- 1Department of Cardiovascular Medicine, The Second Affiliated Hospital of Nanchang University, Nanchang, China
- 2Department of Neurology, The Second Affiliated Hospital of Nanchang University, Nanchang, China
Background: Reported evidence of coronary stent fracture (CSF) has increased in recent years. The purpose of this study was to determine reliable estimates of the overall incidence of CSF.
Methods and results: The MEDLINE, Embase and Cochrane databases were searched until March 18, 2022. Pooled estimates were acquired using random effects models. Meta-regression and subgroup analysis were used to explore sources of heterogeneity, and publication bias was evaluated by visual assessment of funnel plots and Egger’s test. Overall, 46 articles were included in this study. Estimates of CSF incidence were 5.5% [95% confidence interval (CI): 3.7–7.7%] among 39,953 patients based on 36 studies, 4.8% (95% CI: 3.1–6.8%) among 39,945 lesions based on 29 studies and 4.9% (95% CI: 2.5–9.4%) among 19,252 stents based on 8 studies. There has been an obvious increase in the incidence of CSF over the past two decades, and it seems that the duration of stent placement after stent implantation has no impact on incidence estimation.
Conclusion: The incidence of CSF was 5.5% among patients, 4.8% for lesions and 4.9% for stents and increased over the past 20 years. The duration of stent placement after stent implantation was found to have no impact on the incidence of CSF, but drug-eluting stent (DES) types and right coronary artery (RCA) lesions influenced the pooled incidence.
Systematic review registration: [https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022311995], identifier [CRD42022311995].
Introduction
Coronary drug-eluting stents (DES) are widely used in the interventional treatment of coronary heart disease. The material, structure, technology, and thickness of the steel beam and the drug loading model of the stent have changed significantly (1). With the maturity of interventional technology, coronary stents are used not only for simple A-type lesions but also widely used for C-type complex lesions, such as twisted angulation, calcification, and chronic total occlusion (2). Due to pulsation of the heart, metal fatigue, damage, or even coronary stent fracture (CSF) could occur in the coronary stent, which is affected by persistent complicated mechanical functions.
Especially in recent years, the thinner steel beam thickness in the new generation of alloy stents and the wide use of intraluminal imaging technology have led to a significant increase in the incidence of CSF in clinical practice (3–5). In addition, serious clinical cardiovascular events such as in-stent restenosis (ISR), stent thrombus (ST), coronary artery aneurysm and coronary perforation induced by CSF have threatened patients’ health (6–8). In addition, trials assessing the incidence of CSF and related risk factors for CSF have recently been published and added to the evidence base. There is a need for an update on the incidence of CSF and predictive factors of CSF.
Therefore, a systematic review and meta-analysis were performed to highlight the incidence of CSF and to summarize the current knowledge of related factors for CSF. We hypothesized that we could identify the incidence of CSF and the risk factors for CSF.
Materials and methods
This systematic review and meta-analysis were performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines. The complete details for this meta-analysis, including the electronic search strategy, objectives, criteria for study selection, eligibility, data collection, and assessment of study quality, are available in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42022311995) on 25 March 2022.1
Literature search and data extraction
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; internet), MEDLINE, and Embase databases from the inception dates to 18 March 2022 using keywords and Medical Subject Headings (MeSH) terms relevant to the exposure of interest to collect data for the systematic review and meta-analysis: “Strut Fracture,” “Strut Fractures,” “Strut Fractured,” “Strut Fracturing,” “Strut Crack,” “Strut Cracked,” “Strut Cracks,” “Strut Cracking,” “Stent Fracture,” “Stent Fractured,” “Stent Fractures,” “Stent Fracturing,” “Stent Crack,” “Stent Cracked,” “Stent Cracks,” “Stent Cracking,” “DES Fracture,” “DES Fractures,” “DES Fractured,” “DES Fracturing,” “DES Crack,” “DES Cracked,” “DES Cracks,” “DES Cracking,” “BMS Fracture,” “BMS Fractures,” “BMS Fractured,” “BMS Fracturing,” “BMS Crack,” “BMS Cracked,” “BMS Cracks,” and “BMS Cracking.” There were no language restrictions. Clinical randomized trials, controlled before-and-after studies, case control studies, and prospective and retrospective cohort studies were eligible for inclusion in the review. Cross-sectional studies, repeat publications of the same analysis or dataset, case reports, conference abstracts, opinion pieces, books or gray literature were excluded. To acquire qualitative/quantitative analyses, inclusion and exclusion criteria were summarized according to the PICOS (Population, Intervention, Comparator, Outcomes, and Study) approach (Supplementary Table 1). Two researchers (YC and DDL) independently screened titles and abstracts. Disagreements were resolved by consensus. The authors looked through the possible relevant articles for inclusion in the final analysis after excluding non-relevant studies. Supplementary Records of selected studies were also evaluated under available conditions. Data were extracted from the included studies in a standardized form for the assessment of study quality and evidence synthesis. Extracted information included year of publication, study type, study period, population, sample size, participant demographics, baseline characteristics, type of DES and postprocedural outcomes.
Outcomes and analyses
The primary outcome of this meta-analysis was the incidence of CSF. CSF was considered and recorded in the research analysis according to reports of the use of plain fluoroscopy, computed tomography (CT), coronary angiography (CAG), intravascular ultrasound (IVUS) or optical coherence tomography (OCT) in the selected studies. The review was performed in accordance with instructions given in the Cochrane Handbook for Systematic Reviews of Intervention.
Risk of bias
Quality was assessed using the Cochrane Collaboration’s tool for assessing the risk of bias in randomized controlled trials (RCTs). For non-RCTs, the modified Newcastle–Ottawa quality assessment scale was used to assess the quality of the included cohort studies and case control studies. The total score for the modified Newcastle–Ottawa scale for cohort studies is a maximum of nine stars overall with a minimum of zero. A study was considered high quality if it achieved 7 out of 9 stars and moderate if it achieved 5 out of 9 stars (Supplementary Table 2). Overall quality was independently determined by each reviewer, and any discrepancies were resolved by consensus.
Statistical analysis
Review Manager (RevMan, version 5.4) and the R package for Windows (version 4.1.0) (meta package) were used to perform all the meta-analyses. Random effects meta-analyses were performed using the proportions of patients who experienced CSF as the outcome of interest. Because of the anticipated high degree of heterogeneity, predominantly among non-RCTs, an inverse variance (DerSimonian–Laird) random effects model was applied. Following the identification of each study to be included, precise event rates were noted from the reported results in all cases. After transforming the event parameters to a normal distribution, an appropriate estimation method was chosen to estimate the original rate (9). Pooled effect estimates were expressed as risk ratios with 95% confidence intervals (CIs). Heterogeneity was explored within each meta-analysis using a chi-squared test with significance set at P < 0.10. We expressed the percentage of heterogeneity attributable to variation rather than to chance as I2 (10). Heterogeneity was defined as follows: I2 = 25–49%, low heterogeneity; I2 = 50–74%, moderate heterogeneity; and I2 > 75%, high heterogeneity (10). Based on the results of Cochran’s Q test and Higgins I2, the random effects method took into account the variability among the included studies to be as conservative as possible. A subgroup analysis was performed to investigate the relationship of DES types, follow-up evaluation modes or type of included studies with CSF incidence. Meta-regression models were performed by applying inverse variance weighting with a mixed effects model to explore the potential association between DES duration, study year, patient characteristics, lesion characteristics, stent characteristics and CSF incidence. Publication bias was assessed by visual assessment of funnel plots, Egger’s funnel plots and Egger’s test. Sensitivity analyses were conducted with R (metainf command) to calculate the combined proportion value and 95% confidence interval by excluding each included study separately.
