- 1Xiamen Branch, Zhongshan Hospital, Fudan University, Xiamen, China
- 2Zhongshan Hospital, Fudan University, Shanghai, China
- 3Zhongshan Hospital, School of Medicine, Xiamen University, Xiamen, China
- 4Xiamen Hospital of Traditional Chinese Medicine, Xiamen, China
Background: Clostridium difficile (CD) is a major cause of healthcare-associated infections and antibiotic-associated diarrhea in hospitalized patients worldwide. Carriers of toxigenic CD (tCD) have a higher risk of developing CD infections and can transmit CD to the environment and susceptible patients. However, little is known regarding the carriers and transmission of tCD in China.
Methods: A multi-center cross-sectional study of tCD colonization (tCDC) was conducted from October 24 to 31, 2014, at 33 hospitals in Shanghai, China. Rectal swabs or stool samples were collected and tested, and the clinical and demographic status, epidemiological data, and blood parameters of 531 participants were recorded. The status of tCDC was defined by a positive result on the nucleic acid amplification test for the tcdA (toxin A), tcdB (toxin B), and cdtAB (toxin CDT) genes after positive bacterial culture.
Results: The overall prevalence of CD colonization (CDC) was 19.02%, tCDC accounted for 92.08%, and A+B+CDT– was the dominant genotype (87.13%). The CD infection (CDI) prevalence was 1.51%. Potential tCDC-associated factors were admission to secondary grade hospitals, a body mass index <18.5, hospitalization during the previous 30 days, underlying diseases (including hypertension, diabetes mellitus, coronary heart disease, and respiratory failure), diarrhea during the previous 7 days, and exposure to fluoroquinolones or lansoprazole.
Conclusions: This study reveals the prevalence of CDC and tCDC in Shanghai, elucidates several associated factors, contributes to the awareness of the current epidemiology in parts of eastern China and provides new insights for further study and infection control practices.
Introduction
Clostridium difficile infection (CDI) is a persistent clinical challenge for the past four decades, as it has been a worldwide healthcare-associated infection and the major cause of antibiotic-associated diarrhea in hospitalized patients (McFarland et al., 2018). The clinical features vary from no symptoms (asymptomatic), infectious diarrhea, pseudomembranous colitis, toxic megacolon to even death (Ghose, 2013; Steele et al., 2015; Shoaei et al., 2019). Global attention has recently been directed toward the incidence of CD infection (CDI); despite efforts to prevent patient deterioration, CDI results in a worrisome outcome, and it is associated with increased morbidity, mortality, medical costs, and family burdens (Lin et al., 2015; Deshpande et al., 2017; Ho et al., 2017; Peng et al., 2018). In the United States, CDI prolongs hospital stays by 2.8–5.5 days, increases medical costs by $3,006–$15,397 per episode, and results in mortality in 5–10% of cases (Dubberke et al., 2016).
Risk factors for susceptible patients include the following: (a) age over 65 years old, (b) a long duration of hospitalization, (c) a history of prior hospitalization, (d) antimicrobial exposure (especially broad-spectrum second- or third-generation cephalosporins, penicillins, clindamycin, and fluoroquinolones), (e) a history of taking proton pump inhibitors (PPIs) or other antacid treatments, (f) severe illness, and (g) immune suppression (Vonberg et al., 2008; Janarthanan et al., 2012; Nissle et al., 2016). It should be noted that patients carrying toxigenic CD (tCD) upon hospital admission have a risk of subsequent CDI almost 6 times higher than that of non-carriers (Zacharioudakis et al., 2015), and carriers of tCD may become significant reservoirs for transmission to the environment and susceptible patients mainly via direct or indirect contact (Curry et al., 2013; Ghose, 2013). Therefore, to prevent nosocomial CDI transmission, the early recognition of tCD colonization (tCDC) upon admission is essential for the timely implementation of infection control measures, antibiotic stewardship measures, contact isolation precautions, proper hand hygiene procedures, environmental cleaning and disinfection procedures, etc. (Yakob et al., 2014; McDonald et al., 2018). A meta-analysis concluded that the overall pooled CD positivity rate among diarrhea patients was 14.8%, with a higher prevalence in East Asia (19.5%) than in South Asia (10.5%) or the Middle East (11.1%) (Deshpande et al., 2017). Routine detection methods of CD includes toxigenic culture, cell cytotoxic neutralization assay, glutamate dehydrogenase assay, enzyme immunoassays, nucleic acid amplification tests, etc. (Martinez-Melendez et al., 2017). However, the popularization of these methods may be problematic in developing countries, likely due to limitations in awareness, laboratory capacity and capabilities, and surveillance systems (Collins et al., 2013; Forrester et al., 2017). As a result, information on carriers and transmission of tCD in China is scarce. Hence, we conducted a multi-center cross-sectional study to reveal the prevalence of tCDC among intensive care unit (ICU)-hospitalized patients.
