- 1Department of Microbiology and Environmental Toxicology, University of California Santa Cruz, Santa Cruz, CA, USA
- 2Department of Molecular, Cell, and Developmental Biology, University of California Santa Cruz, Santa Cruz, CA, USA
The iron overload disorder hereditary hemochromatosis (HH) predisposes humans to serious disseminated infection with pathogenic Yersinia as well as several other pathogens. Recently, we showed that the iron-sulfur cluster coordinating transcription factor IscR is required for type III secretion in Y. pseudotuberculosis by direct control of the T3SS master regulator LcrF. In E. coli and Yersinia, IscR levels are predicted to be regulated by iron bioavailability, oxygen tension, and oxidative stress, such that iron depletion should lead to increased IscR levels. To investigate how host iron overload influences Y. pseudotuberculosis virulence and the requirement for the Ysc type III secretion system (T3SS), we utilized two distinct murine models of HH: hemojuvelin knockout mice that mimic severe, early-onset HH as well as mice with the HfeC282Y∕C282Y mutation carried by 10% of people of Northern European descent, associated with adult-onset HH. Hjv−∕− and HfeC282Y∕C282Y transgenic mice displayed enhanced colonization of deep tissues by Y. pseudotuberculosis following oral inoculation, recapitulating enhanced susceptibility of humans with HH to disseminated infection with enteropathogenic Yersinia. Importantly, HH mice orally infected with Y. pseudotuberculosis lacking the T3SS-encoding virulence plasmid, pYV, displayed increased deep tissue colonization relative to wildtype mice. Consistent with previous reports using monocytes from HH vs. healthy donors, macrophages isolated from HfeC282Y∕C282Y mice were defective in Yersinia uptake compared to wildtype macrophages, indicating that the anti-phagocytic property of the Yersinia T3SS plays a less important role in HH animals. These data suggest that Yersinia may rely on distinct virulence factors to cause disease in healthy vs. HH hosts.
Introduction
Iron is an essential element for almost all microorganisms, with the exception of some examples including Lactobacillus plantarum and Borrelia burgdorferi (Archibald, 1983; Posey and Gherardini, 2000). Most bacteria require anywhere from 10−6 to 10−7 M free iron to support growth. However, pathogenic bacteria often encounter iron-limiting conditions, particularly during growth within mammalian hosts due to the success of host iron sequestration systems (Weinberg, 1978; Cassat and Skaar, 2013). These systems include binding of iron to the storage protein ferritin, complexing iron with heme, and the tight association of serum iron to transferrin (Cassat and Skaar, 2013). Free iron in humans is further sequestered during infection via inflammation-induced hypoferremia, which includes host production of increased amounts of lactoferrin in an attempt to restrict bacterial growth (Jurado, 1997). To compensate, pathogens employ a number of iron acquisition mechanisms to acquire iron from the host, including siderophores, transferrin/lactoferrin receptors, heme acquisition systems, and other types of iron uptake systems (Cassat and Skaar, 2013).
The ability to sense the iron limiting environment of mammalian hosts not only allows for induction of bacterial iron acquisition systems, but serves as a signal for many pathogens to regulate virulence determinant expression (Skaar, 2010). An important virulence determinant for many Gram-negative pathogens that can be regulated by iron is the type III secretion system (T3SS; Murphy and Payne, 2007; Ellermeier and Slauch, 2008; Gode-Potratz et al., 2010; Chakraborty et al., 2011; Kurushima et al., 2012). This system utilizes a needle-like apparatus to deliver a series of effector proteins directly into host cells leading to modulation of normal host cell processes (Cornelis, 2006). Shigella dysenteriae, Salmonella enterica, Vibrio parahaemolyticus, Bordetella bronchiseptica, and Edwardsiella tarda are a few of the Gram-negative pathogens that regulate their T3SS in response to iron bioavailability within the host (Murphy and Payne, 2007; Ellermeier and Slauch, 2008; Gode-Potratz et al., 2010; Chakraborty et al., 2011; Kurushima et al., 2012). The Yersinia Ysc T3SS, which is encoded on the 70 kb virulence plasmid termed pYV, is modulated in response to temperature, calcium concentration, and host cell contact. Iron has never been demonstrated to modulate expression or function of the Yersinia Ysc T3SS and studies are typically performed under iron replete conditions (Cornelis et al., 1998). Recently, our group identified the iron-sulfur cluster coordinating transcription factor IscR as a novel component of the Yersinia T3SS regulatory cascade; deletion of IscR leads to a dramatic decrease in secretion of T3SS effector proteins (Miller et al., 2014). In that study, we demonstrated that IscR is essential for T3SS expression through direct regulation of lcrF, which encodes an AraC-type DNA binding protein responsible for expression of the majority of T3SS genes (Cornelis et al., 1998). It remains unclear exactly which environmental stimuli influence IscR target gene expression; however, the closely related E. coli IscR has been shown to respond to oxidative stress and oxygen limitation as well as iron starvation (Giel et al., 2006; Yeo et al., 2006; Wu and Outten, 2009).
The fact that IscR regulates the Y. pseudotuberculosis Ysc T3SS suggests that iron may play an important role in modulating expression of Yersinia virulence factors. Indeed, enteropathogenic Yersinia transit from the intestinal lumen, where they may be able to successfully compete for dietary iron, to severely iron restricted distal tissues. It is in these deeper tissue sites where the Yersinia Ysc T3SS has been shown to translocate effector proteins called Yops into cells such as macrophages and neutrophils (Marketon et al., 2005; Koberle et al., 2009; Durand et al., 2010). These Yops act to inhibit phagocytosis and to dampen inflammatory properties of innate immune cells (McCance and Widdowson, 1938; Martin et al., 1987; Miret et al., 2003; Heesemann et al., 2006; Matsumoto and Young, 2009). How host iron availability impacts T3SS utilization and virulence in Yersinia is unclear.
Iron overload disorders such as hereditary hemochromatosis (HH) predispose individuals to Yersinia infection (Jacquenod et al., 1984; Mennecier et al., 2001a; Harris and Paraskevakis, 2012; Quenee et al., 2012). Hereditary hemochromatosis (HH) is a genetic iron overload disorder and is one of the most common genetic disorders in Caucasians (Bahram et al., 1999). Individuals with HH absorb excess dietary iron, which then accumulates in tissues such as the liver. If left untreated, this iron accumulation can lead to organ failure as a result of iron-induced oxidative stress (MacKenzie et al., 2008). Mutations within several different genes, including the high iron Fe (Hfe) and the hemojuvelin (Hjv) genes, have been linked to HH. Both Hfe and Hjv act to control expression of the iron-regulating hormone hepcidin, which regulates iron uptake in the gut and the recycling of senescent red blood cells by macrophages (Feder et al., 1996; Bahram et al., 1999; Huang et al., 2005). The Hfe C282Y mutation is associated with adult-onset HH, while Hjv mutations are rare and associated with more severe, early onset iron overload (Brandhagen et al., 2002; Lanzara et al., 2004). The HfeC282Y∕C282Y and Hjv−∕− mouse models have been developed for the study of HH and mimic a number of the symptoms seen in the human disease, including excess liver iron (Levy et al., 1999; Huang et al., 2005).
Quenee et al. previously showed that an Hjv mutation predisposes mice to infection with a Y. pestis vaccine strain lacking the pgm locus, which encodes the yersiniabactin siderophore iron uptake system. However, the authors observed no difference in the ability of fully virulent Y. pestis to cause disease in wildtype and Hjv−∕− mice (Quenee et al., 2012). While humans with HH are at higher risk for contracting disseminated enteropathogenic Yersinia infection (Piroth et al., 1997; Bergmann et al., 2001; Hopfner et al., 2001; Mennecier et al., 2001b), whether HfeC282Y∕C282Y and Hjv−∕− mice are more susceptible to Y. pseudotuberculosis is unknown. Additionally, based on the knowledge that IscR directly regulates T3SS expression, it is unclear to what extent the Ysc T3SS contributes to Y. pseudotuberculosis pathogenesis in an HH host. This work provides the first evidence for enhanced susceptibility of HfeC282Y∕C282Y and Hjv−∕− mice to Y. pseudotuberculosis. Furthermore, we demonstrate that there is a decreased requirement for the Yersinia Ysc T3SS for disease causation in hosts with hereditary hemochromatosis.
