- 1Department of Neurology, The First People's Hospital of Changde, Changde, China
- 2Department of Science and Education, The First People's Hospital of Changde, Changde, China
- 3Department of Neurosurgery, The First People's Hospital of Changde, Changde, China
Objective: A limited number of scholars concentrated on the relationship between carotid atherosclerosis (CAS) and white matter hyperintensity (WMH) (i.e., CAS-WMH relationship). The current research aimed to clarify the CAS-WMH relationship in Japanese population.
Methods: All participants underwent MRI of head and ultrasonography of the carotid artery. WMH was diagnosed from MRI results. The carotid ultrasound findings, carotid artery plaque score (PS), and plaque number (PN) could be achieved to indicate the severity of CAS. We also employed multivariate logistic regression models to estimate the CAS-WMH relationship. Interaction and stratified analyses were undertaken on the basis of a number of factors (e.g., gender, age, smoking status, drinking habit, and history of chronic diseases).
Results: A total of 1,904 Japanese subjects were included, and the prevalence of WMH was 54.8% (1,044/1,904). It was unveiled that frequency of CAS was greater in cases with WMH. In a fully adjusted model, high PS was associated with the frequency of WMH, followed by high PN. Further analyses revealed a dose-response relationship between PS and incidence of WMH.
Conclusion: PS and PN exhibited the greatest influences on determining the frequency of WMH, highlighting the potentially important pathophysiological role of large artery atherosclerosis in intensifying WMH.
Introduction
Cerebral white matter hyperintensity (WMH) is an extremely frequent finding on magnetic resonance imaging (MRI) or computed tomography (CT) of brain in cases with stroke and dementia (1). Although a number of scholars attempted to concentrate on WMH, efforts were made to identify association between WMH and factors that may influence WMH (2, 3). Previously, pathologic thickening or necrosis of the vessel wall were noted to affect WMH (4, 5), while no information could be attained justifying how large-artery atherosclerosis may influence WMH.
Recently, increasing evidence has indicated a close association between carotid atherosclerosis (CAS) and WMH (i.e., CAS-WMH relationship) (6, 7). Shu et al. (8) demonstrated that CAS could lead to WMH by inducing cerebral hypoperfusion. Insufficient cerebral perfusion due to CAS may affect the severity of WMH and increase the frequency of WMH (9). Moreover, WMH may be partially reversible in patients with carotid artery stenosis (10). These findings highlight an important role of CAS in WMH as an intermediate factor, although this conclusion remains controversial.
Carotid ultrasonography, a non-invasive examination, can be utilized to identify CAS in the early stages. Numerous researches have used intima-media thickness (IMT) as a surrogate marker of carotid stiffness, while few reports have concentrated on plaque score (PS) and plaque number (PN). PS may more precisely represent the atherosclerotic condition of the carotid artery than IMT because a wider range of observations are required to obtain PS compared to IMT, which suggests the superiority of PS in indicating CAS (11).
In the present study, PS and PN were assessed as indicators of CAS to investigate the associations between the severity of CAS and the incidence of WMH in Japanese population. It also was attempted to indicate how vascular risk factors may influence the mentioned association with the assistance of stratified analysis.
Methods
Collection of Data
The DATADRYAD database was employed to attain data. We also made an effort to cite the Dryad data package following protocols released in the Dryad Digital Repository (Tokyo, Japan).
Study Design and Participants
Our research was undertaken via Shinkawa et al.'s findings (12). Their cross-sectional study involved a comprehensive medical checkup of 1,904 asymptomatic participants, including 988 men and 916 women, who were admitted to Shin Takeo Hospital between April 1, 2016, and October 31, 2017, some patients underwent head MRI, blood tests and carotid ultrasonography, and completed standard questionnaires all within this period. Exclusion of subjects was undertaken on the basis of criteria outlined below: presence of (1) a number of devices frequently utilized surgically; (2) a psychiatry-dependent disease; (3) paralysis or a history of stroke, traumatic brain injuries, and other definitive encephalopathies; and (4) poorly recorded carotid ultrasonograms precluding measurement; (5) subjects who were unintended to cooperate. Procedures related to consent to participate and confirming the protocol were detailed in the native manuscript (12).
Extraction and Processing of Data
The comprehensive medical examination included a general inspection (age, sex, body mass index, diastolic blood pressure [DBP], systolic blood pressure [SBP], presence of visceral steatosis [for the determination of metabolic syndrome]), blood and biochemical tests, and a questionnaire on medical history (antihypertensive medicine, antidiabetic medicine, and cholesterol-lowering medications), and lifestyle characteristics, including drinking habits, drinking volume, and smoking habits. Fasting blood samples were analyzed for low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), ratio of LDL-C to HDL-C, triglyceride, fasting plasma glucose, and glycated hemoglobin (HbA1c).
