- 1Tufts Medical Center, Cardiovascular Center for Research and Innovation, Boston, MA, United States
- 2Cardiovascular Research Foundation, New York, NY, United States
- 3New York-Presbyterian, Advanced Heart Failure and Cardiac Transplant, New York, NY, United States
- 4Duke Medical Center, Durham, NC, United States
- 5Henry Ford Health System, Detroit, MI, United States
Background: Right ventricular failure (RVF) is associated with increased mortality among patients receiving left ventricular mechanical circulatory support (LV-MCS) for cardiogenic shock and requires prompt recognition and management. Increased central venous pressure (CVP) is an indicator of potential RVF.
Objectives: We studied whether elevated CVP during LV-MCS for acute myocardial infarction complicated by cardiogenic shock is associated with higher mortality.
Methods: Between January 2014 and June 2019, we analyzed hemodynamic parameters during Impella LV-MCS from 28 centers in the United States participating in the global, prospective catheter-based ventricular assist device (cVAD) study. A total of 132 patients with a documented CVP measurement while on Impella left-sided support for cardiogenic shock were identified.
Results: CVP was significantly higher among patients who died in the hospital (14.0 vs. 11.7 mmHg, p = 0.014), and a CVP >12 identified patients at significantly higher risk for in-hospital mortality (65 vs. 45%, p = 0.02). CVP remained significantly associated with in-hospital mortality even after adjustment in a multivariable model (adjusted OR 1.10 [95% CI 1.02–1.19] per 1 mmHg increase). LV-MCS suction events were non-significantly more frequent among patients with high vs. low CVP (62.11 vs. 7.14 events, p = 0.067).
Conclusion: CVP is a single, readily accessible hemodynamic parameter which predicts a higher rate of short-term mortality and may identify subclinical RVF in patients receiving LV-MCS for cardiogenic shock.
Introduction
Right ventricular failure (RVF) after myocardial infarction, cardiotomy, or left-sided mechanical support is associated with increased morbidity and mortality (1). In these patients, early identification of RVF and deployment of temporary RV support may improve outcomes. Increased central venous pressure (CVP) measured before or during surgical left ventricular assist device (LVAD) support is a well-established clinical indicator of risk for RVF (2). The role for CVP in the setting of short-term mechanical circulatory support is less well-characterized.
The Recover Right (RR) Trial demonstrated the safety and potential efficacy of the Impella RP, a rapidly deployed percutaneous RV assist device, in the setting of cardiogenic shock. RVF in the RR Trial was defined as a cardiac index <2.2 L/min/m2 despite the continuous infusion of high dose inotropes and any of the following: a CVP >15 mmHg, CVP-to-pulmonary capillary wedge pressure (PCWP) ratio >0.63, or moderate to severe global RV dysfunction (3). The Impella RP post-approval study demonstrated improved survival among patients receiving the Impella RP who met the pre-market IDE RR inclusion criteria for RVF compared to those who did not meet these criteria and received the device as a salvage procedure. Furthermore, a recent analysis of the SHOCK Trial and Registry identified that 45 and 38% of patients would have met hemodynamic inclusion criteria for RVF in the RR Trial. These findings and other recent reports suggest that elevated CVP is an important indicator of RVF and early identification and management of RVF may improve outcomes (4).
No studies have explored a role for CVP monitoring in the setting of short-term left ventricular mechanical circulatory support (LV-MCS) with the Impella pump for cardiogenic shock (CS). Furthermore, deciding when to embark on an extensive, multimodality assessment for RV dysfunction remains clinically challenging. We hypothesized that CVP may be a sensitive, readily accessible indicator that could be used to trigger a comprehensive evaluation for RVF in patients receiving LV-MCS. In this study, we utilize data from the catheter-based ventricular assist device (cVAD) registry to assess the relationship between CVP, mortality, and indicators of RV failure among patients receiving left-sided Impella support.
