Abstract
Regular physical activity is widely recognized for its systemic health benefits, extending beyond physical fitness to influence metabolism, immunity, and neurophysiology. Pregnancy is a physiologically unique period characterized by dynamic immunometabolic changes that are crucial for maternal and fetal health. Maternal exercise during this window offers a non-pharmacological strategy to enhance maternal wellbeing and optimize offspring development. This review summarizes recent advances in understanding the effects of maternal exercise on both pregnant women and their offspring. In mothers, exercise improves metabolic profiles, modulates inflammatory responses, supports neuroplasticity, and promotes skeletal health. In offspring, maternal exercise confers long-term benefits including improved glucose metabolism, enhanced neurogenesis, cognitive development, and immune resilience. Mechanistically, these effects are mediated through molecular pathways such as placental superoxide dismutase 3 (SOD3) upregulation, adenosine 5′-monophosphate-activated protein kinase/ten-eleven translocation (AMPK/TET) signaling in the fetal liver, and exercise-induced circulating factors like Apelin and SERPINA3C, which contribute to epigenetic remodeling and tissue-specific programming. Despite growing evidence, gaps remain in understanding the optimal intensity, timing, and molecular mediators of maternal exercise, particularly regarding long-term immune and neurodevelopmental outcomes in offspring. Future studies leveraging multi-omics approaches are needed to elucidate cross-organ signaling mechanisms and identify therapeutic targets to mimic exercise-induced benefits. Overall, maternal exercise emerges as a safe, accessible intervention with significant potential to improve maternal-fetal health and reduce offspring disease risk across the lifespan.
1 Introduction
Regular exercise is a health-promoting lifestyle generally recommended to reduce the risk of various disorders. Growing evidence shows the multiple benefits of exercise, which extend beyond physical fitness and can exert positive effects on the metabolism, immunity, and nervous system (Kusuyama et al., 2020; Hayman et al., 2023; Davenport et al., 2018). However, the deeper underlying mechanism of exercise-induced effects remains unclear, hindering the development of alternative drugs that can reproduce the exercise-induced effects.
The prenatal period encompasses a critical window for the future healthy development of offspring (the Barker Hypothesis). Thus, investigating the effects of maternal exercise during pregnancy on offspring throughout intrauterine and postnatal development is also an interesting topic (Muglia et al., 2022). Emerging evidence supports that moderate exercise by mothers during pregnancy benefits their children. The US Department of Health and Human Services recommends that pregnant women insist on a minimum of 150 min per week of moderate-intensity exercise (Piercy et al., 2018). However, only a minority of pregnant women meet the recommendations (Hayman et al., 2023; Davenport et al., 2018).
In this review, we firstly introduced the benefits of exercise on pregnant women focusing in their common body conditions, which include recent findings of exercise-induced effects on body metabolism, neuron system, immune system, and skeletal system. We also review recent studies of maternal exercise-induced effects on offspring. We also discuss the present challenges and future directions for studying exercise.
2 Benefits of exercise on mothers
Physical exercise is a well-known non-pharmacological treatment to improve various disorders. It produces systemic health benefits by affecting multiple tissues, including the skeletal system, muscle, adipose tissue, liver and brain (Figure 1). Therefore, these exercise-induced effects and the underlying mechanisms must be studied from a holistic perspective.
FIGURE 1
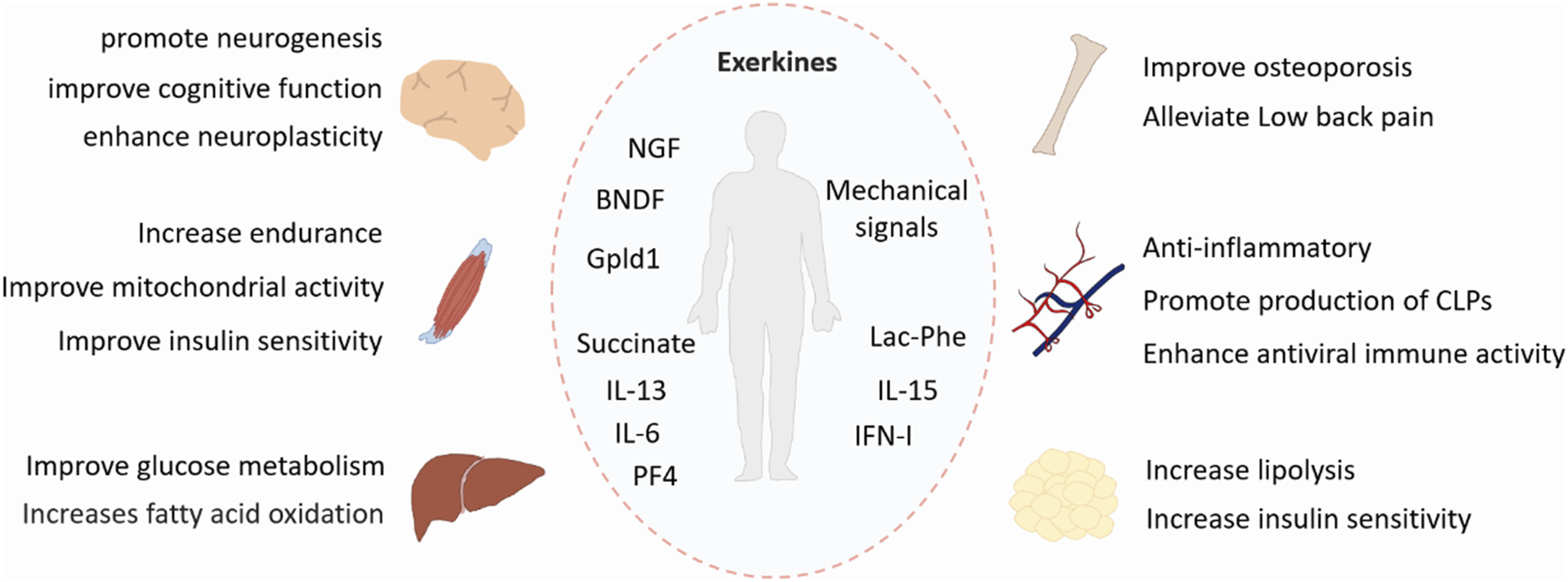
Benefits of exercise. Exercise has systemic positive effects on multiple tissues, including the skeletal system, muscle, adipose tissue, liver, and brain. Exerkines are exercise-induced factors that exert their effects through endocrine, paracrine, or autocrine pathways. BDNF, brain-derived neurotrophic factor; NGF, nerve growth factor; Gpld1, glycosylphosphatidylinositol-specific phospholipase D1; IL-6, interleukin-6; IL-13, interleunkin-13; IL-15, interleukin-15; IFN-I, type I interferon; Lac-Phe, N-lactoyl-phenylalanine; PF4, platelet factor 4; CLPs, common lymphoid progenitors.
2.1 Improve body metabolism
The global incidence of obesity and diabetes has risen sharply, and exercise is a key non-pharmacological intervention to improve metabolic health. Traditionally, the benefits of exercise have been attributed to skeletal muscle (Egan and Zierath, 2013; Jonathon et al., 2023), which releases myokines and metabolites during activity (Martin et al., 2023). For example, succinate (Reddy et al., 2020) and IL-13 (Nelson et al., 2020) are secreted by muscle during exercise and contribute to enhanced glucose tolerance, mitochondrial activity, and endurance.
However, recent studies highlight that other tissues also mediate exercise benefits (Stanford et al., 2015a). Adipose tissue responds to exercise in a time-of-day dependent manner, as shown by Pendergrast et al. (2023), with fat mobilization occurring only during nocturnal activity in mice. Exercise also modulates cardiac metabolism by reducing glycolytic activity (Gibb et al., 2017) and reshapes the gut microbiome (Jonathan et al., 2024), thereby improving endurance. Furthermore, Li et al. identified the metabolite N-lactoyl-phenylalanine (Li et al., 2022), which suppresses appetite and reduces obesity, though its cellular origin remains unclear. A meta-analysis also suggests that combining exercise with metformin enhances glucose regulation in diabetic patients (Zhao et al., 2024).
In humans, maternal exercise during pregnancy lowers gestational weight, reduces cesarean risk (The International Weight Management in Pregnancy (i-WIP) Collaborative Group, 2017; Wang et al., 2017), and decreases the incidence of gestational diabetes mellitus (GDM) (Wang et al., 2017). It also induces long-term liver mitochondrial adaptations in GDM mothers, potentially delaying metabolic complications later in life (Stevanović-Silva et al., 2021).
Advancements in multi-omics technologies have accelerated this field. Sato et al. mapped the exercise-induced metabolome across tissues and time points, while the Molecular Transducers of Physical Activity Consortium developed a comprehensive database spanning transcriptomic to epigenomic changes across multiple tissues during endurance training (Sato et al., 2022). These resources offer powerful tools for deciphering the complex molecular responses to exercise and identifying potential therapeutic targets (MoTrPAC Study GroupLead AnalystsMoTrPAC Study Group, 2024).
