- 1Department of Clinical Laboratory, The Fifth People’s Hospital of Hainan Province, Haikou, China
- 2Department of Pathology, The Fifth People’s Hospital of Hainan Province, Haikou, China
- 3Department of Dermatology, The Fifth People’s Hospital of Hainan Province, Haikou, China
- 4Department of Xiuying Outpatient, The Fifth People’s Hospital of Hainan Province, Haikou, China
Objective: Tinea capitis, a chronic inflammatory disease of the scalp and hair shafts caused by dermatophyte infections, manifests clinically as erythematous lesions, scaling, alopecia, and pustule formation. Hainan, a tropical island province in China, exhibits distinctive climatic conditions and demographic contact patterns that could shape its pathogen spectrum. However, comprehensive epidemiological data remain scarce. This study analyzes the epidemiological characteristics and pathogen spectrum of tinea capitis in Hainan, China.
Methods: This study was undertaken across 11 coastal and inland dermatological centers in Hainan Province (January 2023 to December 2024). A total of 76 tinea capitis patients diagnosed via mycological examination (fungal fluorescence microscopy, fungal culture with species identification) and corroborated by dermatoscopic evaluation were consecutively enrolled. Data on demographic characteristics, exposure history, and clinical manifestations were systematically collated using standardized case report forms. Pathogen subtyping was performed via integrated morphological identification supplemented by molecular biological analysis of the ITS region.
Results: Among 76 enrolled patients, minors (≤15 years) accounted for 80.26% (61/76), with ≤10-year-olds representing 91.8% (56/61) of pediatric cases. The overall male-to-female ratio was recorded as 1:1.05 (37 males vs. 39 females), while pediatric patients (≤15 years) exhibited a 1.07:1 ratio (31 males vs. 29 females). Animal contact history was reported in 31 cases (40.79%), scalp trauma in 3 cases (3.95%), and co-occurring superficial fungal infections in 12 cases (15.79%). Fungal elements were detected via direct microscopic examination in 64 cases (84.21%). Fungal cultures obtained 45 positive isolates (19 strains in 2023, 26 in 2024), with kerion (inflammatory tinea capitis) exhibiting the highest culture positivity rate, followed by tinea alba. The primary pathogens identified were zoophilic Microsporum canis (M. canis) (20 cases, 43.79%) and anthropophilic Trichophyton rubrum (T. rubrum) (9 strains, 19.57%). Clinical manifestations comprised kerion (44 cases, 57.89%), tinea alba (27 cases, 35.53%), and black dot tinea (5 cases, 6.58%). Kerion cases were predominantly linked to M. canis and T. mentagrophytes (interdigital subtype), whereas tinea alba demonstrated infections by M. canis and T. rubrum. Black dot lesions were additionally observed in M. canis infections.
Conclusion: The primary affected group is children ≤10 years old, with the most common pathogenic fungus being zoophilic M. canis, and clinical classification is predominantly kerion. Dermatologists should pay attention to different transmission routes and pathogen spectra.
1 Introduction
Tinea capitis, a chronic dermatophytic infection of the hair and scalp, causes erythema, scaling, alopecia, abscesses, and scarring after lesion resolution. This illness is the most frequent superficial fungal infection in school-aged children worldwide, with higher rates reported in resource-limited settings (Ferguson and Fuller, 2017; Rodríguez-Cerdeira et al., 2021).
The epidemiological distribution of tinea capitis pathogens exhibits regional and temporal heterogeneity, shaped by socioeconomic factors, lifestyle changes, migration trends, and host demographic dynamics. In China, rapid socioeconomic development and lifestyle changes since the 1980s have triggered a transition in predominant pathogens. Before 1985, anthropophilic dermatophytes prevailed; however, after 1985, zoophilic species—particularly M. canis —have predominated, representing >80% of cases (Zhan et al., 2015). Anthropophilic strains such as Trichophyton violaceum remain endemic solely in localized areas of southeastern and northwestern China. The dominant clinical manifestations of tinea capitis in China have shifted chronologically: favus (1950s) was superseded by white tinea (peak prevalence in the 1990s), with black dot tinea emerging as the most prevalent form in the 21st century (Wan and Wu, 2022). Comparable epidemiological trends are observed globally (Rodríguez-Cerdeira et al., 2021). M. canis continues to be the predominant zoophilic pathogen internationally, whereas Trichophyton violaceum and Trichophyton tonsurans are the most epidemiologically significant anthropophilic species.
This study utilized a prospective, descriptive design to prospectively collate data from tinea capitis cases diagnosed across multi-city dermatology departments, specialized hair/scalp clinics, and dermatology prevention/treatment centers in Hainan Province, China (January 2023–December 2024). The investigation systematically examined the age and gender distribution, risk factors, and pathogen spectrum of tinea capitis in Hainan, with the objective of establishing an evidence-based foundation to advance understanding of its epidemiology, diagnosis, and clinical management in the region.
