- 1Section of Infectious Disease, Department of Internal Medicine, Yale University School of Medicine, New Haven, CT, United States
- 2Howard Hughes Medical Institute, Chevy Chase, MD, United States
- 3Department of Microbial Pathogenesis, Yale University, New Haven, CT, United States
Tick-pathogen-host interactions have been closely studied to understand the molecular mechanisms of pathogen transmission for tick-borne diseases, including Lyme disease, babesiosis, spotted fever diseases, and Tick-borne encephalitis, among others. Such studies have yielded insights into disease processes and have identified promising candidates for vaccines against tick-borne diseases (Dai et al., 2009; Schuijt et al., 2011; de la Fuente et al., 2016). In addition to these vaccine targets, the advent of “omics” technologies, such as transcriptomics and proteomics, has opened the doors for discovery of a wide variety of tick bioactive molecules (Francischetti et al., 2005, 2008, 2011; Untalan et al., 2005; Aljamali et al., 2009; Kongsuwan et al., 2010; Karim et al., 2011; Diaz-Martin et al., 2013; Oliveira et al., 2013; Egekwu et al., 2014; Radulovic et al., 2014; Tirloni et al., 2014; Karim and Ribeiro, 2015; Oleaga et al., 2015; Bullard et al., 2016; Kim et al., 2016; Moreira et al., 2017). While some of these bioactive molecules may be applicable for the treatment of tick-borne diseases, many are promising candidates for the treatment of other pathogens or human diseases. Therefore, we propose that careful study of tick bioactive molecules, such as those discovered in “omics” studies, is a promising rich source of novel therapeutics.
Tick-Pathogen Interactions
Tick-borne pathogens have a complex lifecycle that involves both a tick and vertebrate host. Within the natural cycle, uninfected ticks acquire pathogens when taking a blood-meal on an infected host. The microbes enter with the blood into the tick's gut. At this point, some pathogens, such as Anaplasma phagocytophilum (the causative agent of human granulocytic anaplasmosis), migrate to the salivary glands (Hodzic et al., 1998). Others, such as Borrelia burgdorferi (the etiologic agent of Lyme disease) remain in gut (De Silva and Fikrig, 1995). The pathogens are then maintained within the tick organs during molting (De Silva and Fikrig, 1995; Hodzic et al., 1998). Upon the next blood meal, the infectious microbes exit into a vertebrate host with the tick saliva, which is made in the salivary glands (De Silva and Fikrig, 1995; Hodzic et al., 1998). Therefore, microorganisms that remain in the gut through molting must migrate to the salivary glands during the next blood meal.
The complex processes of acquisition and transmission of tick-borne pathogens require specific interactions between the tick, microbe, and host. Indeed, disruption of some tick-pathogen interactions has been shown to decrease transmission (Ramamoorthi et al., 2005; Dai et al., 2009; Zhang et al., 2011; Narasimhan et al., 2014; Coumou et al., 2016). Likewise, vaccination against some tick saliva or salivary gland proteins decreases the ability of the tick to feed on a mammalian host (Gomes et al., 2015; Contreras and de la Fuente, 2016, 2017), which could reduce transmission of pathogens. Therefore, tick proteins that interact with pathogens or facilitate tick feeding have been studied as potential vaccine targets for tick-borne diseases. However, many of these proteins perform biological functions that could also be exploited for therapeutic development.
Tick Bioactive Molecules
Perhaps the best-studied source of tick bioactive molecules is tick saliva. Tick saliva includes a cocktail of potent proteins that aid in the feeding of the tick on a mammalian host and improve pathogen transmission from a tick to a mammalian host. These proteins are known to act as anticoagulants, immunosuppressants and immunomodulators, platelet inhibitors, vasodilators, inhibitors of wound healing, and facilitators of tick attachment (Reviewed in Kazimírová and Štibrániová, 2013). Many of these functions have potential uses in the treatment of disease.
