Abstract
Background:
The role of worsening renal function during acute heart failure (AHF) hospitalization is still debated. Very few studies have extensively evaluated the renal function (RF) trend during hospitalization by repetitive measurements.
Objectives:
To investigate the prognostic relevance of different RF trajectories together with the congestion status in hospitalized patients.
Methods:
This is a post hoc analysis of a multi-center study including 467 patients admitted with AHF who were screened for the Diur-AHF Trial. We recognized five main RF trajectories based on serum creatinine and estimated glomerular filtration rate (eGFR) behavior. According to the RF trajectories our sample was divided into 1-stable (S), 2-transient improvement (TI), 3-permanent improvement (PI), 4-transient worsening (TW), and 5-persistent worsening (PW). The primary outcome was the combined endpoint of 180 days including all causes of mortality and re-hospitalization.
Results:
We recruited 467 subjects with a mean congestion score of 3.5±1.08 and a median creatinine value of 1.28 (1.00–1.70) mg/dl, eGFR 50 (37–65) ml/min/m2 and NTpro B-type natriuretic peptide (BNP) 7,000 (4,200–11,700) pg/ml. A univariate analysis of the RF pattern demonstrated that TI and PW patterns were significantly related to poor prognosis [HR: 2.71 (1.81–4.05); p < 0.001; HR: 1.68 (1.15–2.45); p = 0.007, respectively]. Conversely, the TW pattern showed a significantly protective effect on outcome [HR:0.34 (0.19–0.60); p < 0.001]. Persistence of congestion and BNP reduction ≥ 30% were significantly related to clinical outcome at univariate analysis [HR: 2.41 (1.81–3.21); p < 0.001 and HR:0.47 (0.35–0.67); p < 0.001]. A multivariable analysis confirmed the independently prognostic role of TI, PW patterns, persistence of congestion, and reduced BNP decrease at discharge.
Conclusions:
Various RF patterns during AHF hospitalization are associated with different risk(s). PW and TI appear to be the two trajectories related to worse outcome. Current findings confirm the importance of RF evaluation during and after hospitalization.
Introduction
Renal function (RF) deterioration occurring in acute heart failure (AHF) is one of the most important features during hospitalization with several repercussions on treatment and prognosis. Interventional trials reported a wide percentage of worsening renal function (WRF) ranging from 20 to 40% with a different prognostic impact (1–3). The unreadable relationship existing between WRF appearance and heart failure (HF) outcome is currently under debate, and the question of whether renal function impairment is just a marker or a true component of HF severity remains unanswered (4). Several definitions have been suggested to elucidate the exact role and prevalence of renal dysfunction in the setting of HF (5, 6). Some studies report that WRF is a detrimental factor in HF deterioration and outcome. Other authors described this status as a simple condition linked to decongestion therapy and the high loop diuretic amount administered during the acute phase (7–10). Alternatively, an increased neurohormonal overdrive and altered renal blood flow redistribution may be implicated. Predisposing common risk factors, such as diabetes, smoking, metabolic disorders, and hypertension, could amplify the cardiac and kidney atherosclerosis process and increase neurohormonal overdrive, leading to a final systemic and renal hemodynamic derangement (11). This assessment should be considered during AHF hospitalization and should be interpreted by looking at both the individual patients and their primary cardiac and renal disorder. The bidirectional nature and the specific mechanisms related to the vicious circle of coexisting cardiac and renal deterioration still need to be completely explained. Indeed, the current cardio-renal syndrome type 1 (CRS-1) update only recognizes the primary and secondary organ damage (12, 13). Unfortunately, this definition does not yet provide an extensive interplay regarding the pathophysiological cross-talk, including predisposing factors, haemodynamic derangement, and preliminary kidney condition, implicated in its occurrence and organ deterioration (14). Notably, several studies have shown significant fluctuation of renal function during hospitalization that likely reflects the diversity of the RF pattern in these patients (15–18). Accordingly, a more complete mechanistic approach should include serial measurement, a basal renal evaluation at admission, a serial assessment during hospitalization, and monitoring after discharge (5, 19). Thus, the identification of specific renal trajectories occurring during acute treatment appear mandatory in order to better understand the individual heterogeneity of renal pattern and the related risk. Therefore, a better recognition of RF changes over hospitalization could improve insights into the assessment existing among HF conditions, congestion, and outcome. Notably, we divided our patients according to RF fluctuations during the hospitalization period and we recognized 5 main subtypes. Then, we evaluated each subtypes in relation to the prognosis during a mean follow up period of 6 months.
Methods
Study Design
This is a retrospective post hoc analysis of a multi-center study including 467 patients admitted with AHF who were screened for the Diur-AHF Trial (20) and enrolled in both the Cardiovascular Diseases Unit of Internal Medicine Department and Cardiology Unit of Siena Hospital and the Cardiology Section of Regina Montis Regalis Hospital of Mondovì (Cuneo). The patients screened were over the age of 18 years and were admitted with dyspnoea, evidence of volume overload, and/or clinical signs of HF (peripheral edema, rales, third heart sound, jugular turgor, lung congestion on chest X-ray) in whom a diagnosis of AHF was confirmed by chest X-ray and/or elevated (>1,500 pg/ml) levels of Amino-terminal (NT) pro-B-type natriuretic peptide (BNP). The excluded patients were those with end-stage (serum creatinine levels > 4.0 mg/dL) renal disease or the need for renal replacement therapy (dialysis or ultrafiltration), a recent myocardial infarction (within 30 days of screening), a systolic blood pressure <80 mm Hg, or the needing of vasoactive or inotropic drugs infusion during hospitalization. We did not screen patients with known liver or neoplastic disease or concurrent infective disease. All patients gave their written informed consent. This study was approved by the local ethics committee of Siena Hospital (C.E.A.V.S.E.).
Laboratory Analysis
Renal function parameters, including creatinine, estimated glomerular filtration rate (eGFR), and blood urea nitrogen (BUN), were measured from blood samples taken at admission, 3 days after admission, and before discharge. Renal function parameters were monitored every 48 h. The eGFR was calculated using the four-variable Modification of Diet in Renal Disease (MDRD) formula. Chronic Kidney Disease (CKD) was defined by creatinine > 1.2 mg/dl and/or eGFR <60 ml/min/1.73 m2 before admission (21, 22). A rise in serum creatinine ≥0.3 mg/dl or eGFR reduction ≥ 20% were used according to conventional criteria to define worsening renal function (WRF). NTpro BNP was also measured within 24 h of hospital admission and before discharge.
RF Pattern Definition
We identified 5 main trajectories based on changes in creatinine and GFR summarized in Figure 1:
-
Stable pattern (S) identifies patients with no substantial or minimal differences in renal function;
-
Bump pattern: a transient improvement (TI) of 0.2 or > 20% in creatinine and GFR, respectively, followed by a subsequent deterioration;
-
Permanent improvement (PI) characterized by gradual amelioration of creatinine and GFR during hospitalization;
-
Transient worsening (TW) of Renal function followed by an amelioration before discharge;
-
Persistent worsening (PW) due to progressive impairment of Creatinine and GFR from admission to discharge.