Materials and Methods
All animal use procedures were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the UCSC Institutional Animal Care and Use Committee.
Bacterial Strains and Growth Conditions
Y. pseudotuberculosis strains used in this study are included in Table 1. Unless specified, Y. pseudotuberculosis strains were grown overnight in LB at 26°C with shaking at 250 rpm for animal infections. For macrophage infections, bacteria were grown overnight in 2 × YT at 26°C at 250 rpm, back-diluted to OD600 of 0.2 into low calcium medium (2 × YT with 20 mM sodium oxalate and 20 mM MgCl2), grown for 1.5 h at 26°C at 250 rpm, and then for 1.5 h at 37°C at 250 rpm to induce the T3SS (Auerbuch et al., 2009).
Mouse Infections
HfeC282Y∕C282Y mice were rederived in the UC Santa Cruz vivarium and Hjv−∕− breeding pairs were obtained from Dr. Nancy Andrews (Duke University). Colonies were maintained through a combination of mating pairs including homozygous knockout/homozygous knockout, heterozygous mutant/heterozygous mutant as well as homozygous knockout/heterozygous mutant. Age matched wildtype mice were obtained through wildtype/wildtype as well as the above mentioned heterozygous mutant/heterozygous mutant matings. Genotypes were determined from tail biopsies processed using the DNeasy Blood & Tissue Kit (Qiagen) per the manufacturer's protocol. Wildtype or knockout female and male, 11 to 12-week-old HfeC282Y∕C282Y mice and 5 to 12-week-old Hjv−∕− mice in the 129S6/SvEvTac background from our breeding facilities were used for oral infections as previously described (Auerbuch and Isberg, 2007). Mice were orogastrically inoculated with 2 × 108 colony forming units (CFU) for HfeC282Y∕C282Y infections or 2 × 107 CFU for Hjv−∕− infections in a 200 μl volume of PBS using a feeding needle. Mice were given food containing a standard amount of iron (200 ppm) and water ad libitum and were euthanized at either 3 or 5 days post-inoculation. Peyer's patches, mesenteric lymph nodes (MLN), spleens, and livers were isolated and homogenized for 30 s in PBS followed by serial dilution and plating on LB supplemented with 1 μg mL−1 irgasan for CFU determination.
Tissue Iron Content
Total hepatic iron content was measured in tissue homogenates (tissue homogenized in PBS containing 0.2% NP-40, 1:4 w/v tissue:buffer) processed for analyses by aliquoting into acid-cleaned polyethylene tubes, evaporating to dryness, and digesting in 16N quartz-distilled HNO3 (Optima, Fisher Scientific) at 80°C in a heat block. Following complete digestion, samples were diluted with Milli-Q water (18 Mohm/cm2) for analyses; rhodium was added as an internal standard. Iron levels were measured by high resolution inductively coupled plasma–mass spectrometry (Thermo Scientific Element XR ICP-MS), measuring masses 54Fe, 56Fe, 57Fe, and 103Rh. The analytical detection limit and measurement precision was 5.68 ng/mL 1.04% RSD, respectively.
Peritoneal Macrophages
Peritoneal macrophages were isolated from 129S6/SvEvTac wildtype, HfeC282Y∕C282Y, and Hjv−∕− mice as described in Layoun et al. (2015). Briefly, mice were injected with 1 mL of 3.8% Brewer's thioglycollate media (BD Biosciences). Four days post-injection, mice were euthanized and peritoneal macrophages isolated. Macrophages were seeded at 5 × 105 cells/well into a 24 well plate and allowed to adhere to the plate for 2 h or overnight. Macrophages were then treated with 100 ng ml−1 of lipopolysaccharide (LPS) from Salmonella minnesota (UltraPure), or exposed to either a Y. pseudotuberculosis mutant lacking Yop effectors but otherwise expressing a functional T3SS (T3SS+, Δyop6; Auerbuch et al., 2009) or with T3SS translocon deficient Y. pseudotuberculosis (T3SS-, Δyop6/ΔyopB) at an MOI of 10. Macrophages were treated for 3 h, at which time supernatants were collected and analyzed for cytokine levels as described below.
Inside/Outside Staining
Peritoneal macrophages were isolated as described above. After being allowed to adhere to coverslips for 2 h, macrophages were infected with pYV- Y. pseudotuberculosis at an MOI of 10. After 0.5 h, infected cell monolayers were fixed for 10 min with 4% paraformaldehyde. Infected macrophages were treated with rabbit anti-Yersinia antibody (1:500), generously provided by Dr. Ralph Isberg (Tufts University), for 40 min at 37°C followed by treatment with goat anti-rabbit AlexaFlour 594 antibody (1:100, Life Technologies) for 40 min at 37°C. Macrophages were washed and then treated with ice cold methanol for 10 s to permeabilize. After permeabilizaton, macrophages were treated with rabbit anti-Yersinia antibody (1:500) for 40 min at 37°C followed by treatment with goat anti-rabbit FITC antibody (1:100, Santa Cruz Biotech) and Hoescht stain (1:10,000) for 40 min at 37°C. Coverslips were then mounted onto slides with Prolong Gold Antifade Reagent (Life Technologies). Slides were visualized on a Leica SP5 confocal microscope using Leica Application Suite Advanced Fluorescence software. Nine frames were obtained for each condition, and for each frame, the number of red bacteria and the number of green bacteria were counted using FIJI ImageJ software.
ELISA Cytokine Measurement
Cytokine measurements were performed as previously described (Auerbuch and Isberg, 2007). Briefly, liver homogenates from HfeC282Y∕C282Y and wildtype mice, either uninfected or orogastrically inoculated with 2 × 108 CFU of wildtype Y. pseudotuberculosis, were thawed on ice and centrifuged at 13,000 rpm for 1 min. The mouse inflammatory cytometric bead array kit (BD Biosciences) was used to detect IL-12p70, TNF-α, IFN-γ, MCP-1, IL-10, and IL-6 according to the manufacturer's protocol with the following exceptions. The amount of antibody-conjugated beads was decreased to 4 μl each with 20 μl of sample/standard and 20 μl of detection reagent per reaction. Data were acquired and analyzed using a BD FACS LSRII flow cytometer and BD analysis software, respectively. Cytokine levels detected in the livers of uninfected mice (3 HfeC282Y∕C282Y and 3 wildtype) were averaged and the standard deviations calculated for each cytokine tested (Excel). Standard deviations were added to the averages to determine the baseline cytokine level for uninfected livers. Individual cytokine concentrations in pg per ml−1 from infected samples were plotted against CFU per gram of liver tissue determined at the time of organ harvest.
Statistical Methods
All statistical methods in this study were analyzed using Kaleidagraph v4.1.1 for Windows (Synergy Software). Oral gavage infection studies were analyzed using the unpaired Wilcoxon–Mann–Whitney rank sum test. Measurement of hepatic iron load from tissues and measurement of cytokine levels from liver homogenates and peritoneal macrophages were analyzed using a Student's t-test. The correlation between bacterial burden and cytokine production was analyzed using Pearson's coefficient. Lastly, Student t-test was used for analysis of the uptake assay. Statistical significance for all analyses in this study was defined as p ≤ 0.05.
Results
Host Mutations Associated with Iron Overload, HfeC282Y∕C282Yand Hjv−∕−, Lead to Enhanced Systemic Colonization of Y. pseudotuberculosis
In order to better understand the biological significance of the influence of iron on Y. pseudotuberculosis pathogenesis, we studied susceptibility of murine models of hereditary hemochromatosis (HH) to Y. pseudotuberculosis infection. We began by evaluating the susceptibility of two distinct mouse models of HH, HfeC282Y∕C282Y and Hjv−∕−, to Y. pseudotuberculosis oral infection. While all wildtype mice survived for 5 days (when the experiment was terminated) following an oral inoculation dose of 2 × 108 Y. pseudotuberculosis, 100% of Hjv−∕− mice receiving the same dose had to be euthanized prior to day 5 because of symptoms indicative of more severe disease including hunched appearance and ruffled fur (unpublished observations), in accordance with institutional guidelines. This indicates that Hjv−∕− mice may be more susceptible to oral infection with Y. pseudotuberculosis than normal mice. Using a lower oral inoculation dose of 2 × 107 Y. pseudotuberculosis allowed both wildtype and Hjv−∕− mice to survive 5 days of infection. We observed some differences in Y. pseudotuberculosis colonization of wildtype and Hjv−∕− Peyer's patches and MLN 3 and 5 days post-inoculation (Figures 1A,B). However, more strikingly, while bacterial loads were below the limit of detection from the spleens and livers of all wildtype mice 3 days post-inoculation, the majority of Hjv−∕− mouse spleens and livers contained greater numbers of bacteria relative to wild type (Figure 1A). Furthermore, by 5 days post-inoculation, Hjv−∕− mice had 215- and 380-fold more CFU per gram tissue, on average, in the spleen and liver compared to wildtype mice (Figure 1B). Collectively, these data suggest that Y. pseudotuberculosis colonize deep tissues earlier in Hjv−∕− mice compared to normal mice following the natural oral route of infection.
