- 1College of Life Science, Luoyang Normal University, Luoyang, China
- 2Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Shanghai, China
- 3College of Animal Science and Technology, Henan University of Science and Technology, Luoyang, China
- 4Groupe de Recherche en Écologie Buccale (GREB), Faculté de Médecine Dentaire, Université Laval, Quebec City, QC, Canada
Respiratory infections seriously affect the swine industry worldwide. Co-infections of two vital pathogenic bacteria Streptococcus suis (S. suis) and Actinobacillus pleuropneumoniae (A. pleuropneumoniae), colonizing the respiratory tract often occurs in veterinary clinical practice. Moreover, our previous research found that S. suis and A. pleuropneumoniae can form biofilm in vitro. The formation of a mixed biofilm not only causes persistent infections, but also increases the multiple drug resistance of bacteria, which brings difficulties to disease prevention and control. However, the methods for detecting S. suis and A. pleuropneumoniae in co-infection and biofilm are immature. Therefore, in this study, primers and probes were designed based on the conservative sequence of S. suis gdh gene and A. pleuropneumoniae apxIVA gene. Then, a TaqMan duplex real-time PCR method for simultaneous detection of S. suis and A. pleuropneumoniae was successfully established via optimizing the reaction system and conditions. The specificity analysis results showed that this TaqMan real-time PCR method had strong specificity and high reliability. The sensitivity test results showed that the minimum detection concentration of S. suis and A. pleuropneumoniae recombinant plasmid was 10 copies/μL, which is 100 times more sensitive than conventional PCR methods. The amplification efficiencies of S. suis and A. pleuropneumoniae were 95.9% and 104.4% with R2 value greater than 0.995, respectively. The slopes of the calibration curves of absolute cell abundance of S. suis and A. pleuropneumoniae were 1.02 and 1.09, respectively. The assays were applied to cultivated mixed biofilms and approximately 108 CFUs per biofilm were quantified when 108 CFUs planktonic bacteria of either S. suis or A. pleuropneumoniae were added to biofilms. In summary, this study developed a TaqMan real-time PCR assay for specific, accurate quantification of S. suis or A. pleuropneumoniae in mixed biofilms, which may help for the detection, prevention and control of diseases caused by a bacterial mixed infection involving S. suis and A. pleuropneumoniae.
Introduction
Respiratory system diseases seriously affect the development of the swine industry worldwide. Streptococcus suis (S. suis) and Actinobacillus pleuropneumoniae (A. pleuropneumoniae) are two important respiratory pathogens in the swine industry (Opriessnig et al., 2011). S. suis is a zoonotic pathogen that can cause serotype-dependent diseases in swine and humans, mainly as meningitis (Weinert et al., 2015; Segura et al., 2017). A. pleuropneumoniae is the etiologic agent of porcine contagious pleuropneumonia (PCP) disease in swine, which is considered to be one of the leading causes of economic losses in the swine industry (Mullebner et al., 2018). Meanwhile, S. suis and A. pleuropneumoniae are major pathogens of the Porcine Respiratory Disease Complex (PRDC) (Opriessnig et al., 2011). Clinically, S. suis and A. pleuropneumoniae infections are often detected simultaneously. in vitroBiofilm is a structured surface-bound community formed by microorganisms (Kolter and Greenberg, 2006). Most of the biofilms exist in the form of mixed-species communities (Costerton et al., 1999; Elias and Banin, 2012). The complex interspecies dynamics modulate the development, nature, and survival of biofilms and will affect the pathological process and treatment outcome of biofilm-associated infections, especially by enhancing resistance to host immune defense (Rendueles and Ghigo, 2012; Burmolle et al., 2014). Once the pathogens form a biofilm in the host, they often cause persistent infections and are rather difficult to eliminate (Figueiredo et al., 2017; Wang et al., 2018). In some cases, microbial interactions in mixed biofilms can provide conditions for the exchange of resistance genes or increased survival (Horter et al., 2001; Harriott and Noverr, 2009). In addition, our studies found that S. suis and A. pleuropneumoniae can form two-species biofilms when co-cultured in vitro. When grown in dual-species biofilms in vitro, the genes related to virulence factors in S. suis and A. pleuropneumoniae are significantly up-regulated. Compared with monocultures, the antibiotic resistance of S. suis and A. pleuropneumoniae is both enhanced in the co-culture model (Wang et al., 2020). The mixed biofilm formed by S. suis and A. pleuropneumoniae leads to the increase of drug resistance, which increases the difficulties of disease prevention and control. Furthermore, the detection methods of the mixed biofilm formed by the two bacteria are seldom reported. Therefore, it is urgent to invest efforts and time on investigating mixed biofilms and developing tools for such studies.
Efficient and accurate detection methods play a vital role in the effective prevention and control of the disease. However, traditional diagnosis has some disadvantages in practical application, such as complex cultivation methods, time-consuming and low sensitivity, which cannot quantitatively analyze the pathogenic bacteria in the biofilm. Real-time polymerase chain reaction (PCR) is a powerful and easy to use technology that represents an ideal method for quantifying species in biofilms. Real-time PCR provides high levels of specificity and overcomes some limitations of conventional bacteria quantification methods, because it distinguishes morphologically-similar species by specifically targeting genes of a single species. The real-time PCR assays mainly include the dye method represented by SYBR Green real-time PCR and the probe method represented by TaqMan real-time PCR. SYBR Green real-time PCR uses a fluorescent dye that can bind to any DNA. Whereas, TaqMan real-time PCR uses probe dyes that can specifically bind to target DNA. The specificity of TaqMan real-time PCR is crucial for quantifying the number of individual species in a mixed biofilm (Kirakodu et al., 2008; Cao and Shockey, 2012). Thus, TaqMan real-time PCR should be considered as an ideal method for quantifying bacterial pathogens embedded in a mixed biofilm (Suzuki et al., 2005).
In order to accurately and specifically quantify the clinical co-infection and mixed biofilm formed by S. suis and A. pleuropneumoniae, we developed a specific TaqMan real-time PCR assay that can simultaneously quantify S. suis and A. pleuropneumoniae. The real-time PCR method was evaluated for its specificity, sensitivity, application for detection and diagnosis. This study provides an efficient real-time PCR tool available for the quantify detection for S. suis and A. pleuropneumoniae in co-infection and biofilms, helping for the prevention and control of diseases caused by these two important respiratory pathogens.
Materials and methods
Bacterial strains and growth conditions
S. suis serotype 2 strain HA9801 was isolated from an infected pig in Jiangsu Province and confirmed to be a pathogenic strain in pig model as previously description (Yao et al., 1999). A. pleuropneumoniae CVCC265 (APP) (serotype 1) was purchased from China Veterinary Culture Collection Center (CVCC). S. suis was grown in Tryptic Soy Broth (TSB) or Tryptic Soy Agar (TSA) medium for 12 hours, then diluted with 1:100 and placed in 37°C shaking table at 180 r/min for 8 hours. A. pleuropneumoniae was grown in TSB or TSA supplemented with 0.1 μg/mL of β-nicotinamide-adenine-dinucleotide (NAD) for 18 hours, then dilute it with 1:100 and place it in a 180 r/min 37°C shaking table for 10 hours.
