- 1Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
- 2Department of Radiology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Objective: This study aims to investigate the impact of cardiovascular medications on the coronary flow reserve (CFR) in patients without obstructive coronary artery disease (CAD).
Methods: We searched PubMed, EMBASE, and Cochrane databases from inception to 15 November 2019. Studies were included if they reported CFR from baseline to follow-up after oral drug therapy of patients without obstructive CAD. Data was pooled using random-effects modeling. The primary outcome was change in CFR from baseline to follow-up after oral drug therapy.
Results: A total of 46 studies including 845 subjects were included in this study. Relative to baseline, the CFR was improved by angiotensin-converting enzymes (ACEIs), aldosterone receptor antagonists (ARBs) [standard mean difference (SMD): 1.12; 95% CI: 0.77–1.47], and statins treatments (SMD: 0.61; 95%CI: 0.36–0.85). Six to 12 months of calcium channel blocker (CCB) treatments improved CFR (SMD: 1.04; 95% CI: 0.51–1.58). Beta-blocker (SMD: 0.24; 95% CI: −0.39–0.88) and ranolazine treatment (SMD: 0.31; 95% CI: −0.39–1.01) were not associated with improved CFR.
Conclusions: Therapy with ACEIs, ARBs, and statins was associated with improved CFR in patients with confirmed or suspicious CMD. CCBs also improved CFR among patients followed for 6–12 months. Beta-blocker and ranolazine had no impact on CFR.
Introduction
Patients with angina symptoms without obstructive coronary artery disease (CAD) have been difficult to diagnose and treat. Up to 50–65% of these patients are considered to have coronary microvascular dysfunction (CMD) (1–5), which is associated with diastolic heart failure (6–8). Over the past 20 years, a large number of studies have used invasive and non-invasive imaging techniques to assess coronary microvascular function (9–12) and have increased our understanding of CMD and microvascular ischemia. CMD is a broad term covering four main types and several endotypes, which could be overlap (13–15).
However, there are still no clear guidelines for CMD treatment (16). Although the antihypertensives are not intended for CMD, the recent studies showed that ACEIs, ARBs, and CCBs are potentially useful for improving CRF. Moreover, data on the effectiveness of CMD medications remain scarce. Most studies on this have inconsistent results. Medications including ACEI, statins, and beta-blockers may be used to treat CMD under the current European Society of Cardiology position paper on CMD (17). Several reviews suggest that exercise, controlling risk factors, and medications such as angiotensin-converting enzyme inhibitors (ACEIs), aldosterone receptor antagonists (ARBs), and statins may be effective first-line treatments, and medications including nicorandil and ranolazine can be effective second-line treatments (8, 14, 18, 19).
Statins and ACEIs can improve endothelial dysfunction in patients with hypertension and are also first-line treatments for patients with CMD (20, 21). Ranolazine and ivabradine have been shown to attenuate angina symptoms and improve coronary microvascular function (22–24). Nicorandil is a nitrate and adenosine triphosphate-sensitive potassium channel agonist that can improve peak exercise performance but does not significantly improve the index of microcirculatory resistance (IMR) and ST-induced changes in exercise (22, 25). The proposed treatment algorithm for CMD patients has been summarized by Crea et al. (14). For all patients, efforts should be made to reduce controllable risk factors. Additional traditional and non-traditional anti-ischemic medications are recommended if symptoms are not well-controlled with first-line treatments. Patients with suspected enhanced pain perception should receive pain modulators. For patients with refractory symptoms that significantly restrict the quality of life, treatments such as enhanced external counter pulsation, spinal cord stimulation, and cognitive behavioral therapy should also be considered.
This review focuses on the effects of oral medical therapy on coronary flow reserve (CFR) outcomes in patients with non-obstructed coronary arteries and provides additional evidence to guide physicians in the selection of the optimal pharmaceutical treatment for patients with CMD.
Methods
Search Strategy
This study meticulously followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines. PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials were searched for relevant publications through November 15, 2019. The search strategies were performed using the following MeSH terms and keywords: coronary microvascular dysfunction, coronary microvessel dysfunction, microcirculation, CFR, coronary flow reserve, coronary flow, treatment, management, therapy, oral drug, and pharmacotherapy (Supplementary Table 1).
We also manually screened the reference lists of included manuscripts to identify any relevant studies not identified by the initial search. The search was restricted to peer-reviewed studies on adults. This systematic review protocol has been registered with the PROSPERO (CRD42020158659).
Selection Criteria
Two investigators (JWY and TJF) independently selected studies based on the study eligibility criteria. All randomized and non-randomized studies were considered to be eligible if they (1) included patients without significant stenosis of the epicardial coronary artery determined by invasive angiography or CT coronary angiography or other methods; (2) included patients diagnosed with CMD or CAD [acute, stable coronary syndrome; receiving percutaneous coronary intervention (PCI), or coronary artery bypass grafting (CABG)] or having at least one risk factor for coronary heart disease (such as hypertension, hypercholesterol, and diabetes mellitus); (3) included patients receiving CFR testing to evaluate coronary microvascular function before and after treatment; (4) included patients taking one or more of the following oral drugs: statins, ATP-sensitive potassium channel openers, ranolazine, ivabradine, nitrate, CCBs, beta-blockers, ACEIs, ARBs, or trimetazidine; (5) included patients followed up for at least 3 weeks; and (6) were published in English.
Two researchers independently selected studies based on the study inclusion criteria. Both investigators reviewed the full-text manuscripts of identified articles to assess whether studies met eligibility criteria. We excluded case reports, case series, review, meta-analysis, protocol, comments, abstract, and non-English language studies. Reasons for exclusion during full-text screening included inappropriate population, inappropriate outcome, and insufficient information for outcome assessments, among others. The third opinion (XTS) was consulted to resolve screening discrepancies between the two investigators. The search and screening process is outlined in Figure 1.
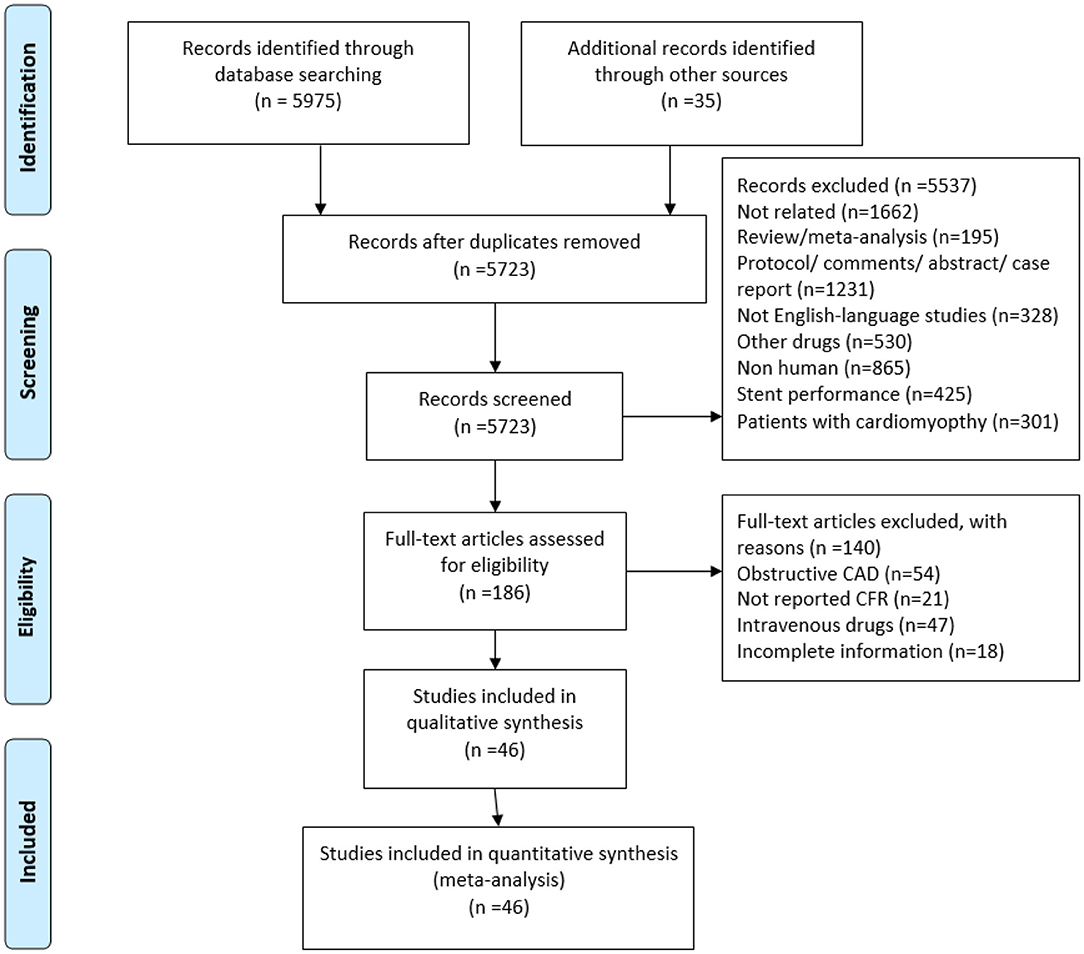
Figure 1. Flowchart of study selection. CMVD, coronary microvascular dysfunction; CFR, coronary flow reserve.
