- 1Department of Cardiology, Subcarpathian Oncological Center, Brzozów, Poland
- 2Department of Pulmonary Circulation, Thromboembolic Diseases and Cardiology, Centre of Postgraduate Medical Education, European Health Centre, Otwock, Poland
Many factors contribute to mortality in lung cancer, including the presence of concomitant cardiovascular disease. In the treatment of early stage of lung cancer, the presence of comorbidities and occurence of cardiotoxicity may be prognostic. The effect of cardiotoxicity of radiotherapy and chemoradiotherapy on overall survival has been documented. Acute arterial and venous thromboembolic events seem to correlate with the degree of the histological malignancy, its clinical advancement, and even with optimal cardiac treatment, they may influence the survival time. In the case of high-grade and advanced lung cancer stage especially in an unresectable stadium, the prognosis depends primarily on the factors related to the histopathological and molecular diagnosis. Electrocardiographic and echocardiographic abnormalities may be prognostic factors, as they seem to correlate with the patient's performance status as well as tumor localization and size.
Introduction
Lung cancer is one of the most common malignancies worldwide and also the most common cause of cancer-related deaths in both men and women. The 5-year survival rates for all stages of lung cancer do not exceed 15–20% (1). There are multiple factors that influence mortality, including the presence of comorbidities. Depending on their duration, these conditions can be classified as chronic and acute.
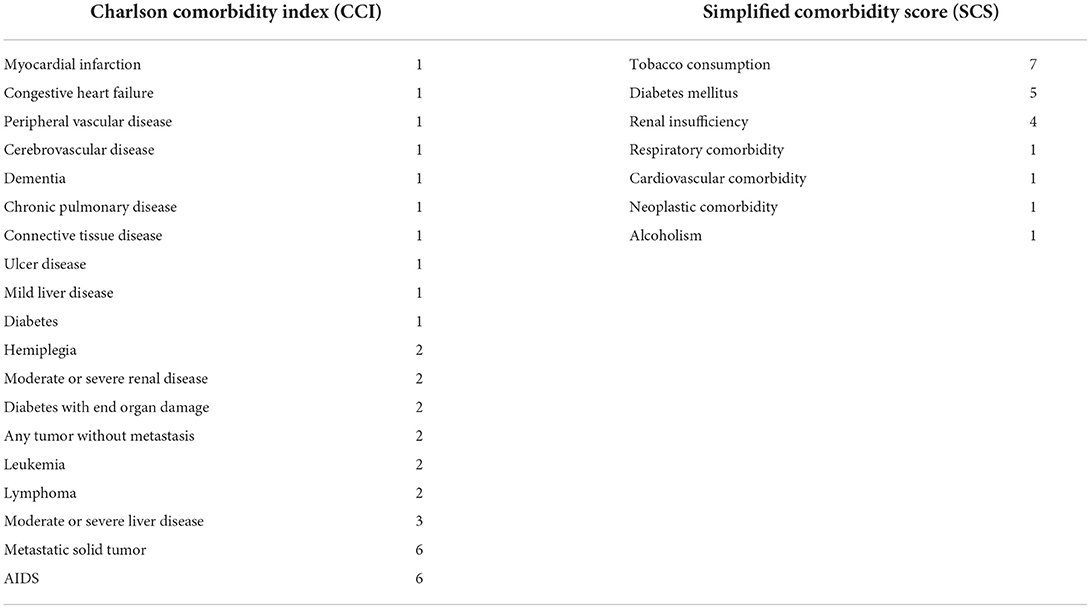
The Charlson Comorbidity Index (CCI) is the most commonly used tool to assess the likelihood of death within a year for a patient with comorbidities, a score of ≥3 is associated with an 80% increase in the risk of death within a year (Table 1) (2). The CCI is important prognostic scale in oncology because it is independent of cancer stage and performance status. Other scale evaluating comorbidities is the Simplified Comorbidity Score (SCS) confirmed in lung cancer as independent determinant of a poor outcome (Table 1) (3). This validation study revealed the strong statistical concordance between CCI and SCS, by univariate analysis of large group of non-small-cell lung cancer (NSCLC) patients with long term follow up, CCI ≥ 3 and SCS > 9 were considered as important for outcome (p = 0.06 and p < 0.01 respectively) with final suggestion of higher prognostic value of SCS. However both CCI and SCS were not predictable for survival, radiological response and toxicity during first-line chemotherapy due to advances lung cancer (4). Similarly in patients with advanced and unrespectable NSCLC treated with radical sequential chemoradiotherapy CCI >4 or SCS >8 were not predictors of survival (5).
In patients with NSCLC, cardiovascular (CV) comorbidities, including coronary artery disease, hypertension, arrhythmias, and peripheral arteriosclerosis, increased the risk of death by 30% compared to patients without these conditions (6). However, some studies have shown no direct effect of concomitant diseases on overall survival (OS) in lung cancer in advanced inoperable stage (7). Retrospective cohort studies have shown that cancer-related mortality rates for biologically aggressive malignancies exceed those for comorbidities (8, 9).
Batra et al. showed that patients with lung cancer and CV diseases were less likely to receive oncological treatment, whether chemotherapy, targeted therapy or radiotherapy (10). It has also been shown that prior CV disease increases the risk of cancer-unrelated death (HR = 1.48; p < 0.0001) and does not contribute to cancer-related mortality. Disqualification from cancer treatment or use of less intensive and consequently less effective cancer treatment due to comorbidities, which may influence on overall survival, also seem an important problem.
The aim of this study was to review literature on the impact of cardiovascular comorbidities on the prognosis in patients with lung cancer with special focus on the more common NSCLC.
Cardiac arrhythmias
The mechanism underlying cardiac arrhythmias and conduction disturbances in cancer patients consists of several elements: patient-related factors (comorbidities, age, genetic predisposition), tumor-related factors (invasion, autonomic system excitation, inflammation), cancer treatment-related factors (electrolyte abnormalities due to gastrointestinal toxicity, cardiac structural or electrical remodeling induced by chemotherapy, targeted therapy, immunotherapy, radiotherapy, supportive medications) (11).
Atrial fibrillation (AF) is the most common arrhythmia, affecting 2–4% of the general population, whose prevalence increases with age (up to 36% at the age of 85 years) (12). Cancer patients have many additional reasons to experience AF (13). The OPERA study (Oulu Project Elucidating Risk of Atherosclerosis) showed that cancer disease may be understood an independent risk factor of AF development, because authors recognized AF in 19% with cancer in comparison to only 9% of subject without cancer (p < 0.001, HR = 2.47; 95%CI: 1.57–3.88) (14). The new-onset AF may be associated with increased relative risk of a diagnosis of cancer disease of the lung, kidney, colon, ovary as well as non-Hodgkin's lymphoma: seven times increased risk for metastatic and 3.5 times for localized cancer disease (15). The time relationship of 90 days between the recognition of AF and cancer is strongly interesting, because in this period the diagnosis of cancer can predict a 3.4 fold increased risk of new AF, however AF occurrence is related to a 1.85 times higher probability of coexisting cancer (16).
The large population-based study proved that not all types of cancer are associated with AF, but certainly hematological and intrathoracic malignancies are associated with AF (17). This study revealed the risk of AF development is more than doubled in patients with esophageal (HR = 2.69) and lung cancer (HR = 2.39), interestingly, lung cancer showed the strongest association with AF in patients aged > 50 years. Among women with new-onset AF the significant age-adjusted risk was observed for colon (HR = 2.36; p < 0.001), breast (HR = 1.35; p = 0.04) and lung cancer (HR = 1.69; p = 0.04) (18).
Particularly high AF percentages are observed among lung cancer patients. One of the Nationwide population-based study showed the highest incidence of AF as number per person years in lung cancer: 58.7/1000 in men and 35.3/1000 in women (19). The large analysis of cardiovascular admissions to hospital in US revealed AF as the main cause of hospitalization in lung cancer patients and significantly increased mortality was noted in lung cancer and AF (aOR = 4.69) (20).