Methods
Between January 2014 and June 2019, we analyzed hemodynamic parameters during Impella LV-MCS from 28 centers in the United States participating in the global, prospective catheter-based ventricular assist device (cVAD) study (5). A total of 132 patients with a documented CVP measurement while on left-sided Impella support for acute myocardial infarction complicated by cardiogenic shock were identified. The diagnosis of acute myocardial infarction (AMI) was made by analysis of ECG changes, cardiac enzymes, and/or identification of an infarct-related coronary occlusion on emergency angiography. The central clinical events committee confirmed the presence of AMICS based on chart information collected. Cardiogenic shock was defined as a (1) systolic blood pressure ≤90 mm Hg or need for inotropes or vasopressors to maintain systolic blood pressures ≥90 mm Hg, (2) signs of peripheral hypoperfusion, and (3) cardiac index <2.2 L/min/m2 and pulmonary capillary wedge pressure ≥15 mm Hg. In order to evaluate the potential utility of CVP as a predictor of death and RV failure, we restricted our analysis to a subset of patients receiving LV-MCS for CS who had a documented CVP during support. When multiple CVP values were recorded prior to initiation of support, we used the value obtained closest to support initiation as the baseline CVP. When multiple CVP values were recorded during support, we report the average of those values as the CVP during support. Baseline characteristics including demographics and medical history as well as laboratory values, hemodynamic parameters and admission characteristics were obtained from the cVAD study. The primary endpoint of the study was in-hospital mortality, which was adjudicated in the registry by an independent clinical events committee.
As an additional validation cohort, a second analysis was performed among patients in the Impella Quality Assurance (IQ) database, a large, HIPAA compliant database of Impella patients maintained by the device manufacturer Abiomed, Inc. (6). Whereas, the cVAD registry contains a relatively small subset of patients with detailed patient information and independently adjudicated events, the IQ database captures nearly all patients treated with an Impella device in the United States but contains less in-depth patient information. Only death or survival to explant are available from the IQ database, so death prior to explant was used as the primary endpoint for the IQ database analysis rather than in-hospital mortality. Patients with AMICS with a CVP available during left-sided Impella support who were treated between October 2011 to June 2019 were identified from the IQ database using the same inclusion criteria as described above.
Statistical Analyses
Continuous variables were reported as means and standard deviations and compared using independent t-tests, while categorical variables were reported as frequency and percentages and compared using Pearson chi-squared tests. Statistical significance was reported using an α level of 0.05. Laboratory values and hemodynamic parameters recorded during mechanical support were compared in the same fashion. To examine the association between mortality and CVP as a continuous variable, we constructed a univariate logistic regression model with in-hospital mortality as the dependent variable and CVP during Impella support as the independent variable. The resulting curve was plotted with 95% confidence limits per point. To determine the optimal cutoff value of CVP which best predicted mortality, we plotted the Receiver Operating Characteristic (ROC) curves of mortality and CVP and identified the optimal point as the point closest to a sensitivity and specificity of (0,0). The Youden index, Mathews correlation coefficient, and total accuracy were also maximized around the selected cutoff point. Various univariate logistic regression models were generated with in-hospital death as the outcome with baseline and procedural characteristics as independent predictors. Variables with statistically significant univariate odds ratios were then included in a multivariable logistic regression model to report adjusted odds ratio with 95% CI for in-hospital mortality.