2.2 Improve nervous system
Exercise exerts profound benefits on the nervous system, influencing both the central and peripheral components. A large body of evidence shows that physical activity promotes neurogenesis, particularly in the hippocampus (Liu et al., 2019). Van Praag et al. demonstrated that running enhances dentate gyrus neurogenesis in mice, improving memory and learning performance (van Praag et al., 1999). Aerobic exercise has been shown to most effectively stimulate adult hippocampal neurogenesis (Nokia et al., 2016), which is also essential for maintaining cognitive function in aging (Zhou et al., 2021). Exercise also enhances neuroplasticity (Yamaguchi et al., 2016), partly by upregulating neurotrophic factors. Notably, exercise increases brain-derived neurotrophic factor (BDNF) expression (Sleiman et al., 2016; Adlard et al., 2005), which supports synapse formation, plasticity, and cognitive enhancement (Hempstead, 2015; Casarotto et al., 2021; Fang et al., 2003; Anastasia et al., 2013; Kowianski et al., 2018). Additionally, nerve growth factor (NGF) activated by exercise binds to TrkA receptors, promoting neuronal survival and synaptic modulation (Chao et al., 2006; Saragovi et al., 1998; Hall et al., 2018). Interestingly, exercise not only acts as a metabolic challenge but also initiates brain-driven metabolic regulation (Hwang et al., 2023; Gautron et al., 2015). For instance, BDNF influences systemic metabolism (Fulgenzi et al., 2020; Xu and Xie, 2016), and exercise stimulates hypothalamic POMC neurons (Kang et al., 2021), leading to thermogenesis via adipose tissue mitochondrial activation. Exercise improves cognitive functions, including memory, learning, and decision-making (Augusto-Oliveira et al., 2023). Horowitz et al. found that plasma from exercise-trained aged mice improves cognition and neurogenesis in sedentary peers, with Gpld1 identified as a key circulating factor (Horowitz et al., 2020). Similarly, platelet factor 4, higher in younger individuals, reduces neuroinflammation and enhances cognition in aged mice (Schroer et al., 2023). Moreover, exercise mitigates neurodegenerative conditions (Zhang et al., 2019). Long-term physical activity alleviates cognitive impairment in Alzheimer’s disease mice by enhancing lysosomal function and promoting amyloid-beta clearance (Wang et al., 2022). Mechanistically, exercise facilitates nuclear translocation of TFEB, increases interaction with AMPK-mediated acetyl-CoA synthetase 2, and boosts lysosomal gene transcription.
In summary, exercise promotes neuronal development, synaptic plasticity, metabolic regulation, and cognitive resilience, highlighting its therapeutic potential for neurodevelopmental and neurodegenerative conditions.
2.3 Improve immunity
The immune system plays essential roles in defense, regulation, and homeostasis, and exercise has emerged as a powerful modulator of immune function (Friedrich, 2008; Watts, 2012). One of the most consistent findings is that regular physical activity helps reduce systemic inflammation (Gleeson et al., 2011), which is particularly beneficial in chronic metabolic disorders such as type 2 diabetes (Papagianni et al., 2023). This anti-inflammatory effect is supported by evidence showing that exercise downregulates pro-inflammatory signaling pathways and enhances anti-inflammatory immune responses, partly through epigenetic and metabolic modulation of immune cells (Nini et al., 2024). Aging-related increases in inflammatory activity can also be attenuated by exercise, highlighting its role in immune rejuvenation (Ling et al., 2023).
Beyond controlling inflammation, exercise promotes immune cell production and activity (Shen et al., 2021). Mechanical stimulation during physical activity can trigger bone marrow niche cells to release factors that support lymphoid progenitor expansion (Tengfei et al., 2024). Additionally, exercise enhances innate antiviral responses, such as increased type I interferon production, and promotes the expansion of regulatory T cells in muscle tissue (Langston et al., 2023). These cells help maintain immune balance and support tissue integrity by preventing excessive inflammatory responses that could lead to cellular damage.
Given these immunomodulatory properties, exercise is increasingly recognized as a valuable adjunctive therapy in cancer (Fiuza-Luces et al., 2024; Kathryn et al., 2019). It has been shown to improve quality of life (Anouk et al., 2024), physical function (Scott et al., 2018), and immune competence (Kurz et al., 2022) in cancer patients. Exercise can lower recurrence risk (Soldato et al., 2024), enhance tumor immune surveillance, and improve treatment outcomes. Mechanistically, this involves the release of cytokines such as IL-15 (Kurz et al., 2022), which supports T cell mobilization and tumor infiltration, strengthening anti-tumor immunity.
Importantly, exercise also contributes to long-term health and longevity, regardless of disease status, by promoting systemic immune balance. Together, these findings support the role of regular physical activity as a low-cost, non-pharmacological strategy to enhance immune defense, reduce chronic inflammation, and support disease prevention and recovery, particularly in aging and cancer contexts (Jessica et al., 2024; Lavery et al., 2023).
2.4 Effects on skeletal system disorders
The skeletal system is fundamental for movement and structural support, and exercise plays a critical role in its development, maintenance, and rehabilitation (Lee et al., 2014; Huiskes et al., 2000). Physical activity has long been recommended for managing skeletal disorders such as osteoporosis and low back pain (LBP) (Kise et al., 2016; Pagnotti et al., 2019; Breda et al., 2021; Owen et al., 2020).
In osteoporosis, particularly among postmenopausal women (Pagnotti et al., 2019; Courteix et al., 1998), various exercise modalities have shown beneficial effects on bone mineral density (BMD) (Mohammad Rahimi et al., 2020). Resistance and impact training are especially effective in improving bone strength and functional performance (Watson et al., 2018), while mind–body exercises (Zhang et al., 2021), such as Tai Chi, have been associated with BMD improvements in the lumbar spine and femoral neck, particularly with long-term practice (Chow et al., 2018; Sun et al., 2016). Mechanistically, bones respond to mechanical loading (Huiskes et al., 2000), where osteoblasts sense strain through mechanosensitive ion channels like PIEZO1/2 (Sun et al., 2019; Wang et al., 2020). In addition to mechanical signaling, moderate exercise has been shown to influence bone formation through sympathetic cholinergic nerve fibers (Gadomski et al., 2022) and epigenetic modifications (Chen et al., 2021), offering insights into how physical activity promotes skeletal adaptation at a molecular level.
For LBP, a condition increasingly prevalent and economically burdensome, intervertebral disc (IVD) degeneration is often a primary cause. Exercise has emerged as a non-invasive strategy for promoting IVD regeneration (Sasaki et al., 2012). Experimental models show that exercise stimulates the proliferation of IVD progenitor cells and increases glycosaminoglycan content (Ueta et al., 2018), enhancing disc hydration and matrix integrity. In humans, early-stage physical activity yields modest but significant improvements in disability related to recent-onset LBP (Fritz et al., 2015). Specific movement therapies, such as motor control exercise (Saragiotto et al., 2016) and moderate intensity aerobic training (Belavý et al., 2017), have shown low to moderate efficacy in reducing chronic LBP symptoms and improving long-term disc function (van Dillen et al., 2021), especially in individuals with less physically demanding occupations (Hayden et al., 2020).
Overall, exercise serves as a mechanically and biologically active intervention for skeletal health, benefiting both bone density and spinal disc integrity, and offers a promising alternative or adjunct to pharmacological and surgical treatments for skeletal disorders.
3 Benefits of maternal exercise on offspring
Maternal exercise during pregnancy exerts multiple beneficial effects on offspring and confers protection against the development of various disorders. However, more studies are required to reveal the maternal-exercise-induced long-term effects on offspring (Figure 2).
FIGURE 2
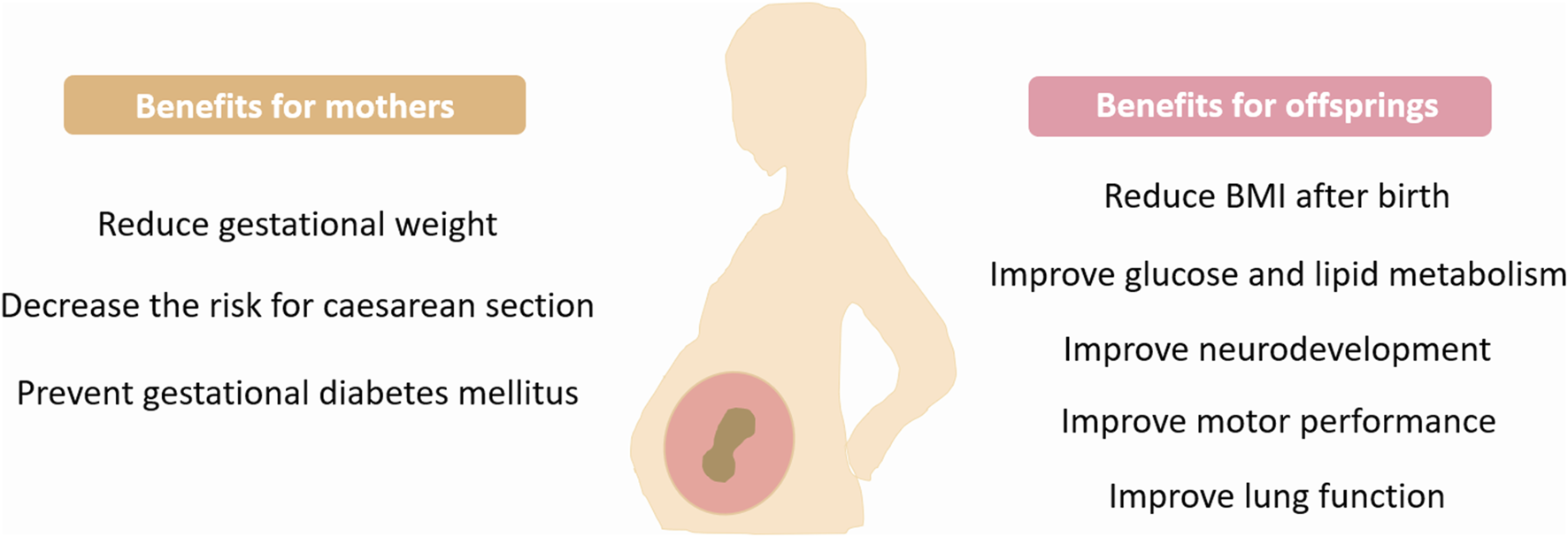
Benefits of maternal exercise. Exercise during pregnancy provides many health benefits for mothers and children. BMI, Body mass index.
