- 1Department of Biomedical Sciences, State University of New York at Albany, Albany, NY, United States
- 2Division of Infectious Diseases, New York State Department of Health, Wadsworth Center, Albany, NY, United States
- 3Institute of Medical Microbiology and Infection Control, University Hospital, Goethe University Frankfurt, Frankfurt, Germany
Lyme disease (LD), which is caused by genospecies of the Borrelia burgdorferi sensu lato complex, is the most common vector-borne disease in the Northern hemisphere. Spirochetes are transmitted by Ixodes ticks and maintained in diverse vertebrate animal hosts. Following tick bite, spirochetes initially establish a localized infection in the skin. However, they may also disseminate hematogenously to several distal sites, including heart, joints, or the CNS. Because they need to survive in diverse microenvironments, from tick vector to mammalian hosts, spirochetes have developed multiple strategies to combat the numerous host defense mechanisms. One of these strategies includes the production of a number of complement-regulator acquiring surface proteins (CRASPs) which encompass CspA, CspZ, and OspE paralogs to blunt the complement pathway. These proteins are capable of preventing complement activation on the spirochete surface by binding to complement regulator Factor H. The genes encoding these CRASPs differ in their expression patterns during the tick-to-host infection cycle, implying that these proteins may exhibit different functions during infection. This review summarizes the recent published reports which investigated the roles that each of these molecules plays in conferring tick-borne transmission and dissemination in vertebrate hosts. These findings offer novel mechanistic insights into LD pathobiology and may facilitate the identification of new targets for preventive strategies against Lyme borreliosis.
Lyme Disease Spirochetes Evade the Vertebrate Hosts' Complement
Lyme disease (LD) is the most common vector-borne disease in the northern hemisphere (Steere et al., 2016). A recent report from the CDC categorizes LD as one of the zoonotic diseases of the greatest concern in the United States. The disease is caused by spirochetes of the Borrelia burgdorferi sensu lato complex (Rosa et al., 2005; Brisson et al., 2012; Radolf et al., 2012). Among the ~20 Borrelia species that comprise the sensu lato complex, at least six have been confirmed to cause LD in humans including Borrelia (B.) burgdorferi sensu stricto (hereafter referred as B. burgdorferi), B. afzelii, B. garinii, B. spielmanii, B. bavariensis, and B. mayonii, all of which are transmitted by Ixodes ticks and maintained in diverse reservoir hosts (mainly small mammals and birds) (Tufts et al., 2019). Upon tick feeding, spirochetes are exposed to host blood and the first line of innate immunity which they must overcome to survive (Hovius et al., 2007; Steere et al., 2016; Figure 1). Spirochetes then migrate through the tick midgut epithelium and the salivary glands and are transmitted to the host skin to establish the infection (Hovius et al., 2007; Steere et al., 2016; Figure 1). In untreated humans, the spirochetes may disseminate hematogenously to distal tissues and organs (Coburn et al., 2013; Hyde, 2017; Bernard et al., 2019; Figure 1).
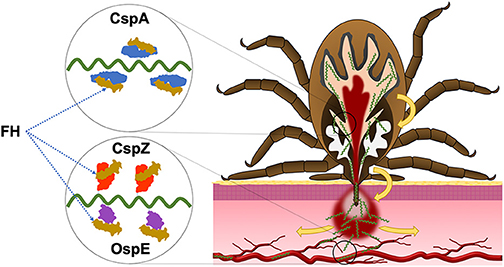
Figure 1. The roles of CRASP proteins in the enzootic cycle of LD spirochetes. During the infection, LD spirochetes require the ability to evade the complement in the vertebrate blood. CspA facilitates spirochete survival in the blood meal of fed ticks and thereby enabling spirochetes to be transmitted to the host. CspZ promotes spirochete survival in the bloodstream of vertebrate animals, allowing in dissemination to distal tissues. While the role that OspE paralogs (OspE) play in enzootic cycle remain unclear, the current evidence supports that these proteins confer spirochete dissemination in the vertebrate animals.
Complement is a central component of the host innate immune system and the first line of defense against bacterial infection. Evasion of the host complement system is essential for Borrelia to successfully establish infection (Caine and Coburn, 2016; Kraiczy, 2016; Marcinkiewicz et al., 2017) (see Sjoberg et al., 2009; Zipfel and Skerka, 2009; Meri, 2016 for more thorough reviews). The complement system is composed of more than 30 proteins and inactive precursors (Zipfel and Skerka, 2009). Activation of complement cascades on the microbial surface is initiated via three distinct pathways (Meri, 2016). Antibody-antigen complexes trigger activation of the classical pathway (CP) whereas the mannose-binding lectin pathway (LP) is activated by recognition of carbohydrate complexes (collectins and ficolins) on microbial surfaces. The alternative pathway (AP) is activated when C3b is bound to the surface of invading microbes. Activation of all three pathways leads to the formation and deposition of C3 and C5 convertases on the microbial surface. This results in the insertion of the pore-forming membrane attack complex (MAC), leading to bacterial cell lysis.
In the absence of invading microbes or cell/tissue damage, vertebrate hosts produce complement regulatory proteins (CRPs) which are deposited on host cells/tissues to avoid non-specific damage by the complement cascade (Sjoberg et al., 2009; Zipfel and Skerka, 2009; Meri, 2016). Factor H (FH) is a CRP that binds to C3b by recruiting the serum protease, factor I. This complex leads to the degradation of C3b and coincidently terminates activation of AP (Zipfel and Skerka, 2009; Zipfel et al., 2013).