Figure 1
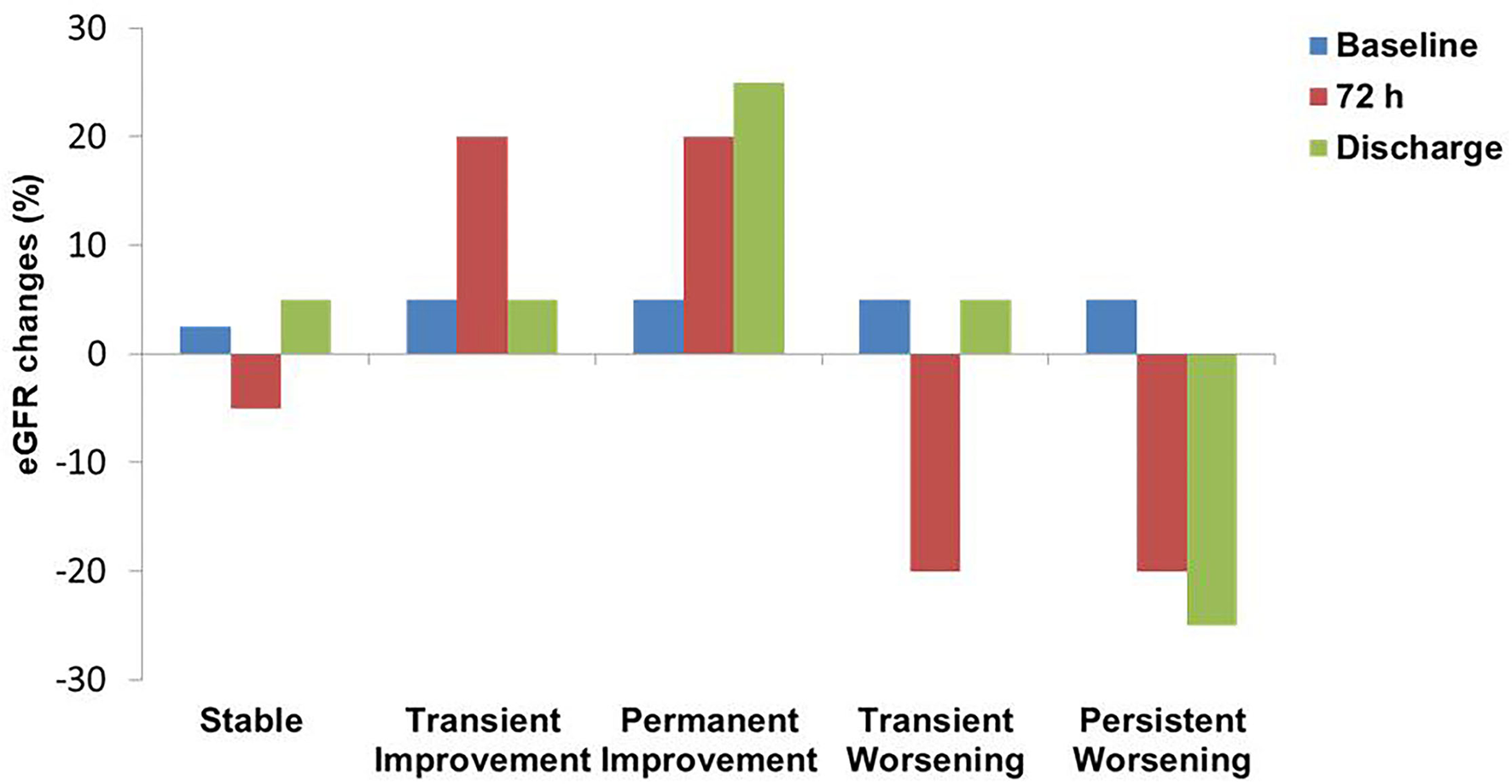
Renal function patterns definition according to eGFR fluctuations. eGFR, estimated Glomerular filtration rate.
Clinical Assessment and Evaluation of Congestion
All these patterns were compared with a clinical evaluation of congestion at admission and before discharge, grading congestion by the assessment of the following clinical signs: pulmonary rales, third heart sound, jugular venous distention, peripheral oedema, hepatomegaly, and dyspnoea at rest or orthopnoea for a total of maximum 6 points based on Gheorghiade criteria (23). Persistence of congestion was defined as the persistence of 3 signs of congestion at discharge or less if the patients did not achieve the complete resolution of two or more clinical signs of HF at discharge.
End Points
(1) To discern among different RF patterns during hospitalization for Acute decompensated Heart failure (ADHF); (2) To evaluate whether various RF subtypes were associated with different outcomes in terms of combined endpoint of mortality and HF rehospitalization during a 180-day follow-up.
Follow-Up
Patients were followed up for 180 days after discharge with clinical visits and telephone contacts. The primary outcome of interest was a composite of all-cause mortality (ACM) or cardiovascular (CV) re-hospitalization.
Statistical Analysis
Categorical data are presented as numbers and percentages and were analyzed with chi-square test. Particularly, normally distributed continuous data were presented as mean ± SD and non-normally distributed continuous variables were presented as median and interquartile range [IQR]. Patients with AHF were grouped by phenotypes according to WRF subtypes, significant decongestion during hospitalization, and CKD. Differences in baseline characteristics for continuous variables were evaluated using appropriate procedures like the T-Student test or Mann Whitney test if two groups were compared. Differences among more than two groups were analyzed with ANOVA and Kruskal-Wallis tests. Kaplan-Meier curves with the log-rank statistics were used to illustrate event rates at the time point of interest. Different multivariable Cox proportional hazard regression models were used to investigate the relationship between WRF subtypes and outcomes. Multivariable models were adjusted for clinical variables of interest (age, gender, hypertension, diabetes, dyslipidemia, coronary artery disease, history of HF, and LVEF) chosen prospectively a priori. Estimates are presented as hazard ratios (HR) with 95% CI. We considered statistically significant results associated with a p ≤ 0.05. We used the SPSS software (version 20.0) for all analyses.
Results
The initial study population consisted of 499 patients enrolled in three different hospitals (the Cardiology Unit of Mondovi Hospital, the Cardiology Unit, and Internal Medicine Unit of Le Scotte Hospital, Siena) with a primary diagnosis of AHF. Twelve patients were lost during the follow-up period and 20 patients were not included for lack of complete clinical and laboratory examination. Among the remaining 467 subjects 55.5% were males with a median age of 78 (67–84) years. Median LVEF was 38% (29–45), mean congestion score was 3.5±1.08, and median creatinine value was 1.28 (1.00–1.70) mg/dl, median eGFR and NTproBNP were 50 (37–65) ml/min /m2 and 7,000 (4,200–11,700) pg/ml, respectively. Complete clinical and laboratory characteristics were divided between patients with adverse events occurrence and those patients free of adverse events are described in Table 1.