Results
Baseline and Admission Characteristics
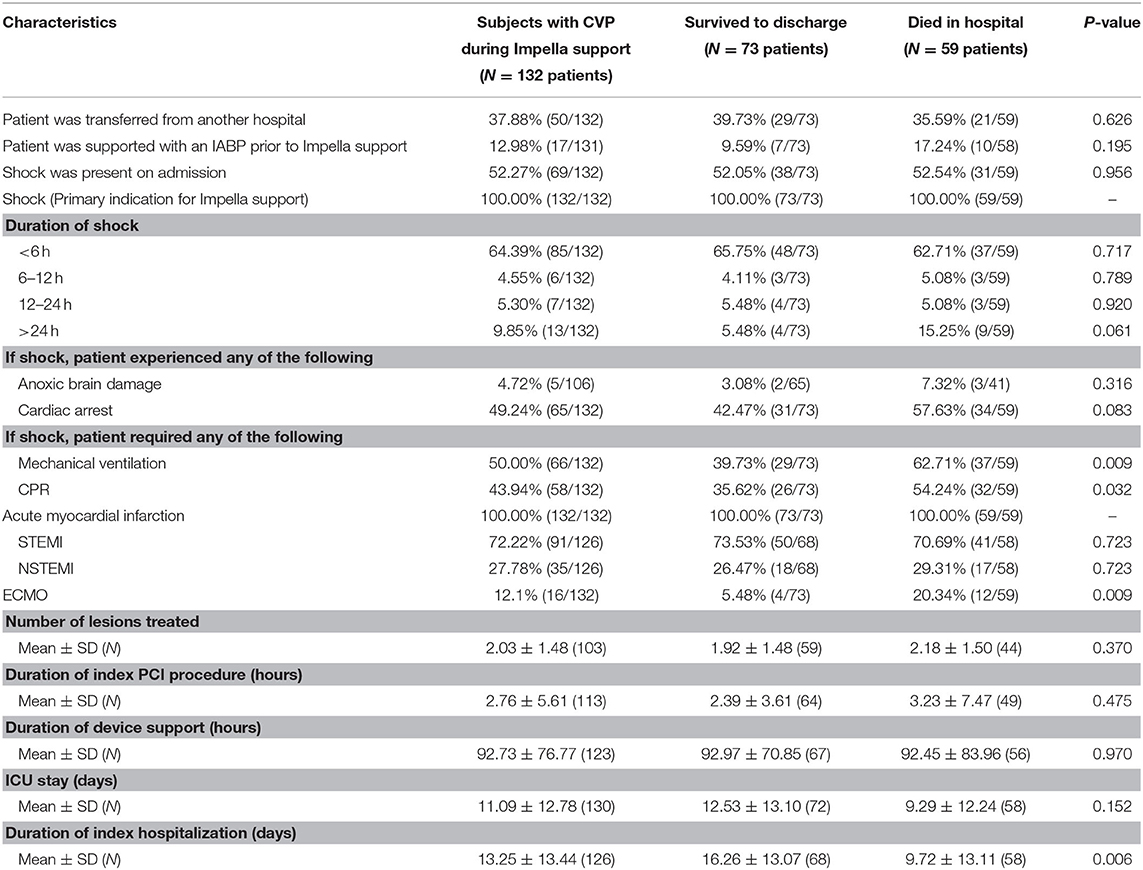
Out of 132 patients receiving LV-MCS for cardiogenic shock with available CVP data from the cVAD registry, 59 died in the hospital and 73 survived to discharge. Baseline characteristics, laboratory values, and hemodynamic parameters obtained before and after initiation of Impella support are displayed in Table 1. Hemodynamic data were more commonly measured after initiation of LV-MCS. Prior to initiation of LV-MCS, mean cardiac index (CI) was 1.9 ± 0.5 L/min/m2, pulmonary capillary wedge pressure (PCWP) was 26.5 ± 11.2 mmHg, and lactate was 6.0 ± 4.6 mmol/L. Compared to baseline values, CI improved significantly to 2.7 ± 0.9 L/min (p = 0.0001) and PCWP improved to 21.7 ± 8.7 mmHg (p = 0.09) with initiation of support. Admission and procedural characteristics are summarized in Table 2. Cardiogenic shock was due to STEMI in 72.2% and NSTEMI in 27.8% of patients, and the mean duration of Impella support was 92.7 ± 76.8 h. Significant differences between those who died in hospital and those who survived to discharge were noted in the rates of CPR (54.2 vs. 35.6%, p = 0.032) and mechanical ventilation (62.7 vs. 39.7%, p = 0.009).
