- Department of Cardiology and Atrial Fibrillation Center, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
Background: Numerous studies have been conducted to investigate the relationship between tea consumption and the risk of cardiovascular diseases (CVD); however, no conclusive results have been achieved. We conducted a Mendelian randomization (MR) study to elucidate the causal associations between tea consumption and several CVD outcomes, including coronary artery disease (CAD), myocardial infarction (MI), atrial fibrillation (AF), and heart failure (HF).
Methods: Independent single-nucleotide polymorphisms (SNPs) genome-wide significantly associated with tea consumption were used as instrumental variables (IVs). Summary statistics for CVD outcomes were obtained from the corresponding genetic consortia and the FinnGen consortium. The inverse-variance weighted (IVW) method was the primary analytical method, and MR estimates from different data sources were combined using fixed-effects meta-analysis. Supplementary MR analyses, including the weighted median, MR-Egger, and the MR pleiotropy residual sum and outlier methods, were conducted to evaluate the robustness of the results. Further MR analyses were repeated by including more genetic variants at a higher P-value threshold.
Results: We found that genetically predicted tea consumption was not causally associated with any CVD outcomes in the IVW method using data from large genetic consortia [CAD: odds ratio (OR) = 1.00, 95% confidence interval (CI), 0.91, 1.10, P = 0.997; MI: OR = 0.98, 95% CI, 0.90, 1.08, P = 0.751; AF: OR = 0.97, 95% CI, 0.92, 1.03, P = 0.350; HF: OR = 0.96, 95% CI, 0.88, 1.05, P = 0.401] or the FinnGen consortium (CAD: OR = 1.06, 95% CI, 0.96, 1.17, P = 0.225; MI: OR = 1.01, 95% CI, 0.89, 1.15, P = 0.882; AF: OR = 1.00, 95% CI, 0.88, 1.14, P = 0.994; HF: OR = 0.96, 95% CI, 0.88, 1.04, P = 0.362). The results were robust and consistent across meta-analysis, supplementary MR analyses, and analyses with more IVs included.
Conclusion: This MR study revealed no causal association between tea consumption and four CVD outcomes, suggesting that tea consumption may not be beneficial for the primary prevention of CVD.
Introduction
Cardiovascular diseases (CVD) is the leading cause of morbidity and mortality worldwide. It was estimated that 17.9 million people died due to cardiovascular-related complications in 2016 (1), and the burden of CVD in terms of diminished quality of life, loss of productivity, and healthcare costs remains enormous (2). In addition to changing well-known risk factors for CVD (such as obesity, diabetes mellitus, high blood pressure, and high cholesterol) (3), recent studies have focused on modifiable lifestyle factors (such as diet, physical activity, and smoking) (4–6).
As one of the most ancient and commonly consumed beverages globally, tea has attracted tremendous attention for its potential beneficial effects on health (7). Although a number of studies have investigated the association between tea consumption and CVD, there have been no conclusive results. To date, no randomized controlled trial (RCT) has investigated the relationship between tea consumption and risk of CVD outcomes, and only one RCT conducted by Sone et al. assessed whether catechin-enriched green tea consumption affected CVD risk factors (8). Several observational studies have suggested that tea consumption reduces the risk of CVD (7, 9, 10), while other studies have failed to show such an association (11, 12). The conflicting findings from observational studies may be due to differences in the study population, tea consumption assessment methods, small sample sizes, and covariates adjusted in the statistical models (such as socioeconomic status and education levels). In addition, other unmeasured confounding factors and reverse causality inherent in traditional epidemiological studies may make the observed associations uncertain (13). Therefore, it is essential to precisely elucidate the causal associations between tea consumption and CVD outcomes free from confounders and reverse causality.
Although RCTs are the gold standard for assessing causality, well-designed RCTs often consume considerable human, financial, and time resources. The Mendelian randomization (MR) approach, a natural genetic counterpart of RCT, has been widely applied in disease epidemiology to investigate the causal relationship between exposures and outcomes. This approach uses genetic variants that have a specific effect on a trait (exposure) as instrumental variables (IVs), with those who inherit or do not inherit the genetic variant assigned to a higher or lower dosage of the specific trait. As it was assumed that genetic variants were unlikely to be affected by disease status and were randomly allocated at meiosis, the MR method avoids confounding factors and reverse causation (14). Given the advantages of the MR approach, we utilized genetic variants that influence tea consumption as IVs to determine the associations between tea consumption and the risk of several CVD outcomes, including coronary artery disease (CAD), myocardial infarction (MI), atrial fibrillation (AF), and heart failure (HF) in a two-sample MR design.
Materials and methods
Study design
This study used a two-sample MR design to estimate the causal inferences between tea consumption and several CVD outcomes (Figure 1) and followed the Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization (STROBE-MR) statement (15). The MR study was based on the following three assumptions. First, the genetic variants selected as IVs were strongly associated with exposure (tea consumption). Second, the IVs were not associated with any confounders. Third, the IVs directly affected the outcomes (CVD) through exposure rather than other pathways.
