- Pediatric Cardiac Surgery Center, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Background: Iron deficiency (ID) is common in patients undergoing cardiac surgery, which is associated with adverse outcomes. However, the relevance of ID in congenital heart disease is still unclear. This study aimed to investigate the characteristics of preoperative ID and its association with clinical outcomes in infants undergoing cardiac surgery with cardiopulmonary bypass.
Methods: In this retrospective study, 314 patients undergoing cardiac surgery were assigned into three groups according to their preoperative ID status. Absolute ID was defined by serum ferritin <12 μg/L, and functional ID was defined by serum ferritin level at 12–30 μg/L and transferrin saturation <20%. Baseline characteristics were compared between groups and multiple logistic regression was used to identify predictors for ID. The association between ID and clinical outcomes, including allogenic blood transfusion requirements, was also evaluated.
Results: Among the 314 patients included, 32.5% were absolute ID and 28.7% were functional ID. Patients with absolute ID were more often of higher weight, cyanotic heart disease, and anemia. The presence of absolute ID was associated with an increase in postoperative blood transfusion (OR 1.837, 95% CI 1.016–3.321, p = 0.044). There was no significant difference in postoperative morbidity, mortality, and the length of hospital stay.
Conclusions: Absolute ID was associated with preoperative anemia and cyanotic heart disease, and was an independent risk factor for postoperative blood transfusion. Further research should better explore the definition of ID and its impact on outcomes in pediatric cardiac surgery.
Introduction
Iron deficiency (ID) is one of the main causes of anemia worldwide and is the most common micronutrient deficiency in children (1, 2). It is associated with adverse outcomes, affecting up to 50% of children in developing countries (1, 3). During early childhood, rapid growth and development exhaust iron stores, leading to increased exposure to ID (3). According to iron stores, ID can be divided into absolute iron deficiency (AID) and functional iron deficiency (FID) (4). In adults, AID is defined in presence of serum ferritin (SF) level <100 μg/L, and FID was defined by SF >100 μg/L combined with transferrin saturation (TAST) <20% (4, 5). Whereas children have different cutoff values depending on age and pathological conditions (1, 6).
Iron deficiency is frequently observed in adult cardiac surgery, which is recognized as a risk factor for increased red blood cell (RBC) transfusion, postoperative fatigue, and mortality (7–9). Currently, the prevalence of ID and its impact on clinical outcomes have not been as comprehensively studied in children, especially in infants. The aims of this study were to investigate the frequency and predictors of preoperative ID in infants undergoing cardiac surgery and to describe the association between ID and clinical outcomes.
Materials and Methods
Study Design and Patients
This was a single-center, retrospective cohort study conducted in Fuwai hospital (Beijing, China). From January 2020 to December 2021, every infant (1–12 months) who underwent initial cardiac surgery with cardiopulmonary bypass (CPB) in our institution qualified for the study. The exclusion criteria included patients with undetermined iron status and aged <6 months. Because there are rapid changes in storage iron concentrations in the first 6 months of life (6), and no appropriate definition of anemia is suitable for this population. Meanwhile, as an acute-phase reaction protein and inflammatory biomarker, high-sensitivity C-reactive protein (HSCRP) >3 mg/L was associated with a high risk of cardiovascular events and systemic inflammatory (10). In order to partially avoid the influence of inflammation on the diagnosis of ID, patients with HSCRP >3 mg/L were also excluded.
The study included 314 consecutive patients transferred to the pediatric intensive care unit (PICU) after cardiac surgery. As there was no comparable report available to develop a satisfactory hypothesis, a formal sample size calculation was not conducted. The sample size of 314 patients analyzed represented the largest cohort of infants (11, 12) and was comparable to the ID study in adults (5).
Definitions
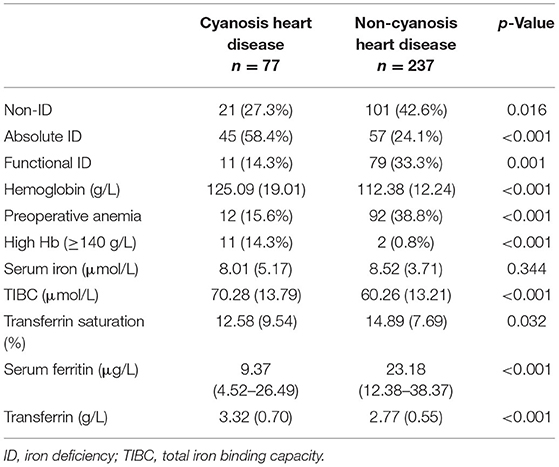
According to the WHO guideline (13), anemia was defined as hemoglobin (Hb) concentration <110 g/L for children 6–59 months of age. Thus, the definition of anemia in our study was Hb <110 g/L, regardless of the type of congenital heart disease. In previous studies on adult cardiac surgery, SF <100 μg/L was defined as AID, and SF >100 μg/L and TAST <20% was defined as FID (5). In children <5 years of age, the WHO (6) recommends that a SF concentration <12 μg/L indicates depleted iron stores, while SF <30 μg/L indicates ID during an acute phase response or chronic disease. Combining the above, AID was defined by SF <12 μg/L, and FID was defined by SF level at 12–30 μg/L and TAST <20% in the present study. The definition of cyanotic heart disease was based on the anatomy of congenital heart disease, including Tetralogy of Fallot (TOF), Double outlet right ventricle (DORV), Pulmonary stenosis (PS), Pulmonary atresia (PA), and Total anomalous pulmonary venous connection (TAPVC).
