Abstract
Genitourinary (GU) cancers are among the most prevalent neoplasms in the world, with bladder cancers constituting 3% of global cancer diagnoses. However, several pathogenetic mechanisms remain controversial and unclear. Claudins, for example, have been shown to play a significant role in several cancers of the human body. Their role in GU cancers has not been extensively studied. Aberrant expression of claudins −1, −2, −3, −4, −7, and −11 has been expressed in urothelial cell carcinomas. In prostate cancers, altered levels of claudins −1, −2, −3, −4, and −5 have been reported. Furthermore, the levels of claudins −1, −2, −3, −4, −6, −7, −8, and −10 have been studied in renal cell carcinomas. Specifically, claudins −7 and −8 have proven especially useful in differentiating between chromophobe renal cell carcinomas and oncocytomas. Several of these claudins also correlate with clinicopathologic parameters and prognosis in GU cancers. Although mechanisms underpinning aberrant expression of claudins in GU cancers are unclear, epigenetic changes, tumor necrosis factor-ɑ, and the p63 protein have been implicated. Claudins also provide therapeutic value through tailored immunotherapy via molecular subtyping and providing therapeutic targets, which have shown positive outcomes in preclinical studies. In this review, we aim to summarize the literature describing aberrant expression of claudins in urothelial, prostatic, and renal cell carcinomas. Then, we describe the mechanisms underlying these changes and the therapeutic value of claudins. Understanding the scope of claudins in GU cancers paves the way for several diagnostic, prognostic, and therapeutic innovations.
1 Introduction
The barrier function of the epithelium is primarily maintained by tight junction proteins composed of zona-occludins, desmosomes, and claudins, among others (Arabi et al., 2023). The claudin family consists of 27 transmembrane proteins (Tsukita et al., 2019). Contrary to other tight junction proteins, some claudins function as paracellular channels and allow for the selective passage of water, anions, and cations (Günzel and Fromm, 2012). Besides their channel and barrier roles, claudins have several biologic effects (Figure 1). Claudins also play a role in cell migration through their effects on matrix metalloproteinases (MMP) (Venugopal et al., 2019). Furthermore, claudins, specifically claudin-2, also control the life cycle of cells and interact with several transcription factors, such as Sp1 and Zonula occludens 1-associated nucleic acid-binding protein (Venugopal et al., 2019). Claudins modulate several signaling pathways as well, including MEK/ERK/1/2 and PI-3K/Bcl-2 pathways (Ahmad et al., 2014; Venugopal et al., 2019). The broad spectrum of claudin functions is further solidified by claudin-knockout models, which lead to atopic dermatitis, renal hypoxia, seizures, and multiple sclerosis (Tsukita et al., 2019).
FIGURE 1
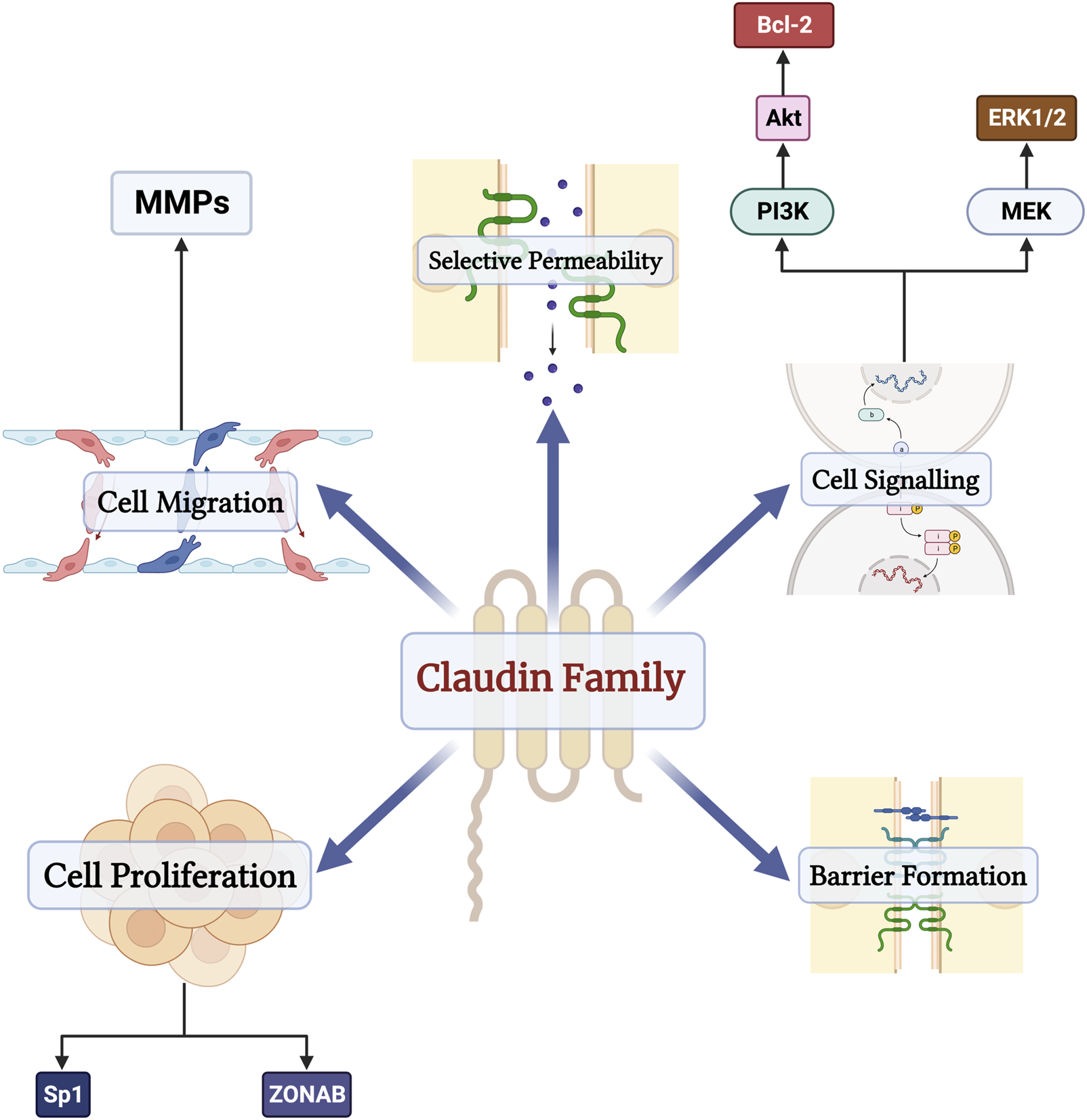
The physiologic functions of the claudin family of proteins in the human body. Besides their functions as barriers and channels, claudins also regulate cell proliferation through their interactions with transcription factors Sp1 and Zonula occludens 1-associated nucleic acid-binding protein (ZONAB), modulate cell migration through matrix metalloproteinases (MMPs), and serve as components of several signaling pathways such as MEK/ERK/1/2 and PI-3K/Bcl-2 pathways.
Emerging evidence has demonstrated that abnormal claudin expression can mediate tumorigenic processes in several cancers, including head and neck, gastrointestinal, breast, and lung neoplasms (Soini, 2011; Zhu et al., 2019; Yadav et al., 2022; Arabi et al., 2023). Additionally, aberrant expression of these proteins can be seen in preneoplastic conditions, highlighting their possible role for early detection and treatment of tumors (Ouban and Arabi, 2023).
Genitourinary (GU) cancers are among the most common cancers worldwide. For example, prostate cancers are the second most common solid tumors in men (Gandaglia et al., 2021). Bladder cancers constitute the 10th most common tumor worldwide, with 3% of all new cancer diagnoses (Bray et al., 2018). Unfortunately, due to higher rates of chemotherapy toxicity and comorbidities, elderly patients suffering from GU cancers often have poor cancer-related outcomes (Singhal et al., 2023). Hence, finding alternative treatment modalities may significantly increase the survival rates of this patient group.
Recent studies have shed some light on the role of claudins in GU cancers. For example, Săndulescu et al. demonstrated that claudin-4 expression is significantly increased in high-grade bladder urothelial carcinomas and those with invasion of the muscularis propria (SĂndulescu et al., 2020). Similarly, elevated levels of claudin-3 have been reported in prostatic cancer patients compared to controls (Ye et al., 2019). Reports of aberrant claudin expression and its role in the development of GU cancers remain limited. Moreover, the mechanisms underlying these changes remain unclear. Therefore, we will gather the latest literature surrounding aberrant expression of claudins and their prognostic implications in GU cancers, including bladder, prostate, and renal cell carcinomas (RCC). Then, we discuss the mechanisms mediating changes in claudin expression and their value as therapeutic targets.
