- 1Department of Prenatal Diagnosis and Fetal Medicine, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
- 2Department of Reproduction Medical Center, The Third Affiliated Hospital of Shenzhen University, Shenzhen, China
Placenta-mediated pregnancy complications (PMPCs), including preeclampsia (PE), fetal growth restriction (FGR), and recurrent spontaneous abortion (RSA), occur in approximately 5% of pregnancies and are caused by abnormal placenta development. The development of effective therapies for PMPCs is still challenging due to the complicated pathogenesis, such as disrupted vascular homeostasis and subsequent abnormal placentation. Synthetic drugs have been recommended for treating PMPCs; however, they tend to cause adverse reactions in the mother and fetus. Salvia miltiorrhiza (S. miltiorrhiza) has potential effects on PMPCs owing to its advantages in treating cardiovascular disorders. S. miltiorrhiza and its active compounds could attenuate the symptoms of PMPCs through anticoagulation, vasodilation, antioxidation, and endothelial protection. Thus, in this review, we summarize the literature and provide comprehensive insights on S. miltiorrhiza and its phytochemical constituents, pharmacological activities, and on PMPCs, which would be valuable to explore promising drugs.
1 Introduction
The human placenta, a specialized organ that mediates exchanges between the mother and fetus, is essential for a successful pregnancy and fetal health. Its development begins during the implantation of the blastocyst (Hemberger et al., 2020). Chorionic villi, as structural and functional units of the placenta, are consisted of two layers of trophoblasts (James et al., 2022). The inner layer is composed of proliferative villous cytotrophoblasts (vCTBs), which can differentiate into outer layer villous syncytiotrophoblasts that form a physical barrier to pathogens (Turco and Moffett, 2019). Cytotrophoblast cells invade the maternal spiral arteries and replace the maternal endothelium. The remodeling of maternal spiral arteries reduces the resistance of blood flow to meet the nutrition transport for fetus (Turco and Moffett, 2019; James et al., 2022). Defective trophoblast differentiation and function cause incomplete spiral artery remodeling, contributing to PMPCs (Staff et al., 2022). However, the physiopathological mechanism of PMPCs has yet to be elucidated (Freitag et al., 2020; Wu et al., 2020; Staff et al., 2022).
PMPCs result in high maternal and neonatal morbidity rates as aforementioned (Kuwabara et al., 2020). Synthetic drugs have been recommended to treat PMPCs (Chappell et al., 2021). In particular, low-dose aspirin can attenuate the symptoms of PE (Hodgetts Morton and Stock, 2022; Walsh and Strauss, 2022). However, synthetic drugs have adverse reactions in the mother and fetus. In contrast, traditional Chinese medicine (TCM), with a long usage history, has drawn increasing attention in recent years due to its fewer side effects (Yang et al., 2022). Clinicians have started treating PMPCs with TCM compounds, achieving satisfactory therapeutic effects (Zhang et al., 2006).
S. miltiorrhiza, known as Danshen in Chinese, is a perennial plant of the Lamiaceae family (Zeng et al., 2017). Modern pharmacological studies have found that S. miltiorrhiza affects the promotion of blood circulation, modulation of vascular endothelial cells, and reduction of immune interactions in the mother-fetus interface. S. miltiorrhiza injection, derived from S. miltiorrhiza extract, plays a remarkable role in treating PMPCs (Bai et al., 2019; Chen and Yang, 2019). Herein, we review the active compounds, potential effects, and the pharmacological mechanisms of S. miltiorrhiza in PMPCs (Figure 1).
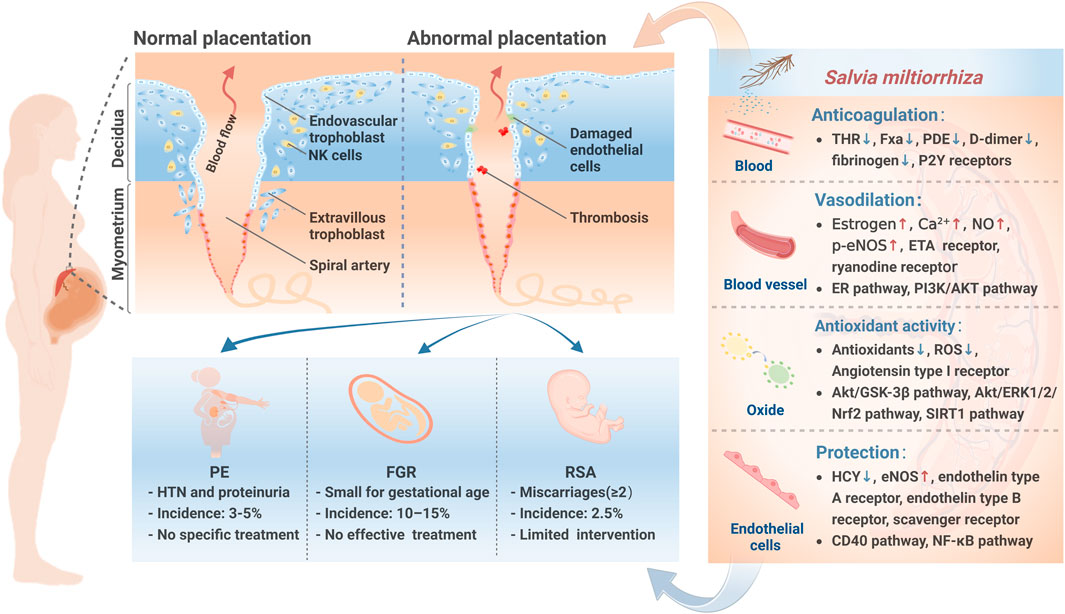
FIGURE 1. PMPCs pathophysiology and the mechanisms of S. miltiorrhiza in PMPCs. During normal placentation, extravillous cytotrophoblasts invade the uterine spiral arteries of the decidua andmyometrium, transforming the spiral arteries into large-caliber vessels. However, during abnormal placentation in PMPCs, failed spiral artery remodeling constrains maternal-fetal interface blood flow, which contributes to the pathogenesis of PMPCs. NK, natural killer; PE, preeclampsia; FGR, fetal growth restriction; RSA, recurrent spontaneous abortion; HTN, hypertension; THR, thrombin; Fxa, factor Xa; NO, nitric oxide; p-eNOS, phosnhorylation of endothelial nitric oxide synthase; ETA, endothelin A; ROS, reactive oxygen species; HCY, homocysteine.