Data Collection
On the day before the operation, the blood sample was systematically collected for blood biochemical examination, including iron status. The following variables were collected and analyzed. Preoperative data: demographics and laboratory assessments including Hb (g/L), serum bilirubin (μmol/L); serum creatinine (μmol/L); blood urea nitrogen (BUN) (mmol/L); HSCRP (mg/L), SF (μg/L) and TAST (%). Intra-operative data: type of surgery; CPB duration; intraoperative RBC consumption (RBC priming and transfusion). Postoperative data: requirement for allogeneic RBC transfusion; length of ICU stay, duration of mechanical ventilation, length of hospitalization, and acute kidney injury (an increase of creatinine >50% vs. preoperative value); inpatient mortality and morbidity. The postoperative morbidity was defined as pulmonary or incision infection, need for peritoneal dialysis, re-intubation or re-thoracotomy, and the requirement of extracorporeal membrane oxygenation. Data were collected through the computerized patient record system.
Clinical Practice
Preoperative nutrition of all patients continued the daily recipes, with no special interventions, including iron supplementation, or dietary restrictions.
The procedure of cardiac surgery followed the standard protocol of the Pediatric Cardiac Surgery Center of Fuwai Hospital and was performed under general anesthesia and CPB. Every patient received a central venous catheter placed in the superior vena cava and a radial or femoral artery puncture. The CPB circuit consisted of a roller pump (Stockert S5, Sorin) and a hollow-fiber membrane oxygenator (Fx05, Terumo). Allogeneic RBC was added to the priming solution if the body weight was <8 kg or preoperative Hb was lower than 120 g/L. All patients underwent conventional ultrafiltration during CPB and modified ultrafiltration after CPB weaning.
Before aortic cannulation, 400 IU/kg heparin was administered to achieve an activated clotting time longer than 480 s, and a protamine dose of 4 mg/kg was administered for the reversal of the heparin effect after weaning of CPB. After surgery, all patients were admitted to PICU, which specialized in congenital heart disease, and received postoperative treatment by a fixed pediatric care team.
The decision to perform an intraoperative RBC transfusion was made if Hb <70 g/L during CPB, and Hb <90 g/L after CPB weaning. Whilst after surgery, the threshold for RBC transfusion was set at 80 or 90 g/L in the presence of signs suggestive of end-organ ischemia and low cardiac output.
Statistical Analysis
To assess baseline characteristics and clinical outcomes, patients were assigned into three groups according to the definition criteria of ID as described above. The normal distribution of continuous variables was assessed visually using histograms and Q–Q plots. The normally distributed data were presented as mean with standard deviation, and non-normally distributed variables were presented as median with inter-quartile range. Differences among groups were compared using the Analysis of Variance or the Kruskal–Wallis test, depending on the normal distribution. Categorical variables were presented as frequency with percentage, and were analyzed with the χ2 test or Fisher exact test. As a post hoc analysis, Bonferroni's multiple comparison test was performed. To further elucidate the distribution of ID, patients were also grouped according to anemia or cyanotic heart disease, comparing differences in the distribution of ID and indexes of iron store among groups.
Multiple logistic regression was applied for the association between variables with AID and FID. Age, gender, and all variables with a p < 0.05 in Table 1 were added into a stepwise variable selection, reporting odds ratio (OR) with 95% confidence interval (CI) for the independent predictors identified. A multivariate logistic regression model was performed on RBC transfusion to determine AID as an independent risk factor. Possible confounders were chosen for clinical reasons, including age, gender, weight, cyanotic heart disease, preoperative Hb, CPB duration, and intraoperative RBC consumption. Moreover, the association of SF and postoperative RBC transfusion was evaluated by the restricted cubic spline model with four knots at the 5th, 35th, 65th, and 95th percentiles of SF.
All analyses were carried out in SPSS 25.0 (IBM Corp., Armonk, NY, USA) and R 4.1.0 (The R Foundation for Statistical Computing, Vienna, Austria). A p-value of <0.05 was considered significant for all statistical tests.
Results
Study Population
From January 2020 to December 2021, the data of 498 infants undergoing cardiac surgery in our institution was collected. A total of 184 patients were excluded from the final analysis. The reasons for exclusion were shown in Figure 1. The median age and weight of included patients (n = 314) were 8.33 ± 1.86 months and 7.46 ± 1.34 kg, respectively. The types of congenital heart disease were as follows: Ventricular septal defect 190 (60.5%), Atrial septal defect 35 (11.2%), TOF 55 (17.5%), DORV 9 (2.9%), Coarctation of the aorta 8 (2.6%), PS 7 (2.2%), Total endocardial cushion defect 5 (1.6%), PA 4 (1.3%), and TAPVC 2 (0.6%). All patients underwent CPB with a median duration of 78 (60–102.5) min (Supplementary Table 1).
