogr.18 (6), 390–400. 10.1089/cell.2016.0025
81
Miyoshi K. Mori H. Mizobe Y. Akasaka E. Ozawa A. Yoshida M. et al (2010). Valproic Acid EnhancesIn VitroDevelopment and Oct-3/4 Expression of Miniature Pig Somatic Cell Nuclear Transfer Embryos. Cell. Reprogr.12 (1), 67–74. 10.1089/cell.2009.0032
82
Mohana Kumar B. Song H.-J. Cho S.-K. Balasubramanian S. Choe S.-Y. Rho G.-J. (2007). Effect of Histone Acetylation Modification with Sodium Butyrate, a Histone Deacetylase Inhibitor, on Cell Cycle, Apoptosis, Ploidy and Gene Expression in Porcine Fetal Fibroblasts. J. Reproduction Dev.53 (4), 903–913. 10.1262/jrd.18180
83
Nashun B. Akiyama T. Suzuki M. G. Aoki F. (2011). Dramatic Replacement of Histone Variants during Genome Remodeling in Nuclear-Transferred Embryos. Epigenetics6 (12), 1489–1497. 10.4161/epi.6.12.18206
84
Ng R. K. Gurdon J. B. (2005). Epigenetic Memory of Active Gene Transcription Is Inherited through Somatic Cell Nuclear Transfer. Proc. Natl. Acad. Sci. U.S.A.102 (6), 1957–1962. 10.1073/pnas.0409813102
85
Ning S.-F. Li Q.-Y. Liang M.-M. Yang X.-G. Xu H.-Y. Lu Y.-Q. et al (2013). Methylation Characteristics and Developmental Potential of Guangxi Bama Minipig (Sus scrofa Domestica) Cloned Embryos from Donor Cells Treated with Trichostatin A and 5-Aza-2′-Deoxycytidine. Zygote21 (2), 178–186. 10.1017/S0967199411000797
86
No J.-G. Hur T.-Y. Zhao M. Lee S. Choi M.-K. Nam Y.-S. et al (2018). Scriptaid Improves the Reprogramming of Donor Cells and Enhances Canine-Porcine Interspecies Embryo Development. Reprod. Biol.18 (1), 18–26. 10.1016/j.repbio.2017.11.001
87
O'Brien J. Hayder H. Zayed Y. Peng C. (2018). Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol.9, 402. 10.3389/fendo.2018.00402
88
Okamoto I. Patrat C. Thépot D. Peynot N. Fauque P. Daniel N. et al (2011). Eutherian Mammals Use Diverse Strategies to Initiate X-Chromosome Inactivation during Development. Nature472 (7343), 370–374. 10.1038/nature09872
89
Ongaratto F. L. Rodriguez-Villamil P. Bertolini M. Carlson D. F. (2020). Influence of Oocyte Selection, Activation with a Zinc Chelator and Inhibition of Histone Deacetylases on Cloned Porcine Embryo and Chemically Activated Oocytes Development. Zygote28 (4), 286–290. 10.1017/S0967199419000856
90
Park C.-H. Jeong Y. H. Jeong Y.-I. Lee S.-Y. Jeong Y.-W. Shin T. et al (2012a). X-Linked Gene Transcription Patterns in Female and Male In Vivo, In Vitro and Cloned Porcine Individual Blastocysts. PLoS One7 (12), e51398. 10.1371/journal.pone.0051398
91
Park S.-J. Park H.-J. Koo O.-J. Choi W.-J. Moon J.-h. Kwon D.-K. et al (2012b). Oxamflatin Improves Developmental Competence of Porcine Somatic Cell Nuclear Transfer Embryos. Cell. Reprogr.14 (5), 398–406. 10.1089/cell.2012.0007
92
Pauli A. Rinn J. L. Schier A. F. (2011). Non-coding RNAs as Regulators of Embryogenesis. Nat. Rev. Genet.12 (2), 136–149. 10.1038/nrg2904
93
Penny G. D. Kay G. F. Sheardown S. A. Rastan S. Brockdorff N. (1996). Requirement for Xist in X Chromosome Inactivation. Nature379 (6561), 131–137. 10.1038/379131a0
94
Prather R. S. Ross J. W. Isom S. C. Green J. A. (2009). Transcriptional, Post-transcriptional and Epigenetic Control of Porcine Oocyte Maturation and Embryogenesis. Soc. Reprod. Fertil. Suppl.66, 165–176.
