- 1Rheumatology Department, Hospital Dos de Maig, Barcelona, Spain
- 2Rheumatology and Autoimmune Diseases Department, Hospital Universitari de la Santa Creu i Sant Pau, Barcelona, Spain
- 3Department of Medicine, Universitat Autònoma de Barcelona (UAB), Barcelona, Spain
- 4Cardiology Department, Hospital Universitari Bellvitge, Hospitalet de Llobregat, Spain
- 5Department of Medicine, Universitat de Barcelona (UB), Barcelona, Spain
- 6Research Unit, Spanish Society of Rheumatology, Madrid, Spain
Objective: Evaluate the evidence on the abnormalities of the aortic root and heart valves, risk and prognostic factors for heart valve disease and valve replacement surgery in spondyloarthritis.
Methods: A systematic literature review was performed using Medline, EMBASE and Cochrane databases until July 2021. Prevalence, incidence, risk and prognostic factors for heart valve disease; dimension, morphology, and pathological abnormalities of the valves were analyzed. Patient characteristics (younger age, history of cardiac disease or longer disease duration) and period of realization were considered for the analysis. The SIGN Approach was used for rating the quality of the evidence of the studies.
Results: In total, 37 out of 555 studies were included. Overall, the level of evidence was low. The incidence of aortic insufficiency was 2.5–3.9‰. Hazard Ratio for aortic insufficiency was 1.8–2.0. Relative risk for aortic valve replacement surgery in ankylosing spondylitis patients was 1.22–1.46. Odds ratio for aortic insufficiency was 1.07 for age and 1.05 for disease duration. Mitral valve abnormalities described were mitral valve prolapse, calcification, and thickening. Aortic valve abnormalities described were calcification, thickening and an echocardiographic “subaortic bump.” Abnormalities of the aorta described were thickening of the wall and aortic root dilatation. The most common microscopic findings were scarring of the adventitia, lymphocytic infiltration, and intimal proliferation.
Conclusions: A higher prevalence and risk of aortic valve disease is observed in patients with ankylosing spondylitis. Studies were heterogeneous and analysis was not adjusted by potential confounders. Most studies did not define accurate outcomes and may have detected small effects as being statistically significant.
Introduction
Spondyloarthropathies are a complex group of rheumatic diseases characterized by inflammation, erosion and new bone formations at peripheral and axial sites. The primary site of inflammation in spondyloarthropathies is thought to be the enthesis.
Another distinctive trait of spondyloarthritis is its overlap with other autoimmune organ diseases such as psoriasis, uveitis and inflammatory bowel disease. Textbooks and classical review articles also consider the heart as another organ frequently involved in spondyloarthritis and associated diseases (1, 2). Cardiac conduction disorders and valvular heart diseases are described as a specific cardiac manifestation of spondyloarthritis.
These statements are based on studies by Bergfeldt and other contemporary investigators. Bergfeldt was not the first investigator to describe cardiac manifestations in spondyloarthritis, but his work originated the concept of “HLA-B27 associated cardiac disease”. These studies were cross-sectional or case series describing HLA-B27 positivity or sacroiliitis in a group of patients with atrioventricular blocks, pacemakers and/or aortic regurgitations. Other frequently cited studies (3–8) were non adjusted case-control studies involving small sample sizes that compared the proportion of patients with valve disease in spondyloarthritis with control subjects. While the results may be interesting, the design and methods of the studies preclude the drawing of any associations between spondyloarthritis with atrioventricular blocks or aortic regurgitations. Furthermore, several recent studies have found no significant association between spondyloarthritis and heart valve diseases (9, 10). Therefore, we find that the evidence of heart valve disease in spondyloarthritis should be systematically reappraised.
Cardiac complications have been a research topic of growing interest over the last two decades. The frequent motto of research into the cardiac complications associated with rheumatic diseases is that chronic systemic inflammation may produce fibrosis in the myocardium and related structures (11). As publication in cardiac complications increased, a wide spectrum of cardiac complications has been described for systemic autoimmune diseases including heart valve diseases and conduction disorders (12, 13). Concurrently, cardiac complications spectrum in spondyloarthritis has also widened to ventricular dysfunction, myocarditis, pericarditis, heart failure or ischaemic heart disease (14). But are some cardiac complications more relevant in different rheumatic diseases?
Just as Bergfeldt and other authors marked a milestone in the investigation of spondyloarthritis, we similarly suspect that there may be a distinctive association between spondyloarthritis with heart valve disease. The aortic valve attachment site and peripheral entheses are known to have histological similarities (1). Two experimental studies observed that enthesis tissue resident T cells were also present in the aortic root and valve (15, 16) while being absent from the myocardium (15). Overexpression of interleukin-23 in vivo resulted in a dense infiltrate of T cells, macrophages and neutrophils in the attachment site of the aortic valve leading to the aortic wall as well as in the enthesis (15, 16). It can be hypothesized that inflammation of the valve attachment site may produce tissue degeneration that leads to aortic valve insufficiency.
This is the first systematic review of the literature to evaluate heart valve disease and valve replacement surgery in spondyloarthritis. The aim of this systematic review of the literature is to evaluate the existing evidence on the abnormalities of the aortic root and heart valves, as well as the risks and prognostic factors for heart valve disease and valve replacement surgery in spondyloarthritis. The objective of this study is to reappraise the presumption that heart valve disease is distinctive in patients with spondyloarthritis.
Materials and Methods
A systematic review was conducted to identify all studies published up to December, 2020. This review was guided by the preferred reporting items for systematic review and meta-analysis (PRISMA) statement (Supplementary Material 1) (17).
Search Strategy
A systematic search strategy was performed using the databases of Pubmed (Medline), EMBASE (Elsevier), and the Cochrane Library (Wiley) by a librarian (MG). The search strategy included MeSH terms and free text using different combinations (Supplementary Material 2). The search was limited to studies published until July 2021 in English, French, Korean, and Spanish. Additional references were manually retrieved by reviewing the references of the included studies. An update of the systematic research was performed before the submission of the manuscript.