Results
Study selection
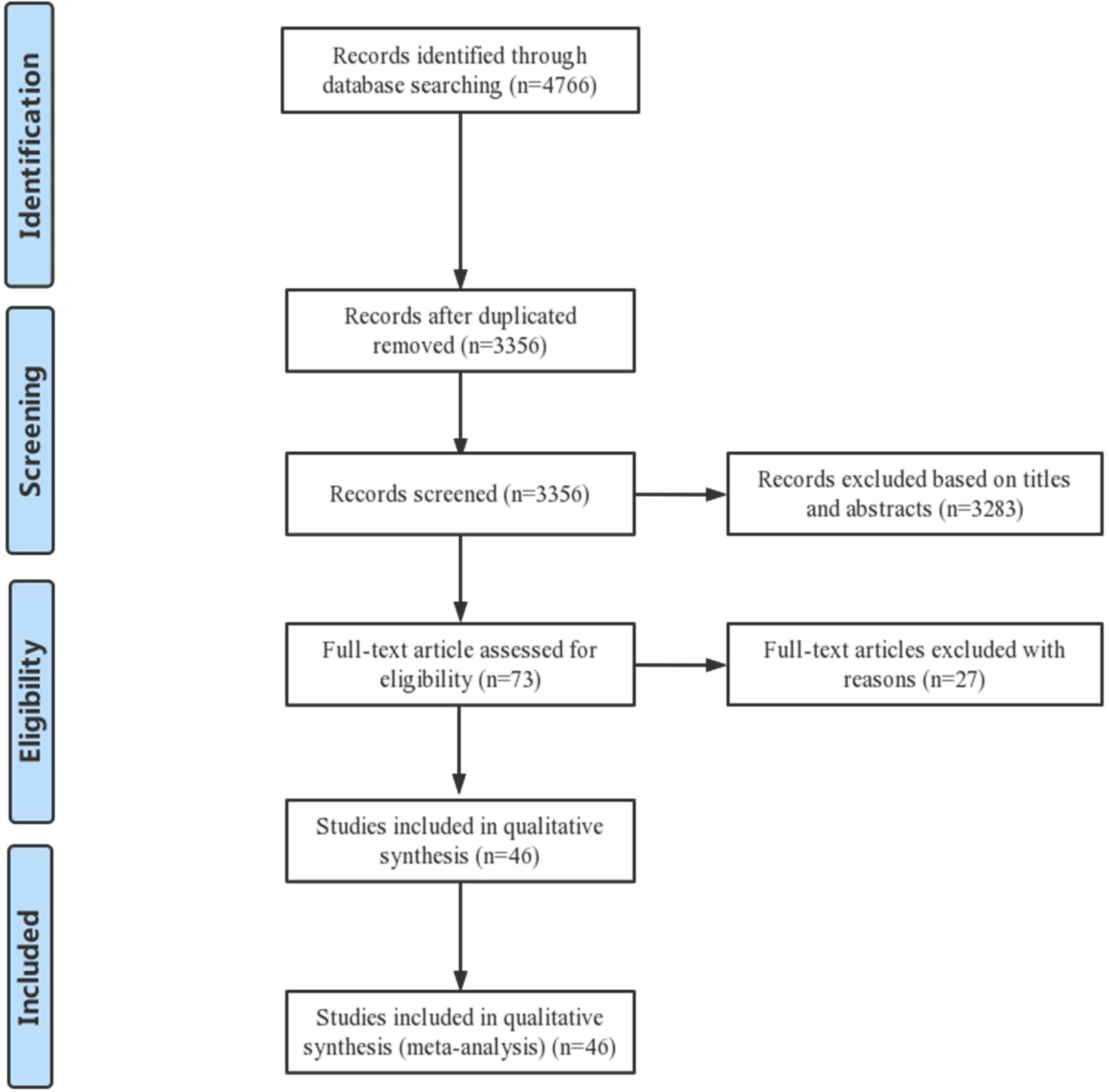
From the systematic review and meta-analysis search, 4,766 potentially eligible records were identified. Titles and abstracts of these records were screened for inclusion. The full texts of 73 records were read, and 46 studies were finally included in the data assessment (3–8, 11–50). Figure 1 shows the PRISMA flow diagram describing the study selection process along with the reasons for exclusion. In brief, a total of 21 prospective studies and 25 retrospective studies were ultimately included in the analysis.
Study characteristics
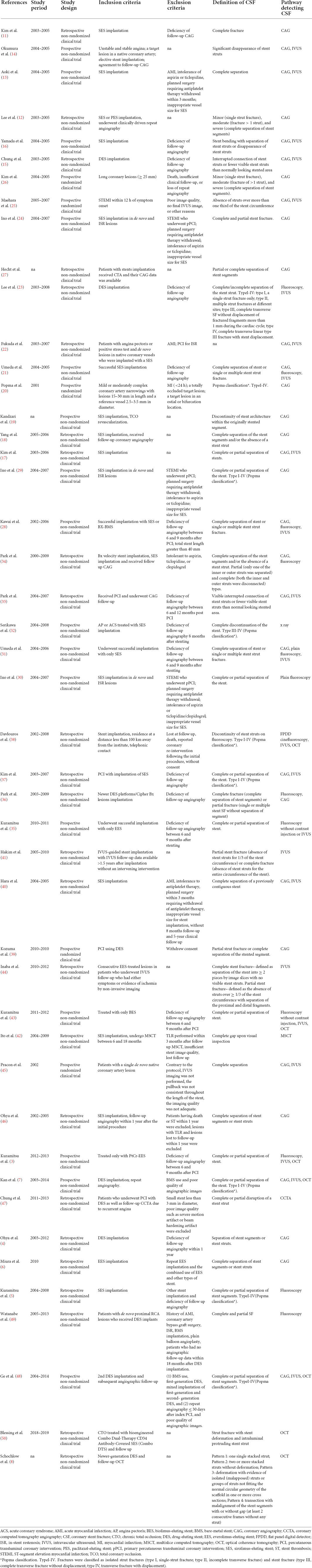
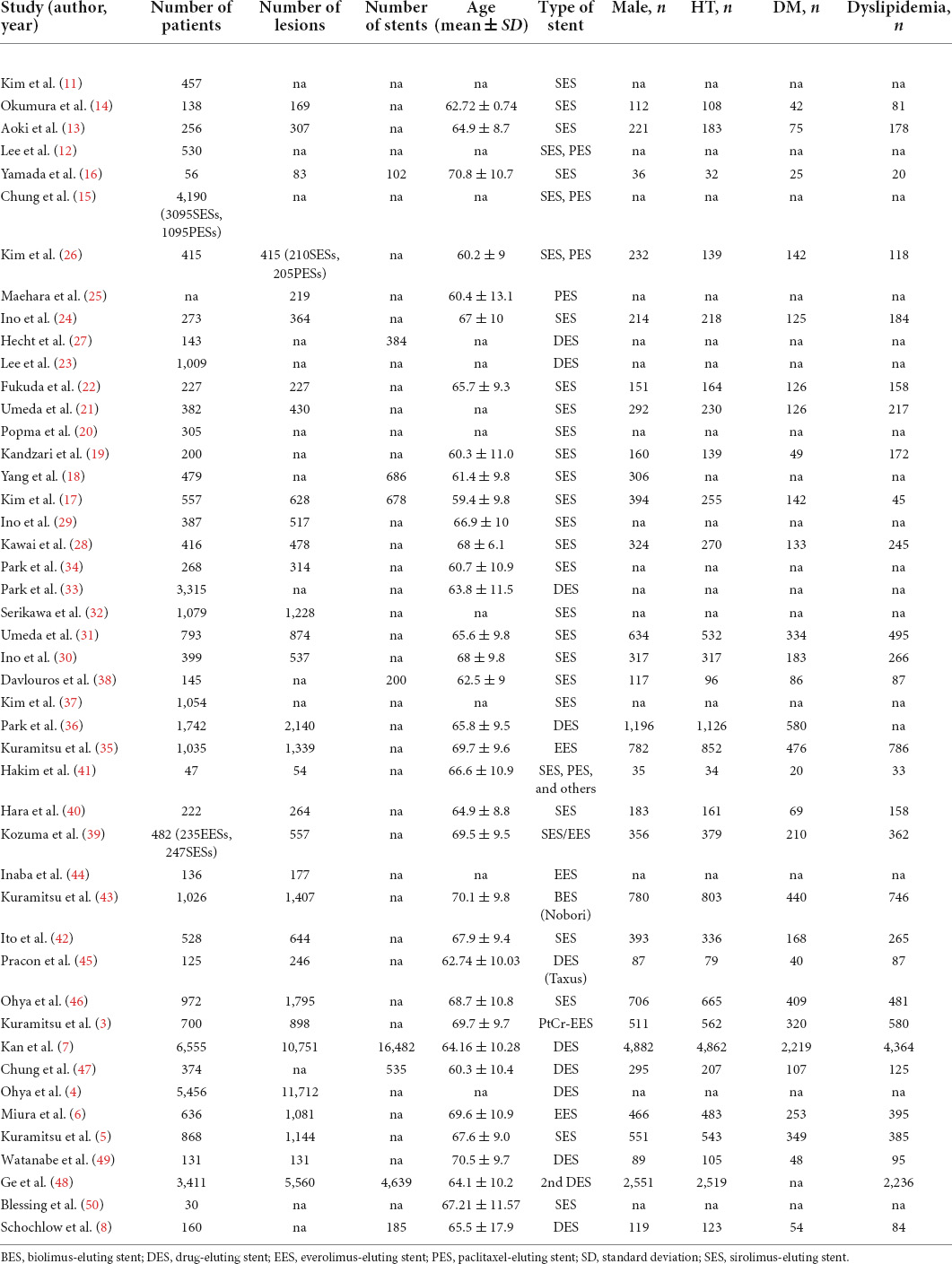
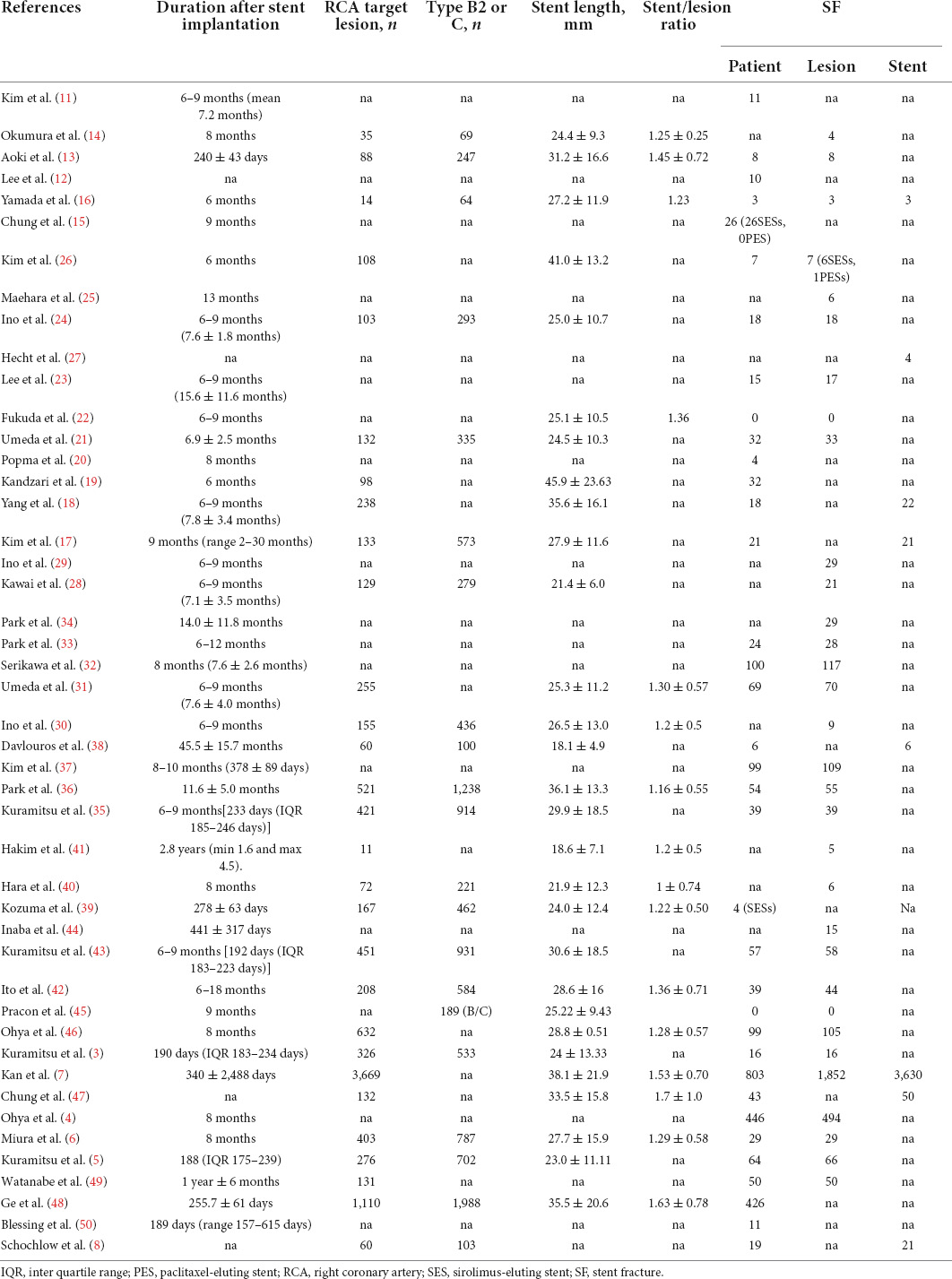
Table 1 shows the main characteristics of the included studies. Table 2 summarizes the study participants’ characteristics. Lesion characteristics and follow-up results are presented in Table 3. All 46 selected papers were published after 2006. The number of included patients for each trial ranged from 30 to 6,555, with the number of included lesions ranging from 54 to 11,712. Among the 46 studies, 36 studies including 39,953 participants showed the incidence of CSF at the patient level, and 29 studies including 39,945 lesions showed the incidence of CSF at the lesion level. In addition, 8 studies including 19,252 stents showed the incidence of CSF at the stent level. Twenty studies included information on sirolimus-eluting stent (SES), and 4 included information on everolimus-eluting stent (EES); 15 studies evaluated CSF based on plain fluoroscopy or CAG, and 31 studies evaluated CSF based on further IVUS, OCT or CT. The total number of included stents was not equal to the sum of the numbers of all kinds of DESs because some studies provided only total numbers rather than DES types in detail. The point incidence of CSF varied from 0 to 38.2% at the patient level, 0 to 38.2% at the lesion level, and 1 to 22% at the stent level.
