- 1Guangdong Provincial Hospital of Chinese Medicine, The Second Affiliated Hospital, Guangzhou University of Chinese Medicine, Guangzhou, China
- 2Shunde Hospital of Guangzhou University of Chinese Medicine, Guangzhou University of Chinese Medicine, Guangzhou, China
- 3The First Clinical Medical College of Guangzhou, University of Chinese Medicine, Guangzhou, China
Objectives: Evidence from systematic reviews/meta-analyses about the efficacy and safety of Qishen Yiqi (QSYQ) dripping pills in chronic heart failure (CHF) remains unclear. This study comprehensively reviewed available systematic reviews on latest evidence to provide reliable information for the clinical use of QSYQ in CHF.
Methods: The systematic review was performed on studies retrieved from six major medical databases. Eligible studies were evaluated in terms of methodological quality and quality of evidence using the Assessment of Multiple Systematic review 2 (AMSTAR-2) tool, the Risk of Bias in Systematic Reviews (ROBIS) was used to assess the risk of bias, and the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) 2020 was utilized for assessing reporting quality. In addition, the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) was used to determine primary outcome indicators’ evidence quality.
Results: A total of 14 systematic reviews were included in this study, based on which it could be concluded that QSYQ combined with conventional medicine (CM) treatment tended to be superior to CM treatment alone in terms of improving cardiac function-related indices (e.g., increasing the left ventricular ejection fraction [LVEF] and reducing the left ventricular end-diastolic dimension [LVEDD] and left ventricular end-systolic internal diameter [LVESD]), improving the total effective rate and 6-min walking distance (6MWD), and reducing N-terminal pro-brain natriuretic peptide (NT-proBNP). Overall, no serious QSYQ-related adverse events were observed. However, the GRADE results showed “very low” to “moderate” evidence for these outcomes, with no high-quality evidence supporting them. Unsatisfactory results were obtained in terms of methodological quality, risk of bias and reporting quality after assessment using the AMSTAR-2, ROBIS, and PRISMA 2020, limited mainly by deficiencies in the following areas: registration of study protocols, explanation of the inclusion of randomized controlled trials (RCTs), complete and detailed search strategy, list of excluded literature, description of funding sources for inclusion in RCTs, investigation of the impact of risk of bias on the results of meta-analysis, and reporting of potential conflicts of interest.
Conclusion: The efficacy and safety of QSYQ adjuvant therapy in CHF remain to be further clarified due to the lack of high-quality evidence provided by current systematic reviews.
Introduction
Chronic heart failure (CHF), a common cardiovascular disease, is a severe manifestation or the end stage of various cardiac conditions (1). In developed countries, approximately 1–2% of people over 80 years old suffer from CHF, which has a 1- and 5-year mortality rate of 20.2 and 56.2%, respectively (2, 3). CHF also negatively impacts patients’ quality of life and productivity and increases health care costs and socioeconomic burdens (4). Studies have shown that health care spending on CHF in the US was approximately $31 billion in 2012 and that the total cost for heart failure treatment would rise by 127% between 2012 and 2030 (5). The main drugs currently used to manage CHF are beta-blockers, diuretics, calcium channel blockers, angiotensin-converting enzyme inhibitors (ACE-Is), and angiotensin receptor antagonists. Patients with CHF may also benefit from emerging drugs such as SGLT-2 inhibitors (6) and sacubitril/valsartan (7). These new drugs were shown to improve the prognosis of heart failure and lower all-cause mortality (8). Despite advances in CHF treatment, CHF remains a leading cause of death or disability (9), patients’ mortality and rehospitalization rates remain high, and there is an urgent need to develop new therapeutic agents and treatment strategies.
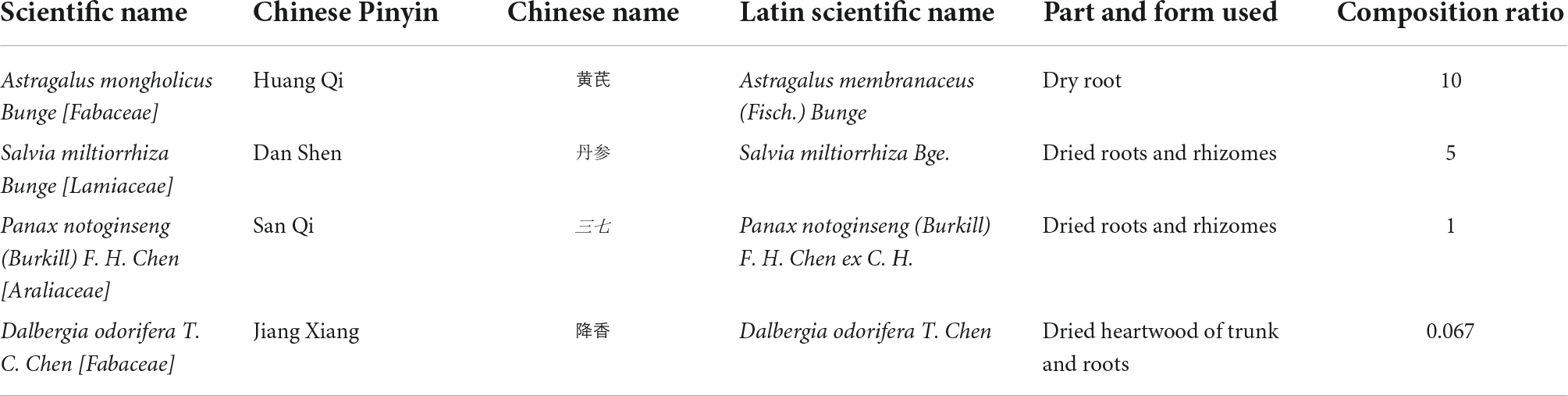
Qishen Yiqi (QSYQ) dripping pill, a well-known Chinese proprietary medicine, was approved by the China Food and Drug Administration (CFDA) in 2003 (Approval number of CFDA: Z20030139) for treating cardiovascular diseases, especially heart failure (10). Its composition is specified in Table 1, of which astragaloside IV, danshensu and salvianolic acids are the major active ingredients (11). A growing body of systematic reviews (10, 12) of randomized controlled trials (RCTs) has investigated the effectiveness of QSYQ in CHF. Although systematic reviews and meta-analyses are widely regarded as the highest level of evidence in the field of evidence-based medicine, the evidence is based on the design and methodology used for assessing the study endpoints (13). While the value of any systematic review depends largely on the number, quality and heterogeneity of the included studies, systematic reviews with serious flaws in methodological quality might mislead decision-makers (14). Although several systematic reviews on QSYQ for CHF have been published, the methodological quality and strength of evidence of these meta-analyses have not yet been adequately assessed. Therefore, the purpose of this present study was to objectively and comprehensively assess current systematic reviews to determine the efficacy and safety of QSYQ in the treatment of CHF.
Methods
The protocol for this overview was registered on the website of Open Science Framework (OSF1) with a registration number of DOI: 10.17605/OSF.IO/JKGP7. Ethical approval was not required because this was a study on systematic reviews.
Inclusion criteria for reviews selection
Types of studies
All peer-reviewed systematic reviews of RCTs evaluating the efficacy and safety of QSYQ for CHF, published in Chinese or English, were included in this study.
Types of participants
Participants aged over 18 and diagnosed with CHF according to existing diagnostic criteria (15–17), with no restrictions on race or sex.
Types of interventions
The basic treatment for patients with CHF in the control and intervention groups was based on the recommended conventional medicine (CM) from relevant guidelines (9), such as ACE-Is, angiotensin II receptor antagonists, beta-blockers, diuretics, and calcium channel blockers. The intervention group was treated with QSYQ in combination with the basic treatment, with no restriction on dose, frequency of treatment, or duration.
Types of outcome measures
The primary outcomes were left ventricular ejection fraction (LVEF) and total effective rate. The latter was defined as the percentage of patients whose signs and symptoms improved during the treatment period and improvement in the New York Heart Association classification by more than one grade according to the guiding principles for clinical research of new drugs in traditional Chinese medicine (18).
The secondary outcomes were adverse events, left ventricular end-diastolic dimension (LVEDD), left ventricular end-systolic internal diameter (LVESD), 6-min walk distance (6MWD), B-type brain natriuretic peptide (BNP), and N-terminal pro-brain natriuretic peptide (NT-proBNP).
Exclusion criteria
Systematic reviews with the following criteria were excluded: (1) non-peer-reviewed systematic reviews; (2) QSYQ combined with other herbal medicines in the intervention group; (3) duplicate published studies; (4) protocol studies; and (5) those with unretrievable full text even after contacting the authors.
