- 1Department of Clinical Pharmacology, Xiangya Hospital, Central South University, Changsha, China
- 2Hunan Key Laboratory of Pharmacogenetics, Institute of Clinical Pharmacology, Central South University, Changsha, China
- 3Engineering Research Center of Applied Technology of Pharmacogenomics, Ministry of Education, Changsha, China
- 4National Clinical Research Center for Geriatric Disorders, Changsha, China
- 5Department of Pharmacy, The Second Xiangya Hospital, Central South University, Changsha, China
- 6Department of Cardiology, Zhuzhou Central Hospital, The Affiliated Zhuzhou Hospital of Xiangya Medical College of Central South University, Zhuzhou, China
Essential Hypertension (EH) results in the burden of cardiovascular disease (CVD) such as Heart Failure (HF) and Ischemic Stroke (IS). A rapidly emerging field involving the role of Wnt/β-catenin signaling pathway in cardiovascular development and dysfunction has recently drawn extensive attention. In the present study, we conducted a genetic association between genomic variants in Wnt/β-catenin signaling pathway and EH, HF, IS. A total of 95 SNPs in 12 Wnt signaling genes (WNT3A, WNT3, WNT4, DKK1, DKK2, LRP5, LRP6, CTNNB1, APC, FZD1, FRZB, SFRP1) were genotyped in 1,860 participants (440 patients with EH, 535 patients with HF, 421 patients with IS and 464 normal control subjects) using Sequenom MassArray technology. WNT3A rs752107(C > T) was strongly associated with an increased risk of EH, HF and IS. Compared with WNT3A rs752107 CC genotype, the CT genotype carriers had a 48% increased risk of EH (OR = 1.48, 95% CI = 1.12–1.96, P = 0.006), the TT genotype conferred a 139% increased risk of EH (OR = 2.39, 95% CI = 1.32–4.34, P = 0.003). Regarding HF and IS, the risk of HF in the T allele carriers (CT + TT) was nearly increased by 58% (OR = 1.58, 95% CI = 1.22–2.04, P = 4.40 × 10−4) and the risk of IS was increased by 37% (OR = 1.37, 95% CI = 1.04–1.79, P = 0.025). Expression quantitative trait loci (eQTL) analysis indicated that rs752107 C allele corresponded to a significant reduction of WNT3A expression. We described a genetic variant of WNT3A rs752107 in Wnt/β-catenin signaling strongly associated with the risk of EH, HF and IS for the first time.
Introduction
Cardiovascular disease (CVD) refers to the pathologies associated with the heart and circulation system, including cerebrovascular, and cardiovascular. CVD is a crisis for human health that plays a vital role on human morbidity and mortality. Substantial evidence suggests that CVD is heritable, and genetic predisposition have long been thought to contribute to the development and progression of this complex disease. With the rapidly evolving of high throughput sequencing, genetic studies have organized large amounts of gene variants across the human genome that are associated with CVD susceptibility (1, 2).
Hypertension is the predominant independent risk factor for CVD. Observational investigations and clinical trials have demonstrated that long periods of uncontrolled hypertension are responsible for the catastrophic events, such as heart attack and stroke (3, 4). Hypertension is caused by the complex interaction of environmental and pathophysiological, as well as genetic predisposition. The evidence for a genetic basis of hypertension provides valuable insights into blood pressure regulation. Based on genome wide association studies (GWAS), over 100 single nucleotide polymorphisms (SNPs) associated with blood pressure phenotypes have been identified (5). Although remarkable progress in genetic or genomic characterization of hypertension and CVD phenotypes has been made, the known genetic markers are still far from explaining the susceptibility or prognosis of these diseases. There is an urge for development of novel markers to enable better prevent, diagnosis, prognosis, and efficient treatment.
Wnt proteins, one family of growth factors, are secreted glycoproteins important for cellular proliferation and differentiation, tissue morphogenesis, and tissue homeostasis (6–8). Wnt signaling pathway is critically important for development of cardiac, vascular, and endothelial cell specification. Evidences showed that both loss- and gain-of-function of Wnt signaling pathway may cause marked alterations of angiogenesis, cardiovascular development and endothelial cell specification (9–11). Normally, few Wnt signaling activity is present in the cardiovascular system of healthy adults. Excessive activation of Wnt signaling in the cardiovascular system may contribute to cardiovascular disease (12). Indisputably, dyfunction of Wnt signaling is factitive to multiple pathologies of heart and blood vessels. Nevertheless, little evidence has described the genetic association between polymorphisms in Wnt signaling pathway and EH, HF, IS.