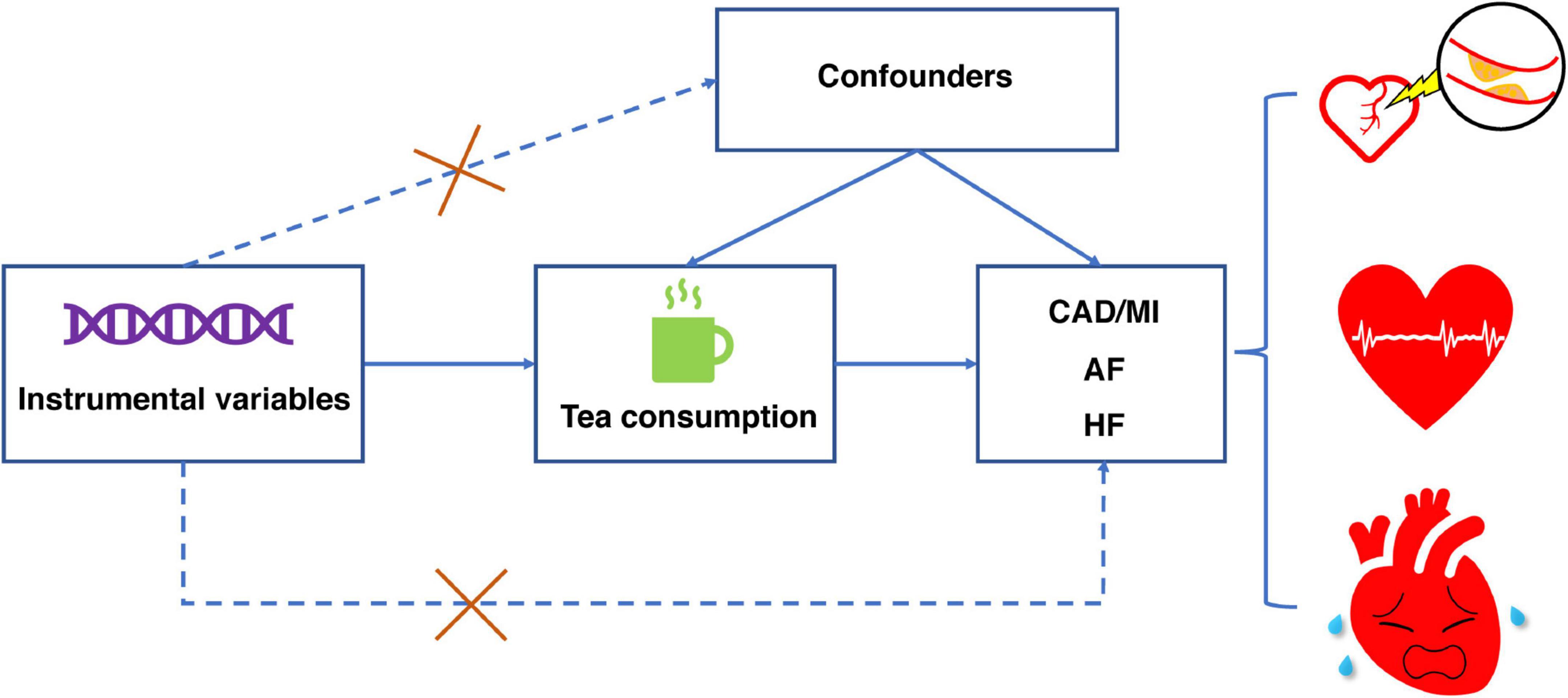
Figure 1. Design of Mendelian randomization study of tea consumption and risk of cardiovascular diseases. This Mendelian randomization study builds upon the assumptions that instrumental variables are associated with tea consumption but not with confounders, and that instrumental variables affect the risk of the four cardiovascular diseases only through tea consumption. CAD, coronary artery disease; MI, myocardial infarction; AF, atrial fibrillation; HF, heart failure.
Outcome data sources
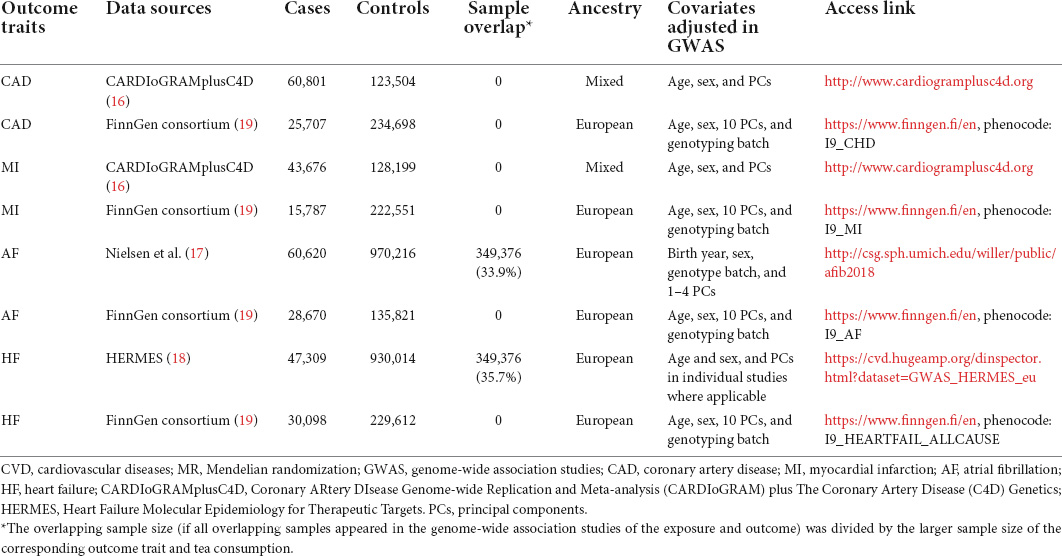
Summary statistics for CAD and MI were obtained from the Coronary ARtery DIsease Genome-wide Replication and Meta-analysis (CARDIoGRAM) plus The Coronary Artery Disease (C4D) Genetics (CardiogramplusC4D) consortium (16). The study included 60,801 patients with CAD (among whom there were 43,676 MI cases) and 123,504 controls. Cases were defined using a broad definition of CAD, including MI, acute coronary syndrome, chronic stable angina, or coronary artery stenosis greater than 50%. As for AF, we obtained summary-level data from the genome-wide association studies (GWAS) meta-analysis conducted by Nielsen et al., with case status defined as paroxysmal or permanent AF or atrial flutter (17). For HF, summary-level data were extracted from the largest GWAS meta-analysis among European individuals performed by the Heart Failure Molecular Epidemiology for Therapeutic Targets (HERMES) Consortium (18). We also obtained summary statistics for CVD outcomes from the FinnGen study (release 6) for replication purposes (19), with the number of cases ranging from 15,787 for MI to 30,098 for HF. Detailed descriptions of the outcome data sources are presented in Table 1.