3.1 Improved metabolic health in offspring
Increasing evidence suggests that an adverse intrauterine environment is strongly associated with a higher risk of obesity and diabetes in offspring (Kusuyama et al., 2020; Sales et al., 2017). In contrast, maternal exercise has emerged as a promising non-pharmacological intervention to improve offspring metabolic health (Harris et al., 2018).
In humans, maternal physical activity during pregnancy is associated with favorable postnatal outcomes, including reduced offspring subcutaneous fat mass (Clapp, 1996), lower BMI (Mourtakos et al., 2015; Jevtovic et al., 2005), and improved body weight regulation into early childhood. Importantly, maternal exercise has been shown to influence the metabolic function of offspring mesenchymal stem cells (MSCs) (Jevtovic et al., 2024), enhancing glucose and lipid metabolism (Chaves et al., 2022), with resistance training showing the most prominent effects (Jevtovic et al., 2023).
Animal studies further confirm that maternal exercise mitigates the adverse metabolic effects of a maternal high-fat diet (Stanford et al., 2017), improving glucose tolerance and liver metabolism in offspring (Zhang et al., 2023). However, the timing of exercise is critical; benefits are most evident when exercise is performed both before and during gestation, but not if limited to either period alone (Stanford et al., 2015b; Sheldon et al., 2016).
At the molecular level, recent research has identified several key pathways through which maternal exercise benefits fetal development. These include the vitamin D receptor-mediated increase in placental superoxide dismutase 3 (SOD3) (Kusuyama et al., 2021), which activates adenosine 5′-monophosphate-activated protein kinase/ten-eleven translocation (AMPK/TET) signaling and promotes DNA demethylation of glucose metabolism genes in fetal liver (Bae-Gartz et al., 2020). Additionally, exercise-induced circulating factors such as Apelin (Jun Seok et al., 2020) and SERPINA3C (Li et al., 2025) play crucial roles in enhancing brown adipose tissue development and reducing inflammation via PI3K-TET1-Klf4 signaling in fetal adipose tissue (Li et al., 2025).
Together, these findings highlight maternal exercise as a powerful modulator of epigenetic programming and cellular metabolism in offspring, offering long-term protection against metabolic disorders.
3.2 Promotion of neuron development in offspring
Maternal exercise during pregnancy has been increasingly recognized to promote not only maternal neurogenesis but also enhance neurodevelopmental outcomes in offspring, particularly in cognitive, behavioral, and motor domains (Wiebe et al., 2015; Labonte-Lemoyne et al., 2017). Studies report that offspring of physically active mothers show more mature neonatal EEG patterns (Labonte-Lemoyne et al., 2017), reduced neural immaturity markers (Clapp et al., 1999), and higher cognitive performance, including elevated IQ levels during infancy (Domingues et al., 2014).
The neuroprotective and neuroenhancement effects of gestational physical activity are likely mediated by multiple mechanisms. Exercise improves fetal cerebral oxygenation (Moreno-Fernandez et al., 2020), promotes synaptogenesis (Yau et al., 2019), and enhances hippocampal neurogenesis, leading to long-term benefits in learning-memory capability (Ayfer et al., 2012), and emotional regulation (Kim et al., 2024). Some findings indicate sex-specific effects, with male and female offspring showing distinct cognitive and neural responses (Yau et al., 2019). Additionally, pre-pregnancy exercise may confer resilience against prenatal stress (Nakahara et al., 2021) and reduce neurodevelopmental issues (Klein et al., 2019) such as sleep or behavioral disturbances (Nakahara et al., 2021).
On a molecular level, maternal exercise has been shown to suppress neurotoxic markers like tau phosphorylation and oxidative stress (Klein et al., 2020), while increasing neurotrophic factors such as BDNF and mature neurotrophic proteins (Park et al., 2021), which contribute to enhanced neurogenesis and synaptic plasticity in the offspring brain (Akhavan et al., 2011). Amyloid precursor proteins (Mohammad et al., 2024) and hippocampal plasticity pathways have also been implicated in mediating these effects.
Motor development benefits have also been reported, with offspring demonstrating improved neuromotor performance in infancy (McMillan et al., 2019) and even into later childhood (Ferrari et al., 2023). These motor improvements may be linked to increased maternal BDNF levels during late pregnancy, which can cross the placenta and influence fetal brain development. However, some findings remain inconsistent, with certain long-term studies reporting no significant differences in motor outcomes (Ellingsen et al., 2020).
Overall, prenatal exercise is a promising, low-risk intervention that supports neural development and functional maturation in offspring. Despite encouraging findings, mechanistic understanding remains limited, highlighting the need for further research into how maternal physical activity programs neurodevelopmental trajectories (Na et al., 2022; Zhou et al., 2022).
3.3 Immunomodulation in offspring
Pregnancy is characterized by trimester-specific immunometabolic adaptations, essential for maintaining maternofetal homeostasis and supporting healthy gestation. These physiological changes include dynamic modulation of inflammatory responses (Mor et al., 2011; Kalagiri et al., 2016), which may be influenced by maternal lifestyle factors such as physical activity. Given the role of exercise-induced cytokines (exerkines) in systemic immunoregulation, maternal exercise could serve as a potential non-pharmacological strategy to modulate inflammation during pregnancy. Exerkines refer to cytokines, peptides, and proteins induced by exercise, which exert their effects throughout the body through blood circulation, regulating various physiological and metabolic processes (Sabaratnam et al., 2022).
Emerging, though limited, evidence suggests that maternal exercise reduces systemic inflammation in pregnant women (Wang et al., 2015; Hawkins et al., 2014). Light to moderate physical activity has been associated with lower levels of C-reactive protein (CRP) (Tinius et al., 2017) and pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) (Acosta-Manzano et al., 2019). However, findings on vigorous exercise remain inconsistent, with some data indicating elevated IL-6 or IL-1β (Acosta-Manzano et al., 2020), emphasizing the importance of exercise intensity and the need for cautious interpretation due to statistical variability. Some studies suggest that moderate-intensity exercise offers the most favorable inflammatory profile, balancing immune activation and suppression (Dhar et al., 2024).
Animal studies further support these findings. Prenatal exercise in rodent models has been shown to increase BDNF and decrease inflammatory markers in offspring exposed to brain injury, suggesting that maternal physical activity confers neuroprotection through enhanced antioxidant and anti-inflammatory pathways (Gorgij et al., 2021).
Despite these promising observations, research on immunomodulation by maternal exercise remains limited, particularly regarding long-term effects on offspring immune and neurodevelopmental health. Future studies are needed to clarify the dose-response relationship between exercise intensity and immune outcomes, and to elucidate the role of maternal exerkines in mediating maternal-fetal immune communication (Acosta-Manzano et al., 2020; Adamo et al., 2024).
3.4 Other benefits of maternal exercise to offspring
Beyond the improvements in metabolic health and neuronal function, maternal exercise has many other beneficial effects on offspring, such as improving hypertensive disorders of pregnancy (Barakat et al., 2016), reducing the risk of cesarean section (Di Mascio et al., 2016; Owe et al., 2016) and heart protection (Reihaneh et al., 2023). Although research in these aspects is not as extensive as that on the effects of maternal exercise on offspring metabolism and neurodevelopment, several randomized clinical trials have reported relevant findings. Musakka et al. (2024) reported as well that maternal exercise during pregnancy, when practiced three or more times per week, is associated with a reduced risk of asthma in offspring. Carlsen et al. (Gudmundsdóttir et al., 2022) suggested that physical activity in the first half of pregnancy is linked to increased lung function in the child. Moreover, Owe et al. (2016) found that regular exercise during pregnancy is associated with reduced risk of acute cesarean section for mothers. Additionally, the meta-analysis from Davenport et al. (2018) indicates that maternal exercise is not associated with adverse childhood complications, but it is associated with reduced odds of macrosomia. Macrosomia refers to infants with a birth weight exceeding 4,000 g, and it is associated with several maternal and fetal complications such as maternal birth canal trauma, shoulder dystocia, and perinatal asphyxia (Araujo Junior et al., 2017; Nguyen and Ouzounian, 2021). Zhang et al. also reported that maternal exercise can alleviate oxidative stress and the impairment of endothelium-dependent vasodilatation, thereby improving vascular function in hypertensive offspring.