2 Aberrant expression of claudins in urothelial carcinomas
Urothelial cells, previously known as transitional epithelial cells, line the lumen of the proximal urethra, bladder, ureters, and renal pelvis (Dalghi et al., 2020). The urothelium consists of several layers including the superficial umbrella cells, followed by the intermediate and basal layers, respectively (Dalghi et al., 2020). Urothelial carcinomas are seventh most common type of neoplasm and arises from the urothelium of the urethra, bladder, and ureters (Giudici et al., 2021). Bladder carcinomas are the most prevalent of urothelial carcinomas, constituting up to 95% of urothelial carcinomas (Giudici et al., 2021). Accordingly, the role of claudins in bladder carcinomas has been studied extensively. Claudin-1 can be detected at the membranes of high-grade urothelial carcinomas of the bladder (Săndulescu et al., 2022). However, a study by Elsen et al. found that both non-invasive bladder carcinoma and normal samples do not express claudin-1 (Elsen et al., 2015). Urothelial papillomas, papillary urothelial neoplasms of low malignant potential, and low-grade urothelial carcinomas of the bladder exhibit significantly less claudin-1 than inverted urothelial papillomas (Székely et al., 2011). Increased claudin-1 expression is also associated with better recurrence-free survival in papillary urothelial neoplasms of low malignant potential (Székely et al., 2011).
Claudin-2 can be seen as perimembranous cytoplasmic granules in the basal layers of nontumorous and tumorous urothelium (Törzsök et al., 2011). However, claudin-2 protein and messenger ribonucleic acid (mRNA) levels are increased in urothelial cell carcinomas compared to controls (Törzsök et al., 2011). Specifically, high-grade urothelial cell carcinomas express higher levels of claudin-2 mRNA compared to their low-grade counterpart (Törzsök et al., 2011). Claudin-3 is expressed in approximately 88% of urothelial carcinomas of the upper urinary tract on the upper epithelial layers (Nakanishi et al., 2008). Increased claudin-3 protein expression is associated with poor staging, grading, and pattern of tumor growth (Nakanishi et al., 2008). Although claudin-3 expression is correlated with overall survival rates in univariate analysis, multivariate analysis has failed to demonstrate a similar effect (Nakanishi et al., 2008).
A study by Chen et al. aimed to assess the alterations of tight junction proteins in bladder carcinoma cells compared to adjacent non-neoplastic tissue (Chen et al., 2020). The authors demonstrated elevated expression of claudin-4 in neoplastic tissue (Chen et al., 2020). However, claudin-4 levels are not associated muscle invasion, lymph node metastasis, or distant metastasis (Chen et al., 2020). Specifically, claudin-4 is overexpressed in low/medium-grade bladder carcinomas and becomes underexpressed in high-grade tumors (Boireau et al., 2007). Alterations in claudin-4 expression have been linked to hypermethylation, with inhibition of methylation enzymes promoting increases in cell polarization and transepithelial resistance (Boireau et al., 2007). Claudin-4 expression has also been studied in upper urinary tract carcinomas (Nakanishi et al., 2008). The protein is localized to the plasma membranes of neoplastic cells and is present in around three-quarters of tumor biopsies. Although claudin-4 has been linked to poor staging, no effect has been reported on disease-free and overall survival in this patient population (Nakanishi et al., 2008).
Gadelmoula et al. studied the expression of claudin-7 proteins and mRNA in urothelial bladder carcinoma patients (Gadelmoula et al., 2013). The authors demonstrated that claudin-7 is significantly downregulated compared to control specimens. Muscle-invasive tumors also exhibit less claudin-7 than their non-invasive counterparts; however, there was no difference in claudin-7 expression between different tumor grades (Gadelmoula et al., 2013). Then, Yamuç et al. examined the correlation between claudin-7 and patient outcomes (Yamuç et al., 2022). Although claudin-7 levels were different between genders, tumor stage, grade, and gender had no impact on the protein. Nevertheless, loss of claudin-7 was associated with tumor recurrence and poor overall survival. Contrarily, the utility of claudin-7 to differentiate between the histological grade of urothelial carcinomas has been reported, where high-grade tumors exhibit lower levels than low-grade ones (Törzsök et al., 2011). Claudin-7 is not associated with clinicopathologic factors, recurrence-free, or overall survival in urothelial carcinomas of the upper urinary tract (Nakanishi et al., 2008). The direct impact of claudin-7 on urothelial carcinomas is controversial; however, claudin-7 appears to play an antineoplastic role in this group of tumors. Further studies are needed to confirm the prognostic role of claudin-7 in urothelial carcinoma patients.
Awsare et al. studied the role of claudin-11 in human bladder carcinomas (Awsare et al., 2011). Claudin-11 mRNA levels were not different between carcinogenic and control samples; however, claudin-11 staining was less in the tumor samples when using immunohistochemistry. Forced expression of claudin-11 in bladder cancer cell lines blunted tumor invasion and increased cell matrix adhesion but increases tumor growth as well. Overall, alterations in the expression of claudins −1, −2, −3, −4, −7, and −11 have been reported in urothelial carcinomas (Table 1). Some of these claudins have shown prognostic value (Table 2), although further research is needed to validify these findings and clarify any contradictions.
TABLE 1
| Location | Type of tumor | Claudin isoform | Level of expression | Type of measured marker | References |
|---|---|---|---|---|---|
| Urinary Tract | Urothelial carcinoma of the lower tract | Claudin-1 | ↑a, ↓b | Protein | Székely et al. (2011), Săndulescu et al. (2022) |
| Claudin-2 | ↑a | Protein and mRNA | Törzsök et al. (2011) | ||
| Claudin-4 | ↓a, ↑b | Protein and mRNA | Boireau et al. (2007) | ||
| Claudin-7 | ↓ | Protein and mRNA | Gadelmoula et al. (2013) | ||
| Claudin-11 | ↓ | Protein and mRNA | Awsare et al. (2011) | ||
| Urothelial papillomas | Claudin-1 | ↓ | Protein | Székely et al. (2011) | |
| Papillary urothelial neoplasms of low malignant potential | Claudin-1 | ↓ | Protein | Székely et al. (2011) | |
| Urothelial carcinoma of the upper tract | Claudin-3 | ↑ | Protein | Nakanishi et al. (2008) | |
| Claudin-4 | ↑ | Protein | Nakanishi et al. (2008) | ||
| Prostate | Prostatic adenocarcinoma | Claudin-1 | ↓ | Protein | Sheehan et al. (2007), Kind et al. (2020) |
| Claudin-2 | ↑ | Protein | Coutinho-Camillo et al. (2011) | ||
| Claudin-3 | ↑; ↓c | Protein | Coutinho-Camillo et al. (2011), Worst et al. (2017), Ye et al. (2019) | ||
| Claudin-4 | ↑ | Protein | Radi and Abd-Elazeem (2016) | ||
| Claudin-5 | ↑; ↓d | Protein | Coutinho-Camillo et al. (2011) | ||
| Claudin-7 | ↓d | Protein | Sheehan et al. (2007) | ||
| Claudin-8 | ↑d | Protein and mRNA | Ashikari et al. (2017) | ||
| Kidney | Clear cell RCC | Claudin-1 | ↓ | Protein | Fritzsche et al. (2008), Shin et al. (2011) |
| Claudin-4 | ↓ | Protein | Lechpammer et al. (2008) | ||
| Claudin-8 | ↓ | Protein and mRNA | Zhu et al. (2020) | ||
| Claudin-10 | ↓ | Protein and mRNA | Yang et al. (2021), Yang et al. (2022) | ||
| Papillary RCC | Claudin-6 | ↓ | Protein | Mikuteit et al. (2022) | |
| Chromophobe RCC | Claudin-6 | ↓ | Protein | Erlmeier et al. (2023) | |
| Claudin-7 | ↑ | Protein | Lechpammer et al. (2008), Li et al. (2008), Gutiérrez et al. (2018) | ||
| Unspecified RCC | Claudin-2 | ↓ | Protein and RNA | Kumar et al. (2021) |
The changes of claudin levels in various neoplasms of the genitourinary tract.
High-grade.
Low-grade.
CRPC.
Conflicting data.
