Abstract
Background:
Epidemiological evidence suggests the association of diabetes with an increased risk of stroke. Clinical studies have investigated the effects of sodium-glucose co-transporter-2 (SGLT2) inhibitors on new-onset stroke (NOS), but the results are inconsistent.
Objectives:
To determine the association between the use of SGLT2 inhibitors and NOS in patients with type 2 diabetes mellitus (DM).
Methods:
We conducted a retrospective longitudinal cohort study based on the Taiwan Health Insurance Review and Assessment Service database (2016–2019). The primary outcome of the assessment was the risk of incident stroke by estimating hazard ratios (HRs) and 95% confidence intervals (CIs). Multiple Cox regression was applied to estimate the adjusted HR of NOS. Subgroup analysis was also conducted.
Results:
Among the 232,101 eligible patients with type 2 DM aged ≥ 20 years, SGLT2-inhibitor users were compared with non-SGLT2-inhibitor users based on age, sex, and the duration of type 2 DM matching at a ratio of 1:2. The event rate per 10 000 person-months was 9.20 (95% CI 8.95 to 9.45) for SGLT2-inhibitor users and 10.5(10.3–10.6) for non-SGLT2-inhibitor users. There was a decreased risk of NOS for SGLT2-inhibitor users (adjusted HR 0.85, 95% CI 0.82–0.88) compared with non-SGLT2-inhibitor users. Results for the propensity score-matched analyses showed similar results (adjusted HR 0.87, 95% CI 0.84–0.91 for both SGLT2-inhibitor users and non-SGLT2-inhibitor users).
Conclusion:
The risk of developing NOS was lower in patients with SGLT2-inhibitor users than in non-SGLT2-inhibitor users. The decreased risk of NOS in patients with type 2 DM was greater among patients with concurrent use of statins, biguanides, thiazolidinediones, and glucagon-like peptide-1 receptor agonists. We, therefore, suggest that the long-term use of SGLT2 inhibitors may help reduce the incidence of NOS in patients with type 2 DM.
Introduction
The global incidence and prevalence of type 2 diabetes mellitus (DM) have increased over the past two decades and caused much health burden across the world (1, 2). Past studies have demonstrated that type 2 DM is associated with an elevated risk of stroke (3, 4). Stroke in patients with type 2 DM has a poor prognosis, which is marked by worse mortality outcomes relative to that in several other diabetes-related comorbidities, including coronary heart diseases (4). It affects approximately 40% of patients with ischemic stroke who had been diagnosed with diabetes in the United States (5). A study reported that controlling glucose levels with intensive diabetes therapy could reduce the risk of stroke by 57% (6).
Sodium-glucose co-transporter-2 (SGLT2) inhibitors are used in patients with type 2 DM as glucose-lowering therapies targeting SGLT2 (7, 8). Although these drugs are primarily indicated for diabetes, several studies have examined their use in the primary and secondary prevention of stroke (9, 10). Animal studies have demonstrated a neuroprotective effect of SGLT2 inhibitors, which play an important role in antioxidant, anti-inflammatory, and anti-apoptotic mechanisms (11–13). SGLT2 inhibitors also improve the endothelial function, prevent remodeling, and exert a protective effect on the neurovascular unit and the blood–brain barrier, which can be promising in stroke therapy (14). However, the results of previous studies are inconsistent in a clinical setting (15–17). Therefore, the objective of the present study was to evaluate the risk of new-onset stroke (NOS) associated with the prescription of SGLT2 inhibitors in a nationwide cohort study of patients with type 2 DM in Taiwan.
Materials and methods
Study design
This is a retrospective study conducted on a population-based cohort using data from the insurance claims provided by the Taiwanese Bureau of National Health Insurance (TBNHI) from January 2004 to December 2019. This database contains anonymized longitudinal medical records that store the claims' information forms in two tables: a visit table and a prescription table. The visit tables contain the patient's identification numbers, sex, age, three diagnostic codes for outpatient and five for inpatient visits, medications, drug doses, medical expenditures, and hospital and physician information. The prescription table contains the quantity and expenditure for all administered drugs, operations, and treatments undertaken.
Patients included in this study were of age at least 20 years, with a newly diagnosed case of type 2 DM with or without prescribed SGLT2 inhibitors between May 2016 and December 2019. SGLT2-inhibitor users were defined as patients who received at least an SGLT2 inhibitor prescription for 180 days during the study period. In contrast, non-SGLT2 inhibitor users were patients who did not receive an SGLT2 inhibitor prescription throughout the study period.
Study population
The study population comprised patients with type 2 DM (ICD-10-CM, E11) who were admitted to the hospital or visited the hospital as an outpatient between May 1, 2016 and December 31, 2019. At least one of the following enrollment criteria was required to be met for inclusion in this study: (1) two or more outpatient visits within 6 months, (2) all antidiabetic drugs were continuously prescribed to the patients for >6 months during the follow-up period, or (3) one or more inpatient admissions with a diagnosis of type 2 DM. The primary endpoint was the development of stroke, which was defined by the time a stroke (ICD-10-CM codes I60, I61, I62, I63, I65, I66, I67.84, G45, G46) code first appeared in the inpatient or outpatient claim records. Comorbidities related to stroke were defined according to the ICD-10-CM code and included coronary heart disease (ICD-10-CM code I20–I25), hypertension (ICD-10-CM code I10), hyperlipidemia (ICD-9-CM code E78.1–E78.5), chronic kidney disease (ICD-10-CM code N18), chronic liver disease (ICD-10-CM code K71, K75, K76), chronic obstructive pulmonary disease (ICD-10-CM code J44), atrial fibrillation and flutter (ICD-10-CM code I48), and rheumatoid arthritis (ICD-9-CM code M05). Patients who fulfilled any of the following criteria were excluded from the study: (1) prior history of stroke before May 1, 2016 and (2) patient age of <20 years. Considering the differences in the baseline characteristics and stroke risk between the SGLT2-inhibitor users and non-SGLT2-inhibitor users, we applied age-, sex-, and type 2 DM duration matching at a ratio of 1:2 for patients with type 2 DM with and without SGLT2 inhibitor use. Finally, the study group comprised 232,101 participants with type 2 DM who were SGLT2 inhibitor users, and the control group included 464,202 randomly selected participants with type 2 DM who were non-SGLT2-inhibitor users (Figure 1). We also conducted propensity score matching with age, sex, duration of type 2 DM, comorbidities, and drug index date at a ratio of 1:1 for sensitivity analysis in patients with type 2 DM with and without the use of an SGLT2 inhibitor (Figure 1).