Based on the identity of Wnt ligands or receptors, Wnt signaling pathway can be subdivided into canonical and non-canonical. The canonical Wnt signaling pathway involves the elevated levels of β-catenin in cells. β-catenin functions as intracellular signaling protein by interacting with transmembrane proteins of the cadherin family. It has been suggested that low-density lipoprotein-related receptor 6(LRP6), a key receptor of Wnt/β-catenin signaling, is involved in the development of cardio-cerebrevascular diseases. Mani et al. found a missense mutation (R611C) in LRP6, which substitutes cysteine for arginine at highly conserved residue of an EGF-like domain and impairs Wnt signaling in vitro, contributes to the metabolic syndrome including early coronary artery disease, hyperlipidemia, hypertension, and diabetes (13). Sarzani et al. indicated that LRP6 I1062V (rs2302685) was associated with carotid artery atherosclerosis (CAA) through retrospective analysis of 334 secondary hypertensive patients and the expression of LRP6 was significantly decreased in atherosclerotic plaque of patients with hypertension (14). Therefore, we hypothesized that the Wnt/β-catenin signaling genes were mainly associated with hypertension and related CVDs. And the aim of this study was to explore the role of the mutated Wnt/β-catenin signaling pathway components on genetic predisposition of EH, HF, and IS.
In the present study, we conducted case–control studies and screened a total of 95 potentially functional variants within 12 Wnt/β-catenin signaling genes (WNT3A, WNT3, WNT4, DKK1, DKK2, LRP5, LRP6, CTNNB1, APC, FZD1, FRZB, SFRP1). At first, we confirmed a genetic association of WNT3A rs752107 with EH susceptibility in exploration cohort (199 patients with EH and 135 healthy subjects) and enlarged validation cohort (440 patients with EH and 464 healthy subjects). Then, WNT3A rs752107 was analyzed in HF cohort (535 patients) and IS cohort (421 patients). We determined the novel association between genomic variants in Wnt/β-catenin signaling pathway and EH and related CVDs.
Materials and Methods
Participants
All participants were recruited from the Xiangya Hospital (Changsha, China). Patients with EH and HF were recruited from Department of Cardiology, Xiangya Hospital, Central South University. According to the universal diagnostic criteria, hypertension was defined as blood pressure above 140/90 mmHg or taking antihypertensive drugs. Here we excluded secondary hypertension patients. Patients with IS were recruited from Department of Neurology, Xiangya Hospital, Central South University. Included patients with IS were ruled out hemorrhagic and cardioembolic stroke. Control group selected from visitors at Physical Examination Center for a routine checkup. Exclusion criteria for health control were hypertension, coronary heart disease, ischemic cardiomyopathy, diabetes, HF, and IS. Medical history, demographic data and anthropometric data were obtained by questionnaire. All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Medical Ethics Committee of Xiangya Hospital, Central South University.
SNP Selection
Candidate genes were chosen from Wnt/β-catenin genetic pathways. The selection of SNPs was based on public information available in dbSNP and Hapmap by Haploview 4.0 with a r2 threshold of 0.8. The criteria for the SNPs were as below: minor allele frequency >5% in Chinese, and with potential functions. A total of 95 SNPs in 12 Wnt/β-catenin signaling genes (WNT3A, WNT3, WNT4, DKK1, DKK2, LRP5, LRP6, CTNNB1, APC, FZD1, FRZB, SFRP1) were selected. The genes, SNP IDs, and their information locations are exhibited in Supplementary Table 1.
SNP Genotyping
Genomic DNA was extracted from 3 ml of venous blood samples using Blood DNA Maxi Kit (OMEGA, D3392) according to the manufacturer's protocol.
In the exploration cohort, genotyping was performed using MassARRAY high-throughput DNA analysis with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Sequenom, Inc., San Diego, CA, USA). 10% of samples were randomly selected for genotyping quality control. Subsequently, SNPs were genotyped using iPLEX Gold technology (Sequenom) followed by an automated data analysis with the TYPER® RT software version 4.0. In the enlarged validation cohort and HF, IS cohort, WNT3A rs752107 was genotyped by DNA sequencing after polymerase chain reaction (PCR).
Expression Quantitative Trait Loci (eQTL) Analysis
To explore the effects of genetic variation in multiple human reference tissues, we used the Genotype-Tissue Expression (GTEx) project database.
Statistical Analysis and Graphical Presentations
The statistical analyses of collected data and clinical results was performed with SPSS version 20.0 and PLINK 1.07 software. Quantitative data were expressed as mean value and standard deviation (SD). Genotype and allele frequencies were compared by direct counting. Categorical data between groups and deviations from Hardy–Weinberg equilibrium were tested by Chi-square test. According to distribution of our data, t-test or Mann–Whitney U Tests were used for between-groups' comparisons. The association between CVD and Wnt/β-catenin signaling genes polymorphism was expressed as the odds ratios (ORs) and 95% confidence intervals (CIs). The Bonferroni correction was used to control for multiple testing. Schematic diagram of Wnt/β-catenin signaling pathway was accomplished by ScienceSlides 6.0.