2 Active compounds of S. miltiorrhiza in PMPCs
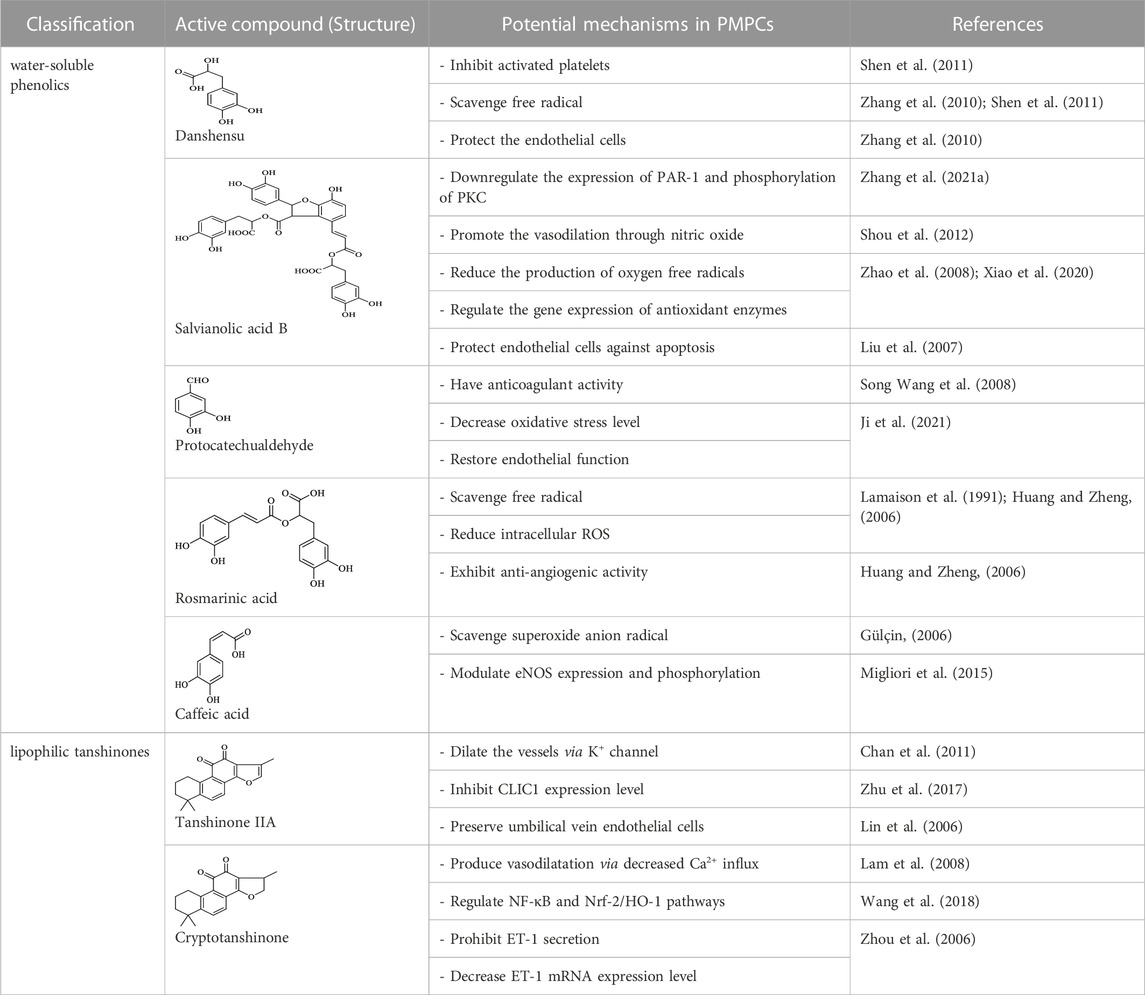
S. miltiorrhiza shows extensive biological activities, including antioxidant, antibacterial and anti-inflammatory. Thus, it is widely used for the treatment of various diseases, containing hyperlipidemia, stroke, and cardiovascular and cerebrovascular diseases (Chong et al., 2019). S. miltiorrhiza is first described in TCM in the Compendium of Materia Medica (Bencao Gangmu, Ming dynasty, 1596 AD). The primary bioactive compounds in S. miltiorrhiza are devided into two major groups of chemicals (Zhang et al., 2022). One group involves water-soluble phenolics, such as salvianolic acid A (Sal A), salvianolic acid B (Sal B), lithospermic acid and rosmarinic acid (Wang et al., 2019). The other group is consisting of lipophilic compounds, such as tanshinone I, tanshinone IIA (Tan IIA), tanshinone IIB, cryptotanshinone, and dihydrotanshinone I (Wang et al., 2020). Hence, we outline the valuable active compounds of S. miltiorrhiza associated with PMPCs, as listed in Table 1.
3 Effects of S. miltiorrhiza in PMPCs
3.1 S. miltiorrhiza ameliorates PE
PE is a serious condition characterized by hypertension and proteinuria after 20 weeks of pregnancy (Vata et al., 2015), with an incidence rate of 3%–5% and at least 42,000 maternal deaths yearly (Chappell et al., 2021). PE is a severe threat to maternal and fetal health during pregnancy and childbirth and increases the long-term risk of cardiovascular diseases in mothers and their fetuses (Staff, 2019). It is divided into early-onset and late-onset types (Lisonkova and Joseph, 2013). PE presents reduced trophoblast invasion and defective spiral artery remodeling, which triggers a series of pathophysiological processes, such as antiangiogenesis, vascular inflammation and oxidative stress, resulting in systemic endothelial dysfunction and clinical manifestations (Ortega et al., 2022). The blood vessels of a patient with PE narrowed because of impaired trophoblast invasion and incomplete spiral artery remodeling. The placenta is deprived of blood and oxygen, resulting in abnormal placentation (Hong et al., 2021). S. miltiorrhiza has been used to treat PE because of its ability to increase blood flow; however, the mechanism is not fully understood (Zhang et al., 2006).
S. miltiorrhiza injection upregulates the serum insulin-like growth factor-1 and placental growth factor, which enhances the invasion and migration abilities of placental trophoblastic cells and improves the condition of patients with early-onset PE and the prognosis of the mother and the fetus (Li and Qiu, 2020). After the treatment, the systolic and diastolic blood pressures and 24 h urine protein levels decrease remarkably. S. miltiorrhiza also improves vascular endothelial function and ischemia-hypoxia status in patients with PE. And there is, abnormal expression of long-chain non-coding RNA (lncRNA) in the placenta of patients with PE (Liang and Meng, 2021). These lncRNAs may cause changes in the expression of downstream regulatory target genes, thereby reducing the invasion of trophoblasts and leading to uterine spiral artery remodeling disorders (Cheng et al., 2019). S. miltiorrhiza injection prevents PE from progressing to severe PE or eclampsia and improves maternal and infant outcomes through downregulating the expression of lncRNAs in the placental tissues (Ma et al., 2021). Furthermore, in PE animal models, S. miltiorrhiza injection effectively lower blood pressure and alleviate proteinuria to normal levels by increasing platelet count and reducing thrombomodulin expression in the placenta (Shen et al., 2011; Peng and Huang, 2021). The decrease of endogenous and exogenous coagulation factors, factor Xa (FXa), D-dimer, and fibrinogen indicates that the coagulation in PE rat models improved. And long-term low-dose Sal A administration exerts better efficacy through enhancing anticoagulant activity (Shen et al., 2011). Therefore, S. miltiorrhiza injection could effectively ameliorate placenta-related indicators and vascular endothelial function in PE (Jiao et al., 2021; Zhang et al., 2021b).