2 Material and methods
2.1 Clinical data
A total of 76 cases of tinea capitis diagnosed via clinical fungal microscopy and culture were included in the study from January 2023 to December 2024. All subjects received written informed consent. The study was conducted under the Declaration of Helsinki and approved by the ethical standards of the Ethics committee of the Fifth People’s Hospital of Hainan Province (IRB no. 2020-020). Records were kept of demographic features, types of tinea capitis, and species of pathogenic fungi, along with skin dermoscopy findings.
2.2 Epidemiological information collection
An epidemiological survey form was filled out with informed consent from patients and their families. The survey form included key information such as age, gender, long-term residence, presence of other diseases, history of animal contact, type of animals contacted, presence of concurrent superficial mycosis, and type of concurrent superficial mycosis among cohabiting and close contacts.
2.3 Fungal fluorescence microscopy
Alcohol (75%) was used to disinfect the affected area, and scales, pus, or broken hairs were collected using a scalpel or forceps. These samples were placed on a slide and directly stained with fungal fluorescence dye (Beckman Coulter). Under a fluorescence microscope, the following fungal microscopy findings were recorded: intramural hyphae, intramural spores, intramural hyphae and spores, extramural hyphae, extramural spores, and extramural hyphae and spores. Multiple types could be present in the same patient’s sample. If none of these were observed, scalp hyphae were recorded.
2.4 Fungal culture and identification
Remaining specimens were inoculated onto Sabouraud dextrose agar (SDA) and incubated at 25-28°C for 14 days. Fungi were preliminarily identified based on colony growth rate, pigmentation, size, conidia morphology, and hyphal characteristics. For rare or difficult-to-identify species, further small-scale cultivation and morphological analysis were performed.
2.5 Molecular biology identification
M. canis was identified by morphology, while the internal transcribed spacer (ITS) regions of the other species were amplified and sequenced, (ITS1 5’-TCCGTAGGTGAACCTGCGG-3’; ITS4 5’-TCCTCCGCTTATTGATATGC-3’) (Chen et al., 2022).
All isolates were subcultured on potato dextrose agar (PDA) for 7 days before sequencing. PCR amplification was performed using universal fungal primers ITS1 and ITS4, followed by sequencing of the amplified products. Reference sequences with high similarity to known sequences were downloaded from BLAST results and compared with rDNA-ITS sequences in the GenBank database to assist in strain identification.
2.6 Statistical analysis
Descriptive statistics were used to analyze the demographic and clinical data of patients diagnosed with tinea capitis, including sex, age groups, clinical subtypes (e.g., kerion, tinea alba), and pathogen groups. Categorical variables (e.g., sex, pathogen species, exposure history) were compared across groups using Fisher’s exact test (employed for small sample sizes as an alternative to the chi-square test), with statistical significance defined as a two-tailed p < 0.05. All statistical analyses were performed using SPSS version 23.0 software (IBM, Armonk, NY, USA).
3 Results
3.1 Epidemiological characteristics
From January 2023 to December 2024, 76 cases of tinea capitis were included, with 37 males and 39 females (male-to-female ratio = 1:1.05). The majority of patients (80.26%, 61/76) were minors (≤15 years old), with 91.8% (56/61) being ≤10 years old. The male-to-female ratio among minors was 1.07:1 (31:29). Thirty-one patients (40.79%) had a history of animal contact, mainly involving cats and dogs (18 and 11 cases, respectively), with 28 children under 10 years old (90.32%) and 3 adults (9.68%). Three patients (3.95%) had a history of scalp trauma, and 12 patients (15.79%) had concurrent superficial mycosis at other sites. Most patients (64.46%, 49/76) were from Haikou City and surrounding areas (Supplementary Table 1).
3.2 Clinical diagnosis and classification
Diagnosis of tinea capitis was based on typical clinical manifestations, dermoscopy, and positive fungal microscopy and culture. Common types of tinea capitis include kerion (57.89%, 44 cases), tinea alba (35.53%, 27 cases), and black dot ringworm (6.58%, 5 cases). No cases of favus were found. Age group did not significantly affect tinea capitis type (P > 0.05). Most of the patients were children (≤15 years old), and most of the patients had more severe pyophyton (Figure 1, Supplementary Table 1).