Results
Baseline Characteristics
The exploration cohort included 199 EH patients and 135 controls. The enlarged validation cohort, which absorbed samples from exploration cohort, included 440 patients with EH and 464 healthy subjects at all. Baseline characteristics of enlarged EH cohort and controls, as well as HF and IS cohorts, are shown in Supplementary Table 2.
Genotype Distribution in EH Cases and Controls
9 SNPs were removed from further analysis for any of the following motives: Monomorphism, call rate below 95%, Minor Allele Frequency (MAF) <5%, and deviation from hardy–weinberg equilibrium. Eighty six valid SNPs were included in the statistics (Supplementary Table 3).
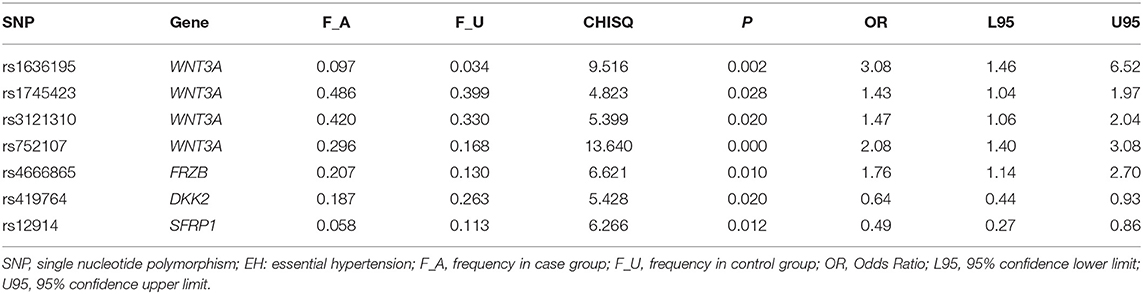
In the exploration cohort, the analysis results showed that seven of candidate SNPs (WNT3A rs752107, rs1636195, rs3121310, rs1745423, FRZB rs4666865, SFRP1 rs12914, DKK2 rs419764) were significantly associated with EH (Table 1). However, after correction for multiple testing, only WNT3A rs752107(C > T) polymorphism remained significant correlation. The frequency of T allele in patients with EH was significantly increased than the controls (29.6 vs. 16.8%; P = 2.2 × 10−4). Therefore, further exploration was performed to confirm the genetic association of WNT3A rs752107 with EH susceptibility in an enlarged validation cohort.
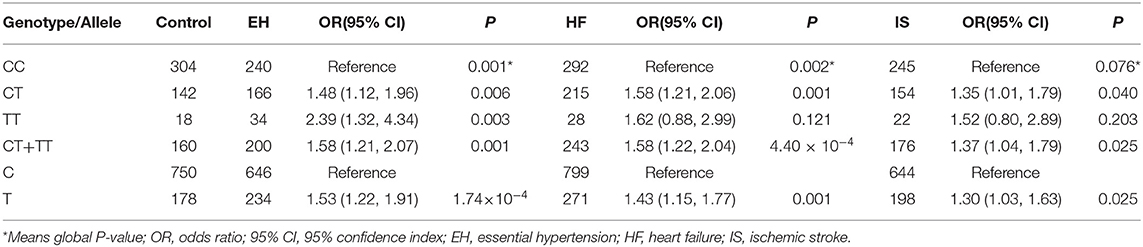
In the enlarged validation cohort, as expected, the frequency of T allele in patients with hypertension was significantly increased than the controls (26.6 vs. 19.2%, P = 1.74 × 10−4). Compared with WNT3A rs752107 CC genotype, the CT genotype carriers had a 48% increased risk of EH (OR = 1.48, 95% CI = 1.12–1.96, P = 0.006), the TT genotype conferred a 139% increased risk of EH (OR = 2.39, 95% CI = 1.32–4.34, P = 0.003) (Table 2).