As described previously (12), it was attempted to extract data outlined below: smoking habits (smoking >100 cigarettes or subsequent smoking for 6 months as well as attempting to smoke in the recent 1 month; drinking status (rarely, sometimes, and daily); and drinking volume (with respect to quantify the consumption of alcoholic beverage/day) in form of <180, 180–360, 360–540 or >540 mL. Hypertension was defined as SBP >140 or DBP > 90 mmHg (or both) on at least two diverse conditions. We also attempted to define hyperlipidemia on the basis of diverse criteria (total plasma cholesterol level >5.7 mmol/L, or levels of LDL-C, HDL-C, and triglyceride >3.4 mmol/L, lower than 1.0 mmol/L, and higher than 1.7 mmol/L, respectively. Diabetes mellitus was defined as a history of diabetes, current anti-diabetic treatment, or if fasting plasma glucose ≥6.1 mmol/L or random blood glucose of ≥11.1 mmol/L.
The carotid arteries were imaged using the LOGIQ S7 Expert and Aplio 400 on the basis of released protocols. We also attempted to carefully search for all carotid artery-dependent associations [i.e., central side, peripheral side, bifurcation of the common carotid artery (CCA) and central site of the internal carotid artery]. The peak value of IMT equal or higher than 1.1 mm was employed for definition of plaque. It was also made an effort to sum thicknesses of plaques for calculation of PS. In the head MRI, MRI scanners were utilized. Estimation of the frequency of WMH could be undertaken with the aid of images of T1- and T2-weighted, as well as fluid-attenuated inversion recovery (FLAIR). WMH was defined as clear hyperintensity in the white matter region, relative to the surrounding white matter on FLAIR images. Two independent experienced scholars analyzed MRI findings in a blinded fashion.
Statistical Analysis
Expression of continuous variables was in form of mean ± SD (for normally distributed outcomes) or median (IQR) (for normally distributed outcomes), and presentation of categorical variables was undertaken as frequencies and percentages. First, one-way analysis of variance and the Chi-square tests we employed to compare continuous variables and categorical variables, respectively. The association of PS or PN with WMH was assessed using multivariable logistic regression models. Non-adjusted, minimally adjusted, and multivariate-adjusted models were employed. The covariances were adjusted according to the following principle: (1) in Model II covariates whose initial regression coefficients changed by at least 10% were included; (2) in Model III covariates in Model II plus previously reported classical vascular risk factors were included; and (3) since the prevalence of WMH strongly increases with age and varies with sex, these two covariates were included in