Data Extraction
For included studies, two investigators (JWY and JFT) extracted the data independently. The primary outcome was CFR before and after oral drug therapy. Extracted information included journal, first author name, publication year, population, clinical characteristics at baseline, and patient CFR data before and after treatment. Publication authors were contacted by email when study source data were unclear or to acquire additional data. The third opinion (XTS) was consulted to resolved any data extraction discrepancies between the two investigators.
Statistical Analysis
Summary results for CFR outcome data are presented as standard mean differences (SMDs) with 95% CI. The I2 test was applied to assess heterogeneity between studies, where I2 < 25% was regarded as no heterogeneity; 25–50%, moderate heterogeneity; and >50%, severe heterogeneity. We calculated pool estimates of the means and standard deviations (SD) of pre-CFR and post-CFR between different drug groups using a random-effects model (Der Simonian and Laird method) to account for uncertainty associated with interstudy variabilities in drug effects. Publication bias was assessed using Egger's linear regression test and visual inspections of funnel plots. Analysis was performed using Stata 11.0 (Stata Corp, College Station, TX) and Review Manager, Version 5.3 (Cochrane Collaboration, Oxford, United Kingdom). A two-tailed P < 0.05 was considered statistically significant.
Results
Study Selection and Characteristics
A total of 5,723 references were identified from database search analyses. Of these, 5,537 were excluded during title and abstract level screening (Figure 1). Of the remaining 186 studies, 140 were excluded for the following reasons: obstructive CAD (n = 54), unclear or missing CFR data (n = 21); use of intravenous drugs (n = 47); and incomplete information (n = 18). Forty-six of the remaining studies reported CFR data and did not meet any other exclusion criteria, of which 28 were randomized controlled trials and 18 were non-randomized studies. The study characteristics are presented in Table 1, and the clinical characteristics of patients are presented in Supplementary Table 2. A total of 845 patients, ranging from 8 to 55 participants per trial, were ultimately included who received coronary microvascular function assessments before and after administration of oral medications. CFR is feasible for coronary microvascular function evaluation (1), and we therefore collected CFR data as an indicator of coronary microvascular function. At present, there is no uniform gold standard for CFR detection methods. Methods for measuring CFR included intracoronary (IC) Doppler flow wire (n = 6), cardiac magnetic resonance imaging (CMRI) (n = 2), positron emission tomography (PET) (n = 11), and Doppler echocardiography (DE) (n = 27). Methods for obtaining stenosis of epicardial coronary artery included invasive angiography (n = 21), CT coronary angiography (n = 6), medical history (n = 8), and DE and treadmill exercise test (n = 11). Follow-up duration varied from 0.75 to 12 months.
Association of ACEIs and ARBs With CFR
A total of 19 studies investigated the effect of ACEIs and ARBs on CFR improvement, including 10 for ACEIs and 9 for ARBs. The CFRs in patients receiving either ACEIs or ARBs improved significantly compared to baseline with a pooled estimate SMD of 1.12 [95% confidence interval (CI): 0.77–1.47, I2 = 71.8%] (Figure 2). Changes in CFR across the follow-up period subgroups were similar: 0–1 month (SMD: 0.93; 95% CI: 0.38–1.47, I2 = 58.8%); 1–3 months (SMD: 1.21; 95% CI: 0.52–1.89, I2 = 70.8%); 3–6 months (SMD: 1.32; 95% CI: 0.41–2.24, I2 = 86.9%); 6–12 months (SMD: 1.23; 95% CI: 0.73–1.72, I2 = 0.0%). For patients with angina symptoms, ACEIs and ARBs can improve the CFR (SMD: 1.95; 95% CI: 0.53–3.38, I2 = 90.6%) (Figure 7). These findings indicate that treatment with ACEIs or ARBs may improve CFR for patients without obstructive CAD.
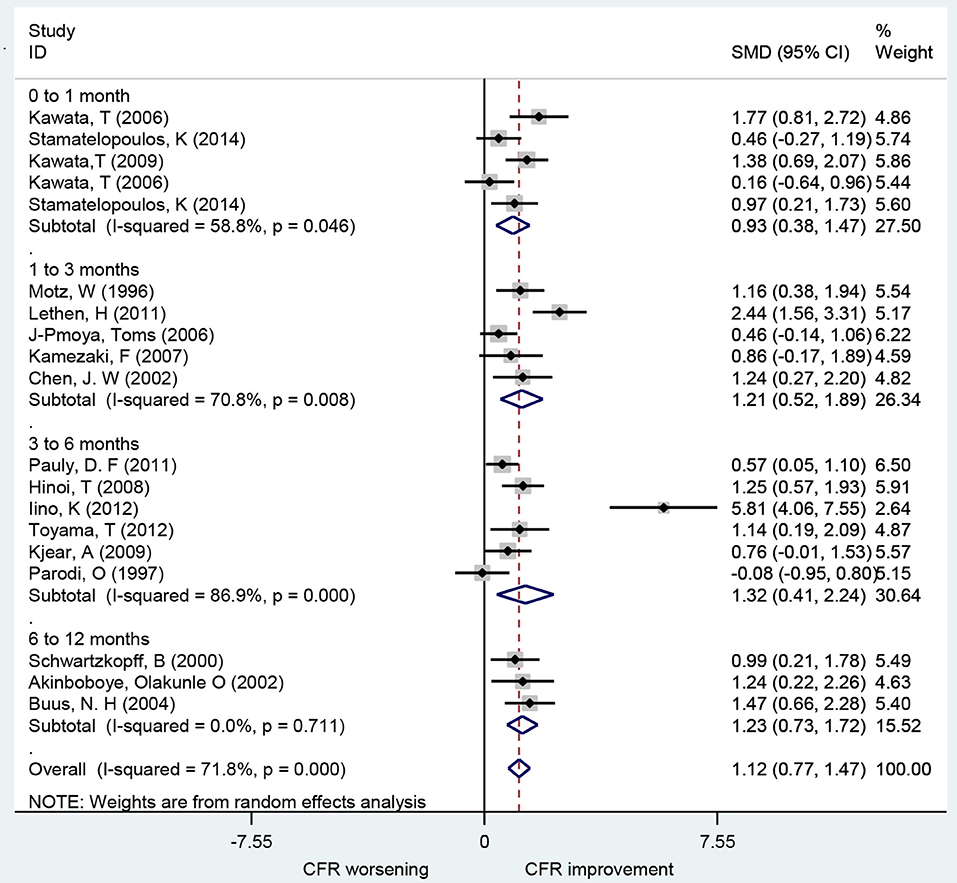
Figure 2. Forest plot of CFR for ACEI and ARB. CFR, coronary flow reserve; ACEI, aldosterone receptor antagonist; ARB, aldosterone receptor antagonist.