Table 1
| Variables | Patients without adverse events*(n. 275) |
Patients with adverse events occurrence*
(n. 192) |
p-value |
|---|---|---|---|
| Age (years) | 78 [67–84] | 78 [67–85] | 0.785 |
| Gender male (%) | 62.2 | 45.8 | <0.001 |
| CV risk factors (%) | |||
| Hypertension | 63.6 | 72.9 | 0.035 |
| Diabetes | 31.4 | 37.5 | 0.170 |
| Dyslipidemia | 21.1 | 23.6 | 0.528 |
| CAD | 48.0 | 66.7 | <0.001 |
| CKD | 48.0 | 59.9 | 0.011 |
| Atrial fibrillation | 17.5 | 35.4 | <0.001 |
| HF etiology | |||
| Hypertensive | 50.9 | 42.2 | 0.063 |
| Ischemic | 20.4 | 25.0 | 0.236 |
| Valvular | 16.7 | 21.9 | 0.162 |
| Primitive | 12.0 | 10.9 | 0.724 |
| Echocardiography | |||
| LVEF (%) | 40 [30–45] | 35 [25–45] | 0.078 |
| LVEDD (mm) | 54 [48–59] | 55 [48–61] | 0.194 |
| LVESD (mm) | 38 [32–45] | 40 [33–47] | 0.127 |
| Basal RVEDD (mm) | 37 [36–43] | 38 [36–44] | 0.161 |
| TAPSE (mm) | 20 [18–22] | 20 [16–22] | 0.025 |
| IVC (mm) | 22 [20–23] | 22 [20–24] | 0.203 |
| PASP (mmHg) | 40 [35–50] | 45 [35–50] | 0.464 |
| E/e' | 15 [13–16] | 15 [14–16] | 0.724 |
| Systolic arterial pressure | 134 [126–140] | 135 [126–145] | 0.402 |
| Admission congestion score | 3.3 [±1.1] | 3.8 [±0.9] | <0.001 |
| Discharge congestion score | 0.8 [±1.1] | 1.9 [±1.0] | <0.001 |
| Admission serum creatinine (mg/dL) | 1.21[0.98–1.62] | 1.43 [1.00–1.86] | 0.007 |
| Discharge serum creatinine (mg/dL) | 1.25 [1.00–1.66] | 1.49 [1.08–2.00] | <0.001 |
| Admission eGFR (mL/min/m2) | 52 [38–66] | 48 [33–63] | 0.049 |
| Discharge eGFR (mL/min/m2) | 52 [36–69] | 45 [30–61] | 0.002 |
| In-hospital IV mean daily furosemide dosage (mg/die) | 100 [80–120] | 125 [120–150] | <0.001 |
| Admission NTproBNP (pg/mL) | 7,100 [4,188–11,297] | 6,837 [4,202–13,269] | 0.784 |
| Discharge NTproBNP (pg/mL) | 2,664 [1,178–6,021] | 4,627 [1,906–7,888] | <0.001 |
| Previous CHF (%) | 52.7 | 61.5 | 0.061 |
| ICD (%) | 12.4 | 12.0 | 0.901 |
| Home therapy (%) | |||
| Loop diuretics | 53.8 | 60.9 | 0.127 |
| ACEis/ARBs/ARNI | 81.8 | 58.9 | <0.001 |
| Beta Blockers | 82.2 | 61.5 | <0.001 |
| MRAs | 6.9 | 13.5 | 0.017 |
| Digoxin | 0.0 | 18.8 | <0.001 |
| Ivabradin | 12.7 | 24.0 | 0.002 |
Clinical and laboratory characteristics according to adverse events development or not.
ACEis, Angiotensin Converting Enzyme Inhibitors; ARBs, Angiotensin receptor blockers; ARNI, Angiontensin Receptor Neprilysin Inhibitor; CV, Cardiovascular; CHF, Chronic heart failure; CKD, Chronic Kidney Disease; CAD, Coronary artery disease; eGFR, estimated Glomerular filtration rate; HF, Heart Failure; ICD, Implantable cardiac defibrillator; IV, Intravenous; IVC, Inferior Cave Vein; LVEDD, Center ventricular end-diastolic diameter; LVEF, Center ventricular ejection fraction; LVESD, Center ventricular end-systolic diameter; MRAs, Mineralocorticoid receptor antagonists; NTproBNP, Aminoterminal pro B-type natriuretic peptide; PASP, Pulmonary artery systolic pressure; RVEDD, Right ventricular end-diastolic diameter; TAPSE, Tricuspid anular plase systolic excursion.
Events are defined as the composite of all-cause mortality (ACM) or cardiovascular (CV) re-hospitalization.
Baseline Characteristics
Baseline characteristics of different trajectories are shown in Table 2. Overall, there are no significant differences among different trajectories regarding risk factor prevalence, except for dyslipidaemia, which is significantly prevalent in patients with PW compared to TW, PI, TI, and stable S (27.5 vs. 14.4, vs. 12.7 vs. 24.6 vs. 26.6%, respectively; p = 0.039). The admission congestion score resulted significantly higher in S, TI, and PW groups with respect to PI and TW groups [3.63 (±1.02) vs. 3.53 (±1.05) vs. 3.80 (±1.10) vs. 3.27 (±1.10) vs. 3.13 (±1.05), respectively; p < 0.001]. Echocardiographic measurements of right ventricle, pulmonary pressures, and left ventricle filling pressures were not different among the groups. CKD was more prevalent in patients who experienced renal function in-hospital improvement (group TI and PI) with respect to S, TW, and PW groups (71.9 and 71.4% vs. 37.3%, 57.1 and 50.5% respectively; p < 0.001). Conversely, patients of S, TW, and PW groups demonstrated better baseline renal function variables (creatinine and eGFR) as reported in Table 2.
Table 2
| Renal function patterns (n. of patients) | ||||||
|---|---|---|---|---|---|---|
| Variables | S (158) | TI (57) | PI (63) | TW (98) | PW (91) | p–value |
| Age (years) | 80 [69–84] | 77 [65–84] | 81 [74–88] | 77 [64–84] | 75 [66–81] | 0.001 |
| Gender Male (%) | 51.3 | 36.8 | 61.9 | 62.2 | 62.6 | 0.008 |
| CV risk factors (%) | ||||||
| Hypertension | 63.3 | 68.4 | 65.1 | 67.3 | 75.8 | 0.362 |
| Diabetes | 32.9 | 42.9 | 33.3 | 33.7 | 30.8 | 0.648 |
| Dyslipidemia | 26.6 | 24.6 | 12.7 | 14.4 | 27.5 | 0.039 |
| CAD | 55.7 | 61.4 | 57.1 | 50.5 | 57.1 | 0.704 |
| CKD | 37.3 | 71.9 | 71.4 | 57.1 | 50.5 | <0.001 |
| Atrial fibrillation | 17.1 | 36.8 | 30.2 | 21.4 | 30.8 | 0.01 |
| Echocardiography | ||||||
| LVEF (%) | 35 [30–50] | 40 [30–45] | 40 [27–50] | 39 [29–45] | 35 [25–45] | 0.467 |
| LVEDD (mm) | 55 [49–59] | 54 [48–59] | 53 [45–58] | 54 [47–60] | 55 [49–63] | 0.279 |
| LVESD (mm) | 39 [33–45] | 39 [33–44] | 39 [31–43] | 38 [31–46] | 40 [34–48] | 0.204 |
| Basal RVEDD (mm) | 38 [36–44] | 37 [36–43] | 37 [36–43] | 36 [35–40] | 38 [35–44] | 0.283 |
| TAPSE (mm) | 20 [18–22] | 20 [18–22] | 20 [16–22] | 21 [19–22] | 20 [16–22] | 0.460 |
| IVC (mm) | 22 [20–23] | 22 [20–23] | 22 [20–24] | 22 [20–23] | 22 [21–24] | 0.223 |
| PASP (mmHg) | 40 [35–50] | 45 [35–45] | 40 [35–50] | 40 [35–45] | 45 [35–50] | 0.169 |
| E/e' | 15 [14–17] | 14 [12–15] | 14 [14–16] | 14 [12–16] | 15 [14–16] | 0.090 |