all adjusted models. In another separate analysis, PN was included as a continuous variable. Unadjusted and adjusted odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. We also used a generalized additive model to identify the non-linear relationships between PS and the frequency of WMH. Interaction and stratified analyses were conducted according to age group (three equal groups), sex, smoking status, drinking status, and history of chronic disease as previously described. We employed R programming and Empower Stats software to conduct the analyses. We set the significance level to P < 0.05.
Results
Subjects' Features
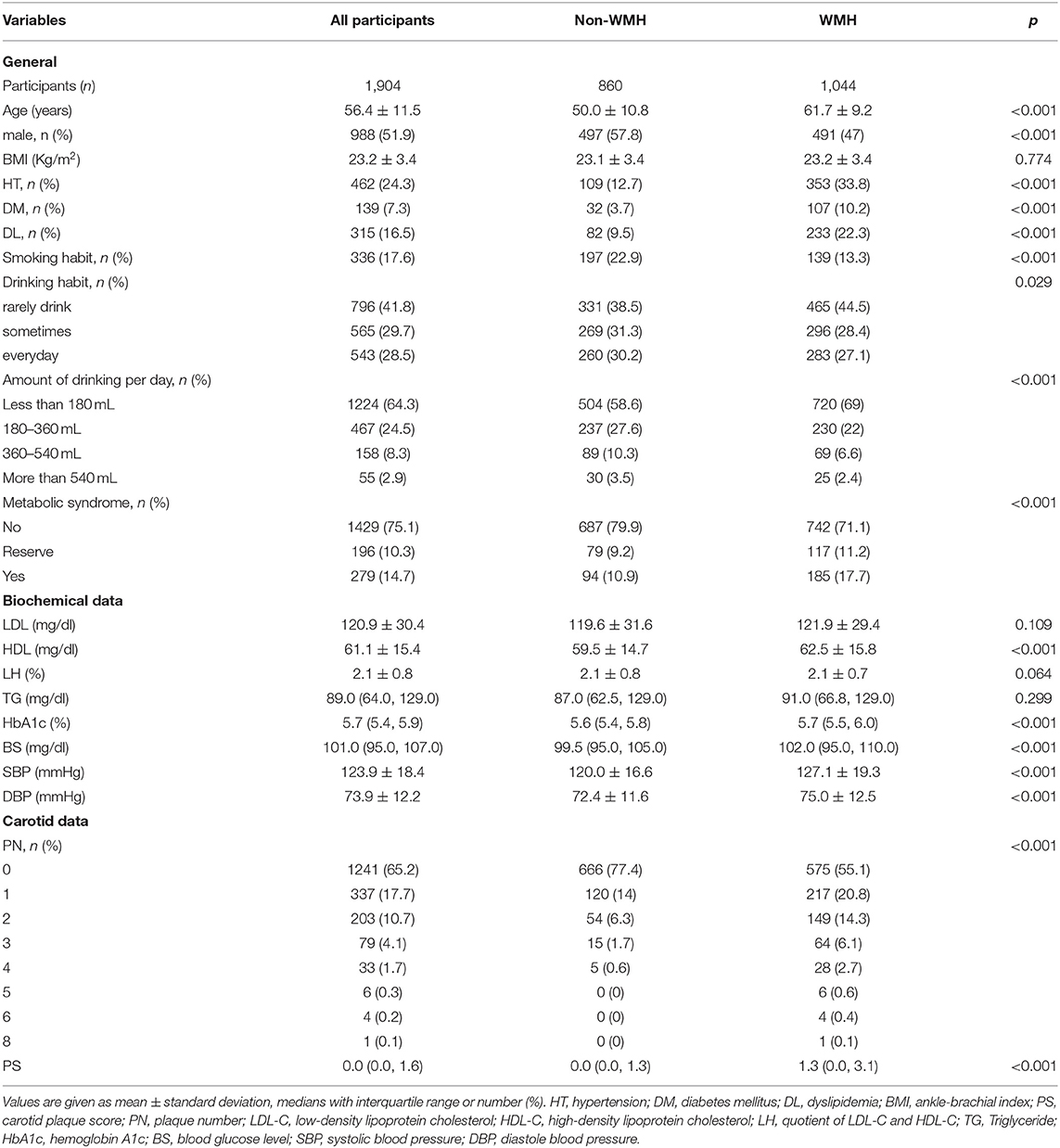
Among the 1,904 participants with a mean age of 56.4 ± 11.5 years old, 1,044 (54.8%) were diagnosed with cerebral WMH from their MRI results. Table 1 illustrates the comparisons' outcomes between WMH and non-WMH groups. Several factors were significantly different between the two groups. The WMH group showed higher age, lower male proportion, smoking habit, and drinking habit ratio, higher incidences of hypertension, hyperlipidemia, and diabetes mellitus, and a higher metabolic syndrome ratio, HDL-C, fasting plasma glucose, HbA1c, SBP and DBP compared to the non-WMH group. No significant difference was detected in levels of triglyceride and LDL-C between the above-mentioned groups. The main findings of carotid ultrasound were markedly greater in the WMH group than those in the non-WMH group [1.3 (0.0, 3.1) and 1.0 (0.0, 2.0), and 0.0 (0.0, 1.3) and 0.0 (0.0, 1.0), respectively; Table 1 and Figure 1].
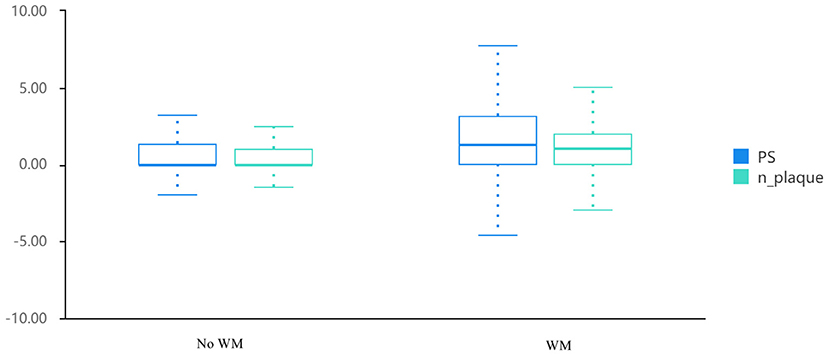
Figure 1. Distribution of PS and PN in patients with white matter hyperintensity and controls. PS, plaque score; PN, plaque number.
Association of PS and PN With WMH
We attempted to conduct multivariable logistic regression analysis for indicating factors that might contribute to the frequency of WMH (Table 2). In the non-adjusted model, odds ratio (OR) was 1.36, and the range of 95% confidence interval (CI) was 1.28 to 1.45. It could be interpreted that a unit increase of PS exhibited a correlation with a 36% increase of incidence of WMH. It was noted that every one unit increase in PS could be associated with a 25% elevation in frequency of WMH. Besides, high PN was also identified to contribute to the incidence of WMH [OR, 1.36 (95% CI: 1.28–1.54)]. To ensure the robustness of our results, we could convert PS value into a categorical variable according to the cut-off points (0, 2.5), and we calculated the P-value for trend. There was an increased risk for the prevalence of WMH as the cutoff point for PS was elevated (P (for trend) <0.001). Participants who had a great PS experienced an elevated risk of the incidence of WMH [OR, 3.68 (95% CI: 2.79–4.85)].
