- 1Department of General Medicine, Beijing Luhe Hospital, Capital Medical University, Beijing, China
- 2Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
- 3Department of Cardiology and Macrovascular Disease, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
Introduction: The real-world treatment of atrial fibrillation (AF) often involves the prescription of new oral anticoagulants (NOACs) using dosing both lower and higher than recommended guidelines. Our study aimed to evaluate the efficacy and safety of non-recommended dosage of NOACs in AF patients.
Methods: A systematic search was performed for relevant studies across multiple electronic databases (PubMed, Embase, Cochrane Library, Clinical Trials Registry) from inception to May 1, 2021. Multicenter randomized trials and observational studies were selected with key reporting measures for inclusion involved efficacy outcomes including stroke or systemic thromboembolism along with safety endpoints assessing major or clinically relevant bleeding events.
Results: A total of 11 eligible studies were included involving 48,648 patients receiving recommended dose of NOACs and 50,116 patients receiving non-recommended dosage. Compared to AF patients treated with recommended dose regimens, administration of low dose of NOACs was associated with higher risk of stroke/systemic embolism (RR = 1.24, 95% CI 1.14–1.35, P < 0.00001), but without reducing bleeding risk (RR = 1.18, 95% CI 0.91–1.53, P = 0.21) and a higher risk of all-cause mortality (RR = 1.58, 95% CI 1.25–1.99, P = 0.0001). Moreover, high dose of NOACs was associated with higher risk of stroke and systemic embolism efficacy (RR = 1.71, 95% CI 1.06–2.76, P = 0.03) and a non-significant trend to a greater risk of major or clinically relevant bleeding (RR = 1.57, 95% CI 0.96–2.58, P = 0.07).
Conclusions: AF patients treated with low dose of NOACs showed equivalent safety but with worse efficacy compared with recommended dose. High dose of NOACs was not superior to recommended dose regimens in preventing stroke/systemic embolism outcomes in AF patients.
Introduction
The past two decades have witnessed the gradual implementation of four new oral anticoagulants (dabigatran, rivaroxaban, apixaban, edoxaban) to prevent stroke in patients with non-valvular AF. These agents have shown greater efficacy and safety compared with vitamin K antagonists (VKAs) (1) and have been widely approved by regulatory bodies including the European Medicines Agency (EMA) (2), the U.S. Food and Drug Administration (FDA) (3), and the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) (4). Nevertheless, in real-world clinical practice, safety concerns among physicians have led to prescribing habits of lower dose of NOACs in patients with high HAS-BLED scores, along with considerations of age, body mass index (BMI), creatinine clearance (CrCl), hepatic function, as well as concomitant disease states (5). Alternatively, patients with significantly high CHA2DS2-VASc scores might be given higher dose of NOACs (6). These scenarios provide concerns that the efficacy and safety of NOACs may be compromised. At present, there are no universal rules for determining the dose regimens in high-risk patients, and no clear risk-benefit analysis was performed to help to adjust NOAC dose regimens. In this study, we performed a meta-analysis of multicenter randomized trials and observational studies to evaluate the efficacy and safety of non-recommended dose compared with recommended dose of NOACs in patients with AF.
Materials and Methods
This systematic review and meta-analysis were performed following the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions and Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement (PRISMA).
Search Strategy
Two investigators independently performed comprehensive searches for all relevant articles against the following databases: PubMed, Embase, Cochrane Library databases and Clinical Trials Registry (www.clinicaltrials.gov). English language articles were searched from inception to May 1, 2021. The search terms keywords used were as follows: atrial fibrillation; new oral anticoagulants, non-vitamin K antagonist, NOAC, oral thrombin inhibitors, factor Xa inhibitors, apixaban, rivaroxaban, edoxaban, dabigatran; non-recommended dose, off-label dose, low-dose, reduced-dose, underdosing, high dose, overdose; stroke; bleeding. Additionally, the reference lists of related review articles were also reviewed to source additional publications of relevance.
Inclusion and Exclusion Criteria
Manually selected studies with the assistance of EndNote software according to the prespecified PICOS criteria. Studies that met the following criteria were selected for inclusion involved: (1) P: patients with atrial fibrillation; (2) Intervention: treated with non-recommended dose (low or high dose) of new oral anticoagulants (NOACs: dabigatran, rivaroxaban, apixaban, edoxaban); (3) Comparison: with recommended dose of NOACs; (4) Outcomes: reported at least one of the following adverse outcomes: stroke/systemic embolism, major bleeding, all-cause mortality, cardiovascular cause of death by dose subgroups; (5) Study design: RCTs and observational studies published as full articles.
The exclusion criteria were as follows: (1) Compared anticoagulation strategies using NOACs vs. warfarin; (2) Other types of studies, including case reports, animal experiments, meta-analysis, reviews, comments, editorials, and conference abstracts; (3) Reported exposure or endpoints not suitable for our analysis.
Data Extraction and Quality Assessment
Two investigators independently extracted data from the eligible studies. Baseline characteristics of the patients (age, sex, creatinine clearance, BMI, CHADS2/CHA2DS2-VASc score, HAS-BLED score), study design, follow-up periods and the prespecified adverse outcomes were retrieved. In addition, they also assessed the quality of the RCTs and observational studies using the Revised Jadad's Scale and Newcastle-Ottawa Scale (NOS), respectively. Any disagreements or uncertainties between the two reviewers in the processes of study selection, data extraction and quality assessment were resolved by discussion with the senior investigator (HL).