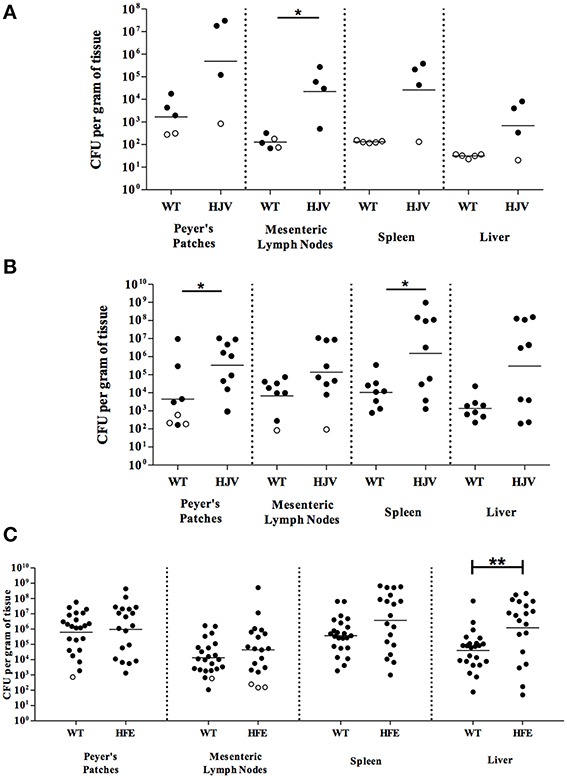
Figure 1. Iron overload leads to enhanced colonization of mice orally infected with Y. pseudotuberculosis. Wildtype (WT) and Hjv−∕− (HJV) mice were infected with 2 × 107 WT Y. pseudotuberculosis via orogastric gavage. At (A) 3 days and (B) 5 days post-inoculation, the Peyer's patches, mesenteric lymph nodes, spleens and livers were collected, homogenized, and CFU determined. (C) WT and HfeC282Y∕C282Y (HFE) mice were infected with 2 × 108 WT Y. pseudotuberculosis via orogastric gavage and tissues harvested 5 days post-inoculation. Each symbol represents data from one organ. Open symbols are set at the limit of detection for each individual organ based on weight and represent CFU that were below this limit. Dashes represent the geometric mean. Shown are data from (A) two, (B) four, and (C) seven independent experiments. *p < 0.05, **p < 0.01 as determined by an unpaired Wilcoxon–Mann–Whitney rank sum test. Dashes represent the geometric mean.
Analysis of the HfeC282Y∕C282Y mice, which display less severe iron overload relative to the Hjv−∕− background (Huang et al., 2005), showed no significant differences in bacterial colonization 3 days post-inoculation compared to wildtype mice (data not shown) and comparable levels of colonization in the Peyer's patches, MLN, and spleens relative to wildtype mice days post-inoculation with 2 × 108 Y. pseudotuberculosis (Figure 1C). However, colonization of the liver 5 days post-inoculation was increased in HfeC282Y∕C282Y mice by 30-fold (p < 0.01). This increased liver colonization of HfeC282Y∕C282Y mice correlated with four-fold more iron in HfeC282Y∕C282Y livers relative to wildtype livers during Yersinia infection (Figure 2).
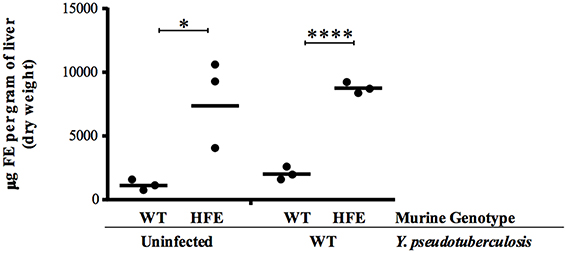
Figure 2. HfeC282Y∕C282Y mice harbor increased hepatic iron. Hepatic iron load was measured from tissues isolated from WT and HfeC282Y∕C282Y (HFE) mice that were left uninfected or were infected 5 days earlier with 2 × 108 WT Y. pseudotuberculosis via orogastric gavage. Each symbol represents data from one organ. *p < 0.05 and ****p < 0.0001 as determined by a Student t-test.
Collectively, these data suggest that both the HfeC282Y∕C282Y and Hjv−∕− mice are effective models for the study of Y. pseudotuberculosis infection in iron overloaded hosts. Based on the knowledge that the HfeC282Y∕C282Y mutation is far more common in humans than mutations in the Hjv gene, and that the Hjv−∕− animals were significantly more challenging to breed, we focused the remainder of our studies on the HfeC282Y∕C282Y mouse model (Brandhagen et al., 2002; Lanzara et al., 2004).
Y. pseudotuberculosis Lacking the T3SS Encoding Virulence Plasmid pYV Display Enhanced Virulence in HfeC282Y∕C282YMice
Excess iron should lead to decreased IscR levels, and therefore decreased expression of the T3SS (Miller et al., 2014). Yet while the Ysc T3SS is required for Yersinia virulence in normal mice (Cornelis, 2002), we observed increased susceptibility of iron overloaded mice to Y. pseudotuberculosis (Figure 1). Thus, we evaluated the susceptibility of HfeC282Y∕C282Y mice to Y. pseudotuberculosis lacking the T3SS-encoding virulence plasmid, pYV. The pYV− strain was better able to colonize livers of HfeC282Y∕C282Y mice compared to wildtype mice; pYV− colonization of HfeC282Y∕C282Y livers was increased eight-fold (p < 0.0001) compared to pYV− infection of wildtype tissues (Figure 3). In fact, while the pYV− strain was able to colonize the liver to levels above the limit of detection in only 50% of the wildtype animals by day 5, this T3SS-deficient strain was able to colonize deep tissues to levels above the limit of detection in 95% of HfeC282Y∕C282Y mice. These findings suggest that there may be a decreased requirement for the Y. pseudotuberculosis Ysc T3SS in iron overloaded animals.