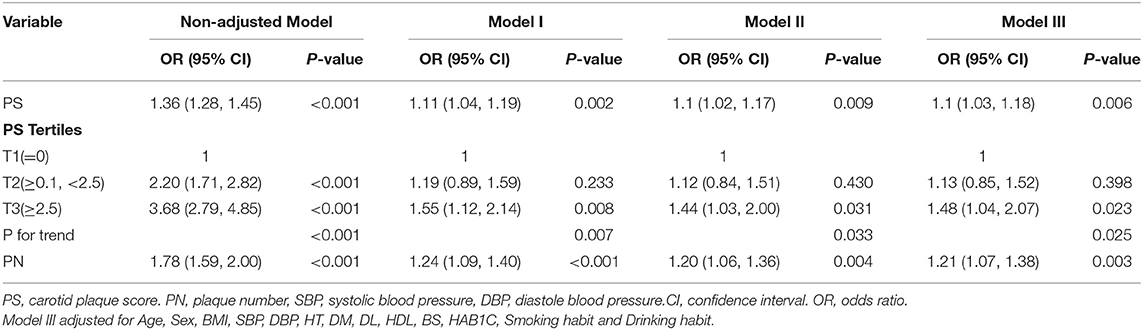
Table 2. Association between PS and incidence of White matter hyperintensity in multiple regression model.
Analysis of a Dose-Response Relationship
The generalized additive model (GAM) model was used to indicate the existence of a non-linear relationship between PS and incidence of WMH. After adjusting for vascular risk factors, a non-significant, moderate, beneficial effect on correlation of high PS with incidence of WMH was identified (Figure 2).
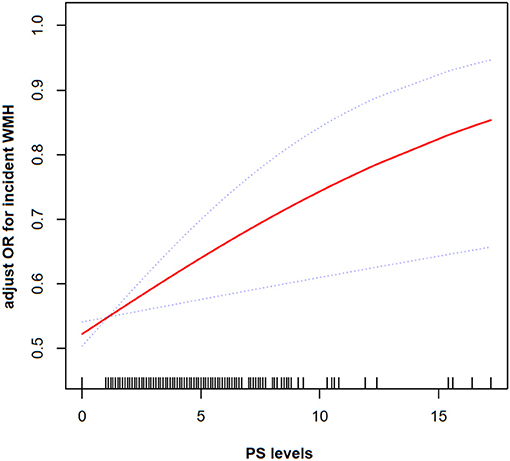
Figure 2. The smoothing curves illustrating the relationship between PS and frequency of WMH. A dose-response relationship between them was detected after adjusting for gender, age, SBP, DBP, BMI, HT, DL, DM, HDL, BS, HAB1C, smoking history, and drinking status.
Subgroup Analysis
Age, gender, smoking status, drinking habit, and history of chronic diseases were identified as confounders of the PS-WMH association. To determine whether the association between PS and frequency of WMH could be detected in diverse subgroups, we made an effort to carry out further analyses. Subgroup analyses stratified by gender, age, smoking status, drinking habit, and history of chronic diseases yielded consistent outcomes (Table 3 and Figure 3). The interaction analysis revealed that age could make a link between PS and incidence of WMH. The incidence of WMH was higher in those cases who aged <65 years old, while we did not detect a significant association in cases who aged > 65 years old. No noticeable interaction was identified for sex, drinking habit, smoking status, history of hypertension, hyperlipidemia, and diabetes mellitus.
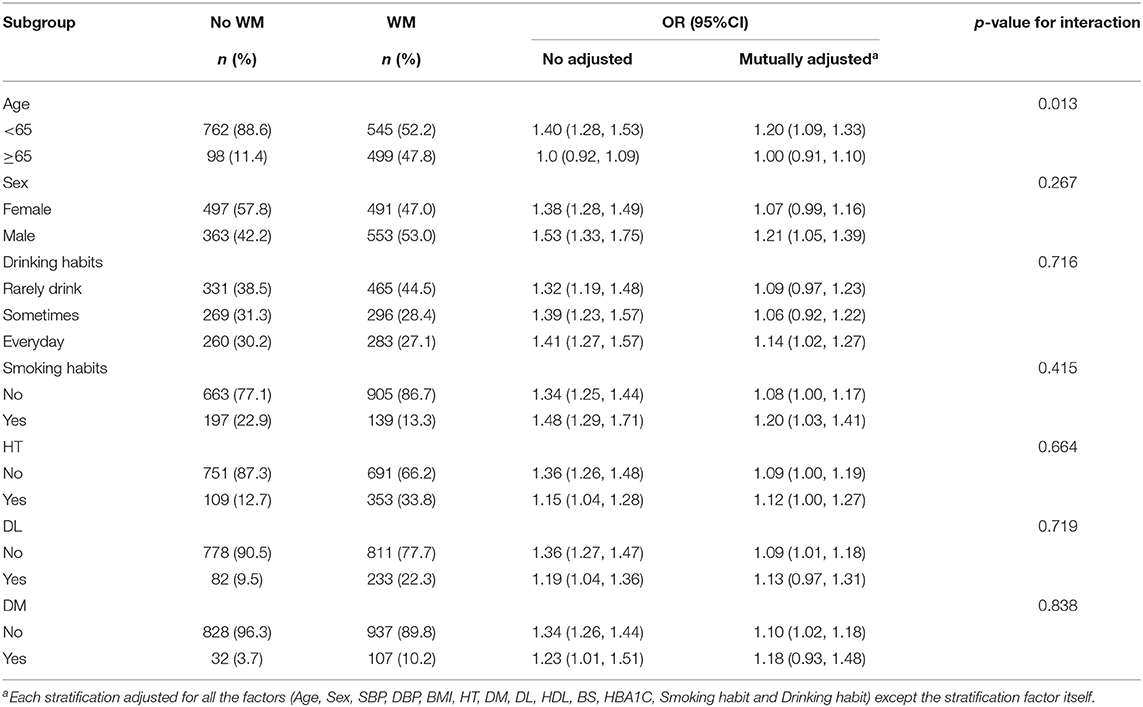
Table 3. Association between PS and White matter hyperintensity according to baseline characteristics.