Materials and Methods
Study Population and Data Collection
A multi-center cross-sectional study was conducted in ICUs from 33 public general hospitals (including 15 tertiary grade A units, 4 tertiary grade B units, and 14 secondary grade units) in Shanghai. Surveys in different hospitals were organized respectively on a single day between Oct 24 and 31, 2014. Hospitals were classified according to National Hospital Grade Accreditation that tertiary hospitals were equipped over 500 beds while secondary were inferior. Subjects were eligible for inclusion if they were over 14 years old and were in one of the study ICUs during the study period. The exclusion criteria were as follows: (a) the patient had incomplete medical records; or (b) redundant data from patients who received repeated inquiry or specimen collection. Patients who may have been on treatment at the time of surveillance for CDI were not excluded, since the CD test may remain positive for a long as 30 days in patients who have resolution of symptoms (Surawicz et al., 2013). A total of 555 patients were hospitalized in the study sites on the survey days, of which 24 patients under 14 years old were excluded. Underlying diseases of the study participants were diagnosed by clinicians in charge, according to the International Classification of Diseases 10th Revision. Stool specimen or rectal swab from single patient was collected on the survey day (rectal swab was sampled from patient with ileus, which had been proven to be equivalent with stool for laboratory test) (Kundrapu et al., 2012). Clinical and demographic status, epidemiological data, and blood parameters (white blood cell counts and creatinine levels) were recorded simultaneously by trained infection control agents, and rechecked by YC and WS, antimicrobial application for perioperative prophylaxis was not collected.
Diagnostic Criteria
The diagnosis of CDI was based on a combination of the following clinical criteria (Gerding and Johoson, 2017): (1) diarrhea (≥3 unformed stools per 24 h for over 2 days) with no other recognized cause; and (2) toxin A or B detected in the stool, toxin-producing C. difficile detected in the stool by PCR or culture, or pseudomembranes seen in the colon. In this study, the history of diarrhea was defined as the occurrence of symptoms that ≥3 unformed stools per 24 h for over 2 days according to the Stool Form Scale (Lewis and Heaton, 1997) within 7 days prior to the survey.
Laboratory Tests
CDI symptoms are mostly mediated by toxins, toxin A (TcdA, enterotoxin, encoded by tcdA), toxin B (TcdB, cytotoxin, encoded by tcdB), and binary toxin (CDT, encoded by cdtAB), have been identified as the major virulence factors (Dayananda and Wilcox, 2019; Shoaei et al., 2019).
All stool and rectal swab samples collected from 33 hospitals were transported by anaerobe transport culture medium (Hopebio, China) at room temperature to the central laboratory in Zhongshan Hospital, Fudan University for subsequent tests. The specimens undergoing CD culture were treated with 75% (v/v) ethanol at a 1:1 ratio for 1 h before inoculation onto ChromID C. difficile agar (CDIF, BioMerieux SA, France), whose sensitivity and specificity were higher than BBL C. difficile selective agar (Han et al., 2014). All agar plates were incubated anaerobically at 37°C for 72 h before identification and DNA extraction. CD were identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (BioMerieux SA, France). Positive CD cultures were analyzed by PCR for gene tcdA, tcdB, and cdtAB. DNA from all CD-positive cultures was extracted and purified using the QIAcube automatic nucleic acid purifier and QIAamp DNA blood kit (Qiagen, Germany) and stored at −80°C until testing. PCR primers sequences were published by Jin et al. (2016). CD ATCC 43255 (A+B+CDT–, RT 087) was used as a control strain.
Statistical Analyses
The proportions of laboratory-confirmed tCDC among patients with different clinical and demographic characteristics were calculated. The Mann–Whitney U-test was used for continuous variables with skewed distributions, and a χ2 test or Fisher's exact test was used for categorical variables. The odds ratios (ORs) with 95% confidence intervals (CIs) were calculated among compared groups to assess the odds of developing tCDC. The statistical analyses were performed using SPSS 19.0 for Windows (SPSS Inc., Chicago, Illinois, USA). A two-sided P-value of <0.05 was considered statistically significant.
Results
Demographic Characteristics of the Study Subjects
Among the 531 study participants, the CDC prevalence was 19.02% (101/531), and the tCDC accounted for 92.08% (93/101). The tcdA, tcdB, and cdtAB genes were tested in all CDC subjects, and all subjects were cdtAB negative. The CDC genotypes were A–B–CDT– (7.92%, 8/101), A+B+CDT– (87.13%, 88/101), and A+B–CDT– (4.95%, 5/101). According to the diagnostic criteria, 8 subjects were diagnosed with CDI, the overall prevalence of CDI was 1.51% (8/531).