Definitions and Outcomes
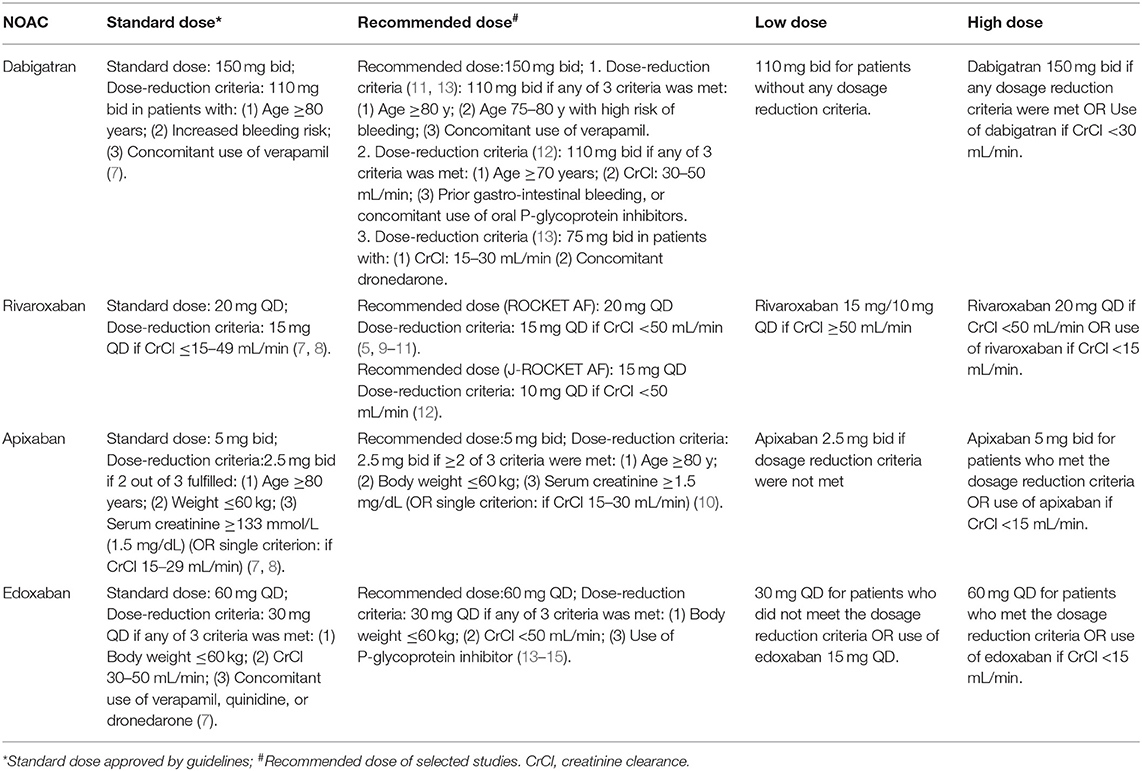
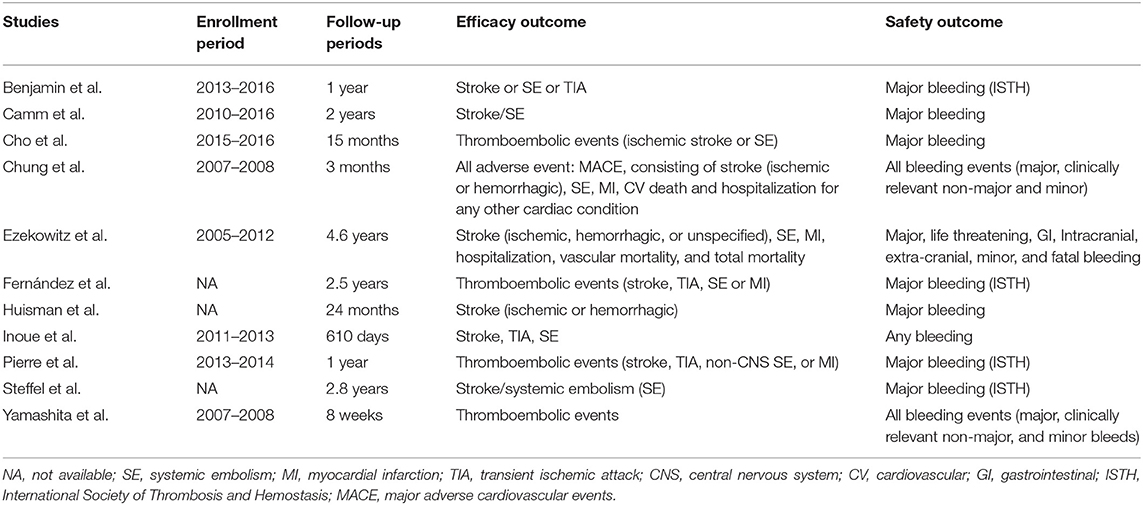
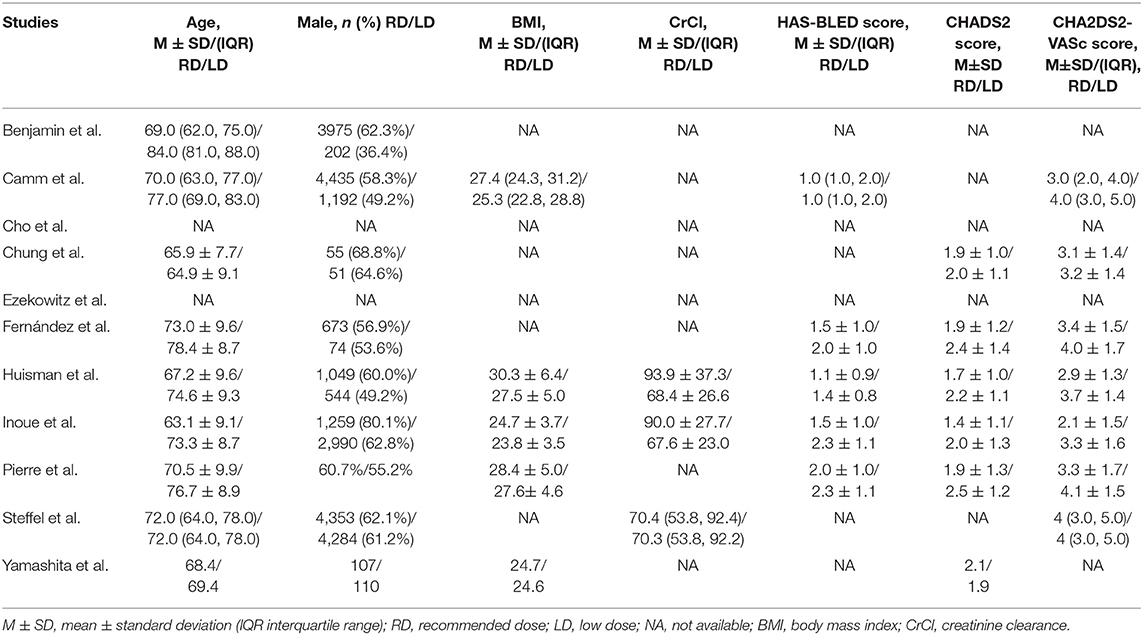
The criteria of standard dose approved by guidelines lately as well as the definitions of recommended/low/high dose of NOACs in our selected studies are listed in Table 1 (5, 7–19). The primary efficacy outcomes were stroke or systemic embolism (SE) and the primary safety outcomes were major or clinically relevant bleeding. For trials reporting only major bleeding, the same data were used for major or clinically relevant bleeding. The definition of major bleeding was in accordance with the International Society on Thrombosis and Hemostasis (ISTH) criteria (10, 20). The secondary outcomes included all-cause mortality, cardiovascular cause of death, if reported data were available.
Statistical Analysis
Comparison of the treatment effects of non-recommended dose of NOACs (low/high dose) vs. recommended dose of NOACs was performed used risk ratios (number of events or the incidence in each treatment group) and respective 95% confidence intervals. The heterogeneity across the studies was assessed by Cochran's Q-test (P < 0.1 was regarded as statistically significant) and I2 statistics, which estimate heterogeneity quantitatively (I2 value < 25% indicates no or mild heterogeneity, I2 > 75% indicates high heterogeneity). If I2 ≤ 50% and P ≥ 0.1, the fixed-effects model was used. If I2 > 50% or P < 0.1, data were pooled used random-effects, according to the Mantel-Haenszel model, and the cause of heterogeneity was sought. Publication bias assessment was made through visual inspection of the asymmetry in funnel plots. All statistical analyses were performed using Review Manager software (Rev- Man) version 5.3 (Cochrane Collaboration 2014, Nordic Cochrane Center, Copenhagen, Denmark). Two-tailed P-values < 0.05 were considered significant.