Figure 1
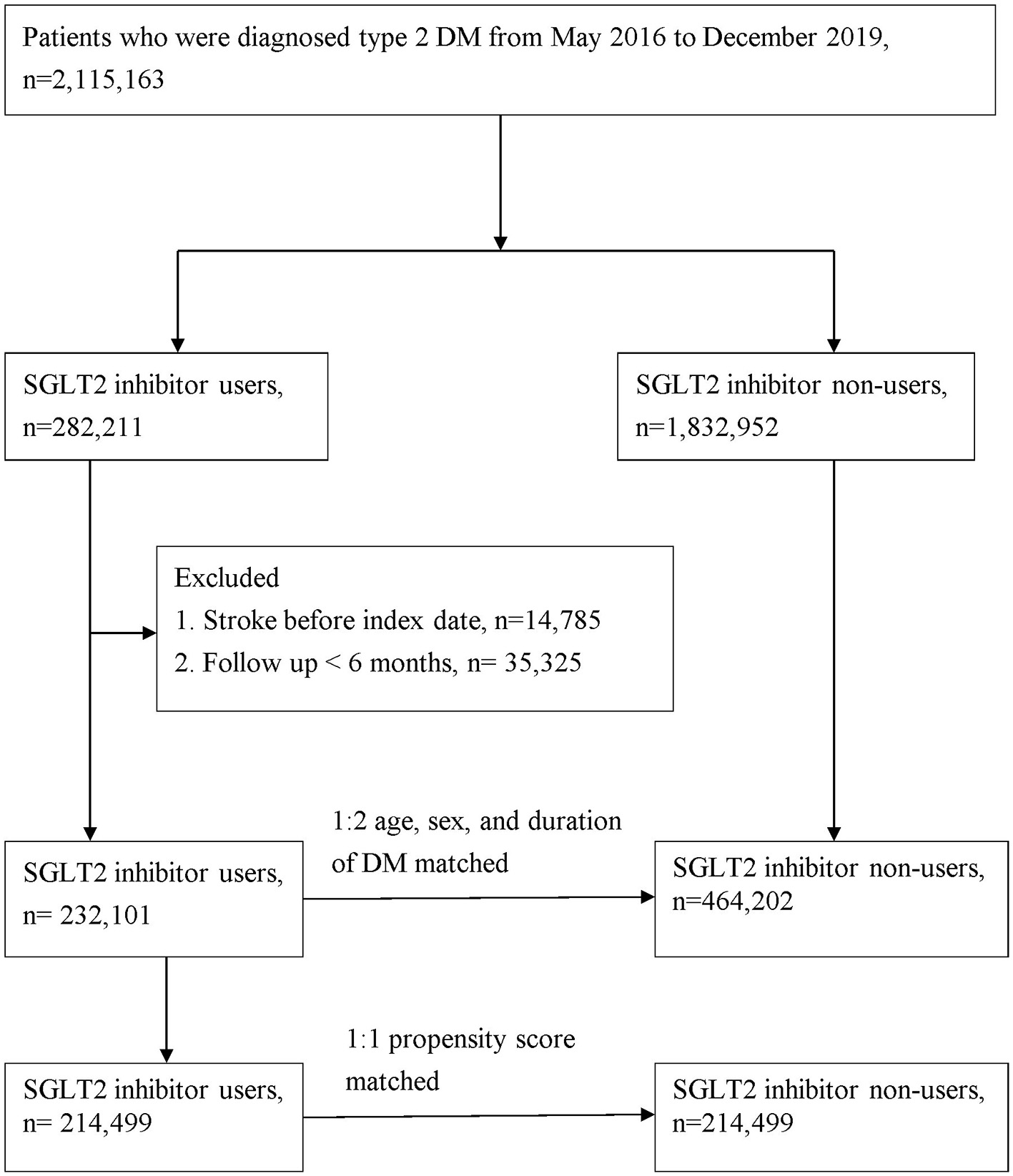
Patient flow chart.
Statistical analysis
Data were presented as valid percentages and the mean values with a standard deviation. Differences in the demographic data and clinical characteristics between SGLT2-inhibitor users and non-SGLT2-inhibitor users were examined using a t-test for continuous variables, whereas Chi-square tests were performed for categorical variables. The Cox proportional hazard regression model was applied to compare the risk of developing study events between the SGLT2 inhibitor group and the non-SGLT2 inhibitor group. Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated after adjusting for important risk factors toward developing the study events, including age, sex, concurrent medication, and comorbidities. The risk of study outcomes over time for the SGLT2 inhibitor group compared with the non-SGLT2 inhibitor group was determined by survival analysis using the Kaplan–Meier method.
We also conducted a sensitivity analysis to test the robustness of our primary findings. Initially, a propensity score was calculated for each patient to minimize confounding by indication, when patients with other risk factors between the SGLT2 inhibitor user group and non-SGLT2 inhibitor user group. Then, the propensity score matching (1:1) and absolute standardized difference (ASD) were performed to estimate the difference between the two groups. An ASD of <0.10 implied a negligible difference in the potential confounders between the two groups.
In addition, we conducted subgroup analyses stratified by sex, age, duration of type 2 DM, presence of comorbidities, and concurrent medication at baseline for the primary outcomes of NOS. Statistical significance was considered at P < 0.05. All statistical calculations were performed using the statistical analysis software, version 9.3 (SAS Institute, Inc., Cary, NC, USA).