Instrumental single-nucleotide polymorphisms selection
Similar to a previous MR study (20), we identified tea consumption-associated single-nucleotide polymorphisms (SNPs) based on the summary-level statistics for untransformed daily cups of tea consumption (Phenotype Code:1488_raw) obtained from GWAS published by the Neale laboratory (GWAS round 2),1 including up to 349,376 individuals of European descent. The covariates adjusted in the GWAS for tea intake were the first 20 principal components + sex + age + age2 + sex × age + sex × age2. Tea intake was obtained at baseline from a dietary questionnaire of “How many cups of tea do you drink each day? (Include black and green tea).” The overall workflow of SNP selection is summarized in Figure 2. We selected autosomal biallelic SNPs with P-values < 5 × 10–8 and minor frequencies > 1%, leaving 2,673 unique SNPs. We then performed clumping function using the TwoSampleMR R package (version 0.5.6) to select genetic variants without any linkage disequilibrium (LD) (r2 < 0.001 across a 10,000 kb window) based on European sample reference data from the 1,000 Genomes Project (21). Finally, 19 independent SNPs associated with tea consumption (P < 5 × 10–8) remained; detailed information on these SNPs is shown in Supplementary Table 1. We further proceeded with the following steps to determine valid IVs: First, the F-statistic was computed to measure the strength of the IVs. The F-statistic of each SNP was > 10 (Supplementary Table 1), indicating a low risk of weak instrument bias; none of these SNPs was excluded in this step (22). Second, we searched for SNPs in the PhenoScanner database2 to assess whether these SNPs were associated with established CVD risk factors (P < 5 × 10–8), such as obesity (23, 24), systolic and diastolic blood pressure (25), lipid traits (26), type 2 diabetes mellitus (27), smoking, and alcohol intake (28). A total of four SNPs showed horizontal pleiotropic effects and were excluded from the MR analyses (Supplementary Table 2). Third, SNPs not available in the outcome datasets were replaced by proxy SNPs in high LD (r2 > 0.8) by searching an online website3 (Supplementary Table 3). Fourth, we applied MR Steiger filtering to test the causal direction of each extracted SNP on the exposure and the outcome. This approach calculated the variance explained in the exposure and outcome by the IV and tested whether the variance in the outcome was less than the exposure. No SNP was removed for false causal direction, which showed evidence of primarily affecting outcomes rather than the exposure.
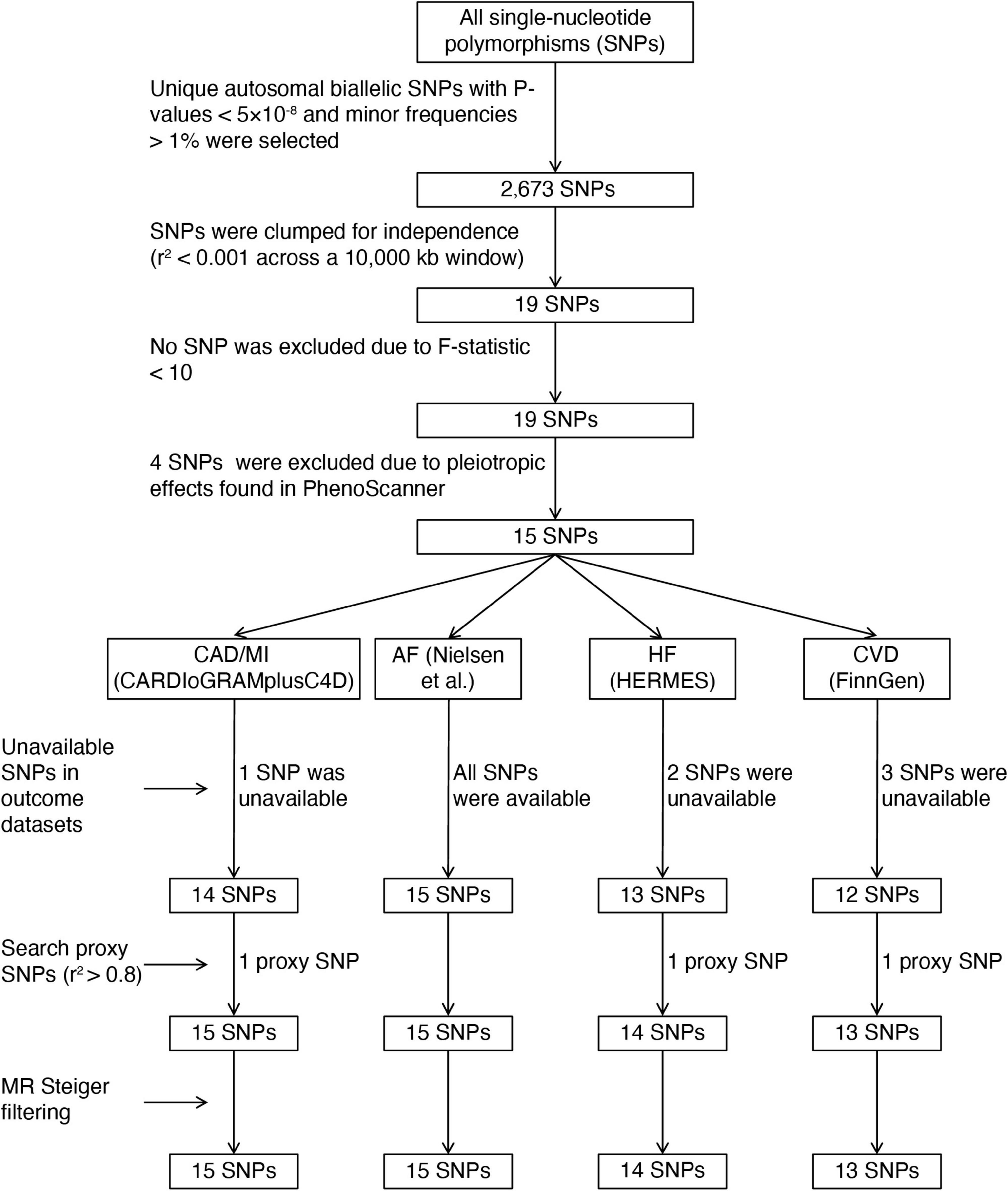
Figure 2. The flowchart of instrumental variables selection. CAD, coronary artery disease; MI, myocardial infarction; AF, atrial fibrillation; HF, heart failure; CVD, cardiovascular diseases; CARDIoGRAMplusC4D, Coronary ARtery DIsease Genome-wide Replication and Meta-analysis (CARDIoGRAM) plus The Coronary Artery Disease (C4D) Genetics; HERMES, Heart Failure Molecular Epidemiology for Therapeutic Targets.