95
Qiao S. Deng Y. Li S. Yang X. Shi D. Li X. (2019). Partially Reprogrammed Induced Pluripotent Stem Cells Using MicroRNA Cluster miR-302s in Guangxi Bama Minipig Fibroblasts. Cell. Reprogr.21 (5), 229–237. 10.1089/cell.2019.0035
96
Qin H. Zhao A. Zhang C. Fu X. (2016). Epigenetic Control of Reprogramming and Transdifferentiation by Histone Modifications. Stem Cell. Rev Rep12 (6), 708–720. 10.1007/s12015-016-9682-4
97
Qu J. Sun M. Wang X. Song X. He H. Huan Y. (2020). Melatonin Enhances the Development of Porcine Cloned Embryos by Improving DNA Methylation Reprogramming. Cell. Reprogr.22 (3), 156–166. 10.1089/cell.2019.0103
98
Ramos-Ibeas P. Sang F. Zhu Q. Tang W. W. C. Withey S. Klisch D. et al (2019). Pluripotency and X Chromosome Dynamics Revealed in Pig Pre-gastrulating Embryos by Single Cell Analysis. Nat. Commun.10 (1), 500. 10.1038/s41467-019-08387-8
99
Reik W. Dean W. Walter J. (2001). Epigenetic Reprogramming in Mammalian Development. Science293 (5532), 1089–1093. 10.1126/science.1063443
100
Rissi V. B. Glanzner W. G. de Macedo M. P. Gutierrez K. Baldassarre H. Gonçalves P. B. D. et al (2019). The Histone Lysine Demethylase KDM7A Is Required for Normal Development and First Cell Lineage Specification in Porcine Embryos. Epigenetics14 (11), 1088–1101. 10.1080/15592294.2019.1633864
101
Rissi V. B. Glanzner W. G. de Macedo M. P. Mujica L. K. S. Campagnolo K. Gutierrez K. et al (2019). Inhibition of RNA Synthesis during Scriptaid Exposure Enhances Gene Reprogramming in SCNT Embryos. Reproduction157 (2), 123–133. 10.1530/REP-18-0366
102
Rissi V. B. Glanzner W. G. Mujica L. K. S. Antoniazzi A. Q. Gonçalves P. B. D. Bordignon V. (2016). Effect of Cell Cycle Interactions and Inhibition of Histone Deacetylases on Development of Porcine Embryos Produced by Nuclear Transfer. Cell. Reprogr.18 (2), 8–16. 10.1089/cell.2015.0052
103
Rollo C. Li Y. Jin X. L. O’Neill C. (2017). Histone 3 Lysine 9 Acetylation Is a Biomarker of the Effects of Culture on Zygotes. Reproduction154 (4), 375–385. 10.1530/REP-17-0112
104
Ruan D. Peng J. Wang X. Ouyang Z. Zou Q. Yang Y. et al (2018). XIST Derepression in Active X Chromosome Hinders Pig Somatic Cell Nuclear Transfer. Stem Cell. Rep.10 (2), 494–508. 10.1016/j.stemcr.2017.12.015
105
Shi B. Gao D. Zhong L. Zhi M. Weng X. Xu J. et al (2020). IRF-1 Expressed in the Inner Cell Mass of the Porcine Early Blastocyst Enhances the Pluripotency of Induced Pluripotent Stem Cells. Stem Cell. Res. Ther.11 (1), 505. 10.1186/s13287-020-01983-2
106
Siriboon C. Li T.-S. Yu C.-W. Chern J.-W. Ju J.-C. (2018). Novel Histone Deacetylase Inhibitors and Embryo Aggregation Enhance Cloned Embryo Development and ES Cell Derivation in Pigs. PLoS One13 (9), e0204588. 10.1371/journal.pone.0204588
107