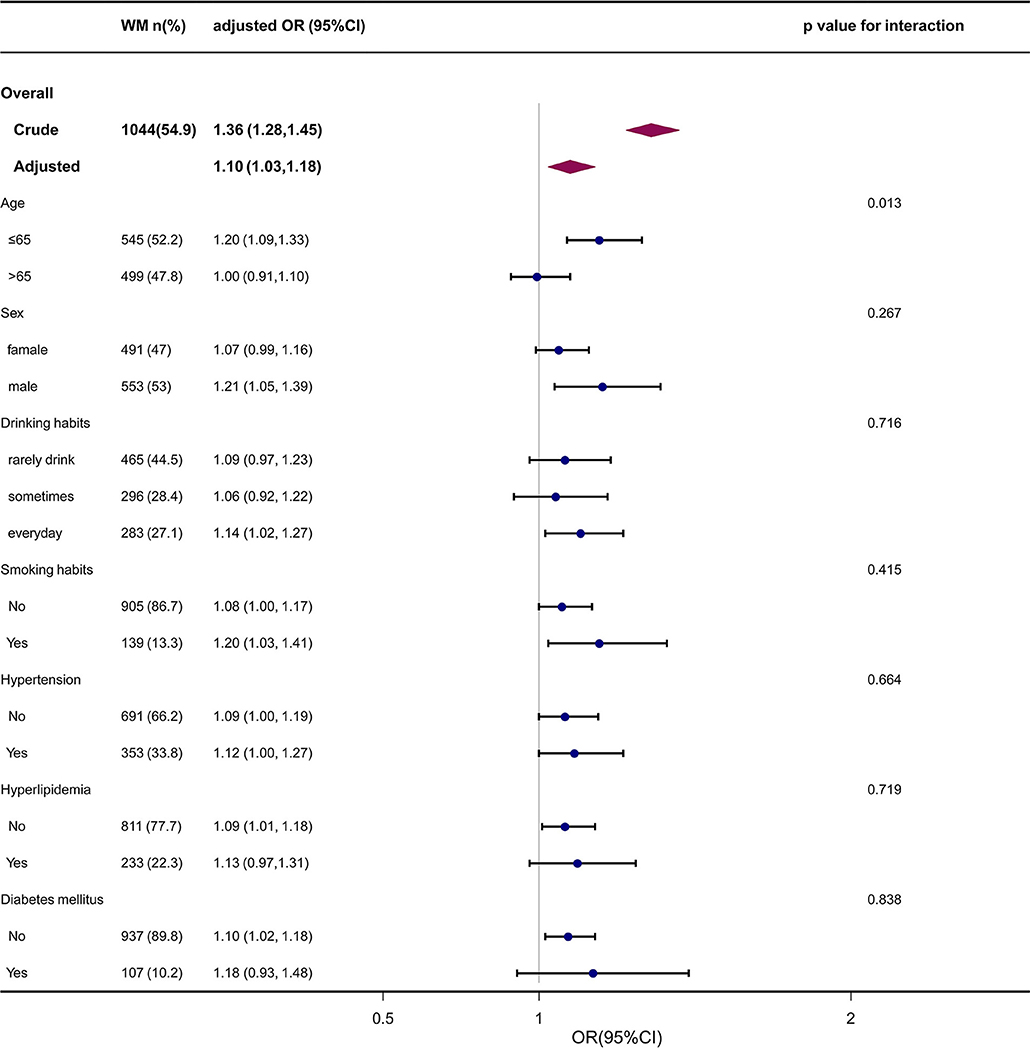
Figure 3. The forest plot of the association between PS and WMH according to baseline characteristics. P-value represents the likelihood of interaction between the variable and the PS. PS, carotid plaque score, WMH, white matter hyperintensity; OR, odds ratio; CI, confidence interval.
Discussion
The current cross-sectional study aimed to clarify the CAS-WMH relationship in Japanese population. The findings of the current research indicated that functional markers of carotid atherosclerosis (high values of PN and PS on the carotid ultrasonography) noticeably influenced determining the frequency of WMH, independent of demographic and vascular risk factors. We additionally revealed a dose-response relationship between PS and incidence of WMH. Even after adjusting for vascular risk factors, subgroup analyses stratified by multiple variables yielded consistent results. The CAS-WMH relationship demonstrated that structural carotid artery remodeling may be important in the pathophysiology of WMH, and it is consistent with that reported previously. Numerous researches have also described a positive association between the number or characteristics of the carotid plaque and incidence of WMH (6, 7, 13–15). The Rotterdam study recruited healthy adults who aged 65–85 years old and investigated an association between WMH and non-invasively assessed atherosclerosis, and found that CAS could be correlated to WMH (6). de Leeuw et al. reported a graded relationship between IMT severity and the presence of plaques in the carotid artery with periventricular WMH, rather than with subcortical WMH (7). Fernando et al. examined a relationship between carotid plaques and severe WMH at the 4-year follow-up, and showed that indicators of CAS, IMT and plaques, were associated with the frequency of severe WMH (14). Moreover, a recent meta-analysis revealed that WMH tended to be associated with carotid plaques and plaques or IMT increased with elevation of WMH severity (13).