Results
Study Selection and Characteristics of the Eligible Studies
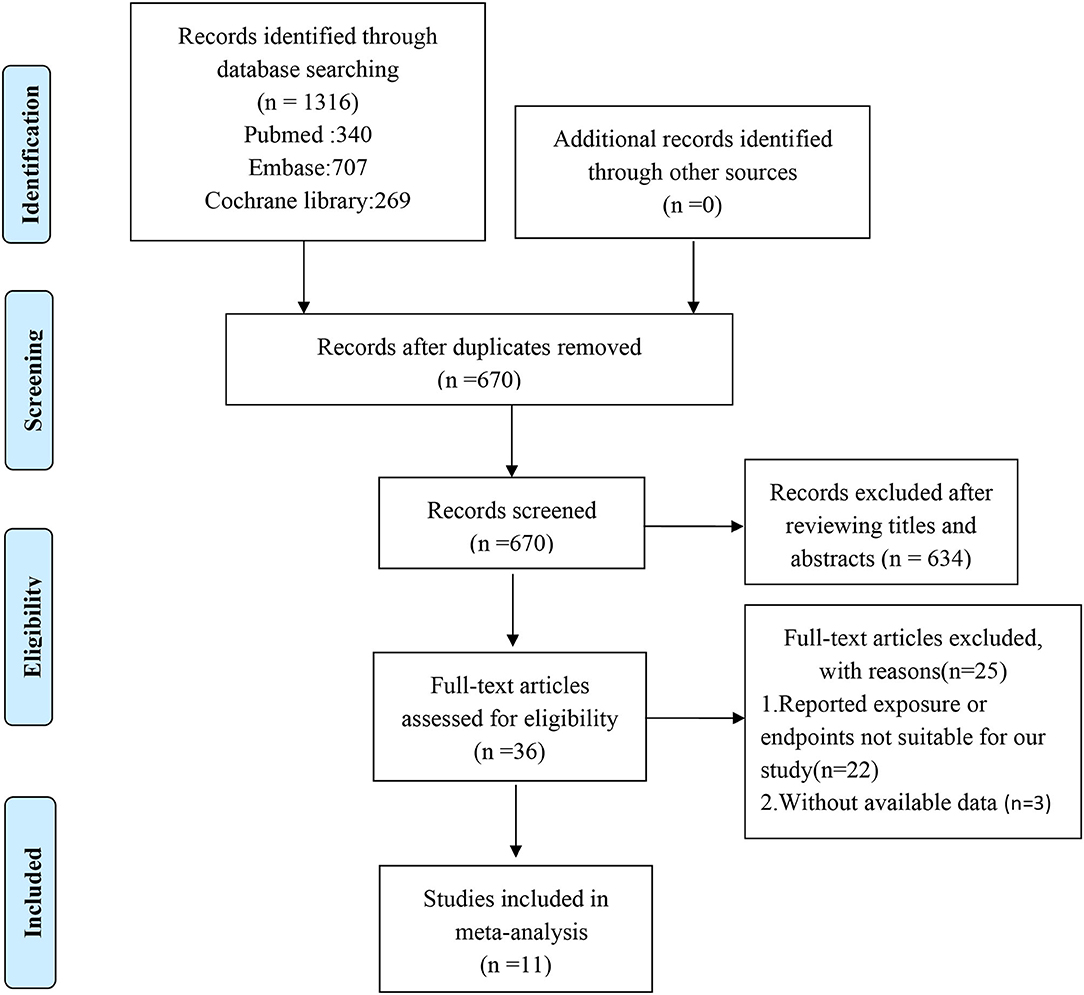
Our search strategy identified 1,316 potentially relevant studies, of which 670 records were retrieved after duplicates were removed. After assessing titles and abstracts, a total of 36 full-text articles were eligible for further screening. Of these, 25 records were excluded for not meeting the eligibility criteria, leaving 11 articles for further consideration (5, 9–15, 17–19). The study selection flowchart is presented in Figure 1.
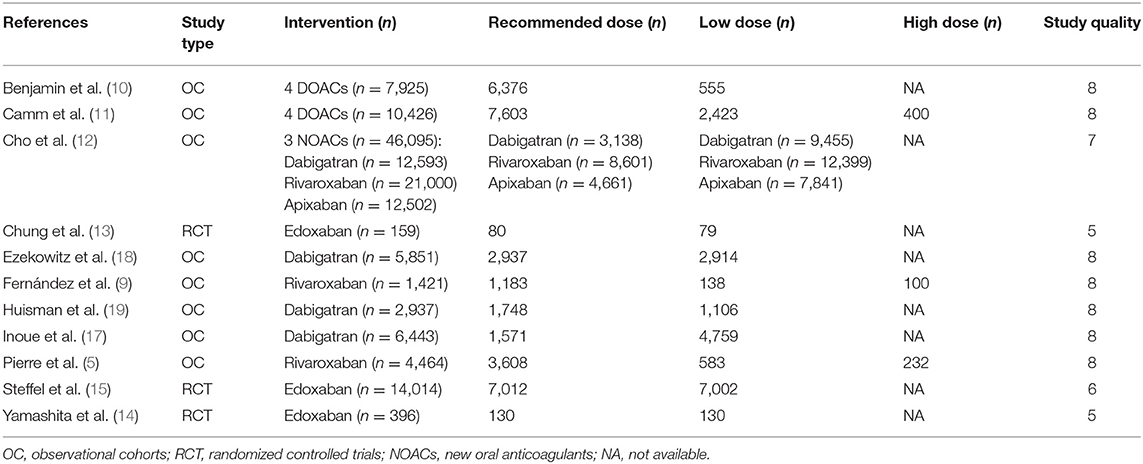
Overall assessment of the 11 eligible studies showed data for 100,131 total patients to be included in the meta-analysis. Of these, 48,648 received recommended dose of NOACs whereas 50,116 received non-recommended dose of NOACs. Among the latter group, 49,384 patients were prescribed lower than recommended dose while 732 patients were given higher dose. Articles were published from 2011 to 2021, and most of them were non-randomized, observational and comparative studies. Importantly, the 11 identified trials were rated good for methodological quality according to assessment by NOS and Revised Jadad's scales. The general features of the 11 studies are summarized in Table 2 with corresponding reported endpoints and follow-up periods listed in Table 3.
Baseline Characteristics of Patients Enrolled in the Eligible Studies
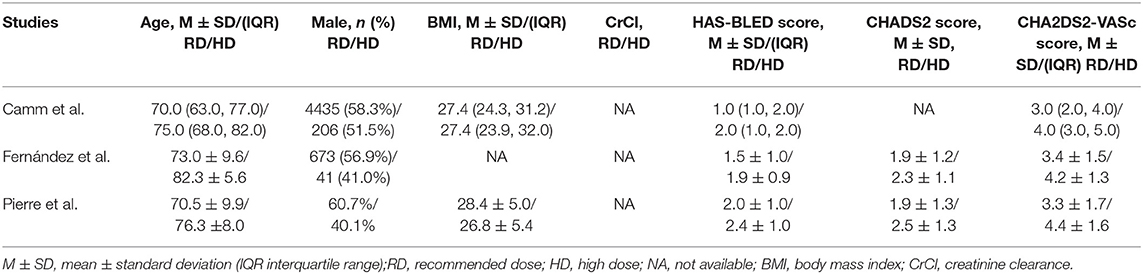
The baseline demographic characteristics of AF patients treated with low/high dose of NOACs vs. recommended dose are presented in Tables 4, 5, respectively. The patients receiving non-recommended dose tended to be elderly, more likely be female, have low body weight, and show high HAS-BLED or CHA2DS2-VASc scores.
