- 1Department of Bio & Medical Big Data (BK4 Program), Division of Life Sciences, Research Institute of Natural Science (RINS), Gyeongsang National University (GNU), Jinju, Republic of Korea
- 2Department of Pharmaceutical Engineering, Kyungnam University, Changwon, Republic of Korea
- 3Division of Applied Life Science (BK21 Four), ABC-RLRC, PMBBRC, Gyeongsang National University, Jinju, Republic of Korea
Orthopoxvirus is one of the most notorious genus amongst the Poxviridae family. Monkeypox (MP) is a zoonotic disease that has been spreading throughout Africa. The spread is global, and incidence rates are increasing daily. The spread of the virus is rapid due to human-to-human and animals-to-human transmission. World Health Organization (WHO) has declared monkeypox virus (MPV) as a global health emergency. Since treatment options are limited, it is essential to know the modes of transmission and symptoms to stop disease spread. The information from host–virus interactions revealed significantly expressed genes that are important for the progression of the MP infection. In this review, we highlighted the MP virus structure, transmission modes, and available therapeutic options. Furthermore, this review provides insights for the scientific community to extend their research work in this field.
1 Introduction to viruses
Viruses are organisms that cause infectious diseases globally and their infections to humans have been observed since ancient times (Chappell and Dermody, 2015). Studies on viruses and viral diseases began in 19th century (Chappell and Dermody, 2015). Reports have shown that at least 219 virus species can cause infections in humans (Woolhouse et al., 2012). The first virus to be identified by Iwanovsky and Beijerinck was the tobacco mosaic virus in 1892 and the foot-and-mouth disease virus by Loeffler and Frosch in 1898 (van Kammen, 1999; Woolhouse et al., 2012; Chappell and Dermody, 2015). In humans, the first discovery was the yellow fever virus in 1901 (Woolhouse et al., 2012; Chappell and Dermody, 2015). Since then, multiple viruses have been discovered.
Several viruses have been infecting humans and posing a challenge in finding effective therapeutics (Wong et al., 2017), such as human immunodeficiency virus (HIV) (Lloyd, 1996; Simon et al., 2006) and SARS-CoV-2 (Dhama et al., 2020; Hu et al., 2021; Rampogu and Lee, 2021a). Particularly, the SARS-CoV-2 has hampered the health system, and this virus also warrants the scientific community to be ready for future pandemics. One such infectious diseases is monkeypox, which has been recently spreading globally.
2 Monkeypox
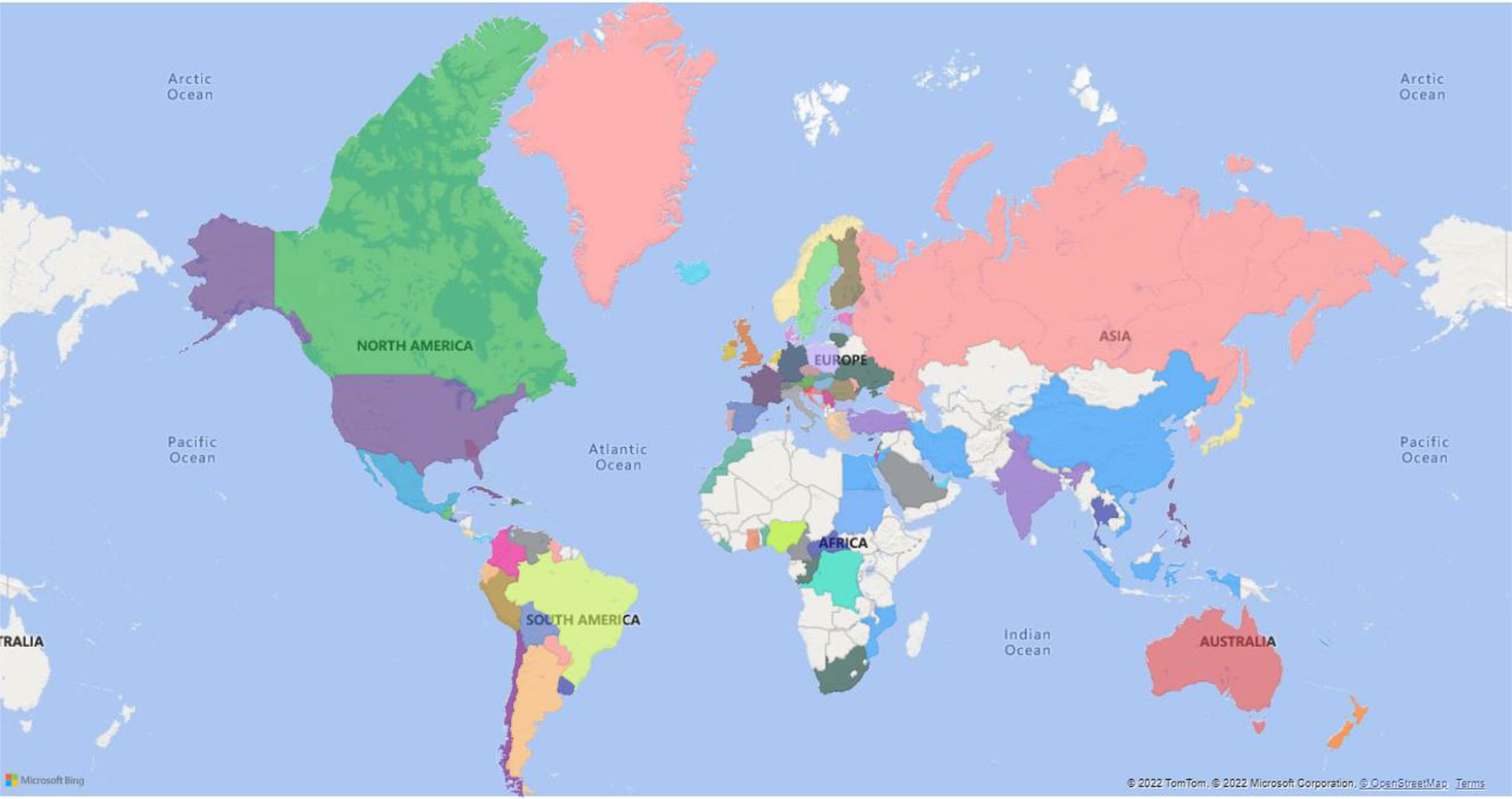
Monkeypox (MP) is a zoonotic disease that demonstrates smallpox-like characteristics in humans and is related to variola virus, the causative agent of small pox (Bunge et al., 2022). Monkeypox virus (MPV) belongs to the family: Poxviridae, subfamily: chordopoxvirinae, genus: orthopoxvirus, and species: Monkeypox virus (Moore et al., 2022). Other members of this genus include cowpox, camelpox, and vaccinia (VV) (Pauli et al., 2010; McCollum and Damon, 2014; Silva et al., 2020). As on 14th October 2022, a total of 73,288 cases have been recorded according to https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.htm. Distribution of MPV infection in different countries (Figure 1).
3 Monkeypox and smallpox
Interestingly, variola virus (VARV) and MPV are related antigenically and genetically (Shchelkunov et al., 2001; Cann et al., 2013). However, these viruses vary in the regions of their sequence for virulence and host-range factors at the genome termini (Shchelkunov et al., 2001; Cann et al., 2013). Upon comparing the VARV strain Japan 1951 (Harper, Masterseed) and MPV strains (Zaire 96-I-16), the length of MPV genome was 10,678 bp larger than Variola virus (Shchelkunov et al., 2001; Esposito et al., 2006; Cann et al., 2013). Furthermore, MPV has displayed mutations that influence the process of translation of interferon resistance genes that code for proteins C3L and E3L. The MPV genome also encodes a secreted interleukin (IL)-1β-binding protein, which is absent in VARV genomes. The absence of this vaccinia virus (VACV) - gene corresponds to amplified pathogenicity (Cann et al., 2013).
4 History of MPV
The advent of MPV was primarily observed in monkeys shipped from Singapore to Copenhagen in 1958 by von Magnus et al. (Cho and Wenner, 1973; Moore et al., 2022). The name monkey virus is coined after its first discovery from monkeys (Bunge et al., 2022). Approximately, 20-30% of animals have manifested clinical illness (Cho and Wenner, 1973; Moore et al., 2022). Notably, out of the 6 cases in 1970, the first human infection was reported in an infant (9 months old) from the Democratic Republic of the Congo (Ladnyj et al., 1972; Cho and Wenner, 1973; Moore et al., 2022). Four other infections were observed in children from Bouduo, Liberia aged 4-9 years (Cho and Wenner, 1973), and the other case was in a male of 24 years from Sierra Leone (Cho and Wenner, 1973). In 2003, MPV cases were recorded in the USA that is outside Africa (Bunge et al., 2022). Notably, this is caused due to animal-to- human transmission (Reed et al., 2004). Between 2018 and 2021, the MPV spread was observed in the USA, the UK, Israel, and Singapore (Mauldin et al., 2022). By 2022, MPV reached 31 countries (Xiang and White, 2022) with no travel history to endemic countries, and the virus isolates are reported as West African clade (Xiang and White, 2022).
5 Genome and structure of MPV
The MPV genome is a linear genome with a size of approximately 197 kb (196,858-base pairs) (Shchelkunov et al., 2001; Alkhalil et al., 2009) comprising ≈190 non-overlapping ORFs >180 nt in length (Kugelman et al., 2014). The central coding region sequence is positioned at ≈56000–120000 nucleotides (Kugelman et al., 2014). This conserved region is flanked by variable ends that comprise inverted terminal repeats (ITRs). The genome encodes biological machinery that is essential for the survival of the virus (Alkhalil et al., 2009). Genes in the central region are necessary for entry, self-replication, and maturation (Alkhalil et al., 2009). The less conserved terminal regions are useful for host-virus interaction (Alkhalil et al., 2009).At the ITR zone, a minimum of 4 ORFs are present (Kugelman et al., 2014).