TABLE 2
| Location | Type of tumor | Claudin isoform | Diagnostic/prognostic significance | References |
|---|---|---|---|---|
| Urinary tract | Urothelial carcinomas of the lower tract | Claudin-7 | Tumor recurrence and poor survival | Gadelmoula et al. (2013) |
| Papillary urothelial neoplasms of low malignant potential | Claudin-1 | Poor recurrence-free survival | Székely et al. (2011) | |
| Urothelial carcinoma of the upper tract | Claudin-3 | Poor staging, grading, and pattern of growth | Nakanishi et al. (2008) | |
| Prostate | Prostatic adenocarcinoma | Claudin-1 | Reduced risk of PSA recurrencea | Sheehan et al. (2007), Kind et al. (2020) |
| Claudin-3 | Distant metastasis; poor disease-free survival and time to progressionb | Coutinho-Camillo et al. (2011), Worst et al. (2017), Ye et al. (2019) | ||
| Claudin-4 | Lymph node metastasis | Radi and Abd-Elazeem (2016) | ||
| Kidney | Clear cell RCC | Claudin-1 | Increased levels associated with metastasis | Fritzsche et al. (2008), Shin et al. (2011) |
| Claudin-8 | Poor overall survival | Zhu et al. (2020) | ||
| Claudin-10 | Poor disease-free and overall survival | Yang et al. (2021), Yang et al. (2022) | ||
| Papillary RCC | Claudin-1 | Increased levels compared to chromophobe RCC | Lechpammer et al. (2008) | |
| Chromophobe RCC | Claudin-7 | Differentiates between other subtypes with 95% sensitivity and 92.3% specificity | Choi et al. (2007), Zhou et al. (2019) | |
| Differentiates between papillary RCC, clear cell RCC, and oncocytomas with 94% sensitivity and 78% specificity, which increases to 100% when combining other markers | ||||
| Claudin-8 | Decreased levels compared to oncocytomas | Lechpammer et al. (2008), Kim et al. (2009), Osunkoya et al. (2009) | ||
| Unspecified RCC | Claudin-2 | Progression and poor survival | Kumar et al. (2021) |
The prognostic and diagnostic significance of claudins in genitourinary tract neoplasms.
Conflicting data.
CRPC.
3 Aberrant expression of claudins in prostate cancer
After analyzing over 17,000 prostate cancer specimens, Kind et al. found claudin-1 protein in 39% of cases, suggesting upregulation during malignant transformation compared to normal glandular cells (Kind et al., 2020). Two other studies, with smaller cohorts of 101 and 141 cases, also reported claudin-1 upregulation in 97% and 41% of tumors relative to normal prostatic glands (Sheehan et al., 2007; Väre et al., 2008). The differences in positive rates between these three studies likely stem from variations in staining protocols and evaluation criteria.
While claudin-1 expression is found normally in the prostate, there is conflicting data regarding its clinical utility. Four studies involving a total of 30, 48, 101, and 141 specimens have reported that the downregulation of claudin-1 is linked to unfavorable tumor characteristics, a poor prognosis, and/or biochemical recurrence (Sheehan et al., 2007; Väre et al., 2008; Seo et al., 2010; Szász et al., 2010). In fact, through multivariate analysis, one study identified reduced claudin-1 expression as an independent predictor of tumor recurrence (Sheehan et al., 2007). Consistent with previous studies, Kind et al. found that claudin-1 upregulation is associated with favorable tumor features, low Gleason grade, and a reduced risk of PSA recurrence (Kind et al., 2020). Particularly, this was most relevant in ERG-positive prostate cancer where increased claudin-1 expression predicts a favorable prognosis (Kind et al., 2020). Nonetheless, multivariate analyses did not reveal an independent association of claudin-1 expression with prognosis (Kind et al., 2020).
However, these findings contradict the research by Krajevska et al. who reported claudin-1 expression in the basal cells of normal prostate glands, but its complete absence in luminal/glandular cells and in 98% of the analyzed cancer samples (Krajewska et al., 2007). This characteristic staining pattern of claudin-1 in the normal prostate was also described by Kind et al. (Kind et al., 2020). It is hypothesized that the loss of claudin-1 expression in prostate cancer could potentially indicate epithelial-mesenchymal transition (Medici et al., 2006; Yang et al., 2006).
In a study exploring the connection between abnormal expressions of claudin-1, claudin-3, claudin-4, and claudin-7 and prognostic factors in prostate cancer, Sheehan et al. determined that, like claudin-1, reduced claudin-7 expression correlated with poorly differentiated histological tumors (Sheehan et al., 2007). Conversely, they found that elevated levels of claudin-3 and claudin-4 were linked to advanced-stage tumors (Sheehan et al., 2007). In contrast, Camillo et al. demonstrated claudins-1 and -7 were equivocal, claudins-2, -3, and -5 were overexpressed, and claudin-4 was downregulated in prostate adenocarcinomas compared with normal samples (Coutinho-Camillo et al., 2011). However, even with all these alterations in claudin levels, they did not find any association with the main clinicopathological parameters.
Similar findings were observed by other studies regarding elevated claudin-3 and higher Gleason grade (Coutinho-Camillo et al., 2011; Worst et al., 2017). Specifically, Ye et al. found claudin-3 levels to be associated with distant metastasis in cancer cells, preoperative PSA levels, tumor diameter, and the pathological stage of the disease (Ye et al., 2019). Interestingly, in castration-resistant prostate cancer (CRPC), claudin-3 expression significantly decreased in samples from patients with high total Gleason scores (≥8) and locally advanced tumors, and this loss of expression was associated with worse disease-free survival and time to clinical progression (Orea et al., 2023). These findings strongly suggest that epigenetic silencing of claudin-3 is a common occurrence in CRPC, potentially serving as a valuable molecular marker for predicting the prognosis of prostate cancer patients and differentiating aggressive from indolent prostate tumors.
The observation by Sheehan et al. (Sheehan et al., 2007) regarding claudin-4 was similar to other studies (Landers et al., 2008; Väre et al., 2008; Szász et al., 2010; Radi and Abd-Elazeem, 2016). One of those studies highlighted high claudin-4 expression as a poor prognosis biomarker due to its association with high tumor grade, lymphovascular invasion, positive lymph node metastasis, and high mean peritumoral lymphatic vessel density (Radi and Abd-Elazeem, 2016). Furthermore, Landers et al. found heightened expression in prostate cancer and metastatic prostate carcinoma compared to prostatic hyperplasia tissues (Landers et al., 2008). One noteworthy difference from the report of Sheehan et al. (Sheehan et al., 2007), Landers et al.’s immunohistochemical analysis of claudin-4 was supported by a comparative evaluation of α-Methylacyl-CoA racemase (AMACR) and prostate-specific membrane antigen (PSMA)—two well-established prostate cancer markers (Landers et al., 2008).
When examining sections containing high-grade prostatic intraepithelial neoplasia (HG-PIN) without associated invasive carcinoma, Landers et al. observed moderate claudin-4 intensity and low PSMA and AMACR levels (Landers et al., 2008). In contrast, HG-PIN cells in conjunction with invasive carcinoma displayed strong claudin-4 staining, but AMACR levels were low and PSMA levels were moderate (Landers et al., 2008). This suggests that claudin-4 may play a role in the early stages of prostate cancer development. The authors also discovered that lower-grade carcinomas exhibit higher claudin-4 expression compared to higher-grade carcinomas, with epithelial cells in benign glands surrounding the cancer sections displaying moderate to strong claudin-4 staining (Landers et al., 2008). This is consistent with loss of cellular organization as cancer progresses leading to a reduction in tight junction function, which aligns with alterations cellular polarity that enhance cellular mobility (Weinstein et al., 1976). Moreover, claudin-4 staining was strongly positive in most metastatic tumors, with no noticeable difference between specimens from patients receiving androgen suppression and those who were not (Landers et al., 2008). Also, there seemed to be no correlation between the metastatic site and the intensity of claudin-4 expression.
Generally, claudin-5 was found to be weakly expressed in prostate cancer compared to normal tissue, particularly in clinically poorly behaving carcinomas (Väre et al., 2008; Seo et al., 2010; Szász et al., 2010). As for claudins −7 and −10, studies showed equivocal results (Väre et al., 2008; Szász et al., 2010; Coutinho-Camillo et al., 2011). Nonetheless, one study found an association between low expression of claudin-7 and higher tumor grade (Sheehan et al., 2007). To note, two forms of claudin-7 exist, a full-length form of CLDN-7 with 211 amino-acid residues and a C-terminal truncated form with 158 amino-acid residues, both of which were found to regulate the expression of PSA (Zheng et al., 2003). Hence, it can be of interest for future studies to explore the difference in expression of both forms of claudin-7 and correlate it to prognostic markers.
While Szász et al. observed a significant decrease in claudin-8 expression, it did not have any clinical correlation (Szász et al., 2010). On the contrary, Ashikari et al. indicated that claudin 8 was overexpressed in prostate cancer clinical samples compared to benign tissues (Ashikari et al., 2017). Moreover, their findings demonstrate that claudin-8 not only regulates intracellular signal transduction and stabilizes the cytoskeleton but also operates as an androgen receptor downstream signal, collectively contributing to the advancement of prostate cancer. Collectively, several claudins are altered in prostate cancers, among which several have clinical significance (Table 1).
4 Aberrant expression of claudins in renal cell carcinomas
Kidney neoplasms are the sixth and 10th most common cancers in men and women, respectively (Bahadoram et al., 2022). RCC, the most common type of renal tumors, constitutes approximately 3% of all adult malignancies (Rini et al., 2009). RCCs are generally divided into five subtypes: clear cell, papillary, chromophobe, collecting duct, and unclassified, among which the first three constitute the majority of RCCs (Truong and Shen, 2011). Although advances in the medical and surgical management of RCCs have been made (Rini et al., 2009; Bazarbashi et al., 2023), the outcomes associated with the tumor remain relatively poor (Badran et al., 2020). Additionally, differentiating between different subtypes of RCC is still difficult. Claudins have been used to fill these gaps as diagnostic, prognostic, and therapeutic tools in RCCs. In this section, we will summarize the evidence discussing these applications (Table 1).