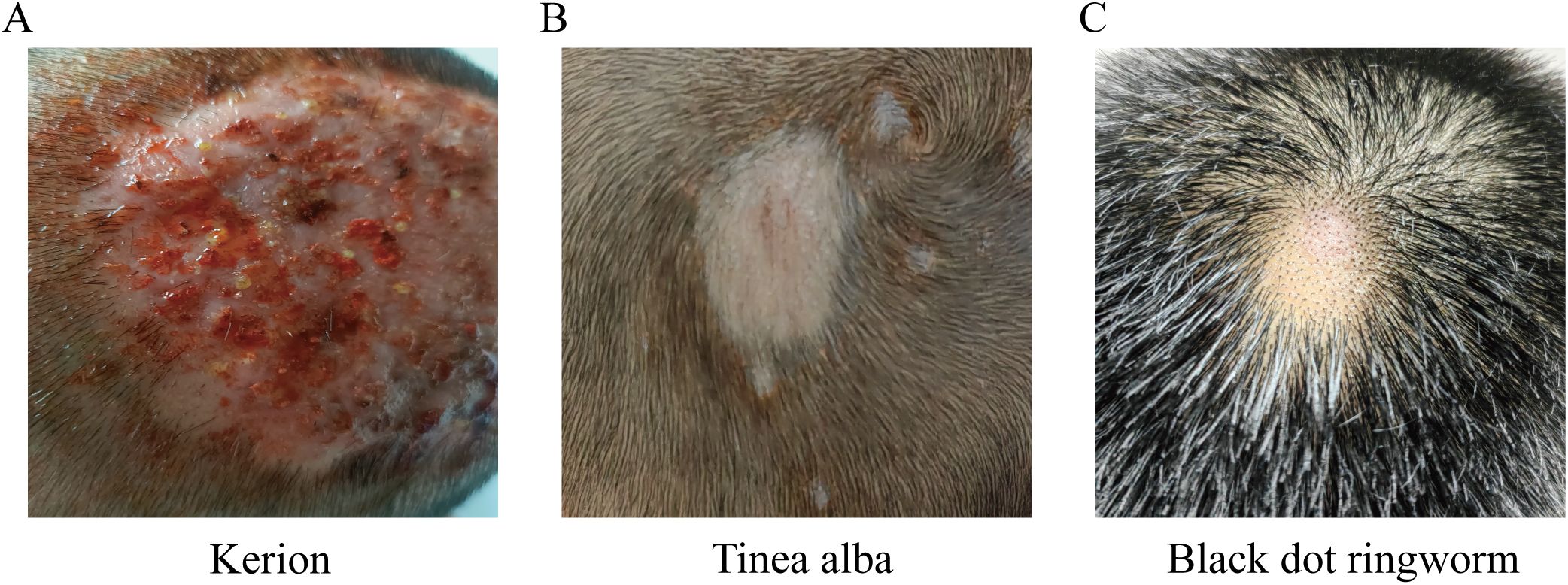
Figure 1. Types of Tinea capitis (A) Kerion: one to several round, erythematous, infiltrative, or raised inflammatory plaques with surface clusters of follicular micropustules, honeycomb-like follicular openings, and expressible purulent discharge upon pressure. Hairs in the affected area are loose and easily extracted. (B) Tinea alba: Scalp lesions present as gray-white scaly patches, round or oval in shape, with possible satellite lesions. (C) Black dot ringworm: Affected hairs break off at the follicular orifice, leaving black, dot-like remnants at the follicular openings.
3.3 Pathogen composition
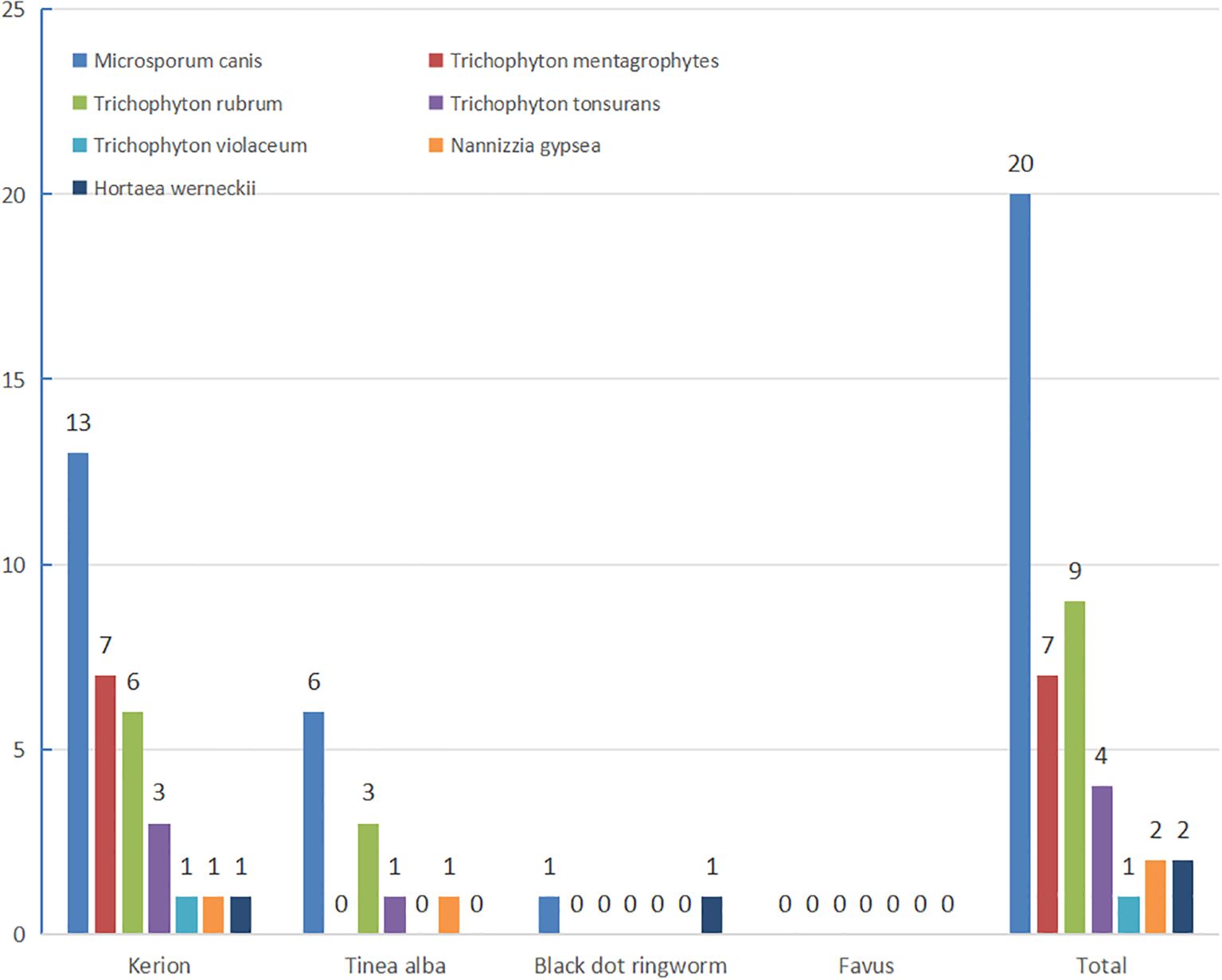
Among 76 clinically diagnosed tinea capitis cases, direct fungal microscopy yielded positive results in 64 cases (84.21% positivity rate). Fungal culture isolation yielded 45 fungal isolates, including 20 presumptive M. canis strains (later confirmed through rice grain medium phenotyping). All 45 strains underwent morphological identification followed by molecular sequencing. Sequence alignment of the rDNA-ITS region with reference strains in the GenBank database demonstrated ≥99.0% similarity for all isolates. The most positive culture was kerion, followed by tinea alba. The most common pathogenic fungi were zoophilic M. canis (43.79%) and anthropophilic T. rubrum Trichophyton 9 (19.57%), There were (17.39%) (7 strains). 4 (8.70%) and 1 (2.17%) strains of zoophilic mentagrophytes (T. mentagrophytes), anthropophilic Trichophyton tonsurans (T. tonsurans) and Trichophyton violaceum (T. violaceum), respectively. Two strains (4.35%) of Nannizzia gypsea were soil ophilic. Two strains of Hortaea werneckii (H. werneckii) were also identified. Kerion was predominantly caused by M. canis (13 strains) and Trichophyton mentagrophytes (7 strains). White tinea (tinea alba) primarily involved M. canis (6 strains) and T. rubrum (3 strains), while a single case of black dot tinea also demonstrated M. canis infection (1 strain) (Figure 2).
There was no significant difference between tinea capitis type and pathogenic fungi (P>0.05). There was no significant difference in pathogenic fungi detected between 2023 and 2024 (P>0.05). In 2023, mycological analysis identified 19 fungal strains, predominantly M. canis (4 strains) and T. rubrum (6 strains). In 2024, 26 strains were isolated, with M. canis (16 strains) and Trichophyton mentagrophytes (3 strains) being the most frequent isolates (Figure 3).