There are two clades present in the MPV: the Congo Basin strain and West African strain (Kindrachuk et al., 2012). The Congo Basin strain is also known as the Central African strain (ZAI-96). The West African strains are SL-V70, COP-58, and WRAIR-6 (Weaver and Isaacs, 2008). These clades demonstrate dissimilar virulence (Kindrachuk et al., 2012) and are geographically, clinically, and genetically different (Kindrachuk et al., 2012). The Congo Basin strain demonstrated a case fatality rates of approximately 10% noticed in the people who are non-vaccinated than the less lethal West African MPV clade (Kindrachuk et al., 2012). Furthermore, the fatality rate differs with the strain, with 3.6% with the West African clade and 10.6% with the Central African clade (Bunge et al., 2022).
The West African strain is linked with lower transmission within the humans (Hutson et al., 2009), with a difference of 0.55-0.56% nucleotide between both the strains (Chen et al., 2005). A difference of 0.01–0.07% nucleotides is observed among the West African strains (Chen et al., 2005). A recent study conducted through shotgun metagenomics, showed that the MPV belongs to clade 3 and presumably has a single origin (Isidro et al., 2022). Another phylogenetic study was conducted on African monkeypox (Nakazawa et al., 2015). The authors examined fairly large genomic sequence data obtained from the MPV isolates across the area of their distribution to determine the relationship between the clades and among the isolates and further improve the phylogenetic analysis (Nakazawa et al., 2015).
Furthermore, the amplified virulence observed in the Central African strains is believed to be due to D14L (complement inhibitor), D10L (host range protein), B14R (interleukin [IL]-1β binding protein), B10R (apoptotic regulator), and B19R (serine protease inhibitor-like protein) genes (Estep et al., 2011) (Karumathil et al., 2018). The West African strains are devoid of D14L (Estep et al., 2011). The orthologs of D10L and B19R orthologs are conserved in both clades (Chen et al., 2005). The orthologs of ZAI-96, B10R and B14R are absent in West African strain (Chen et al., 2005). Additionally, selective repression of the host response is noticed in Central African strain demonstrated by its ability to regulate apoptosis in the host (Kumar et al., 2022).
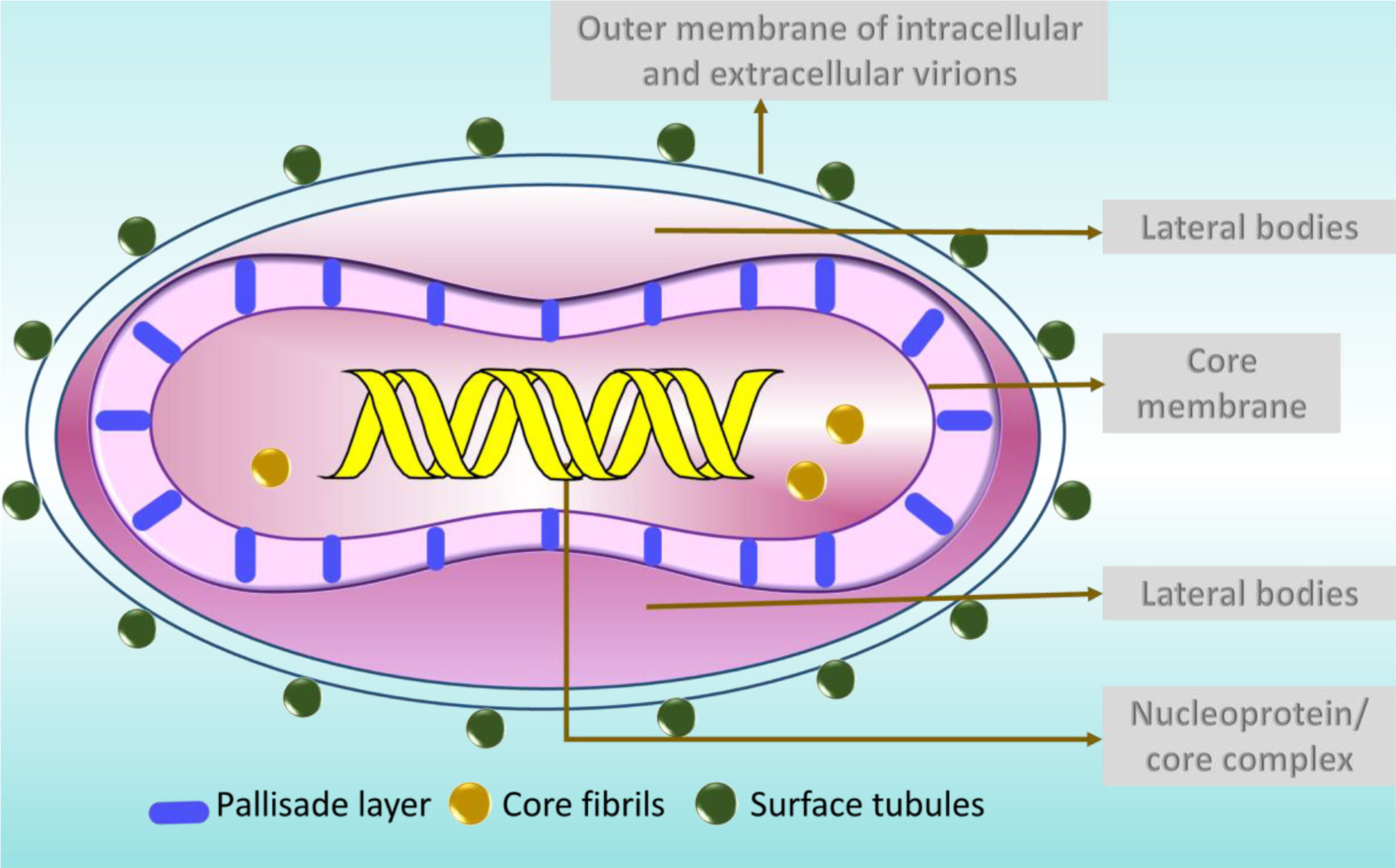
MPV is one of the largest and most highly complex viruses (Barreto-Vieira and Barth, 2015) demonstrating a brick-shaped structure with a length of 220 - 450 nm and a width ranging from 140 - 260 nm (Hyun, 2022). It has four components: core, lateral bodies, outer membrane, and outer lipoprotein envelope (Sklenovská, 2020). The core is the central part, encompassed by core fibrils and double stranded viral DNA. This layer is encircled by a rigid structure called the palisade layer (Sklenovská, 2020). The outer membrane accommodates the palisade layer, lateral bodies and the central core (Sklenovská, 2020). The structures called the surface tubules and are present on the outer surface (Sklenovská, 2020) (Figure 2).
Typically two types of virions are noticed in the negatively stained preparations of orthopoxviruses, namely the M form (mulberry) and C form (capsule) (Jezek and Fenner, 1988). The M-type virions are undamaged, while the C forms are the damaged ones (Jezek and Fenner, 1988). In C type virions, the stain percolates the cell, exposing the outer membrane and partially exposing the internal structures (Jezek and Fenner, 1988). Clinically, the M-types is present in the vesicle fluid and the C type in dried scabs (Jezek and Fenner, 1988).
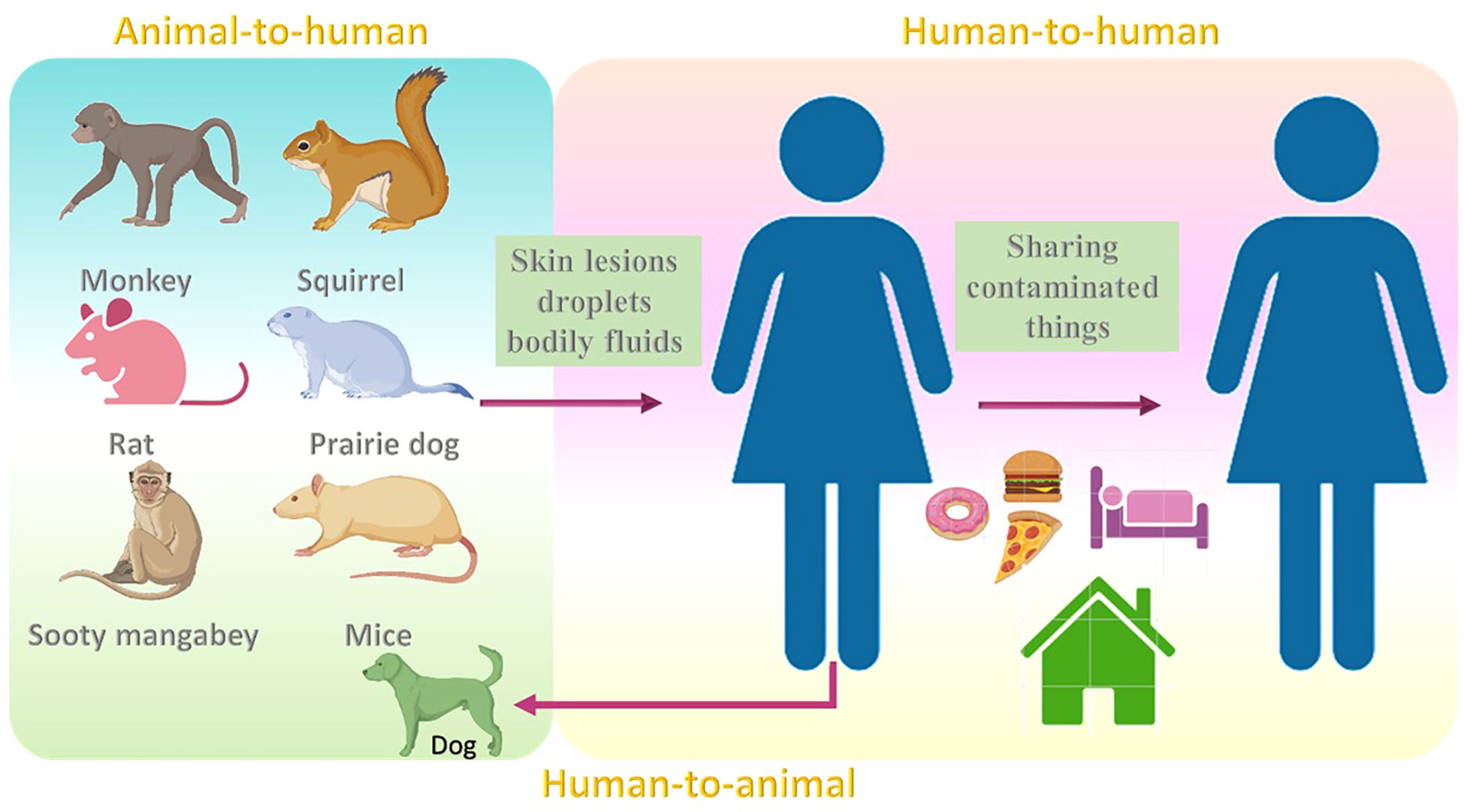
6 Reservoir and transmission
The common reservoir for MPV is thought to be not only monkeys (Moore et al., 2022), but also other animals such as squirrels (Khodakevich et al., 1987), and sooty mangabey. (Radonić et al., 2014). Although the rate at which the virus resides among animals is still obscure, rodents are thought to be the reservoir hosts for this virus (Nolen et al., 2015; Wilson, 2017). Infections may also occur in mice, rats, humans (Learned et al., 2005; Nolen et al., 2016; Besombes et al., 2019), and prairie dogs (Tesh et al., 2004; Moore et al., 2022). MPV transmission may occur when a healthy individual comes into contact with the skin lesions, droplets, and bodily fluids of infected animals. Partially cooked food might also be a reason for the infection (Ahmed et al., 2022). The infection may also result from to contact with contaminated fomites (Moore et al., 2022). Additionally, the infections may also result due to activities that enhance exposure to animals, such as sleeping on the ground outside and habituating near the forest (Fuller et al., 2011; Guagliardo et al., 2020). The risk of infection may also be noted when eating bushmeat or wild game (Kmiec and Kirchhoff, 2022).