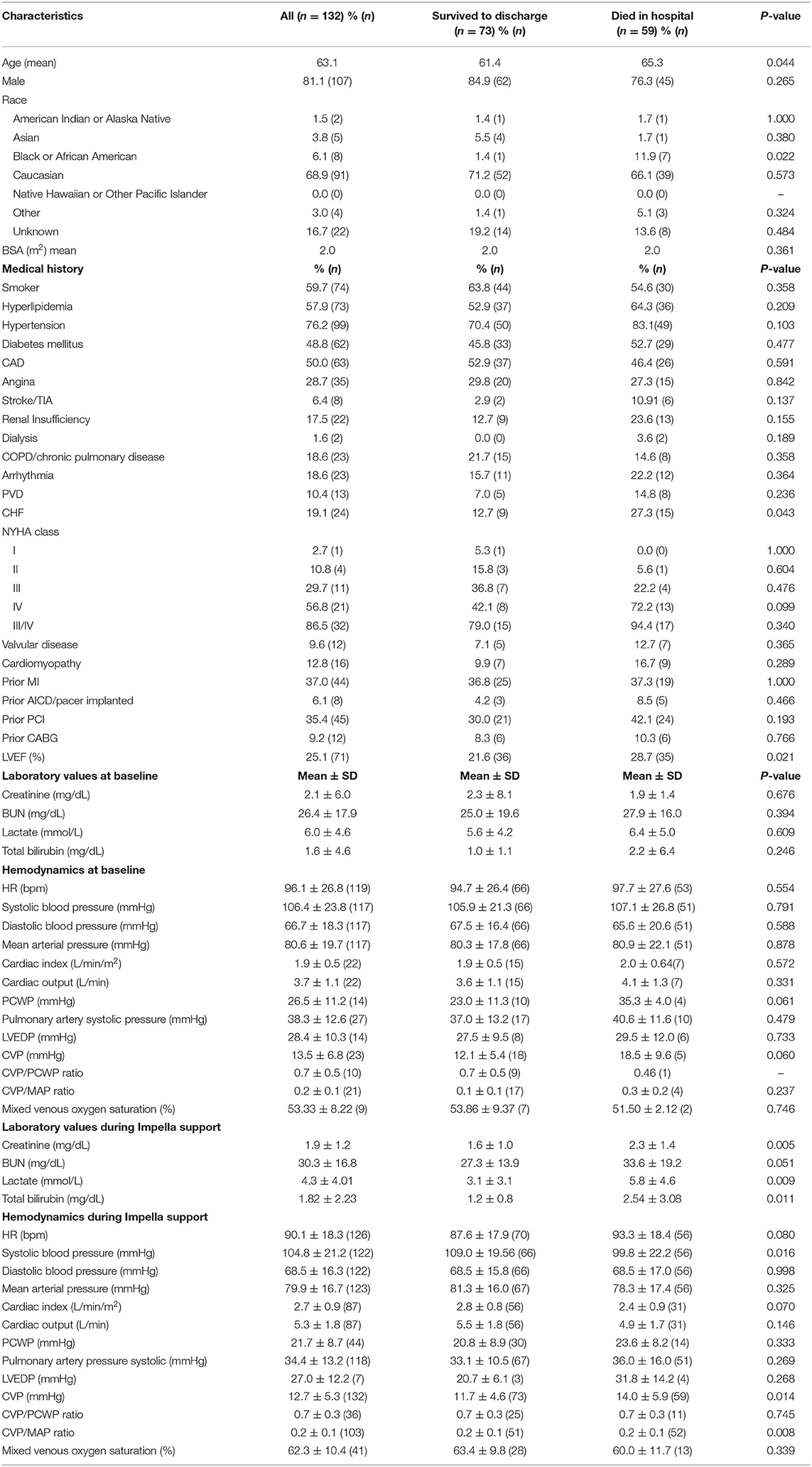
Table 1. Baseline characteristics and laboratory values/hemodynamics before and during Impella support.
Relationship Between CVP and In-Hospital Mortality
CVP was significantly higher among patients who died than among those who survived to discharge (14.0 ± 5.9 vs. 11.7 ± 4.6 mmHg, p = 0.014). The probability of in-hospital mortality increased directly with increased CVP measured during LV-MCS (Figure 1A). Receiver operating curve (ROC) analysis was performed to determine a cutoff point of CVP that best predicted mortality. The area under the receiver operating curve (AUROC) was 0.624 (95% CI 0.525–0.723). A CVP threshold of 12 was selected as the point of intersection between the sensitivity and specificity curves, with a Youden index of 0.196. Sensitivity of a CVP >12 to predict in-hospital mortality was 0.593 with a specificity of 0.602, positive predictive value 0.546, and negative predictive value 0.647. Using this cutoff, in-hospital mortality among patients with a CVP >12 was significantly higher than patients with CVP ≤12 (65 vs. 45%, p = 0.02, Figure 1B). To validate this analysis, we analyzed data from the IQ database and again found that death prior to device explant was significantly higher among patients with CVP >12 compared to those with CVP ≤12 (76 vs. 63%, p < 0.001, Figure 1C).