Statistical analysis
Our primary MR analysis was performed using the multiplicative random-effects inverse-variance weighted (IVW) method, which could provide a robust causal estimate in the presence of heterogeneity (29). The fixed-effects meta-analysis method was applied to combine MR estimates derived from two different data sources. We also performed several supplementary MR methods to evaluate the robustness of the results, including the weighted median (30), MR-Egger regression (31), and MR-pleiotropy residual sum and outlier (MR-PRESSO) (32). The weighted median method can provide consistent causal estimates when more than 50% of the weight in the analysis is derived from valid IVs (30). MR-Egger regression was applied to detect horizontal pleiotropy with P-value for its intercept and generate estimates after correcting for pleiotropy (31). The MR-PRESSO test can detect and correct horizontal pleiotropic outliers in the IVW method and explore significant differences in the causal assessments before and after excluding outliers (32). Cochran’s Q statistic and I2 statistic were used to evaluate the heterogeneity among SNPs, with Cochran’s Q derived P-value < 0.05 or I2 > 25% considered as horizontal pleiotropy. Furthermore, we performed the leave one-out analysis to determine whether any pleiotropic SNPs drove MR estimates. Finally, considering the low number of IVs (P < 5 × 10–8) in the primary MR analysis, which explained the low variance (approximately 0.37%) in tea consumption, we added genetic variants with higher P-values for tea consumption (P < 5 × 10–7) and then repeated the MR analyses stated above to investigate whether the results were robust. A similar selection of IVs with P-values < 5 × 10–7 is shown in Supplementary Figure 1.
Power calculations were performed to evaluate the required effects of exposure on the outcomes at 80% power based on the sample size of each outcome and variance of exposure explained by the IVs on a web-based application.4 The results are presented in (Supplementary Table 4).
Results are presented as odds ratios (ORs), and all reported ORs and corresponding 95% confidence intervals (CIs) of CVD were scaled to per cup increment in daily tea consumption. A Bonferroni-corrected P-value of < 0.013 (correcting for four outcomes) was considered as the threshold of significance. All MR analyses were performed using the TwoSampleMR (33) and MRPRESSO (32) packages in R (version 4.0.2).
Results
Figure 3 shows the associations between genetically determined tea consumption (per cup increment in daily tea consumption) and the risk of CVD outcomes in the primary MR analyses. We observed no causal association between tea consumption and CVD outcomes in the GWAS of large genetic consortia (CAD: OR = 1.00, 95% CI, 0.91, 1.10, P = 0.997; MI: OR = 0.98, 95% CI, 0.90, 1.08, P = 0.751; AF: OR = 0.97, 95% CI, 0.92, 1.03, P = 0.350; HF: OR = 0.96, 95% CI, 0.88, 1.05, P = 0.401; Figure 3) or in the FinnGen consortium (CAD: OR = 1.06, 95% CI, 0.96, 1.17, P = 0.225; MI: OR = 1.01, 95% CI, 0.89, 1.15, P = 0.882; AF: OR = 1.00, 95% CI, 0.88, 1.14, P = 0.994; HF: OR = 0.96, 95% CI, 0.88, 1.04, P = 0.362; Figure 3). Meta-analysis results combining MR estimates from different data sources also revealed no causal inference between tea consumption and CVD (P-values for all CVD outcomes were > 0.05; Figure 3).