In 2018, MPV transmission was observed from an infected patient to a healthcare assistant which might be due to contact with bedding that was contaminated (Vaughan et al., 2020). Human-to-human transmission has also been noted in the Democratic Republic of the Congo (Nolen et al., 2016). Transmission within humans can also occur by sharing the same household and consuming food or water from the same dish as the patient (Bunge et al., 2022). In 2022, the United Kingdom witnessed an increase in MPV cases, categorized into three different events (Vivancos et al., 2022). The first case was in Nigerian imports, the second case was a cluster in a household, and the third case was reported in men with no reported link with previous cases or the travel history (Vivancos et al., 2022). One study reported the first case of MPV transmission from a human to dog (Seang et al., 2022). A recent report showed that out of 528 infections across 16 countries, 98% were bisexual or gay, 75% of the infections were seen within the white, and 41% were positive for HIV (Thornhill et al., 2022) (Figure 3).
7 Replication
To gain insight onto MPV replication, we relied on pox virus replication. Poxvirus replication occurs in the cytoplasm in specific structures known as Guarnieri bodies (Kieser et al., 2020; Kaler et al., 2022). These bodies are also called factories obtained from infecting particles (Kieser et al., 2020). The count (number) of factories depends on the multiplication rate of infection (Moss, 2013). The factory is the site where transcription translation, and assembly of virions take place (Moss, 2013).
Generally, there exists two types of infectious forms of MPV: the mature virion [MV, also called intracellular mature virions (IMV)] and the enveloped virion (EV, also called extracellular enveloped virions (EEVs)) and cell-associated enveloped virions (CEVs) (Chung et al., 2006; Moss, 2012). The MV has a single membrane, and the EV has an additional outer membrane that is cleaved before fusion. When the MV is enclosed within an endosomal membrane or trans-Golgi, it forms a triple-membrane. These are hence termed wrapped virions (WVs, also called intracellular enveloped virions (IEVs) (Moss, 2012). Unenclosed MVs remain free until cell lysis occurs (Chung et al., 2006; Moss, 2012). Both MV and EV are infectious and can spread the disease (Moss, 2012; Sklenovská, 2020). Comparatively, the stable MV transmits the infection between the host animals, while EVs with fragile external membranes are instrumental in spreading the disease within the host (Moss, 2012; Sklenovská, 2020).
The proteins facilitate the attachment of the virus to the cell, fusion of the membrane and entry into the host cell (Kaler et al., 2022). The single membrane in MV and an extra outer membrane in the EV perform the disruption prior to fusion (Kaler et al., 2022). There are a total of four viral proteins connected to the MV that help in the process of attachment of MV to the host cell (Kaler et al., 2022).
The virus attaches to the host via 11 to 12 non-glycosylated transmembrane proteins (4- to 43-kDa) (Moss, 2012; Kaler et al., 2022). In some pox viruses, laminin and heparine sulfate aid in the attachment (Kmiec and Kirchhoff, 2022). After infection DNA synthesis is initiated for no more than 2 hours (Moss, 2013). For replication, MV initially uncoats to gain entry into the cytoplasm (Sklenovská, 2020). The early genes are then expressed after the inactivation of the cells defence mechanisms. This inactivation is achieved by the generation of prepackaged viral proteins and the enzymatic factors (Sklenovská, 2020). Subsequently, the early messenger RNA (mRNA) is synthesized through the DNA-dependent RNA polymerase of the virus. The translated early mRNA assists another uncoating mechanism, replication of DNA, and generation of intermediate transcription factors. (Sklenovská, 2020). Then the transcription and translation of intermediate mRNA occurs to promote the late mRNAs expression. Further, the translation of late mRNAs into structural and nonstructural proteins initiates (Sklenovská, 2020). The proteins that are translated are gathered together with the concatemers of DNA that are processed in the earlier step of replication (Sklenovská, 2020). They are enclosed to form an immature virions (IMVs) that transform to MV which are devoid of external membrane and causes infection when liberated due to the disruption of the cell. (Hiller and Weber, 1985; Bray and Buller, 2004; Roberts and Smith, 2008) They then travel into the inner cell membrane aided by the microtubules and eventually fuses to from the cell-associated virions (CEVs). These trigger the actin polymerization and the development of the filaments. The CEVs exists the cell that are now called the extracellular enveloped virions (EEVs) (Roberts and Smith, 2008) (Figure 4).
8 Symptoms and clinical manifestations of MP
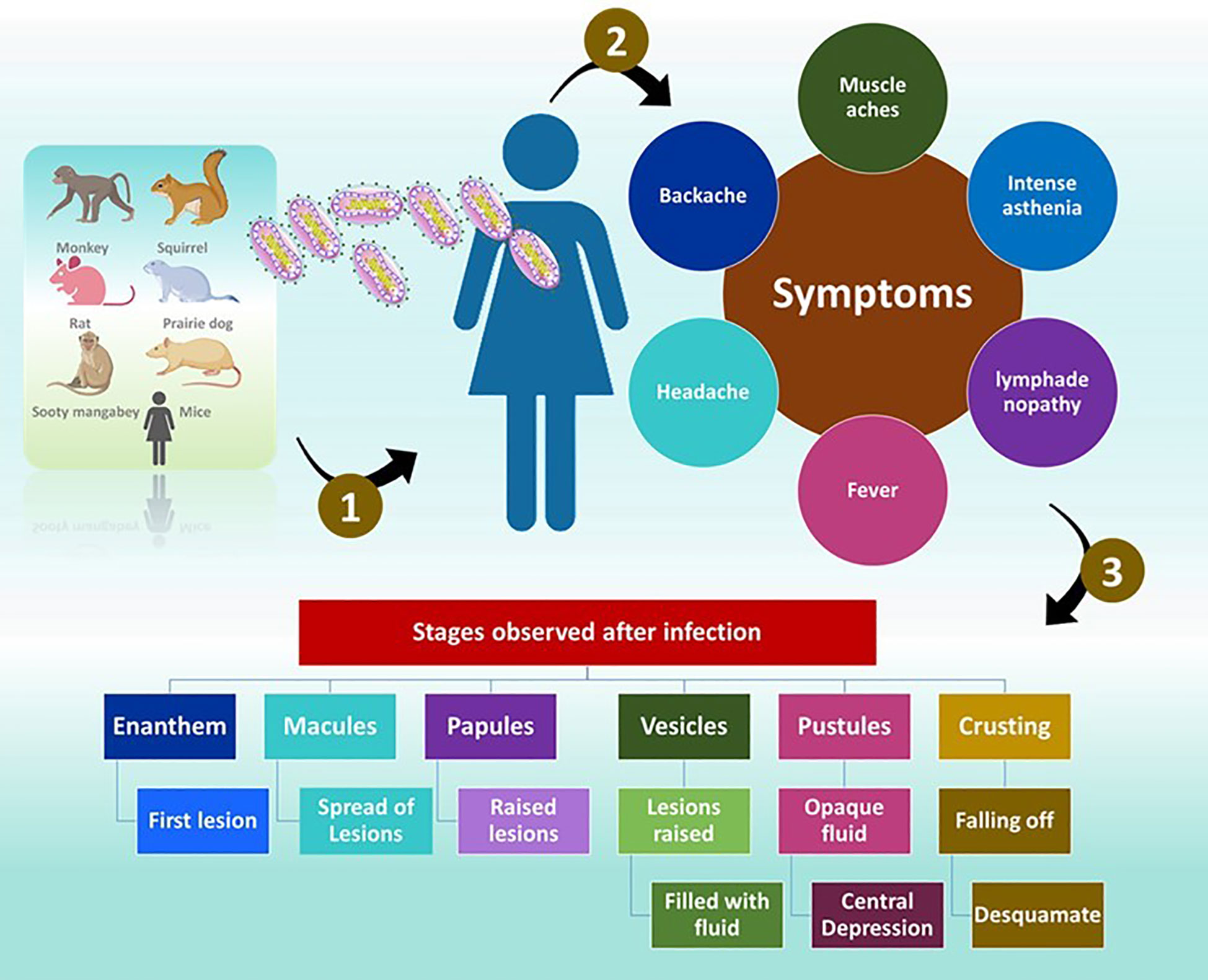
The symptoms of MP are similar to those of smallpox but are generally milder (Ligon, 2004). Once exposed to an infection, the incubation period is between 10 and 14 days and followed by a prodromal period of two days (Weaver and Isaacs, 2008). The individuals demonstrate, muscle aches, backache, headache, intense asthenia, fever between 38.5°C and 40.5°C (McCollum and Damon, 2014) with swollen lymph nodes. Often, patients experience discomfort and exhaustion (Ligon, 2004). The feature that separates monkeypox from smallpox is lymphadenopathy (Moore et al., 2022). Mucosal lesions are observed in the mouth (enanthem) (Kaler et al., 2022) after 1 or 2 days. The skin lesions appear on the face and extremities. According to the World Health Organization (WHO) the face is highly affected, as noticed in 95% of the cases. In 75% of the cases, the palms and soles are affected and in 75% of the cases the oral mucous membranes are affected. The affect is also noticed on genitalia and conjunctivae in 30% and 20% of the cases.