Although current findings tentatively indicate various potential enhancing effects of maternal exercise during pregnancy, yet conclusions are constrained by methodological limitations including small sample sizes and inconsistent assessment protocols. Moreover, research examining the impacts of prenatal exercise on offspring across different offspring age groups remains limited, with underlying mechanisms poorly understood. Therefore, addressing these research gaps holds significant clinical value for establishing evidence-based guidelines for prenatal health management.
4 Risks of maternal exercise during pregnancy
While prior research has established the benefits of maternal exercise for offspring, its potential association with miscarriage risk warrants clarification. Our comprehensive literature review found no evidence that exercise during pregnancy increases miscarriage risk. However, the lack of documented evidence does not preclude this possibility. Previous research indicated that high-intensity exercise can negatively affect placental blood flow (Salvesen et al., 2012). While some studies suggest strenuous exercise could be safe for pregnant women, but only for athletes who are well trained before pregnancy (Titova et al., 2024). Moreover, vigorous leisure activity is associated with reduced birth weight, suggesting a cautious engagement in vigorous exercise during pregnancy (Leet and Flick, 2003; Evenson et al., 2014; Mottola et al., 2018). The Australian guidelines proposed by Brown et al. (2022), aligned with recently published international standards and professional recommendations, outline contraindications and warning signs for prenatal and postnatal physical activity/exercise. Pregnant individuals should undergo individualized risk assessments and prioritize moderate-to-low intensity exercise while monitoring for pregnancy-related complications (Vargas-Terrones et al., 2019; Bull et al., 2020). Absolute contraindications may include: Poorly controlled metabolic disorders (Type 1 diabetes or Thyroid disease); Cardiovascular/Respiratory disorders; Pre-eclampsia; Cervical insufficiency or ruptured membranes; Persistent second or third trimester bleeding; Placenta previa; Intrauterine growth restriction; Multiple gestation (triplets or higher number). Overall, further research is warranted to systematically evaluate exercise-related risks during pregnancy through comprehensive risk stratification and establish standardized risk assessment protocols for prenatal exercise to prevent adverse pregnancy outcomes while optimizing maternal-fetal health outcomes.
5 Conclusion
Regular exercise can improve whole-body health, but the systemic effects and the underlying molecular mechanisms remain incompletely understood. Various sequencing methods that have emerged in recent years can help us further understand the exercise-induced systemic effects. In fact, scientists around the world have provided multiple databases for studying the exercise-induced cross-organ effects under different conditions. These datasets serve as valuable resources for understanding the multi-tissue molecular effects of exercise. However, current studies do not consider the effects of exercise on pregnant mammals and their offspring. Future study may profile the multi-omic sequence across tissues of pregnant mammals with or without exercise, which must be helpful for exploring the molecular effects of exercise on pregnant mothers and their offspring.
Additionally, despite the multiple benefits of exercise introduced here, an active lifestyle, as well as insistence on exercise, may be difficult for most individuals because of busy work or owing to age, disease, or other reasons. Therefore, one of the ultimate goals of sports medicine research is to identify the key regulators and factors (e.g., peptides, metabolites, and cytokines) that are induced after exercise. They may be developed as potential therapeutic agents to mimic beneficial effects in the absence of physical training. Moreover, the multi-omics profiles for exercise under any of the conditions mentioned above may be pivotal for identifying the promising target.
Statements
Author contributions
KW: Conceptualization, Writing – original draft, Writing – review and editing. JZ: Supervision, Validation, Writing – review and editing. YW: Validation, Visualization, Writing – review and editing. ML: Conceptualization, Funding acquisition, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research was fully supported by Humanities and Social Sciences Research Project of Ministry of Education of China (No. 22YJA890013).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Generative AI was used to shorten the length of the manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Acosta-ManzanoP.AcostaF. M.FemiaP.Coll-RiscoI.Segura-JiménezV.Díaz-CastroJ.et al (2020). Association of sedentary time and physical activity levels with immunometabolic markers in early pregnancy: the GESTAFIT project. Scand. J. Med. and Sci. sports30, 148–158. 10.1111/sms.13547
2
Acosta-ManzanoP.Coll-RiscoI.Van PoppelM. N. M.Segura-JiménezV.FemiaP.Romero-GallardoL.et al (2019). Influence of a concurrent exercise training intervention during pregnancy on maternal and arterial and venous cord serum cytokines: the GESTAFIT project. J. Clin. Med.8, 1862. 10.3390/jcm8111862
3
AdamoK. B.GoudreauA. D.CorsonA. E.MacDonaldM. L.O'RourkeN.TzanevaV. (2024). Physically active pregnancies: insights from the placenta. Physiol. Rep.12, e16104. 10.14814/phy2.16104
4
AdlardP. A.PerreauV. M.CotmanC. W. (2005). The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiol. Aging26, 511–520. 10.1016/j.neurobiolaging.2004.05.006
5
AkhavanM. M.ForoutanT.SafariM.Sadighi-MoghaddamB.Emami-AbarghoieM.Rashidy-PourA. (2011). Prenatal exposure to maternal voluntary exercise during pregnancy provides protection against mild chronic postnatal hypoxia in rat offspring. Pak J. Pharm. Sci.25.
6
AnastasiaA.DeinhardtK.ChaoM. V.WillN. E.IrmadyK.LeeF. S.et al (2013). Val66Met polymorphism of BDNF alters prodomain structure to induce neuronal growth cone retraction. Nat. Commun.4, 2490. 10.1038/ncomms3490
7
AnoukE. H.DepenbuschJ.SchmidtM. E.MonninkhofE. M.PelaezM.ClaussD.et al (2024). Supervised, structured and individualized exercise in metastatic breast cancer: a randomized controlled trial. Nat. Med.30, 2957–2966. 10.1038/s41591-024-03143-y
8
Araujo JuniorE.PeixotoA. B.ZamarianA. C.Elito JuniorJ.TonniG. M. (2017). Macrosomia. Best. Pract. Res. Clin. Obstet. Gynaecol.38, 83–96. 10.1016/j.bpobgyn.2016.08.003
9
Augusto-OliveiraM.ArrifanoG. P.Leal-NazaréC. G.Santos-SacramentoL.Lopes-AraújoA.RoyesL. F. F.et al (2023). Exercise reshapes the brain: molecular, cellular, and structural changes associated with cognitive improvements. Mol. Neurobiol.60, 6950–6974. 10.1007/s12035-023-03492-8
10
AyferD.AgilkayaS.OzbalS.CetinF.AksuI.GencogluC.et al (2012). Maternal aerobic exercise during pregnancy can increase spatial learning by affecting leptin expression on offspring's early and late period in life depending on gender. ScientificWorldJournal2012, 429803. 10.1100/2012/429803
11
Bae-GartzI.KasperP.GroßmannN.BreuerS.JanoschekR.KretschmerT.et al (2020). Maternal exercise conveys protection against NAFLD in the offspring via hepatic metabolic programming. Sci. Rep.10, 15424. 10.1038/s41598-020-72022-6
12
BarakatR.PelaezM.CorderoY.PeralesM.LopezC.CoteronJ.et al (2016). Exercise during pregnancy protects against hypertension and macrosomia: randomized clinical trial. Am. J. obstetrics Gynecol.214, 649.e641–e8. 10.1016/j.ajog.2015.11.039
13
BelavýD. L.QuittnerM. J.RidgersN.LingY.ConnellD.RantalainenT. (2017). Running exercise strengthens the intervertebral disc. Sci. Rep.7, 45975. 10.1038/srep45975
14