Inclusion Criteria
Studies were included if they had the characteristics described below:
Population: Patients with a diagnosis of SpA and fulfilling classification criteria of ASAS, ESSG, Amor, modified New York, ARA, Moll and Wright, or CASPAR criteria. Als, patients older than 18 years presenting grade II bilateral sacroiliitis or grade III or superior if unilateral sacroiliitis by X-Ray; and/or HLAB27 positivity; that also fulfilled two or more clinical characteristics (peripheral arthritis, enthesitis, dactylitis, uveitis, inflammatory back pain, psoriasis, inflammatory bowel disease, history of urethritis, or infective diarrhea) were included.
Intervention: valve insufficiency, regurgitation, dilatation, valvular bump, stenosis, annuloplasty, or valve replacement confirmed by cardiac imaging [transthoracic echocardiogram (TTE), transesophageal echocardiogram (TOE), or cardiac magnetic resonance (CMR)], post-mortem and/or surgical pathology or ICD codes.
Outcomes: Frequency measured by prevalence, prevalence ratio, incidence proportion and rate; Risk and prognostic factors measured by hazard ratio (HR), odds ratio (OR), risk ratio (RR), and risk difference (RD).
Study design: Meta-analysis, systematic review of the literature, case-control, cohort, cross sectional studies, and case series with more than five patients.
Exclusion Criteria
Studies carried out in patients younger than 18 years, adolescents and pregnant women; studies not suited to the PICO framework in terms of the patient sample, intervention, comparison group(s), outcome(s) or study design; studies carried out in animals or populations based in ethnic minorities; and abstracts, posters, narrative reviews, letters, editorials, and any type of unpublished study.
Article Selection
A total of 555 citations were peer reviewed by two rheumatologists (H.S.P and A.L) under the assessment of a senior methodologist (P.D and M.A.M). The reviewers independently performed two-stage screening (title/abstract and full-text screening), data extraction and risk of bias assessment. Some studies were not available mainly because of antiquity (Supplementary Material 3). They were previously consulted in two different national university library sources and the documentary collection of the Spanish Society of Rheumatology. The original authors were also contacted if possible. EndNote X8 software was used to manage the literature references.
Data Extraction and Data Analysis
For data extraction, standardized forms from the Critical Appraisal Tools 3.0. Platform (http://www.lecturacritica.com/en/) was used. Risk of bias and quality of the evidence was rated according to the Scottish Intercollegiate Guidelines Network Approach (SIGN; http://www.sign.ac.uk/). Due to the small scale of the majority of the studies and heterogeneity in the design, a qualitative synthesis was carried out rather than a meta-analysis.
Some aspects were considered as critical to integrate the information. Specific characteristics of the population, such as younger age, history of cardiac disease or disease duration were contrasted for the results. A cardiologist specialized in cardiac imaging (J.S.V) was consulted for the interpretation of the data. Valve and aorta involvement was accepted only if confirmed by accurate techniques. Period of execution was also taken into account considering the evolution of imaging techniques throughout time.
Results
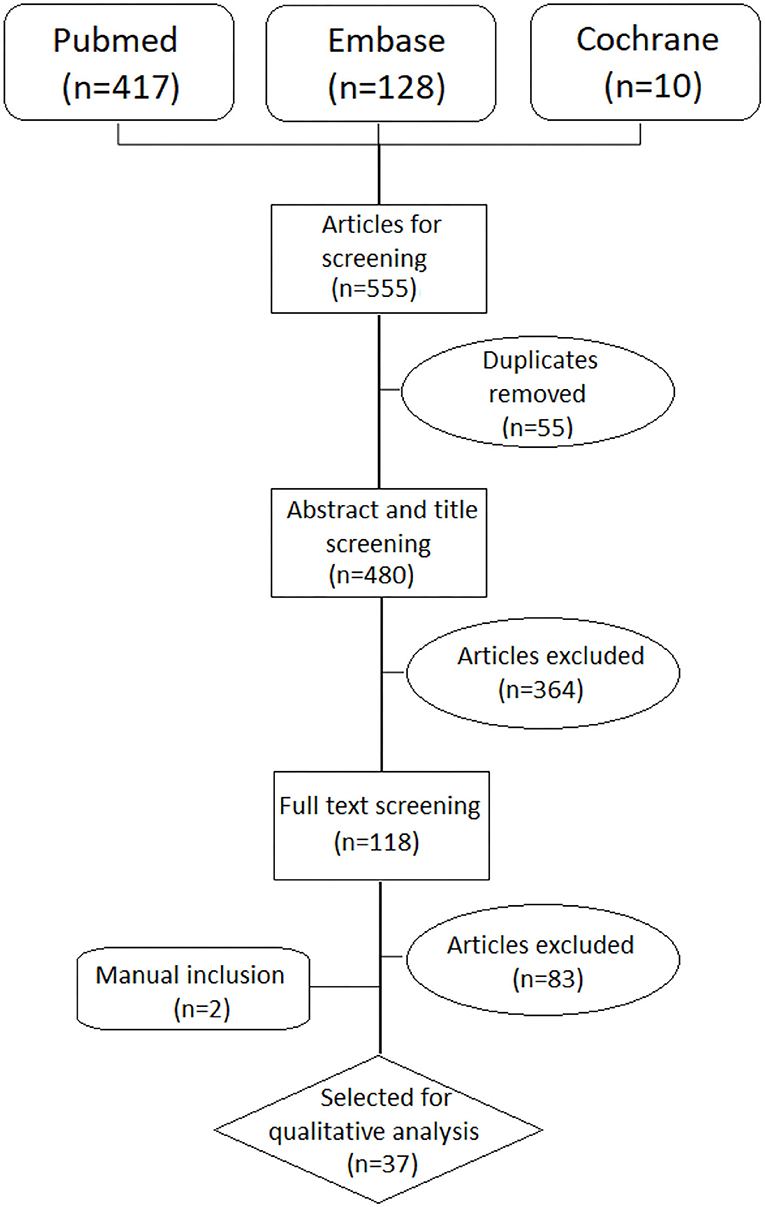
Of the original 555 studies, we selected 118 studies for further reading based on title and abstract screening. After excluding 83 studies following full text reading, we included 37 studies in the analysis, as presented in the PRISMA flow-chart (Figure 1).