Song X. Liu Z. He H. Wang J. Li H. Li J. et al (2017). Dnmt1s in Donor Cells Is a Barrier to SCNT-Mediated DNA Methylation Reprogramming in Pigs. Oncotarget8 (21), 34980–34991. 10.18632/oncotarget.16507
108
Song Y. Hai T. Wang Y. Guo R. Li W. Wang L. et al (2014a). Epigenetic Reprogramming, Gene Expression and In Vitro Development of Porcine SCNT Embryos Are Significantly Improved by a Histone Deacetylase Inhibitor-M-Carboxycinnamic Acid Bishydroxamide (CBHA). Protein Cell.5 (5), 382–393. 10.1007/s13238-014-0034-3
109
Song Z. Ji Q. Zhao H. Nie Y. He Z. Chen Y. et al (2014b). Ectopic Expression of Reprogramming Factors Enhances the Development of Cloned Porcine Embryos. Biotechnol. Lett.36 (10), 1953–1961. 10.1007/s10529-014-1580-8
110
Statello L. Guo C.-J. Chen L.-L. Huarte M. (2021). Gene Regulation by Long Non-coding RNAs and its Biological Functions. Nat. Rev. Mol. Cell. Biol.22 (2), 96–118. 10.1038/s41580-020-00315-9
111
Stresemann C. Lyko F. (2008). Modes of Action of the DNA Methyltransferase Inhibitors Azacytidine and Decitabine. Int. J. Cancer123 (1), 8–13. 10.1002/ijc.23607
112
Sun J. Cui K. Li Z. Gao B. Jiang J. Liu Q. et al (2020). Histone Hyperacetylation May Improve the Preimplantation Development and Epigenetic Status of Cloned Embryos. Reprod. Biol.20 (2), 237–246. 10.1016/j.repbio.2020.02.005
113
Sun J. Cui K. Li Z. Lu X. Xu Z. Liu Q. et al (2017). Suberoylanilide Hydroxamic Acid, a Novel Histone Deacetylase Inhibitor, Improves the Development and Acetylation Level of Miniature Porcine Handmade Cloning Embryos. Reprod. Dom. Anim.52 (5), 763–774. 10.1111/rda.12977
114
Sun J. Liu Q. Lv L. Sun R. Li Z. P. Huang B. et al (2021). HDAC6 Is Involved in the Histone Deacetylation of In Vitro Maturation Oocytes and the Reprogramming of Nuclear Transplantation in Pig. Reprod. Sci.28 (9), 2630–2640. 10.1007/s43032-021-00533-2
115
Tang F. Kaneda M. O’Carroll D. Hajkova P. Barton S. C. Sun Y. A. et al (2007). Maternal microRNAs Are Essential for Mouse Zygotic Development. Genes. Dev.21 (6), 644–648. 10.1101/gad.418707
116
Tao C. Li J. Zhang X. Chen B. Chi D. Zeng Y. et al (2017). Dynamic Reorganization of Nucleosome Positioning in Somatic Cells after Transfer into Porcine Enucleated Oocytes. Stem Cell. Rep.9 (2), 642–653. 10.1016/j.stemcr.2017.06.004
117
Taweechaipaisankul A. Jin J.-X. Lee S. Kim G. A. Suh Y. H. Ahn M. S. et al (2019a). Improved Early Development of Porcine Cloned Embryos by Treatment with Quisinostat, a Potent Histone Deacetylase Inhibitor. J. Reproduction Dev.65 (2), 103–112. 10.1262/jrd.2018-098
118
Taweechaipaisankul A. Kim G. A. Jin J. X. Lee S. Qasim M. Kim E. H. et al (2019b). Enhancement of Epigenetic Reprogramming Status of Porcine Cloned Embryos with Zebularine, a DNA Methyltransferase Inhibitor. Mol. Reprod. Dev.86 (8), 1013–1022. 10.1002/mrd.23178
119