Primer and probe design
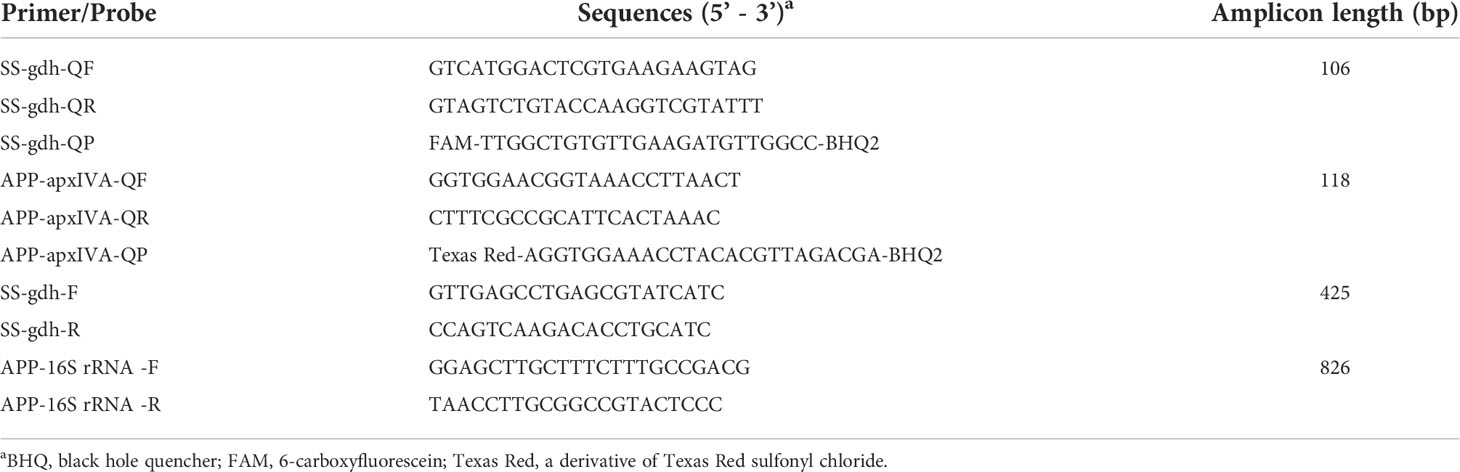
The sequences of the S. suis gdh gene and A. pleuropneumoniae apxIVA gene (GenBank ID: AY853916 and AF021919) were aligned and analyzed using DNASTAR 7.10. Primers and probes (Table 1) for conventional PCR and TaqMan real-time PCR were designed using prime premier 5.0 software. PCR amplification, electrophoresis and sequencing verified that the designed primers can amplify the target sequence.
DNA extraction and construction of recombinant plasmid
DNA extraction from 3 mL bacterial culture solution was performed using the TIANamp Bacteria DNA Kit according to the manufacturer’s instructions with a final elution volume of 50 μL. The concentration and purity of the extracted DNA samples were determined by agarose gel electrophoresis and NanoDrop 2000C (Thermo Fisher Scientific Inc.) spectrophotometry. Genomic DNA of SS and APP strains were used as templates. Target genes of the two strains were amplified by PCR using corresponding primers (Table 1) and inserted into pMD19-T vector. The resultant recombinant plasmids (pMD-gdh and pMD-apxIVA) were transformed into Escherichia coli DH5α, respectively. The positive recombinant plasmid was sequenced by Sangong Bioengineering (Shanghai) Co., LTD., and used as the plasmid standard of the corresponding bacteria.
TaqMan real-time PCR reactions
TaqMan real-time PCR amplification was performed using a Bio-Rad CFX96 Touch real-time PCR instrument. Using the recombinant plasmids pMD-gdh and pMD-apxIVA as templates, the real-time PCR amplifications were conducted using triplicate samples, three no-template controls, and 4 inter-plate calibrators in duplicate. Conventional PCR reaction system: total system 20 μL, 2 × PCR Master Mix 10 μL, 1 μL of upstream and downstream primers, 2 μL of template DNA, ddH2O to obtain a final volume of 20 μL; reaction conditions: pre-denaturation at 95 °C for 5 minutes, denaturation at 95 °C; for 30 s, annealing at 55 °C; for 30 s, extension at 72 °C; for 60 s, for 35 cycles, re-extension at 72 °C; for 10 minutes.
Specificity and cross-reactivity
The specificity of the TaqMan real-time PCR assay was evaluated using bacterial swine pathogens causing respiratory diseases as well as bacteria prevalent in the animal environment. TaqMan real-time PCR was used to amplify the genomic DNA of S. suis, A. pleuropneumoniae, Bordetella bronchiseptica, Staphylococcus aureus, Pseudomonas aeruginosa, Haemophilus parasuis, Pasteurella multocida and Streptococcus agalactiae.
The real-time PCR primer/probe sets were tested for cross reactivity between S. suis and A. pleuropneumoniae. On the one hand, 10-107 copies/μL of A. pleuropneumoniae recombinant plasmids were subjected to TaqMan real-time PCR amplification using SS-gdh-QF/R and SS-gdh-QP. On the other hand, 10-107 copies of S. suis recombinant plasmids were subjected to TaqMan real-time PCR amplification using APP-apxIVA-Q/R and APP-apxIVA-QP.
Standard curve determination of TaqMan real-time PCR
The TaqMan real-time PCR standard curve of S. suis and A. pleuropneumoniae can be plotted based on Cq value and Log10 copy number of 10-fold gradient dilution of recombinant plasmids. Plasmid abundance was calculated by equation (Whelan et al., 2003).
Cmass is the concentration of the plasmid (ng/μL), Lplasmid is the length of the plasmid (bp), Mbasepair is the mass of base pairs assumed to be 660 Da basepairs -1, and NA is the Avogadro’s Constant.
Recombinant plasmids were extracted and their concentration and purity were determined using NanoDrop 2000C ultra-micro spectrometer. The recombinant plasmid was converted into target gene copy number and 10-fold diluted in the range of 103 to 107 copies, which were used as template of TaqMan real-time PCR, respectively. Average Cq values were plotted against the Log10 copy number to calculate the efficiency, slope and y-intercept of each standard curve and obtain a linear equation. Standard curves with an efficiency of 90%-110% were deemed suitable for real-time PCR analysis.
Assessment of sensitivity and repeatability
The recombinant plasmid of S. suis and A. pleuropneumoniae were ten-fold serially diluted to 1×104 copies/μL ~1 copy/μL. The optimized reaction system and conditions were used for TaqMan real-time PCR amplification with serially dilutions of recombinant plasmid and ddH2O as templates to evaluate the sensitivity of the detection method established in this study.
The repeatability of TaqMan real-time PCR was evaluated using recombinant plasmid mixture of S. suis and A. pleuropneumoniae, which were ten-fold serially diluted to 1×106 copies/μL~1×104 copies/μL. Repeatability within the group: amplification of the dilutions was performed by TaqMan real-time PCR in triplicate. For assessing the repeatability between groups, amplification of dilutions was performed by TaqMan real-time PCR for three consecutive weeks at intervals of one week. The coefficient of variation was calculated using Excel 2016 software (Microsoft).
Calibration curve validation
A bacterial calibration curve was built based on the copy number of the target gene quantified by TaqMan real-time PCR and the bacterial concentration of ten-fold serial dilutions. Bacteria cultured overnight were quantified to 108 colony forming units (CFUs), and then ten-fold serially diluted to bring the number of bacteria to 106-102 CFUs. The number of bacterial CFUs was confirmed by plating on TSA plates. DNA was extracted from the samples according to the method described in section 2.2 and quantified using established TaqMan real-time PCR reactions.
Quantification of S. suis and A. pleuropneumoniae in clinical samples
To evaluate the discrimination and applicability of established method for clinical samples, a total of 45 lung samples from porcine suspected to be infected with S. suis and A. pleuropneumoniae were collected from farms in Henan Province, China. Lung tissues were minced by mill, diluted 1:10 in phosphate-buffered saline (PBS, pH 7.4), and genomic DNA of the samples was extracted according to the procedure described above. The genomic DNA extracted from lung tissue samples, the mixed genomic DNA of S. suis and A. pleuropneumoniae, and ddH2O were used as templates. The TaqMan real-time PCR and conventional PCR were used for detection, and the experimental results were statistically analyzed and compared.
Quantification of S. suis and A. pleuropneumoniae biofilm samples in vitro
First, the mixed biofilm samples of S. suis and A. pleuropneumoniae were cultrued. Pure cultures (bacterial culture medium) of S. suis and A. pleuropneumoniae were grown to the exponential phase (OD600, 0.6-0.7) and used to inoculate 24-well polystyrene, flat bottom, tissue culture-treated microplates in a ratio of 1:1 and a final volume added to each well of 2 mL. The microplates were then incubated at 37°C for 48-72 h.