Results
Study population and baseline characteristics
A total of 696,303 patients were enrolled in the present study, with the SGLT2-inhibitor user group consisting of 232,101 individuals from the NHIRD who were diagnosed with type 2 DM from May 2016 through December 2019. This group was compared with 464,202 control patients who were non-SGLT2-inhibitor users at a 1:2 ratio (Figure 1). There were more men (55.94%) than women (44.06%) in this study. At the baseline, patients receiving SGLT2 inhibitor had more comorbidities, except for rheumatoid arthritis, and they used more concurrent medication than those not receiving SGLT2 inhibitor (Table 1).
Table 1
| 2:1 sex, age matching | 1:1 Propensity score matching | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Non- SGLT2 (n = 464,202) | SGLT2 (n = 232,101) | P | Non-SGLT2 (n = 214,499) | SGLT2 (n = 214,499) | ASD | |
| Sex | 1.0000 | 0.00177 | |||||
| Female | 204,534 (44.06%) | 102,267 (44.06%) | 94,518 (44.06%) | 94,707 (44.15%) | |||
| Male | 259,668 (55.94%) | 129,834 (55.94%) | 119,981 (55.94%) | 119,792 (55.85%) | |||
| Age | 1.0000 | 0.00000 | |||||
| <50 | 114,804 (24.73%) | 57,402 (24.73%) | 53,466 (24.93%) | 53,131 (24.77%) | |||
| 51–60 | 138,494 (29.83%) | 69,247 (29.83%) | 64,132 (29.90%) | 64,128 (29.90%) | |||
| 61–70 | 142,538 (30.71%) | 71,269 (30.71%) | 66,008 (30.77%) | 65,896 (30.72%) | |||
| >70 | 68,366 (14.73%) | 34,183 (14.73%) | 30,893 (14.4%) | 31,344 (14.61%) | |||
| Years (Mean ± SD) | 58.34 ± 12.21 | 58.34 ± 12.21 | 1.0000 | 58.44 ± 11.89 | 58.29 ± 12.23 | ||
| DM history | <0.0001 | 0.02967 | |||||
| < =2 years | 133,455 (28.75%) | 59,608 (25.68%) | 54,688 (25.50%) | 55,752 (25.99%) | |||
| 3-4 years | 243,394 (52.43%) | 126,088 (54.32%) | 115,391 (53.80%) | 115,875 (54.02%) | |||
| ≥5 years | 87,353 (18.82%) | 46,405 (19.99%) | 44,420 (20.71%) | 42,872 (19.99%) | |||
| Comorbidities | |||||||
| dv11 | Hypertension | 250,659 (54%) | 139,336 (60.03%) | <0.0001 | 128,819 (60.06%) | 12,738 5(59.39%) | 0.01363 |
| dv13 | Coronary artery disease | 51,129 (11.01%) | 41,448 (17.86%) | <0.0001 | 33,966 (15.84%) | 35,030 (16.33%) | 0.01350 |
| dv14 | Hyperlipidemia | 257,784 (55.53%) | 153,956 (66.33%) | <0.0001 | 142,463 (66.42%) | 140,575 (65.54%) | 0.01858 |
| dv19 | Chronic kidney disease | 104,962 (22.61%) | 59,599 (25.68%) | <0.0001 | 57,593 (26.85%) | 54,907 (25.60%) | 0.02847 |
| dv20 | Chronic liver disease | 50,928 (10.97%) | 26,537 (11.43%) | <0.0001 | 24,725 (11.53%) | 24,501 (11.42%) | 0.00328 |
| dv66 | COPD | 15,910 (3.43%) | 8,446 (3.64%) | <0.0001 | 7,301 (3.40%) | 7,631 (3.56%) | 0.00839 |
| dv29 | Atrial fibrillation and flutter | 4,902 (1.06%) | 3,824 (1.65%) | <0.0001 | 3,087 (1.44%) | 3,149 (1.47%) | 0.00242 |
| Rheumatoid arthritis | 3,188 (0.69%) | 1,285 (0.55%) | 0.01696 | 1,168 (0.54%) | 1,202 (0.56%) | 0.00214 | |
| Concurrent medication | |||||||
| Dr1 | NSAIDs | 263,337 (56.73%) | 133,108 (57.35%) | <0.0001 | 122,355 (57.04%) | 122,768 (57.23%) | 0.00389 |
| Dr2 | Corticosteroids | 88,850 (19.14%) | 45,398 (19.56%) | <0.0001 | 41,286 (19.25%) | 41,608 (19.40%) | 0.00380 |
| Dr3 | PPIs | 35,647 (7.68%) | 18,410 (7.93%) | 0.0002 | 16,619 (7.75%) | 16,739 (7.80%) | 0.00209 |
| Dr4 | H2-receptor antagonists | 120,629 (25.99%) | 61,091 (26.32%) | 0.0027 | 55,435 (25.84%) | 56,109 (26.16%) | 0.00716 |
| Dr5 | Aspirins | 92,245 (19.87%) | 63,518 (27.37%) | <0.0001 | 55,176 (25.72%) | 55,748 (25.99%) | 0.00609 |
| Dr25 | Statins | 240,244 (51.75%) | 162,084 (69.83%) | <0.0001 | 147,212 (68.63%) | 146,131 (68.13%) | 0.01084 |
| Dr13 | Biguanides | 242,784 (52.3%) | 151,068 (65.09%) | <0.0001 | 134,691 (62.79%) | 136,345 (63.56%) | 0.01599 |
| Dr14 | Sulfonylureas | 155,979 (33.6%) | 101,140 (43.58%) | <0.0001 | 91,743 (42.77%) | 90,022 (41.97%) | 0.01624 |
| Dr15 | Alpha glucosidase inhibitors | 45,540 (9.81%) | 43,008 (18.53%) | <0.0001 | 34,432 (16.05%) | 35,391 (16.50%) | 0.01211 |
| Dr16 | Thiazolidinediones | 43,754 (9.43%) | 41,938 (18.07%) | <0.0001 | 34,607 (16.13%) | 34,857 (16.25%) | 0.00316 |
| Dr17 | DPP4 inhibitors | 99,152 (21.36%) | 93,734 (40.39%) | <0.0001 | 80,445 (37.50%) | 79,384 (37.01%) | 0.01023 |
| Dr18 | Insulins | 71,925 (15.49%) | 57,020 (24.57%) | <0.0001 | 48,358 (22.54%) | 48,840 (22.77%) | 0.00537 |
| Dr26 | GLP-1 receptor agonists | 5,101 (1.1%) | 4,244 (1.83%) | <0.0001 | 3,763 (1.75%) | 3,665 (1.71%) | 0.00350 |
Baseline characteristics of all patients.