Efficacy Outcomes
The results of our pooled indicated that the risk of stroke/systemic embolism in AF patients using non-recommended dose of NOACs was significantly higher than patients using the recommended dose (RR = 1.25, 95% CI 1.15–1.36, P < 0.00001). Moreover, similar results were also observed when either low dose (RR = 1.24, 95% CI 1.14–1.35, P < 0.00001) or high dose groups (RR = 1.71, 95% CI 1.06–2.76, P = 0.03) were compared against the recommended dose group (Figure 2).
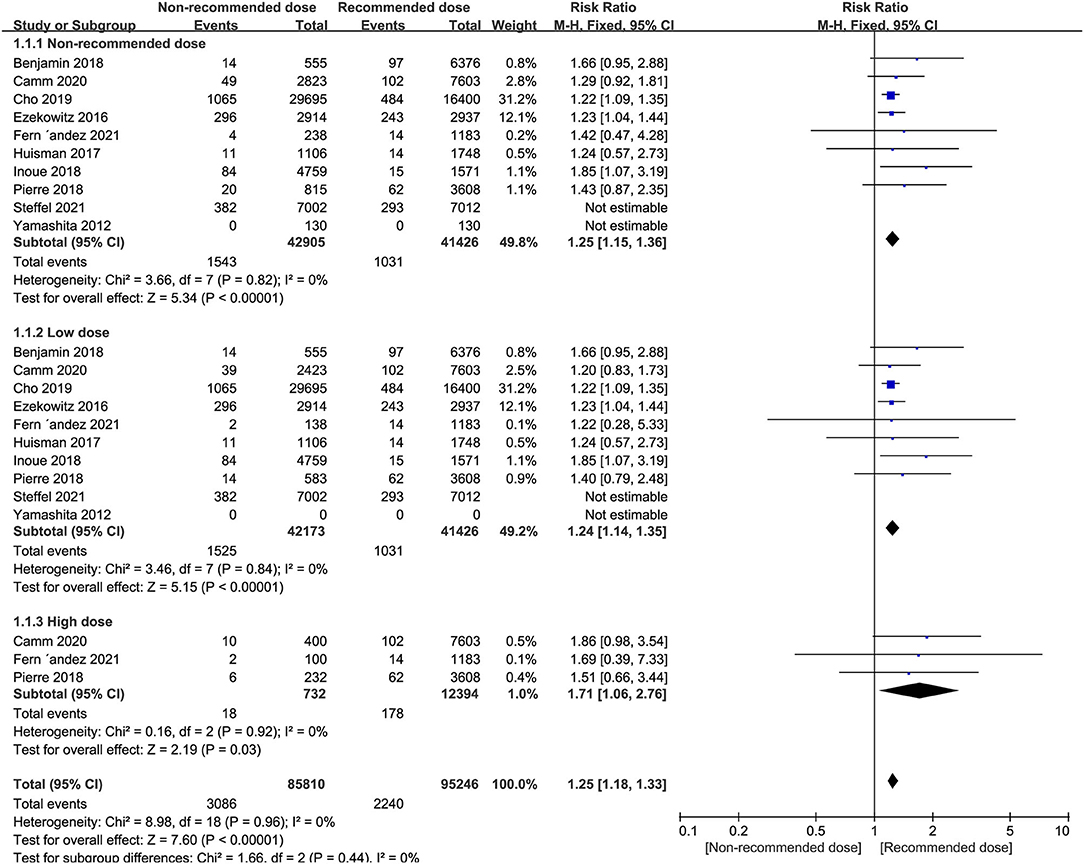
Figure 2. Forest plot for stroke or systemic embolism in non-recommended dose compared to recommended dose of NOACs. NOACs, new oral anticoagulants.
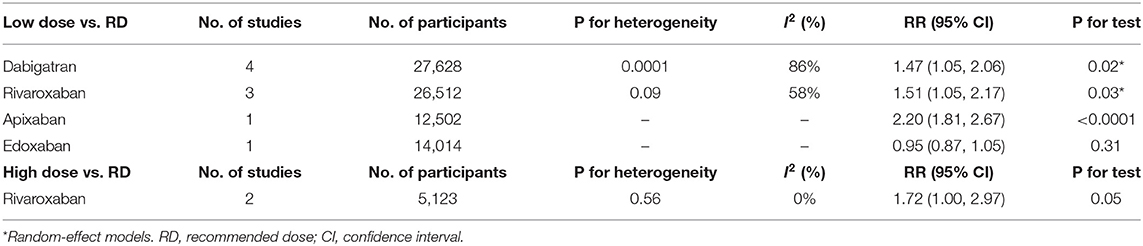
We next conducted subgroup analyses comparing the individual NOAC agents separately between the non-recommended dose and recommend dose categories. Notably, we found that patients prescribed low dose of NOACs, no matter dabigatran, apixaban or edoxaban, showed a higher risk of stroke/systemic embolism than patients administered the recommended dose. In contrast, patients receiving non-recommended dose of rivaroxaban (low/high) were not at greater risk of stroke/systemic embolism than the standard dose (Table 6).
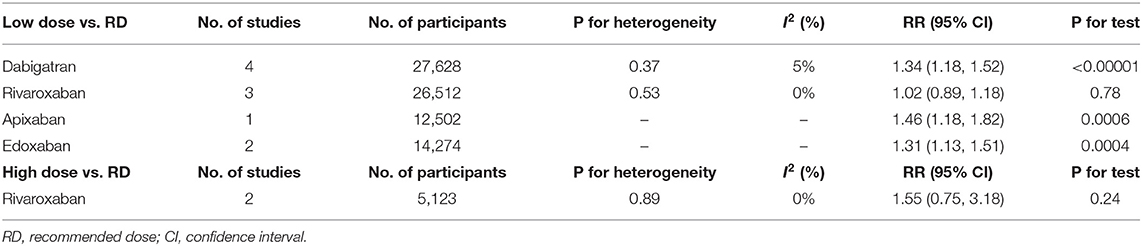
Table 6. Stroke or systemic embolism of low/high dose of individual NOACs compared to recommended dose.
Safety Outcomes
Patients administered non-recommended dose of NOACs did not experience more major or clinically relevant bleeding events than patients treated with recommended dose (RR = 1.22, 95% CI 0.95–1.56, P = 0.12). Similarly, equal safety outcomes were found after dividing patients into either low or high dose groups relative to patients treated with recommended dose (I2 = 87%, RR = 1.18, 95% CI 0.91–1.53, P = 0.21; I2 = 0%, RR = 1.57, 95% CI 0.96–2.58, P = 0.07, respectively) (Figure 3).