Search strategy
Two reviewers conducted a comprehensive search of three Chinese databases (China National Knowledge Infrastructure [CNKI], Wanfang and VIP) and three English databases (PubMed, Embase and Cochrane Library) from their inception to May 3, 2022. The detailed search strategy for each database is shown in Supplementary material 1.
Study selection and data extraction
After removing duplicates, the two authors independently screened the titles and abstracts and evaluated the full text for potentially eligible studies. For studies with insufficient information, the authors of systematic reviews were contacted. Disagreements were resolved through discussion between the two authors. Relevant data were extracted from each eligible review using a standardized form developed by the team, which included first author and year of publication (country), number of trials (subjects), trial quality assessment methods, interventions, main results, and conclusions. For studies with errors or missing data during data extraction, the authors were contacted by email, and if no response was received, this was indicated in the discussion section. The data extraction was independently performed and cross-checked by the two reviewers, and disagreements were resolved by mutual discussion or discussion with a third evaluator.
Assessment of methodological quality
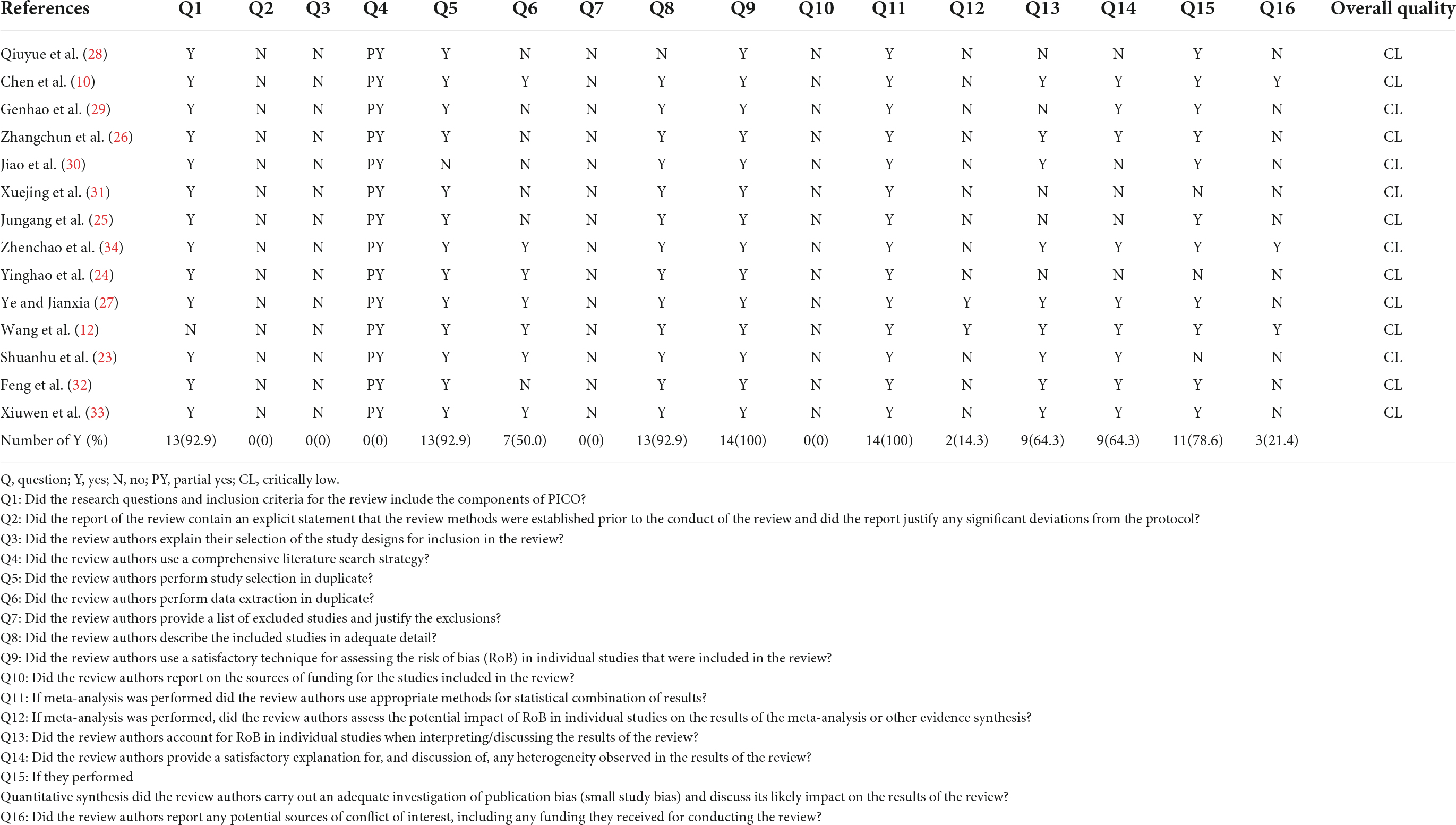
The methodological quality of the included literature was assessed according to the Assessment of Multiple Systematic Review 2 (AMSTAR-2) tool (19), which consists of 16 items, of which items 2, 4, 7, 9, 11, 13, and 15 are critical. The entries were described as “yes,” “partially yes” and “no,” and the literature was classified as “high,” “moderate,” “low” or “critically low” according to the compliance status of the entries. (1) No or one noncritical weakness was assessed as “high” quality; (2) more than one noncritical weakness was assessed as “moderate” quality; (3) one critical weakness with or without noncritical weaknesses was assessed as “low” quality and; (4) more than one critical weakness with or without noncritical weaknesses was assessed as “critically low” quality. The two reviewers independently evaluated the systematic reviews and discussed their quality. A third reviewer was queried when necessary.
Assessment of risk of bias
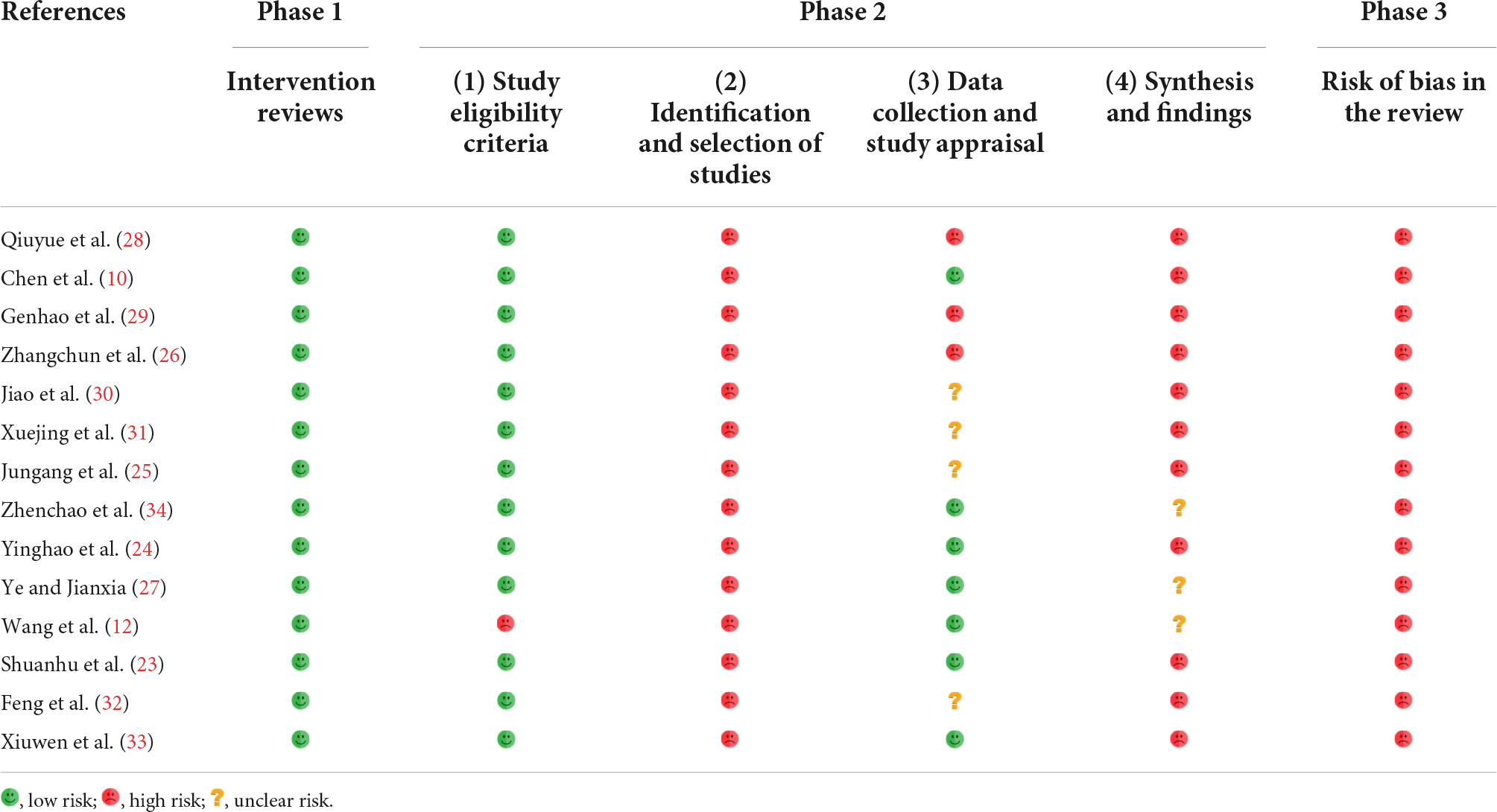
The Risk of Bias in Systematic Reviews (ROBIS) (20) tool is a new tool for assessing the risk of bias in systematic reviews. The tool is divided into three Phases and consists of 24 entries that assist in determining the risk of bias in the review process, results, and conclusions. Responses to landmark questions are indicated by “yes,” “probably yes,” “could be,” “no” and “no information.” The final determination of the risk of bias was classified as “low,” “high” or “uncertain. The risk of bias was considered “low” if all the landmark questions were answered by “maybe” or “could be.” The risk of bias was considered “high” if the answer to any of the landmark questions was “may or may not” or “no,” and “uncertain” if the information provided was insufficient to make a judgment.