Association Between WNT3A rs752107 and HF, IS Susceptibility
Hypertension is the most important contributing cause to other CVDs. Evidences show that long periods of uncontrolled high blood pressure are responsible for the consequent catastrophic events, such as HF and IS. Meanwhile, dyfunction of Wnt Signaling is considered to be a causative factor to vascular and cardiac disease. Reactivation of Wnt signaling is observed in multiple pathologies of heart and blood vessels (12). Therefore, we then explored the association of WNT3A rs752107 with the risk of HF and IS. Baseline Characteristics of IS and HF patients are shown in Supplementary Table 2. The control cohort for IS and HF is in common with controls for EH in the enlarged validation cohort. Interestingly, the results suggested strong links between WNT3A rs752107 and the risk of IS and HF in line with expectation (Table 2). The frequency of T allele in patients with HF and IS was significantly higher than the controls (25.3 vs. 19.2%, P = 0.001; 23.5 vs. 19.2%, P = 0.025; respectively). Compared with WNT3A rs752107 CC genotype, the risk of HF in the CT genotype carriers was nearly increased by 60% (OR = 1.58, 95% CI = 1.21–2.06, P = 0.001) and the risk of IS was increased by 35% (OR = 1.35, 95% CI = 1.01–1.79, P = 0.04).
eQTL Analysis
The rs752107 polymorphism is located in 3'untranslated regions (3'-UTRs) involved in gene expression control by binding to MicroRNAs (miRNAs) (15), which may influence transcription of proximal genes. Mirsnpscore (http://www.bigr.medisin.ntnu.no/mirsnpscore) and MirSNP databases (http://cmbi.bjmu.edu.cn/mirsnp) were used to predict the effect of rs752107 polymorphism on gene regulation. The presence of T allele is predicted to break the binding site for has-miR-892b which may lead to an increased level of secreted Wnt3a ligand (Figure 1A). And C allele is predicted to a stronger miRNA-mRNA interaction which may result in a decreased level of WNT3A gene expression (p = 2.5e−12, Figure 1B).
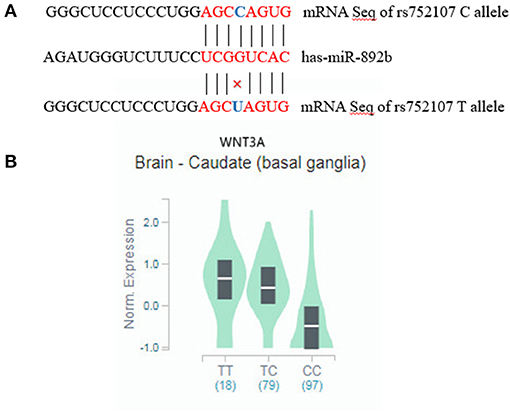
Figure 1. (A) mRNA alignment analysis of has-miR-892b with rs752107. (B) WNT3A mRNA expression level among the different genotypes of rs752107 in brain.
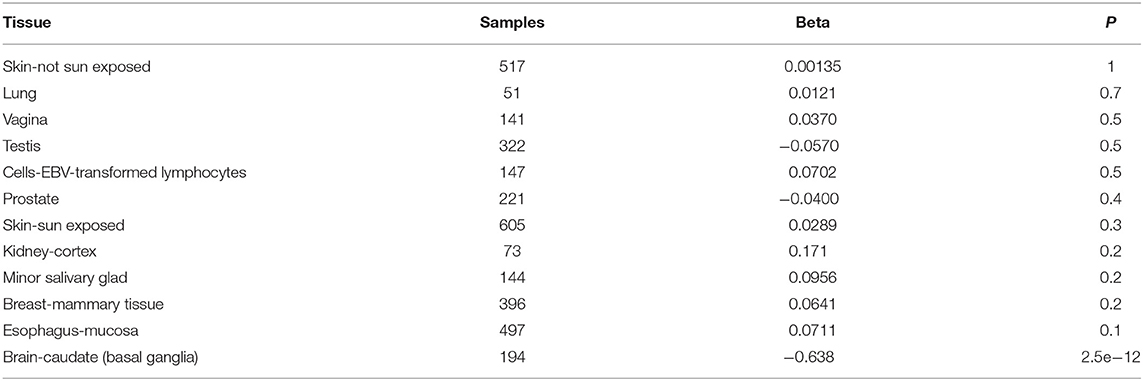
eQTL analysis was used to evaluate the association of rs752107 with gene expression. Using the GTEx database, rs752107 was found to be associated with differential expression of WNT3A in 12 human tissues (Table 3). And C allele corresponded to a significant reduction of WNT3A expression in brain.