COPD, chronic obstructive pulmonary disease; DPP4, Dipeptidyl peptidase 4; GLP-1, Glucagon-like peptide-1; NSAID, Non-steroid anti-inflammatory drug; PPI, proton pump inhibitor; ASD, absolute standardized difference; PSM, propensity score matching; SD, standard deviation.
Analysis of the main TBNHI cohort
During the follow-up, 5,186 and 11,701 NOSs events were recorded in the SGLT2-inhibitor user and non-SGLT2-inhibitor user groups, respectively. The event rate was 9.20 per 10 000 person-months (95% CI 8.95–9.45) for SGLT2-inhibitor users when compared with 10.50 (95% CI 10.30–10.60) for non-SGLT2-inhibitor users. There was a significantly lower the incidence rate of NOS after adjusting for the duration of type 2 DM history, sex, age, comorbidities, and concurrent medication among the SGLT2-inhibitor users when compared to that among the non-SGLT2-inhibitor users (adjusted HR: 0.85; 95% CI: 0.82–0.88) (Table 2). The cumulative incidence rate of developing stroke was also lower in the SGLT2-inhibitor users than in the non-SGLT2-inhibitor in the Kaplan–Meier survival analysis (P < 0.0001; Figure 2A).
Table 2
| 2:1 sex age matching | 1:1 Propensity score matching | |||
|---|---|---|---|---|
| Non- SGLT2 | SGLT2 | Non- SGLT2 | SGLT2 | |
| N | 464,202 | 232,101 | 214,499 | 214,499 |
| Follow up person months | 11,135,130 | 5,634,359 | 5,177,840 | 5,191,193 |
| New case | 11,701 | 5,186 | 5,328 | 4,678 |
| Incidence rate*(95% C.I.) | 10.50 (10.30–10.60) | 9.20 (8.95–9.45) | 10.20 (10.00–10.50) | 9.01 (8.75–9.27) |
| Crude Relative risk (95% C.I.) | Reference | 0.88 (0.85–0.91) | Reference | 0.88 (0.84–0.91) |
| Adjusted HR* (95% C.I.)† | Reference | 0.85 (0.82–0.88) | Reference | 0.87 (0.84–0.91) |
Incidence rate of stroke.
Incidence rate, per 10,000 person-months.
adjusted hazard ratio, the covariates including duration of DM history, sex, age, co-morbidities, and medication at baseline.
Figure 2
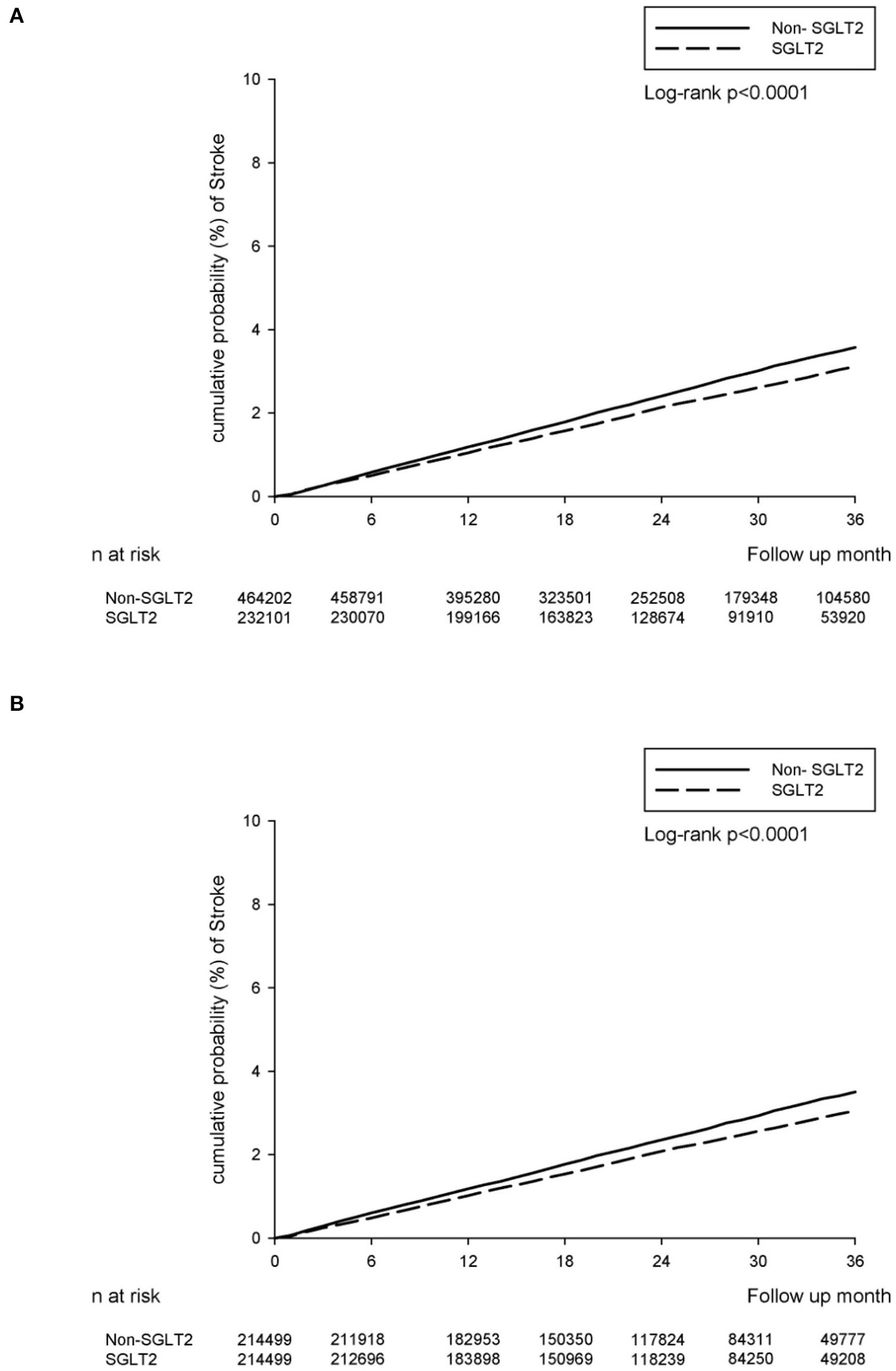
The cumulative incidence rate of developing stroke between SGLT2-inhibitor group and non-SGLT2-inhibitor group. (A) The main TBNHI Cohort. (B) The propensity score-matched cohort.