For example, coagulation is an important process in many cancers, as it supports tumor growth, angiogenesis, and metastasis (Rickles et al., 2001). Additionally, cancer patients often have complications related to coagulation, such as venous thromboembolisms (Karakatsanis et al., 2016). Treatment of some cancers and cancer complications with anticoagulants has been shown to be effective (Rickles et al., 2001; Karakatsanis et al., 2016). Tick saliva is a rich source of novel anticoagulants that could be exploited for the development of anticoagulants for the treatment of diverse cancers. Indeed, Ixolaris and Amblyomin-X, anticoagulant and antiangionenic proteins from Amblyomma cajennense, have shown promising results for the treatment of glioblastoma (Carneiro-Lobo et al., 2009; Barboza et al., 2015), renal cell carcinoma (de Souza et al., 2016), and melanoma (Chudzinski-Tavassi et al., 2010; de Oliveira Ada et al., 2012) in mice. Additionally, complement inhibitors may be useful for disorders of inappropriate complement activation (Baines and Brodsky, 2017) or diseases exacerbated by the complement system, such as cardiovascular disease (Shields et al., 2017). Indeed, Ornithodoros moubata Complement Inhibitor (OmCI) has shown promising results in an in vitro model of the complement disease paroxysmal nocturnal hemoglobinuria (Kuhn et al., 2016) and a porcine model of myocardial infarction (Pischke et al., 2017). Additional uses for salivary gland proteins include treatment of microbial infections (Cabezas-Cruz et al., 2016; Abraham et al., 2017), autoimmune disease (Sá-Nunes et al., 2009; Soltys et al., 2009), and cardiovascular diseases (Abendschein et al., 2001).
Recently, tick—tick microbiome—pathogen interactions have begun to be studied to understand the implications of the tick microbiome in pathogen transmission. Indeed, perturbing the Ixodes scapularis tick microbiome decreases transmission of B. burgdorferi (Narasimhan et al., 2014) and increases transmission of A. phagocytophilum (Abraham et al., 2017). Study of such interactions can lead to the discovery of novel mechanisms of interaction and potential therapeutics. For example, further work into A. phagocytophilum- microbiota interactions determined that A. phagocytophilum modulates the tick microbiome during colonization of I. scapularis, which facilitates its migration from the tick gut to the salivary glands (Abraham et al., 2017). This occurs through the bacterium inducing expression of the tick gut protein I. scapularis antifreeze glycoprotein (IAFGP) (Neelakanta et al., 2010; Abraham et al., 2017), which decreases microbiota biofilms in the tick gut (Abraham et al., 2017). The antibiofilm activity of IAFGP makes it a promising candidate for the treatment of antimicrobial-resistant bacterial pathogens that form biofilms. Indeed, IAFGP expression in flies and mice increases their resistance to bacterial pathogens, such as Staphylococcus aureus (Heisig et al., 2014). Additionally, testing in a catheter model demonstrated that IAFGP coatings can inhibit bacterial biofilm formation on medical devices (Heisig et al., 2014). These studies on IAFGP function and potential highlight that other interactions within the tick, such as those between the ticks, pathogens, and microbiomes, are another rich source of bioactive molecules.