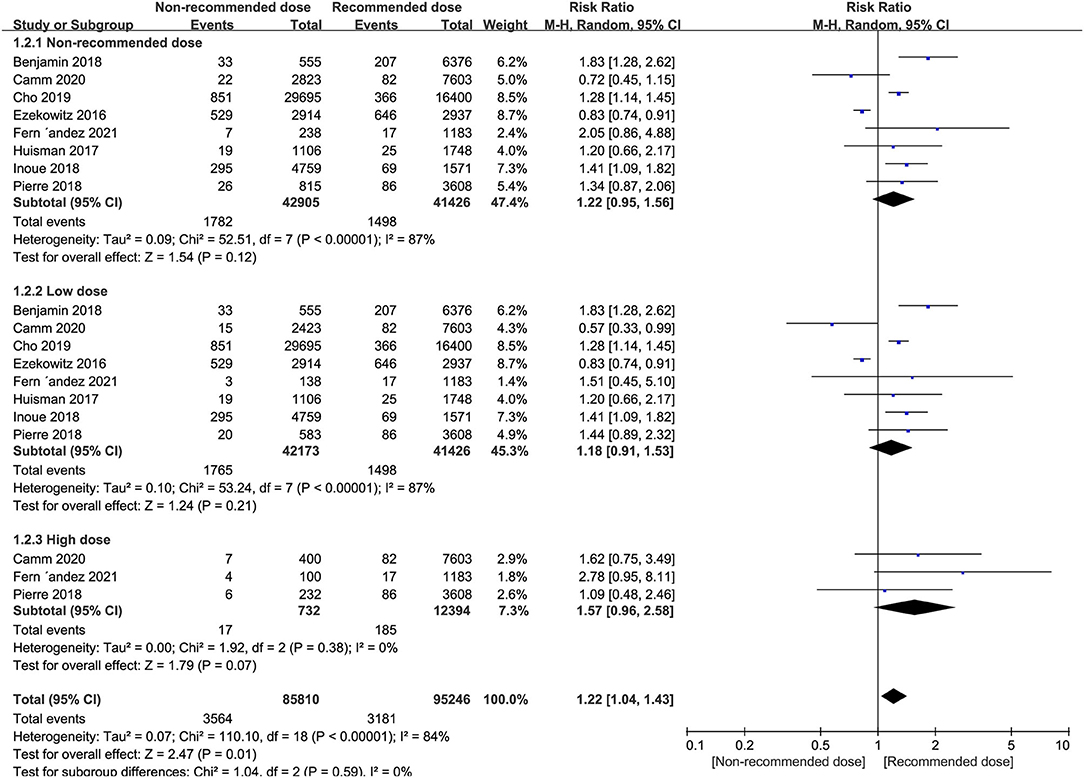
Figure 3. Forest plot for major or clinically relevant bleeding in non-recommended dose compared to recommended dose of NOACs. NOACs, new oral anticoagulants.
However, subgroup analyses revealed that patients receiving low dose rivaroxaban or apixaban, exhibited a significantly higher risk of bleeding compared to those on recommended dose (RR = 1.21, 95% CI 1.04–1.41, P = 0.01; RR = 1.42, 95% CI 1.09–1.85, P = 0.01, respectively). In contrast, superior safety was encountered in the low dose edoxaban group (RR = 0.67, 95% CI 0.58–0.76, P < 0.00001) whereas there were no safety differences found between patients receiving low and recommended dose of dabigatran (I2 = 90%, RR = 1.21, 95% CI 0.81–1.80, P = 0.36). Intriguingly, patients administered high dose rivaroxaban showed a non-significant trend toward increased major or clinically relevant bleeding above the standard dose (I2 = 48%, RR = 1.43, 95% CI 0.75–2.72, P = 0.28) (Table 7).
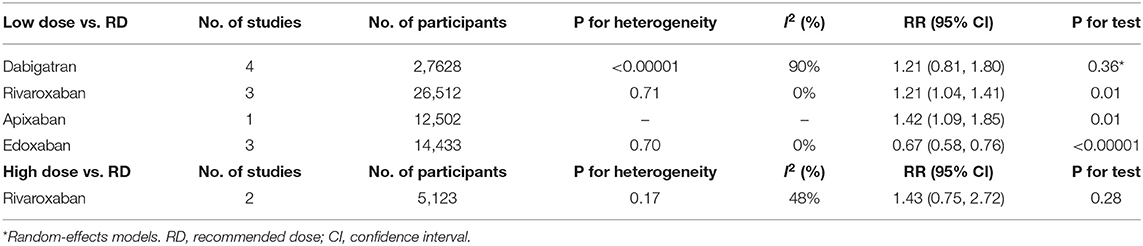
Table 7. Major or clinically relevant bleeding of low/high dose of individual NOACs compared to recommended dose.
All-Cause Mortality
Patients receiving non-recommended dose of NOACs were at significantly higher risk of all-cause mortality than standard dose (RR = 1.57, 95% CI 1.25–1.97, P < 0.0001), with similar outcomes evident in low dose group patients (RR = 1.58, 95% CI 1.25–1.99, P = 0.0001) and high dose group patients (RR = 1.74, 95% CI 1.30–2.33, P = 0.0002) (Figure 4).
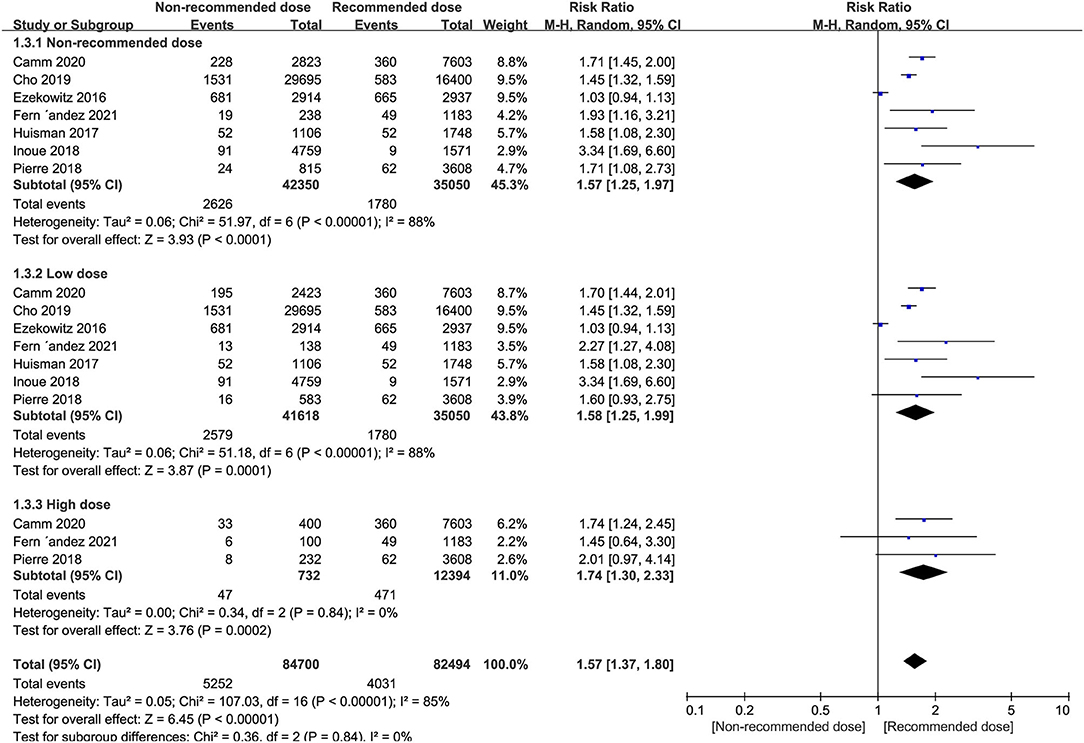
Figure 4. Forest plot for all-cause mortality in non-recommended dose compared to recommended dose of NOACs. NOACs, new oral anticoagulants.