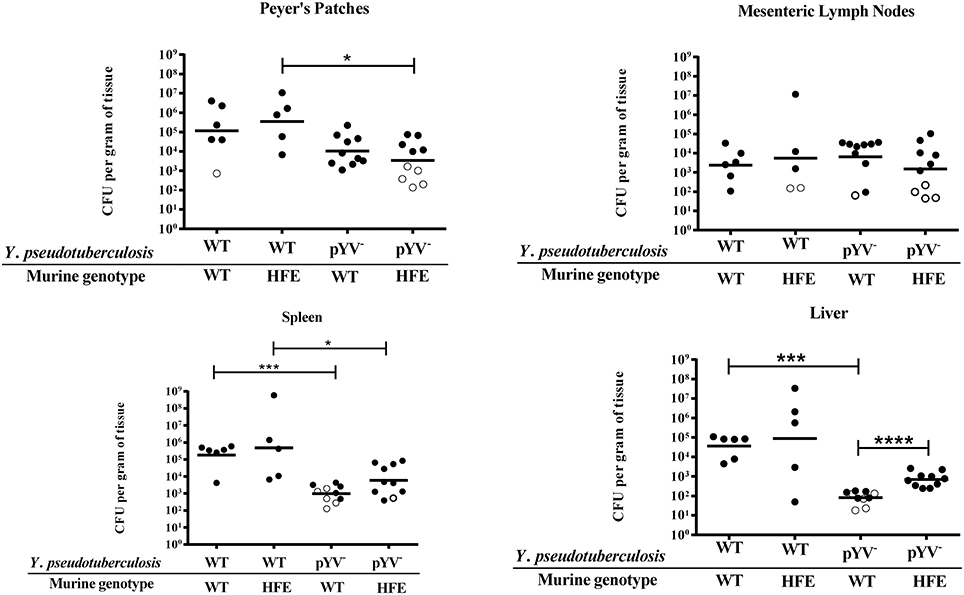
Figure 3. Y. pseudotuberculosis lacking the T3SS encoding virulence plasmid, pYV, are able to better colonize the livers of HfeC282Y∕C282Y mice relative to wildtype mice. WT and HfeC282Y∕C282Y (HFE) mice were infected with 2 × 108 CFU of either WT Y. pseudotuberculosis or the pYV− strain via orogastric gavage. At 5 days post-inoculation, the Peyer's patches, mesenteric lymph nodes, spleens and livers were collected, homogenized, and CFU determined. Each symbol represents data from one organ. Open symbols are set at the limit of detection for each individual organ based on weight and represent CFU that were below this limit. Dashes represent the geometric mean. Shown are data from three independent experiments. The wildtype data presented here is also included in Figure 1C. *p < 0.05, ***p < 0.001, and ****p < 0.0001 as determined by an unpaired Wilcoxon–Mann–Whitney rank sum test.
Peritoneal Macrophages from HH Mice are Not Attenuated in their Cytokine and Chemokine Response to Yersinia
Previous work by Wang et al., demonstrated that peritoneal macrophages isolated from Hfe−∕− mice were defective in their cytokine response to Salmonella through Toll-like receptor 4, as a result of decreased cytokine mRNA translation (Wang et al., 2009). Several Yersinia T3SS effector proteins are known to inhibit production of several cytokines (Pha and Navarro, 2016). Therefore, we sought to examine whether cytokine production was decreased in HH mice during Yersinia infection, as diminished cytokine production may account for both the increased bacterial burden as well as the decreased requirement for the T3SS observed for these mice. We measured the levels of six different cytokines, TNF-α, IFN-γ, IL-6, IL-10, IL-12p70, and MCP-1, in Yersinia-infected mouse tissues. We found levels of IL-10 and IL-12p70 for both wildtype and HfeC282Y∕C282Y mice to be comparable to those of our uninfected controls (data not shown). These findings are not surprising as previous reports demonstrate these cytokines to be present at low levels until very late stages of infection (Auerbuch and Isberg, 2007). Liver TNF-α, IFN-γ, MCP-1, and IL-6 levels were above background in both HfeC282Y∕C282Y and wildtype mice and TNF-α, MCP-1, and IL-6 levels correlated with bacterial burden (Figures 4A,B; Pearson correlation). When WT and HfeC282Y∕C282Y liver cytokine levels were averaged, no significant differences could be detected (Figure 4A). In fact, consistent with many HH livers having higher average CFU burdens than wildtype mice, cytokine levels for a number of the HH livers were actually higher than for wildtype livers (Figure 4B).
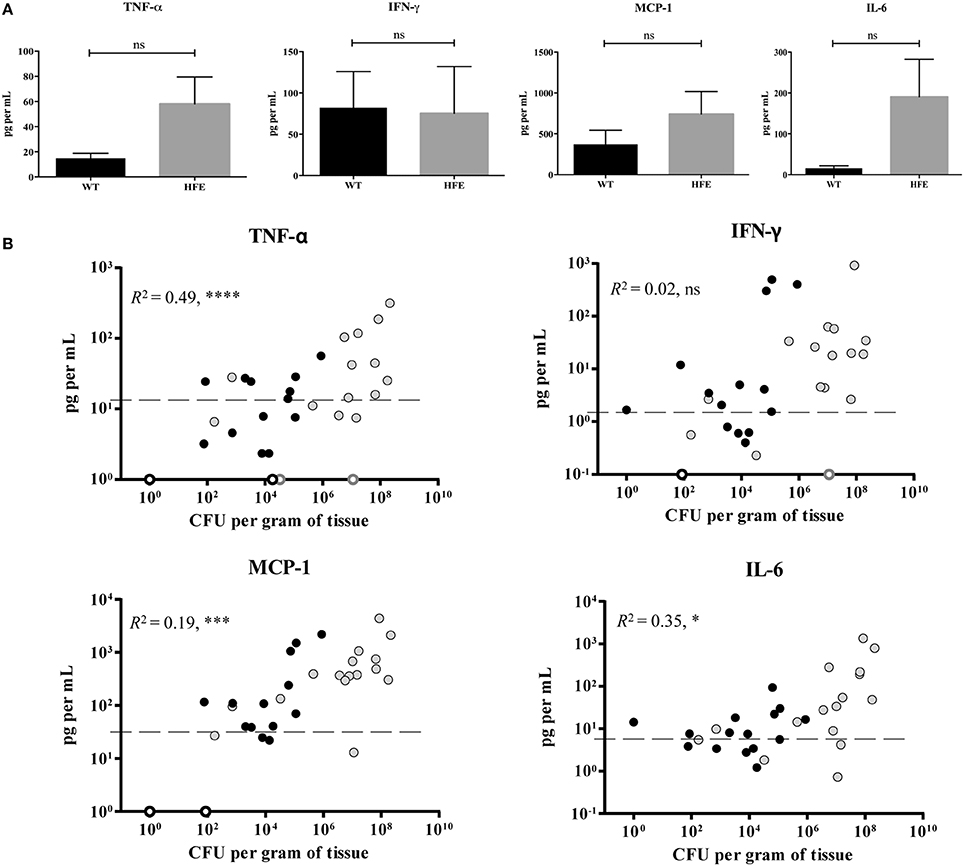
Figure 4. HfeC282Y∕C282Y mice display robust hepatic cytokine production during Y. pseudotuberculosis infection. WT (black) and HfeC282Y∕C282Y (HFE; gray) mice were infected with 2 × 108 WT Y. pseudotuberculosis via orogastric gavage and tissues harvested 5 days post-inoculation. Flow cytometry-based ELISA was used to measure levels of the cytokines IFN-γ, IL-6, and TNF-α and the chemokine MCP-1 from liver homogenates. (A) IFN-γ, IL-6, TNF-α, and MCP-1 levels are displayed as mean pg per ml liver homogenate ± standard error of the mean; ns = no significance as determined by a Student t-test. (B) Individual cytokine concentrations in pg/ml are plotted against CFU per gram of liver tissue determined at the time of organ harvest. Open circles represent samples where CFU were below the limit of detection. Average uninfected cytokine concentrations are displayed as a dashed line and represent the average plus the standard deviation for uninfected liver samples. R2-values were determined based on Pearson's correlation coefficient using combined WT and HFE data; ns, no significance, *p < 0.05, ***p < 0.001, and ****p < 0.0001.
Because CFU burden differences between wildtype and HH mice complicated our cytokine analysis, we isolated thioglycollate-elicited peritoneal macrophages (which serve as a model for tissue macrophages) from wildtype, HfeC282Y∕C282Y, and Hjv−∕− mice, and infected them with Y. pseudotuberculosis either expressing a functional T3SS translocon but lacking the known T3SS effector proteins (Δyop6) or lacking a functional T3SS translocon (Δyop6/ΔyopB). These strains were used because several T3SS effector proteins have been shown to modulate cytokine production upon translocation inside host cells (Bliska et al., 2013). Surprisingly, the amount of IL-6, MCP-1, and TNFα secreted by HfeC282Y∕C282Y or Hjv−∕− macrophages after 3 h was significantly higher than that secreted by wildtype macrophages in response to the Δyop6 or Δyop6/ΔyopB Y. pseudotuberculosis strains or to LPS (Figures 5A,B). Similar results were seen for the cytokine response to the WT and pYV- Yersinia strains (data not shown). Furthermore, incubating peritoneal macrophages for only 2 h following isolation and prior to inoculation, rather than overnight, did not alter these findings (data not shown). These data are in contrast to results from Hfe−∕− C57Bl/6 mouse peritoneal macrophages treated with LPS or Salmonella (Wang et al., 2009), for reasons that remain unclear. However, these data suggest that an attenuated HH cytokine response is not responsible for the increased bacterial burden or decreased requirement for the T3SS during Yersinia infection of HH mice.