Assessment of the reporting quality
The Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) 2020 statement (21) consists of 27 items, including seven areas of specification such as Title, Abstract, Introduction, Methods, Results, Discussion, and Funding. Each item was assessed as “yes,” “no,” and “partially yes” based on the completion of the systematic review.
Assessment of quality of evidence
The Grades of Recommendation, Assessment, Development and Evaluation (GRADE) (22) was used to evaluate the quality of evidence for outcome indicators through five downgrading factors: study limitations, imprecision, inconsistency, non-directivity, and publication bias. After the assessment, evidence was categorized into four levels: “high,” “moderate,” “low,” and “very low.” To reach an objective result, evaluators were trained to reach a consensus before conducting the evaluation. The entire evaluation process was conducted by two independent reviewers, and any disagreements were resolved through consensus or discussion with an experienced and authoritative third reviewer.
Results
Study selection
In total, 70 studies were searched from English and Chinese databases according to predefined search strategies. Twenty-eight studies remained after manually screening out duplicates. After reading the titles and abstracts, 12 studies were excluded as they did not meet the inclusion criteria, of which two studies were conference papers, one study was a research protocol, three studies were not systematic reviews, one study did not use QSYQ, and two studies were not on CHF. Then, the full text of 16 potential articles was further screened, of which one of our previous studies was excluded because it was an RCT. Finally, 14 systematic reviews (10, 12, 23–34) were included. The specific screening process is presented in Figure 1 and Supplementary material 2.
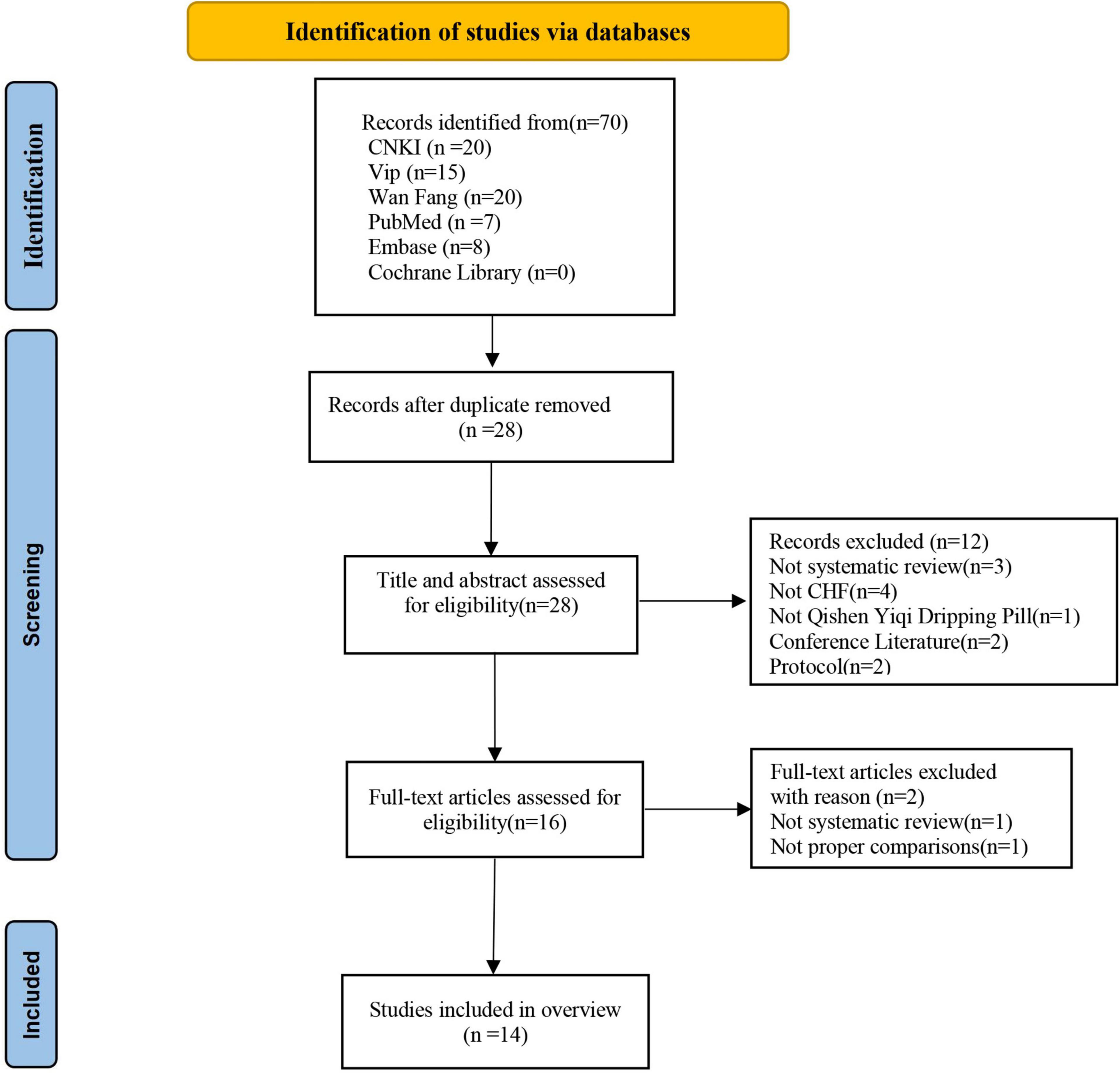
Figure 1. Flow chart of the study selection process. CHF, chronic heart failure; CNKI, China National Knowledge Infrastructure database; Embase, Excerpta Medica database; SR, systematic review.