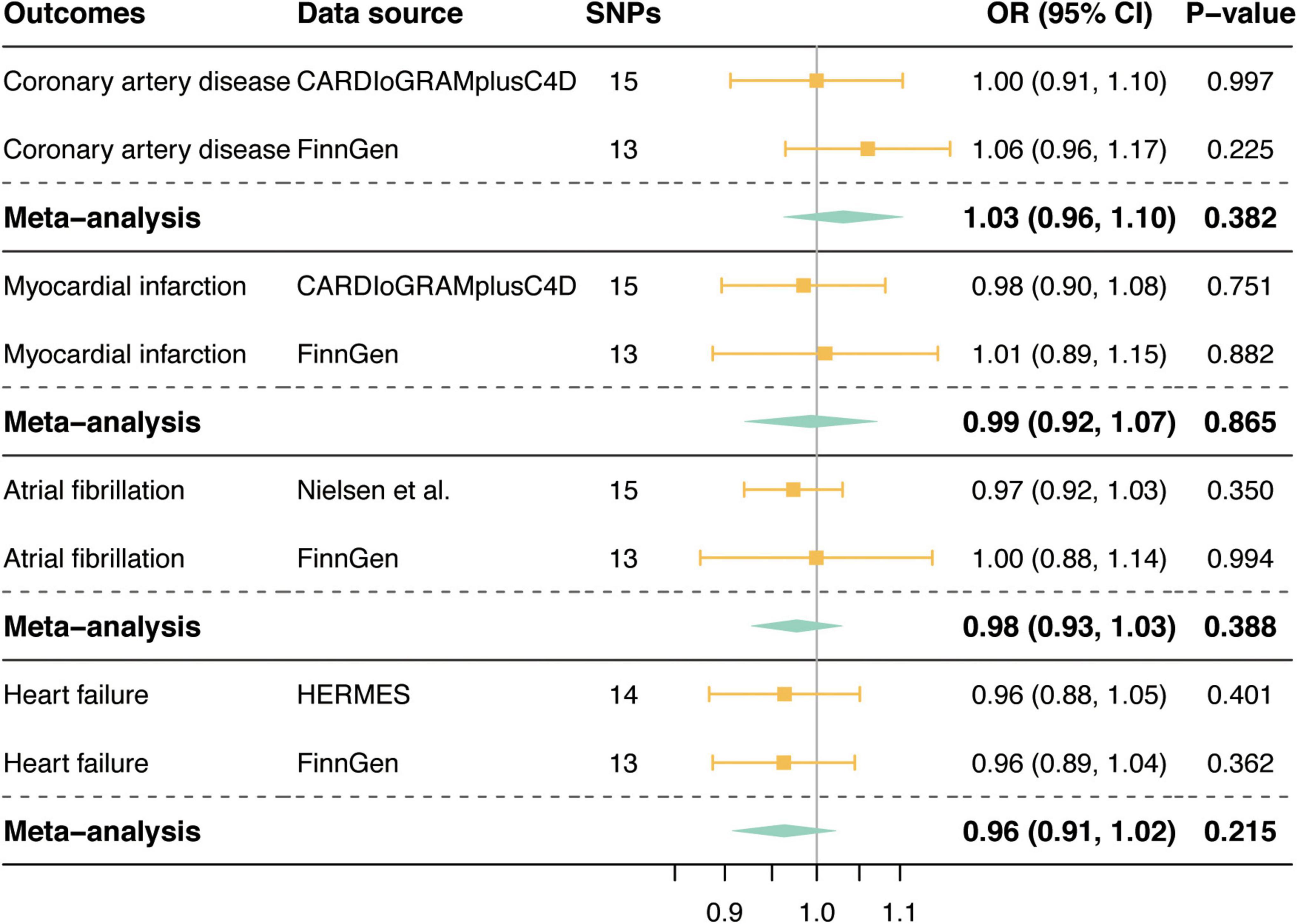
Figure 3. Mendelian randomization associations of tea consumption with risk of cardiovascular diseases. SNPs, single nucleotide polymorphisms; OR, odds ratio; CI, confidence interval; CARDIoGRAMplusC4D, Coronary ARtery DIsease Genome-wide Replication and Meta-analysis (CARDIoGRAM) plus The Coronary Artery Disease (C4D) Genetics; HERMES, Heart Failure Molecular Epidemiology for Therapeutic Targets.
Supplementary MR analyses, including the weighted median and MR-Egger methods, also showed no association between genetically predicted tea consumption and any CVD outcomes (Table 2). There was low to moderate heterogeneity detected in several analyses indicated by PCochran’s Q and I2 statistics (CAD in the CARDIoGRAMplusC4D: PCochran’s Q = 0.097, I2 = 33.9%; AF in the FinnGen: PCochran’s Q = 0.147, I2 = 29.7%; HF in the HERMES: PCochran’s Q = 0.052, I2 = 41.5%; Table 2). Nevertheless, the multiplicative random-effects IVW performed in primary MR analyses could provide a robust causal estimate even in the presence of heterogeneity. Moreover, the P-values for the intercept in the corresponding MR-Egger regression were all above 0.05 (Table 2), suggesting no evidence of pleiotropy. MR-PRESSO only identified one outlier SNP (rs4410790) for CAD in CARDIoGRAMplusC4D (Table 2). After excluding this outlier, there was no evidence of causal association between genetically determined tea consumption and CAD (OR = 0.92, 95% CI, 0.85, 1.00, P = 0.078, Table 2). Furthermore, we performed leave-one-out analyses of all the CVD outcomes from different data sources. The results consistently indicated that no single SNP dramatically biased the non-causal associations between tea consumption and CVD (Supplementary Figures 2, 3).
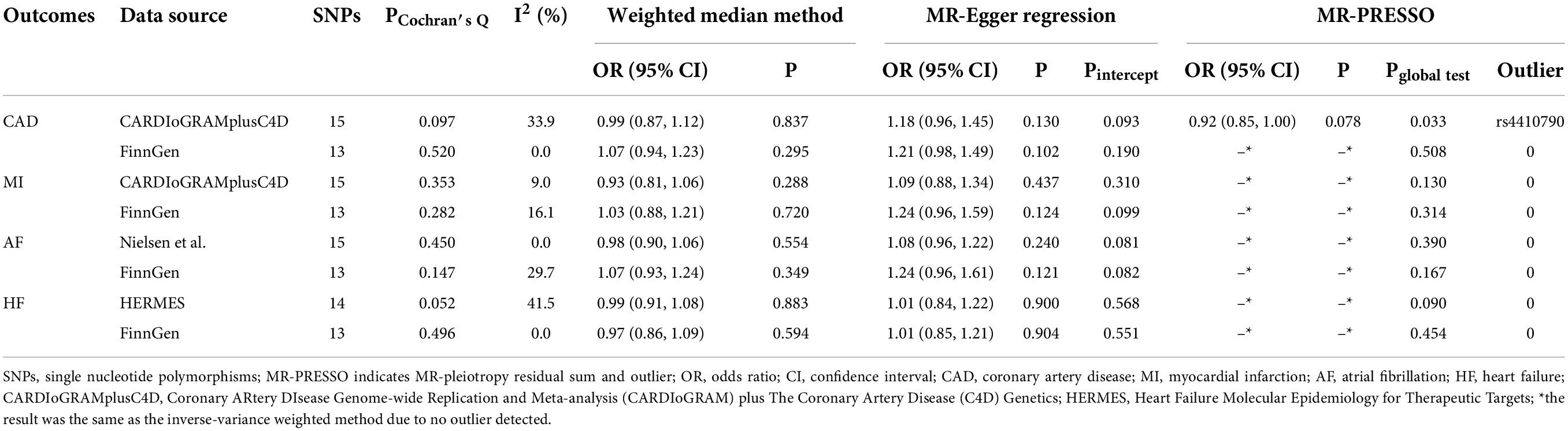
Table 2. Supplementary analyses of the associations between tea consumption and cardiovascular diseases.