“Omics” Studies for the Discovery of Bioactive Molecules
The advent of “omics” technologies, including transcriptomics, proteomics, and genomics, has opened the door for the discovery of new microbial consortium members, host-microbe interactions, and bioactive molecules. Such studies have led to the discovery of many new promising therapeutic candidates, such as animal venom peptides from mollusks (Verdes et al., 2016) and antibiotics from bacteria (Wecke and Mascher, 2011).
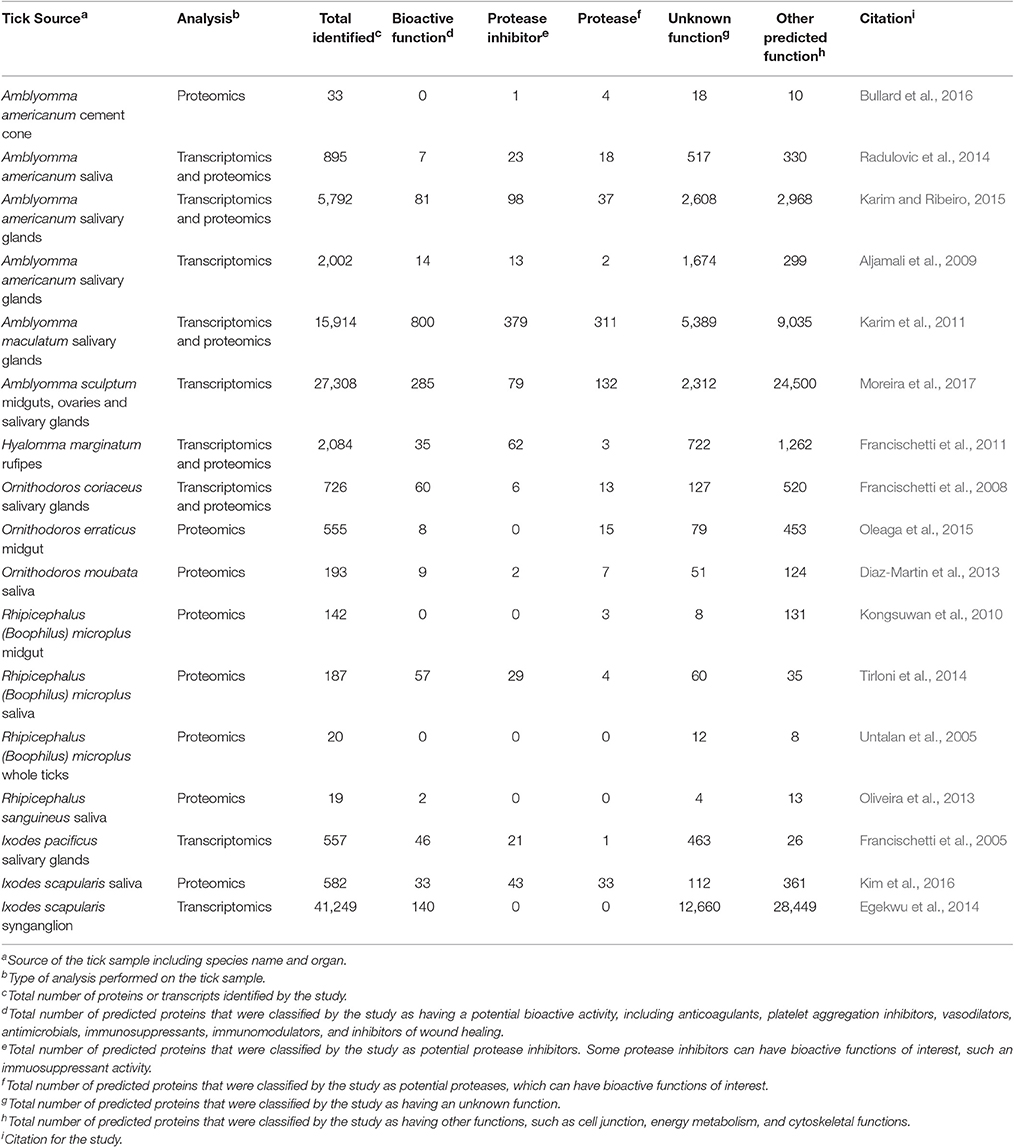
The use of proteomic and transcriptomic analyses has uncovered many novel tick-microbe interactions. Additionally, these studies have yielded a multitude of predicted tick bioactive molecules, such as anticoagulants, platelet aggregation inhibitors, vasodilators, antimicrobials, immunosuppressants, immunomodulators, and inhibitors of wound healing (Table 1; Francischetti et al., 2005, 2008, 2011; Untalan et al., 2005; Aljamali et al., 2009; Kongsuwan et al., 2010; Karim et al., 2011; Diaz-Martin et al., 2013; Oliveira et al., 2013; Egekwu et al., 2014; Radulovic et al., 2014; Tirloni et al., 2014; Karim and Ribeiro, 2015; Oleaga et al., 2015; Bullard et al., 2016; Kim et al., 2016; Moreira et al., 2017). These studies have also identified new classes of protein families as well as many proteins of unknown function (Table 1; Francischetti et al., 2005, 2008, 2011; Untalan et al., 2005; Aljamali et al., 2009; Kongsuwan et al., 2010; Karim et al., 2011; Diaz-Martin et al., 2013; Oliveira et al., 2013; Egekwu et al., 2014; Radulovic et al., 2014; Tirloni et al., 2014; Karim and Ribeiro, 2015; Oleaga et al., 2015; Bullard et al., 2016; Kim et al., 2016; Moreira et al., 2017). The vast majority of these bioactive proteins have not been studied in detail, and it is likely that many may be homologs or overlap in function. Therefore, the actual number of discovered bioactive proteins with divergent mechanisms of action is likely less than the total of these studies. However, these studies highlight that there is a vast array of potential bioactive molecules within tick-microbe interactions awaiting further study.
Development of Bioactive Molecules Into Therapeutics
Although “omics” studies have identified a plethora of potential therapeutics, these studies have not led to FDA approval of any novel drugs. In fact, at the time of this publication, no arthropod compound identified by proteomics, transcriptomics, or genomics is in clinical trials in the United States. As mentioned above, this is partially due to lack of follow-up studies on the mechanisms, uses, and optimization of the drug candidates. However, this is likely also due to issues specific to arthropod compounds.