3.4 Relationship between pathogen species and age groups
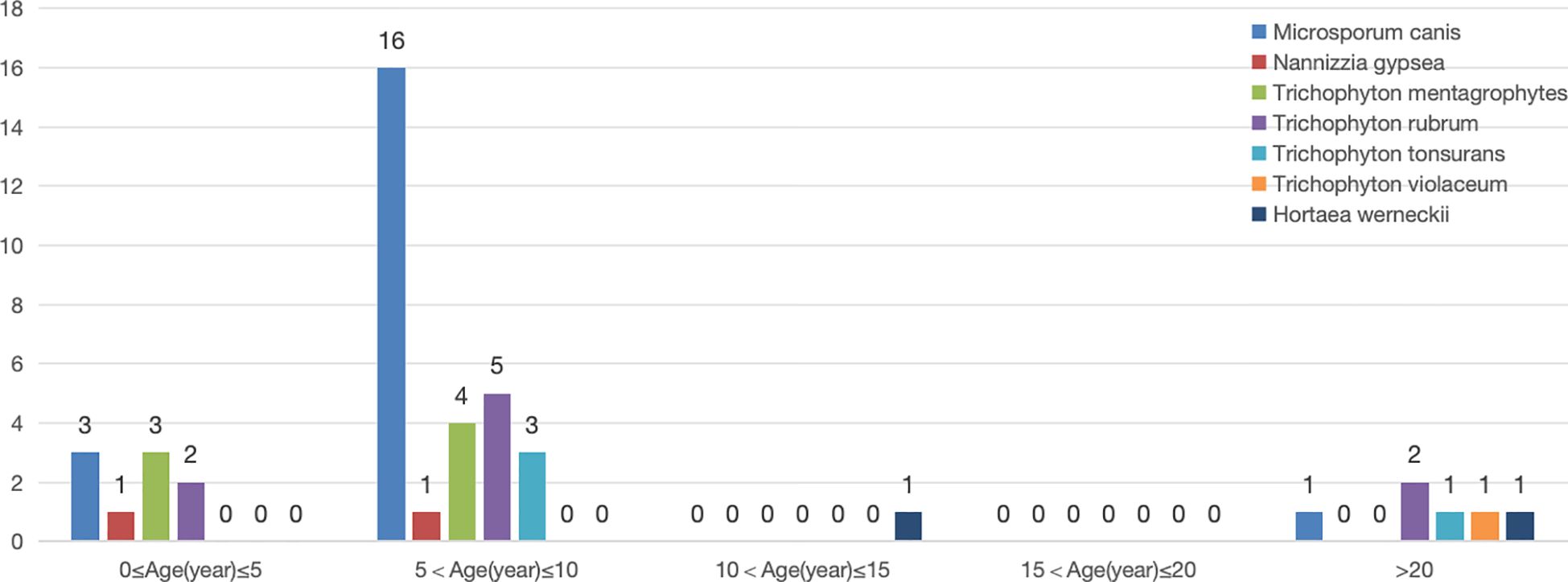
M. canis was most commonly detected in children, particularly in the 5–10 year-old age group (16 cases, 80%). M. canis(20cases), Nannizzia gypsea(2cases) and T. mentagrophytes(7cases) were only found in children aged 0–10 years.
Only 1 strain of Trichophyton violaceum was isolated from an adult with tinea capitis. T. rubrum (9cases) and Trichophyton tonsurans (4cases) were detected in both children and adults. Hortaea werneckii, although not a classic tinea capitis pathogen, was identified in two cases, one from a 13-year-old girl with black dot ringworm and another from a 24-year-old man with kerion. Significant differences were observed in pathogen species distribution across age groups (P < 0.05) (Figure 4).
3.5 Dermoscopy results
Fifteen patients underwent dermoscopy, with 12 cases of tinea alba, 2 cases of black dot ringworm, and 1 case of kerion. In 12 cases of tinea alba, Morse code-like broken hair, external fungal sheath, black dot sign, spiral hair, and comma or question mark-like hair were observed in 3 (25.00%), 1 (8.33%), 10 (83.33%), 3 (25.00%), and 11 (91.67%) cases, respectively. All five features were present in 3 cases (20.00%). Tinea alba exhibited characteristic dermoscopic features in 91.66% (11/12) of cases. In 2 cases of black dot ringworm, external fungal sheath, black dot sign, and comma or question mark-like hair were observed in 1 (50.00%), 1 (50.00%), and 2 (100%) cases, respectively. One case of kerion showed both external fungal sheath and comma or question mark- like hair (Supplementary Table 2).
3.6 Treatment and course of tinea capitis
The main treatment for tinea capitis was systemic therapy, primarily oral terbinafine, itraconazole, and griseofulvin. Of the 76 cases, 19 received oral terbinafine, 23 received oral itraconazole, and 5 received both. Two cases used traditional Chinese medicine, and 27 received no oral medication. The main treatment was for severe kerion cases, with 16 cases adding oral prednisone acetate and 25 cases combining antibiotic therapy. Some cases also used photodynamic therapy. The course of treatment ranged from 4 to 8 weeks, with the shortest being 30 days and the longest 180 days.
3.7 Follow-up protocol and therapeutic efficacy criteria
Follow-up Protocol, Patients were scheduled for biweekly clinical re-evaluations during active treatment. After clinical resolution was achieved, telephone follow-ups were conducted monthly for two consecutive sessions to monitor recurrence and long-term outcomes.
Therapeutic Efficacy Criteria, Final efficacy assessment was based on clinical manifestations combined with mycological examination (direct microscopy and/or culture) at the end of therapy.