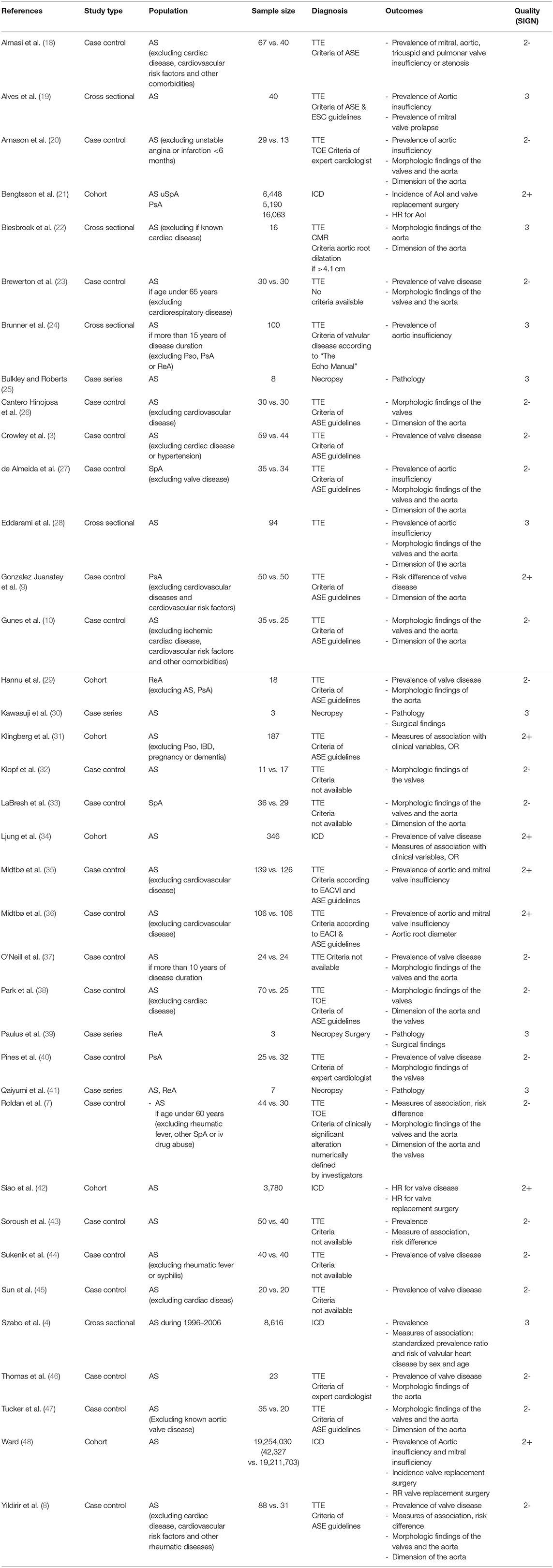
The reasons to exclude the remaining studies after reading the full text are provided in the Supplementary Material 4. Nineteen studies had a level of evidence 2- according to the SIGN grading system which implies high risk of confounding and non-causality. Seven studies were qualified as level 2+ with low risk for confounding and moderate probability for causality. Other eight studies were descriptive studies qualified as level 3. Details for each of the 35 studies are shown in Table 1.
Frequency, Risk, and Prognostic Factors for Heart Valve Disease
Prevalence
The prevalence of valve insufficiency and stenosis were described in patients with ankylosing spondylitis (AS), psoriatic arthritis (PsA), Reiter's Syndrome (ReS) and undifferentiated spondyloarthritis (uSpA). The majority of studies retrieved were small scaled with the exception of some nationwide health databases. Also, some studies were carried out in specific groups: patients without known cardiac disease, patients aged under 60, or with more than 10 years of disease duration. Period of execution and imaging technique were considered for the synthesis of the data.
In the general AS population the reported prevalence of aortic insufficiency was 3.3–18% (19, 23, 28, 31, 32, 43, 44, 46). The prevalence of aortic stenosis was 0–4% (31, 43), of mitral insufficiency was 2–74% (31, 43), of mitral stenosis was 0–2% (19, 32), of tricuspid insufficiency was 0–94% (31, 43), and of pulmonary insufficiency was 0–43% (31, 43). Only one study reported prevalence in relation to the degree of severity (31) which was 4.8% for moderate aortic insufficiency, 0.53% for severe aortic insufficiency, 1% for moderate mitral insufficiency, 3% for moderate tricuspid insufficiency, and 2% for moderate pulmonary insufficiency.
Three studies based on health insurance databases reported a prevalence of 1.1–3.9% which is lower than those based on small samples (4, 21, 34) probably due to the difference in the diagnostic method and severity of valve disease. One health insurance database reported the prevalence of aortic and mitral valve disease by age intervals but did not distinguish insufficiency from stenosis (48). The prevalence of aortic valve disease was 2.6% between 65 and 69 years, 6.7% between 70 and 74 years, 10.9% between 75 and 79 years and 17.1% in age 80 or older (48). The prevalence of mitral valve disease was 3.8% between 65 and 69 years, 9.8% between 70 and 74 years, 14.1% between 75 and 79 years and 19.2% in age 80 or older.
