- 1Department of Cardiology, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 2Department of Cardiology, The Eighth Affiliated Hospital of Sun Yat-sen University, Shenzhen, China
- 3Guangdong Innovative Engineering and Technology Research Center for Assisted Circulation, Shenzhen, China
- 4Department of Cardiology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 5NHC Key Laboratory of Assisted Circulation, Sun Yat-sen University, Guangzhou, China
This study aimed to investigate the effects of pulse pressure (PP) on cognition and the role of white matter lesions (WMLs) in mediating this association. We enrolled 3,009 participants from the SPRINT-MIND study. Of those, 755 participants underwent brain magnetic resonance imaging. Cognitive tests were summarized in five cognition domains, including global cognition, executive function, attention, memory, and language. Multiple linear regression models were employed to analyze PP in association with cognition, and mediation analysis was applied to determine the role of WMLs in the association between PP and cognition. We found that PP was negatively linearly associated with global cognition (β = −0.048, P = 0.008), executive function (β = −0.014, P = 0.040), attention (β = −0.013, P = 0.035), memory (β = −0.021, P = 0.045), and language (β = −0.020, P = 0.001), respectively. Furthermore, PP was not significantly associated with brain component volume changes, except for WMLs (β = 0.029, P = 0.044). Additionally, mediation analysis showed that increased WML volume contributed to 10.8% of global cognition, 9.5% of executive function, 10.6% of memory, and 7.2% of language decline associated with PP. Exposure to higher PP levels was associated with poor cognitive performance, and WMLs partially moderated the influence of PP on cognition.
Introduction
Most previous studies have shown that elevated blood pressure (BP) exacerbates cognitive impairment (1–3). Cognitive decline occurs mostly in middle-aged and older populations, and one of the characteristics of BP in this age group is its tendency toward high systolic blood pressure (SBP) and low diastolic blood pressure (DBP). Therefore, the role of elevated pulse pressure (PP) in the cognitive decline process needs to be investigated.
The association between PP and cognition remains controversial. To our knowledge, a community-based longitudinal study is the first to demonstrate that higher PP is associated with increased risk for Alzheimer's disease and dementia (4). A secondary analysis of the hypertension in the very elderly trial (HYVET) indicated that wider PP may increase the risk of dementia (5). Similar results were reported in other studies (6–8). In contrast, a few studies suggested that higher PP is not independently associated with cognitive decline (9, 10).
In addition, increased brachial PP is an age-independent factor associated with white matter lesions (WMLs) in elderly individuals, while the association between WMLs and cognition is already established (11, 12). A few studies are currently available on the effect of WMLs on the association between PP and cognition domains in stroke-free adults with hypertension. Therefore, in the present study, we assessed whether PP was associated with cognition using Systolic BP Intervention Trial-Memory and cognition IN Decreased hypertension (SPRINT-MIND) baseline data and explored the potential mechanism by which WMLs moderate the association between PP and cognition.
Methods
Study Population
This was a cross-sectional study of SPRINT-MIND data obtained from the National Heart, Lung, and Blood Institute. SPRINT was a multicenter, randomized controlled trial that examined whether intensive BP treatment (SBP <120 mm Hg) would reduce the risk of cardiovascular events and total mortality compared with standard BP treatment (SBP <140 mm Hg) among 9,361 participants aged ≥50 years with hypertension (SBP of 130 to 180 mm Hg). The detailed acceptance criteria and methods have been described in the previous SPRINT design study (13). A subset of 3,009 participants who answered cognitive function questionnaires and 755 participants who underwent brain MRI scan at baseline were enrolled in the MIND cohort (Supplementary Figure 1).
Blood Pressure Measurement
BP was measured at each clinic visit after a rest period using an automated device that reduced potential for observer biases. PP was calculated by SBP minus DBP at baseline. PP was analyzed as a continuous variable and categorical variable, respectively.
Cognitive Tests
The MIND screening battery included Montreal Cognitive Assessment, Logical Memory Test, and Digits Symbol Coding Test, while the MIND extended battery included Hopkins Verbal Learning Test, Trail Making Test, Digit Span Test, Boston Naming Test, and Category Fluency Test—Animals. These cognitive tests were summarized to five specific major cognition domains, including global cognition, executive function, memory, attention, and language (Supplementary Table 1). Individual test results were standardized as z scores added to develop summary cognition domain scores. Lower scores indicate poor cognitive performance.