Subgroup analyses revealed that the risk of all-cause mortality in patients receiving low dose of dabigatran, rivaroxaban or apixaban, was significantly higher than the recommended dose (RR = 1.47, 95% CI 1.05–2.06, P = 0.02 for dabigatran; RR = 1.51, 95% CI 1.05–2.17, P = 0.03 for rivaroxaban, and RR = 2.20, 95% CI 1.81–2.67, P < 0.0001 for apixaban). However, low dose edoxaban produced a similar risk of all-cause mortality in patients to the recommended dose (RR = 0.95, 95% CI 0.87–1.05, P = 0.31). Nevertheless, the all-cause mortality events patients were increased in patients receiving high dose rivaroxaban (RR = 1.72, 95% CI 1.00–2.97, P = 0.05), albeit at marginal significance levels (Table 8).
Cardiovascular Cause of Death
Patients treated with non-recommended dose of NOACs were not more likely to experience a higher risk of cardiovascular death compared to patients receiving standard dose (RR = 1.24, 95% CI 0.76–2.03, P = 0.39), and this result was consistent when the non-recommended dose patients were divided into low and high dose groups (RR = 1.27, 95% CI 0.76–2.12, P = 0.36; RR = 1.52, 95% CI 0.80–2.87, P = 0.20, respectively) (Figure 5).
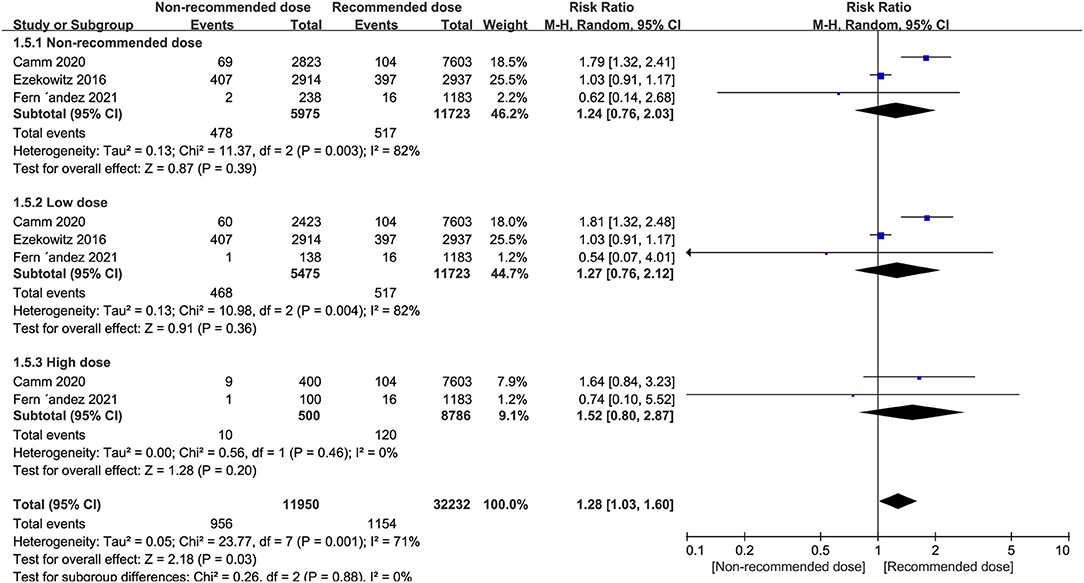
Figure 5. Forest plot for cardiovascular cause of death in non-recommended dose compared to recommended dose of NOACs. NOACs, new oral anticoagulants.
Publication Bias and Sensitivity Analysis
Based on a visual inspection of funnel plots (Supplementary Figures 1–4), there was no obvious evidence of publication bias for the selected studies for either reporting efficacy or safety outcomes. But performance bias in these studies inevitably existed. Random effects model showed similar results to the fixed-effects model. AF patients receiving low dose dabigatran, showed high major or clinically relevant bleeding risk in the sensitivity analysis after excluding Ezekowitz 2016 (I2 = 0%, RR = 1.46, 95% CI 1.22–1.75, P < 0.0001). The study of Ezekowitz 2016 (18) had the longest follow-up of 4.6 years, which completed RE-LY trial and continued into RELYABLE study (1, 21), showed low dose of dabigatran was associated with reduced bleeding risk (RR = 0.83,95% CI 0.74–0.91), suggesting that use of low dose dabigatran may reduce long term bleeding risk (Supplementary Figure 8). With regard to all-cause mortality, the pooled results remained stable after sensitivity analysis.
Discussion
In routine clinical practice, a balance is struck between the risks of stroke and bleeding in AF patients, highlighting the need to optimize interventions using NOACs. Unscheduled dosages of NOACs are often prescribed under real-world conditions, where physicians make judicious decisions according to the patient's age, weight, renal function amongst other performance indicators. Our meta-analysis aimed to investigate the efficacy and safety of using non-recommended dose of NOACs in AF patients. Foremost, our pooled results confirmed that patients receiving scheduled dose of NOACs, no matter dose lower or higher than recommended, had increased risks of stroke/systemic embolism and all-cause mortality. In contrast, there were no significant differences in the risk of bleeding as well as cardiovascular-related death events associated with non-recommended dose of NOACs.
Currently few reported studies have objectively evaluated clinical outcomes in AF patients receiving non-recommended dose of NOACs. In Denmark, Staerk et al. reported that AF patients treated with low dose of NOACs regimens might have a higher risk of stroke and bleeding compared to the recommended dose (22). The ORBIT-AF study showed improved safety outcomes in AF patients with renal indications for NOACs dose reduction where major bleeding outcomes were reduced although there were no significant differences encountered for stroke risk relative to patients receiving recommended dose (23). Yu et al. also suggested that Asian AF patients treated with higher dose of NOACs were at increased risk of stroke or systemic embolism, major bleeding, and all-cause mortality compared with recommended regimens (24).
As shown in our meta-analysis, we found that treating patients with high dose of NOACs produced a trend of higher incidence of stroke or systemic embolism compared with AF patients under standard treatment. This difference may be explained considering the reason underlying the administration of higher dose was likely the associated ischemic comorbidities of these patients. Non-recommended (low or high) dose of NOACs were also significantly associated with a higher risk of death along with composite endpoints (stroke/systemic embolism, major bleeding and death) compared to patients receiving recommended dose. However, after adjustment for baseline characteristics, the composite outcomes, including death risk, were no longer significant, suggested that the higher rates of events associated with non-recommended dose were probably related to the underlying diseases rather than the dose itself (5).