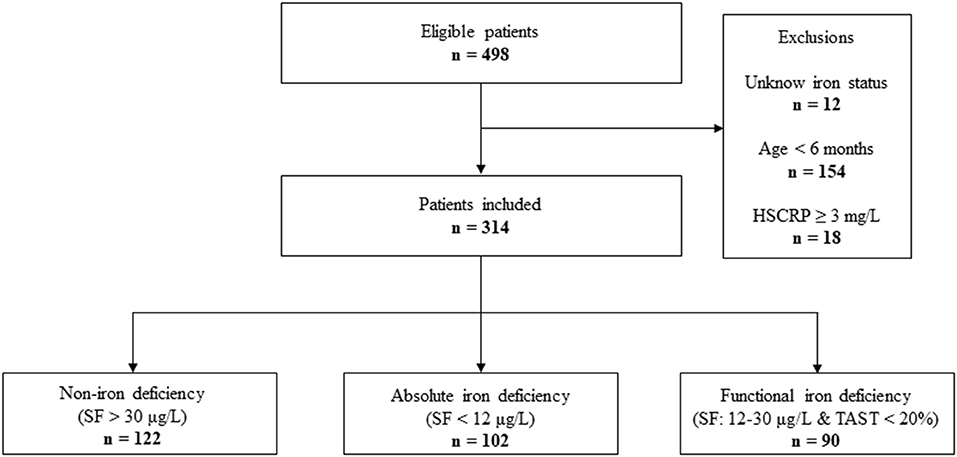
Figure 1. STROBE-style study flow chart outlining recruitment to and exclusion from the analysis sample. HSCRP, hypersensitive C reactive protein; SF, serum ferritin; TAST, transferrin saturation.
Characteristics and Predictors of ID
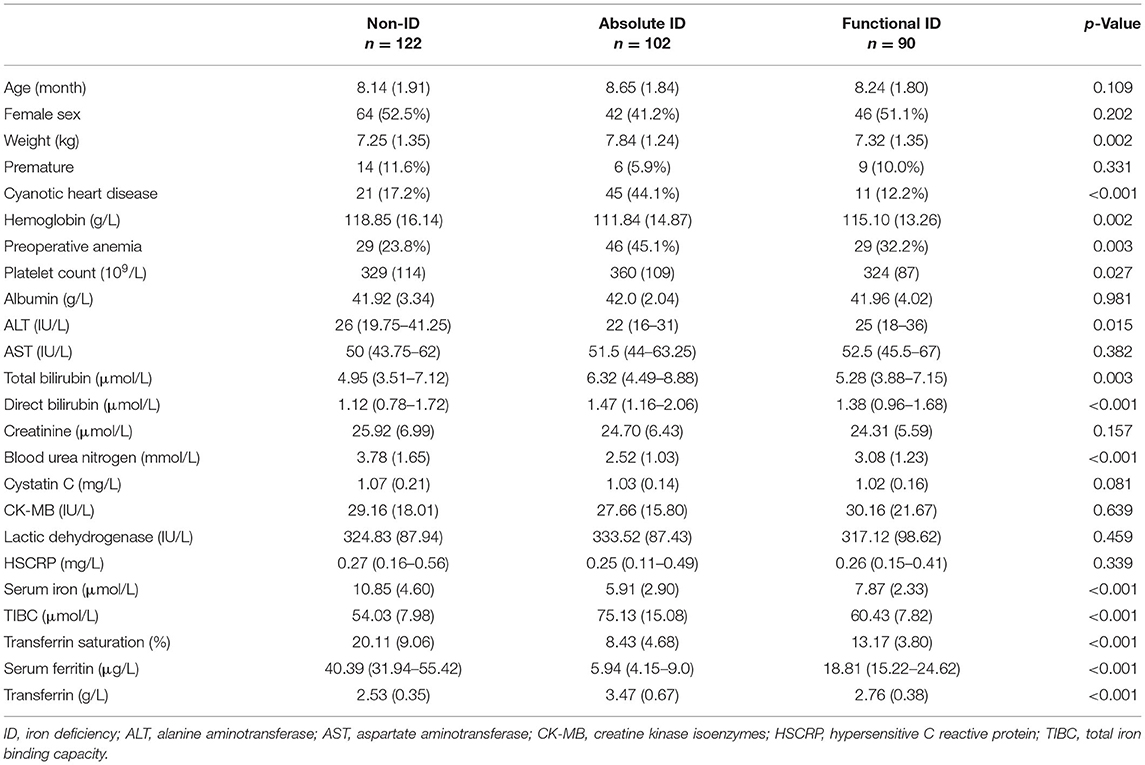
Figure 2 depicted the distribution of SF and TAST before surgery. According to the preoperative status of iron stores, ID was identified in 192 (61.2%) out of the 314 patients. The pattern of FID was found in 90 (28.7%) patients, and 102 (32.5%) patients were identified as AID. The baseline characteristics of patients were reported in Table 1. The group differences showed that patients with AID were more often of higher weight, cyanotic heart disease, and anemia (Hb <110 g/L). Furthermore, the Hb value was significantly lower in patients with AID vs. patients without ID or with FID (p = 0.002). However, there was no significant difference between the FID and non-ID patients. BUN and serum iron concentration was significantly lower in both FID and AID groups with respect to non-ID patients (p < 0.001). As a consequence of the definition of ID, SF levels and TAST were lower in patients with AID or FID vs. patients without ID (p < 0.001).