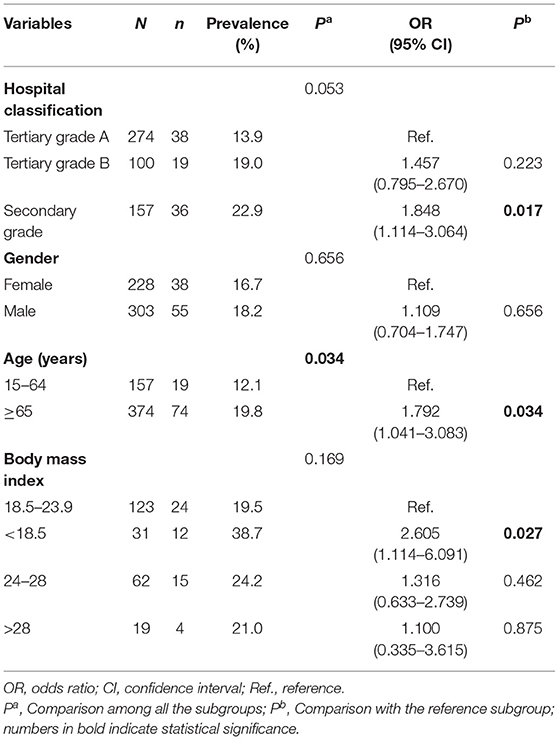
The subjects were recruited from tertiary grade A hospitals (274/531), tertiary grade B hospitals (100/531) and secondary grade hospitals (157/531). Patients hospitalized in secondary grade hospitals were more likely to acquire tCDC than those in tertiary grade A hospitals (OR = 1.848; 95% CI, 1.114–3.064; P = 0.017). The sex ratio (male: female) of the subjects was approximately 1.3:1, and the prevalence of tCDC was 18.15% (55/303) in males and 16.67% (38/228) in females (P = 0.656). The median age of the participants was 74 years old. Compared with the relatively younger group, patients aged over 65 years were more likely to present tCDC (OR = 1.792; 95% CI, 1.041–3.083; P = 0.034). The body mass indexes (BMIs) were investigated for 235 study subjects; the BMI data were unavailable for the remaining 296 patients due to critical conditions (these patients remained bedridden during their hospital stay). Patients with a BMI < 18.5 were more likely to acquire tCDC than patients with a normal BMI (OR = 2.605; 95% CI, 1.114–6.091; P = 0.027) (Table 1).
Clinical Conditions of the Study Subjects
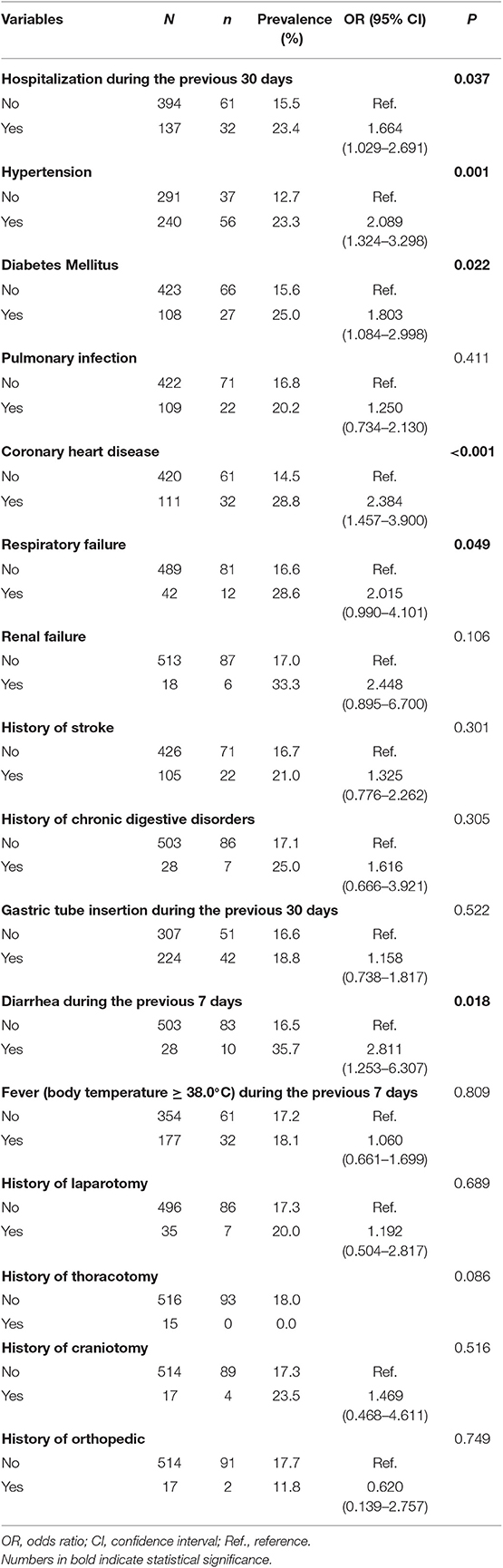
Clinical conditions were investigated for all the study subjects (Table 2). In patients hospitalized during the previous 30 days, the prevalence of tCDC was higher than that in those who were not (23.36 vs. 15.48%, P = 0.037). The prevalence of tCDC was increased in patients with hypertension (23.33 vs. 12.71%, P = 0.001), diabetes mellitus (25.00 vs. 15.60%, P = 0.022), coronary heart disease (28.83 vs. 14.52%, P < 0.001), and respiratory failure (28.57 vs. 16.56%, P = 0.049). The prevalence of tCDC in patients who experienced diarrhea during the previous 7 days was higher than those who did not (35.71 vs. 16.50%, P = 0.018). There were no significant differences in the prevalence of tCDC between groups considering pulmonary infection, renal failure, gastric tube insertion, fever, history of stroke, chronic digestive disorders, laparotomy, thoracotomy, craniotomy, or orthopedic characteristics (P > 0.05). No significant difference was shown in peripheral white blood cell counts and creatinine levels between tCDC patients and others (data not shown).