BredaS. J.OeiE. H. G.ZwerverJ.VisserE.WaarsingE.KrestinG. P.et al (2021). Effectiveness of progressive tendon-loading exercise therapy in patients with patellar tendinopathy: a randomised clinical trial. Br. J. Sports Med.55, 501–509. 10.1136/bjsports-2020-103403
15
BrownW. J.HaymanM.HaakstadL. A. H.LamertonT.MenaG. P.GreenA.et al (2022). Australian guidelines for physical activity in pregnancy and postpartum. J. Sci. Med. sport25, 511–519. 10.1016/j.jsams.2022.03.008
16
BullF. C.Al-AnsariS. S.BiddleS.BorodulinK.BumanM. P.CardonG.et al (2020). World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med.54, 1451–1462. 10.1136/bjsports-2020-102955
17
CasarottoP. C.GirychM.FredS. M.KovalevaV.MolinerR.EnkaviG.et al (2021). Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell.184, 1299–1313.e19. 10.1016/j.cell.2021.01.034
18
ChaoM. V.RajagopalR.LeeF. S. (2006). Neurotrophin signalling in health and disease. Clin. Sci. (Lond)110, 167–173. 10.1042/CS20050163
19
ChavesA.WeyrauchL. A.ZhengD.BiagioniE. M.KrassovskaiaP. M.DavidsonB. L.et al (2022). Influence of maternal exercise on glucose and lipid metabolism in offspring stem cells: ENHANCED by mom. J. Clin. Endocrinol. metabolism107, e3353–e3365. 10.1210/clinem/dgac270
20
ChenX.ZhuX.WeiA.ChenF.GaoQ.LuK.et al (2021). Nrf2 epigenetic derepression induced by running exercise protects against osteoporosis. Bone Res.9, 15. 10.1038/s41413-020-00128-8
21
ChowT. H.LeeB. Y.AngA. B. F.CheungV. Y. K.HoM. M. C.TakemuraS. (2018). The effect of Chinese martial arts Tai Chi Chuan on prevention of osteoporosis: a systematic review. J. Orthop. Transl.12, 74–84. 10.1016/j.jot.2017.06.001
22
ClappJ. F. (1996). Morphometric and neurodevelopmental outcome at age five years of the offspring of women who continued to exercise regularly throughout pregnancy. J. Pediatr.129, 856–863. 10.1016/s0022-3476(96)70029-x
23
ClappJ. F.LopezB.Harcar-SevcikR. (1999). Neonatal behavioral profile of the offspring of women who continued to exercise regularly throughout pregnancy. Am. J. obstetrics Gynecol.180, 91–94. 10.1016/s0002-9378(99)70155-9
24
CourteixD.LespessaillesE.PeresS. L.ObertP.GermainP.BenhamouC. L. (1998). Effect of physical training on bone mineral density in prepubertal girls: a comparative study between impact-loading and non-impact-loading sports. Osteoporos. Int.8, 152–158. 10.1007/bf02672512
25
DavenportM. H.MeahV. L.RuchatS. M.DaviesG. A.SkowR. J.BarrowmanN.et al (2018). Impact of prenatal exercise on neonatal and childhood outcomes: a systematic review and meta-analysis. Br. J. Sports Med.52, 1386–1396. 10.1136/bjsports-2018-099836
26
DharP.SominskyL.O'HelyM.DawsonS.CollierF.TangM. L. K.et al (2024). Physical activity and circulating inflammatory markers and cytokines during pregnancy: a population-based cohort study. Acta obstetricia Gynecol. Scand.103, 1808–1819. 10.1111/aogs.14870
27
Di MascioD.Magro-MalossoE. R.SacconeG.MarhefkaG. D.BerghellaV. (2016). Exercise during pregnancy in normal-weight women and risk of preterm birth: a systematic review and meta-analysis of randomized controlled trials. Am. J. obstetrics Gynecol.215, 561–571. 10.1016/j.ajog.2016.06.014
28
DominguesM. R.MatijasevichA.BarrosA. J. D.SantosI. S.HortaB. L.HallalP. C. (2014). Physical activity during pregnancy and offspring neurodevelopment and IQ in the first 4 years of life. PLoS One9, e110050. 10.1371/journal.pone.0110050
29
EganB.ZierathJ. (2013). Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell. metab.17, 162–184. 10.1016/j.cmet.2012.12.012
30
EllingsenM. S.PettersenA.StafneS. N.MørkvedS.SalvesenK. Å.EvensenK. (2020). Neurodevelopmental outcome in 7-year-old children is not affected by exercise during pregnancy: follow up of a multicentre randomised controlled trial. BJOG Int. J. obstetrics Gynaecol.127, 508–517. 10.1111/1471-0528.16024
31
EvensonK. R.BarakatR.BrownW. J.Dargent-MolinaP.HarunaM.MikkelsenE. M.et al (2014). Guidelines for physical activity during pregnancy: comparisons from around the world. Am. J. lifestyle Med.8, 102–121. 10.1177/1559827613498204
32
FangH.ChartierJ.SodjaC.DesboisA.Ribecco-LutkiewiczM.WalkerP. R.et al (2003). Transcriptional activation of the human brain-derived neurotrophic factor gene promoter III by dopamine signaling in NT2/N neurons. J. Biol. Chem.278, 26401–26409. 10.1074/jbc.M211539200
33
FerrariN.SchmidtN.Bae-GartzI.VohlenC.AlcazarM. A. A.BrockmeierK.et al (2023). Maternal exercise during pregnancy impacts motor performance in 9-year-old children: a pilot study. Child. Basel, Switz.10, 1797. 10.3390/children10111797
34
Fiuza-LucesC.ValenzuelaP. L.GálvezB. G.RamírezM.López-SotoA.SimpsonR. J.et al (2024). The effect of physical exercise on anticancer immunity. Nat. Rev. Immunol.24, 282–293. 10.1038/s41577-023-00943-0
35
FriedrichM. J. (2008). Exercise may boost aging immune system. Jama299, 160–161. 10.1001/jama.2007.56-a
36
FritzJ. M.MagelJ. S.McFaddenM.AscheC.ThackerayA.MeierW.et al (2015). Early physical therapy vs usual care in patients with recent-onset low back pain: a randomized clinical trial. Jama314, 1459–1467. 10.1001/jama.2015.11648
37
FulgenziG.HongZ.Tomassoni-ArdoriF.BarellaL. F.BeckerJ.BarrickC.et al (2020). Novel metabolic role for BDNF in pancreatic β-cell insulin secretion. Nat. Commun.11, 1950. 10.1038/s41467-020-15833-5
38
GadomskiS.FieldingC.García-GarcíaA.KornC.KapeniC.AshrafS.et al (2022). A cholinergic neuroskeletal interface promotes bone formation during postnatal growth and exercise. Cell. Stem Cell.29, 528–544.e9. 10.1016/j.stem.2022.02.008
39
GautronL.ElmquistJ. K.WilliamsK. W. (2015). Neural control of energy balance: translating circuits to therapies. Cell.161, 133–145. 10.1016/j.cell.2015.02.023
40
GibbA.EpsteinP. N.UchidaS.ZhengY.McNallyL. A.ObalD.et al (2017). Exercise-induced changes in glucose metabolism promote physiological cardiac growth. Circulation136, 2144–2157. 10.1161/circulationaha.117.028274
41
GleesonM.BishopN. C.StenselD. J.LindleyM. R.MastanaS. S.NimmoM. A. (2011). The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol.11, 607–615. 10.1038/nri3041
42
GorgijE.FanaeiH.YaghmaeiP.ShahrakiM. R.MirahmadiH. (2021). Treadmill exercise during pregnancy decreased vulnerability to neonatal hypoxia-ischemia through reducing inflammation and increasing antiapoptotic gene expressions and antioxidant capacity in rats. Stroke Res. Treat.2021, 5512745. 10.1155/2021/5512745
43
GudmundsdóttirH. K.CarlsenO. C. L.BainsK. E. S.FärdigM.HaugenG.JonassenC. M.et al (2022). Infant lung function and maternal physical activity in the first half of pregnancy. ERJ open Res.8. 10.1183/23120541.00172-2022
44
HallJ. M.Gomez-PinillaF.SavageL. M. (2018). Nerve growth factor is responsible for exercise-induced recovery of septohippocampal cholinergic structure and function. Front. Neurosci.12, 773. 10.3389/fnins.2018.00773
45
HarrisJ. E.BaerL. A.StanfordK. I. (2018). Maternal exercise improves the metabolic health of adult offspring. Trends Endocrinol. Metab.29, 164–177. 10.1016/j.tem.2018.01.003
46
HawkinsM.PekowP.Chasan-TaberL. (2014). Physical activity, sedentary behavior, and C-reactive protein in pregnancy. Med. Sci. sports Exerc.46, 284–292. 10.1249/MSS.0b013e3182a44767
47
HaydenJ. A.WilsonM. N.StewartS.CartwrightJ. L.SmithA. O.RileyR. D.et al (2020). Exercise treatment effect modifiers in persistent low back pain: an individual participant data meta-analysis of 3514 participants from 27 randomised controlled trials. Br. J. Sports Med.54, 1277–1278. 10.1136/bjsports-2019-101205
48
HaymanM.BrownW. J.BrinsonA.Budzynski-SeymourE.BruceT.EvensonK. R. (2023). Public health guidelines for physical activity during pregnancy from around the world: a scoping review. Br. J. Sports Med.57, 940–947. 10.1136/bjsports-2022-105777
49
HempsteadB. L. (2015). Brain-derived neurotrophic factor: three ligands, many actions. Trans. Am. Clin. Climatol. Assoc.126, 9–19.