Arthropod compounds often have high cytotoxicity and/or are unstable (Ratcliffe et al., 2014). Therefore, the development of some compounds will require basic research into optimization of the compound, dosage, synthesis methods, and delivery mechanism. For example, Cantharidin, a small molecule toxin from beetles in the Meloidae family, has potent anti-cancer activities and has been shown to be effective against a large variety of cancers (Reviewed in, Deng et al., 2013; Puerto Galvis et al., 2013). However, this compound also has significant toxicity in mammals related to its anticancer activity (Deng et al., 2013; Puerto Galvis et al., 2013; Ratcliffe et al., 2014). Extensive studies have been undertaken to reduce this toxicity through modification of the compound (Deng et al., 2013; Puerto Galvis et al., 2013), alternative production and delivery methods (Chang et al., 2008; Han et al., 2013; Yu and Zhao, 2016), or combination therapies (Wu et al., 2015). These efforts highlight that the resolution of issues, such as toxicity, will require the investment of time and money into basic scientific research for the development process.
Additionally, there are concerns with developing individual compounds from a complex mixture, such as tick saliva. Tick saliva contains a cocktail of potent proteins, and the production of these proteins changes throughout tick feeding (Kim et al., 2016). This suggests that saliva proteins may work synergistically within the context of tick feeding for differing functions or similar functions (e.g., various immunosuppressants could work in concert for greater immunosuppression) at specific time points. Additionally, it is possible that separately encoded proteins or subunits may be necessary for proper function. Therefore, studying individual genes or proteins may miss potential therapeutics. In these cases, it would be necessary to consider co-expression of proteins and/or identify interacting partners within the tick saliva to capture the optimal combinations.
It is worth noting that is some instances the lack of progress toward a viable therapeutic candidate is due to the high cost of drug development rather than a lack of follow-up research. For these compounds, investing in the approval process is not attractive for pharmaceutical companies (Shlaes et al., 2004; Kinter and DeGeorge, 2016). This is the case for many antimicrobials, such as arthropod-derived antimicrobial peptides that target bacterial and fungal pathogens (Ratcliffe et al., 2011).
Conclusions
Tick-derived bioactive molecules are a promising source of new therapeutics. However, the discovery and development of such compounds is in its infancy. Although some drug candidates have shown promising pre-clinical results, these compounds could fall into the so-called “Valley of Death,” the gap between basic research and translation into treatments. For some therapeutics, this is due to the broad issues common to potential therapeutics: lack of funding for translational research and/or lack of viable pathways for clinical development (Butler, 2008; Collins et al., 2016). However, as discussed in this article, this can also be due to a lack of basic research assessing biological function, potential uses, or optimization of the compound. For tick bioactive compounds to be successfully developed into therapeutics, it will require the investment of basic researchers into the discovery and approval of therapeutic candidates.
Author Contributions
KEM and EF contributed to the writing and editing of the manuscript.
Funding
KEM was supported by a James Hudson Brown-Alexander Brown Coxe Fellowship from Yale University. This work was supported in part by a gift from the John Monsky and Jennifer Weis Monsky Lyme Disease Research Fund. EF is an investigator supported by the Howard Hughes Medical Institute.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abendschein, D. R., Baum, P. K., Verhallen, P., Eisenberg, P. R., Sullivan, M. E., and Light, D. R. (2001). A novel synthetic inhibitor of factor Xa decreases early reocclusion and improves 24-h patency after coronary fibrinolysis in dogs. J. Pharmacol. Exp. Ther. 296, 567–572.
Abraham, N. M., Liu, L., Jutras, B. L., Yadav, A. K., Narasimhan, S., Gopalakrishnan, V., et al. (2017). Pathogen-mediated manipulation of arthropod microbiota to promote infection. Proc. Natl. Acad. Sci. U.S.A. 114, E781–E790. doi: 10.1073/pnas.1613422114
Aljamali, M. N., Hern, L., Kupfer, D., Downard, S., So, S., Roe, B. A., et al. (2009). Transcriptome analysis of the salivary glands of the female tick Amblyomma americanum (Acari: Ixodidae). Insect Mol. Biol. 18, 129–154. doi: 10.1111/j.1365-2583.2009.00863.x
Baines, A. C., and Brodsky, R. A. (2017). Complementopathies. Blood Rev. doi: 10.1016/j.blre.2017.02.003. [Epub ahead of print].