LD spirochetes produce several outer surface proteins that facilitate host complement evasion (de Taeye et al., 2013; Caine and Coburn, 2016; Kraiczy, 2016; Marcinkiewicz et al., 2017). These proteins include five complement-regulator acquiring surface proteins (BbCRASPs or CRASPs) (Kraiczy and Stevenson, 2013): CspA (CRASP-1, BBA68), CspZ (CRASP-2, BBH06), and OspE paralogs [i.e., ErpP (CRASP-3, BBN38), ErpC (CRASP-4), and ErpA/I/N (CRASP-5, BBP38, BBL39)] (Table 1). While all these proteins bind to FH to inactivate human complement, CspA and CspZ also bind to FH-like protein 1 (FHL-1), the truncated form of FH (Zipfel and Skerka, 1999; Kraiczy and Stevenson, 2013). Additionally, ErpP, ErpC, and ErpA bind to different FH-related proteins (CFHR), a family of CRPs with similar sequence identity and high-resolution structures to that of FH (Zipfel et al., 2002; Kraiczy and Stevenson, 2013). The expression of the genes encoding these outer surface proteins varies at different stages of the infection cycle, e.g., during spirochete transmission and dissemination (Miller et al., 2003; von Lackum et al., 2005; Bykowski et al., 2007; Brissette et al., 2008). These findings suggest that CRASPs play distinct roles in facilitating spirochete survival in ticks and/or vertebrate hosts. However, until recently, the role of these CRASPs in the spirochete infection cycle in vertebrate hosts is still unclear.
In this review, we summarize previous findings regarding the role of CRASPs in the pathobiology and provide mechanistic insights into transmission and dissemination of LD spirochetes in ticks and different vertebrate animals.
CspA Facilitates Spirochete Survival in Ticks' Blood Meal and During Transmission From Ticks To Hosts
During feeding, ticks are vulnerable to the attack by complement present in the blood meal. To neutralize complement and other dangerous constituents, ticks generate a cocktail of diverse immunomodulatory proteins with immunosuppressive, anti-inflammatory, and anti-complement activity in their saliva (Tyson et al., 2007, 2008; Schuijt et al., 2008, 2011; Wagemakers et al., 2016) (see de Taeye et al., 2013 for the review). These proteins shield spirochetes from complement-mediated killing in the ticks' midgut. However, ticks devoid of any one of these anti-complement proteins can still transmit spirochetes to vertebrate animals (Schuijt et al., 2011; Wagemakers et al., 2016). Additionally, LD spirochetes survive at similar levels in the ticks feeding on wild-type or complement-deficient mice (Rathinavelu et al., 2003; Hart et al., 2018). These results suggest that spirochetes have developed additional means to evade complement when residing in fed ticks.
The cspA gene is located on a linear plasmid 54 (lp54) which is essential for LD spirochetes survival in the infection cycle (Purser and Norris, 2000; Table 1). This gene is uniquely expressed in spirochetes residing in ticks, suggesting that CspA plays a role during spirochetal colonization of ticks (von Lackum et al., 2005; Bykowski et al., 2007; Hart et al., 2018; Table 1). Ectopically producing CspA into a non-infectious, serum-sensitive, and cspA-deficient B. burgdorferi strain enables this strain to inactivate complement and survive when exposed to sera from various vertebrate animals in vitro (Kraiczy et al., 2004b; Brooks et al., 2005; Hammerschmidt et al., 2014; Muhleip et al., 2018; Table 1). Conversely, deleting cspA from a low passage and fully infectious B. burgdorferi strain results in the inability of this strain to survive in presence of serum from vertebrate animals and enhances complement activation on spirochete surface (Kenedy et al., 2009; Table 1). These results demonstrate the role of CspA in conferring spirochetal evasion from complement.
Moreover, a previous study demonstrates that CspA also confers protection when spirochetes are exposed to complement components in blood acquired during tick feeding. A recent study shows that a LD Borrelia strain deficient in cspA is eliminated in nymphs after the nymphs feed on wild-type mice (Hart et al., 2018). However, this strain survives in the nymphs feeding on complement deficient mice, indicating that CspA promotes spirochetal evasion of complement in ticks' blood meal (Hart et al., 2018). The CspA-mediated blood meal survival has been attributed to the ability of CspA to bind FH (Hart et al., 2018; Figure 1 and Table 1). CspA orthologs from different LD species differ in their ability to bind to FH from other vertebrate animals including birds, mice, and humans (Bhide et al., 2009; Hart et al., 2018; Muhleip et al., 2018). CspA of B. burgdorferi displays <50% of sequence identity compared to other LD Borrelia species but >95% identity on the intra-species level (von Lackum et al., 2005; Wywial et al., 2009). Further, the sequence variability of CspA orthologs correlates with their ability to interact with FH from humans and other hosts (von Lackum et al., 2005; Bhide et al., 2009; Hammerschmidt et al., 2014; Hart et al., 2018; Muhleip et al., 2018). Of note, one previous study showed that recombinant CspA from B. burgdorferi B31 does not bind to non-human FH in the sera applied on a Far-Western blot (McDowell et al., 2006). This result suggests that those non-human FH variants are required to be maintained as a native form in order to display their ability to bind to CspA. Consistent with the allelic differences in FH-binding activity of CspA, a cspA-deficient B. burgdorferi strain producing CspA from B. garinii was incapable of surviving in nymphs upon feeding on wild-type mice (Hart et al., 2018). That isogenic strains survived in nymphs feeding on the complement-deficient mice, similar to the isogenic strain producing CspA from B. burgdorferi strain B31 (Hart et al., 2018). These findings imply an allelic variation of CspA-mediated FH-binding activity. Such results also lead to an intriguing possibility that CspA determines spirochete host tropism by driving the transmission from ticks to specific hosts (Kurtenbach et al., 2002; Kraiczy, 2016; Tufts et al., 2019).
Recent investigations also revealed that CspA acts in multiple ways to inactivate complement. CspA was shown to inactivate the AP by binding to FH and FHL-1 as well as by binding to complement proteins C7 and C9 to block MAC formation (Hallstrom et al., 2013; Table 1). The presence of CspA on the bacterial surface prevents the formation of MAC, suggesting a FH-independent mechanism to confer complement evasion. However, compared to the high affinity binding to FH (KD < 100 nM), CspA binds only moderately to C7 and C9 (KD > 5 μM). These results raise questions regarding the physiological relevance of CspA-mediated C7- and C9-binding activity (Kraiczy et al., 2004a; Hallstrom et al., 2013; Hart et al., 2018).