Teranishi T. Tanaka M. Kimoto S. Ono Y. Miyakoshi K. Kono T. et al (2004). Rapid Replacement of Somatic Linker Histones with the Oocyte-specific Linker Histone H1foo in Nuclear Transfer. Dev. Biol.266 (1), 76–86. 10.1016/j.ydbio.2003.10.004
120
Wang H. Cui W. Meng C. Zhang J. Li Y. Qian Y. et al (2018). MC1568 Enhances Histone Acetylation during Oocyte Meiosis and Improves Development of Somatic Cell Nuclear Transfer Embryos in Pig. Cell. Reprogr.20 (1), 55–65. 10.1089/cell.2017.0023
121
Wang H. Luo Y. Lin Z. Lee I.-W. Kwon J. Cui X.-S. et al (2015). Effect of ATM and HDAC Inhibition on Etoposide-Induced DNA Damage in Porcine Early Preimplantation Embryos. PLoS One10 (11), e0142561. 10.1371/journal.pone.0142561
122
Wang P. Li X. Cao L. Huang S. Li H. Zhang Y. et al (2017). MicroRNA-148a Overexpression Improves the Early Development of Porcine Somatic Cell Nuclear Transfer Embryos. PLoS One12 (6), e0180535. 10.1371/journal.pone.0180535
123
Wang X. Shi J. Cai G. Zheng E. Liu D. Wu Z. et al (2019). Overexpression of MBD3 Improves Reprogramming of Cloned Pig Embryos. Cell. Reprogr.21 (5), 221–228. 10.1089/cell.2019.0008
124
Wang Y. Li Y. Luan D. Kang J. He R. Zhang Y. et al (2020). Dynamic Replacement of H3.3 Affects Nuclear Reprogramming in Early Bovine SCNT Embryos. Theriogenology154, 43–52. 10.1016/j.theriogenology.2020.05.031
125
Wen D. Banaszynski L. A. Liu Y. Geng F. Noh K.-M. Xiang J. et al (2014a). Histone Variant H3.3 Is an Essential Maternal Factor for Oocyte Reprogramming. Proc. Natl. Acad. Sci. U.S.A.111 (20), 7325–7330. 10.1073/pnas.1406389111
126
Wen D. Banaszynski L. A. Rosenwaks Z. Allis C. D. Rafii S. (2014b). H3.3 Replacement Facilitates Epigenetic Reprogramming of Donor Nuclei in Somatic Cell Nuclear Transfer Embryos. Nucleus5 (5), 369–375. 10.4161/nucl.36231
127
Weng X.-g. Cai M.-m. Zhang Y.-t. Liu Y. Liu C. Liu Z.-h. (2020). Improvement in the In Vitro Development of Cloned Pig Embryos after Kdm4a Overexpression and an H3K9me3 Methyltransferase Inhibitor Treatment. Theriogenology146, 162–170. 10.1016/j.theriogenology.2019.11.027
128
Whitworth K. M. Mao J. Lee K. Spollen W. G. Samuel M. S. Walters E. M. et al (2015). Transcriptome Analysis of PigIn Vivo,In Vitro-Fertilized, and Nuclear Transfer Blastocyst-Stage Embryos Treated with Histone Deacetylase Inhibitors Postfusion and Activation Reveals Changes in the Lysosomal Pathway. Cell. Reprogr.17 (4), 243–258. 10.1089/cell.2015.0022
129
Wilmut I. Schnieke A. E. McWhir J. Kind A. J. Campbell K. H. S. (1997). Viable Offspring Derived from Fetal and Adult Mammalian Cells. Nature385 (6619), 810–813. 10.1038/385810a0
130
Xie B. Zhang H. Wei R. Li Q. Weng X. Kong Q. et al (2016). Histone H3 Lysine 27 Trimethylation Acts as an Epigenetic Barrier in Porcine Nuclear Reprogramming. Reproduction151 (1), 9–16. 10.1530/REP-15-0338
131