In order to evaluate the effectiveness of the established TaqMan real-time PCR assays in a biofilm system, biofilm samples were spiked with S. suis and A. pleuropneumoniae cultures, and then analyzed by real-time PCR method. Four different samples were created in triplicate using a 24-well plate method: i) mixed biofilm, ii) mixed biofilm + 108 CFUs pure culture of S. suis, iii) mixed biofilm + 108 CFUs pure culture of A. pleuropneumoniae, and iv) mixed biofilm + 108 CFUs pure culture of S. suis + 108 CFUs pure culture of A. pleuropneumoniae. Culture biofilm according to the above method. The cultured biofilms were washed three times with PBS, and 200 μL of PBS was added to each well, followed by ultrasonic treatment with an ultrasonic cleaner for 5 min, and finally the amount of each group of samples was adjusted to 200 μL. DNA was extracted from the samples according to the procedure described above, and the DNA samples were amplified by TaqMan real-time PCR. In addition, the biofilm diluent of S. suis and A. pleuropneumoniae were diluted 10-fold with PBS to 102-106 CFUs/mL biofilm diluent, and the biofilm diluent was amplified by TaqMan real-time PCR to determine the minimum detection limit of biofilm state by this method. Cell abundance was calculated using the developed calibration curves.
Statistical analysis
Quantitative variables were expressed as means ± standard error (SE). Calibration curves were calculated using linear regression with 95% confidence intervals. All calculations and plots were completed using GraphPad v7.
Results
Establishment and optimization of TaqMan real-time PCR assay
Based to the bioinformatics analysis, in order to improve the amplification efficiency, we worked on improving the specificity and stability of the primer as well as probes. We optimized the annealing temperature, primer and probe concentration. The final optimal conditions were as follows: 95°C for 2 minutes, followed by 40 cycles of 95°C for 15 s, 60°C for 30 s. Amplification reactions were performed in a 20 µL reaction volume involving of 10 µL Bestar® 2 × qPCR Master Mix, 0.6 μL of each primers (0.3 μM), 0.3 μL of probes (0.15 μM), 1 μL of template DNA, and ddH2O to obtain a final volume of 20 μL. Under these optimization conditions, the S. suis and A. pleuropneumoniae were specifically detected by TaqMan duplex real-time PCR assay.
Specificity analysis and cross-reactivity of TaqMan real-time PCR assay
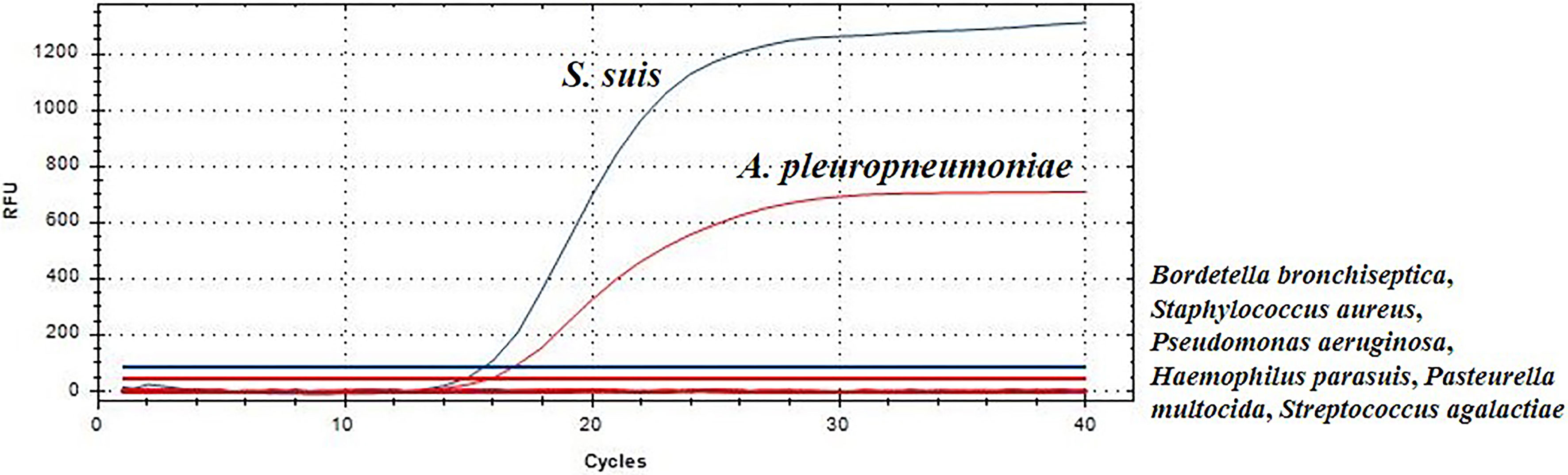
To analyze the specificity of this TaqMan real-time PCR, S. suis, A. pleuropneumoniae, Bordetella bronchiseptica, Staphylococcus aureus, Haemophilus parasuis, Pseudomonas aeruginosa, Pasteurella multocida and Streptococcus agalactiae, were used as templates for TaqMan real-time PCR, respectively. S. suis strain HA9801 and A. pleuropneumoniae strain CVCC265 showed a fluorescent signal (Figure 1). Moreover, other serotypes of S. suis and A. pleuropneumoniae strains also yielded positive results in this TaqMan real-time PCR. Whereas, other bacteria yielded negative results in the duplex real-time PCR, indicating that the TaqMan real-time PCR method is specific. These experiments validate the real-time PCR assays abilities to specifically distinguish S. suis and A. pleuropneumoniae.
Standard curves
The standard curve of the TaqMan real-time PCR assay were determined using the recombinant plasmid standards pMD-gdh and pMD-apxIVA. The copy numbers of recombinant plasmid standards pMD-gdh and pMD-apxIVA were 9.343×1010 copies/μL and 8.644×1010 copies/μL, respectively. The standard curve established with the recombinant plasmids is depicted in Figure 2. In the gradient concentration range of 103 copies/μL to 107 copies/μL, the copy number of S. suis recombinant plasmid has a good linear relationship with the Cq value. The linear equation is Y=-3.57X+47.72, and the amplification efficiency is 95.9% with R2 value 0.997. The copy number of A. pleuropneumoniae recombinant plasmid also had a good linear relationship with the Cq value. The linear equation is Y=-3.14X+40.88, the amplification efficiency is 104.4%, and the R2 value is 0.999.