Antifungal discontinuation: Oral medications were discontinued upon confirmation of mycological negativity (negative microscopy/culture). Cure confirmation: Patients underwent periodic re-evaluation post-discontinuation, with cure defined as 2–3 consecutive negative mycological results.
4 Discussion
Tinea capitis is an ancient chronic infectious disease. Historical records from the Sui Dynasty (610 AD) mention “White baldness” and “Red baldness”. “White baldness” correspond to modern tinea alba and black dot ringworm. “Red baldness” is the modern favus. Tinea capitis is caused by dermatophyte infections of the scalp and hair, classified into kerion, tinea alba, black dot ringworm, and favus based on the causative agent and host response (Hay, 2017; Jin and Min, 2019).
Children are more susceptible due to incomplete development of sebaceous glands and lack of free fatty acids inhibiting fungal growth (Rui et al., 2017). Children also tend to be less clean and more active, and they regularly engage with animals. In this study, 31 patients (40.79%) had a history of animal contact, with 90.32% being children ≤10 years old and 9.68% adults. Concurrent superficial mycosis at additional sites was present in 12 patients (15.79%).
The incidence ranges from infants to the elderly, but it peaks during preschool and school age. Minors (≤15 years old) accounted for 80.26% of cases, with the majority (62.3%, 38/61) in the 5–10 year-old age group. This aligns with studies from other regions (Zhi et al., 2021; Cheng et al., 2022; Chen and Yu, 2023; Zeng et al., 2023). There were 15 adult cases (19.48%), with a greater percentage of females (male:female ratio = 0.6:1). The adult incidence rate is comparable to studies from Hubei Province but slightly higher than the national average (9.04%), suggesting that adult tinea capitis in Hainan is significant (Liang et al., 2020; He et al., 2021). Adult cases often mimic seborrheic dermatitis or alopecia, leading to potential misdiagnosis. Dermoscopy plays a crucial role in diagnosing tinea capitis. Dermoscopy plays a crucial role in diagnosing tinea capitis (Kumar et al., 2022).
In this study, Dermoscopy was performed in 15 patients, revealing subtle clinical features of tinea alba and black dot tinea in 14 cases. In 91.66% of patients, tinea alba displayed distinctive dermoscopic characteristics.
According to classification criteria, 76 cases were primarily kerion (57.89%), followed by tinea alba (35.53%). This corresponds with international studies but differs from some domestic studies (Zhan et al., 2015; Hamroune et al., 2016; Bassyouni et al., 2017; Wen and Xiao, 2018; Chen et al., 2022; Jing, 2022; Wan and Wu, 2022), presumably due to the higher probability of white ringworm and black dot ringworm to develop as kerion. This, combined with the unique geography and climate of Hainan Island, may explain the higher prevalence of kerion.
Pathogens in Hainan Island children’s tinea capitis from 2023–2024 were predominantly zoophilic M. canis and anthropophilic T. rubrum. This contrasts with previous studies where T. mentagrophytes and T. tonsurans were dominant (Farag et al., 2018). This shift aligns with results from southern and northern China = and the 2019–2020 multicenter study (Chen et al., 2022). However, in North Africa (Hamroune et al., 2016; Bassyouni et al., 2017; Farag et al., 2018), East Asia (Lee et al., 2020; Takenaka et al., 2020), Southeast Asia (Tan et al., 2018), and Eastern Europe (Baranová et al., 2018; Colosi et al., 2020), the dominant pathogen is M. canis. In South Asia and Western Asia=, T. violaceum and M. audouinii are predominant, while in the United States, T. tonsurans is the most common (Nguyen et al., 2020; Usman et al., 2021).
With economic development and improved hygiene conditions, pet ownership has increased, leading to a rise in zoophilic M. canis as a pathogen in tinea capitis. Therefore, preventing and managing pet-associated infections is critical. Notably, two cases of H. werneckii were identified, one in a 13-year-old girl with black dot ringworm and another in a 24-year-old man with kerion (Zheng et al., 2024). This halophilic fungus, which causes black dot ringworm, is most commonly seen in tropical and subtropical coastal locations.
Based on predefined therapeutic efficacy criteria, all patients achieved clinical and mycological cure, excluding one pediatric case of Hortaea werneckii-associated tinea nigra lost to follow-up prior to final assessment. Due to inherent limitations in existing diagnostic methods, achieving 100% sensitivity remains elusive for both direct mycological microscopy and fungal culture. Among 76 clinically confirmed tinea capitis cases, direct microscopy detected fungal elements in 64 cases (84.21%), whereas fungal culture confirmed pathogen growth in 45 cases (59.21%). These positivity rates were notably lower than those documented in prior studies (Chen et al., 2022; Zeng et al., 2023), which reported sensitivities exceeding 90% for microscopy and 70% for culture. This discrepancy may be attributed to differences in case numbers across studies, the predominance of kerion (inflammatory tinea capitis) as the clinical subtype, and variations in technical proficiency among laboratory personnel.