The lesions progress through four stages namely, the macular (development of macular lesions), popular (the lesions are a slightly raised), vesicular (the lesions are raised clearly and filled with fluid), and pustula (lesions are filled with opaque fluid and forms a depression at the Centre and remain for about 5 - 7 days) in the next 2 - 4 weeks (Moore et al., 2022). After the pustular phase, the formation of the crust starts and desquamates in the next 7 - 14 day (Moore et al., 2022). Once the crusts are fallen off, the patients are no longer infectious (Moore et al., 2022). Lesions can cause dyspigmented scars in a few instances (Weaver and Isaacs, 2008).
The distribution of the lesion is typically centrifugal and appears firm, deep, well-circumscribed and umbilicated (McCollum and Damon, 2014). Additionally, dissimilarities were observed in the morphology of lesions between vaccinated and unvaccinated people (McCollum and Damon, 2014). In individuals those who were vaccinated <20 years before the infection, the lesions were smaller and fewer, with the centrifugal spreading of the rash (McCollum and Damon, 2014). The skin of patients is firm, inflamed, and painful before the formation of crusts (McCollum and Damon, 2014) (Figure 5).
Sequelae and serious complications have also been noticed usually in unvaccinated individuals of approximately 74% compared to those in the vaccinated individuals (39.5%) (McCollum and Damon, 2014). Patients develop pulmonary distress or bronchopneumonia indicating that an infection of the lungs could be a secondary infection (McCollum and Damon, 2014). In others, vomiting or diarrhoea leading to acute dehydration was noticed (McCollum and Damon, 2014). Encephalitis, ocular infections and septicaemia were also recorded, with an average case-fatality rate of 11% in unvaccinated patients with children being highly vulnerable (McCollum and Damon, 2014).
9 Virus-host interaction
It has been reported that the MPV encompasses a substantial number of accessory genes and notably hasa broader host range (Bonilla-Aldana and Rodriguez-Morales, 2022; Xiang and White, 2022). Interestingly, the loss of an accessory gene in approximately 17% of samples from West African strain demonstrated an association with an upsurge in human-to-human transmission (Xiang and White, 2022).
A gene-expression profile study was conducted to understand the viral-host biology (Alkhalil et al., 2010). The experiment was conducted on kidney epithelial cells (MK2) of Macaca mulatta using the GeneChip rhesus macaque genome microarrays (Alkhalil et al., 2010). The findings from this study demonstrate the function of ion channels, cell cycle regulators, histones, and actin play a role in MPV infection (Alkhalil et al., 2010).
A recent bioinformatics study based on transcriptome analysis was conducted to identify biomarkers and signaling pathways in cells infected with monkeypox (Tang et al., 2022). GSE36854 and GSE11234 were retrieved from Gene Expression Omnibus (GEO) (Tang et al., 2022). The results showed that from the GSE36854 dataset, 84 genes were significantly different. The protein-protein interaction (PPI) interactions and the identification of hub genes has revealed that the genes such as ZC3H12A, IER3, EREG, IFIT2, AREG, IL11, IFIT1 IER2, NFKBIE, and FST as the 10 hub genes. (Tang et al., 2022). The genes IFIT1 and IFIT2 (antiviral genes) were noticed to be remarkably repressed. Furthermore, upon searching the drugs that essentially target the hub genes, itraconazole and AP-26113 were found to stimulate the expression levels of IFIT1 and IFIT2 illuminating their ability as MP therapeutics (Tang et al., 2022).
To discover variations in the expression of genes, co-regulated genes, and the pathways that are of concern during MP progression, another research group worked on in vitro models that were infected with MPV (Xuan et al., 2022). The two models on which several analysis were performed were, rhesus monkey (Macaca mulatta) kidney epithelial (MK2) cells and human immortal epithelial cancer (HeLa) cells (Xuan et al., 2022). The results showed that in the animal cell line model, the prominent regulators were histamine, plasmin and cluster of differentiation 40 (CD40), whereas in the human cell line model, the neutrophil-related signaling pathways and macrophages were noted (Xuan et al., 2022). Additionally, in both models, certain genes that were remarkably expressed during the progression of the infection including, TNFAIP3, IL11, ADORA2A, DUOX1, BIRC3, PTX3, CXCL1, LIF, IER3, IL6, ZC3H12A, EGR1, CSF2, and CCL2. Additionally, epigenetic regulators were also observed, namely, HIST1H3D and HIST1H2BJ (Xuan et al., 2022). Furthermore, a computational study uncovered the over-expression of some histones in both humans and monkeys infected with monkeypox-infected cells (Xuan et al., 2022). The elevated histone members are HIST1H2BB, HIST1H2AK, HIST2H2AB, HIST1H2AC, HIST1H2BM, HIST1H2BJ, HIST1H2BH, HIST1H2AD, HIST1H3D, and HIST1H1B highlighting the possibility of the role epigenetic regulators in monkeypox infection (Xuan et al., 2022).
Another study also has identified the upregulation of histone genes namely, HIST1H2BJ, HIST4H4, HIST1H3I, HIST1H2AD, and, HIST1H1D, while H1F0, the linker histone was downregulated (Alkhalil et al., 2010). Certain genes such as MYCBP2, PRMT3, RARS2, FBXO11 and MYST2 were repressed (Alkhalil et al., 2010). During the infection, the repression of ten ion channels and transporters was observed. The cell cycle regulators and actin MPV infection were noticed to participate in MPV infection (Alkhalil et al., 2010). Furthermore, the disease biomarkers can be discovered through proteomics analysis (Wang et al., 2022).
Bourquain et al, conducted an experiment to determine how poxviruses vary their host cell gene expression. HeLa cells were selected, and the changes were recorded using microarrays that are representative of the whole human genome (Bourquain et al., 2013). HeLa cells were treated with CPXV Brighton Red (BR) reference strain, central African MPV strain MSF-6, or the mouse-pathogenic VACV strain IHD-W (Bourquain et al., 2013). In particular to the MPV results, merely 321 (1.1%) transcripts showed 2-fold expression variations with 219 (68.2%) upregulated transcripts and 102 downregulated transcripts (Bourquain et al., 2013). Interestingly, the results also showed common transcripts among infections (Bourquain et al., 2013). The MPV has regulated 321 host transcripts of which 241 (75.1%) were observed with cowpox virus (CPXV) and 148 (46.1%) within VACV infection (Bourquain et al., 2013). Genes such as DUSP5/6, SPRED1/2, and SPRY2/4 are upregulated along with EGR1 and EGR2. After infection, the EGR1 upregulation was observed via the MAPK-ERK pathway (Bourquain et al., 2013).
In MPV infection, the genes that take part in the negative regulation of MAPK activity and intracellular protein kinase cascade were enriched, and they induced genes associated with chemotaxis or leukocyte activation (Bourquain et al., 2013). Hammarlund et al, reported that monocytes infected with MPV do not recognize antiviral CD4+ and CD8+ T Cells (Hammarlund et al., 2008). MPV can cause infection in primary human monocytes without eliciting the production of inflammatory cytokines (IFNγ or TNFα) (Hammarlund et al., 2008). It was also reported that MPV impedes the activation of T- cell by VV, implying that MPV bears an immunomodulatory protein which is absent in VV (Hammarlund et al., 2008). This inhibition in trans can also obstruct the activation of T - cells by other virus-infected cells that are not precisely MPV-infected, suggesting that MPV is immunosuppressive and immune-evasive (Hammarlund et al., 2008). Cell-associated factor/factors generated in MPV hinder the activation of T - cells autonomously by MHC class I or class II processing or presentation (Hammarlund et al., 2008).