Propensity score-matched analysis
We included 428,998 patients (214,499 in the SGLT2-inhibitor group and 214,499 in the non-SGLT2-inhibitor group) in the propensity score matching, and the baseline characteristics of sex, age, and duration of type 2 DM did not differ (Table 1). At the baseline, the non-SGLT2-inhibitor group had more comorbidities, except for coronary artery disease, chronic obstructive pulmonary disease, atrial fibrillation and flutter, and rheumatoid arthritis than the SGLT2-inhibitor group. However, the SGLT2 inhibitor users used more concurrent medication, except statins, sulfonylureas, dipeptidyl peptidase 4 inhibitors, and glucagon-like peptide-1 receptor agonists than the non-SGLT2 inhibitor users (Table 1).
There were 4,678 and 5,328 NOS events recorded in the SGLT2-inhibitor and non-SGLT2-inhibitor groups, respectively, in the follow-up period. The event rate was 9.01 per 10 000 person-months (95% CI 8.75–9.27) for the SGLT2-inhibitor group compared with 10.20 (95% CI 10.00–10.50) for the non-SGLT2-inhibitor group. The relative risk of NOS after adjusting the duration of type 2 DM history, sex, age, comorbidities, and concurrent medication demonstrated a decreasing risk of incident stroke in the SGLT2 inhibitor group when compared to those in the non-SGLT2-inhibitor group (adjusted HR: 0.87; 95% CI: 0.84–0.91) (Table 2). Similarly, the SGLT2-inhibitor group revealed a significantly lower cumulative incidence rate of developing stroke than the non-SGLT2-inhibitor group as per the Kaplan–Meier survival analysis (P < 0.0001, Figure 2B).
Subgroup analysis
The results of the subgroup analyses revealed that, after adjusting for the duration of type 2 DM history, sex, age, comorbidities, and concurrent medication were partly consistent with the results of the main analyses (Table 3). The two groups were different in terms of their incidental stroke, with the SGLT2 inhibitor users exhibiting a substantially high risk of NOS with male, an adjusted HR = 1.34 (95% CI: 1.30 to 1.39) than female. Compared with younger patients (aged < 50), elderly patients exhibited a significantly higher risk of NOS (aHR 1.59, 95% CI 1.51–1.68 for patients aged 50–60; aHR 2.24, 95% CI 2.13–2.36 for patients aged 60–70; aHR 3.67, 95% CI 3.48–3.88 for patients aged > 70). The duration of type 2 DM history were higher in the < =2 or 2–4 years than in the ≥4 years. Patients with hypertension, chronic kidney disease, chronic obstructive pulmonary disease, atrial fibrillation and flutter, and rheumatoid arthritis were also at significantly higher risks of NOS (aHR = 1.22, 1.17, 1.08, 1.79, and 1.23, respectively). However, patients with hyperlipidemia and chronic liver disease have significantly lower risks of NOS (aHR = 0.77, and 0.81, respectively). Similar findings were also noted for concurrent medication of statins (aHR 0.84, 95% CI 0.81–0.86 in the main TBNHI cohort; aHR 0.88, 95% CI 0.84–0.92 in the propensity score matching), biguanides (aHR 0.77, 95% CI 0.75–0.79 in the main TBNHI cohort; aHR 0.85, 95% CI 0.82–0.89 in the propensity score matching), thiazolidinediones (aHR 0.89, 95% CI 0.85–0.93 in the main TBNHI cohort; aHR 0.93, 95% CI 0.88–0.98 in the propensity score matching), and glucagon-like peptide-1 receptor agonists (aHR 0.84, 95% CI 0.71–0.98 in the main TBNHI cohort; aHR 0.77, 95% CI 0.63–0.93 in the propensity score matching). However, an increased risk of NOS was noted for concurrent medication with non-steroid anti-inflammatory drugs (aHR 1.01, 95% CI 0.98–1.05 in the main TBNHI cohort; aHR 1.05, 95% CI 1.01–1.05 in the propensity score matching), corticosteroids (aHR 1.07, 95% CI 1.03–1.11 in the main TBNHI cohort; aHR 1.08, 95% CI 1.02–1.13 in the propensity score matching), proton pump inhibitors (aHR 1.19, 95% CI 1.13–1.25 in the main TBNHI cohort; aHR 1.20, 95% CI 1.12–1.20 in the propensity score matching), H2-receptor antagonists (aHR 1.05, 95% CI 1.02–1.09 in the main TBNHI cohort; aHR 1.07, 95% CI 1.02–1.12 in the propensity score matching), aspirins (aHR 1.53, 95% CI 1.48–1.59 in the main TBNHI cohort; aHR 1.55, 95% CI 1.49–1.62 in the propensity score matching), sulfonylureas (aHR 1.09, 95% CI 1.06–1.13 in the main TBNHI cohort; aHR 1.14, 95% CI 1.10–1.19 in the propensity score matching), alpha-glucosidase inhibitors (aHR 1.03, 95% CI 0.98–1.07 in the main TBNHI cohort; aHR 1.06, 95% CI 1.01–1.12 in the propensity score matching), Dipeptidyl peptidase 4 inhibitors (aHR 1.05, 95% CI 1.02–1.09 in the main TBNHI cohort; aHR 1.08, 95% CI 1.03–1.12 in the propensity score matching), and insulins (aHR 1.62, 95% CI 1.56–1.68 in the main TBNHI cohort; aHR 1.67, 95% CI 1.60–1.74 in the propensity score matching) (Table 3).