50
HorowitzA. M.FanX.BieriG.SmithL. K.Sanchez-DiazC. I.SchroerA. B.et al (2020). Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science369, 167–173. 10.1126/science.aaw2622
51
HuiskesR.RuimermanR.van LentheG. H.JanssenJ. D. (2000). Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature405, 704–706. 10.1038/35015116
52
HwangE.PortilloB.GroseK.FujikawaT.WilliamsK. W. (2023). Exercise-induced hypothalamic neuroplasticity: implications for energy and glucose metabolism. Mol. Metab.73, 101745. 10.1016/j.molmet.2023.101745
53
JessicaA. L.BoutrosP. C.KnightD.TammelaT.MoskowitzC. S.JonesL. W. (2024). Association of exercise with pan-cancer incidence and overall survival. Cancer Cell.42, 169–171. 10.1016/j.ccell.2023.12.007
54
JevtovicF.CollierD. N.DeVenteJ.MouroS.ClaiborneA.WissemanB.et al (2005). Maternal exercise increases infant resting energy expenditure: preliminary results. Int. J. Obes.48, 1347–1350. 10.1038/s41366-024-01560-0
55
JevtovicF.ZhengD.ClaiborneA.BiagioniE. M.WissemanB. L.KrassovskaiaP. M.et al (2024). Effects of maternal exercise on infant mesenchymal stem cell mitochondrial function, insulin action, and body composition in infancy. Physiol. Rep.12, e16028. 10.14814/phy2.16028
56
JevtovicF.ZhengD.HoumardJ. A.KrassovskaiaP. M.LopezC. A.WissemanB. L.et al (2023). Effects of maternal exercise modes on glucose and lipid metabolism in offspring stem cells. J. Clin. Endocrinol. metabolism108, e360–e370. 10.1210/clinem/dgad059
57
JonathanS.JacobM.TheodoreA.TaraM.AngelaT.Loc-DuyenP.et al (2024). Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med.25. 10.1038/s41591-019-0485-4
58
JonathonA.Kevin AM.KennethA. D.JuleenR. Z. (2023). Exercise metabolism and adaptation in skeletal muscle. Nat. Rev. Mol. Cell. Biol.24, 607–632. 10.1038/s41580-023-00606-x
59
Jun SeokS.ZhaoL.ChenY.ChenK.ChaeS. A.de AvilaJ. M.et al (2020). Maternal exercise via exerkine apelin enhances brown adipogenesis and prevents metabolic dysfunction in offspring mice. Sci. Adv.6, eaaz0359. 10.1126/sciadv.aaz0359
60
KalagiriR. R.CarderT.ChoudhuryS.VoraN.BallardA. R.GovandeV.et al (2016). Inflammation in complicated pregnancy and its outcome. Am. J. perinatology33, 1337–1356. 10.1055/s-0036-1582397
61
KangG.MinS. H.LeeC. H.KimJ. Y.LimH. S.ChoiM. J.et al (2021). Mitohormesis in hypothalamic POMC neurons mediates regular exercise-induced high-turnover metabolism. Cell. metab.33, 334–349.e6. 10.1016/j.cmet.2021.01.003
62
KathrynH. S.CampbellA. M.StuiverM. M.PintoB. M.SchwartzA. L.MorrisG. S.et al (2019). Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA Cancer J. Clin.69, 468–484. 10.3322/caac.21579
63
KimT. W.ParkS. S.KimS. H.KimM. K.ShinM. S.KimS. H. (2024). Exercise before pregnancy exerts protective effect on prenatal stress-induced impairment of memory, neurogenesis, and mitochondrial function in offspring. J. Exerc. rehabilitation20, 2–10. 10.12965/jer.2448068.034
64
KiseN. J.RisbergM. A.StensrudS.RanstamJ.EngebretsenL.RoosE. M. (2016). Exercise therapy versus arthroscopic partial meniscectomy for degenerative meniscal tear in middle aged patients: randomised controlled trial with two year follow-up. Bmj354, i3740. 10.1136/bmj.i3740
65
KleinC. P.HoppeJ. B.SaccomoriA. B.Dos SantosB. G.SaginiJ. P.CrestaniM. S.et al (2019). Physical exercise during pregnancy prevents cognitive impairment induced by amyloid-β in adult offspring rats. Mol. Neurobiol.56, 2022–2038. 10.1007/s12035-018-1210-x
66
KleinC. P.HoppeJ. B.SaccomoriA. B.Gindri Dos SantosB.AugustP. M.KleinI. P.et al (2020). Protective effect of maternal exercise against amyloid-β neurotoxicity in the male rat offspring's cerebellum. J. Dev. Orig. health Dis.11, 521–532. 10.1017/s2040174420000562
67
KowianskiP.LietzauG.CzubaE.WaśkowM.SteligaA.MoryśJ. (2018). BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell. Mol. Neurobiol.38, 579–593. 10.1007/s10571-017-0510-4
68
KurzE.HirschC. A.DaltonT.ShadaloeyS. A.Khodadadi-JamayranA.MillerG.et al (2022). Exercise-induced engagement of the IL-15/IL-15Rα axis promotes anti-tumor immunity in pancreatic cancer. Cancer Cell.40, 720–737.e5. 10.1016/j.ccell.2022.05.006
69
KusuyamaJ.Alves-WagnerA. B.ConlinR. H.MakarewiczN. S.AlbertsonB. G.PrinceN. B.et al (2021). Placental superoxide dismutase 3 mediates benefits of maternal exercise on offspring health. Cell. Metab.33, 939–956.e8. 10.1016/j.cmet.2021.03.004
70
KusuyamaJ.Alves-WagnerA. B.MakarewiczN. S.GoodyearL. J. (2020). Effects of maternal and paternal exercise on offspring metabolism. Nat. Metab.2, 858–872. 10.1038/s42255-020-00274-7
71
Labonte-LemoyneE.CurnierD.EllembergD. (2017). Exercise during pregnancy enhances cerebral maturation in the newborn: a randomized controlled trial. J. Clin. Exp. neuropsychology39, 347–354. 10.1080/13803395.2016.1227427
72
LangstonP. K.SunY.RybackB. A.MuellerA. L.SpiegelmanB. M.BenoistC.et al (2023). Regulatory T cells shield muscle mitochondria from interferon-γ-mediated damage to promote the beneficial effects of exercise. Sci. Immunol.8, eadi5377. 10.1126/sciimmunol.adi5377
73
LaveryJ.BoutrosP. C.ScottJ. M.TammelaT.MoskowitzC. S.JonesL. W. (2023). Pan-cancer analysis of postdiagnosis exercise and mortality. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol.41, 4982–4992. 10.1200/jco.23.00058
74
LeeW.LeddyH. A.ChenY.LeeS. H.ZelenskiN. A.McNultyA. L.et al (2014). Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc. Natl. Acad. Sci. U. S. A.111, E5114–E5122. 10.1073/pnas.1414298111
75
LeetT.FlickL. (2003). Effect of exercise on birthweight. Clin. obstetrics Gynecol.46, 423–431. 10.1097/00003081-200306000-00021
76
LiV. L.HeY.ContrepoisK.LiuH.KimJ. T.WiggenhornA. L.et al (2022). An exercise-inducible metabolite that suppresses feeding and obesity. Nature606, 785–790. 10.1038/s41586-022-04828-5
77
LiY.LiR. Y.ZhuJ. Y.ChenM.MuW. J.LuoH. Y.et al (2025). Maternal exercise prevents metabolic disorders in offspring mice through SERPINA3C. Nat. Metab.7, 401–420. 10.1038/s42255-024-01213-6
78
LingL.KimS.BuckleyM. T.ReyesJ. M.KangJ.TianL.et al (2023). Exercise reprograms the inflammatory landscape of multiple stem cell compartments during mammalian aging. Cell. Stem Cell.30, 689–705.e4. 10.1016/j.stem.2023.03.016
79
LiuY.YanT.ChuJ. M. T.ChenY.DunnettS.HoY. S.et al (2019). The beneficial effects of physical exercise in the brain and related pathophysiological mechanisms in neurodegenerative diseases. Lab. Investig.99, 943–957. 10.1038/s41374-019-0232-y
80
MartinR.ViggarsM.EsserK. (2023). Metabolism and exercise: the skeletal muscle clock takes centre stage. Nat. Rev. Endocrinol.19, 272–284. 10.1038/s41574-023-00805-8
81
McMillanA. G.MayL. E.GainesG. G.IslerC.KuehnD. (2019). Effects of aerobic exercise during pregnancy on 1-month infant neuromotor skills. Med. Sci. sports Exerc.51, 1671–1676. 10.1249/mss.0000000000001958
82
MohammadA.RuegseggerG. N.OlverT. D.MacPhersonR. E. (2024). Gestational physical activity alters offspring brain APP processing in an age-specific manner. Appl. Physiol. Nutr. Metab.49 (11), 1507–1516. 10.1139/apnm-2024-0019
83
Mohammad RahimiG. R.SmartN. A.LiangM. T. C.BijehN.AlbanaqiA. L.FathiM.et al (2020). The impact of different modes of exercise training on bone mineral density in older postmenopausal women: a systematic review and meta-analysis research. Calcif. Tissue Int.106, 577–590. 10.1007/s00223-020-00671-w
84
MorG.CardenasI.AbrahamsV.GullerS. (2011). Inflammation and pregnancy: the role of the immune system at the implantation site. Ann. N. Y. Acad. Sci.1221, 80–87. 10.1111/j.1749-6632.2010.05938.x