10 Treatments against MPV
10.1 Small molecule inhibitors
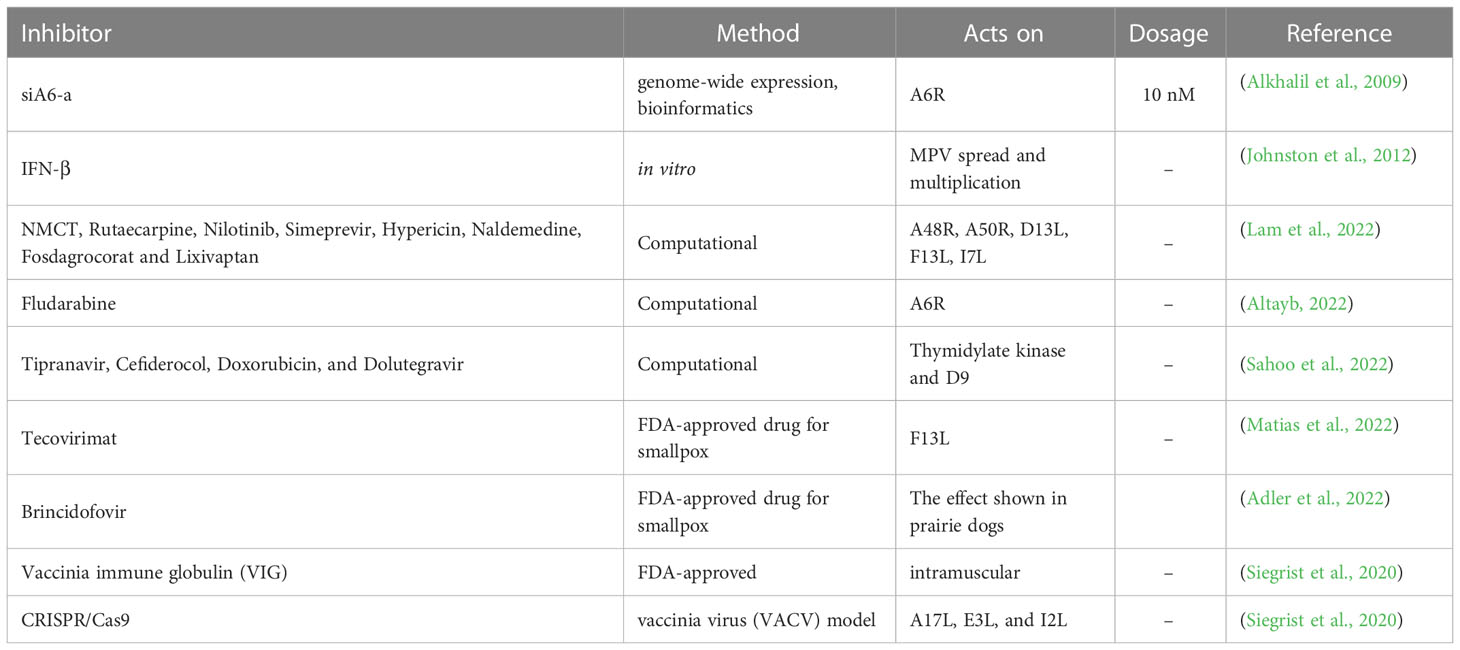
The replication of MPV can be brought about by the RNA interference using genome-wide expression studies combined with a bioinformatics approach (Alkhalil et al., 2009). Accordingly 12 viral genes were targeted using small interfering RNA (siRNAs). Eventually, siA6-a inhibited replication of the virus for 7 days when administered at 10 nM (Alkhalil et al., 2009). Additionally, this study also demonstrated the significance of the A6R gene in replication (Alkhalil et al., 2009). The use of interferon-β (IFN-β) is another approach to curb MPV spread and multiplication (Johnston et al., 2012). IFN-β has been approved by the FDA for treating multiple sclerosis (Johnston et al., 2012). IFN-β administration after 6-8 hours after infection remarkably inhibited the production and spread of MPV, thereby highlighting the potential of IFN-β as an effective therapeutic option against MPV (Johnston et al., 2012). In previous studies, silver-based nanoparticles have been found to repress plaque formation of MPV with a diameter of 10 nm (Rogers et al., 2008), suggesting the role of silver-based nanoparticles as MPV treatment option (Rogers et al., 2008).
Tecovirimat may offer improved survival when administered for up to 8 days after the lethal aerosol MPV challenge in cynomolgus macaques (Russo et al., 2018). It offers a shield from the clinical effects of the disease earlier than 5 days after the challenge (Russo et al., 2018). This FDA approved drug is used to treat smallpox (Smith et al., 2011; Grosenbach et al., 2018; Laudisoit et al., 2018; Russo et al., 2018). The compound tecovirimat is believed to repress the product of F13L gene which is present across orthopoxviruses (Matias et al., 2022) and is used for orthopoxvirus wrapping (Duraffour et al., 2008; Duraffour et al., 2015). The drug brincidofovir, a nucleotide analogue, has demonstrated promising results in animal models when assessed against MPV (Adler et al., 2022). Improved survival was observed in prairie dog models, administered with brincidofovir soon after monkeypox contact (Hutson et al., 2021; Siegrist and Sassine, 2022).
Computational methods are paramount in the development and design of new drugs. The predominant methods include molecular docking (Morris and Lim-Wilby, 2008; Rampogu and Lee, 2021c; Rampogu and Lee, 2021a), and molecular dynamics simulation (Durrant and McCammon, 2011; Rampogu and Lee, 2021a). Pharmacophore modeling can also be adapted when there are any experimentally known ligands/inhibitors or when the X-ray structure of the target protein is present (Yang, 2010; Rampogu et al., 2020). Homology modeling can be used to build a structure when the X-ray structure is not resolved (Muhammed and Aki-Yalcin, 2019). In one study, five targets were investigated, and compounds, such as NMCT, rutaecarpine, nilotinib, simeprevir, hypericin, naldemedine, fosdagrocorat and lixivaptan (Lam et al., 2022), were found to have potential inhibitory activities. These five targets were A48R, A50R, D13L, F13L and I7L (Lam et al., 2022). An in silico study revealed that the compound fludarabine is a potential inhibitor of the MPV target DNA-dependent RNA polymerase subunit (A6R) along with two other targets, protein catalysing the envelopment of intracellular mature virus particles (F13L) and proteins involved in cell entry (D8L) (Altayb, 2022). Sahoo et al., identified four potential inhibitors, Tipranavir, Cefiderocol, Doxorubicin, and Dolutegravir towards the targets thymidylate kinase and D9 (decapping enzyme) (Sahoo et al., 2022) (Table 1).
A study was conducted to evaluate the role of specific genes in viral replication and pathogenicity (Lopera et al., 2015). Correspondingly, a bioinformatics approach has been adapted to discover genomic regions in MPV possessing numerous virulence genes (Lopera et al., 2015). Following this, two regions were selected, and the study was conducted in vitro and in vivo after single deletion and double deletion of the selected genes. The results demonstrated that simultaneous deletion of both genes led to a decrease in replication observed in cell culture (Lopera et al., 2015). When either region was deleted, a remarkable amplified attenuation in vivo was observed (Lopera et al., 2015).
10.2 Vaccination
In addition to small molecules, vaccines are also widely used as a treatment option (Hooper et al., 2004). Notably, the smallpox vaccine has demonstrated protection in Nonhuman Primates against MPV (Hooper et al., 2004). It is reported that smallpox has provided approximately 85% protection against monkeypox (Bunge et al., 2022). JYNNEOS is a modified vaccinia ankara (MVA) vaccine that has been approved for MPV disease in adults ≥18 years of age (Rao et al., 2022). This was a subcutaneous injection administered at two doses with 28 days apart (Rao et al., 2022). In 2017, healthcare individuals were administered with IMVAMUNE® which is a smallpox vaccine (Petersen et al., 2019). This study aimed to understand the immunogenicity, effectiveness, and safety of IMVAMUNE® in patients at high risk for MPV (Petersen et al., 2019). Another smallpox vaccine, the live VACV Dryvax protects against monkeypox (Heraud et al., 2006; Earl et al., 2008). A multivalent DNA vaccine with eight VACVs virus (Western Reserve strain genes: A4L, A27L, A33R, A56R, B5R, F9L, H3L, and L1R) confers protection against MPV in nonhuman primates cynomolgus macaques (Hirao et al., 2011).
It has been observed that, patients vaccinated for smallpox showed immunity (orthopoxvirus [OPXV], IgG and memory B cells) upon exposure to MPV (Nguyen et al., 2021). Interestingly, the smallpox vaccine triggers both humoral and cell-mediated responses towards OPXV which also includes MPV (Nguyen et al., 2021). They target a host of viral elements, thereby hindering viral replication (Nguyen et al., 2021). Vaccinia immune globulin is an approved medication used after contacting the MPV, which is administered intramuscularly (Lederman et al., 2012; Siegrist et al., 2020; Parker et al., 2021). In one study, CRISPR/Cas9 was used to treat orthopoxviruses with VACV, which was used as a model organism (Siegrist et al., 2020). This study focused on the indispensable conserved genes A17L, E3L, and I2L using an adeno-associated virus as a vector (Siegrist et al., 2020). The results have shown a reduction in the viral titre further protecting host cells (Siegrist et al., 2020). An assay based on CRISPR-Cas12a and real-time PCR was used to detect MPV (Li et al., 2006; Sui et al., 2022).
In order to find effective therapeutics, few clinical trials have been conducted. Upon searching for clinical trial information, it was found that nine studies are currently being performed that are either on-going or completed (https://clinicaltrials.gov/)
11 Conclusion and future outlook
Viruses are notorious microorganisms that cause serious human infections. Pox viruses are not new as they exist in reptiles, birds, insects, and mammals. Hence, they are also known as ancient viruses (Alakunle et al., 2020). Human MP is a zoonotic disease that is recently spreading globally. In order to prevent the spread of the disease, the contact tracing is an important step (Titanji et al., 2022). If a person is in close proximity with a confirmed case of monkeypox, then that individual should be observed for development of symptoms for 21 days (Titanji et al., 2022).
Since the treatment options for monkeypox are limited (Kaler et al., 2022), the available research methods, such as computational drug discovery, could be an effective method to identify drugs (Ou-Yang et al., 2012; Sliwoski et al., 2014). This approach is useful for discovering new drugs over a short period (Leelananda and Lindert, 2016). Drug repurposing is another approach that can be used to identify mmediate candidate compounds (Pushpakom et al., 2018; Begley et al., 2021). This approach has been proven to be promising for the treatment of SARS-CoV-2. Remdesivir is one such candidate (Li and Peng, 2021). To prevent the spread of virus in the developing countries the awareness on health hygiene is essentially important as the samples from the excreta are reported to have monkeypox virus DNA (Singla and Shen, 2022). Additionally, the knowledge and treat from the pandemics and similar kind of diseases should be widely spread among all the sections of the people (Poland et al., 2022). Countries need to review their approach and preventive measure are to be taken during the outbreaks and be prepared for any such kind in the future (Poland et al., 2022). To conclude, since the spread of MPV is swift, it demands extra awareness and thoughtfulness to control the transmission and thereby to mitigate the disease and further its future reappearance.
Author contributions
SR and KW conceived the idea. SR wrote the manuscript. KW, S-WK, and YK reviewed the manuscript and revised. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1A5A8029490).