Discussion
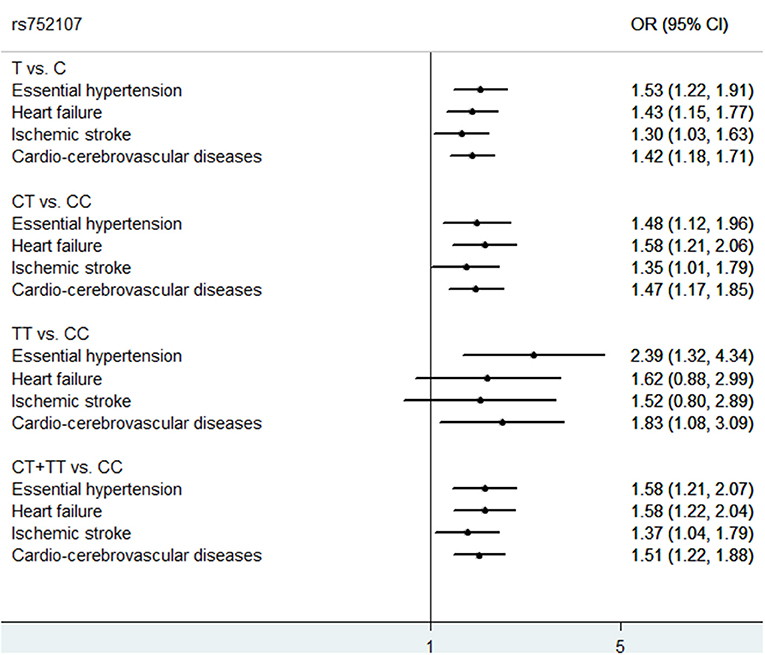
Hypertension is associated with an increased risk of CVD and is the predominant risk factor for all-cause morbidity and mortality globally. Genetic factors are proved to get involve in the development and progression of hypertension and related CVDs. Here, we validated a novel genomic variant of WNT3A rs752107 in Wnt/β-catenin signaling pathway strongly associated with the incidence of EH, HF, and IS. As summarized in forest plot (Figure 2), compared with WNT3A rs752107 C allele, the T allele conferred an increased risk for EH, HF, and IS.
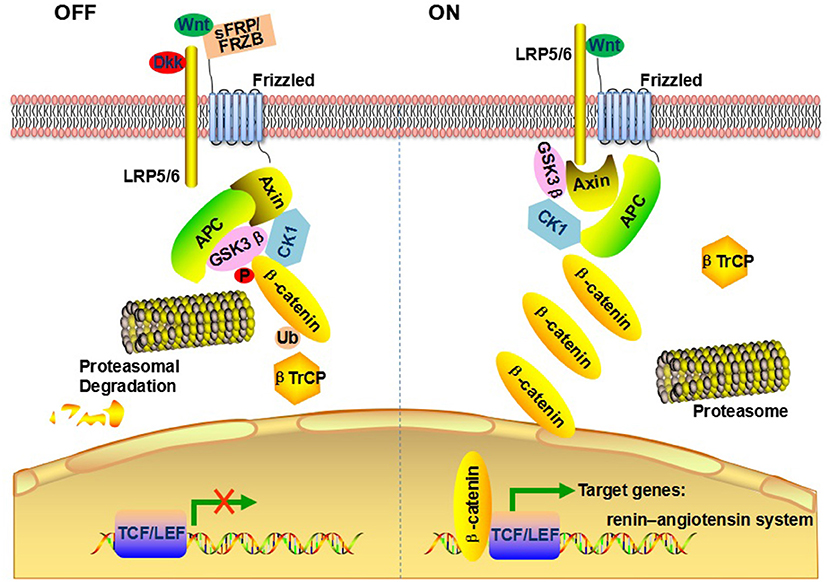
The canonical Wnt/β-catenin pathway involves the transcriptional co-activator β-catenin, as showed in Figure 3. Transmembrane frizzled (FZD) and low-density lipoprotein-related receptor (LRP) act as co-receptor complexes. In the presence of Wnt, FZD, and LRP complexes are triggered resulting in β-catenin accumulation and translocation to the nucleus. In the nucleus, β-catenin binds to T cell factor (TCF)/lymphoid-enhancer binding factor (LEF), activating transcription of Wnt target genes (16). Wnt/β-catenin signaling can be regulated at many different levels. The expression of CTNNB1, the gene encoding β-catenin, could regulate Wnt signaling (17). β-catenin can be destroyed directly by destruction complex in the cytoplasm consisting of axin, adenomatosis polyposis coli (APC), and glycogen synthase kinase3β (GSK3β) (10). The Wnt/β-catenin signaling antagonist includes dickkopf (Dkk)-an endogenous inhibitor of LRP (18), frizzled- related protein (FRZB)- a secreted Wnt antagonist (19), secreted frizzled- related proteins (SFRP)- a competitive antagonist of Wnt (20).