Claudin-1 is expressed in renal biopsies as a membranous pattern, mostly in the Bowman membrane and, to a lesser degree, the epithelium of the distal tubules and collecting ducts (Fritzsche et al., 2008; Lechpammer et al., 2008). On the other hand, the protein is only seen in one-third of RCC samples (Fritzsche et al., 2008). Claudin-1 mainly localizes at the plasma membrane and sometimes in the cytoplasm of neoplastic cells. Specifically, three-quarters of papillary RCCs express claudin-1, while only a quarter of clear cell RCCs exhibit the protein (Fritzsche et al., 2008). In clear cell RCC, claudin-1 expression is associated with metastasis and high-grade tumors (Fritzsche et al., 2008; Shin et al., 2011). Claudin-1 expression correlates with poor disease-specific survival only in asymptomatic clear cell RCC patients but not in all patients (Fritzsche et al., 2008). However, the protein is associated with increased postoperative distant metastasis in clear cell RCC patients (Shin et al., 2011). Contrarily, claudin-1 expression is associated with improved clinicopathologic characteristics in papillary RCC, but not survival (Fritzsche et al., 2008). Claudin-1 immunohistochemistry can also be used to differentiate papillary RCC from its chromophobe counterpart, which exhibits weak expression (Lechpammer et al., 2008).
Claudin-2 is significantly reduced in RCC samples, and loss of the protein is associated with tumor progression and poor survival (Kumar et al., 2021). Claudin-2 overexpression inhibits mesenchymal plasticity, tumorigenic abilities, and tumor growth in murine models (Kumar et al., 2021). Claudin-3 is strongly expressed in the distal tubules and collecting ducts and weakly in the glomeruli and proximal tubules of normal renal samples (Lechpammer et al., 2008). Claudin-3 expression varies from moderate to strong in papillary, chromophobe, and clear cell RCCs (Lechpammer et al., 2008). Claudin-3 is predominantly seen in lower grades of clear cell RCCs and is not associated with tumor grade in other subtypes (Lechpammer et al., 2008). Although claudin-3 is associated with poor survival in univariate analysis, multivariate analysis diminishes this effect (Lechpammer et al., 2008).
Strong expression of claudin-4 in normal renal samples can be seen in the cell membrane and cytoplasm of distal tubules and collecting ducts cells (Lechpammer et al., 2008; Owari et al., 2020). Claudin-4 is not associated with histological grade or staging in RCC patients (Owari et al., 2020). Chromophobe RCCs also exhibit moderate to strong claudin-4 expression, while only half of clear cell RCCs express the protein (Lechpammer et al., 2008). Similar to claudin-3, strong expression of claudin-4 is associated with poor survival in clear cell RCC using univariate but not multivariate analysis (Lechpammer et al., 2008).
Studies reporting the presence of claudin-6 in RCCs are limited. Mikuteit et al. assessed the prognostic role of the protein in papillary RCCs (Mikuteit et al., 2022). Claudin-6 was present in around one-fifth of patients with type 1 and type 2 papillary RCC. Expression of the protein had no impact on survival in these patients. Then, Erlmeier et al. aimed to assess the same in chromophobe RCC patients (Erlmeier et al., 2023). Claudin-6 was only seen in 12.3% of chromophobe tumor samples. Like papillary RCC, claudin-6 expression was not correlated with overall survival (Erlmeier et al., 2023). To the best of the authors’ knowledge, these are the only available reports of claudin-6 expression in RCC samples. Future studies should aim to define its role in clear cell RCCs and other subtypes.
In normal renal samples, claudin-7 is only expressed in the distal convoluted tubules and distal collecting ducts (Lechpammer et al., 2008). Membranous claudin-7 expression can be seen in the majority of chromophobe and, to a lesser extent, papillary RCCs (Lechpammer et al., 2008; Li et al., 2008; Gutiérrez et al., 2018). Claudin-7 has proven useful as a diagnostic tool to differentiate between different renal tumors, especially oncocytomas and chromophobe RCC (Osunkoya et al., 2009; Ohe et al., 2012). The protein has a sensitivity and specificity of 95% and 92.3% in discriminating between chromophobe RCC and other RCC subtypes (Choi et al., 2007). When differentiating chromophobe tumors from papillary RCCs, clear cell RCCs, and oncocytoma, the protein demonstrates a sensitivity and specificity of 94% and 78%, respectively (Zhou et al., 2019). Specificity can be further increased to 100% when combining claudin-7 with additional markers CD117 and CK7 (Zhou et al., 2019).
The prognostic implications of claudin-7 expression are controversial. In a study of 120 clear cell RCC patients, reduced claudin-7 expression predicted poor staging and survival (Li et al., 2018). In line with these findings, forced overexpression of claudin-7 triggers neoplastic cell apoptosis, blunts proliferation, and inhibits tumor invasion and migration (Li et al., 2018). However, another study by Shin et al. found no association between the protein with disease-free and overall survival (Shin et al., 2011). Despite the clear diagnostic practicality of using claudin-7, more investigations are needed into its prognostic role.
Similar to claudin-7, claudin-8 exhibits membranous staining patterns in the distal tubules and collecting ducts (Lechpammer et al., 2008). Clear cell RCCs exhibit lower claudin-8 mRNA and protein levels than normal tissue samples (Zhu et al., 2020). The protein appears to play a role in facilitating pro-neoplastic effects. Zhu et al. studied the effects of claudin-8 on clear cell RCC progression and its prognostic value (Zhu et al., 2020). Claudin overexpression attenuated tumor invasion, migration, and proliferation via its modulatory effects on epithelial-to-mesenchymal transition and Akt pathways (Zhu et al., 2020). Low claudin-8 levels were also independently associated with poor overall survival in their group of patients (Zhu et al., 2020). Furthermore, chromophobe RCCs demonstrate significantly reduced cytoplasmic staining of claudin-8 than oncocytomas, highlighting its diagnostic utility (Lechpammer et al., 2008; Kim et al., 2009; Osunkoya et al., 2009). Whether claudin-8 has any prognostic value in other RCC subtypes remains unstudied.
Claudin-10 expression is significantly blunted in clear cell RCC (Yang et al., 2021). Increased claudin-10 mRNA expression is independently associated with longer disease-free and overall survival in clear cell RCC patients (Yang et al., 2021; Yang et al., 2022). Claudin-10 overexpression induces apoptosis and inhibits migration, invasion, and proliferation by promoting mitochondrial dysfunction (Yang et al., 2022). Moreover, claudin-10 is associated with increased dendritic cell and naïve and memory T-cell tumor infiltration, which has been linked with better outcomes (Yang et al., 2021). Aberrant expression of claudins −14, −17, −19, and −22 has also been reported in RCCs (Men et al., 2015; Singh and Vinod, 2020); however, reports about these proteins are still extremely limited.
5 Mechanisms causing aberrant expression of claudins in GU cancers
The mechanisms underlying aberrant expression of claudins in GU carcinomas are not completely understood. Nevertheless, several mechanisms have been studied and reported (Figure 2). Epigenetic modifications–mainly through DNA methylation–appear to play a crucial role in the induction of aberrant expression. For example, the claudin-4 gene is hypermethylated in bladder carcinomas (Boireau et al., 2007). Treatment with DNA methyltransferase inhibitors reverses the effects of CLDN4 methylation, increasing cell polarization and transepithelial resistance (Boireau et al., 2007). In RCC, promoter hypermethylation is associated with reduced claudin-7 mRNA and protein expression (Li et al., 2018). Hypermethylation of the claudin-7 promoter is associated with advanced tumors and poor survival in these patients (Li et al., 2018). Furthermore, hypermethylation of claudin −8, −9, −10, −14, −19, and −22 promoters has been reported in papillary RCC (Singh and Vinod, 2020).