Drug Exposure of the Study Subjects
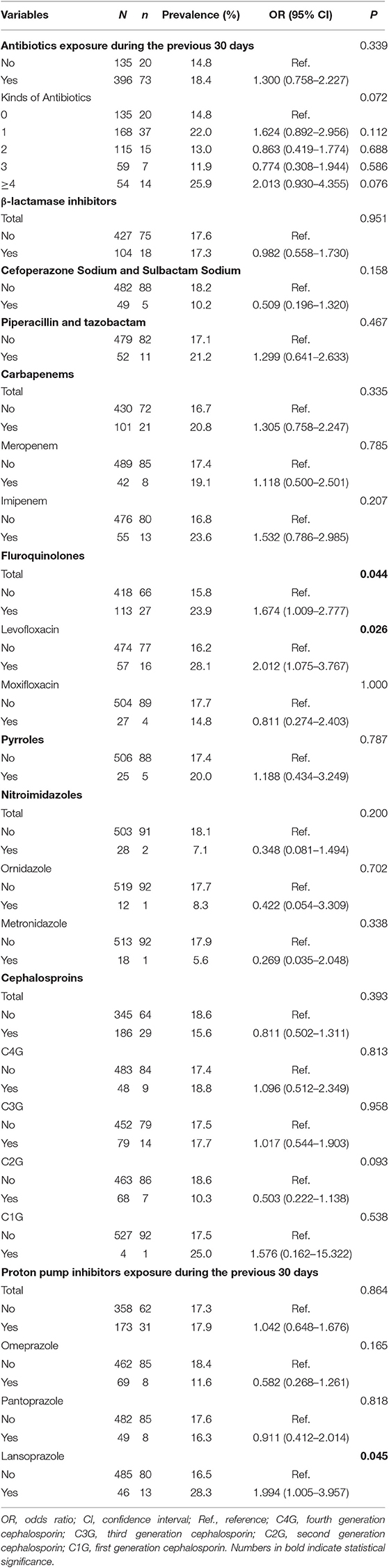
Antibiotic and PPI exposure over the previous 30 days were investigated for all subjects (Table 3). The prevalence of tCDC in patients who were treated with over 4 kinds of antibiotics tended to be higher than those who received no antibiotics treatment (25.93 vs. 14.81%, OR = 2.013, 95% CI, 0.930–4.355, P = 0.076). The prevalence of tCDC was increased in patients who underwent treatment with fluoroquinolones (23.89 vs. 15.79%, OR = 1.674, 95% CI, 1.009–2.777, P = 0.044) and lansoprazole (28.26 vs. 16.49%, OR = 1.994, 95% CI, 1.005–3.957, P = 0.045). There were no significant differences in the prevalence of tCDC considering treatment with other antibiotic treatment.
Discussion
A better understanding of the epidemiological characteristics of tCDC will contribute to the control and prevention of healthcare-associated infections and antibiotic-associated diarrhea. The present study investigated the prevalence of tCDC under different medical conditions among ICU patients in multiple medical institutions. The results showed a prevalence of 19.02% for CDC, 17.51% for tCDC, and 1.51% for CDI overall. Two studies conducted among ICU patients in China showed that the tCDC rates were 17.81 and 16.67%, and the CDI rates were 2.90 and 2.99%, respectively (Hung et al., 2012; Lin et al., 2015). While rates of tCDC of overall inpatients were about 10% (Behar et al., 2017; Jin et al., 2017). ICU patients may be at higher risk of tCDC on account of long-term hospitalization and severe illness. Patients in secondary grade hospitals, patients with a BMI < 18.5, patients who were hospitalized during the previous 30 days, patients who had underlying diseases (hypertension, diabetes mellitus, coronary heart disease, and respiratory failure), patients who experienced diarrhea during the previous 7 days, and patients who were administered fluoroquinolones or lansoprazole had an increased prevalence of tCDC.
In this cross-sectional study, the major genotype for CDC was A+B+CDT– (87.13%), but no A–B+CDT– isolates, which were previously reported to be epidemic in Asia including parts of China, were detected (Collins et al., 2013; Cheng et al., 2016; Jin et al., 2017), showing significant regional variation in the genotype distribution of CD. Similarly, no CDT positive strains were detected, neither in studies conducted in northern China (Jin et al., 2017; Wang et al., 2018).