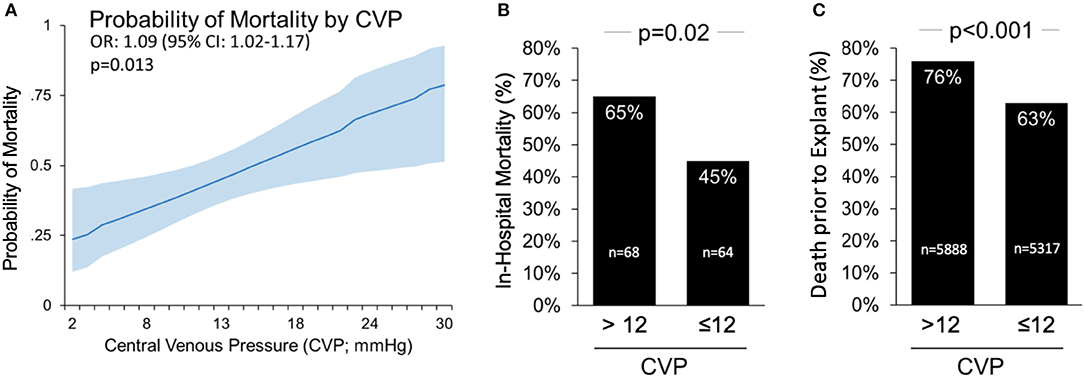
Figure 1. Central Venous Pressure (CVP) >12 mmHg on Impella support is associated with higher mortality in cardiogenic shock. (A) The probability of death based on CVP during left side Impella support; (B) CVP >12 is associated with higher in-hospital mortality rates among patients in the cVAD; and (C) associated with higher rate of death prior to device explant in the IQ Registry.
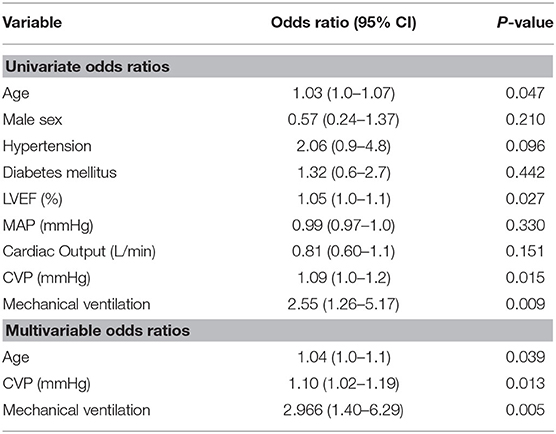
Univariate ORs and 95% confidence intervals are presented in Table 3. Among the variables tested, increasing age, decreasing LVEF, increasing CVP and need for mechanical ventilation were significantly associated with a higher odds of mortality. After adjusting for age, LVEF, and the need for mechanical ventilation, CVP remained significantly associated with in-hospital mortality (OR 1.10 per 1 mmHg increase in CVP, 95% CI 1.02–1.19, p = 0.013).
CVP and Suction Events
We analyzed suction alarm data downloaded from the Automated Impella Controller (AIC) during Impella support, which were available in 21 out of 132 patients from the cVAD registry. Compared to patients with a CVP ≤12 during Impella support, suction events were more common among patients with a CVP >12 (62.11 ± 93.56 vs. 7.14 ± 8.79, number of events, p = 0.067, Figure 2).