FIGURE 2
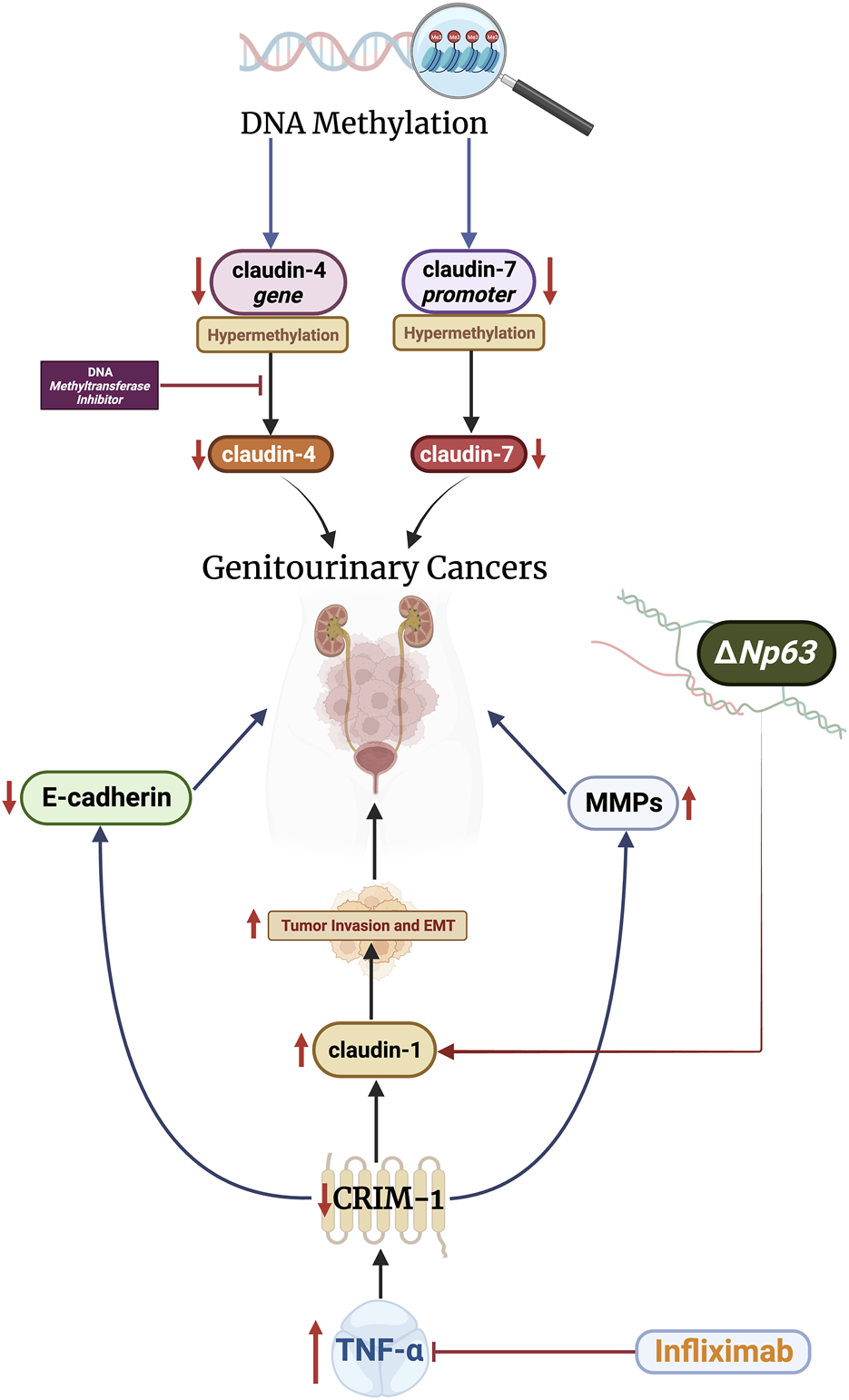
Alterations relating to ΔNp63 and CRIM-1 increase claudin-1 levels, which promote tumor invasion and epithelial-mesenchymal transition (EMT). Claudins-4 and -7 are modulated by DNA methylation. These mechanisms can be targeted therapeutically through DNA methyltransferase inhibitors and infliximab, which have shown promising results.
Transmembrane proteins, specifically cysteine-rich motor neuron-1 (CRIM-1), also regulate the expression of claudins in GU cancers. The CRIM-1 protein is highly expressed in the kidney and placenta cells (Kolle et al., 2000). Ogasawara et al. analyzed the effects of CRIM-1 on proliferation, malignant transformation, and claudin expression in RCCs (Ogasawara et al., 2018). The authors demonstrated that decreased expression of CRIM-1 does not impact tumor growth or proliferation. However, the authors revealed that under-expression of CRIM-1 increases tumor invasion and epithelial-mesenchymal transition by reducing E-cadherin expression and increasing claudin-1 and MMP-2 and -9 expression. Expression of CRIM-1 was blunted by tumor necrosis factor (TNF)-ɑ, indicating a potential therapeutic value of targeting this pathway. In line with this, treatment with infliximab, an anti-TNF-ɑ antibody, resulted in 16% of RCC patients achieving partial response and 16% achieving stable disease in a phase II trial (Harrison et al., 2007). Nevertheless, the efficacy of infliximab in RCC remains controversial and further studies are still needed (Larkin et al., 2010).
The p63 gene, a member of the tumor suppressor p53 family, has two different promotes which produce isotypes that contain or lack (ΔN) an NH2-terminus (Chen et al., 2018). Studies have demonstrated the regulatory effects of p63 on claudin expression in epithelial cells (Kojima et al., 2017). However, this relationship remains unclear in GU cancers. The ΔNp63 isotype is overexpressed in bladder cell carcinomas (Park et al., 2000; Jing et al., 2013). In bladder carcinoma cell lines, ΔNp63 promotes tumor invasion and metastasis through overexpression of claudin-1, and knockdown of the isotype blunts these effects (Jing et al., 2013). Conclusively, epigenetics, transmembrane proteins, and the p63 gene modulate claudin expression in various GU cancers. Studies are still limited in this regard and more investigations are needed to implicate additional signaling pathways.
6 The therapeutic value of claudins in GU cancers
Several therapeutic avenues targeting claudins have been explored in the context of GU carcinomas. Firstly, claudin expression has been used to tailor management and predict the response of patients to certain drugs. Despite claudin-low bladder tumors expressing high levels of immune gene signatures, they also exhibit high levels of immune checkpoint molecules (Kardos et al., 2016). These findings have led some to hypothesize that molecular subtypes can be used to predict immunotherapeutic responses (Kardos et al., 2016). In line with these findings, the PURE-01 study assessed the effects of preoperative pembrolizumab on post-cystectomy remission times. The study revealed that claudin-low muscle-invasive bladder cancer patients exhibit longer event-free survival than other patient categories after pembrolizumab and radical cystectomy (Bandini et al., 2020; Necchi et al., 2020).
Antibodies targeting different claudins have also begun emerging. Among them, Kuwada et al. developed a rat antibody targeting the extracellular domain of the claudin-4 protein (Kuwada et al., 2015). The antibody inhibited bladder cell growth and invasion and promoted apoptosis (Kuwada et al., 2015). Furthermore, the antibody increased cellular permeability to cisplatin, inhibiting tumor growth and improving survival in mice models (Kuwada et al., 2015).
Bufalin–a steroid obtained from toads–exhibits antineoplastic effects in several tumors, such as gastrointestinal, breast, and lung cancers (Jiang et al., 2010; Takai et al., 2012). Bufalin inhibits bladder cancer migration and invasion by reducing expression of several claudins and MMPs, possibly through phosphorylation of the extracellular signal-regulated protein kinase (ERK) pathway (Hong et al., 2013). Diallyl trisulfide–a compound extracted from garlic–also exhibits antineoplastic similar effects to bufalin in bladder cancer cell lines (Shin et al., 2013). Finally, the Clostridium perfringens enterotoxin (CPE), which binds to claudins −3 and −4, induces cell death in low and moderately aggressive bladder cancer cells (Gabig et al., 2016). The non-toxic fragment of CPE also increases cell sensitivity to mitomycin C and dasatinib (Gabig et al., 2016). Claudins have provided promising therapeutic value in both clinical and preclinical studies. Nevertheless, human studies are needed to determine whether direct targeting of claudins is impactful in clinical settings.
7 Conclusion and future perspectives
GU cancers are among the most prevalent neoplasms worldwide. Despite several advances in diagnosis and therapeutics, poor outcomes are seen in a large portion of patients. Claudins have piqued the interest of the scientific community due to the aberrant expression seen in a wide spectrum of tumors. Several studies have expanded on this in the context of GU cancers, such as those in the bladder, prostate, and kidneys. Abnormal expression of claudins, such as claudins −1 and −7, has been linked to clinicopathological characteristics and prognosis in GU cancer patients. Furthermore, claudins have been used to histologically differentiate between different types of renal tumors, specifically chromophobe RCCs and oncocytomas.
This manuscript also summarizes the different pathways involved in promoting aberrant claudin expression. DNA methylation impacts the expression of various claudins, and such effects have been reported in several types of GU tumors. TNF-ɑ modulates the expression of claudin-1 and promotes tumor invasion. Finally, the ΔNp63 isotype exerts pro-neoplastic effects by increasing claudin-1 expression in bladder cancer cells.
In the realm of claudin research within GU neoplasms, there lies a fertile ground for future research, particularly in unraveling the molecular mechanisms of aberrant expression of claudins. The interactions of claudins with other tight junction proteins and their roles in cellular signaling pathways are pivotal areas that demand deeper investigation, especially concerning their contribution to tumorigenesis and metastasis. With the advent of cutting-edge technologies such as CRISPR/Cas9 gene editing and advanced imaging, new dimensions of claudin behavior in cancer cells can be unveiled, potentially leading to more accurate disease models and real-time visualization of tumor progression. Molecular targeted magnetic resonance imaging is capable of detecting claudin-2 in interstitial cystitis mouse models (Smith et al., 2020). Similar imaging techniques could be utilized in the future to accurately detect and track claudin-positive GU neoplasms.