| Systolic arterial pressure | 135 [126–140] | 133 [125–147] | 135 [130–145] | 130 [125–140] | 135 [130–145] | 0.598 |
| Admission congestion score | 3.63 [±1.02] | 3.53 [±1.05] | 3.27 [±1.10] | 3.13 [±1.05] | 3.80 [±1.10] | <0.001 |
| Discharge congestion score | 1.15 [±1.17] | 1.63 [±1.36] | 1.11 [±1.17] | 0.95 [±1.11] | 1.55 [±1.38] | 0.001 |
| Admission serum creatinine (mg/dL) | 1.03 [0.84–1.43] | 1.60 [1.30–2.07] | 1.73 [1.29–2.20] | 1.25 [1.03–1.62] | 1.23 [0.99–1.63] | <0.001 |
| Discharge serum creatinine (mg/dL) | 1.08 [0.90–1.41] | 1.70 [1.40–2.24] | 1.31 [1.00–1.70] | 1.29 [1.00–1.73] | 1.66 [1.27–2.26] | <0.001 |
| Admission eGFR (mL/min/m2) | 58 [46–77] | 39 [32–53] | 35 [25–51] | 49 [36–64] | 49 [38–66] | <0.001 |
| Discharge eGFR (mL/min/m2) | 61 [45–75] | 35 [28–52] | 52 [35–67] | 49 [34–65] | 37 [24–53] | <0.001 |
| In–hospital IV mean daily furosemide dosage (mg/die) | 120 [100–120] | 125 [120–150] | 125 [100–150] | 100 [80–120] | 120 [120–175] | <0.001 |
| Admission NTproBNP (pg/mL) | 6,720 [3,863–11,325] | 6,440 [2,824–10,700] | 7,230 [4,939–14,200] | 7,545 [4,478–11,246] | 7,520 [4,826–16,956] | 0.366 |
| Discharge NTproBNP (pg/mL) | 3,110 [1,184–7,238] | 3,147 [1,187 −9,687] | 3,358 [1,853–5,790] 60.3 | 2,782 [998–6,135] | 4,594 [2,100–7,126] | 0.062 |
| Previous CHF (%) | 53.2 | 60.3 | 66.7 | 58.2 | 50.5 | 0.298 |
| ICD (%) | 12.7 | 14.0 | 11.1 | 5.1 | 18.7 | 0.076 |
| Home therapy (%) | ||||||
| Loop diuretics | 50.0 | 70.2 | 71.4 | 56.1 | 50.5 | 0.007 |
| ACEis/ARBs/ARNI | 72.2 | 71.9 | 68.3 | 81.6 | 65.9 | 0.157 |
| Beta Blockers | 72.0 | 75.4 | 69.8 | 80.6 | 70.3 | 0.446 |
| MRAs | 9.5 | 17.5 | 4.8 | 6.1 | 12.1 | 0.098 |
| Digoxin | 5.7 | 17.5 | 6.3 | 2.0 | 12.0 | 0.003 |
| Ivabradin | 19.0 | 8.8 | 12.7 | 17.3 | 23.1 | 0.180 |
| Death (%) | 22.8 | 40.4 | 31.7 | 10.2 | 25.3 | <0.001 |
| Rehospitalization (%) | 17.1 | 28.1 | 12.7 | 5.1 | 26.4 | <0.001 |
Differences in clinical and laboratory characteristics according to renal function patterns.
ACEis, Angiotensin Converting Enzyme Inhibitors; ARBs, Angiotensin receptor blockers; ARNI, Angiontensin Receptor Neprilysin Inhibitor; CV, Cardiovascular; CHF, Chronic heart failure; CKD, Chronic Kidney Disease; CAD, Coronary artery disease; eGFR, estimated Glomerular filtration rate; ICD, Implantable cardiac defibrillator; IV, Intravenous; IVC, Inferior Cave Vein; LVEDD, Center ventricular end-diastolic diameter; LVEF, Center ventricular ejection fraction; LVESD, Center ventricular end-systolic diameter; MRAs, Mineralocorticoid receptor antagonists; NTproBNP, Aminoterminal pro B-type natriuretic peptide; S, Stable; PASP, Pulmonary artery systolic pressure; PI, Permanent improvement; PW, Persistent worsening; RVEDD, Right ventricular end-diastolic diameter; TAPSE, Tricuspid anular plase systolic excursion; TI, Transient improvement; TW, Transient worsening.
Renal Trajectories and Congestion
Of the 467 recruited patients, 192 (41,1%) encountered the primary composite outcome defined as death or CV hospitalization during 180 days of follow up. Among these patients, 112 died and 80 were re-hospitalized due to CV causes. Patients with an adverse composite outcome had a higher percentage of congestion at discharge compared to events free patients (44.8 vs. 17.5%; p < 0.001). Similarly, patients with NTproBNP decrease at discharge <30% experienced an increased adverse events rate compared to those with NTproBNP decrease more than 30% (52.1 vs. 28.4%; p < 0.001). Among different RF trajectories, TI and PW groups demonstrated a significantly (p < 0.001) higher rate of death (40.4 and 25.3%, respectively) and re-hospitalization (28.1 and 26.4%, respectively) compared to other groups (Table 2).
Composite Outcome
A univariate analysis of the renal function pattern demonstrated that the TI pattern was significantly related to poor prognosis [HR: 2.71 (1.81–4.05); p < 0.001] as was the PW pattern [HR: 1.68 (1.15–2.45); p = 0.007]. Conversely, the TW pattern showed a significantly protective effect on outcome [HR:0.34 (0.19–0.60); p < 0.001]. Persistence of congestion and BNP reduction ≥30% were significantly related to clinical outcome at univariate analysis [HR: 2.41 (1.81–3.21); p < 0.001 and HR:0.47 (0.35–0.67); p < 0.001]. Similarly, in the univariate analysis, CKD was related to poor prognosis [HR: 1.52 (1.14–2.04); p = 0.004; Table 3]. Multivariable analysis including renal function patterns and CKD confirmed the independent prognostic role of TI [HR: 2.39 (1.58–3.60); p < 0.001], TW [HR:0.31 (0.18–0.55); p < 0.001], PW [HR: 1.60 (1.10–2.34); p = 0.015] and CKD [HR: 1.51 (1.11–2,03]; p = 0.007] (Figure 2). A multivariable analysis combining renal function and persistence of congestion pattern confirmed the univariate analysis findings about TI [HR: 2.61 (1.75–3.91); p < 0.001], TW [HR:0.34 (0.20–0.60); p < 0.001], PW [HR: 1.52 (1.04–2.22); p = 0.032] and congestion persistence [HR: 2.29 (1.71–3.05); p < 0.001] (Table 3). Multivariable analysis including renal function patterns, persistence of congestion, BNP reduction ≥30% adjusted for age, gender, previous CHF, CKD, LVEF <50%, and CV risk factors confirmed the independent relation of TI [HR: 2.30 (1.52–3.50); p < 0.001], TW [HR:0.30 (0.17–0.55); p < 0.001], PW [HR: 1.51 (1.02–2.24); p = 0.04], persistence of congestion [HR: 1.87 (1.39–2.52); p < 0.001] and NTproBNP reduction≥30% [HR 0.65 (0.48–0.87); p = 0.004] with clinical outcome (Table 3). Kaplan Meier survival curves showed the significant relation among renal function trajectories and persistence of congestion with adverse events occurrence (p < 0.001) (Figure 3).