Risk of bias and study quality
The risk of bias of the included studies is summarized in the Supplemental Material. Quality assessment using the modified Newcastle–Ottawa scale for observational studies showed a low risk of bias with no low-quality study (Supplementary Table 2). There was no high risk of bias for RCTs in terms of random sequence generation, allocation concealment, blinding of participants or blinding of outcome assessment. The particulars of individual bias domains are shown in the risk of bias (Supplementary Figures 1, 2).
Publication bias
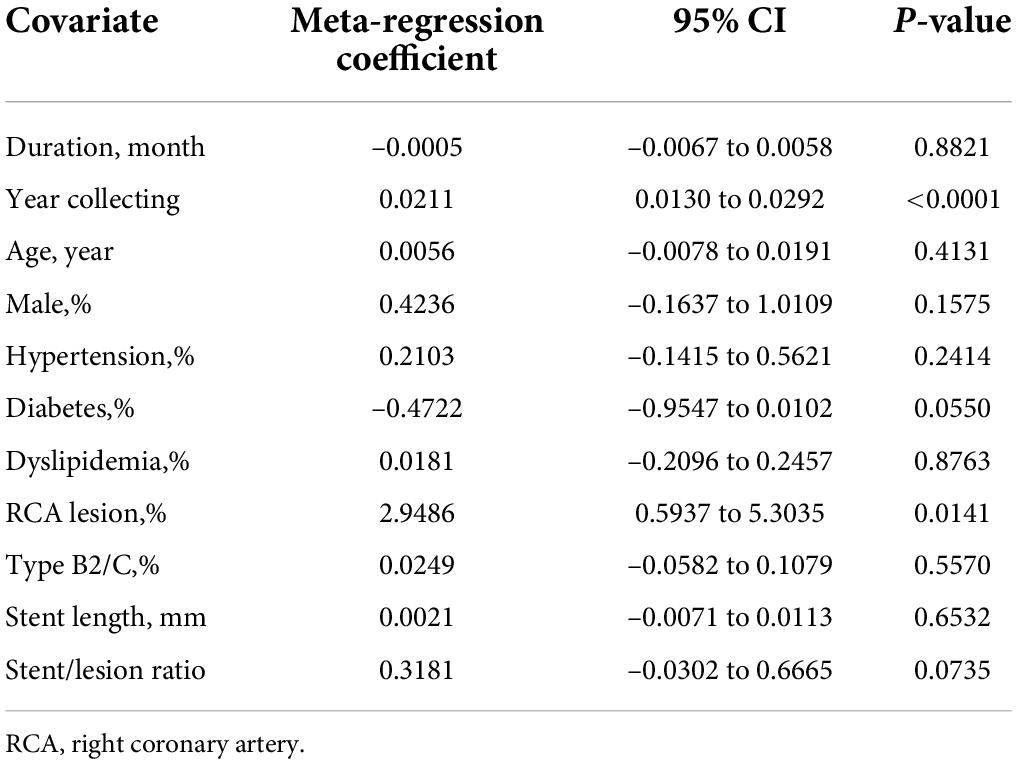
Funnel plots for all the included studies showed symmetrical distributions, indicating no publication bias for the incidence of CSF at the patient and lesion levels (Supplementary Figures 9–12). However, a funnel plot for CSF incidence at the stent level showing an asymmetric distribution indicated likely publication bias (Supplementary Figures 13, 14). Furthermore, analysis using Egger’s test provided similar results at the patient, lesion and stent levels (P = 0.5619, P = 0.0690, P = 0.0008) (Supplementary Tables 6–8).
Pooled incidence rates of coronary stent fracture
The pooled incidence rate for CSF from 36 studies with 39,953 subjects (2,702 patients with CSF) was 5.5% (95% CI: 3.7–7.7%) using a random effects model because of a high level of heterogeneity (98%) (Figure 2). In comparison, the CSF incidence was 4.8% (95% CI: 3.1–6.8%) for 39,945 lesions (3,188 lesions with CSF) in 29 studies and 4.9% (95% CI: 2.5% to 9.4%) for 19,252 stents (3,757 stents with CSF) in 8 studies.