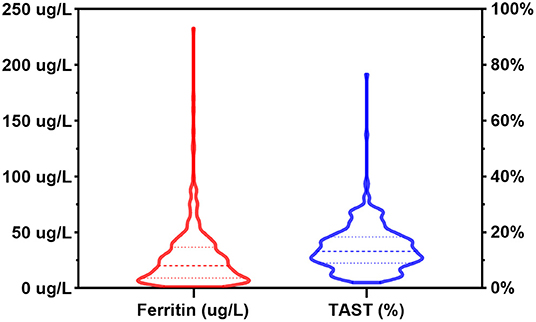
Figure 2. Distribution of serum ferritin levels and transferrin saturation in the patient population. TAST, transferrin saturation.
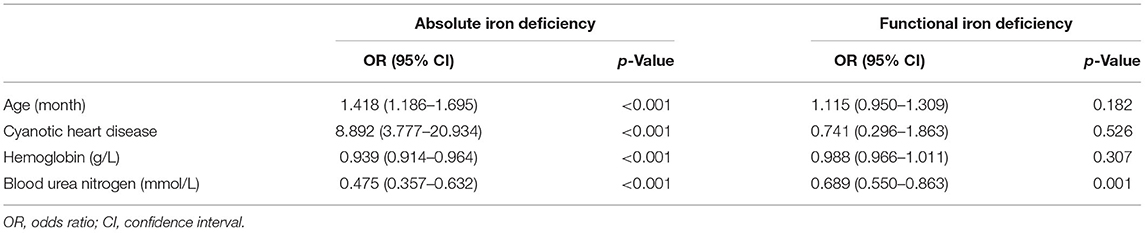
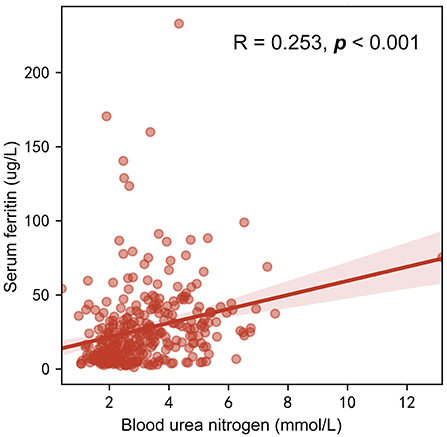
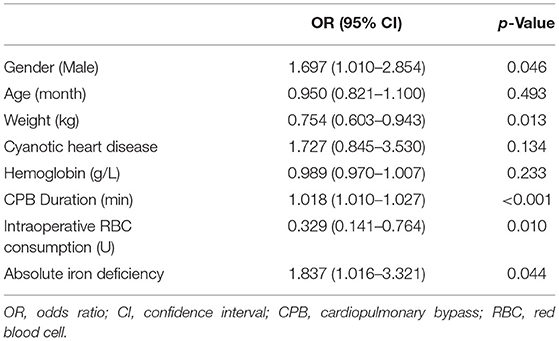
Multiple logistic regression analysis for identification of the independent risk factors for ID was performed, variables including age, gender, and all factors with p-value < 0.05 at the univariate analysis in Table 1. Table 2 reported the results of this analysis: the independent predictors for AID were age, cyanotic heart disease, Hb, and BUN (p < 0.001). BUN was also significantly associated with FID (p = 0.001). Interestingly, BUN seemed to exert a “protective” effect for ID, which was correlated with SF (R = 0.253, p < 0.001; Figure 3), and the receiver operating characteristic (ROC) curve for BUN predicting the presence of ID had an AUC of 0.708 (95%CI 0.649–0.768, p < 0.001). Additionally, when taking anemia into the logistic regression instead of Hb, it was an independent risk factor for AID (OR 4.144, 95% CI 2.002–8.578, p < 0.001).