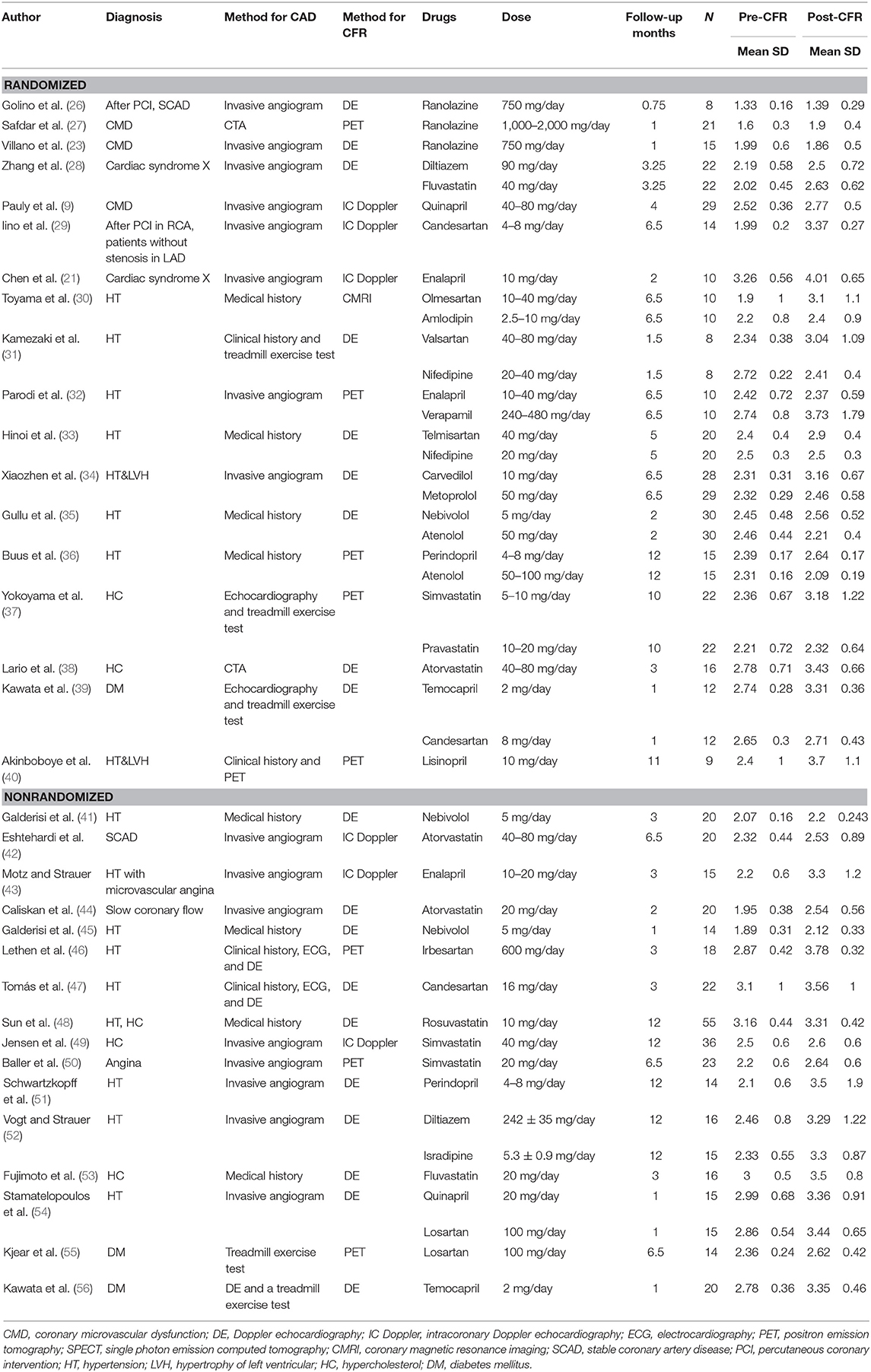
Association of Beta-Blocker With CFR
A total of seven studies that included 166 patients investigated the effects of beta-blocker on CFR. These found no difference in CFR at follow-up compared to baseline (SMD: 0.24; 95% CI: −0.39–0.88, I2 = 87.2%) (Figure 3). There was no statistical difference in CFR change across subgroups for follow-up periods up to 6 months: 0–1 month (SMD: 0.72; 95% CI: −0.05–1.48, I2 =. %); 1–3 months (SMD: 0.07; 95% CI: −0.63–0.77, I2 = 79.1%); 3–6 months (SMD: 0.96; 95% CI: −0.34–2.25, I2 = 90.5%). Change in CFR was significant for the 6–12 months follow-up subgroup; however, only one study reported data on this subgroup (SMD: −1.25; 95% CI: −2.04 to −0.47, I2 =. %). For patients with myocardial ischemic symptoms, beta-blockers had no significant effect on CFR (SMD: 0.96; 95% CI: −0.34–2.25, I2 = 90.5%) (Figure 7).
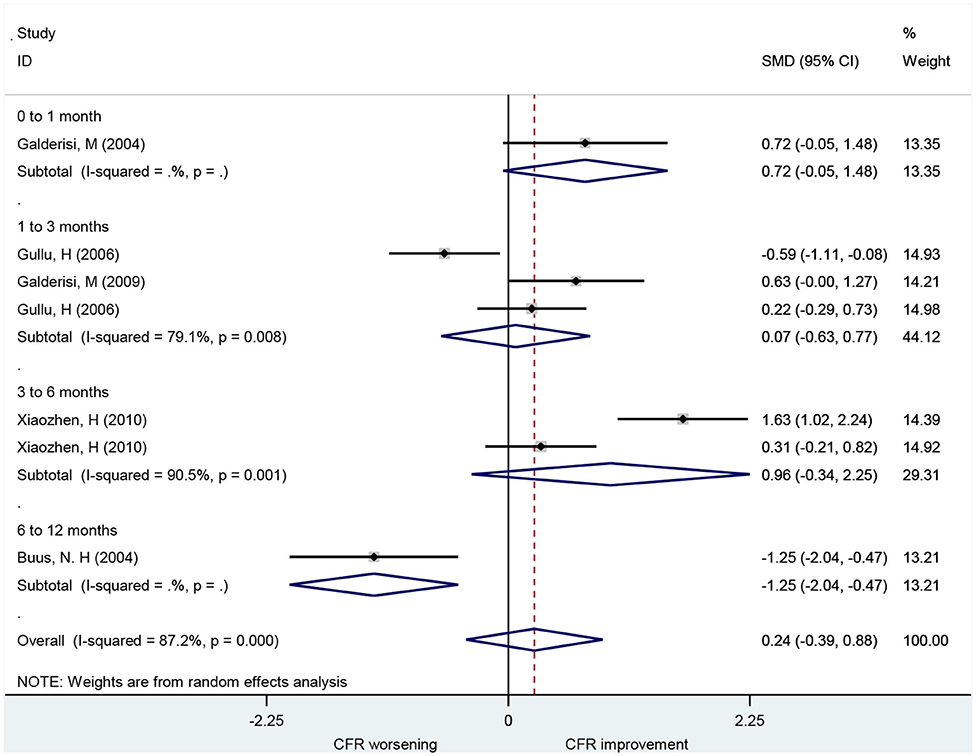
Association of CCB With CFR
A total of seven studies that included 101 patients investigated the use of CCBs for CFR improvement. They showed no difference in CFR at follow-up compared to baseline (SMD: 0.41; 95% CI: −0.06–0.87, I2 = 60.6%) (Figure 4). Changes in CFR were not significantly different across follow-up subgroups for periods up to 6 months: 1–3 months (SMD: −0.96; 95% CI: −2.00–0.08, I2 =. %); 3–6 months (SMD: 0.32; 95% CI: −0.04–0.67, I2 = 0%). However, CCBs were associated with CFR improvement in the 6–12 months follow-up subgroup (SMD: 1.04; 95% CI: 0.51–1.58, I2 = 0%).
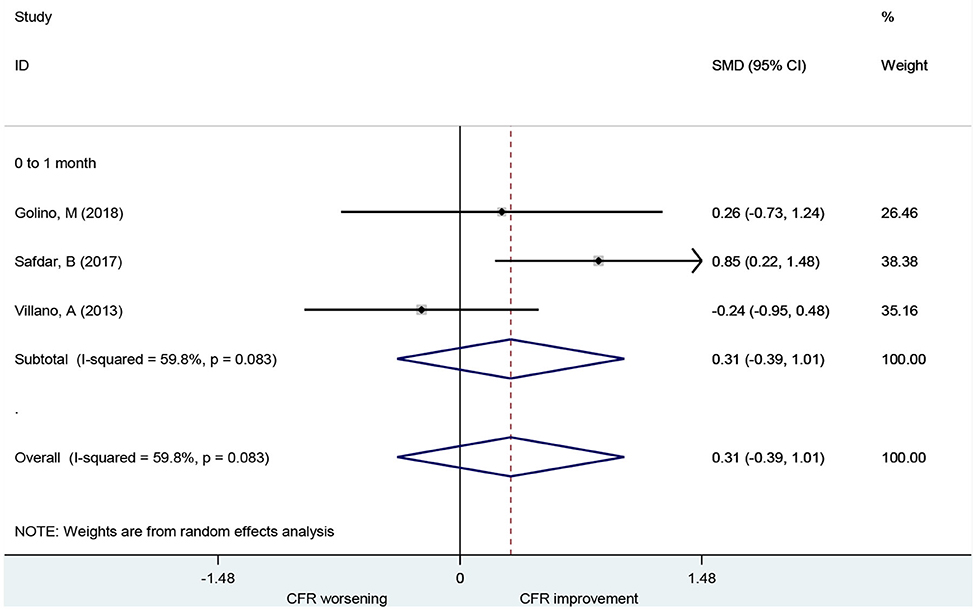
Association of Ranolazine With CFR
A total of three studies including 44 patients investigated the use of ranolazine for CFR improvement. All patients included had symptoms of myocardial ischemia. CFR was not improved significantly after patients received ranolazine for 1 month (SMD, 0.31; 95% CI: −0.39–1.01, I2 = 59.8%) (Figure 5).