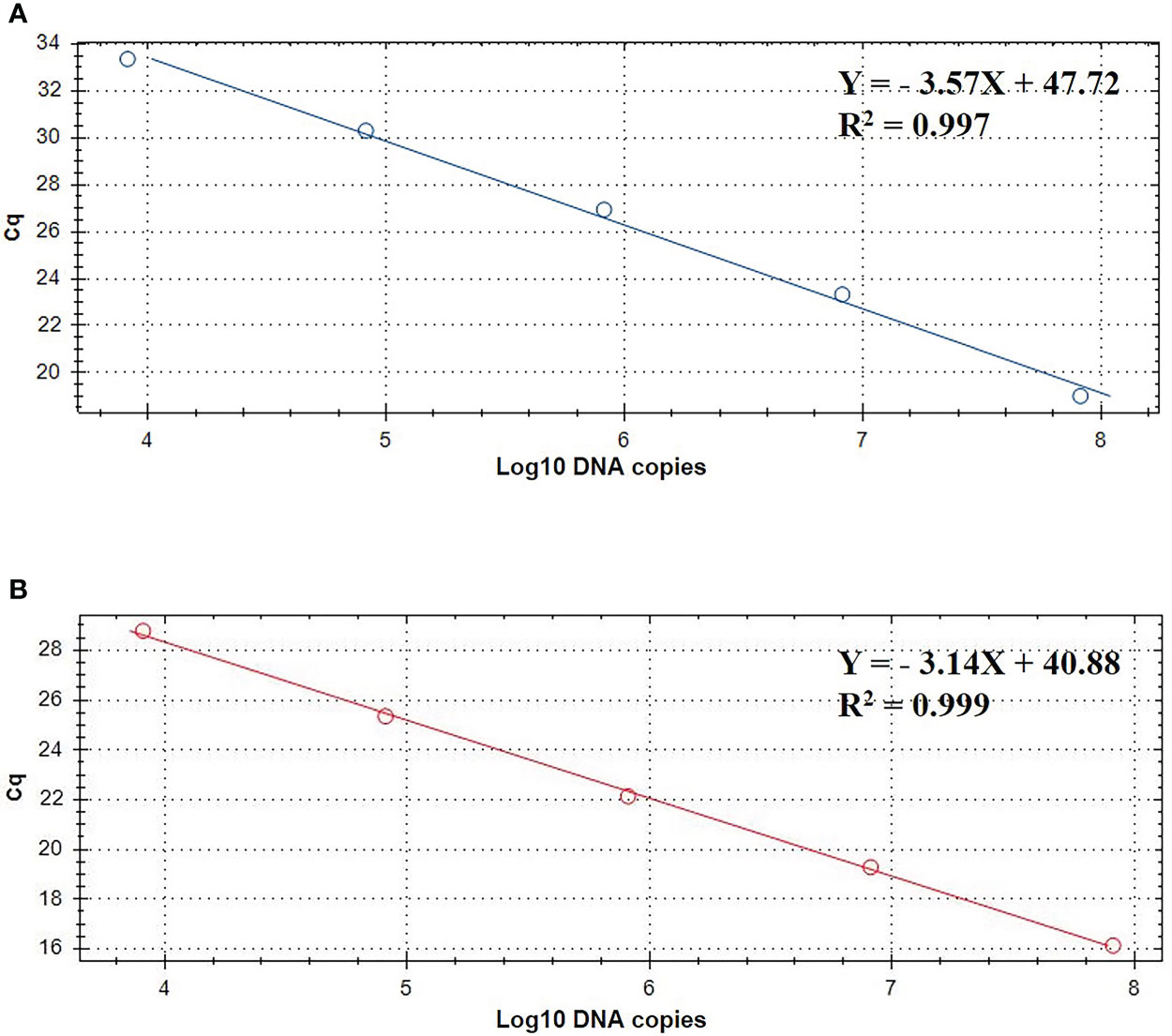
Figure 2 TaqMan standard curve of 10- fold dilutions of linearized plasmids pMD-gdh (A) and pMD-apxIVA (B) ranging from 103 to 107 DNA copies.
Since standard curves are a vital part of real-time PCR analysis, their accuracy and precision must be confirmed. In this study, the standard curve slopes are -3.57, -3.14 for S. suis and A. pleuropneumoniae, respectively. Moreover, the efficiencies are between 90% to 120%, suggesting the standard curve of this real-time PCR is considered reliable.
Sensitivity and reproducibility
The sensitivity of TaqMan real-time PCR was analyzed using 10-fold serial dilutions of recombinant plasmids (pMD-gdh and pMD- apxIVA) DNA (100 copies/μL~104 copies/μL). The limit of detection using TaqMan real-time PCR was 10 copies/μL (Figure 3), while the limit of detection using conventional PCR is 103 copies/μL (Figure 3). Traditional bacterial isolation and identification tests were used to detect the same samples, and the results were consistent with the results of conventional PCR, and all showed true positive. This result indicates that the sensitivity of TaqMan real-time PCR is 100 times that of conventional PCR.
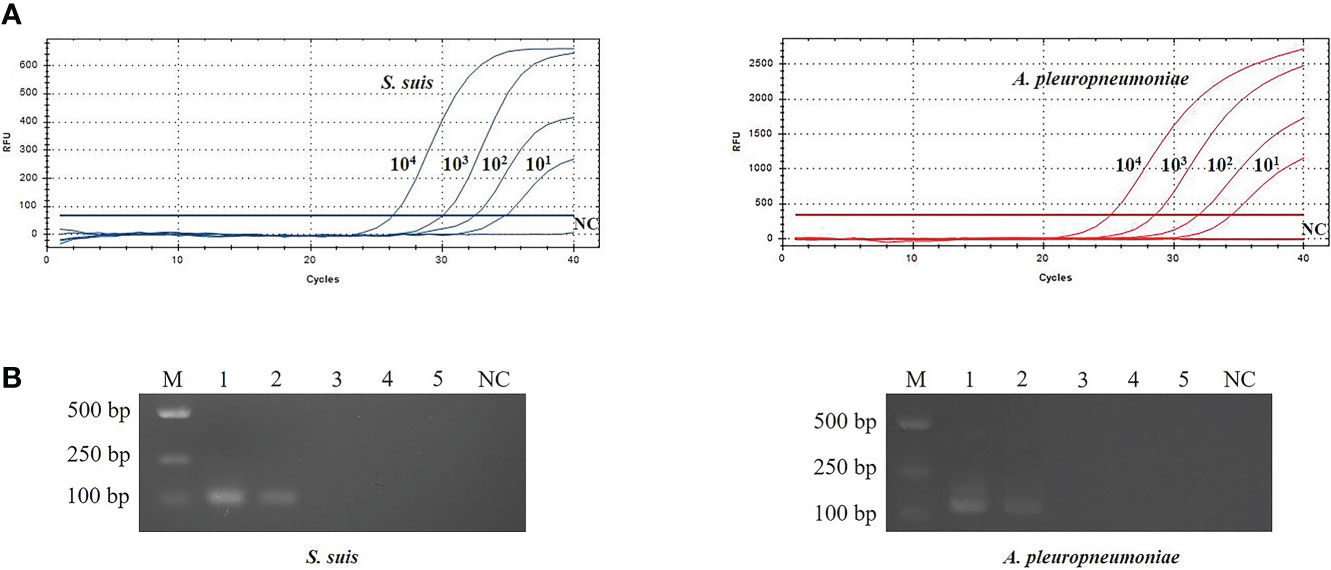
Figure 3 Evaluation of sensitivity for TaqMan real-time PCR (A) and conventional PCR (B).The plasmid template dilution concentrations of S. suis and A. pleuropneumoniae were 7.534×104~7.534×100 copies/μL and 6.555×104~6.555×100 copies/μL, respectively. M: DL2000 Marker; B1-5: 104 copies/μL-100 copies/μL plasmid standards; NC, Negative control.
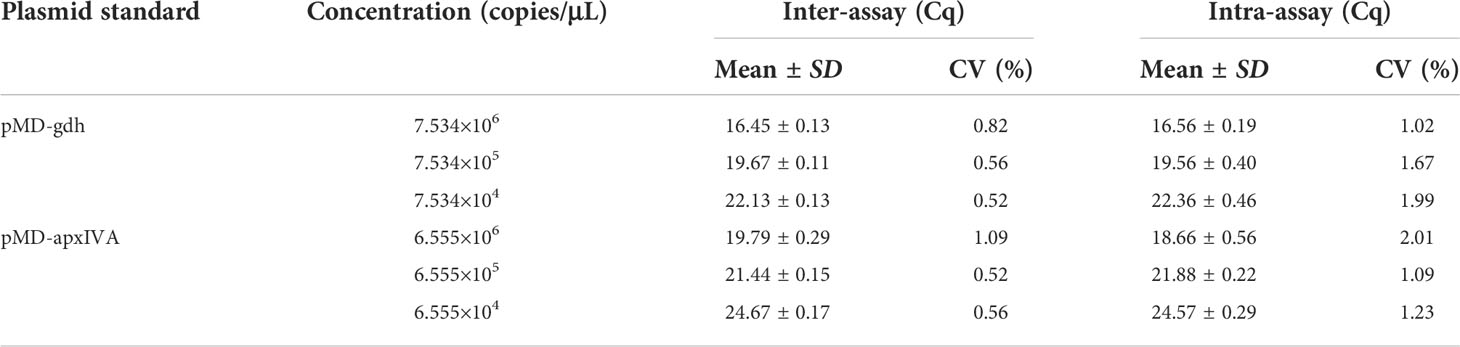
In order to evaluate the reproducibility of TaqMan PCR, the serially diluted recombinant plasmids (pMD-gdh and pMD-apxIVA) were repeatedly tested every other week. As shown in Table 2, the coefficient of variation (CV) of pMD-gdh and pMD-apxIVA in the inter-assay and intra-assay were less than 3%, respectively. The results showed that the TaqMan real-time PCR method established in this study had good reproducibility.