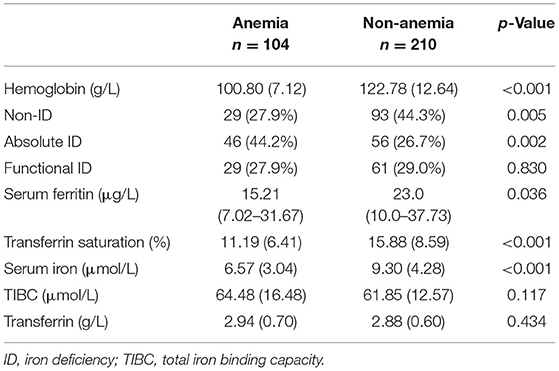
Before surgery, anemia (Hb <110 g/L) was found in 104 (33.1%) of the patient population. Within the patients with preoperative anemia, AID (44.2%) was observed more frequently than FID (27.9%) or non-ID (27.9%; Table 3). In contrast, most patients in non-anemia group presented with non-ID (44.3%; p = 0.003). Patients with anemia had significantly lower values of SF and TAST (p < 0.001), as well as lower levels of serum iron (p = 0.036). After adjusting for age, gender, weight, and cyanotic heart disease, AID was an independent risk factor for anemia (p < 0.001). In addition, patients with cyanotic heart disease were more likely to experience AID (p < 0.001; Figure 4), and with significantly lower levels of SF (p < 0.001) and TAST (p = 0.032; Table 4).
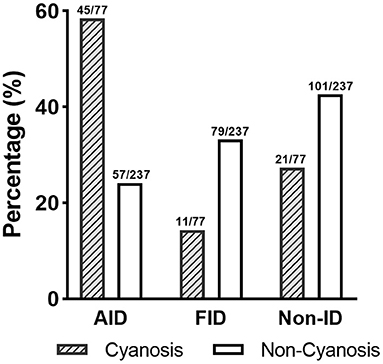
Figure 4. Distribution of ID patterns in patients with cyanotic heart disease. AID, absolute iron deficiency; FID, functional iron deficiency; non-ID, non-iron deficiency.
Effect of ID on Clinical Outcomes
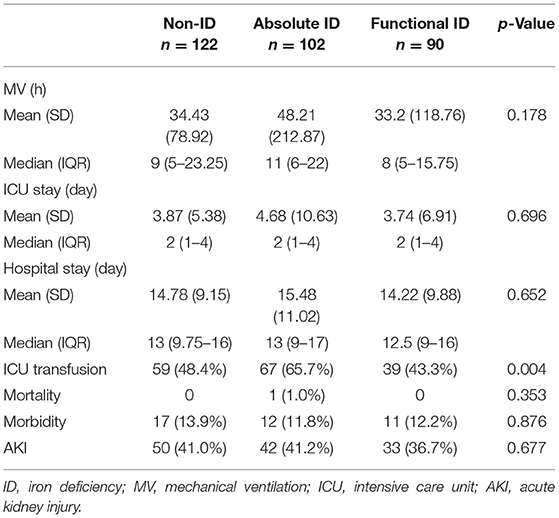
As shown in Table 5, there was no statistic difference in postoperative duration of mechanical ventilation between groups, although it seemed longer in patients with AID, compared with FID and non-ID (p = 0.178). The length of stay in ICU and hospital, mortality, morbidity, and AKI were not different among groups. Patients with AID were more likely to receive a postoperative RBC transfusion (65.7%) compared with FID group (43.3%) and non-ID group (48.4%; p = 0.004).
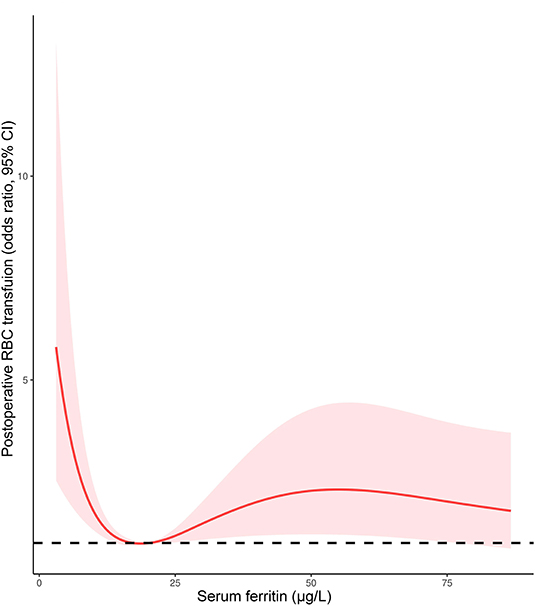
Given the proportion of postoperative RBC transfusion was comparable in patients with FID and non-ID, a multivariate logistic regression model was performed to determine the association of AID with RBC transfusion. After adjusting for age, gender, weight, cyanotic heart disease, preoperative Hb, CPB duration, and intraoperative RBC consumption, AID was an independent risk factor for postoperative RBC transfusion (OR 1.837, 95% CI 1.016–3.321, p = 0.044; Table 6). Additionally, the restricted cubic spline model confirmed a nonlinear association between SF and RBC transfusion (p-Nonlinear = 0.001), with a cutoff value of 20.225 (Figure 5). The ROC curve for SF also showed a prediction power for RBC transfusion (p = 0.035), with an AUC of 0.431 (95% CI 0.368–0.495).