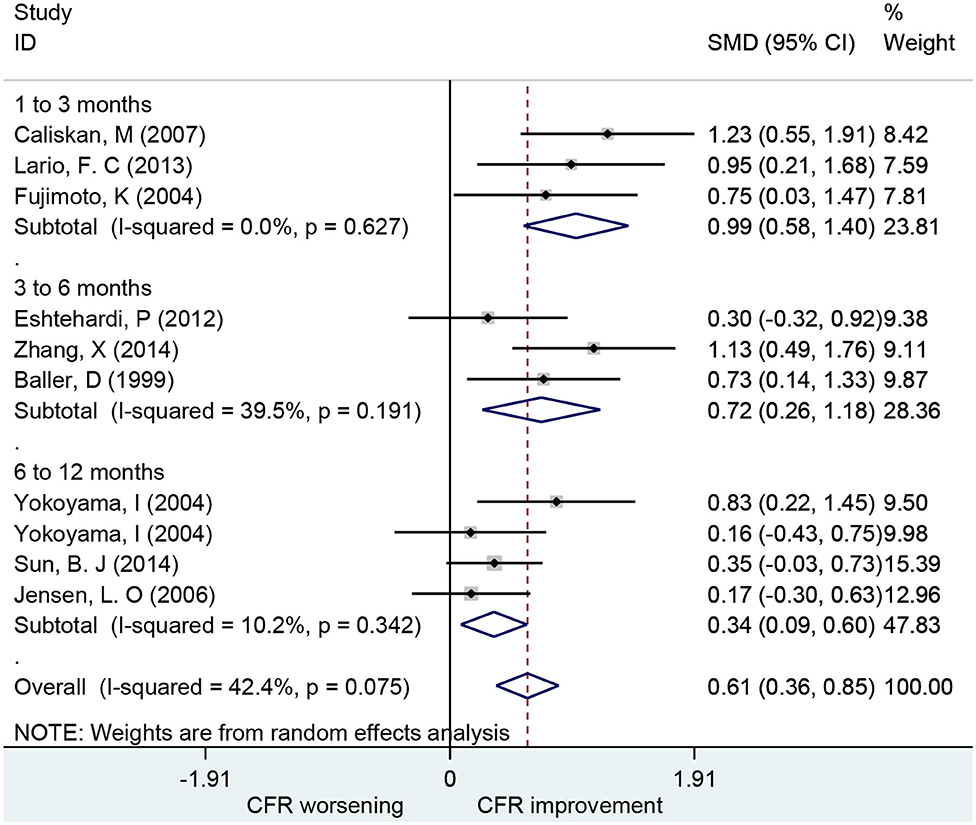
Association of Statins With CFR
Ten studies including 252 patients investigated the use of statins for CFR improvement. Patients receiving statins showed significant improvement in CFR compared to baseline with a pooled estimate SMD of 0.61 (95% CI: 0.36–0.85, I2 = 42.4%) (Figure 6). Subgroup analyses by follow-up time showed that changes in CFR were similar: 1–3 months (SMD: 0.99; 95% CI: 0.58–1.40, I2 = 0%); 3–6 months (SMD: 0.72; 95% CI: 0.26–1.18, I2 = 39.5%); 6–12 months (SMD: 0.34; 95% CI: 0.09–0.60, I2 = 10.2%). Five of these studies showed that statins can improve CFR in patients with symptoms of myocardial ischemia (SMD: 0.68; 95% CI: 0.25–1.11, I2 = 78.7%) (Figure 7). This indicates that statins may improve CFR for patients without coronary stenosis, regardless of follow-up period duration.
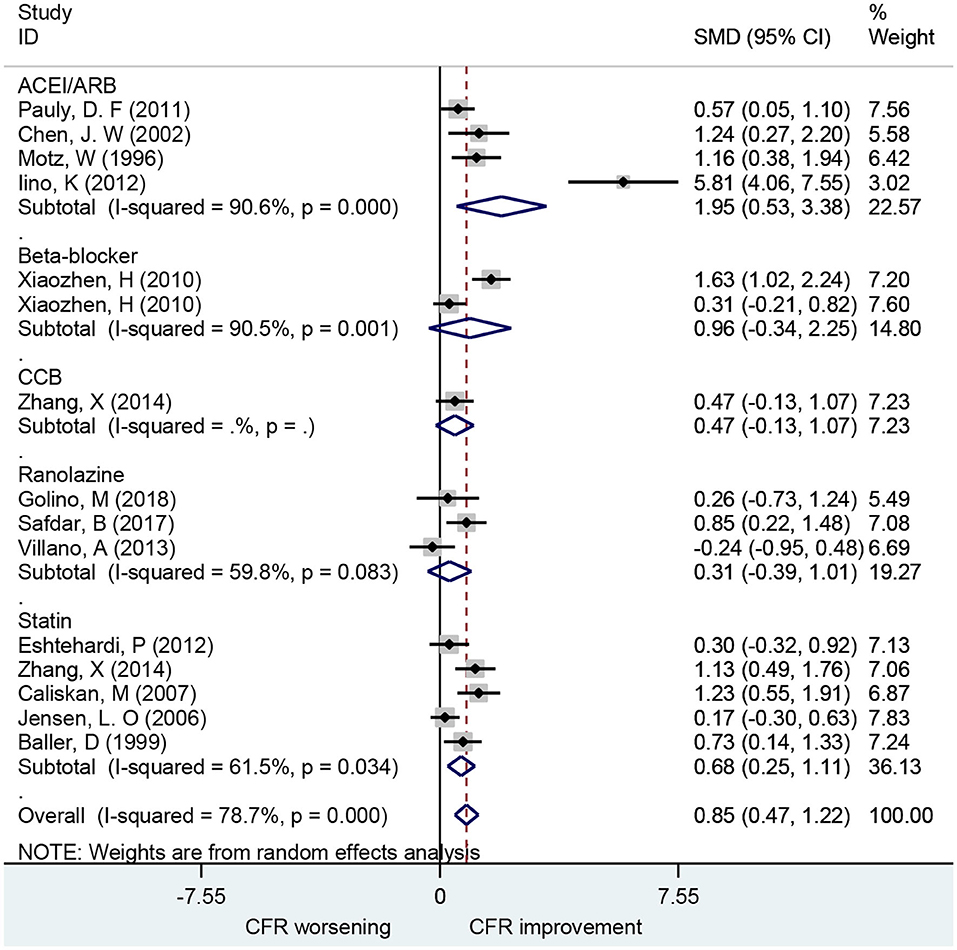
Figure 7. Forest plot of CFR of different drugs in patients with myocardial ischemia symptoms. CFR, coronary flow reserve; ACEI, aldosterone receptor antagonist; ARB, aldosterone receptor antagonist; CCB, calcium channel blocker.
Quality of Studies, Clinical Heterogeneity, and Publication Bias
The quality assessment of included studies is shown in Supplemental Table 3. The quality of the 18 studies that mentioned randomized grouping was primarily assessed. Three studies were considered low risk with respect to randomization (9, 23, 27) because they used computer-designated procedure, and the remaining studies were classified as unclear risk as they did not elaborate on the randomized methods applied. Except for the study of Pauly et al. no other randomized controlled studies reported location concealment in detail and were therefore considered unclear risk. As for the performance bias, 12 randomized controlled studies were considered low risk as they were blinded, and the remaining six studies reported no blinding or unclear blinding methods. For eight randomized controlled studies, no treatment or distribution schemes were known by the outcome observers, and a third party performed outcome analyses. Therefore, detection bias was considered low risk. Attrition bias was considered low risk because the final proportion of those who completed the study in both groups was almost the same as that in the beginning despite participant dropout. Neither reporting bias nor other biases were found in any included randomized controlled study. In addition, we restricted analyses to 17 studies with high methodological quality, as indicated by a Newcastle–Ottawa Scale (NOS) score of 60% or more.
In terms of CFR, statistical heterogeneity as measured by I2 ranged from 42.04 to 87.2%, indicating no to moderate heterogeneity. Some heterogeneity can be explained by differences in follow-up duration, drug dosage, study type, imaging methods used, and patient disease. Considering the potentially high degree of heterogeneity between studies, a subgroup analysis was performed based on follow-up duration. In the sensitivity analysis, the conclusion from recalculating pooled estimates were consistent with the primary analysis, when each study was excluded individually. This was reflected by the 95% CIs of the separate studies that overlap well (Supplementary Figures 1–5).