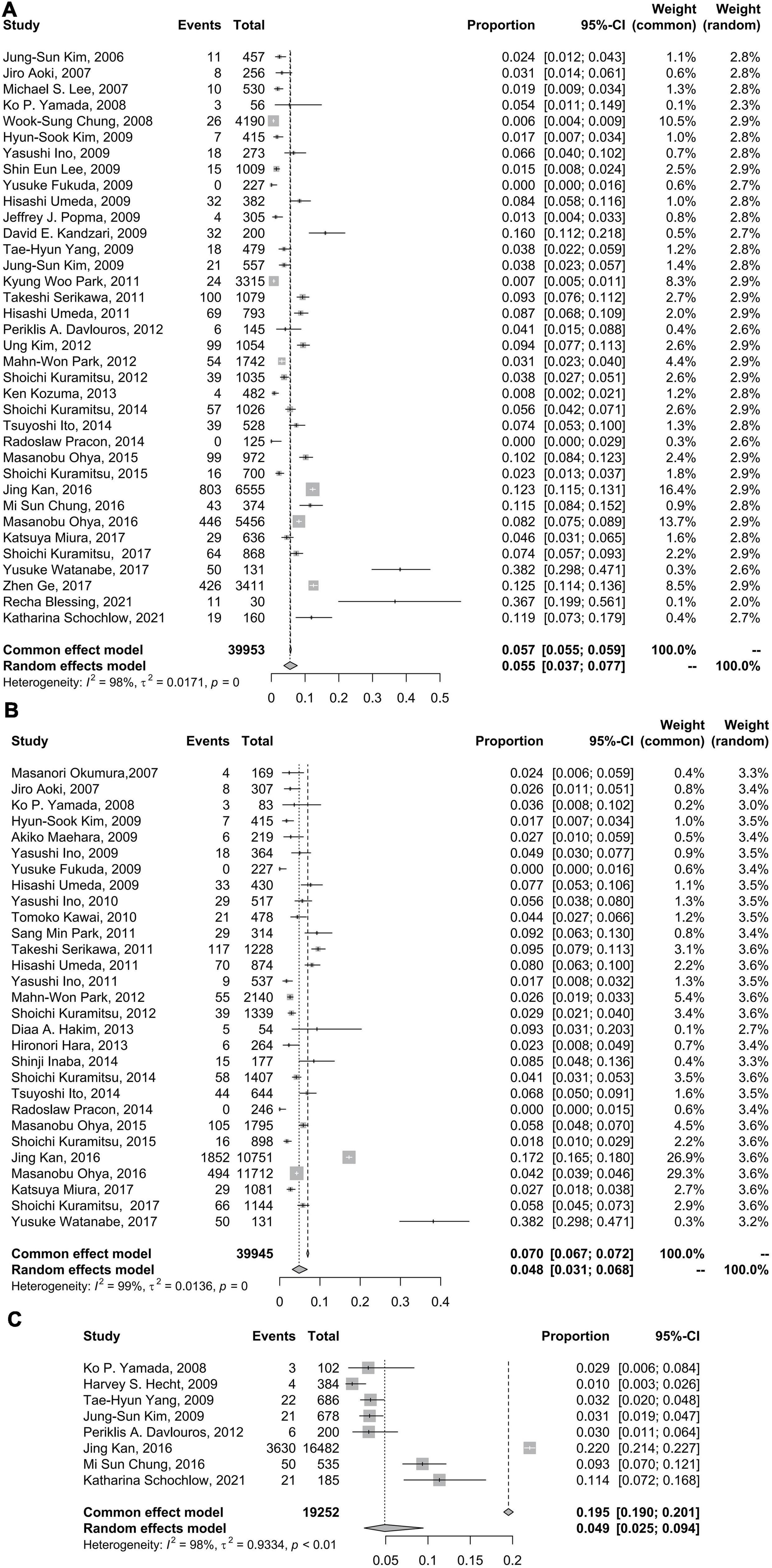
Figure 2. Forest plots of incidence of coronary stent fracture in patient level (A), incidence of stent fracture in lesion level (B) and incidence of stent fracture in stent level (C). CI, confidence interval.
Drug-eluting stent type
The CSF incidence rates for sirolimus-eluting stents (SESs), paclitaxel-eluting stents (PESs), everolimus-eluting stents (EESs), and biolimus-eluting stents (BESs) were 6% (95% CI: 3–8%), 0% (95% CI: 0–0%), 2% (95% CI: 0–5%), and 6% (95% CI: 4–7%), respectively. Tests for subgroup differences in DES type with random effects showed remarkable differences (p < 0.01) (Figure 3).
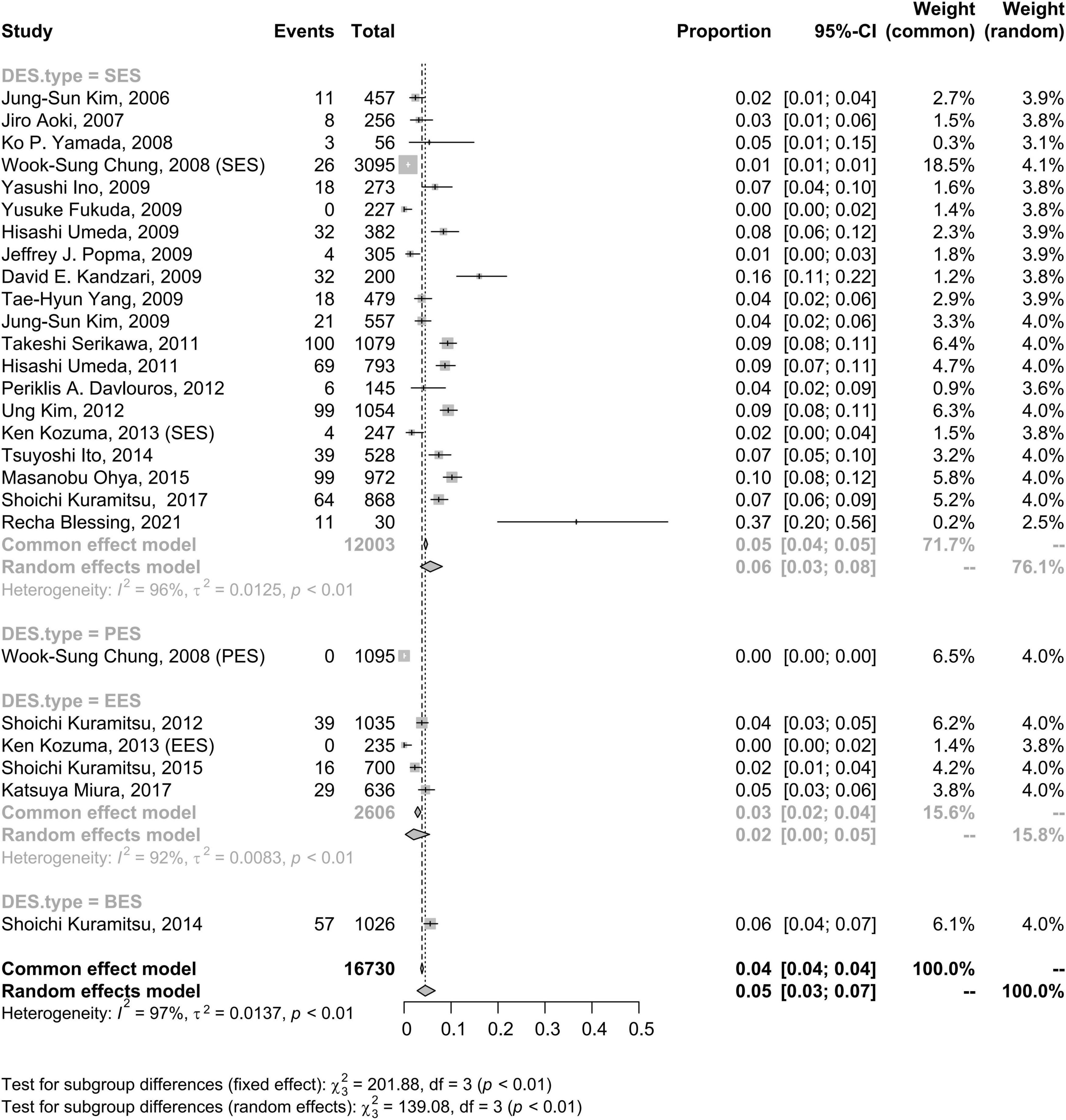
Figure 3. Forest plots of incidence of coronary stent fracture in patient level in different DES type. BES, biolimus-eluting stent; CI, confidence interval; DES, drug-eluting stent; EES, everolimus-eluting stent; PES, paclitaxel-eluting stent; SES, sirolimus-eluting stent.