Discussion
In this study, we demonstrated that AID (SF <12 μg/L), independent of anemia and cyanosis heart disease, is a risk factor for the requirements of RBC transfusion after infant cardiac surgery. As far as we know, this is the first study showing the characteristics and the effect of preoperative ID in infants undergoing cardiac surgery. The frequency of postoperative RBC transfusion was significantly higher for patients with AID than those with FID and without ID. After adjustment for confounders, a preoperative serum ferritin <12 μg/L was associated with 1.8 times increased risk of postoperative RBC transfusion. We chose the confounders based on a review (14) and clinical reasons, including age, gender, weight, cyanotic heart condition, preoperative Hb, and intraoperative RBC consumption. As there were many different types of congenital heart surgery, we used cyanotic heart condition for classification. In addition, as the duration of CPB could partially represent the complexity of the procedure, it was added as a confounder in the logistic regression model.
Iron deficiency is a prevalent malnutrition that affects up to two billion people globally, especially infants (1). Apart from erythropoiesis, iron has integral roles in several organ systems, including oxygen transport and storage, mitochondrial activity, cell signaling, and gene expression (15). Most of the circulating iron is consumed by erythropoiesis. Muscle growth and production of new myoglobin are also important consumers of iron in children (3). Since infants have high iron requirements due to rapid growth and development, they may potentially be at increased risk of ID if not supplemented with adequate iron-rich formulas or supplements (3). Our results showed that AID patients were of higher weight than FID or non-ID, which might also reflect this. The distribution of ID in children undergoing cardiac surgery is not well characterized, particularly in cyanotic children in whom ID can exist even with increased Hb levels (14). This was also shown in our data, where patients with cyanotic heart disease had higher preoperative Hb levels and less anemia, but most of them (58.4%) had AID (Table 4). A possible explanation is that abnormal erythropoiesis in patients with cyanotic heart disease may expose them to more severe iron depletion and consequently lead to an increased risk of AID.
In adults, the definition of ID included two different patterns, AID and FID, defined by SF <100 mg/L and SF <300 mg/L combined with TAST <20%, respectively (4, 5). Whilst in current WHO guideline (6), a cut-off value of 12 mg/L was recommended to define ID in children younger than 5 years old, and 30 mg/L in the presence of inflammation. Consequently, our definition of ID combined the definition of ID in adult cardiac surgery with the characteristics of iron metabolism in children, defining AID as SF <12 mg/L and FID as SF at 12–30 mg/L and TAST <20%. In previous studies in children, ID was defined as the presence of ≥2 of the following 4 criteria: serum iron <50 mg/dl, SF <20 ng/ml, transferrin >300 ng/ml, and TAST <15% (11, 12). However, this definition failed to distinguish between AID and FID, two different patterns of ID that have been generally demonstrated in adults (5, 16). To further clarify the effect of ID, it would be valuable to examine the difference of AID and FID in the associated risk of morbidity, RBC transfusion and response to iron treatment (16).
Our results were in line with previous studies in adult cardiac surgery, which showed ID to be associated with increased transfusions of allogeneic blood products (7, 9). Rossler et al. (8) indicated that postoperative transfusions of allogenic blood products within 90 days were considerably increased in ID (SF <100 mg/L) patients with or without anemia. The effect of ID on RBC transfusion was shown to have an OR of 1.8 (95% CI: 1.2–2.6) in multivariate regression analysis (8), which was similar to our findings. Additionally, even with a higher prevalence of preoperative anemia, patients with FID had a comparable rate of postoperative RBC transfusion with the non-ID group. Furthermore, restricted cubic spline plots showed that the level of SF at the lowest risk for postoperative RBC transfusion is 20 mg/L, which is within the definition of FID. Perhaps a better definition of ID was needed, for example with hepcidin and soluble transferrin receptors, which showed advantages for the diagnosis of ID (6).
In general, the postoperative course of patients with FID was not different from that of non-ID patients (5). Contrary to FID, AID was associated with a worse postoperative course, including increased mortality and prolonged hospital stay (5, 8). Contrary to expectations, we did not find a significant difference in clinical outcomes such as duration of mechanical ventilation and length of ICU stay between groups. Miles et al. (17) also reported the weak association between ID and outcomes. The impact of different patterns of ID on clinical outcomes remains to be further explored.
Patients with heart failure and ID showed symptomatic improvements from intravenous iron administration, irrespective of the presence of anemia (15). Whilst all patients might benefit from the treatment of preoperative ID (4), patients undergoing cardiac surgery could especially do so (18). This might be attributable to the physiological role of iron for the heart, and its essential role for mitochondrial function and cellular bioenergetics (19). Spahn et al. reported that an ultrashort-term treatment of isolated ID resulted in a higher postoperative Hb and reduced transfusions of allogeneic blood products in anemia patients with no significant negative side-effects (18). In addition, oral iron supplementation increased preoperative Hb levels in children undergoing cardiac surgery (20). As allogenic transfusions are associated with an increase of adverse events (21, 22), children undergoing cardiac surgery may benefit from a reduced requirement for postoperative RBC transfusion.