We found evidence of publication bias based on the funnel plots (Supplementary Figure 6) or the Egger test (t = 3.04; P = 0.004).
Discussion
We reviewed 46 studies that assessed CFR improvement in 845 patients without obstructive CAD treated with oral drugs. We found that ACEIs, ARBs, and statins were associated with significant improvement in CFR compared to baseline among patients who were followed up between 0 and 12 months. Moreover, CCBs and β-blockers were also associated with improved CFR when follow-up was extended to 6–12 months. Treatment with ranolazine for 1 month had no significant effect on CFR improvement compared to baseline. Compared with the previous studies, the present study focused on the improvement of microvascular function by oral medication during different follow-up periods.
The 2020 ESC position paper on CMD and 2019 ESC guidelines on chronic coronary syndromes recommend that ACEI/ARB, statins, and beta-blockers can be used for secondary prevention treatment of CMD (17). Several reviews suggest exercise, control of risk factors, and drugs such as ACEIs/ARBs and statins as effective first-line treatments and CCBs, nicorandil, and ranolazine as second-line treatments (8, 14, 18, 19). However, these recommendations are controversial regarding the therapeutic effects of beta-blockers, CCB, and ranolazine and did not give a definite treatment time. Our research affirmed that long-term CCB improved CFR, but beta-blockers and ranolazine did not. We also conducted subgroup analysis of follow-up time, which allowed better guidance of clinical medication time.
Previous studies have found that ACEIs and ARBs can improve microcirculatory function, which corresponds with the findings of this study (9, 54, 57, 58). Studies evaluating the effects of ACEIs and ARBs on Seattle Angina score and E/e' indicate that the CFR improvement is associated with reduced angina and left ventricular filling pressures (9, 30, 54). This mechanism by which ACEIs and ARBs improve CFR may be related to their anti-inflammatory and antioxidant characters as well as their effects of coronary endothelial dysfunction improvement and vasodilatation (29, 54, 59). Thus, ACEI improvements in microcirculatory function and left ventricular diastolic function are not merely dependent on its antihypertensive and antiventricular remodeling effects (46, 54).
We found that early treatment with statins was associated with improved microcirculatory function, similar with the findings of previous studies (38, 42, 48). The mechanisms by which statins protect microcirculatroy function include their anti-inflammatory effect, endothelium protective effect, and antiremodeling effect on left ventricular (60–64). The benefits of statin therapy may also be related to elevated levels of nitric oxide and reduced expression of endothelin-1 (28, 61). These findings suggest that short-term statin therapy is recommended to improve coronary microvascular function.
CCBs showed improved CFR among patients followed up for 0–6 months, but the drug was found to be effective with prolonged follow-up time (34, 41, 65). Therefore, the negative findings from previous studies may be due to insufficient treatment duration (30, 31, 33, 66). CCBs may improve microcirculation through vasodilation.
This study found that β-blockers did not improve CFR. However, many studies have shown that β-blockers have microcirculation protection, which was attributable to its antioxidant and endothelial protection properties (41, 45, 67, 68). We found that ranolazine did not improve CFR, and other studies have yielded similar findings (23, 26, 69–71). However, some studies indicate that ranolazine may improve the coronary microvascular function by improving angina symptoms (18, 72). This inconsistency in study findings may be due to insufficient dosage and treatment time (73). Ranolazine exerts its anti-ischemic effect by affecting the activity of pyruvate dehydrogenase to improve myocardial energy metabolism, by inhibiting the late Na+ current in myocardial cells, and reducing Ca2+ influx, thereby improving diastolic function and myocardial perfusion. However, this is an effect that can only be achieved at high concentrations (23, 26). Additional large-scale follow-up studies are needed to fully clarify this.
Moreover, some studies indicate that nicorandil may improve coronary microvascular function by regulating plasma levels of nitric oxide and endothelin-1 (74–77). However, most of these studies involved intracoronary injections or intravenous drugs and were therefore excluded from evaluation here. Ivabradine and diuretics are also reported to be associated with improved of microcirculation function (12, 18, 78). However, a meta-analysis could not be performed due to the small number of available studies.
Several studies have investigated the effects of medication on CMD without obstructive CAD and concluded that optimal treatment for microvascular angina should focus on relieving symptoms and improving vascular function (18). Exercise and weight loss, in addition to statins, L-arginine, ACEIs, and beta-blockers, can also improve vascular function via restoring endothelial dysfunction and impaired CFR (18). Effective treatment for microvascular angina requires aggressive control of risk factors, and one of the most effective methods is exercise. Further studies should be carried out to determine whether specific treatments are associated with prolonged survival and symptom alleviation (18).
Comparisons of IMR, fractional flow reserve (FFR), coronary microvascular resistance (CMR), myocardial blood flow (MBF), myocardial perfusion reserve index (MPRI), and CFR indicate that CFR is more available for coronary microvascular function evaluation (14, 19, 68). Methods to assess CFR include coronary angiogram, PET, CMR, and transthoracic Doppler echocardiography (TTDE) (79). During coronary angiogram, CFR measurement is made using a Doppler guidewire following administration of intracoronary adenosine (9). During TTDE, PET, and CMR, coronary flow velocity at rest and maximal hyperemia induced by administration of intravenous adenosine (140 lg/kg/min) are recorded to measure CFR (79). Studies have established CFR ranges to assess coronary physiology in patients with an increased risk of coronary heart disease or symptoms of chest pain but with normal coronary angiography (17, 23, 69). Variations in study findings may arise from differences in the indicators selected for evaluation. To avoid this, we chose CFR as the primary and only indicator to evaluate coronary microvascular function improvement.
Limitations
First, due to the fact that we focused on the improvement of microcirculation function by drugs among patients without obvious coronary stenosis, not all patients in this study had CMD (symptoms of chest pain and abnormal CFR). The CFR varied greatly due to the diagnosis and condition of the patients. Second, there was no uniform detection method for measuring CFR and the sensitivity, effectiveness, and accuracy of different detection methods varied across studies. In addition, we realized that despite the same detection methods, the use of different test drugs or different administration methods during measurement can also cause differences. We conducted subgroup analysis based on different detection methods and found that there are indeed differences between the different detection methods (Figure 8). Finally, improvement of symptoms is also an important indicator for evaluating microcirculation disorders. However, due to the fact that only limited numbers of retrieved studies used symptoms as an outcome, we only choose CFR in this meta-analysis.
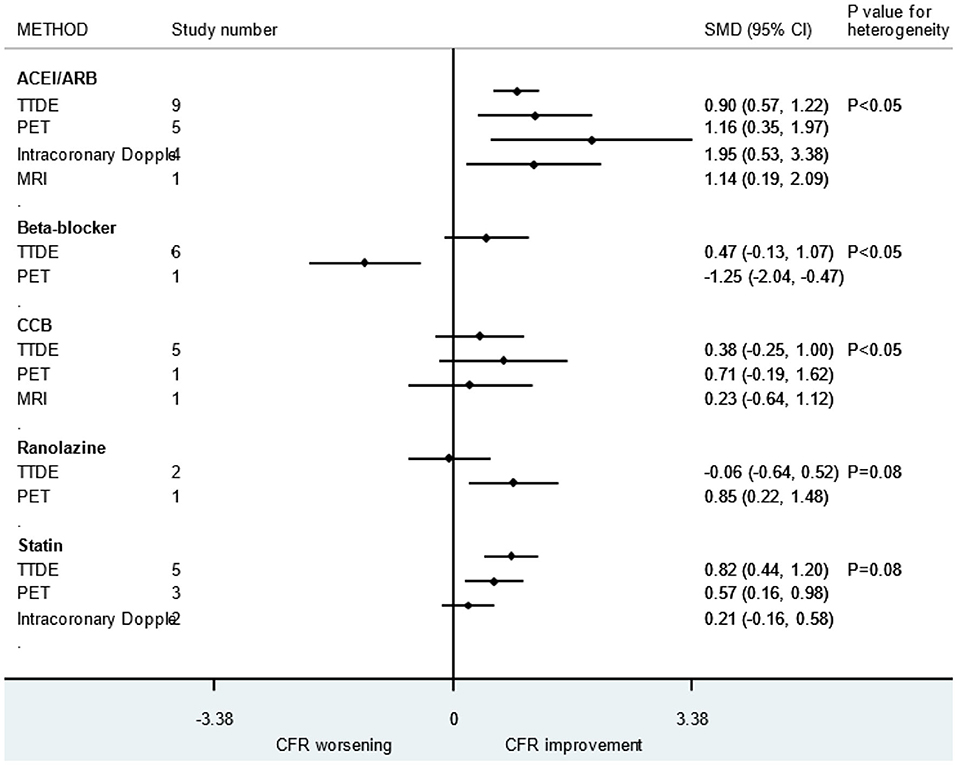
Figure 8. Subgroup analysis of different CFR detection methods. ACEI, aldosterone receptor antagonist; ARB, aldosterone receptor antagonist; CCB, calcium channel blocker; CFR, coronary flow reserve.