The Role of CspZ in Promoting Spirochete Dissemination After Invading Vertebrate Hosts
A previous finding indicates that a B. burgdorferi strain deficient in cspA is capable of surviving at the inoculation site in skin at similar levels to the wild-type parental strain introduced by needle infection (Hart et al., 2018). This suggests that additional proteins confer this phenotype and/or work collaboratively with CspA to facilitate the establishment of infection. In fact, CspZ has been identified as an additional FH/FHL-1-binding protein which is encoded on the linear plasmid 28-3 (lp28-3) of B. burgdorferi B31 (Table 1). During tick-to-host transmission, the expression of cspZ is undetectable when spirochetes reside in ticks, but up-regulated when spirochetes reach the bite site in host skin (Bykowski et al., 2007). Further investigation reveals that cspZ is expressed throughout different infection stages in vertebrate animals (Bykowski et al., 2007; Marcinkiewicz et al., 2019), suggesting that the expression of CspZ and its role in the infection are restricted to the host (Table 1). Similar to CspA, introduction of CspZ into a cspZ-deficient, serum-sensitive borrelial strain allows the transformed strains to survive in vitro in presence of serum from various vertebrate animals by preventing complement activation (Hartmann et al., 2006; Siegel et al., 2008; Table 1). However, a cspZ-deficient strain in the infectious background of B. burgdorferi also survived in sera and colonized mouse tissues at similar levels as the parental strain (Coleman et al., 2008; Marcinkiewicz et al., 2019; Table 1). These findings support the following notions that such indistinguishable phenotypes could be attributed to low expression levels of cspZ in B. burgdorferi (Bykowski et al., 2007; Rogers and Marconi, 2007; Marcinkiewicz et al., 2019). As LD spirochetes produce additional complement interacting proteins that confer evasion during dissemination, delineating CspZ's phenotype can be cumbersome (Kraiczy et al., 2003, 2004a; Alitalo et al., 2004, 2005; Pietikainen et al., 2010; Bhattacharjee et al., 2013; Garcia et al., 2016; Caine et al., 2017).
To amplify the phenotype conferred by these genes, vertebrate blood has been used to cultivate spirochetes as cue to mimic in vivo conditions, possibly due to host-specific nutrients and ions in blood (Tokarz et al., 2004). Several borrelial genes upregulated during transmission can be triggered in vitro by incubation of the spirochetes with host blood (Tokarz et al., 2004). These genes include cspZ. These findings are consistent with additional data showing that a cspZ-deficient strain in an infectious background of B. burgdorferi displays reduced ability to survive when incubated with vertebrate sera (Marcinkiewicz et al., 2019; Table 1). Furthermore, this cspZ mutant strain when pre-treated with blood shows a delayed onset of dissemination and lower burdens in distal tissues, compared to wild-type B. burgdorferi strain, demonstrating CspZ' role in promoting spirochete dissemination (Marcinkiewicz et al., 2019; Figure 1 and Table 1).
Further, several studies examined the role of CspZ (or the plasmid encoding cspZ) in infection cycle. CspZ was shown not essential for spirochetes acquisition from mammalian hosts to ticks (Coleman et al., 2008). However, fewer mice develop antibody reactivity against whole spirochete cell lysates after being fed on by the ticks carrying a B. burgdorferi strain missing lp28-3 plasmid which encodes cspZ, compared to wild-type parental spirochete strain (Dulebohn et al., 2013). These findings suggest that the proteins encoded by lp28-3 (e.g., CspZ) facilitate spirochete to establish an infection and disseminate to distal sites after tick bites. A previous study revealed that LD patients with manifestations (e.g., acrodermatitis, neuroborreliosis, erythema migran) and/or positivity in two-tier LD serological tests elicited antibodies to CspZ, indicating that spirochetes produce this protein during the infection process (Kraiczy et al., 2008; Rogers et al., 2009).
Rogers et al. observed that CspZ shows allelically different ability in binding to human FH (Rogers and Marconi, 2007; Rogers et al., 2009). As CspZ is highly conserved (nearly 98% identical among B. burgdorferi strains and ~70% identical among LD spirochetes), the difference of these variants may convey the observed strain-to-strain variation in binding activity to human FH (Rogers et al., 2009; Brangulis et al., 2014). Several sequence diverse regions in CspZ have been identified (Brangulis et al., 2014). According to a recently reported high-resolution co-crystal structure of CspZ-FH binding complex (Protein Data Bank #6ATG) some of these variable regions are located in the binding site/interface with human FH. These results support the possibility that these variable regions of CspZ mediate the different levels of FH-binding activity and spirochete survival in the infection cycle (Table 1).
The Role of OspE Paralogs in Spirochete Survival During the Infection Cycle Remains Unclear
Not every spirochete strain isolated from ticks feeding on LD spirochetes-infected vertebrate hosts encodes CspZ (Rogers and Marconi, 2007; Kraiczy et al., 2008), supporting that additional FH-binding proteins confer dissemination during infection. In fact, LD spirochetes produce multiple copies of OspE proteins, encoded by several circular plasmids 32 (cp32) (Marconi et al., 1996; Stevenson et al., 1996; Akins et al., 1999; Caimano et al., 2000; Kraiczy and Stevenson, 2013; Table 1). Most of these OspE paralogs bind to FH in vitro and share similar promoter sequences (as known as upstream homology box or “UHB”) to other outer surface proteins on cp32, such as OspF (Marconi et al., 1996; Akins et al., 1999; Caimano et al., 2000; Brissette et al., 2008). Because of these similarities, these OspE/F-related proteins were grouped under the term as Erps (Brissette et al., 2008).