Characteristics of the included reviews
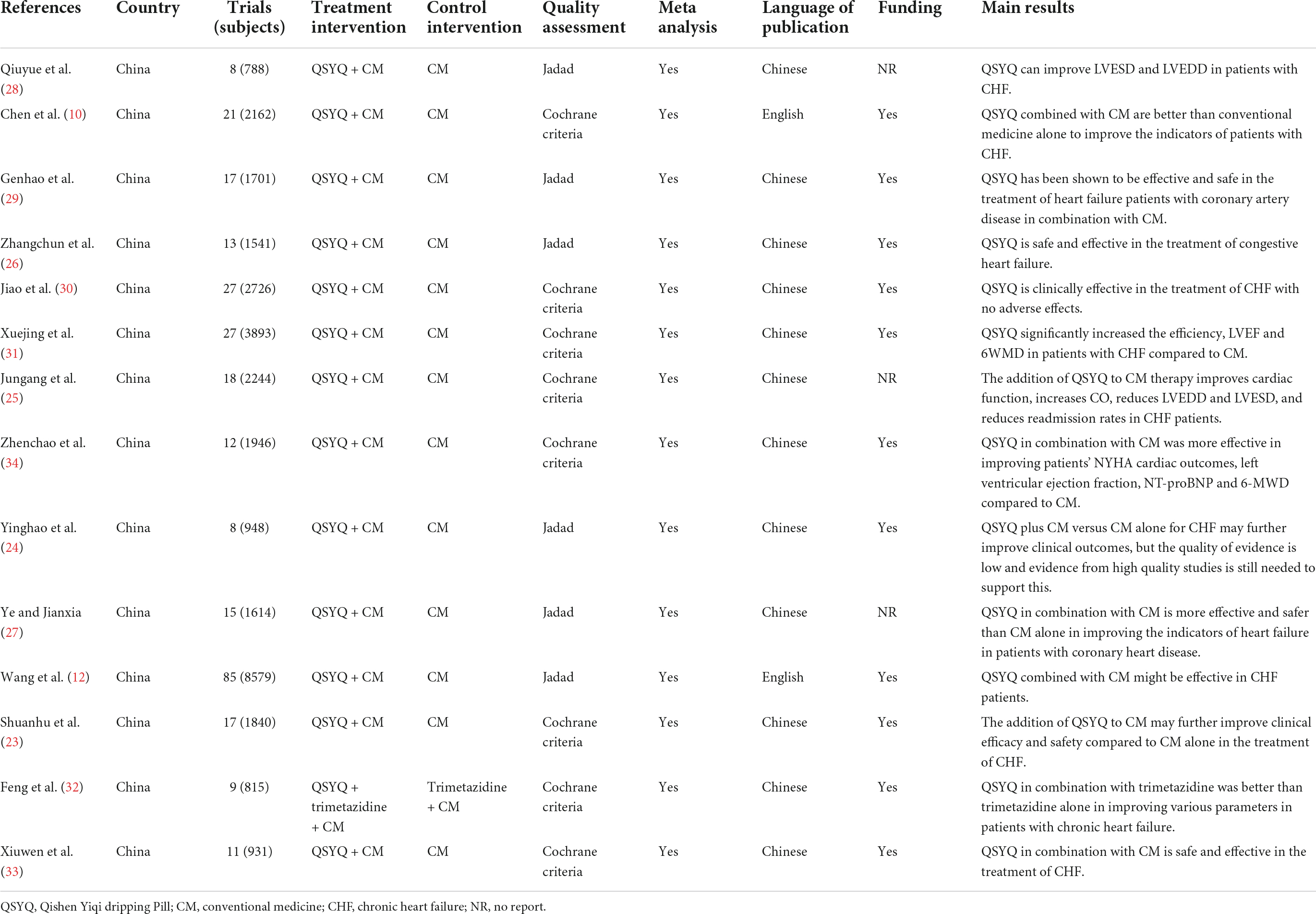
Table 2 summarizes the main characteristics of all included reviews. They were published between 2013 and 2021, with two studies (10, 12) published in English and the remaining (23–34) published in Chinese. All articles were published in peer-reviewed journals. Of the 14 systematic reviews, 13 (10, 12, 23–31, 33, 34) compared QSYQ in combination with CM to CM alone, and one systematic review (32) compared the efficacy of QSYQ in combination with trimetazidine to trimetazidine alone in addition to CM treatment. Eight studies (10, 23, 25, 30–34) assessed the methodological quality of systematic reviews using the Cochrane Collaboration’s risk of bias tool, and six studies (12, 24, 26–29) used the Jadad scale. Eleven studies (10, 12, 23–26, 29, 30, 32–34) reported the funding source, all of which were government-funded. The results of all systematic reviews suggested that QSYQ appeared to have beneficial effects in CHF treatment but required further support from high-quality RCTs.
Methodological quality of the included systematic review
Table 3 shows the results of the AMSTAR-2 evaluation of the methodological quality of the 14 systematic reviews included in this overview. As all studies had more than one key weakness, their methodological quality was determined to be critically low. The methodological quality of the systematic reviews varied considerably, with most of the included studies showing certain limitations. Such as, one study (12) did not report clear inclusion and exclusion criteria, none of the studies had registered study protocols on relevant websites prior to conducting the systematic evaluation, none of the studies explained the rationale for including only RCTs in the systematic reviews, and none of the authors of the systematic reviews used a comprehensive search strategy such as searching gray literature. One studies (30) did not use two-person repeated literature screening, seven studies (25, 26, 28–32) did not use two-person repeated extraction of data, no studies provided full exclusion descriptions for excluding the retrieved literature, one study (28) did not sufficiently detail the essential characteristics of the included RCTs and no studies reported on the source of funding for the included RCTs. When conducting the meta-analysis, 12 studies (10, 23–26, 28–34) did not analyze the impact of RCTs with a high risk of bias on the meta-analysis outcomes, and five studies (24, 25, 28, 29, 31) did not consider the effect of the risk of bias in the inclusion of the original RCT when interpreting and discussing the results of the meta-analysis. Five studies (24, 25, 28, 30, 31) did not provide reasonable explanations or discussions on the heterogeneity of the findings, three studies (23, 24, 31) did not perform analysis or provide reasonable discussions on publication bias, and eleven studies (23–33) did not report on all potential sources of conflicts of interest.
Risk of bias of included systematic review
Risk of bias in systematic reviews assessment results indicated that in Phase 1, where the relevance of the study topic was assessed, all systematic reviews were rated as low risk of bias. In Phase 2, for Domain 1, One study (12) had a high risk of bias due to the lack of clear inclusion criteria, and all other studies had a low risk of bias. In Domains 2, all systematic reviews were at high risk of bias. In Domain 3, four systematic reviews (25, 30–32) had unclear risks, three (26, 28, 29) had a high risk of bias, and only seven (10, 12, 23, 24, 27, 33, 34) had a low risk of bias. In Domains 4, three systematic reviews (12, 27, 34) had unclear risks, 11 (10, 23–26, 28–33) had a high risk of bias. In addition, in Phase 3, all systematic reviews had a high risk of bias. The evaluation details of the included systematic reviews on the ROBIS scale are shown in Table 4 and Supplementary material 3.
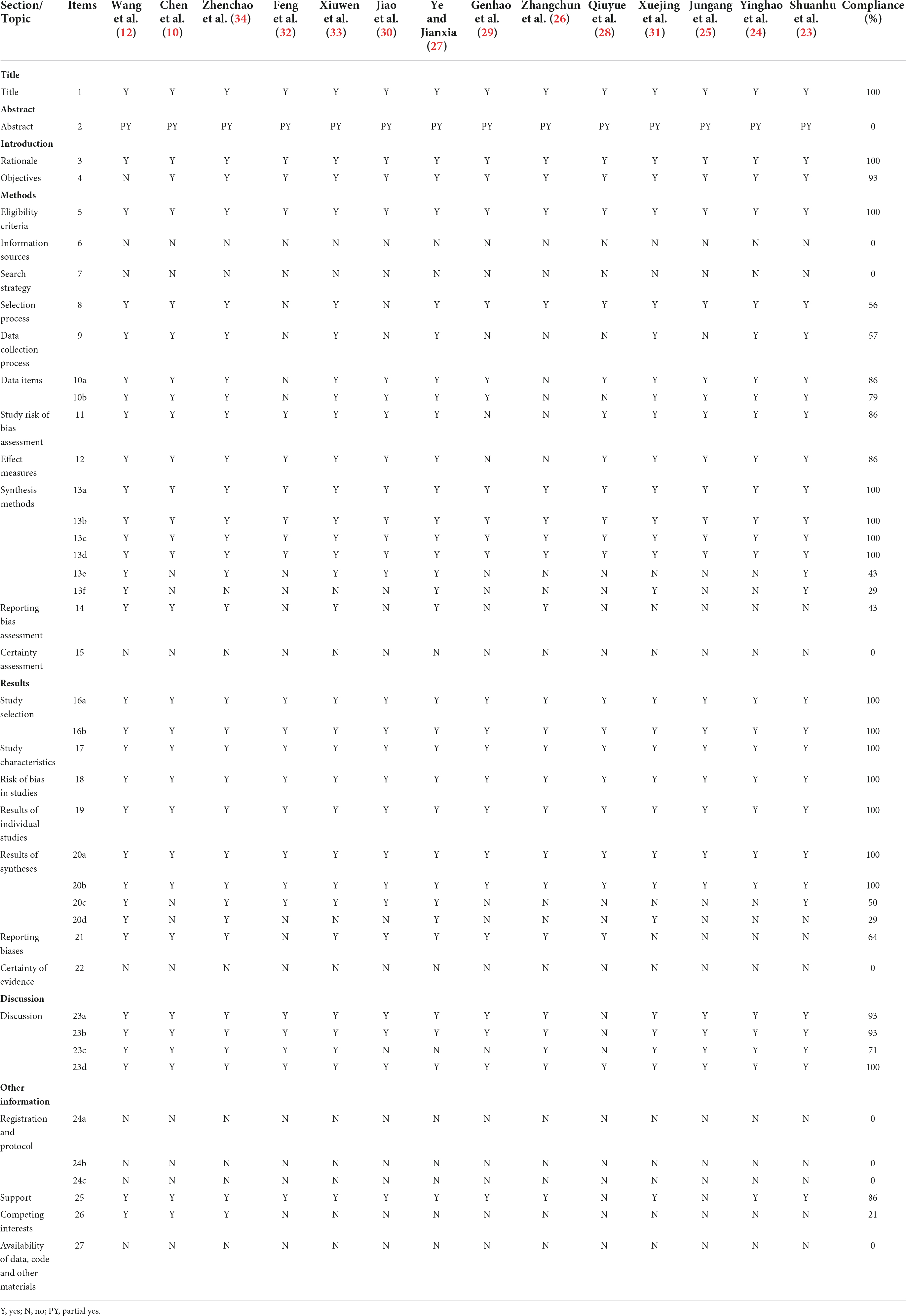
Reporting quality of included systematic review
Table 5 presents the results of the quality of the reports assessed by the PRISMA 2020 checklist. Although Q1 (title), Q3 (rationale), Q5 (eligibility criteria), Q13a, b, c d (synthesis of methods), Q16 (study selection), Q17 (study characteristics), Q18 (risk of bias), Q19 (results of individual studies), Q20a, b (results of syntheses) and Q23d (discussion) were fully reported, some reporting deficiencies were found in other sections. Q2 (abstract), Q6 (information sources), Q7 (search strategy), Q15 (certainty assessment), Q22 (certainty of evidence), Q24 (registration and protocol), and Q27 (availability of data, code and other materials) were reported deficiently (0%). The remaining entries are only partially complete.