Conclusion
Statin, ACEI, and ARB therapy could improve CFR in patients without obstructive CAD from 0 to 12 months. CCBs and beta-blockers were associated with improved CFR in patients followed for 6–12 months. One month of treatment with ranolazine was not associated with improve CFR.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
XS and YH helped to conceive the topic and revise the manuscript. JY and JT contributed to the study selection, data extraction, and manuscript draft. XY and HX contributed to the data analysis. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Capital Health Development Research Project (No. 2018-2-2063) (SOS-moral NCT 03372785), Coronary Microvascular Innovation Foundation (No. 2018-CCA-CMVD-01), 2020 Beijing Municipal Education Commission Science and Technology Plan (No. KM202010025016), 2018 Beijing Excellent Talent Fund (No. 2018000021469G241), National Natural Science Foundation of China (No. 81971569), National Natural Science Foundation of China (No. 81671650), National Natural Science Foundation of China (No. 81670324), and Beijing Lab for Cardiovascular Precision Medicine (No. PXM2017_014226_000037).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank my patient, Ms. Chen, who was suffering from CMD. I raised this research question while treating her. She gave me the motivation to do this research. I hope to help her and people like her.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2020.580419/full#supplementary-material
References
1. Marinescu MA, Löffler AI, Ouellette M, Smith L, Kramer CM, Bourque J. Coronary microvascular dysfunction and microvascular angina: a systematic review of therapies. JACC Cardiovasc Imaging. (2015) 8:210. doi: 10.1016/j.jcmg.2014.12.008
2. Reis SE, Holubkov R, Lee JS, Sharaf B, Reichek N, Rogers WJ, et al. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women's Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol. (1999) 33:1469–75. doi: 10.1016/S0735-1097(99)00072-8
3. Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. (2011) 124:2215–24. doi: 10.1161/CIRCULATIONAHA.111.050427
4. Geltman EM, Henes CG, Senneff MJ, Sobel BE, Bergmann SR. Increased myocardial perfusion at rest and diminished perfusion reserve in patients with angina and angiographically normal coronary arteries. J Am Coll Cardiol. (1990) 16:586–95. doi: 10.1016/0735-1097(90)90347-R
5. Graf S, Khorsand A, Gwechenberger M, Novotny C, Kletter K, Sochor H, et al. Typical chest pain and normal coronary angiogram: cardiac risk factor analysis versus PET for detection of microvascular disease. J Nucl Med. (2007) 48:175–81. doi: 10.1002/jmri.20805
6. Shaw LJ, Merz CNB, Pepine CJ, Reis SE, Bittner V, Kip KE, et al. The economic burden of angina in women with suspected ischemic heart disease. Circulation. (2006) 114:894–904. doi: 10.1161/CIRCULATIONAHA.105.609990
7. Hoenig M, Bianchi C, Rosenzweig A, Sellke F. The cardiac microvasculature in hypertension, cardiac hypertrophy and diastolic heart failure. Curr Vasc Pharmacol. (2008) 6:292–300. doi: 10.2174/157016108785909779
8. Titterington JS, Hung OY, Wenger NK. Microvascular angina: an update on diagnosis and treatment. Future Cardiol. (2015) 11:229–42. doi: 10.2217/fca.14.79
9. Pauly DF, Johnson BD, Anderson RD, Handberg EM, Smith KM, Cooper-DeHoff RM, et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, microvascular dysfunction. angiotensin-converting enzyme inhibition is associated with improved microvascular function: a double-blind randomized study from the National Heart, Lung and Blood Institute Women's Ischemia Syndrome Evaluation (WISE). Am Heart J. (2011) 162:678–84. doi: 10.1016/j.ahj.2011.07.011
10. Yang N, Su YF, Li WW, Wang SS, Zhao CQ, Wang BY, et al. Microcirculation function assessed by adenosine triphosphate stress myocardial contrast echocardiography and prognosis in patients with nonobstructive coronary artery disease. Medicine. (2019) 98:e15990. doi: 10.1097/MD.0000000000015990
11. Taqueti VR, Di Carli MF. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state-of-the-art review. J Am Coll Cardiol. (2018) 72:2625–41. doi: 10.1016/j.jacc.2018.09.042
12. Suhrs HE, Michelsen MM, Prescott E. Treatment strategies in coronary microvascular dysfunction: a systematic review of interventional studies. Microcirculation. (2019) 26:e12430. doi: 10.1111/micc.12430
13. Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U, et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. (2017) 250:16–20. doi: 10.1016/j.ijcard.2017.08.068
14. Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. (2014) 35:1101. doi: 10.1093/eurheartj/eht513
15. Ford TJ, Yii E, Sidik N, Good R, Berry C. Ischemia and no obstructive coronary artery disease: prevalence and correlates of coronary vasomotion disorders. Circ Cardiovas Interv. (2019) 12:e008126. doi: 10.1161/CIRCINTERVENTIONS.119.008126
16. 中华医学会心血管病学分会基础研究学组, 中华医学会心血管病学分会介入心脏病学组, 中华医学会心血管病学分会女性心脏健康学组, and 中华医学会心血管病学分会动脉粥样硬化和冠心病学组, 冠状动脉微血管疾病诊断和治疗的中国专家共识. 中国循环杂志
17. Teresa P, Olivia M, Raffaele B, John C, Edina C, Giuseppe DL, et al. Esc working group on coronary pathophysiology and microcirculation position paper on “coronary microvascular dysfunction in cardiovascular disease”. Cardiovascular Research, (2020) 4:741–755. doi: 10.1093/cvr/cvaa003
18. Duvernoy CS. Evolving strategies for the treatment of microvascular angina in women. Exp Rev Cardiovasc Ther. (2012) 10:1413–9. doi: 10.1586/erc.12.55
19. Ong P, Athanasiadis A, Sechtem U. Pharmacotherapy for coronary microvascular dysfunction. Eur Heart J Cardiovasc Pharmacother. (2015) 1:65–71. doi: 10.1093/ehjcvp/pvu020
20. Meral K, Serdar P, Oguz Y, Hakan K, Can LH, Inan S. Benefits of statin treatment in cardiac syndrome-X1. Acc Cur J Rev. (2004) 13:10–11. doi: 10.1016/j.accreview.2004.02.005
21. Chen JW, Hsu NW, Wu TC, Lin SJ, Chang MS. Long-term angiotensin-converting enzyme inhibition reduces plasma asymmetric dimethylarginine and improves endothelial nitric oxide bioavailability and coronary microvascular function in patients with syndrome X. Am J Cardiol. (2002) 90:974–82. doi: 10.1016/s0002-9149(02)02664-4
22. Zhu H, Xu X, Fang X, Zheng J, Zhao Q, Chen T, et al. Effects of the antianginal drugs ranolazine, nicorandil, and ivabradine on coronary microvascular function in patients with nonobstructive coronary artery disease: a meta-analysis of randomized controlled trials. Clin Ther. (2019) 41:2137–52.e12. doi: 10.1016/j.clinthera.2019.08.008
23. Villano A, Di Franco A, Nerla R, Sestito A, Tarzia P, Lamendola P, et al. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. Am J Cardiol. (2013) 112:8–13. doi: 10.1016/j.amjcard.2013.02.045
24. Mehta PK, Goykhman P, Thomson LEJ, Shufelt C, Wei J, Yang Y, et al. Ranolazine improves angina in women with evidence of myocardial ischemia but no obstructive coronary artery disease. JACC Cardiovasc Imaging. (2011) 4:514–22. doi: 10.1016/j.jcmg.2011.03.007
25. Crea F, Pupita G, Galassi AR, el-Tamimi H, Kaski JC, Davies G, et al. Role of adenosine in pathogenesis of anginal pain. Circulation. (1990) 81:164–72.