When we lowered the P-value threshold of IV selection (P < 5 × 10–7), tea consumption was not significantly associated with CVD outcomes in the random-effects IVW method or in the fixed-effect meta-analysis combining MR estimates from different data sources (Supplementary Figure 4), which was in line with the results of the primary analyses. Likewise, the relationships between tea consumption and CVD outcomes were consistent across the weighted median and MR-Egger regression methods, with modest heterogeneity observed in several analyses. No evidence of pleiotropy was indicated by the intercept from the MR-Egger regression (All Pintercept were > 0.05, Supplementary Table 5). The MR-PRESSO method identified one outlier (rs2117137) for AF in the GWAS meta-analysis by Nielsen et al., and the non-causal association remained after excluding this outlier (Supplementary Table 5).
Discussion
This MR study demonstrated no causal relationship between tea consumption and several CVD outcomes, including CAD, MI, AF, and HF, thus providing little rationale for drinking tea to reduce CVD risk.
Over the last 20 years, many cross-sectional and prospective studies have investigated the relationships between tea consumption and CVD; however, no definite conclusions can be drawn from available data. In a multi-ethnic study involving 6,508 participants, Miller et al. found a statistically significant lower incidence of cardiovascular events in ≥ 1 cup/day tea drinkers (adjusted hazard ratio, 0.71; 95% CI, 0.53, 0.95) (34). A similar inverse association was observed between tea consumption and CVD mortality (35), risk of coronary heart disease (CHD) (36), incident MI (37), and both paroxysmal AF and persistent AF (38). Several studies have suggested that there was no correlation between tea consumption and the risk of developing CVD or CHD (39, 40). In addition, divergent results were obtained for different types of tea. For example, in a study by Mineharu et al. (41), black tea consumption was not associated with CVD-related mortality. In contrast, green tea and oolong tea reduced the mortality risk of CVD. Another meta-analysis, including 13 studies on black tea and five studies on green tea, suggested that green tea consumption was significantly associated with a decreased risk of CAD; on the contrary, black tea consumption did not show such an inverse association (42).
In this MR study, we found no protective effect of tea consumption on comprehensive CVD, which corroborated the results of some traditional observational studies and extended the findings of a previous MR study of tea consumption on the risk of stroke (20). The lack of causality in this MR study indicated that the protective effect of tea consumption on CVD observed in several observational studies may be limited by confounders and reverse causation rather than by identifying a causal correlation. It is worth noting that these epidemiological studies are potentially biased by confounding factors, such as different lifestyles between tea drinkers and non-tea drinkers, baseline flavonoid intake, background health, socioeconomic status, and many other factors (10). Another explanation for the divergent results is that the contents of chemical compounds differ in different types of tea. Tea is rich in polyphenols, particularly flavonoids, and green tea is more abundant than black or oolong tea (43). Catechins, a distinctive polyphenolic compound that can improve redox status, inhibit inflammation, reduce platelet aggregation, and ameliorate hyperlipidemia, constitute 80–90% and 20–30% of total flavonoids in green tea and black tea, respectively (42, 44). Thus, black tea, which is mainly consumed in Europe, may have fewer protective components than green tea, which is principally consumed in Asia (45). As a result, the preference for black tea in the European participants in our study might be the cause of the unbeneficial effect of tea consumption on CVD, although we used summary statistics for tea consumption, including both green tea and black tea. In addition, the null association between tea consumption and CVD risk in our MR study might be due to the balanced impact of cardioprotective and cardio-detrimental components of tea and dietary components. Therefore, further MR studies are warranted to determine the causal effects of different tea subtypes on CVD.
This study has several strengths. First, for the first time, we applied the MR method to investigate the causal associations between tea consumption and the risk of comprehensive CVD outcomes, avoiding potential confounding factors and reverse causation. Second, we utilized summary-level data from several large genetic consortia and the FinnGen consortium, and the results of the different datasets were consistent, assuring the reliability of our findings. Third, our results were less likely to be biased by population stratification, as the analyses were restricted to individuals of European ancestry. Nonetheless, this might limit the generalizability of our conclusions to other ethnicities.
However, this study has potential limitations. First, as mentioned above, the contents of chemical compounds in different types of tea are different, and the commonly consumed types also differ worldwide. Thus, our results might be biased because we could not assess whether the effects of different kinds of tea (black tea or green tea) on CVD risk were similar owing to a lack of detailed information. Second, data on tea consumption were obtained from a dietary questionnaire, which may be susceptible to measurement errors. Third, potential pleiotropy is a major limitation of MR studies. However, we used several sensitivity analyses that were more robust to pleiotropy to minimize the bias from pleiotropy, and the results remained consistent. Fourth, the required ORs of tea consumption on CVD outcomes to achieve 80% statistical power ranged from 0.621 (or 1.399) to 0.811 (or 1.193) (Supplementary Table 4). However, our estimated effect sizes did not reach the threshold to achieve 80% statistical power, indicating that there might be insufficient statistical power to detect the weak associations between tea consumption and CVD outcomes. It might be due to the low variance explained by the SNPs at the genome-wide significance level (approximately 0.37%). In such circumstances, we should be cautious with interpreting the negative results as there remains the possibility that false-negative results may occur. Therefore, we further investigated whether the MR estimates were robust when more genetic variants were selected as IVs, and the results remained consistent. However, increasing the number of genetic variants increases the chance of weak instrument bias to some extent. We used the F-statistic to assess instrument strength and found that there was no weak instrument (F > 10 for each variant). Fifth, there was some sample overlap in the GWAS of tea consumption and the GWAS meta-analysis by Nielsen et al. for AF and GWAS by HERMES for HF (Table 1), leading to some MR estimate bias. However, the F statistic for each IV was sufficiently large, suggesting that our MR estimates were unlikely to be affected by sample overlap bias (46). Furthermore, this sample overlap would not lead to bias if genetic associations with tea consumption were estimated in non-cases only (as is usual for continuous phenotypes) (46). Moreover, MR estimates obtained from the FinnGen consortium (no sample overlap) yielded similar results. Sixth, since we used summary-level data in our MR analyses, we cannot rule out the possibility of a non-linear causal relationship between tea consumption and CVD risk. The potential dose–response causal associations between tea consumption and CVD risk should be evaluated in further MR studies with individual-level data and longitudinal designs.