However, the mechanisms underlying the high incidence of AF in cancer patients are not fully elucidated. In addition to classical risk factors present in the general population (hypertension, diabetes, heart failure, coronary artery disease, obesity, etc.), other factors, i.e., water-electrolyte disturbances, hypoxia, sympathetic over activity due to pain and emotional stress, have also been considered (21, 22). It can be additionally assumed that chronic pulmonary obstructive disease, often coexisting with lung cancer, is also a risk factor for AF, especially if there are episodes of infectious exacerbations and when the size of the left atrium is increased (23). A similar cause can be seen in AF with concomitant pulmonary hypertension associated with hypoxia in lung cancer (24). Compression or infiltration of the tumor mass or metastases to the heart may be another possible cause. AF can also be a complication of systemic treatment, radiotherapy or thoracic surgery (it occurs in 10–20% of patients 2–3 days after surgery) (25).
The prevalence of new onset AF (i.e., first occurrence after the cancer diagnosis) is associated with a higher tumor grade and thus a worse prognosis and higher cardiovascular mortality (26). Poor prognosis has been demonstrated in patients undergoing thoracic surgery for lung cancer who developed AF: increased hospital mortality (6.7 vs. 1.0%, p = 0.024) and higher long-term mortality (HR = 3.75) (27). A significant negative prognostic value of AF (HR = 2.39 for mortality, p = 0.02) in lung cancer patients qualified for systemic cancer treatment has also been demonstrated (7).
Ventricular arrhythmia is another common type of rhythm abnormalities with possible impact on prognosis in the general population. Anker et al. assessed cancer patients free of cardiovascular diseases in comparison to healthy controls using 24-h ECG (28). They showed an increased frequency of non-sustained ventricular tachycardia (nsVT) observed in 6% of NSCLC patients and associated with negative impact on survival (HR = 2.68; p = 0.005). Prognostic value of nsVT was significant independently of type of cancer. Although in the same study single premature ventricular contractions (PVCs) were observed in 42% of NSCLC patients (in 21% in number of 50 or more per 24 h) this arrhythmia did not affect the prognosis for NSCLC patients, but surprisingly it had an effect on the survival of patients with pancreatic and colorectal cancer. As one of possible explanation may be a fact that NSCLC patients receive beta-blockers more frequent which could inhibit PVCs. In the larger study where 24-h ECG recordings from the period of 6 years (2012–2018) were reviewed, the highest frequency of nsVT (33%) was observed in lung cancer patients without cardiac dysfunction (29). Moreover 52% of lung cancer patients had at least 20 PVCs during monitoring. The analysis in whole group of cancer patients revealed the arrhythmias nsVT ≥ 4 beats or PVCs ≥ 20/day were independently associated with higher risk of all-cause mortality (HR = 1.81, p = 0.016 and HR = 1.6, p = 0.0088, respectively in multivariate adjusted analysis).
Elevated resting heart rate alone may be an independent risk factor for death in stable cardiovascular disease in general cardiology (30). In selected cancer diseases irrespective of hemoglobin levels or tumor grade, a similar relationship has been observed (31). Hemu et al. showed that sinus tachycardia (heart rate ≥100 /min.) occurring during cancer treatment is associated with an increase in cardiovascular events and mortality over a 10-year period (32). The prospective study in lung cancer demonstrated the prognostic significance of heart rate, regardless of whether it was sinus rhythm or atrial fibrillation, heart rate > 90/min predicted a higher risk of mortality (HR = 1.67; p = 0.03) (7). The tumor growth effect can be considered a potential explanation, as shorter survival was also observed in patients with right ventricular systolic pressure (RVSP) higher than 39 mmHg (HR = 2.01; p = 0.0045).
On the other hand, asymptomatic sinus bradycardia, defined as heart rate < 50/min, is an adverse effect of ALK inhibitor treatment (such as crizotinib) and may positively correlate with clinical response to treatment (33, 34). However it should be remembered that this slowing of the heart rate is an effect of the cancer drug activity and therefore appears to correlate with its efficacy.
Arterial hypertension
Hypertension (HT) is one of the major single risk factors for cardiovascular (CV) diseases and increased CV mortality (35). A worldwide survey among 1.5 million adults performed in May 2019 showed that 32% of the population had never had their blood pressure measured, 34% had HT of whom 23.3% had untreated or sub-optimally treated HT (36). It is also the most common comorbidity among cancer patients, regardless of the type of malignancy identified in 38% of patients (37). Prospective multicenter study documented HT in 43% of NSCLC patients (38). Similarly Polish study based on one center experience identified HT in 42.3% of metastatic NSCLC EGFR positive patients (39).
There are no papers clearly demonstrating the negative impact of pre-existing HT as a single prognostic factor in lung cancer. Moreover, there are no evidences that lung cancer (except for neuroendocrine type) may lead to HT development.
Lung cancer patients usually experience increase in blood pressure related to cancer therapy. Cisplatin is the most commonly used alkylating agent in various treatment regimens for both NSCLC and small cell lung cancer (SCLC). It increases blood pressure at varying rates, depending on the observation of patients with testicular cancer: 39% in 10-year follow-up (40) or 53% in 11-year follow-up (41). The main mechanisms leading to increased blood pressure include endothelial cell dysfunction or damage, excessive platelet aggregation and reduced nitric oxide availability (42). Anti-VEGF agents, such as bevacizumab and ramucirumab, are other drugs used in the treatment of these tumors that also may contribute to the increase in blood pressure. Yan et al. showed that HT in the treatment with bevacizumab-based regimens for metastatic NSCLC was associated with higher response rates (43). This is a next clear confirmation that HT as effect of cancer drug activity correlate positively with outcome. Supportive therapies, such as steroids, non-steroidal anti-inflammatory drugs or erythropoietin, are also implicated in hypertension (44). Reduction of angiotensin-converting enzyme (ACE) activity in tumor tissue correlating with poor prognosis and tumor metastasis is yet another problem. One retrospective paper has shown a positive effect of RAAS blockers on survival, whereas none has shown its negative effect (45).
Coronary artery disease
Coronary artery disease (CAD) is a condition associated with the formation of atherosclerotic plaques in the coronary arteries, which runs with periods of clinical stability (chronic coronary syndrome) and destabilization (acute coronary syndrome). According to population-based studies, the CAD prevalence increases with age and is 10–12% in women aged 65–84 years, and 12–14% in similarly aged men (46). The prevalence in lung cancer patients seems to be higher because ranges from 10.3% (10) to 33.7% (47), depending on the source. Every third patient (33%) from those with cardiovascular disease had previous myocardial infarction (10).
The high prevalence of CAD and lung cancer is due to common aetiological factors: cigarette smoking (48), advanced age, obesity (49) and the same pathomechanism associated with oxidative stress and chronic inflammation (50, 51). During lung cancer screening by a low-dose CT scan, coronary micro-calcifications indicative of atherosclerotic lesions have been additionally found as often coexisting clinical problem (52, 53). Sun et al. demonstrated a relationship between the severity of CAD (degree of coronary stenosis) and lung cancer, which may broaden the diagnostic scope for this malignancy in the future (54).
The coexistence of CAD can worsen the prognosis of patients undergoing surgery for stage I and II NSCLC (55, 56). On the other hand, other studies have shown no significant effect of CV disease on mortality during primary surgery (57, 58). Assuming that CAD is the most common cause of CV diseases, the conflicting data may reflect the thoracic perioperative risk and prognosis may depend on the severity of CAD and control of ischemic symptoms through effective cardiac treatment.
Acute coronary syndromes (ACS) are another problem. Approximately 15% of patients treated for ACS have a coexisting cancer (59). Non-ST-segment elevation myocardial infarction (NSTEMI) accounted for the majority of ACS in cancer patients, as in the general population (60). The clinical picture of ACS in cancer patients is untypical, with only 33% of patients experiencing chest pain, 44% reporting dyspnoea and 23% developing hypotension (61). ACS can also be triggered by anti-cancer treatment. Cisplatin, gemcitabine and bevacizumab are highly thrombogenic (62). Treatment guidelines for cancer patients are missing, and it is believed that this group should be treated like other patients.