3.2 S. miltiorrhiza mitigates the severity of FGR
FGR is a severe pregnancy-related disease wherein fetuses cannot achieve the expected weight. It is a pivotal cause of stillbirths and an essential factor affecting the long-term health of the fetus. FGR is mainly caused by abnormal placentation due to insufficient placental development and microvascular resistance, which causes blood circulation disorders in 10%–15% of pregnant women (Aplin et al., 2020; Wang et al., 2020; Ortega et al., 2022). It is divided into early-onset and late-onset according to the gestational age at onset. Early-onset FGR occurs before 32 weeks of pregnancy, accounting for 20% of all cases. Late-onset FGR (≥32 weeks) occurs in about 70% of patients and has a weak correlation with hypertension (approximately 10%) (Audette and Kingdom, 2018). FGR can be attributed to maternal (such as malnutrition, hypertension, PE) and fetal factors (such as chromosomal abnormalities and multiple births) and placental dysfunction. However, placental dysfunction is the most frequent underlying cause of FGR (Aplin et al., 2020; Freitag et al., 2020; Melamed et al., 2021). Incomplete invasion of vCTBs results in inhibition of placental growth, impairment of placental function, long-term hypoxia, and fetus malnutrition. The combination of sodium lactate Ringer’s injection and S. miltiorrhiza is more effective than sodium lactate Ringer’s injection alone (Wei et al., 2021).
3.3 S. miltiorrhiza improves the adverse symptoms of RSA
RSA, defined as the failure of two or more clinically recognized pregnancies before 20–24 weeks of gestation, occurs in nearly 2.5% of women trying to conceive. The etiology of RSA is still not fully understood. Trophoblast cells are the most critical cells in placental development, and their proliferation, migration, and invasion are essential for establishing and maintaining a successful pregnancy. Defective trophoblast function impairs uterine spiral artery reconstruction and is implicated in RSA (Wu et al., 2020). Currently, RSA is mainly treated with immunotherapy and anticoagulation, but these therapies have no specificity (Pan et al., 2022).
In RSA mouse models, Tan IIA is ascertained to reduce the rate of embryo loss (Tong et al., 2022). S. miltiorrhiza injection has a specific curative effect on patients with RSA through improving the trophoblast cell function and prethrombotic state (Liu et al., 2021). However, relevant studies remain limited and further studies are needed to elucidate the mechanism of S. miltiorrhiza in RSA.
4 Potential mechanisms of S. miltiorrhiza in PMPCs
4.1 Anticoagulation
The precise regulation of blood coagulation is critical in maintaining a successful pregnancy (True et al., 2022). The blood coagulation cascade is a complicated process regulated by plasma proteins and cofactors affected by different coagulation factors (Davie, 1995). Human pregnancy involves hemochorial placentation wherein the villous covered by a trophoblast layer is subdivided into functional units bathed by maternal blood, ensuring maternal-fetal exchanges (Kohli et al., 2022). Meanwhile, pregnant women are at high risk of hemorrhage, organ-specific thrombosis, and thromboinflammation (Kohli et al., 2022). Decidual thrombosis and spontaneous intrauterine umbilical artery thrombosis are associated with FGR, placental abruption, PE, and preterm birth (Vedmedovska et al., 2011). Thus, inhibiting coagulation is a promising strategy to treat PMPCs (Boeldt and Bird, 2017). Thrombin (THR), which is closely involved with the occurrence of thrombosis and embolism, and FXa, which is a common mediator of intrinsic and extrinsic coagulation, play crucial roles in the coagulation cascade (Yang et al., 2020). Some components of S. miltiorrhiza have been reported in response to these key events. Tan IIA, tanshinone I, dihydrotanshinone I, and cryptotanshinone, act as THR/FXa inhibitors, thereby destroying the coagulation cascade to achieve anticoagulation (Yang et al., 2020). Danshensu, one of the active compounds of S. miltiorrhiza, strongly mitigates blood viscosity and increases hematocrit levels due to its antithrombotic and antiplatelet aggregation effects (Yu et al., 2014). Moreover, S. miltiorrhiza injection substantially improves coagulation in PE rats (Peng and Huang, 2021). Notably, three major active compounds of S. miltiorrhiza (Sal A, B, and C) function by targeting the prothrombotic P2Y1 and P2Y12 receptors (Liu et al., 2018). Sal B inhibits platelet activation by decreasing phosphodiesterase activity and antagonizing the P2Y12 receptors (Liu et al., 2014). Coactivation of both P2Y receptors plays an essential role in ADP-induced platelet aggregation, whereas the inhibition of both receptors has a synergistic effect on antithrombotic therapy (Liu et al., 2018). However, to date, there are currently few commercial drugs targeting the P2Y receptors.
4.2 Vasodilation
A successful pregnancy is associated with dramatic changes in the uterine blood flow, facilitating the maternal-fetal exchanges of respiratory gas and meeting the needs of the developing fetus (Bowman et al., 2021). Impaired endothelium-dependent vasodilation has been implicated in the development of PE. Danshensu directly acts on vascular endothelial and smooth muscle cells to promote vascular relaxation (Wang et al., 2013; Lin et al., 2022). It also dilates the vessels and improves blood circulation to increase renal blood flow and improve renal function in PE rats (Peng and Huang, 2021). The mechanisms underlying pregnancy-associated uterine vasodilation are related to increased estrogen receptor (ER) levels, which drive the production of specific ER-dependent vasodilators in the uterine artery (Bai et al., 2020). Tan IIA exerts its vasodilation effect through activating the ER signal pathway and increasing endothelial nitric oxide synthase (eNOS) gene expression level, nitric oxide (NO) production, ERK1/2 phosphorylation, and Ca2+ mobilization (Fan et al., 2011). It also promotes vasodilatation by decreasing the expression of the endothelin A receptor, which is a primary receptor in modulating vasoconstriction (Chen et al., 2017). Magnesium acetate, an active compound of S. miltiorrhiza, dilates blood vessels through activating the PI3K/AKT pathway and increasing the phosphorylation of eNOS (Liu et al., 2019). Sal B is a potentially effective natural compound to lower blood pressure and alleviate hypertension-associated vascular dysfunctions (Ling et al., 2017). It mediates vasodilation by inhibiting extracellular calcium influx and intracellular calcium release. The calcium release mechanism relies on the ryanodine receptor family, one of the families of calcium release channels (Shou et al., 2012).