Four studies described the prevalence of valve disease in groups of young AS patients aged under 60 (4, 7, 18, 23, 28). They observed aortic insufficiency in 3.3–16%, mitral insufficiency in 0–32%, pulmonary insufficiency in 3% and tricuspid insufficiency in 2–77.6%. Aortic insufficiency was mild in 9.1%, moderate in 6.8–9% (7, 18). Mitral insufficiency was mild in 20.5% and moderate in 9–11.4% (7, 18). Mitral valve stenosis was observed in 1.5% (18).
Eight studies described the prevalence of valve disease in AS patients without known cardiac disease or symptoms (3, 8, 10, 20, 35, 36, 38, 45). They observed aortic insufficiency in 0–34.5% and mitral insufficiency in 0–49%. Moderate aortic insufficiency was observed in 2.3–10.3%.
In two studies based on AS patient groups with more than 10 years of disease duration, aortic insufficiency was observed in 8.3–10% (24, 37) of which moderate in 3% (24). Mitral insufficiency was observed in 29% of which 2% was moderate and 1% severe (24).
As for PsA the reported prevalence was wide ranged. A study based on a nationwide population registry detected 0.4% prevalence of aortic insufficiency. In a study of small samples the prevalence of mild aortic insufficiency was 10%, mild mitral insufficiency was 16% and mild tricuspid insufficiency was 10% (9). In another study no valve disease was observed at all (40).
In a small study of ReA population mild aortic insufficiency was seen in 5.6% and mild mitral insufficiency in 5.6% (29).
One study carried out in a mixed population of spondyloarthritis (AS, PsA, ReA) reported a prevalence of aortic insufficiency of 11.4%, which was mild in 8.5% and moderate in 2.9%. Mild mitral insufficiency was reported in 8.6% (27).
Standardized prevalence ratio for aortic valvular heart disease was 1.59 (95% IC 1.31–1.91) and for non-aortic valvular heart disease was 1.58 (95% IC 1.43–1.74%) (4).
Prevalence difference when compared to control groups showed contrasting results. Two studies carried out in an AS group younger than 60 years without known cardiac disease showed significantly higher prevalence for aortic insufficiency (7, 8) and mitral insufficiency (8) in AS groups.
However, six other studies based on PsA and AS groups without cardiac disease showed no significant difference in valve disease compared to control groups (9, 18, 35, 36, 38, 43).
Incidence
Two studies reported on the incidence of aortic insufficiency for AS, PsA and uSpA based on a nationwide population registry (21, 48). The incidence proportion of aortic insufficiency was 3.8‰ for AS, 2.5‰ for undifferentiated SpA and 3.9‰ for PsA (21). The incidence rate (1,000 person-years at risk) of aortic insufficiency was 0.7 (0.4–0.9) for AS, 0.5 (0.2–0.7) for uSpA, and 0.7 (0.5–0.8) for PsA (21).
Risk of Heart Valve Disease in Patients With SpA
When compared to a control population without AS, age and sex adjusted HR for aortic insufficiency was significantly increased in AS (HR 1.9, 95% IC 1.3–2.9), uSpA (HR 2.0, 95% IC 1.2–3.5), and PsA (HR 1.8, IC 1.4–2.4) (21). Similar results was observed in a nationwide population registry in patients with AS with a HR of 1.67 (95% CI 1.38–2.02, p < 0.001) when adjusted by confounders (28).
Risk or Prognostic Factors of SpA for Heart Valve Disease Manifestation
Three studies presented data about association of clinical SpA features with valve disease presentation (7, 31, 34). Age and disease duration showed association in all three studies. The OR for the duration of symptoms was 1.05 (31, 34) and the OR for age was 1.07 (34). Also male sex (OR 2.57), NSAID treatment (OR 0.37) (34), mSASSS (OR 1.02), history of anterior uveitis (OR 2.72) showed statistically significant association (19). No statistically important association was found for: disease activity, severity, diabetes mellitus or smoking habits (31).
Frequency and Risk for Valve Surgery
Prevalence and Incidence for Valve Surgery
The prevalence of valve replacement surgery in AS was reported as 0.6–2.5% (21, 44). The prevalence of aortic valve surgery in PsA by a nationwide population registry was 0.4% (21).
The incidence of aortic valve replacement or repairing in AS patients was 1.25 per 1,000 person-years in age between 65 and 69, 1.83 per 1,000 person-years in age between 70 and 74, 2.61 per 1,000 person-years in age between 75 and 79 and 2.79 per 1,000 person-year in age 80 or older (48). The incidence of mitral valve surgery was 0.54 per 1,000 person-years in age between 65 and 69, 0.73 per 1,000 person-years in age between 70 and 74, 0.72 per 1,000 person-years in age between 75 and 79 and 0.55 per 1,000 person-year in age 80 or older (48). The incidence increased substantially over time in patients with AS but this increment also occurred in the controls so the risk remained constant over time.
Risk for Valve Surgery
RR of aortic valve repairing or replacement surgery in AS patients compared to the control population was significantly increased at all ages (48). In a nationwide population registry the HR for valve replacement surgery after developing valvular heart disease was higher in AS patients compared to those without AS with a HR of 5.09 (95% CI 1.55–16.68, p = 0.007) (28).
At ages between age 65–69 RR was 1.34 (95% CI 1.10–1.63), between age 70–74 was 1.22 (95% CI 1.05–1.43), between 75 and 59 was 1.25 (95% CI 1.10–1.43) and 1.46 (95% CI 1.32–1.63) in age 80 or older (48). RR of mitral valve surgery in AS patients compared to the control population was not statistically significant in any age group (48). None of the studies retrieved described SpA disease associated risk or prognostic factors for valve repairment surgery.
Morphology and Pathology of the Aortic and Mitral Valves
Morphology
A total of 12 studies reported morphologic abnormalities by cardiac imaging in mitral and aortic valves. Type of imaging technique and period of realization were considered to synthesize the information.