The field also grapples with unresolved questions, notably the heterogeneity of claudin expression in various cancer subtypes and stages, and its implications on prognosis and therapeutic response. The exploration of claudin-based therapeutics, despite its promising outlook, remains in its nascent stage, requiring more rigorous clinical trials and research to ascertain its efficacy and safety. Looking ahead, the integration of claudins into personalized medicine emerges as a critical frontier. Developing diagnostic tools and treatment strategies based on individual claudin profiles could revolutionize patient care. Additionally, the synergy of claudin-targeted therapies with established treatment modalities like chemotherapy, immunotherapy, or radiation presents a promising avenue for enhancing treatment outcomes and overcoming resistance.
Statements
Author contributions
TA: Writing–original draft. NA: Writing–original draft. BS: Visualization, Writing–original draft. AO: Conceptualization, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The figures in this manuscript were made using BioRender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Ahmad R. Chaturvedi R. Olivares-Villagómez D. Habib T. Asim M. Shivesh P. et al (2014). Targeted colonic claudin-2 expression renders resistance to epithelial injury, induces immune suppression, and protects from colitis. Mucosal Immunol.7 (6), 1340–1353. 10.1038/mi.2014.21
2
Arabi T. Z. Algheryafi L. A. Alodah N. A. Enabi H. M. K. Alshehry A. A. Ouban A. (2023). Aberrant expression of claudins in head and neck carcinomas and their prognostic and therapeutic value: a narrative review. Cancers15 (17), 4208. 10.3390/cancers15174208
3
Ashikari D. Takayama K.-i. Obinata D. Takahashi S. Inoue S. (2017). CLDN8, an androgen-regulated gene, promotes prostate cancer cell proliferation and migration. Cancer Sci.108 (7), 1386–1393. 10.1111/cas.13269
4
Awsare N. S. Martin T. A. Haynes M. D. Matthews P. N. Jiang W. G. (2011). Claudin-11 decreases the invasiveness of bladder cancer cells. Oncol. Rep.25 (6), 1503–1509. 10.3892/or.2011.1244
5
Badran A. Elshenawy M. A. Shahin A. Aljubran A. Alzahrani A. Eldali A. et al (2020). Efficacy and prognostic factors of sunitinib as first-line therapy for patients with metastatic renal cell carcinoma in an arab population. JCO Glob. Oncol.6 (6), 19–26. 10.1200/jgo.19.00111
6
Bahadoram S. Davoodi M. Hassanzadeh S. Bahadoram M. Barahman M. Mafakher L. (2022). Renal cell carcinoma: an overview of the epidemiology, diagnosis, and treatment. G. Ital. Nefrol.39 (3), 2022-vol3.
7
Bandini M. Gibb E. A. Gallina A. Raggi D. Marandino L. Bianchi M. et al (2020). Does the administration of preoperative pembrolizumab lead to sustained remission post-cystectomy? First survival outcomes from the PURE-01 study☆. Ann. Oncol.31 (12), 1755–1763. 10.1016/j.annonc.2020.09.011
8
Bazarbashi S. Alzahrani A. Aljubran A. Elshenawy M. Gad A. M. Maraiki F. et al (2023). Combining sunitinib and bevacizumab for the management of advanced renal cell carcinoma: a phase I/II trial. Oncologist28 (5), e254–e262. 10.1093/oncolo/oyac261
9
Boireau S. Buchert M. Samuel M. S. Pannequin J. Ryan J. L. Choquet A. et al (2007). DNA-methylation-dependent alterations of claudin-4 expression in human bladder carcinoma. Carcinogenesis28 (2), 246–258. 10.1093/carcin/bgl120
10
Bray F. Ferlay J. Soerjomataram I. Siegel R. L. Torre L. A. Jemal A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin.68 (6), 394–424. 10.3322/caac.21492
11
Chen B. Bu R. Xu X. (2020). Expression of tight junction proteins is altered in bladder cancer. Anal. Cell. Pathol.2020, 6341256–6341258. 10.1155/2020/6341256
12
Chen Y. Peng Y. Fan S. Li Y. Xiao Z.-X. Li C. (2018). A double dealing tale of p63: an oncogene or a tumor suppressor. Cell. Mol. Life Sci.75 (6), 965–973. 10.1007/s00018-017-2666-y
13
Choi Y. D. Kim K. S. Ryu S. Park Y. Cho N. H. Rha S. H. et al (2007). Claudin-7 is highly expressed in chromophobe renal cell carcinoma and renal oncocytoma. J. Korean Med. Sci.22 (2), 305–310. 10.3346/jkms.2007.22.2.305
14
Coutinho-Camillo C. M. Lourenço S. V. da Fonseca F. P. Soares F. A. (2011). Claudin expression is dysregulated in prostate adenocarcinomas but does not correlate with main clinicopathological parameters. Pathology43 (2), 143–148. 10.1097/PAT.0b013e3283428099
15
Dalghi M. G. Montalbetti N. Carattino M. D. Apodaca G. (2020). The urothelium: life in a liquid environment. Physiol. Rev.100 (4), 1621–1705. 10.1152/physrev.00041.2019
16
Elsen S. Lerut E. Van Cleynenbreugel B. van der Aa F. van Poppel H. de Witte P. A. (2015). Biodistribution of Evans blue in an orthotopic AY-27 rat bladder urothelial cell carcinoma model: implication for the improved diagnosis of non-muscle-invasive bladder cancer (NMIBC) using dye-guided white-light cystoscopy. BJU Int.116 (3), 468–477. 10.1111/bju.13113
17
Erlmeier F. Zschäbitz S. Mikuteit M. Autenrieth M. Weichert W. Hartmann A. et al (2023). The role of claudin-6 in chromophobe renal cell carcinoma. Histol. Histopathol.38 (4), 403–407. 10.14670/hh-18-520
18
Fritzsche F. R. Oelrich B. Johannsen M. Kristiansen I. Moch H. Jung K. et al (2008). Claudin-1 protein expression is a prognostic marker of patient survival in renal cell carcinomas. Clin. Cancer Res.14 (21), 7035–7042. 10.1158/1078-0432.Ccr-08-0855
19
Gabig T. G. Waltzer W. C. Whyard T. Romanov V. (2016). Clostridium perfringens enterotoxin as a potential drug for intravesical treatment of bladder cancer. Biochem. Biophys. Res. Commun.478 (2), 887–892. 10.1016/j.bbrc.2016.08.046
20
Gadelmoula M. Fukumori T. Nakatsuji H. Elgammal M. Toida K. Kanayama H.-O. (2013). Down-regulated claudin-7 immunoexpression in urothelial carcinoma of the urinary bladder. Arab J. Urology11 (2), 182–186. 10.1016/j.aju.2013.04.006
21
Gandaglia G. Leni R. Bray F. Fleshner N. Freedland S. J. Kibel A. et al (2021). Epidemiology and prevention of prostate cancer. Eur. Urol. Oncol.4 (6), 877–892. 10.1016/j.euo.2021.09.006
22
Giudici N. Bonne F. Blarer J. Minoli M. Krentel F. Seiler R. (2021). Characteristics of upper urinary tract urothelial carcinoma in the context of bladder cancer: a narrative review. Transl. Androl. Urol.10 (10), 4036–4050. 10.21037/tau-20-1472
23
Günzel D. Fromm M. (2012). Claudins and other tight junction proteins. Compr. Physiol.2 (3), 1819–1852. 10.1002/cphy.c110045
24
Gutiérrez F. J. Q. Panizo Á. Tienza A. Rodriguez I. Sola J. J. Temprana-Salvador J. et al (2018). Cytogenetic and immunohistochemical study of 42 pigmented microcystic chromophobe renal cell carcinoma (PMChRCC). Virchows Arch.473 (2), 209–217. 10.1007/s00428-018-2389-y
25
Harrison M. L. Obermueller E. Maisey N. R. Hoare S. Edmonds K. Li N. F. et al (2007). Tumor necrosis factor alpha as a new target for renal cell carcinoma: two sequential phase II trials of infliximab at standard and high dose. J. Clin. Oncol.25 (29), 4542–4549. 10.1200/jco.2007.11.2136