Xie Y. Cao H. Zhang Z. Zhang S. Wang H. (2019). Molecular Network of miR-1343 Regulates the Pluripotency of Porcine Pluripotent Stem Cells via Repressing OTX2 Expression. RNA Biol.16 (1), 82–92. 10.1080/15476286.2018.1559688
132
Xu W. Li Z. Yu B. He X. Shi J. Zhou R. et al (2013). Effects of DNMT1 and HDAC Inhibitors on Gene-specific Methylation Reprogramming during Porcine Somatic Cell Nuclear Transfer. PLoS One8 (5), e64705. 10.1371/journal.pone.0064705
133
Yamanaka K.-i. Sugimura S. Wakai T. Kawahara M. Sato E. (2009). Acetylation Level of Histone H3 in Early Embryonic Stages Affects Subsequent Development of Miniature Pig Somatic Cell Nuclear Transfer Embryos. J. Reproduction Dev.55 (6), 638–644. 10.1262/jrd.20245
134
Yang L. Liu X. Song L. Di A. Su G. Bai C. et al (2020). Transient Dux Expression Facilitates Nuclear Transfer and Induced Pluripotent Stem Cell Reprogramming. EMBO Rep.21 (9), e50054. 10.15252/embr.202050054
135
Yang X. Wu X. Yang Y. Gu T. Hong L. Zheng E. et al (2019). Improvement of Developmental Competence of Cloned Male Pig Embryos by Short Hairpin Ribonucleic Acid (shRNA) Vector-Based but Not Small Interfering RNA (siRNA)-Mediated RNA Interference (RNAi) of Xist Expression. J. Reproduction Dev.65 (6), 533–539. 10.1262/jrd.2019-070
136
Yu S. Zhang R. Shen Q. Zhu Z. Zhang J. Wu X. et al (2021). ESRRB Facilitates the Conversion of Trophoblast-like Stem Cells from Induced Pluripotent Stem Cells by Directly Regulating CDX2. Front. Cell. Dev. Biol.9, 712224. 10.3389/fcell.2021.712224
137
Yuan L. Wang A. Yao C. Huang Y. Duan F. Lv Q. et al (2014). Aberrant Expression of Xist in Aborted Porcine Fetuses Derived from Somatic Cell Nuclear Transfer Embryos. Ijms15 (12), 21631–21643. 10.3390/ijms151221631
138
Yun Y. Zhao G.-m. Wu S.-j. Li W. Lei A.-m. (2012). Replacement of H1 Linker Histone during Bovine Somatic Cell Nuclear Transfer. Theriogenology78 (6), 1371–1380. 10.1016/j.theriogenology.2012.06.004
139
Zeng F. Huang Z. Yuan Y. Shi J. Cai G. Liu D. et al (2016). Effects of RNAi-Mediated Knockdown of Xist on the Developmental Efficiency of Cloned Male Porcine Embryos. J. Reproduction Dev.62 (6), 591–597. 10.1262/jrd.2016-095
140
Zhai Y. Zhang Z. Yu H. Su L. Yao G. Ma X. et al (2018). Dynamic Methylation Changes of DNA and H3K4 by RG108 Improve Epigenetic Reprogramming of Somatic Cell Nuclear Transfer Embryos in Pigs. Cell. Physiol. Biochem.50 (4), 1376–1397. 10.1159/000494598
141
Zhang J. Hao L. Wei Q. Zhang S. Cheng H. Zhai Y. et al (2020). TET3 Overexpression Facilitates DNA Reprogramming and Early Development of Bovine SCNT Embryos. Reproduction160 (3), 379–391. 10.1530/REP-20-0021
142
Zhang J. Qu P. Zhou C. Liu X. Ma X. Wang M. et al (2017a). MicroRNA-125b Is a Key Epigenetic Regulatory Factor that Promotes Nuclear Transfer Reprogramming. J. Biol. Chem.292 (38), 15916–15926. 10.1074/jbc.M117.796771