Although some Erps have been shown to bind FH and confer complement evasion, their role in spirochete survival during the infection remains less clear. A serum-sensitive B. burgdorferi strain which expresses erpP or erpA (the genes encoding OspE paralogs in B. burgdorferi B31) driven by the endogenous promoters, remains susceptible to complement-mediated killing in human serum (Siegel et al., 2010; Hammerschmidt et al., 2012; Table 1). This result is consistent with other B. burgdorferi strains (i.e., the cspA-deficient strain) encoding erpP and erpA under the control by the endogenous promoters which remain susceptible to human serum. However, when those genes are expressed ectopically in a serum-sensitive B. burgdorferi strain using a strong and constitutive promoter, these spirochetes inactivate complement and survive when incubated with human serum (Kenedy and Akins, 2011; Table 1). These results imply that high expression levels of OspE are needed for complement inactivation and serum resistance.
The genes encoding OspE paralogs are not expressed when spirochetes are in post-molting flat nymphs whereas they are upregulated immediately after blood meals (Hefty et al., 2001; Miller et al., 2003). Additionally, the expression of ospE is maintained throughout different stages of infection after spirochete transmission from ticks to hosts (Hefty et al., 2001; Miller et al., 2003, 2005; Table 1). Consistent with the expression profiles of these ospE genes, spirochete burdens are reduced in nymphs feeding on mice passively immunized with anti-OspE IgG, but remain unaffected when feeding on mice inoculated with Ig isotype control (Nguyen et al., 1994). Further, the transposon-inserted erpA mutant in an infectious B. burgdorferi strain causes a 2-week delay in dissemination to distal tissues when co-infected with a library of other transposon-inserted mutants (Lin et al., 2012; Table 1). These findings suggest that OspE paralogs may play a role in conferring tick-to-host transmission of spirochetes as well as facilitating rapid dissemination to distal tissues (Figure 1). However, the off-target silencing by antibody-dependent deletion or transposon insertion methodologies may be the confounding effects of these results. Generating the deletion mutant of ospE paralogs could be the favorable approach to address this caveat, but multiple copies of OspE present in LD spirochetes could be cumbersome. Thus, the gain-of-function approach such as producing these OspE paralogs in a serum-sensitive strain and evaluating bloodstream survival during a short-term infection may be a suitable approach to address these technical hurdles (Caine and Coburn, 2015).
OspE paralogs among different strains have highly variable sequences (Marconi et al., 1996; Sung et al., 1998; Akins et al., 1999; Caimano et al., 2000; Stevenson and Miller, 2003; Brissette et al., 2008). These variants differ in their ability to bind to vertebrate animals' FH (Stevenson et al., 2002; McDowell et al., 2003; Hovis et al., 2006). These results imply potential roles of OspE paralogs in promoting LD spirochetes complement evasion in a host-specific manner. Besides FH, OspE also binds to different isotypes of CFHR (Zipfel et al., 2002; Siegel et al., 2010; Kraiczy and Stevenson, 2013; Skerka et al., 2013; Jozsi et al., 2015). However, the physiological importance of CFHR-binding activity of OspE proteins is unclear and warrants further investigation.
Conclusion
To survive their complex life cycle, LD spirochetes have developed several strategies to evade the host immune system that they encounter in ticks during feeding (blood meal) and in the bloodstream of vertebrate animals. A key evasion mechanism is to circumvent the complement components by producing complement- or CRP-binding proteins, including CRASPs, which facilitate complement inactivation. These CRASPs have been shown to confer spirochete transmission from ticks to hosts and promote infection and dissemination in vertebrate hosts. However, the concurrent production of CRASPs increases the complexity in delineating the contribution of these proteins individually in each of the stages within the infection cycle. Elucidating such mechanisms will provide new insights into how spirochetes survive in two distinct environments, ticks, and vertebrate hosts. Such information will provide foundation for the development of preventions through targeting CRASPs to block these infection mechanisms, which will ultimately reduce LD burdens in humans.
Author Contributions
Y-PL, AF, TN, and PK wrote the manuscript. TN and Y-PL prepared the figures.
Funding
This work was supported by NSF IOS1755286 (to Y-PL, AF, and TN), DoD TB170111 (to Y-PL, AF, and TN), NIAID 75N93019C00040 (to Y-PL, AF, and TN), New York State Department of Health Wadsworth Center Start-Up Grant (to Y-PL, AF, and TN), and NIH R01AI121401 (to PK).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Klemen Strle, Ashley Marcinkiewicz, and Thomas Hart for critical reading of the manuscript.
Abbreviations
CRASPs, Complement regulator acquiring surface proteins; OspE, OspE paralogs; CP, Classical Pathway; LP, Mannose-binding lectin pathway; AP, Alternative pathway; TP, Terminal pathway; MAC, Membrane attacking complex; CRPs, Complement regulatory proteins; FH, Factor H; BbCRASPs, Borrelia burgdorferi sensu lato complement regulator acquiring surface proteins; FHL-1, Factor H like protein 1; CFHR, Factor H related protein; lp54, Linear plasmid 54; lp28-3, Linear plasmid 28-3; cp32, Circular plasmid 32; UHB, Upstream homology box; LD, Lyme diseases.