In this study, cases were primarily concentrated in Haikou, with sporadic cases in other cities. This limitation might be due to Haikou’s status as a central hub for economics, culture, and transportation, attracting a dense population. Other cities may have fewer cases due to transportation barriers and economic constraints. Additionally, the study focused on dermatology prevention and treatment centers rather than comprehensive hospitals. Limited funding also restricted in-depth investigations in rural areas. Furthermore, no comprehensive fungal testing was conducted on other body parts or animals, limiting the understanding of transmission routes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics committee of the Fifth People’s Hospital of Hainan Province. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
WZ: Writing – original draft, Writing – review & editing. FQ: Writing – original draft, Writing – review & editing. XY: Writing – original draft, Writing – review & editing. XT: Writing – original draft, Writing – review & editing. CL: Writing – original draft, Writing – review & editing. QC: Writing – original draft, Writing – review & editing. ZP: Writing – original draft, Writing – review & editing. RC: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work is supported by the Construction Project of Hainan Province Clinical Medical Center and the Hainan provincial health industry scientific research project (No.22A200285).
Acknowledgments
We are very grateful to all the volunteer subjects who participated in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1590315/full#supplementary-material
References
Baranová, Z., Kampe, T., Dorko, E., and Rimárová, K. (2018). Epidemiological and clinical aspects of dermatophytoses in Eastern Slovakia: a retrospective three-year study. Cent. Eur. J. Public Health 26, S72–S75. doi: 10.21101/cejph.a5279
Bassyouni, R. H., El-Sherbiny, N. A., Abd El Raheem, T. A., and Mohammed, B. H. (2017). Changing in the epidemiology of tinea capitis among school children in Egypt. Ann. Dermatol. 29, 13–19. doi: 10.5021/ad.2017.29.1.13
Chen, X. Q. and Yu, J. (2023). Global demographic characteristics and pathogen spectrum of tinea capitis. Mycopathologia 188, 433–447. doi: 10.1007/s11046-023-00710-8
Chen, X. Q., Zheng, D. Y., Xiao, Y. Y., Dong, B. L., Cao, C. W., Ma, L., et al. (2022). Aetiology of tinea capitis in China: A multicentre prospective study. Br. J. Dermatol. 186, 705–712. doi: 10.1111/bjd.20875
Cheng, Z., Meng, X., Hai, Z., Yan, S., Yuan, Z., and Xiao, Z. (2022). Clinical epidemiological characteristics of tinea capitis in 331 cases. Chin. J. Mycology 17, 373–376.
Colosi, I. A., Cognet, O., Colosi, H. A., Sabou, M., and Costache, C. (2020). Dermatophytes and dermatophytosis in Cluj-Napoca, Romania: a 4-year cross-sectional study. J. Fungi (Basel Switzerland) 6, 154. doi: 10.3390/jof6030154
Farag, A. G. A., Hammam, M. A., Ibrahem, R. A., Mahfouz, R. Z., Elnaidany, N. F., Qutubuddin, M., et al. (2018). Epidemiology of dermatophyte infections among school children in Menoufia Governorate, Egypt. Mycoses 61, 321–325. doi: 10.1111/myc.12743
Ferguson, L. and Fuller, L. C. (2017). Spectrum and burden of dermatophytes in children. J. Infection 74, S54–S60. doi: 10.1016/S0163-4453(17)30192-5
Hamroune, Z., Mazouz, A., Benelmouffok, A. B., and Kellou, D. (2016). Évolution des teignes du cuir chevelu observées au laboratoire de mycologie de l’institut Pasteur d’Algérie de 1995 à 2015 [Evolution of tinea capitis observed in mycology laboratory of institute Pasteur of Algeria from 1995 to 2015. J. Mycologie Médicale 26, 337–344. doi: 10.1016/j.mycmed.2016.06.005
Hay, R. J. (2017). Tinea capitis: current status. Mycopathologia 182, 87–93. doi: 10.1007/s11046-016-0058-8
He, M., Zeng, J., Mao, Y., Zheng, Y., Lian, X., and Chen, H. (2021). Aetiological changes of tinea capitis in the Hubei area in 60 years: Focus on adult tinea capitis. Mycoses 64, 1527–1534. doi: 10.1111/myc.13305
Jin, Y. and Min, Z. (2019). Chinese guidelines for diagnosis and treatment of tinea capitis (Revised 2018). Chin. J. Mycology 14, 4–6.