Many studies show a worse prognosis for patients with ACS and cancer (63–65). In particular, lung cancer patients are at risk of arterial thromboembolism like myocardial infarction and in this way the risk of mortality is increased three times (66). Lung cancer is one of the four most common types of cancer disease with highest frequency of myocardial infarction, only 21.0% of those patients were treated by coronary intervention, lung cancer was associated with the highest in-hospital mortality, major adverse cardiovascular and cerebrovascular complications (67). The large “real world” data on prognosis after STEMI presented lung cancer as one of the strongest independent determinants of all-cause mortality (HR = 2.04), next advanced peripheral artery disease (HR = 1.78), metastasis (HR = 1.72), previous stroke (HR = 1.44) (68).
Heart failure
Heart failure (HF) affects 1–2% of adults in the general population, and its prevalence increases with age (69). There are 23 million people worldwide with HF (70) and many of them may experience of lung cancer.
Current treatment of HF improves patients' survival, which means that more of these patients will have cancer. In an analysis performed by the Women's Health Initiative, in postmenopausal women HF was shown to be associated with an increased incidence of obesity-related cancers (HR = 1.24), and even more with the risk of developing lung cancer (HR = 1.58) (71). The prevalence of HF in patients with lung cancer is estimated between 7.6% (72) and 17.5% (47), depending on the source.
HF patients were less likely to undergo surgery and chemotherapy than patients without HF. HF is significantly correlated with increased perioperative mortality in lung cancer (OR = 6.0) (73). More and more aggressive anticancer treatment will increase the number of patients with newly diagnosed HF (74). Both old and newer drugs recommended in lung cancer seem to predispose to HF development through different patomechanism (75, 76).
Patients with known lung cancer hospitalized for HF have a higher mortality rate (5.9%) compared to those cancer-free (3.3%) (77). In the same study, as a very optimistic observation, it should be considered that over the years from 2003 to 2014, mortality among patients hospitalized due to HF decreased, but very importantly, the decrease in mortality was the highest among patients with accompanying lung cancer (8.1 to 4.6%; p < 0.001).
Valvular heart disease
Valvular heart disease (VHD) may occur in cancer patients for several reasons: due to pre-existing valvular defects and as a complication of anti-cancer treatment: after radiotherapy, due to infective endocarditis in the course of chemotherapy-induced severe infection and secondary to cardiac dysfunction (78, 79).
Degenerative aortic valve stenosis is the most common primary valvular heart defect in the general population (80). The majority of patients with active cancer are disqualified from classical cardiac surgery (surgical aortic valve replacement, SAVR) due to the high risk of perioperative complications such as bleeding, arrhythmias, infections or coagulation disorders (81). Transcatheter aortic valve replacement (TAVR) seems to be an alternative solution. According to current European recommendations, the procedure should be performed in patients with expected survival of at least 1 year (82), but only a small percentage of patients with advanced lung cancer meets such criteria. In a study by Landes et al. compared the survival of patients with and without cancer who underwent TAVR, lung cancer patients constituted a small group - only 11% (83). The authors showed a worse prognosis in oncology patients, with tumor stage being the strongest predictor of late mortality. Similar findings were also published in several other papers (84–86). However it is worth to consider concomitant cardiac surgery as treatment option for valvular defects in patients with early stage lung cancer scheduled for thoracic surgery (87).
The prevalence of radiotherapy-induced VHD is described as frequent, affecting approximately 10% of treated patients (88, 89), but it occurs late (median time to diagnosis is 22 years) (90). The short expected survival time of lung cancer patients does not allow for the manifestation of late cardiac toxicity, which is a typical complication of radiation therapy.
Cancer patients have a higher risk of developing infective endocarditis (IE) due to immunosuppression (e.g., secondary to chemotherapy) or the presence of a central line or a vascular port (91). In most studies, Staphylococcus aureus was the predominant aetiological agent, with one native valve (aortic or mitral, less frequently tricuspid) most frequently involved (92). A higher mortality in the course of IE was also demonstrated in all cancer patients in comparison to the control group (also associated with tumor progression). Cardiac surgery was performed in approximately 50% of patients (93, 94).
Venous thromboembolism
Venous thromboembolism (VTE) as deep vein thrombosis (DVT) and pulmonary embolism (PE) is the common clinical worldwide problem in general population, because last data showed the annual incidence rate of VTE in Europe ranged from 104 to 183 per 100000 person-years (95). Prevalence of VTE varies greatly from region to region but generally fluctuates 39–115/100000 for PE, and 53–162/100000 for DVT (96). Acute PE remains the third most common cause of acute cardiovascular syndromes, its incidence increases (97, 98).
Major surgery (OR = 18.95) and active cancer (OR = 14.64) belong to the strongest independent risk factors for DVT or PE (99). Lung cancer is the sixth most frequent reason of PE among malignancies (100). Lung cancer, especially adenocarcinoma, predisposes to PE more than other malignancies, especially within 3 months of the diagnosis (101). It has also been shown that lung cancer patients are six times more likely to develop PE than those cancer-free in the 12 months preceding the diagnosis (102). Risk of PE diagnosis correlates with a moment of cancer occurrence: for NSCLC: HR = 9.7 during 6 months prior to cancer diagnosis, HR = 20.0 during 6 months after cancer diagnosis and HR = 17.4 during 12 months after cancer diagnosis and for SCLC: HR = 6.9 and HR = 14.8 and HR = 16.1 respectively (103).
PE most often accompanies advanced-stage lung cancer (stages III to IV) (104). The Vienna Cancer and Thrombosis Study by multivariable Cox proportional hazards analysis confirmed that lung is one of the high risk tumor site associated with VTE (HR = 4.3; p < 0.001) together with high tumor grade, tumor histology (adenocarcinoma) and elevated D-dimer level (105). PE is diagnosed in a high percentage of cancer patients incidentally as unprovoked PE or asymptomatic PE (the increased risk of incidental PE in cancer was calculated as OR = 1.80) (106). PE can be recognized during diagnostic imaging for staging or evaluation of response to cancer treatment. In lung cancer such correlation with asymptomatic/incidental PE ranges from 29.4 to 63% of patients (107). Colorectal cancer and lung cancer appears to be two cancer diseases with the most frequent incidental VTE (108).
Lung cancer treatment can induce new episodes of VTE (107). PE developed during lung cancer treatment rather does not affect survival (p = 0.206) (101). Subsequent cancer remission resulted from cancer therapy and control of cancer-related coagulation state seem to reduce the occurrence of VTE important for prognosis (109).
Nichols et al. showed in their post-mortem studies in lung cancer patients that PE was the direct cause of death in 10% of cases, however from a pathophysiologic perspective, PE may be an additional contributing cause of death in many other cases (110). PE significantly worsens the prognosis in lung cancer (p < 0.0005) and as a possible explanation authors discussed more advanced stage of cancer disease (III or IV) and more frequent used only supportive care without anticancer therapy (111). In the prospective cohort study in older patients (age ≥ 65 years) with lung cancer it was documented significantly shorter survival in subgroup with PE (4.3 vs. 9.2 months, p = 0.0015), there were significant differences in PE-related mortality (15.1 vs. 0%) but insignificant differences in tumor-related mortality (75.5 vs. 66.0%) (112). It should be highlighted that PE is associated with shorter survival when is recognized synchronous with lung cancer (113).
No difference in mortality between symptomatic vs. asymptomatic PE in lung cancer was documented, with both forms worsening the prognosis due to haemorrhagic complications and VTE recurrences (there were similar patients' age and frequency of metastatic disease) (114). There are data that even 55% of lung cancer with unsuspected PE did not receive anticoagulation therapy which leaded to premature death (HR = 4.1) (115).