Calibration curves
Calibration curves for bacterial quantity and gene copy number were created for absolute quantification of cells. The calibration curve for S. suis bacteria was Y=1.02X-1.06 and the R2 value was 0.98 (Figure 4). The A. pleuropneumoniae concentration calibration curve was Y=1.09X-0.48 and the R2 value was 0.98 (Figure 4).
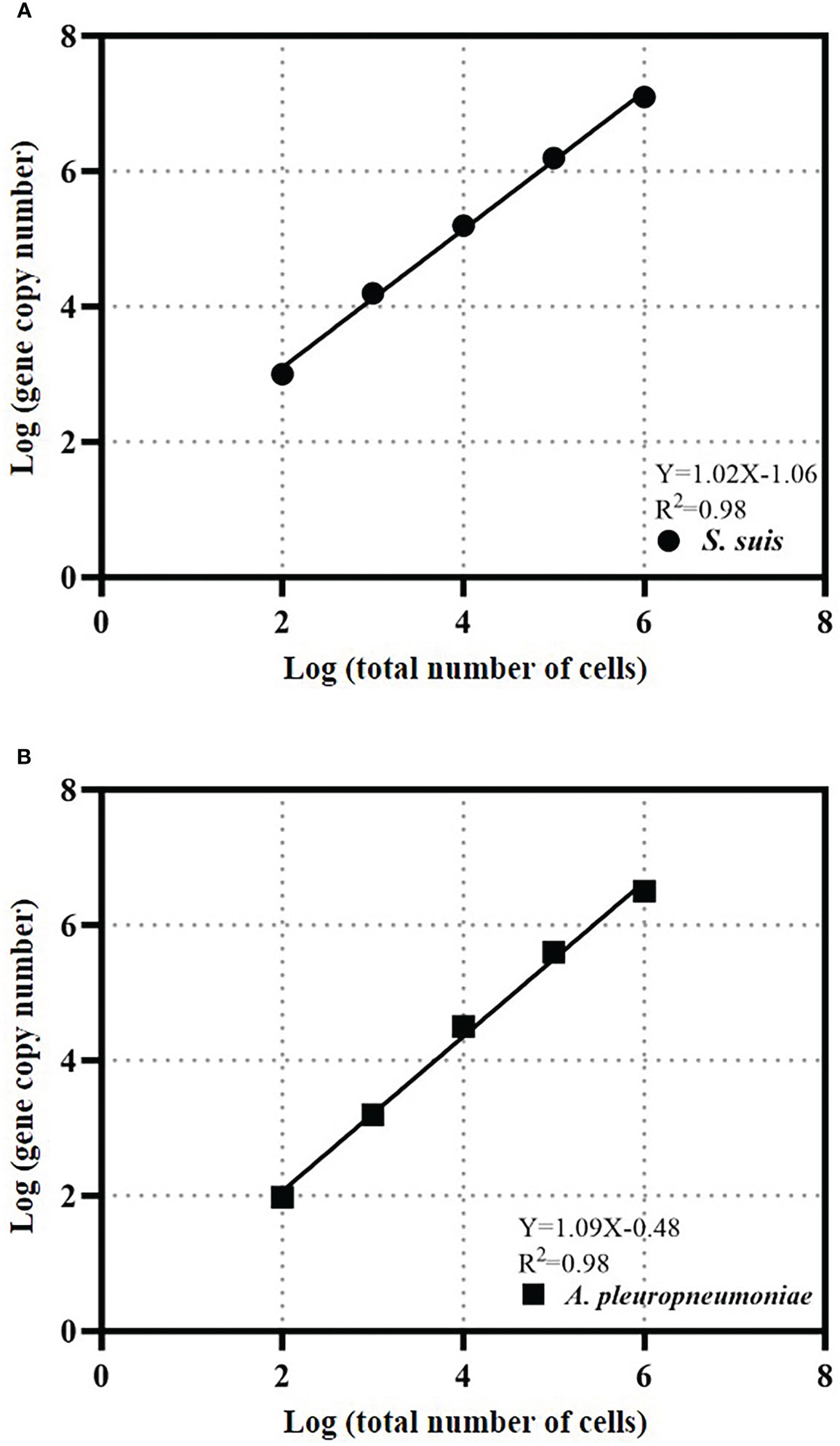
Figure 4 TaqMan real-time PCR estimates gene copy abundance and bacterial abundance of S. suis (A) or A. pleuropneumoniae (B). The data is expressed as an average with 95% confidence interval band (n = 3).
The cell calibration curve can avoid deviations that may be caused by the gene copy number or DNA extraction efficiency of each bacterium. The slopes of the cell calibration curves of S. suis and A. pleuropneumoniae are close to 1, which indicates that the gene copy number increases proportionally to the bacterial concentration in the test range, and the gene copy number of each bacterium does not change. The R2 values that the gdh gene of S. suis and copy number of apxIVA gene of A. pleuropneumoniae can be used to quantify bacterial quantity.
Quantification of S. suis and A. pleuropneumoniae in clinical samples
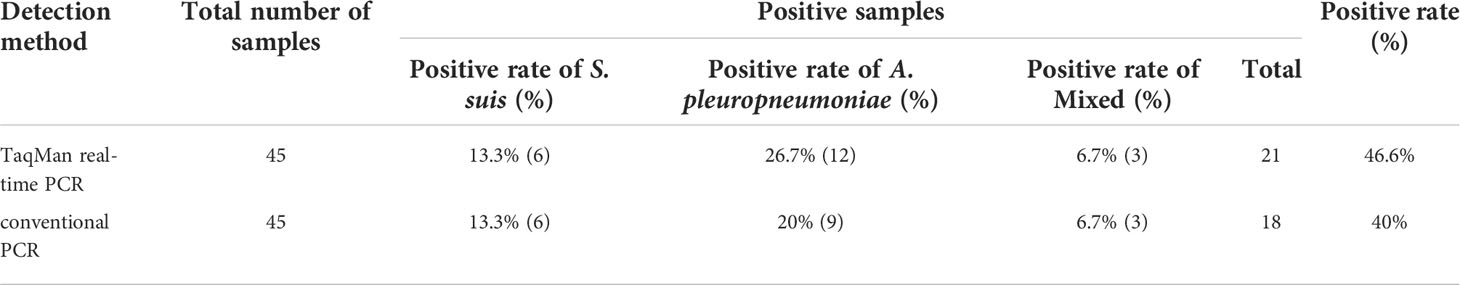
A total of 45 clinical samples were used for evaluating the real-time PCR assay developed in this study. The suspected samples were analyzed by both TaqMan real-time PCR and conventional PCR. The results are presented as the percentage of positive samples. As reported in Table 3, the positive rate of TaqMan real-time PCR was 46.6% (21/45), including 13.3% (6/45) of S. suis infection, 26.7% (12/45) of A. pleuropneumoniae infection and 6.7% (3/45) of S. suis and A. pleuropneumoniae mixed infection. However, the positive rate of conventional PCR was 40% (18/45), including 13.3% (6/45) of S. suis infection, 20% (9/45) of A. pleuropneumoniae infection and 6.7% (3/45) of S. suis and A. pleuropneumoniae mixed infection. Traditional bacterial isolation and PCR identification tests were used to detect the same samples, and the results were consistent with TaqMan real-time PCR. The sensitivity of TaqMan real-time PCR was higher than that of conventional PCR.