CDI occurs frequently in hospitals with a high level of antimicrobial use; often, the environment is contaminated by CD spores (Ghose, 2013; Gerding and Johoson, 2017). In this study, the prevalence of tCDC was relatively lower in the tertiary grade hospitals than that in the secondary grade hospitals. There were more advanced medical resources and specialists in the tertiary grade hospitals; therefore, tertiary grade hospitals may provide more professional medical care and sustain a better antibiotic stewardship programme, which is strongly recommended to minimize unnecessary high-risk antibiotic administration and the number of antibiotic agents prescribed to reduce CDI risk (McDonald et al., 2018). Moreover, education for cleaning personnel of regular environmental cleaning and disinfection is essential (Vonberg et al., 2008). On the other hand, patients who are admitted to secondary grade hospitals are usually located in the suburbs, suggesting their poor economic capabilities and decreased concerns about health, which may result in a higher susceptibility to infections to some extent.
The prevalence of tCDC was also increased in underweight patients (BMI < 18.5). Underweight could reflect malnutrition and was noted to be an independent predictor of tCDC (Behar et al., 2017), which may increase susceptibility to infections. Moreover, underweight may be a reflection of poor economic conditions, which results in poor hygienic status. The relationship between underweight and risk of infection was most prevalent in developing countries (Dobner and Kaser, 2018).
Our results showed an increased prevalence (23.36%) of tCDC in patients who were hospitalized during the previous 30 days, as well as in patients with underlying diseases (including hypertension, diabetes mellitus, coronary heart disease, and respiratory failure). A previous hospital stay was shown to be a risk factor for CDC (Behar et al., 2017), and it could be inferred that frequent contact with the nosocomial environment increased the odds of infection. Additionally, a previous hospital stay may represent a poor health condition or an underlying disease, increasing the susceptibility to tCDC.
An increased prevalence of tCDC was observed in patients who experienced diarrhea during the previous 7 days. Diarrhea is a clinical symptom of CDI (Schaffler and Breitruck, 2018); the differential diagnosis of diarrhea should include CDI, and CDI-associated potential life-threatening conditions, such as pseudomembranous colitis or toxic megacolon, should receive increased attention. The early detection of CD and CD toxins is critical, as this allows earlier treatment that can significantly reduce the morbidity, mortality, medical cost, and family burden of CDI. For nosocomial infection prevention and control, the early detection of CD in patients who experienced recent diarrhea would be of great value for early case identification in clinical practice, subsequently followed by isolation, cohort nursing, antimicrobial stewardship, surveillance, education for targeted populations, reinforced environmental disinfection and early therapy to limit the spread of the infection (Vonberg et al., 2008; Yakob et al., 2014).
In addition to exposure to tCD and an inadequate host immune response, exposure to antimicrobial agents, which likely resulted in a disruption of the normal gastrointestinal microbiota, is a key event in the development of CDI (Gerding and Johoson, 2017). Fluoroquinolones have been found to be one of the primary precipitating antimicrobials associated with CDI, along with broad-spectrum cephalosporins, ampicillin, and clindamycin (Dubberke et al., 2016). According to our results, the prevalence of tCDC was increased in patients previously exposed to fluoroquinolones (especially levofloxacin). Levofloxacin is one of the most commonly used fluoroquinolones and was reported to increase the odds of CDI by 9.3 times (Wong-McClure et al., 2013). Different from previous studies reporting that exposures of broad-spectrum second-, third-generation cephalosporins, or penicillins were risk factors of CDI (Vonberg et al., 2008; Janarthanan et al., 2012; Nissle et al., 2016), no significant association between broad-spectrum cephalosporins and tCDC was observed in this study. This difference may be interpreted by a cross-sectional study design, of which no causal relationships can be deduced, as well as by a different time interval (30 days) between the antibiotics exposure and the survey day. Additionally, clindamycin is one kind of known high-risk antibiotics, but it was not analyzed since only 4 of 531 subjects received clindamycin treatment.
Several studies demonstrated that PPI use had a clinical association with CDI (Dubberke et al., 2007; Janarthanan et al., 2012; Kwok et al., 2012; Lin et al., 2015), but the mechanism remains unclear since no randomized controlled trials or quasi-experimental studies have studied the relationship between discontinuing or avoiding PPI use and the risk of CDI (McDonald et al., 2018). The relationship between PPI exposure and CDI remains controversy, even a global review of guidelines could not extract a conclusion of it (Balsells et al., 2016). No association between PPI use and tCDC was observed in our study, but interestingly, patients who received lansoprazole treatment acquired tCDC more often than patients who did not (28.26 vs. 16.49%, OR = 1.994, 95% CI, 1.005–3.957). Lansoprazole was previously reported to be associated with tCDC in an experimental study (Kaur et al., 2007). US Food and Drug Administration data also reported 2 signals of disproportionate reporting (SDR) for CD associated with lansoprazole, while no SDRs for omeprazole, pantoprazole and other PPIs were reported (Hauben et al., 2007). Consistent with this monitoring data, our finding provided clues for further study of the relationship between PPI exposure and CDI.