Jing, Z. (2022). Clinical analysis of 60 cases of tinea cephalus in Jilin children. Jilin: Jilin University. doi: 10.27162/d.cnki.gjlin.2022.004937
Kumar, P., Pandhi, D., Bhattacharya, S. N., and Das, S. (2022). Trichoscopy as a diagnostic tool for tinea capitis: a prospective, observational study. Int. J. Trichology 12, 68–74. doi: 10.4103/ijt.ijt_30_20
Lee, W. J., Lee, E. H., Bang, Y. J., and Jun, J. B. (2020). Retrospective two-centre study on prepubertal children with tinea capitis in Korea. Mycoses 63, 52–57. doi: 10.1111/myc.13014
Liang, G., Zheng, X., Song, G., Zhang, M., Liu, J., Zang, X., et al. (2020). Adult tinea capitis in China: A retrospective analysis from 2000 to 2019. Mycoses 63, 876–888. doi: 10.1111/myc.13102
Nguyen, C. V., Collier, S., Merten, A. H., Maguiness, S. M., and Hook, K. P. (2020). Tinea capitis: a single-institution retrospective review from 2010 to 2015. Pediatr. Dermatol. 37, 305–310. doi: 10.1111/pde.14092
Rodríguez-Cerdeira, C., Martínez-Herrera, E., Szepietowski, J. C., Pinto-Almazán, R., Frías-De-León, M. G., Espinosa-Hernández, V. M., et al. (2021). A systematic review of worldwide data on tinea capitis: analysis of the last 20 years. J. Eur. Acad. Dermatol. Venereology 35, 844–883. doi: 10.1111/jdv.16951
Rui, S., Hui, W., and Zhe, W. (2017). Analysis of 92 cases of tinea cephalus in Kaifeng. Chin. J. Integrated TCM Dermatol. Venereology 16, 323–324.
Takenaka, M., Murota, H., and Nishimoto, K. (2020). Epidemiological survey of 42–403 dermatophytosis cases examined at Nagasaki University Hospital from 1966 to 2015. J. Dermatol. 47, 615–621. doi: 10.1111/1346-8138.15340
Tan, C. W., Foong, A. Y., Liew, H. M., and Koh, M. J. (2018). A review of tinea capitis in a cohort of Asian children. Ann. Acad. Medicine Singapore 47, 156–158. doi: 10.47102/annals-acadmedsg.
Usman, B., Rehman, A., Naz, I., and Anees, M. (2021). Prevalence and antifungal drug resistance of dermatophytes in clinical samples from Pakistan. Acta Microbiologica Immunologica Hungarica 68, 217–226. doi: 10.1556/030.2021.01461
Wan, J. and Wu, Q. H. (2022). Review and prospect of the research on the prevention and treatment of tinea capitis ever since the founding of the People’s Republic of China in 1949. J. Hubei Polytechnic Univ. 39, 67–75. doi: 10.3969/j.issn.2095-4662.2022.06.012
Wen, Z. and Xiao, T. (2018). Clinical and pathogenic bacteria analysis of 35 cases of tinea cephalus in Hainan. Chin. J. Leprosy Dermatol. 34, 357–359.
Zeng, J., Wang, S., Guo, L., Lv, S., Shan, B., Liu, Z., et al. (2023). Pediatric tinea capitis in Jilin Province: analyzing previous results from a new perspective. Mycopathologia 188, 515–522. doi: 10.1007/s11046-023-00718-0
Zhan, P., Li, D., Wang, C., Sun, J., Geng, C., Xiong, Z., et al. (2015). Epidemiological changes in tinea capitis over the sixty years of economic growth in China. Med. Mycology 53, 691–698. doi: 10.1093/mmy/myv057
Zheng, W., Zhang, M., Wu, W., Tang, X., and Pan, Z. (2024). The likelihood of resistant tinea capitis caused by Hortaea werneckii: a case report. Clinical Cosmetic Investigational Dermatol. 17, 2653–2657. doi: 10.2147/CCID.S489139
Keywords: tinea capitis, prospective, microsporum canis, epidemiology, pathogenic fungi
Citation: Zheng W, Qiao F, Yang X, Tang X, Lin C, Chen Q, Pan Z and Chen R (2025) Tinea capitis in Hainan: a prospective study. Front. Cell. Infect. Microbiol. 15:1590315. doi: 10.3389/fcimb.2025.1590315
Received: 11 March 2025; Accepted: 09 June 2025;
Published: 23 June 2025.
Edited by:
Regina Helena Pires, University of Franca, BrazilReviewed by:
Maria Gloria Teixeira Sousa, University of São Paulo, BrazilMaria José Soares Mendes Giannini, São Paulo State University, Brazil
Copyright © 2025 Zheng, Qiao, Yang, Tang, Lin, Chen, Pan and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenai Zheng, d2VuYWlAMTYzLmNvbQ==; Rongqiang Chen, bTE4MDg5ODY1NTMwMUAxNjMuY29t
 Wenai Zheng1*
Wenai Zheng1* Zhaobing Pan
Zhaobing Pan