Brain Magnetic Resonance Imaging
In the SPRINT-MIND study, 755 participants completed brain MRI at baseline. Several 3.0-T MRI scanner models from manufacturers (Siemens, Philips, and GE Healthcare) were used to perform the brain MRI. At least one trained and certified technician was responsible for MRI quality control at each participating field center. The image data were transmitted from the field center to the MRI reading center at the University of Pennsylvania for review. Using a label fusion method, the brain tissue was divided into several anatomical regions of interest (14). The WMLs were characterized from fluid-attenuated inversion recovery and T1-weighted images by applying a deep learning-based segmentation technique (15).
Statistical Analysis
All variables at SPRINT-MIND baseline were summarized using standard descriptive statistics, and stratified by PP quartile.
Using a multivariate linear regression model, we examined the association of PP with cognitive tests and brain MRI variables. Individual tests results were standardized as z scores. In addition, we adjusted for covariates, including age, gender, race, education, smoking, drinking, body mass index, cardiovascular disease (CVD), cholesterol, fasting plasma glucose, estimated glomerular filtration rate, and medication use (statin, aspirin, and antihypertensive). Analyses involving brain MRI variables were additionally adjusted for scanner type, intracranal volume, and brain volume.
Mediation analysis was conducted to characterize the cognitive effects of PP that could be explained by WMLs. That is, analyses were used to identify and explain the mechanism pathways that underlie an observed relationship between an independent variable (PP) and outcome variables (cognition parameters) via a mediator (brain MRI variables). It allows estimation of the direct and indirect effects and the proportion mediated. The proportion can be calculated by dividing the indirect effect by the total effect. In this study, all analyses were performed using SPSS software, version 22 (Chicago, IL, USA).