As part of our meta-analysis, we considered the effects of the individual agents as subgroup analyses. We found that patients treated with low dose dabigatran had higher associated risks of stroke/systemic embolism, but nevertheless, exhibited equivalent safety, compared with recommended dose. Consistently, Beyer-Westendorf et al. showed that low dose dabigatran produced increased risks of bleeding and stroke compared with AF patients receiving 150 mg bid (25).
Regarding rivaroxaban, we found patients who received non-recommended dosing showed comparable efficacy to standard regimens, no matter low or high. Nevertheless, low dose rivaroxaban was associated with higher bleeding risk in patients, whereas the higher dose group showed equivalent safety compared to the recommended dose. This contrasts with the findings of the XAPASS study where the incidence rate of stroke/systemic embolism was significantly higher in patients who received low dose rivaroxaban. Moreover, after adjustment for baseline characteristics, the authors reported that the rates of major bleeding were similar in for patients receiving either low or recommended dose of rivaroxaban (26). Other comparisons can be made with the EXPAND and SAKURA AF registry studies where the incidence rates of stroke/systemic embolism and major bleeding were both comparable between rivaroxaban interventions using low and recommended dose (6, 27).
Our subgroup analysis of apixaban-treated patients showed lower dose were associated with significantly higher risk of stroke and systemic embolism, major or clinically relevant bleeding compared to patients receiving the recommended dose. Our data are partly consistent with a prior study showing that low dose apixaban in patients with no renal indication for dose reduction was associated with significantly higher risk of stroke but without a reduction of major bleeding compared to patients taken standard dose of apixaban (23).
Lastly, the risk of stroke or systemic embolism in AF patients receiving low dose edoxaban was higher than patients treated with the recommended dose, but nonetheless this regimen had a superior safety outcome. This notion is supported by a published study reporting that the rate of ischemic stroke was higher with a low dose edoxaban regimen, but with greater risk reduction in bleeding compared to warfarin (28).
In summary, our pooled meta-analysis showed low dose of NOACs had worse efficacy for stroke/systemic embolism prevention, but without reducing in major bleeding, and with increased all-cause mortality compared to standard use. Moreover, high dose of NOACs did not show better outcomes in stroke/systemic embolism prevention in AF patients. Therefore, non-recommended dose of NOACs should be carefully prescribed since off-label use of any NOAC may cause different clinical outcomes. Furthermore, there may be subtle differences among the individual NOACs, such as pharmacologic properties, frequency of use, dose, interaction between drugs or other currently undefined aspects to consider. So, direct comparative studies are warranted to determine whether these are real differences in clinical efficacy and safety outcomes between individual NOACs.
Limitations
Some limitations should be acknowledged of this meta-analysis. First, pooled data were collected from different trials with differences in their design, including baseline participant characteristics, agents and dose regimens, definitions of efficacy and safety outcomes, along with differences in the length of follow-up time. Any combination of these variables could account for the source of moderate to high degree of statistically heterogeneity in our meta-analysis. This point needs further validation and it is also important to consider that heterogeneity can occur by chance and would almost certainly be found with meta-analysis involving many large studies (29). Furthermore, most of the studies included in our meta-analysis were non-randomized observational studies and were limited by potential selection and ascertainment bias. Moreover, the efficacy or safety outcomes in some of the included studies were not fully adjusted with medical records or baseline characteristics. Therefore, clinical events might be associated with off-label use but not causally related to dosing, thus introducing a possible risk of bias and misclassification of the end points (12), indicating the need for caution in interpreting outcomes.
Conclusions
Based on the available data, our study concludes that AF patients treated with non-recommended dose of NOACs may be at higher risk of stroke/systemic embolism and all-cause mortality compared with recommended dose, albeit the benefit of without reducing in major bleeding. Of particular interest, dabigatran, rivaroxaban, apixaban, edoxaban all differed in their efficacy and safety outcomes with non-recommended dose regimens.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
Data analysis, interpretation, and manuscript writing were performed by XK. Literature search, study selection, data extraction, and quality assessment were performed by XK, LP, YZ, SM, LZ, and WZ. YZ and HL were responsible for the conception and design of the study. YZ and HL revised the manuscript carefully. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the grant from Beijing Lab for Cardiovascular Precision Medicine, Beijing, China (PXM2020_014226_000017_00377132_FCG).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.774109/full#supplementary-material
Abbreviations
AF, atrial fibrillation; NOACs, new oral anticoagulants; VKA, vitamin K antagonists; EMA, European Medicines Agency; FDA, U.S. Food and Drug Administration; PMDA, Japanese Pharmaceuticals and Medical Devices Agency; BMI, body mass index; CrCl, creatinine clearance; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement; NOS, Newcastle-Ottawa Scale; SE, systemic embolism; ISTH, International Society on Thrombosis and Hemostasis; RR, risk ratios; OC, observational cohorts; RCT, randomized controlled trials; NA, not available; MI, myocardial infarction; TIA, transient ischemic attack; CNS, central nervous system; CV, cardiovascular; GI, gastrointestinal; MACE, major adverse cardiovascular events; M ± SD, mean ± standard deviation; IQR, interquartile range; RD, recommended dose; LD, low dose; HD, high dose; CI, confidence interval.