4.3 Antioxidant
Oxidative stress is widely believed to disrupt the balance between ROS and the antioxidant system (Hussain et al., 2021). During pregnancy, nutritional deficiencies result in adverse offspring outcomes (Sebastiani et al., 2019). Excessive oxidative stress impairs maternal and placental functions by limiting the antioxidant supply and eventually results in the decreased metabolic health of offspring (Schoots et al., 2018; Nadeem et al., 2019; Burton et al., 2021). Cryptotanshinone improves doxorubicin-induced oxidative damage and apoptosis through inhibiting the opening of the mitochondrial permeability transition pore via the Akt/GSK-3β pathway (Wang et al., 2021). Danshensu has a protective effect against oxidative stress during ischemia-reperfusion injury through ROS scavenging, and it enhances the activity of endogenous antioxidants, such as superoxide dismutase, catalase and malondialdehyde, through activating the Akt/ERK1/2/Nrf2 signaling pathway (Yu et al., 2015). Tan ⅡA exerts robust antioxidant activity through the SIRT1 signaling pathway (Feng et al., 2016). It also reduces the accumulation of free radicals in radioactive brain injuries (Sun et al., 2017). Sal A is essential to protect cells from damage caused by toxic stimuli (Wang and Xu, 2005). Sal B protects against oxidative damage by upregulating the Nrf2 antioxidant signaling pathway, which may be regulated by activating the SIRT1 pathway (Zhang et al., 2018). Through angiotensin type I receptors, angiotensin II activates the reduced nicotinamide adenine dinucleotide phosphate, which results in the formation of ROS in the vasculature (Gonzaga et al., 2018). In this progress, Sal B also downregulates angiotensin type I receptors in the vessel wall to alleviate the deleterious effect of angiotensin II, an essential stimulant for the production of ROS in the vascular system (Ling et al., 2017).
4.4 Endothelial protective effect
The endothelium, formed by a single endothelial cell layer, lines all blood vessels, such as arterioles, venules and veins (Lee et al., 2022). The endothelium regulates blood homeostasis via controlling blood fluidity, continuity, and fibrinolysis (Triggle et al., 2012). Endothelial cells have proteins involved in the various functions of leukocytes (Panés and Granger, 1998). Dysfunction and altered structure of the endothelial layer during pregnancy are associated with PMPCs (Ghafourian et al., 2022). Danshensu protects endothelial cells via the CD40 pathway and inhibition of apoptosis by downregulating the proportion of cells in the G(0)/G(1) phase (Yang et al., 2009). In addition, it reduces the serum levels of homocysteine, a substance damaging endothelial cells (Yang et al., 2010). Tan ⅡA inhibits endothelial cell apoptosis by reducing the expression of related apoptotic proteins via the NF-κB signaling pathway, thereby exerting a protective effect on vascular endothelial cells (Liu et al., 2021). It also protects endothelial function through inhibiting endothelin-1 expression, decreasing endothelin type A receptors, increasing endothelin type B receptors, and upregulating eNOS (Chen et al., 2017). Cryptotanshinone’s endothelium protective action is mainly associated with the reduction of endothelial inflammation. In particular, cryptotanshinone blocks the scavenger receptor LOX1-mediated pro-inflammatory response in endothelial cells, preventing monocyte adhesion to endothelial cells (Li et al., 2018). Further researches are also necessary to determine the potential impact of cryptotanshinone on other crucial aspects of endothelium protection.
The above effects and mechanisms suggest that S. miltiorrhiza ameliorates adverse cardiovascular symptoms. Since PMPCs are characterized by insufficient blood perfusion, vascular endothelial dysfunction, and abnormal coagulation, S. miltiorrhiza and its active compounds can be applied in PMPCs (Bai et al., 2019). Thus, we profile the potential mechanisms of S. miltiorrhiza for PMPCs in Table 1.
5 Conclusion
In summary, PMPCs are a heterogeneous disease with similar mechanisms, including reduced trophoblast cell invasion and insufficient spiral artery remodeling, which results in placental hypoperfusion, endothelial dysfunction, and abnormal coagulation. S. miltiorrhiza effectively attenuates the symptoms of PMPCs through anticoagulation, vasodilation, inhibition of free radical formation, and protection of endothelial cells. Notebly, S. miltiorrhiza and its active compounds have been shown to treat PE, mitigate the severity of FGR, and improve the adverse symptoms of RSA. Hence, S. miltiorrhiza may be used to improve the pregnancy outcomes of pregnant women with PMPCs effectively. However, the specific effects of S. miltiorrhiza on PMPCs still need clinical verification, although animal models have provided much more valuable clues. In vitro and in vivo studies are required to clarify the related signaling pathways of. New techniques are needed to study human placental development and provide optimal therapy for patients with PMPCs.
Author contributions
MC and YL conceived the context. JK, SL, MC, and YL drafted the manuscript. JK prepared the figure and the table. All authors crucially revised the manuscript for important intellectual content and approved the final version to be published.
Funding
This study was partly supported by the Guangzhou Science and Technology Program (No. 202102010129).