Table 3
| Variables | Univariate HR [CI] | p–value | Multivariablea HR[CI] | p–value | Multivariableb HR[CI] | p–value |
|---|---|---|---|---|---|---|
| Renal function patterns | ||||||
| TI | 2.71 [1.81–4.05] | <0.001 | 2.61 [1.75–3.91] | <0.001 | 2.30 [1.52–3.50] | <0.001 |
| PI | 1.22 [0.78–1.91] | 0.375 | 1.29 [0.83–2.02] | 0.256 | 1.13 [0.71–1.80] | 0.594 |
| TW | 0.34 [0.19–0.60] | <0.001 | 0.34 [0.20–0.60] | <0.001 | 0.30 [0.17–0.55] | <0.001 |
| PW | 1.68 [1.15–2.45] | 0.007 | 1.52 [1.04–2.22] | 0.032 | 1.51 [1.02–2.24] | 0.040 |
| S | Ref. | – | Ref | – | Ref | – |
| Persistence of congestion | 2.41 [1.81–3.21] | <0.001 | 2.29 [1.71–3.05] | <0.001 | 1.87 [1.39–2.52] | <0.001 |
| Δ NTproBNP reduction ≥ 30% | 0.47 [0.35–0.67] | <0.001 | – | – | 0.65 [0.48–0.87] | 0.004 |
| CKD | 1.52 [1.14–2.04] | 0.004 | – | – | 1.33 [0.98–1.82] | 0.067 |
Univariate and multivariable analysis for 180 days outcome prediction.
Analysis including renal function trajectories and persistence of congestion
Analysis adjusted for Age, Gender, previous CHF, LVEF <50% and CV risk factors
CKD, Chronic Kidney Disease; CI, Confidence Interval; HR, Hazard Ratio; NTproBNP, Aminoterminal pro B-type natriuretic peptide; PI, Permanent improvement; PW, P ersistent worsening; S, Stable; TI, Transient improvement; TW, Transient worsening.
Figure 2
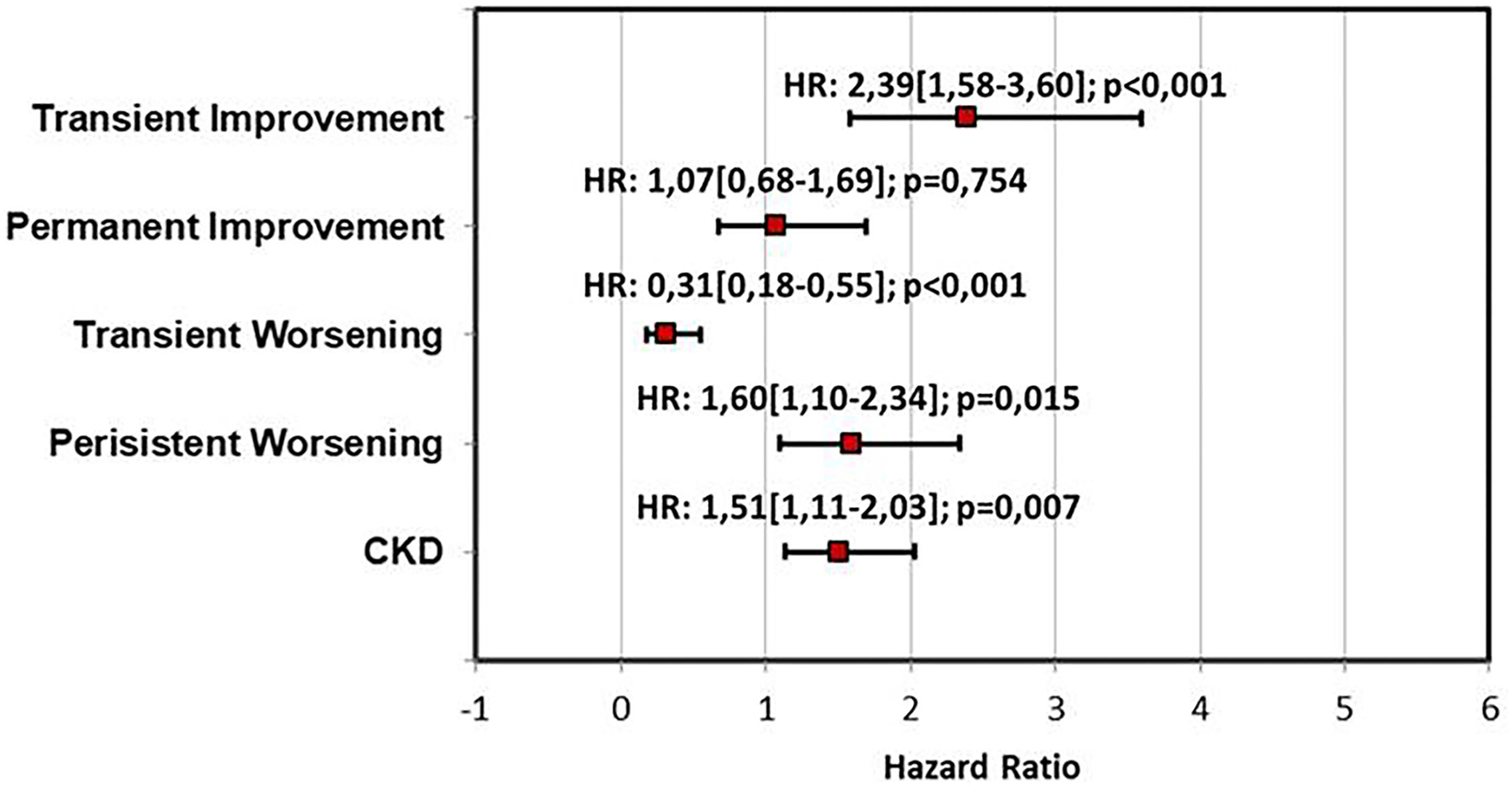
Multivariable analysis for outcome prediction including renal function patterns and CKD. CKD, Chronic Kidney Disease; HR, Hazard ratio.