Mitral valve abnormalities described were mitral valve prolapse (8, 19, 32, 37, 40), mitral valve calcification (23, 27) and thickening of the mitral valve (7, 8, 27, 28). Mitral valve prolapse had a prevalence between 4.17 and 56%. In studies published in the 80's the prevalence ranged from 10 to 36.4% in AS (19, 32) and 56% in PsA (40). The other two studies published after the 90;s showed much lower prevalence of 4.17–5.7% but were carried out in AS without known cardiac disease (8, 37). When compared to a control group the differences in prevalence were irrelevant (8, 37).
Mitral valve calcification was observed in two studies based on AS and SpA population without known valve disease with a prevalence of 6.67–8.57%.
Thickening of the mitral valve was observed in two studies carried out on patients without known valve disease with a prevalence of 2.22–22.86% (8, 27, 28) and on patients younger than 60 years (7) with a prevalence of 34%. The latter study was carried out by TOE which is the most suitable imaging modality for mitral valve anatomy. Mitral valve thickening was more prevalent in AS patients when compared to a control group (7). The mean mitral valve thickening was 0.12–0.36 cm (7, 38).
Aortic valve abnormalities described were calcification, thickening and an echocardiographic “subaortic bump.” It is a distinctive finding most described in spondyloarthritis that consists in a thickening of the aortomitral junction from the aortic cusps to the anterior mitral leaflet.
Calcification of the aortic valve was seen in 8.57% of SpA patients without known cardiac disease (27).
Thickening of the aortic valve in the AS and SpA population without known cardiac disease was 10–20% by TTE (27, 33) and 41–77% by TOE (7, 38). Two studies showed higher prevalence of thickening in AS patients when compared to control groups (7, 38). The mean aortic valve thickness was 0.18–0.32 cm (7, 38).
Subaortic bump was studied by five studies. The range of prevalence was broad. Two studies reported a prevalence of 25–77% when measured with TOE (7, 20). Other three studies described a lower prevalence of 4–31% measured by TTE (26, 33, 47). When compared to a control group, subaortic bump was more prevalent in AS patients (7, 20).
Pathology
Macroscopic findings were thickening of aortic valve cusps (25, 30, 39, 41). The most common microscopic findings were scarring of the adventitia, lymphocytic infiltration and intimal proliferation of valve cusps (25, 30, 39).
Morphology, Dimension, and Pathology of the Aorta
Morphology
Morphologic abnormalities of the aorta observed were thickening of the aortic wall and aortic root dilatation. The thickening of the aortic wall was evaluated in two studies (7, 37). The thickening of the posterior aortic wall had a prevalence of 4.17% by TTE (37) and 61.4% by TOE (7), but only the latter provided the definition of thickening as >2.2 mm. Aortic root dilatation prevalence was between 1.11 and 26.1% by TTE, 25% by TOE and 0% by CMR (7, 8, 10, 22, 23, 28, 29, 46).
Some reported lower prevalence between 2.99 and 4.5% (8, 10, 23, 29) and others reported higher prevalence between 12.5 and 26.1% (7, 22, 46). This wideness of range in prevalence was not explained by differences in population, year of publication, cardiac imaging test or criteria used to diagnose dilatation. Disease duration showed correlation with aortic root dilatation (8) but disease severity and spine deformity showed no significant difference of aortic root dilatation (46).
Dimension
Fourteen studies reported measurement data on aortic root diameter, ascending aorta diameter and aortic wall thickness.
Mean aortic root diameter in AS patients was 3.2–3.4 cm (8, 20, 36) and in PsA patients was 3 cm (9). Other studies described dimension at different sites of the aortic root: at the annulus 1.6–3.1 cm (7, 26, 33, 38, 47), at the sinotubular junction 2.4–3.3 (7, 12) and at the sinuses of Valsalva 3.4–3.7 cm (8, 26, 31). Some measured in mid cusp and above the cusp without specifying the exact place of measurement even though most followed American Society of Echocardiography guidelines. In mid cusp the diameter was 1.9–3.3 cm (26, 33, 47) and above the cusp 2.0–3.4 cm (26, 33, 47).
Case control studies also reported diameter in control groups (7, 9, 20, 26, 33, 36, 38, 47). Only two studies concluded that the difference was statistically significant with a mean difference of 0.2 cm (7, 8). The diameter was similar for AS patients regarding positivity for HLA-B27, presence of inflammatory activity or severe sacroiliitis (26). One study found a trend for increasing age and larger aortic root dimension but no significant relation for the duration of disease symptoms (47).
Ascending aorta diameters were reported in 5 studies. Mean diameters ranged from 2.4 to 4.7cm (10, 22, 27, 28, 38) in AS patients. Similar diameters were reported in ReA 2.7 cm and in PsA 2.9 cm (27).
Aortic wall thickness at the anterior wall ranged from 0.24 to 0.25 cm (7, 20) and at the posterior wall from 0.27 to 0.38 cm (7, 20).
Pathology
Macroscopic findings of the aorta were dilatation of the aortic root and ascending aorta (25, 30, 39, 41). Most common microscopic findings were scarring of the adventitia, lymphocytic infiltration and intimal proliferation (25, 30).
Discussion
Prevalence and incidence of valve heart disease had a wide range of variability due to the small number of samples, heterogeneity in sample and outcome definitions. Some studies found a higher prevalence of valve disease when compared to control populations with low grade of evidence (7, 8) while others did not (9, 18, 35, 36, 38, 43). Key aspects in valve degeneration are age, mechanism of valve disease and grade of severity but very few studies provided these information (49, 50).
Non-adjusted studies based on nationwide health databases showed higher HR for aortic insufficiency and slightly higher RR for valve replacement surgery compared to the general population (7, 8, 21, 48). According to some studies, age and disease duration showed association with valve disease with weak evidence for causality (7, 31, 34). These may be potential confounders but this possibility was not analyzed.