Comorbidity or multi-morbidity
The outcome and quality of life in lung cancer can be determine not only by coexisting cardiovascular diseases, the importance of other age-, obesity- and tobacco-related diseases should be taken into account. Number of comorbidities in lung cancer is so high that authors from Spain proposed the term multi-morbidity when at least two chronic diseases coexist with lung cancer and documented the highest mortality in patients with multi-morbidity (p = 0.002) in comparison to patients with one or no comorbidity (40% higher mortality) (116). It is worth emphasizing that the prevalence of multi-morbidity correlated with older patients' age and history of smoking.
Apart from cardiovascular diseases, chronic obstructive pulmonary disease is most often associated with lung cancer (117). Among other serious co-morbidities another cancer (10–20%) and diabetes mellitus (5–25%) seem to be essential for prognosis (118). Generally, mortality in lung cancer was defined as 1.1–1.5 times higher for patients with comorbidity (119). In lung cancer 19 comorbidities were found as independent predictors of survival (72). The Nebraska Hospital Discharge Data showed survival in lung cancer may negatively depend on congestive HF, diabetes, liver disease, dementia, renal disease, cerebrovascular disease, the greatest difference in survival in patients with and without comorbidities was seen at low grades: HR = 1.316 for localized, HR = 1.228 for regional and HR = 1.075 for metastatic lung cancer (120). Due to frequent follow-up, patients with comorbidities were more likely to be diagnosed at an early stages of each cancer disease.
Conclusion
Acute cardiac conditions, such as pulmonary embolism or myocardial infarction, clearly worsen the prognosis in lung cancer. Lung cancer belongs to such malignancies where the risk of venous and arterial thromboembolic complications correlates with tumor advancement (Figure 1) (105, 121). Often an arterial or venous thromboembolic event occurs at the onset of the neoplastic disease (122, 123).
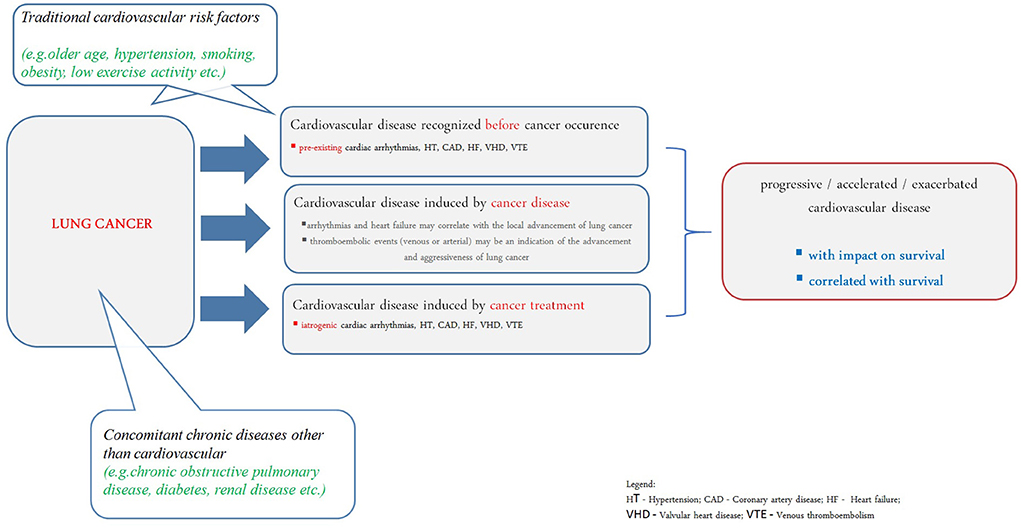
Figure 1. Understanding of cardiovascular disease in prognosis of lung cancer patients. HT, hypertension; CAD, coronary artery disease; HF, heart failure; VHD, valvular heart disease; VTE, venous thromboembolism.
Systemic treatment and radiotherapy in lung cancer may cause cardiovascular complications (124, 125). It has been shown that in patients over 65 years of age undergoing chemotherapy, the risk of developing CAD or HF was increased; cardiac disorders were also more common in patients undergoing radiotherapy, especially if the left lung was irradiated. The greatest risk of cardiotoxicity was found in patients undergoing chemo-radiotherapy (126). ARIC Study revealed that lung cancer survivors have higher risk of cardiovascular disease development (especially HF) even they do not have traditional cardiovascular risk factors (Figure 1) (127).
Lung cancer patients have the highest prevalence of cardiovascular comorbidities compared to other cancers (128). At least one concomitant cardiovascular disease was present in 67.2% of patients with NSCLC (129). The hypothesis that the effect of chronic cardiac comorbidities on mortality is dominant in early stages of lung cancer seems most plausible. Data on 95 167 NSCLC patients showed that cardiovascular disease can increase mortality when the cancer stage is in the range I-III B, while it is not important for survival in stage IV (10). Worse prognosis was associated with concomitant heart failure, myocardial infarction, and arrhythmias diagnosed during follow-up, although the risk still varied depending on the stage of the disease and the treatment method. For stage I-IIIB disease, concomitant cardiovascular diseases increased the risk of mortality by as much as 2.59 (p < 0.001) for chemotherapy and by 2.20 (p < 0.001) for chemotherapy and radiotherapy.
The impact of cardiovascular comorbidities on prognosis is limited in advanced stages of lung cancer. Cardiac arrhythmias (especially atrial fibrillation) and echocardiographic changes suggesting the development of pulmonary hypertension (right ventricular systolic pressure increase) and dysfunction of the right ventricle rather result from the advancement of neoplastic disease, correlate with decreased performance status and predict shorter overall survival (Figure 1) (7).
Author contributions
All authors have design and conception, writing of manuscript, editing and reviewing the manuscript and final approval of the version to be published.
Conflict of interest
SS declares speaker fee or advisory board fee or travel and meeting support: Angelini, Amgen, Astra-Zeneca, Bayer, BMS, Gilead, Pfizer, TEVA (all outside of this study).