Figure 3
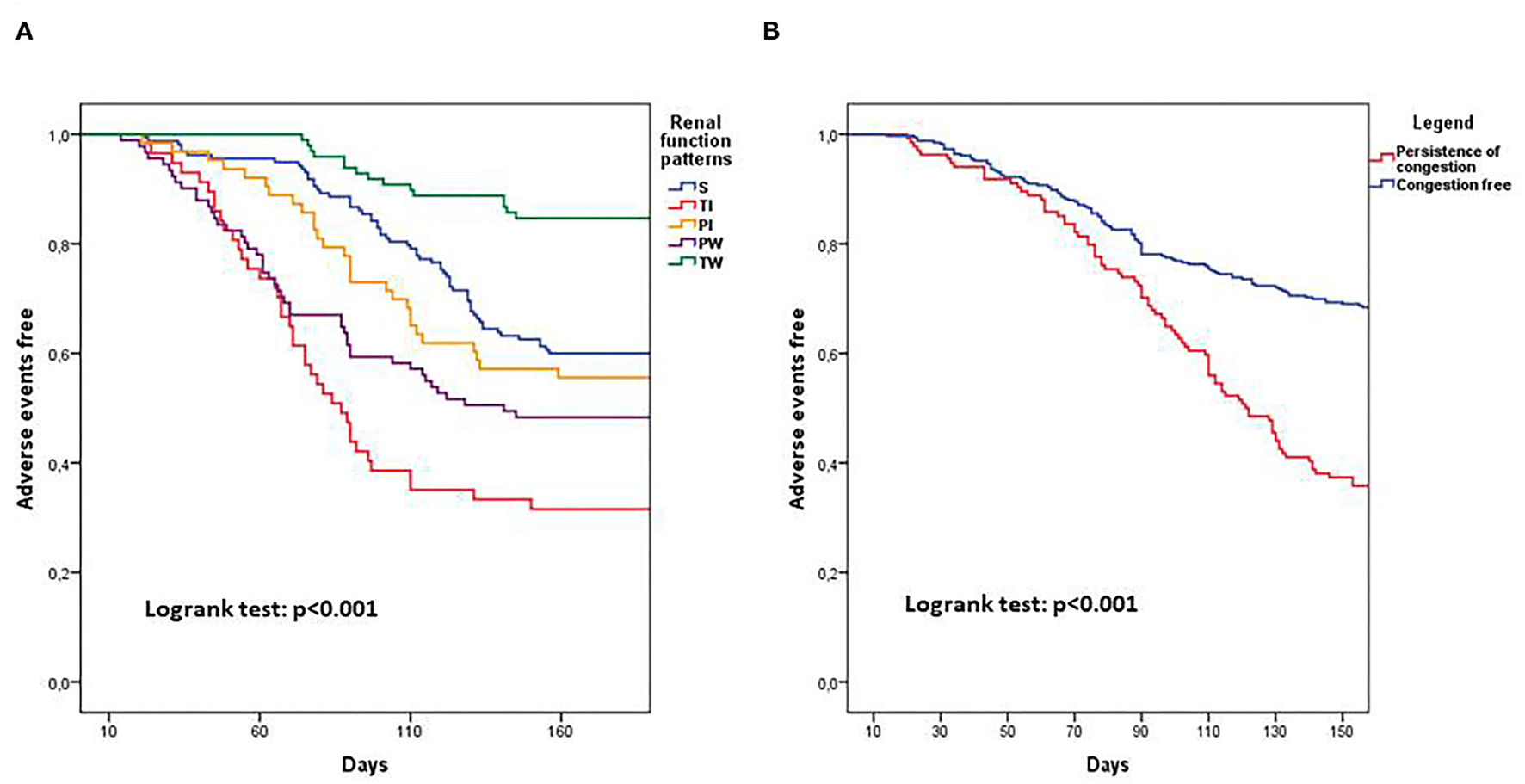
Kaplan Meier curves showing 180 days prognosis dividing patients for renal function patterns (A) and for persistence of clinical congestion (B). PI, Permanent improvement; PW, Persistent worsening; S, Stable; TI, Transient improvement; TW, Transient worsening.
Discussion
The current analysis has demonstrated that there are multiple subtypes of RF and each one likely comprises different mechanisms related to intrinsic kidney conditions, diuretic response, and congestion profile (24, 25). The study also explains some contrasting findings identified in the multiple analyses previously published, revealing a/the different impact of WRF. Indeed, the term WRF likely includes many patterns with different trajectories and specific significance (18, 19). Our results demonstrated that the TW group has a better outcome, similar to stable RF, whereas both permanent WRF and TI patterns showed a worse outcome. Our findings demonstrated that patients with permanent WRF and TI have a higher prevalence of unfavorable conditions, such as CAD, worse LVEF, and increased congestion, that could potentially impair outcome. Thus, the current findings may partially depend on adverse risk profile before admission. The recognition of different trajectories may be achieved only by repetitive blood sample measurements during the whole hospitalization period. Conversely, the simple evaluation of RF at admission and discharge as reported in most studies, is not sufficient to identify the real trend. A different RF pattern could be influenced by several features, such as the treatment adopted during the acute phase, baseline characteristics, presence of baseline CKD, and the congestion status (26). Notably, the far more dangerous pattern we identified needs to be contextualized according to the RF time course, CKD degree, systemic clinical conditions, haemodynamic status, and neurohormonal overdrive. Therefore, the intrinsic renal status may affect the different RF pattern. Hence, systemic blood pressure and kidney perfusion, increased central and renal venous pressure, tubulo glomerular feedback, and medullary and tubular state, are all potentially contributing factors (27, 28). Importantly, no universal agreement exists on when the renal blood marker must be measured in order to define and monitor RF in Acute decompensated Heart failure (ADHF) patients. Many trials have demonstrated that 30–40% of the patients hospitalized with HF experienced some WRF degrees (29, 30). However, not all studies agree about its prognostic role: an observational study showed that creatinine changes during hospitalization were independently associated with a higher risk of one-year mortality only in subjects with basal CKD (31). In the PROTECT Trial which included patients with some degree of CKD, many patients had a creatinine increase during hospitalization, and those experiencing more severe CKD showed a higher mortality during the 60 days follow-up (32). More recently, Holgado et al. demonstrated that AHF patients with more severe AKI degree had poorer prognosis (33). Interestingly, when WRF is associated with hemoconcentration or reduction in NTproBNP, it is not connected to an increased adverse event rate (34). Therefore, in a post hoc analysis of PROTECT in which serial measurements of RF were evaluated and defined in relation to specific trajectories, the authors did not find a remarkable difference among the different patterns (18). It is noteworthy that Testani et al., in a post hoc analysis, showed that patients with Improved Renal Function (IRF) had the worse prognosis compared with those with WRF (16). Similar findings have been recently replaced by Sai et al. and could be related to previous renal function deterioration before hospitalization or dynamic changes in central venous pressure during acute and post discharge phase (35). These contrasting findings suggest that RF should be evaluated during and after discharge to determine specific trends with their associated clinical characteristics and to detect those subtypes with increased risk (8).
Looking at congestion analysis, we showed that residual clinical congestion score before discharge is higher in permanent WRF and TI groups which are related to poor prognosis. Moreover, at multivariable analysis, the pre-discharge congestion is significantly related to adverse outcome. Although our findings may be influenced by the intravenous diuretic amount during the hospitalization period and population heterogeneity, they highlight the relevance of the concomitant measurements of hydro-saline retention and diuretic efficiency monitorization in the context of RF trajectories. Probably, in these groups, the persistence of congestion and in particular the presence of clinical signs of both pulmonary and peripheral congestion are the main driver of worse outcome but should be related also to diuretic resistance which was confirmed by the higher dosage of intravenous diuretics used during hospitalization (36). A proof of these theories appears to be confirmed by two post hoc analyses from PROTECT and RELAX-AHF trials, evaluating diuretic response during the hospitalization phase (37, 38). Accordingly, our data confirmed the strict relation among poor diuretic response, renal dysfunction, and congestion. Obviously, a multi-parametric assessment of congestion status through imaging integration of clinical congestion signs would have been most accurate in terms outcome prediction and fluid retention definition in this analysis (23). However, admission echocardiographic parameters such as pulmonary artery systolic pressures and inferior cave vein did not differ among groups as well as the index of left ventricle overload (E/e'). This finding of the current study confirmed the pivotal role of renal trajectories and clinical congestion in outcome prediction.
Interestingly, our analysis revealed that CKD was more prevalent in the groups with RF improvement (both transient and persistent). CKD severity appears related to adverse event occurrence, however, its impact may differ among different RF subtypes during hospitalization This data highlights the relevance of basal renal function status as one of the most important prognostic variables, and it suggests a need to look at both baseline RF and RF trajectories for better patient recognition. This appearance also confirms a previous metanalysis showing that CKD had a much greater unfavorable impact compared to WRF (39).