Acknowledgments
Written informed consent was obtained from the BioRender (https://biorender.com/) for the publication of any potentially identifiable images or data included in this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aplin, J. D., Myers, J. E., Timms, K., and Westwood, M. (2020). Tracking placental development in health and disease. Nat. Rev. Endocrinol. 16 (9), 479–494. doi:10.1038/s41574-020-0372-6
Audette, M. C., and Kingdom, J. C. (2018). Screening for fetal growth restriction and placental insufficiency. Semin. Fetal Neonatal Med. 23 (2), 119–125. doi:10.1016/j.siny.2017.11.004
Bai, J., Qi, Q. R., Li, Y., Day, R., Makhoul, J., Magness, R. R., et al. (2020). Estrogen receptors and estrogen-induced uterine vasodilation in pregnancy. Int. J. Mol. Sci. 21 (12), 4349. doi:10.3390/ijms21124349
Bai, X., Huang, G., and Zhao, S. (2019). Clinical application prospect of Salvia miltiorrhiza combined with low molecular weight heparin in recurrent abortion based on prethrombotic state. World Latest Med. Inf. 19 (07), 6–8. doi:10.19613/j.cnki.1671-3141.2019.07.004
Boeldt, D. S., and Bird, I. M. (2017). Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J. Endocrinol. 232 (1), R27–R44. doi:10.1530/joe-16-0340
Bowman, C. E., Arany, Z., and Wolfgang, M. J. (2021). Regulation of maternal-fetal metabolic communication. Cell. Mol. Life Sci. 78 (4), 1455–1486. doi:10.1007/s00018-020-03674-w
Burton, G. J., Cindrova-Davies, T., Yung, H. W., and Jauniaux, E. (2021). Hypoxia and reproductive health: Oxygen and development of the human placenta. Reproduction 161 (1), F53–f65. doi:10.1530/rep-20-0153
Chan, P., Liu, I. M., Li, Y. X., Yu, W. J., and Cheng, J. T. (2011). Antihypertension induced by tanshinone IIA isolated from the roots of Salvia miltiorrhiza. Evid. Based Complement. Altern. Med. 2011, 392627. doi:10.1093/ecam/nep056
Chappell, L. C., Cluver, C. A., Kingdom, J., and Tong, S. (2021). Pre-eclampsia. Lancet 398 (10297), 341–354. doi:10.1016/s0140-6736(20)32335-7
Chen, L., Guo, Q. H., Chang, Y., Zhao, Y. S., Li, A. Y., and Ji, E. S. (2017). Tanshinone IIA ameliorated endothelial dysfunction in rats with chronic intermittent hypoxia. Cardiovasc Pathol. 31, 47–53. doi:10.1016/j.carpath.2017.06.008
Chen, W., and Yang, K. (2019). The influence of compound Danshen injection combined with low-molecular-weight heparin for treating women with early onset severe preeclampsia on their heart kidney function, and its mechanism. Chin. J. Fam. Plan. 27 (10), 1321–1325. doi:10.3969/j.issn.1004-8189.2007.04.027
Cheng, D., Jiang, S., Chen, J., Li, J., Ao, L., and Zhang, Y. (2019). Upregulated long noncoding RNA Linc00261 in pre-eclampsia and its effect on trophoblast invasion and migration via regulating miR-558/TIMP4 signaling pathway. J. Cell. Biochem. 120 (8), 13243–13253. doi:10.1002/jcb.28598
Chong, C. M., Su, H., Lu, J. J., and Wang, Y. (2019). The effects of bioactive components from the rhizome of Salvia miltiorrhiza (Danshen) on the characteristics of Alzheimer's disease. Chin. Med. 14, 19. doi:10.1186/s13020-019-0242-0
Davie, E. W. (1995). Biochemical and molecular aspects of the coagulation cascade. Thromb. Haemost. 74 (1), 001–006. doi:10.1055/s-0038-1642645
Fan, G., Zhu, Y., Guo, H., Wang, X., Wang, H., and Gao, X. (2011). Direct vasorelaxation by a novel phytoestrogen tanshinone IIA is mediated by nongenomic action of estrogen receptor through endothelial nitric oxide synthase activation and calcium mobilization. J. Cardiovasc Pharmacol. 57 (3), 340–347. doi:10.1097/FJC.0b013e31820a0da1
Feng, J., Li, S., and Chen, H. (2016). Tanshinone IIA inhibits myocardial remodeling induced by pressure overload via suppressing oxidative stress and inflammation: Possible role of silent information regulator 1. Eur. J. Pharmacol. 791, 632–639. doi:10.1016/j.ejphar.2016.09.041
Freitag, N., Tirado-Gonzalez, I., Barrientos, G., Powell, K. L., Boehm-Sturm, P., Koch, S. P., et al. (2020). Galectin-3 deficiency in pregnancy increases the risk of fetal growth restriction (FGR) via placental insufficiency. Cell. Death Dis. 11 (7), 560. doi:10.1038/s41419-020-02791-5
Ghafourian, M., Mahdavi, R., Akbari Jonoush, Z., Sadeghi, M., Ghadiri, N., Farzaneh, M., et al. (2022). The implications of exosomes in pregnancy: Emerging as new diagnostic markers and therapeutics targets. Cell. Commun. Signal 20 (1), 51. doi:10.1186/s12964-022-00853-z
Gonzaga, N. A., do Vale, G. T., Parente, J. M., Yokota, R., De Martinis, B. S., Casarini, D. E., et al. (2018). Ethanol withdrawal increases blood pressure and vascular oxidative stress: A role for angiotensin type 1 receptors. J. Am. Soc. Hypertens. 12 (7), 561–573. doi:10.1016/j.jash.2018.03.012
Gülçin, I. (2006). Antioxidant activity of caffeic acid (3, 4-dihydroxycinnamic acid). Toxicology 217 (2-3), 213–220. doi:10.1016/j.tox.2005.09.011
Hemberger, M., Hanna, C. W., and Dean, W. (2020). Mechanisms of early placental development in mouse and humans. Nat. Rev. Genet. 21 (1), 27–43. doi:10.1038/s41576-019-0169-4
Hodgetts Morton, V., and Stock, S. J. (2022). Low-dose aspirin for the prevention of preterm birth: More questions than answers. PLoS Med. 19 (2), e1003908. doi:10.1371/journal.pmed.1003908
Hong, K., Kim, S. H., Cha, D. H., and Park, H. J. (2021). Defective uteroplacental vascular remodeling in preeclampsia: Key molecular factors leading to long term cardiovascular disease. Int. J. Mol. Sci. 22 (20), 11202. doi:10.3390/ijms222011202
Huang, S. S., and Zheng, R. L. (2006). Rosmarinic acid inhibits angiogenesis and its mechanism of action in vitro. Cancer Lett. 239 (2), 271–280. doi:10.1016/j.canlet.2005.08.025
Hussain, T., Murtaza, G., Metwally, E., Kalhoro, D. H., Kalhoro, M. S., Rahu, B. A., et al. (2021). The role of oxidative stress and antioxidant balance in pregnancy. Mediat. Inflamm. 2021, 9962860. doi:10.1155/2021/9962860
James, J. L., Lissaman, A., Nursalim, Y. N. S., and Chamley, L. W. (2022). Modelling human placental villous development: Designing cultures that reflect anatomy. Cell. Mol. Life Sci. 79 (7), 384. doi:10.1007/s00018-022-04407-x
Ji, B., Yuan, K., Li, J., Ku, B. J., Leung, P. S., and He, W. (2021). Protocatechualdehyde restores endothelial dysfunction in streptozotocin-induced diabetic rats. Ann. Transl. Med. 9 (8), 711. doi:10.21037/atm-21-1431
Jiao, S., Sun, S., Ban, X., Zhang, Y., and Xing, H. (2021). Effect of compound danshen injection combined with magnesium sulfate on placental circulation in patients with preeclampsia. China Pharm. 30 (05), 45–47. doi:10.1155/2021/9026223
Kohli, S., Shahzad, K., Jouppila, A., Holthöfer, H., Isermann, B., and Lassila, R. (2022). Thrombosis and inflammation-A dynamic interplay and the role of glycosaminoglycans and activated protein C. Front. Cardiovasc Med. 9, 866751. doi:10.3389/fcvm.2022.866751
Kuwabara, Y., Yonezawa, M., Kubota, Y., Ichikawa, T., Ohashi, R., and Takeshita, T. (2020). Unique clinical and histological features of placental mesenchymal dysplasia complicated by severe preeclampsia in the midtrimester. AJP Rep. 10 (1), e113–e117. doi:10.1055/s-0040-1709186
Lam, F. F., Yeung, J. H., Chan, K. M., and Or, P. M. (2008). Mechanisms of the dilator action of cryptotanshinone on rat coronary artery. Eur. J. Pharmacol. 578 (2-3), 253–260. doi:10.1016/j.ejphar.2007.09.040
Lamaison, J. L., Petitjean-Freytet, C., and Carnat, A. (1991). Medicinal Lamiaceae with antioxidant properties, a potential source of rosmarinic acid. Pharm. Acta Helv. 66 (7), 185–188.