References
Akins, D. R., Caimano, M. J., Yang, X., Cerna, F., Norgard, M. V., and Radolf, J. D. (1999). Molecular and evolutionary analysis of Borrelia burgdorferi 297 circular plasmid-encoded lipoproteins with OspE- and OspF-like leader peptides. Infect. Immun. 67, 1526–1532. doi: 10.1128/IAI.67.3.1526-1532.1999
Alitalo, A., Meri, T., Chen, T., Lankinen, H., Cheng, Z. Z., Jokiranta, T. S., et al. (2004). Lysine-dependent multipoint binding of the Borrelia burgdorferi virulence factor outer surface protein E to the C terminus of factor H. J. Immunol. 172, 6195–6201. doi: 10.4049/jimmunol.172.10.6195
Alitalo, A., Meri, T., Comstedt, P., Jeffery, L., Tornberg, J., Strandin, T., et al. (2005). Expression of complement factor H binding immunoevasion proteins in Borrelia garinii isolated from patients with neuroborreliosis. Eur. J. Immunol. 35, 3043–3053. doi: 10.1002/eji.200526354
Bernard, Q., Thakur, M., Smith, A. A., Kitsou, C., Yang, X., and Pal, U. (2019). Borrelia burgdorferi protein interactions critical for microbial persistence in mammals. Cell Microbiol. 21:e12885. doi: 10.1111/cmi.12885
Bhattacharjee, A., Oeemig, J. S., Kolodziejczyk, R., Meri, T., Kajander, T., Lehtinen, M. J., et al. (2013). Structural basis for complement evasion by Lyme disease pathogen Borrelia burgdorferi. J. Biol. Chem. 288, 18685–18695. doi: 10.1074/jbc.M113.459040
Bhide, M. R., Escudero, R., Camafeita, E., Gil, H., Jado, I., and Anda, P. (2009). Complement factor H binding by different Lyme disease and relapsing fever Borrelia in animals and human. BMC Res. Notes 2:134. doi: 10.1186/1756-0500-2-134
Brangulis, K., Petrovskis, I., Kazaks, A., Bogans, J., Otikovs, M., Jaudzems, K., et al. (2014). Structural characterization of CspZ, a complement regulator factor H and FHL-1 binding protein from Borrelia burgdorferi. FEBS J. 281, 2613–2622. doi: 10.1111/febs.12808
Brissette, C. A., Cooley, A. E., Burns, L. H., Riley, S. P., Verma, A., Woodman, M. E., et al. (2008). Lyme borreliosis spirochete Erp proteins, their known host ligands, and potential roles in mammalian infection. Int. J. Med. Microbiol. 298(Suppl. 1), 257–267. doi: 10.1016/j.ijmm.2007.09.004
Brisson, D., Drecktrah, D., Eggers, C. H., and Samuels, D. S. (2012). Genetics of Borrelia burgdorferi. Annu. Rev. Genet. 46, 515–536. doi: 10.1146/annurev-genet-011112-112140
Brooks, C. S., Vuppala, S. R., Jett, A. M., Alitalo, A., Meri, S., and Akins, D. R. (2005). Complement regulator-acquiring surface protein 1 imparts resistance to human serum in Borrelia burgdorferi. J. Immunol. 175, 3299–3308. doi: 10.4049/jimmunol.175.5.3299
Bykowski, T., Woodman, M. E., Cooley, A. E., Brissette, C. A., Brade, V., Wallich, R., et al. (2007). Coordinated expression of Borrelia burgdorferi complement regulator-acquiring surface proteins during the Lyme disease spirochete's mammal-tick infection cycle. Infect. Immun. 75, 4227–4236. doi: 10.1128/IAI.00604-07
Caimano, M. J., Yang, X., Popova, T. G., Clawson, M. L., Akins, D. R., Norgard, M. V., et al. (2000). Molecular and evolutionary characterization of the cp32/18 family of supercoiled plasmids in Borrelia burgdorferi 297. Infect. Immun. 68, 1574–1586. doi: 10.1128/IAI.68.3.1574-1586.2000
Caine, J. A., and Coburn, J. (2015). A short-term Borrelia burgdorferi infection model identifies tissue tropisms and bloodstream survival conferred by adhesion proteins. Infect. Immun. 83, 3184–3194. doi: 10.1128/IAI.00349-15
Caine, J. A., and Coburn, J. (2016). Multifunctional and redundant roles of Borrelia burgdorferi outer surface proteins in tissue adhesion, colonization, and complement evasion. Front. Immunol. 7:442. doi: 10.3389/fimmu.2016.00442
Caine, J. A., Lin, Y. P., Kessler, J. R., Sato, H., Leong, J. M., and Coburn, J. (2017). Borrelia burgdorferi outer surface protein C (OspC) binds complement component C4b and confers bloodstream survival. Cell Microbiol. 12:e12786. doi: 10.1111/cmi.12786
Coburn, J., Leong, J., and Chaconas, G. (2013). Illuminating the roles of the Borrelia burgdorferi adhesins. Trends Microbiol. 21, 372–379. doi: 10.1016/j.tim.2013.06.005
Coleman, A. S., Yang, X., Kumar, M., Zhang, X., Promnares, K., Shroder, D., et al. (2008). Borrelia burgdorferi complement regulator-acquiring surface protein 2 does not contribute to complement resistance or host infectivity. PLoS ONE 3:3010e. doi: 10.1371/journal.pone.0003010
de Taeye, S. W., Kreuk, L., Van Dam, A. P., Hovius, J. W., and Schuijt, T. J. (2013). Complement evasion by Borrelia burgdorferi: it takes three to tango. Trends Parasitol. 29, 119–128. doi: 10.1016/j.pt.2012.12.001
Dulebohn, D. P., Bestor, A., and Rosa, P. A. (2013). Borrelia burgdorferi linear plasmid 28–3 confers a selective advantage in an experimental mouse-tick infection model. Infect. Immun. 81, 2986–2996. doi: 10.1128/IAI.00219-13
Garcia, B. L., Zhi, H., Wager, B., Hook, M., and Skare, J. T. (2016). Borrelia burgdorferi BBK32 inhibits the classical pathway by blocking activation of the C1 complement complex. PLoS Pathog. 12:e1005404. doi: 10.1371/journal.ppat.1005404