85
Moreno-FernandezJ.OchoaJ. J.Lopez-FriasM.Diaz-CastroJ. (2020). Impact of early nutrition, physical activity and sleep on the fetal programming of disease in the pregnancy: a narrative review. Nutrients12, 3900. 10.3390/nu12123900
86
MoTrPAC Study GroupLead AnalystsMoTrPAC Study Group (2024). Temporal dynamics of the multi-omic response to endurance exercise training. Nature629, 174–183. 10.1038/s41586-023-06877-w
87
MottolaM. F.DavenportM. H.RuchatS. M.DaviesG. A.PoitrasV. J.GrayC. E.et al (2018). 2019 Canadian guideline for physical activity throughout pregnancy. Br. J. Sports Med.52, 1339–1346. 10.1136/bjsports-2018-100056
88
MourtakosS. P.TambalisK. D.PanagiotakosD. B.AntonogeorgosG.ArnaoutisG.KarteroliotisK.et al (2015). Maternal lifestyle characteristics during pregnancy, and the risk of obesity in the offspring: a study of 5,125 children. BMC pregnancy childbirth15, 66. 10.1186/s12884-015-0498-z
89
MugliaL. J.BenhalimaK.TongS.OzanneS. (2022). Maternal factors during pregnancy influencing maternal, fetal, and childhood outcomes. BMC Med.20, 418. 10.1186/s12916-022-02632-6
90
MusakkaE. R.YlilauriM. P. T.JalankaJ.KarvonenA. M.TäubelM.HantunenS.et al (2024). Maternal exercise during pregnancy is associated with reduced risk of asthma in the child: a prospective birth cohort study. Med6, 100514. 10.1016/j.medj.2024.09.003
91
NaX.RajaR.PhelanN. E.TadrosM. R.MooreA.WuZ.et al (2022). Mother's physical activity during pregnancy and newborn's brain cortical development. Front. Hum. Neurosci.16, 943341. 10.3389/fnhum.2022.943341
92
NakaharaK.MichikawaT.MorokumaS.OgawaM.KatoK.SanefujiM.et al (2021). Influence of physical activity before and during pregnancy on infant's sleep and neurodevelopment at 1-year-old. Sci. Rep.11, 8099. 10.1038/s41598-021-87612-1
93
NelsonH. K.StanyaK. J.HydeA. L.ChalomM. M.AlexanderR. K.LiouY. H.et al (2020). Interleukin-13 drives metabolic conditioning of muscle to endurance exercise. Science368, eaat3987. 10.1126/science.aat3987
94
NguyenM. T.OuzounianJ. G. (2021). Evaluation and management of fetal macrosomia. Obstet. Gynecol. Clin. North Am.48, 387–399. 10.1016/j.ogc.2021.02.008
95
NiniZ.WangX.FengM.LiM.WangJ.YangH.et al (2024). Early-life exercise induces immunometabolic epigenetic modification enhancing anti-inflammatory immunity in middle-aged male mice. Nat. Commun.15, 3103. 10.1038/s41467-024-47458-3
96
NokiaM. S.LensuS.AhtiainenJ. P.JohanssonP. P.KochL. G.BrittonS. L.et al (2016). Physical exercise increases adult hippocampal neurogenesis in male rats provided it is aerobic and sustained. J. Physiol.594, 1855–1873. 10.1113/JP271552
97
OweK. M.NystadW.StigumH.VangenS.BøK. (2016). Exercise during pregnancy and risk of cesarean delivery in nulliparous women: a large population-based cohort study. Am. J. obstetrics Gynecol.215, 791.e791–791. 10.1016/j.ajog.2016.08.014
98
OwenP. J.MillerC. T.MundellN. L.VerswijverenS. J. J. M.TagliaferriS. D.BrisbyH.et al (2020). Which specific modes of exercise training are most effective for treating low back pain? Network meta-analysis. Br. J. Sports Med.54, 1279–1287. 10.1136/bjsports-2019-100886
99
PagnottiG. M.StynerM.UzerG.PatelV. S.WrightL. E.NessK. K.et al (2019). Combating osteoporosis and obesity with exercise: leveraging cell mechanosensitivity. Nat. Rev. Endocrinol.15, 339–355. 10.1038/s41574-019-0170-1
100
PapagianniG.PanayiotouC.VardasM.BalaskasN.AntonopoulosC.TachmatzidisD.et al (2023). The anti-inflammatory effects of aerobic exercise training in patients with type 2 diabetes: a systematic review and meta-analysis. Cytokine164, 156157. 10.1016/j.cyto.2023.156157
101
ParkS. S.KimC. J.KimS. H.KimT. W.LeeS. J. (2021). Maternal swimming exercise during pregnancy improves memory through enhancing neurogenesis and suppressing apoptosis via wnt/β-catenin pathway in autistic mice. Int. Neurourol. J.25, S63–S71. 10.5213/inj.2142338.169
102
PendergrastL. A.LundellL. S.EhrlichA. M.AshcroftS. P.SchönkeM.BasseA. L.et al (2023). Time of day determines postexercise metabolism in mouse adipose tissue. Proc. Natl. Acad. Sci. U. S. A.120, e2218510120. 10.1073/pnas.2218510120
103
PiercyK. L.TroianoR. P.BallardR. M.CarlsonS. A.FultonJ. E.GaluskaD. A.et al (2018). The physical activity guidelines for Americans. Jama320, 2020–2028. 10.1001/jama.2018.14854
104
ReddyA.BoziL. H.YaghiO. K.MillsE. L.XiaoH.NicholsonH. E.et al (2020). pH-gated succinate secretion regulates muscle remodeling in response to exercise. Cell.183 (1), 62–67. 10.1016/j.cell.2020.08.039
105
ReihanehM.KomakiA.KarimiS. A.BehzadM.HeidarisasanS.SalehiI. (2023). Maternal high-intensity interval training as a suitable approach for offspring's heart protection in rat: evidence from oxidative stress and mitochondrial genes. Front. Physiol.14, 1117666. 10.3389/fphys.2023.1117666
106
SabaratnamR.WojtaszewskiJ. F. P.HojlundK. (2022). Factors mediating exercise-induced organ crosstalk. Acta Physiol. (Oxf)234, e13766. 10.1111/apha.13766
107
SalesV. M.Ferguson-SmithA. C.PattiM. E. (2017). Epigenetic mechanisms of transmission of metabolic disease across generations. Cell. Metab.25, 559–571. 10.1016/j.cmet.2017.02.016
108
SalvesenK.HemE.Sundgot-BorgenJ. (2012). Fetal wellbeing may be compromised during strenuous exercise among pregnant elite athletes. Br. J. Sports Med.46, 279–283. 10.1136/bjsm.2010.080259
109
SaragiottoB. T.MaherC. G.YamatoT. P.CostaL. O. P.Menezes CostaL. C.OsteloR. W. J. G.et al (2016). Motor control exercise for chronic non-specific low-back pain. Cochrane Database Syst. Rev.2016, Cd012004. 10.1002/14651858.Cd012004
110
SaragoviH. U.ZhengW.MaliartchoukS.DiGugliemoG. M.MawalY. R.KamenA.et al (1998). A TrkA-selective, fast internalizing nerve growth factor-antibody complex induces trophic but not neuritogenic signals. J. Biol. Chem.273, 34933–34940. 10.1074/jbc.273.52.34933
111
SasakiN.HenrikssonH. B.RunessonE.LarssonK.SekiguchiM.KikuchiS. i.et al (2012). Physical exercise affects cell proliferation in lumbar intervertebral disc regions in rats. Spine (Phila Pa 1976)37, 1440–1447. 10.1097/BRS.0b013e31824ff87d
112
SatoS.DyarK. A.TreebakJ. T.JepsenS. L.EhrlichA. M.AshcroftS. P.et al (2022). Atlas of exercise metabolism reveals time-dependent signatures of metabolic homeostasis. Cell. metab.34, 329–345.e8. 10.1016/j.cmet.2021.12.016
113
SchroerA. B.VenturaP. B.SucharovJ.MisraR.ChuiM. K. K.BieriG.et al (2023). Platelet factors attenuate inflammation and rescue cognition in ageing. Nature620, 1071–1079. 10.1038/s41586-023-06436-3
114
ScottJ.ZaborE. C.SchwitzerE.KoelwynG. J.AdamsS. C.NilsenT. S.et al (2018). Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol.36, 2297–2305. 10.1200/jco.2017.77.5809
115
SheldonR. D.Nicole BlaizeA.FletcherJ. A.PearsonK. J.DonkinS. S.NewcomerS. C.et al (2016). Gestational exercise protects adult male offspring from high-fat diet-induced hepatic steatosis. J. hepatology64, 171–178. 10.1016/j.jhep.2015.08.022
116
ShenB.TasdoganA.UbellackerJ. M.ZhangJ.NosyrevaE. D.DuL.et al (2021). A mechanosensitive peri-arteriolar niche for osteogenesis and lymphopoiesis. Nature591, 438–444. 10.1038/s41586-021-03298-5
117
SleimanS. F.HenryJ.Al-HaddadR.El HayekL.Abou HaidarE.StringerT.et al (2016). Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body beta-hydroxybutyrate. Elife5, e15092. 10.7554/eLife.15092
118
SoldatoD.MichielsS.HavasJ.Di MeglioA.PagliucaM.FranzoiM. A.et al (2024). Dose/Exposure relationship of exercise and distant recurrence in primary breast cancer. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol.42, 3022–3032. 10.1200/jco.23.01959