The remaining author declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. (2021) 71:7–33. doi: 10.3322/caac.21654
2. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. (1987) 40:373–83. doi: 10.1016/0021-9681(87)90171-8
3. Colinet B, Jacot W, Bertrand D, Lacombe S, Bozonnat MC, Daurès JP, et al. oncoLR health network. A new simplified comorbidity score as a prognostic factor in non-small-cell lung cancer patients: description and comparison with the Charlson's index. Br J Cancer. (2005) 93:1098–105. doi: 10.1038/sj.bjc.6602836
4. Singh N, Singh PS, Aggarwal AN, Behera D. Comorbidity assessment using charlson comorbidity index and simplified comorbidity score and its association with clinical outcomes during first-line chemotherapy for lung cancer. Clin Lung Cancer. (2016) 17:205–213.e1. doi: 10.1016/j.cllc.2015.10.002
5. Zaborowska-Szmit M, Olszyna-Serementa M, Kowalski DM, Szmit S, Krzakowski M. Elderly patients with locally advanced and unresectable non-small-cell lung cancer may benefit from sequential chemoradiotherapy. Cancers. (2021) 13:4534. doi: 10.3390/cancers13184534
6. Iachina M, Jakobsen E, Møller H, Lüchtenborg M, Mellemgaard A, Krasnik M, et al. The effect of different comorbidities on survival of non-small cells lung cancer patients. Lung. (2015) 193:291–7. doi: 10.1007/s00408-014-9675-5
7. Medrek S, Szmit S. Baseline electrocardiographic and echocardiographic assessment may help predict survival in lung cancer patients-a prospective cardio-oncology study. Cancers. (2022) 14:2010. doi: 10.3390/cancers14082010
8. Kendal WS. Dying with cancer: the influence of age, comorbidity, and cancer site. Cancer. (2008) 112:1354–62. doi: 10.1002/cncr.23315
9. Zaorsky NG, Churilla TM, Egleston BL, Fisher SG, Ridge JA, Horwitz EM, et al. Causes of death among cancer patients. Ann Oncol. (2017) 28:400–7. doi: 10.1093/annonc/mdw604
10. Batra A, Sheka D, Kong S, Cheung WY. Impact of pre-existing cardiovascular disease on treatment patterns and survival outcomes in patients with lung cancer. BMC Cancer. (2020) 20:1004. doi: 10.1186/s12885-020-07487-9
11. Farmakis D, Filippatos G. Arrhythmias in cancer: rhythm is gonna get you! Eur J Heart Fail. (2021) 23:154–6. doi: 10.1002/ejhf.2079
12. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. (2019) 139:e56–e528. doi: 10.1161/CIR.0000000000000659
13. Chu G, Versteeg HH, Verschoor AJ, Trines SA, Hemels MEW, Ay C, et al. Atrial fibrillation and cancer - An unexplored field in cardiovascular oncology. Blood Rev. (2019) 35:59–67. doi: 10.1016/j.blre.2019.03.005
14. Kattelus H, Kesäniemi YA, Huikuri H, Ukkola O. Cancer increases the risk of atrial fibrillation during long-term follow-up (OPERA study). PLoS ONE. (2018) 13:e0205454. doi: 10.1371/journal.pone.0205454
15. Ostenfeld EB, Erichsen R, Pedersen L, Farkas DK, Weiss NS, Sørensen HT. Atrial fibrillation as a marker of occult cancer. PLoS ONE. (2014) 9:e102861. doi: 10.1371/journal.pone.0102861
16. Saliba W, Rennert HS, Gronich N, Gruber SB, Rennert G. Association of atrial fibrillation and cancer: Analysis from two large population-based case-control studies. PLoS ONE. (2018) 13:e0190324. doi: 10.1371/journal.pone.0190324
17. Yun JP, Choi EK, Han KD, Park SH, Jung JH, Park SH, et al. Risk of atrial fibrillation according to cancer type: a nationwide population-based study. JACC CardioOncol. (2021) 3:221–32. doi: 10.1016/j.jaccao.2021.03.006
18. Conen D, Wong JA, Sandhu RK, Cook NR, Lee IM, Buring JE, et al. Risk of malignant cancer among women with new-onset atrial fibrillation. JAMA Cardiol. (2016) 1:389–96. doi: 10.1001/jamacardio.2016.0280
19. Jakobsen CB, Lamberts M, Carlson N, Lock-Hansen M, Torp-Pedersen C, Gislason GH, et al. Incidence of atrial fibrillation in different major cancer subtypes: a Nationwide population-based 12 year follow up study. BMC Cancer. (2019) 19:1105. doi: 10.1186/s12885-019-6314-9
20. Matetic A, Mohamed M, Miller RJH, Kolman L, Lopez-Mattei J, Cheung WY, et al. Impact of cancer diagnosis on causes and outcomes of 59 million US patients with cardiovascular admissions. Int J Cardiol. (2021) 341:76–83. doi: 10.1016/j.ijcard.2021.07.054
21. Farmakis D, Parissis J, Filippatos G. Insights into onco-cardiology: atrial fibrillation in cancer. J Am Coll Cardiol. (2014) 63:945–53. doi: 10.1016/j.jacc.2013.11.026
22. Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. (2014) 114:1500–15. doi: 10.1161/CIRCRESAHA.114.303772
23. Grymonprez M, Vakaet V, Kavousi M, Stricker BH, Ikram MA, Heeringa J, et al. Chronic obstructive pulmonary disease and the development of atrial fibrillation. Int J Cardiol. (2019) 276:118–24. doi: 10.1016/j.ijcard.2018.09.056
24. Wanamaker B, Cascino T, McLaughlin V, Oral H, Latchamsetty R, Siontis KC. Atrial Arrhythmias in Pulmonary Hypertension: Pathogenesis, Prognosis and Management. Arrhythm Electrophysiol Rev. (2018) 7:43–8. doi: 10.15420/aer.2018.3.2
25. Bandyopadhyay D, Ball S, Hajra A, Chakraborty S, Dey AK, Ghosh RK, et al. Impact of atrial fibrillation in patients with lung cancer: Insights from National Inpatient Sample. Int J Cardiol Heart Vasc. (2019) 22:216–7. doi: 10.1016/j.ijcha.2019.02.012
26. Yang X, Li X, Yuan M, Tian C, Yang Y, Wang X, et al. Anticancer therapy-induced atrial fibrillation: electrophysiology and related mechanisms. Front Pharmacol. (2018) 9:1058. doi: 10.3389/fphar.2018.01058
27. Imperatori A, Mariscalco G, Riganti G, Rotolo N, Conti V, Dominioni L. Atrial fibrillation after pulmonary lobectomy for lung cancer affects long-term survival in a prospective single-center study. J Cardiothorac Surg. (2012) 7:4. doi: 10.1186/1749-8090-7-4
28. Anker MS, von Haehling S, Coats AJS, Riess H, Eucker J, Porthun J, et al. Ventricular tachycardia, premature ventricular contractions, and mortality in unselected patients with lung, colon, or pancreatic cancer: a prospective study. Eur J Heart Fail. (2021) 23:145–53. doi: 10.1002/ejhf.2059
29. Albrecht A, Porthun J, Eucker J, Coats AJS, von Haehling S, Pezzutto A, et al. Spontaneous non-sustained ventricular tachycardia and premature ventricular contractions and their prognostic relevance in patients with cancer in routine care. Cancers. (2021) 13:2303. doi: 10.3390/cancers13102303
30. Lonn EM, Rambihar S, Gao P, Custodis FF, Sliwa K, Teo KK, et al. Heart rate is associated with increased risk of major cardiovascular events, cardiovascular and all-cause death in patients with stable chronic cardiovascular disease: an analysis of ONTARGET/TRANSCEND. Clin Res Cardiol. (2014) 103:149–59. doi: 10.1007/s00392-013-0644-4
31. Anker MS, Ebner N, Hildebrandt B, Springer J, Sinn M, Riess H, et al. Resting heart rate is an independent predictor of death in patients with colorectal, pancreatic, and non-small cell lung cancer: results of a prospective cardiovascular long-term study. Eur J Heart Fail. (2016) 18:1524–34. doi: 10.1002/ejhf.670
32. Hemu M, Chiang CJ, Bhatt PK, Ahmed A, Hein KZ, Mourad T, et al. Associations between sinus tachycardia and adverse cardiovascular outcomes and mortality in cancer patients. J Thorac Dis. (2021) 13:4845–52. doi: 10.21037/jtd-21-779
33. Ou SH, Tong WP, Azada M, Siwak-Tapp C, Dy J, Stiber JA. Heart rate decrease during crizotinib treatment and potential correlation to clinical response. Cancer. (2013) 119:1969–75. doi: 10.1002/cncr.28040
34. Ou SH, Azada M, Dy J, Stiber JA. Asymptomatic profound sinus bradycardia (heart rate ≤ 45) in non-small cell lung cancer patients treated with crizotinib. J Thorac Oncol. (2011) 6:2135–7. doi: 10.1097/JTO.0b013e3182307e06
35. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76:2982–3021.