Hallstrom, T., Siegel, C., Morgelin, M., Kraiczy, P., Skerka, C., and Zipfel, P. F. (2013). CspA from Borrelia burgdorferi inhibits the terminal complement pathway. MBio 4:e00481-13. doi: 10.1128/mBio.00481-13
Hammerschmidt, C., Hallstrom, T., Skerka, C., Wallich, R., Stevenson, B., Zipfel, P. F., et al. (2012). Contribution of the infection-associated complement regulator-acquiring surface protein 4 (ErpC) to complement resistance of Borrelia burgdorferi. Clin. Dev. Immunol. 2012:349657. doi: 10.1155/2012/349657
Hammerschmidt, C., Koenigs, A., Siegel, C., Hallstrom, T., Skerka, C., Wallich, R., et al. (2014). Versatile roles of CspA orthologs in complement inactivation of serum-resistant Lyme disease spirochetes. Infect. Immun. 82, 380–392. doi: 10.1128/IAI.01094-13
Hart, T., Nguyen, N. T. T., Nowak, N. A., Zhang, F., Linhardt, R. J., Diuk-Wasser, M., et al. (2018). Polymorphic factor H-binding activity of CspA protects Lyme borreliae from the host complement in feeding ticks to facilitate tick-to-host transmission. PLoS Pathog. 14:e1007106. doi: 10.1371/journal.ppat.1007106
Hartmann, K., Corvey, C., Skerka, C., Kirschfink, M., Karas, M., Brade, V., et al. (2006). Functional characterization of BbCRASP-2, a distinct outer membrane protein of Borrelia burgdorferi that binds host complement regulators factor H and FHL-1. Mol. Microbiol. 61, 1220–1236. doi: 10.1111/j.1365-2958.2006.05318.x
Hefty, P. S., Jolliff, S. E., Caimano, M. J., Wikel, S. K., Radolf, J. D., and Akins, D. R. (2001). Regulation of OspE-related, OspF-related, and Elp lipoproteins of Borrelia burgdorferi strain 297 by mammalian host-specific signals. Infect. Immun. 69, 3618–3627. doi: 10.1128/IAI.69.6.3618-3627.2001
Hovis, K. M., Tran, E., Sundy, C. M., Buckles, E., McDowell, J. V., and Marconi, R. T. (2006). Selective binding of Borrelia burgdorferi OspE paralogs to factor H and serum proteins from diverse animals: possible expansion of the role of OspE in Lyme disease pathogenesis. Infect. Immun. 74, 1967–1972. doi: 10.1128/IAI.74.3.1967-1972.2006
Hovius, J. W., Van Dam, A. P., and Fikrig, E. (2007). Tick-host-pathogen interactions in Lyme borreliosis. Trends Parasitol. 23, 434–438. doi: 10.1016/j.pt.2007.07.001
Hyde, J. A. (2017). Borrelia burgdorferi keeps moving and carries on: a review of borrelial dissemination and invasion. Front. Immunol. 8:114. doi: 10.3389/fimmu.2017.00114
Jozsi, M., Tortajada, A., Uzonyi, B., Goicoechea De Jorge, E., and Rodriguez De Cordoba, S. (2015). Factor H-related proteins determine complement-activating surfaces. Trends Immunol. 36, 374–384. doi: 10.1016/j.it.2015.04.008
Kenedy, M. R., and Akins, D. R. (2011). The OspE-related proteins inhibit complement deposition and enhance serum resistance of Borrelia burgdorferi, the lyme disease spirochete. Infect. Immun. 79, 1451–1457. doi: 10.1128/IAI.01274-10
Kenedy, M. R., Vuppala, S. R., Siegel, C., Kraiczy, P., and Akins, D. R. (2009). CspA-mediated binding of human factor H inhibits complement deposition and confers serum resistance in Borrelia burgdorferi. Infect. Immun. 77, 2773–2782. doi: 10.1128/IAI.00318-09
Kraiczy, P. (2016). Hide and seek: how lyme disease spirochetes overcome complement attack. Front. Immunol. 7:385. doi: 10.3389/fimmu.2016.00385
Kraiczy, P., Hartmann, K., Hellwage, J., Skerka, C., Kirschfink, M., Brade, V., et al. (2004a). Immunological characterization of the complement regulator factor H-binding CRASP and Erp proteins of Borrelia burgdorferi. Int. J. Med. Microbiol. 293(Suppl. 37), 152–157. doi: 10.1016/S1433-1128(04)80029-9
Kraiczy, P., Hellwage, J., Skerka, C., Becker, H., Kirschfink, M., Simon, M. M., et al. (2004b). Complement resistance of Borrelia burgdorferi correlates with the expression of BbCRASP-1, a novel linear plasmid-encoded surface protein that interacts with human factor H and FHL-1 and is unrelated to Erp proteins. J. Biol. Chem. 279, 2421–2429. doi: 10.1074/jbc.M308343200
Kraiczy, P., Hellwage, J., Skerka, C., Kirschfink, M., Brade, V., Zipfel, P. F., et al. (2003). Immune evasion of Borrelia burgdorferi: mapping of a complement-inhibitor factor H-binding site of BbCRASP-3, a novel member of the Erp protein family. Eur. J. Immunol. 33, 697–707. doi: 10.1002/eji.200323571
Kraiczy, P., Seling, A., Brissette, C. A., Rossmann, E., Hunfeld, K. P., Bykowski, T., et al. (2008). Borrelia burgdorferi complement regulator-acquiring surface protein 2 (CspZ) as a serological marker of human Lyme disease. Clin. Vaccine Immunol. 15, 484–491. doi: 10.1128/CVI.00415-07
Kraiczy, P., and Stevenson, B. (2013). Complement regulator-acquiring surface proteins of Borrelia burgdorferi: structure, function and regulation of gene expression. Ticks Tick Borne Dis. 4, 26–34. doi: 10.1016/j.ttbdis.2012.10.039
Kurtenbach, K., De Michelis, S., Etti, S., Schafer, S. M., Sewell, H. S., Brade, V., et al. (2002). Host association of Borrelia burgdorferi sensu lato–the key role of host complement. Trends Microbiol. 10, 74–79. doi: 10.1016/S0966-842X(01)02298-3