To the best of our knowledge, this is the first multi-center study to report the prevalence of tCDC among ICU patients in Eastern China. However, this study has several limitations that need to be addressed. First, this study was conducted in 2014, the variation trend of CDI or CDC from then on was scarcely possible to estimate due to unachievable popularization of routine CD detection methods in China and a paucity of data reported about CDI or CDC genotype distribution. Additionally, infection control programmes targeting the carbapenem-resistant A. baumannii and carbapenem-resistant Enterobacteriaceae were continuously implemented in Shanghai in the past decade, and the strategies might have stable influence on the prevalence of CDI or CDC. A longitudinal study would be worthwhile in future to further understand the variation trend of CDI or CDC. Second, tCD was detected using PCR in this study; PCR may be oversensitive because extremely few or insignificant genetic residues were detected. It is hard to distinguish contamination from true colonization. Third, as this study was conducted in ICUs, a considerable part of patients had lost the ability of movement at admission, of whom the height and weight data could not be collected. Thus the association between BMI and tCDC were analyzed in only 44.3% (235/531) of the participants. The demographic characteristics between patients with and without BMI data were compared and showed no significant difference. Thus the missing values of BMI data might have little effect on the conclusion that low BMI was associated with tCDC. Certainly a larger sample size to verify this association is imperative. Fourth, the disease severity of ICU patients was not analyzed due to the lack of a unified evaluation standard in our all study sites. Finally, associations between tCDC and antibiotics were discussed insufficiently. We entered all kinds of documented antibiotics in this study into the factorial analysis, but the sample size for each combination was insufficient to achieve significant results. A larger sample size would be worthy of further study to determine the association between a specific combination of antibiotics and tCDC.
In conclusion, we surveyed the prevalence of CDC, tCDC, and the CDI rate (%) among ICU patients in Shanghai and demonstrated the epidemic genotype distribution of CD. The findings highlighted the prevalence among different hospitals grades, elucidated some risk factors related to tCDC, and provided new insights for further study and infection control practices. To prevent the spread of nosocomial CDI, awareness of the epidemiology, risk factors and infection control strategies should be increased reinforced.
Data Availability Statement
All datasets generated for this study are included in the article.
Ethics Statement
This study was approved by the Ethics Committee of Zhongshan Hospital, Fudan University, and was in compliance with national legislation and the Declaration of Helsinki guidelines. Written informed consent was obtained from the patient or, from next of kin or an independent patient advocate if the patient lacked this capacity.
Author Contributions
HM, RB, and YX wrote the main manuscript text. YC and WS collected samples and information of study subjects. YS and QS contributed to laboratory tests. XC and JL did the statistical analysis. BH and XG conceived the study and revised the manuscript.
Funding
This work was supported by the 4th three-year Action Plan for Public Health of Shanghai [grant number 15GWZK0101], and the Projects of Xiamen Science and Technology Program [grant number 3502Z20184057]. The funders played no role in the study design, data collection, or analyses, the decision to publish, or manuscript preparation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge Profs. Xiaoping Ni and Hui Jin in Hangzhou Center for Disease Control and Prevention, for their professional advice, as well as their support in this project.
References
Balsells, E., Filipescu, T., Kyaw, M. H., Wiuff, C., Campbell, H., and Nair, H. (2016). Infection prevention and control of Clostridium difficile: a global review of guidelines, strategies, and recommendations. J. Glob. Health 6:020410. doi: 10.7189/jogh.06.020410
Behar, L., Chadwick, D., Dunne, A., Jones, C. I., Proctor, C., Rajkumar, C., et al. (2017). Toxigenic Clostridium difficile colonization among hospitalised adults; risk factors and impact on survival. J. Infect. 75, 20–25. doi: 10.1016/j.jinf.2017.04.006
Cheng, J. W., Xiao, M., Kudinha, T., Kong, F., Xu, Z. P., Sun, L. Y., et al. (2016). Molecular epidemiology and antimicrobial susceptibility of Clostridium difficile isolates from a university teaching hospital in China. Front. Microbiol. 7:1621. doi: 10.3389/fmicb.2016.01621
Collins, D. A., Hawkey, P. M., and Riley, T. V. (2013). Epidemiology of Clostridium difficile infection in Asia. Antimicrob. Resist. Infect. Control 2:21. doi: 10.1186/2047-2994-2-21
Curry, S. R., Muto, C. A., Schlackman, J. L., Pasculle, A. W., Shutt, K. A., Marsh, J. W., et al. (2013). Use of multilocus variable number of tandem repeats analysis genotyping to determine the role of asymptomatic carriers in Clostridium difficile transmission. Clin. Infect. Dis. 57, 1094–1102. doi: 10.1093/cid/cit475
Dayananda, P., and Wilcox, M. H. (2019). A review of mixed strain Clostridium difficile colonization and infection. Front. Microbiol. 10:692. doi: 10.3389/fmicb.2019.00692
Deshpande, A., Borren, N. Z., Ghadermarzi, S., Hutfless, S., and Ananthakrishnan, A. N. (2017). The emergence of Clostridium difficile infection in Asia: a systematic review and meta-analysis of incidence and impact. PLoS ONE 12:e0176797. doi: 10.1371/journal.pone.0176797
Dobner, J., and Kaser, S. (2018). Body mass index and the risk of infection - from underweight to obesity. Clin. Microbiol. Infect. 24, 24–28. doi: 10.1016/j.cmi.2017.02.013
Dubberke, E. R., Carling, P., Carrico, R., Donskey, C. J., Loo, V. G., McDonald, L. C., et al. (2016). Strategies to prevent clostridium difficile infections in acute care hospitals: 2014 update. Infect. Control Hosp. Epidemiol. 35, S48–S65. doi: 10.1017/S0899823X00193857
Dubberke, E. R., Reske, K. A., Yan, Y., Olsen, M. A., McDonald, L. C., and Fraser, V. J. (2007). Clostridium difficile–associated disease in a setting of endemicity: identification of novel risk factors. Clin. Infect. Dis. 45, 1543–1549. doi: 10.1086/523582
Forrester, J. D., Cai, L. Z., Mbanje, C., Rinderknecht, T. N., and Wren, S. M. (2017). Clostridium difficile infection in low- and middle-human development index countries: a systematic review. Trop. Med. Int. Health 22, 1223–1232. doi: 10.1111/tmi.12937
Gerding, N. D, and Johoson, S. (2017). “Clostridium difficile infection, including pseudomembranous colitis,” in Harrison's Infectious Diseases, 3rd Edn., eds L. D. Kasper and S. A. Fauci (New York, NY: McGraw-Hill Education), 294–298.