Results
Participant Characteristics
At the SPRINT-MIND study baseline, 3,009 participants (of whom 755 underwent brain MRI) had available PP values and completed the MIND questionnaires including dementia screening and extended cognitive battery. The mean age was 68.6 ± 8.7 years, 1,103 (36.7%) were female, 1,798 (59.8%) were white, and 2,226 (74.8%) had advanced education. The mean PP level was 61.64 ± 14.62 mm Hg. There were 610 (20.3%) participants with CVD history and 939 (31.2%) participants with CKD history.
Compared with participants with low or normal PP (PP ≤ 60 mm Hg), participants with PP > 60 mm Hg were more likely to be older, female, former smoker, and had a higher CVD risk score and CKD history (Table 1, Supplementary Table 2).
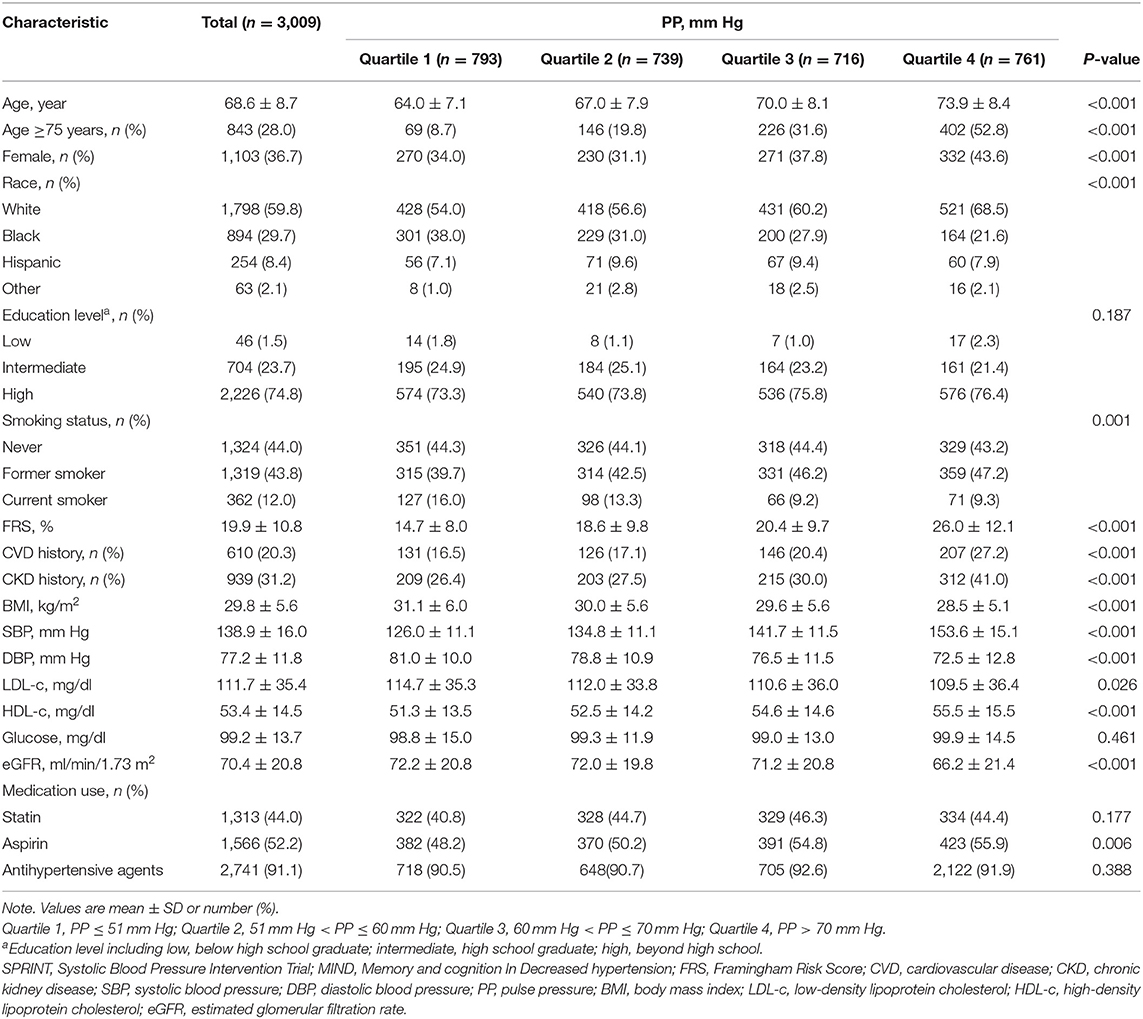
Table 1. Baseline characteristics of Systolic BP Intervention Trial-Memory and cognition IN Decreased hypertension (SPRINT-MIND) participants classified by pulse rressure (PP) quartile.
Association Between Pulse Pressure and Cognition
Unadjusted performance comparisons on individual cognitive tests by PP categories are shown in Table 2. For the mean scores of all cognitive tests, statistical differences were observed across the PP strata.
Table 3 shows the association between PP as a continuous variable and cognition domains using multiple linear regression models. PP was negatively linearly associated with the global cognition summary score in regression models adjusted for demographics [estimate (SEM): −0.03 (0.01); P < 0.01] and clinical characteristics [estimate (SEM): −0.05 (0.02); P < 0.01]. Similarly, this negative linear correlation between PP and other cognition domains including executive function, attention, memory, and language was demonstrated. In addition, we examined the association between PP and individual cognitive test (Supplementary Table 3).
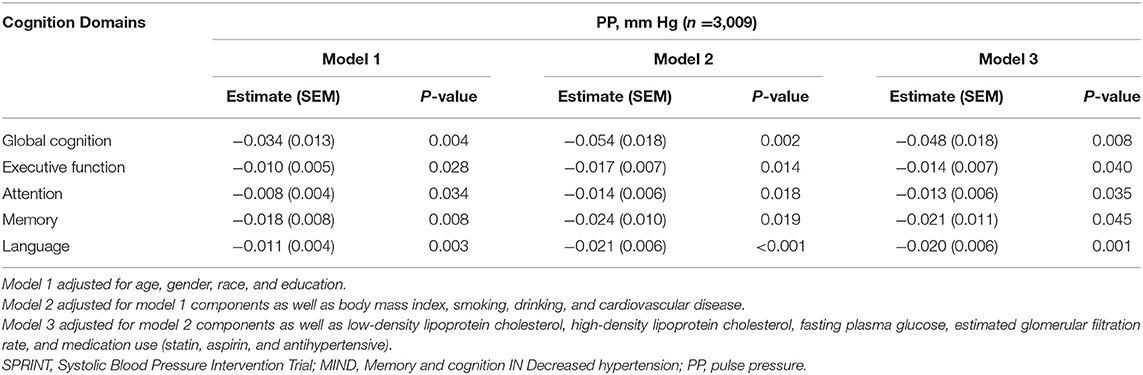
Table 3. Association between continuous PP and summary cognition domains in SPRINT-MIND participants.