A rapidly emerging field involving the role of Wnt/β-catenin signaling pathway in cardiovascular development and dysfunction has recently drawn extensive attention. Indisputably, pathway mutations of Wnt/β-catenin Signaling pathway are factitive to multiple pathologies of cardiovascular disease. As the common risk factor for CVD, hypertension has been focused by us for a long time (21–23). To define whether the genetic variants of Wnt/β-catenin signaling pathway correlated with hypertension, we conducted case–control studies and screened a total of 95 potentially functional variants within 12 Wnt/β-catenin signaling genes (WNT3A, WNT3, WNT4, DKK1, DKK2, LRP5, LRP6, CTNNB1, APC, FZD1, FRZB, SFRP1) mentioned above (Supplementary Table 1). In the exploration cohort of 334 individuals (199 patients with EH and 135 healthy subjects), after Bonferroni correction, we preliminarily validated a genetic association of WNT3A rs752107 with EH susceptibility. Then, this genetic association was further confirmed in an enlarged validation cohort of 904 individuals (440 patients with EH and 464 normal control subjects). Interactions between Wnt/β-catenin pathway and renin-angiotensin system (RAS), which plays an essential role in the maintenance of blood pressure homeostasis, may be an interesting way to better understand the function of Wnt/β-catenin pathway during hypertension. Wnt/β-catenin pathway and RAS regulate positively each other during hypertension (24, 25). Zhou et al. demonstrated that Wnt/β-catenin signaling is a master regulator controlling multiple RAS genes, such as AGT, Renin, ACE, AT1, AT2 (26).
Wnt/β-catenin signaling also get involved in abnormal cardiac remodeling in heart failure (27, 28). In the mouse model of cardiac hypertrophy and heart failure, the cardiac lesions were accompanied with upregulation of multiple Wnt ligands and activation of β-catenin and RAS (29). Malekar et al. found that activation of Wnt signaling is critical and sufficient for maladaptive myocardial hypertrophy and cardiomyopathy (30). Data showed that inhibition of glycogen synthase kinase 3 beta(GSK-3β) during heart failure is protective (31). Besides, a multiancestry GWAS meta-analysis identified a SNP in WNT2B (rs12037987) as the novel stroke risk loci (32). Therefore, we then explored the association of WNT3A rs752107 with the risk of HF and IS. Interestingly, the results suggest strong links between WNT3A rs752107 and the risk of HF and IS in line with expectation.
Wnt3a, as the ligands of Wnt signaling, plays a direct role in the activation of Wnt/β-catenin signaling pathway. Functional mutations in WNT3A gene may affect the pathogenesis of cardio-cerebrovascular diseases. WNT3A rs752107 polymorphism was identified to be associated with cleft palate and bone mineral density variation (33, 34). The rs752107 polymorphism is located in 3'untranslated regions (3'-UTRs) involved in gene expression control by binding to MicroRNAs (miRNAs) (15). Based on publicly available data, the presence of C allele predicted a stronger binding site for has-miR-892b resulting in a decreased level of WNT3A gene expression. Then, eQTL analysis validated that rs752107 C allele is significantly associated with reduced WNT3A expression in brain (Table 3). Since Wnt/β-catenin pathway and RAS regulate positively each other during hypertension, the decreased WNT3A expression may lead to inactivation of RAS which has a major role in the pathophysiology of hypertension. Otherwise, previous research indicated the inhibition of Wnt signaling could attenuate Wnt3a-induced central blood pressure regulation by downregulating GSK-3β pathway (35).
Limitations of the present study should also be considered. The first limitation is generalizability, while we examined only individuals with Chinese ancestry and results may not be generalizable to other ethnic groups. Additionally, WNT3A rs752107 was just predicted to disrupt a miRNA-binding site by web-based tools while lacking experimental validation. Further functional studies are needed to determine the exact implications of this polymorphism.