Ghose, C. (2013). Clostridium difficile infection in the twenty-first century. Emerg. Microbes Infect. 2:e62. doi: 10.1038/emi.2013.62
Han, S. B., Chang, J., Shin, S. H., Park, K. G., Lee, G. D., Park, Y. G., et al. (2014). Performance of chromID Clostridium difficile Agar compared with BBL C. difficile selective Agar for detection of C. difficile in stool specimens. Annu. Lab. Med. 34:376. doi: 10.3343/alm.2014.34.5.376
Hauben, M., Horn, S., Reich, L., and Younus, M. (2007). Association between gastric acid suppressants and Clostridium difficile colitis and community-acquired pneumonia: analysis using pharmacovigilance tools. Int. J. Infect. Dis. 11, 417–422. doi: 10.1016/j.ijid.2006.11.004
Ho, J., Dai, R. Z. W., Kwong, T. N. Y., Wang, X., Zhang, L., Ip, M., et al. (2017). Disease burden of Clostridium difficile infections in adults, Hong Kong, China, 2006-2014. Emerg. Infect. Dis. 23, 1671–1679. doi: 10.3201/eid2310.170797
Hung, Y. P., Tsai, P. J., Hung, K. H., Liu, H. C., Lee, C. I., Lin, H. J., et al. (2012). Impact of toxigenic Clostridium difficile colonization and infection among hospitalized adults at a district hospital in southern Taiwan. PLoS ONE 7:e42415. doi: 10.1371/journal.pone.0042415
Janarthanan, S., Ditah, I., Adler, D. G., and Ehrinpreis, M. N. (2012). Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am. J. Gastroenterol. 107, 1001–1010. doi: 10.1038/ajg.2012.179
Jin, D., Luo, Y., Huang, C., Cai, J., Ye, J., Zheng, Y., et al. (2017). Molecular Epidemiology of Clostridium difficile Infection in hospitalized patients in Eastern China. J. Clin. Microbiol. 55, 801–810. doi: 10.1128/JCM.01898-16
Jin, H., Gao, X., Chen, L., Gan, T., Ren, S., Li, F., et al. (2016). Characteristics of Clostridium difficile harboring toxin genes in some Chinese cities. Chin. J. Nosocomiol. 26:2418. doi: 10.11816/cn.ni.2016-161136
Kaur, S., Vaishnavi, C., Prasad, K. K., Ray, P., and Kochhar, R. (2007). Comparative role of antibiotic and proton pump inhibitor in experimental Clostridium difficile infection in mice. Microbiol. Immunol. 51, 1209–1214. doi: 10.1111/j.1348-0421.2007.tb04016.x
Kundrapu, S., Sunkesula, V. C. K., Jury, L. A., Sethi, A. K., and Donskey, C. J. (2012). Utility of perirectal swab specimens for diagnosis of Clostridium difficile infection. Clin. Infect. Dis. 55, 1527–1530. doi: 10.1093/cid/cis707
Kwok, C. S., Arthur, A. K., Anibueze, C. I., Singh, S., Cavallazzi, R., and Loke, Y. K. (2012). Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am. J. Gastroenterol. 107, 1011–1019. doi: 10.1038/ajg.2012.108
Lewis, S. J., and Heaton, K. W. (1997). Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 32, 920–924. doi: 10.3109/00365529709011203
Lin, H. J., Hung, Y. P., Liu, H. C., Lee, J. C., Lee, C. I., Wu, Y. H., et al. (2015). Risk factors for Clostridium difficile-associated diarrhea among hospitalized adults with fecal toxigenic C. difficile colonization. J. Microbiol. Immunol. Infect. 48, 183–189. doi: 10.1016/j.jmii.2013.08.003
Martinez-Melendez, A., Camacho-Ortiz, A., Morfin-Otero, R., Maldonado-Garza, H. J., Villarreal-Trevino, L., and Garza-Gonzalez, E. (2017). Current knowledge on the laboratory diagnosis of Clostridium difficile infection. World J. Gastroenterol. 23, 1552–1567. doi: 10.3748/wjg.v23.i9.1552
McDonald, L. C., Gerding, D. N., Johnson, S., Bakken, J. S., Carroll, K. C., Coffin, S. E., et al. (2018). Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 66, e1–e48. doi: 10.1093/cid/ciy149