Furthermore, we examined the association between PP and cognition involving a subset of 755 participants who had undergone brain MRI. As Supplementary Table 4 shows, there were negative linear correlations between PP and cognition domains including global cognition, executive function, memory, and language consistent in three adjustment models. No obvious linear correlation between PP and attention was observed in this study (P = 0.31).
Association Between Pulse Pressure and Brain Magnetic Resonance Imaging Variables
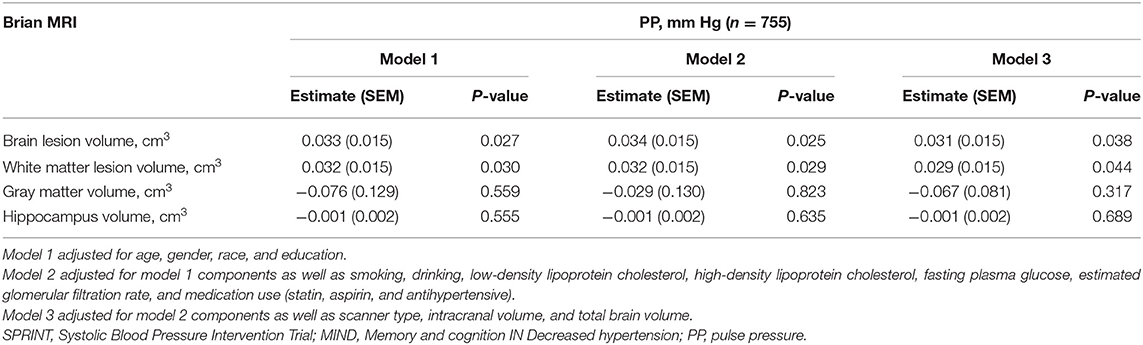
For the MRI subgroup (n = 755), unadjusted performance comparisons on brain MRI variables by PP categories are shown in Table 4. PP levels and brain MRI variables including WML volume, gray matter, hippocampus, brain volume, brain lesion volume, and cerebrospinal fluid showed statistical differences. Multiple linear regression analyses indicated that PP is positively correlated with brain lesion volume [estimate (SEM): 0.03 (0.02); P = 0.04] and WML volume [estimate (SEM): 0.03 (0.02); P = 0.04]. There was no statistically significant linear association between PP and gray matter, hippocampus after adjusting for confounding factors (Table 5).