Barboza, T., Gomes, T., Mizurini, D. M., Monteiro, R. Q., Konig, S., Francischetti, I. M., et al. (2015). (99m)Tc-ixolaris targets glioblastoma-associated tissue factor: in vitro and pre-clinical applications. Thromb. Res. 136, 432–439. doi: 10.1016/j.thromres.2015.05.032
Bullard, R., Allen, P., Chao, C. C., Douglas, J., Das, P., Morgan, S. E., et al. (2016). Structural characterization of tick cement cones collected from in vivo and artificial membrane blood-fed Lone Star ticks (Amblyomma americanum). Ticks Tick Borne Dis. 7, 880–892. doi: 10.1016/j.ttbdis.2016.04.006
Butler, D. (2008). Translational research: crossing the valley of death. Nature 453, 840–842. doi: 10.1038/453840a
Cabezas-Cruz, A., Tonk, M., Bouchut, A., Pierrot, C., Pierce, R. J., Kotsyfakis, M., et al. (2016). Antiplasmodial activity is an ancient and conserved feature of tick defensins. Front. Microbiol. 7:1682. doi: 10.3389/fmicb.2016.01682
Carneiro-Lobo, T. C., Konig, S., Machado, D. E., Nasciutti, L. E., Forni, M. F., Francischetti, I. M., et al. (2009). Ixolaris, a tissue factor inhibitor, blocks primary tumor growth and angiogenesis in a glioblastoma model. J. Thromb. Haemost. 7, 1855–1864. doi: 10.1111/j.1538-7836.2009.03553.x
Chang, C. C., Liu, D. Z., Lin, S. Y., Liang, H. J., Hou, W. C., Huang, W. J., et al. (2008). Liposome encapsulation reduces cantharidin toxicity. Food Chem. Toxicol. 46, 3116–3121. doi: 10.1016/j.fct.2008.06.084
Chudzinski-Tavassi, A. M., De-Sa-Junior, P. L., Simons, S. M., Maria, D. A., de Souza Ventura, J., Batista, I. F., et al. (2010). A new tick Kunitz type inhibitor, Amblyomin-X, induces tumor cell death by modulating genes related to the cell cycle and targeting the ubiquitin-proteasome system. Toxicon 56, 1145–1154. doi: 10.1016/j.toxicon.2010.04.019
Collins, J. M., Reizes, O., and Dempsey, M. K. (2016). Healthcare commercialization programs: improving the efficiency of translating healthcare innovations from academia into practice. IEEE J. Transl. Eng. Health Med. 4:3500107. doi: 10.1109/JTEHM.2016.2609915
Contreras, M., and de la Fuente, J. (2016). Control of Ixodes ricinus and Dermacentor reticulatus tick infestations in rabbits vaccinated with the Q38 Subolesin/Akirin chimera. Vaccine 34, 3010–3013. doi: 10.1016/j.vaccine.2016.04.092
Contreras, M., and de la Fuente, J. (2017). Control of infestations by Ixodes ricinus tick larvae in rabbits vaccinated with aquaporin recombinant antigens. Vaccine 35, 1323–1328. doi: 10.1016/j.vaccine.2017.01.052
Coumou, J., Narasimhan, S., Trentelman, J. J., Wagemakers, A., Koetsveld, J., Ersoz, J. I., et al. (2016). Ixodes scapularis dystroglycan-like protein promotes Borrelia burgdorferi migration from the gut. J. Mol. Med. 94, 361–370. doi: 10.1007/s00109-015-1365-0
Dai, J., Wang, P., Adusumilli, S., Booth, C. J., Narasimhan, S., Anguita, J., et al. (2009). Antibodies against a tick protein, Salp15, protect mice from the Lyme disease agent. Cell Host Microbe 6, 482–492. doi: 10.1016/j.chom.2009.10.006
de la Fuente, J., Kopacek, P., Lew-Tabor, A., and Maritz-Olivier, C. (2016). Strategies for new and improved vaccines against ticks and tick-borne diseases. Parasite Immunol. 38, 754–769. doi: 10.1111/pim.12339
Deng, L., Dong, J., and Wang, W. (2013). Exploiting protein phosphatase inhibitors based on cantharidin analogues for cancer drug discovery. Mini Rev. Med. Chem. 13, 1166–1176. doi: 10.2174/1389557511313080005
de Oliveira Ada, S., Lima, L. G., Mariano-Oliveira, A., Machado, D. E., Nasciutti, L. E., Andersen, J. F., et al. (2012). Inhibition of tissue factor by ixolaris reduces primary tumor growth and experimental metastasis in a murine model of melanoma. Thromb. Res. 130, e163–170. doi: 10.1016/j.thromres.2012.05.021
De Silva, A. M., and Fikrig, E. (1995). Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am. J. Trop. Med. Hyg. 53, 397–404.