Quantification of S. suis and A. pleuropneumoniae in biofilm samples
Then, this TaqMan real-time PCR assay were also used for quantification of S. suis and A. pleuropneumoniae in biofilm system. For this purpose, a known amount of S. suis and A. pleuropneumoniae were added to a biofilm sample, and then amplified to assess the performance of the designed TaqMan real-time PCR assays in a biofilm system. The minimum detection limit of S. suis and A. pleuropneumoniae biofilm was 103 CFUs/mL (Figure 5). The TaqMan real-time PCR assays revealed the initial abundance of S. suis and A. pleuropneumoniae in the biofilm to be approximately 9.56×106 copies/mL and 7.89×106 copies/mL, respectively. In the sample with 108 CFUs/mL of S. suis added to the biofilm, 3.37×108 copies/mL S. suis was detected with no detection of any additional A. pleuropneumoniae compared to the initial abundance in the biofilm (P > 0.05) (Figure 6). 108 CFUs bacterial of A. pleuropneumoniae were added to the biofilm sample, and 2.89×108 copies/mL A. pleuropneumoniae was detected. The addition of 108 CFUs/mL S. suis and 108 CFUs/mL A. pleuropneumoniae in biofilm samples gave the very similar results as when added alone (P> 0.05), which were 4.88×108 copies/mL and 1.56×108 copies/mL, respectively. It was thus concluded that each bacterium could be accurately quantitative in a biofilm sample.
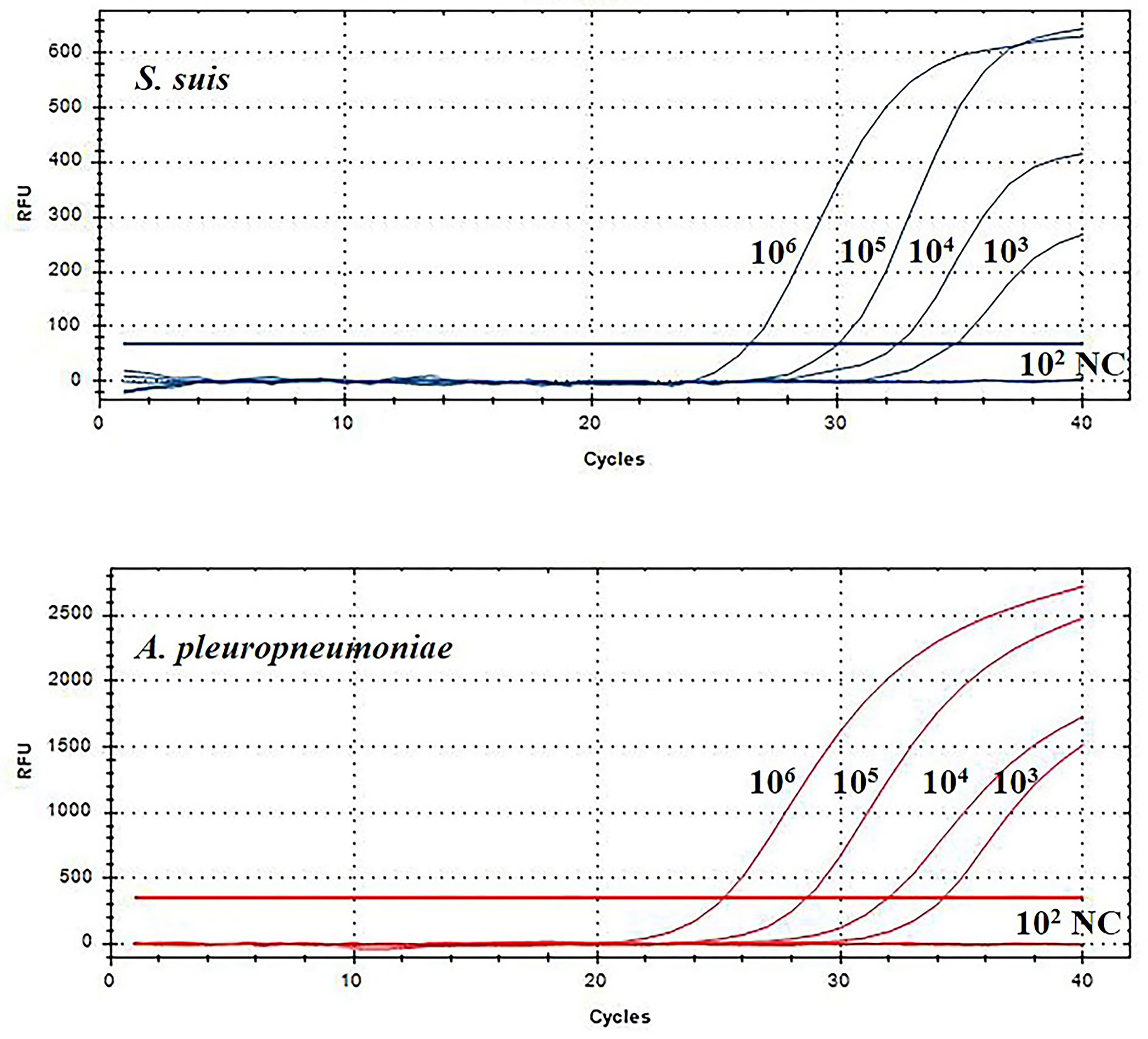
Figure 5 TaqMan real-time PCR analysis of biofilm sensitivity. The biofilm dilution multiple of S. suis and A. pleuropneumoniae was 102-106 CFUs/mL; NC, Negative control.
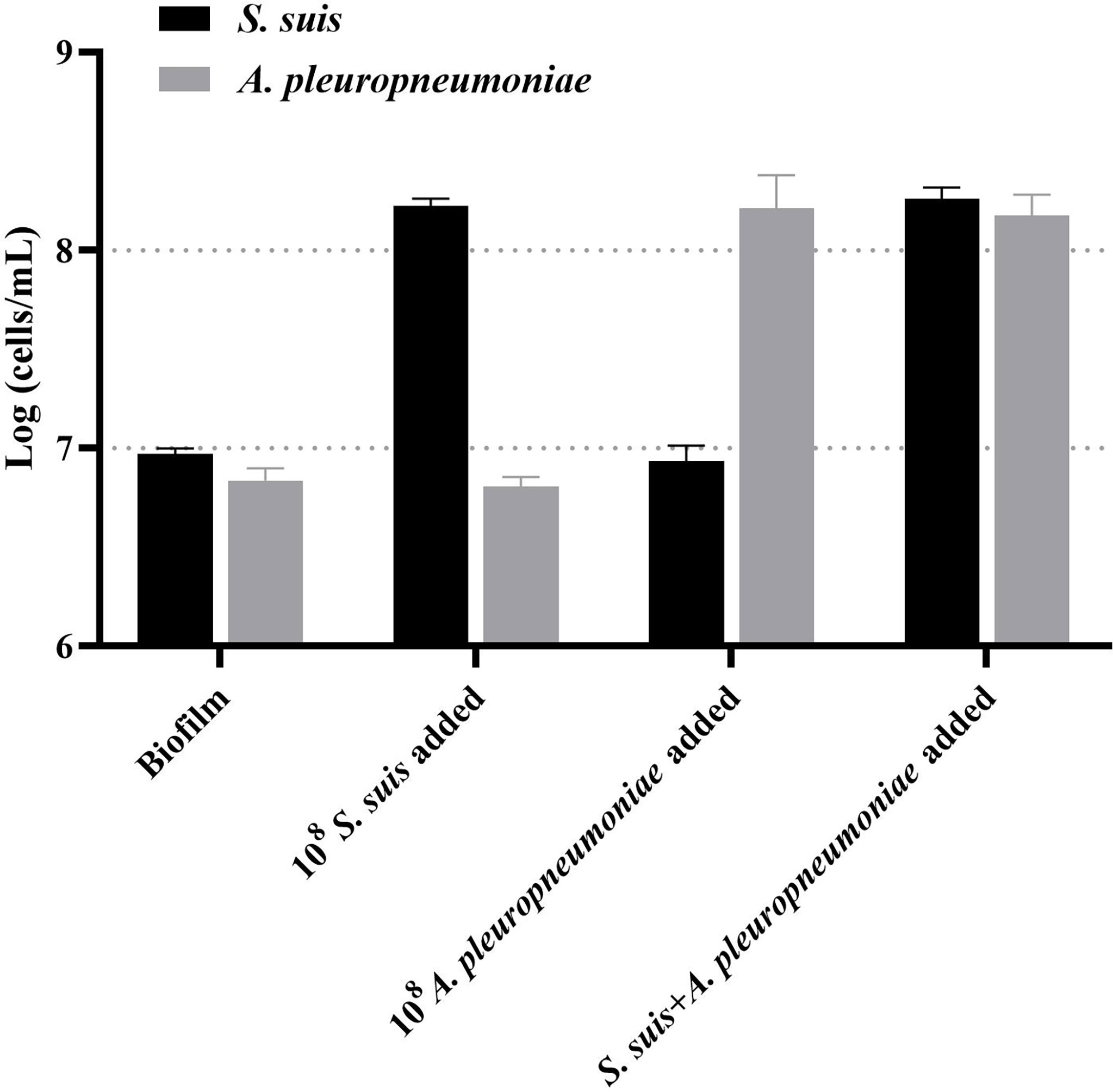
Figure 6 TaqMan real-time PCR analysis of biofilm samples, including: no added planktonic bacteria, 108 CFUs/mL S. suis, 108 CFUs/mL A. pleuropneumoniae, or the two bacterial species; data are expressed as mean ± standard deviation (n = 3).