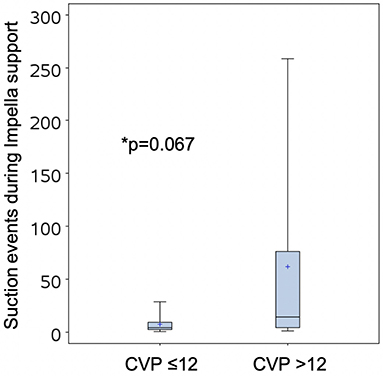
Figure 2. Suction events recorded by the Automated Impella Controller in patients with CVP >12 or ≤12.
Discussion
We report for the first time that an elevated CVP during LV-MCS for cardiogenic shock is associated with in-hospital mortality. We identified that CVP was higher among patients who died in the hospital compared to those that survived to discharge in the cVAD registry. A CVP >12 among patients receiving LV-MCS predicted a higher odds of in-hospital mortality, even after adjustment for other variables. We further observed that suction events, which indicate reduced LV preload, were paradoxically more frequent among patients with a higher CVP, suggesting that a higher rate of impaired RV function may in part account for the higher short-term mortality observed among patients with high CVP. Collectively, these data suggest that identification of an elevated CVP during LV-MCS should trigger further evaluation of RV function with echocardiography or a pulmonary artery catheter.
While mechanical RV support devices such as the Impella RP can be used to stabilize patients with acute RVF, prompt recognition of RV dysfunction is paramount to prevent rapid deterioration and death. In contrast to LV failure where pulmonary edema is often readily apparent, right sided congestion indicating RV failure may be clinically silent, reinforcing the need for a high clinical suspicion and readily accessible bedside indicators which can be used to identify incipient RVF. Most well-validated hemodynamic indices of RVF such as the pulmonary artery pulsatility index (PAPi), CVP/PCWP ratio, and pulmonary vascular resistance (PVR) require use of a pulmonary artery catheter (PAC), and thus a more accessible bedside parameter is needed to trigger a formal evaluation for RVF.
On the basis of our findings, we propose that a CVP >12 in a patient receiving left sided mechanical support should prompt a formal hemodynamic and echocardiographic assessment of RV function to assess the need for decongestive therapies or RV mechanical support. The concurrent presence of frequent suction events in the face of adequate volume should further raise suspicion for RV pump failure. Prior studies including the Recover Right trial have proposed specific criteria for initiation of mechanical RV support including a CVP/PCWP ratio >0.63 or PAPi <0.9 in conjunction with echocardiographic indicators of RV dysfunction, though future studies will be needed to confirm the benefits of such an algorithm prospectively (3, 7).
Several limitations of our study must be acknowledged. First, these data are retrospective, and the limitations of cVAD data are such that the exact timing of laboratory and hemodynamic values relative to initiation of Impella support cannot be ascertained. Additionally, while we have proposed that the increased mortality observed in patients with high CVP is due at least in part to RVF, this connection cannot be definitively established due to a lack of high-resolution data on the specific causes of death among patients in this sample. Alternative causes of increased CVP that would also likely increase odds of mortality include hypervolemia, pulmonary hypertension, progressive LV failure, cardiac tamponade, renal failure, and the need for mechanical ventilation with high positive end-expiratory pressure. Accordingly, these results should be considered hypothesis generating, and warrant confirmation in larger, higher-resolution prospective studies. Finally, we did not have granular data on patient outcomes other than mortality, so some patients who survived in this analysis may have been bridged to durable VAD or transplant.
In conclusion, we report data from the cVAD registry showing that a CVP >12 predicts mortality in patients receiving left-sided aMCS and propose that a CVP >12 should prompt formal hemodynamic assessment for RV failure, especially in the presence of frequent suction events. Future studies will be needed to confirm these findings and refine hemodynamic criteria for mechanical RV support.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by WIRB and Institutional IRBs for CVAD Registry. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
EW generated figures and tables, and drafted the manuscript. KT assisted with generation of figures and editing of the manuscript. DB contributed to conception and design of the research as well as editing of the manuscript. NU and WO'N contributed to conception and design of the project. EO contributed to conception and design of the project, and editing of the manuscript. NK contributed to conception and design of the research, generation of figures and tables, and drafting/editing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant from the National Institutes of Health (R01HL139785-01 and R01HL133215-01) to NK and from Abiomed Inc. to Tufts Medical Center. Abiomed Inc. funded the study, provided data, and assisted with statistical analysis. They had no role in the interpretation of the data, decision to publish, or preparation of the manuscript.