Morphologic abnormalities of the mitral and aortic valves were: calcification, thickening, subaortic bump and mitral valve prolapse (7, 20, 26, 33, 47). Subaortic bump, mitral and aortic valve thickening were more frequent in SpA patients when compared to control groups (7, 33, 38). Morphologic abnormalities of the aorta were aortic root dilatation and thickening of the posterior aortic wall. The prevalence of these findings was wide ranging. No difference was observed when sorted by population, year of publication or type of cardiac imaging used.
The diameter of the aortic root and ascending aorta were similar to control groups (7, 9, 20, 26, 33, 36, 38, 47). No significant difference was found when comparing mean aortic root diameter regarding different AS disease characteristics.
Several critical points are raised for the interpretation of the results. Very few studies were adjusted by potential confounders. Nationwide databases adjusted by sex and age but lacked clinical information to analyze potential confounders. Smaller studies lacked power or did not use adjusted models for design. Results may be spurious or may not have detected association. Another important point to consider is the absence of clinically relevant outcome definition of valve disease and abnormality. The grade of severity, mechanism of valve disease and relevant abnormalities considering age-related degeneration are very important information that most of the studies omitted. Some studies found statistically significant differences in clinically irrelevant results. Some other potentially interesting results are difficult to interpret due to imprecise description of the results. The evidence is weak due to the low quality of the majority of the studies.
This is the first systematic review of the literature regarding heart valve disease and aortic abnormalities in spondyloarthritis. A cardiac imaging specialist was consulted for strict interpretation of clinically relevant information. Some limitations are to be considered. Meta-analysis was not possible due to the heterogeneity in the design and clinical measures employed. The incomplete retrieval of the bibliography may affect some of the results but the majority of the missing studies were old and probably of lower evidence quality.
Conclusions
The conclusion of our study is that further studies with larger sample sizes, multivariate analysis, and a better definition of outcomes are needed. Data to assess valve and aortic diseases in spondyloarthritis is weak and may be spurious because of possible confounders.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
MG-R performed the systematic literature search. HSP and AL were involved in data screening, extraction, analysis, and drafted the manuscript. PD, HC, and MM-M contributed substantially to the study conceptions, design, and critical revision of the article. JS-V was consulted for interpretation of the extracted data as a referent cardiac imaging expert. All authors read and approved the final manuscript.
Conflict of Interest
AL has received speaker fees/honoraria from Abbvie, Lilly, Novartis, Pfizer, and UCB. HC has received speaker fees/honoraria from BMS, Gebro, MSD, Lilly, Novartis, Pfizer, Roche, Sanofi, and UCB and has participated in consulting for Abbvie, Amgen, Biogen, Celgene, Gilead, Kern, Pfizer, and Sanofi.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank David Bridgewater and David Moore for the English editing of the article.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.719523/full#supplementary-material
Abbreviations
AS, Ankylosing Spondylitis; ReA, Reactive arthritis; PsA, Psoriatic Arthritis; uSpA, undifferentiated spondyloarthritis; SpA, spondyloarthritis; TTE, Transthoracic echocardiography; TOE, Transesophageal echocardiography; ASE, American Society of Echocardiography; EACVI, European Association of Cardiovascular Imaging.
References
1. Gensler LS. Axial spondyloarthritis: the heart of the matter. Clin Rheumatol. (2015) 34:995–8. doi: 10.1007/s10067-015-2959-1
3. Crowley JJ, Donnelly SM, Tobin M, FitzGerald O, Bresnihan B, Maurer BJ, et al. Doppler echocardiographic evidence of left ventricular diastolic dysfunction in ankylosing spondylitis. Am J Cardiol. (1993) 71:1337–40. doi: 10.1016/0002-9149(93)90551-M
4. Szabo SM, Levy AR, Rao SR, Kirbach SE, Lacaille D, Cifaldi M, et al. Increased risk of cardiovascular and cerebrovascular diseases in individuals with ankylosing spondylitis: a population-based study. Arthritis Rheum. (2011) 63:3294–304. doi: 10.1002/art.30581
5. Bergfeldt L. HLA-B27-associated cardiac disease. Ann Intern Med. (1997) 15:621–9. doi: 10.7326/0003-4819-127-8_Part_1-199710150-00007