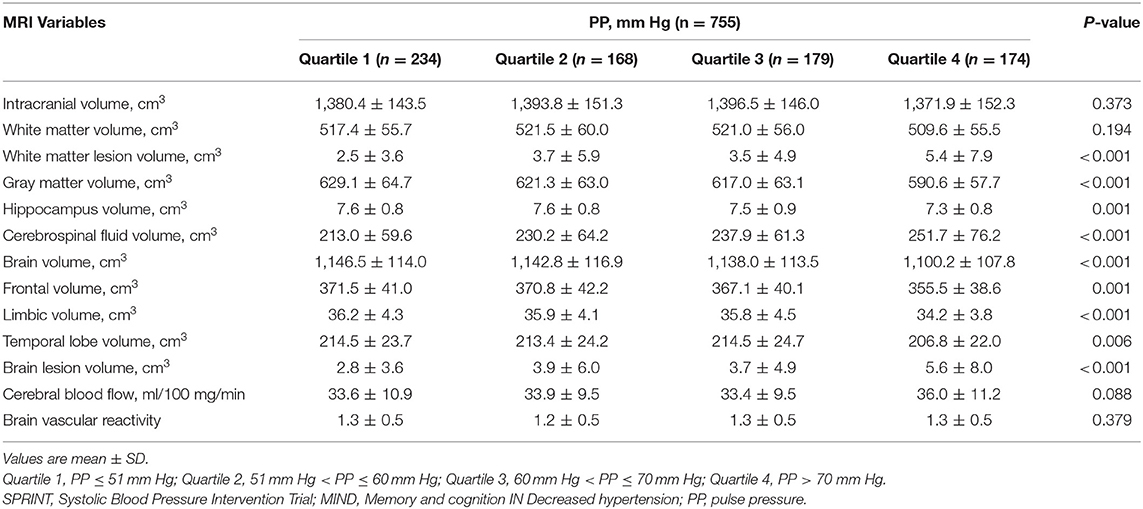
Table 4. Performance on brain magnetic resonance imaging (MRI) variables by PP quartile in SPRINT-MRI subgroup.
Association Between White Matter Lesions and Cognition
Supplementary Table 5 showed the association between WMLs and cognition. After adjusted for all covariates, WML volume was negatively correlated with cognition including global cognitive function [estimate (SEM): −0.20 (0.05); P < 0.01], executive function [estimate (SEM): −0.06 (0.02); P < 0.01], attention [estimate (SEM): −0.04 (0.02); P < 0.05], memory [estimate (SEM): −0.11 (0.03); P < 0.01], and language [estimate (SEM): −0.04 (0.02); P < 0.05].
Mediation Analysis
Given the association between PP and both WML volume and cognition, mediation analysis was conducted to better understand the extent of interactions. As observed in Table 6, a fraction of cognition domain changes including global cognition, executive function, memory, and language caused by PP was partly explained by combined increases in WML volume (mediation percentage 10.8, 9.48, 10.6, and 7.2%, respectively).
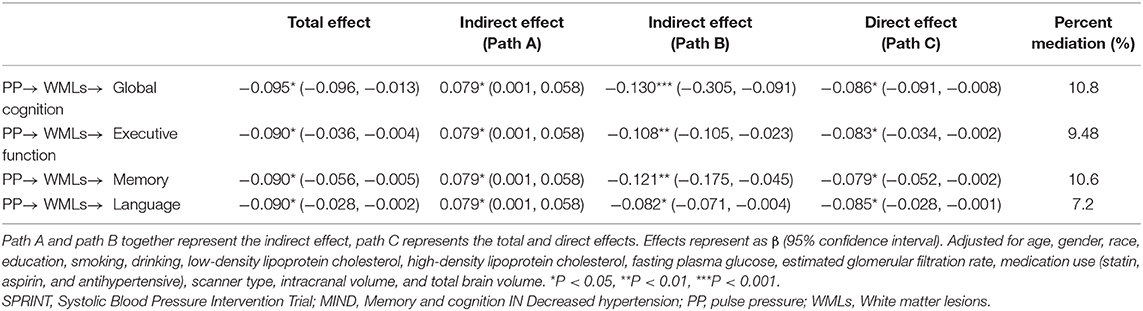
Table 6. Mediation effect by white matter lesions (WMLs) in the association between PP and cognition in SPRINT-MRI subgroup.
Discussion
In a large cohort of stroke-free adults with hypertension, we confirmed that PP was negatively associated with cognition, an association mediated partly by WMLs.
There is support for the notion that patients with optimal SBP control may still have an increased risk for CVD and cognitive impairment. The impact of higher PP on target organ damage has been underestimated. In fact, higher PP as a CVD risk factor has also been shown to have a similar relationship with cognitive decline. Cognitive decline is a part of a specific hypertensive microvascular target organ damage (16). Elevated PP is a marker for increased arterial stiffness or atherosclerosis (17). Therefore, as a consequence of reduced damping of the arterial waveforms, the small vessels in the brain remodeling are exposed to high pulsating pressure. This pathological remodeling may result in impaired cerebral autoregulation accompanying endothelial dysfunction, nitric oxide synthase decrease, and oxidative stress increase, which potentially contribute to the pathogenesis and development of cerebral microvascular damage, leading to WML progression (18–22). Moreover, the Rotterdam Scan Study showed that progression of small vessel disease was paralleled with a decline in cognitive function (23). Another clinical study related arterial stiffness to cerebral WMLs (24). Furthermore, WMLs, as a marker of impaired microcirculation, increased the risk of stroke, vascular dementia, and mortality (1, 25, 26).