Conflict of Interest
NK receives consulting/speaker honoraria and institutional grant support from: Abbott Laboratories, Abiomed Inc., Boston Scientific, Medtronic, LivaNova, MDStart, and Precardia. DB has received an unrestricted educational research grant from Abiomed Inc. Abiomed Inc. funded the study, provided data, and assisted with statistical analysis. They had no role in the interpretation of the data, decision to publish, or preparation of the manuscript.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2020.00155/full#supplementary-material
Abbreviations
CVP, central venous pressure; RHF, right heart failure; RVF, right ventricular failure; RR, Recover Right trial.
References
1. Konstam MA, Kiernan MS, Bernstein D, Bozkurt B, Jacob M, Kapur NK, et al. Evaluation and management of right-sided heart failure: a scientific statement from the American Heart Association. Circulation. (2018) 137:e578–22. doi: 10.1161/CIR.0000000000000560
2. Bellavia D, Iacovoni A, Scardulla C, Moja L, Pilato M, Kushwaha SS, et al. Prediction of right ventricular failure after ventricular assist device implant: systematic review and meta-analysis of observational studies. Eur J Heart Fail. (2017). 19:926–46. doi: 10.1002/ejhf.733
3. Anderson MB, Goldstein J, Milano C, Morris LD, Kormos RL, Bhama J, et al. Benefits of a novel percutaneous ventricular assist device for right heart failure: the prospective RECOVER RIGHT study of the Impella RP device. J Heart Lung Transplant. (2015) 34:1549–60. doi: 10.1016/j.healun.2015.08.018
4. Kapur NK, Esposito ML, Bader Y, Morine KJ, Kiernan MS, Pham DT, et al. Mechanical circulatory support devices for acute right ventricular failure. Circulation. (2017) 136:314–26. doi: 10.1161/CIRCULATIONAHA.116.025290
5. Vetrovec GW, Anderson M, Schreiber T, Popma J, Lombardi W, Maini B, et al. The cVAD registry for percutaneous temporary hemodynamic support: a prospective registry of Impella mechanical circulatory support use in high-risk PCI, cardiogenic shock, and decompensated heart failure. Am Heart J. (2018) 199:115–21. doi: 10.1016/j.ahj.2017.09.007
6. O'Neill WW, Grines C, Schreiber T, Moses J, Maini B, Dixon SR, et al. Analysis of outcomes for 15,259 US patients with acute myocardial infarction cardiogenic shock (AMICS) supported with the Impella device. Am Heart J. (2018) 202:33–8. doi: 10.1016/j.ahj.2018.03.024
Keywords: central venous pressure, right heart failure, Impella RP, cardiogenic shock, mechanical circulatory support
Citation: Whitehead EH, Thayer KL, Burkhoff D, Uriel N, Ohman EM, O'Neill W and Kapur NK (2020) Central Venous Pressure and Clinical Outcomes During Left-Sided Mechanical Support for Acute Myocardial Infarction and Cardiogenic Shock. Front. Cardiovasc. Med. 7:155. doi: 10.3389/fcvm.2020.00155
Received: 19 May 2020; Accepted: 22 July 2020;
Published: 28 August 2020.
Edited by:
Chris J. Pemberton, University of Otago, New ZealandReviewed by:
Philip Adamson, Department of Medicine, University of Otago, New ZealandEisuke Amiya, The University of Tokyo Hospital, Japan
Copyright © 2020 Whitehead, Thayer, Burkhoff, Uriel, Ohman, O'Neill and Kapur. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Navin K. Kapur, bmthcHVyQHR1ZnRzbWVkaWNhbGNlbnRlci5vcmc=
 Evan H. Whitehead
Evan H. Whitehead Katherine L. Thayer
Katherine L. Thayer Daniel Burkhoff
Daniel Burkhoff Nir Uriel3
Nir Uriel3 Navin K. Kapur
Navin K. Kapur