Table 3
| aHR (95% CI) | ||
|---|---|---|
| 2:1 sex, age matching | 1:1 propensity score matching | |
| Sex | ||
| Female | reference | reference |
| Male | 1.34(1.30–1.39) | 1.33(1.27–1.38) |
| Age | ||
| <50 | reference | reference |
| 51–60 | 1.59(1.51–1.68) | 1.51(1.41–1.63) |
| 61–70 | 2.24(2.13–2.36) | 2.17(2.02–2.32) |
| >70 | 3.67(3.48–3.88) | 3.55(3.31–3.82) |
| Duration of type 2 DM history | ||
| < =2 years | 1.21(1.14–1.28) | 1.27(1.11–1.37) |
| 2–4 years | 1.16(1.11–1.23) | 1.20(1.12–1.28) |
| >=4 years | reference | reference |
| Comorbidity(ref: non-comorbidity) | ||
| Hypertension | 1.22(1.18–1.26) | 1.28(1.23–1.34) |
| Coronary artery disease | 1.02(0.97–1.06) | 1.02(0.97–1.07) |
| Hyperlipidemia | 0.77(0.74–0.79) | 0.80(0.77–0.83) |
| Chronic kidney disease | 1.17(1.13–1.21) | 1.16(1.11–1.21) |
| Chronic liver disease | 0.81(0.77–0.85) | 0.79(0.74–0.85) |
| Malignancy | 1.02(0.96–1.08) | 1.03(0.95–1.13) |
| COPD | 1.08(1.01–1.16) | 1.06(0.97–1.15) |
| Atrial fibrillation and flutter | 1.79(1.64–1.95) | 1.82(1.64–2.02) |
| Rheumatoid Arthritis | 1.23(1.04–1.44) | 1.15(0.91–1.45) |
| Medication (reference: non-medication) | ||
| NSAIDs | 1.00(0.97–1.04) | 1.05(1.01–1.09) |
| Corticosteroids | 1.07(1.03–1.11) | 1.08(1.02–1.13) |
| PPIs | 1.19(1.13–1.25) | 1.20(1.12–1.28) |
| H2-receptor antagonists | 1.05(1.02–1.09) | 1.07(1.02–1.12) |
| Aspirins | 1.53(1.48–1.59) | 1.55(1.49–1.62) |
| Statins | 0.84(0.81–0.86) | 0.88(0.84–0.92) |
| Biguanides | 0.77(0.75–0.79) | 0.85(0.82–0.89) |
| Sulfonylureas | 1.09(1.06–1.13) | 1.14(1.10–1.19) |
| Alpha glucosidase inhibitors | 1.03(0.98–1.07) | 1.06(1.01–1.12) |
| Thiazolidinediones | 0.89(0.85–0.93) | 0.93(0.88–0.98) |
| DPP4 inhibitors | 1.05(1.02–1.09) | 1.08(1.03–1.12) |
| Insulins | 1.62(1.56–1.68) | 1.67(1.60–1.74) |
| GLP-1 receptor agonists | 0.84(0.71–0.98) | 0.77(0.63-y0.93) |
Multiple Cox regression to estimate the hazard ratio for subgroup analysis.
COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; DPP4, Dipeptidyl peptidase 4; GLP-1, Glucagon-like peptide-1; NSAID, Non-steroid anti-inflammatory drug; PPI, proton pump inhibitor.
Discussion
The present findings suggest that the incidence of NOS was decreased in type 2 DM patients who were SGLT2-inhibitor users compared with those who were not. Sensitivity analysis was also consistent with the main analysis. The subgroups analysis identified the concurrent use of statins, biguanides, thiazolidinediones, and glucagon-like peptide-1 receptor agonists as having a protective effect against developing NOS. However, we observed the increased risk based on whether non-steroid anti-inflammatory drugs, corticosteroids, proton pump inhibitors, H2-receptor antagonists, aspirins, sulfonylureas, alpha-glucosidase inhibitors, dipeptidyl peptidase 4 inhibitors, and insulins were prescribed for concurrent use with an SGLT2 inhibitor.
Hypertension, type 2 DM, and obesity are identified as the most important risk factors for stroke (18). Several experimental studies reported improvements in these risk factors in diabetic and obese or stroke-prone mice and rats after treatment with SGLT2 inhibitors (11–13, 19). In vitro data has shown that the SGLT2 inhibitor significantly increased survival (67%) of spontaneously hypertensive stroke-prone rats when compared with controls (13). The authors observed that SGLT2 inhibitor-treated rats had weight and blood pressure reduction, which could explain the reduced stroke risk and increased survival. However, the effects of SGLT2 inhibitors on stroke prevention were contradictory in different clinical trials. In the Empagliflozin Cardiovascular Outcomes and Mortality in Type 2 Diabetes (EMPA-REG OUTCOME) trial (17), empagliflozin users were found to be associated with an insignificantly increased risk of stroke when compared to empagliflozin non-users (HR, 1.18; 95% CI, 0.89–1.56; P = 0.26). On the other hand, canagliflozin users were found to be associated with an insignificantly decreased risk of stroke relative to canagliflozin non-users (HR, 0.87; 95% CI, 0.69–1.09) in the Cardiovascular and Renal Events in Type 2 Diabetes (CANVAS) trial (20). However, several meta-analyses have demonstrated that SGLT2 inhibitors may lower the risk of embolic stroke (9, 21, 22). Their results were the same as ours and they suggested a possible protective effect of SGLT2 inhibitors including different populations and the level of renal functions.