Nevertheless, a retrospective study showed supplementation with intravenous iron did not improve outcomes in patients with iron deficiency anemia and the transfusion requirement was significantly higher than the non-anemic cohort (23). Myles et al. (24) even believed that intravenous iron therapy should not be recommended since it was costly and offered a modest effect on the reduction in transfusion requirements. When the diagnosis of iron deficiency anemia is made preoperatively, treatment with oral iron supplements may be inadequate to achieve timely correction in children (14). However, as the iron regulatory hormone, hepcidin increases in inflammatory state, regardless of the presence or absence of infection (25). It impairs the function of the ferroportin, preventing the transport of iron across basement membranes, and thereby influencing the role of ID therapy in preoperative anemia (16). Newer intravenous iron preparations, like ferumoxytol and ferric iron gluconate, offer safer means for rapid iron replacement (26). Currently, larger studies to clarify the effect of preoperative treatment of ID are warranted in children undergoing cardiac surgery. Furthermore, regardless of the efficacy of the treatment, ID should be considered an additional risk factor for cardiac surgery.
There were some limitations in our study. First, as a single-center study, the external validity of our results might be limited. Second, although we excluded patients with high baseline HSCRP in order to partially avoid the impact of the inflammatory on SF. Unfortunately, the lack of data on CRP or hepcidin made the diagnosis of ID less accurate. In addition, specific reasons for RBC transfusion, and the causes of anemia or coagulation/bleeding disorders were not analyzed in current study due to the limitation of data. The effect of ID on postoperative RBC transfusion remains to be further studied. Finally, the small sample might limit our statistical power to detect significant differences in clinical outcomes. Therefore, it was difficult to further compare the effects of different patterns of ID on outcomes in anemic and non-anemic patients. Future studies should include more patients and extend the current analysis by distinguishing between different types of congenital heart disease.
Conclusion
Preoperative ID was a common comorbidity in infants undergoing cardiac surgery, including AID and FID. There was almost no difference between FID and non-ID patients. Whereas AID was an independent risk factor for postoperative RBC transfusion, with its own predictors including age, cyanosis heart disease, anemia, and BUN. Since patients might benefit from the treatment of ID and thus improved clinical outcomes, Further studies should better explore the definition of ID and its impact on outcomes in pediatric cardiac surgery.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Fuwai Hospital. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
PG, XW, PZ, YJ, LB, YL, WW, and JL contributed to conception and design of the study. PG, XW, and YJ organized the database. PG and PZ performed the statistical analysis. PG wrote the first draft of the manuscript. XW and JL revised the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study was supported by CAMS Innovation Fund for Medical Sciences (CIFMS) (Project Number: 2020-I2M-C&T-B-063).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.887535/full#supplementary-material
References
1. Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. (2007) 370:511–20. doi: 10.1016/S0140-6736(07)61235-5
2. Kyu HH, Pinho C, Wagner JA, Brown JC, Bertozzi-Villa A, Charlson FJ, et al. Global and National Burden of Diseases and Injuries among children and adolescents between 1990 and 2013: findings from the global burden of disease 2013 study. JAMA Pediatr. (2016) 170:267–87. doi: 10.1001/jamapediatrics.2015.4276
3. Cerami C. Iron nutriture of the fetus, neonate, infant, and child. Ann Nutr Metab. (2017) 71:8–14. doi: 10.1159/000481447
4. Muñoz M, Acheson AG, Auerbach M, Besser M, Habler O, Kehlet H, et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia. (2017) 72:233–47. doi: 10.1111/anae.13773
5. Tramarin R, Pistuddi V, Maresca L, Pavesi M, Castelvecchio S, Menicanti L, et al. Patterns and determinants of functional and absolute iron deficiency in patients undergoing cardiac rehabilitation following heart surgery. Eur J Prev Cardiol. (2017) 24:799–807. doi: 10.1177/2047487317689975
6. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. Available online at: https://www.who.int/publications-detail-redirect/9789240000124 (accessed March 1, 2022).
7. Piednoir P, Allou N, Driss F, Longrois D, Philip I, Beaumont C, et al. Preoperative iron deficiency increases transfusion requirements and fatigue in cardiac surgery patients: a prospective observational study. Eur J Anaesthesiol. (2011) 28:796–801. doi: 10.1097/EJA.0b013e32834ad97b
8. Rössler J, Schoenrath F, Seifert B, Kaserer A, Spahn GH, Falk V, et al. Iron deficiency is associated with higher mortality in patients undergoing cardiac surgery: a prospective study. Br J Anaesth. (2020) 124:25–34. doi: 10.1016/j.bja.2019.09.016
9. Hubert M, Gaudriot B, Biedermann S, Gouezec H, Sylvestre E, Bouzille G, et al. Impact of preoperative iron deficiency on blood transfusion in elective cardiac surgery. J Cardiothorac Vasc Anesth. (2019) 33:2141–50. doi: 10.1053/j.jvca.2019.02.006
10. Yousuf O, Mohanty BD, Martin SS, Joshi PH, Blaha MJ, Nasir K, et al. High-sensitivity C-reactive protein and cardiovascular disease: a resolute belief or an elusive link? J Am Coll Cardiol. (2013) 62:397–408. doi: 10.1016/j.jacc.2013.05.016
11. Puri K, Price JF, Spinner JA, Powers JM, Denfield SW, Cabrera AG, et al. Iron deficiency is associated with adverse outcomes in pediatric heart failure. J Pediatr. (2020) 216:58–66.e1. doi: 10.1016/j.jpeds.2019.08.060
12. Puri K, Jeewa A, Adachi I, Denfield SW, Cabrera AG, Price JF, et al. Prevalence of anemia and iron deficiency in pediatric patients on ventricular assist devices. ASAIO J. (2018) 64:795–801. doi: 10.1097/MAT.0000000000000725
13. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Available online at: https://www.who.int/publications/i/item/WHO-NMH-NHD-MNM-11.1 (accessed May 7, 2022).