26. Golino M, Spera FR, Manfredonia L, De Vita A, Di Franco A, Lamendola P, et al. Microvascular ischemia in patients with successful percutaneous coronary intervention: effects of ranolazine and isosorbide-5-mononitrate. Eur Rev Med Pharmacol Sci. (2018) 22:6545–50. doi: 10.1093/eurheartj/ehx493.5929
27. Safdar B, D'Onofrio G, Dziura J, Russell RR, Johnson C, Sinusas AJ. Ranolazine and microvascular angina by PET in the emergency department: results from a pilot randomized controlled trial. Clin Ther. (2017) 39:55–63. doi: 10.1016/j.clinthera.2016.12.002
28. Zhang X, Li Q, Zhao J, Li X, Sun X, Yang H, et al. Effects of combination of statin and calcium channel blocker in patients with cardiac syndrome X. Coron Artery Dis. (2014) 25:40–4. doi: 10.1097/MCA.0000000000000054
29. Iino K, Watanabe H, Iino T, Katsuta M, Koyama T, Kosaka T, et al. Candesartan improves impaired endothelial function in the human coronary artery. Coron Artery Dis. (2012) 23:278–83. doi: 10.1097/MCA.0b013e328351ab42
30. Toyama T, Sato C, Koyama K, Kasama S, Murakami J, Yamashita E, et al. Olmesartan improves coronary flow reserve of hypertensive patients using coronary magnetic resonance imaging compared with amlodipine. Cardiology. (2012) 122:230–6. doi: 10.1159/000339762
31. Kamezaki F, Tasaki H, Yamashita K, Shibata K, Hirakawa N, Tsutsui M, et al. Angiotensin receptor blocker improves coronary flow velocity reserve in hypertensive patients: comparison with calcium channel blocker. Hypertens Res. (2007) 30:699–706. doi: 10.1291/hypres.30.699
32. Parodi O, Neglia D, Palombo C, Sambuceti G, Giorgetti A, Marabotti C, et al. Comparative effects of enalapril and verapamil on myocardial blood flow in systemic hypertension. Circulation. (1997) 96:864–73. doi: 10.1161/01.CIR.96.3.864
33. Hinoi T, Tomohiro Y, Kajiwara S, Matsuo S, Fujimoto Y, Yamamoto S, et al. Telmisartan, an angiotensin II type 1 receptor blocker, improves coronary microcirculation and insulin resistance among essential hypertensive patients without left ventricular hypertrophy. Hypertens Res. (2008) 31:615–22. doi: 10.1291/hypres.31.615
34. Xiaozhen H, Yun Z, Mei Z, Yu S. Effect of carvedilol on coronary flow reserve in patients with hypertensive left-ventricular hypertrophy. Blood Press. (2010) 19:40–7. doi: 10.3109/08037050903450492
35. Gullu H, Erdogan D, Caliskan M, Tok D, Yildirim I, Sezgin AT, et al. Different effects of atenolol and nebivolol on coronary flow reserve. Heart. (2006) 92:1690–1. doi: 10.1136/hrt.2005.084079
36. Buus NH, Bottcher M, Jorgensen CG, Christensen KL, Thygesen K, Nielsen TT, et al. Myocardial perfusion during long-term angiotensin-converting enzyme inhibition or beta-blockade in patients with essential hypertension. Hypertension. (2004) 44:465–70. doi: 10.1161/01.HYP.0000141273.72768.b7
37. Yokoyama I, Inoue Y, Moritan T, Ohtomo K, Nagai R. Impaired myocardial vasodilatation during hyperaemic stress is improved by simvastatin but not by pravastatin in patients with hypercholesterolaemia. Eur Heart J. (2004) 25:671–9. doi: 10.1016/j.ehj.2004.02.017
38. Lario FC, Miname MH, Tsutsui JM, Santos RD, Kowatsch I, Sbano JC, et al. Atorvastatin treatment improves myocardial and peripheral blood flow in familial hypercholesterolemia subjects without evidence of coronary atherosclerosis. Echocardiography. (2013) 30:64–71. doi: 10.1111/j.1540-8175.2012.01810.x
39. Kawata T, Daimon M, Hasegawa R, Teramoto K, Toyoda T, Sekine T, et al. Effect on coronary flow velocity reserve in patients with type 2 diabetes mellitus: comparison between angiotensin-converting enzyme inhibitor and angiotensin II type 1 receptor antagonist. Am Heart J. (2006) 151:798.e9–15. doi: 10.1016/j.ahj.2005.09.014
40. Akinboboye OO, Ru-Ling C, Bergmann SR. Augmentation of myocardial blood flow in hypertensive heart disease by angiotensin antagonists: a comparison of lisinopril and losartan. J Am Coll Cardiol. (2002) 40:703–709. doi: 10.1016/S0735-1097(02)02033-8
41. Galderisi M, D'Errico A, Sidiropulos M, Innelli P, de Divitiis O, de Simone G. Nebivolol induces parallel improvement of left ventricular filling pressure and coronary flow reserve in uncomplicated arterial hypertension. J Hypertens. (2009) 27:2108–15. doi: 10.1097/HJH.0b013e32832ea925
42. Eshtehardi P, McDaniel MC, Dhawan SS, Binongo JN, Krishnan SK, Golub L, et al. Effect of intensive atorvastatin therapy on coronary atherosclerosis progression, composition, arterial remodeling, microvascular function. J Invasive Cardiol. (2012) 24:522–9. doi: 10.1016/j.hlc.2012.07.004
43. Motz W, Strauer BE. Improvement of coronary flow reserve after long-term therapy with enalapril. Hypertension. (1996) 27:1031–8. doi: 10.1161/01.HYP.27.5.1031
44. Caliskan M, Erdogan D, Gullu H, Topcu S, Ciftci O, Yildirir A, et al. Effects of atorvastatin on coronary flow reserve in patients with slow coronary flow. Clin Cardiol. (2007) 30:475–9.
45. Galderisi M, Cicala S, D'Errico A, de Divitiis O, de Simone G. Nebivolol improves coronary flow reserve in hypertensive patients without coronary heart disease. J Hypertens. (2004) 22:2201–8. doi: 10.1097/00004872-200411000-00024
46. Lethen H, Tries HP, Kersting S, Bramlage P, Lambertz H. Improvement of coronary microvascular function after angiotensin receptor blocker treatment with irbesartan in patients with systemic hypertension. J Clin Hypertens. (2011) 13:155–61. doi: 10.1111/j.1751-7176.2010.00401.x
47. Tomás JP, Moya JL, Barrios V, Campuzano R, Guzman G, Megías A, et al. Effect of candesartan on coronary flow reserve in patients with systemic hypertension. J Hypertens. (2006) 24:2109–14. doi: 10.1097/01.hjh.0000244962.77609.57
48. Sun BJ, Hwang E, Jang JY, Kim DH, Song JM, Kang DH. Effect of rosuvastatin on coronary flow reserve in patients with systemic hypertension. Am J Cardiol. (2014) 114:1234–7. doi: 10.1016/j.amjcard.2014.07.046
49. Jensen LO, Thayssen P, Pedersen KE, Haghfelt T. Effect of simvastatin on coronary flow reserve in patients with atherosclerosis and hypercholesterolemia: an intracoronary Doppler study. Coron Artery Dis. (2006) 17:51–6. doi: 10.1097/00019501-200602000-00009
50. Baller D, Notohamiprodjo G, Gleichmann U, Holzinger J, Weise R, Lehmann J. Improvement in coronary flow reserve determined by positron emission tomography after 6 months of cholesterol-lowering therapy in patients with early stages of coronary atherosclerosis. Circulation. (1999) 99:2871–5. doi: 10.1161/01.CIR.99.22.2871
51. Schwartzkopff B, Brehm M, Mundhenke M, Strauer BE. Repair of coronary arterioles after treatment with perindopril in hypertensive heart disease. Hypertension. (2000) 36:220–5. doi: 10.1161/01.HYP.36.2.220
52. Vogt M, Strauer BE. Response of hypertensive left ventricular hypertrophy and coronary microvascular disease to calcium antagonists. Am J Cardiol. (1995) 76:24d−30. doi: 10.1016/S0002-9149(99)80488-3
53. Fujimoto K, Hozumi T, Watanabe H, Shimada K, Takeuchi M, Sakanoue Y, et al. Effect of fluvastatin therapy on coronary flow reserve in patients with hypercholesterolemia. Am J Cardiol. (2004) 93:1419–21. doi: 10.1016/j.amjcard.2004.02.046
54. Stamatelopoulos K, Bramos D, Manios E, Alexaki E, Kaladaridou A, Georgiopoulos G, et al. Pleiotropic effects of the acute and chronic inhibition of the renin-angiotensin system in hypertensives. J Hum Hypertens. (2014) 28:378–83. doi: 10.1038/jhh.2013.125