de Souza, J. G., Morais, K. L., Angles-Cano, E., Boufleur, P., de Mello, E. S., Maria, D. A., et al. (2016). Promising pharmacological profile of a Kunitz-type inhibitor in murine renal cell carcinoma model. Oncotarget 7, 62255–62266. doi: 10.18632/oncotarget.11555
Diaz-Martin, V., Manzano-Roman, R., Valero, L., Oleaga, A., Encinas-Grandes, A., and Perez-Sanchez, R. (2013). An insight into the proteome of the saliva of the argasid tick Ornithodoros moubata reveals important differences in saliva protein composition between the sexes. J. Proteomics 80, 216–235. doi: 10.1016/j.jprot.2013.01.015
Egekwu, N., Sonenshine, D. E., Bissinger, B. W., and Roe, R. M. (2014). Transcriptome of the female synganglion of the black-legged tick Ixodes scapularis (Acari: Ixodidae) with comparison between Illumina and 454 systems. PLoS ONE 9:e102667. doi: 10.1371/journal.pone.0102667
Francischetti, I. M., Anderson, J. M., Manoukis, N., Pham, V. M., and Ribeiro, J. M. (2011). An insight into the sialotranscriptome and proteome of the coarse bontlegged tick, Hyalomma marginatum rufipes. J. Proteomics 74, 2892–2908. doi: 10.1016/j.jprot.2011.07.015
Francischetti, I. M., Meng, Z., Mans, B. J., Gudderra, N., Hall, M., Veenstra, T. D., et al. (2008). An insight into the salivary transcriptome and proteome of the soft tick and vector of epizootic bovine abortion, Ornithodoros coriaceus. J. Proteomics 71, 493–512. doi: 10.1016/j.jprot.2008.07.006
Francischetti, I. M., My Pham, V., Mans, B. J., Andersen, J. F., Mather, T. N., Lane, R. S., et al. (2005). The transcriptome of the salivary glands of the female western black-legged tick Ixodes pacificus (Acari: Ixodidae). Insect Biochem. Mol. Biol. 35, 1142–1161. doi: 10.1016/j.ibmb.2005.05.007
Gomes, H., Moraes, J., Githaka, N., Martins, R., Isezaki, M., Vaz Ida, S. Jr., et al. (2015). Vaccination with cyclin-dependent kinase tick antigen confers protection against Ixodes infestation. Vet. Parasitol. 211, 266–273. doi: 10.1016/j.vetpar.2015.05.022
Han, W., Wang, S., Liang, R., Wang, L., Chen, M., Li, H., et al. (2013). Non-ionic surfactant vesicles simultaneously enhance antitumor activity and reduce the toxicity of cantharidin. Int. J. Nanomedicine 8, 2187–2196. doi: 10.2147/IJN.S43568
Heisig, M., Abraham, N. M., Liu, L., Neelakanta, G., Mattessich, S., Sultana, H., et al. (2014). Antivirulence properties of an antifreeze protein. Cell Rep. 9, 417–424. doi: 10.1016/j.celrep.2014.09.034
Hodzic, E., Fish, D., Maretzki, C. M., De Silva, A. M., Feng, S., and Barthold, S. W. (1998). Acquisition and transmission of the agent of human granulocytic ehrlichiosis by Ixodes scapularis ticks. J. Clin. Microbiol. 36, 3574–3578.