14. Cholette JM, Faraoni D, Goobie SM, Ferraris V, Hassan N. Patient blood management in pediatric cardiac surgery: a review. Anesth Analg. (2018) 127:1002–16. doi: 10.1213/ANE.0000000000002504
15. von Haehling S, Jankowska EA, van Veldhuisen DJ, Ponikowski P, Anker SD. Iron deficiency and cardiovascular disease. Nat Rev Cardiol. (2015) 12:659–69. doi: 10.1038/nrcardio.2015.109
16. Miles LF, Story DA. Blood and iron: associations with poor outcome after cardiac surgery. Br J Anaesth. (2020) 124:1–3. doi: 10.1016/j.bja.2019.09.019
17. Miles LF, Kunz SA, Na LH, Braat S, Burbury K, Story DA. Postoperative outcomes following cardiac surgery in non-anaemic iron-replete and iron-deficient patients - an exploratory study. Anaesthesia. (2018) 73:450–8. doi: 10.1111/anae.14115
18. Spahn DR, Schoenrath F, Spahn GH, Seifert B, Stein P, Theusinger OM, et al. Effect of ultra-short-term treatment of patients with iron deficiency or anaemia undergoing cardiac surgery: a prospective randomised trial. Lancet. (2019) 393:2201–12. doi: 10.1016/S0140-6736(18)32555-8
19. Melenovsky V, Petrak J, Mracek T, Benes J, Borlaug BA, Nuskova H, et al. Myocardial iron content and mitochondrial function in human heart failure: a direct tissue analysis. Eur J Heart Fail. (2017) 19:522–30. doi: 10.1002/ejhf.640
20. Otsuka Y, Naraine N, Switzer T, Faraoni D. Preoperative iron supplementation in pediatric cardiac surgical patients: a preliminary single-center experience. J Cardiothorac Vasc Anesth. (2022) 36:1565–70. doi: 10.1053/j.jvca.2021.12.022
21. Székely A, Cserép Z, Sápi E, Breuer T, Nagy CA, Vargha P, et al. Risks and predictors of blood transfusion in pediatric patients undergoing open heart operations. Ann Thorac Surg. (2009) 87:187–97. doi: 10.1016/j.athoracsur.2008.09.079
22. Salvin JW, Scheurer MA, Laussen PC, Wypij D, Polito A, Bacha EA, et al. Blood transfusion after pediatric cardiac surgery is associated with prolonged hospital stay. Ann Thorac Surg. (2011) 91:204–10. doi: 10.1016/j.athoracsur.2010.07.037
23. Quarterman C, Shaw M, Hughes S, Wallace V, Agarwal S. Anaemia in cardiac surgery - a retrospective review of a centre's experience with a pre-operative intravenous iron clinic. Anaesthesia. (2021) 76:629–38. doi: 10.1111/anae.15271
24. Myles PS, Richards T, Klein A. Preoperative intravenous iron for cardiac surgery. Lancet. (2020) 396:1883–4. doi: 10.1016/S0140-6736(20)32406-5
25. Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. (2004) 306:2090–3. doi: 10.1126/science.1104742
Keywords: iron deficiency, cardiac surgery, infants, blood transfusion, congenital heart disease
Citation: Gao P, Wang X, Zhang P, Jin Y, Bai L, Wang W, Li Y and Liu J (2022) Preoperative Iron Deficiency Is Associated With Increased Blood Transfusion in Infants Undergoing Cardiac Surgery. Front. Cardiovasc. Med. 9:887535. doi: 10.3389/fcvm.2022.887535
Received: 01 March 2022; Accepted: 09 May 2022;
Published: 02 June 2022.
Edited by:
Ariane Willems, Leiden University Medical Center, NetherlandsReviewed by:
Jill Marie Cholette, Golisano Children's Hospital, United StatesMohsen Saleh Elalfy, Ain Sham University, Egypt
Marianne Nellis, NewYork-Presbyterian, United States
Copyright © 2022 Gao, Wang, Zhang, Jin, Bai, Wang, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinping Liu, bGl1amlucGluZ0BmdXdhaS5jb20=
 Peng Gao
Peng Gao Xu Wang
Xu Wang Peiyao Zhang
Peiyao Zhang Liting Bai
Liting Bai Wenting Wang
Wenting Wang Jinping Liu
Jinping Liu