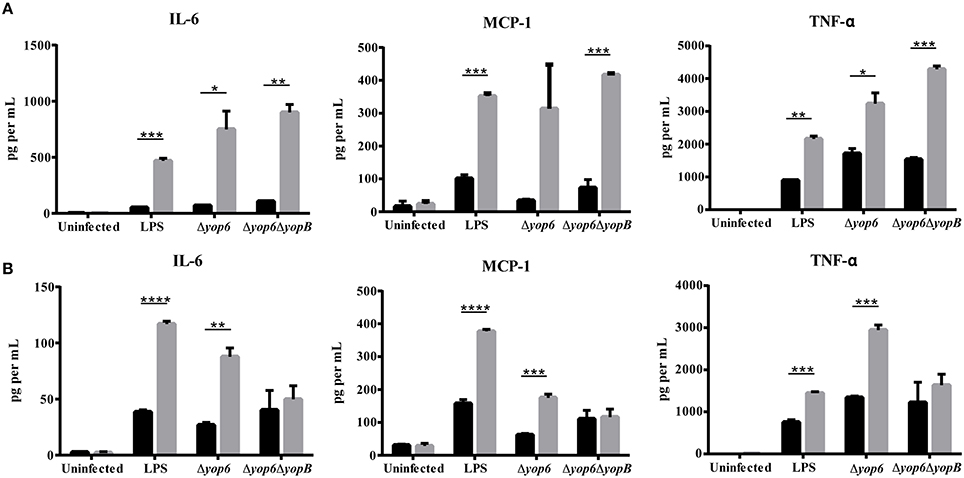
Figure 5. Peritoneal macrophages isolated from hemochromatosis mice produce elevated levels of IL-6, MCP-1, and TNF-α in response to Y. pseudotuberculosis compared to macrophages from wildtype mice. Elicited peritoneal macrophages from naïve WT (black bars), (A) HfeC282Y∕C282Y (gray bars), and (B) Hjv−∕− (gray bars) mice were either left untreated, treated with 100 ng ml−1 of lipopolysaccharide (LPS) from Salmonella minnesota (UltraPure), or exposed to either Δyop6, a Y. pseudotuberculosis mutant lacking Yop effectors but expressing a functional T3SS translocon, or with Δyop6/ΔyopB, a T3SS translocon-deficient Y. pseudotuberculosis at an MOI of 10. Flow cytometry-based ELISA was used to measure levels of the cytokines IL-6 and TNF-α and the chemokine MCP-1 in the supernatant after 3 h. Data is displayed as the average cytokine concentration from the macrophages of two mice that had been pooled and analyzed in triplicate. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 as determined by a Student t-test.
Peritoneal Macrophages from HH Mice are Defective in their Ability to take up Yersinia
Previous studies indicated that phagocytic cells isolated from human HH patients were defective in phagocytosis (van Asbeck et al., 1984; Moura et al., 1998). As the Yersinia T3SS has potent anti-phagocytic activity (Pha and Navarro, 2016), we examined the ability of peritoneal macrophages to take up pYV− Y. pseudotuberculosis. Consistent with previous studies, HfeC282Y∕C282Y macrophages contained two-fold fewer intracellular bacteria than wildtype macrophages (Figure 6). These data indicate that the effector function defect of HH mouse phagocytes partly negates the virulence requirement of the Yersinia T3SS.
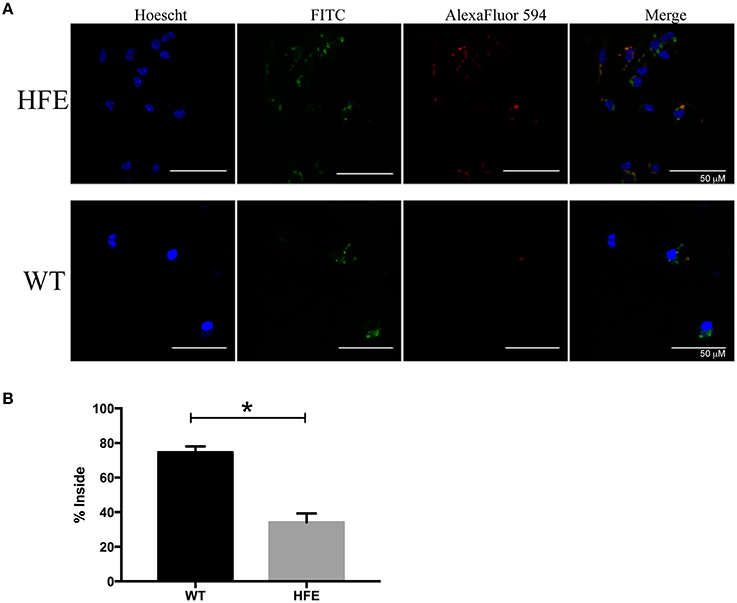
Figure 6. Peritoneal macrophages isolated from hemochromatosis mice are defective in Yersinia uptake compared to wildtype macrophages. Elicited peritoneal macrophages from naïve WT and HfeC282Y∕C282Y mice were infected with pYV− Y. pseudotuberculosis and percent of bacterial uptake quantitated after 30 min by immunofluorescence microscopy. Extracellular bacteria were stained both red and green, while internalized bacteria were only stained green. (A) Representative images are shown in addition to the (B) average % internalized bacteria ± standard deviation of 17–20 frames from two biological replicates. *p < 0.02 as determined by a Student t-test.
Discussion
In this study, we demonstrate that two HH mouse models are more susceptible to disseminated infection with fully virulent Y. pseudotuberculosis than are wildtype mice, consistent with clinical data on humans with HH. Furthermore, Y. pseudotuberculosis lacking the T3SS-encoding virulence plasmid pYV are able to colonize a higher percentage of HH hosts and disseminate into deeper tissues. As peritoneal macrophages isolated from HH mice were less phagocytic toward pYV− Yersinia than their wildtype counterparts, we propose that the requirement for the Yersinia T3SS, which is strongly anti-phagocytic, is diminished in HH hosts compared to healthy hosts.
Hereditary hemochromatosis is characterized by an increase in intestinal absorption of iron (Hanson et al., 2001). As there is no physiological process to rid the body of this excess iron, it accumulates in organs throughout the body such as the heart, pancreas, and liver, leading to tissue damage and decreased immune response (Hanson et al., 2001; Wang et al., 2008; Hentze et al., 2010; Pietrangelo, 2010; Ekanayake et al., 2015; Rishi et al., 2015). This condition causes an increased susceptibility to serious infection with specific bacterial pathogens including enteropathogenic Yersinia. However, it is unclear whether this increase in susceptibility of HH hosts is due to increased virulence of the pathogen, enhanced bacterial fitness due to increased iron availability, or a combination of these factors (Sinkovics et al., 1980; Christopher, 1985; Abbott et al., 1986; Bullen et al., 1991; Mennecier et al., 2001a; Harris and Paraskevakis, 2012). In this study, we demonstrate that two murine models of HH, carrying HfeC282Y∕C282Y or Hjv−∕− mutations, display increased susceptibility to Y. pseudotuberculosis infection. The more severely iron overloaded Hjv−∕− mice were significantly more susceptible to Yersinia infection than the HfeC282Y∕C282Y mice with milder iron overload, as Hjv−∕− but not HfeC282Y∕C282Y mice or wildtype mice showed overt signs of disease 5 days post-inoculation with 2 × 108 Y. pseudotuberculosis, while HfeC282Y∕C282Y mice had higher liver CFU than wildtype mice. This defect in resistance of Hjv−∕− mice to Y. pseudotuberculosis is consistent with the enhanced susceptibility of these mice to a pgm− vaccine strain of Y. pestis (Quenee et al., 2012).