To demonstrate the association between PP and cognition, we summarized eight cognitive tests into five cognitive function domains, finding consistent results. Our findings extend further than most previous cross-sectional studies, relating higher PP to WMLs and lower performance on cognitive screening tests among non-stroke individuals. The negative association between PP and cognition is consistent with previous reports. In a dementia-free elderly cohort that was followed up from 0.1 to 8.3 years, higher PP was associated with an increased risk for Alzheimer disease (4). This association was confirmed in very old populations, and a study including 148 younger participants (mean age 64 years) with suboptimal BP control revealed that elevated PP during the day or night correlates with cognitive impairment (16). In addition, a U-shaped relationship between PP and cognitive decline has been observed in both healthy elderly and stroke patients (4, 8). The participants included in our study were middle-aged and older individuals aged >50 years, with an average age of 68 years, and were, therefore, broadly representative. In view of the limited sample size of participants with low PP, we did not further investigate the relationship between low PP and cognition. However, using correlation analysis, we found that participants with PP ≤ 51 mm Hg had higher cognitive test scores than those with PP > 51 mm Hg.
In previous studies, both higher SBP and DBP were strongly associated with WML severity (27–29). Kim reported for the first time that increased brachial PP is an age-independent factor associated with WMLs in asymptomatic elderly individuals11. This was an association that we also observed. We investigated whether brain MRI variables including brain lesion volume, WMLs, gray matter, and hippocampus are related to PP; results showed that only brain lesion volume including WMLs was positively correlated with PP, without significant correlations for the rest. Therefore, we further conducted mediation analysis to verify the hypothesis that WMLs mediate the association of PP and cognition. As a result, WMLs were found to underlie the adverse relationship between PP and multiple cognition domains, including global cognition, executive function, memory, and language.
The strengths of our study include concurrent BP measurement, brain MRI, and cognitive function tests. In addition, a large number of cognitive questionnaires were used in this study, allowing us to distinguish subtypes of cognitive deficits associated with high PP levels. Also, the study population was a large sample size of stroke-free participants with hypertension, so as to avoid the interference of stroke on study results.
Limitations
There are several limitations in this study. First, this is a cross-sectional analysis, and the causality link between PP and WMLs cannot be inferred. Therefore, a longitudinal cohort analysis should be conducted in a further study. Second, although BP was measured using automated devices, a single BP measurement did not represent the usual BP. Thus, it is necessary to perform an ambulatory BP check to obtain the PP index. This would further extend our study results.
Conclusions
Our study demonstrates that cognitive decline is more frequent in patients with higher PP and is related to the severity of PP. Furthermore, WMLs partially moderate the association of PP and cognition, including executive function, memory, and language. A longitudinal study should be conducted to consolidate our results and further verify the causality between PP, WMLs, and cognition.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://biolincc.nhlbi.nih.gov/studies/sprint/.
Ethics Statement
The studies involving human participants were reviewed and approved by The Ethics Review Committee of The First Affiliated Hospital of Sun Yat-sen University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
GW and XZ provided the conception and design for the study. XZ provided the study materials or patients. JZ and JS contributed to the development of the methodology and wrote the manuscript. JL analyzed the acquired data. XZ, WW, and CY were responsible for the interpretation of statistical results. GW revised the manuscript. All authors contributed to the article and approved the final submitted version.