In our study, subgroups analyses demonstrated that the patients' concurrent use of statins, biguanides, thiazolidinediones, and glucagon-like peptide-1 receptor agonists had a protective effect against developing NOS, whereas patients' concurrent use of non-steroid anti-inflammatory drugs, corticosteroids, proton pump inhibitors, H2-receptor antagonists, aspirins, sulfonylureas, alpha-glucosidase inhibitors, dipeptidyl peptidase 4 inhibitors, and insulins showed an increased risk of developing NOS. This result demonstrates that different drugs may play a major role in lowering or increasing the risk of NOS when combined with SGLT2 inhibitors for patients with type 2 DM, which conforms to previous reports (23–27).
Other than antidiabetic effects, SGLT2 inhibitors also promoted natriuresis and osmotic diuresis to lower blood pressure in patients with cardiovascular disease and heart failure (28–30). As evidence of the efficacy of SGLT-2 inhibitors continued to grow, many trails and meta-analysis on these drugs have expanded their prescriptions from diabetes patients only to also include patients with HF without type 2 DM (28–32). Furthermore, the safety and dose-response relationship of SGLT2 inhibitors were recommended in the clinical practice (33–35).
In summary, there is negative association between the use of SGLT2 inhibitors and the risk of NOS in patients with type 2 DM. The decreased risk of NOS in patients with type 2 DM was greater among patients with concurrent use of statins, biguanides, thiazolidinediones, and glucagon-like peptide-1 receptor agonists. Therefore, we suggest that the long-term use of SGLT2 inhibitors may help reduce the incidence of NOS in patients with type 2 DM.
Funding
This study was supported by grants (CSH-2021-C-001) from Chung Shan Medical University Hospital.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the Ethics Committee of the Chung Shan Medical University Hospital (CS1-21037). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
T-KL and M-CC: conceptualization, methodology, formal analysis, and writing–original draft. Y-HC and J-YH: formal analysis and validation. P-LL: formal analysis. T-KL, Y-HC, J-YH, and M-CC: data curation. L-FP and G-PJ: conceptualization, investigation, writing–review and editing, supervision, project administration, and funding acquisition. All authors read the study and approved the manuscript for publication.
Acknowledgments
The authors gratefully acknowledge expert technical assistance in data management by Dry Lab of the Chung Shan Medical University Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
TinajeroMGMalikVS. An update on the epidemiology of type 2 diabetes: a global perspective. Endocrinol Metab Clin North Am. (2021) 50:337–55. 10.1016/j.ecl.2021.05.013
2.
IshikawaYLewisRDLaingEMAndersonAKZhangDQuyyumiAAet al. Prevalence and trends of type 2 diabetes mellitus and prediabetes among community-dwelling heart failure patients in the United States. Diabetes Res Clin Pract. (2022) 184:109191. 10.1016/j.diabres.2022.109191
3.
ZhouZLindleyRIRådholmKJenkinsBWatsonJPerkovicVet al. Canagliflozin and stroke in type 2 diabetes mellitus. Stroke. (2019) 50:396–404. 10.1161/STROKEAHA.118.023009
4.
LiuYLiJDouYMaH. Impacts of type 2 diabetes mellitus and hypertension on the incidence of cardiovascular diseases and stroke in China real-world setting: a retrospective cohort study. BMJ Open. (2021) 11:e053698. 10.1136/bmjopen-2021-053698
5.
MichaRPeñalvoJLCudheaFImamuraFRehmCDMozaffarianD. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA. (2017) 317:912–24. 10.1001/jama.2017.0947
6.
LachinJMOrchardTJNathanDMDCCT/EDIC ResearchGroup. Update on cardiovascular outcomes at 30 years of the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. (2014) 37:39–43. 10.2337/dc13-2116
7.
CowieMRFisherM. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol. (2020) 17:761–72. 10.1038/s41569-020-0406-8
8.
Al HamedFAElewaH. Potential therapeutic effects of sodium glucose-linked cotransporter 2 inhibitors in stroke. Clin Ther. (2020) 42:e242–9. 10.1016/j.clinthera.2020.09.008
9.
ZhouZJardine MJ LiQNeuenBLCannonCPde ZeeuwDet al. Effect of SGLT2 inhibitors on stroke and atrial fibrillation in diabetic kidney disease: results from the CREDENCE trial and meta-analysis. Stroke. (2021) 52:1545–56. 10.1161/STROKEAHA.120.031623
10.
TsaiWHChuangSMLiuSCLeeCCChienMNLeungCHet al. Effects of SGLT2 inhibitors on stroke and its subtypes in patients with type 2 diabetes: a systematic review and meta-analysis. Sci Rep. (2021) 11:15364. 10.1038/s41598-021-94945-4
11.
Sa-nguanmooPTanajakPKerdphooSJaiwongkamTPratchayasakulWChattipakornNet al. SGLT2-inhibitor and DPP-4 inhibitor improve brain function via attenuating mitochondrial dysfunction, insulin resistance, inflammation, and apoptosis in HFD-induced obese rats. Toxicol Appl Pharmacol. (2017) 333:43–50. 10.1016/j.taap.2017.08.005
12.
MillarPPathakNParthsarathyVBjoursonAJO'KaneMPathakVet al. Metabolic and neuroprotective effects of dapagliflozin and liraglutide in diabetic mice. J Endocrinol. (2017) 234:255–67. 10.1530/JOE-17-0263
13.
ZhangWWelihindaAMechanicJDingHZhuLLuYet al. EGT1442, a potent and selective SGLT2 inhibitor, attenuates blood glucose and HbA1c levels in db/db mice and prolongs the survival of stroke-prone rats. Pharmacol Res. (2011) 63:284–93. 10.1016/j.phrs.2011.01.001
14.
PawlosABroncelMWozniakEGorzelak-PabiśP. Neuroprotective effect of SGLT2 inhibitors. Molecules. (2021) 26:7213. 10.3390/molecules26237213
15.
O'BrienMJKaramSLWalliaAKangRHCooperAJLanckiNet al. Association of second-line antidiabetic medications with cardiovascular events among insured adults with type 2 diabetes. JAMA Network Open. (2018) 1:e186125. 10.1001/jamanetworkopen.2018.6125
16.