Follow-up evaluation modes
The incidence rate of CSF at the patient level was not significantly different when the follow-up evaluation was executed with IVUS, OCT or CT (5%, 95% CI: 3–8%) compared to when the follow-up evaluation was executed with plain fluoroscopy or CAG (6%, 95% CI: 3–10%) (Figure 4).
Figure 4. Forest plots of morbidity of coronary stent fracture in patient level in different follow up modes. CAG, coronary angiography; CI, confidence interval; CT, computed tomography; CTA, computed tomography angiography; IVUS, intravascular ultrasound; OCT, optical coherence tomography.
Type of included studies
Overall, the pooled estimate for the subgroup including randomized studies differed appreciably from the combined result from observational studies. The CSF pooled incidence in randomized studies was 1% (95% CI: 0–2%); in observational studies was 6% (95% CI: 4–9%) (Figure 5).
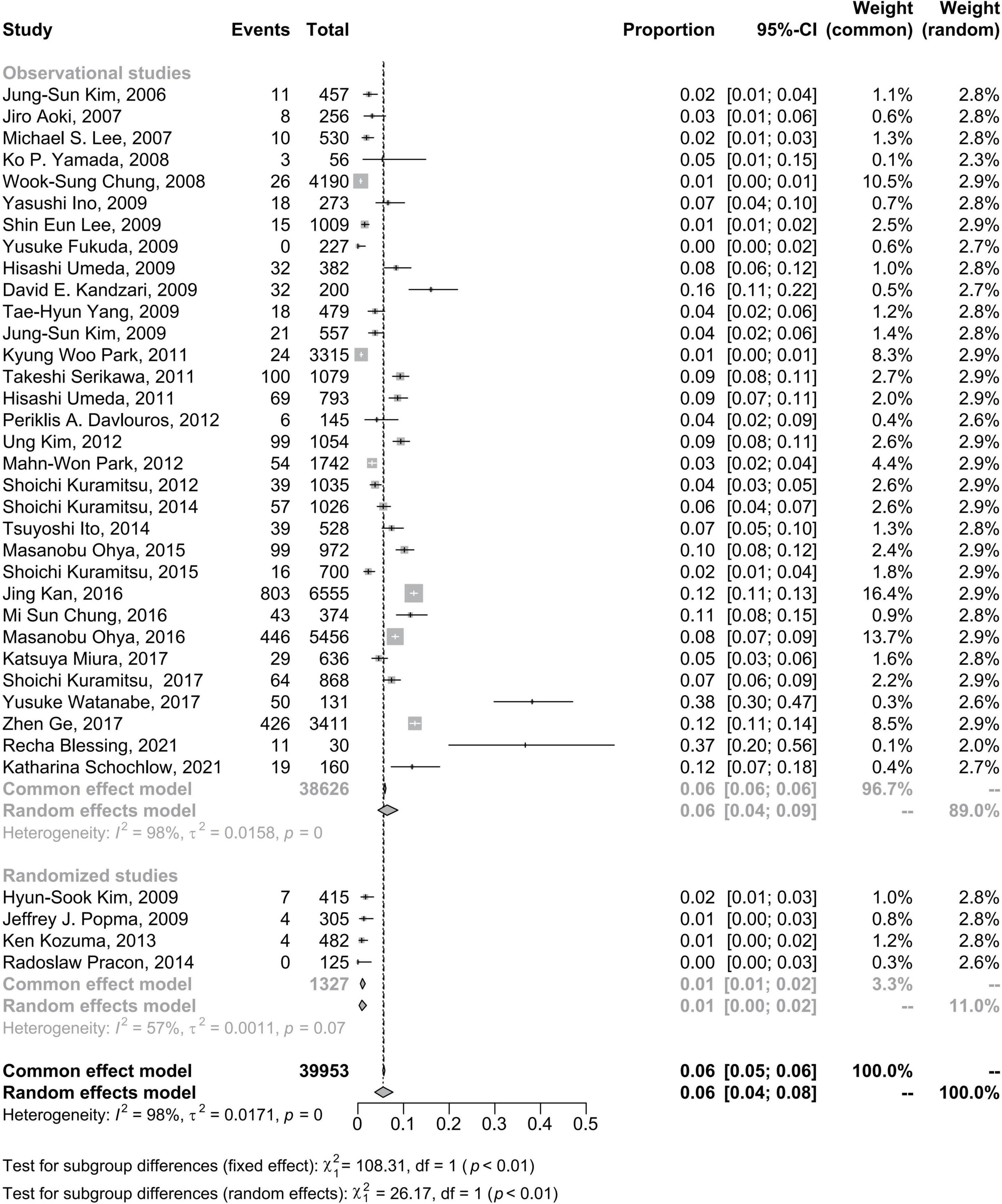
Figure 5. Forest plots of morbidity of coronary stent fracture in patient level in randomized and observational studies. CI, confidence interval.
Meta-regression
Generally, pulsation of the heart can result in metal fatigue and CSF. We evaluated the duration after stent implantation and the incidence of CSF with meta-regression. In the meta-regression analyses, patients’ overall CSF estimates were not modified by the duration of follow-up, which lasted for nearly 5 years (P = 0.8821, Table 4 and Supplementary Figure 4, Supplementary Table 4). The meta-regression results and scattered bubble plot of the incidence of CSF at the patient level between 2000 and 2020 showed a general upwards trend (P < 0.0001, Table 4 and Supplementary Figure 5, Supplementary Table 5). These data indicated that the CSF incidence rate increased from 2000 to 2020. Furthermore, the meta-regression analyses confirmed that RCA lesions were a risk factor for CSF incidence (Table 4). However, the analysis did not identify other potential effects for the incidence of CSF at the lesion level (Table 4).
Sensitivity analyses
Fixed effects models did not change the results of CSF incidence at the patient level but had a slight impact on the CSF incidence at the lesion level and materially changed the results of CSF incidence at the stent level (Figure 2). In the sensitivity analysis using the R command metainf, there was no prominent change when omitting each included study separately at the patient level and lesion level but a relatively prominent change when omitting a study (7), at the stent level for CSF incidence (Supplementary Figures 6–8).
Discussion
This systematic review and meta-analysis were conducted to estimate the incidence rates of CSF, characterize the epidemiology of CSF, compare CSF incidence rates between the different DES types and follow-up evaluation modes, and investigate the potential risk factors for the incidence of CSF. Several CSF characteristics were identified. First, the CSF incidence rate was not as low as expected (5.5% among patients, 4.8% among lesions, and 4.9% among stents). Second, the pooled CSF incidence rates of SES and EES were 6 and 2%, respectively. Third, the duration of the stent after stent implantation seemed to have no significant effect on the incidence of CSF. Fourthly, the incidence of CSF increased with time in recent decades. Finally, RCA lesions contributed significantly to CSF at the lesion level.
To our knowledge, coronary DES fracture was first reported in 2004 (51), and the first study (13) designed to explicitly investigate the incidence of coronary SES fracture occurred in 2007, with an estimate of 2.6% at the lesion level. Compared with the previous meta-analysis of CSF and combined with the meta-regression of our study, there was an increase in the incidence of CSF with time. On the one hand, the closed-cell design and stainless steel stent struts of first-generation DESs resulted in poor flexibility and conformability. However, second-generation stents, characterized by thinner struts, an open-cell design and increased radial strength, tolerated fatigue and reduced the incidence of CSF, as clinical studies have identified (6, 7, 52, 53). On the other hand, some new-generation DESs, such as Promus Element, had a significantly higher risk of longitudinal compression, especially when employed for ostial lesions (47). Furthermore, the incidence may have increased due to the expansion of revisit rates prompting the discovery of asymptomatic CSF and the extensive use of coronary stents in complex lesions (such as twisted angulation, calcification, and chronic total occlusion) in which there was need for longer stents, aggressive post-dilation, stents overlap or multiple stents implantation. Besides, advance in endovascular imaging technology and possibly regional specificity, demographic, sociocultural, and socioeconomic changes may also give rise to the increased incidence. Factors that may have contributed to a more revisit rates include progressive improvements in coverage of post-procedure education, or more widespread coverage of available medical resource.
Previous studies (15, 21, 39, 52, 54) have identified that CSF occurs more frequently in lesions treated with SES. There are some reasons for this. First, an inchoate SES is more likely to cause CSF because of its closed-cell design and stainless steel material with low flexibility and conformability (35). Second, compared with other stents, such as EESs, SESs are more prone to ISR (39, 55–57), which indicates a new hinge point in the stent. With cardiac impulse, the hinge point in the stent segment can be displaced or twisted, leading to stent fracture.