Funding
This study was founded by Shenzhen Key Medical Discipline Construction Fund (No. SZXK002), National Key R& D Program of China (No. 2020YFC2004400), National Natural Science Foundation of China (No. 81970367), Natural Science Foundation of Guangdong Province (No. 2018A030313807), the Science and Technology Planning Project of Shenzhen Municipality (No. JCYJ20180306180229307) and Sun Yat-sen University Clinical Medicine Research 5010 Cultivation Project (No. 2018027).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the National Heart, Lung, and Blood Institute for providing the data. We are also grateful to all the participants in the survey design and data collection as well as the SPRINT research team for collecting high-quality, nationally representative data. This research does not necessarily represent the opinions of the study investigators or NHLBI.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.654522/full#supplementary-material
References
1. Hajjar I, Quach L, Yang F, Chaves PH, Newman AB, Mukamal K, et al. Hypertension, white matter hyperintensities, and concurrent impairments in mobility, cognition, and mood: the Cardiovascular Health Study. Circulation. (2011) 123:858–65. doi: 10.1161/CIRCULATIONAHA.110.978114
2. Launer LJ, Masaki K, Petrovitch H, Foley DJ, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function: the honolulu-Asia aging study. JAMA. (1995) 274:1846–51. doi: 10.1001/jama.274.23.1846
3. Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. (2005) 4:487–99. doi: 10.1016/S1474-4422(05)70141-1
4. Qiu C, Winblad B, Viitanen M, Fratiglioni L. Pulse pressure and risk of Alzheimer disease in persons aged 75 years and older: a community-based, longitudinal study. Stroke. (2003) 34:594–9. doi: 10.1161/01.STR.0000060127.96986.F4
5. Peters R, Beckett N, Fagard R, Thijs L, Wang JG, Forette F, et al. Increased pulse pressure linked to dementia: further results from the Hypertension in the Very Elderly Trial - HYVET. J Hypertens. (2013) 31:1868–75. doi: 10.1097/HJH.0b013e3283622cc6
6. Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. (2008) 51:99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674
7. Yasar S, Ko JY, Nothelle S, Mielke MM, Carlson MC. Evaluation of the effect of systolic blood pressure and pulse pressure on cognitive function: the Women's Health and Aging Study II. PLoS ONE. (2011) 6:e27976. doi: 10.1371/journal.pone.0027976
8. Wang Z, Wong A, Liu W, Yang J, Chu WC, Au L, et al. Pulse pressure and cognitive decline in stroke patients with white matter changes. J Clin Hypertens. (2015) 17:694–8. doi: 10.1111/jch.12583
9. Freitag MH, Peila R, Masaki K, Petrovitch H, Ross GW, White LR, et al. Midlife pulse pressure and incidence of dementia: the Honolulu-Asia Aging Study. Stroke. (2006) 37:33–7. doi: 10.1161/01.STR.0000196941.58869.2d
10. Morris MC, Scherr PA, Hebert LE, Glynn RJ, Bennett DA, Evans DA. Association of incident Alzheimer disease and blood pressure measured from 13 years before to 2 years after diagnosis in a large community study. Arch Neurol. (2001) 58:1640–6. doi: 10.1001/archneur.58.10.1640
11. Kim CK, Lee SH, Kim BJ, Ryu WS, Yoon BW. Age-independent association of pulse pressure with cerebral white matter lesions in asymptomatic elderly individuals. J Hypertens. (2011) 29:325–9. doi: 10.1097/HJH.0b013e3283408ffb
12. Semplicini A. Cerebral White Matter Lesions as a Clinically Relevant Intermediate Target of Cerebrovascular Prevention. J Clin Hypertens. (2015) 17:699–700. doi: 10.1111/jch.12584
13. Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. (2014) 11:532–46. doi: 10.1177/1740774514537404
14. Tamura MK, Pajewski NM, Bryan RN, Weiner DE, Diamond M, Van Buren P, et al. Chronic kidney disease, cerebral blood flow, and white matter volume in hypertensive adults. Neurology. (2016) 86:1208–16. doi: 10.1212/WNL.0000000000002527