In summary, we took a pathway-based candidate gene analysis approach to verify the relationship between Wnt/β-catenin signaling and hypertension and related CVDs. And our data highlighted that WNT3A rs752107(C > T) was associated with incidence of EH and related CVDs for the first time. However, more representative and comprehensive studies in people of different cohorts and ethnic groups should be considered in the near future. In addition, it will need more in vitro and in vivo experimental validation to reveal the role of WNT3A rs752107 in the function of Wnt3a and Wnt/β-catenin signaling.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Xiangya Hospital, Central South University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
X-PC, H-HZ, W-HH, and WZ conceptualized and designed the study protocol. FO and LC collected clinical data and blood samples. HR and J-QL analyzed the data and prepared the manuscript. All authors helped in reviewing the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81573511, 81874329, 81522048, 81703623), Science and technology innovation project of Hunan province (2018SK2129), Wu Jieping Medical Foundation (320.6750.2020-04-14), Central South University Innovation Foundation for Postgraduate (2019zzts346) and the Scientific Foundation of Hunan (No. 2018JJ3719).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to express our gratitude to all the participants in this project.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.675222/full#supplementary-material
References
1. Schunkert H. Genetics of CVD in 2017: expanding the spectrum of CVD genetics. Nat Rev Cardiol. (2018) 15:77–8. doi: 10.1038/nrcardio.2017.209
2. Arking DE, Chakravarti A. Understanding cardiovascular disease through the lens of genome-wide association studies. Trends Genet. (2009) 25:387–94. doi: 10.1016/j.tig.2009.07.007
3. Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet. (2007) 370:591–603. doi: 10.1016/S0140-6736(07)61299-9
4. Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. (2020) 75:285–92. doi: 10.1161/HYPERTENSIONAHA.119.14240
5. Padmanabhan S, Aman A, Dominiczak AF. Genomics of hypertension. Pharmacol Res. (2017) 121:219–29. doi: 10.1016/j.phrs.2017.04.031
6. Rosenbluh J, Wang X, Hahn WC. Genomic insights into WNT/beta-catenin signaling. Trends Pharmacol Sci. (2014) 35:103–9. doi: 10.1016/j.tips.2013.11.007
7. Nusse R, Clevers H. Wnt/beta-Catenin signaling, disease, and emerging therapeutic modalities. Cell. (2017) 169:985–99. doi: 10.1016/j.cell.2017.05.016
8. Rao TP, Kuhl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res. (2010) 106:1798–806. doi: 10.1161/CIRCRESAHA.110.219840
9. Dejana E. The role of wnt signaling in physiological and pathological angiogenesis. Circ Res. (2010) 107:943–52. doi: 10.1161/CIRCRESAHA.110.223750
10. Marinou K, Christodoulides C, Antoniades C, Koutsilieris M. Wnt signaling in cardiovascular physiology. Trends Endocrinol Metab. (2012) 23:628–36. doi: 10.1016/j.tem.2012.06.001
11. Zhou Y, Wang Y, Tischfield M, Williams J, Smallwood PM, Rattner A, et al. Canonical WNT signaling components in vascular development and barrier formation. J Clin Invest. (2014) 124:3825–46. doi: 10.1172/JCI76431
12. Foulquier S, Daskalopoulos EP, Lluri G, Hermans KCM, Deb A, Blankesteijn WM. WNT signaling in cardiac and vascular disease. Pharmacol Rev. (2018) 70:68–141. doi: 10.1124/pr.117.013896
13. Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. (2007) 315:1278–82. doi: 10.1126/science.1136370
14. Sarzani R, Salvi F, Bordicchia M, Guerra F, Battistoni I, Pagliariccio G, et al. Carotid artery atherosclerosis in hypertensive patients with a functional LDL receptor-related protein 6 gene variant. Nutr Metab Cardiovasc Dis. (2011) 21:150–6. doi: 10.1016/j.numecd.2009.08.004
15. Hernando B, Pena-Chilet M, Ibarrola-Villava M, Martin-Gonzalez M, Gomez-Fernandez C, Ribas G, et al. Genetic 3'UTR variation is associated with human pigmentation characteristics and sensitivity to sunlight. Exp Dermatol. (2017) 26:896–903. doi: 10.1111/exd.13333
16. Schaefer KN, Peifer M. Wnt/Beta-Catenin signaling regulation and a role for biomolecular condensates. Dev Cell. (2019) 48:429–44. doi: 10.1016/j.devcel.2019.01.025
17. Kim G, Kurnit KC, Djordjevic B, Singh C, Munsell MF, Wang WL, et al. Nuclear beta-catenin localization and mutation of the CTNNB1 gene: a context-dependent association. Mod Pathol. (2018) 31:1553–9. doi: 10.1038/s41379-018-0080-0
18. Baetta R, Banfi C. Dkk (Dickkopf) proteins. Arterioscler Thromb Vasc Biol. (2019) 39:1330–42. doi: 10.1161/ATVBAHA.119.312612