Previous reports clearly showed that the high IMT observed by carotid ultrasonography is strongly associated with WMH incidence. However, the validity of atherosclerosis measurements should be first clarified. Increased carotid IMT according to the Mannheim consensus may not be an indicator of atherosclerosis (16). Increased carotid IMT without plaques, as determined by ultrasonography, is only associated with cardiovascular risk factors (17). Moreover, neither progression (18) nor regression (19) of IMT predicted the influences of cardiovascular risk factors. In a 6-year follow up study, IMT did not predict coronary risk, whereas total plaque area was noted as a robust predictor of coronary risk (20). In contrast, PS was associated with coronary heart disease (21), and PS more closely represented the atherosclerotic condition of the carotid artery compared to IMT (11) because of its wider range of observations in terms of carotid arteries. In the current research, it was unveiled how high PS could be markedly associated with WMH, and we also identified a dose-response relationship between frequency of WMH and PS, independent of factors mentioned earlier. It is noteworthy that we uniquely attempted to investigate the dose-response relationship that may further confirm WMH-CAS relationship.
The mechanisms underlying the WMH-CAS association are speculative. Factors that may interfere with cerebral blood flow, such as unstable microatheroma at the origin of perforator arteries, may play a role (22). Cerebral emboli originating from ruptured or unstable carotid plaques might intensify development of WMH, although they may not be major contributors to a population-based setting (1, 22). Other mechanisms could include large-artery hypertrophic remodeling and capillary rarefaction, continually and passively, to highly pulsatile pressure and flow, causing chronic ischemic lesions (23, 24). Non-stenotic CAS, defined as IMT or carotid plaque, was associated with larger ipsilateral intracranial artery remodeling (25), which in turn may distally influence hemodynamics, thereby affecting intracranial arterial remodeling. There may be a possible balance between changes in the cardiovascular homeostasis of the brain tissue, which may lead to white matter lesions when lost (26). Otherwise, the carotid plaque may also reflect the overall effect of the shared vascular risk factors, or lifetime exposure to factors, including age, smoking status, and atrial fibrillation, which are also associated with WMH according to previous researches (27, 28). A possible explanation is that atrial fibrillation could modulate pathogenesis of WMH through chronic silent microembolization (27, 29).
The outcomes of the current research unveiled how PS could be associated with the frequency of WMH, while age factor was excluded. It was illuminated that a high PS was positively associated with WMH incidence in subjects aged <65 years old, rather than in senior adults. As the most significant risk factor, a number of scholars concentrated on the role of aging in WMH was documented noteworthy (30), and older subjects typically experience a higher incidence of WMH; thus, some “non-ischemic” WMH may partly explain the results. Additionally, “non-ischemic” WMH may simply be a CT or MRI manifestation of the dilation of the perivascular spaces, which were filled with cerebrospinal fluid or atrophic changes that include gliosis or the loss of the myelinated axons seen in the normal, aging brain (31, 32). Consequently, bias was inevitable when we analyzed the association between PS and the prevalence of WMH in senior subjects.
The strengths of our study include the use of a large cohort of asymptomatic individuals in Japan in a single-center, which realized collecting data systematically and uniformly. In addition, strict statistical adjustments were employed to minimize unmeasured confounding. Moreover, standardized ultrasound scanning and PS were utilized as reliable measures of atherosclerosis rather than IMT used in a previous research (11). Finally, experienced scholars analyzed MRI findings in a blinded fashion, which might attenuate information bias.
The shortcomings of the current research should be acknowledged. First, no data regarding the severity of WMH could be presented. Second, this was a cross-sectional study and no data on correlation of the aggravation of CAS and the extension of WMH could be provided. Third, blood tests and ultrasonic scanning were undertaken on the basis of a single measurement, which might cause regression dilution bias. In addition, differentiating the “non-ischemic” or “natural” hyperintensity from the WMH region of interest is typically difficult, and a software could better indicate the spectrum of small vessel illnesses. Finally, as the current research was undertaken in a single-center, generalizability of the results is questionable. Hence, it is essential to conduct further researches to eliminate the above-mentioned shortcomings.