15. Lao Z, Shen D, Liu D, Jawad AF, Melhem ER, Launer LJ, et al. Computer-assisted segmentation of white matter lesions in 3D MR images using support vector machine. Acad Radiol. (2008) 15:300–13. doi: 10.1016/j.acra.2007.10.012
16. Yaneva-Sirakova T, Tarnovska-Kadreva R, Traykov L. Pulse pressure and mild cognitive impairment. J Cardiovasc Med. (2012) 13:735–40. doi: 10.2459/JCM.0b013e328357ba78
17. Safar ME. Pulse pressure in essential hypertension: clinical and therapeutical implications. J Hypertens. (1989) 7:769–76. doi: 10.1097/00004872-198910000-00001
18. Poels MMF, Zaccai K, Verwoert GC, Vernooij MW, Hofman A, van der Lugt A, et al. Arterial stiffness and cerebral small vessel disease: the Rotterdam Scan Study. Stroke. (2012) 43:2637–42. doi: 10.1161/STROKEAHA.111.642264
19. O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. (2005) 46:200–4. doi: 10.1161/01.HYP.0000168052.00426.65
20. Mitchell GF, Vita JA, Larson MG, Parise H, Keyes MJ, Warner E, et al. Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: the Framingham Heart Study. Circulation. (2005) 112:3722–8. doi: 10.1161/CIRCULATIONAHA.105.551168
21. Hoth KF, Tate DF, Poppas A, Forman DE, Gunstad J, Moser DJ, et al. Endothelial function and white matter hyperintensities in older adults with cardiovascular disease. Stroke. (2007) 38:308–12. doi: 10.1161/01.STR.0000254517.04275.3f
22. Lehoux S. Redox signalling in vascular responses to shear and stretch. Cardiovasc Res. (2006) 71:269–79. doi: 10.1016/j.cardiores.2006.05.008
23. van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke. (2008) 39:2712–19. doi: 10.1161/STROKEAHA.107.513176
24. Ohmine T, Miwa Y, Yao H, Yuzuriha T, Takashima Y, Uchino A, et al. Association between arterial stiffness and cerebral white matter lesions in community-dwelling elderly subjects. Hypertens Res. (2008) 31:75–81. doi: 10.1291/hypres.31.75
25. Luo DH, Tseng WI, Chang YL. White matter microstructure disruptions mediate the adverse relationships between hypertension and multiple cognitive functions in cognitively intact older adults. Neuroimage. (2019) 197:109–19. doi: 10.1016/j.neuroimage.2019.04.063
26. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. (2010) 341:c3666. doi: 10.1136/bmj.c3666
27. Liao D, Cooper L, Cai J, Toole JF, Bryan NR, Hutchinson RG, et al. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control. The ARIC Study. Atherosclerosis Risk in Communities Study. Stroke. (1996) 27:2262–70. doi: 10.1161/01.STR.27.12.2262
28. de Leeuw FE, de Groot JC, Oudkerk M, Witteman JC, Hofman A, van Gijn J, et al. A follow-up study of blood pressure and cerebral white matter lesions. Ann Neurol. (1999) 46:827–33. doi: 10.1002/1531-8249(199912)46:6<827::aid-ana4>3.3.co;2-8
Keywords: pulse pressure, cognition, white matter lesions, sprint, mediation analysis
Citation: Zang J, Shi J, Liang J, Zhang X, Wei W, Yao C, Zhuang X and Wu G (2021) Pulse Pressure, Cognition, and White Matter Lesions: A Mediation Analysis. Front. Cardiovasc. Med. 8:654522. doi: 10.3389/fcvm.2021.654522
Received: 16 January 2021; Accepted: 22 March 2021;
Published: 04 May 2021.
Edited by:
Guido Iaccarino, University of Naples Federico II, ItalyReviewed by:
Claudio Ferri, University of L'Aquila, ItalyGiovambattista Desideri, University of L'Aquila, Italy
Copyright © 2021 Zang, Shi, Liang, Zhang, Wei, Yao, Zhuang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaodong Zhuang, emh1YW5neGQzQG1haWwuc3lzdS5lZHUuY24=; Guifu Wu, d3VndWlmdUBtYWlsLnN5c3UuZWR1LmNu
†These authors have contributed equally to this work
 Jiabin Zang
Jiabin Zang Jian Shi
Jian Shi Jianwen Liang
Jianwen Liang Xiaocong Zhang2,3
Xiaocong Zhang2,3 Xiaodong Zhuang
Xiaodong Zhuang Guifu Wu
Guifu Wu