Lin, T., Gao, L., Zhang, C., Odeh, E., Jacobs, M. B., Coutte, L., et al. (2012). Analysis of an ordered, comprehensive STM mutant library in infectious Borrelia burgdorferi: insights into the genes required for mouse infectivity. PLoS ONE 7:e47532. doi: 10.1371/journal.pone.0047532
Marcinkiewicz, A., Kraiczy, P., and Lin, Y.-P. (2017). There is a method to the madness: strategies to study host complement evasion by Lyme disease and relapsing fever spirochetes. Front. Microbiol. 8:328. doi: 10.3389/fmicb.2017.00328
Marcinkiewicz, A. L., Dupuis, A. P. II., Zamba-Campero, M., Nowak, N., Kraiczy, P., Ram, S., Kramer, L. D., et al. (2019). Blood treatment of Lyme borreliae demonstrates the mechanism of CspZ-mediated complement evasion to promote systemic infection in vertebrate hosts. Cell Microbiol. 21:e12998. doi: 10.1111/cmi.12998
Marconi, R. T., Sung, S. Y., Hughes, C. A., and Carlyon, J. A. (1996). Molecular and evolutionary analyses of a variable series of genes in Borrelia burgdorferi that are related to ospE and ospF, constitute a gene family, and share a common upstream homology box. J. Bacteriol. 178, 5615–5626. doi: 10.1128/JB.178.19.5615-5626.1996
McDowell, J. V., Hovis, K. M., Zhang, H., Tran, E., Lankford, J., and Marconi, R. T. (2006). Evidence that the BBA68 protein (BbCRASP-1) of the Lyme disease spirochetes does not contribute to factor H-mediated immune evasion in humans and other animals. Infect. Immun. 74, 3030–3034. doi: 10.1128/IAI.74.5.3030-3034.2006
McDowell, J. V., Wolfgang, J., Tran, E., Metts, M. S., Hamilton, D., and Marconi, R. T. (2003). Comprehensive analysis of the factor h binding capabilities of borrelia species associated with lyme disease: delineation of two distinct classes of factor h binding proteins. Infect. Immun. 71, 3597–3602. doi: 10.1128/IAI.71.6.3597-3602.2003
Meri, S. (2016). Self-nonself discrimination by the complement system. FEBS Lett. 590, 2418–2434. doi: 10.1002/1873-3468.12284
Miller, J. C., Narayan, K., Stevenson, B., and Pachner, A. R. (2005). Expression of Borrelia burgdorferi erp genes during infection of non-human primates. Microb. Pathog. 39, 27–33. doi: 10.1016/j.micpath.2005.04.001
Miller, J. C., von Lackum, K., Babb, K., Mcalister, J. D., and Stevenson, B. (2003). Temporal analysis of Borrelia burgdorferi Erp protein expression throughout the mammal-tick infectious cycle. Infect. Immun. 71, 6943–6952. doi: 10.1128/IAI.71.12.6943-6952.2003
Muhleip, J. J., Lin, Y. P., and Kraiczy, P. (2018). Further insights into the interaction of human and animal complement regulator factor H with viable lyme disease spirochetes. Front. Vet. Sci. 5:346. doi: 10.3389/fvets.2018.00346
Nguyen, T. P., Lam, T. T., Barthold, S. W., Telford, S. R. III., Flavell, R. A., and Fikrig, E. (1994). Partial destruction of Borrelia burgdorferi within ticks that engorged on OspE- or OspF-immunized mice. Infect. Immun. 62, 2079–2084. doi: 10.1128/IAI.62.5.2079-2084.1994
Pietikainen, J., Meri, T., Blom, A. M., and Meri, S. (2010). Binding of the complement inhibitor C4b-binding protein to Lyme disease Borreliae. Mol. Immunol. 47, 1299–1305. doi: 10.1016/j.molimm.2009.11.028
Purser, J. E., and Norris, S. J. (2000). Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. U.S.A. 97, 13865–13870. doi: 10.1073/pnas.97.25.13865
Radolf, J. D., Caimano, M. J., Stevenson, B., and Hu, L. T. (2012). Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat. Rev. Microbiol. 10, 87–99. doi: 10.1038/nrmicro2714
Rathinavelu, S., Broadwater, A., and De Silva, A. M. (2003). Does host complement kill Borrelia burgdorferi within ticks? Infect. Immun. 71, 822–829. doi: 10.1128/IAI.71.2.822-829.2003
Rogers, E. A., Abdunnur, S. V., McDowell, J. V., and Marconi, R. T. (2009). Comparative analysis of the properties and ligand binding characteristics of CspZ, a factor H binding protein, derived from Borrelia burgdorferi isolates of human origin. Infect. Immun. 77, 4396–4405. doi: 10.1128/IAI.00393-09
Rogers, E. A., and Marconi, R. T. (2007). Delineation of species-specific binding properties of the CspZ protein (BBH06) of Lyme disease spirochetes: evidence for new contributions to the pathogenesis of Borrelia spp. Infect. Immun. 75, 5272–5281. doi: 10.1128/IAI.00850-07
Rosa, P. A., Tilly, K., and Stewart, P. E. (2005). The burgeoning molecular genetics of the Lyme disease spirochaete. Nat. Rev. Microbiol. 3, 129–143. doi: 10.1038/nrmicro1086
Schuijt, T. J., Coumou, J., Narasimhan, S., Dai, J., Deponte, K., Wouters, D., et al. (2011). A tick mannose-binding lectin inhibitor interferes with the vertebrate complement cascade to enhance transmission of the lyme disease agent. Cell Host Microbe 10, 136–146. doi: 10.1016/j.chom.2011.06.010
Schuijt, T. J., Hovius, J. W., Van Burgel, N. D., Ramamoorthi, N., Fikrig, E., and Van Dam, A. P. (2008). The tick salivary protein Salp15 inhibits the killing of serum-sensitive Borrelia burgdorferi sensu lato isolates. Infect. Immun. 76, 2888–2894. doi: 10.1128/IAI.00232-08
Siegel, C., Hallstrom, T., Skerka, C., Eberhardt, H., Uzonyi, B., Beckhaus, T., et al. (2010). Complement factor H-related proteins CFHR2 and CFHR5 represent novel ligands for the infection-associated CRASP proteins of Borrelia burgdorferi. PLoS ONE 5:e13519. doi: 10.1371/journal.pone.0013519