119
StanfordK. I.LeeM. Y.GetchellK. M.SoK.HirshmanM. F.GoodyearL. J. (2015b). Exercise before and during pregnancy prevents the deleterious effects of maternal high-fat feeding on metabolic health of male offspring. Diabetes64, 427–433. 10.2337/db13-1848
120
StanfordK. I.MiddelbeekR. J. W.TownsendK. L.LeeM. Y.TakahashiH.SoK.et al (2015a). A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes64, 2002–2014. 10.2337/db14-0704
121
StanfordK. I.TakahashiH.SoK.Alves-WagnerA. B.PrinceN. B.LehnigA. C.et al (2017). Maternal exercise improves glucose tolerance in female offspring. Diabetes66, 2124–2136. 10.2337/db17-0098
122
Stevanović-SilvaJ.BelezaJ.CoxitoP.PereiraS.RochaH.GasparT. B.et al (2021). Maternal high-fat high-sucrose diet and gestational exercise modulate hepatic fat accumulation and liver mitochondrial respiratory capacity in mothers and male offspring. Metabolism Clin. Exp.116, 154704. 10.1016/j.metabol.2021.154704
123
SunW.ChiS.LiY.LingS.TanY.XuY.et al (2019). The mechanosensitive Piezo1 channel is required for bone formation. Elife8, e47454. 10.7554/eLife.47454
124
SunZ.ChenH.BergerM. R.ZhangL.GuoH.HuangY. (2016). Effects of tai chi exercise on bone health in perimenopausal and postmenopausal women: a systematic review and meta-analysis. Osteoporos. Int.27, 2901–2911. 10.1007/s00198-016-3626-3
125
TengfeiR.HeJ.ZhangT.NiuA.YuanY.ZuoY.et al (2024). Exercise activates interferon response of the liver via Gpld1 to enhance antiviral innate immunity. Sci. Adv.10, eadk5011. 10.1126/sciadv.adk5011
126
The International Weight Management in Pregnancy (i-WIP) Collaborative Group (2017). Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: meta-analysis of individual participant data from randomised trials. Bmj358, j3991. 10.1136/bmj.j3991
127
TiniusR. A.CahillA. G.CadeW. T. (2017). Low-intensity physical activity is associated with lower maternal systemic inflammation during late pregnancy. J. Obes. and weight loss Ther.7, 343. 10.4172/2165-7904.1000343
128
TitovaJ.DavenportM. H.HumphrysA.HaymanM. (2024). Barriers and enablers encountered by elite athletes during preconception and pregnancy: a mixed-methods systematic review. Br. J. Sports Med.–2024-108380. 10.1136/bjsports-2024-108380
129
UetaR. H. S.TariniV. A. F.FrancioziC. E. S.TamaokiM. J. S.MedeirosV. P.NaderH. B.et al (2018). Effects of training and overtraining on intervertebral disc proteoglycans. Spine (Phila Pa 1976)43, E1–e6. 10.1097/brs.0000000000002368
130
van DillenL. R.LanierV. M.Steger-MayK.WallendorfM.NortonB. J.CivelloJ. M.et al (2021). Effect of motor skill training in functional activities vs strength and flexibility exercise on function in people with chronic low back pain: a randomized clinical trial. JAMA Neurol.78, 385–395. 10.1001/jamaneurol.2020.4821
131
van PraagH.ChristieB. R.SejnowskiT. J.GageF. H. (1999). Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. U. S. A.96, 13427–13431. 10.1073/pnas.96.23.13427
132
Vargas-TerronesM.NagpalT. S.BarakatR. (2019). Impact of exercise during pregnancy on gestational weight gain and birth weight: an overview. Braz. J. Phys. Ther.23, 164–169. 10.1016/j.bjpt.2018.11.012
133
WangC.WeiY.ZhangX.ZhangY.XuQ.SunY.et al (2017). A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am. J. obstetrics Gynecol.216, 340–351. 10.1016/j.ajog.2017.01.037
134
WangL.YouX.LotinunS.ZhangL.WuN.ZouW. (2020). Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat. Commun.11, 282. 10.1038/s41467-019-14146-6
135
WangX.ZhuY. T.ZhuY.SunY. L.HuangJ.LiZ.et al (2022). Long-term running exercise alleviates cognitive dysfunction in APP/PSEN1 transgenic mice via enhancing brain lysosomal function. Acta Pharmacol. Sin.43, 850–861. 10.1038/s41401-021-00720-6
136
WangY.Cupul-UicabL. A.RoganW. J.EggesboM.TravlosG.WilsonR.et al (2015). Recreational exercise before and during pregnancy in relation to plasma C-reactive protein concentrations in pregnant women. J. Phys. activity and health12, 770–775. 10.1123/jpah.2013-0390
137
WatsonS. L.WeeksB. K.WeisL. J.HardingA. T.HoranS. A.BeckB. R. (2018). High-intensity resistance and impact training improves bone mineral density and physical function in postmenopausal women with osteopenia and osteoporosis: the LIFTMOR randomized controlled trial. J. Bone Min. Res.33, 211–220. 10.1002/jbmr.3284
138
WattsG. (2012). Exercise boosts immunity--up to a point. Bmj344, e218. 10.1136/bmj.e218
139
WiebeH. W.BouléN. G.ChariR.DavenportM. H. (2015). The effect of supervised prenatal exercise on fetal growth: a meta-analysis. Obstetrics Gynecol.125, 1185–1194. 10.1097/aog.0000000000000801
140
XuB.XieX. (2016). Neurotrophic factor control of satiety and body weight. Nat. Rev. Neurosci.17, 282–292. 10.1038/nrn.2016.24
141
YamaguchiM.SekiT.ImayoshiI.TamamakiN.HayashiY.TatebayashiY.et al (2016). Neural stem cells and neuro/gliogenesis in the central nervous system: understanding the structural and functional plasticity of the developing, mature, and diseased brain. J. Physiol. Sci.66, 197–206. 10.1007/s12576-015-0421-4
142
YauS.LeeT. H. Y.FormoloD. A.LeeW. L.LiL. C. K.SiuP. M.et al (2019). Effects of maternal voluntary wheel running during pregnancy on adult hippocampal neurogenesis, temporal order memory, and depression-like behavior in adult female and male offspring. Front. Neurosci.13, 470. 10.3389/fnins.2019.00470
143
ZhangL.ZouW.HuY.WuH.GaoY.ZhangJ.et al (2023). Maternal voluntary wheel running modulates glucose homeostasis, the gut microbiota and its derived fecal metabolites in offspring. Clin. Sci. Lond. Engl. 1979137, 1151–1166. 10.1042/cs20230372
144
ZhangS.HuangX.ZhaoX.LiB.CaiY.LiangX.et al (2021). Effect of exercise on bone mineral density among patients with osteoporosis and osteopenia: a systematic review and network meta-analysis. J. Clin. Nurs.31, 2100–2111. 10.1111/jocn.16101
145
ZhangX.HeQ.HuangT.ZhaoN.LiangF.XuB.et al (2019). Treadmill exercise decreases aβ deposition and counteracts cognitive decline in APP/PS1 mice, possibly via hippocampal microglia modifications. Front. aging Neurosci.11, 78. 10.3389/fnagi.2019.00078
146
ZhaoT.YangQ.FeuerbacherJ. F.YuB.BrinkmannC.ChengS.et al (2024). Effects of exercise, metformin and their combination on glucose metabolism in individuals with abnormal glycaemic control: a systematic review and network meta-analysis. Br. J. sports Med.58, 1452–1460. 10.1136/bjsports-2024-108127
147
ZhouX. A.BlackmoreD. G.ZhuoJ.NasrallahF. A.ToX.KurniawanN. D.et al (2021). Neurogenic-dependent changes in hippocampal circuitry underlie the procognitive effect of exercise in aging mice. iScience24, 103450. 10.1016/j.isci.2021.103450
148
ZhouY.GuoX.MuJ.LiuJ.YangH.CaiC. (2022). Current research trends, hotspots, and frontiers of physical activity during pregnancy: a bibliometric analysis. Int. J. Environ. Res. public health19, 14516. 10.3390/ijerph192114516
Summary
Keywords
maternal exercise, offspring, metabolism, neuron development, immunity
Citation
Wang K, Zhao J, Wang Y and Liu M (2025) Exercise benefits yourself and your offspring: a mini-review. Front. Cell Dev. Biol. 13:1606790. doi: 10.3389/fcell.2025.1606790
Received
06 April 2025
Accepted
22 May 2025
Published
30 May 2025
Volume
13 - 2025
Edited by
Julio J. Ochoa, University of Granada, Spain
Reviewed by
Jacob Peedicayil, Christian Medical College and Hospital, India
Updates
Copyright
© 2025 Wang, Zhao, Wang and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mairu Liu, liumairu@ccnu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.