Quality of evidence in the included systematic reviews
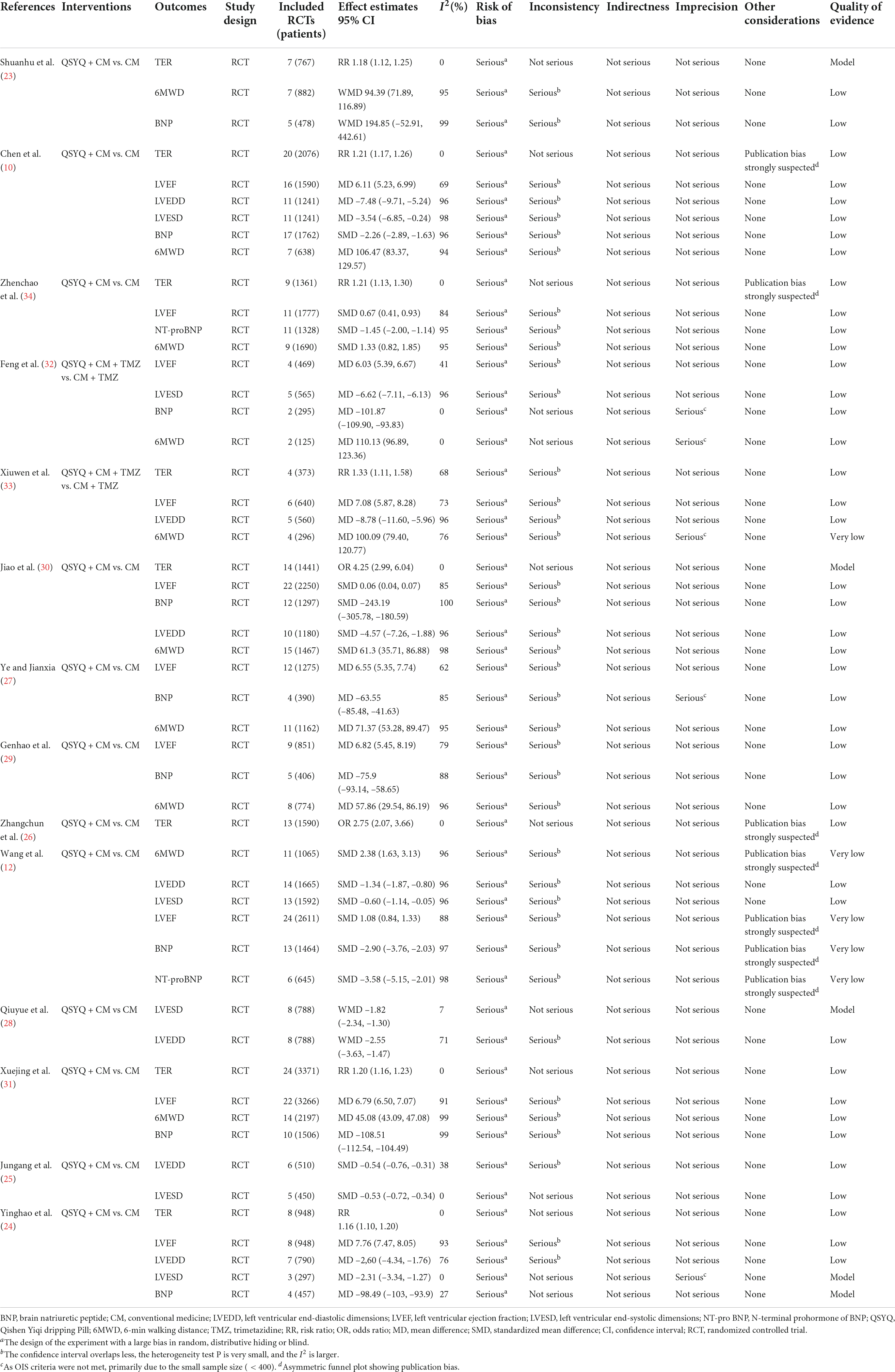
The details of the 14 systematic reviews containing a total of 52 outcomes for GRADE assessment are shown in Table 6. The results showed that 4 (4/52, 7.69%), 43 (43/52, 82.69%), and 5 (5/52, 9.62%) outcomes were rated as moderate, low and very low quality, respectively. No high-quality evidence results were found. Significant risks of bias in the systematic reviews were due to the design of the original RCTs (52/52, 100.00%), which was the most important factor contributing to lower quality of evidence, followed by inconsistency (39/52, 75.00%), publication bias (7/52, 13.46%), and imprecision (5/52, 9.62%).
Efficacy evaluation with evidence quality
Left ventricular ejection fraction
Ten systematic reviews (10, 12, 24, 27, 29–34) reported on LVEF outcomes, and all meta-analyses showed that combined QSYQ treatment was significantly better than CM alone in improving LVEF in patients with CHF. However, most of the results (9/10, 90%) (10, 12, 24, 27, 29–31, 33, 34) were significantly heterogeneous, which further decreased the strength of the evidence. This outcome in most of the meta-analyses (9/10, 90%) (10, 24, 27, 29–34) was evaluated as a “low” strength of evidence, with one (12) being “very low.” The study with the largest sample size included 22 RCTs (31), with a total of 3,266 patients (MD = 6.79, 95% CI = 6.50 to 7.07, p < 0.00001, I2 = 91%, random effect model). The results were statistically and clinically significant.
Total effective rate
Eight systematic reviews (10, 23, 24, 26, 30, 31, 33, 34) compared the total effective rate of QSYQ combined with CM versus CM alone, and all of the meta-analyses found a higher total effective rate in the combined treatment group than in the CM group, with all demonstrating low heterogeneity (I2 = 0%). Regarding the quality of evidence, two meta-analyses (23, 30) showed a “moderate” quality of evidence for the results, and six (10, 24, 26, 31, 33, 34) were determined as “low” quality. The study with the largest sample size included 24 RCTs (31), with a total of 3,371 patients (RR = 1.20, 95% CI = 1.16 to 1.23, p < 0.00001, I2 = 0%, fixed effect model).
Left ventricular end-diastolic dimension
Seven systematic reviews (10, 12, 24, 25, 28, 30, 33) evaluated the potential benefits of QSYQ combined with CM on LVEDD, and all of the meta-analyses showed that QSYQ combined with CM could improve LVEDD better than CM alone. However, most results(6/7, 85.71%) (10, 12, 24, 28, 30, 33) showed high heterogeneity. All studies had a “low” quality of evidence for the results. The study with the largest sample size included 14 RCTs (12), with a total of 1,665 patients (SMD = –1.34, 95% CI = –1.87 to –0.80, p < 0.00001, I2 = 96%, random effect model).
Left ventricular end-systolic internal diameter
Six systematic reviews (10, 12, 24, 25, 28, 32) evaluated the potential benefits of QSYQ combined with CM on LVESD, and all of the meta-analyses showed that QSYQ combined with CM improved LVESD better than CM alone. The results of three studies (10, 12, 32) demonstrated high heterogeneity and the quality of evidence was rated as “moderate” for one outcome (28) and “low” for the rest. The study with the largest sample size included 13 RCTs (12), with a total of 1,592 patients (SMD = –0.60, 95%CI = –1.14 to –0.05, p < 0.00001, I2 = 96%, random effect model).