26
Hong S. H. Kim G.-Y. Chang Y.-C. Moon S.-K. Kim W.-J. Choi Y. H. (2013). Bufalin prevents the migration and invasion of T24 bladder carcinoma cells through the inactivation of matrix metalloproteinases and modulation of tight junctions. Int. J. Oncol.42 (1), 277–286. 10.3892/ijo.2012.1683
27
Jiang Y. Zhang Y. Luan J. Duan H. Zhang F. Yagasaki K. et al (2010). Effects of bufalin on the proliferation of human lung cancer cells and its molecular mechanisms of action. Cytotechnology62 (6), 573–583. 10.1007/s10616-010-9310-0
28
Jing P. Zou J. Zhang J. Jiang X. (2013). ΔNp63 promotes UM-UC-3 cell invasiveness and migration through claudin-1 in vitro. Mol. Med. Rep.7 (3), 1026–1030. 10.3892/mmr.2013.1271
29
Kardos J. Chai S. Mose L. E. Selitsky S. R. Krishnan B. Saito R. et al (2016). Claudin-low bladder tumors are immune infiltrated and actively immune suppressed. JCI Insight1 (3), e85902. 10.1172/jci.insight.85902
30
Kim S. S. Choi Y. D. Jin X. M. Cho Y. M. Jang J. J. Juhng S. W. et al (2009). Immunohistochemical stain for cytokeratin 7, S100A1 and claudin 8 is valuable in differential diagnosis of chromophobe renal cell carcinoma from renal oncocytoma. Histopathology54 (5), 633–635. 10.1111/j.1365-2559.2009.03271.x
31
Kind S. Büscheck F. Höflmayer D. Hube-Magg C. Kluth M. Tsourlakis M. C. et al (2020). Claudin-1 upregulation is associated with favorable tumor features and a reduced risk for biochemical recurrence in ERG-positive prostate cancer. World J. Urology38 (9), 2185–2196. 10.1007/s00345-019-03017-w
32
Kojima T. Kohno T. Kubo T. Kaneko Y. Kakuki T. Kakiuchi A. et al (2017). Regulation of claudin-4 via p63 in human epithelial cells. Ann. N. Y. Acad. Sci.1405 (1), 25–31. 10.1111/nyas.13456
33
Kolle G. Georgas K. Holmes G. P. Little M. H. Yamada T. (2000). CRIM1, a novel gene encoding a cysteine-rich repeat protein, is developmentally regulated and implicated in vertebrate CNS development and organogenesis. Mech. Dev.90 (2), 181–193. 10.1016/S0925-4773(99)00248-8
34
Krajewska M. Olson A. H. Mercola D. Reed J. C. Krajewski S. (2007). Claudin-1 immunohistochemistry for distinguishing malignant from benign epithelial lesions of prostate. Prostate67 (9), 907–910. 10.1002/pros.20578
35
Kumar B. Ahmad R. Giannico G. A. Zent R. Talmon G. A. Harris R. C. et al (2021). Claudin-2 inhibits renal clear cell carcinoma progression by inhibiting YAP-activation. J. Exp. Clin. Cancer Res.40 (1), 77. 10.1186/s13046-021-01870-5
36
Kuwada M. Chihara Y. Luo Y. Li X. Nishiguchi Y. Fujiwara R. et al (2015). Pro-chemotherapeutic effects of antibody against extracellular domain of claudin-4 in bladder cancer. Cancer Lett.369 (1), 212–221. 10.1016/j.canlet.2015.08.019
37
Landers K. A. Samaratunga H. Teng L. Buck M. Burger M. J. Scells B. et al (2008). Identification of claudin-4 as a marker highly overexpressed in both primary and metastatic prostate cancer. Br. J. Cancer99 (3), 491–501. 10.1038/sj.bjc.6604486
38
Larkin J. M. G. Ferguson T. R. Pickering L. M. Edmonds K. James M. G. Thomas K. et al (2010). A phase I/II trial of sorafenib and infliximab in advanced renal cell carcinoma. Br. J. Cancer103 (8), 1149–1153. 10.1038/sj.bjc.6605889
39
Lechpammer M. Resnick M. B. Sabo E. Yakirevich E. O Greaves W. T Sciandra K. et al (2008). The diagnostic and prognostic utility of claudin expression in renal cell neoplasms. Mod. Pathol.21 (11), 1320–1329. 10.1038/modpathol.2008.116
40
Li L. Yao J. L. di Sant'Agnese P. A. Bourne P. A. Picken M. M. Young A. N. et al (2008). Expression of claudin-7 in benign kidney and kidney tumors. Int. J. Clin. Exp. Pathol.1 (1), 57–64.
41
Li Y. Gong Y. Ning X. Peng D. Liu L. He S. et al (2018). Downregulation of CLDN7 due to promoter hypermethylation is associated with human clear cell renal cell carcinoma progression and poor prognosis. J. Exp. Clin. Cancer Res.37 (1), 276. 10.1186/s13046-018-0924-y
42
Medici D. Hay E. D. Goodenough D. A. (2006). Cooperation between snail and LEF-1 transcription factors is essential for TGF-beta1-induced epithelial-mesenchymal transition. Mol. Biol. Cell17 (4), 1871–1879. 10.1091/mbc.e05-08-0767
43
Men W. Martin T. A. Ruge F. Zhang N. Du P. Yang Y. et al (2015). Expression of claudins in human clear cell renal cell carcinoma. Cancer Genomics Proteomics12 (1), 1–8.
44
Mikuteit M. Zschäbitz S. Stöhr C. Herrmann E. Polifka I. Agaimy A. et al (2022). The prognostic impact of Claudin 6 in papillary renal cell carcinoma. Pathology - Res. Pract.231, 153802. 10.1016/j.prp.2022.153802
45
Nakanishi K. Ogata S. Hiroi S. Tominaga S. Aida S. Kawai T. (2008). Expression of occludin and claudins 1, 3, 4, and 7 in urothelial carcinoma of the upper urinary tract. Am. J. Clin. Pathology130 (1), 43–49. 10.1309/u77a6btexvca5d0e
46
Necchi A. Raggi D. Gallina A. Ross J. S. Farè E. Giannatempo P. et al (2020). Impact of molecular subtyping and immune infiltration on pathological response and outcome following neoadjuvant pembrolizumab in muscle-invasive bladder cancer. Eur. Urol.77 (6), 701–710. 10.1016/j.eururo.2020.02.028
47
Ogasawara N. Kudo T. Sato M. Kawasaki Y. Yonezawa S. Takahashi S. et al (2018). Reduction of membrane protein CRIM1 decreases E-cadherin and increases claudin-1 and MMPs, enhancing the migration and invasion of renal carcinoma cells. Biol. Pharm. Bull.41 (4), 604–611. 10.1248/bpb.b17-00990
48
Ohe C. Kuroda N. Takasu K. Senzaki H. Shikata N. Yamaguchi T. et al (2012). Utility of immunohistochemical analysis of KAI1, epithelial-specific antigen, and epithelial-related antigen for distinction of chromophobe renal cell carcinoma, an eosinophilic variant from renal oncocytoma. Med. Mol. Morphol.45 (2), 98–104. 10.1007/s00795-011-0546-3
49
Orea M. J. Angulo J. C. González-Corpas A. Echegaray D. Marvá M. Lobo M. V. T. et al (2023). Claudin-3 loss of expression is a prognostic marker in castration-resistant prostate cancer. Int. J. Mol. Sci.24 (1), 803. 10.3390/ijms24010803
50
Osunkoya A. O. Cohen C. Lawson D. Picken M. M. Amin M. B. Young A. N. (2009). Claudin-7 and claudin-8: immunohistochemical markers for the differential diagnosis of chromophobe renal cell carcinoma and renal oncocytoma. Hum. Pathol.40 (2), 206–210. 10.1016/j.humpath.2008.07.002
51
Ouban A. Arabi T. Z. (2023). Expression of claudins in preneoplastic conditions of the gastrointestinal tract: a review. Cancers15 (16), 4095. 10.3390/cancers15164095
52
Owari T. Sasaki T. Fujii K. Fujiwara-Tani R. Kishi S. Mori S. et al (2020). Role of nuclear claudin-4 in renal cell carcinoma. Int. J. Mol. Sci.21 (21), 8340. 10.3390/ijms21218340
53
Park B.-J. Lee S.-J. Kim J. I. Lee S.-J. Lee C.-H. Chang S.-G. et al (2000). Frequent alteration of p63 expression in human primary bladder carcinomas. Cancer Res.60 (13), 3370–3374.