IVUS and OCT have been used as more sensitive methods for the detection of stent fracture due to their high resolution (16, 58, 59). In addition, multislice computed tomography (CT) (42) and CT angiography (27, 60, 61) have been identified as ideal imaging modalities for CSF than CAG. Because coronary stents are small in size and minimally radiopaque, and the 2D technique of CSF detection is viewing angle-dependent, the fractures occurring on one side of the stent could be missed (62). Additionally, an autopsy investigation showed a higher rate of DES fractures (29%) than that in normal clinical studies (53). This result implies that CSF may be underdiagnosed by fluoroscopy as a result of its limited sensitivity to detect stent fractures. However, there was no significant difference between fluoroscopy and IVUS/OCT/CT/CTA based on the current data. It needs to be emphasized that the results were affected by many issues, such as differences in diagnostic criteria, study population, duration of stent, stent types, management condition, and many hidden factors. Besides, not all patients in the group using OCT/IVUS were diagnosed with OCT/IVUS. Most studies stated that the IVUS/OCT was used when performer thought IVUS or OCT was in need. In fact, most follow-up evaluations were often performed using coronary angiography first, with IVUS or OCT being considered for complex lesions. Practically speaking, the number of patients in the group using CAG was much higher than the number of patients in the group using IVUS/OCT. The imbalanced sample size of the two groups may cause a potential bias and the small sample size of the group using IVUS/OCT may limit the power of the study to detect differences between the two groups. According to current knowledge, IVUS and OCT are still the gold standards for CSF diagnosis. Additionally, high heterogeneity was observed and this heterogeneity was still presented in subgroup analyses. More homogeneous studies need to be conducted in the future to explore the problem.
The quality control data of coronary stents has shown that stents can be used consistently for more than 10 years when they are expanded to the maximum diameter on the condition of simulative physiological heart beats (72 beats/min, 40 million beats/year), and no fatigue or fracture occurs. Combined with our analysis results of the association between the duration of the stent after stent implantation, it may be unnecessary for patients with stent implantation to undergo longer repeated evaluations for the occurrence of CSF. However, the best time range to detect CSF remains undefined.
In general, there is greater curvature and range of motion in the right coronary artery (RCA) than in the left coronary artery, especially in the proximal-to-middle segment of the RCA, indicating more severe cardiac motion and angulation, a classic risk factor for metal fatigue (63, 64). Additionally, overlapping stents, saphenous vein grafts, longer stent lengths, stainless stents and multiple stents are universally accepted to be related to CSF (13–15, 19, 54, 65–68). Among these, Kuramitsu et al. (35) revealed that hinge motion and tortuosity contributed the most to the incidence of CSF, suggesting that RCA lesions may be a more important risk factor for CSF.
However, there are some contradictions between our results and previous consensus. The indeterminate definition of CSF, stent type, study participants, incompleteness of follow-up and other factors in the included studies may account for the negative results of the meta-regression analysis of the association of follow-up modes, stent length and lesion type with the incidence of CSF.
Overall, our study findings support that the incidence of CSF has increased in recent years and that CSF remains an inevitable issue. Accordingly, it is vital for clinicians to be aware of the incidence and predictors of CSF. More importantly, it is useful to learn about the characteristics of stents for proper use of current DESs based on lesion characteristics, and an appropriate detection mode according to the complexity of the lesion is also essential. In addition, it seems unnecessary to carry out longer follow-ups to detect the occurrence of CSF.
This study has several limitations. First, studies including only participants with ST or ISR were excluded for their high heterogeneity, which may result in an underestimate of the incidence of CSF among all patients treated with stents, as CSF occurs more frequently among participants with ST or ISR. Second, there were few studies investigating the occurrence of CSF at the stent level, and the funnel plots showed an asymmetrical trend, which may indicate publication bias or be a result of small-study effects, that is, the tendency for smaller studies to show larger treatment effects. Third, even though we tried to account for the high heterogeneity with an inverse variance (DerSimonian–Laird) random effects model, it is difficult to interpret the results. Fourthly, analyses to assess the competing risk were not performed, meaning that the occurrence of the event could have been impacted by competing events. Finally, standards of diagnostic criteria for CSF were not established, and not all information was available, possibly leading to an underestimation of the incidence of CSF. Consequently, large prospective randomized trials are needed to better analyze and understand the phenomenon.
Conclusion
CSF is a common phenomenon in patients treated with DES, and the incidence of CSF has increased in the past two decades. This meta-analysis provided reliable estimates of the incidence of CSF among patients with coronary heart disease treated with stent implantation. The point incidence of CSF was found to be 5.5% among patients, 4.8% among lesions, and 4.9% among stents, and the data had high heterogeneity. The results indicated that the duration of stent placement after stent implantation had no impact on the incidence of CSF. In addition, DES type and RCA lesions influenced the incidence of CSF. The potential mechanisms are multifactorial. It is important to understand the relevant features of stents and select appropriate stent types based on the type of lesion. Further research to design appropriate strategies and protocols for the monitoring, management, and prevention of CSF should be a matter of thorough investigation.
Data availability statement
All datasets analyzed for this study are included in the article/Supplementary material.
Author contributions
YC, DL, and RY conceived the study design and wrote the manuscript. YC, HL, XY, and JD performed the data collection and analyses. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81960081), the Jiangxi Thousand Talents Plan Project (No. jxsq2019201029), and the “5511” Science and Technology Innovation Talent Program of Jiangxi (No. 20171BCB18004). The source of funding did not have any impact on study design; collection, analysis, and interpretation of data or writing of the manuscript.
Acknowledgments
We would like to thank the patients included in the studies we reviewed.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.925912/full#supplementary-material
Footnotes
References
1. Schmidt T, Abbott JD. Coronary stents: History, design, and construction. J Clin Med. (2018) 7:126. doi: 10.3390/jcm7060126
2. Torii S, Jinnouchi H, Sakamoto A, Kutyna M, Cornelissen A, Kuntz S, et al. Drug-eluting coronary stents: Insights from preclinical and pathology studies. Nat Rev Cardiol. (2020) 17:37–51. doi: 10.1038/s41569-019-0234-x
3. Kuramitsu S, Hiromasa T, Enomoto S, Shinozaki T, Iwabuchi M, Mazaki T, et al. Incidence and clinical impact of stent fracture after PROMUS element platinum chromium everolimus-eluting stent implantation. JACC Cardiovasc Interv. (2015) 8:1180–8. doi: 10.1016/j.jcin.2015.02.029
4. Ohya M, Kadota K, Kubo S, Tada T, Habara S, Shimada T, et al. Incidence, predictive factors, and clinical impact of stent recoil in stent fracture lesion after drug-eluting stent implantation. Int J Cardiol. (2016) 214:123–9. doi: 10.1016/j.ijcard.2016.03.013
5. Kuramitsu S, Jinnouchi H, Shinozaki T, Hiromasa T, Matsumura Y, Yamaji Y, et al. Incidence and long-term clinical impact of late-acquired stent fracture after sirolimus-eluting stent implantation in narrowed coronary arteries. Am J Cardiol. (2017) 120:55–62. doi: 10.1016/j.amjcard.2017.03.259
6. Miura K, Tada T, Kuwayama A, Shimada T, Ohya M, Amano H, et al. Stent fracture and peri-stent contrast staining after everolimus-eluting stent implantation-5-year outcomes. Circ J. (2017) 81:1514–21. doi: 10.1253/circj.CJ-17-0236
7. Kan J, Ge Z, Zhang J, Liu Z, Tian N, Ye F, et al. Incidence and clinical outcomes of stent fractures on the basis of 6,555 patients and 16,482 drug-eluting stents from 4 centers. JACC Cardiovasc Interv. (2016) 9:1115–23. doi: 10.1016/j.jcin.2016.02.025