Perhaps most intriguing is the finding that the increased bacterial burden observed for HH mice can occur in the absence of the Ysc T3SS, which is required for disseminated Yersinia infection of wildtype mice (Cornelis, 2002). Wang et al. described an attenuated inflammatory response of macrophages isolated from HH mice to Salmonella (Wang et al., 2008). In addition, macrophages and polymorphonuclear leukocytes from HH humans have been previously shown to have decreased phagocytic and microbicidal properties (van Asbeck et al., 1984; Weiss et al., 1994; Moura et al., 1998; Walker and Walker, 2000). As these host defenses are also targeted by Yersinia T3SS effector proteins, it is possible that in HH mice, several host defense pathways that must normally be inactivated by the Ysc T3SS to enable Yersinia growth are already compromised as a result of the downstream consequences of iron overload. However, we could not detect any statistically significant differences in production of TNF-α, MCP-1, IFN-γ, or IL-6 in the livers of wildtype and HH mice infected with Y. pseudotuberculosis, although the elevated colonization of HH mice compared to wildtype mice complicated this analysis, as cytokine/chemokine level correlated with CFU load. Furthermore, elicited peritoneal macrophages from naïve HH mice actually produced increased levels of TNF-α, MCP-1, or IL-6 in response to Yersinia compared to wildtype macrophages. However, we did observe a defect in the ability of HH peritoneal macrophages to take up Yersinia compared to wildtype macrophages. Therefore, it is possible that the phagocytic properties of tissue macrophages in the HH mice are compromised, rendering the anti-phagocytic activity of the Ysc T3SS less important in these tissues. Interestingly, there was no decrease in Ly6G+ cell infiltration in infected Hjv−∕− mouse livers compared to infected WT livers (data not shown). Collectively, these data suggest that phagocytes are recruited to sites of infection in HH animals and produce cytokines in response to microbial PAMPS, yet are ineffective in microbial uptake.
Our data support the proposed model that an increase in iron availability might dampen T3SS expression through IscR control of the T3SS master regulator LcrF (Miller et al., 2014), as loss of the T3SS in iron overloaded mice would not be as detrimental to Y. pseudotuberculosis virulence as it is in wildtype mice. Furthermore, with increased emphasis on identification of novel antimicrobial strategies and research on chemical inhibitors of the T3SS as virulence blockers, our data suggests that, while T3SS inhibitors may one day be used to prevent or treat Yersinia infection, they may not be effective in the context of host iron overload. Recently there was a case of lethal laboratory-acquired plague in a researcher with undiagnosed HH who became infected while working with pgm− Y. pestis, a strain with diminished virulence (Quenee et al., 2012). Yersinia strains lacking the T3SS are also considered to have decreased pathogenic potential; however, based on the data presented here, HH hosts may be more susceptible to these strains as well.
Huang et al. showed that Hjv−∕− mice contained less splenic non-heme iron compared to wildtype mice, in contrast to the elevated non-heme iron observed in Hjv−∕− livers (Huang et al., 2005). Quenee et al. also found iron deposits in the livers, but not the spleens, of Hjv−∕− mice. Macrophages play an important role in the recycling of iron from senescent red blood cells, and Huang et al. demonstrated that the increased iron recycling in Hjv−∕− mice leads to higher extracellular iron and lower macrophage intracellular iron (Huang et al., 2005). As the spleen contains numerous macrophages (Cesta, 2006), it is possible that decreased macrophage iron in Hjv−∕− mice contributes to the lower overall splenic iron level (Hentze et al., 2010). Interestingly, we observed an increase in bacterial burden in both the liver and spleen of Hjv−∕− mice compared to wildtype mice. As we did not perform perfusion prior to organ harvesting, as per standard practice in the field, it is possible that enhanced bacterial colonization of Hjv−∕− spleens reflects enhanced Y. pseudotuberculosis growth in Hjv−∕− blood. Unlike HfeC282Y∕C282Y mice, which have been shown to have comparable serum iron concentrations relative to wild type mice, Hjv−∕− mice have elevated iron levels in the blood relative to wild type (Zhou et al., 1998; Gkouvatsos et al., 2014). Indeed, Hjv−∕− mice displayed enhanced Yersinia spleen colonization but HfeC282Y∕C282Y mice did not.
Interestingly, Quenee et al. showed that a recombinant protein vaccine that targets the T3SS needle tip protein LcrV protects Hjv−∕− mice against fully virulent plague or against a live, attenuated vaccine strain of Y. pestis (Quenee et al., 2012). The rV10 vaccine was previously shown to elicit antibodies to LcrV, and anti-LcrV antibodies are known to inhibit translocation of Yop effectors into host cells (DeBord et al., 2006; Quenee et al., 2010). These data suggest that anti-LcrV antibody targeting of Y. pestis protects HH mice against otherwise lethal plague either by inactivation of the T3SS and/or through opsonization of Yersinia (Quenee et al., 2010). Given our data showing that the Y. pseudotuberculosis T3SS is dispensable for disseminated infection, it is possible that the increased bacterial load that we observed also occurs in the model of survival utilized by Quenee et al. but that the HH mice are able to clear the infection.
Our findings as well as those of Quenee et al. (2012) suggest that the increased susceptibility of HH hosts to Yersinia infection is likely a result of excess iron available to promote bacterial growth. Indeed, Y. pestis strains lacking the ability to synthesize yersiniabactin, an iron scavenging siderophore, are fully virulent in HH hosts (Quenee et al., 2012). Additionally, mice infected with Y. enterocolitica or Y. pestis that were given either iron-dextran or the siderophore Desferal, which can be used by Yersinia as an iron source, displayed reduced lethal doses as well as a more severe yersiniosis (Burrows and Jackson, 1956; Robins-Browne and Prpic, 1985; Galvan et al., 2010; Quenee et al., 2012). Collectively, these results and the data shown here suggest that the progression and pathology of yersiniosis may differ greatly in iron overloaded vs. non-iron overloaded hosts because of excess bioavailable iron, deficiency in the phagocytic properties of immune cells, and differences in bacterial virulence factors required to cause disease.
Author Contributions
HM and LS contributed equally to this work. VA and HM designed the study. HM, LS, and WA performed the experiments. HM and VA wrote the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Martha Zuniga for critical review of the manuscript, Donald Smith and Rob Franks for technical support with the tissue iron measurement, Nancy Andrews for the Hjv−∕− mice, Armen Shamamian in the UCSC vivarium for rederivation of the HFEC282Y∕C282Y mice, Bari Holm Nazario in the UCSC Flow Cytometry Facility for technical support with the flow-cytometry assay, Ben Abrams in the UCSC Life Sciences Microscopy Center for technical support with microscopy, Richard Frothingham for useful discussion on HH mouse infection, Ralph Isberg for the anti-Yersinia antibody, and the UCSC vivarium staff for their assistance with animal care and maintenance. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R21AI099747 and R01AI119082 (to VA). LS was supported by the National Institutes of Health training grant T32GM008646.
References
Abbott, M., Galloway, A., and Cunningham, J. L. (1986). Hemochromatosis presenting with a double yersinia infection. J. Infect. 13, 143–145. doi: 10.1016/S0163-4453(86)92869-0
Archibald, F. (1983). Lactobacillus plantarum, an organism not requiring Iron. FEMS Microbiol. Lett. 19, 29–32. doi: 10.1111/j.1574-6968.1983.tb00504.x
Auerbuch, V., Golenbock, D. T., and Isberg, R. R. (2009). Innate immune recognition of Yersinia pseudotuberculosis type III secretion. PLoS Pathog. 5:e1000686. doi: 10.1371/journal.ppat.1000686
Auerbuch, V., and Isberg, R. R. (2007). Growth of Yersinia pseudotuberculosis in mice occurs independently of Toll-like receptor 2 expression and induction of interleukin-10. Infect. Immun. 75, 3561–3570. doi: 10.1128/IAI.01497-06
Bahram, S., Gilfillan, S., Kuhn, L. C., Moret, R., Schulze, J. B., Lebeau, A., et al. (1999). Experimental hemochromatosis due to MHC class I HFE deficiency: immune status and iron metabolism. Proc. Natl. Acad. Sci. U.S.A. 96, 13312–13317. doi: 10.1073/pnas.96.23.13312
Bergmann, T. K., Vinding, K., and Hey, H. (2001). Multiple hepatic abscesses due to Yersinia enterocolitica infection secondary to primary haemochromatosis. Scand. J. Gastroenterol. 36, 891–895. doi: 10.1080/003655201750313450
Bliska, J. B., Guan, K. L., Dixon, J. E., and Falkow, S. (1991). Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc. Natl. Acad. Sci. U.S.A. 88, 1187–1191. doi: 10.1073/pnas.88.4.1187
Bliska, J. B., Wang, X., Viboud, G. I., and Brodsky, I. E. (2013). Modulation of innate immune responses by Yersinia type III secretion system translocators and effectors. Cell. Microbiol. 15, 1622–1631. doi: 10.1111/cmi.12164
Brandhagen, D. J., Fairbanks, V. F., and Baldus, W. (2002). Recognition and management of hereditary hemochromatosis. Am. Fam. Physician 65, 853–860.