54
Radi D. A. Abd-Elazeem M. A. (2016). Prognostic significance of lymphatic vessel density detected by D2-40 and its relation to claudin-4 expression in prostatic adenocarcinoma. Int. J. Surg. Pathology24 (3), 219–226. 10.1177/1066896915611488
55
Rini B. I. Campbell S. C. Escudier B. (2009). Renal cell carcinoma. Lancet373 (9669), 1119–1132. 10.1016/s0140-6736(09)60229-4
56
Săndulescu A. Stepan A. E. MărgĂritescu C. Badiu A. M. Matei M. Simionescu C. E. (2020). Claudin-4 immunoexpression in urothelial carcinomas. Curr. Health Sci. J.46 (4), 379–382. 10.12865/chsj.46.04.08
57
Săndulescu A. Ş. Stepan A. E. Mărgăritescu C. Enăchescu V. Mitroi G. Simionescu C. E. (2022). The role of cell adhesion molecules in the progression of bladder urothelial carcinomas. Romanian J. Morphol. Embryology63 (1), 145–151. 10.47162/rjme.63.1.15
58
Seo K. W. Kwon Y. K. Kim B. H. Kim C. I. Chang H. S. Choe M. S. et al (2010). Correlation between claudins expression and prognostic factors in prostate cancer. Korean J. Urol.51 (4), 239–244. 10.4111/kju.2010.51.4.239
59
Sheehan G. M. Kallakury B. V. S. Sheehan C. E. Fisher H. A. G. Kaufman R. P. Ross J. S. (2007). Loss of claudins-1 and -7 and expression of claudins-3 and -4 correlate with prognostic variables in prostatic adenocarcinomas. Hum. Pathol.38 (4), 564–569. 10.1016/j.humpath.2006.11.007
60
Shin D. Cha H.-J. Kim G.-Y. Kim W.-J. Choi Y. (2013). Inhibiting invasion into human bladder carcinoma 5637 cells with diallyl trisulfide by inhibiting matrix metalloproteinase activities and tightening tight junctions. Int. J. Mol. Sci.14 (10), 19911–19922. 10.3390/ijms141019911
61
Shin H. I. Kim B. H. Chang H. S. Kim C. I. Jung H. R. Park C. H. (2011). Expression of claudin-1 and -7 in clear cell renal cell carcinoma and its clinical significance. Korean J. Urology52 (5), 317–322. 10.4111/kju.2011.52.5.317
62
Singh N. P. Vinod P. K. (2020). Integrative analysis of DNA methylation and gene expression in papillary renal cell carcinoma. Mol. Genet. Genomics295 (3), 807–824. 10.1007/s00438-020-01664-y
63
Singhal S. Marwell J. G. Khaki A. R. (2023). Geriatric assessment in the older adult with genitourinary cancer: a narrative review. Front. Oncol.13, 1124309. 10.3389/fonc.2023.1124309
64
Smith N. Saunders D. Lerner M. Zalles M. Mamedova N. Cheong D. et al (2020). In vivo and ex vivo assessment of bladder hyper-permeability and using molecular targeted magnetic resonance imaging to detect claudin-2 in a mouse model for interstitial cystitis. PLOS ONE15 (10), e0239282. 10.1371/journal.pone.0239282
65
Soini Y. (2011). Claudins in lung diseases. Respir. Res.12 (1), 70. 10.1186/1465-9921-12-70
66
Szász A. M. Nyirády P. Majoros A. Szendrõi A. Szûcs M. Székely E. et al (2010). beta-catenin expression and claudin expression pattern as prognostic factors of prostatic cancer progression. BJU Int.105 (5), 716–722. 10.1111/j.1464-410X.2009.08808.x
67
Székely E. Törzsök P. Riesz P. Korompay A. Fintha A. Székely T. et al (2011). Expression of claudins and their prognostic significance in noninvasive urothelial neoplasms of the human urinary bladder. J. Histochem. Cytochem.59 (10), 932–941. 10.1369/0022155411418829
68
Takai N. Kira N. Ishii T. Yoshida T. Nishida M. Nishida Y. et al (2012). Bufalin, a traditional oriental medicine, induces apoptosis in human cancer cells. Asian Pac J. Cancer Prev.13 (1), 399–402. 10.7314/apjcp.2012.13.1.399
69
Törzsök P. Riesz P. Kenessey I. Székely E. Somorácz Á. Nyirády P. et al (2011). Claudins and ki-67: potential markers to differentiate low- and high-grade transitional cell carcinomas of the urinary bladder. J. Histochem. Cytochem.59 (11), 1022–1030. 10.1369/0022155411424606
70
Truong L. D. Shen S. S. (2011). Immunohistochemical diagnosis of renal neoplasms. Archives Pathology Laboratory Med.135 (1), 92–109. 10.1043/2010-0478-RAR.1
71
Tsukita S. Tanaka H. Tamura A. (2019). The claudins: from tight junctions to biological systems. Trends Biochem. Sci.44 (2), 141–152. 10.1016/j.tibs.2018.09.008
72
Väre P. Loikkanen I. Hirvikoski P. Vaarala M. H. Soini Y. (2008). Low claudin expression is associated with high Gleason grade in prostate adenocarcinoma. Oncol. Rep.19 (1), 25–31. 10.3892/or.19.1.25
73
Venugopal S. Anwer S. Szászi K. (2019). Claudin-2: roles beyond permeability functions. Int. J. Mol. Sci.20 (22), 5655. 10.3390/ijms20225655
74
Weinstein R. S. Merk F. B. Alroy J. (1976). “The structure and function of intercellular junctions in cancer,” in Advances in cancer research. Editors KlelnG.WeinhouseS.HaddowA. (Cambridge: Academic Press), 23–89.
75
Worst T. S. Von Hardenberg J. Gross J. C. Erben P. Schnölzer M. Hausser I. et al (2017). Database-augmented mass spectrometry analysis of exosomes identifies claudin 3 as a putative prostate cancer biomarker. Mol. Cell. Proteomics16 (6), 998–1008. 10.1074/mcp.m117.068577
76
Yadav R. Kumar Y. Dahiya D. Bhatia A. (2022). Claudins: the newly emerging targets in breast cancer. Clin. Breast Cancer22 (8), 737–752. 10.1016/j.clbc.2022.09.001
77
Yamuç E. Barışık N. Şensu S. Tarhan F. Barışık C. C. (2022). Correlation of REG1A, Claudin 7 and Ki67 expressions with tumor recurrence and prognostic factors in superficial urothelial urinary bladder carcinomas. Indian J. Pathol. Microbiol.65 (2), 355–361. 10.4103/ijpm.Ijpm_914_20
78
Yang J. Mani S. A. Weinberg R. A. (2006). Exploring a new twist on tumor metastasis. Cancer Res.66 (9), 4549–4552. 10.1158/0008-5472.Can-05-3850
79
Yang W. Li L. Zhang K. Ma K. Gong Y. Zhou J. et al (2021). CLDN10 associated with immune infiltration is a novel prognostic biomarker for clear cell renal cell carcinoma. Epigenomics13 (1), 31–45. 10.2217/epi-2020-0256
80
Yang W. Zhang K. Zhang Z. Zhou J. Li L. Xu Y. et al (2022). Claudin-10 overexpression suppresses human clear cell renal cell carcinoma growth and metastasis by regulating ATP5O and causing mitochondrial dysfunction. Int. J. Biol. Sci.18 (6), 2329–2344. 10.7150/ijbs.70105
81
Ye X. Zhao L. Kang J. (2019). Expression and significance of PTEN and Claudin-3 in prostate cancer. Oncol. Lett.17, 5628–5634. 10.3892/ol.2019.10212
82
Zheng J. Y. Yu D. Foroohar M. Ko E. Chan J. Kim N. et al (2003). Regulation of the expression of the prostate-specific antigen by claudin-7. J. Membr. Biol.194 (3), 187–197. 10.1007/s00232-003-2038-4
83
Zhou J. Yang X. Zhou L. Zhang P. Wang C. (2019). Combined immunohistochemistry for the “three 7” markers (CK7, CD117, and claudin-7) is useful in the diagnosis of chromophobe renal cell carcinoma and for the exclusion of mimics: diagnostic experience from a single institution. Dis. Markers2019, 4708154. 10.1155/2019/4708154
84
Zhu L. Han J. Li L. Wang Y. Li Y. Zhang S. (2019). Claudin family participates in the pathogenesis of inflammatory bowel diseases and colitis-associated colorectal cancer. Front. Immunol.10, 1441. 10.3389/fimmu.2019.01441
85
Zhu Z. Xu C. Lin L. Lv T. Cai T. Lin J. (2020). Prognostic value and potential biological functions of CLDN8 in patients with clear cell renal cell carcinoma. Onco Targets Ther.13, 9135–9145. 10.2147/ott.S266846
Summary
Keywords
claudins, carcinoma, urothelial cell, prostate, renal cell carcinoma, treatment, prognosis, diagnosis
Citation
Arabi TZ, Ashraf N, Sabbah BN and Ouban A (2023) Claudins in genitourinary tract neoplasms: mechanisms, prognosis, and therapeutic prospects. Front. Cell Dev. Biol. 11:1308082. doi: 10.3389/fcell.2023.1308082
Received
05 October 2023
Accepted
11 December 2023
Published
22 December 2023
Volume
11 - 2023
Edited by
Igor Jakovcevski, Witten/Herdecke University, Germany
Reviewed by
Kranthi Kiran Kishore Tanagala, Columbia University, United States
Panagiota S. Filippou, Teesside University, United Kingdom
Updates
Copyright
© 2023 Arabi, Ashraf, Sabbah and Ouban.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abderrahman Ouban, aouban@alfaisal.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.