Lee, H. W., Shin, J. H., and Simons, M. (2022). Flow goes forward and cells step backward: Endothelial migration. Exp. Mol. Med. 54 (6), 711–719. doi:10.1038/s12276-022-00785-1
Li, J., and Qiu, R. (2020). Study on effect of compound Salvia miltiorrhiza injection on levels of IGF-1 and PLGF and pregnancy outcome of preeclampsia patients with critical early onset. Chin. Archives Traditional Chin. Med. 38 (11), 251–254. doi:10.13193/j.issn.1673-7717.2020.11.060
Li, Z. M., Xu, S. W., and Liu, P. Q. (2018). Salvia miltiorrhizaBurge (danshen): A golden herbal medicine in cardiovascular therapeutics. Acta Pharmacol. Sin. 39 (5), 802–824. doi:10.1038/aps.2017.193
Liang, X., and Meng, Q. (2021). Efficacy of compound Salvia miltiorrhiza injection combined with magnesium sulfate in the treatment of preeclampsia and its effect on serum PLGF, sFlt-1 and sEng. Popular Sci. Technol. 23 (09), 56–59. doi:10.7759/cureus.17322
Lin, R., Wang, W. R., Liu, J. T., Yang, G. D., and Han, C. J. (2006). Protective effect of tanshinone IIA on human umbilical vein endothelial cell injured by hydrogen peroxide and its mechanism. J. Ethnopharmacol. 108 (2), 217–222. doi:10.1016/j.jep.2006.05.004
Lin, Y. K., Chen, Y. J., Li, J. Y., Chen, Y. L., He, D., Zuo, R., et al. (2022). Salvianolic acid A from Danhong Injection induces vasorelaxation by Regulating L-type calcium channel in isolated mouse arteries. J. Ethnopharmacol. 296, 115431. doi:10.1016/j.jep.2022.115431
Ling, W. C., Liu, J., Lau, C. W., Murugan, D. D., Mustafa, M. R., and Huang, Y. (2017). Treatment with salvianolic acid B restores endothelial function in angiotensin II-induced hypertensive mice. Biochem. Pharmacol. 136, 76–85. doi:10.1016/j.bcp.2017.04.007
Lisonkova, S., and Joseph, K. S. (2013). Incidence of preeclampsia: Risk factors and outcomes associated with early-versus late-onset disease. Am. J. Obstet. Gynecol. 209 (6), 541–544. e512. doi:10.1016/j.ajog.2013.08.019
Liu, C. L., Xie, L. X., Li, M., Durairajan, S. S., Goto, S., and Huang, J. D. (2007). Salvianolic acid B inhibits hydrogen peroxide-induced endothelial cell apoptosis through regulating PI3K/Akt signaling. PLoS One 2 (12), e1321. doi:10.1371/journal.pone.0001321
Liu, L., Li, J., Zhang, Y., Zhang, S., Ye, J., Wen, Z., et al. (2014). Salvianolic acid B inhibits platelets as a P2Y12 antagonist and PDE inhibitor: Evidence from clinic to laboratory. Thromb. Res. 134 (4), 866–876. doi:10.1016/j.thromres.2014.07.019
Liu, X., Feng, J., Zhou, D., and Yu, G. (2021a). The protective effect of Tanshinone ⅡA on vascular endothelial cells in septic rats by regulating NF-κB signaling pathway. Chin. J. Difficult Complicat. Cases 20 (09), 935–938. doi:10.1080/17512433.2021.1878877
Liu, X., Gao, Z. G., Wu, Y., Stevens, R. C., Jacobson, K. A., and Zhao, S. (2018). Salvianolic acids from antithrombotic Traditional Chinese Medicine Danshen are antagonists of human P2Y(1) and P2Y(12) receptors. Sci. Rep. 8 (1), 8084. doi:10.1038/s41598-018-26577-0
Liu, Y., Liu, W., Ma, S., Kang, N., He, Y., and Zhang, W. (2021b). Clinical study on the therapeutic effect of Salvia miltiorrhiza injection in the early treatment of recurrent abortion. Guangming J. Chin. Med. 36 (06), 851–854. doi:10.3969/j.issn.1003-8914.2021.06.001
Liu, Y. L., Zhou, X. Y., and Xuan, L. J. (2019). Magnesium lithospermate B ameliorates microcirculation perfusion in rats by promoting vascular NO production via activating the PI3K/AKT pathway. Acta Pharmacol. Sin. 40 (8), 1010–1018. doi:10.1038/s41401-018-0203-7
Ma, Y., Liang, X., Wu, H., Li, J., Chen, H., and Li, P. (2021). Clinical efficacy of Danshen injection in the treatment of preeclampsia and its effect on lncRNA NR_002794 of placenta. Chin. J. Woman Child Health Res. 32 (04), 571–577. doi:10.3969/j.issn.1673-5293.2021.04.020
Melamed, N., Baschat, A., Yinon, Y., Athanasiadis, A., Mecacci, F., Figueras, F., et al. (2021). FIGO (international federation of gynecology and obstetrics) initiative on fetal growth: Best practice advice for screening, diagnosis, and management of fetal growth restriction. Int. J. Gynaecol. Obstet. 152 (1), 3–57. doi:10.1002/ijgo.13522
Migliori, M., Cantaluppi, V., Mannari, C., Bertelli, A. A., Medica, D., Quercia, A. D., et al. (2015). Caffeic acid, a phenol found in white wine, modulates endothelial nitric oxide production and protects from oxidative stress-associated endothelial cell injury. PLoS One 10 (4), e0117530. doi:10.1371/journal.pone.0117530
Nadeem, A., Ahmad, S. F., Al-Harbi, N. O., Attia, S. M., Alshammari, M. A., Alzahrani, K. S., et al. (2019). Increased oxidative stress in the cerebellum and peripheral immune cells leads to exaggerated autism-like repetitive behavior due to deficiency of antioxidant response in BTBR T + tf/J mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 89, 245–253. doi:10.1016/j.pnpbp.2018.09.012
Ortega, M. A., Fraile-Martínez, O., García-Montero, C., Sáez, M. A., Álvarez-Mon, M. A., Torres-Carranza, D., et al. (2022). The pivotal role of the placenta in normal and pathological pregnancies: A focus on preeclampsia, fetal growth restriction, and maternal chronic venous disease. Cells 11 (3), 568. doi:10.3390/cells11030568
Pan, Q., Xu, H., Xu, L., and Mo, Z. (2022). Analysis on the effect of fuzheng huatan huoxue recipe in treating recurrent abortion of kidney deficiency and blood stasis type online first. Chin. Archives Traditional Chin. Med. 2022, 1–9. doi:10.3969/j.issn.1004-8189.2019.05.006