Conclusions
The current research enabled us to identify how an increase in values of PS and PN could be independently correlated with a higher incidence of WMH in an asymptomatic population. It was confirmed that PN and PS exhibited the greatest influences on determining the incidence of WMH, independent of demographic and vascular risk factors. Furthermore, a dose-response relationship between PS and incidence of WMH was noted. Our findings highlighted the potentially important pathophysiological role of large artery atherosclerosis in intensifying WMH. However, the mentioned outcomes should be confirmed by conducting further researches.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethical review committee of Shin Takeo Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LZ contributed to the drafting of the manuscript and analysis and interpretation of the data. JX contributed to the conception, critical revision of the manuscript, analysis, and interpretation of data, and approved the final version of the submitted manuscript. LZ and JX read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by grants from the general program of Changde city Science Foundation (2020S014), and Foundation of Hunan Science and Technology Department-Clinical Medical Technology Demonstration Base for Neurosurgery in Hunan Province (2018SK4003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.665573/full#supplementary-material
Abbreviations
CT, computed tomography; DBP, diastolic blood pressure; FLAIR, fluid-attenuated inversion recovery; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; IMT, intima-media thickness; LDL-C, low-density lipoprotein cholesterol; MRI, magnetic resonance imaging; PN, plaque number; PS, carotid plaque score; SBP, systolic blood pressure; WMH, white matter hyperintensity.
References
1. Wardlaw JM, Valdés Hernández MC, Muñoz-Maniega S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J Am Heart Assoc. (2015) 4:001140. doi: 10.1161/JAHA.114.001140
2. Wisniewska M, Devuyst G, Bogousslavsky J, Ghika J, van Melle G. What is the significance of leukoaraiosis in patients with acute ischemic stroke. Arch Neurol. (2000) 57:967–73. doi: 10.1001/archneur.57.7.967
3. Longstreth WT Jr, Arnold AM, Beauchamp NJ Jr, Manolio TA, Lefkowitz D, Jungreis C, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. (2005) 36:56–61. doi: 10.1161/01.STR.0000149625.99732.69
4. Patel B, Markus HS. Magnetic resonance imaging in cerebral small vessel disease and its use as a surrogate disease marker. Int J Stroke. (2011) 6:47–59. doi: 10.1111/j.1747-4949.2010.00552.x
5. Lammie GA, Brannan F, Slattery J, Warlow C. Nonhypertensive cerebral small-vessel disease. An autopsy study. Stroke. (1997) 28:2222–9. doi: 10.1161/01.STR.28.11.2222
6. Bots ML, van Swieten JC, Breteler MM, de Jong PT, Gijn JV, Hofman A, et al. Cerebral white matter lesions and atherosclerosis in the Rotterdam Study. Lancet. (1993) 341:1232–7. doi: 10.1016/0140-6736(93)91144-B
7. de Leeuw FE, de Groot JC, Bots ML, Witteman JC, Oudkerk M, Hofman A, et al. Carotid atherosclerosis and cerebral white matter lesions in a population-based magnetic resonance imaging study. J Neurol. (2000) 247:291–6. doi: 10.1007/s004150050586
8. Shu M, Zhang JJ, Dong Y, Zhang ZP. Significance of increased CIMT with coexisting carotid plaques in cerebral white matter lesions in elders. J Huazhong Univ Sci Technol Med Sci. (2013) 33:69–74. doi: 10.1007/s11596-013-1073-3
9. Kobari M, Meyer JS, Ichijo M, Oravetz WT. Leukoaraiosis: correlation of MR and CT findings with blood flow, atrophy, and cognition. Am J Neuroradiol. (1990) 11:273–81.
10. Yamada K, Sakai K, Owada K, Mineura K, Nishimura T. Cerebral white matter lesions may be partially reversible in patients with carotid artery stenosis. Am J Neuroradiol. (2010) 31:1350–2. doi: 10.3174/ajnr.A1873
11. Morito N, Inoue Y, Urata M, Yahiro E, Kodama S, Fukuda N, et al. Increased carotid artery plaque score is an independent predictor of the presence and severity of coronary artery disease. J Cardiol. (2008) 51:25–32. doi: 10.1016/j.jjcc.2007.09.003
12. Shinkawa Y, Yoshida T, Onaka Y, Ichinose M, Ishii K. Mathematical modeling for the prediction of cerebral white matter lesions based on clinical examination data. PLoS ONE. (2019) 14:e0215142. doi: 10.1371/journal.pone.0215142
13. Liao SQ, Li JC, Zhang M, Wang YJ, Li BH, Yin YW, et al. The association between leukoaraiosis and carotid atherosclerosis: a systematic review and meta-analysis. Int J Neurosci. (2015) 125:493–500. doi: 10.3109/00207454.2014.949703
14. Pico F, Dufouil C, Lévy C, Besançon V, Kersaint-Gilly AD, Bonithon-Kopp C, et al. Longitudinal study of carotid atherosclerosis and white matter hyperintensities: the EVA-MRI cohort. Cerebrovasc Dis. (2002) 14:109–15. doi: 10.1159/000064741