Bullen, J. J., Spalding, P. B., Ward, C. G., and Gutteridge, J. M. C. (1991). Hemochromatosis, Iron, and Septicemia caused by Vibrio vulnificus. Arch. Intern. Med. 151, 1606–1609. doi: 10.1001/archinte.1991.00400080096018
Burrows, T. W., and Jackson, S. (1956). The virulence-enhancing effect of iron on nonpigmented mutants of virulent strains of Pasteurella pestis. Br. J. Exp. Pathol. 37, 577–583.
Cassat, J. E., and Skaar, E. P. (2013). Iron in infection and immunity. Cell Host Microbe 13, 510–520. doi: 10.1016/j.chom.2013.04.010
Cesta, M. F. (2006). Normal structure, function, and histology of the spleen. Toxicol. Pathol. 34, 455–465. doi: 10.1080/01926230600867743
Chakraborty, S., Sivaraman, J., Leung, K. Y., and Mok, Y. K. (2011). Two-component PhoB-PhoR regulatory system and ferric uptake regulator sense phosphate and iron to control virulence genes in type III and VI secretion systems of Edwardsiella tarda. J. Biol. Chem. 286, 39417–39430. doi: 10.1074/jbc.M111.295188
Christopher, G. W. (1985). Escherichia coli bacteremia, meningitis, and hemochromatosis. Arch. Intern. Med. 145, 1908–1908. doi: 10.1001/archinte.1985.00360100178031
Cornelis, G. R. (2002). The Yersinia Ysc-Yop 'type III' weaponry. Nat. Rev. Mol. Cell Biol. 3, 742–752. doi: 10.1038/nrm932
Cornelis, G. R. (2006). The type III secretion injectisome. Nat. Rev. Microbiol. 4, 811–825. doi: 10.1038/nrmicro1526
Cornelis, G. R., Boland, A., Boyd, A. P., Geuijen, C., Iriarte, M., Neyt, C., et al. (1998). The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62, 1315–1352.
DeBord, K. L., Anderson, D. M., Marketon, M. M., Overheim, K. A., DePaolo, R. W., Ciletti, N. A., et al. (2006). Immunogenicity and protective immunity against bubonic plague and pneumonic plague by immunization of mice with the recombinant V10 antigen, a variant of LcrV. Infect. Immun. 74, 4910–4914. doi: 10.1128/IAI.01860-05
Durand, E. A., Maldonado-Arocho, F. J., Castillo, C., Walsh, R. L., and Mecsas, J. (2010). The presence of professional phagocytes dictates the number of host cells targeted for Yop translocation during infection. Cell. Microbiol. 12, 1064–1082. doi: 10.1111/j.1462-5822.2010.01451.x
Ekanayake, D., Roddick, C., and Powell, L. W. (2015). Recent advances in hemochromatosis: a 2015 update: a summary of proceedings of the 2014 conference held under the auspices of Hemochromatosis Australia. Hepatol. Int. 9, 174–182. doi: 10.1007/s12072-015-9608-2
Ellermeier, J. R., and Slauch, J. M. (2008). Fur regulates expression of the Salmonella pathogenicity island 1 type III secretion system through HilD. J. Bacteriol. 190, 476–486. doi: 10.1128/JB.00926-07
Feder, J. N., Gnirke, A., Thomas, W., Tsuchihashi, Z., Ruddy, D. A., Basava, A., et al. (1996). A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat. Genet. 13, 399–408. doi: 10.1038/ng0896-399
Galvan, E. M., Nair, M. K., Chen, H., Del Piero, F., and Schifferli, D. M. (2010). Biosafety level 2 model of pneumonic plague and protection studies with F1 and Psa. Infect. Immun. 78, 3443–3453. doi: 10.1128/IAI.00382-10
Giel, J. L., Rodionov, D., Liu, M., Blattner, F. R., and Kiley, P. J. (2006). IscR-dependent gene expression links iron-sulphur cluster assembly to the control of O2-regulated genes in Escherichia coli. Mol. Microbiol. 60, 1058–1075. doi: 10.1111/j.1365-2958.2006.05160.x
Gkouvatsos, K., Fillebeen, C., Daba, A., Wagner, J., Sebastiani, G., and Pantopoulos, K. (2014). Iron-dependent regulation of hepcidin in Hjv-/- mice: evidence that hemojuvelin is dispensable for sensing body iron levels. PLoS ONE 9:e85530. doi: 10.1371/journal.pone.0085530
Gode-Potratz, C. J., Chodur, D. M., and McCarter, L. L. (2010). Calcium and iron regulate swarming and type III secretion in Vibrio parahaemolyticus. J. Bacteriol. 192, 6025–6038. doi: 10.1128/JB.00654-10
Hanson, E. H., Imperatore, G., and Burke, W. (2001). HFE gene and hereditary hemochromatosis: a HuGE review. Am. J. Epidemiol. 154, 193–206. doi: 10.1093/aje/154.3.193
Harris, S., and Paraskevakis, I. (2012). Multiple liver abscesses due to Yersinia enterocolitica uncovering hemochromatosis. J. Investig. Med. 60, 364–364. doi: 10.1080/00365520117816
Heesemann, J., Sing, A., and Trulzsch, K. (2006). Yersinia's stratagem: targeting innate and adaptive immune defense. Curr. Opin. Microbiol. 9, 55–61. doi: 10.1016/j.mib.2005.10.018
Hentze, M. W., Muckenthaler, M. U., Galy, B., and Camaschella, C. (2010). Two to tango: regulation of Mammalian iron metabolism. Cell 142, 24–38. doi: 10.1016/j.cell.2010.06.028
Hopfner, M., Nitsche, R., Rohr, A., Harms, D., Schubert, S., and Folsch, U. R. (2001). Yersinia enterocolitica infection with multiple liver abscesses uncovering a primary hemochromatosis. Scand. J. Gastroenterol. 36, 220–224. doi: 10.1080/003655201750066004
Huang, F. W., Pinkus, J. L., Pinkus, G. S., Fleming, M. D., and Andrews, N. C. (2005). A mouse model of juvenile hemochromatosis. J. Clin. Invest. 115, 2187–2191. doi: 10.1172/JCI25049
Jacquenod, P., Poitrine, A., Loiseau, D., and Naveau, S. (1984). Yersinia enterocolitica septicemia and idiopathic hemochromatosis - role of iron overload. Gastroenterol. Clin. Biol. 8, 293–294.
Jurado, R. L. (1997). Iron, infections, and anemia of inflammation. Clin. Infect. Dis. 25, 888–895. doi: 10.1086/515549
Koberle, M., Klein-Gunther, A., Schutz, M., Fritz, M., Berchtold, S., Tolosa, E., et al. (2009). Yersinia enterocolitica targets cells of the innate and adaptive immune system by injection of Yops in a mouse infection model. PLoS Pathog. 5:e1000551. doi: 10.1371/journal.ppat.1000551
Kurushima, J., Kuwae, A., and Abe, A. (2012). Iron starvation regulates the type III secretion system in Bordetella bronchiseptica. Microbiol. Immunol. 56, 356–362. doi: 10.1111/j.1348-0421.2012.00442.x
Lanzara, C., Roetto, A., Daraio, F., Rivard, S., Ficarella, R., Simard, H., et al. (2004). Spectrum of hemojuvelin gene mutations in 1q-linked juvenile hemochromatosis. Blood 103, 4317–4321. doi: 10.1182/blood-2004-01-0192
Layoun, A., Samba, M., and Santos, M. M. (2015). Isolation of murine peritoneal macrophages to carry out gene expression analysis upon Toll-like receptors stimulation. J. Vis. Exp. e52749. doi: 10.3791/52749
Levy, J. E., Montross, L. K., Cohen, D. E., Fleming, M. D., and Andrews, N. C. (1999). The C282Y mutation causing hereditary hemochromatosis does not produce a null allele. Blood 94, 9–11.