Panés, J., and Granger, D. N. (1998). Leukocyte-endothelial cell interactions: Molecular mechanisms and implications in gastrointestinal disease. Gastroenterology 114 (5), 1066–1090. doi:10.1016/s0016-5085(98)70328-2
Peng, Q., and Huang, M. (2021). Effect of Compound Danshen Injection on coagulation and renal function in preeclampsia rats. J. Mod. Med. Health 37 (23), 3989–3992. doi:10.3892/etm.2018.6173
Schoots, M. H., Gordijn, S. J., Scherjon, S. A., van Goor, H., and Hillebrands, J. L. (2018). Oxidative stress in placental pathology. Placenta 69, 153–161. doi:10.1016/j.placenta.2018.03.003
Sebastiani, G., Herranz Barbero, A., Borrás-Novell, C., Alsina Casanova, M., Aldecoa-Bilbao, V., Andreu-Fernández, V., et al. (2019). The effects of vegetarian and vegan diet during pregnancy on the health of mothers and offspring. Nutrients 11 (3), 557. doi:10.3390/nu11030557
Shen, Y., Hu, Y., and Zhang, Y. (2011). Favorable maternal and fetal effects of danshensu in an experimental mice model of preeclampsia. Hypertens. Pregnancy 30 (4), 465–480. doi:10.3109/10641955.2010.507842
Shou, Q., Pan, Y., Xu, X., Xu, J., Wang, D., Ling, Y., et al. (2012). Salvianolic acid B possesses vasodilation potential through NO and its related signals in rabbit thoracic aortic rings. Eur. J. Pharmacol. 697 (1-3), 81–87. doi:10.1016/j.ejphar.2012.09.044
Song Wang, Z. G., Li, E., Su, C., Zhu, H., and Zhu, H. (2008). The application with protocatechualdehyde to improve anticoagulant activity and cell affinity of silk fibroin. Appl. Surf. Sci. 255, 486–488. doi:10.1016/j.apsusc.2008.06.156
Staff, A. C., Fjeldstad, H. E., Fosheim, I. K., Moe, K., Turowski, G., Johnsen, G. M., et al. (2022). Failure of physiological transformation and spiral artery atherosis: Their roles in preeclampsia. Am. J. Obstet. Gynecol. 226 (2), S895–s906. doi:10.1016/j.ajog.2020.09.026
Staff, A. C. (2019). The two-stage placental model of preeclampsia: An update. J. Reprod. Immunol. 134-135, 1–10. doi:10.1016/j.jri.2019.07.004
Sun, Y., Xin, Q., Li, C., and Wang, N. (2017). Protective effect of Tan ⅡA on brain injury induced by radiation in mice and its molecular mechanism. Pharmacol. Clin. Chin. Materia Medica 33 (01), 66–70. doi:10.13412/j.cnki.zyyl.2017.01.019
Tong, X., Guo, Y., Nie, X., Yin, Y., and Tan, Y. (2022). Effects of tanshinone II A on the expression of annexin A2 mRNA and protein in decidua of mice with recurrent spontaneous abortion. China J. Traditional Chin. Med. Pharm. 2014, 308976. doi:10.1155/2014/308976
Triggle, C. R., Samuel, S. M., Ravishankar, S., Marei, I., Arunachalam, G., and Ding, H. (2012). The endothelium: Influencing vascular smooth muscle in many ways. Can. J. Physiol. Pharmacol. 90 (6), 713–738. doi:10.1139/y2012-073
True, H., Blanton, M., Sureshchandra, S., and Messaoudi, I. (2022). Monocytes and macrophages in pregnancy: The good, the bad, and the ugly. Immunol. Rev. 308 (1), 77–92. doi:10.1111/imr.13080
Turco, M. Y., and Moffett, A. (2019). Development of the human placenta. Development 146 (22), dev163428. doi:10.1242/dev.163428
Vata, P. K., Chauhan, N. M., Nallathambi, A., and Hussein, F. (2015). Assessment of prevalence of preeclampsia from Dilla region of Ethiopia. BMC Res. Notes 8, 816. doi:10.1186/s13104-015-1821-5
Vedmedovska, N., Rezeberga, D., Teibe, U., Melderis, I., and Donders, G. G. (2011). Placental pathology in fetal growth restriction. Eur. J. Obstet. Gynecol. Reprod. Biol. 155 (1), 36–40. doi:10.1016/j.ejogrb.2010.11.017
Walsh, S. W., and Strauss, J. F. (2022). Pregnancy-specific expression of protease-activated receptor 1: A therapeutic target for prevention and treatment of preeclampsia? Am. J. Obstet. Gynecol. 226 (2), S945–s953. doi:10.1016/j.ajog.2021.11.1367
Wang, D., Fan, G., Wang, Y., Liu, H., Wang, B., Dong, J., et al. (2013). Vascular reactivity screen of Chinese medicine danhong injection identifies Danshensu as a NO-independent but PGI2-mediated relaxation factor. J. Cardiovasc Pharmacol. 62 (5), 457–465. doi:10.1097/FJC.0b013e3182a29657
Wang, J., Xu, J., Gong, X., Yang, M., Zhang, C., and Li, M. (2019). Biosynthesis, chemistry, and pharmacology of polyphenols from Chinese Salvia species: A review. Molecules 24 (1), 155. doi:10.3390/molecules24010155
Wang, P., Li, H., and Wang, X. (2020a). Clinical study of Danshen Injection combined with magnesium sulfate in treatment of fetal growth restriction. Drug Eval. Res. 43 (11), 2263–2267. doi:10.7501/j.issn.1674-6376.2020.11.020
Wang, W., Wang, X., Zhang, X. S., and Liang, C. Z. (2018). Cryptotanshinone attenuates oxidative stress and inflammation through the regulation of nrf-2 and NF-κB in mice with unilateral ureteral obstruction. Basic Clin. Pharmacol. Toxicol. 123 (6), 714–720. doi:10.1111/bcpt.13091
Wang, X., Sun, Q., Jiang, Q., Jiang, Y., Zhang, Y., Cao, J., et al. (2021). Cryptotanshinone ameliorates doxorubicin-induced cardiotoxicity by targeting akt-GSK-3β-mPTP pathway in vitro. Molecules 26 (5), 1460. doi:10.3390/molecules26051460
Wang, X., and Xu, J. (2005). Salvianic acid A protects human neuroblastoma SH-SY5Y cells against MPP+-induced cytotoxicity. Neurosci. Res. 51 (2), 129–138. doi:10.1016/j.neures.2004.10.001