36. Beaney T, Schutte AE, Stergiou GS, Borghi C, Burger D, Charchar F, et al. May measurement month 2019: the global blood pressure screening campaign of the international society of hypertension. Hypertension. (2020) 76:333–41. doi: 10.1161/HYPERTENSIONAHA.120.14874
37. Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. (2004) 291:2441–7. doi: 10.1001/jama.291.20.2441
38. Herrero Rivera D, Nieto-Guerrero Gómez JM, Cacicedo Fernández de Bobadilla J, Delgado D, Rivin Del Campo E, Praena-Fernández JM, et al. Cardiovascular disease and survival in non-small cell lung cancer: a multicenter prospective assessment. Clin Transl Oncol. (2019) 21:1220–30. doi: 10.1007/s12094-019-02047-5
39. Zaborowska-Szmit M, Kowalski DM, Piórek A, Krzakowski M, Szmit S. A decrease in D-dimer concentration and an occurrence of skin rash as iatrogenic events and complementary predictors of survival in lung cancer patients treated with EGFR tyrosine kinase inhibitors. Pharmacol Rep. (2016) 68:1140–8. doi: 10.1016/j.pharep.2016.07.003
40. Meinardi MT, Gietema JA, van der Graaf WT, van Veldhuisen DJ, Runne MA, Sluiter WJ, et al. Cardiovascular morbidity in long-term survivors of metastatic testicular cancer. J Clin Oncol. (2000) 18:1725–32. doi: 10.1200/JCO.2000.18.8.1725
41. Sagstuen H, Aass N, Fosså SD, Dahl O, Klepp O, Wist EA, et al. Blood pressure and body mass index in long-term survivors of testicular cancer. J Clin Oncol. (2005) 23:4980–90. doi: 10.1200/JCO.2005.06.882
42. Soultati A, Mountzios G, Avgerinou C, Papaxoinis G, Pectasides D, Dimopoulos MA, et al. Endothelial vascular toxicity from chemotherapeutic agents: preclinical evidence and clinical implications. Cancer Treat Rev. (2012) 38:473–83. doi: 10.1016/j.ctrv.2011.09.002
43. Yan LZ, Dressler EV, Adams VR. Association of hypertension and treatment outcomes in advanced stage non-small cell lung cancer patients treated with bevacizumab or non-bevacizumab containing regimens. J Oncol Pharm Pract. (2018) 24:209–17. doi: 10.1177/1078155217690921
44. Grossman A, Messerli FH, Grossman E. Drug induced hypertension–An unappreciated cause of secondary hypertension. Eur J Pharmacol. (2015) 763:15–22. doi: 10.1016/j.ejphar.2015.06.027
45. Rachow T, Schiffl H, Lang SM. Risk of lung cancer and renin-angiotensin blockade: a concise review. J Cancer Res Clin Oncol. (2021) 147:195–204. doi: 10.1007/s00432-020-03445-x
46. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. (2020) 41:407–77. doi: 10.1093/eurheartj/ehz425
47. Kravchenko J, Berry M, Arbeev K, Lyerly HK, Yashin A, Akushevich I. Cardiovascular comorbidities and survival of lung cancer patients: Medicare data based analysis. Lung Cancer. (2015) 88:85–93. doi: 10.1016/j.lungcan.2015.01.006
48. Alberg AJ, Samet JM. Epidemiology of lung cancer. Chest. (2003) 123:21S−49S. doi: 10.1378/chest.123.1_suppl.21S
49. Shields M, Carroll MD, Ogden CL. Adult obesity prevalence in Canada and the United States. NCHS Data Brief. (2011) 56:1–8.
50. Barrera G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. (2012) 2012:137289. doi: 10.5402/2012/137289
51. Kamp DW, Shacter E, Weitzman SA. Chronic inflammation and cancer: the role of the mitochondria. Oncology. (2011) 25:400–10.
52. Arcadi T, Maffei E, Sverzellati N, Mantini C, Guaricci AI, Tedeschi C, et al. Coronary artery calcium score on low-dose computed tomography for lung cancer screening. World J Radiol. (2014) 6:381–7. doi: 10.4329/wjr.v6.i6.381
53. Mendoza DP, Kako B, Digumarthy SR, Shepard JO, Little BP. Impact of significant coronary artery calcification reported on low-dose computed tomography lung cancer screening. J Thorac Imaging. (2020) 35:129–35. doi: 10.1097/RTI.0000000000000458
54. Sun M, Yang Q, Li M, Jing J, Zhou H, Chen Y, et al. Association between the severity of coronary artery disease and lung cancer: a pilot cross-sectional study. Arq Bras Cardiol. (2022) 118:478–85. doi: 10.36660/abc.20200478
55. Licker M, de Perrot M, Höhn L, Tschopp JM, Robert J, Frey JG, et al. Perioperative mortality and major cardio-pulmonary complications after lung surgery for non-small cell carcinoma. Eur J Cardiothorac Surg. (1999) 15:314–9. doi: 10.1016/S1010-7940(99)00006-8
56. Ambrogi V, Pompeo E, Elia S, Pistolese GR, Mineo TC. The impact of cardiovascular comorbidity on the outcome of surgery for stage I and II non-small-cell lung cancer. Eur J Cardiothorac Surg. (2003) 23:811–7. doi: 10.1016/S1010-7940(03)00093-9
57. Mishra PK, Pandey R, Shackcloth MJ, McShane J, Grayson AD, Carr MH, et al. Cardiac comorbidity is not a risk factor for mortality and morbidity following surgery for primary non-small cell lung cancer. Eur J Cardiothorac Surg. (2009) 35:439–43. doi: 10.1016/j.ejcts.2008.10.029
58. Takenaka T, Katsura M, Shikada Y, Tsukamoto S, Takeo S. The impact of cardiovascular comorbidities on the outcome of surgery for non-small-cell lung cancer. Interact Cardiovasc Thorac Surg. (2013) 16:270–4. doi: 10.1093/icvts/ivs489
59. Chen HY, Saczynski JS, McManus DD, Lessard D, Yarzebski J, Lapane KL, et al. The impact of cardiac and noncardiac comorbidities on the short-term outcomes of patients hospitalized with acute myocardial infarction: a population-based perspective. Clin Epidemiol. (2013) 5:439–48. doi: 10.2147/CLEP.S49485
60. Guha A, Dey AK, Jneid H, Addison D. Acute coronary syndromes in cancer patients. Eur Heart J. (2019) 40:1487–90. doi: 10.1093/eurheartj/ehz267
61. Banasiak W, Zymliński R, Undas A. Optimal management of cancer patients with acute coronary syndrome. Pol Arch Intern Med. (2018) 128:244–53. doi: 10.20452/pamw.4254
62. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. (2016) 37:2768–801. doi: 10.1093/eurheartj/ehw211
63. Landes U, Kornowski R, Bental T, Assali A, Vaknin-Assa H, Lev E, et al. Long-term outcomes after percutaneous coronary interventions in cancer survivors. Coron Artery Dis. (2017) 28:5–10. doi: 10.1097/MCA.0000000000000429
64. Rohrmann S, Witassek F, Erne P, Rickli H, Radovanovic D. Treatment of patients with myocardial infarction depends on history of cancer. Eur Heart J Acute Cardiovasc Care. (2018) 7:639–45. doi: 10.1177/2048872617729636
65. Potts JE, Iliescu CA, Lopez Mattei JC, Martinez SC, Holmvang L, Ludman P, et al. Percutaneous coronary intervention in cancer patients: a report of the prevalence and outcomes in the United States. Eur Heart J. (2019) 40:1790–800. doi: 10.1093/eurheartj/ehy769
66. Grilz E, Königsbrügge O, Posch F, Schmidinger M, Pirker R, Lang IM, et al. Frequency, risk factors, and impact on mortality of arterial thromboembolism in patients with cancer. Haematologica. (2018) 103:1549–56. doi: 10.3324/haematol.2018.192419
67. Bharadwaj A, Potts J, Mohamed MO, Parwani P, Swamy P, Lopez-Mattei JC, et al. Acute myocardial infarction treatments and outcomes in 65 million patients with a current or historical diagnosis of cancer in the USA. Eur Heart J. (2020) 41:2183–93. doi: 10.1093/eurheartj/ehz851