MacKenzie, E. L., Iwasaki, K., and Tsuji, Y. (2008). Intracellular iron transport and storage: from molecular mechanisms to health implications. Antioxid. Redox Signal. 10, 997–1030. doi: 10.1089/ars.2007.1893
Marketon, M. M., DePaolo, R. W., DeBord, K. L., Jabri, B., and Schneewind, O. (2005). Plague bacteria target immune cells during infection. Science 309, 1739–1741. doi: 10.1126/science.1114580
Martin, R. B., Savory, J., Brown, S., Bertholf, R. L., and Wills, M. R. (1987). Transferrin binding of Al3+ and Fe3+. Clin. Chem. 33, 405–407.
Matsumoto, H., and Young, G. M. (2009). Translocated effectors of Yersinia. Curr. Opin. Microbiol. 12, 94–100. doi: 10.1016/j.mib.2008.12.005
McCance, R. A., and Widdowson, E. M. (1938). The absorption and excretion of iron following oral and intravenous administration. J. Physiol. 94, 148–154. doi: 10.1113/jphysiol.1938.sp003669
Mennecier, D., Lapprand, M., Hernandez, E., Minvielle, F., Bredin, C., Potier, V., et al. (2001a). Liver abscess caused by Yersinia pseudotuberculosis revealing genetic hemochromatosis. Gastroenterol. Clin. Biol. 25, 1113–1115.
Mennecier, D., Lapprand, M., Hernandez, E., Minvielle, F., Bredin, C., Potier, V., et al. (2001b). Liver abscesses due to Yersinia pseudotuberculosis discloses a genetic hemochromatosis. Gastroenterol. Clin. Biol. 25, 1113–1115. doi: 10.1371/journal.ppat.1004194
Miller, H. K., Kwuan, L., Schwiesow, L., Bernick, D. L., Mettert, E., Ramirez, H. A., et al. (2014). IscR is essential for Yersinia pseudotuberculosis type III secretion and virulence. PLoS Pathog. 10:e1004194. doi: 10.1371/journal.ppat.1004194
Miret, S., Simpson, R. J., and McKie, A. T. (2003). Physiology and molecular biology of dietary iron absorption. Annu. Rev. Nutr. 23, 283–301. doi: 10.1146/annurev.nutr.23.011702.073139
Moura, E., Verheul, A. F., and Marx, J. J. (1998). A functional defect in hereditary haemochromatosis monocytes and monocyte-derived macrophages. Eur. J. Clin. Invest. 28, 164–173. doi: 10.1046/j.1365-2362.1998.00263.x
Murphy, E. R., and Payne, S. M. (2007). RyhB, an iron-responsive small RNA molecule, regulates Shigella dysenteriae virulence. Infect. Immun. 75, 3470–3477. doi: 10.1128/IAI.00112-07
Pha, K., and Navarro, L. (2016). Yersinia type III effectors perturb host innate immune responses. World J. Biol. Chem. 7, 1–13. doi: 10.4331/wjbc.v7.i1.1
Pietrangelo, A. (2010). Hereditary hemochromatosis: pathogenesis, diagnosis, and treatment. Gastroenterology 139, e391–e392. doi: 10.1053/j.gastro.2010.06.013
Piroth, L., Meyer, P., Bielefeld, P., and Besancenot, J. F. (1997). [Yersinia bacteremia and iron overload]. Rev. Med. Int. 18, 932–938. doi: 10.1016/S0248-8663(97)80112-9
Posey, J. E., and Gherardini, F. C. (2000). Lack of a role for iron in the Lyme disease pathogen. Science 288, 1651–1653. doi: 10.1126/science.288.5471.1651
Quenee, L. E., Berube, B. J., Segal, J., Elli, D., Ciletti, N. A., Anderson, D., et al. (2010). Amino acid residues 196-225 of LcrV represent a plague protective epitope. Vaccine 28, 1870–1876. doi: 10.1016/j.vaccine.2009.11.076
Quenee, L. E., Hermanas, T. M., Ciletti, N., Louvel, H., Miller, N. C., Elli, D., et al. (2012). Hereditary hemochromatosis restores the virulence of plague vaccine strains. J. Infect. Dis. 206, 1050–1058. doi: 10.1093/infdis/jis433
Rishi, G., Wallace, D. F., and Subramaniam, V. N. (2015). Hepcidin: regulation of the master iron regulator. Biosci. Rep. 35:e00192 doi: 10.1042/BSR20150014
Robins-Browne, R. M., and Prpic, J. K. (1985). Effects of iron and desferrioxamine on infections with Yersinia enterocolitica. Infect. Immun. 47, 774–779.
Sinkovics, J. G., Cormia, F., and Plager, C. (1980). Hemochromatosis and Listeria Infection. Arch. Intern. Med. 140, 284–284. doi: 10.1001/archinte.1980.00330140142049
Skaar, E. P. (2010). The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog. 6:e1000949. doi: 10.1371/journal.ppat.1000949
van Asbeck, B. S., Marx, J. J., Struyvenberg, A., and Verhoef, J. (1984). Functional defects in phagocytic cells from patients with iron overload. J. Infect. 8, 232–240. doi: 10.1016/S0163-4453(84)93955-0
Walker, E. M. Jr., and Walker, S. M. (2000). Effects of iron overload on the immune system. Ann. Clin. Lab. Sci. 30, 354–365.
Wang, L., Harrington, L., Trebicka, E., Shi, H. N., Kagan, J. C., Hong, C. C., et al. (2009). Selective modulation of TLR4-activated inflammatory responses by altered iron homeostasis in mice. J. Clin. Invest. 119, 3322–3328. doi: 10.1172/jci39939
Wang, L. J., Johnson, E. E., Shi, H. N., Walker, W. A., Wessling-Resnick, M., and Cherayil, B. J. (2008). Attenuated inflammatory responses in hemochromatosis reveal a role for iron in the regulation of macrophage cytokine translation. J. Immunol. 181, 2723–2731. doi: 10.4049/jimmunol.181.4.2723
Weiss, G., Werner-Felmayer, G., Werner, E. R., Grunewald, K., Wachter, H., and Hentze, M. W. (1994). Iron regulates nitric oxide synthase activity by controlling nuclear transcription. J. Exp. Med. 180, 969–976. doi: 10.1084/jem.180.3.969
Wu, Y., and Outten, F. W. (2009). IscR controls iron-dependent biofilm formation in Escherichia coli by regulating type I fimbria expression. J. Bacteriol. 191, 1248–1257. doi: 10.1128/JB.01086-08
Yeo, W. S., Lee, J. H., Lee, K. C., and Roe, J. H. (2006). IscR acts as an activator in response to oxidative stress for the suf operon encoding Fe-S assembly proteins. Mol. Microbiol. 61, 206–218. doi: 10.1111/j.1365-2958.2006.05220.x
Keywords: Yersinia pseudotuberculosis, type III secretion system, IscR, hemochromatosis, HFE, hemojuvelin
Citation: Miller HK, Schwiesow L, Au-Yeung W and Auerbuch V (2016) Hereditary Hemochromatosis Predisposes Mice to Yersinia pseudotuberculosis Infection Even in the Absence of the Type III Secretion System. Front. Cell. Infect. Microbiol. 6:69. doi: 10.3389/fcimb.2016.00069
Received: 23 February 2016; Accepted: 08 June 2016;
Published: 24 June 2016.
Edited by:
Matthew S. Francis, Umeå University, SwedenReviewed by:
Matthew B. Lawrenz, University of Louisville School of Medicine, USADanielle Malo, McGill University, Canada
Yinon Levy, Israel Institute for Biological Research, Israel
Copyright © 2016 Miller, Schwiesow, Au-Yeung and Auerbuch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Victoria Auerbuch, dmFzdG9uZUB1Y3NjLmVkdQ==
†These Authors have contributed equally to this work.
 Halie K. Miller1†
Halie K. Miller1† Leah Schwiesow
Leah Schwiesow Victoria Auerbuch
Victoria Auerbuch