Discussion
In recent years, respiratory diseases constitute one of the most important health issues affecting the swine industry worldwide. Polymicrobial respiratory diseases have also been threatening the swine industry worldwide. S. suis and A. pleuropneumoniae are important pathogens associated with PRDC. Their co-occurrence in the same site of infection has been frequently reported (Opriessnig et al., 2011), resulting in serious mixed infection of pigs, increasing the lethality, which has seriously affected the development of swine industry. Although the traditional detection methods such as PCR and ELISA provide a reliable method to distinguish S. suis and A. pleuropneumoniae respectively, it is labor intensive, complex and time consuming (Srinivasan et al., 2016; Teshima et al., 2017; Xia et al., 2017; Srijuntongsiri et al., 2022). Nowadays, reducing cost and time of experiment is critical for pathogen detection. Therefore, the rapid and accurate detection method has the potential to be of great significance for the diagnosis and preventing the co-infection of S. suis and A. pleuropneumoniae. Through literature review and practice, we found that real-time PCR is a powerful and easy to use technology (Kralik and Ricchi, 2017). Thus, this study developed a TaqMan real-time PCR assay that simultaneously detected and differentiated S. suis and A. pleuropneumoniaeare, which exhibited efficiently identification in clinical samples and mixed biofilms.
In this study, a TaqMan real-time PCR assay targeting the gdh gene of S. suis and the apxIVA gene of A. pleuropneumoniae was designed and validated. First, the gdh gene of S. suis and the apxIVA gene of A. pleuropneumoniae were sequenced to confirm the identity of the two bacterial species used for this study. Following this step, the confirmed gene sequences were used to design bacterial species-specific real-time PCR primers and probes. Then, the real-time PCR reaction system was established and optimized, and standard curves for the real-time PCR assays were developed. Finally, the specificity, sensitivity and reproducibility of the assay were evaluated, and the assay was validated by cross-reactivity tests and measuring known concentrations of each bacterium in biofilm samples. The results showed that the method has high sensitivity, high-throughput and strong specificity. This method can accurately quantify not only S. suis and A. pleuropneumoniae in mixed infection, but also S. suis and A. pleuropneumoniae in mixed biofilm samples. In order to prevent the long-term coexistence of S. suis and A. pleuropneumoniae resulting in the formation of biofilms, it provides a new technical means for the rapid detection of the co-infection of S. suis and A. pleuropneumoniae (Wang et al., 2020). The results of this study serve as a proof of concept and exemplifies successful application of TaqMan real-time PCR for the specific quantification of S. suis and A. pleuropneumoniae in mixed-community biofilms in vitro for the first time. At the same time, by quantifying and comparing the biomass of each species in a mixed biofilm, we can better understand the interaction between the species in the mixed biofilm. With the demand for faster and more accurate detection of clinical materials, this TaqMan real-time PCR detection method may become an invaluable tool.
The methods commonly used to study mixed biofilms have several limitations. For example, crystal violet staining and viable cell count have poor reproducibility when analyzing multi-species biofilms due to bacterial coaggregation (Stepanovic et al., 2000; Martinez and Casadevall, 2007). The method combining laser confocal scanning microscopy (CLSM) and fluorescence in situ hybridization (FISH) is only suitable for qualitative studies of biofilm, but is not suitable for high-throughput comparative screening studies (Bridier et al., 2014; Reichhardt and Parsek, 2019). Accurate detection of S. suis and A. pleuropneumoniae in biofilm samples showed that the designed TaqMan real-time PCR assay is an effective method for quantifying biofilms. On the contrary of the crystal violet staining method, this procedure can quantify each species in a mixed biofilm. Live cell counting can also quantify individual species in a mixed biofilm, but it has many influencing factors. For example, live bacteria are affected by sample processing, and consequently there is an error in the counting process. The method established in this study also has some limitations. One is that it cannot distinguish between live and dead bacteria. The second is that the method established in this study may not be applicable to all strains. Consequently, it is very important that various methods can verify and complement each other when studying mixed biofilms. We can use these three methods of crystal violet staining, living cell counting and TaqMan real-time PCR together to complement each other.
Further studies are necessary in order to fully highlight the impact of mixed bacterial infections detection in disease prevention. In the future, our research group will focus on increase the number of primers, optimize the reaction conditions, improve the amplification efficiency and reduce the detection limit. TaqMan real-time PCR is combined with other molecular biology techniques to further improve the detection sensitivity, detection range and detection specificity. This qPCR technique can be widely used for the detection of bacterial mixed infection, it may represent a reference for the rapid detection of bacterial pathogens in the breeding industry, and finally it lays a foundation for the prevention and control of porcine respiratory diseases.
Data availability statement
The original contributions presented in the study are included in the article/supplementary files. Further inquiries can be directed to the corresponding authors.