WuJHFooteCBlomsterJToyamaTPerkovicVSundströmJet al. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. (2016) 4:411–9. 10.1016/S2213-8587(16)00052-8
17.
ZinmanBWannerCLachinJMFitchettDBluhmkiEHantelSet al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. (2015) 373:2117–28. 10.1056/NEJMoa1504720
18.
HeCWangWChenQShenZPanESunZet al. Factors associated with stroke among patients with type 2 diabetes mellitus in China: a propensity score matched study. Acta Diabetol. (2021) 58:1513–23. 10.1007/s00592-021-01758-y
19.
MehtaVKumarAJaggiASSinghN. Restoration of the attenuated neuroprotective effect of ischemic postconditioning in diabetic mice by SGLT inhibitor phlorizin. Curr Neurovasc Res. (2020) 17:706–18. 10.2174/1567202617666201214112016
20.
RådholmKFigtreeGPerkovicVSolomonSDMahaffeyKWde ZeeuwDet al. Canaglifozin and heart failure in type 2 diabetes mellitus: results from the CANVAS Program. Circulation. (2018) 138:458–68. 10.1161/CIRCULATIONAHA.118.034222
21.
LiHLLipGYHFengQFeiYTseYKWuMZet al. Sodium-glucose cotransporter 2 inhibitors (SGLT2i) and cardiac arrhythmias: a systematic review and meta-analysis. Cardiovasc Diabetol. (2021) 20:100. 10.1186/s12933-021-01293-8
22.
GiuglianoDLongoMSignorielloSMaiorinoMISolerteBChiodiniPet al. The efect of DPP-4 inhibitors, GLP-1 receptor agonists and SGLT-2 inhibitors on cardiorenal outcomes: a network meta-analysis of 23 CVOTs. Cardiovasc Diabetol. (2022) 21:42. 10.1186/s12933-022-01474-z
23.
WangWZhangB. Statins for the prevention of stroke: a meta-analysis of randomized controlled trials. PLoS ONE. (2014) 9:e92388. 10.1371/journal.pone.0092388
24.
EkströmNSvenssonAMMiftarajMFranzénSZetheliusBEliassonBet al. Cardiovascular safety of glucose-lowering agents as add-on medication to metformin treatment in type 2 diabetes: report from the Swedish national diabetes register. Diabetes Obes Metab. (2016) 18:990–8. 10.1111/dom.12704
25.
BethelMAPatelRAMerrillPLokhnyginaYBuseJBMentzRJet al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. (2018) 6:105–13. 10.1016/S2213-8587(17)30412-6
26.
MartoJPStramboDLivioFMichelP. Drugs associated with ischemic stroke: a review for clinicians. Stroke. (2021) 52:e646–59. 10.1161/STROKEAHA.120.033272
27.
Castilla-GuerraLFernandez-MorenoMDCLeon-JimenezDCarmona-NimoE. Antidiabetic drugs and stroke risk. Current evidence Eur J Intern Med. (2018) 48:1–5. 10.1016/j.ejim.2017.09.019
28.
McMurrayJJSolomonSDInzucchiSEKøberLKosiborodMNMartinezFAet al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. (2019) 381:1995–2008.
29.
PackerMAnkerSDButlerJFilippatosGPocockSJCarsonPet al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. (2020) 383:1413–24.
30.
ZannadFFerreiraJPPocockSJAnkerSDButlerJFilippatosGet al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. (2020) 396:819–29. 10.1016/S0140-6736(20)31824-9
31.
Shi FH LiHShenLXuLGeHGuZCet al. Beneficial effect of sodium-glucose co-transporter 2 inhibitors on left ventricular function. J Clin Endocrinol Metab. (2022) 107:1191–203. 10.1210/clinem/dgab834
32.
RyanPBBuseJBSchuemieMJDeFalcoFYuanZStangPEet al. Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: a real-world meta-analysis of 4 observational databases (OBSERVE-4D). Diabetes Obes Metab. (2018) 20:2585–97. 10.1111/dom.13424
33.
Shi FH LiHShenLZhangZJiangYHHuYMet al. Appraisal of non-cardiovascular safety for sodium-glucose co-transporter 2 inhibitors: a systematic review and meta-analysis of placebo-controlled randomized clinical trials. Front Pharmacol. (2019) 10:1066. 10.3389/fphar.2019.01066
34.
Shi FH LiHShenLFuJJMaJGuZCet al. High-dose sodium-glucose co-transporter-2 inhibitors are superior in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. (2021) 23:2125–36. 10.1111/dom.14452
35.
ShiFHLiHYueJJiangYHGuZCMaJet al. Clinical adverse events of high-dose vs low-dose sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of 51 randomized clinical trials. J Clin Endocrinol Metab. (2020) 105:dgaa586. 10.1210/clinem/dgaa586
Summary
Keywords
new-onset stroke, SGLT2 inhibitor, type 2 DM, concurrent medication, ischemic stroke, hemorrhagic stroke
Citation
Lin T-K, Chen Y-H, Huang J-Y, Liao P-L, Chen M-C, Pan L-F and Jong G-P (2022) Sodium-glucose co-transporter-2 inhibitors reduce the risk of new-onset stroke in patients with type 2 diabetes: A population-based cohort study. Front. Cardiovasc. Med. 9:966708. doi: 10.3389/fcvm.2022.966708
Received
11 June 2022
Accepted
22 July 2022
Published
09 August 2022
Volume
9 - 2022
Edited by
Annalisa Capuano, University of Campania Luigi Vanvitelli, Italy
Reviewed by
Mei Qiu, Shenzhen Longhua District Central Hospital, China; Hao Li, Shanghai Jiao Tong University, China
Updates
Copyright
© 2022 Lin, Chen, Huang, Liao, Chen, Pan and Jong.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lung-Fa Pan panlungfa@gmail.comGwo-Ping Jong cgp8009@yahoo.com.tw
†These authors have contributed equally to this work
This article was submitted to Cardiovascular Therapeutics, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.