55. Kjaer A, Kristoffersen US, Tarnow L, Parving HH, Hesse B. Short-term oral treatment with the angiotensin II receptor antagonist losartan does not improve coronary vasomotor function in asymptomatic type 2 diabetes patients. Dibetes Res Clin Pract. (2009) 84:34–8. doi: 10.1016/j.diabres.2009.01.010
56. Kawata T, Daimon M, Hasegawa R, Teramoto K, Toyoda T, Sekine T, et al. Effect of angiotensin-converting enzyme inhibitor on serum asymmetric dimethylarginine and coronary circulation in patients with type 2 diabetes mellitus. Int J Cardiol. (2007) 132:286–8. doi: 10.1016/j.ijcard.2007.08.066
57. Neglia D, Fommei E, Varela-Carver A, Mancini M, Ghione S, Lombardi M, et al. Perindopril and indapamide reverse coronary microvascular remodelling and improve flow in arterial hypertension. J Hypertens. (2011) 29:364–72. doi: 10.1097/HJH.0b013e328340a08e
58. Gunes Y, Tuncer M, Guntekin U, Ceylan Y, Sahin M, Simsek H. Regional functions of the left ventricle in patients with coronary slow flow and the effects of nebivolol. Ther Adv Cardiovasc Dis. (2009) 3:441–6. doi: 10.1177/1753944709345926
59. Engholm M, Mulvany MJ, Eftekhari A, Mathiassen ON, Buus NH, Christensen KL. Positive effects of aggressive vasodilator treatment of well-treated essential hypertensive patients. J Hum Hypertens. (2016) 30:690–6 doi: 10.1038/jhh.2016.13
60. Kabaklic A, Fras Z. Moderate-dose atorvastatin improves arterial endothelial function in patients with angina pectoris and normal coronary angiogram: a pilot study. Arch Med Sci. (2017) 13:827–36. doi: 10.5114/aoms.2017.68238
61. Ishida K, Geshi T, Nakano A, Uzui H, Mitsuke Y, Okazawa H, et al. Beneficial effects of statin treatment on coronary microvascular dysfunction and left ventricular remodeling in patients with acute myocardial infarction. Int J Cardiol. (2012) 155:442–7. doi: 10.1016/j.ijcard.2011.11.015
62. Hong SJ, Choi SC, Kim JS, Shim WJ, Park SM, Ahn CM, et al. Low-dose versus moderate-dose atorvastatin after acute myocardial infarction: 8-month effects on coronary flow reserve and angiogenic cell mobilisation. Heart. (2010) 96:756–64. doi: 10.1136/hrt.2009.182683
63. Ling MC, Ruddy TD, deKemp RA, Ukkonen H, Duchesne L, Higginson L, et al. Early effects of statin therapy on endothelial function and microvascular reactivity in patients with coronary artery disease. Am Heart J. (2005) 149:1137. doi: 10.1016/j.ahj.2005.02.033
64. Kayikcioglu M, Payzin S, Yavuzgil O, Kultursay H, Can LH, Soydan I. Benefits of statin treatment in cardiac syndrome-X1. Eur Heart J. (2003) 24:1999–2005. doi: 10.1016/S0195-668X(03)00478-0
65. Sugioka K, Hozumi T, Takemoto Y, Ehara S, Ogawa K, Iwata S, et al. Relation of early improvement in coronary flow reserve to late recovery of left ventricular function after beta-blocker therapy in patients with idiopathic dilated cardiomyopathy. Am Heart J. (2007) 153:1080.e1–6. doi: 10.1016/j.ahj.2007.03.014
66. Caliskan M, Ciftci O, Gullu H, Muderrisoglu H. The effect of carvedilol therapy on coronary flow reserve in patients with idiopathic dilated cardiomyopathy. Turk Kardiyoloji Dernegi arsivi. (2008) 36:247–52.
67. Hung OY, Molony D, Corban MT, Rasoul-Arzrumly E, Maynard C, Eshtehardi P, et al. Comprehensive assessment of coronary plaque progression with advanced intravascular imaging, physiological measures, and wall shear stress: a pilot double-blinded randomized controlled clinical trial of nebivolol versus atenolol in nonobstructive coronary artery disease. J Am Heart Assoc. (2016) 5:e002764. doi: 10.1161/JAHA.115.002764
68. Galderisi M, D'Errico A. Beta-blockers and coronary flow reserve: the importance of a vasodilatory action. Drugs. (2008) 68:579–90.
69. Ong P, Athanasiadis A, Sechtem U. Treatment of angina pectoris associated with coronary microvascular dysfunction. Cardiovasc Drugs Ther. (2016) 30:351–6. doi: 10.1007/s10557-016-6676-z
70. Saha S, Ete T, Kapoor M, Jha PK, Megeji RD, Kavi G, et al. Effect of ranolazine in patients with chest pain and normal coronaries- a hospital based study. J Clin Diagn Res. (2017) 11:Oc14–16. doi: 10.7860/JCDR/2017/24405.9617
71. Tagliamonte E, Rigo F, Cirillo T, Astarita C, Quaranta G, Marinelli U, et al. Effects of ranolazine on noninvasive coronary flow reserve in patients with myocardial ischemia but without obstructive coronary artery disease. Echocardiography. (2015) 32:516–21. doi: 10.1111/echo.12674
72. Osovska NY, Kuzminova NV. Efficiency of ranolazine in the patient with microvascular angina, atrial fibrillation and migraine. Wiad Lek. (2016) 69:832–7.
73. Ahmed B, Mondragon J, Sheldon M, Clegg S. Impact of ranolazine on coronary microvascular dysfunction (MICRO) study. Cardiovasc Revasc Med. (2017) 18:431–5. doi: 10.1016/j.carrev.2017.04.012
74. Ishibuchi K, Fujii K, Otsuji S, Takiuchi S, Hasegawa K, Tamaru H, et al. Utility and validity of intracoronary administration of nicorandil alone for the measurement of fractional flow reserve in patients with intermediate coronary stenosis. Circ J. (2019) 83:2010–6. doi: 10.1253/circj.CJ-19-0421
75. Kostic J, Djordjevic-Dikic A, Dobric M, Milasinovic D, Nedeljkovic M, Stojkovic S, et al. The effects of nicorandil on microvascular function in patients with ST segment elevation myocardial infarction undergoing primary PCI. Cardiovasc Ultrasound. (2015) 13:26. doi: 10.1186/s12947-015-0020-9
76. Chen Z, Chen X, Li S, Huo X, Fu X, Dong X. Nicorandil improves myocardial function by regulating plasma nitric oxide and endothelin-1 in coronary slow flow. Coron Artery Dis. (2015) 26:114–20. doi: 10.1097/MCA.0000000000000179
77. Hirohata A, Yamamoto K, Hirose E, Kobayashi Y, Takafuji H, Sano F, et al. Nicorandil prevents microvascular dysfunction resulting from PCI in patients with stable angina pectoris: a randomised study. EuroIntervention. (2014) 9:1050–6. doi: 10.4244/EIJV9I9A178
78. Tagliamonte E, Cirillo T, Rigo F, Astarita C, Coppola A, Romano C, et al. Ivabradine and bisoprolol on doppler-derived coronary flow velocity reserve in patients with stable coronary artery disease: beyond the heart rate. Adv Ther. (2015) 32:757–67. doi: 10.1007/s12325-015-0237-x
Keywords: oral drug, coronary microvascular, microvascular function, coronary flow reserve (CFR), therapy
Citation: Yong J, Tian J, Yang X, Xing H, He Y and Song X (2020) Effects of Oral Drugs on Coronary Microvascular Function in Patients Without Significant Stenosis of Epicardial Coronary Arteries: A Systematic Review and Meta-Analysis of Coronary Flow Reserve. Front. Cardiovasc. Med. 7:580419. doi: 10.3389/fcvm.2020.580419
Received: 15 July 2020; Accepted: 14 September 2020;
Published: 30 October 2020.
Edited by:
Ailin Barseghian, University of California, Irvine, United StatesReviewed by:
Akshaya Srikanth Bhagavathula, United Arab Emirates University, United Arab EmiratesHack-Lyoung Kim, Seoul Metropolitan Government—Seoul National University Boramae Medical Center, South Korea
Copyright © 2020 Yong, Tian, Yang, Xing, He and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiantao Song, c29uZ3hpYW50YW8wOTI5QHFxLmNvbQ==; Yi He, aGV5aTEzOUBzaW5hLmNvbQ==
†These authors have contributed equally to this work
 Jingwen Yong
Jingwen Yong Jinfan Tian
Jinfan Tian Xueyao Yang1
Xueyao Yang1