Acknowledgments
The icons are taken from BioRender.com and the figures are created accordingly.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adler, H., Gould, S., Hine, P., Snell, L. B., Wong, W., Houlihan, C. F., et al. (2022). Clinical features and management of human monkeypox: A retrospective observational study in the UK. Lancet Infect. Dis 22, 1153–1162. doi: 10.1016/S1473-3099(22)00228-6
Ahmed, M., Naseer, H., Arshad, M., Ahmad, A. (2022). Monkeypox in 2022: A new threat in developing. Ann. Med. Surg. 78, 103975. doi: 10.1016/j.amsu.2022.103975
Alakunle, E., Moens, U., Nchinda, G., Okeke, M. I. (2020). Monkeypox virus in Nigeria: Infection biology, epidemiology, and evolution. Viruses 12, 1–29. doi: 10.3390/v12111257
Alkhalil, A., Hammamieh, R., Hardick, J., Ichou, M. A., Jett, M., Ibrahim, S. (2010). Gene expression profiling of monkeypox virus-infected cells reveals novel interfaces for host-virus interactions. Virol. J. 7, 173. doi: 10.1186/1743-422X-7-173
Alkhalil, A., Strand, S., Mucker, E., Huggins, J. W., Jahrling, P. B., Ibrahim, S. M. (2009). Inhibition of monkeypox virus replication by RNA interference. Virol. J. 6, 188. doi: 10.1186/1743-422X-6-188
Altayb, H. N. (2022). Fludarabine, a potential DNA-dependent RNA polymerase inhibitor, as a prospective drug against monkeypox virus: A computational approach. Pharmaceuticals 15, 1–18. doi: 10.3390/ph15091129
Barreto-Vieira, D. F., Barth, O. M. (2015). Negative and positive staining in transmission electron microscopy for virus diagnosis. Microbiol. Agric. Hum. Heal. 45–56. doi: 10.5772/60511
Begley, C. G., Ashton, M., Baell, J., Bettess, M., Brown, M. P., Carter, B., et al. (2021). Drug repurposing: Misconceptions, challenges, and opportunities for academic researchers. Sci. Transl. Med. 13, eabd5524. doi: 10.1126/scitranslmed.abd5524
Besombes, C., Gonofio, E., Konamna, X., Selekon, B., Grant, R., Gessain, A., et al. (2019). Intrafamily transmission of monkeypox virus, central African republi. Emerg. Infect. Dis. 25, 1602–1604. doi: 10.3201/eid2508.190112
Bonilla-Aldana, D. K., Rodriguez-Morales, A. J. (2022). Is monkeypox another reemerging viral zoonosis with many animal hosts yet to be defined? Vet. Q. 42, 148–150. doi: 10.1080/01652176.2022.2088881
Bourquain, D., Dabrowski, P. W., Nitsche, A. (2013). Comparison of host cell gene expression in cowpox, monkeypox or vaccinia virus-infected cells reveals virus-specific regulation of immune response genes. Virol. J. 10, 61. doi: 10.1186/1743-422X-10-61
Bray, M., Buller, M. (2004). Looking back at smallpox. Clin. Infect. Dis. 38, 882–889. doi: 10.1086/381976
Bunge, E. M., Hoet, B., Chen, L., Lienert, F., Weidenthaler, H., Baer, L. R., et al. (2022). The changing epidemiology of human monkeypox-a potential threat? A systematic review. PLos Negl. Trop. Dis. 16, e0010141. doi: 10.1371/journal.pntd.0010141
Cann, J. A., Jahrling, P. B., Hensley, L. E., Wahl-Jensen, V. (2013). Comparative pathology of smallpox and monkeypox in man and macaques. J. Comp. Pathol. 148, 6–21. doi: 10.1016/j.jcpa.2012.06.007
Chappell, J. D., Dermody, T. S. (2015). Biology of viruses and viral diseases. Mand. Douglas Bennett’s Princ. Pract. Infect. Dis. 2, 1681–1693.e4. doi: 10.1016/B978-1-4557-4801-3.00134-X
Chen, N., Li, G., Liszewski, M. K., Atkinson, J. P., Jahrling, P. B., Feng, Z., et al. (2005). Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology 340, 46–63. doi: 10.1016/j.virol.2005.05.030
Cho, C. T., Wenner, H. A. (1973). Monkeypox virus. Bacteriol. Rev. 37, 1–18. doi: 10.1128/br.37.1.1-18.1973
Chung, C.-S., Chen, C.-H., Ho, M.-Y., Huang, C.-Y., Liao, C.-L., Chang, W. (2006). Vaccinia virus proteome: Identification of proteins in vaccinia virus intracellular mature virion particles. J. Virol. 80, 2127–2140. doi: 10.1128/JVI.80.5.2127-2140.2006
Dhama, K., Khan, S., Tiwari, R., Sircar, S., Bhat, S., Malik, Y. S., et al. (2020). Coronavirus disease 2019-COVID-19. Clin. Microbiol. Rev. 33, 1–48. doi: 10.1128/CMR.00028-20
Duraffour, S., Lorenzo, M. M., Zöller, G., Topalis, D., Grosenbach, D., Hruby, D. E., et al. (2015). ST-246 is a key antiviral to inhibit the viral F13L phospholipase, one of the essential proteins for orthopoxvirus wrapping. J. Antimicrob. Chemother. 70, 1367–1380. doi: 10.1093/jac/dku545
Duraffour, S., Vigne, S., Vermeire, K., Garcel, A., Vanstreels, E., Daelemans, D., et al. (2008). Specific targeting of the F13L protein by ST-246 affects orthopoxvirus production differently. Antivir. Ther. 13, 977–990. doi: 10.1177/135965350801300817
Durrant, J. D., McCammon, J. A. (2011). Molecular dynamics simulations and drug discovery. BMC Biol. 9, 71. doi: 10.1186/1741-7007-9-71
Earl, P. L., Americo, J. L., Wyatt, L. S., Espenshade, O., Bassler, J., Gong, K., et al. (2008). Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus. Proc. Natl. Acad. Sci. U. S. A. 105, 10889–10894. doi: 10.1073/pnas.0804985105
Esposito, J. J., Sammons, S. A., Frace, A. M., Osborne, J. D., Olsen-Rasmussen, M., Zhang, M., et al. (2006). Genome sequence diversity and clues to the evolution of variola (smallpox) virus. Science 313, 807–812. (80-. ). doi: 10.1126/science.1125134
Estep, R. D., Messaoudi, I., O’Connor, M. A., Li, H., Sprague, J., Barron, A., et al. (2011). Deletion of the monkeypox virus inhibitor of complement enzymes locus impacts the adaptive immune response to monkeypox virus in a nonhuman primate model of infection. J. Virol. 85, 9527–9542. doi: 10.1128/JVI.00199-11
Fuller, T., Thomassen, H. A., Mulembakani, P. M., Johnston, S. C., Lloyd-Smith, J. O., Kisalu, N. K., et al. (2011). Using remote sensing to map the risk of human monkeypox virus in the Congo basin. Ecohealth 8, 14–25. doi: 10.1007/s10393-010-0355-5
Grosenbach, D. W., Honeychurch, K., Rose, E. A., Chinsangaram, J., Frimm, A., Maiti, B., et al. (2018). Oral tecovirimat for the treatment of smallpox. N. Engl. J. Med. 379, 44–53. doi: 10.1056/NEJMoa1705688
Guagliardo, S. A. J., Doshi, R. H., Reynolds, M. G., Dzabatou-Babeaux, A., Ndakala, N., Moses, C., et al. (2020). Do monkeypox exposures vary by ethnicity? Comparison of aka and Bantu suspected monkeypox cases. Am. J. Trop. Med. Hyg. 102, 202. doi: 10.4269/ajtmh.19-0457
Hammarlund, E., Dasgupta, A., Pinilla, C., Norori, P., Früh, K., Slifka, M. K. (2008). Monkeypox virus evades antiviral CD4+ and CD8+ T cell responses by suppressing cognate T cell activation. Proc. Natl. Acad. Sci. 105, 14567–14572. doi: 10.1073/pnas.0800589105
Heraud, J.-M., Edghill-Smith, Y., Ayala, V., Kalisz, I., Parrino, J., Kalyanaraman, V. S., et al. (2006). Subunit recombinant vaccine protects against monkeypox. J. Immunol. 177, 2552–2564. doi: 10.4049/jimmunol.177.4.2552
Hiller, G., Weber, K. (1985). Golgi-derived membranes that contain an acylated viral polypeptide are used for vaccinia virus envelopment. J. Virol. 55, 651–659. doi: 10.1128/jvi.55.3.651-659.1985
Hirao, L. A., Draghia-Akli, R., Prigge, J. T., Yang, M., Satishchandran, A., Wu, L., et al. (2011). Multivalent smallpox DNA vaccine delivered by intradermal electroporation drives protective immunity in nonhuman primates against lethal monkeypox challenge. J. Infect. Dis. 203, 95–102. doi: 10.1093/infdis/jiq017
Hooper, J. W., Thompson, E., Wilhelmsen, C., Zimmerman, M., Ichou, M. A., Steffen, S. E., et al. (2004). Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J. Virol. 78, 4433–4443. doi: 10.1128/jvi.78.9.4433-4443.2004
Hu, B., Guo, H., Zhou, P., Shi, Z.-L. (2021). Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 19, 141–154. doi: 10.1038/s41579-020-00459-7
Hutson, C. L., Kondas, A. V., Mauldin, M. R., Doty, J. B., Grossi, I. M., Morgan, C. N., et al. (2021). Pharmacokinetics and efficacy of a potential smallpox therapeutic, brincidofovir, in a lethal monkeypox virus animal model. mSphere 6, 1–15. doi: 10.1128/mSphere.00927-20
Hutson, C. L., Olson, V. A., Carroll, D. S., Abel, J. A., Hughes, C. M., Braden, Z. H., et al. (2009). A prairie dog animal model of systemic orthopoxvirus disease using West African and Congo basin strains of monkeypox virus. J. Gen. Virol. 90, 323–333. doi: 10.1099/vir.0.005108-0
Isidro, J., Borges, V., Pinto, M., Sobral, D., Santos, J. D., Nunes, A., et al. (2022). Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat. Med. 28, 1569–1572. doi: 10.1038/s41591-022-01907-y
Hyun, J. (2022). Poxvirus under the eyes of electron microscope. Appl. Microsc, 52 (11), 1–9. doi: 10.1186/s42649-022-00080-3
Jezek, Z., Fenner, F. (1988). Human Monkeypox. Monogr Virol. Basel, Karger, 17, 6–32. doi: 10.1159/000416457
Johnston, S. C., Lin, K. L., Connor, J. H., Ruthel, G., Goff, A., Hensley, L. E. (2012). In vitro inhibition of monkeypox virus production and spread by interferon-β. Virol. J. 9, 5. doi: 10.1186/1743-422X-9-5
Kaler, J., Hussain, A., Flores, G., Kheiri, S., Desrosiers, D. (2022). Monkeypox: A comprehensive review of transmission, pathogenesis, and manifestation. Cureus 14. doi: 10.7759/cureus.26531
Karumathil, S., Raveendran, N. T., Ganesh, D., Kumar Ns, S., Nair, R. R., Dirisala, V. R. (2018). Evolution of synonymous codon usage bias in West African and central African strains of monkeypox virus. Evol. Bioinform. Online 14, 1176934318761368. doi: 10.1177/1176934318761368
Khodakevich, L., Szczeniowski, M., Manbu-ma-Disu, Jezek, Z., Marennikova, S., Nakano, J., et al. (1987). The role of squirrels in sustaining monkeypox virus transmission. Trop. Geogr. Med. 39, 115–122.