8. Schochlow K, Weissner M, Blachutzik F, Boeder NF, Tröbs M, Lorenz L, et al. Coronary stent strut fractures: Classification, incidence and clinical associations. J Clin Med. (2021) 10:1765. doi: 10.3390/jcm10081765
9. Luo M, Tan H, Zhou Q, Wang S, Cai C, Guo Y, et al. Realizing the meta-analysis of single rate in R software. J Evid Based Med. (2013) 13:181–4. doi: 10.3969/j.issn.1671-5144.2013.03.014
10. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
11. Kim J, Yoon YW, Hong B, Kwon HM, Cho J, Choi D, et al. Delayed stent fracture after successful sirolimus-eluting stent(Cypher®) implantation. Korean Circ J. (2006) 36:443. doi: 10.4070/kcj.2006.36.6.443
12. Lee MS, Jurewitz D, Aragon J, Forrester J, Makkar RR, Kar S. Stent fracture associated with drug-eluting stents: Clinical characteristics and implications. Catheterizat Cardiovasc Interv. (2007) 69:387–94. doi: 10.1002/ccd.20942
13. Aoki J, Nakazawa G, Tanabe K, Hoye A, Yamamoto H, Nakayama T, et al. Incidence and clinical impact of coronary stent fracture after sirolimus-eluting stent implantation. Catheterizat Cardiovasc Interv. (2007) 69:380–6. doi: 10.1002/ccd.20950
14. Okumura M, Ozaki Y, Ishii J, Kan S, Naruse H, Matsui S, et al. Restenosis and stent fracture following sirolimus-eluting stent (SES) implantation. Circ J. (2007) 71:1669–77. doi: 10.1253/circj.71.1669
15. Chung W, Park C, Seung K, Kim P, Lee J, Koo B, et al. The incidence and clinical impact of stent strut fractures developed after drug-eluting stent implantation. Int J Cardiol. (2008) 125:325–31. doi: 10.1016/j.ijcard.2007.02.033
16. Yamada KP, Koizumi T, Yamaguchi H, Kaneda H, Bonneau HN, Honda Y, et al. Serial angiographic and intravascular ultrasound analysis of late stent strut fracture of sirolimus-eluting stents in native coronary arteries. Int J Cardiol. (2008) 130:255–9. doi: 10.1016/j.ijcard.2007.08.082
17. Kim J, Lee S, Lee JM, Yoon YW, Ahn C, Kim M, et al. Significant association of coronary stent fracture with in-stent restenosis in sirolimus-eluting stents. Coronary Artery Dis. (2009) 20:59–63. doi: 10.1097/MCA.0b013e32830fd101
18. Yang T, Kim D, Park S, Seo J, Cho H, Seol S, et al. Clinical characteristics of stent fracture after sirolimus-eluting stent implantation. Int J Cardiol. (2009) 131:212–6. doi: 10.1016/j.ijcard.2007.10.059
19. Kandzari DE, Rao SV, Moses JW, Dzavik V, Strauss BH, Kutryk MJ, et al. Clinical and angiographic outcomes with sirolimus-eluting stents in total coronary occlusions. The ACROSS/TOSCA-4 (Approaches to Chronic Occlusions With Sirolimus-Eluting Stents/Total Occlusion Study of Coronary Arteries-4) trial. JACC Cardiovasc Interv. (2009) 2:97–106. doi: 10.1016/j.jcin.2008.10.013
20. Popma JJ, Tiroch K, Almonacid A, Cohen S, Kandzari DE, Leon MB. A qualitative and quantitative angiographic analysis of stent fracture late following sirolimus-eluting stent implantation. Am J Cardiol. (2009) 103:923–9. doi: 10.1016/j.amjcard.2008.12.022
21. Umeda H, Gochi T, Iwase M, Izawa H, Shimizu T, Ishiki R, et al. Frequency, predictors and outcome of stent fracture after sirolimus-eluting stent implantation. Int J Cardiol. (2009) 133:321–6. doi: 10.1016/j.ijcard.2007.12.067
22. Fukuda Y, Shirai K, Miura S, Ike A, Takamiya Y, Kuwano T, et al. The impact of angulated lesions on angiographic late loss in patients with drug-eluting stent implantation. J Cardiol. (2009) 53:396–401. doi: 10.1016/j.jjcc.2009.01.007
23. Lee SE, Jeong MH, Kim IS, Ko JS, Lee MG, Kang WY, et al. Clinical outcomes and optimal treatment for stent fracture after drug-eluting stent implantation. J Cardiol. (2009) 53:422–8. doi: 10.1016/j.jjcc.2009.02.010
24. Ino Y, Toyoda Y, Tanaka A, Ishii S, Kusuyama Y, Kubo T, et al. Predictors and prognosis of stent fracture after sirolimus-eluting stent implantation. Circ J. (2009) 73:2036–41. doi: 10.1253/circj.CJ-09-0343
25. Maehara A, Mintz GS, Lansky AJ, Witzenbichler B, Guagliumi G, Brodie B, et al. Volumetric intravascular ultrasound analysis of paclitaxel-eluting and bare metal stents in acute myocardial infarction. Circulation. (2009) 120:1875–82. doi: 10.1161/CIRCULATIONAHA.109.873893
26. Kim H, Kim Y, Lee S, Park D, Lee CW, Hong M, et al. Incidence and predictors of drug-eluting stent fractures in long coronary disease. Int J Cardiol. (2009) 133:354–8. doi: 10.1016/j.ijcard.2008.01.005
27. Hecht HS, Polena S, Jelnin V, Jimenez M, Bhatti T, Parikh M, et al. Stent gap by 64-detector computed tomographic angiography. J Am Coll Cardiol. (2009) 54:1949–59. doi: 10.1016/j.jacc.2009.06.045
28. Kawai T, Umeda H, Ota M, Hattori K, Ishikawa M, Okumura M, et al. Do drug elution components increase the risk of fracture of sirolimus-eluting stents? Coronary Artery Dis. (2010) 21:298–303. doi: 10.1097/MCA.0b013e32833aa6a1
29. Ino Y, Toyoda Y, Tanaka A, Ishii S, Kusuyama Y, Kubo T, et al. Serial angiographic findings and prognosis of stent fracture site without early restenosis after sirolimus-eluting stent implantation. Am Heart J. (2010) 160:771–5. doi: 10.1016/j.ahj.2010.07.010
30. Ino Y, Kubo T, Kitabata H, Shimamura K, Shiono Y, Orii M, et al. Impact of hinge motion on in-stent restenosis after sirolimus-eluting stent implantation. Circ J. (2011) 75:1878–84. doi: 10.1253/circj.CJ-10-1182
31. Umeda H, Kawai T, Misumida N, Ota T, Hayashi K, Iwase M, et al. Impact of sirolimus-eluting stent fracture on 4-year clinical outcomes. Circ Cardiovasc Interv. (2011) 4:349–54. doi: 10.1161/CIRCINTERVENTIONS.110.958306
32. Serikawa T, Kawasaki T, Koga H, Orita Y, Ikeda S, Goto Y, et al. Late catch-up phenomenon associated with stent fracture after sirolimus-eluting stent implantation: Incidence and outcome. J Interv Cardiol. (2011) 24:165–71. doi: 10.1111/j.1540-8183.2010.00614.x
33. Park KW, Park JJ, Chae I, Seo J, Yang H, Lee H, et al. Clinical characteristics of coronary drug-eluting stent fracture: Insights from a two-center DES registry. J Korean Med Sci. (2011) 26:53. doi: 10.3346/jkms.2011.26.1.53
34. Park SM, Kim J, Hong B, Lee BK, Min P, Rim S, et al. Predictors of stent fracture in patients treated with closed-cell design stents. Coronary Artery Dis. (2011) 22:40–4. doi: 10.1097/MCA.0b013e328341bb04
35. Kuramitsu S, Iwabuchi M, Haraguchi T, Domei T, Nagae A, Hyodo M, et al. Incidence and clinical impact of stent fracture after everolimus-eluting stent implantation. Circ Cardiovasc Interv. (2012) 5:663–71. doi: 10.1161/CIRCINTERVENTIONS.112.969238
36. Park M, Chang K, Her SH, Lee J, Choi Y, Kim D, et al. Incidence and clinical impact of fracture of drug-eluting stents widely used in current clinical practice: Comparison with initial platform of sirolimus-eluting stent. J Cardiol. (2012) 60:215–21. doi: 10.1016/j.jjcc.2012.07.011
37. Kim U, Kim D, Kim D, Seol S, Jang J, Yang T, et al. Long-term clinical and angiographic outcomes of patients with sirolimus-eluting stent fracture. Int J Cardiol. (2012) 158:83–7. doi: 10.1016/j.ijcard.2011.01.013
38. Davlouros PA, Chefneux C, Xanthopoulou I, Papathanasiou M, Zaharioglou E, Tsigkas G, et al. Flat panel digital detector cinefluoroscopy late following SES or BMS implantation for detection of coronary stent fracture in asymptomatic patients. Int J Cardiol. (2012) 156:277–82. doi: 10.1016/j.ijcard.2010.11.002
39. Kozuma K, Kimura T, Kadota K, Suwa S, Kimura K, Iwabuchi M, et al. Angiographic findings of everolimus-eluting as compared to sirolimus-eluting stents: Angiographic sub-study from the randomized evaluation of sirolimus-eluting versus everolimus-eluting stent Trial (RESET). Cardiovasc Interv Ther. (2013) 28:344–51. doi: 10.1007/s12928-013-0179-7
40. Hara H, Aoki J, Tanabe K, Tanimoto S, Nakajima Y, Yahagi K, et al. Incidence and predictors for late target lesion revascularization after sirolimus-eluting stent implantation. Circ J. (2013) 77:988–94. doi: 10.1253/circj.CJ-12-0570
41. Hakim DA, Mintz GS, Sanidas E, Rusinova R, Weisz G, Leon MB, et al. Serial gray scale intravascular ultrasound findings in late drug-eluting stent restenosis. Am J Cardiol. (2013) 111:695–9. doi: 10.1016/j.amjcard.2012.11.027
42. Ito T, Kimura M, Ehara M, Terashima M, Nasu K, Kinoshita Y, et al. Impact of sirolimus-eluting stent fractures without early cardiac events on long-term clinical outcomes: A multislice computed tomography study. Eur Radiol. (2014) 24:1006–12. doi: 10.1007/s00330-014-3118-9
43. Kuramitsu S, Iwabuchi M, Yokoi H, Domei T, Sonoda S, Hiromasa T, et al. Incidence and clinical impact of stent fracture after the nobori biolimus-eluting stent implantation. J Am Heart Assoc. (2014) 3:e703. doi: 10.1161/JAHA.113.000703
44. Inaba S, Mintz GS, Yun KH, Yakushiji T, Shimizu T, Kang SJ, et al. Mechanical complications of everolimus-eluting stents associated with adverse events: An intravascular ultrasound study. Eurointervention. (2014) 9:1301–8. doi: 10.4244/EIJV9I11A220
45. Pracon R, Opolski MP, Mintz GS, Pregowski J, Kruk M, Popma JJ, et al. Serial intravascular ultrasound analysis of stent strut distribution and fracture: An integrated analysis of the taxus IV, V, and VI trials. J Invasive Cardiol. (2014) 26:501.