68. Lange SA, Feld J, Kühnemund L, Köppe J, Makowski L, Engelbertz CM, et al. Acute and long-term outcomes of ST-elevation myocardial infarction in cancer patients, a 'real world' analysis with 175,000 patients. Cancers. (2021) 13:6203. doi: 10.3390/cancers13246203
69. Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail. (2014) 1:4–25. doi: 10.1002/ehf2.12005
70. Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. (2011) 8:30–41. doi: 10.1038/nrcardio.2010.165
71. Leedy DJ, Reding KW, Vasbinder AL, Anderson GL, Barac A, Wactawski-Wende J, et al. The association between heart failure and incident cancer in women: an analysis of the Women's Health Initiative. Eur J Heart Fail. (2021) 23:1712–21. doi: 10.1002/ejhf.2207
72. Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. Impact of comorbidity on lung cancer survival. Int J Cancer. (2003) 103:792–802. doi: 10.1002/ijc.10882
73. Dominguez-Ventura A, Allen MS, Cassivi SD, Nichols FC, Deschamps C, Pairolero PC. Lung cancer in octogenarians: factors affecting morbidity and mortality after pulmonary resection. Ann Thorac Surg. (2006) 82:1175–9. doi: 10.1016/j.athoracsur.2006.04.052
74. Anker MS, von Haehling S, Landmesser U, Coats AJS, Anker SD. Cancer and heart failure-more than meets the eye: common risk factors and co-morbidities. Eur J Heart Fail. (2018) 20:1382–4. doi: 10.1002/ejhf.1252
75. Jain P, Gutierrez Bugarin J, Guha A, Jain C, Patil N, Shen T, et al. Cardiovascular adverse events are associated with usage of immune checkpoint inhibitors in real-world clinical data across the United States. ESMO Open. (2021) 6:100252. doi: 10.1016/j.esmoop.2021.100252
76. Batra A, Patel B, Addison D, Baldassarre LA, Desai N, Weintraub N, et al. Cardiovascular safety profile of taxanes and vinca alkaloids: 30 years FDA registry experience. Open Heart. (2021) 8:e001849. doi: 10.1136/openhrt-2021-001849
77. Ram P, Tiu A, Lo KB, Parikh K, Shah M. Trends in the prevalence of malignancy among patients admitted with acute heart failure and associated outcomes: a nationwide population-based study. Heart Fail Rev. (2019) 24:989–95. doi: 10.1007/s10741-019-09808-y
78. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. (2014) 15:1063–93. doi: 10.1093/ehjci/jeu192
79. Hering D, Faber L, Horstkotte D. Echocardiographic features of radiation-associated valvular disease. Am J Cardiol. (2003) 92:226–30. doi: 10.1016/S0002-9149(03)00546-0
80. Iung B, Delgado V, Rosenhek R, Price S, Prendergast B, Wendler O, et al. Contemporary Presentation and Management of Valvular Heart Disease: The EURObservational Research Programme Valvular Heart Disease II Survey. Circulation. (2019) 140:1156–69. doi: 10.1161/CIRCULATIONAHA.119.041080
81. Chan J, Rosenfeldt F, Chaudhuri K, Marasco S. Cardiac surgery in patients with a history of malignancy: increased complication rate but similar mortality. Heart Lung Circ. (2012) 21:255–9. doi: 10.1016/j.hlc.2012.02.004
82. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. (2022) 43:561–632. doi: 10.1093/ejcts/ezac209
83. Landes U, Iakobishvili Z, Vronsky D, Zusman O, Barsheshet A, Jaffe R, et al. Transcatheter Aortic Valve Replacement in Oncology Patients With Severe Aortic Stenosis. JACC Cardiovasc Interv. (2019) 12:78–86. doi: 10.1016/j.jcin.2018.10.026
84. Watanabe Y, Kozuma K, Hioki H, Kawashima H, Nara Y, Kataoka A, et al. Comparison of results of transcatheter aortic valve implantation in patients with versus without active cancer. Am J Cardiol. (2016) 118:572–7. doi: 10.1016/j.amjcard.2016.05.052
85. Berkovitch A, Guetta V, Barbash IM, Fink N, Regev E, Maor E, et al. Favorable Short-Term and Long-Term Outcomes Among Patients With Prior History of Malignancy Undergoing Transcatheter Aortic Valve Implantation. J Invasive Cardiol. (2018) 30:105–9.
86. Mangner N, Woitek FJ, Haussig S, Holzhey D, Stachel G, Schlotter F, et al. Impact of active cancer disease on the outcome of patients undergoing transcatheter aortic valve replacement. J Interv Cardiol. (2018) 31:188–96. doi: 10.1111/joic.12458
87. Ming S, Gang L, Wei S. Thoracoscopic Lung Cancer Resection with Simultaneous Heart Valve Procedure. Heart Surg Forum. (2021) 24:E628–30. doi: 10.1532/hsf.3937
88. Hull MC, Morris CG, Pepine CJ, Mendenhall NP. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of hodgkin lymphoma treated with radiation therapy. JAMA. (2003) 290:2831–7. doi: 10.1001/jama.290.21.2831
89. Malanca M, Cimadevilla C, Brochet E, Iung B, Vahanian A, Messika-Zeitoun D. Radiotherapy-induced mitral stenosis: a three-dimensional perspective. J Am Soc Echocardiogr. (2010) 23:108.e1–2. doi: 10.1016/j.echo.2009.08.006
90. Glanzmann C, Huguenin P, Lütolf UM, Maire R, Jenni R, Gumppenberg V. Cardiac lesions after mediastinal irradiation for Hodgkin's disease. Radiother Oncol. (1994) 30:43–54. doi: 10.1016/0167-8140(94)90008-6
91. Kim K, Kim D, Lee SE, Cho IJ, Shim CY, Hong GR, et al. Infective endocarditis in cancer patients - causative organisms, predisposing procedures, and prognosis differ from infective endocarditis in non-cancer patients. Circ J. (2019) 83:452–460. doi: 10.1253/circj.CJ-18-0609
92. Cosyns B, Roosens B, Lancellotti P, Laroche C, Dulgheru R, Scheggi V, et al. Cancer and infective endocarditis: characteristics and prognostic impact. Front Cardiovasc Med. (2021) 8:766996. doi: 10.3389/fcvm.2021.766996
93. San Román JA, López J, Vilacosta I, Luaces M, Sarriá C, Revilla A, et al. Prognostic stratification of patients with left-sided endocarditis determined at admission. Am J Med. (2007) 120:369.e1–7. doi: 10.1016/j.amjmed.2006.05.071
94. Habib G, Badano L, Tribouilloy C, Vilacosta I, Zamorano JL, Galderisi M, et al. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr. (2010) 11:202–19. doi: 10.1093/ejechocard/jeq004
95. Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. (2016) 41:3–14. doi: 10.1007/s11239-015-1311-6
96. Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ Res. (2016) 118:1340–7. doi: 10.1161/CIRCRESAHA.115.306841
97. Raskob GE, Angchaisuksiri P, Blanco AN, Buller H, Gallus A, Hunt BJ, et al. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol. (2014) 34:2363–71. doi: 10.1161/ATVBAHA.114.304488
98. Keller K, Hobohm L, Ebner M, Kresoja KP, Münzel T, Konstantinides SV, et al. Trends in thrombolytic treatment and outcomes of acute pulmonary embolism in Germany. Eur Heart J. (2020) 41:522–9. doi: 10.1093/eurheartj/ehz236
99. Barsoum MK, Heit JA, Ashrani AA, Leibson CL, Petterson TM, Bailey KR. Is progestin an independent risk factor for incident venous thromboembolism? A population-based case-control study. Thromb Res. (2010) 126:373–8. doi: 10.1016/j.thromres.2010.08.010
100. Shinagare AB, Guo M, Hatabu H, Krajewski KM, Andriole K, Van den Abbeele AD, et al. Incidence of pulmonary embolism in oncologic outpatients at a tertiary cancer center. Cancer. (2011) 117:3860–6. doi: 10.1002/cncr.25941