Six-minutes walking distance
Ten systematic reviews (10, 12, 23, 27, 29–34) showed that QSYQ combined with CM treatment was better than CM in improving the 6MWD of patients with CHF, but most of the results (9/10, 90%) (10, 12, 23, 27, 29–31, 33, 34) were significantly heterogeneous. The quality of evidence was “low” to “very low.” The study with the largest sample size included 14 RCTs (31), comprising a total of 2,197 patients (MD = 45.08, 95% CI = 43.09–47.08, p < 0.00001, I2 = 99%, random effect model).
Brain natriuretic peptide
Nine systematic reviews (10, 12, 23, 24, 27, 29–32) evaluated the potential benefits of QSYQ combined with CM on decreased BNP. Among the eight systematic reviews (10, 12, 24, 27, 29–32), QSYQ combined with CM decreased BNP better than CM alone. The study with the largest sample size included 17 RCTs (10), comprising a total of 1,762 patients (SMD = –2.26, 95% CI –2.89 to –1.63, p < 0.00001, I2 = 96%, random effect model). However, there was no significant difference was observed in one study (23) (5 RCTs, SMD = 194.85, 95% CI = –52.91 to 442.61, P < 0.001, I2 = 99%, random effect model, “low” quality of evidence).
N-terminal pro-brain natriuretic peptide
Two systematic reviews (12, 34) evaluated the potential benefits of QSYQ combined with CM on decreasing NT-proBNP, and all meta-analyses showed that QSYQ combined with CM decreased NT-proBNP better than CM alone. However, there was significant heterogeneity in all of these results, with one study (34) having “low” quality of evidence and another (12) with “very low” quality of evidence. The study with the largest sample size included 11 RCTs (34), comprising a total of 1,328 patients (SMD = –1.45, 95% CI = –2.00 to –1.14, p < 0.00001, I2 = 95%, random effect model).
Safety of Qishen Yiqi for chronic heart failure
Of the 14 included systematic reviews, 11 (11/14, 78.57%) (10, 23–27, 29, 31–34) reported adverse effects associated with QSYQ, which mainly included hypotension, dry cough, dizziness and headache. Overall, the results suggested that the combination of QSYQ adjuvant therapy with CM treatment was safe and did not significantly increase the risk of adverse events compared with CM only. However, it should be noted that most of the original RCTs included in the 10 systematic reviews (10, 23–25, 27, 29, 31–34) did not report adverse reactions.
Discussion
In China, many patients with CHF undergo adjunctive treatment with Chinese proprietary medicines, such as QSYQ, due to unsatisfactory treatment of symptoms, reduced quality of life, or side effects of conventional treatment (35). This has aroused the interest of investigators, and many related RCTs were conducted. Previous works demonstrated that the combination of QSYQ appeared to be more effective and had a good safety profile in the treatment of CHF in addition to conventional treatment (36). Related meta-analyses (10, 12) have also been published more frequently, but the results remained controversial regarding the clinical effectiveness and safety of QSYQ for CHF. Therefore, we conducted this review, retrieved relevant systematic reviews of all corresponding RCTs of QSYQ for CHF, evaluated the methodological quality using the AMSTAR-2 tool, and GRADE evaluated the level of evidence.
Summary of findings
In this present review, the evidence on the efficacy and safety of QSYQ for the treatment of CHF was derived from 14 systematic reviews. Overall, the available evidence strongly suggested that QSYQ was effective as an adjunctive treatment for CHF, as evidenced by greater benefits in improving cardiac function (e.g., increasing LVEF and reducing LVEDD and LVESD), increasing the total effective rate and 6MWD, and decreasing NT-proBNP. In terms of decreasing BNP, while eight meta-analyses showed QSYQ to be effective, one meta-analysis (23) reported no advantage compared with controls. No serious adverse events were associated with QSYQ. However, the overall methodological quality and data reporting quality of the original RCTs included in these systematic reviews were generally poor, and the lack of large-sample, multicenter, placebo studies contributed to the inability of almost all included systematic reviews to draw firm and reliable conclusions about the efficacy and safety of QSYQ in CHF. In addition, the methodological and evidentiary quality of most systematic reviews was unsatisfactory, as shown by the results of the AMSTAR-2, PRISMA 2020, ROBIS and GRADE assessments. Therefore, there is an urgent need for future RCTs and systematic reviews to further improve the methodological design to accurately determine the true effectiveness and safety of QSYQ for the treatment of CHF.
High-quality systematic reviews can provide clinicians, patients, and other decision-makers with a reliable scientific basis (37). The methodological quality of the systematic review was assessed using the AMSTAR-2 tool, and our results indicated that the methodological quality of these systematic reviews was “critical low.” The following are the potential considerations for improving the quality of future studies: (1) none of the systematic reviews included in the QSYQ for CHF were preregistered prior to the study initiation, which was also reflected in the original RCT. Systematic reviews or RCTs are often performed with substantial financial support; thus, duplicate or similar studies might waste resources, and registration would allow researchers to check if similar topics already exist or are “in progress” at the planning stage of the study to determine if it is necessary to proceed with a similar project (38). In addition, the transparency of research, accuracy and completeness of test methods should be improved once the results are published, thereby reducing selective reporting of results and publication bias and improving the authenticity of the research (39). It is worth noting that clinical trial registration is an ethical imperative for medical research, as well as a responsibility and obligation of trial investigators (40). Therefore, we call for future systematic reviews or RCTs on the effectiveness of QSYQ in CHF to be preregistered on relevant websites. (2) All of the included reviews selected only RCTs but did not explain the specific reasons for the choice of study type. Although RCTs are the gold standard for assessing new drugs, systematic reviews of non-randomized intervention studies can also complement their role when there are few RCTs, missing outcome indicators and insufficient statistical effects (41). In addition to justifying the choice of study type, it is equally important to justify the reasons for the choice. (3) A high-quality systematic review requires a thorough, objective and reproducible search and screening of relevant studies after developing a research strategy and exhaustive inclusion criteria (42). However, none of the meta-analyses included in this overview searched gray literature. In addition, most of the systematic reviews did not present the full search strategy for all databases and websites in detail, including the filters and qualifiers used, and some studies did not specify a screening process in which at least two researchers independently screened the literature and extracted the data, which reduced the rigor and reproducibility of the corresponding studies. (4) Most of the reviews did not investigate the potential impact of the risk of bias of the inclusion of original RCTs on the meta-analysis results by subgroup analysis or sensitivity analysis. In the case of including only high-quality RCTs, there may be little discussion of the potential impact of bias on outcomes. However, none of the RCTs on QSYQ for CHF were of high quality, indicating the need to assess the impact of the risk of bias in RCTs on the review results. (5) Another important finding was that most of the authors of the systematic review (11/14, 78.57%) did not report all sources of potential conflicts of interest, which also contributed to the low quality of the methodology. Several lines of evidence (43) have demonstrated that pharmaceutical company-funded systematic reviews were more likely to yield effective interventions than unfunded studies and investigators should report the direct source of funding even if they do not receive funding but still have a relationship with the company whose product is involved in the systematic evaluation. Similar to the AMSTAR-2results, the PRISMA 2020 evaluation showed that the included systematic reviews also had the deficiencies mentioned above. The risk of bias assessment on the results ROBIS scale indicated that all systematic reviews were at high risk of bias. Further analysis showed that inadequate interpretation of risk of bias, risk of study identification and selection bias and inadequate assessment of publication bias were the main factors contributing to the high risk of bias.
The present overview assessed the quality of evidence of systematic reviews using the GRADE system. Our results showed that most outcome indicators (e.g., LVEF, total effective rate, LVEDD, LVESD, 6MWD, and NT-proBNP) indicated QSYQ was beneficial as an adjunctive treatment for patients with CHF. However, it is noteworthy that these evidence quality grades ranged from “very low” to “moderate.” Risk of bias, publication bias, and inconsistency were the primary reasons for the low quality of the evidence. The original RCTs included in the systematic reviews all had significant bias in their trial design, such as unclear study randomization schemes, lack of allocation concealment information, and failure to implement blinding. Therefore, future RCTs should strictly follow the “CONSORT Extension for Chinese Herbal Medicine Formulas 2017” statement (44) to further standardize their design in terms of random allocation, allocation concealment, and blinding. In addition, another factor leading to lower quality of evidence was inconsistency, with large heterogeneity of outcome indicators in most studies. Future meta-analyses should conduct subgroup analyses and sensitivity analyses of the more heterogeneous outcome indicators to identify sources of heterogeneity, and if the heterogeneity still cannot be reduced, descriptive analyses might be considered. Moreover, the lack of description of how sample sizes were determined in the original RCTs included in the systematic reviews and the small sample size reduced the precision, contributing to the reduced quality of evidence. Lastly, we also noted a lack of focus on the impact of QSYQ on reducing the morbidity, mortality and readmission rates of CHF patients in the RCTs. CHF is the end stage of various heart diseases with relatively high annual mortality rates; therefore, reducing mortality and readmission rates are the endpoint goals in the treatment of CHF (9), and future studies should focus on evaluating long-term efficacy indicators.