6. Bergfeldt L, Insulander P, Lindblom D, Möller E, Edhag O. An important genetic risk factor for lone aortic regurgitation and severe conduction system abnormalities. Am J Med. (1988) 82:12–8. doi: 10.1016/0002-9343(88)90497-4
7. Roldan CA, Chavez J, Wiest PW, Qualls CR, Crawford MH. Aortic root disease and valve disease associated with ankylosing spondylitis. J Am Coll Cardiol. (1998) 32:1397–404. doi: 10.1016/S0735-1097(98)00393-3
8. Yildirir A, Aksoyek S, Calguneri M. Echocardiographic evidence of cardiac involvement in ankylosing spondylitis. Clin Rheumatol. (2002) 21:129–34. doi: 10.1007/s10067-002-8271-x
9. Gonzalez-Juanatey C, Amigo-Diaz E, Miranda-Filloy JA, Testa A, Revuelta J, Garcia-Porrua C, et al. Lack of echocardiographic and Doppler abnormalities in psoriatic arthritis patients without clinically evident cardiovascular disease or classic atherosclerosis risk factors. Semin Arthritis Rheum. (2006) 35:333–9. doi: 10.1016/j.semarthrit.2005.12.002
10. Gunes Y, Tuncer M, Guntekin U, Sahin M, Yazmalar L. Effects of ankylosing spondylitis on the heart. Acta Cardiol. (2009) 64:385–92. doi: 10.2143/AC.64.3.2038026
11. Nurmohamed MT, Heslinga M, Kitas GD. Cardiovascular comorbidity in rheumatic diseases. Nat Rev Rheumatol. (2015) 11:693–704. doi: 10.1038/nrrheum.2015.112
12. Miner JJ, Kim AH. Cardiac manifestations of systemic lupus erythematosus. Rheum Dis Clin North Am. (2014) 40:51–60. doi: 10.1016/j.rdc.2013.10.003
13. Corrao S, Messina S, Pistone G, Calvo L, Scaglione R, Licata G, et al. Heart involvement in rheumatoid arthritis: systematic review and meta-analysis. Int J Cardiol. (2013) 167:2031–8. doi: 10.1016/j.ijcard.2012.05.057
14. Liew JW, Ramiro S, Gensler LS. Cardiovascular morbidity and mortality in ankylosing spondylitis and psoriatic arthritis. Best Pract Res Clin Rheumatol. (2018) 32:369–89. doi: 10.1016/j.berh.2019.01.002
15. Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4–CD8– entheseal resident T cells. Nat Med. (2012) 18:1069–76. doi: 10.1038/nm.2817
16. Reinhardt A, Yevsa T, Worbs T, Lienenklaus S, Sandrock I, Oberdörfer L, et al. Interleukin-23–dependent γ/δ T cells produce interleukin-17 and accumulate in the enthesis, aortic valve, and ciliary body in mice. Arthritis Rheumatol. (2016) 68:2476–86. doi: 10.1002/art.39732
17. Liberati A, Altman DG, Tetzlaff J, Mulrow C, GÃtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
18. Almasi S, Farahani B, Samiei N, Rezaei Y, Mahmoodi H, Qorbani M, et al. Echocardiographic and electrocardiographic findings in patients with ankylosing spondylitis without cardiovascular risk factors. J Teh Uni H Center. (2020) 15:43–9. doi: 10.18502/jthc.v15i2.4182
19. Alves MG, Espirito-Santo J, Queiroz MV, Madeira H, Macieira-Coelho E. Cardiac alterations in ankylosing spondylitis. Angiology. (1988) 39:567–71. doi: 10.1177/000331978803900702
20. Arnason JA, Patel AK, Rahko PS, Sundstrom WR. Transthoracic and transesophageal echocardiographic evaluation of the aortic root and subvalvular structures in ankylosing spondylitis. J Rheumatol. (1996) 23:120–3.
21. Bengtsson K, Forsblad-d'Elia H, Lie E, Klingberg E, Dehlin M, Exarchou S, et al. Risk of cardiac rhythm disturbances and aortic regurgitation in different spondyloarthritis subtypes in comparison with general population: a register-based study from Sweden. Ann Rheum Dis. (2018) 77:541–8. doi: 10.1136/annrheumdis-2017-212189
22. Biesbroek PS, Heslinga SC, Konings TC, van der Horst-Bruinsma IE, Hofman M, van de Ven PM, et al. Insights into cardiac involvement in ankylosing spondylitis from cardiovascular magnetic resonance. Heart. (2017) 103:745–52. doi: 10.1136/heartjnl-2016-310667
23. Brewerton DA, Gibson DG, Goddard DH, Jones TJ, Moore RB, Pease CT, et al. The myocardium in ankylosing spondylitis. A clinical, echocardiographic, and histopathological study. Lancet. (1987) 1:995–8. doi: 10.1016/S0140-6736(87)92268-9
24. Brunner F, Kunz A, Weber U, Kissling R. Ankylosing spondylitis and heart abnormalities: do cardiac conduction disorders, valve regurgitation and diastolic dysfunction occur more often in male patients with diagnosed ankylosing spondylitis for over 15 years than in the normal population? Clin Rheumatol. (2006) 25:24–9. doi: 10.1007/s10067-005-1117-6
25. Bulkley BH, Roberts WC. Ankylosing spondylitis and aortic regurgitation. Description of the characteristic cardiovascular lesion from study of eight necropsy patients. Circulation. (1973) 48:1014–27. doi: 10.1161/01.CIR.48.5.1014
26. Cantero Hinojosa J, Salvatierra Ossorio J, Raya Alvarez E, Miras Parra F, Salvatierra Ríos D. An echocardiographic study of the aortic root in ankylosing spondylitis. An Med Interna. (1997) 14:565–8.
27. de Almeida FA, Albanesi Filho FM, de Albuquerque EM, Magalhaes EC, de Menezes ME. Echocardiography in the evaluation of cardiac involvement in seronegative spondylo-arthropathies. Medicina. (1995) 22:231–6.