Wang, X., Yang, Y., Liu, X., and Gao, X. (2020b). Pharmacological properties of tanshinones, the natural products from Salvia miltiorrhiza. Adv. Pharmacol. 87, 43–70. doi:10.1016/bs.apha.2019.10.001
Wei, M., Wang, J., and Fu, J. (2021). Effect of danshen injection on fetal growth restriction and its influence on ultrasound parameters. J. Rare Uncommon Dis. 28 (01), 103–104. doi:10.3969/j.issn.1672-5131.2021.01.046
Wu, L., Cheng, B., Liu, Q., Jiang, P., and Yang, J. (2020). CRY2 suppresses trophoblast migration and invasion in recurrent spontaneous abortion. J. Biochem. 167 (1), 79–87. doi:10.1093/jb/mvz076
Xiao, Z., Liu, W., Mu, Y. P., Zhang, H., Wang, X. N., Zhao, C. Q., et al. (2020). Pharmacological effects of salvianolic acid B against oxidative damage. Front. Pharmacol. 11, 572373. doi:10.3389/fphar.2020.572373
Yang, G. D., Zhang, H., Lin, R., Wang, W. R., Shi, X. L., Liu, Y., et al. (2009). Down-regulation of CD40 gene expression and inhibition of apoptosis with Danshensu in endothelial cells. Basic Clin. Pharmacol. Toxicol. 104 (2), 87–92. doi:10.1111/j.1742-7843.2008.00342.x
Yang, R., Huang, S., Yan, F., Lu, X., Xing, Y., Liu, Y., et al. (2010). Danshensu protects vascular endothelia in a rat model of hyperhomocysteinemia. Acta Pharmacol. Sin. 31 (10), 1395–1400. doi:10.1038/aps.2010.167
Yang, Y. Y., Wu, Z. Y., Zhang, H., Yin, S. J., Xia, F. B., Zhang, Q., et al. (2020). LC-MS-based multivariate statistical analysis for the screening of potential thrombin/factor Xa inhibitors from Radix Salvia Miltiorrhiza. Chin. Med. 15, 38. doi:10.1186/s13020-020-00320-2
Yang, Z., Lin, S., Liu, Y., Ren, Q., Ge, Z., Wang, C., et al. (2022). Traditional Chinese medicine in coronary microvascular disease. Front. Pharmacol. 13, 929159. doi:10.3389/fphar.2022.929159
Yu, C., Qi, D., Lian, W., Li, Q. Z., Li, H. J., and Fan, H. Y. (2014). Effects of danshensu on platelet aggregation and thrombosis: In vivo arteriovenous shunt and venous thrombosis models in rats. PLoS One 9 (11), e110124. doi:10.1371/journal.pone.0110124
Yu, J., Wang, L., Akinyi, M., Li, Y., Duan, Z., Zhu, Y., et al. (2015). Danshensu protects isolated heart against ischemia reperfusion injury through activation of Akt/ERK1/2/Nrf2 signaling. Int. J. Clin. Exp. Med. 8 (9), 14793–14804.
Zeng, H., Su, S., Xiang, X., Sha, X., Zhu, Z., Wang, Y., et al. (2017). Comparative analysis of the major chemical constituents in Salvia miltiorrhiza roots, stems, leaves and flowers during different growth periods by UPLC-TQ-MS/MS and HPLC-ELSD methods. Molecules 22 (5), 771. doi:10.3390/molecules22050771
Zhang, J., Wu, T. X., and Liu, G. J. (2006). Chinese herbal medicine for the treatment of pre-eclampsia. Cochrane Database Syst. Rev. 2006 (2), Cd005126. doi:10.1002/14651858.CD005126.pub2
Zhang, N., Zou, H., Jin, L., Wang, J., Zhong, M. F., Huang, P., et al. (2010). Biphasic effects of sodium danshensu on vessel function in isolated rat aorta. Acta Pharmacol. Sin. 31 (4), 421–428. doi:10.1038/aps.2010.24
Zhang, T., Liu, M., Gao, Y., Li, H., Song, L., Hou, H., et al. (2021a). Salvianolic acid B inhalation solution enhances antifibrotic and anticoagulant effects in a rat model of pulmonary fibrosis. Biomed. Pharmacother. 138, 111475. doi:10.1016/j.biopha.2021.111475
Zhang, W., Li, J., Yang, P., Wang, G., Yue, Y., Zhong, Y., et al. (2022). Efficacy and safety of Salvia miltiorrhiza for treating chronic kidney diseases: A systematic review and meta-analysis. Evid. Based Complement. Altern. Med. 2022, 2117433. doi:10.1155/2022/2117433
Zhang, X., Wu, Q., Lu, Y., Wan, J., Dai, H., Zhou, X., et al. (2018). Cerebroprotection by salvianolic acid B after experimental subarachnoid hemorrhage occurs via Nrf2-and SIRT1-dependent pathways. Free Radic. Biol. Med. 124, 504–516. doi:10.1016/j.freeradbiomed.2018.06.035
Zhang, Y., Hao, Y., Li, Y., and Gao, Q. (2021b). Clinical analysis of compound Danshen injection combined with magnesium sulfate and nifedipine in the treatment of preeclampsia. Chin. J. Hum. Sex. 30 (03), 108–111. doi:10.3969/j.issn.1672-1993.2021.03.033
Zhao, G. R., Zhang, H. M., Ye, T. X., Xiang, Z. J., Yuan, Y. J., Guo, Z. X., et al. (2008). Characterization of the radical scavenging and antioxidant activities of danshensu and salvianolic acid B. Food Chem. Toxicol. 46 (1), 73–81. doi:10.1016/j.fct.2007.06.034
Zhou, Z., Wang, S. Q., Liu, Y., and Miao, A. D. (2006). Cryptotanshinone inhibits endothelin-1 expression and stimulates nitric oxide production in human vascular endothelial cells. Biochim. Biophys. Acta 1760 (1), 1–9. doi:10.1016/j.bbagen.2005.09.009
Keywords: placental pregnancy complications, Salvia miltiorrhiza, active compounds, placental development, preeclampsia
Citation: Kong J, Li S, Li Y and Chen M (2023) Effects of Salvia miltiorrhiza active compounds on placenta-mediated pregnancy complications. Front. Cell Dev. Biol. 11:1034455. doi: 10.3389/fcell.2023.1034455
Received: 01 September 2022; Accepted: 03 January 2023;
Published: 13 January 2023.
Edited by:
Huashan Zhao, Shenzhen Institutes of Advanced Technology (CAS), ChinaReviewed by:
Ruan Degong, The University of Hong Kong, Hong Kong SAR, ChinaZhen Dong, Southwest University, China
Copyright © 2023 Kong, Li, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Chen, ZWRjaGVuOTlAZ21haWwuY29t
†These authors have contributed equally to this work.
 Jingyin Kong
Jingyin Kong Songjun Li
Songjun Li Yingting Li
Yingting Li Min Chen
Min Chen