Karakatsanis, S. J., Roumpi, A., and Syrigos, K. N. (2016). The use of novel oral anticoagulants in cancer patients with venous thromboembolism. Semin. Oncol. 43, 655–665. doi: 10.1053/j.seminoncol.2016.11.009
Karim, S., and Ribeiro, J. M. (2015). An Insight into the Sialome of the Lone Star Tick, Amblyomma americanum, with a glimpse on its time dependent gene expression. PLoS ONE 10:e0131292. doi: 10.1371/journal.pone.0131292
Karim, S., Singh, P., and Ribeiro, J. M. (2011). A deep insight into the sialotranscriptome of the gulf coast tick, Amblyomma maculatum. PLoS ONE 6:e28525. doi: 10.1371/journal.pone.0028525
Kazimírová, M., and Štibrániová, I. (2013). Tick salivary compounds: their role in modulation of host defences and pathogen transmission. Front. Cell. Infect. Microbiol. 3:43. doi: 10.3389/fcimb.2013.00043
Kim, T. K., Tirloni, L., Pinto, A. F., Moresco, J., Yates, J. R. III., da Silva Vaz, I. Jr., et al. (2016). Ixodes scapularis tick saliva proteins sequentially secreted every 24 h during blood feeding. PLoS Negl. Trop. Dis. 10:e0004323. doi: 10.1371/journal.pntd.0004323
Kinter, L. B., and DeGeorge, J. J. (2016). Scientific knowledge and technology, animal experimentation, and pharmaceutical development. ILAR J. 57, 101–108. doi: 10.1093/ilar/ilw027
Kongsuwan, K., Josh, P., Zhu, Y., Pearson, R., Gough, J., and Colgrave, M. L. (2010). Exploring the midgut proteome of partially fed female cattle tick (Rhipicephalus (Boophilus) microplus). J. Insect Physiol. 56, 212–226. doi: 10.1016/j.jinsphys.2009.10.003
Kuhn, N., Schmidt, C. Q., Schlapschy, M., and Skerra, A. (2016). PASylated coversin, a C5-Specific complement inhibitor with extended pharmacokinetics, shows enhanced anti-hemolytic activity in vitro. Bioconjug. Chem. 27, 2359–2371. doi: 10.1021/acs.bioconjchem.6b00369
Moreira, H. N., Barcelos, R. M., Vidigal, P. M., Klein, R. C., Montandon, C. E., Maciel, T. E., et al. (2017). A deep insight into the whole transcriptome of midguts, ovaries and salivary glands of the Amblyomma sculptum tick. Parasitol. Int. 66, 64–73. doi: 10.1016/j.parint.2016.10.011
Narasimhan, S., Rajeevan, N., Liu, L., Zhao, Y. O., Heisig, J., Pan, J., et al. (2014). Gut microbiota of the tick vector Ixodes scapularis modulate colonization of the Lyme disease spirochete. Cell Host Microbe 15, 58–71. doi: 10.1016/j.chom.2013.12.001
Neelakanta, G., Sultana, H., Fish, D., Anderson, J. F., and Fikrig, E. (2010). Anaplasma phagocytophilum induces Ixodes scapularis ticks to express an antifreeze glycoprotein gene that enhances their survival in the cold. J. Clin. Invest. 120, 3179–3190. doi: 10.1172/JCI42868
Oleaga, A., Obolo-Mvoulouga, P., Manzano-Roman, R., and Perez-Sanchez, R. (2015). Midgut proteome of an argasid tick, Ornithodoros erraticus: a comparison between unfed and engorged females. Parasit. Vectors 8:525. doi: 10.1186/s13071-015-1148-z
Oliveira, C. J., Anatriello, E., de Miranda-Santos, I. K., Francischetti, I. M., Sa-Nunes, A., Ferreira, B. R., et al. (2013). Proteome of Rhipicephalus sanguineus tick saliva induced by the secretagogues pilocarpine and dopamine. Ticks Tick Borne Dis. 4, 469–477. doi: 10.1016/j.ttbdis.2013.05.001
Pischke, S. E., Gustavsen, A., Orrem, H. L., Egge, K. H., Courivaud, F., Fontenelle, H., et al. (2017). Complement factor 5 blockade reduces porcine myocardial infarction size and improves immediate cardiac function. Basic Res. Cardiol. 112:20. doi: 10.1007/s00395-017-0610-9
Puerto Galvis, C. E., Vargas Mendez, L. Y., and Kouznetsov, V. V. (2013). Cantharidin-based small molecules as potential therapeutic agents. Chem. Biol. Drug Des. 82, 477–499. doi: 10.1111/cbdd.12180
Radulovic, Z. M., Kim, T. K., Porter, L. M., Sze, S. H., Lewis, L., and Mulenga, A. (2014). A 24-48 h fed Amblyomma americanum tick saliva immuno-proteome. BMC Genomics 15:518. doi: 10.1186/1471-2164-15-518
Ramamoorthi, N., Narasimhan, S., Pal, U., Bao, F., Yang, X. F., Fish, D., et al. (2005). The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 436, 573–577. doi: 10.1038/nature03812