Limitations
This is a retrospective, observational multicenter study conducted in three tertiary hospitals where patients admitted for AHF were usually older and with more comorbidities than those enrolled in interventional clinical trials. This item might explain the high rate of adverse events in terms of re-hospitalization and mortality during follow-up (34). Also, only consenting patients considered suitable for the DIUR-AHF trial were screened and enrolled in this study, which introduces further selection bias. However, with respect to similar studies, our analysis was not influenced by additional drug and study protocols because our sample was treated according to guideline recommendations. In addition, treating physicians were not blinded to the clinical congestion assessment and renal function modifications that occurred during hospitalization, and different therapeutic choices might have influenced results. The RF definition was arbitrary although it reflects another similar study, and therefore, in our estimation, the definition of WRF does not exactly match the recent classification that indicates deterioration over a longer period of time (5, 18). The RF trend may be influenced by the change in diuretic infusion amount and diuretic response that were not included in our analysis. Importantly, a comprehensive clinical evaluation might also take time, and the diuretic dosage amount and infusional timing period might have influenced congestion degree and renal patterns. Congestion evaluation has been performed by clinical assessment and NTproBNP values, and a more integrated study should comprise a detailed ultrasound evaluation by B-lines, cava vein, and peripheral impedance examinations. In this study, there is the lack of laboratory assessment of multi-organ and hepatic dysfunction which are usually a typical feature of patients with HF in the decompensation phases. Finally, our renal function patterns and screening were based on the in-hospital trend that reflect a relatively short observational period, and a longer evaluation, with 3- and 6-month creatinine values measurement after discharge, should help us to identify further renal fluctuations potentially responsible for different outcomes.
Conclusions
Given the contrasting results regarding the prognostic relevance of different RF trajectories during AHF hospitalization, it becomes of paramount importance to identify the pattern with much more clinical relevance. The application of algorithms evaluating both different renal function changes and clinical congestion during the hospitalization period may help to distinguish subgroups with increased risk. Persistent deterioration and transient improvement appear to be the two patterns associated with increased risk. Further studies might be warranted to determine whether the contemporary assessment of these features could become an appropriate target for CRS-1 recognition and management.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
Study data will be available after an official request to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by CEAVSE. The patients/participants provided their written informed consent to participate in this study.
Author contributions
APal: conception and design and drafting of the manuscript. FC: statistical analysis and support for data interpretation. APag, AR, LL, and AB: data collection and interpretation. MF: final approval of the manuscript and critical revision. NG: data curation and interpretation. GR: design and drafting of the manuscript, statistical analysis and support for data interpretation, and critical revision. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.779828/full#supplementary-material
Supplementary Figure 1Study design of DIUR-AHF trial (20).
Supplementary Figure 2Scheme of loop diuretic infusion in DIUR-AHF trial (20).
- ADHF
Acute decompensated heart failure
- AHF
Acute heart failure
- AKI
Acute kidney injury
- ACM
All-cause mortality
- NT-proBNP
Amino terminal pro B-type natriuretic peptide
- CRS-1
Cardio-renal syndrome type 1
- CV
Cardiovascular
- CAD
Coronary artery disease
- CKD
Chronic kidney disease
- eGFR
Estimated Glomerular filtration rate
- HR
Hazard ratio
- HF
Heart failure
- IQR
Interquartile range
- LVEF
Left ventricular ejection fraction
- MDRD
Modification of Diet in Renal Disease
- PI
Permanent improvement
- PW
Persistent worsening
- RF
Renal function
- RD
Renal dysfunction
- S
Stable
- TI
Transient improvement
- TW
Transient worsening
- WRF
Worsening renal function.
Abbreviations
References
1.
Heywood JT Fonarow GC . High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE Database. J Card Fail. (2007) 13:422–30. 10.1016/j.cardfail.2007.03.011
2.
Abraham WT Fonarow GC Albert NM Stough WG Gheorghiade M Greenberg BH et al . OPTIMIZE-HF investigators and coordinators. predictors of in-hospital mortality in patients hospitalized for heart failure: insights from the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE-HF). J Am Coll Cardiol. (2008). 52:347–56. 10.1016/j.jacc.2008.04.028
3.
Smith GL Lichtman JH Bracken MB Shlipak MG Phillips CO DiCapua P et al . Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. (2006) 47:1987–96. 10.1016/j.jacc.2005.11.084
4.
Damman K Tang WH Testani JM McMurray JJ . Terminology and definition of changes renal function in heart failure. Eur Heart J. (2014) 35:3413–6. 10.1093/eurheartj/ehu320
5.
Mullens W Damman K Testani JM Martens P Mueller C Lassus J et al . Evaluation of kidney function throughout the heart failure trajectory - a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. (2020) 22:584–603. 10.1002/ejhf.1697
6.
Palazzuoli A Lombardi C Ruocco G Padeletti M Nuti R Metra M et al . Chronic kidney disease and worsening renal function in acute heart failure: different phenotypes with similar prognostic impact?Eur Heart J Acute Cardiovasc Care. (2016) 5:534–4810.1177/2048872615589511
7.
Nohria A Hasselblad V Stebbins A Pauly DF Fonarow GC Shah M et al . Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. (2008) 51:1268–74. 10.1016/j.jacc.2007.08.072
8.
Feng S Janwanishstaporn S Teerlink JR Metra M Cotter G Davison B et al . Association of left ventricular ejection fraction with worsening renal function in patients with acute heart failure: insights from the RELAX-AHF-2 study. Eur J Heart Fail. (2021) 23:58–67. 10.1002/ejhf.2012
9.
Klein L Massie BM Leimberger JD O'Connor CM Piña IL Adams KF Jr et al . Admission or changes in renal function during hospitalization for worsening heart failure predict postdischarge survival: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF). Circulation Heart Fail. (2008) 1:25–3310.1161/CIRCHEARTFAILURE.107.746933
10.
Metra M Davison B Bettari L Sun H Edwards C Lazzarini V et al . Is worsening renal function an ominous prognostic sign in patients with acute heart failure? the role of congestion and its interaction with renal function. Circ Heart Fail. (2012) 5:54–6210.1161/CIRCHEARTFAILURE.111.963413
11.
Cleland JG Carubelli V Castiello T Yassin A Pellicori P Antony R . Renal dysfunction in acute and chronic heart failure: prevalence, incidence and prognosis. Heart Fail Rev. (2012) 17:133–49. 10.1007/s10741-012-9306-2
12.
Damman K Testani JM . The kidney in heart failure: an update. Eur Heart J. (2015) 36:1437–44. 10.1093/eurheartj/ehv010
13.
Ronco C Cicoira M McCullough PA . Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. JACC (2012) 60:1031–4210.1016/j.jacc.2012.01.077
14.
Rangaswami J Bhalla V Blair JEA Chang TI Costa S Lentine KL et al . Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the american heart association. Circulation. (2019) 139:e840–78. 10.1161/CIR.0000000000000664
15.
Damman K Jaarsma T Voors AA Navis G Hillege HL van Veldhuisen DJ . COACH investigators. Both in- and out-hospital worsening of renal function predict outcome in patients with heart failure: results from the Coordinating Study Evaluating Outcome of Advising and Counseling in Heart Failure (COACH). Euro J Heart Fail. (2009) 11:847–54. 10.1093/eurjhf/hfp108
16.
Testani JM McCauley BD Chen J Coca SG Cappola TP Kimmel SE . Clinical characteristics and outcomes of patients with improvement in renal function during the treatment of decompensated heart failure. J Card Fail. (2011) 17:993–1000. 10.1016/j.cardfail.2011.08.009
17.
McCallum W Tighiouart H Testani JM Griffin M Konstam MA Udelson JE et al . Acute kidney function declines in the context of decongestion in acute decompensated heart failure. JACC Heart Fail. (2020) 8:537–47. 10.1016/j.jchf.2020.03.009
18.