28. Eddarami J, Azzouzi H, Ichchou L. Heart involvement in a moroccan population with spondyloarthritis: a cross-sectional study. J Saudi H Assoc. (2021) 3:191–6. doi: 10.37616/2212-5043.1258
29. Hannu T, Nieminen MS, Swan H, Leirisalo-Repo M. Cardiac findings of reactive arthritis: an observational echocardiographic study. Rheumatol Int. (2002) 21:169–72. doi: 10.1007/s00296-001-0154-y
30. Kawasuji M, Hetzer R, Oelert H, Stauch G, Borst HG. Aortic valve replacement and ascending aorta replacement in ankylosing spondylitis: report of three surgical cases and review of the literature. Thorac Cardiovasc Surg. (1982) 30:310–4. doi: 10.1055/s-2007-1022414
31. Klingberg E, Sveälv BG, Täng MS, Bech-Hanssen O, Forsblad-d'Elia H, Bergfeldt L. Aortic regurgitation is common in ankylosing spondylitis: time for routine echocardiography evaluation? Am J Med. (2015) 128:1244–50.e1. doi: 10.1016/j.amjmed.2015.04.032
32. Klopf D, Maisch B, Raab B, Auer I, Sprotte G, Kochsiek K. Cardiac involvement in chronic polyarthritis and ankylosing spondylitis - an echocardiographic and immunological study. Eur H J. (1987) 8:95–100. doi: 10.1093/eurheartj/8.suppl_J.95
33. LaBresh KA, Lally EV, Sharma SC, Ho G Jr. Two-dimensional echocardiographic detection of preclinical aortic root abnormalities in rheumatoid variant diseases. Am J Med. (1985) 78:908–12. doi: 10.1016/0002-9343(85)90211-6
34. Ljung L, Sundström B, Smeds J, Ketonen M, Forsblad-d'Elia H. Patterns of comorbidity and disease characteristics among patients with ankylosing spondylitis—a cross-sectional study. Clin Rheumatol. (2018) 37:647–53. doi: 10.1007/s10067-017-3894-0
35. Midtbø H, Gerdts E, Berg IJ, Rollefstad S, Jonsson R, Semb AG. Ankylosing spondylitis is associated with increased prevalence of left ventricular hypertrophy. J Rheumatol. (2018) 45:1249–55. doi: 10.3899/jrheum.171124
36. Midtbø H, Semb AG, Matre K, Rollefstad S, Berg IJ, Gerdts E. Left ventricular systolic myocardial function in ankylosing spondylitis. Arthr Care Res. (2019) 71:1276–83. doi: 10.1002/acr.23765
37. O'Neill TW, King G, Graham IM, Molony J, Bresnihan B. Echocardiographic abnormalities in ankylosing spondylitis. Ann Rheum Dis. (1992) 51:652–4. doi: 10.1136/ard.51.5.652
38. Park SH, Sohn IS, Joe BH, Hwang HJ, Park CB, Jin ES. Early cardiac valvular changes in ankylosing spondylitis: a transesophageal echocardiography study. J Cardiovasc Ultrasound. (2012) 20:30–6. doi: 10.4250/jcu.2012.20.1.30
39. Paulus HE, Pearson CM, Pitts W Jr. Aortic insufficiency in five patients with Reiter's syndrome. A detailed clinical and pathologic study. Am J Med. (1972) 53:464–72. doi: 10.1016/0002-9343(72)90142-8
40. Pines A, Ehrenfeld M, Fisman EZ, Kaplinsky N, Samra Y, Ronnen M, et al. Mitral valve prolapse in psoriatic arthritis. Arch Intern Med. (1986) 146:1371–3. doi: 10.1001/archinte.1986.00360190149021
41. Qaiyumi S, Hassan ZU, Toone E. Seronegative spondyloarthropathies in lone aortic insufficiency. Arch Intern Med. (1985) 145:822–4. doi: 10.1001/archinte.1985.00360050066010
42. Siao WZ, Liu CH, Wang YH, Wei JC, Jong GP. Increased risk of valvular heart disease in patients with ankylosing spondylitis: a nationwide population-based longitudinal cohort study. Ther Adv Musculoskel Dis. (2021) 13:1–10. doi: 10.1177/1759720X211021676
43. Soroush M, Mominzadeh M, Ghelich Y, Soroosh S, Pasha MA. Investigation of cardiac complications and their incidence in patients with ankylosing spondylitis. Med Arch. (2016) 70:35–8. doi: 10.5455/medarh.2016.70.35-38
44. Sukenik S, Pras A, Buskila D, Katz A, Snir Y, Horowitz J. Cardiovascular manifestations of ankylosing spondylitis. Clin Rheumatol. (1987) 6:588–92. doi: 10.1007/BF02330598
45. Sun JP, Khan MA, Farhat AZ, Bahler RC. Alterations in cardiac diastolic function in patients with ankylosing spondylitis. Int J Cardiol. (1992) 37:65–72. doi: 10.1016/0167-5273(92)90133-N
46. Thomas D, Hill W, Geddes R, Sheppard M, Arnold J, Fritzsche J, et al. Early detection of aortic dilatation in ankylosing spondylitis using echocardiography. Aust N Z J Med. (1982) 12:10–3. doi: 10.1111/j.1445-5994.1982.tb02416.x
47. Tucker CR, Fowles RE, Calin A, Popp RL. Aortitis in ankylosing spondylitis: early detection of aortic root abnormalities with two dimensional echocardiography. Am J Cardiol. (1982) 49:680–6. doi: 10.1016/0002-9149(82)91946-4
48. Ward MM. Lifetime risks of valvular heart disease and pacemaker use in patients with ankylosing spondylitis. J Am Heart Assoc. (2018) 7:e010016. doi: 10.1161/JAHA.118.010016
49. Iung B, Vahanian A. Epidemiology of valvular heart disease in adults. Nat Rev Cardiol. (2011) 8:162–72. doi: 10.1038/nrcardio.2010.202
Keywords: systematic (literature) review, HVD (heart valve disease), spondyloarthritis, echocardiography, cardiac image, epidemiology, psoriatic arthritis, valve (lesions, repair, replacement)
Citation: Park H-S, Laiz A, Sanchez-Vega J, Díaz del Campo P, Martín-Martínez MA, Guerra-Rodríguez M and Corominas H (2021) Valve Abnormalities, Risk Factors for Heart Valve Disease and Valve Replacement Surgery in Spondyloarthritis. A Systematic Review of the Literature. Front. Cardiovasc. Med. 8:719523. doi: 10.3389/fcvm.2021.719523
Received: 02 June 2021; Accepted: 17 August 2021;
Published: 24 September 2021.
Edited by:
Natalia Lopez-Andres, NavarraBiomed, SpainReviewed by:
Jiancheng Han, Capital Medical University, ChinaDelia Reina, Hospital of Sant Joan Despí Moisès Broggi, Spain
Copyright © 2021 Park, Laiz, Sanchez-Vega, Díaz del Campo, Martín-Martínez, Guerra-Rodríguez and Corominas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hector Corominas, aGNvcm9taW5hc0BzYW50cGF1LmNhdA==
 Hye-Sang Park
Hye-Sang Park Ana Laiz2,3
Ana Laiz2,3