19. Wang S, Krinks M, Lin K, Luyten FP, Moos M. Jr. Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell. (1997) 88:757–66. doi: 10.1016/S0092-8674(00)81922-4
20. Finch PW, He X, Kelley MJ, Uren A, Schaudies RP, Popescu NC, et al. Purification and molecular cloning of a secreted, Frizzled-related antagonist of Wnt action. Proc Natl Acad Sci USA. (1997) 94:6770–5. doi: 10.1073/pnas.94.13.6770
21. Luo JQ, He FZ, Wang ZM, Sun NL, Wang LY, Tang GF, et al. SLCO1B1 variants and angiotensin converting enzyme inhibitor (Enalapril)-induced cough: a pharmacogenetic study. Sci Rep. (2015) 5:17253. doi: 10.1038/srep17253
22. Luo JQ, He FZ, Luo ZY, Wen JG, Wang LY, Sun NL, et al. Rs495828 polymorphism of the ABO gene is a predictor of enalapril-induced cough in Chinese patients with essential hypertension. Pharmacogenet Genom. (2014) 24:306–13. doi: 10.1097/FPC.0000000000000050
23. Luo JQ, Wang LY, He FZ, Sun NL, Tang GF, Wen JG, et al. Effect of NR3C2 genetic polymorphisms on the blood pressure response to enalapril treatment. Pharmacogenomics. (2014) 15:201–8. doi: 10.2217/pgs.13.173
24. Vallee A, Levy BL, Blacher J. Interplay between the renin-angiotensin system, the canonical WNT/beta-catenin pathway and PPARgamma in hypertension. Curr Hypertens Rep. (2018) 20:62. doi: 10.1007/s11906-018-0860-4
25. Xiao L, Xu B, Zhou L, Tan RJ, Zhou D, Fu H, et al. Wnt/beta-catenin regulates blood pressure and kidney injury in rats. Biochim Biophys Acta Mol Basis Dis. (2019) 1865:1313–22. doi: 10.1016/j.bbadis.2019.01.027
26. Zhou L, Li Y, Hao S, Zhou D, Tan RJ, Nie J, et al. Multiple genes of the renin-angiotensin system are novel targets of Wnt/beta-catenin signaling. J Am Soc Nephrol. (2015) 26:107–20. doi: 10.1681/ASN.2014010085
27. Bergmann MW. WNT signaling in adult cardiac hypertrophy and remodeling: lessons learned from cardiac development. Circ Res. (2010) 107:1198–208. doi: 10.1161/CIRCRESAHA.110.223768
28. Hou N, Ye B, Li X, Margulies KB, Xu H, Wang X, et al. Transcription factor 7-like 2 mediates canonical Wnt/beta-Catenin signaling and c-Myc upregulation in heart failure. Circ Heart Fail. (2016) 9:e003010. doi: 10.1161/CIRCHEARTFAILURE.116.003010
29. Zhao Y, Wang C, Hong X, Miao J, Liao Y, Hou FF, et al. Wnt/beta-catenin signaling mediates both heart and kidney injury in type 2 cardiorenal syndrome. Kidney Int. (2019) 95:815–29. doi: 10.1016/j.kint.2018.11.021
30. Malekar P, Hagenmueller M, Anyanwu A, Buss S, Streit MR, Weiss CS, et al. Wnt signaling is critical for maladaptive cardiac hypertrophy and accelerates myocardial remodeling. Hypertension. (2010) 55:939–45. doi: 10.1161/HYPERTENSIONAHA.109.141127
31. Hirotani S, Zhai P, Tomita H, Galeotti J, Marquez JP, Gao S, et al. Inhibition of glycogen synthase kinase 3beta during heart failure is protective. Circ Res. (2007) 101:1164–74. doi: 10.1161/CIRCRESAHA.107.160614
32. Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. (2018) 50:524–37. doi: 10.1038/s41588-018-0058-3
33. Yao T, Yang L, Li PQ, Wu H, Xie HB, Shen X, et al. Association of Wnt3A gene variants with non-syndromic cleft lip with or without cleft palate in Chinese population. Arch Oral Biol. (2011) 56:73–8. doi: 10.1016/j.archoralbio.2010.09.002
34. Velazquez-Cruz R, Garcia-Ortiz H, Castillejos-Lopez M, Quiterio M, Valdes-Flores M, Orozco L, et al. WNT3A gene polymorphisms are associated with bone mineral density variation in postmenopausal mestizo women of an urban Mexican population: findings of a pathway-based high-density single nucleotide screening. Age. (2014) 36:9635. doi: 10.1007/s11357-014-9635-2
Keywords: essential hypertension, heart failure, ischemic stroke, Wnt3a, genetic polymorphisms
Citation: Ren H, Luo J-Q, Ouyang F, Cheng L, Chen X-P, Zhou H-H, Huang W-H and Zhang W (2021) WNT3A rs752107(C > T) Polymorphism Is Associated With an Increased Risk of Essential Hypertension and Related Cardiovascular Diseases. Front. Cardiovasc. Med. 8:675222. doi: 10.3389/fcvm.2021.675222
Received: 04 March 2021; Accepted: 31 May 2021;
Published: 12 July 2021.
Edited by:
Georges Nemer, Hamad bin Khalifa University, QatarReviewed by:
Lazaros Lataniotis, University of California, San Francisco, United StatesRaj Sewduth, VIB KU Leuven Center for Cancer Biology, Belgium
Copyright © 2021 Ren, Luo, Ouyang, Cheng, Chen, Zhou, Huang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zhang, Y3N1emhhbmd3ZWlAY3N1LmVkdS5jbg==
 Huan Ren1,2,3,4
Huan Ren1,2,3,4 Xiao-Ping Chen
Xiao-Ping Chen Wei Zhang
Wei Zhang