References
1. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. (2009) 361:1139–51. doi: 10.1056/NEJMoa0905561
2. Lindo VS, Kakkar VV, Melissari E. Cleaved antithrombin (ATc): a new marker for thrombin generation and activation of the coagulation system. Br J Haematol. (1995) 89:157–62. doi: 10.1111/j.1365-2141.1995.tb08923.x
3. Krawczak M, Smith-Sorensen B, Schmidtke J, Kakkar VV, Cooper DN, Hovig E. Somatic spectrum of cancer-associated single basepair substitutions in the TP53 gene is determined mainly by endogenous mechanisms of mutation and by selection. Hum Mutat. (1995) 5:48–57. doi: 10.1002/humu.1380050107
4. Patel Y, Kakkar VV, Authi KS. Calpain-induced down-regulation of protein kinase C inhibits dense-granule secretion in human platelets. Inhibition of platelet aggregation or calpain activity preserves protein kinase C and restores full secretion. Biochim Biophys Acta. (1994) 1224:480–8. doi: 10.1016/0167-4889(94)90285-2
5. Amarenco P, Haas S, Hess S, Kirchhof P, Lambelet M, Bach M, et al. Outcomes associated with non-recommended dosing of rivaroxaban. Eur Heart J Cardiovasc Pharmacother. (2019) 5:70–9. doi: 10.1093/ehjcvp/pvy041
6. Murata N, Okumura Y, Yokoyama K, Matsumoto N, Tachibana E, Kuronuma K, et al. Clinical outcome of off-label dosing of direct oral anticoagulant therapy among Japanese patients with atrial fibrillation identified from the SAKURA AF registry. Circ J. (2019) 83:727–35. doi: 10.1253/circj.CJ-18-0991
7. Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG, et al. 2021 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace. (2021) 23:1612–76. doi: 10.1093/europace/euab065
8. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. (2020) 42:373–49. doi: 10.1093/eurheartj/ehab648
9. Fernández MS, Marín F, Rafols C, Arribas F, Barrios V, Cosín-Sales J, et al. Thromboembolic and bleeding events with rivaroxaban in clinical practice in Spain: impact of inappropriate doses (the EMIR study). J Comp Eff Res. (2021) 10:583–93. doi: 10.2217/cer-2020-0286
10. Steinberg BA, Shrader P, Pieper K, Thomas L, Allen LA, Ansell J, et al. Frequency and outcomes of reduced dose non–vitamin k antagonist anticoagulants: results from ORBIT-AF II (The Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II). J Am Heart Assoc. (2018) 7:e007633. doi: 10.1161/JAHA.117.007633
11. Camm AJ, Cools F, Virdone S, Bassand JP, Fitzmaurice DA, ArthurFox KA, et al. GARFIELD-AF investigators. mortality in patients with atrial fibrillation receiving nonrecommended doses of direct oral anticoagulants. J Am Coll Cardiol. (2020) 76:1425–36. doi: 10.1016/j.jacc.2020.07.045
12. Cho MS, Yun JE, Park JJ, Kim YJ, Lee J, Kim H, et al. Outcomes after use of standard- and low-dose non–vitamin k oral anticoagulants in asian patients with atrial fibrillation. Stroke. (2019) 50:110–8. doi: 10.1161/STROKEAHA.118.023093
13. Chung N, Jeon HK, Lien LM, Lai WT, Tse HF, Chung WS, et al. Safety of edoxaban, an oral factor Xa inhibitor, in Asian patients with non-valvular atrial fibrillation. Thrombosis and Haemostasis. (2011) 105:535–44. doi: 10.1160/TH10-07-0451
14. Yamashita T, Koretsune Y, Yasaka M, Inoue H, Kawai Y, Yamaguchi T, et al. Randomized, multicenter, warfarin-controlled phase II study of edoxaban in Japanese patients with non-valvular atrial fibrillation. Circ J. (2012) 76:1840–7. doi: 10.1253/circj.CJ-11-1140
15. Steffel J, Ruff CT, Yin O, Braunwald E, Park JG, Murphy SA, et al. Randomized, double-blind comparison of half-dose versus full-dose edoxaban in 14,014 patients with atrial fibrillation. J Am Coll Cardiol. (2021) 77:1197–207. doi: 10.1016/j.jacc.2020.12.053
16. Chan YH, Chao TF, Chen SW, Lee HF, Yeh YH, Huang YC, et al. Off-label dosing of non–vitamin K antagonist oral anticoagulants and clinical outcomes in Asian patients with atrial fibrillation. Heart Rhythm. (2020) 17:1–9. doi: 10.1016/j.hrthm.2020.07.022
17. Inoue H, Uchiyama S, Atarashi H, Okumura K, Koretsune Y, Yasaka M, et al. Effectiveness and safety of long-term dabigatran among patients with non-valvular atrial fibrillation in clinical practice: J-dabigatran surveillance. J Cardiol. (2019) 73:507–14. doi: 10.1016/j.jjcc.2018.12.013
18. Ezekowitz1MD, Eikelboom J, Oldgren J, Reilly PA, Brueckmann M, Kent AP, et al. Long-term evaluation of dabigatran 150 vs. 110mg twice a day in patients with non-valvular atrial fibrillation. Europace. (2016) 18:973–8. doi: 10.1093/europace/euv312
19. Huisman MV, Rothman KJ, Paquette M, Teutsch C, Diener HC, Dubner SJ, et al. Two-year follow-up of patients treated with dabigatran for stroke prevention in atrial fibrillation: GLORIA-AF registry. Am Heart J. (2018) 198:55–63. doi: 10.1016/j.ahj.2017.08.018
20. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. (2005) 3:692–4. doi: 10.1111/j.1538-7836.2005.01204.x
21. Connolly SJ, Wallentin L, Ezekowitz MD, Eikelboom J, Oldgren J, Reilly PA, et al. The long-term multicenter observational study of dabigatran treatment in patients with atrial fibrillation (RELY-ABLE) study. Circulation. (2013) 128:237–43. doi: 10.1161/CIRCULATIONAHA.112.001139
22. Staerk L, Gerds TA, Lip GYH, Ozenne B, Bonde AN, Lamberts M, et al. Standard and reduced doses of dabigatran, rivaroxaban and apixaban for stroke prevention in atrial fibrillation: a nationwide cohort study. J Intern Med. (2018) 283:45–55. doi: 10.1111/joim.12683
23. Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non-vitamin K antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J Am Coll Cardiol. (2017) 69:2779–90. doi: 10.1016/j.jacc.2017.03.600
24. Yu HT, Yang PS, Jang E, Kim TH, Uhm JS, Kim JY, et al. Label adherence of direct oral anticoagulants dosing and clinical outcomes in patients with atrial fibrillation. J Am Heart Assoc. (2020) 9:e014177. doi: 10.1161/JAHA.119.014177
25. BeyerWestendorf J, Ebertz F, Förster K, Gelbricht V, Michalski F, Köhler C, et al. Effectiveness and safety of dabigatran therapy in daily-care patients with atrial fibrillation results from the dresden NOAC registry. Thromb Haemost. (2015) 113:1–11. doi: 10.1160/TH14-11-0954
26. Ikeda T, Ogawa S, Kitazono T, Nakagawara J, Minematsu K, Miyamoto S, et al. Outcomes associated with under-dosing of rivaroxaban for management of non-valvular atrial fibrillation in real-world Japanese clinical settings. J Thromb Thrombolysis. (2019) 48:653–60. doi: 10.1007/s11239-019-01934-6
27. Shimokawa H, Yamashita T, Uchiyama S, Kitazono T, Shimizu W, Ikeda T, et al. The EXPAND study: efficacy and safety of rivaroxaban in Japanese patients with non-valvular atrial fibrillation. Int J Cardiol. (2018) 258:126–32. doi: 10.1016/j.ijcard.2018.01.141
28. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. (2013) 369:2093–104. doi: 10.1056/NEJMoa1310907
Keywords: atrial fibrillation, new oral anticoagulants, non-recommended dose, bleeding, stroke, meta-analysis
Citation: Kong X, Zhu Y, Pu L, Meng S, Zhao L, Zeng W, Sun W, Wu G and Li H (2021) Efficacy and Safety of Non-recommended Dose of New Oral Anticoagulants in Patients With Atrial Fibrillation: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 8:774109. doi: 10.3389/fcvm.2021.774109
Received: 11 September 2021; Accepted: 29 November 2021;
Published: 23 December 2021.
Edited by:
Xiaofeng Yang, Temple University, United StatesReviewed by:
Christopher J. Boos, Poole Hospital NHS Foundation Trust, United KingdomYajing Wang, Thomas Jefferson University, United States
Copyright © 2021 Kong, Zhu, Pu, Meng, Zhao, Zeng, Sun, Wu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Li, ZHJfbGlob25nQHNpbmEuY29t
 Xiangyun Kong
Xiangyun Kong Yong Zhu
Yong Zhu Lianmei Pu
Lianmei Pu Shuai Meng3
Shuai Meng3