Conclusion
In conclusion, this MR study found no causal association between tea consumption and several CVD outcomes, including CAD, MI, AF, and HF, suggesting that tea consumption may not benefit the primary prevention of CVD.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethics approval or informed consent was not required since this study was based on publicly available databases.
Author contributions
LC and LZ designed the study. LC and XS analyzed the data, prepared the original draft, and revised and edited the manuscript. LZ supervised the study and acquired funding for the work. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (No. 81873484).
Acknowledgments
We thank the UK Biobank, CARDIoGRAMplusC4D, Nielsen et al., HERMES consortium, and FinnGen study for providing summary-level data. We also thank Editage (www.editage.com) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.870972/full#supplementary-material
Footnotes
- ^ http://www.nealelab.is/uk-biobank
- ^ http://www.phenoscanner.medschl.cam.ac.uk
- ^ http://snipa.helmholtz-muenchen.de/snipa3/
- ^ http://cnsgenomics.com/shiny/mRnd/
References
1. Evans MA, Sano S, Walsh K. Cardiovascular disease, aging, and clonal hematopoiesis. Annu Rev Pathol. (2020) 15:419–38. doi: 10.1146/annurev-pathmechdis-012419-032544
2. Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic impact goal through 2020 and beyond. Circulation. (2010) 121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703
3. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. (2016) 133:e38–360.
4. Gaziano TA. Lifestyle and cardiovascular disease: more work to do. J Am Coll Cardiol. (2017) 69:1126–8. doi: 10.1016/j.jacc.2016.12.019
5. Chen L, Sun X, Wang Z, Lu Y, Chen M, He Y, et al. The impact of plasma vitamin C levels on the risk of cardiovascular diseases and Alzheimer’s disease: a Mendelian randomization study. Clin Nutr. (2021) 40:5327–34. doi: 10.1016/j.clnu.2021.08.020
6. Zhuo C, Zhao J, Chen M, Lu Y. Physical activity and risks of cardiovascular diseases: a Mendelian randomization study. Front Cardiovasc Med. (2021) 8:722154. doi: 10.3389/fcvm.2021.722154
7. Liu J, Liu S, Zhou H, Hanson T, Yang L, Chen Z, et al. Association of green tea consumption with mortality from all-cause, cardiovascular disease and cancer in a Chinese cohort of 165,000 adult men. Eur J Epidemiol. (2016) 31:853–65. doi: 10.1007/s10654-016-0173-3
8. Sone T, Kuriyama S, Nakaya N, Hozawa A, Shimazu T, Nomura K, et al. Randomized controlled trial for an effect of catechin-enriched green tea consumption on adiponectin and cardiovascular disease risk factors. Food Nutr Res. (2011) 55:8326. doi: 10.3402/fnr.v55i0.8326
9. Zhao LG, Li HL, Sun JW, Yang Y, Ma X, Shu XO, et al. Green tea consumption and cause-specific mortality: results from two prospective cohort studies in China. J Epidemiol. (2017) 27:36–41. doi: 10.1016/j.je.2016.08.004
10. Deka A, Vita JA. Tea and cardiovascular disease. Pharmacol Res. (2011) 64:136–45. doi: 10.1016/j.phrs.2011.03.009
11. Sesso HD, Paffenbarger RS Jr, Oguma Y, Lee IM. Lack of association between tea and cardiovascular disease in college alumni. Int J Epidemiol. (2003) 32:527–33. doi: 10.1093/ije/dyg103
12. Hertog MG, Sweetnam PM, Fehily AM, Elwood PC, Kromhout D. Antioxidant flavonols and ischemic heart disease in a Welsh population of men: the Caerphilly Study. Am J Clin Nutr. (1997) 65:1489–94. doi: 10.1093/ajcn/65.5.1489
13. Qian Y, Ye D, Huang H, Wu DJH, Zhuang Y, Jiang X, et al. Coffee consumption and risk of stroke: a Mendelian randomization study. Ann Neurol. (2020) 87:525–32. doi: 10.1002/ana.25693
14. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. (2008) 27:1133–63. doi: 10.1002/sim.3034
15. Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236
16. Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, et al. A comprehensive 1,000 genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. (2015) 47:1121–30. doi: 10.1038/ng.3396
17. Nielsen JB, Thorolfsdottir RB, Fritsche LG, Zhou W, Skov MW, Graham SE, et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet. (2018) 50:1234–9. doi: 10.1038/s41588-018-0171-3
18. Shah S, Henry A, Roselli C, Lin H, Sveinbjörnsson G, Fatemifar G, et al. Genome-wide association and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat Commun. (2020) 11:163.
19. FinnGen Consortium. FinnGen Data Freeze 6. (2022). Available online at: https://www.finngen.fi/ (accessed Feb 4, 2022).