Over the past decades, several in vivo studies have attempted to elucidate the mechanism of action of QSYQ in the treatment of heart failure (45). In a mouse model of high-fat diet-induced heart failure with preserved ejection fraction (HFpEF), it was observed that QSYQ significantly improved cardiac function and myocardial remodeling in mice, possibly through the inhibition of microvascular endothelial inflammation and activation of the NO-cGMP-PKG pathway (46). In addition, it was also reported that, in coronary artery ligation-induced ischemic CHF rats, QSYQ intervention reduced myocardial infarct size and apoptosis and improved myocardial fibrosis (47). Interestingly, it was found that QSYQ also prevented doxorubicin-induced cardiotoxicity in mice, which may be closely related to enhanced cardiac angiogenesis (48). Altogether, the above preclinical studies suggest that the mechanism of action of QSYQ in the treatment of heart failure might be mediated through multiple targets and pathways.
The safety of Chinese medicines has been a widespread concern, and safety is an important outcome indicator in clinical studies of interventions (49). However, three systematic reviews (12, 28, 30) did not report adverse reactions associated with QSYQ. Although the conclusions of the original RCTs included in the meta-analysis indicated that QSYQ was safe with no major adverse events, it is important to note that these RCTs were vague in their descriptions of adverse events that occurred during the study, focused only on the discomforts exhibited, and lacked biochemical testing indicators. For instance, chronic liver and kidney damage was a frequently reported adverse effect of Chinese medicines (49), but patients with mild symptoms might not have reported these manifestations or felt the need to inform the investigators, thus unless proper serum biomarkers and abdominal ultrasounds are performed, these conditions might have been under-reported. Therefore, we suggest that future studies should adequately describe the specific methods and potential basis for QSYQ safety assessment. Details of all adverse events (e.g., time of occurrence, number or frequency, severity, the number of cases withdrawn, and/or dose reduction) should be reported. Lastly, the underlying cause or potential trigger should be discussed for any adverse events.
Limitations
This is the first overview to examine the quality of evidence for the safety and efficacy of QSYQ in CHF patients using the AMSTAR-2, PRISMA 2020, ROBIS, and GRADE approaches. However, there were some limitations to our study. First, only studies published in English and Chinese were included in this study; considering that Chinese proprietary medicine is also popular in Asian countries, such as Korea and Japan, the inclusion of relevant systematic reviews might have provided new insights into the study findings. Second, this review did not conduct any quantitative analysis, which might have led to biased conclusions. Third, most of the systematic reviews included in this study were of poor quality, and all RCTs were conducted in China, which reduced the credibility of the evidence reported.
Conclusion
Despite the reported efficacy and safety of QSYQ, current evidence limits our ability to confirm the benefits of QSYQ in CHF, given the poor methodological quality and low quality of evidence in most of the investigated systematic reviews. Thus, better-designed and high-quality clinical studies and systematic reviews are still needed to provide clear indications about the clinical significance of QSYQ in CHF.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
YL, DW, and WC designed this study. DW and JY performed the search. XY and YW collected the data. JC, XY, and YW rechecked the data. JC, YL, and WC performed the analysis. WC and YW drafted the manuscript. DW and YL revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was funded by the grants from the National Natural Science Foundation of China (No. 82174156), Guangzhou Science and Technology Project (No. 202002030432), Guangdong Basic and Applied Basic Research Foundation (2022A1515011701), and Key-Area Research and Development Program of Guangdong Province (No. 2020B1111100004).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1001072/full#supplementary-material
Abbreviations
6MWD, 6-min walking distance; RCTs, randomized controlled trials; AMSTAR 2, A Measurement Tool to Assess Systematic Reviews 2; ACE-Is, angiotensin-converting enzyme inhibitors; BNP, B-type brain natriuretic peptide; CHF, chronic heart failure; CM, conventional medicine; GRADE, the Grades of Recommendation, Assessment, Development, and Evaluation; HFpEF, heart failure with preserved ejection fraction; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic internal diameter; NT-proBNP, N-terminal pro-brain natriuretic peptide; QSYQ, Qishen Yiqi.
Footnotes
References
1. Coronel R, de Groot JR, van Lieshout JJ. Defining heart failure. Cardiovasc Res. (2001) 50:419–22. doi: 10.1016/s0008-6363(01)00284-x
2. Townsend N, Kazakiewicz D, Lucy Wright F, Timmis A, Huculeci R, Torbica A, et al. Epidemiology of cardiovascular disease in Europe. Nat Rev Cardiol. (2022) 19:133–43. doi: 10.1038/s41569-021-00607-3
3. Jones NR, Roalfe AK, Adoki I, Hobbs FDR, Taylor CJ. Survival of patients with chronic heart failure in the community: a systematic review and meta-analysis. Eur J Heart Fail. (2019) 21:1306–25. doi: 10.1002/ejhf.1594
4. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. (2014) 63:1123–33. doi: 10.1016/j.jacc.2013.11.053
5. Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. (2016) 133:e38–360. doi: 10.1161/CIR.0000000000000350
6. Seferovic PM, Fragasso G, Petrie M, Mullens W, Ferrari R, Thum T, et al. Heart failure association of the European society of cardiology update on sodium-glucose co-transporter 2 inhibitors in heart failure. Eur J Heart Fail. (2020) 22:1984–6. doi: 10.1002/ejhf.2026
7. Kaplinsky E. Sacubitril/valsartan in heart failure: latest evidence and place in therapy. Ther Adv Chronic Dis. (2016) 7:278–90. doi: 10.1177/2040622316665350
8. Fiuzat M, Lowy N, Stockbridge N, Sbolli M, Latta F, Lindenfeld J, et al. Endpoints in Heart failure drug development: history and future. JACC Heart Fail. (2020) 8:429–40. doi: 10.1016/j.jchf.2019.12.011
9. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. (2017) 70:776–803. doi: 10.1016/j.jacc.2017.04.025
10. Chen L, Wang R, Liu H, Wei S, Jing M, Wang M, et al. Clinical efficacy and safety of Qishen Yiqi dropping pill combined with conventional western medicine in the treatment of chronic heart failure: a systematic review and meta-analysis. Evid Based Complement Alternat Med. (2021) 2021:6612653. doi: 10.1155/2021/6612653
11. Yunfei L, Haibin Q, Yiyu C. Identification of major constituents in the traditional Chinese medicine “Qi-Shen-Yi-Qi” dropping pill by high-performance liquid chromatography coupled with diode array detection-electrospray ionization tandem mass spectrometry. J Pharm Biomed Anal. (2008) 47:407–12. doi: 10.1016/j.jpba.2007.12.037
12. Wang H, Li L, Qing X, Zhang S, Li S. Efficacy of Qishen Yiqi drop pill for chronic heart failure: an updated meta-analysis of 85 studies. Cardiovasc Ther. (2020) 2020:8138764. doi: 10.1155/2020/8138764
13. Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ. (1996) 312:71–2. doi: 10.1136/bmj.312.7023.71
14. LeLorier J, Gregoire G, Benhaddad A, Lapierre J, Derderian F. Discrepancies between meta-analyses and subsequent large randomized, controlled trials. N Engl J Med. (1997) 337:536–42. doi: 10.1056/NEJM199708213370806
15. Zhang J, Zhang Y. Chinese guidelines for the diagnosis and treatment of heart failure 2014. Chin J Cardiol. (2014) 42:98–122. doi: 10.3760/cma.j.issn.0253-3758.2014.02.004
16. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC) developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. (2016) 37:2129–200. doi: 10.1093/eurheartj/ehw128
17. Wu X. 2007 version of guidelines for chronic heart failure in China. Chin J Internal Med. (2008) 47:267–8. doi: 10.3321/j.issn:0578-1426.2008.04.003
18. Xiaoyu Z. Guiding Principles for Clinical Research of New Chinese Medicine. Beijing: China Medical Science and Technology Publishing (2002).
19. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. Amstar 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008. doi: 10.1136/bmj.j4008
20. Whiting P, Savovic J, Higgins JP, Caldwell DM, Reeves BC, Shea B, et al. Robis: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. (2016) 69:225–34. doi: 10.1016/j.jclinepi.2015.06.005
21. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
22. Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. Grade guidelines: 3. rating the quality of evidence. J Clin Epidemiol. (2011) 64:401–6. doi: 10.1016/j.jclinepi.2010.07.015
23. Shuanhu W, Yuan MJ, Yazhu H, Jiaying W, Xianliang W, Zhijun L. Routine western medicine treatment plus Qishen Yiqi dripping pill for treating patients with chronic heart failure: a systematic review of randomized control trials. Chin J Integrated Traditional Western Med. (2013) 33:1468–75. doi: 10.7661/CJIM.2013.11.1468
24. Yinghao P, Cuiling Z, Mingjun Z, Kuipo Y, Xiaoxu C. Systematic evaluation on efficacy and safety of Qishenyiqi droping pill for chronic heart failure. J Emerg Traditional Chin Med. (2013) 22:1472–5.
25. Jungang L, Wanhong G, Xiaoshuan L, Xixiang L, Qingjie H. Meta-analysis of Qishenyiqi drop pills treatment for chronic heart failure. Chin J New Drugs Clin Remedies. (2014) 33:189–95. doi: 10.19540/j.cnki.cjcmm.20220510.502
26. Zhangchun G, Guoliang X, Ling Q. Meta analysis of the efficacy and safety of Qishenyiqi drip pill on chronic congestive heart failure. J Emerg Traditional Chin Med. (2014) 23:232–4. doi: 10.3969/j.issn.1004-745X.2014.02.018
27. Ye T, Jianxia G. Meta-analysis of Qishen Yiqi drops in the treatment of heart failure in coronary artery disease. J Emerg Traditional Chin Med. (2016) 25:1725–7+42. doi: 10.3969/j.issn.1004-745X.2016.09.025
28. Qiuyue S, Wen Z, Lu L, Lingyan L, He S, Zhixin G. Meta-analysis of the effect of inhibiting ventricular remodeling in patients with chronic heart failure with Qishen Yiqi drops. Contemporary Med Forum. (2017) 15:71–3.
29. Genhao F, Zuoying X, Zhaoqi C, Mengmeng Z, Yucai H, Anshe Z, et al. Systematic review of Qishen Yiqi dropping pills in the treatment of coronary heart disease combined with heart failure. Chin J Basic Med Traditional Chin Med. (2020) 26:932–5+97. doi: 10.3969/j.issn.1006-3250.2020.07.022
30. Jiao G, Xincan L, Tianfu S. Clinical efficacy and safety of Qishenyiqi gutta pills combined with western medicine in treatment of chronic heart failure. Chin J Evidence Bases Cardiovasc Med. (2020) 12:519–24. doi: 10.3969/j.issn.1674-4055.2020.05.03
31. Xuejing L, Huiling Z, Xiaoli W, Shengnan G, Yang L, Guoqiang L. Evidence-based pharmacoeconomic evaluation of Qishen Yiqi dripping pills in the treatment of chronic heart failure. Eval Anal Drug Use Hospitals China. (2020) 20:1472–7+82. doi: 10.14009/j.issn.1672-2124.2020.12.017
32. Feng X, Guangjing D, Bin W, Peifeng W, Lin C, Min L. Effect of Qishen Yiqi dripping pills combined with trimetazidine on treatment of chronic heart failure: a meta analysis. J Hainan Med Univ. (2021) 27:689–94+700. doi: 10.13210/j.cnki.jhmu.20200814.004
33. Xiuwen Z, Yanyan D, Zongliang W, Haoxin Y, Yu X. Meta-analysis of Qishen Yiqi dripping pills combined with trimexazidine in the treatment of chronic heart failure. World J Integrated Traditional Western Med. (2021) 16:23–8+33. doi: 10.13935/j.cnki.sjzx.210105
34. Zhenchao N, Wenyong L, Yiping L, Tingting Q, Xiaolong W. Meta-analysis of clinical effects according to syndrome differentiation of Qishen Yiqi dropping pills on chronic heart failure. Drug Eval Res. (2021) 44:1076–87. doi: 10.7501/j.issn.1674-6376.2021.05.027
35. Hao P, Jiang F, Cheng J, Ma L, Zhang Y, Zhao Y. Traditional Chinese medicine for cardiovascular disease: evidence and potential mechanisms. J Am Coll Cardiol. (2017) 69:2952–66. doi: 10.1016/j.jacc.2017.04.041
36. Mao J, Zhang J, Lam CSP, Zhu M, Yao C, Chen S, et al. Qishen Yiqi dripping pills for chronic ischaemic heart failure: results of the CACT-IHF randomized clinical trial. ESC Heart Fail. (2020) 7:3881–90. doi: 10.1002/ehf2.12980
37. Gopalakrishnan S, Ganeshkumar P. Systematic reviews and meta-analysis: understanding the best evidence in primary healthcare. J Family Med Prim Care. (2013) 2:9–14. doi: 10.4103/2249-4863.109934
38. Stewart L, Moher D, Shekelle P. Why prospective registration of systematic reviews makes sense. Syst Rev. (2012) 1:7. doi: 10.1186/2046-4053-1-7
39. PLoS Medicine Editors,. Best practice in systematic reviews: the importance of protocols and registration. PLoS Med. (2011) 8:e1001009. doi: 10.1371/journal.pmed.1001009
40. Tharyan P, Ghersi D. Registering clinical trials in India: a scientific and ethical imperative. Natl Med J India. (2008) 21:31–4.
41. Schunemann HJ, Tugwell P, Reeves BC, Akl EA, Santesso N, Spencer FA, et al. Non-randomized studies as a source of complementary, sequential or replacement evidence for randomized controlled trials in systematic reviews on the effects of interventions. Res Synth Methods. (2013) 4:49–62. doi: 10.1002/jrsm.1078
42. Booth A. Searching for qualitative research for inclusion in systematic reviews: a structured methodological review. Syst Rev. (2016) 5:74. doi: 10.1186/s13643-016-0249-x
43. Yank V, Rennie D, Bero LA. Financial ties and concordance between results and conclusions in meta-analyses: retrospective cohort study. BMJ. (2007) 335:1202–5. doi: 10.1136/bmj.39376.447211.BE
44. Cheng CW, Wu TX, Shang HC, Li YP, Altman DG, Moher D, et al. Consort extension for Chinese herbal medicine formulas 2017: recommendations, explanation, and elaboration. Ann Intern Med. (2017) 167:112–21. doi: 10.7326/M16-2977
45. Luo Y, Chen J, Chen Y, Su Y, Wu X, Zheng W, et al. Qishen Yiqi dropping pills improve isoproterenol-induced cardiomyocyte hypertrophy by regulating X-inactive specific transcript (Xist) expression in rats. J Thorac Dis. (2022) 14:2213–23. doi: 10.21037/jtd-22-606
46. Huang Y, Zhang K, Liu M, Su J, Qin X, Wang X, et al. An herbal preparation ameliorates heart failure with preserved ejection fraction by alleviating microvascular endothelial inflammation and activating NO-cGMP-PKG pathway. Phytomedicine. (2021) 91:153633. doi: 10.1016/j.phymed.2021.153633
47. Lu Y, Wang D, Yuan X, Wang M, Li Z, Bao X, et al. Protective effect of Qishen Yiqi dropping pills on the myocardium of rats with chronic heart failure. Exp Ther Med. (2019) 17:378–82. doi: 10.3892/etm.2018.6957
48. Wang L, Wang L, Zhou X, Ruan G, Yang G. Qishen Yiqi dropping pills ameliorates doxorubicin-induced cardiotoxicity in mice via enhancement of cardiac angiogenesis. Med Sci Monit. (2019) 25:2435–44. doi: 10.12659/MSM.915194
Keywords: Qishen Yiqi drop pill, chronic heart failure, systematic review, meta-analyses, overview
Citation: Chen W, Chen J, Wang Y, Yan J, Yan X, Wang D and Liu Y (2022) The role of Qishen Yiqi dripping pills in treating chronic heart failure: An overview of systematic reviews and meta-analyses. Front. Cardiovasc. Med. 9:1001072. doi: 10.3389/fcvm.2022.1001072
Received: 22 July 2022; Accepted: 03 October 2022;
Published: 24 October 2022.
Edited by:
Yuling Zhang, Sun Yat-sen Memorial Hospital, ChinaReviewed by:
Ailan Chen, First Affiliated Hospital of Guangzhou Medical University, ChinaXiao Hong Wei, Peking University, China
Copyright © 2022 Chen, Chen, Wang, Yan, Yan, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuntao Liu, aWFtbGl1eXVudGFvQDE2My5jb20=; Dawei Wang, ZGF2aWRAZ3p1Y20uZWR1LmNu
 Wensheng Chen1
Wensheng Chen1 Yuanping Wang
Yuanping Wang Yuntao Liu
Yuntao Liu