Siegel, C., Schreiber, J., Haupt, K., Skerka, C., Brade, V., Simon, M. M., et al. (2008). Deciphering the ligand-binding sites in the Borrelia burgdorferi complement regulator-acquiring surface protein 2 required for interactions with the human immune regulators factor H and factor H-like protein 1. J. Biol. Chem. 283, 34855–34863. doi: 10.1074/jbc.M805844200
Sjoberg, A. P., Trouw, L. A., and Blom, A. M. (2009). Complement activation and inhibition: a delicate balance. Trends Immunol. 30, 83–90. doi: 10.1016/j.it.2008.11.003
Skerka, C., Chen, Q., Fremeaux-Bacchi, V., and Roumenina, L. T. (2013). Complement factor H related proteins (CFHRs). Mol. Immunol. 56, 170–180. doi: 10.1016/j.molimm.2013.06.001
Steere, A. C., Strle, F., Wormser, G. P., Hu, L. T., Branda, J. A., Hovius, J. W., et al. (2016). Lyme borreliosis. Nat. Rev. Dis. Primers 2:16090. doi: 10.1038/nrdp.2016.90
Stevenson, B., El-Hage, N., Hines, M. A., Miller, J. C., and Babb, K. (2002). Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect. Immun. 70, 491–497. doi: 10.1128/IAI.70.2.491-497.2002
Stevenson, B., and Miller, J. C. (2003). Intra- and interbacterial genetic exchange of Lyme disease spirochete erp genes generates sequence identity amidst diversity. J. Mol. Evol. 57, 309–324. doi: 10.1007/s00239-003-2482-x
Stevenson, B., Tilly, K., and Rosa, P. A. (1996). A family of genes located on four separate 32-kilobase circular plasmids in Borrelia burgdorferi B31. J. Bacteriol. 178, 3508–3516. doi: 10.1128/JB.178.12.3508-3516.1996
Sung, S. Y., Lavoie, C. P., Carlyon, J. A., and Marconi, R. T. (1998). Genetic divergence and evolutionary instability in ospE-related members of the upstream homology box gene family in Borrelia burgdorferi sensu lato complex isolates. Infect. Immun. 66, 4656–4668. doi: 10.1128/IAI.66.10.4656-4668.1998
Tokarz, R., Anderton, J. M., Katona, L. I., and Benach, J. L. (2004). Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect. Immun. 72, 5419–5432. doi: 10.1128/IAI.72.9.5419-5432.2004
Tufts, D. M., Hart, T. M., Chen, G. F., Kolokotronis, S. O., Diuk-Wasser, M. A., and Lin, Y. P. (2019). Outer surface protein polymorphisms linked to host-spirochete association in Lyme borreliae. Mol. Microbiol. 111, 868–882. doi: 10.1111/mmi.14209
Tyson, K., Elkins, C., Patterson, H., Fikrig, E., and De Silva, A. (2007). Biochemical and functional characterization of Salp20, an Ixodes scapularis tick salivary protein that inhibits the complement pathway. Insect Mol. Biol. 16, 469–479. doi: 10.1111/j.1365-2583.2007.00742.x
Tyson, K. R., Elkins, C., and De Silva, A. M. (2008). A novel mechanism of complement inhibition unmasked by a tick salivary protein that binds to properdin. J. Immunol. 180, 3964–3968. doi: 10.4049/jimmunol.180.6.3964
von Lackum, K., Miller, J. C., Bykowski, T., Riley, S. P., Woodman, M. E., Brade, V., et al. (2005). Borrelia burgdorferi regulates expression of complement regulator-acquiring surface protein 1 during the mammal-tick infection cycle. Infect. Immun. 73, 7398–7405. doi: 10.1128/IAI.73.11.7398-7405.2005
Wagemakers, A., Coumou, J., Schuijt, T. J., Oei, A., Nijhof, A. M., Van 'T Veer, C., et al. (2016). An Ixodes ricinus tick salivary lectin pathway inhibitor protects Borrelia burgdorferi sensu lato from human complement. Vector Borne Zoonotic Dis. 16, 223–228. doi: 10.1089/vbz.2015.1901
Wywial, E., Haven, J., Casjens, S. R., Hernandez, Y. A., Singh, S., Mongodin, E. F., et al. (2009). Fast, adaptive evolution at a bacterial host-resistance locus: the PFam54 gene array in Borrelia burgdorferi. Gene 445, 26–37. doi: 10.1016/j.gene.2009.05.017
Zipfel, P. F., Hallstrom, T., and Riesbeck, K. (2013). Human complement control and complement evasion by pathogenic microbes–tipping the balance. Mol. Immunol. 56, 152–160. doi: 10.1016/j.molimm.2013.05.222
Zipfel, P. F., and Skerka, C. (1999). FHL-1/reconectin: a human complement and immune regulator with cell-adhesive function. Immunol. Today 20, 135–140. doi: 10.1016/S0167-5699(98)01432-7
Zipfel, P. F., and Skerka, C. (2009). Complement regulators and inhibitory proteins. Nat. Rev. Immunol. 9, 729–740. doi: 10.1038/nri2620
Keywords: Borrelia, complement, Factor H, CspA, CspZ, OspE, tick, host-pathogen interaction
Citation: Lin Y-P, Frye AM, Nowak TA and Kraiczy P (2020) New Insights Into CRASP-Mediated Complement Evasion in the Lyme Disease Enzootic Cycle. Front. Cell. Infect. Microbiol. 10:1. doi: 10.3389/fcimb.2020.00001
Received: 29 November 2019; Accepted: 06 January 2020;
Published: 30 January 2020.
Edited by:
Ryan Oliver Marino Rego, Institute of Parasitology (ASCR), CzechiaReviewed by:
Juan Anguita, Center for Cooperative Research in Biosciences, SpainJon Blevins, University of Arkansas for Medical Sciences, United States
Copyright © 2020 Lin, Frye, Nowak and Kraiczy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi-Pin Lin, WWktUGluLkxpbkBoZWFsdGgubnkuZ292; Peter Kraiczy, S3JhaWN6eUBlbS51bmktZnJhbmtmdXJ0LmRl
 Yi-Pin Lin
Yi-Pin Lin Amber M. Frye
Amber M. Frye Tristan A. Nowak
Tristan A. Nowak Peter Kraiczy
Peter Kraiczy