20. Wang M, Bai Y, Wang Z, Zhang Z, Liu D, Lian X. Higher tea consumption is associated with decreased risk of small vessel stroke. Clin Nutr. (2021) 40:1430–5. doi: 10.1016/j.clnu.2020.08.039
21. Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, et al. A global reference for human genetic variation. Nature. (2015) 526:68–74.
22. Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. (2011) 40:740–52. doi: 10.1093/ije/dyq151
23. Elagizi A, Kachur S, Lavie CJ, Carbone S, Pandey A, Ortega FB, et al. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. (2018) 61:142–50. doi: 10.1016/j.pcad.2018.07.003
24. Khan SS, Ning H, Wilkins JT, Allen N, Carnethon M, Berry JD, et al. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. (2018) 3:280–7. doi: 10.1001/jamacardio.2018.0022
25. Malik R, Georgakis MK, Vujkovic M, Damrauer SM, Elliott P, Karhunen V, et al. Relationship between blood pressure and incident cardiovascular disease: linear and nonlinear Mendelian randomization analyses. Hypertension. (2021) 77:2004–13. doi: 10.1161/HYPERTENSIONAHA.120.16534
26. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. (2017) 38:2459–72.
27. Liu B, Mason AM, Sun L, Di Angelantonio E, Gill D, Burgess S. Genetically predicted type 2 diabetes mellitus liability, glycated hemoglobin and cardiovascular diseases: a wide-angled Mendelian randomization study. Genes. (2021) 12:1644. doi: 10.3390/genes12101644
28. Rosoff DB, Davey Smith G, Mehta N, Clarke TK, Lohoff FW. Evaluating the relationship between alcohol consumption, tobacco use, and cardiovascular disease: a multivariable Mendelian randomization study. PLoS Med. (2020) 17:e1003410. doi: 10.1371/journal.pmed.1003410
29. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. (2017) 36:1783–802. doi: 10.1002/sim.7221
30. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
31. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
32. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
33. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife. (2018) 7:e34408. doi: 10.7554/eLife.34408
34. Miller PE, Zhao D, Frazier-Wood AC, Michos ED, Averill M, Sandfort V, et al. Associations of coffee, tea, and caffeine intake with coronary artery calcification and cardiovascular events. Am J Med. (2017) 130:188–97.e5. doi: 10.1016/j.amjmed.2016.08.038
35. Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, et al. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki Study. JAMA. (2006) 296:1255–65. doi: 10.1001/jama.296.10.1255
36. de Koning Gans JM, Uiterwaal CSPM, van der Schouw YT, Boer JMA, Grobbee DE, Verschuren WMM, et al. Tea and coffee consumption and cardiovascular morbidity and mortality. Arterioscler Thromb Vasc Biol. (2010) 30:1665–71. doi: 10.1161/ATVBAHA.109.201939
37. Geleijnse JM, Launer LJ, Van der Kuip DAM, Hofman A, Witteman JCM. Inverse association of tea and flavonoid intakes with incident myocardial infarction: the Rotterdam Study. Am J Clin Nutr. (2002) 75:880–6. doi: 10.1093/ajcn/75.5.880
38. Liu D-C, Yan J-J, Wang Y-N, Wang Z-M, Xie Z-Y, Ma Y, et al. Low-dose green tea intake reduces incidence of atrial fibrillation in a Chinese population. Oncotarget. (2016) 7:85592–602. doi: 10.18632/oncotarget.12243
39. Beresniak A, Duru G, Berger G, Bremond-Gignac D. Relationships between black tea consumption and key health indicators in the world: an ecological study. BMJ Open. (2012) 2:e000648. doi: 10.1136/bmjopen-2011-000648
40. Brown CA, Bolton-Smith C, Woodward M, Tunstall-Pedoe H. Coffee and tea consumption and the prevalence of coronary heart disease in men and women: results from the Scottish Heart Health Study. J Epidemiol Community Health. (1993) 47:171–5. doi: 10.1136/jech.47.3.171
41. Mineharu Y, Koizumi A, Wada Y, Iso H, Watanabe Y, Date C, et al. Coffee, green tea, black tea and oolong tea consumption and risk of mortality from cardiovascular disease in Japanese men and women. J Epidemiol Community Health. (2011) 65:230–40. doi: 10.1136/jech.2009.097311
42. Wang Z-M, Zhou B, Wang Y-S, Gong Q-Y, Wang Q-M, Yan J-J, et al. Black and green tea consumption and the risk of coronary artery disease: a meta-analysis. Am J Clin Nutr. (2011) 93:506–15. doi: 10.3945/ajcn.110.005363
43. Khan N, Mukhtar H. Tea polyphenols in promotion of human health. Nutrients. (2018) 11:39. doi: 10.3390/nu11010039
44. Babu PVA, Liu D. Green tea catechins and cardiovascular health: an update. Curr Med Chem. (2008) 15:1840–50. doi: 10.2174/092986708785132979
45. McKay DL, Blumberg JB. The role of tea in human health: an update. J Am Coll Nutr. (2002) 21:1–13. doi: 10.1080/07315724.2002.10719187
Keywords: tea consumption, coronary artery disease, myocardial infarction, atrial fibrillation, heart failure, Mendelian randomization
Citation: Chen L, Sun X and Zheng L (2022) No causal effect of tea consumption on cardiovascular diseases: A two-sample Mendelian randomization study. Front. Cardiovasc. Med. 9:870972. doi: 10.3389/fcvm.2022.870972
Received: 07 February 2022; Accepted: 15 August 2022;
Published: 07 September 2022.
Edited by:
Jun Ren, Fudan University, ChinaReviewed by:
Zhiyong Cui, Peking University Third Hospital, ChinaHaixia Xu, Fudan University, China
Copyright © 2022 Chen, Sun and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liangrong Zheng, MTE5MTA2NkB6anUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Lu Chen†
Lu Chen† Xingang Sun
Xingang Sun Liangrong Zheng
Liangrong Zheng