46. Ohya M, Kadota K, Tada T, Habara S, Shimada T, Amano H, et al. Stent fracture after sirolimus-eluting stent implantation: 8-year clinical outcomes. Circ Cardiovasc Interv. (2015) 8:e2664. doi: 10.1161/CIRCINTERVENTIONS.115.002664
47. Chung MS, Yang DH, Kim Y, Roh J, Song J, Kang J, et al. Stent fracture and longitudinal compression detected on coronary CT angiography in the first- and new-generation drug-eluting stents. Int J Cardiovasc Imaging. (2016) 32:637–46. doi: 10.1007/s10554-015-0798-4
48. Ge Z, Liu Z, Kan J, Zhang J, Li S, Tian N, et al. Stent fracture is associated with a higher mortality in patients with type-2 diabetes treated by implantation of a second-generation drug-eluting stent. Int J Cardiovasc Imaging. (2017) 33:1873–81. doi: 10.1007/s10554-017-1194-z
49. Watanabe Y, Takagi K, Naganuma T, Kawamoto H, Fujino Y, Ishiguro H, et al. Independent predictors of in-stent restenosis after drug-eluting stent implantation for ostial right coronary artery lesions. Int J Cardiol. (2017) 240:108–13. doi: 10.1016/j.ijcard.2017.04.083
50. Blessing R, Ahoopai M, Geyer M, Brandt M, Zeiher AM, Münzel T, et al. The bioengineered combo dual-therapy CD34 antibody-covered sirolimus-eluting coronary stent in patients with chronic total occlusion evaluated by clinical outcome and optical coherence tomography imaging analysis. J Clin Med. (2021) 10:80. doi: 10.3390/jcm10010080
51. Sianos G, Hofma S, Ligthart JMR, Saia F, Hoye A, Lemos PA, et al. Stent fracture and restenosis in the drug-eluting stent era. Catheterizat Cardiovasc Interv. (2004) 61:111–6. doi: 10.1002/ccd.10709
52. Alexopoulos D, Xanthopoulou I. Coronary stent fracture: How frequent it is? Does it matter? Hell J Cardiol. (2011) 52:1–5.
53. Nakazawa G, Finn AV, Vorpahl M, Ladich E, Kutys R, Balazs I, et al. Incidence and predictors of drug-eluting stent fracture in human coronary artery. J Am Coll Cardiol. (2009) 54:1924–31. doi: 10.1016/j.jacc.2009.05.075
54. Chakravarty T, White AJ, Buch M, Naik H, Doctor N, Schapira J, et al. Meta-analysis of incidence, clinical characteristics and implications of stent fracture. Am J Cardiol. (2010) 106:1075–80. doi: 10.1016/j.amjcard.2010.06.010
55. Habib A, Karmali V, John MC, Polavarapu R, Nakazawa G, Pachura K, et al. Everolimus-eluting stents improve vascular response in a diabetic animal model. Circ Cardiovasc Interv. (2014) 7:526–32. doi: 10.1161/CIRCINTERVENTIONS.113.001023
56. Kaul U, Bangalore S, Seth A, Arambam P, Abhaichand RK, Patel TM, et al. Paclitaxel-eluting versus everolimus-eluting coronary stents in diabetes. New Engl J Med. (2015) 373:1709–19. doi: 10.1056/NEJMoa1510188
57. Bangalore S, Toklu B, Feit F. Outcomes with coronary artery bypass graft surgery versus percutaneous coronary intervention for patients with diabetes mellitus. Circ Cardiovasc Interv. (2014) 7:518–25. doi: 10.1161/CIRCINTERVENTIONS.114.001346
58. Barlis P, Sianos G, Ferrante G, Del FF, D’Souza S, Di Mario C. The use of intra-coronary optical coherence tomography for the assessment of sirolimus-eluting stent fracture. Int J Cardiol. (2009) 136:e16–20. doi: 10.1016/j.ijcard.2008.04.076
59. Damelou A, Davlouros PA, Alexopoulos D. Late asymptomatic sirolimus-eluting stent fracture in a female with systemic lupus erythematosus. Int J Cardiol. (2011) 149:e72–4. doi: 10.1016/j.ijcard.2009.03.116
60. Lim HB, Hur G, Kim SY, Kim YH, Kwon SU, Lee WR, et al. Coronary stent fracture: Detection with 64-section multidetector CT angiography in patients and in vitro. Radiology. (2008) 249:810–9. doi: 10.1148/radiol.2493088035
61. Pang JH, Kim D, Beohar N, Meyers SN, Lloyd-Jones D, Yaghmai V. Detection of stent fractures: A comparison of 64-slice CT, conventional cine-angiography, and intravascular ultrasonography. Acad Radiol. (2009) 16:412–7. doi: 10.1016/j.acra.2008.10.010
62. Carroll JD. Coronary stent fracture: The hidden truth of a problem more common than stent thrombosis. Catheterizat Cardiovasc Interv. (2009) 73:88–9. doi: 10.1002/ccd.21905
63. Leong DP, Dundon BK, Puri R, Yeend RAS. Very late stent fracture associated with a sirolimus-eluting stent. Heart Lung Circ. (2008) 17:426–8. doi: 10.1016/j.hlc.2007.06.004
64. Ramegowda RT, Chikkaswamy SB, Bharatha A, Radhakrishna J, Krishnanaik GB, Nanjappa MC, et al. Circumferential stent fracture: Novel detection and treatment with the use of stentboost. Tex Heart Inst J. (2012) 39: 431–4.
65. Park JS, Cho IH, Kim YJ. Stent fracture and restenosis after zotarolimus-eluting stent implantation. Int J Cardiol. (2011) 147:e29–31. doi: 10.1016/j.ijcard.2009.01.030
66. Schömig A, Dibra A, Windecker S, Mehilli J, Suárez De Lezo J, Kaiser C, et al. A meta-analysis of 16 randomized trials of sirolimus-eluting stents versus paclitaxel-eluting stents in patients with coronary artery disease. J Am Coll Cardiol. (2007) 50:1373–80. doi: 10.1016/j.jacc.2007.06.047
67. Canan T, Lee MS. Drug-eluting stent fracture: Incidence, contributing factors, and clinical implications. Catheterizat Cardiovasc Interv. (2010) 75:237–45. doi: 10.1002/ccd.22212
Keywords: stent fracture, drug-eluting stent, meta-analysis, meta-regression, incidence
Citation: Chen Y, Li D, Liao Y, Yao X, Ruan Y, Zou K, Liao H, Ding J, Qin H, Yu Z, Zhao Y, Hu L and Yang R (2022) Incidence of coronary drug-eluting stent fracture: A systematic review and meta-analysis. Front. Cardiovasc. Med. 9:925912. doi: 10.3389/fcvm.2022.925912
Received: 22 April 2022; Accepted: 04 August 2022;
Published: 23 August 2022.
Edited by:
Rajeev Gupta, Medicilinic, United Arab EmiratesReviewed by:
Yeela Talmor Barkan, Rabin Medical Center, IsraelDamiano Regazzoli, Humanitas Research Hospital, Italy
Mohammed Najeeb Osman, University Hospitals Cleveland Medical Center, United States
Copyright © 2022 Chen, Li, Liao, Yao, Ruan, Zou, Liao, Ding, Qin, Yu, Zhao, Hu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renqiang Yang, eWFuZ3JlbnFpYW5nY25AMTYzLmNvbQ==
 Yang Chen
Yang Chen Dandan Li2
Dandan Li2 Longlong Hu
Longlong Hu