Kieser, Q., Noyce, R. S., Shenouda, M., Lin, Y.-C. J., Evans, D. H. (2020). Cytoplasmic factories, virus assembly, and DNA replication kinetics collectively constrain the formation of poxvirus recombinants. PLos One 15, e0228028. doi: 10.1371/journal.pone.0228028
Kindrachuk, J., Arsenault, R., Kusalik, A., Kindrachuk, K. N., Trost, B., Napper, S., et al. (2012). Systems kinomics demonstrates Congo basin monkeypox virus infection selectively modulates host cell signaling responses as compared to West African monkeypox virus. Mol. Cell. Proteomics 11, M111.015701. doi: 10.1074/mcp.M111.015701
Kmiec, D., Kirchhoff, F. (2022). Monkeypox: A new threat? Int. J. Mol. Sci. 23, 7866. doi: 10.3390/ijms23147866
Kugelman, J. R., Johnston, S. C., Mulembakani, P. M., Kisalu, N., Lee, M. S., Koroleva, G., et al. (2014). Genomic variability of monkeypox virus among humans, democratic republic of the Congo. Emerg. Infect. Dis. 20, 232–239. doi: 10.3201/eid2002.130118
Kumar, N., Acharya, A., Gendelman, H. E., Byrareddy, S. N. (2022). The 2022 outbreak and the pathobiology of the monkeypox virus. J. Autoimmun. 131, 102855. doi: 10.1016/j.jaut.2022.102855
Ladnyj, I. D., Ziegler, P., Kima, E. (1972). A human infection caused by monkeypox virus in basankusu territory, democratic republic of the Congo. Bull. World Health Organ. 46, 593–597.
Lam, H. Y. I., Guan, J. S., Mu, Y. (2022). In silico repurposed drugs against monkeypox virus. bioRxiv. doi: 10.1101/2022.07.17.500371
Laudisoit, A., Tepage, F., Colebunders, R. (2018). Oral tecovirimat for the treatment of smallpox. N. Engl. J. Med. 379, 2084–2085. doi: 10.1056/NEJMc1811044
Learned, L. A., Reynolds, M. G., Wassa, D. W., Li, Y., Olson, V. A., Karem, K., et al. (2005). Extended interhuman transmission of monkeypox in a hospital community in the republic of the Congo 2003. Am. J. Trop. Med. Hyg. 73, 428–434. doi: 10.4269/ajtmh.2005.73.428
Lederman, E. R., Davidson, W., Groff, H. L., Smith, S. K., Warkentien, T., Li, Y., et al. (2012). Progressive vaccinia: Case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J. Infect. Dis. 206, 1372–1385. doi: 10.1093/infdis/jis510
Leelananda, S. P., Lindert, S. (2016). Computational methods in drug discovery. Beilstein J. Org. Chem. 12, 2694–2718. doi: 10.3762/bjoc.12.267
Ligon, B. L. (2004). Monkeypox: A review of the history and emergence in the Western hemisphere. Semin. Pediatr. Infect. Dis. 15, 280–287. doi: 10.1053/j.spid.2004.09.001
Li, Y., Olson, V. A., Laue, T., Laker, M. T., Damon, I. K. (2006). Detection of monkeypox virus with real-time PCR assays. J. Clin. Virol. 36, 194–203. doi: 10.1016/j.jcv.2006.03.012
Li, X., Peng, T. (2021). Strategy, progress, and challenges of drug repurposing for efficient antiviral discovery. Front. Pharmacol. 12. doi: 10.3389/fphar.2021.660710
Lopera, J. G., Falendysz, E. A., Rocke, T. E., Osorio, J. E. (2015). Attenuation of monkeypox virus by deletion of genomic regions. Virology 475, 129–138. doi: 10.1016/j.virol.2014.11.009
Matias, W. R., Koshy, J. M., Nagami, E. H., Kovac, V., Moeng, L. R., Shenoy, E. S., et al. (2022). Tecovirimat for the treatment of human monkeypox: An initial series from Massachusetts, united states. Open Forum Infect. Dis. 9, ofac377. doi: 10.1093/ofid/ofac377
Mauldin, M. R., McCollum, A. M., Nakazawa, Y. J., Mandra, A., Whitehouse, E. R., Davidson, W., et al. (2022). Exportation of monkeypox virus from the African continent. J. Infect. Dis. 225, 1367–1376. doi: 10.1093/infdis/jiaa559
McCollum, A. M., Damon, I. K. (2014). Human monkeypox. Clin. Infect. Dis. 58, 260–267. doi: 10.1093/cid/cit703
Moore, M. J., Rathish, B., Zahra, F. (2022). Monkeypox (Treasure Island (FL), StatPearls Publishing).
Morris, G. M., Lim-Wilby, M. (2008). Molecular docking. Methods Mol. Biol 443, 365–382. doi: 10.1007/978-1-59745-177-2_19
Moss, B. (2012). Poxvirus cell entry: How many proteins does it take? Viruses 4, 688–707. doi: 10.3390/v4050688
Moss, B. (2013). Poxvirus DNA replication. Cold Spring Harb. Perspect. Biol. 5. doi: 10.1101/cshperspect.a010199
Muhammed, M. T., Aki-Yalcin, E. (2019). Homology modeling in drug discovery: Overview, current applications, and future perspectives. Chem. Biol. Drug Des. 93, 12–20. doi: 10.1111/cbdd.13388
Nakazawa, Y., Mauldin, M. R., Emerson, G. L., Reynolds, M. G., Lash, R. R., Gao, J., et al. (2015). A phylogeographic investigation of African monkeypox. Viruses 7, 2168–2184. doi: 10.3390/v7042168
Nguyen, P.-Y., Ajisegiri, W. S., Costantino, V., Chughtai, A. A., MacIntyre, C. R. (2021). Reemergence of human monkeypox and declining population immunity in the context of urbanization, nigeria 2017–2020. Emerg. Infect. Dis. 27, 1007–1014. doi: 10.3201/eid2704.203569
Nolen, L. D., Osadebe, L., Katomba, J., Likofata, J., Mukadi, D., Monroe, B., et al. (2015). Introduction of monkeypox into a community and household: Risk factors and zoonotic reservoirs in the democratic republic of the Congo. Am. J. Trop. Med. Hyg. 93, 410–415. doi: 10.4269/ajtmh.15-0168
Nolen, L. D., Osadebe, L., Katomba, J., Likofata, J., Mukadi, D., Monroe, B., et al. (2016). Extended human-to-Human transmission during a monkeypox outbreak in the democratic republic of the Congo. Emerg. Infect. Dis. 22, 1014–1021. doi: 10.3201/eid2206.150579
Ou-Yang, S., Lu, J., Kong, X., Liang, Z., Luo, C., Jiang, H. (2012). Computational drug discovery. Acta Pharmacol. Sin. 33, 1131–1140. doi: 10.1038/aps.2012.109
Parker, S., D’Angelo, J., Buller, R. M., Smee, D. F., Lantto, J., Nielsen, H., et al. (2021). A human recombinant analogue to plasma-derived vaccinia immunoglobulin prophylactically and therapeutically protects against lethal orthopoxvirus challenge. Antiviral Res. 195, 105179. doi: 10.1016/j.antiviral.2021.105179
Pauli, G., Blümel, J., Burger, R., Drosten, C., Gröner, A., Gürtler, L., et al. (2010). Orthopox viruses: Infections in humans. Transfus. med. hemotherapy. 37, 351–364. doi: 10.1159/000322101
Petersen, B. W., Kabamba, J., McCollum, A. M., Lushima, R. S., Wemakoy, E. O., Muyembe Tamfum, J.-J., et al. (2019). Vaccinating against monkeypox in the democratic republic of the Congo. Antiviral Res. 162, 171–177. doi: 10.1016/j.antiviral.2018.11.004
Poland, G. A., Kennedy, R. B., Tosh, P. K. (2022). Prevention of monkeypox with vaccines: a rapid review. Lancet Infect. Dis 22, E349–E358. doi: 10.1016/S1473-3099(22)00574-6
Pushpakom, S., Iorio, F., Eyers, P. A., Escott, K. J., Hopper, S., Wells, A., et al. (2018). Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discovery 18, 41–58. doi: 10.1038/nrd.2018.168
Radonić, A., Metzger, S., Dabrowski, P. W., Couacy-Hymann, E., Schuenadel, L., Kurth, A., et al. (2014). Fatal monkeypox in wild-living sooty mangabey, cote d’Ivoire 2012. Emerg. Infect. Dis. 20, 1009. doi: 10.3201/eid2006.131329
Rampogu, S., Kim, S. M., Son, M., Baek, A., Park, C., Lee, G., et al. (2020). A computational approach with biological evaluation: Combinatorial treatment of curcumin and exemestane synergistically regulates ddx3 expression in cancer cell lines. Biomolecules 10, 1–17. doi: 10.3390/biom10060857
Rampogu, S., Lee, K. W. (2021a). Old drugs for new purpose–fast pace therapeutic identification for SARS-CoV -2 infections by pharmacophore guided drug repositioning approach. Bull. Korean Chem. Soc 42, 212–226. doi: 10.1002/bkcs.12171
Rampogu, S., Lee, K. W. (2021c). Pharmacophore modelling-based drug repurposing approaches for SARS-CoV-2 therapeutics. Front. Chem. 9. doi: 10.3389/fchem.2021.636362