Author contributions
YW and SW designed the study and approved the manuscript. LY, LS, DG, MG and MJ developed the multiplex PCR method, analyzed data and drafted the manuscript. MJ, HW and QF contribute to reagents/materials/analysis tools. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (32172852, 31902309 and 31972654), the National Key Research and Development Program of China (2018YFD0500104), and Excellent Youth Foundation of He’nan Scientific Committee (222300420005), the Scientific and Technical Innovation Project of the Chinese Academy of Agricultural Sciences (SHVRI-ASTIP-2014-8).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bridier, A., Briandet, R., Bouchez, T., Jabot, F. (2014). A model-based approach to detect interspecific interactions during biofilm development. Biofouling 30 (7), 761–771. doi: 10.1080/08927014.2014.923409
Burmolle, M., Ren, D., Bjarnsholt, T., Sorensen, S. J. (2014). Interactions in multispecies biofilms: Do they actually matter? Trends Microbiol. 22 (2), 84–91. doi: 10.1016/j.tim.2013.12.004
Cao, H., Shockey, J. M. (2012). Comparison of TaqMan and SYBR green qPCR methods for quantitative gene expression in tung tree tissues. J. Agric. Food Chem. 60 (50), 12296–12303. doi: 10.1021/jf304690e
Costerton, J. W., Stewart, P. S., Greenberg, E. P. (1999). Bacterial biofilms: a common cause of persistent infections. Science 284 (5418), 1318–1322. doi: 10.1126/science.284.5418.1318
Elias, S., Banin, E. (2012). Multi-species biofilms: living with friendly neighbors. FEMS Microbiol. Rev. 36 (5), 990–1004. doi: 10.1111/j.1574-6976.2012.00325.x
Figueiredo, A. M. S., Ferreira, F. A., Beltrame, C. O., Cortes, M. F. (2017). The role of biofilms in persistent infections and factors involved in ica-independent biofilm development and gene regulation in Staphylococcus aureus. Crit. Rev. Microbiol. 43 (5), 602–620. doi: 10.1080/1040841X.2017.1282941
Harriott, M. M., Noverr, M. C. (2009). Candida albicans and Staphylococcus aureus form polymicrobial biofilms: Effects on antimicrobial resistance. Antimicrob. Agents Chemother. 53 (9), 3914–3922. doi: 10.1128/AAC.00657-09
Horter, D., Chang, C. C., Pogranichnyy, R., Zimmerman, J., Yoon, K. J. (2001). Persistence of porcine reproductive and respiratory syndrome in pigs. Adv. Exp. Med. Biol. 494, 91–94. doi: 10.1007/978-1-4615-1325-4_14
Kirakodu, S. S., Govindaswami, M., Novak, M. J., Ebersole, J. L., Novak, K. F. (2008). Optimizing qPCR for the quantification of periodontal pathogens in a complex plaque biofilm. Open Dent. J. 2, 49–55. doi: 10.2174/1874210600802010049
Kolter, R., Greenberg, E. P. (2006). Microbial sciences: the superficial life of microbes. Nature 441 (7091), 300–302. doi: 10.1038/441300a
Kralik, P., Ricchi, M. (2017). A basic guide to real time PCR in microbial diagnostics: definitions, parameters, and everything. Front. Microbiol. 8. doi: 10.3389/fmicb.2017.00108
Martinez, L. R., Casadevall, A. (2007). Cryptococcus neoformans biofilm formation depends on surface support and carbon source and reduces fungal cell susceptibility to heat, cold, and UV light. Appl. Environ. Microbiol. 73 (14), 4592–4601. doi: 10.1128/AEM.02506-06
Mullebner, A., Sassu, E. L., Ladinig, A., Frombling, J., Miller, I., Ehling-Schulz, M., et al. (2018). Actinobacillus pleuropneumoniae triggers IL-10 expression in tonsils to mediate colonisation and persistence of infection in pigs. Vet. Immunol. Immunopathol. 205, 17–23. doi: 10.1016/j.vetimm.2018.10.008
Opriessnig, T., Gimenez-Lirola, L. G., Halbur, P. G. (2011). Polymicrobial respiratory disease in pigs. Anim. Health Res. Rev. 12 (2), 133–148. doi: 10.1017/S1466252311000120
Reichhardt, C., Parsek, M. R. (2019). Confocal laser scanning microscopy for analysis of Pseudomonas aeruginosa biofilm architecture and matrix localization. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.00677
Rendueles, O., Ghigo, J. M. (2012). Multi-species biofilms: how to avoid unfriendly neighbors. FEMS Microbiol. Rev. 36 (5), 972–989. doi: 10.1111/j.1574-6976.2012.00328.x
Segura, M., Fittipaldi, N., Calzas, C., Gottschalk, M. (2017). Critical Streptococcus suis virulence factors: Are they all really critical? Trends Microbiol. 25 (7), 585–599. doi: 10.1016/j.tim.2017.02.005
Srijuntongsiri, G., Mhoowai, A., Samngamnim, S., Assavacheep, P., Bosse, J. T., Langford, P. R., et al. (2022). Novel DNA markers for identification of actinobacillus pleuropneumoniae. Microbiol. Spectr. 10 (1), e0131121. doi: 10.1128/spectrum.01311-21
Srinivasan, V., McGee, L., Njanpop-Lafourcade, B. M., Moisi, J., Beall, B. (2016). Species-specific real-time PCR assay for the detection of Streptococcus suis from clinical specimens. Diagn. Microbiol. Infect. Dis. 85 (2), 131–132. doi: 10.1016/j.diagmicrobio.2016.02.013
Stepanovic, S., Vukovic, D., Dakic, I., Savic, B., Svabic-Vlahovic, M. (2000). A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 40 (2), 175–179. doi: 10.1016/s0167-7012(00)00122-6
Suzuki, N., Yoshida, A., Nakano, Y. (2005). Quantitative analysis of multi-species oral biofilms by TaqMan real-time PCR. Clin. Med. Res. 3 (3), 176–185. doi: 10.3121/cmr.3.3.176
Teshima, K., Lee, J., To, H., Kamada, T., Tazumi, A., Hirano, H., et al. (2017). Application of an enzyme-linked immunosorbent assay for detection of antibodies to Actinobacillus pleuropneumoniae serovar 15 in pig sera. J. Vet. Med. Sci. 79 (12), 1968–1972. doi: 10.1292/jvms.17-0374
Wang, Y., Gong, S., Dong, X., Li, J., Grenier, D., Yi, L. (2020). In vitro mixed biofilm of Streptococcus suis and Actinobacillus pleuropneumoniae impacts antibiotic susceptibility and modulates virulence factor gene expression. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.00507
Wang, L., Li, Y., Wang, L., Zhang, H., Zhu, M., Zhang, P., et al. (2018). Extracellular polymeric substances affect the responses of multi-species biofilms in the presence of sulfamethizole. Environ. Pollut. 235, 283–292. doi: 10.1016/j.envpol.2017.12.060
Weinert, L. A., Chaudhuri, R. R., Wang, J., Peters, S. E., Corander, J., Jombart, T., et al. (2015). Genomic signatures of human and animal disease in the zoonotic pathogen Streptococcus suis. Nat. Commun. 6, 6740. doi: 10.1038/ncomms7740
Whelan, J. A., Russell, N. B., Whelan, M. A. (2003). A method for the absolute quantification of cDNA using real-time PCR. J. Immunol. Methods 278 (1-2), 261–269. doi: 10.1016/S0022-1759(03)00223-0
Xia, X. J., Wang, L., Shen, Z. Q., Qin, W., Hu, J., Jiang, S. J., et al. (2017). Development of an indirect dot-PPA-ELISA using glutamate dehydrogenase as a diagnostic antigen for the rapid and specific detection of Streptococcus suis and its application to clinical specimens. Antonie. Van. Leeuwenhoek. 110 (4), 585–592. doi: 10.1007/s10482-016-0825-z
Keywords: Streptococcus suis, Actinobacillus pleuropneumoniae, TaqMan real-time PCR, co-infection, biofilm
Citation: Yi L, Jin M, Gao M, Wang H, Fan Q, Grenier D, Sun L, Wang S and Wang Y (2022) Specific quantitative detection of Streptococcus suis and Actinobacillus pleuropneumoniae in co-infection and mixed biofilms. Front. Cell. Infect. Microbiol. 12:898412. doi: 10.3389/fcimb.2022.898412
Received: 17 March 2022; Accepted: 12 July 2022;
Published: 03 August 2022.
Edited by:
Anders P Hakansson, Lund University, SwedenReviewed by:
Janet MacInnes, University of Guelph, CanadaAdina R. Bujold, University of Guelph, Canada
Copyright © 2022 Yi, Jin, Gao, Wang, Fan, Grenier, Sun, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Wang, d2FuZ3lvY2VhbkAxNjMuY29t; Shaohui Wang, c2h3YW5nMDgyN0AxMjYuY29t
†These authors have contributed equally to this work
 Li Yi
Li Yi Manyu Jin
Manyu Jin Mengxia Gao
Mengxia Gao Haikun Wang
Haikun Wang Qingying Fan
Qingying Fan Daniel Grenier
Daniel Grenier Liyun Sun
Liyun Sun Shaohui Wang
Shaohui Wang Yang Wang
Yang Wang