101. Chuang YM Yu CJ. Clinical characteristics and outcomes of lung cancer with pulmonary embolism. Oncology. (2009) 77:100–6. doi: 10.1159/000229503
102. van Herk-Sukel MP, Shantakumar S, Penning-van Beest FJ, Kamphuisen PW, Majoor CJ, Overbeek LI, et al. Pulmonary embolism, myocardial infarction, and ischemic stroke in lung cancer patients: results from a longitudinal study. Lung. (2013) 191:501–9. doi: 10.1007/s00408-013-9485-1
103. Mulder FI, Horváth-Puhó E, van Es N, van Laarhoven HWM, Pedersen L, Moik F, et al. Venous thromboembolism in cancer patients: a population-based cohort study. Blood. (2021) 137:1959–69. doi: 10.1182/blood.2020007338
104. Cui YQ, Tan XM, Liu B, Zheng Y, Zhang LY, Chen ZA, et al. Analysis on risk factors of lung cancer complicated with pulmonary embolism. Clin Respir J. (2021) 15:65–73. doi: 10.1111/crj.13270
105. Ahlbrecht J, Dickmann B, Ay C, Dunkler D, Thaler J, Schmidinger M, et al. Tumor grade is associated with venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol. (2012) 30:3870–5. doi: 10.1200/JCO.2011.40.1810
106. Dentali F, Ageno W, Becattini C, Galli L, Gianni M, Riva N, et al. Prevalence and clinical history of incidental, asymptomatic pulmonary embolism: a meta-analysis. Thromb Res. (2010) 125:518–22. doi: 10.1016/j.thromres.2010.03.016
107. Li Y, Shang Y, Wang W, Ning S, Chen H. Lung cancer and pulmonary embolism: what is the relationship? A review. J Cancer. (2018) 9:3046–57. doi: 10.7150/jca.26008
108. Giustozzi M, Connors JM, Ruperez Blanco AB, Szmit S, Falvo N, Cohen AT, et al. Clinical characteristics and outcomes of incidental venous thromboembolism in cancer patients: Insights from the Caravaggio study. J Thromb Haemost. (2021) 19:2751–9. doi: 10.1111/jth.15461
109. Canonico ME, Santoro C, Avvedimento M, Giugliano G, Mandoli GE, Prastaro M, et al. Venous thromboembolism and cancer: a comprehensive review from pathophysiology to novel treatment. Biomolecules. (2022) 12:259. doi: 10.3390/biom12020259
110. Nichols L, Saunders R, Knollmann FD. Causes of death of patients with lung cancer. Arch Pathol Lab Med. (2012) 136:1552–7. doi: 10.5858/arpa.2011-0521-OA
111. Ma L, Wen Z. Risk factors and prognosis of pulmonary embolism in patients with lung cancer. Medicine. (2017) 96:e6638. doi: 10.1097/MD.0000000000006638
112. Junjun L, Pei W, Ying Y, Kui S. Prognosis and risk factors in older patients with lung cancer and pulmonary embolism: a propensity score matching analysis. Sci Rep. (2020) 10:1272. doi: 10.1038/s41598-020-58345-4
113. Malgor RD, Bilfinger TV, Labropoulos N. A systematic review of pulmonary embolism in patients with lung cancer. Ann Thorac Surg. (2012) 94:311–6. doi: 10.1016/j.athoracsur.2012.03.025
114. Shinagare AB, Okajima Y, Oxnard GR, Dipiro PJ, Johnson BE, Hatabu H, et al. Unsuspected pulmonary embolism in lung cancer patients: comparison of clinical characteristics and outcome with suspected pulmonary embolism. Lung Cancer. (2012) 78:161–6. doi: 10.1016/j.lungcan.2012.08.007
115. Sun JM, Kim TS, Lee J, Park YH, Ahn JS, Kim H, et al. Unsuspected pulmonary emboli in lung cancer patients: the impact on survival and the significance of anticoagulation therapy. Lung Cancer. (2010) 69:330–6. doi: 10.1016/j.lungcan.2009.11.015
116. Niksic M, Redondo-Sanchez D, Chang YL, Rodriguez-Barranco M, Exposito-Hernandez J, Marcos-Gragera R, et al. The role of multimorbidity in short-term mortality of lung cancer patients in Spain: a population-based cohort study. BMC Cancer. (2021) 21:1048. doi: 10.1186/s12885-021-08801-9
117. Janssen-Heijnen ML, Schipper RM, Razenberg PP, Crommelin MA, Coebergh JW. Prevalence of co-morbidity in lung cancer patients and its relationship with treatment: a population-based study. Lung Cancer. (1998) 21:105–13. doi: 10.1016/S0169-5002(98)00039-7
118. Coebergh JW, Janssen-Heijnen ML, Post PN, Razenberg PP. Serious co-morbidity among unselected cancer patients newly diagnosed in the southeastern part of The Netherlands in 1993-1996. J Clin Epidemiol. (1999) 52:1131–6. doi: 10.1016/S0895-4356(99)00098-0
119. Søgaard M, Thomsen RW, Bossen KS, Sørensen HT, Nørgaard M. The impact of comorbidity on cancer survival: a review. Clin Epidemiol. (2013) 5:3–29. doi: 10.2147/CLEP.S47150
120. Islam KM, Jiang X, Anggondowati T, Lin G, Ganti AK. Comorbidity and survival in lung cancer patients. Cancer Epidemiol Biomarkers Prev. (2015) 24:1079–85. doi: 10.1158/1055-9965.EPI-15-0036
121. Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. (2017) 70:926–38. doi: 10.1016/j.jacc.2017.06.047
122. Giustozzi M, Curcio A, Weijs B, Field TS, Sudikas S, Katholing A, et al. Variation in the association between antineoplastic therapies and venous thromboembolism in patients with active cancer. Thromb Haemost. (2020) 120:847–56. doi: 10.1055/s-0040-1709527
123. Herrmann J. Vascular toxic effects of cancer therapies. Nat Rev Cardiol. (2020) 17:503–22. doi: 10.1038/s41569-020-0347-2
124. Zaborowska-Szmit M, Krzakowski M, Kowalski DM, Szmit S. Cardiovascular complications of systemic therapy in non-small-cell lung cancer. J Clin Med. (2020) 9:1268. doi: 10.3390/jcm9051268
125. Mitchell JD, Cehic DA, Morgia M, Bergom C, Toohey J, Guerrero PA, et al. Cardiovascular manifestations from therapeutic radiation: a multidisciplinary expert consensus statement from the international cardio-oncology society. JACC CardioOncol. (2021) 3:360–80. doi: 10.1016/j.jaccao.2021.06.003
126. Steingart RM, Yadav N, Manrique C, Carver JR, Liu J. Cancer survivorship: cardiotoxic therapy in the adult cancer patient; cardiac outcomes with recommendations for patient management. Semin Oncol. (2013) 40:690–708. doi: 10.1053/j.seminoncol.2013.09.010
127. Florido R, Daya NR, Ndumele CE, Koton S, Russell SD, Prizment A, et al. Cardiovascular disease risk among cancer survivors: the atherosclerosis risk in communities (ARIC) study. J Am Coll Cardiol. (2022) 80:22–32. doi: 10.1016/j.jacc.2022.04.042
128. Al-Kindi SG, Oliveira GH. Prevalence of preexisting cardiovascular disease in patients with different types of cancer: the unmet need for onco-cardiology. Mayo Clin Proc. (2016) 91:81–3. doi: 10.1016/j.mayocp.2015.09.009
Keywords: lung cancer, cardio-oncology, survival, prognosis, heart failure, thromboembolism
Citation: Mędrek S and Szmit S (2022) Are cardiovascular comorbidities always associated with a worse prognosis in patients with lung cancer? Front. Cardiovasc. Med. 9:984951. doi: 10.3389/fcvm.2022.984951
Received: 02 July 2022; Accepted: 01 September 2022;
Published: 23 September 2022.
Edited by:
Rohit Moudgil, Cleveland Clinic, United StatesReviewed by:
Mario Enrico Canonico, University of Naples Federico II, ItalyAaron L. Sverdlov, University of Newcastle, Australia
Copyright © 2022 Mędrek and Szmit. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sabina Mędrek, c2FicEB3cC5wbA==
 Sabina Mędrek
Sabina Mędrek Sebastian Szmit
Sebastian Szmit