Rao, A. K., Petersen, B. W., Whitehill, F., Razeq, J. H., Isaacs, S. N., Merchlinsky, M. J., et al. (2022). Use of JYNNEOS (Smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: Recommendations of the advisory committee on immunization practices - united states 2022. MMWR. Morb. Mortal. Wkly. Rep. 71, 734–742. doi: 10.15585/mmwr.mm7122e1
Reed, K. D., Melski, J. W., Graham, M. B., Regnery, R. L., Sotir, M. J., Wegner, M. V., et al. (2004). The detection of monkeypox in humans in the Western hemisphere. N. Engl. J. Med. 350, 342–350. doi: 10.1056/NEJMoa032299
Roberts, K. L., Smith, G. L. (2008). Vaccinia virus morphogenesis and dissemination. Trends Microbiol. 16, 472–479. doi: 10.1016/j.tim.2008.07.009
Rogers, J. V., Parkinson, C. V., Choi, Y. W., Speshock, J. L., Hussain, S. M. (2008). A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale Res. Lett. 3, 129–133. doi: 10.1007/s11671-008-9128-2
Russo, A. T., Grosenbach, D. W., Brasel, T. L., Baker, R. O., Cawthon, A. G., Reynolds, E., et al. (2018). Effects of treatment delay on efficacy of tecovirimat following lethal aerosol monkeypox virus challenge in cynomolgus macaques. J. Infect. Dis. 218, 1490–1499. doi: 10.1093/infdis/jiy326
Sahoo, A. K., Augusthian, P. D., Muralitharan, I., Vivek-Ananth, R. P., Kumar, K., Kumar, G., et al. (2022). In silico identification of potential inhibitors of vital monkeypox virus proteins from FDA approved drugs. doi: 10.21203/rs.3.rs-1983080/v1
Seang, S., Burrel, S., Todesco, E., Leducq, V., Monsel, G., Le Pluart, D., et al. (2022). Evidence of human-to-dog transmission of monkeypox virus. Lancet 400, 658–659. doi: 10.1016/S0140-6736(22)01487-8
Shchelkunov, S. N., Totmenin, A. V., Babkin, I. V., Safronov, P. F., Ryazankina, O. I., Petrov, N. A., et al. (2001). Human monkeypox and smallpox viruses: Genomic comparison. FEBS Lett. 509, 66–70. doi: 10.1016/S0014-5793(01)03144-1
Siegrist, C. M., Kinahan, S. M., Settecerri, T., Greene, A. C., Santarpia, J. L. (2020). CRISPR/Cas9 as an antiviral against orthopoxviruses using an AAV vector. Sci. Rep. 10, 19307. doi: 10.1038/s41598-020-76449-9
Siegrist, E. A., Sassine, J. (2022). Antivirals with activity against monkeypox: A clinically oriented review. Clin. Infect. Dis., 76, ciac622. doi: 10.1093/cid/ciac622
Silva, N. I. O., de Oliveira, J. S., Kroon, E. G., Trindade, G., de, S., Drumond, B. P. (2020). Here, there, and everywhere: The wide host range and geographic distribution of zoonotic orthopoxviruses. Viruses 13, 1–20. doi: 10.3390/v13010043
Simon, V., Ho, D. D., Abdool Karim, Q. (2006). HIV/AIDS epidemiology, pathogenesis, prevention, and treatment. Lancet (London England) 368, 489–504. doi: 10.1016/S0140-6736(06)69157-5
Singla, R. K., Shen, B. (2022). Monkeypox- a global emergency: What nations should learn from recent COVID-19 pandemic? – correspondence. Int. J. Surg. 106, 106955. doi: 10.1016/j.ijsu.2022.106955
Sklenovská, N. (2020). “Monkeypox virus BT - animal-origin viral zoonoses, Animal-Origin Viral Zoonoses”. Eds. Malik, Y. S., Singh, R. K., Dhama, K. (Singapore: Springer Singapore), 39–68. doi: 10.1007/978-981-15-2651-0_2
Sliwoski, G., Kothiwale, S., Meiler, J., Lowe, E. W. (2014). Computational methods in drug discovery. Pharmacol. Rev. 66 (1), 334–395. doi: 10.1124/pr.112.007336
Smith, S. K., Self, J., Weiss, S., Carroll, D., Braden, Z., Regnery, R. L., et al. (2011). Effective antiviral treatment of systemic orthopoxvirus disease: ST-246 treatment of prairie dogs infected with monkeypox virus. J. Virol. 85, 9176–9187. doi: 10.1128/JVI.02173-10
Sui, Y., Xu, Q., Liu, M., Zuo, K., Liu, X., Liu, J. (2022). CRISPR-Cas12a-based detection of monkeypox virus. J. Infect. doi: 10.1016/j.jinf.2022.08.043
Tang, Z., Mao, Y., Meng, Y., Qiu, X., Bajinka, O., Wu, G., et al. (2022). A bioinformatics approach to systematically analyze the molecular patterns of monkeypox virus-host cell interactions. bioRxiv 85, 702–769. doi: 10.1101/2022.10.12.511850
Tesh, R. B., Watts, D. M., Sbrana, E., Siirin, M., Popov, V. L., Xiao, S.-Y. (2004). Experimental infection of ground squirrels (Spermophilus tridecemlineatus) with monkeypox virus. Emerg. Infect. Dis. 10, 1563–1567. doi: 10.3201/eid1009.040310
Thornhill, J. P., Barkati, S., Walmsley, S., Rockstroh, J., Antinori, A., Harrison, L. B., et al. (2022). Monkeypox virus infection in humans across 16 countries—April–June 2022. N. Engl. J. Med 387, 679–691. doi: 10.1056/NEJMoa2207323
Titanji, B. K., Tegomoh, B., Nematollahi, S., Konomos, M., Kulkarni, P. A. (2022). Monkeypox: A contemporary review for healthcare professionals. Open Forum Infect. Dis. 9, ofac310. doi: 10.1093/ofid/ofac310
van Kammen, A. (1999). “Beijerinck’s contribution to the virus concept — an introduction BT,” in 100 years of virology: The birth and growth of a discipline. Eds. Calisher, C. H., Horzinek, M. C. (Vienna: Springer Vienna), 1–8. doi: 10.1007/978-3-7091-6425-9_1
Vaughan, A., Aarons, E., Astbury, J., Brooks, T., Chand, M., Flegg, P., et al. (2020). Human-to-Human transmission of monkeypox virus, united kingdom, October 2018. Emerg. Infect. Dis. 26, 782–785. doi: 10.3201/eid2604.191164
Vivancos, R., Anderson, C., Blomquist, P., Balasegaram, S., Bell, A., Bishop, L., et al. (2022). Community transmission of monkeypox in the united kingdom, April to may 2022. Eurosurveillance 27, 2200422. doi: 10.2807/1560-7917.ES.2022.27.22.2200422
Wang, Z., Tober-Lau, P., Farztdinov, V., Lemke, O., Schwecke, T., Steinbrecher, S., et al. (2022). The human host response to monkeypox infection: A proteomic case series study. EMBO Mol. Med., 14, e16643. doi: 10.15252/emmm.202216643
Weaver, J. R., Isaacs, S. N. (2008). Monkeypox virus and insights into its immunomodulatory proteins. Immunol. Rev. 225, 96–113. doi: 10.1111/j.1600-065X.2008.00691.x
Wilson, M. E. (2017). Re-emerging diseases: Overview (Oxford: Academic Press), 269–277. S. R. B. T.-I. E. @ of P. H. (Second E. Quah. doi: 10.1016/B978-0-12-803678-5.00374-X
Wong, J. P., Viswanathan, S., Wang, M., Sun, L.-Q., Clark, G. C., D’Elia, R. V. (2017). Current and future developments in the treatment of virus-induced hypercytokinemia. Future Med. Chem. 9, 169–178. doi: 10.4155/fmc-2016-0181
Woolhouse, M., Scott, F., Hudson, Z., Howey, R., Chase-Topping, M. (2012). Human viruses: discovery and emergence. Philos. Trans. R. Soc London. Ser. B Biol. Sci. 367, 2864–2871. doi: 10.1098/rstb.2011.0354
Xiang, Y., White, A. (2022). Monkeypox virus emerges from the shadow of its more infamous cousin: Family biology matters. Emerg. Microbes Infect. 11, 1768–1777. doi: 10.1080/22221751.2022.2095309
Xuan, D. T. M., Yeh, I.-J., Wu, C.-C., Su, C.-Y., Liu, H.-L., Chiao, C.-C., et al. (2022). Comparison of transcriptomic signatures between monkeypox-infected monkey and human cell lines. J. Immunol. Res. 2022, 3883822. doi: 10.1155/2022/3883822
Keywords: monkeypox virus, replication, MPV, drugs, viral structure, transmission mode
Citation: Rampogu S, Kim Y, Kim S-W and Lee KW (2023) An overview on monkeypox virus: Pathogenesis, transmission, host interaction and therapeutics. Front. Cell. Infect. Microbiol. 13:1076251. doi: 10.3389/fcimb.2023.1076251
Received: 21 October 2022; Accepted: 10 January 2023;
Published: 10 February 2023.
Edited by:
Saadullah Khattak, Henan University, ChinaReviewed by:
Sumbul Saeed, Huazhong Agricultural University, ChinaMohd Ahmar Rauf, University of Michigan, United States
Faizan Uddin, National Centre for Biological Sciences, India
Copyright © 2023 Rampogu, Kim, Kim and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seon-Won Kim, c3draW1AZ251LmFjLmty; Keun Woo Lee, a3dsZWVAZ251LmFjLmty
 Shailima Rampogu
Shailima Rampogu Yongseong Kim2
Yongseong Kim2 Seon-Won Kim
Seon-Won Kim