15. Devantier TA, Nørgaard BL, Poulsen MK, Garde E, Øvrehus KA, Marwan M, et al. White matter lesions, carotid and coronary atherosclerosis in late-onset depression and healthy controls. Psychosomatics. (2016) 57:369–77. doi: 10.1016/j.psym.2016.02.005
16. Finn AV, Kolodgie FD, Virmani R. Correlation between carotid intimal/medial thickness and atherosclerosis: a point of view from pathology. Arterioscler Thromb Vasc Biol. (2010) 30:177–81. doi: 10.1161/ATVBAHA.108.173609
17. Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. (2007) 115:459–67. doi: 10.1161/CIRCULATIONAHA.106.628875
18. Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Völzke H, Tuomainen TP, et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet. (2012) 379:2053–62. doi: 10.1016/S0140-6736(12)60441-3
19. Costanzo P, Perrone-Filardi P, Vassallo E, Paolillo S, Cesarano P, Brevetti G, et al. Does carotid intima-media thickness regression predict a reduction of cardiovascular events? A meta-analysis of 41 randomized trials. J Am Coll Cardiol. (2010) 56:2006–20. doi: 10.1016/j.jacc.2010.05.059
20. Johnsen SH, Mathiesen EB, Joakimsen O, Stensland E, Wilsgaard T, Løchen ML, et al. Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: a 6-year follow-up study of 6226 persons: the Tromsø Study. Stroke. (2007) 38:2873–80. doi: 10.1161/STROKEAHA.107.487264
21. Zhang H, Liu M, Ren T, Wang XQ, Liu DD, Xu ML, et al. Associations between carotid artery plaque score, carotid hemodynamics and coronary heart disease. Int J Environ Res Public Health. (2015) 12:14275–84. doi: 10.3390/ijerph121114275
22. Manolio TA, Burke GL, O'Leary DH, Evans G, Beauchamp N, Knepper L, et al. Relationships of cerebral MRI findings to ultrasonographic carotid atherosclerosis in older adults : the Cardiovascular Health Study. CHS Collaborative Research Group. Arterioscler Thromb Vasc Biol. (1999) 19:356–65. doi: 10.1161/01.ATV.19.2.356
23. O'Rourke MF, Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. (2005) 46:200–4. doi: 10.1161/01.HYP.0000168052.00426.65
24. Laurent S, Briet M, Boutouyrie P. Large and small artery cross-talk and recent morbidity-mortality trials in hypertension. Hypertension. (2009) 54:388–92. doi: 10.1161/HYPERTENSIONAHA.109.133116
25. Gutierrez J, Elkind MS, Gomez-Schneider M, DeRosa JT, Cheung K, Bagci A, et al. Compensatory intracranial arterial dilatation in extracranial carotid atherosclerosis: the Northern Manhattan study. Int J Stroke. (2015) 10:843–8. doi: 10.1111/ijs.12464
26. Rundek T, Della-Morte D, Gardener H, Dong CH, Markert MS, Gutierrez J, et al. Relationship between carotid arterial properties and cerebral white matter hyperintensities. Neurology. (2017) 88:2036–42. doi: 10.1212/WNL.0000000000003951
27. de Leeuw FE, de Groot JC, Oudkerk M, Kors JA, Hofman A, Gijn JV, et al. Atrial fibrillation and the risk of cerebral white matter lesions. Neurology. (2000) 54:1795–801. doi: 10.1212/WNL.54.9.1795
28. Arntz RM, van den Broek SM, van Uden IW, Ghafoorian M, Platel B, Rutten-Jacobs LC, et al. Accelerated development of cerebral small vessel disease in young stroke patients. Neurology. (2016) 87:1212–9. doi: 10.1212/WNL.0000000000003123
29. Mayasi Y, Helenius J, McManus DD, Goddeau RP Jr, Jun-O'Connell AH, Moonis M, et al. Atrial fibrillation is associated with anterior predominant white matter lesions in patients presenting with embolic stroke. J Neurol Neurosurg Psychiatry. (2018) 89:6–13. doi: 10.1136/jnnp-2016-315457
30. Liao D, Cooper L, Cai J, Toole J, Bryan N, Burke G, et al. The prevalence and severity of white matter lesions, their relationship with age, ethnicity, gender, and cardiovascular disease risk factors: the ARIC Study. Neuroepidemiology. (1997) 16:149–62. doi: 10.1159/000368814
Keywords: carotid artery plaque score, carotid atherosclerosis, carotid plaque number, white matter hyperintensity, cross-sectional study
Citation: Zhang L, Zhou Q, Shao LH, Wen J and Xia J (2021) Association of Carotid Atherosclerosis With White Matter Hyperintensity in an Asymptomatic Japanese Population: A Cross-Sectional Study. Front. Cardiovasc. Med. 8:665573. doi: 10.3389/fcvm.2021.665573
Received: 10 February 2021; Accepted: 06 April 2021;
Published: 29 April 2021.
Edited by:
Gen-Min Lin, Hualien Armed Forces General Hospital, TaiwanReviewed by:
Yu-Kai Lin, Tri-Service General Hospital, TaiwanDejan Jakimovski, Buffalo Neuroimaging Analysis Center, United States
Copyright © 2021 Zhang, Zhou, Shao, Wen and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Xia, NjE2Njk1MDhAcXEuY29t
 Li Zhang
Li Zhang Quan Zhou2
Quan Zhou2 Jun Xia
Jun Xia