McFarland, L. V., Ship, N., Auclair, J., and Millette, M. (2018). Primary prevention of Clostridium difficile infections with a specific probiotic combining Lactobacillus acidophilus, L. casei, and L. rhamnosus strains: assessing the evidence. J. Hosp. Infect. 99, 443–452. doi: 10.1016/j.jhin.2018.04.017
Nissle, K., Kopf, D., and Rosler, A. (2016). Asymptomatic and yet C. difficile-toxin positive? Prevalence and risk factors of carriers of toxigenic Clostridium difficile among geriatric in-patients. BMC Geriatr. 16:185. doi: 10.1186/s12877-016-0358-3
Peng, Z., Ling, L., Stratton, C. W., Li, C., Polage, C. R., Wu, B., et al. (2018). Advances in the diagnosis and treatment of Clostridium difficile infections. Emerg. Microbes Infect. 7:15. doi: 10.1038/s41426-017-0019-4
Schaffler, H., and Breitruck, A. (2018). Clostridium difficile- from colonization to infection. Front. Microbiol. 9:646. doi: 10.3389/fmicb.2018.00646
Shoaei, P., Shojaei, H., Jalali, M., Khorvash, F., Hosseini, S. M., Ataei, B., et al. (2019). Clostridium difficile isolated from faecal samples in patients with ulcerative colitis. BMC Infect. Dis. 19:361. doi: 10.1186/s12879-019-3965-8
Steele, S. R., McCormick, J., Melton, G. B., Paquette, I., Rivadeneira, D. E., Stewart, D., et al. (2015). Practice parameters for the management of Clostridium difficile infection. Dis. Colon. Rectum. 58, 10–24. doi: 10.1097/DCR.0000000000000289
Surawicz, C. M., Brandt, L. J., Binion, D. G., Ananthakrishnan, A. N., Curry, S. R., Gilligan, P. H., et al. (2013). Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am. J. Gastroenterol. 108, 478–498. doi: 10.1038/ajg.2013.4
Vonberg, R. P., Kuijper, E. J., Wilcox, M. H., Barbut, F., Tull, P., Gastmeier, P., et al. (2008). Infection control measures to limit the spread of Clostridium difficile. Clin. Microbiol. Infect. 14, 2–20. doi: 10.1111/j.1469-0691.2008.01992.x
Wang, B., Lv, Z., Zhang, P., and Su, J. (2018). Molecular epidemiology and antimicrobial susceptibility of human Clostridium difficile isolates from a single institution in Northern China. Medicine 97:e11219. doi: 10.1097/MD.0000000000011219
Wong-McClure, R. A., Ramirez-Salas, E., Mora-Brenes, N., Aguero-Sandi, L., Morera-Sigler, M., Badilla-Vargas, X., et al. (2013). Long term effect of infection control practices and associated factors during a major Clostridium difficile outbreak in Costa Rica. J. Infect. Dev. Ctries. 7, 914–921. doi: 10.3855/jidc.2854
Yakob, L., Riley, T. V., Paterson, D. L., Marquess, J., and Clements, A. C. (2014). Assessing control bundles for Clostridium difficile: a review and mathematical model. Emerg. Microbes Infect. 3:e43. doi: 10.1038/emi.2014.43
Keywords: Clostridium difficile, colonization, toxigenic, infection control, cross-sectional study
Citation: Mi H, Bao R, Xiao Y, Cui Y, Sun W, Shen Y, Shi Q, Chen X, Lin J, Hu B and Gao X (2020) Colonization of Toxigenic Clostridium difficile Among Intensive Care Unit Patients: A Multi-Centre Cross-Sectional Study. Front. Cell. Infect. Microbiol. 10:12. doi: 10.3389/fcimb.2020.00012
Received: 04 September 2019; Accepted: 13 January 2020;
Published: 30 January 2020.
Edited by:
Yi-Wei Tang, Cepheid, United StatesReviewed by:
Wen-Chien Ko, National Cheng Kung University, TaiwanChunhui Li, Central South University, China
Copyright © 2020 Mi, Bao, Xiao, Cui, Sun, Shen, Shi, Chen, Lin, Hu and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bijie Hu, aHUuYmlqaWVAenMtaG9zcGl0YWwuc2guY24=; Xiaodong Gao, Z2FveGQ1QHZpcC5zaW5hLmNvbQ==
†These authors have contributed equally to this work
 Hongfei Mi
Hongfei Mi Rong Bao2†
Rong Bao2† Yao Xiao
Yao Xiao