Beldhuis IE Streng KW van der Meer P Ter Maaten JM O'Connor CM Metra M et al . Trajectories of changes in renal function in patients with acute heart failure. J Card Fail. (2019) 25:866–74. 10.1016/j.cardfail.2019.07.004
19.
Sinkeler SJ Damman K van Veldhuisen DJ Hillege H Navis G . A re-appraisal of volume status and renal function impairment in chronic heart failure: combined effects of pre-renal failure and venous congestion on renal function. Heart Failure Rev. (2011) 17:263–70. 10.1007/s10741-011-9233-7
20.
Palazzuoli A Ruocco G Vescovo G Valle R Di Somma S Nuti R . Rationale and study design of intravenous loop diuretic administration in acute heart failure: DIUR-AHF. ESC Heart Fail. (2017) 4:479–8610.1002/ehf2.12226
21.
Levey AS Coresh J Greene T Stevens LA Zhang YL Hendriksen S et al . Chronic kidney disease epidemiology collaboration. using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. (2006) 145:247–54. 10.7326/0003-4819-145-4-200608150-00004
22.
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. (2013) 3:1–150. 10.7326/0003-4819-158-11-201306040-00007
23.
Gheorghiade M Follath F Ponikowski P Barsuk JH Blair JE Cleland JG et al . European Society of Cardiology; European Society of Intensive Care Medicine. Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail. (2010) 12:423–3310.1093/eurjhf/hfq045
24.
Palazzuoli A Ruocco G Pellicori P Incampo E Di Tommaso C Favilli R et al . The prognostic role of different renal function phenotypes in patients with acute heart failure. Int J Cardiol. (2019) 276:198–203. 10.1016/j.ijcard.2018.11.108
25.
Shirakabe A Hata N Kobayashi N Okazaki H Matsushita M Shibata Y et al . Worsening renal function definition is insufficient for evaluating acute renal failure in acute heart failure. ESC Heart Fail. (2018) 5:322–33. 10.1002/ehf2.12264
26.
Valente MA Voors AA Damman K Van Veldhuisen DJ Massie BM O'Connor CM et al . Diuretic response in acute heart failure: clinical characteristics and prognostic significance. Eur Heart J. (2014) 35:1284–9310.1093/eurheartj/ehu065
27.
Testani JM Coca SG McCauley BD Shannon RP Kimmel SE . Impact of changes in blood pressure during the treatment of acute decompensated heart failure on renal and clinical outcomes. Euro J Heart Failure. (2011) 13:877–8410.1093/eurjhf/hfr070
28.
Palazzuoli A Testani JM Ruocco G Pellegrini M Ronco C Nuti R . Different diuretic dose and response in acute decompensated heart failure: clinical characteristics and prognostic significance. Intern Journal Cardiol. (2016) 224:213–910.1016/j.ijcard.2016.09.005
29.
Beldhuis IE Streng KW Ter Maaten JM Voors AA van der Meer P Rossignol P et al . Renin-Angiotensin system inhibition, worsening renal function, and outcome in heart failure patients with reduced and preserved ejection fraction: a meta-analysis of published study data. Circ Heart Fail. (2017) 10:e003588. 10.1161/CIRCHEARTFAILURE.116.003588
30.
Metra M Cotter G Senger S Edwards C Cleland JG Ponikowski P et al . Prognostic significance of creatinine increases during an acute heart failure admission in patients with and without residual congestion: a post hoc analysis of the PROTECT data. Circ Heart Fail. (2018) 11:e004644. 10.1161/CIRCHEARTFAILURE.117.004644
31.
Núñez J Garcia S Núñez E Bonanad C Bodí V Miñana G et al . Early serum creatinine changes and outcomes in patients admitted for acute heart failure: the cardio-renal syndrome revisited. Eur Heart J Acute Cardiovasc Care. (2017) 6:430–440. 10.1177/2048872614540094
32.
Voors AA Dittrich HC Massie BM DeLucca P Mansoor GA Metra M et al . Effects of the adenosine A1 receptor antagonist rolofylline on renal function in patients with acute heart failure and renal dysfunction: results from PROTECT (Placebo-Controlled Randomized Study of the selective adenosine a1 receptor antagonist rolofylline for patients hospitalized with acute decompensated heart failure and volume overload to assess treatment effect on congestion and renal function). J Am Coll Cardiol. (2011) 57:1899–907. 10.1016/j.jacc.2010.11.057
33.
Holgado JL Lopez C Fernandez A Sauri I Uso R Trillo JL et al . Acute kidney injury in heart failure: a population study. ESC Heart Fail. (2020) 7:415–22. 10.1002/ehf2.12595
34.
Salah K Kok WE Eurlings LW Bettencourt P Pimenta JM Metra M et al . Competing risk of cardiac status and renal function during hospitalization for acute decompensated heart failure. JACC Heart Fail. (2015) 3:751–61. 10.1016/j.jchf.2015.05.009
35.
Sai S Seo Y Nakagawa D Nakatsukasa T Kawamatsu N Sugano A et al . Clinical impacts of changes of renal function during hospitalization depend on grades of renal dysfunction in acute decompensated heart failure. Heart Vessels. (2020) 35:509–20. 10.1007/s00380-019-01511-0
36.
Sokolska JM Sokolski M Zymliński R Biegus J Siwołowski P Nawrocka-Millward S et al . Distinct clinical phenotypes of congestion in acute heart failure: characteristics, treatment response, and outcomes. ESC Heart Fail. (2020) 7:3830–40. 10.1002/ehf2.12973
37.
O'Connor CM Mentz RJ Cotter G Metra M Cleland JG Davison BA et al . The PROTECT in-hospital risk model: 7-day outcome in patients hospitalized with acute heart failure and renal dysfunction. Eur J Heart Fail. (2012) 14:605–1210.1093/eurjhf/hfs029
38.
Voors AA Davison BA Teerlink JR Felker GM Cotter G Filippatos G et al . RELAX-AHF Investigators. Diuretic response in patients with acute decompensated heart failure: characteristics clinical outcome–an analysis from RELAX-AHF. Eur J Heart Fail. (2014) 16:1230–40. 10.1002/ejhf.170
39.
Damman K Valente MA Voors AA O'Connor CM van Veldhuisen DJ Hillege HL . Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. (2014) 35:455–69. 10.1093/eurheartj/eht386
Summary
Keywords
acute heart failure (AHF), renal dysfunction, congestion, outcome, worsening renal function (WRF)
Citation
Palazzuoli A, Crescenzi F, Luschi L, Brazzi A, Feola M, Rossi A, Pagliaro A, Ghionzoli N and Ruocco G (2022) Different Renal Function Patterns in Patients With Acute Heart Failure: Relationship With Outcome and Congestion. Front. Cardiovasc. Med. 9:779828. doi: 10.3389/fcvm.2022.779828
Received
19 September 2021
Accepted
07 January 2022
Published
07 March 2022
Volume
9 - 2022
Edited by
Laurent Calvier, University of Texas Southwestern Medical Center, United States
Reviewed by
Robert Zymliński, Wroclaw Medical University, Poland; Ingrid Inge Prkacin, University of Zagreb, Croatia
Updates
Copyright
© 2022 Palazzuoli, Crescenzi, Luschi, Brazzi, Feola, Rossi, Pagliaro, Ghionzoli and Ruocco.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gaetano Ruocco gmruocco@virgilio.it
This article was submitted to Heart Failure and Transplantation, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.