Ratcliffe, N. A., Mello, C. B., Garcia, E. S., Butt, T. M., and Azambuja, P. (2011). Insect natural products and processes: new treatments for human disease. Insect Biochem. Mol. Biol. 41, 747–769. doi: 10.1016/j.ibmb.2011.05.007
Ratcliffe, N., Azambuja, P., and Mello, C. B. (2014). Recent advances in developing insect natural products as potential modern day medicines. Evid. Based Complement. Alternat. Med. 2014:904958. doi: 10.1155/2014/904958
Rickles, F. R., Shoji, M., and Abe, K. (2001). The role of the hemostatic system in tumor growth, metastasis, and angiogenesis: tissue factor is a bifunctional molecule capable of inducing both fibrin deposition and angiogenesis in cancer. Int. J. Hematol. 73, 145–150. doi: 10.1007/BF02981930
Sá-Nunes, A., Bafica, A., Antonelli, L. R., Choi, E. Y., Francischetti, I. M., Andersen, J. F., et al. (2009). The immunomodulatory action of sialostatin L on dendritic cells reveals its potential to interfere with autoimmunity. J. Immunol. 182, 7422–7429. doi: 10.4049/jimmunol.0900075
Schuijt, T. J., Narasimhan, S., Daffre, S., DePonte, K., Hovius, J. W., Van't Veer, C., et al. (2011). Identification and characterization of Ixodes scapularis antigens that elicit tick immunity using yeast surface display. PLoS ONE 6:e15926. doi: 10.1371/journal.pone.0015926
Shields, K. J., Mollnes, T. E., Eidet, J. R., Mikkelsen, K., Almdahl, S. M., Bottazzi, B., et al. (2017). Plasma complement and vascular complement deposition in patients with coronary artery disease with and without inflammatory rheumatic diseases. PLoS ONE 12:e0174577. doi: 10.1371/journal.pone.0174577
Shlaes, D. M., Projan, S. J., and Edwards, J. E. Jr. (2004). Antibiotic discovery: state of the state. ASM News 70, 275–281.
Soltys, J., Kusner, L. L., Young, A., Richmonds, C., Hatala, D., Gong, B., et al. (2009). Novel complement inhibitor limits severity of experimentally myasthenia gravis. Ann. Neurol. 65, 67–75. doi: 10.1002/ana.21536
Tirloni, L., Reck, J., Terra, R. M., Martins, J. R., Mulenga, A., Sherman, N. E., et al. (2014). Proteomic analysis of cattle tick Rhipicephalus (Boophilus) microplus saliva: a comparison between partially and fully engorged females. PLoS ONE 9:e94831. doi: 10.1371/journal.pone.0094831
Untalan, P. M., Guerrero, F. D., Haines, L. R., and Pearson, T. W. (2005). Proteome analysis of abundantly expressed proteins from unfed larvae of the cattle tick, Boophilus microplus. Insect Biochem. Mol. Biol. 35, 141–151. doi: 10.1016/j.ibmb.2004.10.009
Verdes, A., Anand, P., Gorson, J., Jannetti, S., Kelly, P., Leffler, A., et al. (2016). From mollusks to medicine: a venomics approach for the discovery and characterization of therapeutics from terebridae peptide toxins. Toxins (Basel). 8:117. doi: 10.3390/toxins8040117
Wecke, T., and Mascher, T. (2011). Antibiotic research in the age of omics: from expression profiles to interspecies communication. J. Antimicrob. Chemother. 66, 2689–2704. doi: 10.1093/jac/dkr373
Wu, W., Su, M., Li, T., Wu, K., Wu, X., and Tang, Z. (2015). Cantharidin-induced liver injuries in mice and the protective effect of vitamin C supplementation. Int. Immunopharmacol. 28, 182–187. doi: 10.1016/j.intimp.2015.06.003
Yu, M., and Zhao, Y. (2016). Cantharis by photosynthetic bacteria biotransformation: reduced toxicity and improved antitumor efficacy. J. Ethnopharmacol. 186, 151–158. doi: 10.1016/j.jep.2016.03.058
Keywords: tick, pathogen, microbiota, vector-borne disease, bioactive molecules, therapeutics
Citation: Murfin KE and Fikrig E (2017) Tick Bioactive Molecules as Novel Therapeutics: Beyond Vaccine Targets. Front. Cell. Infect. Microbiol. 7:222. doi: 10.3389/fcimb.2017.00222
Received: 04 March 2017; Accepted: 15 May 2017;
Published: 06 June 2017.
Edited by:
Sarah Irène Bonnet, Institut National de la Recherche Agronomique (INRA), FranceReviewed by:
Patricia Anne Nuttall, University of Oxford, United KingdomJose Ribeiro, National Institute of Allergy and Infectious Diseases, United States
Copyright © 2017 Murfin and Fikrig. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erol Fikrig, ZXJvbC5maWtyaWdAeWFsZS5lZHU=
 Kristen E. Murfin
Kristen E. Murfin Erol Fikrig
Erol Fikrig