143
Zhang L. Huang Y. Wu Y. Si J. Huang Y. Jiang Q. et al (2017b). Scriptaid Upregulates Expression of Development-Related Genes, Inhibits Apoptosis, and Improves the Development of Somatic Cell Nuclear Transfer Mini-Pig Embryos. Cell. Reprogr.19 (1), 19–26. 10.1089/cell.2016.0033
144
Zhang Y. Li J. Villemoes K. Pedersen A. M. Purup S. Vajta G. (2007). An Epigenetic Modifier Results in Improved In Vitro Blastocyst Production after Somatic Cell Nuclear Transfer. Cloning Stem Cells9 (3), 357–363. 10.1089/clo.2006.0090
145
Zhang Y. Wang Q. Liu K. Gao E. Guan H. Hou J. (2018a). Treatment of Donor Cells with Recombinant KDM4D Protein Improves Preimplantation Development of Cloned Ovine Embryos. Cytotechnology70 (5), 1469–1477. 10.1007/s10616-018-0224-6
146
Zhang Z. Zhai Y. Ma X. Zhang S. An X. Yu H. et al (2018b). Down-Regulation of H3K4me3 by MM-102 Facilitates Epigenetic Reprogramming of Porcine Somatic Cell Nuclear Transfer Embryos. Cell. Physiol. Biochem.45 (4), 1529–1540. 10.1159/000487579
147
Zhao J. Hao Y. Ross J. W. Spate L. D. Walters E. M. Samuel M. S. et al (2010). Histone Deacetylase Inhibitors ImproveIn VitroandIn VivoDevelopmental Competence of Somatic Cell Nuclear Transfer Porcine Embryos. Cell. Reprogr.12 (1), 75–83. 10.1089/cell.2009.0038
148
Zhao J. Ross J. W. Hao Y. Spate L. D. Walters E. M. Samuel M. S. et al (2009). Significant Improvement in Cloning Efficiency of an Inbred Miniature Pig by Histone Deacetylase Inhibitor Treatment after Somatic Cell Nuclear Transfer1. Biol. Reprod.81 (3), 525–530. 10.1095/biolreprod.109.077016
149
Zhou C. Wang Y. Zhang J. Su J. An Q. Liu X. et al (2019). H3K27me3 Is an Epigenetic Barrier while KDM6A Overexpression Improves Nuclear Reprogramming Efficiency. FASEB J.33 (3), 4638–4652. 10.1096/fj.201801887R
Summary
Keywords
pig, cloning, SCNT, embryo development, chromatin, epigenetics, histone acetylation, histone methylation
Citation
Glanzner WG, de Macedo MP, Gutierrez K and Bordignon V (2022) Enhancement of Chromatin and Epigenetic Reprogramming in Porcine SCNT Embryos—Progresses and Perspectives. Front. Cell Dev. Biol. 10:940197. doi: 10.3389/fcell.2022.940197
Received
10 May 2022
Accepted
20 June 2022
Published
11 July 2022
Volume
10 - 2022
Edited by
Sadie L. Marjani, Central Connecticut State University, United States
Reviewed by
Bojiang Li, Shenyang Agricultural University, China
Jingyue Ellie Duan, Cornell University, United States
Updates
Copyright
© 2022 Glanzner, de Macedo, Gutierrez and Bordignon.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vilceu Bordignon, vilceu.bordignon@mcgill.ca
This article was submitted to Epigenomics and Epigenetics, a section of the journal Frontiers in Cell and Developmental Biology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.